Abstract
Improvement in survival in patients living with human immunodeficiency virus (PLHIV) has led to increased prevalence of cardiovascular disease. Whether HIV-associated immune dysfunction is associated with preclinical left ventricular (LV) dysfunction despite normal LV ejection fraction (LVEF) is unclear. Accordingly, we investigated the relation of immune status and LV function in PLHIV. Global longitudinal strain (GLS) analyses were performed retrospectively on all echocardiograms for PLHIV who had available HIV-1 RNA viral load, nadir, and proximal CD4 cell count data at Duke University Medical Center between 2001 and 2012. The relation between HIV-1 RNA viral load, nadir, and proximal CD4 count and GLS as a continuous dependent variable was assessed with unadjusted and adjusted linear regression. GLS was calculated for 253 PLHIV. Median GLS in our cohort was — 15.1% with interquartile range from (−16.7 to −13.6). All participants had an LVEF ≥50%. In adjusted analyses, proximal CD4 <500 cells/mm3 and nadir CD4 <250 cells/mm3 were significantly inversely correlated with GLS (p = 0.01 and p = 0.004, respectively). In PLHIV, patient with plasma HIV RNA < 400 copies/ml at baseline had a trend toward significantly more negative values of GLS compared with those patients without viral suppression at baseline (p = 0.08). In conclusion, this study is the first to demonstrate such a high prevalence of abnormal GLS in PLHIV, and the first to identify that proximal and nadir CD4 cell count are independently associated with GLS despite normal LVEF.
With the advent of antiretroviral therapy (ART), human immunodeficiency virus (HIV) infection has become a long-term health condition, with near normal life expectancy.1 Improvement in survival has led to a shift in mortality and increased prevalence of serious nonacquired immune deficiency syndrome related conditions, including cardiovascular disease (CVD).2,3 Although the underlying mechanism leading to the increased risk of CVD in patients living with HIV (PLHIV) is most likely multifactorial and includes co-morbid conditions and toxicity from ART,4 chronic HIV-associated inflammation, viral replication, and immune dysfunction are recognized as key factors.5,6 Early detection of myocardial dysfunction has predominantly relied upon serial cardiac imaging to identify a reduction in LV function before signs or symptoms of heart failure (HF) develop (stage B HF).7 Accordingly, we investigated the prevalence of abnormal global longitudinal strain (GLS) in PLHIV with normal left ventricle ejection fraction (LVEF) and HIV-associated immune dysfunction. We also explored whether GLS could serve as a predictor of preclinical abnormalities in PLHIV, who might be candidates for enhanced CVD prevention.
METHODS
This was a single-center, retrospective, observational cohort study. The data sources for the present study included the Duke Echocardiography Laboratory Database, Duke HIV Clinical Database, and Duke Enterprise Data Unified Content Explorer.8 For the purpose of the present study, data from Duke HIV Clinical Database were linked to Duke Echocardiography Laboratory Database to form a cohort of PLHIV who had at least one transthoracic echocardiography (TTE). All PLHIV with available viral loads and CD4 counts were included in the study. Clinical data, including medical and cardiovascular history, as well as CVD risk factors (i.e., hypertension, diabetes, and hyperlipidemia) were obtained from the Duke Enterprise Data Unified Content Explorer, a web-based electronic medical record query tool. All 2 dimensional (2D) TTE examinations which had been acquired using standard clinical protocols were uploaded to an independent software package (2D Cardiac Performance Analysis version 4.5, Tom Tec Imaging Systems, Unterschleissheim, Germany) at frame rate ≥50/s for offline speckle tracking echocardiography (STE) analyses. All analyses were interpreted by a single experienced reader blinded to the clinical, HIV specific markers, and immune system status [F.A]. LV volumes and LVEF were calculated by the modified biplane Simpson’s method, according to the American Society of Echocardiographic.9 For STE, LV endocardial border was manually traced in end-systole. The integrity of speckle tracking was visually ascertained. In case of insufficient tracking, manual correction of the endocardial tracing was attempted and if still unsatisfactory, or inadequate image quality (defined as poor visualization or poor tracking of >2 ventricular segments, segment dropout, missing view, or significant foreshortening of the LV) the study was excluded from the final analysis. GLS was the average of the 3 apical peak longitudinal strain measurements (apical 4, 3, and 2 chamber views).10 Intraobserver variability was also tested.
Baseline clinical characteristics were stratified by quartiles of GLS (−24.5% to −16.65%, −16.65% to −15.1%, −15.1% to −13.6%, and −13.6% to −3.9%). Continuous variables are presented as medians and twenty-fifth to seventy-fifth percentiles. Categorical variables are presented as frequencies and percentages. Baseline TTE characteristics are presented similarly. HIV-associated variables are also presented stratified by GLS quartile. The relation between HIV-1 RNA viral load, proximal CD4, and nadir CD4 cell count with GLS (as a continuous dependent variable) was assessed with unadjusted and adjusted linear regression. Adjustment variables believe to be potential confounders were prespecified by the investigators and included: age, race, hypertension, hyperlipidemia, and diabetes. Exploration for nonlinear relations between continuous CD4 measurements with GLS yielded significant non-linear relations for proximal CD4 and nadir CD4 measurements in relation to continuous GLS. Investigators implemented use of piece-wise linear splines with single cut-points of 500 and 250 cells/mm3 for proximal and nadir CD4, respectively. Scatterplots with regression lines including 95% confidence intervals were provided to visually demonstrate the linear relations between continuous GLS and piece-wise splines for proximal and nadir CD4 cell counts. Boxplots for distribution of continuous GLS for levels of plasma HIV RNA, proximal CD4, and nadir CD4 were provided to visually demonstrate mean GLS comparisons across groups of categorical variables. Analyses were performed using SAS software version 9.4 and a p value <0.05 was considered statistically significant.
RESULTS
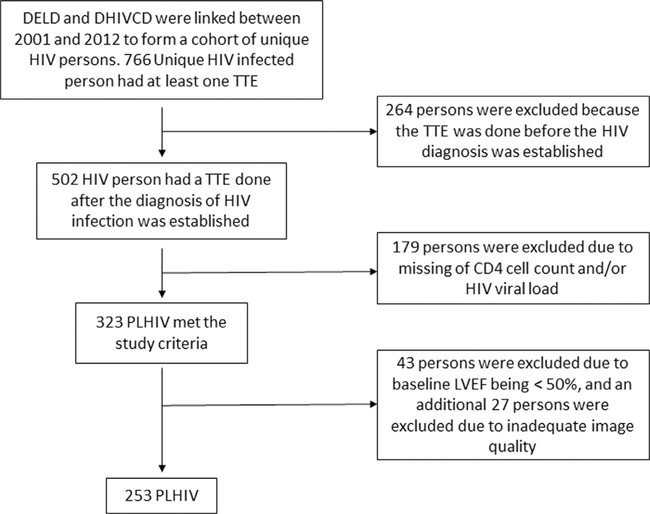
The database yielded 323 unique patients. From that group, 43 patients were excluded due to baseline LVEF being <50%, and an additional 27 patients were excluded due to inadequate image quality for speckle tracking-based assessment of GLS, providing a final analysis cohort of 253 PLHIV (Figure 1). Of the 253 patients included in the analysis, 72% were Black, 38% were female, and the median age was 44 years (interquartile range [IQR] 37 to 50). Forty percent of cohort members had hypertension at baseline and 19% had a diagnosis of diabetes. In addition, 42% of cohort members actively smoked cigarettes at the time of their echocardiogram. The median CD4 count for the cohort was 295 cells/mm3 (IQR 118 to 469). On assessing for cardiac strain, the median cohort GLS was −15.1% with IQR ranging from −16.7% to −13.6% (Table 1).
Figure 1.
Flow chart for extracting Duke HIV unique cohort (n = 253). Abbreviations: CD4 = cluster of differentiation 4; DELD = Duke Echocardiography Laboratory Database; DHIVCD Duke HIV Clinical Database; HIV = human immunodeficiency virus; PLHIV patients living with HIV; STE = speckle tracking echocardiography; TTE = transthoracic echocardiography.
Table 1.
Distribution of baseline characteristics across quartiles of global longitudinal strain
| Global longitudinal strain (GLS) quartile | ||||||
|---|---|---|---|---|---|---|
| Characteristic | All patients (n = 253) | GLS 1st quartile [−24.5 to−16.65] (n = 64) | GLS 2nd quartile [−16.65 to −15.1] (n = 63) | GLS 3rd quartile [−15.1 to −13.6] (n = 66) | GLS 4th quartile [−13.6 to −3.9] (n = 60) | p Value |
| Age (years), median Interquartile range (IQR) number of missing cases (nmiss) | 44 (37–50)[253] | 47 (38–53) [64] | 41 (35–48) [63] | 44 (36–50) [66] | 44 (39–49) [60] | 0.191 |
| Women | 96/252 (38%) | 29/63 (46%) | 25/63 (40%) | 23/66 (35%) | 19/60 (32%) | 0.377 |
| Black | 181/253 (72%) | 42/64 (66%) | 46/63 (73%) | 51/66(77%) | 42/60 (70%) | |
| White/non-Hispanic | 55/253 (22%) | 15/64 (23%) | 12/63 (19%) | 12/66(18%) | 16/60 (27%) | |
| Hispanic | 11/253 (4%) | 5/64 (7%) | 2/63 (3%) | 3/66 (5%) | 1/60 (2%) | |
| Asian | 0/253 (0%) | 0/64 (0%) | 0/63 (0%) | 0/66 (0%) | 0/60 (0%) | |
| Other | 6/253 (2%) | 2/64 (3%) | 3/63 (5%) | 0/66 (0%) | 1/60 (2%) | |
| Hypertension | 102/253 (40%) | 29/64 (45%) | 18/63 (29%) | 28/66 (42%) | 27/60 (45%) | 0.176 |
| Hyperlipidemia | 52/253 (21%) | 14/64 (22%) | 15/63 (24%) | 13/66 (20%) | 10/60 (17%) | 0.786 |
| Diabetes mellitus | 47/253 (19%) | 14/64 (22%) | 10/63 (16%) | 9/66 (14%) | 14/60 (23%) | 0.436 |
| Any cocaine use | 80/252 (32%) | 19/63 (30%) | 18/63 (29%) | 20/66 (30%) | 23/60 (38%) | 0.652 |
| Any tobacco use | ||||||
| None | 108/251 (43%) | 26/63 (41%) | 28/63 (44%) | 30/66 (46%) | 24/59 (41%) | 0.588 |
| Current | 105/251 (42%) | 23/63 (37%) | 26/63 (41%) | 30/66 (46%) | 26/59 (44%) | |
| Former | 38/251 (15%) | 14/63 (22%) | 9/63 (14%) | 6/66 (9%) | 9/59 (15%) | |
| Duration of HIV Infection at time of ECHO (yr), median (IQR) (nmiss) | 7 (3–12) [253] | 8(3–13) [64] | 7(4–13) [63] | 7 (2–10) [66] | 6(3–12) [60] | 0.620 |
| Median CD4 count (cells/mm3), (IQR) (nmiss) | 295 (118–469) [80] | 319(155–498) [22] | 356 (223–540) [18] | 246 (20–450) [20] | 244 (59–370) [20] | 0.314 |
| Coronary heart disease | 14/253 (6%) | 6/64 (9%) | 2/63 (3%) | 2/66 (3%) | 4/60 (7%) | 0.367 |
| Hepatitis C diagnosis | 52/253 (21%) | 18/64 (28%) | 10/63 (16%) | 13/66 (20%) | 11/60(18%) | 0.347 |
| Angiotensin converting enzyme inhibitors or angiotensin II receptor blockers use | 57/253 (23%) | 17/64 (27%) | 11/63 (18%) | 12/66(18%) | 17/60 (28%) | 0.334 |
| Beta blocker use | 52/253 (21%) | 10/64 (16%) | 8/63 (13%) | 14/66 (21%) | 20/60 (33%) | 0.025 |
| Antiretroviral therapy use | 199/253 (78%) | 49/64 (77%) | 54/63 (86%) | 50/66 (76%) | 46/60 (77%) | 0.474 |
| Abacavir (Ziagen) use | 54/253 (21%) | 7/64(11%) | 14/63 (22%) | 18/66(27%) | 15/60 (25%) | 0.111 |
| Didanosine/Stavudine/Zalcitabine use | 65/253 (26%) | 17/64 (27%) | 15/63 (24%) | 18/66(27%) | 15/60 (25%) | 0.970 |
| Zidovudine (AZT, Retovir) use | 80/253 (32%) | 17/64 (27%) | 29/63 (46%) | 15/66 (23%) | 19/60 (32%) | 0.030 |
| Protease inhibitor use | 134/253 (53%) | 34/64 (53%) | 37/63 (59%) | 29/66 (44%) | 34/60 (57%) | 0.344 |
| Body mass index (kg/m2), median (IQR) | 26.70 (23.00–31.40) [179] | 27.34 (23.10–31.70) [49] | 26.90 (24.07–30.24) [51] | 25.15 (22.50–31.07) [40] | 26.40 (23.10–30.04) [39] | 0.932 |
| Left ventricle end-diastolic volume index (ml/m2), median (IQR) [nobs] | 109 (97–124)[231] | 102 (88–118) [57] | 113 (108–123) [59] | 109 (97–123)[62] | 120(102–129) [53] | 0.005 |
| Left ventricle end-systolic volume index (ml/m2), median (IQR) (nmiss) | 43 (32–54) [231] | 37 (30–48) [57] | 43 (34–54) [59] | 43 (30–49) [62] | 52 (42–68) [53] | <0.001 |
| Left ventricle ejection fraction (%), median (IQR) (nmiss) | 55 (55–55) [253] | 55 (55–55) [64] | 55 (55–55) [63] | 55 (55–55) [66] | 55 (55–55) [60] | 0.201 |
| Global longitudinal strain (%), median (IQR) (nmiss) | −15.1 (−16.7 to−13.6) [253] | −18.3 (−19.2 to−17.3) [64] | −15.6 (−16.3 to−15.2) [63] | −14.4 (−14.6 to −13.9) [66] | −12.1 (−13.3 to−11.2) [60] | <0.001 |
In the unadjusted analysis, higher proximal CD4 counts up to 500 cells/mm3 were associated with more negative (“further in the normal range”) GLS values (p = 0.008). There was no further correlation between CD4 counts and more negative GLS values for CD4 counts above 500 cells/ mm3. In analysis of continuous GLS with categorical proximal CD4, patients with proximal CD4 < 200 cells/mm3 had less negative values of GLS compared with patients with proximal CD4 > 200 cells/mm3, but this difference was not statistically significant (p=0.08). Higher CD4 nadir counts were associated with more negative GLS values up to a CD4 count of 250. There was no correlation between CD4 counts higher than 250 cells/mm3 and GLS. Viral suppression (defined as viral load less than 400 copies/ml) also trended towards an association with more negative GLS values (p = 0.08) (Table 2).
Table 2.
Relation of global longitudinal strain with viral load and CD4 cell count in unadjusted and adjusted analyses.
| Unadjusted |
Adjusted* |
||||||
|---|---|---|---|---|---|---|---|
| Variable | Sample size | Slope estimate | 95% Confidence interval | p Value | Slope estimate | 95% Confidence interval | p Value |
| Continuous proximal cluster of differentiation (CD4) count | |||||||
| Proximal CD4 < 500, per 50 | 234 | −0.16 | −0.28 to −0.04 | 0.008 | −0.16 | −0.28 to −0.04 | 0.011 |
| Proximal CD4 ≥ 500, per 50 Continuous nadir CD4 count | 0.05 | −0.08 to 0.18 | 0.444 | 0.07 | −0.06 to 0.20 | 0.306 | |
| Continuous nadir CD4 count | |||||||
| Nadir CD4 < 250, per 50 | 251 | −0.38 | −0.63 to −0.14 | 0.002 | −0.39 | −0.64 to −0.14 | 0.004 |
| Nadir CD4 ≥ 250, per 50 | 0.13 | −0.02 to 0.28 | 0.096 | 0.14 | −0.01 to 0.30 | 0.069 | |
| Proximal CD4 count < 200 cells/mm | 234 | 0.66 | −0.09 to 1.41 | 0.0845 | 0.59 | −0.20 to 1.38 | 0.140 |
| CD4 Nadir < 200 cells/mm3 | 251 | 0.42 | −0.34 to 1.18 | 0.275 | 0.37 | −0.40 to 1.15 | 0.344 |
| Plasma HIV ribonucleic acid (RNA) < 400 consistently for 6 months or more prior to echo | 219 | −0.70 | −1.48 to 0.08 | 0.077 | −0.76 | −1.60 to 0.08 | 0.076 |
| Log10 (Viremia copy years), per 1 | 20 | −0.17 | −0.86 to 0.53 | 0.622 | −0.07 | −0.92 to 0.78 | 0.855 |
| Years of known untreated Viremia | 70 | −0.07 | −0.22 to 0.09 | 0.376 | −0.08 | −0.25 to 0.09 | 0.348 |
Adjusted for age, black race, hypertension, hyperlipidemia and diabetes.
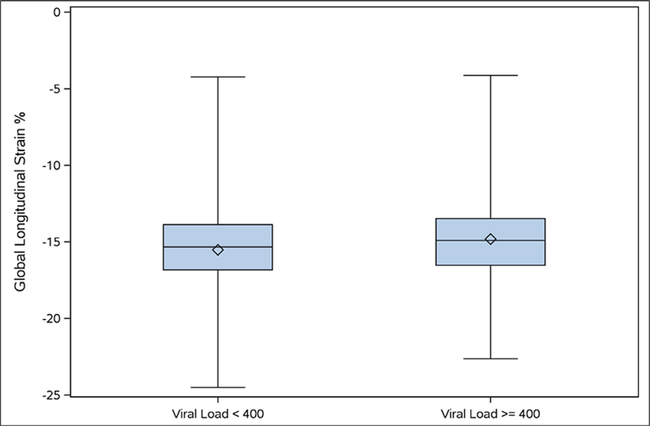
After adjusting for relevant cofounding covariates, higher CD4 counts were independently associated with more negative GLS values up to a threshold of 500 cells/mm3 (Table 2). When using the diagnostic cutoff of proximal CD4 cell count >200 cells/mm3, there was no significant association between values of GLS in patients with proximal CD4 <200 cells/mm3 compared to patients with proximal CD4 >200 cells/mm3 (Table 2). Also, in the adjusted analysis, GLS was significantly inversely correlated with continuous nadir CD4 cell count <250 cells/mm3, such that increasing nadir CD4 cell count was associated with more negative GLS values (p = 0.004). We found no significant linear relation with GLS for continuous nadir CD4 ≥250 cells/mm3 (Table 2). Finally, after adjustment, there was no change in relation between continuous GLS and plasma HIV RNA <400 copies/ml (p = 0.08) (Table 2 and Figure 2) and (Supplemental material: Figures 3 to 5).
Figure 2.
Boxplot of mean GLS for viral load (plasma HIV RNA) (<400 vs ≥400). Abbreviations: GLS = global longitudinal strain; HIV = human immunodeficiency virus; RNA = ribonucleic acid.
In unadjusted and adjusted linear regression analyses we found no significant correlation or association between GLS and viremia copy-years, or years of known untreated viremia (Table 2). Of the 280 study subjects, 27 were excluded from the final analysis because of inadequate image quality for STE analysis (feasibility of LV STE function assessment > 90%). In a subset of 25 subjects, intraobserver variability was tested with no significant systematic difference observed and an intraclass coefficient of correlation of 0.70 (95% confidence interval 0.74 to 0.97).
DISCUSSION
We studied a population of PLHIV who were treated with ART, and had normal LVEF. This study is the first to demonstrate such a high prevalence of abnormal GLS in PLHIV, a group now recognized to be at higher risk for major CAD events and sudden cardiac death. In addition, we identify that proximal and nadir CD4 cell counts are independently associated with GLS despite normal LV ejection fraction. Recent evidence implicates that long-term inflammation and immune dysfunction despite effective ART are strongly linked to CVD,11 a phenomenon noted globally.12 This study suggests that patients with low HIV-1 RNA viral load <400 copies/ml tend to have normal LV function as assessed by more negative values of GLS compared to those with higher HIV-1 RNA viral load. This finding may supports the aggressive control of HIV-1 RNA viral load as a potential means to limit myocardial injury over the lifespan of PLHIV. A large epidemiological study has also recently shown that HIV-1 RNA viral load of at least 500 copies/ml compared with <500 copies/ ml is a risk factor for LV dysfunction.13
Previous study demonstrated that compared with CD4 cell count >500 cells/mm3, CD4 cell count <200 cells/ mm3 is a risk factor for HF with preserved or reduced EF.13 Our study goes a step further by studying the correlation of CD4 cell count with preclinical evidence of myocardial dysfunction and demonstrated for the first time that GLS is significantly inversely correlated with proximal CD4 cell count, with increasing proximal CD4 being associated with more negative values of GLS. In addition, we find that CD4 cell count <200 cells/mm3 is significantly associated with abnormal values of LV function assessed by less negative values of GLS. Our study findings suggest that immune status as represented by proximal and nadir CD4 cell count are independent risk factors for subclinical myocardial dysfunction. Low CD4 nadirs have been associated with increased MI events presumably through increased atherosclerotic plaque burden associated with long-term inflammation and increased immune turnover.14
There are limited literature and guidelines on the appropriate method and timing to detect early myocardial changes in PLHIV. Although, several imaging modalities can be employed in the evaluation of myocardial function, the benefit of echocardiography comes from its versatility, lower cost, and ability to assess more than ventricular function.15 Although LVEF measurements pump efficiency, it does not assess contractility and is limited in its ability to detect subtle regional myocardial abnormalities with a low sensitivity for incident HF. More recently, several studies show that GLS changes occur before, or even in the absence of, changes in traditional diastolic parameters.16
The strength of the GLS parameter also includes the ability to more readily detect regional abnormalities in LV function, improved measurement reproducibility due to the semiautomated nature of some measurement systems, and the ability to forecast subsequent LV dysfunction. Our study showed that more than two-thirds of PLHIV have an abnormal LV GLS, despite having normal LVEF. This is although the cohort was relatively young (median age 44), only 40% of the cohort had diagnosed hypertension and only about 20% had diabetes. This study is the first to demonstrate such a high prevalence of GLS in PLHIV a group now recognized to be at higher risk for major CAD events and sudden cardiac death. Our study also showed that patients with less negative LV GLS have higher LV end-diastolic and end-systolic volumes compared to those with more negative GLS, supporting that solely relying on deterioration of standard echocardiographic measurements of LVEF may be misguided and can miss early cardiac dysfunction.17 Much remains to be understood about the role of GLS in the identification and management of myocardial dysfunction PLHIV. Whether strain measurements are required at multiple time points, the natural history of GLS in PLHIV with viral suppression and the predictive value of GLS for future cardiac outcomes need to be determined.
The strengths of this study include being the first to demonstrate a correlation between immunologic parameters of HIV infection and myocardial function as identified by STE. In addition, this is the largest observational cohort study on the prevalence of GLS in PLHIV where the feasibility of GLS measurement was high (> 90%). Nevertheless, some limitations should also be considered when interpreting our results. The TTEs were acquired for clinical indications which would potentially bias our results towards detecting more abnormalities compared with the group in whom providers did not identify a reason to request a TTE. Twenty-seven studies were excluded due to an adequate echogenicity. Lastly, this is a single-center study performed at an academic health center which limits generalizability.
In conclusion, in a large HIV clinic, analyzing clinically obtained echocardiograms over a 10-year period, PLHIV had a high prevalence of abnormal GLS and proximal and nadir CD4 cell count are independently correlated with LV GLS despite normal LV ejection fraction. STE analysis may be a useful adjunct for diagnosing subclinical LV dysfunction and allow targeted therapies to prevent incident cardiovascular syndromes in PLHIV.
Supplementary Material
Footnotes
Supplementary materials
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.amjcard.2019.06.013.
References
- 1.Palella FJ Jr., Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 1998;338:853–860. [DOI] [PubMed] [Google Scholar]
- 2.Palella FJ Jr., Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, Holmberg SD, Investigators HIVOS. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr 2006;43:27–34. [DOI] [PubMed] [Google Scholar]
- 3.Greene M, Justice AC, Lampiris HW, Valcour V. Management of human immunodeficiency virus infection in advanced age. JAMA 2013;309:1397–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grinspoon SK, Grunfeld C, Kotler DP, Currier JS, Lundgren JD, Dube MP, Lipshultz SE, Hsue PY, Squires K, Schambelan M, Wilson PW, Yarasheski KE, Hadigan CM, Stein JH, Eckel RH. State of the science conference: Initiative to decrease cardiovascular risk and increase quality of care for patients living with HIV/AIDS: executive summary. Circulation 2008;118:198–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med 2011;62:141–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torriani FJ, Komarow L, Parker RA, Cotter BR, Currier JS, Dube MP, Fichtenbaum CJ, Gerschenson M, Mitchell CK, Murphy RL, Squires K, Stein JH, Team AsS. Endothelial function in human immunodeficiency virus-infected antiretroviral-naive subjects before and after starting potent antiretroviral therapy: The ACTG (AIDS Clinical Trials Group) Study 5152s. J Am Coll Cardiol 2008;52:569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C, Levine GN, O’Gara PT, Halperin JL, Al-Khatib SM, Birtcher KK, Brindis RG, Cigarroa JE, Curtis LH, Fleisher LA, Gentile F, Gidding S, Hlatky MA, Ikonomidis J, Joglar J, Pressler SJ, Wijeysundera DN. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the heart failure society of America. J Card Fail 2017;23:628–651. [DOI] [PubMed] [Google Scholar]
- 8.Velazquez EJ, Samad Z, Al-Khalidi HR, Sangli C, Grayburn PA, Massaro JM, Stevens SR, Feldman TE, Krucoff MW. The MitraClip and survival in patients with mitral regurgitation at high risk for surgery: A propensity-matched comparison. Am Heart J 2015;170 1050–1059 e3. [DOI] [PubMed] [Google Scholar]
- 9.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39 e14. [DOI] [PubMed] [Google Scholar]
- 10.Yingchoncharoen T, Agarwal S, Popovic ZB, Marwick TH. Normal ranges of left ventricular strain: a meta-analysis. J Am Soc Echocardiogr 2013;26:185–191. [DOI] [PubMed] [Google Scholar]
- 11.Hsue PY, Deeks SG, Hunt PW. Immunologic basis of cardiovascular disease in HIV-infected adults. J Infect Dis 2012;205 (Suppl 3):S375–S382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bloomfield GS, Alenezi F, Barasa FA, Lumsden R, Mayosi BM, Velazquez EJ. Human immunodeficiency virus and heart failure in low- and middle-income countries. JACC Heart Fail 2015;3:579–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freiberg MS, Chang CH, Skanderson M, Patterson OV, DuVall SL, Brandt CA, So-Armah KA, Vasan RS, Oursler KA, Gottdiener J, Gottlieb S, Leaf D, Rodriguez-Barradas M, Tracy RP, Gibert CL, Rimland D, Bedimo RJ, Brown ST, Goetz MB, Warner A, Crothers K, Tindle HA, Alcorn C, Bachmann JM, Justice AC, Butt AA. Association between HIV infection and the risk of heart failure with reduced ejection fraction and preserved ejection fraction in the antiretroviral therapy era: results from the veterans aging cohort study. JAMA Cardiol 2017;2:536–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grinspoon SK. Cardiovascular disease in HIV: traditional and nontraditional risk factors. Top Antivir Med 2014;22:676–679. [PMC free article] [PubMed] [Google Scholar]
- 15.Okeke NL, Alenezi F, Bloomfield GS, Dunning A, Clement ME, Shah SH, Naggie S, Velazquez EJ. Determinants of left ventricular hypertrophy and diastolic dysfunction in an HIV clinical cohort. J Card Fail 2018;24:496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Cohen V, Gosavi S, Carver JR, Wiegers SE, Martin RP, Picard MH, Gerszten RE, Halpern EF, Passeri J, Kuter I, Scherrer-Crosbie M. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol 2011;107:1375–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Telli ML, Hunt SA, Carlson RW, Guardino AE. Trastuzumab-related cardiotoxicity: calling into question the concept of reversibility. J Clin Oncol 2007;25:3525–3533. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.