Abstract
Background
Tactical Combat Casualty Care guidelines for hemorrhage recommend resuscitation to systolic blood pressure (SBP) of 85±5 mm Hg during prehospital care. Success depends on transport to definitive care within the ‘golden hour’. As future conflicts may demand longer prehospital/transport times, we sought to determine safety of prolonged permissive hypotension (PH).
Methods
Adult male swine were randomized into three experimental groups. Non-shock (NS)/normotensive underwent anesthesia only. NS/PH was bled to SBP of 85±5 mm Hg for 6 hours of prolonged field care (PFC) with SBP maintained via crystalloid, then recovered. Experimental group underwent controlled hemorrhage to mean arterial pressure 30 mm Hg until decompensation (Decomp/PH), followed by 6 hours of PFC. Hemorrhaged animals were then resuscitated with whole blood and observed for 24 hours. Physiologic variables, blood, tissue samples, and neurologic scores were collected.
Results
Survival of all groups was 100%. Fluid volumes to maintain targeted SBP in PFC were significantly higher in the hemorrhage group than sham groups. After 24 hours’ recovery, no significant differences were observed in neurologic scores or cerebrospinal fluid markers of brain injury. No significant changes in organ function related to treatment were observed during PFC through recovery, as assessed by serum chemistry and histological analysis.
Conclusions
After 6 hours, a prolonged PH strategy showed no detrimental effect on survival or neurologic outcome despite the increased ischemic burden of hemorrhage. Significant fluid volume was required to maintain SBP—a potential logistic burden for prehospital care. Further work to define maximum allowable time of PH is needed.
Study type
Translational animal model.
Level of evidence
N/A.
Keywords: hypotension, hemorrhagic shock, prolonged field care, full resuscitation
Background
Traumatic injuries with hemorrhage are the leading cause of potentially survivable death in both the military and civilian sectors.1 2 The goal of initial prehospital hemorrhage control and resuscitative measures is to stop bleeding when possible, determine patient status, and delay progression into irreversible shock by providing intravenous fluids sparingly, to partially restore the patient’s hemodynamics until definitive surgical control can be achieved.3–6 The current Tactical Combat Casualty Care (TCCC) Clinical Practice Guideline (CPG) for the prehospital (Role 1 and Role 2) treatment of casualties in shock, without traumatic brain injury, is to resuscitate until a palpable radial pulse is restored, mental status is improved, or a ‘permissively hypotensive’ systolic blood pressure (SBP) of 85±5 mm Hg is present, preferably with blood products.7–10 If these criteria are met, resuscitation should pause. Implementation of this hypotensive resuscitation within the ‘golden hour’ has improved casualty survival in recent conflicts.11 12
Future conflicts are predicted to occur in more resource-constrained environments, not conducive to ‘golden hour’ medical planning or its preferred resuscitative methods. A prolonged field care (PFC) strategy is anticipated to be required for casualty care over extended durations, up to 72 hours. Hypotensive resuscitation acutely reduces risk of clot rupture at the site of vascular injury and maintains perfusion to vital organs while decreasing risk of death from hemorrhage.9 10 12 However, it is well established that sustained hypotension will consume and eventually deplete an individual’s compensatory reserve to the point that vital organ perfusion is compromised, especially the brain.13–17 While cerebral blood flow (CBF) is affected, it is generally maintained, at least over the short term, by activation of compensatory mechanisms and preserved by cerebral autoregulation. Still, many studies have demonstrated that prolonged hypotension impairs cerebral autoregulation and reduces CBF and/or oxygenation to critical brain regions.14 16 18–20
The aim of this study was to improve our current understanding of the implications and limitations of PFC permissive hypotensive resuscitation. We hypothesize that a strategy of 6 hours of permissive hypotension (PH) after severe decompensated hemorrhage will lead to clinically acceptable outcomes, as evidenced by physiologic and neurologic parameters.
Materials and methods
Preoperative preparation
Fifteen male Yorkshire/Landrace swine (Sus scrofa domesticus) weighing 70–90 kg were randomized into three experimental groups: (1) anesthesia sham (non-shock/normotensive (NS/NT), n=5); (2) hypotensive sham (NS/PH, n=5); or (3) decompensated hemorrhage+hypotension (Decomp/PH, n=5). Male swine were used in this protocol because males comprise the majority of battlefield injuries, and regardless of species, exhibit more deleterious complications and higher mortality trauma and hemorrhage than females.21 22
Animals were sedated with tiletamine/zolazepam (6.0 mg/kg; Fort Dodge Animal Health, Overland Park, KS), premedicated with analgesic (buprenorphine HCl 0.01 mg/kg; Reckitt & Colman Pharmaceuticals, Richmond, VA), and intubated for ventilation and anesthesia, maintained on 1% to 3% isoflurane. Core body temperature was monitored via rectal temperature probe and maintained between 36.0°C and 38.0°C.
Four catheters were placed percutaneously. Briefly, a femoral venous catheter (8Fr; Arrow, Morrisville, NC) for fluid infusion, and a jugular vein triple lumen catheter (7Fr; Cook Medical, Bloomington, IN) for central venous pressure monitoring and continuous infusion anesthesia. Femoral arteries were cannulated with either 8Fr catheter for hemorrhage or 5Fr catheter (Cook Medical) for blood pressure (BP) monitoring and sampling. Additionally, a urinary bladder catheter (14 G needle or pediatric Foley catheter) was placed percutaneously to characterize urine output.
Hemorrhagic shock
After baseline (BSLN) blood samples were drawn, hemorrhage was initiated by free-flow of arterial blood until mean arterial pressure (MAP) reached 30 mm Hg. Additional blood was withdrawn to maintain a MAP of 30–35 mm Hg. End of shock (EOS) was defined as the animal being unable to maintain MAP >30 mm Hg for 10 minutes. Hemorrhaged blood was collected in a blood donor bag containing anticoagulant citrate phosphate dextrose adenine solution at a 1:10 ratio for subsequent resuscitation.
To establish BSLN data and segregate effects of anesthesia alone, animals in NS/NT and NS/PH (sham groups) were maintained at NT levels (MAP≥70 mm Hg) during a 30-minute simulated shock period by administering supplemental fluid (lactated Ringer’s solution; LR) in the absence of hemorrhage.
Prolonged field care
At EOS, the 6-hour PFC resuscitation phase commenced. Isoflurane was gradually reduced to 0.4% to 0.5%, as intravenous combination of propofol (3–10 mg/kg/h) and buprenorphine (2–8 µg/kg/h) started. Resuscitation commenced for Decomp/PH according to current TCCC guidelines, assuming absence of whole blood or blood component availability. Briefly, two 500 mL LR boluses were administered, followed by LR at a continuous rate to target SBP of 85±5 mm Hg. Hypotension was induced in NS/PH during the PFC period by discontinuing supplemental fluids and removing blood to a target SBP of 85±5 mm Hg. For both PH groups, if animals dropped below or climbed above the target range of 85±5 mm Hg, LR was infused or blood was removed, respectively, to maintain SBP range.
Resuscitation
At the end of PFC (END PFC), animals entered a simulated 2-hour hospital phase. Anesthesia was returned to 1% to 3% isoflurane, with cessation of intravenous anesthesia. Decomp/PH animals were resuscitated to MAP>70 mm Hg by autologous transfusion of 100% shed blood, intravenously infused over 30 minutes using a Belmont Rapid Infuser (Belmont Instrument, Billerica, MA). Calcium gluconate (10–15 mL; 23% solution; 2.14 g Ca2+/100 mL) was intravenously infused immediately prior to reinfusion of shed blood to counteract anticoagulant. Additional resuscitation maintenance fluid (LR) was infused, per TCCC guidelines, at 6 mL/h for the first 20 kg and an additional 1 mL/kg/h, over the final 90 minutes of hospital care to maintain MAP >70 mm Hg.
NS/NT and NS/PH groups received maintenance fluids as described Decomp/PH for the full hospital phase. Buprenorphine Sustained-Release (0.06–0.2 mg/kg) subcutaneous was administered 30 minutes prior to the end of hospital care (EHC) to assure all animals received excellent pain control during the 24-hour survival period.
Recovery and euthanasia
At EHC, all instrumentation was removed before recovering animals. Animals were transferred to a recovery pen and observed for 24 hours, with buprenorphine HCl (0.01–0.03 mg/kg) intramuscular, as deemed necessary by veterinarian for acute pain. Animals were monitored by veterinarian hourly for general condition and every 4 hours for pain management. After the 24-hour recovery period, animals underwent a neurological assessment adapted from Alam et al,23 before being sedated as described in Preoperative preperation, and final blood draws taken. Swine were then humanely euthanized with pentobarbital sodium and phenytoin sodium (Euthasol, 390 mg/mL, Virbac, Fort Worth, TX, USA).
Blood draws and laboratory analysis
Whole blood was collected at BSLN, EOS, PFC (hour) 1, 2, 4, END PFC, EHC, and Final. Arterial blood gas parameters were assessed using a GEM Premier 4000 (Instrumentation Laboratory, Bedford, MA). Basic metabolic panels and liver-associated enzymes were evaluated on Catalyst One Chemistry Analyzers (IDEXX Laboratories, Westbrook, ME).
Cerebrospinal fluid
Cerebrospinal fluid (CSF) was collected during necropsy and analyzed via enzyme-linked immunosorbent assay (Elabscience Biotechnology, Houston, TX) for S100 calcium-binding protein B (S100B), glial fibrillary acidic protein (GFAP), and neuron-specific enolase (NSE).
Pathology/histology
At time of euthanasia, necropsy was performed to collect representative sections of brain (hippocampus, cerebellum, frontal cortex), heart, lungs, liver, kidney, spleen, ileum, adrenal glands, and mesenteric lymph nodes for histopathologic evaluation. Tissue sections were fixed and stained with H&E, and then examined by a veterinarian pathologist blinded to the experimental groups, using an Olympus BX51 upright brightfield microscope (Olympus Scientific Solutions, Waltham, MA). Tissues were scored for severity of pathology as detailed below (table 4).
Data and statistical analysis
Statistical analyses were performed using Prism V.7 (GraphPad Software, La Jolla, CA). Data are presented as mean±SD. Single time point analyses were performed by one-way analysis of variance (ANOVA) with Tukey’s post hoc, and multiple time point analyses were analyzed by two-way repeated measures ANOVA with Bonferroni correction post hoc analysis. Neurocognitive and histological assessment scores were analyzed via Kruskal-Wallis test with Dunn’s multiple comparisons post hoc test. P values <0.05 were considered to be statistically significant. A priori and post hoc power analyses were performed using G*Power V.3.1 Statistical Power Analysis program (Heinrich Heine Universitat, Dusseldorf, Germany), to determine power for neurocognitive scores, lactate, pH, bicarbonate (HCO3), base excess (BE), and aspartate aminotransferase (AST). With 100% survival across all three groups and analyses showing average power calculations of 0.88, it was determined that sufficient group sizes were met to answer the hypothesis with a large effect size.
Results
Demographics, blood loss, fluid volumes, and survival
There were no significant differences in age or weight between groups. Experimental timeline occurred as shown in figure 1A. The mean time until decompensation for the decompensated hemorrhage+hypotension group (Decomp/PH) was 67.6±23.1 minutes, with a mean volume per weight blood loss of 34.4±3.1 mL/kg. Decomp/PH and sham groups (anesthesia sham=NS/NT, hypotensive sham=NS/PH) demonstrated 100% survival (data not shown). As Decomp/PH was unable to achieve and maintain targeted SBP of 85±5 mm Hg, continuous infusion of crystalloid throughout PFC resulted in significantly higher fluid volumes than sham groups (2.1±4.6 vs. 17.3±8.0 vs. 78.1±8.9 mL/kg).
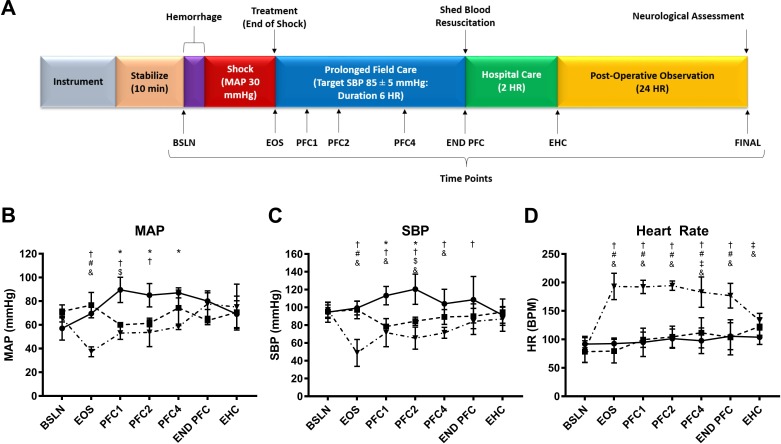
Figure 1.
Timeline and vitals. (A) Schematic depicting experimental timeline, with time points running from instrumentation, through hemorrhage, prolonged field care, hospital care, postoperative observation and euthanasia. Blood draws were obtained at each indicated time point for labs (arterial blood gas and serum chemistries) at baseline (BSLN), end of shock (EOS), prolonged field care (PFC1), PFC2, PFC4, END PFC, end of hospital care (EHC) and euthanasia (Final). Vitals were continuously monitored throughout the operational stages of the protocol. Data are presented as mean±SD for (B) mean arterial pressure (MAP), (C) systolic blood pressure (SBP), and (D) heart rate. Lines indicate groups: group 1 (NS/NT, n=5, solid line); group 2 (NS/PH, n=5, dashed line); group 3 (Decomp/PH, n=5, dashed/dotted line). P values <0.05 were considered to be statistically significant. Significant differences between groups are indicated as: *NS/NT versus NS/PH, †NS/NT versus Decomp/PH, #NS/PH versus Decomp/PH, $NS/NT versus BSLN, ‡NS/PH versus BSLN, &Decomp/PH versus BSLN. BPM, beats per minute; END PFC, end of prolonged field care; HR, heart rate; NS, non-shock; NT, normotensive; PH, permissive hypotension
Hemodynamics, heart rate, and respiratory function
Hemodynamics were equivalent between all groups at BSLN. At EOS, Decomp/PH MAP was significantly lower (37.3±4.2 mm Hg) compared with BSLN (65.7±3.2 mm Hg) and sham groups (NS/NT: 69.5±0.7, NS/PH: 76.7±10.7 mm Hg) (figure 1B). SBP was significantly lower than NS/NT from PFC1 through PFC4 (figure 1C). Mean SBPs over PFC were 109.2±12.3 (NS/NT), 87.7±1.6 (NS/PH), and 69.7±4.3 (Decomp/PH) mm Hg. While Decomp/PH demonstrated lower mean SBPs than NS/PH through PFC, this difference was not significant. Decomp/PH demonstrated significant tachycardia in response to hemorrhage compared with BSLN through END EHC and compared with sham groups through END PFC (figure 1D). At EHC, all changes in BPs and heart rate between groups resolved, with no persistent significant differences.
End-tidal carbon dioxide was significantly lower in hemorrhaged groups compared with BSLN at EOS (data not shown). These differences resolved during PFC and remained equivalent to BSLN in all groups. In all groups, the PaO2/FiO2 ratio remained above 300 throughout (data not shown).
Arterial blood gas
Severity of shock was evaluated via multiple parameters (table 1), including lactate, pH, BE and HCO3. At EOS, Decomp/PH demonstrated significant differences in levels of all shock parameters compared with sham groups. Differences in pH were resolved by END PFC, and all other parameters of shock resolved after hospital resuscitation (table 1).
Table 1.
Arterial gas parameters
| NS/NT | NS/PH | Decomp/PH | |
| n=5 | n=5 | n=5 | |
| pH | |||
| BSLN | 7.54±0.02 | 7.53±0.03 | 7.54±0.02 |
| EOS | 7.57±0.04 | 7.49±0.02 | 7.44±0.081 |
| PFC1 | 7.54±0.07 | 7.51±0.05 | 7.34±0.0911,2 |
| PFC2 | 7.52±0.02 | 7.50±0.07 | 7.36±0.1011,2 |
| PFC4 | 7.56±0.03 | 7.51±0.04 | 7.42±0.0711,2 |
| END PFC | 7.55±0.03 | 7.52±0.04 | 7.47±0.06 |
| EHC | 7.55±0.05 | 7.53±0.05 | 7.50±0.04 |
| Final | 7.40±0.04 | 7.40±0.04 | 7.43±0.04 |
| Lactate (mmol/L) | |||
| BSLN | 1.8±0.4 | 2.4±0.7 | 2.0±0.4 |
| EOS | 1.8±0.4 | 2.2±0.5 | 7.8±2.911,2 |
| PFC1 | 2.1±1.1 | 2.7±1.8 | 10.3±3.411,2 |
| PFC2 | 1.6±0.5 | 3.5±3.9 | 10.7±4.311,2 |
| PFC4 | 1.3±0.1 | 3.6±2.8 | 8.1±4.911,2 |
| END PFC | 1.1±0.2 | 2.0±0.8 | 4.9±3.61 |
| EHC | 1.1±0.1 | 1.5±0.5 | 2.6±2.4 |
| Final | 1.5±0.3 | 1.1±0.3 | 1.2±0.5 |
| Base excess (mmol/L) | |||
| BSLN | 13.3±1.3 | 12.4±1.2 | 12.9±0.9 |
| EOS | 13.6±1.6 | 11.7±1.2 | 0.2±5.211,2 |
| PFC1 | 12.5±2.4 | 11.2±2.9 | −0.46±5.011,2 |
| PFC2 | 12.8±1.3 | 10.1±4.8 | 0.2±6.211,2 |
| PFC4 | 13.6±2.3 | 9.5±3.8 | 3.3±6.411,2 |
| END PFC | 14.0±2.2 | 10.7±1.4 | 7.2±6.01 |
| EHC | 13.6±2.8 | 12.5±2.2 | 11.8±4.5 |
| Final | 10.2±2.3 | 10.8±2.1 | 11.2±3.3 |
| HCO3 (mmol/L) | |||
| BSLN | 37.3±1.3 | 36.6±1.5 | 36.8±0.9 |
| EOS | 36.9±1.5 | 36.4±1.6 | 24.0±4.211,2 |
| PFC1 | 36.4±1.7 | 35.5±2.4 | 25.5±3.811,2 |
| PFC2 | 37.0±1.3 | 34.3±4.1 | 25.8±5.011,2 |
| PFC4 | 37.0±2.3 | 33.3±3.6 | 28.1±5.511,2 |
| END PFC | 37.8±2.1 | 34.5±1.4 | 31.4±5.51 |
| EHC | 37.2±2.5 | 36.4±2.3 | 35.9±4.4 |
| Final | 36.7±2.5 | 37.2±2.2 | 37.0±3.0 |
Results are presented as mean±SD. Statistical significance is achieved by p<0.05. Values significantly different from BSLN are denoted in italics. Superscript numbers indicate value is significantly different from the corresponding column listed.
BSLN, baseline; EHC, end of hospital care; END PFC, end of prolonged field care; EOS, end of shock; NS, non-shock; NT, normotensive; PFC, prolonged field care; PH, permissive hypotension.
Neurological assessment, organ function, and histology
No groups showed neurologic impairment prior to undergoing the protocol. After procedure, and prior to euthanasia, all animals continued to be neurologically intact. No group demonstrated any significant change in score compared with either their BSLN or sham control groups (table 2). Additionally, no markers of neurological damage were detected in CSF (table 3).
Table 2.
Neurological assessment outcomes
| NS/NT | NS/PH | Decomp/PH | P value | ||
| Assessment | Score range | n=5 | n=5 | n=5 | |
| Level of consciousness | 0–3 | 0±0 | 0.2±0.5 | 0±0 | >0.9999 |
| Behavior | 0–3 | 0±0 | 0±0 | 0.4±0.6 | 0.2857 |
| Feeding/drinking | 0–2 | 0±0 | 0±0 | 0±0 | 0.2857 |
| Standing position | 0–4 | 0±0 | 0.4±0.9 | 0.2±0.5 | >0.9999 |
| Head position | 0–2 | 0±0 | 0±0 | 0±0 | NA |
| Utterance | 0–1 | 0±0 | 0±0 | 0±0 | NA |
| Gait | 0–3 | 0±0 | 0.8±1.3 | 0.2±0.5 | 0.5055 |
| Forelimb, left | 0–4 | 0±0 | 0.2±0.5 | 0±0 | >0.9999 |
| Forelimb, right | 0–4 | 0±0 | 0.2±0.5 | 0.2±0.5 | >0.9999 |
| Hindlimb, left | 0–4 | 0±0 | 0.6±1.3 | 0±0 | >0.9999 |
| Hindlimb, right | 0–4 | 0±0 | 0.6±1.3 | 0.4±0.9 | >0.9999 |
| Sum | 0–34 | 0±0 | 3.0±4.8 | 1.4±2.2 | 0.3773 |
Results are presented as mean±SD. Statistical significance is achieved by p<0.05.
NA, not applicable; NS, non-shock; NT, normotensive; PH, permissive hypotension.
Table 3.
Organ function parameters
| NS/NT | NS/PH | Decomp/PH | |
| n=5 | n=5 | n=5 | |
| S100B (ng/mL) | 0.26±0.45 | 0.04±0.06 | 0.48±0.39 |
| GFAP (ng/mL) | 0.00±0.00 | 0.10±0.18 | 0.00±0.00 |
| NSE (ng/mL) | 0.12±0.27 | 0.00±0.00 | 0.19±0.42 |
| BUN (mg/dL) | |||
| BSLN | 6.2±2.4 | 9.2±3.3 | 5.4±3.2 |
| EOS | 6.2±2.4 | 9.2±3.3 | 7.0±3.5 |
| PFC1 | 7.4±2.1 | 9.8±3.4 | 7.2±3.6 |
| PFC2 | 8.8±2.5 | 11.4±3.6 | 8.8±3.8 |
| PFC4 | 10.6±2.3 | 13.8±3.8 | 11.4±4.3 |
| END PFC | 12.0±2.5 | 15.4±3.6 | 13.2±4.3 |
| EHC | 13.4±2.3 | 15.0±5.5 | 11.4±3.2 |
| Final | 8.2±3.0 | 9.4±3.4 | 6.6±4.3 |
| CREA (mg/dL) | |||
| BSLN | 1.3±0.2 | 1.3±0.2 | 1.0±0.2 |
| EOS | 1.3±0.23 | 1.3±0.23 | 1.3±0.111,2 |
| PFC1 | 1.2±0.23 | 1.3±0.23 | 1.2±0.211,2 |
| PFC2 | 1.2±0.23 | 1.2±0.13 | 1.2±0.211,2 |
| PFC4 | 1.2±0.23 | 1.2±0.23 | 1.1±0.21,2 |
| END PFC | 1.2±0.2 | 1.1±0.13 | 1.0±0.12 |
| EHC | 1.1±0.23 | 1.2±0.13 | 1.1±0.21,2 |
| Final | 1.1±0.1 | 1.2±0.1 | 0.9±0.2 |
| AST (U/L) | |||
| BSLN | 22.0±5.2 | 22.6±5.4 | 19.2±2.5 |
| EOS | 23.8±8.2 | 22.6±4.9 | 20.6±3.4 |
| PFC1 | 22.4±4.0 | 23.2±7.0 | 36.6±15.0 |
| PFC2 | 20.4±4.3 | 23.4±4.9 | 114.6±131.0 |
| PFC4 | 25.8±8.7 | 27.6±8.4 | 296.4±375.6 |
| END PFC | 33.8±18.63 | 35.0±15.43 | 472.2±603.511,2 |
| EHC | 42.0±22.33 | 51.2±34.63 | 426.0±395.911,2 |
| Final | 353.6±278.93 | 550.6±223.4 | 794.0±600.01 |
| ALT (U/L) | |||
| BSLN | 53.0±15.2 | 57.2±14.7 | 54.4±7.9 |
| EOS | 51.0±15.3 | 59.4±15.3 | 53.0±7.3 |
| PFC1 | 51.8±15.4 | 62.4±11.3 | 46.8±4.5 |
| PFC2 | 52.2±13.5 | 59.4±13.8 | 54.6±9.2 |
| PFC4 | 51.4±13.7 | 58.4±14.7 | 70.4±24.2 |
| END PFC | 53.2±12.4 | 54.2±10.7 | 75.6±32.9 |
| EHC | 53.0±12.0 | 57.6±13.7 | 88.2±18.3 |
| Final | 153.4±55.6 | 151.8±39.5 | 202.8±80.7 |
Results are presented as mean±SD. Statistical significance is achieved by p<0.05. Values significantly different from BSLN are denoted in italics. Superscript numbers indicate value is significantly different from the corresponding column listed.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BSLN, baseline; BUN, blood urea nitrogen; CREA, creatinine; EHC, end of hospital care; END PFC, end of prolonged field care; EOS, end of shock; GFAP, glial fibrillary acidic protein; NS, non-shock; NSE, neuron-specific enolase; NT, normotensive; PFC, prolonged field care; PH, permissive hypotension.
Kidney function was assessed by blood urea nitrogen (BUN) and creatinine (CREA; table 3). Compared with BSLN, BUN, NS/NT and NS/PH were significantly elevated from PFC4 through EHC, and Decomp/PH was significantly elevated from PFC2 through EHC. Decomp/PH was significantly increased from BSLN at EOS through PFC2. Fold changes of CREA were significantly higher for Decomp/PH than NS/NT from EOS through PFC4, and at EHC, and significantly higher compared with NS/PH from EOS through EHC. All groups demonstrated significant changes in BUN/CREA from BSLN at PFC4 through EHC (data not shown). BUN and BUN/CREA demonstrated no significant group effects.
Acute liver function was assessed via AST and alanine aminotransferase (ALT; table 3). AST levels for Decomp/PH were significantly higher than BSLN at END PFC through euthanasia. Additionally, all groups demonstrated significant increase from BSLN at euthanasia. Decomp/PH had significantly higher levels at END PFC through EHC compared with sham groups, and remained significantly higher than NS/NT at euthanasia. All groups demonstrated a significant increase in ALT from BSLN at euthanasia only, and no significant group effects were seen.
Nine different tissue samples were scored for histology. While 5 of the 31 lesion scores showed significant differences between groups, no acute markers of inflammation or organ failure were identified as having changed due to injury or treatment (table 4).
Table 4.
Histological scores
| NS/NT | NS/PH | Decomp/PH | P value | |
| n=5 | n=5 | n=5 | ||
| Lung, cranial lobe | ||||
| Alveolar histiocytes | 0.2±0.4 | 0±0 | 0±0 | >0.9999 |
| Hemorrhage | 0.1±0.2 | 0±0 | 0.4±0.9 | >0.9999 |
| Pleural expansion | 0.8±0.8 | 0±0 | 0.6±0.9 | 0.2857 |
| Alveolar edema | 0.2±0.4 | 1.0±1.4 | 0.2±0.4 | 0.5255 |
| Atelectasis | 0±0 | 0.5±0.6 | 1.2±1.1 | 0.0899 |
| Alveolar septae fracture | 0.4±0.5 | 0±0 | 0±0 | 0.2857 |
| Congestion | 1.0±0 | 1.3±0.53 | 0.2±0.42 | 0.0050 |
| Heart, left ventricle | ||||
| Mineralization | 0±03 | 0±03 | 0.8±0.41,2 | 0.0110 |
| Adipose tissue on epicardium | 0.6±1.3 | 0.8±1.5 | 0±0 | 0.7253 |
| Necrosis and degeneration | 0.8±0.8 | 0.5±0.6 | 1.0±0.7 | 0.5810 |
| Ileum | ||||
| Crypt abscess | 0±0 | 0±0 | 0±0 | >0.9999 |
| Eos/Lpcyt inflammation | 1.7±0.7 | 1.0±1.2 | 1.0±0 | 0.2462 |
| Lamina propria edema | 0±0 | 0.5±0.6 | 0±0 | 0.0659 |
| Liver | ||||
| Periportal inflammation | 0.2±0.4 | 0.8±0.5 | 0.4±0.5 | 0.3340 |
| Sinusoid dilation | 0.6±0.5 | 0.5±0.6 | 0±0 | 0.1508 |
| Hepatic cord atrophy | 0.2±0.4 | 0.3±0.5 | 0±0 | 0.7253 |
| Glycogenosis | 2.6±0.3 | 2.3±1.03 | 0±01,2 | 0.0015 |
| Lymphoplasmacytic infiltrates | 0±02 | 0.8±0.51,3 | 0±02 | 0.0110 |
| Hepatocyte necrosis | 0±0 | 0.3±0.5 | 0.6±0.9 | 0.3773 |
| Regenerative nodules | 0±0 | 0.3±0.5 | 0±0 | 0.2857 |
| Spleen | ||||
| Eosinophilic/neutrophilic drainage | 0±0 | 0±0 | 0±0 | >0.9999 |
| Diffuse congestion | 2.4±1.33 | 2.3±1.0 | 0±01 | 0.0117 |
| Kidney | ||||
| Tubular epithelium vacuolation | 0.8±0.8 | 0±0 | 0±0 | 0.0659 |
| Interstitial congestion | 0.2±0.4 | 0±0 | 0±0 | >0.9999 |
| Tubular necrosis and degeneration | 0.4±0.9 | 0±0 | 0±0 | >0.9999 |
| Proteinosis | 0.4±0.5 | 0±0 | 0±0 | 0.2857 |
| Brain | ||||
| Hippocampus neuronal degeneration/necrosis | 0±0 | 0±0 | 0±0 | >0.9999 |
| Cerebellum neuronal degeneration/necrosis | 0±0 | 0±0 | 0±0 | >0.9999 |
| Frontal cortex neuronal degeneration/necrosis | 0±0 | 0±0 | 0±0 | >0.9999 |
| Mesenteric lymph node | ||||
| Eosinophilic/neutrophilic drainage | 1.8±0.8 | 2.0±1.2 | 0.4±0.5 | 0.0327 |
| Adrenal gland | ||||
| Lymphoplasmacytic infiltrates | 0±0 | 0.3±0.5 | 0±0 | 0.2857 |
Tissues were scored as: 0=none, 0.5=minimal, 1=mild, 2=moderate, 3=severe. Results are presented as mean±SD. Statistical significance is achieved by p<0.05 and is denoted in italics. Superscript numbers indicate value is significantly different from the corresponding column listed.
NS, non-shock; NT, normotensive; PH, permissive hypotension.
Discussion
As future military conflicts are expected to enter more austere and resource-limited environments, it becomes increasingly necessary to understand the limitations of the ‘golden hour’, and how to adapt this successful short-term hemorrhage control resuscitation strategy for longer periods. As TCCC CPGs for resuscitation after hemorrhage target a hypotensive SBP of 85±5 mm Hg, we sought to determine the physiologic and neurologic limits of prolonged hypotension through initial crystalloid infusion, followed by full resuscitation with blood. Our study required a severe model of shock, as determined via hemorrhage volumes and physiologic parameters assessed.24–26 To achieve this, we chose a pure hemorrhage, pressure-targeted model; previous work has demonstrated that even in absence of a soft tissue injury, without intervention, mortality rates of animals reach 100% within 2 hours after shock.13 27 Additionally, while our animals were not splenectomized, several swine studies have demonstrated that splenectomy may have minimal effect on BP in response to hemorrhage, and by allowing our animals to decompensate physiologically, we exhaust the resuscitative effects of the contractile spleen.24 28 Other studies on hypotensive resuscitation in swine using controlled hemorrhage over a 5-hour PFC demonstrate worse survival, though these models use concomitant contusions, and normal saline rather than LR.29–31 Survival in this study at 6 hours of PFC is similar to those seen in the recent study by Skarda et al, which used a pure hemorrhage, pressure-targeted injury and TCCC-directed resuscitation.13 However, their posthospital survival is worse, which may result from differences in recovery, as animals in their study were kept anesthetized and ventilated for 24 hours after recovery, during which time roughly 30% of their mortality occurs.
TCCC recommends blood-based resuscitation whenever possible and the recent consensus statement from the Trauma Hemostasis and Oxygenation Research Network position paper states shock in casualties with life-threatening hemorrhage should be reversed as soon as possible using a blood-based hemostatic resuscitation fluid with a target SBP of 100 mm Hg.32 The authors here agree with this position, however, in a predicted military PFC scenario, limited availability of resuscitative products may be unable to achieve and maintain these desired goals. Thus, our study focused on ‘pre-hospital’ resuscitation, and demonstrates that hypotensive resuscitation after decompensated shock is permissible over a 6-hour period, before hospital intervention can be achieved, crucial information for the PFC environment. Critically, this methodology resulted in 100% survival, without significant neurologic impact or histological evidence of ischemic injury, neuronal degradation, or necrosis of the brain. Increasing evidence suggests that levels of S100B, NSE, and GFAP rise in the CSF before development of clinically observable brain injury.33 At the point of euthanasia in this study, however, we observed no significant differences in CSF levels of any of these biomarkers.
While Decomp/PH animals developed lactatemia by EOS, this resolved by END PFC. With crystalloid resuscitation restricted to the volume necessary to achieve the resuscitation goal of SBP of 85±5 mm Hg, animals were able to maintain sufficient perfusion to avoid acidosis after 6 hours. This is also reflected in the trend of BE and HCO3 returning towards BSLN by END PFC and completely by EHC. The impact of this may be reflected in the severity of end-organ damage. Kidney function was maintained during the entirety of the study. While BUN and CREA increased during shock and PFC, they decreased appropriately with resuscitation. Importantly, there was no significant increase in CREA at euthanasia, 26 hours after END PFC, indicating no animals developed reduced renal clearance resulting from prolonged hypotension. Histology also demonstrated kidney preservation (eg, no tubular necrosis). Hepatic function in this model was more sensitive to ischemic injury. There was earlier rise of serum AST by the END PFC for Decomp/PH, though all groups showed a significant increase from their BSLNs over the experimental course including sham groups. Overall increase in ALT levels after recovery was independent of injury or resuscitation strategy. Despite the increase in AST and ALT, histology demonstrated no significant findings of liver damage. Across examined tissues, differences in histological scores are more reflective of crystalloid dilution than acute inflammation or organ failure.
Limitations
The use of a pressure-targeted, pure hemorrhage model represents one possible limitation here. Despite lacking a soft tissue injury, blast, or other major trauma insult, models of pressure-targeted hemorrhage are still extremely useful for advancing the treatment of patients in hemorrhagic shock.24 34–36 Adding an injury would undoubtedly change the profile of shock, and we thought that this model approximates our military injury population, representing a penetrating injury with significant hemorrhage, as seen in combat operations. Additionally, the PFC-targeted SBP of 85±5 mm Hg was not achieved in the hemorrhage group. While not significantly different from the NS/PH sham during PFC, lower pressure ranges may be indicative of more severe hypotension. Conversely, they may be a more accurate hypotension level given that swine models demonstrate consistently lower BPs than humans.25
Furthermore, while useful for our experimental model, large volumes of crystalloid in austere environments would represent logistical challenges, being well beyond the limited weight that individual military medics carry. The evidence of acceptable clinical outcomes under prolonged hypotension, despite large volumes of crystalloid to maintain targeted pressure, further highlights the need for development of low-volume resuscitation strategies. Future studies may also include BP adjuncts such as vasopressors to help define their role in hypotensive resuscitation.
Conclusion
We indicate here that after severe hemorrhage and decompensated shock, current standards of hypotensive resuscitation targeting SBP of 85±5 mm Hg over 6 hours can be used to achieve 100% survival, even in situations where there may be limited blood product availability. While the methodology of unlimited crystalloid is unrealistic, prolonged hypotension itself does not induce observed negative clinical outcomes related to neurologic function, brain or other organ function. This represents a partial answer to a critical gap in current knowledge, and future work will be required to describe outcomes out to 72 hours.
Acknowledgments
The authors acknowledge the contributions of Darren Fryer and Kassandra Ozuna of the NAMRU-SA Expeditionary and Trauma Medicine Department, as well as Carrie Crane, MAJ Kamala Rapp-Santos, Charlene Gebauer and Ashley Arredondo of the NAMRU-SA Veterinary Sciences Department. The authors also recognize the pathology support of LTC Michelle Thompson from the USAF 711th HPW/RHDV.
Footnotes
Presented at: Portions of this work were presented at the 14th Annual Academic Surgical Congress, February 7, 2019, Houston, TX.
Contributors: CGM: study design and implementation, data acquisition and interpretation, article drafting and revisions. LEN: study implementation, data analysis and interpretation, article drafting and revisions. ENH: data interpretation and analysis, article revisions. GJR and LJS: study implementation, data acquisition and analysis, article revisions. SC: data interpretation, article revisions. JJG: study design and implementation, data acquisition and analysis, article revisions.
Funding: This work was supported by the Assistant Secretary of Defense for Health Affairs, through the Fiscal Year 2016 Defense Medical Research and Development Program Prolonged Field Care Research Award–Intramural; LOG number: DM167139.
Disclaimer: The views expressed in this article reflect the results of research conducted by the author and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the US Government. In conducting research using animals, the investigators adhered to the laws of the USA and the regulations of the Department of Agriculture. The experiments reported herein were conducted in compliance with the Animal Welfare Act and in accordance with the principles set forth in the 'Guide for the Care and Use of Laboratory Animals', Institute of Laboratory Animals Resources, National Research Council, National Academy Press, 2011.
Competing interests: SC, CGM, and LEN are civilian employees of the US Government. GJR and LJS are contract employees of the US Government. JJG and ENH are military service members and this work was prepared as part of their official duties. Title 17 USC §105 provides that ‘copyright protection under this title is not available for any work of the US Government.’ Title 17 USC §101 defines a US Government work as a work prepared by a military service member or employee of the US Government as part of that person’s official duties.
Patient consent for publication: Not required.
Ethics approval: The study protocol was reviewed and approved by the 711th HPW/RHD JBSA-Fort Sam Houston Institutional Animal Care and Use Committee (IACUC) in compliance with all applicable federal regulations governing the protection of animals in research. All procedures were performed in AAALAC International accredited facilities.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article.
References
- 1.Eastridge BJ, Mabry RL, Seguin P, Cantrell J, Tops T, Uribe P, Mallett O, Zubko T, Oetjen-Gerdes L, Rasmussen TE, et al. Death on the battlefield (2001-2011): implications for the future of combat casualty care. J Trauma Acute Care Surg 2012;73:S431–7. 10.1097/TA.0b013e3182755dcc [DOI] [PubMed] [Google Scholar]
- 2.Rhee P, Joseph B, Pandit V, Aziz H, Vercruysse G, Kulvatunyou N, Friese RS. Increasing trauma deaths in the United States. Ann Surg 2014;260:13–21. 10.1097/SLA.0000000000000600 [DOI] [PubMed] [Google Scholar]
- 3.Bickell WH, Wall MJ, Pepe PE, Martin RR, Ginger VF, Allen MK, Mattox KL. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med 1994;331:1105–9. 10.1056/NEJM199410273311701 [DOI] [PubMed] [Google Scholar]
- 4.Bickell WH, Bruttig SP, Millnamow GA, O'Benar J, Wade CE. Use of hypertonic saline/dextran versus lactated Ringer's solution as a resuscitation fluid after uncontrolled aortic hemorrhage in anesthetized swine. Ann Emerg Med 1992;21:1077–85. 10.1016/S0196-0644(05)80648-1 [DOI] [PubMed] [Google Scholar]
- 5.Bickell WH, Bruttig SP, Millnamow GA, O'Benar J, Wade CE. The detrimental effects of intravenous crystalloid after aortotomy in swine. Surgery 1991;110:529–36. [PubMed] [Google Scholar]
- 6.Bickell WH, Bruttig SP, Wade CE. Hemodynamic response to abdominal aortotomy in the anesthetized swine. Circ Shock 1989;28:321–32. [PubMed] [Google Scholar]
- 7.Spinella PC, Holcomb JB. Resuscitation and transfusion principles for traumatic hemorrhagic shock. Blood Rev 2009;23:231–40. 10.1016/j.blre.2009.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pruitt BA, Rasmussen TE. Vietnam (1972) to Afghanistan (2014): the state of military trauma care and research, past to present. J Trauma Acute Care Surg 2014;77:S57–65. 10.1097/TA.0000000000000419 [DOI] [PubMed] [Google Scholar]
- 9.Duchesne JC, Barbeau JM, Islam TM, Wahl G, Greiffenstein P, McSwain NE. Damage control resuscitation: from emergency department to the operating room. Am Surg 2011;77:201–6. [DOI] [PubMed] [Google Scholar]
- 10.Morrison CA, Carrick MM, Norman MA, Scott BG, Welsh FJ, Tsai P, Liscum KR, Wall MJ, Mattox KL. Hypotensive resuscitation strategy reduces transfusion requirements and severe postoperative coagulopathy in trauma patients with hemorrhagic shock: preliminary results of a randomized controlled trial. J Trauma 2011;70:652–63. 10.1097/TA.0b013e31820e77ea [DOI] [PubMed] [Google Scholar]
- 11.Palm K, Apodaca A, Spencer D, Costanzo G, Bailey J, Blackbourne LH, Spott MA, Eastridge BJ. Evaluation of military trauma system practices related to damage-control resuscitation. J Trauma Acute Care Surg 2012;73:S459–64. 10.1097/TA.0b013e3182754887 [DOI] [PubMed] [Google Scholar]
- 12.Duke MD, Guidry C, Guice J, Stuke L, Marr AB, Hunt JP, Meade P, McSwain NE, Duchesne JC. Restrictive fluid resuscitation in combination with damage control resuscitation: time for adaptation. J Trauma Acute Care Surg 2012;73:674–8. 10.1097/TA.0b013e318265ce1f [DOI] [PubMed] [Google Scholar]
- 13.Skarda DE, Mulier KE, George ME, Bellman GJ. Eight hours of hypotensive versus normotensive resuscitation in a porcine model of controlled hemorrhagic shock. Acad Emerg Med 2008;15:845–52. 10.1111/j.1553-2712.2008.00202.x [DOI] [PubMed] [Google Scholar]
- 14.Sun N, Li LZ, Luo W, Luo Q. Cerebral hemodynamic change and metabolic alteration in severe hemorrhagic shock. Adv Exp Med Biol 2014;812:217–23. 10.1007/978-1-4939-0620-8_29 [DOI] [PubMed] [Google Scholar]
- 15.Tamura H, Witoszka MM, Hopkins RW, Simeone FA. The nervous system in experimental hemorrhagic shock: morphology of the brain. J Trauma 1972;12:869–75. 10.1097/00005373-197210000-00006 [DOI] [PubMed] [Google Scholar]
- 16.Kovách AG, Sándor P. Cerebral blood flow and brain function during hypotension and shock. Annu Rev Physiol 1976;38:571–96. 10.1146/annurev.ph.38.030176.003035 [DOI] [PubMed] [Google Scholar]
- 17.Simeone FA, Witoszka M. The central nervous system in experimental hemorrhagic shock. the cerebrospinal fluid pressure. Am J Surg 1970;119:427–32. 10.1016/0002-9610(70)90145-5 [DOI] [PubMed] [Google Scholar]
- 18.Simeone FA, Witoszka MM. Changes in composition of cerebrospinal fluid in hemorrhagic shock. Surg Forum 1970;21:53–5. [PubMed] [Google Scholar]
- 19.Wan Z, Sun S, Ristagno G, Weil MH, Tang W. The cerebral microcirculation is protected during experimental hemorrhagic shock. Crit Care Med 2010;38:928–32. 10.1097/CCM.0b013e3181cd100c [DOI] [PubMed] [Google Scholar]
- 20.Taccone FS, De Backer D. Is cerebral microcirculation really preserved in shock states? Crit Care Med 2010;38:1008–9. 10.1097/CCM.0b013e3181d16958 [DOI] [PubMed] [Google Scholar]
- 21.Fries M, Nolte K, Demir F, Kottmann K, Timper A, Coburn M, Weis J, Rossaint R. Neurocognitive performance after cardiopulmonary resuscitation in pigs. Crit Care Med 2008;36:842–7. 10.1097/CCM.0B013E3181653041 [DOI] [PubMed] [Google Scholar]
- 22.Wohltmann CD, Franklin GA, Boaz PW, Luchette FA, Kearney PA, Richardson JD, Spain DA. A multicenter evaluation of whether gender dimorphism affects survival after trauma. Am J Surg 2001;181:297–300. 10.1016/S0002-9610(01)00582-7 [DOI] [PubMed] [Google Scholar]
- 23.Halaweish I, Bambakidis T, He W, Linzel D, Chang Z, Srinivasan A, Dekker SE, Liu B, Li Y, Alam HB, et al. Early resuscitation with fresh frozen plasma for traumatic brain injury combined with hemorrhagic shock improves neurologic recovery. J Am Coll Surg 2015;220:809–19. 10.1016/j.jamcollsurg.2015.01.057 [DOI] [PubMed] [Google Scholar]
- 24.Gómez H, Mesquida J, Hermus L, Polanco P, Kim HK, Zenker S, Torres A, Namas R, Vodovotz Y, Clermont G, et al. Physiologic responses to severe hemorrhagic shock and the genesis of cardiovascular collapse: can irreversibility be anticipated? J Surg Res 2012;178:358–69. 10.1016/j.jss.2011.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hannon JP, Bossone CA, Wade CE. Normal physiological values for conscious pigs used in biomedical research. Lab Anim Sci 1990;40:293–8. [PubMed] [Google Scholar]
- 26.Sondeen JL, Dubick MA, Holcomb JB, Wade CE. Uncontrolled hemorrhage differs from volume- or pressure-matched controlled hemorrhage in swine. Shock 2007;28:426–33. 10.1097/shk.0b013e31804a5791 [DOI] [PubMed] [Google Scholar]
- 27.How RA, Glaser JJ, Schaub LJ, Fryer DM, Ozuna KM, Morgan CG, Sams VG, Cardin S. Prehospital adenosine, lidocaine, and magnesium has inferior survival compared with tactical combat casualty care resuscitation in a porcine model of prolonged hemorrhagic shock. J Trauma Acute Care Surg 2019;87:68–75. 10.1097/TA.0000000000002308 [DOI] [PubMed] [Google Scholar]
- 28.Wade CE, Hannon JP. Confounding factors in the hemorrhage of conscious swine: a retrospective study of physical restraint, splenectomy, and hyperthermia. Circ Shock 1988;24:175–82. [PubMed] [Google Scholar]
- 29.Feinstein AJ, Cohn SM, King DR, Sanui M, Proctor KG. Early vasopressin improves short-term survival after pulmonary contusion. J Trauma 2005;59:876–83. 10.1097/01.ta.0000187654.24146.22 [DOI] [PubMed] [Google Scholar]
- 30.Patel MB, Feinstein AJ, Saenz AD, Majetschak M, Proctor KG. Prehospital HBOC-201 after traumatic brain injury and hemorrhagic shock in swine. J Trauma 2006;61:46–56. 10.1097/01.ta.0000219730.71206.3a [DOI] [PubMed] [Google Scholar]
- 31.Sanui M, King DR, Feinstein AJ, Varon AJ, Cohn SM, Proctor KG. Effects of arginine vasopressin during resuscitation from hemorrhagic hypotension after traumatic brain injury. Crit Care Med 2006;34:433–8. 10.1097/01.CCM.0000196206.83534.39 [DOI] [PubMed] [Google Scholar]
- 32.Woolley T, Thompson P, Kirkman E, Reed R, Ausset S, Beckett A, Bjerkvig C, Cap AP, Coats T, Cohen M, et al. Trauma hemostasis and oxygenation research network position paper on the role of hypotensive resuscitation as part of remote damage control resuscitation. J Trauma Acute Care Surg 2018;84:S3–13. 10.1097/TA.0000000000001856 [DOI] [PubMed] [Google Scholar]
- 33.Böhmer AE, Oses JP, Schmidt AP, Perón CS, Krebs CL, Oppitz PP, D'Avila TT, Souza DO, Portela LV, Stefani MA, et al. Neuron-Specific enolase, S100B, and glial fibrillary acidic protein levels as outcome predictors in patients with severe traumatic brain injury. Neurosurgery 2011;68:1624–31. 10.1227/NEU.0b013e318214a81f [DOI] [PubMed] [Google Scholar]
- 34.Chiara O, Pelosi P, Brazzi L, Bottino N, Taccone P, Cimbanassi S, Segala M, Gattinoni L, Scalea T. Resuscitation from hemorrhagic shock: experimental model comparing normal saline, dextran, and hypertonic saline solutions. Crit Care Med 2003;31:1915–22. 10.1097/01.CCM.0000074725.62991.42 [DOI] [PubMed] [Google Scholar]
- 35.Soller BR, Khan T, Favreau J, Hsi C, Puyana JC, Heard SO. Investigation of muscle pH as an indicator of liver pH and injury from hemorrhagic shock. J Surg Res 2003;114:195–201. 10.1016/s0022-4804(03)00251-8 [DOI] [PubMed] [Google Scholar]
- 36.Szebeni J, Baranyi L, Savay S, Götze O, Alving CR, Bünger R, Mongan PD. Complement activation during hemorrhagic shock and resuscitation in swine. Shock 2003;20:347–55. 10.1097/01.shk.0000082444.66379.17 [DOI] [PubMed] [Google Scholar]