Abstract
We present the case of ceftazidime-induced immune-mediated haemolysis with associated acute kidney injury in a 43-year-old woman. The patient initially presented to the regional cystic fibrosis centre for treatment of an infective exacerbation of cystic fibrosis. After initiation of ceftazidime (a third-generation cephalosporin), renal function rapidly deteriorated and a fall in haemoglobin was noted. On transfer to our care, a haemolysis screen identified immune-mediated haemolysis, and renal biopsy confirmed the finding of acute tubular necrosis secondary to haem pigment. The patient’s renal function deteriorated such that she required haemodialysis, although she subsequently recovered and is now dialysis-independent. Although acute haemolytic reactions are recognised with third-generation cephalosporins, this is the first reported case of ceftazidime-induced immune-mediated haemolysis with acute kidney injury. Given the increased frequency of cephalosporin usage, it is important for both nephrologists and general physicians to be aware of this rare but very serious complication.
Keywords: contraindications and precautions, haematology (drugs and medicines), renal system, unwanted effects / adverse reactions, acute renal failure
Background
Drug-induced nephrotoxicity is a common problem in clinical medicine, and a recognised cause of substantial morbidity and mortality. The severity of renal injury ranges from subtle and transient renal dysfunction to overt renal failure. The pathophysiological mechanisms through which drug-induced nephrotoxicity is mediated are equally diverse. Cephalosporins are a widely prescribed class of antibiotic, with the Annual Epidemiological Report 2017 (Centre for Disease Prevention and Control) placing ‘cephalosporins and other beta-lactams’ as the second-most commonly used antibacterial group in the US hospital sector behind penicillins.1 Although generally well tolerated, adverse reactions to third-generation cephalosporins are well documented, and drug-induced immune haemolytic anaemia (DIIHA) is an increasingly recognised phenomenon with second-generation and third-generation cephalosporins.2 To the authors’ knowledge, this report represents the first case of ceftazidime-induced immune haemolytic anaemia presenting with acute kidney failure in an adult patient. Given the increasing frequency of cephalosporin use, and the rapidity with which acute renal failure may develop in the context of acute haemolytic reactions, it is important that physicians are aware of this rare but potentially catastrophic complication.
Case presentation
A 43-year-old woman with cystic fibrosis was treated with outpatient intravenous antibiotic therapy for recurrent respiratory infections. After 2 months of intermittent outpatient treatment, she was admitted to the regional cystic fibrosis centre for further antibiotic therapy and optimisation of lung function. She also had a prior medical history significant for Mycobacterium avium intracellulare, Aspergillus fumigatus and gastro-oesophageal reflux disease. Bloods on admission demonstrated a baseline creatinine of 40 μmol/L, C-reactive protein (CRP) 67 mg/L and haemoglobin 123 g/L. She was commenced on intravenous ceftazidime 3 g three times per day starting on day 2 of admission, after the first administration of which she developed a temperature of 39°C and felt unwell. She had previously been exposed to ceftazidime, with mild nausea being the only adverse complaint. Blood tests on day 3 demonstrated acute kidney injury (AKI) with a creatinine of 375 μmol/L, haemoglobin of 113 g/L, platelets 38×109/L (from 222×109/L) and a CRP of 154 mg/L. Eosinophils were 0.2×109/L. Urine dip demonstrated 4+blood and 2+protein. Antibiotics were discontinued that afternoon. Blood pressure was normal and, with exception of the initial fever, there were no systemic features of note. Her creatinine the next day had risen to 594 µmol/L, a renal tract ultrasound was normal and she was transferred to our centre.
Investigations
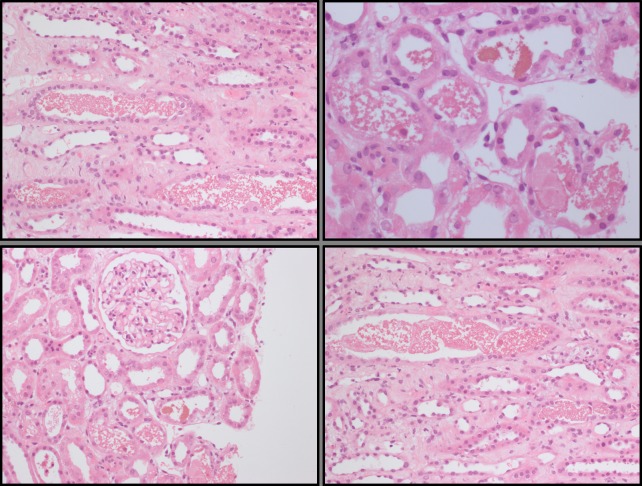
On transfer, the patient’s creatinine was 712 μmol/L, haemoglobin 97 g/L, platelets 56×109/L, haptoglobin 0.4 g/L (normal range 0.5–2.6 g/L), lactate dehydrogenase (LDH) of 562 U/L (normal range 120–246 U/L), bilirubin 4 umol/L, with a positive direct antiglobulin test. A blood film did not demonstrate any fragments. ADAM metallopeptidase with thrombospondin type 1 motif 13 (ADAMTS-13) activity was normal and immunology unremarkable. A renal biopsy was performed (figure 1), demonstrating extensive acute tubular injury with tubules containing eosinophilic material, consistent with intravascular haemolysis. Glomeruli and blood vessels were normal.
Figure 1.
Histology. Diffuse acute tubular injury and intratubular material with appearances consistent with haemogloblin.
By the following day, creatinine had risen to 890 μmol/L, she remained oligoanuric, and was commenced on haemodialysis. By this point, her markers of haemolysis and platelet count had normalised. She had no further episodes of fever after day 2.
Differential diagnosis
The first challenge on presentation was to elucidate the underlying cause of this patient’s renal deterioration and accompanying drop in both haemoglobin and platelets. An initial haemolysis screen, identifying low haptoglobin, elevated LDH and positive direct antiglobulin test, was instrumental in determining the cause of haemolysis, but of course does not provide a specific aetiology. Our first concern was of a thrombotic microangiopathy, possibly drug-induced, given the patient’s haemolytic anaemia, thrombocytopaenia and renal failure. However, the absence of fragments on blood film made this diagnosis less likely and a normal ADAMTS-13 activity assay excluded thrombotic thrombocytopaenic purpura (TTP). Ultimately, the thrombocytopaenia transpired to be artefactual—review of blood films identified platelet clumping, inducing an artefactually low platelet count by the automated counter. Her markers of haemolysis rapidly normalised after cessation of the offending drugs—even while her renal function continued to deteriorate as a consequence of the pigment-induced tubular injury. Renal biopsy was instrumental in determining a direct nephrotoxic injury from haem pigment as the underlying aetiology, and supports the diagnosis of a drug-induced immune-mediated haemolytic reaction.
Outcome and follow-up
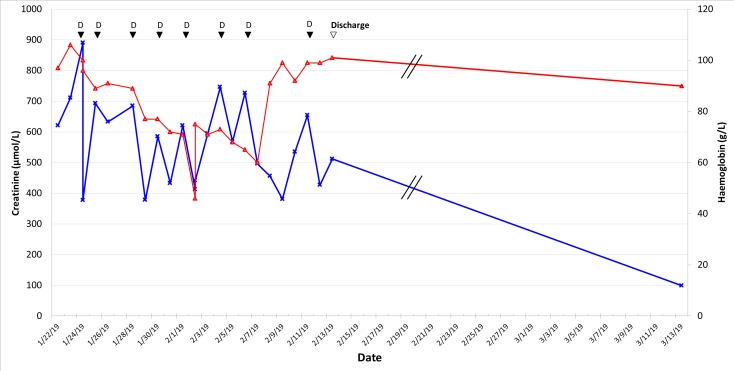
One-week post-transfer, the patient remained oligo-anuric and dialysis-dependent. A tunnelled line was inserted to allow ongoing dialysis after discharge. Renal function recovered over the following few weeks, and dialysis was discontinued 3 weeks later (figure 2).
Figure 2.
Change in haemoglobin concentration and creatinine over time following transfer. D, dialysis episode; red open triangle, haemoglobin concentration (g/L); blue cross, Creatinine concentration (μmol/L).
Discussion
DIIHA is increasingly reported in the literature with the use of second-generation and third-generation cephalosporins. Although data on DIIHA incidence are currently lacking, an estimate of ~1 case per million of the population exposed has been suggested.2 Haemolysis may be induced through either drug-dependent or drug-independent mechanisms, defined by the ability of causative antibodies to react in vitro with red blood cells (RBCs) in either the presence or absence of the drug, respectively.2 Three mechanisms of drug-dependent haemolysis exist. In the first, the causative drug induces antidrug antibodies that bind collectively to the drug and RBC membrane on drug–RBC interaction, resulting in extravascular haemolysis. In the second mechanism of drug-dependent haemolysis, the drug initially combines with antidrug antibody to form an immune complex that may then interact with the RBC membrane to induce complement activation. Third, drug binding induces RBC membrane modification that forms a ‘neoantigen’ to which antidrug antibodies may bind, again leading to complement activation and haemolysis.3 Drug-independent mechanisms of haemolysis (mechanisms of haemolysis which do not require the persistent presence of the drug) remain less well characterised. Proposed mechanisms include drug-mediated modification of RBC antigens so that they are no longer recognised as ‘self-antigens’ following drug adsorption, drug-induced IgG aggregation and subsequent RBC binding, drug-directed antibody formation against RBC membrane components, and direct modification of the immune system, perhaps through suppression of regulatory T cell function.3 Both drug-dependent and drug-independent mechanisms of haemolysis have been described following administration of cephalosporins.
Bywaters and Beall elegantly described the first documented cases of pigment-induced direct tubular injury from myoglobin as a cause of acute renal dysfunction in 1941.4 In this paper, they described and carefully plotted physiological parameters, renal function and histology in four patients who had experienced differing extents of crush injuries, and correctly identified muscle necrosis as the common aetiological factor causing renal injury. A recent interrogation of an animal model of haemoglobinuria provided some clarity on the mechanism of haem-induced tubular injury. During severe haemoglobinuria, local oxidative processes lead to accumulation of ferric haemoglobin and subsequent release of free haem within the tubular system. Haem, as a consequence of its pro-oxidative nature, may activate the unfolded protein response in tubular epithelial cells, contributing to ferroptosis (an iron-dependent mechanism of cell death characterised by failure of antioxidant defences) and acute renal injury.5
The finding of thrombocytopaenia in our case was a significant confounding factor in formulating the correct diagnosis, raising the possibility of differentials including TTP, atypical haemolytic uraemic syndrome and Evans syndrome (an immune-mediated haemolysis affecting both RBCs and platelets). Review of the patient’s blood smear identified platelet clumping, resulting in pseudothrombocytopaenia during determination of platelet count by the automated counter. This is not an uncommon occurrence, and its clinical relevance resides only in its lack of recognition and potential for misdiagnosis. The most common cause is the presence of a naturally occurring anti-GPIIb/IIIa autoantibody, which precipitates platelet aggregation on exposure of GPIIb/IIIa epitopes with ETDA.6
Between 1990 and 2018, just three cases of ceftazidime-induced haemolytic anaemia have been reported.7–9 The presenting symptoms in these cases were jaundice, haematuria, fever, hypotension and tachycardia. To our knowledge, this is the first reported case of ceftazidime-induced DIIHA with pigment-induced acute tubular necrosis and renal failure as the presenting feature. Two cases report the use of glucocorticoids (either dexamethasone or prednisolone) in management of DIIHA.7 9 Our patient was already receiving oral prednisolone as an adjunct to her management of infective exacerbation of cystic fibrosis—additional glucocorticoids were not prescribed.
The British Society for Haematology has produced guidelines on management of drug-induced immune-mediated haemolytic anaemia.10 Broadly, the inciting drug should be discontinued, folic acid should be prescribed and the patient should be considered for thromboembolism prophylaxis, given increased risk of thrombosis with haemolysis. The mainstay of management is supportive. Indications for transfusion do not differ from other causes of acute blood loss. The addition of glucocorticoids to management is of equivocal benefit, and the decision to commence should be individualised according to the severity of haemolysis and certainty of diagnosis. It is recognised that subsequent haemolytic reactions are more severe following administration of similar drugs, and so repeated exposure should be avoided.2
In summary, DIIHA is an increasingly recognised phenomenon with second-generation and third-generation cephalosporins. We present here the first case of DIIHA presenting with acute renal failure, secondary to acute tubular necrosis from a direct nephrotoxic insult of haem pigment. This case highlights the value of renal biopsy in investigating causes of acute renal deterioration and in directing appropriate management. Given the increasing frequency of cephalosporin usage, it is important for all physicians to be aware of this rare complication.
Learning points.
Drug-induced immune haemolytic anaemia (DIIHA) is a rare but potentially serious drug reaction, which may present with acute kidney injury.
Second-generation and third-generation cephalosporins, and piperacillin/tazobactam, are the most frequently associated drugs with DIIHA.
The most important differential diagnosis is a microangiopathic haemolytic anaemia (thrombotic thrombocytopaenic purpura and haemolytic uraemic syndrome).
Current guidelines strongly recommend not administering offending drugs of the same class, as there is a tendency for repeat reactions to be more severe.
Acknowledgments
We wish to acknowledge Dr Anna Paterson, Consultant Histopathologist at Addenbrookes Hospital, for her contribution to formalising the diagnosis and for providing histology slides for the final manuscript.
Footnotes
Contributors: AFr devised the project. AFe and AFr performed the literature review and produced the initial manuscript. MG was instrumental in analysis of histopathological specimens and provided images for the manuscript. RS provided input on diagnosis and contributed to refining the manuscript discussion. Moreover, all authors contributed to critical feedback and manuscript revision.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. European Centre for Disease Prevention and Control Antimicrobial consumption. Annual epidemiological report 2017. Stockholm: ECDC, 2018. [Google Scholar]
- 2. Garratty G. Drug-induced immune hemolytic anemia. Hematology 2009;2009:73–9. 10.1182/asheducation-2009.1.73 [DOI] [PubMed] [Google Scholar]
- 3. Haley KM, Russell TB, Boshkov L, et al. Fatal carboplatin-induced immune hemolytic anemia in a child with a brain tumor. J Blood Med 2014;5:55–8. 10.2147/JBM.S59192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bywaters EG, Beall D. Crush injuries with impairment of renal function. Br Med J 1941;1:427–32. 10.1136/bmj.1.4185.427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Deuel JW, Schaer CA, Boretti FS, et al. Hemoglobinuria-related acute kidney injury is driven by intrarenal oxidative reactions triggering a heme toxicity response. Cell Death Dis 2016;7:e2064 10.1038/cddis.2015.392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. George J. Primary immune thrombocytopaenia : Kitchens C, Konkle B, Kessler C, Consultative haemostasis and thrombosis. England: Elsevier, 2013: 117–31. [Google Scholar]
- 7. Chen F, Zhan Z. Severe drug-induced immune haemolytic anaemia due to ceftazidime. Blood Transfus 2014;12:435–7. 10.2450/2014.0237-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chambers LA, Donovan LM, Kruskall MS. Ceftazidime-induced hemolysis in a patient with drug-dependent antibodies reactive by immune complex and drug adsorption mechanisms. Am J Clin Pathol 1991;95:393–6. 10.1093/ajcp/95.3.393 [DOI] [PubMed] [Google Scholar]
- 9. Yong J, Frost F, Nazareth D, et al. Case report: haemolytic anaemia with ceftazidime use in a patient with cystic fibrosis. F1000Res 2018;7 10.12688/f1000research.14505.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hill QA, Stamps R, Massey E, et al. Guidelines on the management of drug-induced immune and secondary autoimmune, haemolytic anaemia. Br J Haematol 2017;177:208–20. 10.1111/bjh.14654 [DOI] [PubMed] [Google Scholar]