Abstract
Vaccines that autonomously transfer among individuals have been proposed as a strategy to control infectious diseases within inaccessible wildlife populations. However, rates of vaccine spread and epidemiological efficacy in real world systems remain elusive. Here, we investigated whether topical vaccines that transfer among individuals through social contacts can control vampire bat rabies, a medically and economically important zoonosis in Latin America. Field experiments in 3 Peruvian bat colonies which used fluorescent biomarkers as a proxy for the bat-to-bat transfer and ingestion of an oral vaccine revealed that vaccine transfer would increase population-level immunity up to 2.6 times beyond the same effort using conventional, non-spreadable vaccines. Mathematical models demonstrated that observed levels of vaccine transfer would reduce the probability, size, and duration of rabies outbreaks, even at low, but realistically achievable levels of vaccine application. Models further predicted that existing vaccines provide substantial advantages over culling bats, the policy currently implemented in North, Central, and South America. Linking field studies with biomarkers to mathematical models can inform how spreadable vaccines may combat pathogens of health and conservation concern prior to costly investments in vaccine design and testing.
Keywords: intervention, Chiroptera, Lyssavirus, poxvirus, recombinant, reservoir, RNA virus, vaccination, wildlife epidemiology, zoonosis
Introduction
Infectious diseases of wildlife cause threats to human and animal health globally [1]. Controlling these pathogens within their natural animal hosts can offer substantial health, economic, and conservation benefits. For example, baited vaccines targeting wildlife reservoirs eliminated fox rabies from western Europe [2] and currently confine raccoon rabies to the eastern United States [3]. However, for many important wildlife diseases, delivery systems to vaccinate a sufficient proportion of host populations to control pathogens are unavailable, and direct (i.e., individual-based) vaccination is logistically prohibitive. Interventions that spread from treated to untreated individuals are increasingly used to control arthropod-borne diseases [4, 5, 6] and have been proposed as a solution to mass vaccinate wildlife since each unit of vaccine deployed would immunise multiple individuals [7, 8]. However, as seen with poliovirus eradication efforts, vaccines that sustain transmission may revert to virulent phenotypes [9], and in wildlife, vaccine shedding may have unanticipated ecological or evolutionary impacts on competing pathogens or host species [10]. Vaccines with deliberately constrained capacity to transmit are therefore currently the preferred candidates for real world applications. Encouragingly, theoretical models suggest that such weakly-transmissible vaccines consistently outperform individual-based vaccination, increasing the potential for disease eradication [11]. Despite this theoretical promise, spreadable vaccines have only rarely been tested in natural systems (i.e., rabbit hemorrhagic disease and myxomavirus in rabbits [12]). This gap between theory and practice reflects a number of limiting factors: vaccines may be unavailable; epidemiological knowledge of the target pathogen or the dynamics of vaccine spread may be insufficient to guide deployment or predict benefits; and losses incurred under existing management strategies may be considered insufficient to warrant the real or perceived risks of novel interventions.
Vampire bat rabies (VBR), a universally lethal viral zoonosis found throughout Latin America, represents a tractable system to explore the implementation of spreadable vaccines to protect human and animal health. Where common vampire bats (Desmodus rotundus) routinely feed on human blood, VBR is estimated to cause up to 960 deaths/100,000 people [13]. Losses from livestock mortality exceed $50 million annually and disproportionately affect impoverished, rural communities [14, 15]. Existing management strategies have been unable to mitigate the burden of VBR. Vaccines for humans and livestock are protective, but high costs and inaccessibility to remote areas limit uptake [16]. Rabies control programs also cull vampire bats using anticoagulant poisons ('vampiricide') which are applied in topical gels that spread among bats through social contacts and are ingested during grooming (here termed 'orotopical transfer') [17]. While culling reduces bat bites on humans and livestock, effects on rabies transmission remain controversial [18, 19]. Moreover, heightened bat dispersal following culls is predicted to exacerbate VBR transmission by increasing the mixing of bat colonies, analogous to the increased transmission of bovine tuberculosis induced through effects of culling on badger home range size [20, 21]. Oral rabies vaccines that spread by the same orotopical mechanism as vampiricide offer an alternative approach. These recombinant virally-vectored vaccines can indirectly immunise untreated bats in captivity, but have never been tested in wild populations [22, 23, 24, 25]. Several unresolved questions must be answered prior to deploying vaccines for large scale bat rabies control: (1) how efficiently would vaccines transfer among wild bats?, (2) are certain demographic groups of bats especially difficult to vaccinate or especially effective disseminators of vaccines?, (3) would the resulting degree of immunisation significantly reduce rabies transmission?, and (4) would vaccines reduce human and livestock rabies risk more effectively than the current policy of culling? We address these questions by coupling field studies that used fluorescent biomarkers to quantify contact networks and orotopical transfer among wild vampire bats with mathematical models that simulated how vaccines and vampiricide, which spread by identical mechanisms, would impact the size, duration, and probability of rabies outbreaks.
Results
Biomarker transfer and ingestion shows potential for high vaccine coverage in wild vampire bats
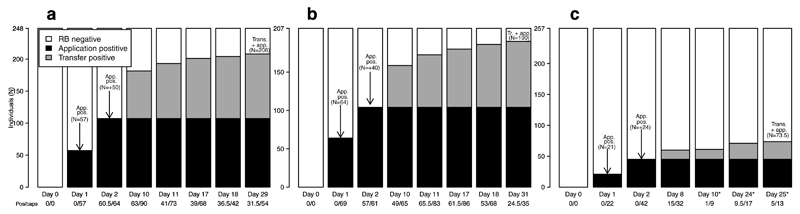
We estimated the potential for a spreadable vaccine to transfer among bats using Rhodamine b (RB), a biomarker that when ingested leads to long-lasting fluorescence in hair follicles in diverse mammalian species [26, 27, 28]. After applying a gel-formulation of RB topically to bats in three colonies in Peru (colony sizes: 207-257 individuals, sex ratios: 43.1-50.6% male), orotopical transfer and ingestion was monitored by fluorescent microscopic analysis of hair samples collected in subsequent capture sessions, with fluorescence indicating RB consumption (Supplementary Table 1). At two sites (LMA5 & LMA6), an estimated 84 and 92% of bats ingested RB, either following topical application or transfer from treated bats (Fig. 1). The third colony (LMA12) relocated to an undocumented roost soon after RB treatment, which diminished captures during the monitoring period relative to the estimated colony size (Supplementary Table 1); consequently, the overall estimated coverage dropped to 28.8% (Fig. 1). Nevertheless, the percentage of sampled LMA12 bats at the end of the monitoring period that were RB positive (48.3%, aggregating days 24 and 25), was not statistically different from the percentages at the final capture dates in the other two colonies (58.3 and 70.0%; Chi-squared test, χ2 = 3.2, df = 2, p = 0.21). We further characterized patterns of RB uptake among demographic groups of bats. The sex ratios of transfer positive bats became slightly more male biased (3-11% increases, depending on the colony) relative to the sex ratios of bats that were treated with RB, suggesting elevated transfer to males; however these increases were not statistically significant (χ2 tests, all p > 0.05; Supplementary Figure 1). We observed RB transfer to untreated bats in all three age classes. Across all colonies, 73.4% of sampled adults (N = 351, averaged across microscopy readings of independent observers), 57.5% of sampled juveniles (N = 30.5), and 89.9% of sampled subadults (N = 34.5) became RB positive through transfer during the monitoring period. Consequently, these results implied that vaccines deployed over only two days of captures (17-50% of total colony size) would yield high levels of population immunity across age classes due to orotopical transfer.
Figure 1.
Transfer and ingestion of an orotopically spread gel biomarker in three vampire bat colonies. In each panel, LMA5 (a), LMA6 (b), and LMA12 (c), x-axes are the days since RB application with the number of transfer positive bats over total captures in subtext. The y-axis is the number of bats in each colony within three categories RB negative (white), application positive (black), or transfer positive (gray). Asterisks (*) on and after day 10 from LMA12 indicate captures from the relocated roost. Data are the mean of microscopy readings from two observers, except where noted otherwise. Transfer positive bats from day 2 had RB applied and are included in the black bar to visualize the total force of application, but were included as transfers in statistical analyses.
Contact heterogeneities among demographic groups of vampire bats
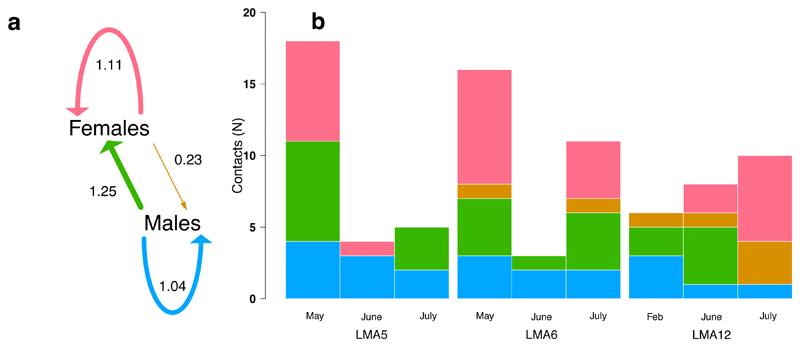
We next examined whether contact heterogeneities might make certain demographic groups of bats especially effective or ineffective spreaders of vaccines using ultraviolet (UV) powder marking, wherein different age/sex groups of bats were treated with different colors of UV powder, and transfer to untreated bats was monitored over two subsequent capture nights [29, 30]. Across 3 replicate UV treatments per colony, we documented 78 instances of UV powder transfer, leading to estimated contact rates ranging from 0.23–1.25 per treated bat (Fig. 2). Male bats had significantly higher contact rates than females (Wilcoxon rank sum test, W = 91, p = 0.025; mean = 1.14 versus 0.67) and had similar rates of male-to-male and male-to-female contacts (Wilcoxon rank sum test, W = 42, p = 0.93). In contrast, females preferentially contacted other females (Fig. 2a). Transfer to juveniles could not be reliably quantified because these bats were mostly too young to forage independently and our capture method during the monitoring period required bats to fly out of roosts. Nevertheless, a single juvenile bat captured had UV transfer from a female. In contrast, transfer from juveniles to adults should have been detectable if it occurred due to the greater ease of capturing adults. However, none of the 27 marked juveniles transferred UV powder to adults. Together with the high observed rates of juvenile exposure to RB, these findings suggest that vaccine deployments should target adults rather than juveniles. Targeting adults would further be logistically advantageous since it would minimize social disruption of colonies that results from entering roosts to capture juveniles.
Figure 2.
Bat contact heterogeneity revealed by UV powder transfers. a, Mean new contacts per marked bat, by sex. Arrow thickness is proportional to contact rate. b, Number and directionality of contacts by sex, location, and sampling date. Contacts to juveniles are not shown since the juveniles in the colonies we studied were too young to feed independently and would have been underestimated by our capture method during monitoring.
Epidemiological models show spreadable vaccines outperform culling for rabies control
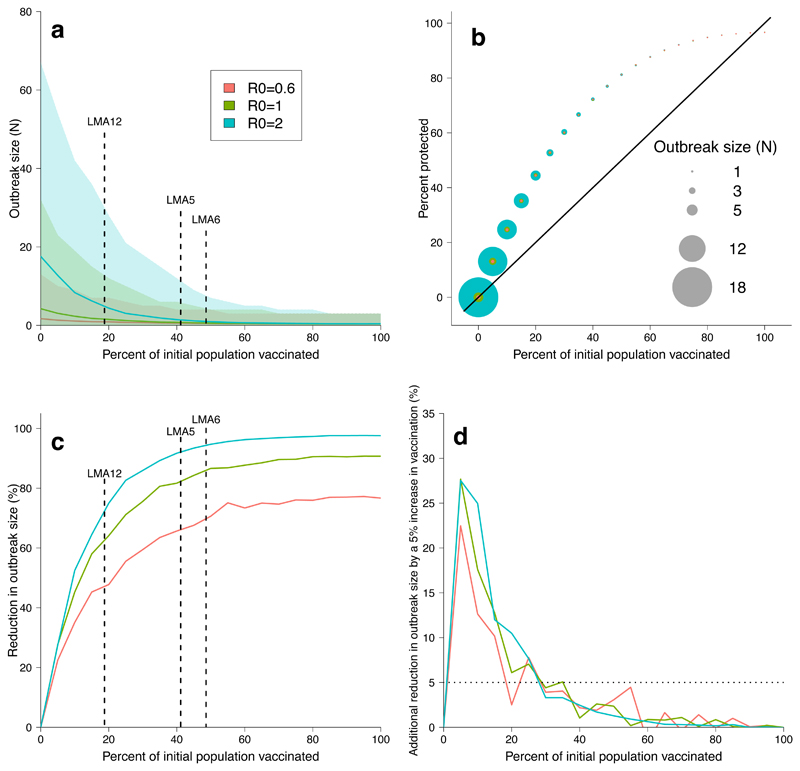
We adapted a deterministic compartmental model of VBR persistence [20] to incorporate an orotopically spread vaccine and used least-squares (Fig. 3b) to estimate expected per capita vaccine transfer rates from the time series of RB transfers observed in our field studies, assuming that RB transfer equated to lifelong protection. This analysis revealed that each treated bat transferred RB to 1.45–2.11 untreated individuals, up to a 2.6-fold increase in population level coverage relative to the coverage that would be expected using conventional, non-spreading vaccines (Fi.g 4b, Supplementary Figure 2 and Supplementary Table 2). We simulated the ability of spreadable vaccines to control rabies across the range of R0 values (0.6 to 2) suggested in the rabies literature [20, 34, 35]. Applying vaccines to approximately 20% of bats vaccinated 40% of the population and reduced rabies outbreak size by 45 to 75%, depending on the assumed R0 of rabies (Fig. 4a,b,c). However, applying vaccines to a higher proportion of bats had diminishing returns for both the proportion of the colony that was ultimately protected and for rabies control. If vaccines were applied to >30% of bats, additional reductions in rabies outbreak sizes were less than 5%, meaning a 5% increase in initial application led to less than a 5% reduction in outbreak sizes (Fig. 4d). The greatest benefit (reduction in outbreak size relative to effort) occurred at vaccination levels below 15%.
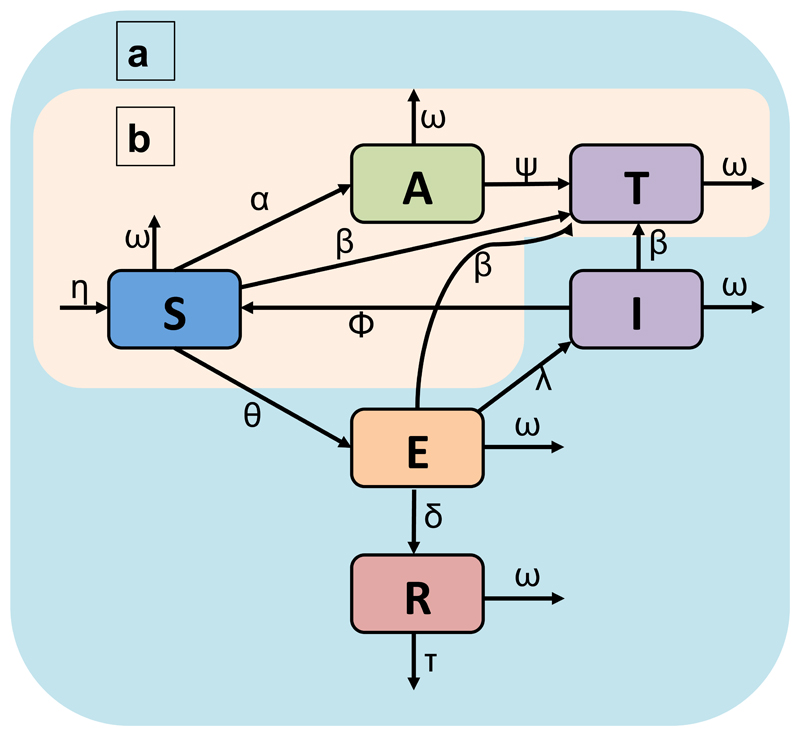
Figure 3.
Dynamic models of rabies transmission and spreadable vaccination. a, The full model used for outbreak analyses includes orotopical transfer and rabies transmission. Classes comprise susceptible (S), application positive (A), transfer positive (T), immune (I), exposed to rabies (E), and rabid (R). b, The biomarker transfer model structure for fitting β. In the vaccination model, the I and T classes both provide immunity from rabies but the T class has permanent immunity. Model parameters describe rates of: natural births (η) and deaths (ω); orotopical gel application (α), persistence (ψ), and transfer (β); rabies transmission (θ); waning of immunity (ϕ); rabies induced mortality (τ); and the probabilities of succumbing to rabies (δ) or surviving (λ) following exposure. Supplementary Table 3 provides further details and references for parameter values.
Figure 4.
Simulating rabies outbreaks with vaccination. a, Mean rabies outbreak sizes after a single rabid bat is introduced to the colony one week following release of a spreadable vaccine. Colors represent varying degrees of rabies R0, with 95% confidence intervals calculated from 5000 simulations. Dashed lines indicate the percent of bats that RB was applied to in our study sites. Supplementary Figure 5 shows results calculated only from simulations where outbreaks occurred. b, Percent of bats ultimately protected by initial vaccine release. Circle size indicates outbreak size under the three rabies R0 values. Solid line represents the 1:1 line; points over the line represent the added benefit of vaccine transfer. c, Reduction in rabies outbreak size (% fewer cases) under varying initial vaccination levels and rabies R0 values. d, Percent of additional rabies cases prevented by increasing the initial vaccine release effort by 5% (i.e., the rate of change in rabies reduction from the panel c).
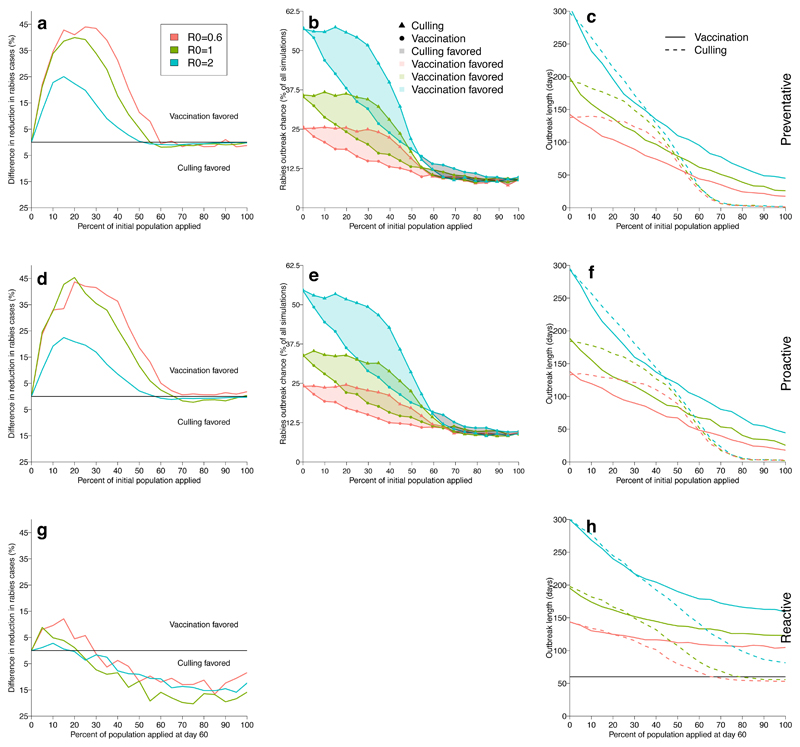
We next compared the relative efficacy of vaccination and culling across three epidemiological scenarios [21]. representing different management strategies: (1) a 'preventative' approach, where vaccine/-vampiricide was applied to prevent VBR invasion into historically rabies–free bat populations [33, 36]; (2) a 'proactive' approach, which represented an intervention in a VBR endemic area, but in a colony that was not currently infected; and (3) a 'reactive' approach where intervention followed 60 days after a single VBR-infected bat was introduced to the colony (Supplementary Figure 4). Although we simulated outcomes across the full possible range of application effort (i.e., 0-100% of bats treated), we focused on lower application levels since capturing large proportions of bats across large geographic areas would be impractical for rabies control campaigns. Indeed, mark-recapture studies across multiple vampire bat colonies in Peru suggested that on average, <10% of colonies were captured in a single night [19]. At realistic levels of application, vaccination consistently reduced the probability of viral invasion, outbreak size, and outbreak duration more effectively than culling, regardless of whether control was preventative, proactive, or reactive (Fig. 5). Culling was only favored when at least 25% of the colony was treated, and only in reactive scenarios. However, the advantage of culling on outbreak size was relatively small - a maximum of a 20% greater reduction - relative to the larger advantages observed when vaccination was favored (up to 45% greater reduction), and differences in outbreak duration were negligible until much larger proportions of bats were culled (Fig. 5). In preventative and proactive scenarios, culling required capturing and treating much larger proportions of vampire bat populations (e.g., >60%) to match the reduction in outbreak size and duration achieved by vaccination (Fig. 5). In fact, the only discernible difference at higher application levels was a greater reduction in the duration of outbreaks by culling; however, this was due to near complete extinction of bat colonies. Even if this degree of bat culling were achievable and ethically acceptable, it may not be a favorable long-term strategy since populations that recovered from culls would be entirely susceptible to rabies, potentially causing larger future outbreaks [37].
Figure 5.
Comparing effects of culling and vaccination on rabies transmission. Rows group results from preventative (top), proactive (middle), and reactive (lower) strategies and columns group metrics of impacts on transmission. a,d,g, The difference in the reduction of rabies cases between equal levels of effort in vaccination versus culling. Values above and below 0 favor vaccination and culling, respectively. b,e, The probability of a rabies outbreak, defined as the percentage of simulations (N = 5000) where VBRV introduction led to onward transmission. Shaded regions represent the difference between vaccination (circles) and culling (triangles); culling is favored in grey regions and vaccination is favored in blue, green, or red regions. The probability of outbreaks was not modelled for reactive control since, by definition, outbreaks had already occurred. c,f,h, The duration of rabies outbreaks under vaccination and culling. The horizontal line in panel H indicates day 60, when reactive control measures were implemented. In all panels, colors correspond to different assumed R0 values for rabies.
Our per capita transfer rates likely represented lower bounds of vaccine and vampiricide spread since the relatively high percentage of bats initially treated with RB left few others available to be exposed via transfer in two of our colonies and relocation of the third colony reduced capture rates during the monitoring period. Indeed, some studies have suggested higher transfer rates of vampiricide [17, 38]. We therefore conducted a sensitivity analysis where both vaccines and vampiricide spread up to 10-fold more efficiently than our RB estimates, values that exceeded the largest transfer rates suggested from vampiricide releases [17, 38]. Additionally, we considered transfer rates that were up to 75% less efficient than our RB estimates. This analysis demonstrated that low-level vaccination remained favored under preventative and proactive approaches even if both the vaccine and vampiricide spread up to 3-fold greater than observed in our field studies (Supplementary Figures 7-9). If both interventions spread less effectively than RB, vaccination was either superior or equivalent to culling except when large proportions of bat colonies were reactively culled (Supplementary Figure 6). Under realistic levels of application (application ≤ 25%), even if vampiricide spread 3-fold better than a vaccine, it was unable to outperform vaccination under preventative or proactive approaches when R0 was less than 2. Under reactive scenarios, culling was favored if vampiricide spread 2-3-fold better than a vaccine or if VBR R0 was 2 (Supplementary Figure 9). Given that existing oral rabies vaccines use replication-competent viral vectors with potential for lower effective doses than chemical poisons [24, 25], heightened vampiricide transfer is less likely than the converse where vaccines spread better [8]. The high R0 scenarios where culling was favored are also unlikely, as the estimated VBR R0 is considerably lower than 2 [20]. Our results therefore support previous suggestions that culling may require near-elimination of bats to locally benefit rabies prevention [18] and reveal spreadable vaccines as efficient tools to reduce the size, duration, and probability of rabies outbreaks in Latin America.
Discussion
This study demonstrates proof-of-principle that at operationally-achievable levels of deployment and empirically-quantified rates of bat-to-bat spread, orotopical vaccines should reduce rabies transmission more effectively than culling, the current policy employed across Latin America. Since VBR persistence requires inter-colony spread for viral dispersal, even modest reductions in outbreak size are likely to have epidemiologically important impacts at the larger geographic scales over which disease control campaigns are implemented. In particular, by reducing the number of infected bats and the probability of viral invasion, vaccination of a limited number of colonies would disproportionately benefit regional rabies elimination by favoring stochastic viral extinctions. Because male dispersal spreads rabies between colonies, vaccination might further benefit from targeting male bats [33]. Although higher rates of social grooming among females was expected to undermine this strategy [38, 39], we found that males have equal or greater inter- and intra-sex contact rates, a possible consequence of attempted mating with females or fighting among males. Importantly, because self-grooming is common [40], any vaccine transferred through these interactions would ultimately be ingested.
Designing large-scale campaigns to deploy spreadable rabies vaccines requires additional research in several areas. First, to optimize the number of vaccine doses to apply to each bat, captive and field studies should quantify individual heterogeneity in transfer rates using actual vaccines in addition to biomarkers. Second, the costs of vaccination must be estimated in economic terms in addition to the epidemiological assessment provided here. Unfortunately, vaccines are currently produced only for research and costs of large-scale production are unavailable. Third, vaccination of vampire bats without population reduction will be unacceptable to some stakeholders since uncontrolled bat depredation sustains exposures to non-rabies pathogens [41] and anemia from bites may reduce livestock productivity independently of rabies [42]. Given that culling shifts bat populations towards younger, more rabies susceptible individuals, which could enhance rabies transmission [19], future research should develop tools for reproductive suppression as an alternative to culling [43]. Finally, metapopulation maintenance of rabies provides opportunities for more efficient, epidemiologically-informed vaccination [44]. For example, vaccines might be deployed with prior knowledge of rabies presence from livestock surveillance systems (e.g., ring vaccination) or preventatively in areas where the locations and timing of outbreaks are predictable [36]. Spatially-explicit rabies transmission models will be an important next step to design these interventions, but will require a more quantitative understanding of bat dispersal than is currently available. Excitingly, once strategies are developed, the operational capacity for their implementation is already available in most Latin American countries through decades of experience with culling campaigns.
These results provide evidence that spreadable vaccines may contribute to pathogen management within wild bats. VBR provided an ideal case study because the epidemiological mechanisms underlying viral maintenance are understood and candidate vaccines are available [20, 25, 36, 45]. While the exact parameter estimates and models developed here should not be applied directly to other bat pathogens, the framework linking biomarkers to mathematical models can guide future research. For several bat pathogens of public health or conservation concern such as White Nose Syndrome, Hendra virus, and Marburg virus, epidemiological models have been proposed [46, 47, 48] and vaccines for bats either exist or have precedents encouraging their development [49, 50, 51]. In these cases, our approach could be implemented over relatively short timescales to evaluate the prospects for vaccines to aid management and the immunological and epidemiological characteristics that would be required for success before investing resources in vaccine development. For other bat pathogens with greater uncertainty in reservoir hosts and transmission biology, such as Ebolaviruses [52], implementation will require greater fundamental knowledge of viral transmission cycles. We encourage further development of virally-vectored vaccines for bats and highlight the need to quantify their spread and efficacy in the wild.
Methods
Field studies of biomarker transfer and ingestion
Field studies were carried out between January and July 2017 in three vampire bat roosts in the Barranca (LMA5, -10.6415, -77.8160), Huaura (LMA6, -11.0555, -77.4594), and Lima (LMA12, -12.1833, -76.8500) provinces of the Department of Lima, Peru (Supplementary Table 1). Two roosts (LMA5 and LMA6) had been monitored since 2007, while the third (LMA12) was examined here for the first time [19]. All roosts were man-made tunnels that formed part of crop irrigation systems. Diurnal captures were carried out to mark bats and estimate sex ratios and colony sizes. Diurnal captures involved teams entering caves and catching bats with hand nets (BioQuip, Tropics Net). In addition, 2.5-meter mist nets (Ecotone) were placed at each end of tunnels to catch bats that attempted to escape. Diurnal capture effort was set to 1 hour across sampling dates and localities. Colony sizes were estimated using the Schnabel method [53]. Nocturnal captures were carried out in the same roosts to monitor biomarker spread. Nets placed at each roost exit were checked every 30 minutes for 4 hours per night at varying hours depending on the lunar cycle. Following removal from mist nets, bats were placed in individual cloth bags until processing. All captured bats were given an individually numbered, 4 digit incoloy wing band (3.5mm Porzana Inc.) to identify recaptures. Age was classified as juvenile, subadult, or adult based on the degree of fusion of the phylangeal epiphyses [54]. In total, we recorded 1777 captures of 709 individually-marked bats, with the average bat captured 2.39 times (range=1-9).
Studies of vaccine transfer and ingestion used RB powder (50mg) mixed with glycerine jelly (44.5ml, Carolina Biological Supply Company) and water (55.5ml) to form a gel. On days 1 and 2, RB was administered orally to confirm fluorescence in RB-treated bats (ca. 0.05ml via needle-free syringe) and applied topically (ca. 0.45ml, rubbed into the dorsal fur) to all captured bats. Uptake in un-treated bats was monitored using hair plucked from bats captured over 4-5 subsequent sessions per colony, carried out up to 31 days after initial application (Supplementary Table 1). Hair samples were examined with a Nikon SMZ1270 microscope at 15x using a fluorescence filter with excitation wavelength 540 nm, emission wavelength 625 nm. Each sample was examined by two individuals to minimize misclassification, except at LMA12 on days 8 & 10, where only one individual examined the hair. The presence of fluorescence in hair was interpreted to indicate transfer and consumption of RB, but was not considered a quantitative measure of the volume of RB consumption. Because bats had identification tags, we were able to distinguish those that were positive due to transfer from RB treated bats (”transfer positives”) from those that had RB applied by experimenters (”application positives”). Hair samples were collected under the Peruvian collection permit, 028-2017-SERFOR/DGGSPFFS and exported to the United States under export permit, 3235-SERFOR. This research was performed under approval of University of Glasgow School of Veterinary Medicine Animal Ethics Committee (Project 25A/18).
Contact heterogeneities among demographic groups of vampire bats
Powder marking was replicated 3 times per colony (total of 9 marking sessions) and bats were monitored for two nights following each marking session (Supplementary Table 1). During each session, red, green, blue, or orange UV powder (DayGlo Corp.) was rubbed into the fur of the bat across the entire body using a toothbrush, with colors dependent on age and sex. UV colors were rotated between groups at different capture dates to control for potential differences in detection probability. UV powder markings were recorded by examining each captured bat for 30s using handheld UV lights (Glowtech Ltd.) prior to removal from mist nets. After removing UV marked bats from the recaptures, directional contact rates for each sex (e.g. female-to-male contacts per marked female) were calculated using equation 1:
| (1) |
where NposX is the number of bats of a certain sex testing positive for the UV color in question, UMX is the number of unmarked bats of that sex captured at this time point, NUMX is the number of unmarked bats of that sex in the entire colony, and MX is the number of initially marked bats from that sex. Example calculations are provided in the Supplementary Information (Eqs. 2 & 3).
Sex biases in UV transfer were tested by comparing all estimated rates from males to all estimated rates from females, treating each site, month, and recipient sex combination as independent observations (N = 36). We used a non-parametric Wilcoxon rank sum test since rates were not normally distributed, even after log transformation (Shapiro-Wilk test, p = 0.01).
Parameter estimation and mathematical modeling
Per capita rates of orotopical transfer and ingestion, defined as the estimated number of bat-to-bat transfers per treated individual, were estimated using the data from our RB field study. Specifically, we incorporated a susceptible (S), application positive (A), and transfer positive (T) deterministic compartmental model (Fig. 3b) using least-squares methods in the statistical software R. A 2-day transfer period was integrated with the number of RB application and transfer positives across time to estimate the expected transfer rate of orotopical vaccines or poisons (β). A 6-day RB transfer period was also considered to examine variation in β across time (Supplementary Table 2, Supplementary Information). We assumed that successful transfer led to death in culling models and lifelong protection against VBR in vaccination models (ca. 3.5 years of protection given the lifespan of D. rotundus, Supplementary Table 3). Importantly, waning of vaccine-induced immunity would not alter the results shown here which focused on single outbreaks.
Mathematical models of rabies control used a stochastic model that simulated both rabies transmission and vaccine transfer. A susceptible (S), application positive (A), transfer positive (T), exposed to rabies (E), immune (I), and rabid (R) model, with a daily time-step, was simulated for 5000 iterations using a Gillespie algorithm (Fig. 3a, Supplementary Information). Following previous models of vampire bat rabies [20] and consistent with the absence of strong relationships between colony size and rabies seroprevalence [19], we utilized a frequency-dependent rabies transmission function. We used 237 bats as the colony size (the mean size from our three field sites). The base model without vaccination or culling followed the mathematical structure and parameter values used by Blackwood et al. [20], with the simplifications of a single infectious class and modeling a single introduction of rabies rather than sustained introductions via immigration. This model generated similar outbreak dynamics to the Blackwood et al. [20] model, characterized by short lived outbreaks (less than 1 year) followed by viral extinction, persistence of the bat population, and seroprevalence levels consistent with field observations, particularly at values of R0 > 0.6 (Supplementary Figure 3). Since we modelled our vaccine spread on a recombinant Raccoonpox virus-vectored vaccine that appears unlikely to spread via an infectious process (i.e., from indirectly vaccinated bats) [45], vaccines were modelled to spread only from those bats to which the vaccine was applied, creating a single generation of transmission. Based on the very low prevalence of rabies in free-flying bats (>1%) and infrequent dispersal in vampire bats [55, 56], we simulated introduction of a single rabid bat to the population. Given that sex differences in RB transfer were non-significant and age-biased transfer was difficult to quantify, we opted against more complex age and sex structured models of rabies and vaccine/vampiricide spread.
Models comparing the efficacy of vampiricide to vaccination used the same model structure with the exception that bats in the exposed class died from ingesting vampiricide, while those that consumed the vaccine were not protected (see Eqs. 8 & 9 in the Supplementary Information). This was because post-exposure vaccination has not been evaluated in bats. We generally assumed equal transfer rates of vaccines and vampiricide based on their identical mechanism of transfer; however, we relaxed this assumption in the Supplementary Information (Supplementary Figures 6-9). We also assumed that both spread over relatively short time periods since vampire bats are exceptional groomers and would quickly ingest vaccine or vampiricide [40]. Importantly, our focal vaccine remains viable over these timescales [57]. After two years (730 days) the cumulative number of newly infected bats was considered to be the outbreak size. Outbreak duration was defined as the total number of days with at least one bat in the exposed class. For preventative and proactive approaches, we quantified the probability of an outbreak as the proportion of simulations where at least 1 new bat became infected after a single rabid bat was introduced.
Supplementary Material
Acknowledgements
K.M.B. was supported by NIH award F32AI134016 and computational resources were provided by NIH award U01GM110712. D.G.S. was supported by a Sir Henry Dale Fellowship, jointly funded by the Wellcome Trust and Royal Society (102507/Z/13/Z). Additional funding was provided by a Challenge Grant from the Royal Society to D.G.S., T.E.R., J.E.O., C.S. and N.F. (CH160097). The authors are grateful to D. Walsh, M. Viana, and the Streicker group for comments on earlier versions of this manuscript. Any use of trade, product, or firm names does not imply endorsement by the U.S. Government.
Footnotes
Author Contributions
D.G.S., T.E.R., and J.E.O. conceived and designed the experiments; R.C.A., C.T., and J.C. performed the experiments; K.M.B. and D.G.S. analyzed the data; T.E.R., J.E.O., W.V., C.S., and N.F. contributed materials/analysis tools; K.M.B. and D.G.S. wrote the first draft of the paper and all authors contributed revisions.
Data Availability
The UV transfer and RB transfer data are available on Dryad (doi: 10.5061/dryad.64t161m). These data were used to generate Figs. 1 and 2, and Supplementary Figures 1 and 2.
Code Availability
The R scripts used to estimate RB transfer rates shown in Supplementary Figure 2 and Supplementary Table 2 and to carry out the epidemiological modeling shown in Figs. 4 and 5 and Supplementary Figures 3 and 5-9 are provided as Supplementary Software 1 and Supplementary Software 2.
Competing Interest Statement
The authors declare no competing interests.
Contributor Information
Tonie E. Rocke, Email: trocke@usgs.gov.
Jorge E. Osorio, Email: jorge.osorio@wisc.edu.
Rachel C. Abbott, Email: rabbott@usgs.gov.
Carlos Tello, Email: carlos.tello.ch@gmail.com.
Jorge Carrera, Email: jecarrerag@gmail.com.
William Valderrama, Email: wvalderrama@illariy.org.
Carlos Shiva, Email: carlos.shiva@upch.pe.
Nestor Falcon, Email: nestor.falcon@upch.pe.
References
- 1.Daszak P, Cunningham AA, Hyatt AD. Emerging infectious diseases of wildlife–threats to biodiversity and human health. Science. 2000;287(5452):443–449. doi: 10.1126/science.287.5452.443. [DOI] [PubMed] [Google Scholar]
- 2.Brochier B, Kieny M, Costy F, Coppens P, Bauduin B, Lecocq J, et al. Large-scale eradication of rabies using recombinant vaccinia-rabies vaccine. Nature. 1991;354(6354):520. doi: 10.1038/354520a0. [DOI] [PubMed] [Google Scholar]
- 3.Slate D, Algeo TP, Nelson KM, Chipman RB, Donovan D, Blanton JD, et al. Oral rabies vaccination in North America: opportunities, complexities, and challenges. PLoS Neglected Tropical Diseases. 2009;3(12):e549. doi: 10.1371/journal.pntd.0000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffmann A, Montgomery B, Popovici J, Iturbe-Ormaetxe I, Johnson P, Muzzi F, et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011;476(7361):454. doi: 10.1038/nature10356. [DOI] [PubMed] [Google Scholar]
- 5.Sinkins SP, Gould F. Gene drive systems for insect disease vectors. Nature Reviews Genetics. 2006;7(6):427–435. doi: 10.1038/nrg1870. [DOI] [PubMed] [Google Scholar]
- 6.Aliota MT, Peinado SA, Velez ID, Osorio JE. The wMel strain of Wolbachia reduces transmission of Zika virus by Aedes aegypti. Scientific Reports. 2016;6 doi: 10.1038/srep28792. srep28792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basinski AJ, Varrelman TJ, Smithson MW, May RH, Remien CH, Nuismer SL. Evaluating the promise of recombinant transmissible vaccines. Vaccine. 2018;36(5):675–682. doi: 10.1016/j.vaccine.2017.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bull JJ, Smithson MW, Nuismer SL. Transmissible Viral Vaccines. Trends in Microbiology. 2018;26(1):6–15. doi: 10.1016/j.tim.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minor P. Vaccine-derived poliovirus (VDPV): impact on poliomyelitis eradication. Vaccine. 2009;27(20):2649–2652. doi: 10.1016/j.vaccine.2009.02.071. [DOI] [PubMed] [Google Scholar]
- 10.Behdenna A, Lembo T, Calatayud O, Cleaveland S, Halliday JEB, Packer C, et al. Transmission ecology of canine parvovirus in a multi-host, multi-pathogen system. Proceedings of the Royal Society B: Biological Sciences. 2019;286(1899) doi: 10.1098/rspb.2018.2772. 20182772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nuismer SL, Althouse BM, May R, Bull JJ, Stromberg SP, Antia R. Eradicating infectious disease using weakly transmissible vaccines. Proceedings of the Royal Society of London B: Biological Sciences. 2016;283(1841) doi: 10.1098/rspb.2016.1903. 20161903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torres JM, Sánchez C, Ramirez MA, Morales M, Bárcena J, Ferrer J, et al. First field trial of a transmissible recombinant vaccine against myxomatosis and rabbit hemorrhagic disease. Vaccine. 2001;19(31):4536–4543. doi: 10.1016/s0264-410x(01)00184-0. [DOI] [PubMed] [Google Scholar]
- 13.Schneider MC, Santos-Burgoa C, Aron J, Munoz B, Ruiz-Velazco S, Uieda W. Potential force of infection of human rabies transmitted by vampire bats in the Amazonian region of Brazil. American Journal of Tropical Medicine and Hygiene. 1996;55(6):680–684. doi: 10.4269/ajtmh.1996.55.680. [DOI] [PubMed] [Google Scholar]
- 14.Belotto A, Leanes LF, Schneider MC, Tamayp H, Correa E. Overview of rabies in the Americas. Virus Research. 2005;111(1):5–12. doi: 10.1016/j.virusres.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Benavides JA, Paniagua ER, Hampson K, Valderrama W, Streicker DG. Quantifying the burden of vampire bat rabies in Peruvian livestock. PLoS Neglected Tropical Diseases. 2017;11(12):e0006105. doi: 10.1371/journal.pntd.0006105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schneider MC, Romijn PC, Uieda W, Tamayo H, Silva DFd, Belotto A, et al. Rabies transmitted by vampire bats to humans: an emerging zoonotic disease in Latin America? Revista Panamericana de Salud Pública. 2009;25(3):260–269. doi: 10.1590/s1020-49892009000300010. [DOI] [PubMed] [Google Scholar]
- 17.Linhart SB, Flores Crespo R, Mitchell GC. Control of vampire bats by topical application of an anticoagulant, chlorophacinone. Boletin of the OSP. 1972;6(2):31–38. [PubMed] [Google Scholar]
- 18.Fornes A, Lord RD, Kuns ML, Larghi OP, Fuenzalida E, Lazar L. Control of bovine rabies through vampire bat control. Journal of Wildlife Diseases. 1974;10(4):310–316. doi: 10.7589/0090-3558-10.4.310. [DOI] [PubMed] [Google Scholar]
- 19.Streicker DG, Recuenco S, Valderrama W, Benavides JG, Vargas I, Pacheco V, et al. Ecological and anthropogenic drivers of rabies exposure in vampire bats: Implications for transmission and control. Proceedings of the Royal Society B: Biological Sciences. 2012;279(1742):3384–3392. doi: 10.1098/rspb.2012.0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blackwood JC, Streicker DG, Altizer S, Rohani P. Resolving the roles of immunity, pathogenesis, and immigration for rabies persistence in vampire bats. Proceedings of the National Academy of Sciences. 2013;110(51):20837–20842. doi: 10.1073/pnas.1308817110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donnelly CA, Woodroffe R, Cox D, Bourne J, Gettinby G, Le Fevre AM, et al. Impact of localized badger culling on tuberculosis incidence in British cattle. Nature. 2003;426(6968):834. doi: 10.1038/nature02192. [DOI] [PubMed] [Google Scholar]
- 22.Aguilar-Setién A, Brochier B, Tordo N, De Paz O, Desmettre P, Péharpré D, et al. Experimental rabies infection and oral vaccination in vampire bats (Desmodus rotundus) Vaccine. 1998;16(11–12):1122–1126. doi: 10.1016/s0264-410x(98)80108-4. [DOI] [PubMed] [Google Scholar]
- 23.Aguilar-Setién A, Campos YL, Cruz ET, Kretschmer R, Brochier B, Pastoret PP. Vaccination of vampire bats using recombinant vaccinia-rabies virus. Journal of Wildlife Diseases. 2002;38(3):539–544. doi: 10.7589/0090-3558-38.3.539. [DOI] [PubMed] [Google Scholar]
- 24.Stading B, Ellison JA, Carson WC, Satheshkumar PS, Rocke TE, Osorio JE. Protection of bats (Eptesicus fuscus) against rabies following topical or oronasal exposure to a recombinant raccoon poxvirus vaccine. PLoS Neglected Tropical Diseases. 2017;11(10):e0005958. doi: 10.1371/journal.pntd.0005958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Almeida M, Martorelli L, Aires C, Barros R, Massad E. Vaccinating the vampire bat Desmodus rotundus against rabies. Virus Research. 2008;137(2):275–277. doi: 10.1016/j.virusres.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 26.Cagnacci F, Massei G, Coats J, De Leeuw A, Cowan DP. Long-lasting systemic bait markers for Eurasian badgers. Journal of Wildlife Diseases. 2006;42(4):892–896. doi: 10.7589/0090-3558-42.4.892. [DOI] [PubMed] [Google Scholar]
- 27.Fry TL, Atwood T, Dunbar MR. Evaluation of rhodamine B as a biomarker for raccoons. Human-Wildlife Interactions. 2010;4(2):275–282. [Google Scholar]
- 28.Fernandez JRR, Rocke TE. Use of Rhodamine B as a biomarker for oral plague vaccination of Prairie Dogs. Journal of Wildlife Diseases. 2011;47(3):765–768. doi: 10.7589/0090-3558-47.3.765. [DOI] [PubMed] [Google Scholar]
- 29.Clay CA, Lehmer EM, Previtali A, Jeor S, Dearing MD. Contact heterogeneity in deer mice: Implications for Sin Nombre virus transmission. Proceedings of the Royal Society B: Biological Sciences. 2009;276(1660):1305–1312. doi: 10.1098/rspb.2008.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoyt JR, Langwig KE, White JP, Kaarakka HM, Redell JA, Kurta A, et al. Cryptic connections illuminate pathogen transmission within community networks. Nature. 2018;563(7733):710–713. doi: 10.1038/s41586-018-0720-z. [DOI] [PubMed] [Google Scholar]
- 31.George DB, Webb CT, Farnsworth ML, O'Shea TJ, Bowen RA, Smith DL, et al. Host and viral ecology determine bat rabies seasonality and maintenance. Proceedings of the National Academy of Sciences. 2011;108(25):10208–10213. doi: 10.1073/pnas.1010875108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilkinson GS. The social organization of the common vampire bat. Behavioral Ecology and Sociobiology. 1985;17(2):123–134. [Google Scholar]
- 33.Streicker DG, Winternitz JC, Satterfield DA, Condori-Condori RE, Broos A, Tello C, et al. Host–pathogen evolutionary signatures reveal dynamics and future invasions of vampire bat rabies. Proceedings of the National Academy of Sciences. 2016;113(39):10926–10931. doi: 10.1073/pnas.1606587113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hampson K, Dushoff J, Cleaveland S, Haydon DT, Kaare M, Packer C, et al. Transmission dynamics and prospects for the elimination of canine rabies. PLoS Biology. 2009;7(3):e1000053. doi: 10.1371/journal.pbio.1000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amengual B, Bourhy H, López-Roíg M, Serra-Cobo J. Temporal dynamics of European bat lyssavirus type 1 and survival of Myotis myotis bats in natural colonies. PLoS ONE. 2007;2(6) doi: 10.1371/journal.pone.0000566. 0000566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benavides JA, Valderrama W, Streicker DG. Spatial expansions and travelling waves of rabies in vampire bats. Proceedings of the Royal Society of London B: Biological Sciences. 2016;283(1832) 20160328. [Google Scholar]
- 37.Choisy M, Rohani P. Harvesting can increase severity of wildlife disease epidemics. Proceedings of the Royal Society B: Biological Sciences. 2006;273(1597):2025–2034. doi: 10.1098/rspb.2006.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gomes MN, Uieda W, Latorre M. Influence of sex differences in the same colony for chemical control of vampire Desmodus rotundus (Phyllostomidae) populations in the state of Sao Paulo, Brazil. Pesquisa Veterinária Brasileira. 2006;26(1):38–43. [Google Scholar]
- 39.Wilkinson GS. Social grooming in the common vampire bat, Desmodus rotundus. Animal Behaviour. 1986;34(6):1880–1889. [Google Scholar]
- 40.Carter G, Leffer L. Social grooming in bats: are vampire bats exceptional? PLoS One. 2015;10(10):e0138430. doi: 10.1371/journal.pone.0138430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bergner LM, Orton RJ, da Silva Filipe A, Shaw AE, Becker DJ, Tello C, et al. Using noninvasive metagenomics to characterize viral communities from wildlife. Molecular ecology resources. 2019;19(1):128–143. doi: 10.1111/1755-0998.12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thompson RD, Elias DJ, Mitchell GC. Effects of vampire bat control on bovine milk production. The Journal of Wildlife Management. 1977:736–739. [Google Scholar]
- 43.Hardy C, Hinds L, Kerr P, Lloyd M, Redwood A, Shellam G, et al. Biological control of vertebrate pests using virally vectored immunocontraception. Journal of Reproductive Immunology. 2006;71(2):102–111. doi: 10.1016/j.jri.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 44.Beyer HL, Hampson K, Lembo T, Cleaveland S, Kaare M, Haydon DT. The implications of metapopulation dynamics on the design of vaccination campaigns. Vaccine. 2012;30(6):1014–1022. doi: 10.1016/j.vaccine.2011.12.052. [DOI] [PubMed] [Google Scholar]
- 45.Stading BR, Osorio JE, Velasco-Villa A, Smotherman M, Kingstad-Bakke B, Rocke TE. Infectivity of attenuated poxvirus vaccine vectors and immunogenicity of a raccoonpox vectored rabies vaccine in the Brazilian Free-tailed bat (Tadarida brasiliensis) Vaccine. 2016;34(44):5352–5358. doi: 10.1016/j.vaccine.2016.08.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Langwig KE, Hoyt JR, Parise KL, Frick WF, Foster JT, Kilpatrick AM. Resistance in persisting bat populations after white-nose syndrome invasion. Philosophical Transactions of the Royal Society B: Biological Sciences. 2017;372(1712) doi: 10.1098/rstb.2016.0044. 20160044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Plowright RK, Foley P, Field HE, Dobson AP, Foley JE, Eby P, et al. Urban habituation, ecological connectivity and epidemic dampening: the emergence of Hendra virus from flying foxes (Pteropus spp.) Proceedings of the Royal Society B: Biological Sciences. 2011;278(1725):3703–3712. doi: 10.1098/rspb.2011.0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hayman DT. Biannual birth pulses allow filoviruses to persist in bat populations. Proceedings of the Royal Society B: Biological Sciences. 2015;282(1803) doi: 10.1098/rspb.2014.2591. 20142591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rocke TE, Kingstad-Bakke B, Wüthrich M, Stading B, Abbott RC, Isidoro-Ayza M, et al. Virally-vectored vaccine candidates against white-nose syndrome induce anti-fungal immune response in little brown bats (Myotis lucifugus) Scientific reports. 2019;9(1):6788. doi: 10.1038/s41598-019-43210-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marzi A, Murphy AA, Feldmann F, Parkins CJ, Haddock E, Hanley PW, et al. Cytomegalovirus-based vaccine expressing Ebola virus glycoprotein protects nonhuman primates from Ebola virus infection. Scientific Reports. 2016;6 doi: 10.1038/srep21674. 21674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pallister J, Middleton D, Wang LF, Klein R, Haining J, Robinson R, et al. A recombinant Hendra virus G glycoprotein-based subunit vaccine protects ferrets from lethal Hendra virus challenge. Vaccine. 2011;29(34):5623–5630. doi: 10.1016/j.vaccine.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Olival K, Hayman D. Filoviruses in bats: current knowledge and future directions. Viruses. 2014;6(4):1759–1788. doi: 10.3390/v6041759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schnabel ZE. The estimation of total fish population of a lake. The American Mathematical Monthly. 1938;45(6):348–352. [Google Scholar]
- 56.Anthony ELP. Age determination in bats. In: Kunz TH, editor. Ecological and Behavioral Methods for the Study of Bats. Washington D.C.: Smithsonian Institution Press; 1988. pp. 47–58. [Google Scholar]
- 55.Constantine DG, Tierkel ES, Kleckner MD, Hawkins DM. Rabies in New Mexico cavern bats. Public Health Reports. 1968;83 [PMC free article] [PubMed] [Google Scholar]
- 56.Delpietro H, Russo R, Carter G, Lord R, Delpietro G. Reproductive seasonality, sex ratio and philopatry in Argentina's common vampire bats. Royal Society Open Science. 2017;4(4) doi: 10.1098/rsos.160959. 160959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stading B. Development of a novel Rabies mosaic antigen and the use of attenuated Poxviruses as vaccine vectors in bats. University of Wisconsin, Madison; 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.