Abstract
Statin-induced immune-mediated necrotising myopathy (IMNM) is a rare but increasingly recognised myositis. Many cases have positive antibodies to 3-hydroxy-3-methylglutaryl coenzyme A reductase (anti-HMGCR). The current treatment is ceasing the statin, but often immunosuppressive therapy is required as the antibodies persist, causing muscle necrosis. Despite the use of immunosuppressive medications, most commonly prednisolone, methotrexate, plasma exchange and/or intravenous immunoglobulin, some patients do not respond. We report the successful treatment with rituximab therapy for three patients with IMNM with positive anti-HMGCR antibodies. All three patients with statin-induced IMNM were elderly, with a disease history of 7–9 years, and had failed several immunosuppressive agents. They responded well to rituximab (induction and maintenance) therapy. They remain in remission with no symptoms and normal creatine kinase. One patient had normalisation of anti-HMGCR antibody level, and one patient’s antibody level reduced significantly. Rituximab is an effective immunosuppressive treatment for patients with refractory IMNM.
Keywords: neurology (drugs and medicines), muscle disease, neuromuscular disease
Background
Since the discovery of antibodies to 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR) in patients with immune-mediated necrotising myopathy (IMNM) in 2010, many cases have been reported in the literature, and most have been related to statin use.1–4 Approximately 1 in every 10 000 statin-treated persons per year progress to develop a severe myopathy marked by weakness and elevated creatine kinase (CK), while 2 to 3 in every 100 000 develop an IMNM.5
As a rare disease, no randomised controlled trials examining treatment efficacy are available. Current management is cessation of the statin and initiation of immunosuppressive therapy. The commonly used immunosuppressive therapies include corticosteroids, methotrexate, intravenous immunoglobulin (IVIG) and, less commonly, azathioprine, cyclosporine, therapeutic plasma exchange (TPX) and mycophenolate mofetil.2 6–9 In a review of 100 cases of statin-associated necrotising autoimmune myopathy (SANAM), many patients required multiple combination therapies, commonly steroid and methotrexate and an addition of IVIG; 84% of patients required two or more immunosuppressants.4 Although it is rational to use the anti-CD20 antibody rituximab for this antibody-mediated disorder as adjunct therapy, it has rarely been reported. Of the 100 cases of SANAM reviewed, 57 cases were tested for anti-HMGCR antibody and were all positive, and among them, only two cases were treated with rituximab.4
Case presentation
We report three consecutive cases from a neuromuscular clinic. The follow-up period varied between 7 and 9 years from their initial presentation through to July 2018.
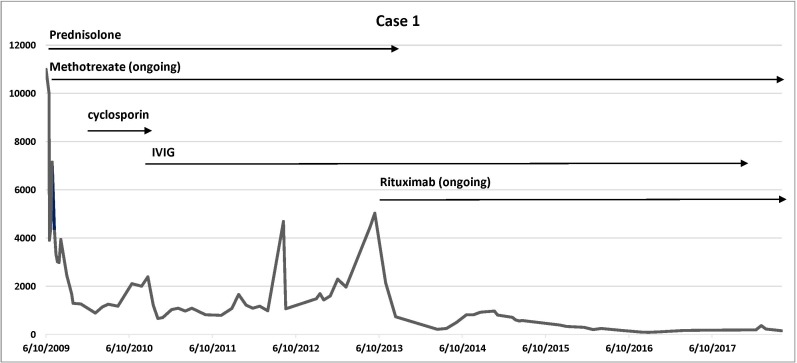
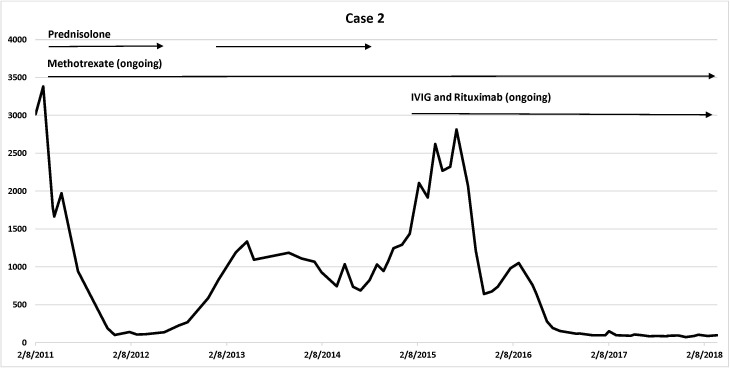
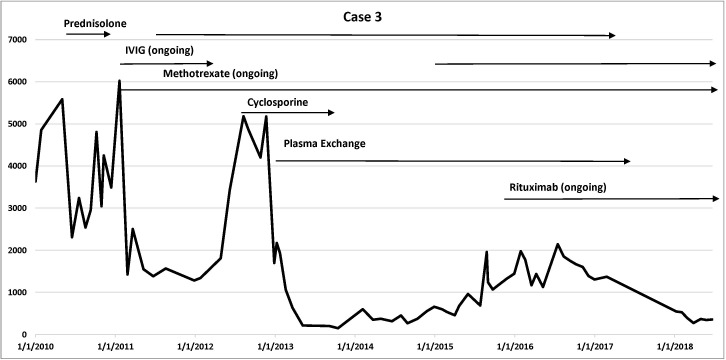
Cases details are summarised in table 1. Disease trajectory with corresponding CK levels and medications use are presented in figure 1 (case 1), figure 2 (case 2) and figure 3 (case 3).
Table 1.
Summarisation of cases
| Cases | Case 1 | Case 2 | Case 3 |
| Age of onset (years) | 60 | 68 | 62 |
| Gender | Female | Female | Male |
| Statin | Atorvastatin and rosuvastatin | Atorvastatin | Atorvastatin |
| Duration from symptom onset to presentation | 2 months | 1 month | 12 months |
| Duration from commencement of statin to symptom onset | Atorvastatin: few months Rosuvastatin: 2 months |
Atorvastatin: 3 years | Atorvastatin: 3 years |
| Pattern of weakness | Proximal upper and lower limbs | Proximal upper and lower limbs | Proximal, especially hip girdle |
| Initial peak CK | 11 003 | 3900 | 5000 |
| Anti-HMGCR antibody at presentation | 54.4 RU (normal range <11.0 RU) | 49.0 RU (normal range <11.0 RU) | 0.84 (normal <0.24 OD units) |
| Muscle biopsy | Focal segmental necrotising myopathy with minimal inflammation | Moderate necrotising myopathy with minimal inflammation | Necrotising myopathy |
| Immunotherapies trialled | Prednisolone Methotrexate Cyclosporine IVIG |
Prednisolone Methotrexate Cyclosporine IVIG |
Prednisolone Methotrexate Cyclosporine IVIG Plasma exchange |
| Current immunotherapy | Methotrexate Rituximab |
Methotrexate IVIG Rituximab |
Methotrexate IVIG Rituximab |
| Current status | Stable CK (<100 U/L) Normal anti-HMGCR antibody level Mild weakness |
Stable CK (200~300 U/L) Reduced anti-HMGCR antibody level Mild weakness |
Stable CK (100~300 U/L) Anti-HMGCR antibody level still elevated Mild weakness |
anti-HMGCR, antibodies to 3-hydroxy-3-methylglutaryl coenzyme A reductase; CK, creatine kinase; IVIG, intravenous immunoglobulin; OD, Optical Density; RU, Reference Unit.
Figure 1.
Case 1 disease trajectory: corresponding CK levels and medications use. Vertical axis: CK level; horizontal axis: timeline. CK, creatine kinase; IVIG, intravenous immunoglobulin.
Figure 2.
Case 2 disease trajectory: corresponding CK levels and medications use. Vertical axis: CK level; horizontal axis: timeline. CK, creatine kinase; IVIG, intravenous immunoglobulin.
Figure 3.
Case 3 disease trajectory: corresponding CK levels and medications use. Vertical axis: CK level; horizontal axis: timeline. CK, creatine kinase; IVIG, intravenous immunoglobulin.
All cases had muscle biopsy and positive anti-HMGCR antibody. All cases had been screened for malignancy and other potential causes of secondary myositis.
The rituximab regimen followed a ‘lymphoma protocol’ with an induction dose of 375 mg/m2 every week for 4 weeks, followed by a maintenance dose of 375 mg/m2 every month. Further dose frequency reduction was tailored to individual patients. Rituximab has been well tolerated in all patients with no adverse side effects reported as part of their combination therapy.
Case 1 was a 60-year-old woman who presented in 2009 with a 2 months’ history of progressive proximal limb weakness and muscle pain. At presentation, she had significant functional impairment, with difficulty walking upstairs or inclining and getting out of a bed, chair or car. She had a mild waddling gait and a moderate degree of proximal weakness of both upper and lower limbs. There was mild thinning of the quadriceps muscles bilaterally. The muscle strength testing was performed as per the Medical Research Council of the United Kingdom. In the upper limbs, shoulder abduction, elbow flexion and elbow extension power were 3/5. In the lower limbs, hip flexion was 3/5; hip extension was 4/5; knee flexion was 4/5; and knee extension was 3/5. Distal strength at the ankles was normal. Reflexes were depressed at 1+ at the knees and ankles, and sensation testing was normal.
She had well-controlled diabetes mellitus, psoriatic arthritis, hypertension and sleep apnoea. Ten years previously, atorvastatin had been prescribed for dyslipidaemia, but it was ceased due abnormal liver function tests. Two months prior to symptom onset, rosuvastatin and pioglitazone were commenced, which were then ceased shortly after the onset of muscle symptoms.
At presentation, her peak CK was elevated at 11 003 U/L (normal range<200 U/L). Muscle biopsy of the left vastus lateralis showed evidence of a focal segmental necrotising myopathy with a number of degenerative and regenerating fibres and minimal inflammation.
There was a significant improvement in both CK level and muscle strength, with a 3-day pulse of intravenous methylprednisolone 1 g and then oral prednisolone 50 mg daily, as well as oral methotrexate 22.5 mg once a week with folate supplementation. With a mild relapse, she received oral cyclosporine 50 mg daily with no benefit. She then received an induction dose of IVIG, followed by a maintenance dose of IVIG every 4 weeks. She remained in remission for 3 years with a combination therapy of oral methotrexate 22.5 mg once a week and regular IVIG every 4 weeks. Prednisolone was gradually weaned and ceased due to her diabetes mellitus.
In late 2012, she had another relapse with significant worsening weakness and elevation of CK to 5000 U/L. She was commenced on rituximab induction therapy using the ‘lymphoma induction protocol’, which was followed by maintenance dose every month for 6 months, and the frequency of dosing was then reduced to every 3 months and later extended to every 6 months as she clinically improved. Her IVIG was weaned and discontinued in 2017.
She has been followed up for 9 years. She responded very well with good improvement in strength, normalisation of CK and reduction of the anti-HMGCR antibody level from 54.4 reference unit (RU) (normal range<11.0 RU) in 2014 to 8.9 RU in May 2018.
Case 2 was a 68-year-old woman who had been taking atorvastatin for 3 years for hyperlipidaemia. She presented with a 2 months’ history of progressive proximal limbs weakness. Her initial symptoms progressed rapidly from full independence to a moderate degree of proximal weakness within 1 month. The atorvastatin was ceased. She was unable to get out of bed or to mobilise independently. There was mild muscle wasting of the proximal muscles in the thigh. Detailed muscle power examination of the upper limbs demonstrated bilateral shoulder abduction of 3/5 and elbow flexion and extension of 3/5. The power assessment in the lower limbs showed hip abduction power of 4/5, hip flexion of 3/5, and hip extension, knee flexion and extension of 4/5 with normal distal power at the ankles. The reflexes in the upper and lower limbs were depressed, and there was normal sensation.
The peak CK at presentation was 3900 U/L. The muscle biopsy of the left vastus lateralis muscle was reported as a necrotising myopathy with minimal inflammation.
She had good clinical response to oral prednisolone 60 mg daily and oral methotrexate 25 mg every week with folate supplementation for 4 years. Several months after the prednisolone was weaned off, she had a relapse with increasing CK and worsening muscle weakness. Another trial of prednisolone was avoided due to severe osteoporosis. She was commenced on IVIG every 4 weeks and rituximab induction therapy, which was followed by maintenance rituximab dose every 8 weeks.
She reached remission again with rituximab in combination with methotrexate 20 mg orally once per week and IVIG every 4 weeks with only mild proximal weakness and normalisation of CK at 7 years of follow-up. Her anti-HMGCR antibody level dropped from 49 RU (normal range<11.0 RU) at presentation to 15 RU after therapy.
Case 3 was a 62-year-old man who presented in 2009 with a 12 months’ history of proximal lower limb weakness, particularly affecting the hip girdle. Atorvastatin had been commenced for management of coronary artery disease 3 years earlier. He developed tightness and stiffness in the hamstring muscles without obvious weakness, followed by progressive weakness in the hip girdle muscles with difficulty getting in and out of his truck or walk up an incline. Atorvastatin was ceased in November 2009, with an initial CK of 2000 U/L, but the CK rose further to 5000 U/L in May 2010. He was managed with a short course of prednisolone 50 mg daily for 3 months, during which period he noticed a mild increase in proximal leg weakness. Prednisolone was then ceased. He was not on any immunosuppressive medications until early 2011.
His neurological examination at presentation to our neuromuscular clinic in 2011 showed mild bilateral proximal upper limbs and proximal hip girdle muscle weakness. Power assessment of shoulder abduction and elbow extension was 4/5; hip flexion was 3/5; and hip extension, abduction and adduction were 4/5. Distal strength of both upper and lower limbs was normal. Reflexes were mildly depressed and sensation was normal.
A muscle biopsy of the left vastus lateralis muscle was reported as consistent with a necrotising myopathy.
He had a good response to combination therapy with recommencement of high-dose oral prednisolone at 50 mg daily with the addition of oral methotrexate 20 mg once per week with folate supplementation, and induction and then regular IVIG (every 4 weeks). After a few relapses during the attempts to wean prednisolone, methotrexate was changed to parenteral dosing once a week, and the addition of cyclosporine 75 mg daily showed no sustained benefit; therefore, cyclosporine was ceased. Since then, an antibody-mediated process, TPX, was trialled while continuing IVIG after TPX every 4 weeks, which achieved some benefit. However, he had another relapse in 2016, when rituximab was added to the combination therapy of methotrexate and IVIG, which allowed a gradual reduction and then complete cessation of prednisolone.
He also has responded well with normalisation of CK and has been stable for 3 years since the addition of rituximab. His previous anti-HMGCR antibody level was 0.82 optical density (OD) (normal range<0.24 OD), and his recent anti-HMGCR antibody remained positive at 29.5 RU (normal range<11.0 RU), but this was tested at a different laboratory, which made direct comparison difficult.
Discussion
This case series highlights the typical clinical presentation and disease trajectory of statin-induced IMNM, which can be a relapsing remitting condition with difficulties achieving clinical remission in patients with standard myositis therapies. This case series also supports the use of B cell depletion therapy in cases with refractory anti-HMGCR antibody-associated IMNM.
This condition is newly identified and rare. Christopher-Stine et al reviewed a cohort of 26 patients with necrotising myopathy with no clear diagnosis despite extensive investigations and identified a unique autoantibody with specificity against 200 and 100 kDa proteins in 16 subjects.1 This antibody was later identified as anti-HMGCR antibody and highly specific for statin-induced necrotising autoimmune myopathy.2 Apart from the presence of the antibody, several other features of SANAM support its immunological aetiology, including the responsiveness to immunosuppressive therapies, the tendency to clinical relapses with weaning immunotherapy and the presence of major histocompatibility complex I on the surface of non-necrotic fibres.1 10
The management of this condition can be complex, given its commonly reported tendency to relapse when weaning immunotherapy. The need for maintenance IVIG for patients can be expensive and difficult to access long term. There has been a growing interest in exploring alternate or adjunct therapies. Recently, B cell depletion therapy has also been found to be effective in diseases with myositis-specific antibodies such as Jo-1-positive myopathy and myopathy associated with signal recognition particle antibodies (anti-SRP).11 12 The levels of Jo-1 and anti-SRP autoantibodies were reduced with rituximab therapy.11 12 Rituximab has also been reported as adjunctive therapy in SANAM. In a systematic review of 100 cases with SANAM, rituximab was used in only six cases as part of a combination therapy, and all cases achieved resolution.4
In our case series, all three patients responded well to rituximab-based combination therapy, and two cases had either normalisation or significant reduction of the levels of anti-HMGCR antibody since the introduction of rituximab therapy. Methotrexate and IVIG were part of the combination therapy, which alone did not achieve remission before rituximab was introduced. All patients were able to cease corticosteroids with rituximab therapy, and one patient has successfully ceased IVIG now the rituximab maintenance therapy has increased to every 6-month intervals. The benefit obtained from adding rituximab suggests that B cells may have a pathogenic role in the inflammatory process of statin-induced IMNM, and rituximab may be very useful in manipulating this immune response to achieve clinical and serological remission.
The rituximab regimen used in our case series followed the ‘lymphoma induction protocol’, which is a more aggressive approach than the ‘rheumatology protocol’ of two doses of 1000 mg given 2 weeks apart, then repeated every 6 months if required. Maintenance doses of IVIG every month with rituximab in this combination therapy reduced the risk of hypogammaglobulinaemia. There is no current literature to suggest which dose should be used in managing patients with anti-HMGCR-positive IMNM. Our experience from this case series suggests the efficacy and safety of this more aggressive dosage approach.
Learning points.
Statin-related anti-HMGCR antibody-positive immune-medicated necrotising myopathy is often responsive to immunotherapy, usually requiring combination therapies.
Relapses are common and difficult to manage.
Rituximab can be an effective adjunctive therapy for refractory cases and may reduce the need for maintenance therapy with IVIG.
The benefit of rituximab and different protocols warrants investigation in prospective trials.
Footnotes
Contributors: WZ reviewed and summarised all case files and drafted the manuscript. KR and HMP have provided medical care to all patients and provided suggestions and amendments to the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Christopher-Stine L, Casciola-Rosen LA, Hong G, et al. A novel autoantibody recognizing 200-kd and 100-kd proteins is associated with an immune-mediated necrotizing myopathy. Arthritis Rheum 2010;62:2757–66. 10.1002/art.27572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mammen AL, Chung T, Christopher-Stine L, et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum 2011;63:713–21. 10.1002/art.30156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Allenbach Y, Drouot L, Rigolet A, et al. Anti-HMGCR autoantibodies in European patients with autoimmune necrotizing myopathies: inconstant exposure to statin. Medicine 2014;93:150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nazir S, Lohani S, Tachamo N, et al. Statin-Associated autoimmune myopathy: a systematic review of 100 cases. J Clin Rheumatol 2017;23:149–54. [DOI] [PubMed] [Google Scholar]
- 5. Mammen AL, Myopathy S-AA. Statin-Associated autoimmune myopathy. N Engl J Med 2016;374:664–9. 10.1056/NEJMra1515161 [DOI] [PubMed] [Google Scholar]
- 6. Grable-Esposito P, Katzberg HD, Greenberg SA, et al. Immune-Mediated necrotizing myopathy associated with statins. Muscle Nerve 2010;41:185–90. [DOI] [PubMed] [Google Scholar]
- 7. Werner JL, Christopher-Stine L, Ghazarian SR, et al. Antibody levels correlate with creatine kinase levels and strength in anti-3-hydroxy-3-methylglutaryl-coenzyme a reductase-associated autoimmune myopathy. Arthritis Rheum 2012;64:4087–93. 10.1002/art.34673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Woolley M, Stebbings S, Highton J. Statin-associated immune-mediated necrotising myopathy: a new Zealand case series showing possible overrepresentation in Pacific Islanders. Intern Med J 2018;48:32–6. 10.1111/imj.13575 [DOI] [PubMed] [Google Scholar]
- 9. Wu Y, Lach B, Provias JP, et al. Statin-associated autoimmune myopathies: a pathophysiologic spectrum. Can. J. Neurol. Sci. 2014;41:638–47. 10.1017/cjn.2014.22 [DOI] [PubMed] [Google Scholar]
- 10. Ramanathan S, Langguth D, Hardy TA, et al. Clinical course and treatment of anti-HMGCR antibody–associated necrotizing autoimmune myopathy. Neurol Neuroimmunol Neuroinflamm 2015;2:e96 10.1212/NXI.0000000000000096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sultan SM, KP N, Edwards JC, et al. Clinical outcome following B cell depletion therapy in eight patients with refractory idiopathic inflammatory myopathy. Clin Exp Rheumatol 2008;26:887–93. [PubMed] [Google Scholar]
- 12. Valiyil R, Casciola-Rosen L, Hong G, et al. Rituximab therapy for myopathy associated with anti-signal recognition particle antibodies: a case series. Arthritis Care Res 2010;62:1328–34. 10.1002/acr.20219 [DOI] [PMC free article] [PubMed] [Google Scholar]