Abstract
Parvimonas micra (P. micra) is a Gram-positive anaerobic cocci, normally found in the oral cavity and rarely causes severe infections. We describe a rare clinical presentation of P. micra as spondylodiscitis and psoas abscess with haematogenous spread in an adult patient. MRI lumbar spine detected L2 and L3 spondylodiscitis. Blood cultures were positive at 48 hours of incubation and P. micra was identified on anaerobic culture after 72 hours. Isolates from bone biopsy confirms P. micra. She was successfully treated with ceftriaxone, followed by oral metronidzole for a total of 8 weeks. The suspected origin of her P. micra was a dental cavity. Anaerobic bacteria tend to be underestimated in spondylodiscitis. In cases of slow growing organisms, we emphasise the importance of performing accurate identification including anaerobic bacteria to guide management. P. micra should be considered in patients with spondylodiscitis who had recent dental intervention or perioral infection.
Keywords: bone and joint infections, unwanted effects / adverse reactions, dentistry and oral medicine, orthopaedics
Background
Spondylodiscitis (also termed vertebral osteomyelitis) most often results from haematogenous seeding from a distant focus, as the disc is avascular.1 2 Staphylococcus aureus is the most common microorganism implicated in spondylodiscitis due to its virulence, followed by Enterococcus coli (E. coli).2–4 Gram-positive anaerobic bacteria, such as Parvimonas micra (P. micra), are uncommon causes for spondylodiscitis. P. micra is normally found in the oral cavity, gastrointestinal and genitourinary tract. It is recognised as an important pathogen in intraoral infections and deep organ abscesses.5
Case presentation
A 77-year-old woman with a background history of cerebrovascular accident, hypertension, hyperlipidemia and osteoporosis, presented to our hospital complaining of acute on chronic lower back pain, aggravated by recent heavy lifting. She has no history of diabetes mellitus, immunosuppressive disorders or cancer. Her mobility was limited with difficulty in weight bearing due to lower back pain, compared with her baseline which was independent in all activities of daily living. There was minimal relief from regular ibuprofen and paracetamol. She had no sensory loss, bladder dysfunction or fevers. She had completed a course of ofloxacin 3 weeks ago for a urinary tract infection with urine cultures that grew E. coli. Neurological examination was normal with intact perianal sensation and normal anal tone. Straight leg raise of her left leg elicited pain in her lower back and there was tenderness on palpation of her lumbar spine at L2 and L3. There was no evidence of skin or soft tissue infections.
Investigations
Her laboratory findings were as follows: white cell count (WCC), 16.48×109/L; haemoglobin count, 115 g/L; platelet count, 338×109/L; C reactive protein (CRP) level, 90 mg/L; erythrocyte sediment rate (ESR) level, 77 mm/hour; serum total protein level, 61 g/L; albumin, 30 g/L; blood urea nitrogen level, 5.1 mmol/L; and creatinine level, 49 µmol/L. Urine cultures showed no growth. X-ray of lumbar spine was normal. T1-weighted MRI lumbar spine showed hypointensity at L2 and L3 vertebral bodies and L2–L3 disc space, suggestive of spondylodiscitis with the infection extending laterally causing a psoas abscess (figure 1A and B). There was no central spinal canal or neural foraminal stenosis. Initial blood cultures were positive for coagulase-negative staphylococci in the aerobic bottle only which was deemed likely to be a contaminant as the patient did not have any prosthetic material. A repeat set of blood cultures were positive at 48-hour incubation and Gram stain demonstrated Gram-positive cocci. P. micra was identified on anaerobic culture after 72 hours and was susceptible to penicillin, metronidazole and clindamycin based on European Committee on Antimicrobial Susceptibility Testing (EUCAST) methodology. Transcutaneous vertebral bone biopsy and disc aspirate confirmed the same organism as in blood cultures, P. micra.
Figure 1.
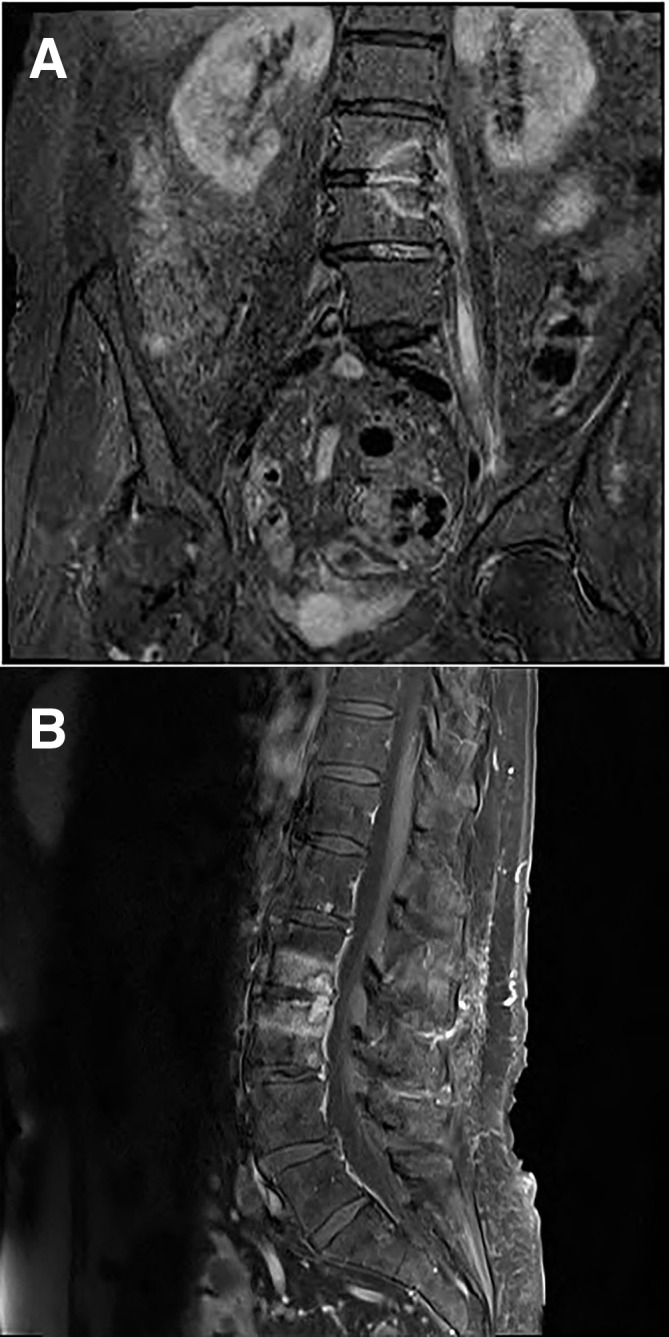
(A) MRI T2 fat suppressed coronal image of the lumbar spine showing L2–L3 high signal abnormality with involvement of the left psoas muscle in keeping with acute spondylodiscitis and psoas abscess formation. (B) MRI T1 fat suppressed sagittal image of the lumbar spine showing L2–L3 high signal abnormality with involvement of the left psoas muscle in keeping with acute spondylodiscitis and psoas abscess formation.
Differential diagnosis
Given her localised back pain aggravated by movements and elevated inflammatory markers, the main differential diagnosis was vertebral osteomyelitis. The patient was afebrile throughout her admission. Fever, however, is an inconsistent finding in bacterial vertebral osteomyelitis and is only present in ~45% of patients.1 In addition, this patient was taking regular analgesics with antipyretic effects. An epidural abscess was high in the differential diagnosis as spinal tenderness was noted on palpation. Pain aggravated by hip movements also increased our clinical suspicion of a psoas abscess. Vertebral compression fracture was also likely given her history of osteoporosis, but there was no wedging on her lumbar spine X-ray. Herniated disc was considered as her pain was aggravated after recent heavy lifting but this did not explain the high CRP and ESR.
Treatment
Following bone biopsy, the patient was started on intravenous ceftriaxone 2 g once daily (OD) and intravenous metronidazole to target the P. micra. Her decline in mobility from being independent of all activities to needing assistance to transfer out of bed warranted regular physiotherapy. Her pain was a limiting factor and an adequate pain control regime was commenced with regular paracetamol, opioids, non-steroid anti-inflammatory agents and gabapentin. She did not require surgical intervention and was transferred to rehabilitation unit to improve her mobility. She was continued on dual cover with intravenous ceftriaxone and oral metronidazole to facilitate outpatient parenteral antimicrobial therapy.
Outcome and follow-up
The patient completed an 8-week course of ceftriaxone and 5-week course of metronidazole. Her WCC and CRP trended down to normal within 1 week of antibiotics. A follow-up MRI lumbar spine after 6 weeks of antibiotics showed resolution of her psoas abscess and marked improvement in her spondylodiscitis (figure 2A and B). Prior to discharge, she regained her confidence in walking and was back to baseline. Her recovery was complicated by Clostridium difficile infection (CDI) which occurred 3 weeks after completion of her prolonged course of antimicrobial therapy. She was treated with oral metronidazole. She was seen in the outpatient 1 and 4 months after discharge. She was doing well and was discharged back to her primary care physician.
Figure 2.
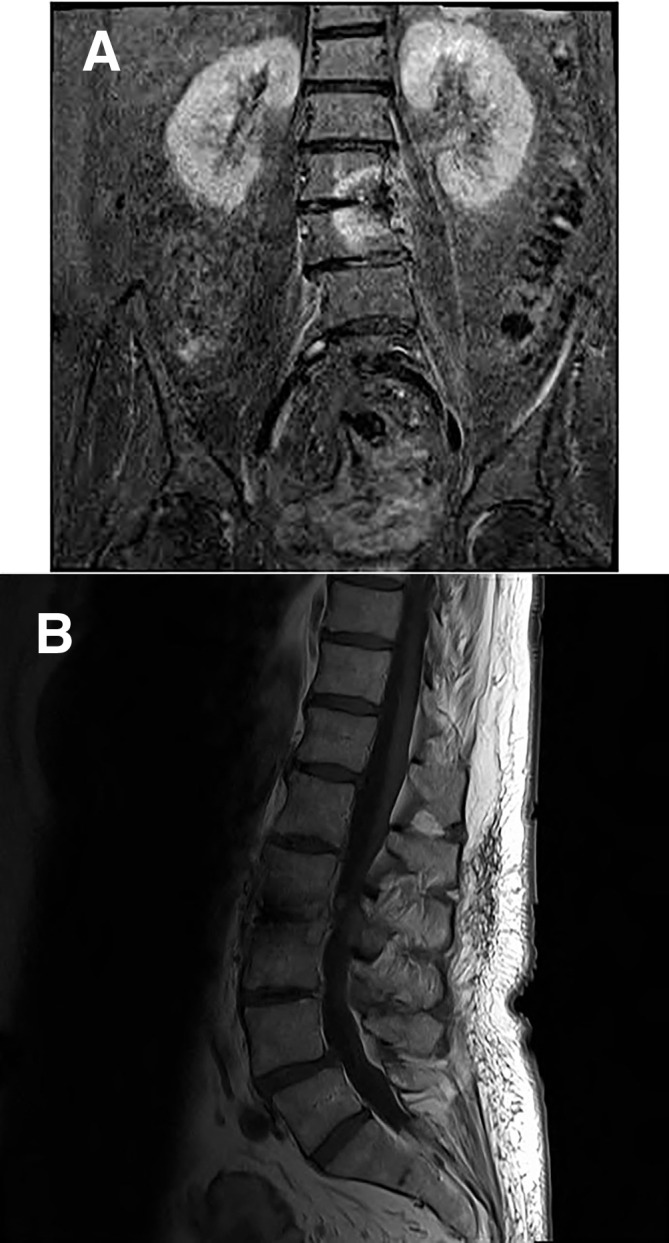
(A) MRI T2 fat suppressed coronal image of the lumbar spine post 6-week antibiotic treatment showing interval improvement with resolution of psoas abscess. (B) MRI T1 non-fat suppressed sagittal image of the lumbar spine post 6-week antibiotic treatment showing interval improvement with resolution of psoas abscess.
Discussion
P. micra is a Gram-positive anaerobic cocci that was originally known as Peptostreptococcus micros, the organism was renamed to the Micromonas genus in 1999, and then reclassified within the Parvimonas genus in 2006.6 Papasian et al 7 described the first reported vertebral osteomyelitis secondary to P. micra in 1986. Since then, there have been 16 reported cases of this rare pathogen in spinal infections.8 9 The diagnosis of this anaerobic bacterium can be challenging because they are slow growing. In this case, the blood cultures required 48 hours of incubation before flagging and the organism only grew on the anaerobic enrichment culture in the bone biopsy and aspirate. First-generation fluoroquinolones are inactive or marginally active against anaerobes,10 hence the course of ofloxacin was unlikely to have suppressed P. micra on her initial blood cultures. Owing to the widespread use of diagnostic technology such as matrix-assisted laser desorption ionisation-time of flight mass spectrometry and 16S rRNA sequencing,11 12 there has been an increasing number of P. micra being identified.8 13–17 Recently, Higashi et al described the melting temperature (Tm) mapping method which successfully detected P. micra in 3 hours, concluding it as a useful method in cases of bacterial infections where organisms are difficult to culture.8
Guidelines have suggested holding empiric antimicrobial therapy until a microbiological diagnosis has been reached in patients with stable haemodynamics and neurological examination, with an aim to increase the sensitivity of establishing a microbiological diagnosis.1 This case highlights the importance of appropriate investigations including blood cultures and bone biopsy to confirm the diagnosis and direct therapy as empiric therapy may not have been appropriate for P. micra. This indicates the importance of anaerobic culture as well as bacterial identification using modern diagnostic technology to allow rapid and accurate identification of pathogenic microorganisms from clinical specimens to guide management, especially when P. micra is rarely identified with our limited knowledge on its epidemiological background.
The Infectious Diseases Society of America 2015 guidelines recommend 6-week duration of parenteral antimicrobial therapy for most patients with bacterial vertebral osteomyelitis.1 Previous published case reports on P. micra spondylodiscitis involved antibiotic treatment with a mean total duration of 9 weeks (4–14 weeks); 15 cases involved treatment for >6 weeks and 5 cases were treated for >12 weeks.8 9 Murdoch5 reported that the strong proteolytic activity of P. micra could be important in abscess development. Dual cover with ceftriaxone and metronidazole was given initially in this patient due to the presence of a psoas abscess. She was stepped down to monotherapy to complete a total of 8 weeks of antimicrobials as she was not suitable for drainage of her psoas abscess.18
The use of broad-spectrum antimicrobials, multiple antibiotic agents and increased duration of antibiotic therapy contributed to her increased risk of CDI. The risk of hospital acquired-CDI remains greatest especially for third-generation cephalosporins19 and this patient was treated with ceftriaxone. A case–control study suggested that the risk for CDI was increased during antibiotic therapy and up to 3 months after cessation of therapy; the risk was highest during and in the first month after antibiotic use.20
The association of P. micros with oral infections has been reported previously.8 9 13 15–17 21 It is recognised as an important pathogen in intraoral infections.5 In this case, there was a tooth cavity identified that necessitated urgent extraction. The underlying cause for her P. micra spondylodiscitis was deemed secondary to a dental cavity.
To conclude, we describe herein a case of spondylodiscitis and psoas abscess due to P. micra secondary to a dental cavity, which was diagnosed based on blood cultures and bone biopsy. It highlights the importance of diagnostics to direct treatment for complex infections that will require prolonged antimicrobial therapy with inherent risks.
Learning points.
In patients with spondylodiscitis, it is reasonable to hold empiric antimicrobial therapy until the underlying microorganism is identified only if neurological examination and haemodynamics are stable.
Appropriate investigations are crucial to diagnose spondylodiscitis and to direct antimicrobial therapy, including use of modern diagnostic technology to allow rapid bacterial identification in cases of slow growing organisms.
Parvimonas micra should be considered in patients with spondylodiscitis who had recent dental intervention or perioral infection.
Prolonged antimicrobial therapy with broad spectrum antimicrobial therapy can lead to adverse events such as Clostridium difficile infection, with the highest risk during and in the first month after antibiotic use.
Footnotes
Contributors: LJHY drafted the initial manuscript. MDZ and MO were involved in direct patient care, edited and reviewed redrafts of the manuscript. RW reviewed drafts and contributed to writing and revision. All authors approved the final approval of the version to be published.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Berbari EF, Kanj SS, Kowalski TJ, et al. 2015 infectious diseases Society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clinical Infectious Diseases 2015;61:e26–46. 10.1093/cid/civ482 [DOI] [PubMed] [Google Scholar]
- 2. Zimmerli W. Clinical practice. vertebral osteomyelitis. N Engl J Med 2010;362:1022–9. 10.1056/NEJMcp0910753 [DOI] [PubMed] [Google Scholar]
- 3. Mylona E, Samarkos M, Kakalou E, et al. Pyogenic vertebral osteomyelitis: a systematic review of clinical characteristics. Semin Arthritis Rheum 2009;39:10–17. 10.1016/j.semarthrit.2008.03.002 [DOI] [PubMed] [Google Scholar]
- 4. Lew DP, Waldvogel FA. Osteomyelitis. The Lancet 2004;364:369–79. 10.1016/S0140-6736(04)16727-5 [DOI] [PubMed] [Google Scholar]
- 5. Murdoch DA. Gram-Positive anaerobic cocci. Clin Microbiol Rev 1998;11:81–120. 10.1128/CMR.11.1.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tindall BJ, Euzéby JP. Proposal of Parvimonas gen. nov. and Quatrionicoccus gen. nov. as replacements for the illegitimate, prokaryotic, generic names Micromonas Murdoch and Shah 2000 and Quadricoccus Maszenan et al. 2002, respectively. Int J Syst Evol Microbiol 2006;56:2711–3. 10.1099/ijs.0.64338-0 [DOI] [PubMed] [Google Scholar]
- 7. Papasian CJ, McGregor DH, Hodges GR, et al. Peptostreptococcal vertebral osteomyelitis. J Clin Microbiol 1986;24:633–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Higashi Y, Nakamura S, Niimi H, et al. Spondylodiscitis due to Parvimonas micra diagnosed by the melting temperature mapping method: a case report. BMC Infect Dis 2017;17:584 10.1186/s12879-017-2690-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Duijvenbode DC, Kuiper JWP, Holewijn RM, et al. Parvimonas micra spondylodiscitis: a case report and systematic review of the literature. J Orthop Case Rep 2018;8:67–71. 10.13107/jocr.2250-0685.1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Appelbaum PC. Quinolone activity against anaerobes. Drugs 1999;58:60–4. 10.2165/00003495-199958002-00012 [DOI] [PubMed] [Google Scholar]
- 11. Riggio MP, Lennon A, Smith A. Detection of Peptostreptococcus micros DNA in clinical samples by PCR. J Med Microbiol 2001;50:249–54. 10.1099/0022-1317-50-3-249 [DOI] [PubMed] [Google Scholar]
- 12. Veloo ACM, Erhard M, Welker M, et al. Identification of gram-positive anaerobic cocci by MALDI-TOF mass spectrometry. Syst Appl Microbiol 2011;34:58–62. 10.1016/j.syapm.2010.11.005 [DOI] [PubMed] [Google Scholar]
- 13. Uemura H, Hayakawa K, Shimada K, et al. Parvimonas micra as a causative organism of spondylodiscitis: a report of two cases and a literature review. International Journal of Infectious Diseases 2014;23:53–5. 10.1016/j.ijid.2014.02.007 [DOI] [PubMed] [Google Scholar]
- 14. Pilmis B, Israel J, Le Monnier A, et al. Spondylodiscitis due to anaerobic bacteria about a case of Parvimonas micra infection. Anaerobe 2015;34:156–7. 10.1016/j.anaerobe.2015.05.013 [DOI] [PubMed] [Google Scholar]
- 15. Endo S, Nemoto T, Yano H, et al. First confirmed case of spondylodiscitis with epidural abscess caused by Parvimonas micra. Journal of Infection and Chemotherapy 2015;21:828–30. 10.1016/j.jiac.2015.06.002 [DOI] [PubMed] [Google Scholar]
- 16. George IA, Pande A, Parsaei S. Delayed infection with Parvimonas micra following spinal instrumentation. Anaerobe 2015;35:102–4. 10.1016/j.anaerobe.2015.08.004 [DOI] [PubMed] [Google Scholar]
- 17. Jones SL, Riordan JW, Glasgow AL, et al. Two cases of spondylodiscitis caused by Parvimonas micra. Intern Med J 2015;45:1090–1. 10.1111/imj.12877 [DOI] [PubMed] [Google Scholar]
- 18. Park K-H, Cho O-H, Lee JH, et al. Optimal duration of antibiotic therapy in patients with hematogenous vertebral osteomyelitis at low risk and high risk of recurrence. Clin Infect Dis. 2016;62:1262–9. 10.1093/cid/ciw098 [DOI] [PubMed] [Google Scholar]
- 19. Slimings C, Riley TV. Antibiotics and hospital-acquired Clostridium difficile infection: update of systematic review and meta-analysis. J Antimicrob Chemother 2014;69:881–91. 10.1093/jac/dkt477 [DOI] [PubMed] [Google Scholar]
- 20. Hensgens MPM, Goorhuis A, Dekkers OM, et al. Time interval of increased risk for Clostridium difficile infection after exposure to antibiotics. J Antimicrob Chemother 2012;67:742–8. 10.1093/jac/dkr508 [DOI] [PubMed] [Google Scholar]
- 21. Gahier M, Cozic C, Bourdon S, et al. Spinal infections caused by Parvimonas micra. Médecine et Maladies Infectieuses 2015;45:397–8. 10.1016/j.medmal.2015.07.006 [DOI] [PubMed] [Google Scholar]


