Abstract
Plasma cell neoplasms may exhibit variations in morphology and immunophenotype, which can mimic mature B-cell lymphoproliferative disorders and pose diagnostic challenges. This case illustrates a rare entity of plasma cell myeloma, where the entire plasma cell population exhibited lymphoid morphology, negativity for CD138, positivity for CD20 and cyclin D1, and positive fluorescence in situ hybridisation for t(11;14) and del(17 p), mimicking a mature B-cell lymphoproliferative disorder, in particular mantle cell lymphoma. In this case, a careful analysis of flow cytometry gating strategies and use of other ancillary tests were keys for correct diagnosis. In addition to the diagnostic implications due to its rarity, CD138-negative plasma cell myeloma may represent a unique entity, which is associated with ‘stem cell’-like clonogenic properties, more aggressive clinical behaviour and resistance to chemotherapy.
Keywords: haematology (incl blood transfusion), immunology, malignant and benign haematology, pathology
Background
Plasma cell neoplasms may exhibit variations in morphology and immunophenotype, which can mimic mature B-cell lymphoproliferative disorders and pose diagnostic challenges. Distinguishing these disease entities is essential as they have different treatment implications. This case illustrates a rare entity of plasma cell myeloma with essentially entire plasma cell population exhibiting lymphoid morphology, negativity for CD138, positivity for CD20 and fluorescence in situ hybridisation (FISH) positivity for t(11;14) and del (17 p). It highlights the importance of a comprehensive diagnostic approach incorporating different ancillary tests to arrive at the correct diagnosis.
Case presentation
A 79-year-old female patient presented with fatigue and shortness of breath on exertion. Her blood work showed mild cytopaenia (haemoglobin 95 g/L, mean corpuscular volume 111 fL, neutrophils 1.2×109/L, platelets 151×109/L). Serum electrophoresis (SPEP) showed 11.3 g/L monoclonal protein, which was characterised as IgG lambda. Serum-free light chains were 33.0 mg/L for lambda light chains and 10.4 mg/L for kappa light chains. Kappa to lambda free light chain ratio was 0.32. Calcium and creatinine were normal at 2.39 mmol/L and 84 µmol/L, respectively. There were no focal lytic lesions identified by skeletal survey. The patient’s performance status was grade 1 by the Eastern Cooperative Oncology Group scale.
Investigations
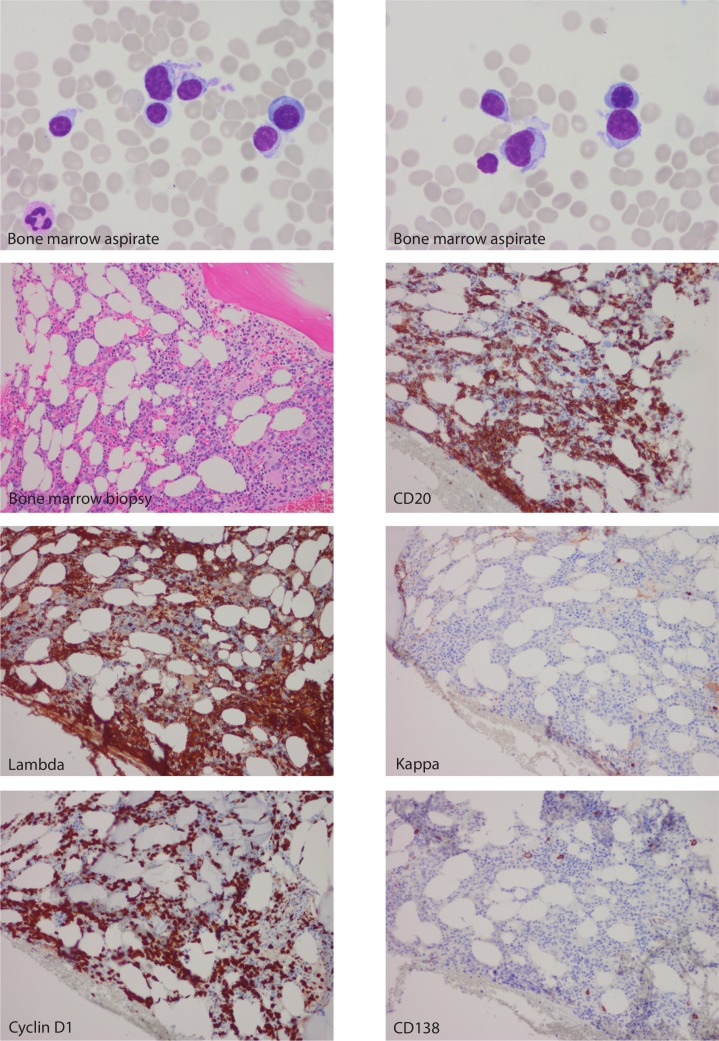
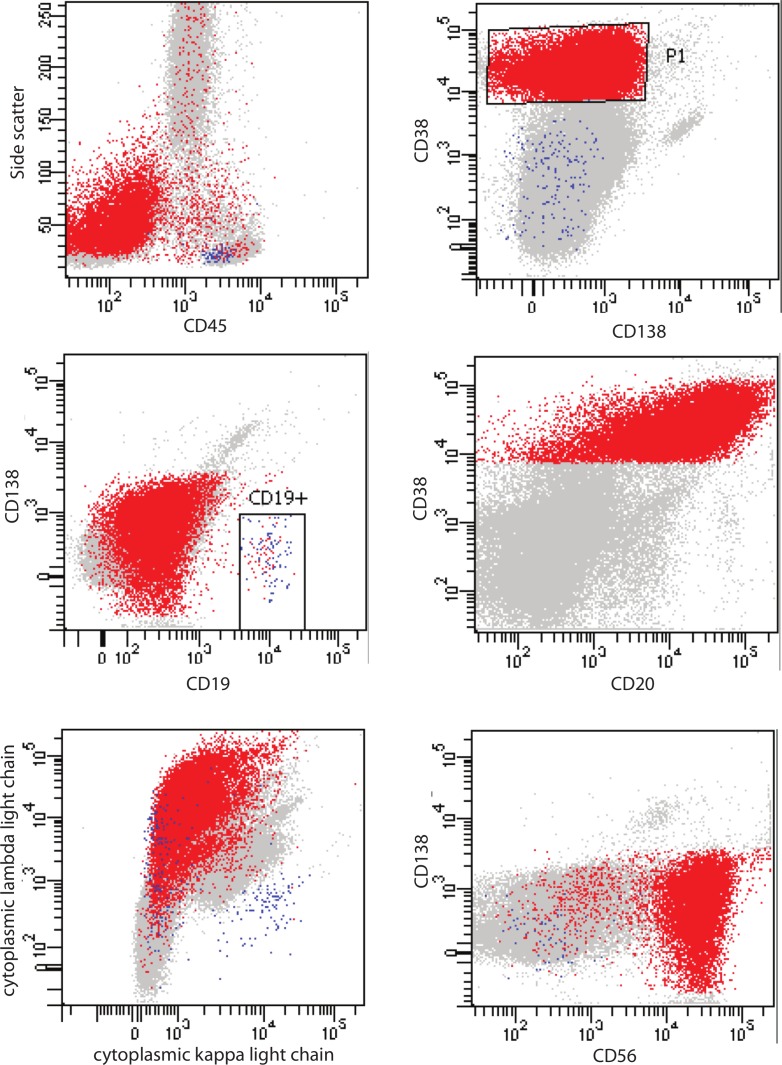
Bone marrow aspirate and biopsy showed atypical cell infiltrate which exhibited lymphoid morphology, accounting for 40%–50% of marrow cellularity (figure 1). These cells were small to medium in size with eccentric nuclei, clumped chromatin, and some forms exhibiting nuclear folds and small cytoplasmic projections. Immunohistochemical stain confirmed that essentially the entire population of abnormal plasma cells was negative for CD138 but strongly positive for CD20 and cyclin D1 (figure 1). Stain for Epstein-Barr virus latent membrane protein-1 was negative. Flow cytometry of the bone marrow aspirate showed that this abnormal cell population was lambda restricted, CD45−, CD19−, CD38+, CD20+, CD27+, CD56+, CD117+ and CD81− (figure 2). This immunophenotype was consistent with lambda clonal plasma cells, but they uniformly lacked CD138 expression. No clonal B-cell population was detected by flow cytometry. Plasma cell-targeted FISH analysis using simultaneous cytoplasmic light chain staining was positive for translocation t(11;14) involving the IGH and CCND1 loci. In addition, FISH performed on the bone marrow cell pellet showed the presence of deletion 17 p involving TP53 gene. Given the 17 p deletion, normal beta-2-microglobulin of 2.3 mg/L, albumin of 43 g/L and slightly elevated lactate dehydrogenase (272 U/L), the Revised International Staging System for this patient was stage II.
Figure 1.
Bone marrow aspirate under high power (×100) magnification (Wright-Giemsa stain), bone marrow biopsy under low power (×20) magnification (H&E stain) and immunohistochemical stains highlighting the plasma cell infiltrate.
Figure 2.
Flow cytometry plots showing immunophenotype of the neoplastic plasma cells (red population).
Differential diagnosis
The lymphoid morphology, uniform lack of CD138 expression, strong positivity of CD20 and cyclin D1 by immunohistochemical stains raise the diagnostic consideration of mature B-cell lymphoproliferative disorder such as mantle cell lymphoma. However, the lack of CD45 and CD19 expression by flow cytometry, in combination with other aberrant plasma cell markers support the diagnosis of CD138-negative plasma cell myeloma.
Treatment
The patient was diagnosed with plasma cell myeloma and started on treatment with cyclophosphamide, bortezomib and dexamethasone (CyBorD) combination chemotherapy.
Outcome and follow-up
A ≥50% reduction of serum M-protein to 4.9 g/L was noted at 1 month follow-up. The patient had a complete response after nine cycles of the CyBorD therapy with no detectable monoclonal band and normal serum-free light chain ratio at the end of treatment. However, her subsequent bloodwork 2 months post-therapy showed reappearance of a faint monoclonal IgG lambda band on SPEP.
Discussion
CD138, also known as syndecan-1 (SDC1), is a marker expressed in terminally differentiated plasma cells. CD138 is often used to characterise plasma cells either by flow cytometry or immunohistochemistry. Historically, the diagnosis of plasma cell myeloma relies mainly on bone marrow morphology, immunohistochemistry, the presence of monoclonal protein and clinical symptoms (hypercalcaemia, renal failure, anaemia and bony lytic lesions). Flow cytometry is not routinely performed for diagnosis, but recently it has become standard in the workup of patients with plasma cell dyscrasias, especially given the recent advances in minimal residual disease testing. In this case, the uniform lack of CD138, positivity for cyclin D1 and CD20, in combination with the lymphoid-like morphology of plasma cells may pose diagnostic challenge for plasma cell myeloma by mimicking B-cell lymphoproliferative disorder, in particular, mantle cell lymphoma. This case highlights the important role of complete immunophenotypic profiling by flow cytometry in establishing the diagnosis of atypical plasma cell myeloma, which could potentially be misdiagnosed as B-cell lymphoma by morphology and immunohistochemistry alone.
Although CD138 is positive in the vast majority of plasma cell myeloma cases, some studies have reported decreased expression of CD138 in myeloma.1–5 These CD138− plasma cell subsets are associated with ‘stem cell’-like, clonogenic and self-renewal properties resembling less differentiated postgerminal centre B cells.1–5 A term ‘preplasma cells (pre-PCs)’ has been proposed to describe these CD19−/CD138− plasma cells, which represent an intermediate form between CD19+/CD138− plasmablasts and CD19−/CD138+ mature plasma cells within the hierarchy of plasma cell maturation.4 This is supported by the observation that pre-PCs are able to generate plasma cells but not plasmablasts in xenograft assays.4 Differences in morphological as well as gene expression patterns have also been described in literature. Unlike classic plasma cells, these pre-PCs were significantly smaller in size, resembling small lymphocytes with little or no visible Golgi apparatus, similar to those observed in our case.4 The CD138-negative plasma cells also have high expression levels of B cell-specific transcription factor BCL6 and PAX5 as well as CD319 (a Signaling Lymphocytic Activation Molecule (SLAM) family receptor highly expressed in normal and malignant plasma cells).2 6 Lower expression of plasma cell-specific transcription factors (IRF4, PRDM1, and XBP1) has been observed in CD138− myeloma cells in comparison to the CD138+ cells in murine2 and human multiple myeloma cell lines.6 To the best of our knowledge, the majority of studies describe myeloma cases in which only a subset of myeloma cells at diagnosis was either CD138-negative or exhibited diminished CD138 expression or cases where CD138 expression on plasma cells decreased after therapy/at relapse. Our case is a rare case where essentially the entire population of neoplastic plasma cells was CD138-negative at initial diagnosis.
The number of studies looking at the prognostic and predictive significance of the CD138− myeloma is limited due to the rarity of such cases. A recent study analysing prognostic value of surface antigen expression in myeloma showed that decreased expression of CD38 and/or CD138 was associated with decreased progression free survival (PFS) and overall survival (OS) compared with cases with bright CD38 and/or CD138 staining in newly diagnosed plasma cell myeloma patients.7 Flow cytometric analysis of primary multiple myeloma cell lines revealed an enrichment of CD138-negative cells in relapsed or refractory myeloma patients compared with the newly diagnosed patients.6 Plasma cell myeloma with reduction or loss of CD138 expression has also been associated with increased resistance to chemotherapy.4–8 A retrospective study reported a worse OS, more frequent disease progression and resistance to lenalidomide in the CD138 negative myeloma compared with CD138-positive myeloma.5 6 This is in part due to the increased expression of ABCG2/BCRP drug transporter and aldehyde dehydrogenase.5 At the same time, no difference in sensitivity to bortezomib was observed.6 Furthermore, the patients with CD138-negative myeloma receiving high-dose chemotherapy followed by autologous stem-cell transplantation (SCT) also had worse prognosis than the CD138-positive counterparts.6 A unique targeted therapeutic strategy may be needed to overcome the chemoresistance of this subset. A recent phase Ib clinical trial showed that pretargeting CD138−/CD20+clonogenic myeloma cells with bispecific antibody-armed T cells before autologous SCT was able to reduce the clonogenic myeloma cell levels and induced cellular and humoral antimyeloma immunity that could be boosted after SCT.9 Another study found that the histone methyltransferase inhibitor DZNep showed more efficacy in the CD138-negative compared with the CD138-positive myeloma cells.10 In addition, since CD138-negative myeloma cells are known to have high RARα2 expression, a study by Yang et al showed that al-trans retinoic acid (ATRA) preferentially induced apoptosis in the CD138-negative subset.11 Telomerase inhibitor is another agent of interest which has an inhibitory activity against clonogenic myeloma stem cells.12
Gene expression and cytogenetic profiles of CD138-negative myeloma are not very well understood, in part since these techniques tend to only capture predominant clone or exclude the CD138-negative subset from analysis (many plasma cell enrichment methods selectively target CD138-positive plasma cells).13 Although a subset of plasma cell myelomas with lymphoid morphology, CD20 and cyclin D1 expression has been reported, the lack of CD138 expression is highly unusual in this entity and can further complicate the diagnosis.14 Dysregulation of Cyclin D1 expression is thought to be an early event in myeloma, present in committed plasma cell precursors in germinal centres and in clonal plasma cells in monoclonal gammopathy of undetermined significance (MGUS).15 It is detected in approximately one-fifth of myeloma cases.16 Many of those cases involve translocation between cyclin D1 gene on chromosome 11 and immunoglobulin heavy chain gene on chromosome 14. t(11;14) myelomas have been associated with CD20 expression on plasma cells, as seen in this case, and have been thought to have better prognosis.12 A recent small study of 20 t(11;14)-translocated myelomas showed that of 15 cases where CD138 expression was assessed by flow cytometry, two cases were negative for CD138, but the clinical significance of this finding is unclear due to rarity of such cases.17 Another recent case report identified deletions of TP53 and CCND1 genes in addition to gains of CKS1B and FGFR3 in a single case of CD138-negative myeloma.18 In our case, using non-enriched bone marrow cell pellets, we detected t(11;14) and a high-risk chromosomal abnormality (del (17 p) involving TP53 gene). It is unclear whether this high-risk chromosomal abnormality is enriched in the CD138-negative subtype and whether it affects prognosis in such patients. Further studies will be of interest to characterise any recurring cytogenetic abnormalities and gene expression profile in CD138-negative myeloma.
These observations suggest that CD138-negative plasma cell myeloma may represent a unique biological entity. From the diagnostic standpoint, the lymphoid-like morphology associated with this subtype may pose a pitfall by mimicking low grade B-cell lymphoproliferative disorder, especially in combination with t(11;14). A thorough workup, including careful assessment of flow cytometry gating strategies and other markers is warranted to fully characterise these plasma cells. This may also have implications in the assessment of minimal residual disease in myeloma by flow cytometry, in which one should be mindful of the gating strategies, which should account for residual CD138-negative clones. From a clinical perspective, this clonogenic subset has been associated with poorer prognosis and resistance to chemotherapy, which may potentially benefit from targeted therapies that are selectively toxic to myeloma stem cells. Longer term follow-up and larger number of cases are required to establish the prognostic behaviour of this subset. Finally, patients with CD138-negative myeloma will likely benefit from a more effective therapeutic strategy to improve their poor outcome.
Learning points.
Plasma cell neoplasms may exhibit morphological and immunophenotypic variants, which can mimic mature B-cell lymphoma and pose diagnostic challenges.
Comprehensive diagnostic workup including careful analysis of flow cytometry gating strategies and use of other ancillary tests are keys for correct diagnosis.
CD138-negative plasma cell myeloma is a rare and unique entity which is associated with ‘stem cell’-like, clonogenic properties with poorer prognosis and resistance to chemotherapy.
Myeloma with t(11;14) is usually associated with better prognosis. However, the prognostic significance of CD138 negativity and concurrent del(17 p) in this subset is unclear due to rarity of such cases.
Footnotes
Contributors: AFS reviewed the patient’s bone marrow specimen, analysed flow cytometry data, conducted literature search and wrote the case report. YS reviewed the case, created the figures, conducted literature search and contributed to writing the case report. All authors approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Next of kin consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Hosen N, Matsuoka Y, Kishida S, et al. CD138-negative clonogenic cells are plasma cells but not B cells in some multiple myeloma patients. Leukemia 2012;26:2135–41. 10.1038/leu.2012.80 [DOI] [PubMed] [Google Scholar]
- 2. Van Valckenborgh E, Matsui W, Agarwal P, et al. Tumor-Initiating capacity of CD138- and CD138+ tumor cells in the 5T33 multiple myeloma model. Leukemia 2012;26:1436–9. 10.1038/leu.2011.373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kawano Y, Hata H. Clinical and biological significance of surface molecules in myeloma. Int J Myeloma 2014;4:1–6. [Google Scholar]
- 4. Chaidos A, Barnes CP, Cowan G, et al. Clinical drug resistance linked to interconvertible phenotypic and functional states of tumor-propagating cells in multiple myeloma. Blood 2013;121:318–28. 10.1182/blood-2012-06-436220 [DOI] [PubMed] [Google Scholar]
- 5. Matsui W, Wang Q, Barber JP, et al. Clonogenic multiple myeloma progenitors, stem cell properties, and drug resistance. Cancer Res 2008;68:190–7. 10.1158/0008-5472.CAN-07-3096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kawano Y, Fujiwara S, Wada N, et al. Multiple myeloma cells expressing low levels of CD138 have an immature phenotype and reduced sensitivity to lenalidomide. Int J Oncol 2012;41:876–84. 10.3892/ijo.2012.1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arana P, Paiva B, Cedena M-T, et al. Prognostic value of antigen expression in multiple myeloma: a PETHEMA/GEM study on 1265 patients enrolled in four consecutive clinical trials. Leukemia 2018;32:971–8. 10.1038/leu.2017.320 [DOI] [PubMed] [Google Scholar]
- 8. Zlei M, Egert S, Wider D, et al. Characterization of in vitro growth of multiple myeloma cells. Exp Hematol 2007;35:1550–61. 10.1016/j.exphem.2007.06.016 [DOI] [PubMed] [Google Scholar]
- 9. Lum LG, Thakur A, Kondadasula SV, et al. Targeting CD138-/CD20+ clonogenic myeloma precursor cells decreases these cells and induces transferable Antimyeloma immunity. Biol Blood Marrow Transplant 2016;22:869–78. 10.1016/j.bbmt.2015.12.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reghunathan R, Bi C, Liu SC, et al. Clonogenic multiple myeloma cells have shared stemness signature associated with patient survival. Oncotarget 2013;4:1230–40. 10.18632/oncotarget.1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang Y, Shi J, Tolomelli G, et al. RARα2 expression confers myeloma stem cell features. Blood 2013;122:1437–47. 10.1182/blood-2013-02-482919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brennan SK, Wang Q, Tressler R, et al. Telomerase inhibition targets clonogenic multiple myeloma cells through telomere length-dependent and independent mechanisms. PLoS One 2010;5:e12487 10.1371/journal.pone.0012487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Mel S, Lim SH, Tung ML, et al. Implications of heterogeneity in multiple myeloma. Biomed Res Int 2014;2014:1–12. 10.1155/2014/232546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Avet-Loiseau H, Attal M, Moreau P, et al. Genetic abnormalities and survival in multiple myeloma: the experience of the Intergroupe Francophone Du Myélome. Blood 2007;109:3489–95. 10.1182/blood-2006-08-040410 [DOI] [PubMed] [Google Scholar]
- 15. Kuehl WM, Bergsagel PL. Molecular pathogenesis of multiple myeloma and its premalignant precursor. J Clin Invest 2012;122:3456–63. 10.1172/JCI61188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. An G, Xu Y, Shi L, et al. t(11;14) multiple myeloma: a subtype associated with distinct immunological features, immunophenotypic characteristics but divergent outcome. Leuk Res 2013;37:1251–7. 10.1016/j.leukres.2013.06.020 [DOI] [PubMed] [Google Scholar]
- 17. Yokoi S, Sakai H, Uchida A, et al. Cytogenetic Study and Analysis of Protein Expression in Plasma Cell Myeloma with t(11;14)(q13;q32): Absence of BCL6 and SOX11, and Infrequent Expression of CD20 and PAX5. J Clin Exp Hematop 2015;55:137–43. 10.3960/jslrt.55.137 [DOI] [PubMed] [Google Scholar]
- 18. Shuai W, Li S. CD138− plasma cell myeloma. Blood 2019;134:906 10.1182/blood.2019001845 [DOI] [PubMed] [Google Scholar]