Abstract
This work provides a summary of the state-of-the-art in inertial-based adaptive stimulation strategies for treatment of movement disorders like Parkinson’s disease and Essential Tremor. A review of recent technical and clinical neuroscience is provided to give a state-of-the-art overview. We then propose a closed-loop system concept using an accelerometer and a phase locked loop algorithm as a conceptual methodology for enhanced therapy systems. The remaining challenges to practical implementation are then briefly described.
Keywords: Control systems, inertial sensors, movement disorders, Parkinson’s disease, Essential Tremor
Introduction
Tremor is associated with a broad range of neurological disorders such as Parkinson’s disease (PD), Essential Tremor (ET), multiple sclerosis, stroke and traumatic brain injury. The common signature of these different tremor pathologies is the excessive rhythmicity of the neural activity in the motor nuclei. ET is the most common form of pathological tremor, affecting up to 6% of the population. Tremor is also a common feature of PD, observed in 70% of the patients. As drug therapy remains ineffective for the long-term management of tremor, there is a need for alternative therapies.
Deep brain stimulation (DBS) is an effective surgical treatment, and involves long-term electrical stimulation of key nuclei in the motor circuit via chronically implanted depth electrodes. High frequency stimulation (130-180 Hz) disrupts pathological rhythmic firing of the motor nuclei, and simultaneously suppresses tremor. However, stimulation may give rise to side effects such as speech, balance and gait impairment in up to 50% of the implanted patients [1], [2]. This is because the form of stimulation presently applied does not distinguish between diseased and normal neural activity patterns. While reducing the number of pulses delivered per second during continuous stimulation appears to minimize stimulation-induced side effects, continuous low frequency DBS is not effective in suppressing disease symptoms in its present form [3]. Furthermore, DBS is not energy efficient since state of the art stimulators for movement disorders do not respond to changes in patient’s symptom severity and continuously applies stimulation even when symptoms are not present.
Closed loop stimulation, during which a disease biomarker is tracked to determine when to stimulate, has the potential to increase power-efficiency of DBS. Moreover, it could be possible to reduce both disease symptoms and stimulation-induced side effects during low frequency stimulation if delivered at fixed phases of the tremor cycle.
Pilot Tremor Detection and Titration Algorithms
The most common closed loop DBS strategy would be to apply stimulation at a pre-determined frequency and intensity when onset of a tremor episode is predicted from limb movement, commonly captured using an accelerometer, and muscle contractions, measured using electromyography (EMG) [4]–[6]. The concept for this controller is illustrated in Fig 1. Essential tremor is usually present during movement and when patients’ assume a posture, while parkinsonian tremor is usually observed when the patient’s limbs are resting and is suppressed during movement. Delivering stimulation only when a tremor episode is detected can potentially reduce the total stimulation time by 50% [7]. Tremor severity might also be used to control stimulation intensity, increasing stimulation amplitude as tremor worsens [4].
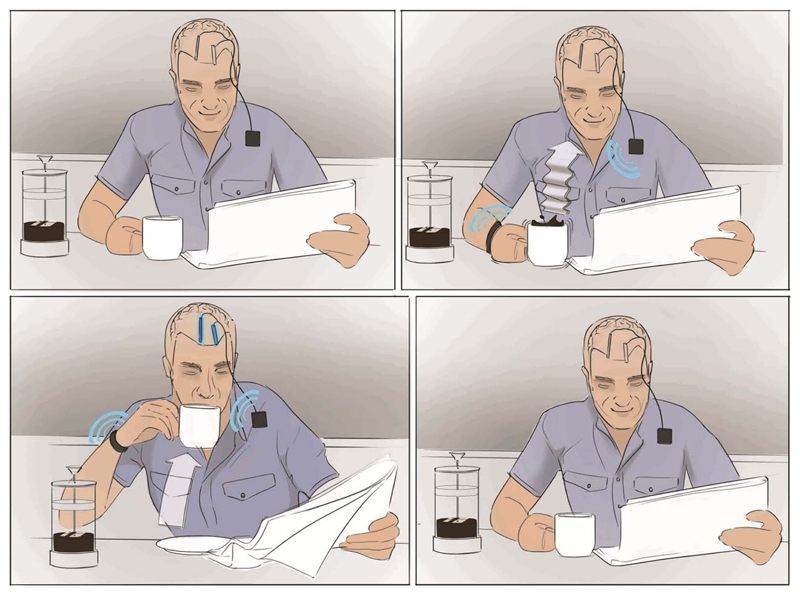
Figure 1.
Concept of an inertial measurement system to drive stimulation. When a tremor is detected, the implantable system initiates the appropriate stimulation.
Prediction algorithms exploit changes in EMG entropy [8], EMG [7] and accelerometer power [6] to estimate tremor onset. In these algorithms, EMG and accelerometer signals are decomposed into different frequency bands. Gradual power changes and regularity of muscle contractions are used to distinguish tremor episodes from voluntary movement and can estimate tremor onset with 85% accuracy in ET patients and 80% accuracy in PD patients [6]–[8].
Scientific Basis for General Synchronization Strategies
Synchronized periodic firing of the motor nuclei drives rhythmic involuntary muscle contractions that generate tremor. Phase alignment between different neural populations determines the degree of synchrony and the efficacy of information exchange [9]. Communication is believed to be most effective when two neural populations are in phase with one another. Conversely, if intrinsic activity patterns of different brain regions are out of phase, input from one would have minimal impact on the neural activity of the other, resulting in a reduction in connection strength [9]. This fundamental property could potentially be incorporated into the design of novel stimulation strategies to selectively weaken exacerbated neural communication by disrupting the phase alignment between different brain regions and pushing the stimulated neural population into either an “anti-phase” or potentially uncorrelated relationship with respect to other brain regions.
It should be noted that tremor is a unique motor symptom, which can be used as a peripheral control signal to determine when to stimulate the pathological neural populations. This is due to the similarities between tremor frequency and rhythmicity of neural activity patterns. Other motor symptoms such as bradykinesia and rigidity, driven by exaggerated neural oscillations in the beta frequency band, cannot be used as peripheral control signals for phasic stimulation due to the frequency mismatch between the peripheral symptom (<1 Hz) and the neural activity. The concepts described in this paper are therefore limited to tremor control, and other feedback loops will be required for suppressing other symptoms.
The effect of precise stimulation timing was explored in a group of ET and PD patients based on the hypothesis that stimulation delivered at certain parts of a tremor cycle should either enhance or disrupt the periodic neural activity driving tremor [10], [11]. To this end, patients with chronic DBS electrodes were stimulated at a frequency approximating the patient’s own tremor frequency, allowing stimulation to slowly drift in and out of phase with respect to tremor to reveal instantaneous effects of stimulation timing.
The tremor amplitude was modulated differentially, resulting in significant tremor amplification or suppression, depending on the timing of stimulation pulses with respect to the tremor cycle in the ET patient group [10]. Crucially, there was a steep reduction in tremor severity if preceding stimulation pulses also coincided with the part of the tremor cycle promoting tremor suppression [10], as illustrated in Fig 2. Cumulative effects induced by stimulation could be driven by short-term synaptic plasticity, which if further exploited could potentially induce long-term suppression of neural oscillations driving disease symptoms even in the absence of stimulation.
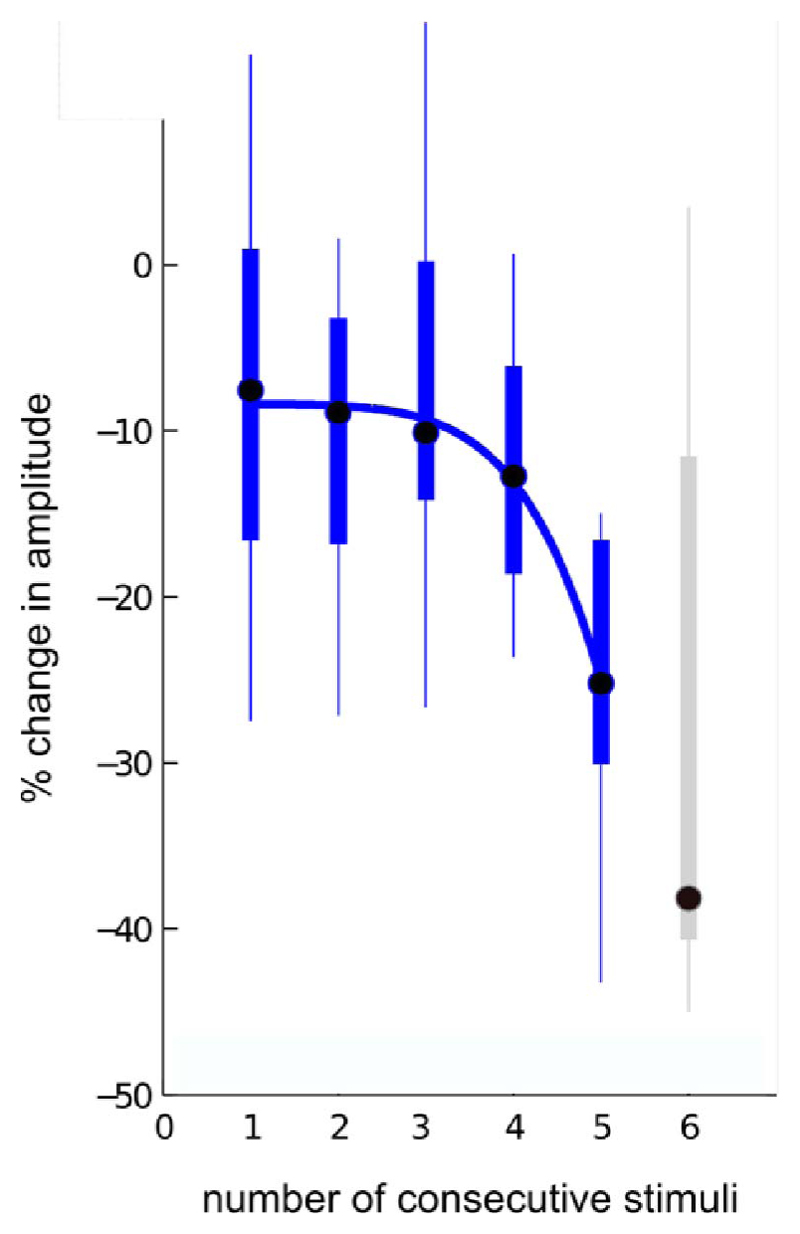
Figure 2.
Steep reduction in essential tremor severity with consecutive stimulation applied at tremor phases promoting suppression (data derived from nine subjects with essential tremor; sixth consecutive stimulation is shown in grey since only six subjects could contribute to this point; figure has been adapted from Cagnan et al. 2013 [10])
Stimulation timing did not induce any instantaneous changes in tremor severity in the PD patient group, potentially due to stimulation location (see below) or tremor circuit complexity [11]. The central and peripheral circuit that gives rise to PD tremor has a broad frequency tolerance with respect to the one driving ET [11], [12]. These differences determine how easily the central tremor drive can be entrained and modulated with phasic stimulation [11], [12].
However stimulation applied over motor cortex was able to induce instantaneous changes in PD tremor severity indicating that stimulation location may be important in determining tremor response to phasic stimulation [13]. The instantaneous effects of stimulation timing on tremor severity suggest that tremor phase could be used to control the precise timing of stimulation. The potential of this novel stimulation strategy was demonstrated in a group of PD patients using transcranial alternating current stimulation (TACS) [13].
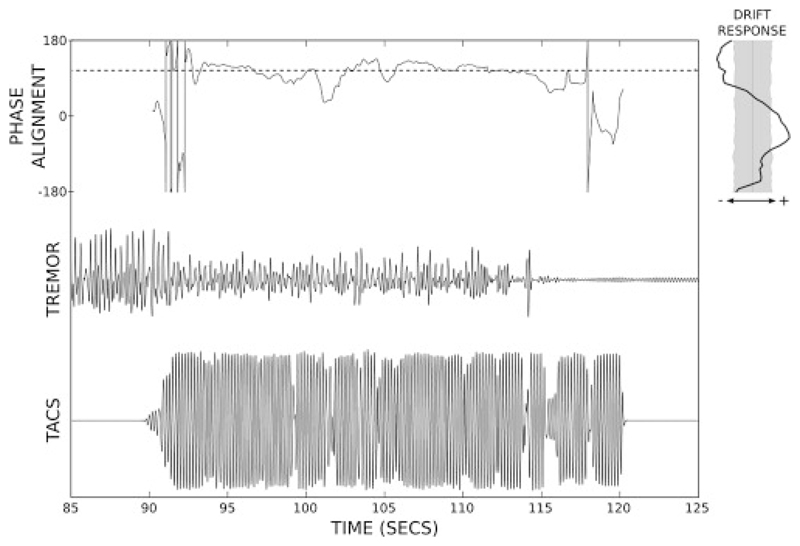
TACS provides a non-invasive and low cost potential alternative to DBS for treatment of tremor. Stimulation is delivered via two electrodes; one placed on the patient’s scalp and one non-cephalic. Brittain et al. successfully demonstrated that tremor phase can be tracked in real-time to control stimulation [13]. When stimulation was applied over the motor cortex at a fixed phase relationship with respect to tremor, up to 50% tremor suppression was achieved in a group of PD patients [13], as illustrated in Fig 3. Tremor severity was measured with an accelerometer placed on the patient’s index finger. Tremor phase was derived from the dominant movement axis using a one second long moving window Fast Fourier Transform and was used to control the phase-offset of the sinusoidal stimulation applied over the motor cortex.
Figure 3.
Tremor phase locked TACS results in significant tremor suppression when the phase alignment between patient’s tremor and the stimulation signal is sustained; figure has been adapted from Brittain et al. 2013 [13].
One of the most striking results of this study was that tremor remained suppressed after stimulation was terminated. These long-term changes are of great interest since they can be exploited to further improve power efficacy of phase locked stimulation and prolong the time periods during which stimulation is not required.
Both DBS and TACS aim to suppress tremor by interacting with the central tremor drive (i.e. pathological neural network). An alternative approach is to apply stimulation directly to muscle groups in the most affected limb. Prochazka and Javidan piloted such a system and demonstrated that tremor can be significantly suppressed in three patient groups (ET, PD and multiple sclerosis) by applying out of phase stimulation to the tremorgenic muscle groups [14]. Functional electrical stimulation of muscle groups reduced tremor severity when it was restricted to a single movement plane since only one agonist-antagonist muscle group could be stimulated at a time. However, long-term usage of functional electrical stimulation for tremor control might be limited due to muscle fatigue induced by prolonged electrical stimulation.
Emerging Closed-Loop Paradigms for Phase-Locked Stimulation
The observations of phase-triggered stimulation suppressing tremor through either DBS or TACS motivates the use of a phase-locked stimulation loop. Phase can be estimated in real time to control stimulation [13] in a computationally efficient manner [15]. Tremor phase-locked stimulation has the advantage of delivering stimulation not only when tremor is present but also at a much lower frequency, reducing stimulation frequency from 130 Hz to tremor frequency (4–8 Hz). This, in turn potentially reduces the likelihood of frequency dependent side-effects, a benefit which might be further promoted by the locking of stimulation to tremor phase and not to the phase of any physiological activities of similar frequency.
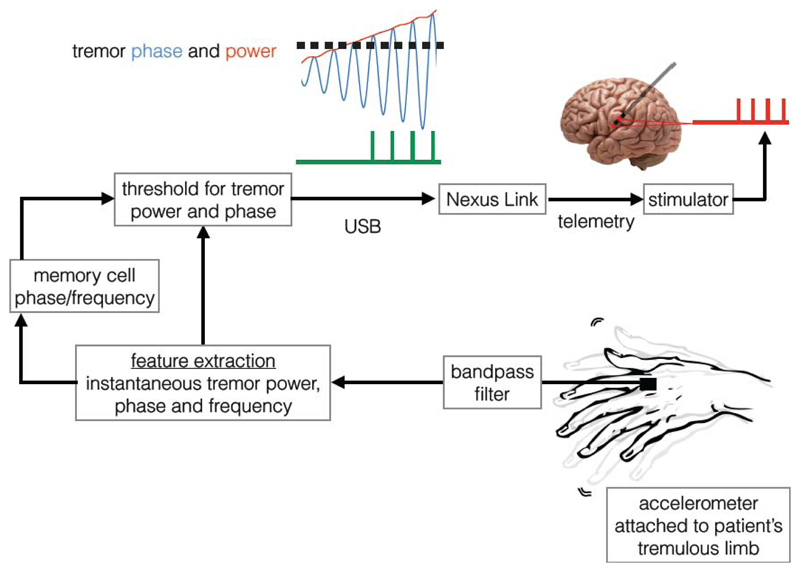
We propose the following closed loop stimulation system that uses tremor phase, derived from the principal tremor axis, to control stimulation timing delivered to key motor nuclei involved in tremor generation. Tremor severity is measured using an accelerometer and the principal tremor axis is defined as the tremor axis with the highest power following projection of the tremor signal on to the x, y and z planes (taking accelerometer position on the limb as the reference point). Signal derived from the principal tremor axis is then band-pass filtered around the mean tremor frequency prior to estimating instantaneous tremor phase and frequency [13], [15]. Stimulation is applied when the tremor amplitude exceeds a threshold and the tremor phase reaches the optimal stimulation angle (pre-determined by allowing tremor and stimulation to drift in and out of phase as in [10], [13]).
The chronically implanted DBS stimulator can be switched on for a pre-determined period using investigational tools like the “Nexus link” described in [16], an investigational stimulator programmer. The communication between the stimulator and algorithm is established telemetrically. For the duration the stimulator is switched on, a pulse train is delivered to the stimulation site at a pre-determined frequency, pulse width and amplitude (possible stimulation sites for DBS: subthalamic nucleus or thalamus for PD; thalamus for essential and dystonic tremor; TACS: motor cortex or cerebellum for PD and ET).
When stimulation is effective, the control signal (i.e. tremor) will be suppressed. An integration/memory cell can be used to store a copy of the control signal based on the average tremor characteristics (e.g. tremor phase and frequency derived from the last 30 tremor cycles) in order to estimate stimulation phase in the absence of the actual control signal. As tremor re-emerges (but remains below the amplitude threshold), the phase and frequency of the control signal is updated and stimulation is applied once the tremor amplitude threshold is exceeded and the tremor phase reaches the optimal stimulation angle.
Additional Challenges
Several challenges exist for using tremor as a control signal. (1) Tremor complexity is a major challenge for tremor phase locked stimulation protocols. Tremor can manifest in a single movement plane or multiple planes and in multiple sites of the body. In certain conditions such as PD, dystonia and multiple sclerosis, the predominant tremor axis can also change over time. It remains to be explored whether the same stimulation phase would be effective in suppressing tremor, even when the tremor axis changes. Likewise the frequency of tremor can drift. These non-stationary features of tremor directly impact the stability and reliability of tremor phase as a control signal. (2) Similarly movement can potentially alter the tremorgenic muscle groups and could impact the phase relationship with respect to the central tremor drive. Impact of voluntary movement on tremor is of particular importance for the treatment of ET, multiple sclerosis and dystonia, since tremor can be present during voluntary movement. (3) Communication with chronically implanted stimulators can be achieved telemetrically using investigational stimulator programmers [16]. However, streaming telemetry would not be a long-term power efficient solution for closed-loop stimulation control, or at a minimum would require a rechargeable system and potentially increase the management burden of the patient. Incorporating an accelerometer on the implantable circuit board can potentially circumvent this problem, if movement of a limb can be accurately captured from a sensing device placed on the trunk; implantable systems with micropower accelerometers exist today that demonstrate the technical feasibility of the concept. (4) Another key issue is that the control signal is the same as the symptom, which will be suppressed when stimulation is clinically effective. A phase locked loop with an integration/memory cell, as proposed in the prior section, could potentially provide a stable chronic stimulation reference even in the absence of the control signal, which would constantly be updated from any residual tremor signal as tremor reemerges. (5) It remains to be explored how precise the phase locking should be for tremor suppression. A certain amount of noise might need to be incorporated to the stimulation timing to prevent habituation to phasic stimulation.
While the concepts discussed here focused on inertial systems, the lessons learned from phasic tremor control might be extended for treatment of other oscillopathies by using implantable stimulators capable of sensing and stimulating from the same electrode (e.g. Activa PC+S) to derive stimulation phase directly from neural activity patterns.
Figure 4.
Tremor phase locked stimulation system
Acknowledgements
HC is funded by the Medical Research Council. PB is funded by the National Institute of Health Research, Oxford Biomedical Research Centre and Medical Research Council.
References
- [1].Kleiner-Fisman G, et al. Long-term follow up of bilateral deep brain stimulation of the subthalamic nucleus in patients with advanced Parkinson disease. Collections. 2009 May;113(3):489–495. doi: 10.3171/jns.2003.99.3.0489. [DOI] [PubMed] [Google Scholar]
- [2].Rodriguez-Oroz MC, et al. Bilateral deep brain stimulation in Parkinson’s disease: a multicentre study with 4 years follow-up. Brain J Neurol. 2005 Oct;128(10):2240–2249. doi: 10.1093/brain/awh571. [DOI] [PubMed] [Google Scholar]
- [3].Pedrosa DJ, et al. Verbal Fluency in Essential Tremor Patients: The Effects of Deep Brain Stimulation. Brain Stimulat. 2014 May;7(3):359–364. doi: 10.1016/j.brs.2014.02.012. [DOI] [PubMed] [Google Scholar]
- [4].Herron J, Chizeck HJ. Prototype closed-loop deep brain stimulation systems inspired by Norbert Wiener. 2014 IEEE Conference on Norbert Wiener in the 21st Century (21CW); 2014. pp. 1–6. [Google Scholar]
- [5].Yamamoto T, et al. On-Demand Control System for Deep Brain Stimulation for Treatment of Intention Tremor. Neuromodulation Technol Neural Interface. 2013 May;16(3):230–235. doi: 10.1111/j.1525-1403.2012.00521.x. [DOI] [PubMed] [Google Scholar]
- [6].Basu I, et al. Pathological tremor prediction using surface electromyogram and acceleration: potential use in ‘ON–OFF’ demand driven deep brain stimulator design. J Neural Eng. 2013 Jun;10(3) doi: 10.1088/1741-2560/10/3/036019. p. 036019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Graupe D, et al. Adaptively controlling deep brain stimulation in essential tremor patient via surface electromyography. Neurol Res. 2010 Nov;32(9):899–904. doi: 10.1179/016164110X12767786356354. [DOI] [PubMed] [Google Scholar]
- [8].Basu I, et al. Adaptive control of deep brain stimulator for Essential Tremor: Entropy-based tremor prediction using surface-EMG. 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBC; 2011. pp. 7711–7714. [DOI] [PubMed] [Google Scholar]
- [9].Womelsdorf T, et al. Modulation of Neuronal Interactions Through Neuronal Synchronization. Science. 2007 Jun;316(5831):1609–1612. doi: 10.1126/science.1139597. [DOI] [PubMed] [Google Scholar]
- [10].Cagnan H, et al. Phase dependent modulation of tremor amplitude in essential tremor through thalamic stimulation. Brain. 2013 Oct;136(10):3062–3075. doi: 10.1093/brain/awt239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cagnan H, et al. The nature of central oscillator circuits in parkinsonian and essential tremor. Brain. 2014 Dec;137(12):3223–3234. doi: 10.1093/brain/awu250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Brittain JS, et al. Distinguishing the Central Drive to Tremor in Parkinson’s Disease and Essential Tremor. J Neurosci. 2015 Jan;35(2):795–806. doi: 10.1523/JNEUROSCI.3768-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Brittain JS, et al. Tremor suppression by rhythmic transcranial current stimulation. Curr Biol CB. 2013 Mar;23(5):436–440. doi: 10.1016/j.cub.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Javidan M, et al. Attenuation of pathological tremors by functional electrical stimulation II: Clinical evaluation. Ann Biomed Eng. 1992 Mar;20(2):225–236. doi: 10.1007/BF02368522. [DOI] [PubMed] [Google Scholar]
- [15].Jackson J, et al. Computationally efficient, configurable, causal, real-time phase detection applied to local field potential oscillations. 7th Int IEEE EMBS Conf Neural Eng; 2015. [Google Scholar]
- [16].Afhshar P, et al. A Translational Platform for Prototyping Closed-Loop Neural Systems. Frontiers of Neural Circuits. 2013 Jan; doi: 10.3389/fncir.2012.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]