Abstract
Anastrozole is an aromatase inhibitor that has been used more frequently over the last decade especially for oestrogen receptor-positive breast cancer. It has a relatively safe side effect profile. However, occasionally it has been associated with serious adverse events. Here, we present the case of a 58-year-old woman who presented with significantly elevated liver enzymes 4 years after starting anastrozole. She was not taking any other medications and an extensive workup did not reveal any other cause for her liver injury. The patient’s liver enzymes normalised after discounting the anastrozole. She scored 4 on the updated Roussel Uclaf Causality Assessment Method grading system which was possible for drug-induced liver injury. A review of the literature revealed six prior cases of anastrozole-related liver injury. Anastrozole should be considered as a possible culprit in patients who develop an unexplained acute liver injury.
Keywords: drug interactions, gastrointestinal system, liver disease
Background
Anastrozole is an aromatase inhibitor and functions by blocking the peripheral conversion of androgens to estrogens in the peripheral tissues. It has been used extensively over the last decade or so, as an adjuvant chemotherapeutic agent in patients diagnosed with oestrogen receptor-positive breast cancer. It is recommended for postmenopausal females as a first-line adjuvant therapy. Though anastrozole can increase susceptibility to fractures and osteoporosis, especially in postmenopausal females, it is known to have a good safety profile. It was part of the Arimidex Tamoxifen Alone or in Combination (ATAC) regimen in the ATAC trial, and was found to have a good liver safety profile in that study.1 However, with its increasing use, there have been case reports of liver injury and have been severe in some cases.2–4
Our case highlights an important complication, that should be taken into account when prescribing anastrozole.
Case presentation
A 58-year-old Caucasian woman, with a history of breast cancer treated 4 years earlier with lumpectomy and radiation, was evaluated for progressively elevated liver tests (LTs). Her tumour had been found to be oestrogen receptor positive at the time of diagnosis and she had been started on anastrozole at that point. The patient had normal LTs at baseline. She was having regular follow-up with her oncologist and primary care physician, and during one of the follow-up appointments 4 years after her diagnosis she was found to have elevated liver enzymes. The patient at that point was asymptomatic and had not reported any symptoms or acute illness. She denied any influenza-like illness, and also denied drinking any alcohol. She denied any intravenous drug use. She did have a tattoo done a few years prior. She had a normal body mass index (BMI) and did not have any history of hyperlipidemia or diabetes. She did not have risk factors for chronic liver disease. She was in remission from cancer. LTs progressively increased in the next 3 weeks despite discontinuing the probiotics that she had been taking. She did not start any other new medications over the last 6 months. Her physical exam was unremarkable.
Investigations
When the patient was initially seen in the clinic for screening labs, she was found to have elevated liver enzymes. The patient’s labs are presented in table 1.
Table 1.
The patients’ liver tests, renal functions and her complete blood count
| Alanine transaminase | 1676 IU | Haemoglobin | 145 g/L |
| Aspartate transaminase | 831 IU | White cell count | 7.6×103/µL |
| Alkaline phosphatases | 207 U/L | Platelet count | 309×103/µL |
| Gamma glutamyl transferase | 59 U/L | Serum creatinine | 0.7 mg/dL |
| Total bilirubin | 1.6 mg/dL | ||
| Direct bilirubin | 0.5 mg/dL | ||
| INR | 0.9 | ||
| Albumin | 4.5 g/dL |
INR, international normalized ratio.
An extensive hepatitis workup was conducted to rule out viral hepatitis, including hepatitis A virus IgM antibodies, hepatitis B surface antigen, hepatitis C virus antibodies, hepatitis E virus, HIV, cytomegalovirus/Epstein-Bar Virus serology. Autoimmune workup revealed a normal antinuclear antibodies and anti-smooth muscle antibodies or metabolic liver diseases. This is depicted in table 2.
Table 2.
The other workup the patient underwent to find a cause of the liver injury
| Antibody test | Result | Antibody test | Result |
| HAV IgM antibodies | -ve | Anti-nuclear antibodies | -ve |
| HBS antigen | -ve | Anti-smooth muscle antibodies | <16 units (normal>20 U) |
| HBV IgM core antibodies | -ve | Anti-Liver, Kidney, Muscle antibodies | 2.0 units (normal>25 U) |
| HCV antibodies | -ve | Anti-Mitochondrial antibody | 3.6 units (normal>20 U) |
| HEV antibodies | -ve | ||
| CMV IgM antibodies | <8 AU/mL (normal<30) | ||
| EBV IgM antibodies | <10 AU/mL (normal<36) | ||
| VZV IgM antibodies | <5 AU/mL (normal<30) |
CMV, cytomegalovirus; EBV, Epstein-Bar virus; HAV, hepatitis A virus; HBS, hepatitis B surface; HBV, hepatitis B virus; HCV, hepatitis C virus; HEV, hepatitis E virus; VZV, varicella-zoster virus.
An abdominal CT was performed to check for any liver pathology and it was normal. A liver biopsy revealed features of chronic hepatitis with mild periportal fibrosis, grade II, stage II. Such portal tracts have been associated with chronic inflammation and interface hepatitis. Scattered foci of necrosis were noted. The inflammatory infiltration consisted primarily of lymphocytes with a few scattered eosinophils which most often associated with medications or toxicity. An autoimmune aetiology is also possible; however, plasma cells are rare (figure 1).
Figure 1.
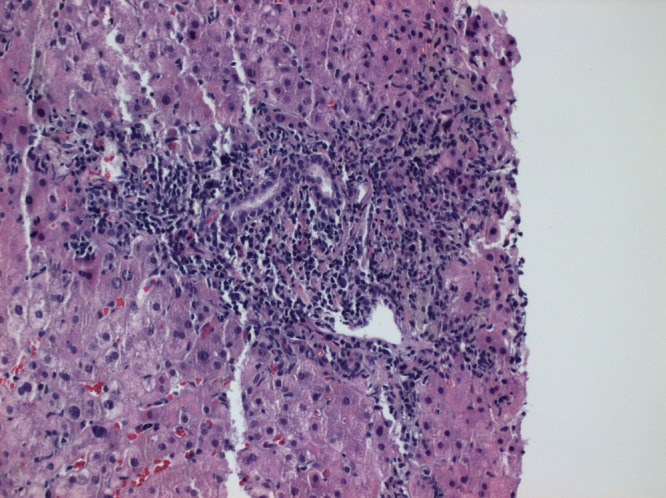
Liver biopsy revealed features of chronic hepatitis with mild periportal fibrosis. The inflammatory infiltration consisted primarily of lymphocytes with a few scattered eosinophils.
Differential diagnosis
At the time of presentation, the top potential diagnosis on the differential list was liver metastasis from her breast cancer. Cancer metastasis to the liver can present both with a cholestatic or hepatocellular pattern. Imaging ruled this out. Imaging also ruled out any gallbladder or bile duct pathology too. The patient denied any significant drinking and her liver enzymes were not consistent with alcoholic liver disease either. Other differentials at this point included autoimmune hepatitis, primary biliary cholangitis and infectious/viral hepatitis. These were subsequently ruled out by further workup. At this point, drug-induced hepatitis became the main differential and that was supported by improvement after stopping anastrozole and the biopsy findings. She scored 4 on the updated Roussel Uclaf Causality Assessment Method (RUCAM) grading system which suggested a possible drug-induced liver injury (table 3).5 However, it must be noted that we did not take the decline in alanine transaminase (ALT) in consideration while calculating this score, as the patient also received steroids at the time of diagnoses, and it was difficult to tell if discontinuation of the offending drug or starting the steroids was more responsible for the decline in ALT. If the decline in ALT was attributable to only discontinuation of the offending drug, then the patient would have scored 7 on the RUCAM scale (probable drug-induced liver injury).
Table 3.
RUCAM (Roussel Uclaf Causality Assessment Method) scoring for assessing the probable casual relationship
| S no | Category | Sub-category | Score |
| 1 | Time of onset from beginning the drug | (>90 days) | +1 |
| 2 | The course of ALT after cessation of the drug | >50% reduction in 8 days | NA |
| 3 | Risk factors | Age >55 years | +1 |
| 4 | Concomitant drug | None | 0 |
| 5 | Search for an alternative cause | All 7 causes of the group-I ruled out (HAV, HCV, HEV, alcoholism-AST/ALT level, sonography, Doppler of hepatic vessels and a recent episode of hypotension. All 5 causes of the group-II ruled out (VZV, CMV, EBV, HSV< and underlying precipitating condition) |
+2 |
| 6 | Previous hepatotoxicity to the same drug | Unknown | 0 |
| 7 | Response to unintentional re-exposure | None | 0 |
| Total score | 1–2=unlikely 3–5=possible 6–8=probable 9 or more=highly probable |
+4 (3–5=possible cause) |
ALT, alanine transaminase; AST, aspartate transaminase; CMV, cytomegalovirus; EBV, Epstein-Barr virus; HAV, hepatitis A virus; HBV, hepatitis B virus; HCV, hepatitis C virus;HEV, hepatitis E virus; HSV, herpes simplex virus; VZV, varicella-zoster virus.
The patient was also taking over the counter probiotics at the time of diagnosis. Her LTs did not improve with the discontinuation of this. RUCAM score for probiotics was only 1, which made this an unlikely culprit. (Time of onset +1, decrease in LTs <50%–2, risk factors age >55 +1, concomitant drugs −1, search for an alternative cause +2, previous hepatotoxicity to same drug 0, accidental re-exposure 0.)5
Treatment
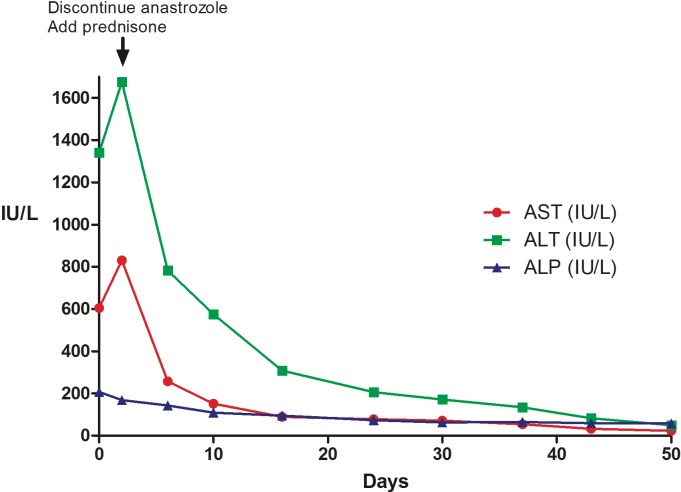
The patient was managed as an outpatient since despite her having deranged LTs, her vital signs and other labs were unremarkable. The patient was started on prednisone 40 mg daily and the anastrozole was also discontinued. The patient was regularly followed up in the clinic with liver function tests. Over the next few weeks, the patients LTs markedly improved, and normalised over the following 7 weeks. This is depicted in figure 2. The prednisone was tapered off and discontinued completely 6 weeks after diagnosis.
Figure 2.
After discontinuing anastrozole and starting prednisone, the LTs markedly improved and normalised over the following 7 weeks. ALT, alanine transaminase; AST, aspartate transaminase; LTs, liver tests.
Outcome and follow-up
The patient was followed for a year by our hepatology service with frequent liver enzyme checks. The patient was asymptomatic at throughout her follow-up and her liver enzymes remained at her baseline. She patient had already received 4 years of therapy and was in remission, so she refused further cancer therapy.
Discussion
Drug-induced liver injury can be divided into two categories. First is the intrinsic, predictable pattern where drugs directly affect the liver in a dose-dependent fashion. Second is the idiosyncratic, unpredictable pattern where drugs result in liver damage via metabolic and immune-mediated mechanisms. The progression of idiosyncratic drug-induced liver injury is variable and it could occur with a long latency without immediate clinical manifestations. The potential mechanism of anastrozole-induced liver injury is not clear, but the idiosyncratic pattern is more usual.6 Only a few rare case reports of anastrozole-induced liver injury have been reported, and LTs elevation occurred after 2–6 months of medication exposure.7 8
We performed an extensive literature review and found six prior cases of anastrozole-induced liver injury. One of the cases was presented as an abstract with limited data, but details of the remaining five cases are presented in the table 4.2–4 7–9 Liver injury has also been reported with similar medications like letrozole.10 Most of the patients were noted to have liver injury within a few months of starting anastrozole, but our patient developed liver injury 4 years after starting anastrozole.
Table 4.
The previous reported cases of anastrozole-induced liver injury
| Study | Sex/age | Latency/other medications | ALT/ AST (peak) IU |
ALP/GGT IU |
INR/albumin | Direct/indirect/total bilirubin | Imaging | Biopsy | Serology | Treatment | Outcome |
| Islam et al 7 | 66/F | 6 months/none | 621/428 | 185/624 | 1.4/30 | Total 42 µmol/L | Ultrasound normal | Heavy portal tract mixed inflammatory cell infiltrate, of plasma cells and lymphocytes. Scattered eosinophils and neutrophils | ANA 1;160, SMA 1;80 |
NAC, Vit K initially prednisone on relapse |
Resolved |
| de la Cruz et al 2 | 58/F | 3 weeks/ doxorubicin(6 cycles) cyclophosphamide (3 months) | 2012/1062 | 1186/714 | Total 187 µmol/L, direct 66 µmol/L | CXR with BL pleural effusions | Diffuse liver cell necrosis in acinar zone 3, without infl ammatory changes, and mild mixed steatosis and cholestasis; portal tracts were normal |
-ve/-ve | Symptomatic | Died of septic shock 1 month after diagnosis | |
| Lacey and Evans8 | 48/F | 2 months/ | 98/ | 385/ | 1.9/25 | Direct 567 µmol/L | CT showed fatty liver | Severe extensive macrovesicular steatosis with marked lobulitis. Marked mixed inflammation in most of the portal tracts, including numerous neutrophils, lymphocytes, plasma cells and eosinophils |
-ve/-ve | Prednisone 40 mg × 2 weeks, 20 mg × 6 weeks | Recovered |
| Zapata et al 4 | 89/F | 3 months/ diuretics (long term) | 410/255 | 231/364 | Total 3.55 mg/dL, direct 1.63 mg/dL | US and CT were nromal | Not available | -ve/-ve | Symptomatic | Recovered (labs normalsed in 2 weeks after stopping) | |
| Inno et al 3 | 70/F | 4 months /ramipril, bisoprolol, manidipine (3 years) | 1344/640 | 355/ | Total 3.54 mg/dL, direct 2.29 mg/dL | CT abdomen showed mild dilatation of the intrahepatic biliary tract without | A pattern of mild steatosis (10%), with moderate inflammatory activity and moderate to severe fibrosis, totalising a 5–6 score according to Ishak’s classification | 1;80 (speckled) | Symptomatic | Recovered | |
| Our case | 66/F | 4 years/no other medications | 1676/831 | 207/ | 0.9/4.5 | Total 1.6 mg/dL, direct 0.5 mg/dL |
CT and US abdomen unremarkable | -ve/-ve | Prednisone | Recovered |
ALP, alkaline phosphatase; ANA, antinuclear antibodies; AST, aspartate transaminase; CXR, chest X-ray; GGT, gamma glutamyl transferase; NAC, N-acetyl cysteine; SMA, smooth muscle antibody.
The exact mechanism by which anastrozole causes liver injury is still debatable, as the biopsy and lab findings in the previous reported cases have varied. As described previously, it is associated with an idiosyncratic response causing liver injury, and it does not appear to be dose dependent. Immune mediated and metabolic damage along with individual susceptibilty are likely to play a role. The analysis of the biopsy reports and other labs point to a different possible mechanism. Two of the prior cases by Islam et al and Inno et al report the presence of a positive ANA, which in the case of Islam et al was at a titer of 1:160. This patient also had a positive antismooth muscle antibody and had a mixed inflammatory inflitrate with plasma cells. This led the authors to question if anastrozole had led to an autoimmune hepatitis. In the case of Inno et al, the positive ANA disappeared after discontinuing the anastrozole. In addition to this three other cases also mention some degree of steatosis, with one case reported by Lacey et al showed severe extensive macrovesicular steatosis with marked lobulitis. Alcoholic hepatitis was not thought to be the aetiology in these cases. This suggest that steatohepatits may be an associated pattern of injury in patients with anastrozole-induced liver injury. Drug-induced steatohepatits has previously been reported with some medications most notably with amiodarone, in which case the drug is thought to interfere with ATP synthesis and lipid metabolism.11
Analysis of the previous cases showed that there is not a single pattern of liver injury associated with anastrozole, as most of the prior cases have a mixed hepatocellular and cholestatic picture on labs. Lacey et al did report a case that had a more cholestatic pattern. Our patient also had a mixed injury pattern demonstrated by elevated transaminases, bilirubin and alkaline phosphatase.
In the prior cases, most of the patients had some form of imaging, either in the form of an ultrasound or a CT of the abdomen. However, in most cases, imaging was unremarkable, and in one case described by Lacey et al that had evidence of steatohepatitis on biopsy, imaging showed diffuse fatty infiltration of the liver.8
Most of the patients described previously made a successful recovery after stopping anastrozole. One patient described by de la Cruz et al, did pass away after developing an acute abdomen and sepsis.2 Though the liver injury may have contributed to the patient’s demise, it was unlikely to be the cause of it. Most of the prior cases were managed symptomatically and with the discontinuation of anastrozole. Our patient and two prior reported patients were also treated with prednisone, though it is difficult to say how much of their improvement can be attributed to prednisone and how much just from discontinuing the anastrozole.
Learning points.
Anastrozole is an aromatase inhibitor that has been used more frequently over the last decade especially for oestrogen receptor-positive breast cancer.
It has a relatively safe side effect profile in the most part, the most concerning being predisposing patients to osteoporosis.
Review of the literature revealed six prior cases of anastrozole-related liver injury.
Anastrozole should be considered as a possible culprit in patients who are taking this medication and developed acute liver injury, especially if it cannot be explained by any other aetiology.
Footnotes
Contributors: CX and HMAA were responsible for writing the discussion and background parts. MA, EQ and MN were responsible for the summary and case presentation.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Buzdar A, Howell A, Cuzick J, et al. Comprehensive side-effect profile of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: long-term safety analysis of the ATAC trial. Lancet Oncol 2006;7:633–43. 10.1016/S1470-2045(06)70767-7 [DOI] [PubMed] [Google Scholar]
- 2. de la Cruz L, Romero-Vazquez J, Jiménez-Sáenz M, et al. Severe acute hepatitis in a patient treated with anastrozole. Lancet 2007;369:23–4. 10.1016/S0140-6736(07)60017-8 [DOI] [PubMed] [Google Scholar]
- 3. Inno A, Basso M, Vecchio FM, et al. Anastrozole-related acute hepatitis with autoimmune features: a case report. BMC Gastroenterol 2011;11:32 10.1186/1471-230X-11-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zapata E, Zubiaurre L, Bujanda L, et al. Anastrozole-induced hepatotoxicity. Eur J Gastroenterol Hepatol 2006;18:1233–4. 10.1097/01.meg.0000243868.64078.af [DOI] [PubMed] [Google Scholar]
- 5. Danan G, Teschke R. RUCAM in drug and herb induced liver injury: the update. Int J Mol Sci 2016;17:14 10.3390/ijms17010014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim S-H, Naisbitt DJ. Update on advances in research on idiosyncratic drug-induced liver injury. Allergy Asthma Immunol Res 2016;8:3–11. 10.4168/aair.2016.8.1.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Islam MS, Wright G, Tanner P, et al. A case of anastrazole-related drug-induced autoimmune hepatitis. Clin J Gastroenterol 2014;7:414–7. 10.1007/s12328-014-0512-4 [DOI] [PubMed] [Google Scholar]
- 8. Lacey R, Evans A. An unusual cause of jaundice in a patient with breast cancer. Case Rep Child Meml Hosp Chic 2014;2014 10.1136/bcr-2014-205764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Klapko O, Ghoulam E, Jakate S, et al. Anastrozole-induced autoimmune hepatitis: a rare complication of breast cancer therapy. Anticancer Res 2017;37:4173–6. 10.21873/anticanres.11805 [DOI] [PubMed] [Google Scholar]
- 10. Gharia B, Seegobin K, Maharaj S, et al. Letrozole-Induced hepatitis with autoimmune features: a rare adverse drug reaction with review of the relevant literature. Oxf Med Case Reports 2017;2017 10.1093/omcr/omx074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stravitz RT, Sanyal AJ. Drug-induced steatohepatitis. Clin Liver Dis 2003;7:435–51. 10.1016/S1089-3261(03)00027-8 [DOI] [PubMed] [Google Scholar]