Abstract
We present a case of a 63-year-old woman with an acute history of abdominal distension and shortness of breath. She had no risk factors for liver disease though her prior medical history was positive for breast carcinoma, in remission for 14 years. Examination and investigations were initially consistent with decompensated cirrhosis, thought to be due to subclinical autoimmune hepatitis. Imaging revealed hepatic contour irregularity, atrophy of the liver parenchyma and numerous lesions highly suggestive for multifocal hepatocellular carcinoma. Surprisingly, tissue histology revealed no evidence of cirrhosis, but recurrence of breast cancer which had mimicked cirrhosis. Pseudocirrhosis may be indistinguishable from true cirrhosis without histopathology. It has previously been linked to chemotherapy-induced hepatic injury and nodular regenerative hyperplasia, although our case illustrates an uncommon pathophysiology. Pseudocirrhosis often represents a poor prognosis even with a good baseline performance status, and early involvement of palliative care specialists may be advisable.
Keywords: Cirrhosis, Breast cancer
Background
Breast cancer and decompensated cirrhosis are common presentations to acute medical teams worldwide. Although pseudocirrhosis has been previously described in case studies, which have predominantly linked it to ongoing chemotherapy for known cancers, our case illustrates an atypical presentation which led to diagnostic uncertainty. In presenting this case, we hope to raise awareness of pseudocirrhosis, which can mimic true cirrhosis and can only truly be excluded with histopathological analysis. As described, even with a good performance status it appears to confer a poor prognosis.
Case presentation
A 63-year-old woman was referred to our gastroenterology outpatient clinic with deranged liver function tests, progressive abdominal distension and shortness of breath. She denied weight loss and changes to appetite or bowel habits, and no other symptoms were identified. There was no personal or family history of liver disease, no history of excessive alcohol consumption, no risk factors for non-alcoholic fatty liver disease, or relevant drug history. Body Mass Index (BMI) in clinic was 22.5 kg/m2. WHO performance status was 1.
Her prior medical history was significant for carcinoma of the left breast diagnosed 14 years previously (T2 G2 N1, human epidermal growth factor receptor 2 negative, progesterone receptor positive 30%, no Allred score documented), which was treated radically with four cycles of neoadjuvant FEC-T and mastectomy with axillary clearance (7/21 lymph node positive, N1 staging per guidelines at the time, N2 per current classification). She subsequently completed 5 years of adjuvant hormonal therapy with 1 year of tamoxifen changed to anastrozole for a further 4 years due to menopausal status. She was discharged from follow-up in 2009 with no evidence of recurrence.
Investigations
Examination identified a mildly distended abdomen, shifting dullness consistent with ascites and a non-tender firm mass on deep palpation of the right upper abdominal quadrant. There were no peripheral stigmata of chronic liver disease. She was admitted for further diagnostic tests and symptom management. Liver function tests (table 1) were deranged in a mixed obstructive/hepatitic pattern, but synthetic function was unaffected. Serum IgA was elevated, and autoantibody tests were positive only for antinuclear antibodies (IgG titre 1:160; speckled pattern). Viral screening was negative, and tumour markers showed an elevated CA-125 but normal alpha-fetoprotein. Paracentesis showed transudative fluid, with a serum-ascites albumin gradient of 2.2 g/dL.
Table 1.
Initial tests on admission
| Test (unit) | Result (range) |
| PT (s) | 13.3 (9.8–14.6) |
| PLT (x109/L) | 198 (150–450) |
| Albumin (g/L) | 35 (35–50) |
| ALT (IU/L) | 96 (1–34)* |
| AST (IU/L) | 141 (12–34)* |
| Bilirubin (μmol/L) | 50 (3–21)* |
| GGT (IU/L) | 908 (0–37)* |
| ALP (IU/L) | 955 (30–130)* |
| IgG (g/L) | 14.5 (6–16) |
| IgA (g/L) | 4.9 (0.8–4) |
| IgM (g/L) | 1.3 (0.5–2) |
| ANA | 1:160, speckled* |
| Anti-DNA (IU/mL) | 1 (0–15) |
| Anti-ENA | -ve |
| AMA | -ve |
| ASMA | -ve |
| Anti-GPC | -ve |
| Anti LKM-1 | -ve |
| Hepatitis B | -ve |
| Hepatitis C | -ve |
| HIV | -ve |
| CMV | -ve |
| EBV | -ve |
| AFP (kU/L) | 3 (0–7) |
| CA-125 (kU/L) | 1632 (0–35)* |
*Abnormal results.
AFP, Alphafetoprotein; ALT, Alanine Aminotransferase; ANA, Anti Nuclear Antibodies; ASMA, Anti Smooth Muscle Antibodies; AST, Aspartate Aminotransferase; CMV, Cytomegalovirus; ENA, Extractable Nuclear Antigen; GGT, Gamma-glutamyl Transferase; GPC, Gastric Parietal Cell; LKM-1, Anti Liver Kidney Microsomal type 1 antibody; PLT, Platelet Count; PT, Prothrombin Time.
Abdominal ultrasound showed a heterogeneous liver with irregular contour and focal change in the left lobe suggestive for a mass lesion, marked ascites, a patent portal vein, no varices and no splenomegaly. A chest radiograph showed a large left-sided pleural effusion. A staging CT showed a lobulated shrunken liver with a large mass in the left lobe segment 2–3, and multiple hypoechoic lesions of varying size and shape associated with significant ascites but no splenomegaly (figure 1). In addition to the left-sided pleural effusion, two lesions were identified in the right lung, along with several sclerotic vertebral lesions. MRI confirmed the CT findings, with additional obliteration of the left branch of the portal vein. The hepatic contour irregularity and atrophy of the liver parenchyma, combined with numerous liver lesions involving all hepatic segments (figure 2) were initially suggestive of multifocal hepatocellular carcinoma with possible lung metastases. The working diagnosis was a hepatocellular carcinoma on a background of liver cirrhosis possibly due to idiopathic end-stage subclinical autoimmune hepatitis. Following a multidisciplinary team discussion, the patient underwent therapeutic paracentesis and percutaneous ultrasound-guided liver biopsy.
Figure 1.
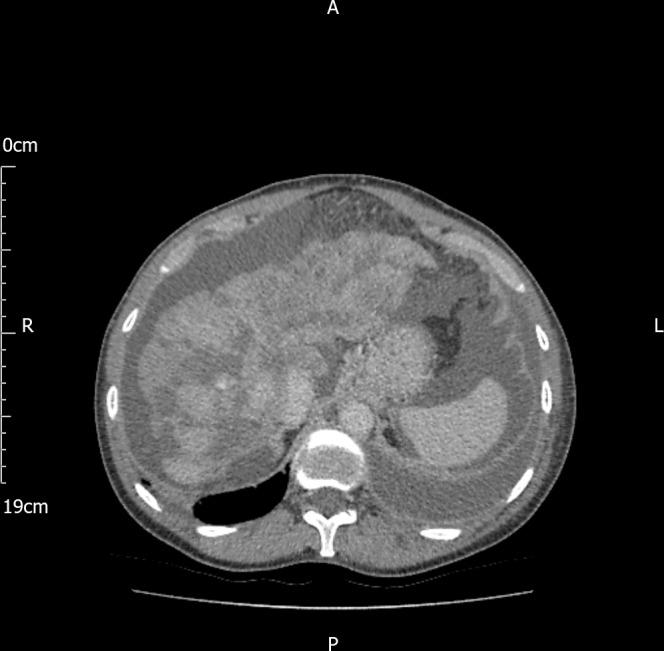
CT showed a lobulated liver with multiple hypoechoic lesions associated with significant ascites.
Figure 2.
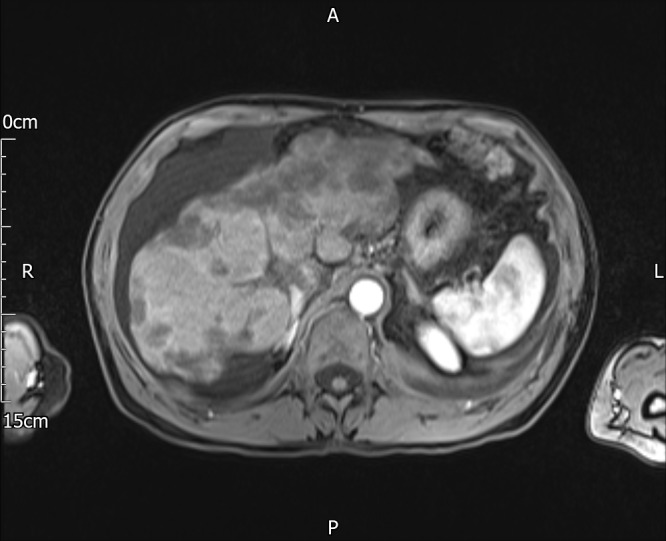
MRI showed numerous liver lesions involving with atrophy of the liver parenchyma and irregularity of the contour reported as marked liver cirrhosis.
Microscopy showed clusters of cells with high nuclear:cytoplasmic ratio and cytoplasmic vacuolation. Immunohistochemistry tested positive for cytokeratin 7 (CK7) and oestrogen receptor, and negative for thyroid transcription factor 1 (TTF1), in keeping with adenocarcinoma of the breast (figures 3 and 4). Reticulin and trichrome stains showed no evidence of established cirrhosis in the background liver (figure 5). A diagnosis of metastatic breast cancer with pseudocirrhosis was established.
Figure 3.
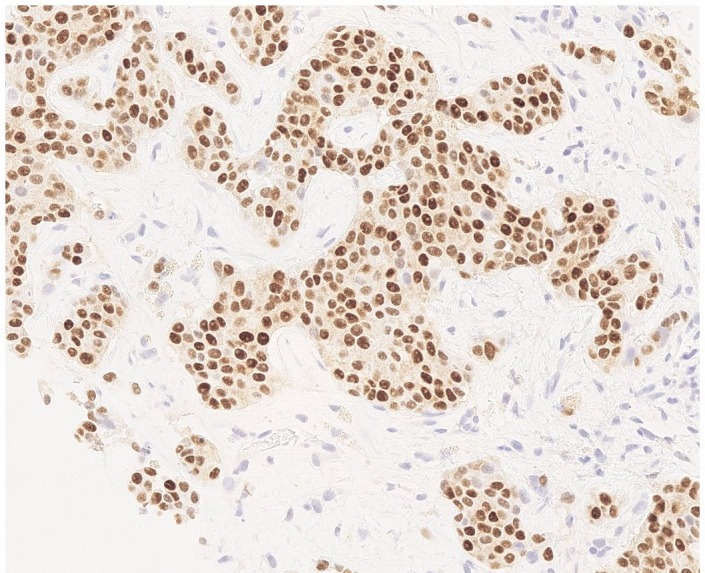
Liver biopsy, oestrogen receptor (ER) immunohistochemistry. Strong nuclear ER expression in metastatic breast carcinoma.
Figure 4.
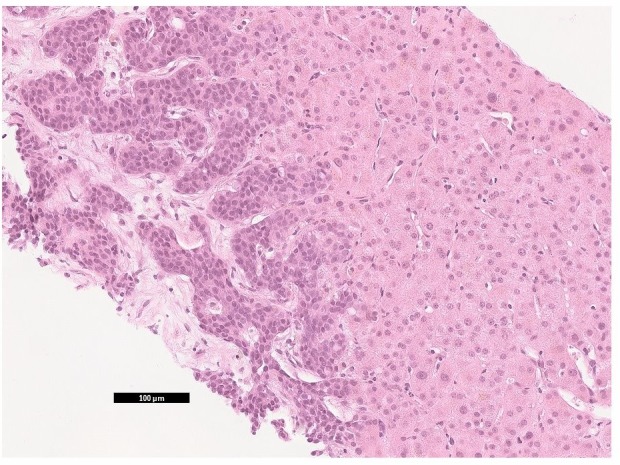
Liver biopsy H&E staining. Metastatic breast carcinoma (left) in liver parenchyma.
Figure 5.
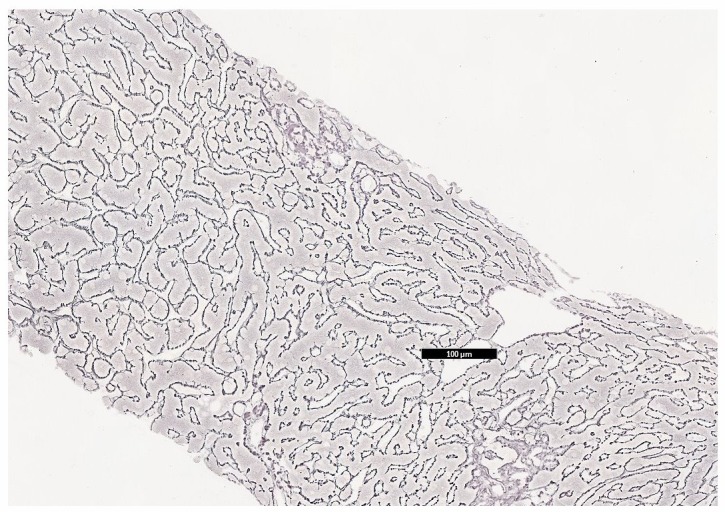
Liver biopsy, reticulin stain. Demonstrating otherwise normal liver architecture.
Outcome and follow-up
The patient was discharged from gastroenterology and started on endocrine manipulation therapy. She was readmitted with refractory hydropneumothorax and following video-assisted thoracoscopic pleurodesis subsequently died of hepatorenal syndrome 5 weeks after her initial presentation.
Discussion
Pseudocirrhosis is a radiological diagnosis which refers to the morphological hepatic contour changes and diffuse nodularity seen in malignant disease,1 which may be indistinguishable from cirrhosis in the absence of histopathology and previous imaging.2 The term itself can be misleading, as patients present with clinical symptoms of portal hypertension. In a reported case series (n=6) of patients with established breast carcinoma, abdominal distension with ascites was the most common presenting complaint.3 It has been reported in several different cancers, including oesophageal,4 pancreatic,5 colorectal6 and thyroid,7 though the majority of cases have been seen with breast cancer primary.
Studies to date have been limited; however, some evidence suggests that isolated hepatic contour abnormalities without clinical portal hypertension are not uncommon findings in known breast cancer with liver involvement.8 An early retrospective analysis of serial CT scans from female breast cancer patients with liver involvement reported varying severity of hepatic contour abnormalities in 74% (n=68) of patients.9
The pathophysiological mechanisms accounting for pseudocirrhosis have not been reliably elicited, due to the relative rarity of the condition, and absence of histopathological correlation in case reports and retrospective studies. The prevalent theory is that chemotherapy induces hepatic ischaemic atrophy, with secondary nodular regenerative hyperplasia (NRH) in areas of preserved blood flow.10 Young et al retrospectively reviewed images for 22 patients with breast cancer and liver metastases undergoing systemic chemotherapy, in whom abdominal CT images were consistent with pseudocirrhosis.11 In serial CT scans, progression of disease was seen over weeks, suggestive of hepatotoxicity rather than true cirrhosis, which develops more slowly. Tissue analysis was available for six patients. Histopathology showed three cases of confirmed, and three cases highly suggestive for NRH. No patients had any evidence of bridging portal fibrosis, and all specimens had residual tumour.
Tumour shrinkage in response to chemotherapy, with subsequent severe desmoplastic fibrosis around liver metastases has also been proposed as a mechanism.2 In a reported case series of breast cancer patients with pseudocirrhosis, all five patients developed liver failure despite decreases in the liver tumour burden postchemotherapy.12
In the majority of published cases, pseudocirrhosis arose in the context of progression of known malignancy undergoing systemic chemotherapy. This case illustrates an atypical presentation due to the predominance of presenting symptoms consistent with portal hypertension and chronic liver disease, and that our patient had not been exposed to chemotherapy agents or endocrine manipulators for 14 and 9 years, respectively. As such, chemotherapy-induced hepatic injury and NRH could not account for this presentation. This is further supported by the absence of fibrotic tissue in the residual liver seen on biopsy. Rather, we believe that the large burden of macro and micro deposits may have resulted in sinusoidal obstruction and secondary obstructive portal hypertension. This led to clinical ascites and ultimately death from hepatorenal syndrome, which is otherwise almost exclusively seen in cirrhosis and fulminant liver failure.13
Gastroenterologists and radiologists should remain aware of conditions which successfully mimic cirrhosis due to their clinical presentation and imaging findings. As seen in both this and previously reported cases, pseudocirrhosis appears to confer poor prognostic outcomes and early recognition may allow for closer disease monitoring, and early involvement of palliative care specialists with greater focus on symptomatic control.
Learning points.
Pseudocirrhosis is a rare diagnosis which may be indistinguishable from true cirrhosis without tissue analysis.
Clinically, patients can present with evidence of decompensated liver disease and portal hypertension.
It has previously been linked to chemotherapy-induced hepatic injury and secondary nodular regenerative hyperplasia, although our case illustrates a different pathophysiology.
Pseudocirrhosis is indicative of poor prognosis even with a good baseline performance status, and early involvement of palliative care specialists may be advisable.
Footnotes
Contributors: MA and IT were responsible for conception, consent and manuscript drafting. MWG was responsible for data and imaging acquisition and manuscript review. GM was the patient’s lead consultant and was responsible for manuscript review and clinical accuracy.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Jha P, Poder L, Wang ZJ, et al. Radiologic mimics of cirrhosis. AJR Am J Roentgenol 2010;194:993–9. 10.2214/AJR.09.3409 [DOI] [PubMed] [Google Scholar]
- 2. Mamone G, Cortis K, Sarah A, et al. Hepatic morphology abnormalities: beyond cirrhosis. Abdom Radiol (NY) 2018;43:1612–26. 10.1007/s00261-017-1351-9 [DOI] [PubMed] [Google Scholar]
- 3. Adike A, Karlin N, Menias C, et al. Pseudocirrhosis: a case series and literature review. Case Rep Gastroenterol 2016;10:381–91. 10.1159/000448066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kobashigawa C, Nakamoto M, Hokama A, et al. Pseudocirrhosis in metastatic esophageal cancer. South Med J 2010;103:488–9. 10.1097/SMJ.0b013e3181d82d50 [DOI] [PubMed] [Google Scholar]
- 5. Kang SP, Taddei T, McLennan B, et al. Pseudocirrhosis in a pancreatic cancer patient with liver metastases: a case report of complete resolution of pseudocirrhosis with an early recognition and management. World J Gastroenterol 2008;14:1622–4. 10.3748/wjg.14.1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Battisti S, Guida FM, Pagliara E, et al. Pseudocirrhosis after anti-EGFR-based neoadjuvant therapy for hepatic metastasis from colon cancer: a different point of view. Clin Colorectal Cancer 2014;13:e13–15. 10.1016/j.clcc.2014.06.003 [DOI] [PubMed] [Google Scholar]
- 7. Harry BL, Smith ML, Burton JR, et al. Medullary thyroid cancer and pseudocirrhosis: case report and literature review. Curr Oncol 2012;19:e36–41. 10.3747/co.19.840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fennessy FM, Mortele KJ, Kluckert T, et al. Hepatic capsular retraction in metastatic carcinoma of the breast occurring with increase or decrease in size of subjacent metastasis. AJR Am J Roentgenol 2004;182:651–5. 10.2214/ajr.182.3.1820651 [DOI] [PubMed] [Google Scholar]
- 9. Qayyum A, Lee GK, Yeh BM, et al. Frequency of hepatic contour abnormalities and signs of portal hypertension at CT in patients receiving chemotherapy for breast cancer metastatic to the liver. Clin Imaging 2007;31:6–10. 10.1016/j.clinimag.2006.09.028 [DOI] [PubMed] [Google Scholar]
- 10. Jeong WK, Choi S-Y, Kim J. Pseudocirrhosis as a complication after chemotherapy for hepatic metastasis from breast cancer. Clin Mol Hepatol 2013;19:190–4. 10.3350/cmh.2013.19.2.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Young ST, Paulson EK, Washington K, et al. Ct of the liver in patients with metastatic breast carcinoma treated by chemotherapy: findings simulating cirrhosis. AJR Am J Roentgenol 1994;163:1385–8. 10.2214/ajr.163.6.7992734 [DOI] [PubMed] [Google Scholar]
- 12. Sonnenblick A, Appelbaum L, Peretz T. Liver failure on the background of pseudocirrhosis in patients with liver metastasis from breast cancer, who responded to treatment. Onkologie 2011;34:199–201. 10.1159/000327010 [DOI] [PubMed] [Google Scholar]
- 13. Wadei HM, Mai ML, Ahsan N, et al. Hepatorenal syndrome: pathophysiology and management. Clin J Am Soc Nephrol 2006;1:1066–79. 10.2215/CJN.01340406 [DOI] [PubMed] [Google Scholar]


