Abstract
Background
Frailty is characterized by increased vulnerability to adverse health outcomes. The prevalence of frailty across neurodegenerative disorders (NDD) is largely unknown. Symptoms of frailty and NDD overlap, calling into question a tautology in some frailty instruments. Our objectives were 1) to construct a Frailty Index (FI) independent of NDD symptoms, and 2) to estimate frailty prevalence in a broad NDD cohort using both the Frailty Phenotype (FP) and the constructed FI as measures.
Methods
Data from the Canadian COMPASS-ND cohort study were assessed for applicability to FI construction. Frailty status according to FI and FP criteria were ascertained for each participant.
Results
81 items were selected for the FI. In the cohort (150 participants; 46% women; mean age 73.6±7.0; 10 NDD subgroups), frailty was identified in 11% and 14% of participants according to the FI and FP, respectively. The difference between estimates was not significant. The FP classified most participants (84%) as pre-frail.
Conclusion
The presence of frailty elements, regardless of whether they are part of NDD, is likely to influence health status. Given the FP identified a large proportion of the cohort as pre-frail or frail, it is likely worthwhile to identify frailty in the context of NDD.
Keywords: frailty, neurodegeneration, frailty index, frailty phenotype, dementia
INTRODUCTION
Frailty, a clinically recognizable state, is characterized by declining reserve and function across multiple physiologic systems, resulting in a compromised ability to deal with acute stressors.(1) Numerous adverse outcomes are associated with frailty including increased vulnerability to falls, disability, hospital admission, institutionalization, and mortality. (2) Identifying frailty is important for addressing care needs and implementing strategies to mitigate or reverse frailty.(3)
The link between frailty and neurodegeneration, particularly cognitive impairment, has been repeatedly demonstrated. (4,5) Adding cognitive impairment to frailty models improves the predictive validity for a range of adverse outcomes; however, including cognitive impairment as a component of frailty has not been done consistently.(6) Compared to robust individuals, frail persons are eight times more likely to have some form of dementia.(5) Dementia commonly arises from neurodegenerative disorders (NDD; a group of motor and cognitive conditions in which progressive degeneration or death of nerve cells results in debilitating diseases).(7) While frailty may accelerate cognitive deterioration, addressing frailty might slow the onset of dementia and its progression.(8)
The literature suggests the prevalence of frailty in NDD has only been reported in Alzheimer’s disease (AD) and Parkinson’s disease (PD), using varied methodologies for determining frailty status. A systematic review of mild-to-moderate AD reported frailty prevalence estimates ranging from 11.1–50.0%.(9) The few frailty prevalence studies available for PD reported results ranging from 22.2–69.4% and did not distinguish between cognitive subtypes.(10,11) Overall, little is known about the prevalence of frailty across neurodegenerative disorders. Establishing these estimates is necessary for developing frailty prevention and treatment programs in NDD populations.
Determining the prevalence of frailty in NDD populations is complicated given that many characteristics of NDD, including clinical features, prediction of outcomes, and risk factors, overlap with frailty.(8) In frailty literature, two of the most popular approaches to assess frailty include: 1) a phenotype model referred to as the Frailty Phenotype (FP), and 2) the Frailty Index (FI), which captures accumulated health deficits.(12,13) The FP is used to stratify individuals into risk profiles (e.g., robust, prefrail, or frail) according to five clinical criteria: shrinking, weakness, exhaustion, slowness, and low activity.(12) The utility of the FP in patients with comorbidities has been questioned, given the overlap of pathological processes on aspects of the phenotype—an overlap which may artificially inflate frailty estimates. For this reason, individuals with Parkinson’s disease, cognitive impairment, stroke, and those taking antidepressants were excluded from the original study.(12,14) An alternative approach, the FI is a flexible instrument without set criteria that can be constructed from most aging-related databases; variables that overlap between pathological processes and frailty can be excluded to allow estimates of frailty that are independent of disease. As long as a minimum of 30–40 health deficits meeting FI criteria are present, prediction of adverse outcomes has been shown to be sufficiently accurate.(15) The thorough nature of the assessments made to calculate an FI dually serve to capture a sufficient number of variables to ensure frailty risk calculations are robust.(16) To the best of our knowledge, frailty prevalence has never before been estimated broadly in NDD using the FP and FI.
The objectives of this study were to 1) construct an FI independent of NDD features, and 2) to examine frailty in participants from the Comprehensive Assessment of Neurodegeneration and Dementia (COMPASS-ND) study using the FI and FP. We hypothesized that frailty prevalence estimates made by the FP, with its fixed content, would be higher than those made by an FI, customized as to be independent of NDD disease states.
METHODS
Overview
This study analyzed the first data release of the COMPASS-ND study (release date October 01, 2018; 150 participants from 12 Canadian sites). All available data were used in this analysis; future data releases are planned by the sponsor pending completion of data monitoring procedures. A detailed methodology has been published previously.(17) Briefly, the cross-sectional observational study collects clinical, neuropsychological, MRI imaging, biomarkers, and sociodemographic information from participants with subjective cognitive impairment, mild cognitive impairment, subcortical ischemic vascular mild cognitive impairment, AD, dementia of mixed etiology, frontotemporal dementia, PD, PD with mild cognitive impairment, PD with dementia, and Lewy body dementia. Local ethics approval was obtained from the research ethics committee of each participating centre.
Operational Definition: Frailty Phenotype
Minor adaptations were necessary to operationalize FP criteria for use with COMPASS-ND study data (Table 1). The shrinking criterion was met if participants reported unintentional weight loss of ≥10 lbs or 5% in the past year. Weakness was determined by grip strength performance. Participants performed three trials of grip strength using a handheld dynamometer with their dominant hand. Cut-off points set by Fried et al.(12) were used to determine whether the average of each participant’s three trials met frailty criteria. Exhaustion was measured via two self-report questions that were adapted from Fried et al.(12) related to constant fatigue. A response indicating that at least a moderate amount of time (3–4 days during the past week) was spent feeling like “everything… was an effort” or that the participant “could not get going” was scored as exhausted. Slowness criterion was met if the average usual comfortable walking speed of participants over three trials was recorded at less than 1 m/s as previously validated and implemented in frailty research.(18–20) The COMPASS-ND gait protocol has been published previously.(21) Activity was determined using an adapted version of the Physical Activity Scale for the Elderly.(22) Scores lower than 64 for men and 52 for women met frailty criteria.(20)
TABLE 1.
Frailty Phenotype—operationalized definition
| Frailty Characteristic | COMPASS-ND Study Operational Definitions | |
|---|---|---|
| Shrinking | “Has the participant experienced any unintentional weight loss (≥ 10 lbs. or 5% of body weight) in the past year?” If yes, then point for weight loss criterion given. | |
| Weakness | Grip strength assessed over three trials. Average of trials compared to cut-off points (stratified by gender and body mass index quartiles) determined by Fried et al.(12) If failure to meet cut-off, point for weakness criterion given. | |
| Exhaustion | Participants asked: 1) How often during the last week did you feel that “everything you did was an effort”? and 2) How often during the last week did you feel that “you could not get going?” If participants answer “a moderate amount of the time (3–4 days)” or “most of the time”, then point for exhaustion criteria given. | |
| Slowness | Walking speed assessed over three timed 6m walking trials. If average walking speed is assessed at less than 1 m/s, point for slowness criteria given. | |
| Low | Activity Point given if men score less than 64 and if women score less than 52 on the adapted Physical Activity Scale for the Elderly. | |
|
| ||
| Scoring | 0 points | Robust |
| 1–2 points | Pre-frail | |
| 3+ points | Frail | |
Per Fried et al.,(12) the following overall frailty scoring was applied: 1) Where no criteria were met, individuals were considered robust; 2) Where one or two criteria were met, individuals were considered pre-frail; 3) If three or more criteria were met, participants were classified as frail. Individuals with missing data for one or more FP criteria were excluded from the analysis if the missing data point(s) could potentially shift the participant between risk profiles.
Operational Definition: Frailty Index
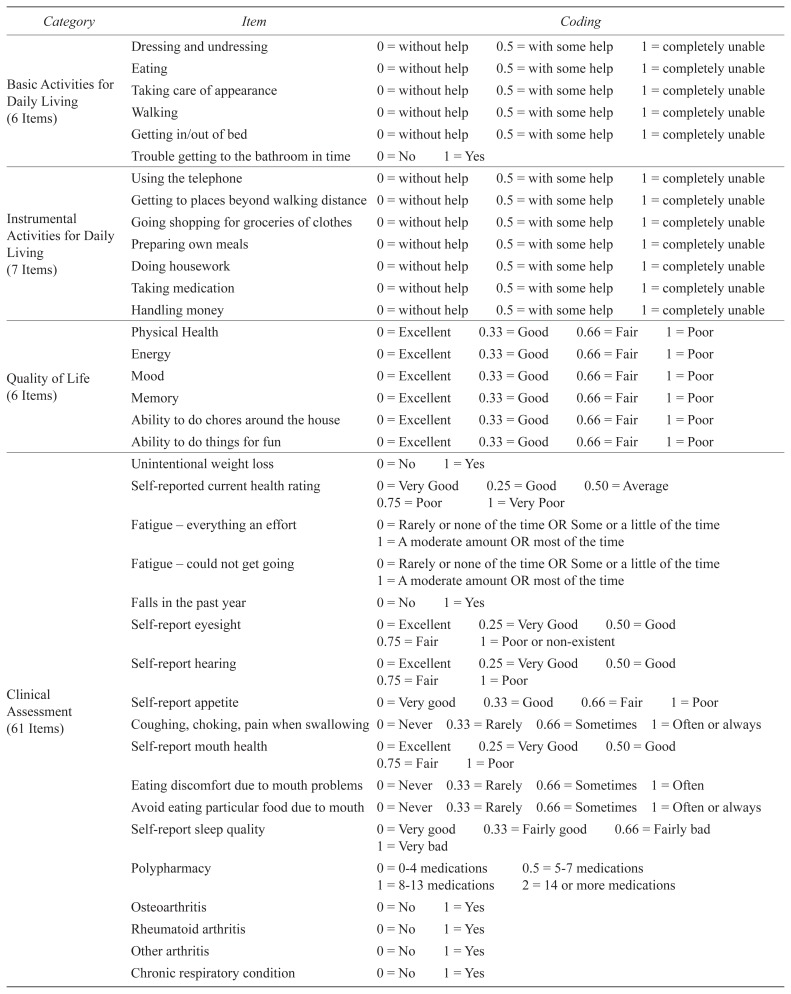
Eighty-one items were selected for the COMPASS-ND FI (Table 2), according to expert opinion and FI construction guidelines.(15) For the purpose of creating an unbiased index across NDD, items related to neurological disease were excluded (e.g., parkinsonism). Item coding was applied according to the convention of ‘0’ indicating deficit absence and ‘1’ indicating deficit presence. For ordinal and continuous variables, intermediate scores were determined by expert opinion and/or self-evident cut-points. The proportion of health deficits present in an individual out of the total number of age-related health variables considered was used to calculate an FI score. The FI was considered in two ways: 1) as a continuous variable per the intent of the original author, and 2) dichotomized in accordance with a previously established cut-off value, such that individuals with an FI score ≥ 0.25 were considered frail.(23)
TABLE 2.
Frailty Index—operationalized definition
| Category | Item | Coding | |||
|---|---|---|---|---|---|
| Basic Activities for Daily Living (6 Items) | Dressing and undressing | 0 = without help | 0.5 = with some help | 1 = completely unable | |
| Eating | 0 = without help | 0.5 = with some help | 1 = completely unable | ||
| Taking care of appearance | 0 = without help | 0.5 = with some help | 1 = completely unable | ||
| Walking | 0 = without help | 0.5 = with some help | 1 = completely unable | ||
| Getting in/out of bed | 0 = without help | 0.5 = with some help | 1 = completely unable | ||
| Trouble getting to the bathroom in time | 0 = No | 1 = Yes | |||
|
| |||||
| Instrumental Activities for Daily Living (7 Items) | Using the telephone | 0 = without help | 0.5 = with some help | 1 = completely unable | |
| Getting to places beyond walking distance | 0 = without help | 0.5 = with some help | 1 = completely unable | ||
| Going shopping for groceries of clothes | 0 = without help | 0.5 = with some help | 1 = completely unable | ||
| Preparing own meals | 0 = without help | 0.5 = with some help | 1 = completely unable | ||
| Doing housework | 0 = without help | 0.5 = with some help | 1 = completely unable | ||
| Taking medication | 0 = without help | 0.5 = with some help | 1 = completely unable | ||
| Handling money | 0 = without help | 0.5 = with some help | 1 = completely unable | ||
|
| |||||
| Quality of Life (6 Items) | Physical Health | 0 = Excellent | 0.33 = Good | 0.66 = Fair | 1 = Poor |
| Energy | 0 = Excellent | 0.33 = Good | 0.66 = Fair | 1 = Poor | |
| Mood | 0 = Excellent | 0.33 = Good | 0.66 = Fair | 1 = Poor | |
| Memory | 0 = Excellent | 0.33 = Good | 0.66 = Fair | 1 = Poor | |
| Ability to do chores around the house | 0 = Excellent | 0.33 = Good | 0.66 = Fair | 1 = Poor | |
| Ability to do things for fun | 0 = Excellent | 0.33 = Good | 0.66 = Fair | 1 = Poor | |
|
| |||||
| Clinical Assessment (61 Items) | Unintentional weight loss | 0 = No | 1 = Yes | ||
| Self-reported current health rating | 0 = Very Good | 0.25 = Good | 0.50 = Average | ||
| 0.75 = Poor | 1 = Very Poor | ||||
| Fatigue – everything an effort | 0 = Rarely or none of the time OR Some or a little of the time | ||||
| 1 = A moderate amount OR most of the time | |||||
| Fatigue – could not get going | 0 = Rarely or none of the time OR Some or a little of the time | ||||
| 1 = A moderate amount OR most of the time | |||||
| Falls in the past year | 0 = No | 1 = Yes | |||
| Self-report eyesight | 0 = Excellent | 0.25 = Very Good | 0.50 = Good | ||
| 0.75 = Fair | 1 = Poor or non-existent | ||||
| Self-report hearing | 0 = Excellent | 0.25 = Very Good | 0.50 = Good | ||
| 0.75 = Fair | 1 = Poor | ||||
| Self-report appetite | 0 = Very good | 0.33 = Good | 0.66 = Fair | 1 = Poor | |
| Coughing, choking, pain when swallowing | 0 = Never | 0.33 = Rarely | 0.66 = Sometimes | 1 = Often or always | |
| Self-report mouth health | 0 = Excellent | 0.25 = Very Good | 0.50 = Good | ||
| 0.75 = Fair | 1 = Poor | ||||
| Eating discomfort due to mouth problems | 0 = Never | 0.33 = Rarely | 0.66 = Sometimes | 1 = Often | |
| Avoid eating particular food due to mouth | 0 = Never | 0.33 = Rarely | 0.66 = Sometimes | 1 = Often or always | |
| Self-report sleep quality | 0 = Very good | 0.33 = Fairly good | 0.66 = Fairly bad | ||
| 1 = Very bad | |||||
| Polypharmacy | 0 = 0–4 medications | 0.5 = 5–7 medications | |||
| 1 = 8–13 medications | 2 = 14 or more medications | ||||
| Osteoarthritis | 0 = No | 1 = Yes | |||
| Rheumatoid arthritis | 0 = No | 1 = Yes | |||
| Other arthritis | 0 = No | 1 = Yes | |||
| Chronic respiratory condition | 0 = No | 1 = Yes | |||
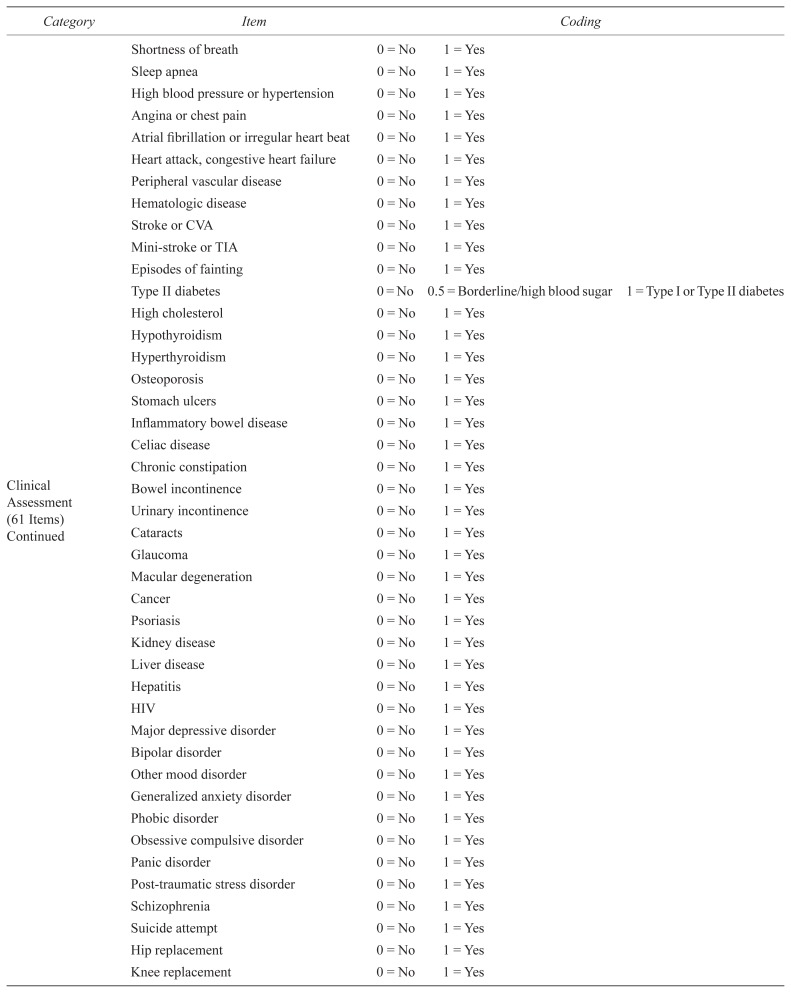
| Shortness of breath | 0 = No | 1 = Yes | |||
| Sleep apnea | 0 = No | 1 = Yes | |||
| High blood pressure or hypertension | 0 = No | 1 = Yes | |||
| Angina or chest pain | 0 = No | 1 = Yes | |||
| Atrial fibrillation or irregular heart beat | 0 = No | 1 = Yes | |||
| Heart attack, congestive heart failure | 0 = No | 1 = Yes | |||
| Peripheral vascular disease | 0 = No | 1 = Yes | |||
| Hematologic disease | 0 = No | 1 = Yes | |||
| Stroke or CVA | 0 = No | 1 = Yes | |||
| Mini-stroke or TIA | 0 = No | 1 = Yes | |||
| Episodes of fainting | 0 = No | 1 = Yes | |||
| Type II diabetes | 0 = No | 0.5 = Borderline/high blood sugar | 1 = Type I or Type II diabetes | ||
| High cholesterol | 0 = No | 1 = Yes | |||
| Hypothyroidism | 0 = No | 1 = Yes | |||
| Hyperthyroidism | 0 = No | 1 = Yes | |||
| Osteoporosis | 0 = No | 1 = Yes | |||
| Stomach ulcers | 0 = No | 1 = Yes | |||
| Inflammatory bowel disease | 0 = No | 1 = Yes | |||
| Celiac disease | 0 = No | 1 = Yes | |||
| Chronic constipation | 0 = No | 1 = Yes | |||
| Bowel incontinence | 0 = No | 1 = Yes | |||
| Urinary incontinence | 0 = No | 1 = Yes | |||
| Cataracts | 0 = No | 1 = Yes | |||
| Glaucoma | 0 = No | 1 = Yes | |||
| Macular degeneration | 0 = No | 1 = Yes | |||
| Cancer | 0 = No | 1 = Yes | |||
| Psoriasis | 0 = No | 1 = Yes | |||
| Kidney disease | 0 = No | 1 = Yes | |||
| Liver disease | 0 = No | 1 = Yes | |||
| Hepatitis | 0 = No | 1 = Yes | |||
| HIV | 0 = No | 1 = Yes | |||
| Major depressive disorder | 0 = No | 1 = Yes | |||
| Bipolar disorder | 0 = No | 1 = Yes | |||
| Other mood disorder | 0 = No | 1 = Yes | |||
| Generalized anxiety disorder | 0 = No | 1 = Yes | |||
| Phobic disorder | 0 = No | 1 = Yes | |||
| Obsessive compulsive disorder | 0 = No | 1 = Yes | |||
| Panic disorder | 0 = No | 1 = Yes | |||
| Post-traumatic stress disorder | 0 = No | 1 = Yes | |||
| Schizophrenia | 0 = No | 1 = Yes | |||
| Suicide attempt | 0 = No | 1 = Yes | |||
| Hip replacement | 0 = No | 1 = Yes | |||
| Knee replacement | 0 = No | 1 = Yes | |||
|
| |||||
| Low Activity (1 Item) | Physical activity (PASE Score) | 0 = Score ≥64 for men or ≥52 for women | |||
| 1 = Score <64 for men or <52 for women | |||||
|
| |||||
| Scoring | Frailty Index = Proportion of health deficits present in an individual out of the total number of age-related health variables considered | ||||
| Frailty Index = Total Health Deficits Score / 81 Variables Considered | |||||
| Frailty Index | Frail: ≥ 0.25 | ||||
| Pre-frail: 0.09–0.24 | |||||
| Robust: ≤ 0.08 | |||||
Sources of Funding
This work was supported by the Canadian Consortium on Neurodegeneration in Aging (CCNA) which itself is supported by a grant from the Canadian Institutes of Health Research with funding from several partners (www.ccna-ccnv.ca). The CCNA designed the COMPASS-ND study, and funded subject recruitment and data collection. The authors were funded by the CCNA through involvement in various CCNA team activities. This project was a collaboration between members of Team 8 (Lewy Bodies, Aging and Dementia), Team 12 (Mobility, Exercise and Cognition), and Team 14 (How Multi-Morbidity Modifies the Risk of Dementia and the Patterns of Disease Expression). The CCNA and its partners had no role in the design, analysis, or preparation of this paper.
Statistical Analysis
Descriptive statistics (counts, percentages, mean, and standard deviation) were used to determine baseline characteristics overall and by NDD subgroup. Continuous FI score range, mean, and median were determined according to FP classification and for the overall cohort. We compared mean FI scores between FP groups (robust, pre-frail, frail) using a one-way analysis of variance (ANOVA). Multiple comparisons were performed with the Tukey’s post hoc test. A cross tabulation of frailty diagnoses made by the FP and FI was created with raw agreement calculated. McNemar’s test was used to determine if the proportion of individuals classified as frail by the FP and FI differed. We also investigated the difference in mean FI scores between sexes using an independent samples t-test. P values < .05 were considered statistically significant. Analyses were performed using SPSS Version 25.0 (IBM Corp., Armonk, NY).
RESULTS
The analysis included 150 participants from 10 NDD subgroups (Table 3). Frailty prevalence was estimated to be 11% (95% CI, 5.8–15.8) and 14% (95% CI, 8.0–19.0) by the FI and FP, respectively. Estimates did not significantly differ (McNemar’s test, p = .57). The FP identified 84% of participants as pre-frail. The overall mean FI score was 0.15 ± 0.07 (Table 4). Mean FI scores did not differ significantly between sexes (t-test, p = .13), and approximately equal numbers of men and women were identified as frail by the FP (9 women vs. 11 men). Mean FI scores differed significantly between FP groups (robust, pre-frail, and frail) as determined by one-way ANOVA (p = .01). During post hoc analysis, a significant difference was found in mean FI scores between the pre-frail and frail FP groups (p < .01). However, there were no statistically significant differences between the robust and frail groups or the robust and pre-frail groups. Table 5 shows a cross tabulation of frailty classifications made by the two instruments. The FI and FP tools agreed on 120 of 148 classifications (raw agreement, 81%).
TABLE 3.
Demographic characteristics of older adults with neurodegenerative disorders
| Neurodegenerative Disorder Subgroup | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||
| Overall | SCI | MCI | V-MCI | AD | PD | PD-MCI | PDD | LBD | FTD | Mixed | |
| Group Size (n) | 150 | 24 | 64 | 6 | 21 | 8 | 6 | 3 | 7 | 3 | 8 |
| Women | |||||||||||
| N (%) | 69 (46) | 18 (75) | 35 (55) | 1 (17) | 4 (19) | 2 (25) | 1 (17) | 0 (0) | 2 (29) | 2 (67) | 4 (50) |
| Age (years) | |||||||||||
| Mean (SD) | 73.6 (7.0) | 68.3 (5.5) | 73.6 (6.7) | 79.3 (3.0) | 76.3 (7.3) | 66.7 (6.0) | 74.6 (6.6) | 80.0 (2.6) | 75.3 (4.9) | 70.9 (7.3) | 80.7 (3.7) |
| Education (years) | |||||||||||
| Mean (SD) | 15.6 (3.4) | 17.2 (3.3) | 15.5 (3.1) | 17.3 (5.0) | 15.2 (2.8) | 14.8 (3.0) | 15.0 (2.7) | 15.0 (2.6) | 13.4 (5.8) | 16.0 (3.5) | 14.4 (3.9) |
| MoCA | |||||||||||
| Mean (SD) | 23.1 (4.5) | 27.2 (2.1) | 24.0 (3.1) | 24.3 (3.6) | 19.1 (3.3) | 27.8 (1.2) | 22.2 (2.6) | 17.7 (7.1) | 17.0 (3.5) | 20.7 (6.0) | 17.5 (3.3) |
| Gait Speed (m/s) | |||||||||||
| Mean (SD) | 1.0a (0.3) | 0.9 (0.2) | 0.9a (0.2) | 0.9 (0.1) | 1.1 (0.3) | 0.8 (0.1) | 0.9 (0.2) | 1.3 (0.1) | 1.2 (0.6) | 0.9 (0.2) | 1.2 (0.2) |
| Hoehn & Yahr Stage | |||||||||||
| Mean (SD) | - | - | - | - | - | 1.9 (0.4) | 1.8 (1.0) | 2.5 (0.7) | 2.3 (0.8) | - | - |
Data unavailable for 2 MCI.
SCI = subjective cognitive impairment; MCI = mild cognitive impairment; V-MCI = subcortical ischemic vascular mild cognitive impairment; AD = Alzheimer’s disease; PD = Parkinson’s disease; PD-MCI = Parkinson’s disease with mild cognitive impairment; PDD = Parkinson’s disease with dementia; FTD = frontotemporal dementia; Mixed = dementia of mixed etiology; SD = standard deviation.
TABLE 4.
Frailty Index score range, mean, and median by Frailty Phenotype classification group for neurodegenerative disorders overall
| Frailty Index Score | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| N | Range | Mean (SD) | 95% Confidence Interval for Mean | Median | ||
| Frailty Phenotype Classification | Robust | 4 | 0.07–0.18 | 0.13 (0.05) | 0.06–0.20 | 0.14 |
| Pre-frail | 124 | 0.02–0.35 | 0.15 (0.07) | 0.14–0.16 | 0.15 | |
| Frail | 20 | 0.12–0.37 | 0.20 (0.07) | 0.17–0.23 | 0.18 | |
|
| ||||||
| Overall | 148 | 0.02–0.37 | 0.15 (0.07) | 0.14–0.16 | 0.15 | |
TABLE 5.
Agreement and disagreement on frailty classifications made by the Frailty Phenotype and Frailty Index
| Frailty Index Classification | ||||
|---|---|---|---|---|
|
|
||||
| Non-frail | Frail | Total | ||
| Frailty Phenotype Classification | Robust or Pre-frail | 116 | 12 | 128 |
| Frail | 16 | 4 | 20 | |
|
| ||||
| Total | 132 | 16 | 148 | |
Two MCI participants were excluded from the FP analysis for missing gait data that were needed to definitively determine risk profile. Regarding FP criteria, 72.3% of participants met criteria for slowness, 34.7% for weakness, 26.0% for low physical activity, 20.5% for exhaustion, and 6.0% for shrinking. The FI analysis included all 150 participants as the minimum criteria of 30–40 health deficits were universally satisfied. On average, 80.4 variables were used to calculate FI score. For 130 participants, all 81 variables were available to calculate frailty score. Among the other 20 participants, 80 variables were available for 11 participants, 79 variables were available for 4 participants, and the remaining 5 participants had constructed FIs from 77, 72, 66, 62, and 60 variables, respectively.
DISCUSSION
In this study, we examined frailty in NDD using two approaches. We obtained frailty prevalence estimates of 11% and 14% for the FI and FP, respectively. As hypothesized, the FP estimated higher frailty prevalence than the FI. Even so, the similarity in frailty prevalence estimates reported here supports previous research showing considerable convergence between the FP and FI approaches to frailty.(24) The presence of frailty elements, regardless of whether or not they are part of a neurodegenerative disorder, is likely to influence an individual’s health status; so it is likely still worthwhile to identify frailty in the context of NDD. Further, frailty (as deficit accumulation) appears to increase the risk of cognitive impairment— including dynamically—and modify the relationship between Alzheimer neuropathology and dementia.(25,26) For example, even people with low levels of plaques and tangles are at risk for dementia if their frailty levels are high.(26)
Compared to previous frailty estimates in AD (11.1–50.0%)(9) and PD (22.2–69.4%),(10,11) there was less frailty in our sample than expected. Although it is difficult to compare our findings with previous studies, given varying frailty measures and disease severities, our low frailty estimates suggest a volunteer bias of less frail participants may have resulted from the demanding protocol of the COMPASS-ND study. By nature, the COMPASS-ND cohort recruits patients with milder disease despite its “all-comers” approach to NDD.(27) As such, our participants may be unusually high-functioning, possibly resulting in an underestimation of the true prevalence of frailty among patients with NDD. Conversely, the applicability of our findings is increased by having examined frailty in a broad cohort of NDD patients, as individuals within this population commonly have uncertain diagnoses, overlapping pathology, or are fluctuating or transitioning between cognitive states. At this time, data in individual neurodegenerative groups were insufficient for subgroup analysis. Similarly, we believe the lack of statistically significant difference in mean FI score between the robust group and the other groups may be attributable to the small sample size identified as robust (n = 4). Future analyses to be completed on the full dataset (to be comprised of 1,650 participants across 30 sites recruited by September 2020) will examine how expressions of frailty and frailty risk may differ between neurodegenerative subgroups; ultimately, longitudinal follow-up should determine the impact of frailty on progression of NDD.
Guidelines currently recommend screening for frailty in all individuals over 70 years of age and all individuals with significant weight loss (≥5%) due to chronic disease. (3) Our findings suggest these guidelines are suboptimal for NDD patients. In our cohort, slowness was the most likely FP criterion to be met by NDD patients, with 65% of frail participants and 76% of pre-frail participants satisfying this criterion. This result supports previous findings that gait speed can be used as a screening method to detect pre-frail individuals at risk of dementia, adverse events, and future functional decline.(28) One notable approach to frailty screening, as introduced in the United Kingdom, may be to integrate an electronic FI into existing electronic medical records systems for routine identification and management of frailty. (29) Given that estimates of pre-frailty in this study were approximately double those seen in non-neurodegenerative populations,(30) we recommend screening all NDD patients for frailty as part of regular care.
We have provided operationalized definitions for future examinations of frailty in COMPASS-ND cohorts and other studies of NDD. Frailty screening using appropriate criteria is a public health priority as it provides clinicians with the opportunity to implement interventions that may serve to prevent frailty and benefit cognition, driving down healthcare costs and increasing patient quality of life. Although the FP and FI diagnosed frailty comparably in NDD, as health systems increasingly move to electronic medical records, the ability to integrate the FI into existing databases may be a significant advantage for detecting frailty in populations with and without comorbidities.
ACKNOWLEDGEMENTS
We would like to thank the COMPASS-ND study volunteers, study partners, and participating sites who made this work possible. Thank you to Krista Nelles for her helpful comments regarding the manuscript.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
Kenneth Rockwood founded and is President and Chief Science Officer of DGI Clinical, which has contracts with Shire, Roche, Otsuka, Biogen, and Hollister for individualized outcome measurement and data analytics. He co-authored the Frailty Index methodology. The remaining authors declare no conflicts of interest.
REFERENCES
- 1.Xue Q. The frailty syndrome: definition and natural history [Internet] Clin Geriatr Med. 2011;27(1):1–15. doi: 10.1016/j.cger.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbasi M, Rolfson D, Khera AS, et al. Identification and management of frailty in the primary care setting [Internet] Can Med Assoc J. 2018;190(38):E1134–40. doi: 10.1503/cmaj.171509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morley JE, Vellas B, Van Kan GA, et al. Frailty consensus: a call to action [Internet] J Am Med Dir Assoc. 2013;14(6):392–97. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Searle SD, Rockwood K. Frailty and the risk of cognitive impairment [Internet] Alzheimer’s Res Ther. 2015;7(1):54. doi: 10.1186/s13195-015-0140-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kulmala J, Nykänen I, Mänty M, et al. Association between frailty and dementia: a population-based study [Internet] Gerontol. 2014;60(1):16–21. doi: 10.1159/000353859. [DOI] [PubMed] [Google Scholar]
- 6.Ávila-Funes JA, Amieva H, Barberger-Gateau P, et al. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: the three-city study [Internet] J Am Geriatr Soc. 2009;57(3):453–61. doi: 10.1111/j.1532-5415.2008.02136.x. [DOI] [PubMed] [Google Scholar]
- 7.EU Joint Programme—Neurodegenerative Disease Research. What Is Neurodegenerative Disease? [Internet] Brussels, Belgium: JPND; Cited Oct 9, 2018. Available from: https://www.neurodegenerationresearch.eu/about/what/ [Google Scholar]
- 8.Sampson EL. Frailty and dementia: common but complex comorbidities [Internet] Aging Mental Health. 2012;16(3):269–72. doi: 10.1080/13607863.2012.657158. Available from: http://www.tandfonline.com/doi/abs/10.1080/13607863.2012.657158. [DOI] [PubMed] [Google Scholar]
- 9.Kojima G, Liljas A, Iliffe S, et al. Prevalence of frailty in mild to moderate Alzheimer’s disease: a systematic review and meta-analysis [Internet] Curr Alzheimer Res. 2017;14(12):1256–63. doi: 10.2174/1567205014666170417104236. [DOI] [PubMed] [Google Scholar]
- 10.Peball M, Mahlknecht P, Werkmann M, et al. Prevalence and associated factors of sarcopenia and frailty in Parkinson’s disease: a cross-sectional study [Internet] Gerontol. 2019;65(3):216–228. doi: 10.1159/000492572. [DOI] [PubMed] [Google Scholar]
- 11.Tan AH, Hew YC, Lim S, et al. Altered body composition, sarcopenia, frailty, and their clinico-biological correlates, in Parkinson’s disease [Internet] Parkinsonism Relat Disord. 2018;56:58–64. doi: 10.1016/j.parkreldis.2018.06.020. [DOI] [PubMed] [Google Scholar]
- 12.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype [Internet] J Gerontol Series A. 2001;56(3):M146–57. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 13.Jones DM, Song X, Rockwood K. Operationalizing a frailty index from a standardized comprehensive geriatric assessment [Internet] J Am Geriatr Soc. 2004;52(11):1929–33. doi: 10.1111/j.1532-5415.2004.52521.x. [DOI] [PubMed] [Google Scholar]
- 14.Bieniek J, Wilczyński K, Szewieczek J. Fried frailty phenotype assessment components as applied to geriatric inpatients [Internet] Clin Intervent Aging. 2016;11:453. doi: 10.2147/CIA.S101369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Searle SD, Mitnitski A, Gahbauer EA, et al. A standard procedure for creating a frailty index [Internet] BMC Geriatr. 2008;8(1):24. doi: 10.1186/1471-2318-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rockwood K, Mitnitski A. How might deficit accumulation give rise to frailty? [Internet] J Frailty Aging. 2012;1(1):8–12. doi: 10.14283/jfa.2012.2. [DOI] [PubMed] [Google Scholar]
- 17.Canadian Consortium on Neurodegeneration and Aging. The Comprehensive Assessment of Neurodegeneration and Dementia (COMPASS-ND) study. [Internet] CCNA; 2019. Cited Mar 26, 2019. Available from: http://ccna-ccnv.ca/compass-nd-study/ [Google Scholar]
- 18.Montero-Odasso M, Schapira M, Soriano ER, et al. Gait velocity as a single predictor of adverse events in healthy seniors aged 75 years and older [Internet] J Gerontol Series A. 2005;60(10):1304–09. doi: 10.1093/gerona/60.10.1304. [DOI] [PubMed] [Google Scholar]
- 19.Montero-Odasso M, Muir SW, Hall M, et al. Gait variability is associated with frailty in community-dwelling older adults [Internet] J Gerontol Series A. 2011;66(5):568–76. doi: 10.1093/gerona/glr007. [DOI] [PubMed] [Google Scholar]
- 20.Montero-Odasso MM, Barnes B, Speechley M, et al. Disentangling cognitive-frailty: results from the gait and brain study [Internet] J Gerontol Series A. 2016;71(11):1476–82. doi: 10.1093/gerona/glw044. [DOI] [PubMed] [Google Scholar]
- 21.Cullen S, Montero-Odasso M, Bherer L, et al. Guidelines for gait assessments in the Canadian Consortium on Neurodegeneration in Aging (CCNA) [Internet] Can Geriatr J. 2018;21(2):157. doi: 10.5770/cgj.21.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Washburn RA, Smith KW, Jette AM, et al. The Physical Activity Scale for the Elderly (PASE): development and evaluation [Internet] J Clin Epidemiol. 1993;46(2):153–62. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 23.Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation [Internet] J Am Geriatr Soc. 2010;58(4):681–87. doi: 10.1111/j.1532-5415.2010.02764.x. [DOI] [PubMed] [Google Scholar]
- 24.Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people [Internet] J Gerontol Series A. 2007;62(7):738–43. doi: 10.1093/gerona/62.7.738. [DOI] [PubMed] [Google Scholar]
- 25.Godin J, Armstrong JJ, Rockwood K, et al. Dynamics of frailty and cognition after age 50: Why it matters that cognitive decline is mostly seen in old age [Internet] J Alzheimer’s Dis. 2017;58(1):231–42. doi: 10.3233/JAD-161280. [DOI] [PubMed] [Google Scholar]
- 26.Wallace LM, Theou O, Godin J, et al. Investigation of frailty as a moderator of the relationship between neuropathology and dementia in Alzheimer’s disease: a cross-sectional analysis of data from the Rush Memory and Aging Project [Internet] The Lancet Neurol. 2019;18(2):177–84. doi: 10.1016/S1474-4422(18)30371-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chertkow HC, Borrie M, Whitehead V. The Comprehensive Assessment of Neurodegeneration and Dementia: Canadian cohort study [Internet] Can J Neurological Sci. 2019;46(5):499–511. doi: 10.1017/cjn.2019.27. [DOI] [PubMed] [Google Scholar]
- 28.Montero-Odasso M, Speechley M, Muir-Hunter SW, et al. Motor and cognitive trajectories before dementia: results from gait and brain study [Internet] J Am Geriatr Soc. 2018;66(9):1676–83. doi: 10.1111/jgs.15341. [DOI] [PubMed] [Google Scholar]
- 29.Brundle C, Heaven A, Brown L, et al. Convergent validity of the electronic frailty index [Internet] Age Ageing. 2018;48(1):152–56. doi: 10.1093/ageing/afy162. [DOI] [PubMed] [Google Scholar]
- 30.Collard RM, Boter H, Schoevers RA, et al. Prevalence of frailty in community-dwelling older persons: a systematic review [Internet] J Am Geriatr Soc. 2012;60(8):1487–92. doi: 10.1111/j.1532-5415.2012.04054.x. [DOI] [PubMed] [Google Scholar]