Abstract
A 23-year-old woman was referred to the allergy and immunology clinic for recurrent abdominal, cutaneous and joint swelling and pain with a history of mucosal infections since childhood. Her history and clinical findings were suggestive of two rare and complex disorders, hereditary angioedema (HAE) and Ehlers-Danlos syndrome (EDS). Her recurrent episodes of abdominal and joint pain were initially misattributed to more common diagnoses such as esophagitis, depression and chronic pain syndrome. However, the coexistence of HAE and EDS likely contributed to a delay in diagnoses as the combination of these two rare but overlapping disorders is less understood by physicians. She had persistently low levels of C4 and C1-esterase inhibitor (C1-INH) with low to low-normal C1-esterase function, normal C1Q and no C1Q antibodies. In the setting of recurrent abdominal pain with cutaneous swelling, this supported the diagnosis of HAE type I. The increase in joint extensibility with recurrent shoulder subluxations since childhood was a manifestation of EDS. Although no known genetic mutations were identified for EDS, her diagnosis was confirmed by a geneticist based on her clinical phenotype. Before the diagnosis of HAE and EDS, our patient had at least 100 visits/year to the emergency department/hospitalisations for these recurrent symptoms. After starting on C1-INH replacement therapy, the frequency has decreased 10-fold. She also noted a 70% improvement in her quality of life. Familiarity with these rare disorders will assist healthcare providers in recognising HAE and EDS and include them as part of their differential diagnoses. Early diagnosis is important for a patient’s well-being as both these chronic disorders have been associated with poor quality of life. Additionally, proper diagnoses will reduce healthcare costs by preventing unnecessary procedures due to misdiagnoses. Proper treatment will help to decrease hospitalisations and avoidance of life-threatening consequences (such as asphyxiation from fatal laryngeal attacks of HAE and rupture of aneurysms in EDS).
Keywords: immunology, medical management, connective tissue disease
Background
Hereditary angioedema (HAE) is a rare disease that is caused by mutations in the C1-inhibitor gene resulting in reduced levels or dysfunctional C1-esterase inhibitor (C1-INH). HAE is characterised by recurrent episodes of subcutaneous or submucosal oedema. Worldwide, it is estimated to affect about 1 in 50 000 individuals.1 Areas that are most commonly affected are the extremities and abdomen. Over half of the patients with HAE will experience at least one episode of oropharyngeal oedema. Laryngeal oedema with asphyxiation contributes to 30% of the mortality in HAE. Abdominal and laryngeal oedema can result in disastrous consequences if HAE remains undiagnosed.
Ehlers-Danlos syndrome (EDS) is a heterogeneous connective tissue disorder caused by mutations in genes encoding for collagen and its processing and extracellular matrix components. It is estimated to affect about 1 in 5000 individuals worldwide.2 Often times, patients will have chronic joint pains with recurrent non-traumatic subluxations and dislocations resulting in poor quality of life.3 The highest mortality is related to arterial, uterine and intestinal rupture.4 Other consequences include joint instability, tissue fragility resulting in bleeding with minimal trauma, poor wound healing and postoperative instability and recurrent failed procedures.5–7 Extra-articular manifestations include orthostatic tachycardia, gastric emptying abnormalities and neuropsychiatric conditions.7 There is limited literature regarding immunological dysregulation in EDS.8–10
Clinical expression of both disorders is variable resulting in under-recognition of these disease entities leading to missed diagnoses, especially if these two disorders coexist in the same patient (such as in the case presented here). An observational study by Zanichelli et al reported that about 50% of patients with HAE were misdiagnosed.11 Misdiagnosis increases a patient’s burden of disease and unintended consequences from delay in treatment. Mortality rates from asphyxiation by fatal laryngeal attacks were higher in patients with undiagnosed HAE.12 Awareness of these unusual disorders will lead to early diagnosis and prevention of deadly outcomes.
Case presentation
In 2014, a 23-year-old woman with non-Hashimoto hypothyroidism (controlled by levothyroxine) and chronic pain syndrome presented to the allergy and immunology clinic for further evaluation of recurrent mucosal infections (4–7 episodes of sinusitis per year that improved as she got older but without recurrent pneumonia or meningitis), sharp, crampy abdominal pain and diffuse joint pain and subluxations since childhood. These symptoms were accompanied by diarrhoea and abdominal and joint swelling. Any light touch in these areas would result in excruciating pain. These episodes were not associated with food or her menstrual cycle. These symptoms usually lasted for 2–3 days but had increased to 5–7 days over the last year prior to consultation. She would‘go to bed skinny and wake up fat.’ She noted that any trauma would result in severe musculoskeletal pain and swelling and that she easily bruised.
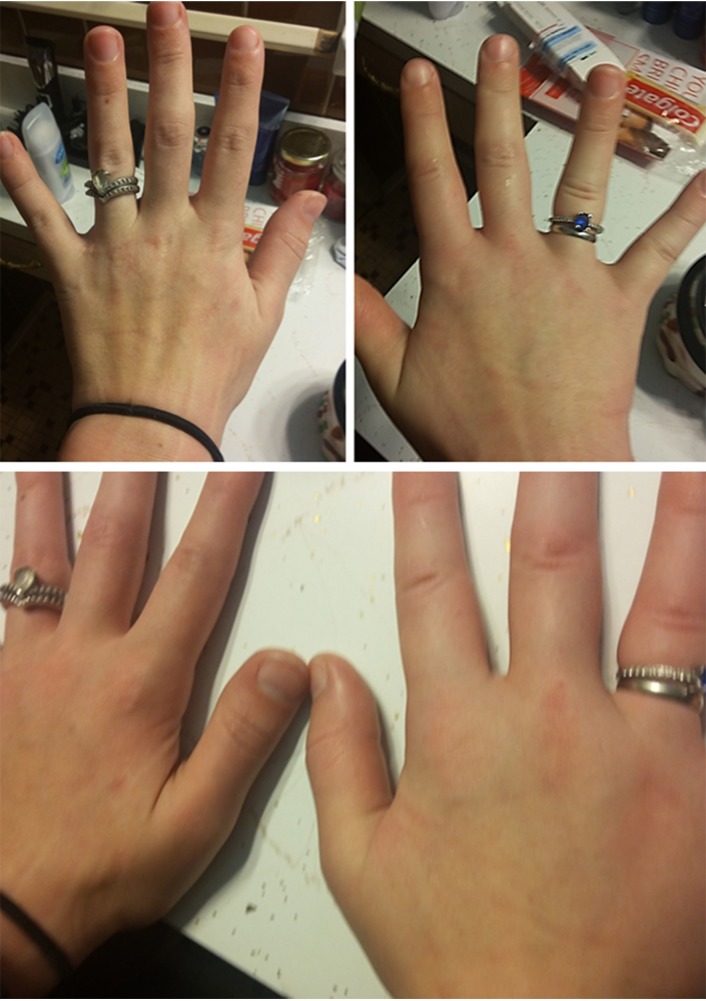
She also described her skin swelling as feeling ‘tight and taut’ making it difficult for her to flex her fingers and wear her shoes comfortably (figure 1). Intermittently, she would also have episodes of facial swelling with throat tightness impacting her ability to eat. These episodes would be preceded by facial numbness before the abrupt onset of facial oedema. When she ate, she would have recurrent episodes of non-bilious, non-bloody emesis consisting of undigested food particles. She admitted that there were times when she was afraid that her ‘throat would close’ and was afraid of eating during her episodes of throat swelling. She noted early satiety with nausea. She denied pruritus or rash. Narcotics and antiemetics only provided minimal relief prompting her to visit the emergency department seeking answers during her severe episodes.
Figure 1.
Swelling of the digits, more pronounced in the right third and fourth digits compared with the left hand.
Not only were these symptoms anxiety provoking, they also impacted her activities of daily living. She had episodes of palpitations and tachycardia which were initially related to her anxiety. These were not associated with syncope or orthostatic intolerance. Eventually, atrial flutter was diagnosed and resolved with cardiac ablation.
Her mother has a history of asthma, allergic rhinitis and recurrent sinusitis, fibromyalgia and rheumatoid arthritis. Our patient had three gestations with two term pregnancies followed by one ruptured ectopic pregnancy requiring laparoscopic right salpingectomy in 2013. Her youngest daughter (who is currently 8 years old) is experiencing similar symptoms. These symptoms include recurrent sinusitis, otitis media, chronic diffuse joint swelling and pain along with chronic abdominal pain with marked distension (evaluation has been delayed due to insurance coverage). The rest of her family history was unremarkable.
On physical examination, she appeared well-nourished, alert and oriented, and sat in the chair with no acute distress. No abnormal facies was noted. Pale, non-oedematous turbinates with clear rhinorrhea were present. An atrophic scar on her right arm (from an old burn) and left hand (from a pet bite) and few striae on her upper thighs were observed. Two laparoscopic scars were seen in the right upper and lower quadrants. Bowel sounds were present and abdomen was non-tender to palpation. No organomegaly was noted. Increase in joint extensibility (Beighton score 6/9) without skin laxity and no hypertrophic scars were noted. Her skin had a soft, doughy texture. The remainder of the physical examination was unremarkable.
Investigations
Prior to referral to the allergy and immunology
Her abdominal pain was previously attributed to biliary dyskinesia but cholecystectomy performed in 2014 did not improve her symptoms. She stated that ‘if anything, it has gotten worse’. This was followed by an exploratory laparoscopy for lysis of adhesions. A gastric emptying study revealed gastroparesis. Biopsies from esophagogastroduodenoscopy and colonoscopy demonstrated reflux oesophagitis and colitis. She was started on a proton pump inhibitor without any improvement. CT-abdomen/pelvis with contrast showed prominent bilateral adnexal vasculature suggestive of pelvic congestion syndrome along with a 3.5 cm abdominal aortic aneurysm. Echocardiogram was within normal limits.
New investigations
Her recurrent respiratory mucosal infection was concerning for an antibody deficiency but laboratory tests were unremarkable. She did have CD8 lymphopenia, likely resulting in recurrent viral infections.
Proton pump inhibitors provided no improvement in her gastrointestinal symptoms. Helicobacter pylori IgA and IgM were positive with negative IgG levels. Breath-Tek test was also positive. She completed a course of treatment with minimal improvements in her symptoms. Due to her history of antibiotic use, there was concern for an infectious cause of her abdominal pain after no improvement was seen with H. pylori treatment. However, comprehensive stool examination (including ova and parasites) was unremarkable. No defining antibodies were detected for Celiac’s disease (including biopsy), both Celiac and inflammatory bowel disease gene studies were negative, and a food diary was unremarkable. Repeat CT-abdomen and pelvis during her acute episodes of abdominal pain showed fluid in the peritoneal cavity with oedema of the bowel walls along with perisplenic fluid collections.
The presence of hyperextensible joints with recurrent joint pain and subluxations since childhood suggested an underlying autoimmune rheumatologic disease or connective tissue disorder. Antinuclear antibody, extractable nuclear antigen antibodies, rheumatoid factor (RF), erythrocyte sedimentation rate and C reactive protein were negative. This increased our suspicion of EDS. Unfortunately, insurance did not cover genetic testing for EDS, and so our patient paid out of her pocket for the test. Although no known EDS mutations were detected, a heterozygous variant of uncertain significance in PLOD1 and COL1A2 were identified. The c.975C>T variant in exon 9 of PLOD1 has not been reported but may affect splicing and mRNA processing since it affects the last nucleotide. The biological significance of the c.3853A>C variant in exon 51 of COL1A2 is also unknown. Based on the clinical findings along with her history, a geneticist confirmed the diagnosis of EDS with suspicion for the classic or hypermobile type. Only 50% of those with classic EDS have an identifiable mutation and the mutations for the hypermobile type are not well established. Note that a duplication/deletion study was recommended but due to out-of-pocket costs, it was not completed. After diagnosis of EDS, multiple abdominal aneurysms were detected eventually requiring intervention. Vascular phenotypes (such as the presence of aneurysms) are most common with the vascular type of EDS but are also present in 11%–25% of the classical type.3
Other laboratory findings were remarkable for low C4 (11 mg/dL with repeat of 8 mg/dL 2 months later) and low C1-INH level (14 mg/dL with repeat of 7 mg/dL 2 months later) and function and normal levels of C1Q with no antibodies present. Her recurrent symptoms of abdominal pain with swelling, nausea, and at times diarrhoea, ‘tight and taut’ swollen skin, recurrent face and lip swelling and intermittent episodes of throat tightness/swelling in the presence of low C1-INH levels supported the diagnosis of HAE type I.
Differential diagnosis
Mast cell activation syndrome (MCAS) has been reported to occur in EDS and was on the differential diagnoses list for our patient. Clinical manifestations of MCAS can be non-specific and can overlap with various other disease entities, such as HAE. Based on the most recent guidelines, criteria for MCAS include (1) the presence of recurrent symptoms related to mast cell activation (such as urticaria, flushing, pruritus, angioedema, diarrhoea), (2) elevated tryptase levels during or within 4 hours of a symptomatic period and (3) response to therapy against mast cells or their mediators (such as histamine receptor antagonists).13
Although, our patient exhibited non-specific manifestations of MCAS (primarily, recurrent angioedema and diarrhoea without any skin lesions or pruritus), there was no response to histamine receptor antagonists that her prior care team had placed her on before referral to our office. Additionally, tryptase levels during her symptomatic episodes were normal. The presence of low C1-INH and low C4 supported HAE over MCAS.
Treatment
Hereditary angioedema type I
General guidelines from the World Allergy Organization and European Academy of Allergy and Clinical Immunology emphasise tailoring treatment for each individual with HAE.14 Although there are various Food and Drug Administration (FDA)-approved options available in the USA (table 1), treatment options are based on insurance coverage which can differ widely. Note that options may differ in other countries.
Table 1.
Hereditary angioedema (HAE), a brief overview in the management of HAE in adults based on the Food and Drug Administration-approved options in the USA.15–17
| Acute management of HAE attacks | |
| Preferred options | Intravenous C1-esterase inhibitor (C1-INH) concentrate (Berinert, Ruconest) Ecallantide (kallikrein inhibitor) Icatibant (bradykinin-receptor antagonist) |
| Alternatives | Fresh frozen plasma |
| P re procedural prophylaxis | |
| No specific C1-INH concentrate is currently indicated in the USA (options are dependent on insurance formulary and coverage). | |
| L ong-term prophylaxis | |
| First-Iine options | Intravenous C1-INH concentrate (Cinryze) Subcutaneous C1-INH concentrate (Haegarda) Lanadelumab (antikallkrien monoclonal antibody) |
| Alternatives | Androgens Tranexamic acid* |
*Tranexamic acid should be used when no other alternatives are available.
Some patients who are on only on-demand treatment for acute HAE attacks may also require short-term prophylaxis in the setting of acute stress from surgery or invasive dental procedures. Due to the lack of controlled studies, there are no drugs approved for preprocedural prophylaxis in the USA.15 However, current guidelines in the USA recommend intravenous C1-INH formulations 1–12 hours before the scheduled procedure.16 In Europe, C1-INH concentrates, Berinert and Cinryze, are approved for preprocedural prophylaxis. 14
Patients who are only receiving on-demand treatment may require short-term prophylaxis before surgical or invasive dental procedures or stressful life events that may trigger an attack. Although several observational studies have been conducted, education, along with discussion, was held with our patient regarding HAE. She was started on human C1-INH replacement (Cinryze at 1000 units every 3–4 days) as prophylaxis for HAE. She was trained on self-injection with recombinant C1-INH concentrate (Ruconest) for management of her acute flare-ups at home. When HAE flare-ups were not possible to be managed at home, a treatment plan was given to the local hospital. Depending on the hospital formulary, acute episodes of angioedema can be managed with C1-INH concentrates (such as Berinert or Ruconest), ecallantide, or icatibant. If these options are not available, fresh frozen plasma can be used. It is important to note that antihistamines, corticosteroids and epinephrine are ineffective in treating HAE.
Our patient had multiple procedures requiring preoperative prophylaxis. Acute HAE attacks may be triggered by trauma such as dental work, elective medical procedures or surgery and prophylaxis to prevent an attack is generally recommended. Our patient typically receives Cinryze (1000 units or 20 units/kg) 1 hour before her scheduled procedures and/or surgeries with remarkable success in preventing or ameliorating her acute HAE episodes. It is also advisable to have two doses of rescue/acute management medication available for use in the event that an acute HAE episode still occurs. Due to the anxiety associated with these symptoms, she was given an anxiolytic to be used as needed.
Ehlers-Danlos syndrome
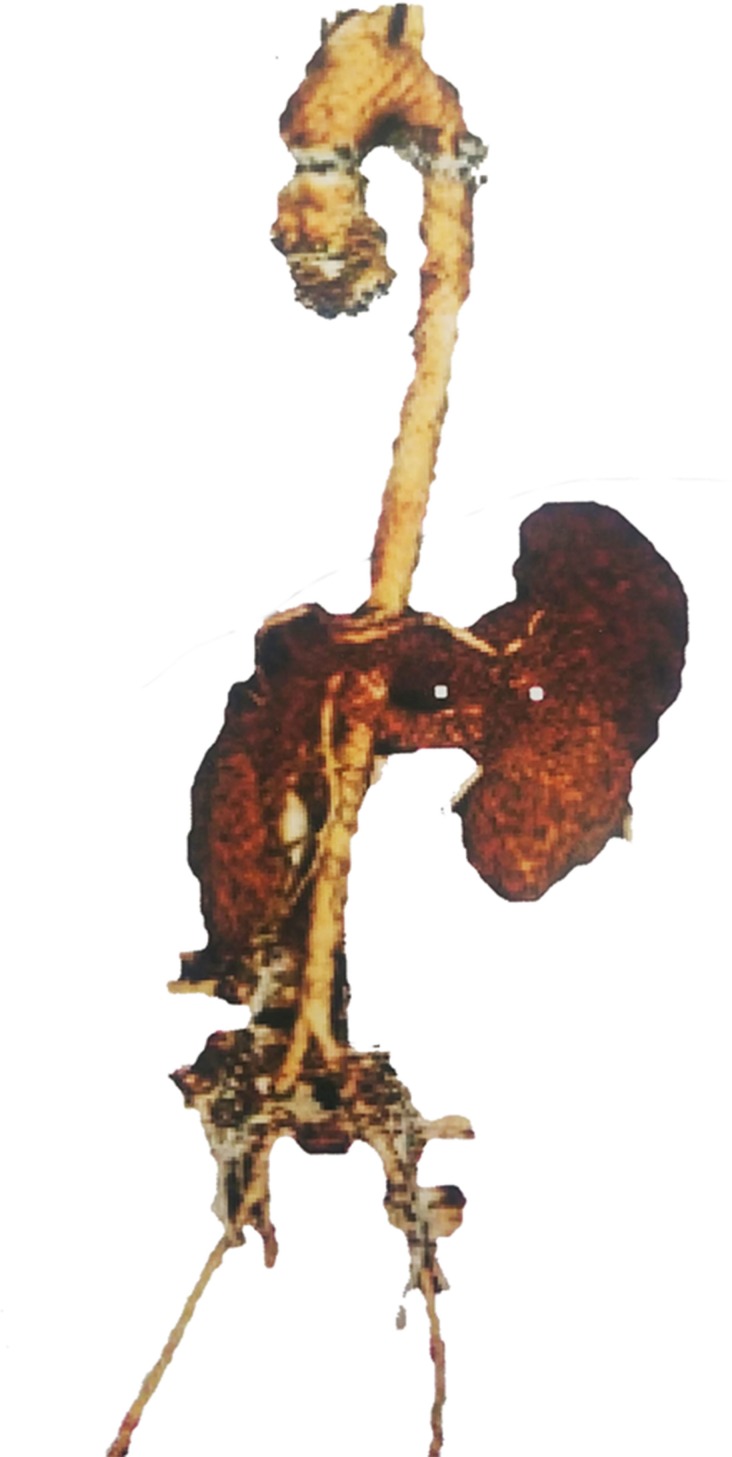
After the initial diagnosis of EDS, multiple abdominal aneurysms were detected. A dilated 3.5 cm aortic root was also noted, which was atypical for the patient’s young age and female gender (figure 2). Dilated gonadal blood vessels required intervention with venous embolisation of gonadal veins by vascular surgery (17 interventions to date). The 3.5 cm abdominal aortic aneurysm continues to be monitored yearly for growth. Advanced degenerative joint disease for her age was identified in her shoulders, hips and knees requiring multiple arthroscopic debridement, bilateral labrum repairs and bilateral patella stabilisation. In August 2018, she had an anterior cruciate ligament reconstruction/repair in her left knee. Recent arthroscopy by orthopaedics for her left shoulder was complicated by joint instability due to severe osteopenia. She sees rheumatology for further management of her EDS. Her current pain regimen consists of a combination of acetaminophen-codeine, acetaminophen-oxycodone and hydromorphone as needed (in collaboration with pain management).
Figure 2.
Three dimensional reconstruction of the aorta from a CT scan showing a dilated aortic root with prominent ascending thoracic aorta (3.5 cm). These findings were atypical for the patient’s young age and female gender.
Outcome and follow-up
Prior to her diagnosis of HAE, our patient had about 100 visits per year to the emergency department/hospitalisations for recurrent swelling and pain in her abdomen and joints. After starting the human C1-INH replacement (Cinryze) for long-term prophylaxis, she noted improvement in her quality of life and reduced number of sick days. Her rate of hospitalisation also decreased 10-fold. Currently, she has around 10 hospitalisations/year. Most of her breakthrough symptoms are managed successfully at home with recombinant C1-INH concentrate, Ruconest. At this time, due to insurance coverage, there has been a delay in the evaluation of her youngest daughter who has symptoms reminiscent of our patient.
A multidisciplinary approach between her primary care physician, rheumatologist, vascular surgeon, orthopedist, gastroenterologist and immunologist has been important in decreasing the risk of complications from these two complex disorders while ensuring that her quality of life is maintained.
Discussion
Our patient’s complex presentation of two coexisting rare disorders resulted in the under-recognition of these disease entities and misdiagnoses. Due to her frequent visits to the emergency department for complaints of recurrent abdominal and joint pain, she was misdiagnosed with chronic pain syndrome with possible drug-seeking tendencies. This initial bias lead healthcare providers to overlook other clinical manifestations (such as the abdominal distension and swelling of the digits) that would suggest alternative diagnoses. In regards to her recurrent abdominal pain, she had a cholecystectomy for suspected biliary dyskinesia and an exploratory laparotomy with lysis of adhesions. However, these interventions had minimal impact on resolving her recurrent abdominal pain.
Decades later, when a physician noted that she was hypermobile, EDS became a part of the differential diagnosis. Her arthralgias were attributed to the frequent joint hypermobility and subluxations. A spectrum of gastrointestinal manifestations is also present with EDS, such as abdominal pain with nausea, vomiting and diarrhoea (an irritable bowel-like syndrome) and gastroparesis, similar to her presentation. However, EDS could not explain all of her clinical manifestations such as the digital and facial swelling. This led our patient to seek out an immunologist to help provide further answers.
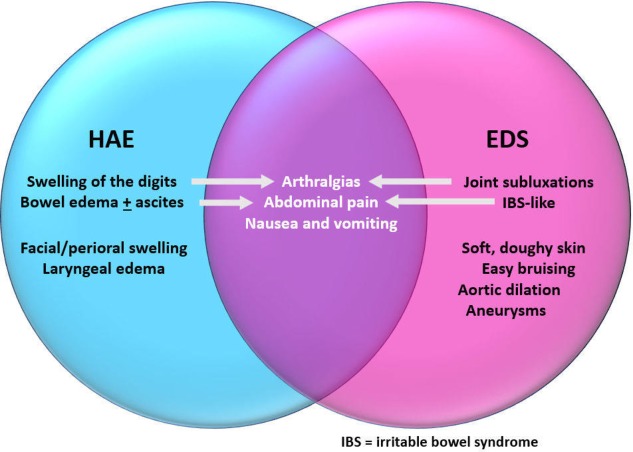
The recurrent gastrointestinal and musculoskeletal symptoms were overlapping manifestations of both HAE and EDS, with each disease process exacerbating the other (figure 3). In addition to these overlapping manifestations, she also had clinical characteristics of each disease entity. Although she did not exhibit the classical skin hyperextensibility, she had soft, doughy skin with recurrent subluxations since childhood. Additionally, she has multiple abdominal aneurysms requiring embolisation and a dilated aorta, all clues to EDS. The sensation of her ‘throat closing’ was likely related to laryngeal oedema for HAE. Fortunately, she had no acute laryngeal attacks requiring intubation.
Figure 3.
Manifestations of hereditary angioedema (HAE) and Ehlers-Danlos syndrome (EDS) in our patient with overlapping symptoms.
Manifestations of HAE result from the increase in vascular permeability due to excessive bradykinin production. Normally, C1-INH limits bradykinin production by inhibiting kallikrein and factor XIIa (both of which are needed to produce bradykinin from kininogen). However, in HAE where C1-INH is low or is dysfunctional, the pathway will favour production of bradykinin resulting in angioedema.1
Defects in collagen synthesis contribute to recurrent joint pain with subluxations/dislocations in EDS. In our patient, her musculoskeletal symptoms from EDS were exacerbated by subcutaneous oedema due to HAE. Extra-articular manifestations of EDS that she experienced included irritable bowel like syndrome (abdominal pain with diarrhoea) and multiple abdominal aneurysms – both of which have been documented in various forms of EDS.2 4 5 7
There is limited literature regarding immunological dysfunctions/disorders in EDS, particularly HAE. In a small study by Rodgers et al, they observed that HAE was more prevalent in the EDS population than the general US population.3 At this time, the association between these two disorders is not known due to lack of studies. In many retrospective analyses of EDS, only one article included HAE as part of their parameter of interest.
EDS and HAE are both rare disorders with adverse consequences if left undiagnosed. Both disorders have variable clinical expressions making it difficult to identify affected patients. Additionally, only a small percentage have known mutations in these disorders and the lack of finding a mutation does not exclude a clinical diagnosis. Awareness of these unusual disorders will result in early diagnosis with minimisation of negative outcomes. On average, the delay in diagnosis of HAE and EDS is 8–16 years.11 15 In addition to preventing adverse consequences from asphyxiation (from HAE) or arterial rupture (from EDS), early diagnosis is important in patient’s well-being as both of these chronic disorders have been associated with poor quality of life. Proper diagnoses will also reduce healthcare costs by preventing unnecessary procedures from misdiagnoses and decreasing hospitalisations.
Patient’s perspective.
I have struggled with pain and my body not working properly since I can remember. I would go to bed ‘skinny’ and wake up so ‘fat’ and swollen the next day. I had tons of abdominal swelling and pain and struggled to poop normally. My mom would find me in the middle of the night covered in throw up. As a kid I puked all the time and had severe painful diarrhoea, abdominal cramps and severe abdominal swelling. My ankles always rolled and gave out on me, my shoulders popped and cracked. I continued to struggle with pain and fatigue and different issues but the doctors kept thinking I was making it up or depressed. I found ways to deal with it, by watching what I ate, sleeping a lot, heating pads, baths etc. and was miserable but could somewhat function. I suffered amniotic fluid leak in both my pregnancies, severe tachycardia and abnormal heart rhythms. I was in constant pain, my joints were unstable, could barely eat, and my abdomen was swollen and in pain. I have had CT abdomen in the past reported as inflamed bowel walls but no explanations as to why. In 2014, I was still miserable so they took my gallbladder out and noted I had adhesion on my appendix, my colon, and my liver and gallbladder. I was still miserable and it took forever to heal. Obstetrics and Gynecology did an exploratory laparotomy after that and lysed some adhesions but could not find a reason. I was hospitalised again and they still could not figure it out. I went to a rheumatologist; he said a lab value was off but since my lips were not swollen enough I did not have it (did not say what it was) and that I was hyperflexible, but who cares, I am fine. I went to doctor after doctor and was constantly in the Emergency Department and begged people to help me. Finally I went to an amazing rheumatologist who listened to me and fully examined me and pulled out his book of rheumatoid rarities and said yes it sounds like you have Ehlers-Danlos syndrome and it explained some of my GI issues and the joints popping out of place but not everything. I kept searching and found blood draws that showed my C1 and C4 were low, I asked him about it and he said not sure and go see an immunologist. I found the most amazing immunologist and she examined me thoroughly and agreed I had EDS and thought my other symptoms sounded like hereditary angioedema so she repeated the blood test twice and both times I tested positive for HAE type 1 (low C4 and low C1). She also reviewed my ultrasound of the pelvis and concluded that my chronic pelvic pain was as a result of pelvic congestion syndrome. She started the process to get HAE meds approved and also ordered genetic testing for EDS. The EDS testing came back with PLOD1 and COL1A2 abnormal and she sent me to a geneticist at Children's Hospital. They agreed I have Ehlers-Danlos syndrome based on symptoms and clinical evaluation. I scored 6/9 on the Beighton/Brighton score and had the GI issues, joint issues, easy bruising, abnormal scarring, doughy soft pale skin, vascular issues etc. I finally got approved for Cinryze and Ruconest but I was still in the hospital every weekend after work and just cried myself to sleep every night and could not function. So my immunologist put me off work for 6 weeks, then 12, then permanently and things got better due to reduced stress. After starting the Cinryze 1000 units every Monday and Thursday and the Ruconest as needed for acute flare-ups, I went from over twelve acute episodes of angioedema a month and not working to now about 8–10 times per year. I suffered from severe pelvic congestion with very painful intercourse. I had 17 pelvic vein embolisations for dilated gonadal veins. My amazing immunologist together with my gastroenterologist, rheumatologist and orthopaedic doctor have helped me so much. I have had 16 surgeries in total. I had labrum repairs and fix impingement on both my hips, stabilise my patella in both knees, and stabilise and fix tears in both my shoulders, removal of cysts and clean up erosions in my right wrist, cardiac ablation, two MediPort surgeries etc. Some of my surgeries are not permanent fixes, they are just Band-Aids and may need to be repeated. All of which would not have been possible without preprocedural prophylaxis with my Cinryze since every little trauma or injury would cause me to swell and stay in the hospital for many days in the past. I have osteopenia. Having Ehlers-Danlos Syndrome and hereditary angioedema is hard at times. I sublux something and then the trauma triggers a massive swelling/pain. My abdomen swells and it makes my gastroparesis worse which then triggers a swelling, it can be a viscous and painful cycle. I am so lucky to have amazing doctors who help me manage everything but it is still a crapshoot when you go to the hospital. Because it is rare, and causes pain, and flares up a lot, doctors consider you a drug seeker. So you try to avoid the hospital. We need more awareness, so when you are that sick you get treated with care and consideration, not be made to feel bad when you are already in super bad shape. The Cinryze does help significantly but I still do get swelling because of everything else I have going on. And the Ruconest helps too but sometimes it works for a few hours then the swelling rebounds because of repeated trauma. However, overall, my HAE is much better controlled to the extent that I can avoid the hospital and have some quality in my life which was never the case before. I am so grateful to all my doctors specially my immunologist who was able to put all my symptoms and diagnoses together, and with my medications I am better managed now than I was but I am still debilitated and cannot work and cannot live the full life I want with my family. We need more research and awareness like this so we can figure out why someone has two rare genetic illnesses with no family history (now my daughter has EDS) and how to cure or at least better manage them. I have found a few other woman all over the world who have both EDS and HAE like me and I am fascinated to find out why and more of the science behind it. Thanks so much for taking the time to write this and for all of those reading this. Just remember sometimes you hear hoof-boot sounds but it is a zebra.
Learning points.
The clinical variability of hereditary angioedema (HAE) and Ehlers-Danlos syndrome (EDS) can decrease awareness of these disorders (on average, resulting in a delay of 8–16 years in diagnosis).
Lack of known genetic mutation does not negate a clinical diagnosis in EDS.
Recurrent joint pain with subluxations and hypermobility should prompt physicians to assess for underlying connective tissue disorders like EDS.
Although a rare diagnosis, healthcare providers should consider HAE in patients with recurrent abdominal pain and swelling, facial swelling along with complaints of ‘tight and taut’ skin, especially in the setting of low C4 and C1-esterase inhibitor levels.
If undiagnosed, detrimental consequences, including asphyxiation (from HAE) and arterial rupture (from EDS), can occur along with unnecessary procedures and increase in healthcare costs.
Footnotes
Contributors: Evaluation and diagnoses of this case were made by our attending physician, AA-M. M-TTD drafted the article with revisions of content by AA and AA-M. All authors gave final approval of this version of the article to be published.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Kerrigan MJ, Naik P. C1 Esterase Inhibitor Deficiency. [Updated 2019 Feb 5] : StatPearls [Internet]. Treasure Island (FL). StatPearls Publishing, 2019. https://www.ncbi.nlm.nih.gov/books/NBK538166/ [PubMed] [Google Scholar]
- 2. Syndrome E-D, Reference GH. Us national library of medicine., 2017. Available: https://ghr.nlm.nih.gov/condition/ehlers-danlos-syndrome [Accessed Last accessed March 22, 2019].
- 3. Rodgers KR, Gui J, Dinulos MBP, et al. Ehlers-Danlos syndrome hypermobility type is associated with rheumatic diseases. Sci Rep 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. D'hondt S, Van Damme T, Malfait F. Vascular phenotypes in nonvascular subtypes of the Ehlers-Danlos syndrome: a systematic review. Genet Med 2018;20:562–73. 10.1038/gim.2017.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shirley ED, Demaio M, Bodurtha J. Ehlers-Danlos syndrome in orthopaedics: etiology, diagnosis, and treatment implications. Sports Health 2012;4:394–403. 10.1177/1941738112452385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fogel S. Surgical failures: is it the surgeon or the patient? the all too often missed diagnosis of Ehlers-Danlos syndrome. Am Surg 2013;79:608–13. [PubMed] [Google Scholar]
- 7. Botrus G, Baker O, Borrego E, et al. Spectrum of gastrointestinal manifestations in joint hypermobility syndromes. Am J Med Sci 2018;355:573–80. 10.1016/j.amjms.2018.03.001 [DOI] [PubMed] [Google Scholar]
- 8. Bonamichi-Santos R, Yoshimi-Kanamori K, Giavina-Bianchi P, et al. Association of postural tachycardia syndrome and Ehlers-Danlos syndrome with mast cell activation disorders. Immunol Allergy Clin N Am 2018;38:497–504. 10.1016/j.iac.2018.04.004 [DOI] [PubMed] [Google Scholar]
- 9. Araki M, Lin Y, Ono H, et al. Application of immunotherapy for neurological manifestations in hypermobile Ehlers–Danlos syndrome. Ther Adv Neurol Disord 2018;11:1–5. 10.1177/1756286418793766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seneviratne SL, Maitland A, Afrin L. Mast cell disorders in Ehlers-Danlos syndrome. Am J Med Genet Part C Semin Med Genet 2017;175:226–36. 10.1002/ajmg.c.31555 [DOI] [PubMed] [Google Scholar]
- 11. Zanichelli A, Longhurst HJ, Maurer M, et al. Misdiagnosis trends in patients with hereditary angioedema from the real-world clinical setting. Ann Allergy Asthma Immunol 2016;117:394–8. 10.1016/j.anai.2016.08.014 [DOI] [PubMed] [Google Scholar]
- 12. Bork K, Hardt J, Witzke G. Fatal laryngeal attacks and mortality in hereditary angioedema due to C1-INH deficiency. J Allergy Clin Immunol 2012;130:692–7. 10.1016/j.jaci.2012.05.055 [DOI] [PubMed] [Google Scholar]
- 13. Valent P, Akin C, Arock M, et al. Definitions, criteria and global classification of mast cell disorders with special reference to mast cell activation syndromes: a consensus proposal. Int Arch Allergy Immunol 2012;157:215–25. 10.1159/000328760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maurer M, Magerl M, Ansotegui I, et al. The International WAO/EAACI guideline for the management of hereditary angioedema-The 2017 revision and update. Allergy 2018;73:1575–96. 10.1111/all.13384 [DOI] [PubMed] [Google Scholar]
- 15. Craig T, Busse P, Gower RG, et al. Long-Term prophylaxis therapy in patients with hereditary angioedema with C1 inhibitor deficiency. Ann Allergy Asthma Immunol 2018;121:673–9. 10.1016/j.anai.2018.07.025 [DOI] [PubMed] [Google Scholar]
- 16. Zuraw BL, Banerji A, Bernstein JA, et al. Us hereditary angioedema association medical Advisory board 2013 recommendations for the management of hereditary angioedema due to C1 inhibitor deficiency. J Allergy Clin Immunol Pract 2013;1:458–67. 10.1016/j.jaip.2013.07.002 [DOI] [PubMed] [Google Scholar]
- 17. Bova M, De Feo G, Parente R, et al. Hereditary and acquired angioedema: heterogeneity of pathogenesis and clinical phenotypes. Int Arch Allergy Immunol 2018;175:126–35. 10.1159/000486312 [DOI] [PubMed] [Google Scholar]