Figure 1. NGF induces a rapid bout of fission followed by maintenance of a new steady state of mitochondria length and density.
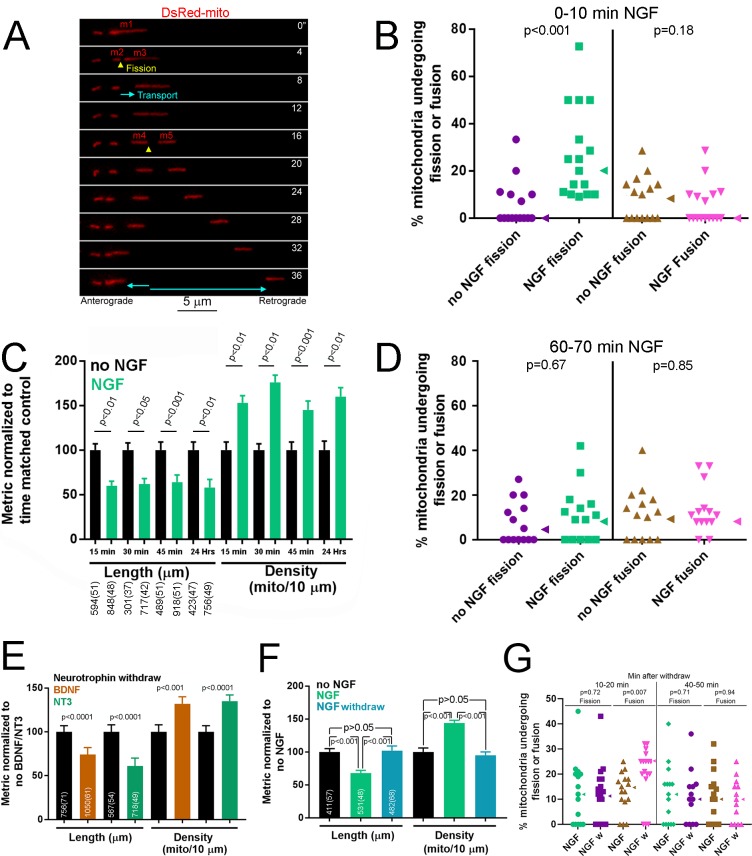
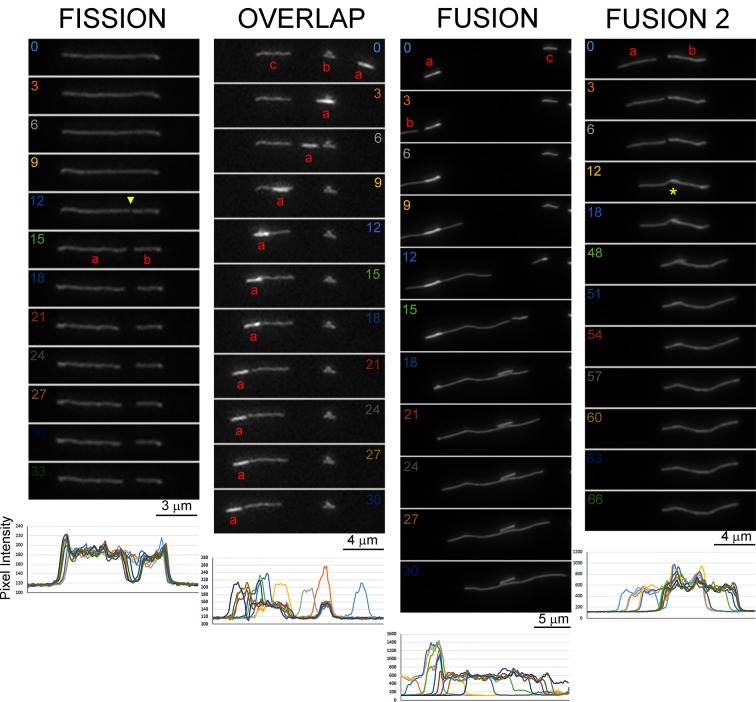
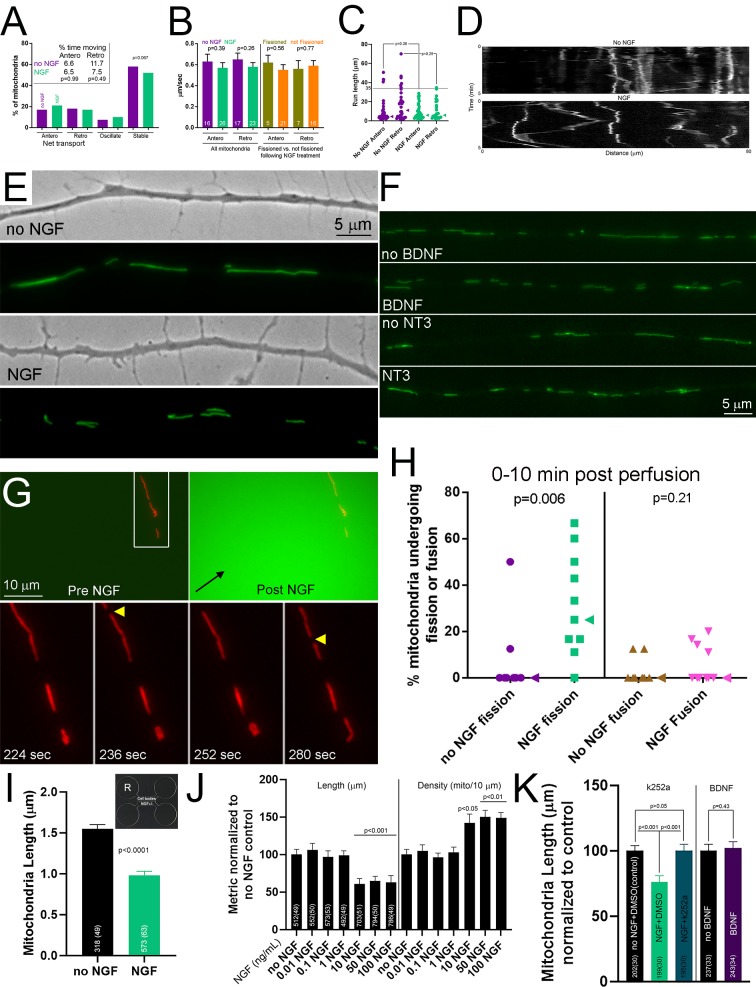
(A) Example of mitochondria labeled by expression of mitochondrially targeted DsRed (DsRed-mito) undergoing fission and subsequent transport during the first 5–10 min of NGF treatment (40 ng/mL throughout). By 4’ the mitochondrion labeled m1 undergoes fission giving rise to m2 and m3. The two emergent mitochondria move away from each other and at 16’ m3 fissions again to give rise to m4 and m5, and again both move away from each other. (B) Determination of the percentage of mitochondria that underwent fission or fusion during an initial 10 min treatment with NGF. Each data point reflects one axon (n = 95 and 100 mitochondria from 15 and 16 axons for no NGF and NGF groups respectively; Mann-Whitney test). Arrowheads to the right of data points denote the median. (C) Mitochondria length and densities in distal 50 μm of axons, excluding growth cones, after multiple durations of NGF treatment (sample sizes shown below bars using an x(y) format where x and y denote the number of mitochondria and axons respectively). Mean and SEM shown, Bonferroni posthoc tests. (D) Percentage of mitochondria that underwent fission or fusion during a 10 min period after a 1 hr treatment with NGF (n = 110 and 126 mitochondria from 14 axons/group for no NGF and NGF respectively; Mann-Whitney test). Arrowheads to the right of data points denote the median. (E) A 45 min treatment with either BDNF or NT3 (40 ng/mL for both) decreases mitochondria length and increases density (n shown in bars using the same format as panel C; Mean and SEM shown; Welch t-test for densities, Mann-Whitney test for length). (F) Removal of NGF with inclusion of a function blocking NGF antibody after a 30 min treatment (NGF withdraw) restores mitochondria length and density to no NGF treatment levels (no NGF) by 3.5 hr. In contrast, in the continued presence of NGF (NGF) mitochondria exhibit the same trends as expected based on the data in panel C. (n shown in bars using the same format as panel C; Bonferroni posthoc tests for density and Dunn’s posthoc tests for length). (G) Percentage of mitochondria that underwent fission or fusion during a 10 min period starting at 10 min or 40 min after NGF withdraw (NGF w) following an initial 30 min treatment with NGF. The time matched control groups (NGF) underwent similar medium exchanges as the NGF w group but the medium contained NGF. Mann-Whitney tests.