Abstract
Cryoneurolysis is the deliberate application of cold temperatures to nerves for therapeutic purposes. The idea of treating pain with this technique is thousands of years old and has evolved over time through the application of surgical techniques, nerve stimulation and/or landmark guidance, and through device development. Recent integration of the interventional radiology skill set to this space has unlocked a myriad of opportunities—primarily through a unique ability to percutaneously access deep structures in the body with accuracy and precision, and the capacity to monitor ablation zones. Understanding of the specific neurohistological process that follows targeted cryoneurolysis leads to new options for treating patients in pain without drugs and opens doors for the potential modification of a wide array of disease states.
Keywords: interventional, cryoneurolysis, cryoanalgesia, cryoablation
Cryoneurolysis is the deliberate application of cold to nerves for therapeutic purposes. The idea of treating pain with this technique is thousands of years old, and has evolved over time through the application of surgical techniques, nerve stimulator and/or landmark guidance, and through device development. Recent integration of the interventional radiology skill set to this space has unlocked a myriad of opportunities—primarily through a unique ability to percutaneously access deep structures in the body with accuracy and precision, and the associated capacity to document and monitor ablation zones. Understanding of the specific neurohistological process that follows targeted cryoneurolysis leads to new options for treating patients in pain without drugs, and opens doors for the potential modification of a wide array of disease states through percutaneous image-guided cryoneurolysis.
The integration of interventional radiology to the long-standing clinical concept of applying cold temperatures to nerves has illuminated several new procedural options and potential applications for the therapy. First, interventional radiology allows for percutaneous access to many nervous system structures in the body that are otherwise unreachable, or reachable only with significant associated costs and morbidity (as with many surgical approaches) or risks (as with landmark or nerve stimulation-guided approaches). Second, the evolution of understanding regarding specific neuroregenerative processes that follow specific deliberate cryothermal insults to nerves allows for tailored therapies and potential applications of percutaneous image-guided cryoneurolysis that exist beyond pain.
What Is the Same?
The analgesic properties of cold for medicinal use date back to Hippocrates in 460 BC. 1 2 Avicenna of Persia subsequently reported the use of cold to decrease pain related to surgery, and physicians from Napoleon's army noted decreased pain during amputation in soldiers exposed to extreme cold. 3 Modern-day implementation of device-mediated cold to target pain generators began with a landmark report by Lloyd et al in 1976, during which investigators targeted a variety of peripheral nerves in 64 patients using a gas-cooled cryoprobe guided by an integrated nerve stimulator—noting that compared with other methods of nerve interruption, cryoneurolysis had the advantages of reversibility, repeatability, and decreased incidence of complications such as neuroma formation or neuritis. 4
Over time, cryoablation of nerves under direct visualization has been widely reported in the setting of thoracotomy and/or during inguinal hernia repairs. 5 6 7 For example, a recent randomized trial by Graves et al demonstrated that intercostal nerve cryoablation during surgical correction of pectus excavatum decreases length of hospital stay and opiate analgesic requirement compared with thoracic epidural analgesia, without complications. 8 The cryoablation group's median length-of-stay was decreased by 2 days and opioid analgesic requirements were reduced by 52 to 82% compared with the thoracic epidural treatment group. A similar retrospective study of 26 patients drew similar conclusions. 9 Pastor et al described a double-blind prospective randomized study of 100 patients undergoing posterolateral thoracotomy. Patients who underwent intercostal cryoanalgesia ( n = 55) had significantly less pain, less analgesic requirement, and increased pulmonary function compared with those who did not. 10
Similarly, Wood et al described cryoablation of the ilioinguinal nerve under direct visualization following hernia repair in 1979, with a follow-up three-arm randomized study in 1981 describing superior pain relief with cryoneurolysis. 3 11 12 Fanelli et al demonstrated a 78% decrease in postoperative pain with associated decreased analgesic use and increased physical activity capacity following nerve cryoablation during herniorrhaphy. 6 Several other open cryoablation investigations in this setting have also been published without clear statistically significant effect. 3 13 14
Nonsurgical targeting of nerves has been reported using anatomical landmark guidance. In 1981, Evans et al reported positioning of patients and cryoprobes to treat perineal pain by targeting lower sacral nerve roots based on anatomical landmarks. Forty patients underwent a total of 71 treatments for a variety of underlying conditions causing perineal pain, with 78% reporting significant improvement. “The treatment was more successful in relieving symptoms in patients suffering from pelvic cancer … [and] best results were obtained in those patients who received numerous freeze applications or prolonged freezing.” 15 Radnovich et al described placement of cryoprobes targeting genicular nerves about painful, osteoarthritic knees in 2017 using only anatomical landmarks. 16 Their randomized, double-blind, multicenter trial enrolled 180 patients, with 121 randomized to a cryoneurolysis group. Results demonstrated that percutaneous cryoneurolysis of the infrapatellar branch of the saphenous nerve is a safe and effective treatment for pain related to knee osteoarthritis with a statistically significant reduction in knee pain for up to 150 days posttreatment.
Percutaneous targeting of nerves has also been performed using stimulator guidance. A 2015 retrospective study by Kim et al demonstrated that cryoneurolysis of the occipital nerves, using surface landmarks and nerve stimulation to assist nerve targeting, is an effective therapy for occipital neuralgia. 17 The 38-patient treatment group reported an average reduction in pain of 57.9% for an average duration of 6.1 months. Similarly, Moesker et al used nerve stimulator guidance to localize amputated nerves corresponding to phantom limb pain descriptions in five patients for cryoablation. This study was a retrospective review with an average follow-up of 32 months postablation. No complications were found, three patients reported 90 to 100% pain relief, one patient reported a 40% pain relief, and one patient reported a 20% pain relief. 18
What Is Different?
The integration of advanced imaging guidance through interventional radiology unlocks a myriad of potential applications for this technology using a percutaneous approach. The interventional radiologists routinely interact with oncologists, surgical oncologists, and palliative care physicians—relationships that may lead to opportunities for care of patients who may benefit from cryoneurolysis. For example, patients who suffer with pain related to neoplastic involvement of a nerve present a difficult clinical challenge. These patients are often not surgical candidates, cannot be radiated effectively and/or safely, and present with lesions deep in the body that cannot be safely reached using landmark or nerve stimulator guidance. The integration of advanced imaging guidance (computed tomography [CT], magnetic resonance imaging [MRI], and/or ultrasound) has helped overcome many of these challenges and allows for new treatment options, many times in a single session ( Figs. 1 and 2 ).
Fig. 1.

( a ) Single axial PET-CT image from a patient with metastatic urothelial cancer who presented with intractable right lower extremity pain demonstrates abnormal radiopharmaceutical activity involving the right portion of the L5 vertebral body and pedicle (arrows). ( b ) Slightly caudal image from the same exam demonstrates direct involvement of the right lumbosacral trunk (arrow). ( c, d ) Intraprocedural axial CT images demonstrate placement of a cryoablation probe via the right pedicle of L5 to the affected vertebral body as well as a second probe targeting the neoplastic focus involving the ipsilateral lumbosacral trunk (arrows indicate visualized ice).
Fig. 2.

( a ) Sagittal T1-weighted fat-saturated post-contrast MRI image of the lumbar spine in a patient with metastatic breast cancer who presented with intractable L4 radiculopathy demonstrates an enhancing metastatic lesion involving the pedicle and vertebral body (arrows) impressing on the exiting nerve root (arrowhead). ( b ) Corresponding intraprocedural CT image demonstrates a cryoablation probe positioned to include the lesion and the exiting nerve root (star).
One illustrative example can be found with patients who are hospitalized with intractable pain secondary to locally advanced neoplastic disease in the pelvis. Palliative radiation therapy is often exhausted, direct ablation of the mass may not be feasible, and most of these patients are not surgical candidates—creating a formidable barrier to timely palliation. 19 20 Given that the pudendal nerve transmits pain signals from the pelvis, 21 22 23 24 interruption of those signals may lead to palliation of intractable pelvic pain related to mass lesions, again providing a different option for patients with a difficult-to-manage clinical syndrome ( Fig. 3 ).
Fig. 3.
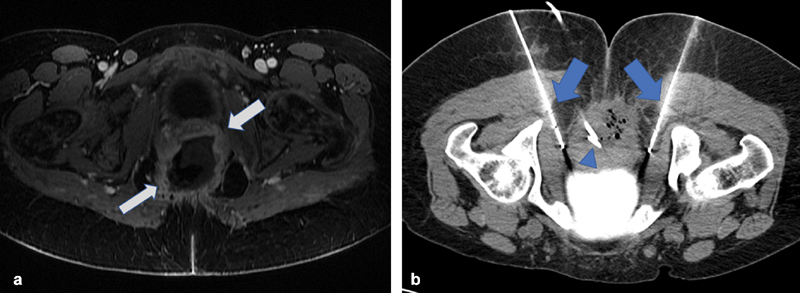
( a ) Axial T1-weighted fat-saturated post-contrast MRI image in the pelvis demonstrates a large pelvic mass (arrows) with a necrotic center in a patient with known advanced cervical carcinoma hospitalized for intractable pain. ( b ) Axial intraprocedural image demonstrates placement of two cryoablation probes (arrows) adjacent to the proximal pudendal nerves. Incidental note is made of a percutaneously placed drainage catheter (arrowhead).
In the same way, nonneoplastic painful conditions may be managed percutaneously with cryoneurolysis if the interventional radiology skill set is applied. As examples, the use of CT guidance for percutaneous cryoneurolysis in amputees has been reported for the management of phantom limb pain. 25 Fritz and coworkers have described MRI-guided cryoneurolysis of the sural nerve and posterior femoral cutaneous nerves to manage pain. 26 27 Kim and Ferrante reported using image guidance to cryoablate the obturator nerve for treatment of adductor spasticity. 28 Yoon et al reported the cryoneurolysis of peripheral nerves localized with ultrasound guidance for a variety of conditions. 29 Targeted nerves included the ilioinguinal, posterior tibial, saphenous, gluteal, sural, genicular, and digital nerves (and three plantar neuromas). Patients reported a significant decrease in self-reported pain scores following the cryoablation to 12 months—demonstrating the ability to reach nerves with imaging guidance for the purposes of cryoablation. In addition, ultrasound has been used to localize and cryoablate the peroneal, suprascapular, superior cluneal, femoral, sciatic, ilioinguinal, genitofemoral, and saphenous nerves, 30 31 32 33 and several reports have described the safety and efficacy of image-guided cryoneurolysis for the management of facet syndrome in the cervical and lumbar spines. 34 35 36 37 38
Potential complications associated with cryoneurolysis procedures performed in interventional radiology include standard risks associated with percutaneous procedures such as bleeding and infection. In addition, nontarget ablation must be considered and avoided in a similar fashion as undertaken for safe tumor ablation—employing positioning and/or dissection techniques when necessary. Partial ablation may also result in unwanted postprocedure symptoms such as allodynia or acute worsening of pain. Care must be taken to impart a uniform thermal lesion (without mechanically disrupting the nerve) for an adequate amount of time. Lastly, the desired mechanism of injury during cryoneurolysis—as explained later—is separate from the osmotic shift-related mechanism employed for cancer cell destruction, such that associated “active thaws” are not necessary and may result in unwanted damage and/or potential adverse sequela.
What Is New?
The new elements regarding cryoneurolysis that underpin its value and expansion in modern times include the integration of advanced image guidance, and the understanding of neural processes that follow cold exposure. To illustrate, again consider interventions that target the pudendal nerve. The pudendal nerve is derived from the S2–S4 nerve roots and follows a well-described course deep in the pelvis—giving rise to three branches along the way—which supply 39 sensation from the external genitalia, perineum, rectum, and skin around the anus. The pudendal nerve is implicated in a well-described, notoriously difficult-to-treat clinical syndrome presenting with pain related to pudendal nerve damage. 21 22 Without cross-sectional imaging guidance, cryoneurolysis is not an option for these patients, given the morbidity and cost of open surgical approaches, lack of precision and associated risk of landmark or fluoroscopically guided percutaneous access, and inability to monitor the ablation zone. With cross-sectional guidance, a cryoablation probe can be precisely placed in the adjacent ischiorectal fat for safe cryoablation of the pudendal nerve, illustrating how the application of imaging guidance creates novel opportunities for the management of existing, difficult-to-manage clinical conditions ( Fig. 4 ). Similarly, patients suffering from inguinodynia related to prior surgical intervention represent a population of patients with a well-characterized pain syndrome that has limited treatment options. 39 40 Using imaging guidance, the confluence of the ilioinguinal and genitofemoral nerves may be targeted safely for the management of pain related to entrapment or postoperative fibrosis ( Fig. 5 ).
Fig. 4.
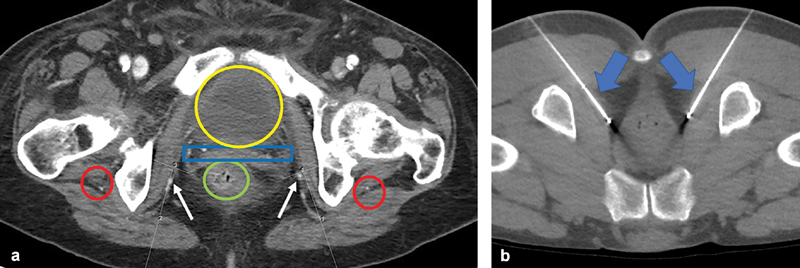
( a ) Single axial contrast-enhanced CT at the level of the pudendal canal, demonstrating anatomy associated with percutaneous access to the pudendal nerve (numbered lines). Without cross-sectional guidance, operators risk damage to the adjacent sciatic nerves (red circles), rectum (green circle), uterus (blue rectangle), pudendal artery and vein (arrows), and/or urinary bladder (yellow oval)—precluding any ablative procedure under landmark or fluoroscopic guidance. ( b ) Intraprocedural axial CT image at the level of the pudendal canal in a separate patient, demonstrating bilateral cryoablation probes percutaneously placed in the ischiorectal fat (arrows).
Fig. 5.

( a ) Single axial T1-weighted fat-saturated post-contrast MRI image at the level of the inguinal ligament demonstrates fibrosis and entrapment (arrow) of the genitofemoral and ilioinguinal nerves between the femoral and inferior epigastric arteries, deep to implanted herniorrhaphy mesh (arrowhead). ( b ) Corresponding intraprocedural CT image demonstrates ablation of the entrapped nerves and fibrous tissue via safe percutaneous placement of a cryoablation probe (arrow).
Precise percutaneous access to peripheral nerves may also lend itself to modification of disease states unrelated to pain. Premature ejaculation (PE) is the most common sexual disorder in men worldwide. 41 The application of CT guidance affords safe access to the dorsal penile nerve (a branch of the pudendal nerve) and/or the proximal pudendal nerve for cryoablation—which has been shown to improve symptoms for men diagnosed with PE, potentially providing a large population of patients with a difficult-to-treat condition a brand new option. 42 In the same way, our group has targeted the posterior vagal trunk—percutaneously with CT guidance—for purposes of attenuating hunger in patients with Class I or Class II obesity who hope to lose weight with calorie restriction. 43
Regarding the mechanism of cryoneurolysis, historically the premise upon which nerve cryoablation procedures have been founded is the induction of a specific, reversible nerve injury. 2 3 44 Nerve injury classifications are classically described and correlated with clinical course following trauma (crush, stretch, laceration, etc.), the most widely cited of which are the classifications of Seddon and Sunderland. 45 46 47 Depending on the diameter and composition of the nerve to be targeted, specific time and temperature combinations can result in a Sunderland 2 injury.
Importantly, this precise neural injury results in several well-described events that may lead to favorable clinical outcomes—cessation of conduction, 48 induced Wallerian degeneration, and predictable regeneration of axons upon an intact connective tissue scaffold. 2 44 49 The translation of these events (beyond conduction cessation) has been documented as nerve function recovery in animal studies and in humans 50 51 52 53 54 —which may have implications for interventional radiologists as neuroregenerative inductive therapies ( Fig. 6 ).
Fig. 6.

( a ) Illustration of a peripheral nerve demonstrating connective tissue components that serve as an axon scaffold. ( b–d ) Following cryoablation, a predictable sequence of degeneration with subsequent regeneration can be induced—potentially repairing damaged nerves.
Going forward, interventional radiologists have an extraordinary opportunity to define a new landscape by improving existing techniques and addressing new clinical conditions—pain related or otherwise—using percutaneous image-guided cryoneurolysis. We are uniquely positioned to answer the call for nonopioid procedural alternatives for pain management, 48 55 as well as to develop solutions for non–pain-related neurogenic clinical conditions.
Footnotes
Conflict of Interest Dr. Prologo reports grants and personal fees from BTG/Galil Medical, grants from Endocare/Healthtronics, outside the submitted work.
References
- 1.Cooper S M, Dawber R P. The history of cryosurgery. J R Soc Med. 2001;94(04):196–201. doi: 10.1177/014107680109400416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ilfeld B M, Preciado J, Trescot A M. Novel cryoneurolysis device for the treatment of sensory and motor peripheral nerves. Expert Rev Med Devices. 2016;13(08):713–725. doi: 10.1080/17434440.2016.1204229. [DOI] [PubMed] [Google Scholar]
- 3.Trescot A M. Cryoanalgesia in interventional pain management. Pain Physician. 2003;6(03):345–360. [PubMed] [Google Scholar]
- 4.Lloyd J W, Barnard J DW, Glynn C J.Cryoanalgesia. A new approach to pain relief Lancet 19762(7992):932–934. [DOI] [PubMed] [Google Scholar]
- 5.Wood G J, Lloyd J W, Bullingham R E, Britton B J, Finch D R. Postoperative analgesia for day-case herniorrhaphy patients. A comparison of cryoanalgesia, paravertebral blockade and oral analgesia. Anaesthesia. 1981;36(06):603–610. doi: 10.1111/j.1365-2044.1981.tb10324.x. [DOI] [PubMed] [Google Scholar]
- 6.Fanelli R D, DiSiena M R, Lui F Y, Gersin K S. Cryoanalgesic ablation for the treatment of chronic postherniorrhaphy neuropathic pain. Surg Endosc. 2003;17(02):196–200. doi: 10.1007/s00464-002-8840-8. [DOI] [PubMed] [Google Scholar]
- 7.Nelson K M, Vincent R G, Bourke R S et al. Intraoperative intercostal nerve freezing to prevent postthoracotomy pain. Ann Thorac Surg. 1974;18(03):280–285. doi: 10.1016/s0003-4975(10)64357-3. [DOI] [PubMed] [Google Scholar]
- 8.Graves C E, Moyer J, Zobel M Jet al. Intraoperative intercostal nerve cryoablation during the Nuss procedure reduces length of stay and opioid requirement: a randomized clinical trial J Pediatr Surg 2019 10.1016/j.jpedsurg.2019.02.057. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keller B A, Kabagambe S K, Becker J C et al. Intercostal nerve cryoablation versus thoracic epidural catheters for postoperative analgesia following pectus excavatum repair: Preliminary outcomes in twenty-six cryoablation patients. J Pediatr Surg. 2016;51(12):2033–2038. doi: 10.1016/j.jpedsurg.2016.09.034. [DOI] [PubMed] [Google Scholar]
- 10.Pastor J, Morales P, Cases E et al. Evaluation of intercostal cryoanalgesia versus conventional analgesia in postthoracotomy pain. Respiration. 1996;63(04):241–245. doi: 10.1159/000196553. [DOI] [PubMed] [Google Scholar]
- 11.Wood G J, Lloyd J W, Bullingham R E, Britton B J, Finch D R. Postoperative analgesia for day-case herniorrhaphy patients. A comparison of cryoanalgesia, paravertebral blockade and oral analgesia. Anaesthesia. 1981;36(06):603–610. doi: 10.1111/j.1365-2044.1981.tb10324.x. [DOI] [PubMed] [Google Scholar]
- 12.Wood G J, Lloyd J W, Evans P J, Bullingham R E, Britton B J, Finch D R.Cryoanalgesia and day-case herniorrhaphy Lancet 19792(8140):479. [DOI] [PubMed] [Google Scholar]
- 13.Khiroya R C, Davenport H T, Jones J G. Cryoanalgesia for pain after herniorrhaphy. Anaesthesia. 1986;41(01):73–76. doi: 10.1111/j.1365-2044.1986.tb12709.x. [DOI] [PubMed] [Google Scholar]
- 14.Callesen T, Bech K, Thorup J et al. Cryoanalgesia: effect on postherniorrhaphy pain. Anesth Analg. 1998;87(04):896–899. doi: 10.1097/00000539-199810000-00028. [DOI] [PubMed] [Google Scholar]
- 15.Evans P J, Lloyd J W, Jack T M. Cryoanalgesia for intractable perineal pain. J R Soc Med. 1981;74(11):804–809. doi: 10.1177/014107688107401106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radnovich R, Scott D, Patel A T et al. Cryoneurolysis to treat the pain and symptoms of knee osteoarthritis: a multicenter, randomized, double-blind, sham-controlled trial. Osteoarthritis Cartilage. 2017;25(08):1247–1256. doi: 10.1016/j.joca.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Kim C H, Hu W, Gao J, Dragan K, Whealton T, Julian C. Cryoablation for the treatment of occipital neuralgia. Pain Physician. 2015;18(03):E363–E368. [PubMed] [Google Scholar]
- 18.Moesker A A, Karl H W, Trescot A M. Treatment of phantom limb pain by cryoneurolysis of the amputated nerve. Pain Pract. 2014;14(01):52–56. doi: 10.1111/papr.12020. [DOI] [PubMed] [Google Scholar]
- 19.Cameron M G, Kersten C, Vistad I, Fosså S, Guren M G. Palliative pelvic radiotherapy of symptomatic incurable rectal cancer - a systematic review. Acta Oncol. 2014;53(02):164–173. doi: 10.3109/0284186X.2013.837582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caraceni A, Martini C, Zecca E et al. Breakthrough pain characteristics and syndromes in patients with cancer pain. An international survey. Palliat Med. 2004;18(03):177–183. doi: 10.1191/0269216304pm890oa. [DOI] [PubMed] [Google Scholar]
- 21.Hibner M, Desai N, Robertson L J, Nour M. Pudendal neuralgia. J Minim Invasive Gynecol. 2010;17(02):148–153. doi: 10.1016/j.jmig.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Wadhwa V, Hamid A S, Kumar Y, Scott K M, Chhabra A. Pudendal nerve and branch neuropathy: magnetic resonance neurography evaluation. Acta Radiol. 2017;58(06):726–733. doi: 10.1177/0284185116668213. [DOI] [PubMed] [Google Scholar]
- 23.Prologo J D, Lin R C, Williams R, Corn D. Percutaneous CT-guided cryoablation for the treatment of refractory pudendal neuralgia. Skeletal Radiol. 2015;44(05):709–714. doi: 10.1007/s00256-014-2075-3. [DOI] [PubMed] [Google Scholar]
- 24.Rhame E E, Levey K A, Gharibo C G. Successful treatment of refractory pudendal neuralgia with pulsed radiofrequency. Pain Physician. 2009;12(03):633–638. [PubMed] [Google Scholar]
- 25.Prologo J D, Gilliland C A, Miller M et al. Percutaneous image-guided cryoablation for the treatment of phantom limb pain in amputees: a pilot study. J Vasc Interv Radiol. 2017;28(01):24–340000. doi: 10.1016/j.jvir.2016.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonham L W, Phelps A, Rosson G D, Fritz J. MR imaging-guided cryoneurolysis of the sural nerve. J Vasc Interv Radiol. 2018;29(11):1622–1624. doi: 10.1016/j.jvir.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Joshi D H, Thawait G K, Del Grande F, Fritz J. MRI-guided cryoablation of the posterior femoral cutaneous nerve for the treatment of neuropathy-mediated sitting pain. Skeletal Radiol. 2017;46(07):983–987. doi: 10.1007/s00256-017-2617-6. [DOI] [PubMed] [Google Scholar]
- 28.Kim P S, Ferrante F M. Cryoanalgesia: a novel treatment for hip adductor spasticity and obturator neuralgia. Anesthesiology. 1998;89(02):534–536. doi: 10.1097/00000542-199808000-00036. [DOI] [PubMed] [Google Scholar]
- 29.Yoon J H, Grechushkin V, Chaudhry A, Bhattacharji P, Durkin B, Moore W. Cryoneurolysis in patients with refractory chronic peripheral neuropathic pain. J Vasc Interv Radiol. 2016;27(02):239–243. doi: 10.1016/j.jvir.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 30.Ilfeld B M, Gabriel R A, Trescot A M. Ultrasound-guided percutaneous cryoneurolysis for treatment of acute pain: could cryoanalgesia replace continuous peripheral nerve blocks? Br J Anaesth. 2017;119(04):703–706. doi: 10.1093/bja/aex142. [DOI] [PubMed] [Google Scholar]
- 31.Gabriel R A, Finneran J J, IV, Trescot A M, Ilfeld B M. Ultrasound-guided percutaneous cryoneurolysis for postoperative analgesia after limb amputation: a case series. A A Pract. 2019;12(07):231–234. doi: 10.1213/XAA.0000000000000893. [DOI] [PubMed] [Google Scholar]
- 32.Friedman T, Richman D, Adler R. Sonographically guided cryoneurolysis: preliminary experience and clinical outcomes. J Ultrasound Med. 2012;31(12):2025–2034. doi: 10.7863/jum.2012.31.12.2025. [DOI] [PubMed] [Google Scholar]
- 33.Campos N A, Chiles J H, Plunkett A R. Ultrasound-guided cryoablation of genitofemoral nerve for chronic inguinal pain. Pain Physician. 2009;12(06):997–1000. [PubMed] [Google Scholar]
- 34.Birkenmaier C, Veihelmann A, Trouillier H et al. Percutaneous cryodenervation of lumbar facet joints: a prospective clinical trial. Int Orthop. 2007;31(04):525–530. doi: 10.1007/s00264-006-0208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolter T, Deininger M, Hubbe U, Mohadjer M, Knoeller S. Cryoneurolysis for zygapophyseal joint pain: a retrospective analysis of 117 interventions. Acta Neurochir (Wien) 2011;153(05):1011–1019. doi: 10.1007/s00701-011-0966-9. [DOI] [PubMed] [Google Scholar]
- 36.Wolter T, Kleinmann B, Knoeller S. Cryoneurolysis for the treatment of cervical facet joint syndrome: a technical note. J Pain Res. 2018;11:1165–1169. doi: 10.2147/JPR.S161053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bärlocher C B, Krauss J K, Seiler R W.Kryorhizotomy: an alternative technique for lumbar medial branch rhizotomy in lumbar facet syndrome J Neurosurg 200398(1, Suppl):14–20. [DOI] [PubMed] [Google Scholar]
- 38.Staender M, Maerz U, Tonn J C, Steude U. Computerized tomography-guided kryorhizotomy in 76 patients with lumbar facet joint syndrome. J Neurosurg Spine. 2005;3(06):444–449. doi: 10.3171/spi.2005.3.6.0444. [DOI] [PubMed] [Google Scholar]
- 39.Amid P K. Causes, prevention, and surgical treatment of postherniorrhaphy neuropathic inguinodynia: triple neurectomy with proximal end implantation. Hernia. 2004;8(04):343–349. doi: 10.1007/s10029-004-0247-0. [DOI] [PubMed] [Google Scholar]
- 40.Lee K S, Sin J M, Patil P P et al. Ultrasound-guided microwave ablation for the management of inguinal neuralgia: a preliminary study with 1-year follow-up. J Vasc Interv Radiol. 2019;30(02):242–248. doi: 10.1016/j.jvir.2018.10.031. [DOI] [PubMed] [Google Scholar]
- 41.Martin C, Nolen H, Podolnick J, Wang R. Current and emerging therapies in premature ejaculation: where we are coming from, where we are going. Int J Urol. 2017;24(01):40–50. doi: 10.1111/iju.13202. [DOI] [PubMed] [Google Scholar]
- 42.David Prologo J, Snyder L L, Cherullo E, Passalacqua M, Pirasteh A, Corn D. Percutaneous CT-guided cryoablation of the dorsal penile nerve for treatment of symptomatic premature ejaculation. J Vasc Interv Radiol. 2013;24(02):214–219. doi: 10.1016/j.jvir.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 43.Prologo J DLE, Lin E, Horesh Bergquist S et al. Percutaneous CT-guided cryovagotomy in patients with Class I or Class II obesity: a pilot trial. Obesity (Silver Spring) 2019;27(08):1255–1265. doi: 10.1002/oby.22523. [DOI] [PubMed] [Google Scholar]
- 44.Burnett M G, Zager E L. Pathophysiology of peripheral nerve injury: a brief review. Neurosurg Focus. 2004;16(05):E1. doi: 10.3171/foc.2004.16.5.2. [DOI] [PubMed] [Google Scholar]
- 45.Caillaud M, Richard L, Vallat J M, Desmoulière A, Billet F. Peripheral nerve regeneration and intraneural revascularization. Neural Regen Res. 2019;14(01):24–33. doi: 10.4103/1673-5374.243699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sunderland S. A classification of peripheral nerve injuries producing loss of function. Brain. 1951;74(04):491–516. doi: 10.1093/brain/74.4.491. [DOI] [PubMed] [Google Scholar]
- 47.Seddon H J.Classification of nerve injuries BMJ 19422(4260):237–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.The U.S. Department of Health and Human Services.Pain Management Best Practices Inter-Agency Task Force Report 2019. Available at:https://www.hhs.gov/ash/advisory-committees/pain/index.html. Accessed August 16, 2019
- 49.Moorjani N, Zhao F, Tian Y, Liang C, Kaluba J, Maiwand M O. Effects of cryoanalgesia on post-thoracotomy pain and on the structure of intercostal nerves: a human prospective randomized trial and a histological study. Eur J Cardiothorac Surg. 2001;20(03):502–507. doi: 10.1016/s1010-7940(01)00815-6. [DOI] [PubMed] [Google Scholar]
- 50.Kilcoyne A, Frenk N E, Arellano R S. Percutaneous cryoablation of a metastatic right external iliac lymph node with associated injury to the femoral nerve. J Vasc Interv Radiol. 2016;27(04):611–612. doi: 10.1016/j.jvir.2015.12.750. [DOI] [PubMed] [Google Scholar]
- 51.Auloge P, Cazzato R L, Rousseau C et al. Complications of percutaneous bone tumor cryoablation: a 10-year experience. Radiology. 2019;291(02):521–528. doi: 10.1148/radiol.2019181262. [DOI] [PubMed] [Google Scholar]
- 52.Johnson C MJ, Manyapu S, Hawkins C, Singer A, Prologo J D. Abstract No 410 Natural history of motor nerve cryoablation: a retrospective cohort analysis. J Vasc Interv Radiol. 2019;30(03):s176. doi: 10.1016/j.jvir.2019.11.026. [DOI] [PubMed] [Google Scholar]
- 53.Kingery W S, Lu J D, Roffers J A, Kell D R. The resolution of neuropathic hyperalgesia following motor and sensory functional recovery in sciatic axonometric mononeuropathies. Pain. 1994;58(02):157–168. doi: 10.1016/0304-3959(94)90196-1. [DOI] [PubMed] [Google Scholar]
- 54.Hsu M, Stevenson F F. Wallerian degeneration and recovery of motor nerves after multiple focused cold therapies. Muscle Nerve. 2015;51(02):268–275. doi: 10.1002/mus.24306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dowell D, Haegerich T M, Chou R. CDC guideline for prescribing opioids for chronic pain--United States, 2016. JAMA. 2016;315(15):1624–1645. doi: 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]


