Abstract
Excessive accumulation of triacylglycerol is the common denominator of a wide range of clinical pathologies of liver diseases, termed non-alcoholic fatty liver disease. Such excessive triacylglycerol deposition in the liver is also referred to as hepatic steatosis. Although liver steatosis often resolves over time, it eventually progresses to steatohepatitis, liver fibrosis and cirrhosis, with associated complications, including liver failure, hepatocellular carcinoma and ultimately death of affected individuals. From the disease etiology it is obvious that a tight regulation between lipid uptake, triacylglycerol synthesis, hydrolysis, secretion and fatty acid oxidation is required to prevent triacylglycerol deposition in the liver. In addition to triacylglycerol, also a tight control of other neutral lipid ester classes, i.e. cholesteryl esters and retinyl esters, is crucial for the maintenance of a healthy liver. Excessive cholesteryl ester accumulation is a hallmark of cholesteryl ester storage disease or Wolman disease, which is associated with premature death. The loss of hepatic vitamin A stores (retinyl ester stores of hepatic stellate cells) is incidental to the onset of liver fibrosis. Importantly, this more advanced stage of liver disease usually does not resolve but progresses to life threatening stages, i.e. liver cirrhosis and cancer. Therefore, understanding the enzymes and pathways that mobilize hepatic neutral lipid esters is crucial for the development of strategies and therapies to ameliorate pathophysiological conditions associated with derangements of hepatic neutral lipid ester stores, including liver steatosis, steatohepatitis, and fibrosis. This review highlights the physiological roles of enzymes governing the mobilization of neutral lipid esters at different sites in liver cells, including cytosolic lipid droplets, endoplasmic reticulum, and lysosomes. This article is part of a Special Issue entitled Molecular Basis of Disease: Animal models in liver disease.
Keywords: liver, neutral lipid metabolism, lipase, triacylglycerol, cholesterol, retinol, vitamin A, steatosis, fibrosis, non-alcoholic fatty liver disease
Brief overview on liver cell types in neutral lipid storage and mobilization
The liver consists of several cell types, which are differentially involved in the uptake, storage and mobilization of neutral lipids. The vast majority of liver cells are parenchymal cells or termed hepatocytes. These cells account for around 80% of total liver volume [1]. They are known to perform most of the liver’s manifold functions in amino acid, carbohydrate, lipid and xenobiotic turnover [2]. In terms of neutral lipid homeostasis, hepatocytes play a pivotal role in the uptake of neutral lipid-rich chylomicron remnants [3], the storage of neutral lipids in cytosolic lipid droplets (LDs), and the mobilization and secretion of neutral lipids via the hydrolysis of cellular neutral lipid esters and the assembly and release of lipoprotein particles [3,4].
Besides hepatocytes, the liver harbors a variety of other cell types, which are collectively termed as non-parenchymal cells. They consist of endothelial cells (including cholangiocytes), Kupffer cells, and hepatic stellate cells (HSCs). In total, non-parenchymal cells account for around 6% of the liver volume [1], the rest (~14%) being the extracellular/vascular space. Endothelial cells do not exert a prominent role in neutral lipid turnover, since they do not take up and store large amounts of neutral lipids [5]. On a cellular basis, Kupffer cells show a higher uptake of chylomicron remnants than hepatocytes [5]. However, since they are much less in number, they do not contribute much to total hepatic neutral lipid uptake [3]. Together, Kupffer cells and endothelial cells contribute to around 1/3 of chylomicron remnant uptake [6]. For Kupffer cells it has been hypothesized that they may contribute to the clearance of rather atherogenic lipoprotein particles [3] and thus may be more important in the development of liver pathologies [7].
The third cell type belonging to the group of non-parenchymal cells, HSCs, are known to play a prominent and specific role in hepatic neutral lipid storage [8]. They are essential for the maintenance of vitamin A homeostasis [9]. These cells account for around 2% of the liver volume [10]. Initially, they have been termed perisinoidal cells, interstitial cells, lipocytes or fat-storing cells, to refer to their occurrence and their prominent role in fat (in particular vitamin A) storage. HSCs store large quantities of neutral lipid esters. These neutral lipid ester stores are comprised of comparable amounts of triacylglycerol (TAG), cholesteryl ester (CE), and retinyl ester (RE) [11–13]. Merely HSCs and not parenchymal cells contain large amounts of REs [8]. To date the physiological role of the TAG and CE stores of HSCs in hepatic neutral lipid turnover is not understood. The role of the vitamin A store of HSCs, however, is essential for the maintenance of constant plasma retinol concentration and the retinol supply of peripheral tissues. In total, HSCs harbor the largest vitamin A reserves of all tissues, which account for around 80% of the body’s total vitamin A content [14]. In times of insufficient nutritional vitamin A supply, these stores are mobilized for the maintenance of constant plasma retinol levels [15,16]. The mobilization of hepatic vitamin A stores for whole body vitamin A supply has also been demonstrated in genetic mouse models. Mice with a genetic disruption of the Lecithin:retinol acyl transferase gene (LRAT-ko), the essential acyltransferase for RE synthesis in HSCs) lack hepatic vitamin A stores [17,18]. Mice with a genetic disruption of the gene encoding Retinol-binding protein 4 (the specific retinol transport protein in the circulation) exert largely reduced circulating retinol (~10-times lower) and are defective in mobilizing hepatic vitamin A stores [19,20]. Both mouse models depend on a constant dietary supply with retinol and when fed a vitamin A deficient diet they quickly develop symptoms of vitamin A-deficiency, such as impaired vision [18,21]. Furthermore, HSCs exert a prominent role in the pathology of liver disease [22,23]. Upon advanced liver injury, when liver steatosis turns into a fibrotic state, HSCs get activated and lose their neutral lipid ester content and transform into myofibroblast-like cells. This activation, however, is independent of the vitamin A store of these cells, since also Lrat knockout mice, lacking hepatic vitamin A stores, develop liver fibrosis after chemically induced liver injury [17].
Overview on neutral lipids of the liver
Neutral lipids are a group of lipid classes, which are all together uncharged and hydrophobic in nature. Historically they have been analyzed by one-dimensional thin layer chromatography using silica plates and a hydrophobic solvent system (e.g. hexane : diethyl ether : acetic acid, 80 : 20 : 1) [24,25]. In the order of separation of hepatic lipid extracts (from furthermost to least), the following neutral lipid classes can be visualized on silica plates: hydrocarbons, CEs, TAGs, free fatty acids (FFAs), cholesterol, 1,3- and 1,2-diacylglycerols (DAGs), and monoacylglycerols (MAGs). Acidification of the solvent system for extraction and separation is necessary to protonate FFAs, which turns them into non-charged, hydrophobic lipids – at basic pH, FFAs would exist deprotonated as charged lipids.
The major neutral lipid esters classes of most tissues are TAGs and CEs, with FFAs esterified to the backbones of glycerol and cholesterol, respectively [26]. The liver also contains significant amounts of REs, with retinol as backbone for FA esterification. From the glycerides with free alcohol groups and the non-esterified neutral lipids, free cholesterol is more abundant, while FFAs, DAGs, and MAGs are less abundant or barely detectable. This is expected since FFAs, DAGs, and MAGs are intermediates of anabolic and catabolic reactions and thus do not accumulate to a large extent.
Within cells, FFAs and cholesterol are transported through binding proteins such as fatty acid and oxysterol-binding proteins, respectively, and shuttle between different organelles [27,28]. FFAs and cholesterol integrate into bio-membranes, while this is not the case for neutral lipid esters, since they are so hydrophobic in nature. That’s why neutral lipid esters only exist densely backed in the hydrophobic core of lipoproteins or LDs. These LDs constitute inert depots for bioactive lipids, such as FFAs, cholesterol and retinol, which are liberated on demand and ensure body’s requirements. The liver is known to have a high capacity to take up, store and release large amounts of neutral lipids. This process is highly dependent on the metabolic state. Upon fasting, the liver takes up large amounts of FFAs from the circulation, which are packed into lipoproteins and secreted in form of very low-density lipoprotein (VLDL) particles. Whereas, in the postprandial state, the liver clears large portions of chylomicron remnants from the circulation.
In general, neutral lipid esters function as storage molecules for FFAs, cholesterol, retinol, and glycerol. FFAs serve several functions: They are (i) energy substrates in mitochondrial beta-oxidation and subsequent oxidative phosphorylation, (ii) building blocks for lipid synthesis and protein modifications, and (iii) signaling molecules, acting as ligands for nuclear receptors thereby transactivating gene expression. Cholesterol integrates into biomembranes, thereby affecting membrane fluidity and function. Cholesterol is also the precursor for the biosynthesis of various steroid hormones as well as bile acids. Retinol is the precursor for the biosynthesis of 9-cis retinal and all-trans as well as 9-cis retinoic acid. 9-cis retinal is the hνacceptor of the visual cycle, thereby facilitating vision. all-trans and 9-cis retinoic acid are ligands of the ligand-activated nuclear receptors, the retinoic acid receptors and retinoid X receptors, respectively, thereby regulating gene expression. After phosphorylation, glycerol can undergo re-esterification, thereby serving as backbone for the name giving glycero-lipids, including phospholipids, neutral lipids, and glyco-phospholipids. In addition, glycerol-3-phosphate also serves as energy substrate: It may enter glycolysis or gluconeogenesis, depending on the metabolic state.
General concept of lipid droplet formation
Irrespective of the cell type, cells store neutral lipids by forming a hydrophobic neutral lipid-containing core, which is surrounded by a phospholipid monolayer. These cellular structures are called LDs and are nowadays understood as cellular organelles. LDs typically reside in the cytosol of cells in close proximity to membranes of the endoplasmic reticulum (ER), in proximity to the perinuclear space. In liver, LDs have been isolated from the lumen of the ER, demonstrating that LDs also reside within the lumen of the ER [29]. The formation process of nascent cytosolic LDs is known to occur at the ER. Irrespective of the exact mechanism how nascent LDs are formed at the ER (for review see [30]), neutral lipids are fed into a phospholipid monolayer, which stays attached to the outer leaflet of the ER membranes. While the nascent lipid droplet grows, LDs bud into the cytosol. In hepatocytes, a portion of ER-associated TAG is also utilized for VLDL assembly and for the formation of microsome-associated luminal LDs [31]. Interestingly, LDs usually stay attached to the ER membrane and thereby can exchange phospholipids but also proteins. Eventually LDs may entirely detach from the ER membrane. The phospholipid monolayer of LDs contains a number of embedded proteins, which exert different functions. One group of proteins exerts shielding functions and protects neutral lipids from enzymatic degradation. These proteins belong to the PAT domain containing proteins, the perilipin protein family [32]. Other proteins localize to LDs, which exhibit lipid modifying activity and act in anabolic or catabolic reactions. The one type of proteins, which promotes anabolic reactions, exhibits acyl-transferase activity and drives the growth of LDs. Members of this proteins are diacylglycerol-acyltransferases 1 and 2 (DGAT1/2), which are required for the formation of TAGs [33]. DGAT2 but not DGAT1 localizes to LDs, while both DGAT enzymes localize to ER membranes [34]. In addition to TAG synthesis, DGAT1 also catalyzes the synthesis of REs [35]. In contrast to DGAT2, other acyltransferases, such as acyl-CoA:cholesterol acyltransferase 1 and 2 (ACAT1/2) and LRAT, which catalyze the synthesis of CEs and REs, respectively, do not localize directly to the LDs but remain attached to ER membranes.
Proteins involved in hepatic neutral lipid mobilization
The classical ATGL-HSL-MGL axis of TAG catabolism
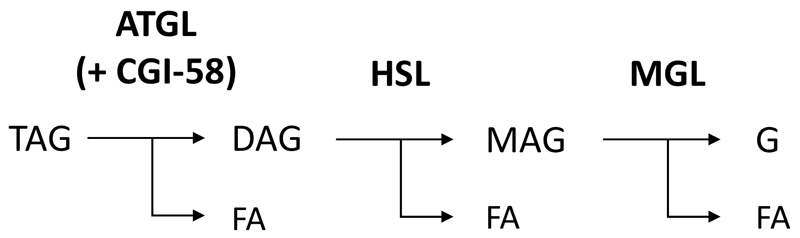
Upon requirement, neutral lipid ester stores are mobilized by the action of lipid ester hydrolases, generally termed lipases. Since centuries the liver is known to contain catalytic activities for TAG, CE and RE hydrolysis [36]. However, the identity of proteins, which are responsible for the mobilization of neutral lipid esters, are less clear. In the last decade, the breakdown of TAG has been intensively investigated (in particular in the adipose tissue (for a recent reviews see [37,38]). It is now well established that the TAG breakdown is a three-step process (figure 1), which involves three different enzymes that act in a cascade, releasing in each step one FA from the glycerol backbone [39]. Adipose triglyceride lipase (ATGL, annotated as Patatin-like phospholipase domain containing 2, also termed Desnutrin [40] or Phospholipase A2ζ [41], performs the first and rate-limiting step in TAG hydrolysis, generating FFAs and DAGs. The second step is performed by Hormone-sensitive lipase (HSL), generating FFAs and MAGs. The third step is performed by Monoglyceride lipase (MGL), generating FFAs and glycerol. All three enzymes are considered rate-limiting for their designated steps. This is evident from genetically modified mice, globally lacking either ATGL, HSL, or MGL. Atgl-, Hsl-, and Mgl knockout mice accumulate TAG, DAG, and MAG, respectively, in many tissues [42–44]. ATGL and MGL exhibit substrate specificity for the designated steps in TAG hydrolysis [45,46]. This is not the case for HSL. HSL is also capable of degrading TAG and MAG, although its relative specific activity for DAG hydrolysis is in the order of one magnitude higher, and thus HSL is a much better DAG hydrolase [47]. In fact, the limiting role of HSL for DAG and not for TAG or MAG catabolism is evident from Hsl knockout mice, which exhibit DAG accumulation in multiple tissues, including white adipose tissue (WAT), testis and muscle tissues [43]. Interestingly, however, Hsl knockout mice do not accumulate DAG in the liver, which suggests that not HSL but other enzymes are more important in hepatic DAG catabolism.
Figure 1. Sequential hydrolysis of triacylglycerol.
The degradation of triacylglycerol (TAG) is a three-step process, catalyzed by Adipose triglyceride lipase (ATGL), Hormone-sensitive lipase (HSL), and Monoglyceride lipase (MGL), forming diacylglycerol (DAG) and monoacylglycerol (MAG) as intermediates, releasing in each step one fatty acid (FA) and in the last step glycerol (G).
Other enzymes in hepatic TAG catabolism
In addition to the classical ATGL-HSL-MGL axis in TAG hydrolysis, other intracellular proteins have been shown to contribute to hepatic TAG catabolism in genetic mouse models: These enzymes include Adiponutrin/Patatin-like phospholipase domain containing 3 (PNPLA3), Carboxylesterase 1 and 3 (Ces1 and Ces3), and Lysosomal acid lipase (LAL). PNPLA3 localizes to cytosolic LDs [48,49] and may therefore contribute to the breakdown of TAG of cytosolic LDs. Ces1 and Ces3, also termed Esterase-x and Triglyceride hydrolase (TGH), respectively, localize to the ER and to cytosolic LDs [50,51]. ER localization of carboxylesterases is not very surprising since most members of the carboxylesterase superfamily encode a C-terminal ER-retention signal (HVEL) [52], which retains them in the lumen of the ER. Thus, Ces1 and Ces3 may interact with luminal LDs and in that may affect channeling of lipids for storage or for VLDL assembly and secretion. LAL is known to reside in the lysosomes and to be required for the breakdown of endocytosed neutral lipids, including TAG, CE, and RE [53]. Apart from the endocytic pathway, LAL has also a prominent role in lipophagy, a specific type of autophagy, where the lipase hydrolyzes various lipid species under acidic conditions, after fusion of cytosolic LDs with autophagosomes. This process is upregulated upon fasting [54].
Proteins involved in the hydrolysis of CE and RE
Several of the aforementioned proteins have also been found to exhibit CE and RE hydrolase activities:
For CE hydrolysis, HSL, Ces3, and LAL have been demonstrated in mouse models to interfere with hepatic CE homeostasis [55–57]. Interestingly, these three enzymes localize to three different cellular compartments. HSL resides in the cytoplasm und translocates upon Protein kinase A activation and subsequent phosphorylation onto cytosolic LDs. Ces3 localizes to the ER and LDs. Because of its ER retention signal Ces3 is likely to act in the lumen of the ER, hydrolyzing luminal CEs (e.g. CE within luminal LDs), thereby counteracting esterification and storage of CEs. As a consequence, Ces3 activity in the ER promotes secretion of CEs via VLDL synthesis and export [58]. The role of LAL in hepatic CE catabolism is evident from humans carrying mutations in the LAL alleles. Patients with loss of function mutations, where LAL entirely lacks catalytic activity, suffer from Wolman disease and die prematurely [59]. Humans with mutated LAL, which retains residual catalytic activity, present cholesterol ester storage disease [60]. Both diseases have in common that affected individuals exhibit massive accumulation of CE in various tissues, most prominently in the liver [61]. Because of the disease severity, LAL apparently is the rate-limiting enzyme in CE catabolism of endocytosed chylomicron remnants within lysosomes.
For RE hydrolysis, ATGL, PNPLA3, HSL, and LAL have been reported to exhibit catalytic activity against REs and have been investigated in mouse models (see below). ATGL, PNPLA3, and HSL act on cytosolic LDs and are expressed in hepatocytes and hepatic stellate cells [11,62,63]. LAL has been reported to be involved in the hydrolysis of lysosomal REs of hepatocytes [53]. In addition, LAL also plays an important role in RE breakdown of hepatic stellate cells [64]. Upon activation of hepatic stellate cells, LAL may be involved in the hydrolysis of cytosolic LDs of hepatic stellate cells, in the process of lipophagy [65]. Genetic knockout mouse models for all four genes (Atgl, Pnpla3, Hsl, and Lal) suggest that none of these enzymes is rate-limiting for hepatic RE mobilization, since none of the mutant mouse models shows hepatic REs accumulation. Furthermore, several members of the large carboxylesterase family have been shown to exhibit RE hydrolase activity [66]. Yet, only esterase 22 has been shown in cell experiments to affect cellular RE content. Since esterase 22 localizes to the ER, it was hypothesized that it may counteract LRAT driven RE deposition in cytosolic LDs, thereby promoting retinol secretion [67]. However, the role of any of the carboxylesterases in RE metabolism has so far not been investigated in mouse models.
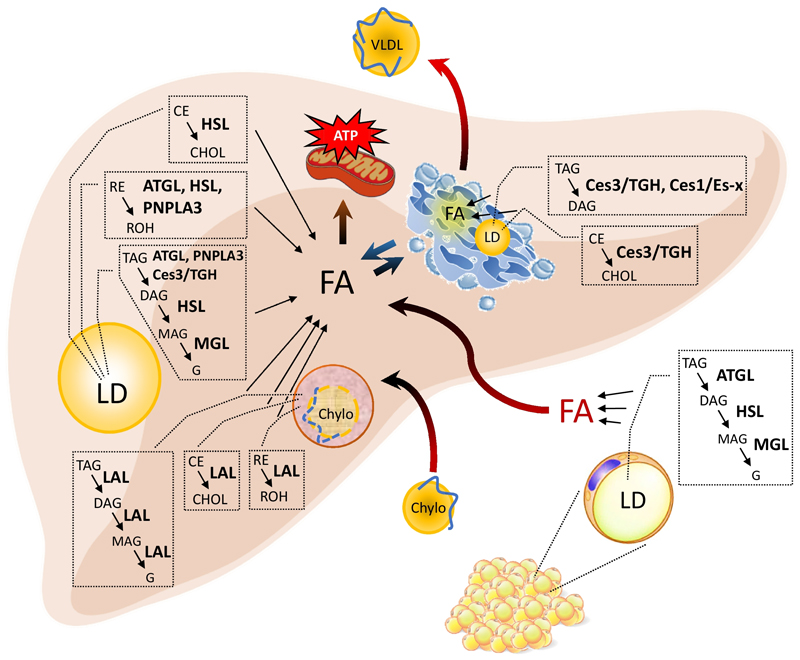
Figure 2 depicts the hepatic uptake of FAs supplied by adipose tissue lipolysis, the mobilization of FAs by proposed lipases at different organelles, and the utilization of FAs for ATP production as well as for the synthesis and release of VLDL. Enzymes, which have been demonstrated in mouse models to interfere with neutral lipid mobilization, are indicated.
Figure 2. Enzymes involved in hepatic neutral lipid ester mobilization.
The liver takes up neutral lipid esters (triacylglycerol, TAG; cholesteryl ester, CE; retinyl ester, RE) via the endocytosis of chylomicron remnants (Chylo) or fatty acids (FA), which are released from adipose tissue lipolysis into the circulation. Intracellularly, Chylo-associated neutral lipid esters are degraded by the action of lysosomal acid lipase (LAL), releasing FAs, glycerol (G), cholesterol (CHOL), and retinol (ROH). At the cytosolic lipid droplets (LD), TAGs are hydrolyzed by concerted action of Adipose triglyceride lipase (ATGL), Hormone-sensitive lipase (HSL), and Monoglyceride lipase (MGL), forming the intermediates diacylglycerol (DAG) and monoacylglycerol (MAG), releasing FA and G. Carboxylesterase 3/Triglyceride hydrolase (Ces3/TGH) and Patatin-like phospholipase domain containing 3 (PNPLA3) may contribute to TAG hydrolysis of cytosolic LD. ATGL, HSL, and PNPLA3 also exhibit hydrolytic activity towards RE. HSL also hydrolyses CE. At the ER, Ces3/TGH and presumably Carboxylesterase 1/Esterase-x (Ces1/Es-x) hydrolyze TAG, while Ces3/TGH presumably also hydrolyzes CE. Released FA can be used as building blocks and/or for mitochondrial FA oxidation and ATP production. FAs in the cytosol as well as of the ER affect VLDL synthesis and secretion.
Targeting ATGL and pharmacological inhibition of ATGL
The role of lipolysis in hepatic TAG homeostasis remained enigmatic until the discovery of ATGL as a novel neutral TAG lipase [45]. The generation of ATGL-deficient mice demonstrated ATGL’s dominant role in TAG hydrolysis, since these mice exhibited TAG accumulation in cytosolic LDs of many organs and tissues, including the liver [42]. Several follow up studies, where ATGL expression was down regulated or increased in mice via injection of recombinant adenovirus, suggested a role for ATGL in liver TAG catabolism and the supply of FAs as oxidative fuel [68,69]. The critical role of ATGL in hepatic TAG homeostasis was evident from the liver-specific deletion of Atgl, which provoked progressive macrovesicular and microvesicular hepatic steatosis [70], accompanied by massive hepatic TAG accumulation in mice fed either a chow or a high fat diet (HFD). Impaired lipolysis in these mice was linked to decreased FA oxidation, which may further promote hepatic TAG deposition. LDs were also present within cholangiocytes, implicating that ATGL is required for lipolysis in these cells. The observation that TAG accumulation was relatively mild in hepatic tissue of mice globally lacking ATGL [42] as compared to hepatic ATGL-deficiency [70] suggests that impaired adipose tissue FA mobilization and consequently reduced FA flux to the liver prevented severe hepatic TAG accumulation in mice globally lacking ATGL. Particularly interesting, severe hepatic steatosis caused by hepatic ATGL-deficiency was benign and not associated with liver fibrosis and inflammation. Furthermore, impaired hepatic lipolysis did not significantly interfere with VLDL secretion and hepatic insulin sensitivity. These findings suggest that i), lipoprotein TAG incorporation does not depend on the release of FAs from cytosolic LDs and that ii), hepatic TAG accumulation per se is not the main trigger in the development of hepatic insulin resistance, at least in ATGL-mutant mice. Although speculative, impaired TAG catabolism together with reduced FA oxidation (FAO) may lower levels of FFAs in mice with hepatic Atgl-disruption, thereby counteracting the detrimental consequences of increased cellular FFA levels, also referred to as lipotoxicity [71]. In line with such an assumption, the study by Fuchs et al. [72] demonstrated that the global lack of ATGL protected mice from tunicamycin-induced ER stress and hepatic inflammation despite hepatic TAG accumulation. This study also showed that the ratio of palmitic acid versus oleic acid was lower in the hepatic TAG pool of ATGL-deficient mice. As saturated FAs can trigger ER stress in hepatocyte cell lines [73], the authors hypothesized that the decrease in the relative amount of palmitic acid may counteract the development of ER stress in mice globally lacking ATGL. However, a study by the same laboratory reported that hepatic inflammation increased in ATGL-deficient mice, kept either on a methionine-choline-deficient (MCD) diet to induce NASH or upon injection of lipopolysaccharide to induce acute hepatic inflammation [74]. Interestingly, dietary supplementation with the peroxisome proliferator-activated receptor alpha (PPARα) agonist fenofibrate mitigates hepatic inflammation in both, MCD fed or lipopolysaccharide-injected ATGL-deficient mice. These findings implicate that impaired PPARα signaling, which was shown to exacerbate hepatic inflammation in the MCD mouse model [75], may promote hepatic inflammation in the absence of ATGL.
Together these data indicate that hepatic steatosis in ATGL-deficient mice can be a precondition that enhances hepatic inflammation under specific conditions of hepatic stress. Yet, these studies were performed in mice globally lacking ATGL and do not allow to delineate whether the observed changes in hepatic inflammation originate from the lack of ATGL in the liver and/or adipose tissue. In line with this question, we could show that impaired adipose tissue lipolysis due to deletion of ATGL or its co-activator CGI-58 solely in adipose tissue profoundly interferes with hepatic PPARα signaling, encompassing a robust decrease in Fibroblast growth factor 21 (FGF21) expression and reduced ER stress on HFD [76]. Besides its established role as a TAG hydrolase, the study by Taschler et al. [62] demonstrated that REs are also a substrate for ATGL and importantly, inhibition of ATGL enzyme activity increased RE content in HSCs. Moreover, ATGL also efficiently hydrolyzed RE in parallel to TAG, implicating that both TAG and RE can compete for ATGL binding. However, RE levels were unchanged in mice globally lacking ATGL indicating that other enzymes participate in hepatic RE catabolism. Nevertheless, the stimulation of lipolysis in HSCs is linked to the activation and proliferation of HSCs, which is considered to contribute to hepatic fibrosis development [22]. However, feeding mice globally lacking ATGL with a MCD diet led to increased collagen 1 alpha expression, rather indicative for induction of HSC activation than protection of disease progression (from liver steatosis to fibrosis) [74]. Accordingly, it would be interesting to examine whether liver-specific ATGL deletion or overexpression affects hepatic fibrosis progression in mice on MCD diet or upon chemical induction of liver fibrosis likely via changes in HSC metabolism.
Increased circulating and/or intracellular FFAs levels are tightly linked to numerous metabolic disorders including NAFLD. The discovery of ATGL as an essential TAG lipase in many if not all cell types offers a promising pharmacological target to interfere with whole body and organ-specific FA homeostasis. This strategy was followed in a collaboration with the lab of R. Breinbauer at the University of Technology, Graz and our lab, where a small-molecule ATGL inhibitor (designated as “Atglistatin”) was developed and demonstrated to specifically and efficiently inhibit ATGL lipolysis in vitro and in vivo [77]. Intriguingly, dietary administration of Atglistatin protected mice from HFD-induced hepatic steatosis and insulin resistance [78]. Moreover, NAFLD score (number of inflammatory cells and areas as well as the degree of liver cell ballooning) was strongly reduced in Atglistatin treated mice, which was accompanied by lower plasma alanine-aminotransferase levels and decreased expression of inflammatory and fibrosis marker genes. The administration of Atglistatin resulted in similar distribution of the inhibitor in white adipose and liver tissue. The substantial decline in hepatic TAG levels was unexpected, as liver-specific ATGL ablation provokes massive TAG accumulation. The protection from hepatic TAG accumulation may be the consequence of ATGL inhibition in adipose tissue causing impaired TAG mobilization and reduced FFA flux to the liver. It is also conceivable that inhibition of ATGL activity in the liver can be compensated by the induction of other TAG hydrolyzing enzymes and/or lipophagy, which awaits further clarification. Nevertheless and as aforementioned, decrease hepatic TAG levels, as observed in HFD fed mice specifically lacking ATGL in the adipose tissues [76], strongly indicates that Atglistatin treatment protects from NAFLD via inhibition of adipose lipolysis. Notably, Atglistatin administration does not affect cardiac lipid homeostasis and energy metabolism, which is a hallmark of ATGL-deficient mice, elucidating the administration of Atglistatin as a promising strategy to counteract NAFLD development in humans.
Together, genetically engineered mice lacking ATGL either in the liver or in adipose tissue as well as mice treated with Atglistatin are interesting animal models to study pathways that induce or protect from NAFLD development and eventually the progression to NASH.
Targeting ATGL co-regulators
Targeting the coactivator CGI-58
The most prominent ATGL co-regulator represents Comparative gene identification-58 (also designated as Abhydrolase domain containing 5, ABHD5). CGI-58 was initially discovered in a comparative genomic study as one of 150 human genes harboring homologues in the proteome of Caenorhabditis elegans [79]. In 2001, Lefèvre and colleagues [80] demonstrated a linkage of mutated Cgi-58 alleles to the development of neutral lipid storage disease with ichthyosis, also known as the Chanarin-Dorfman syndrome. The clinical picture of neutral lipid storage disease with ichthyosis encompasses ichthyosis (a defect in epidermal skin barrier formation) and typically severe hepatic steatosis. We could show that CGI-58 acts as a potent activator of ATGL-mediated TAG hydrolysis and that CGI-58 mutations, as present in neutral lipid storage disease with ichthyosis patients, fail to stimulate ATGL activity [81]. Interestingly, the clinical manifestations differ in patients carrying mutated Atgl alleles, where skin development is normal and severe (cardio)myopathy is the main clinical picture leading to the designation neutral lipid storage disease with myopathy [82,83]. In mice, global Cgi-58 deletion provokes a severe skin barrier defect and the mice die several hours after birth due to rapid dehydration [84]. Newborn CGI-58 deficient mice already showed massive TAG accumulation in the liver demonstrating that CGI-58 plays a central role in hepatic TAG homeostasis. However, the short life span of the mice hindered to further study the role of CGI-58 in hepatic lipid metabolism and steatosis development. To circumvent early postnatal lethality and to study the impact of CGI-58-deficiency on hepatic lipid metabolism in adult mice, an alternative strategy was followed utilizing antisense oligonucleotides (ASO). The injection of ASOs targeting Cgi-58 mRNA expression resulted in a dual > 90% knockdown of Cgi-58 mRNA expression in white adipose tissue and liver, respectively [85]. The ASO-mediated knockdown of Cgi-58 expression provoked an approximate 4-fold increase in hepatic TAG levels on both, on regular chow diet or on HFD. Unexpectedly, and with regard to the established role of CGI-58 in stimulating ATGL-mediated TAG breakdown, the ASO-mediated knockdown of Cgi-58 in adipose tissue (and liver) protected mice from HFD induced obesity. Moreover, mice showed increased insulin sensitivity despite a significant increase in DAG and ceramide levels in the liver, which is considered to trigger the development of hepatic insulin resistance. Hyperinsulinemic-euglycemic-clamp studies corroborate findings showing sustained insulin sensitivity upon injection of Cgi-58 ASOs in mice on HFD [86]. Increased insulin resistance in control mice was linked to elevated DAG levels specifically in the membrane fraction thereby activating Protein kinase Cɛ as an inducer of liver insulin resistance. In contrast, Cgi-58 knockdown led to DAG sequestration within cytosolic LDs and/or lipid-associated ER rather than in the membrane, and hence impeding Protein kinase Cɛ translocation to the plasma membrane and consequently the induction of insulin resistance. Besides hepatic steatosis and insulin sensitivity, the Cgi-58 knock down provoked a marked reduction in hepatic TAG and CE secretion rates, whereas FC secretion was normal. This finding might suggest that impaired TAG catabolism due to the ASO-mediated knockdown of Cgi-58 in the liver impairs hepatic lipolysis and FA incorporation into VLDL-TAG and CE. However, the dual knock down of CGI-58 in adipose tissue and liver upon ASO injection hinders to delineate the tissue specific role of CGI-58 in VLDL production as both, changes in adipose and/or hepatic lipolysis could affect VLDL TAG/CE secretion rates. Reduced plasma FA levels in fasted (and fed) ASO treated mice rather suggested that low adipose tissue FA supply to the liver affects hepatic VLDL production. In line with this assumption, the liver-specific deletion of Cgi-58 via Cre-LoxP recombination provoked massive hepatic steatosis and a 50-fold increase in hepatic TAG levels (compared to a 4-fold increase applying Cgi-58 ASOs), which was well compatible with normal VLDL secretion. We could demonstrate that ATGL-deficiency protects mice from HFD-induced obesity via impaired peroxisomal-proliferator activated receptor gamma (PPARγ)-induced fat synthesis and deposition in adipose tissue [87] independent of impaired adipose lipolysis, which is expected to rather increase adipose TAG deposition. Although speculative, it is conceivable that resistance to HFD-induced obesity of Cgi-58 ASO injected mice similarly involves impaired PPARγ activation due to inefficient stimulation of ATGL-mediated TAG hydrolysis in the absence of its co-activator CGI-58. As aforementioned, and very similar to humans carrying Cgi-58 mutations, liver-specific Cgi-58 deletion via Cre-mediated recombination of Cgi-58 floxed alleles provokes severe macrovesicular and microvesicular steatosis encompassing a more than 50-fold increase in liver TAG content even on a normal chow diet [88]. Intriguingly, and in strong contrast to hepatic ATGL-deficiency [70], the lack of liver CGI-58 led to progression of NASH, showing increased inflammation, ER stress and fibrosis. Notably, increased hepatic inflammation was also a main characteristic of ASO-mediated Cgi-58 knockdown in the liver [89]. Moreover, liver-specific Cgi-58 deletion led to an increase in levels of free and esterified cholesterol [88], which was not observed in mice with hepatic ATGL-deficiency [70]. In line with the divergent NAFLD outcome of mice with hepatic ATGL- compared to CGI-58-deficiency, the application of CGI-58-specific antisense oligonucleotides led to an additional pronounced increase in liver TAG levels of ATGL-deficient mice and the induction of hepatic inflammation [90]. These studies clearly suggest an ATGL-independent and CGI-58-specific role in hepatic lipid metabolism. One possible scenario would be that CGI-58 co-activates and/or recruits other lipid hydrolyzing or lipid modifying enzyme(s) and thereby affects neutral lipid homeostasis. Taken together, mice with hepatic CGI-58 deficiency are an important and promising model to study NAFLD progression to NASH. Of note, for Cre-mediated liver-specific deletion of floxed alleles the preferential Cre expressing system is the Albumin-Cre mouse model [91]. Hepatic deletion of floxed alleles applying the so-called AlfpCre mouse has been shown to promote hepatic steatosis indirectly via changes in hypothalamic growth hormone metabolism [92]. We also used the AflpCre mouse to delete Cgi-58 in hepatocytes and found that AlfpCre mice already display mild hepatic steatosis and obesity compared to wildtype controls (unpublished observation).
Targeting the shielding protein Perilipin 5
Albeit the molecular mechanism how CGI-58 activates the enzymatic activity of ATGL is unknown, several studies have demonstrated that the CGI-58-dependent stimulation of ATGL lipolytic activity is under regulation of the Perilipins, a family of LD-associated proteins found in adipose and non-adipose tissues [32,93]. In oxidative organs including the liver, CGI-58 is recruited by Perilipin 5 (Plin5) at the surface of LDs and this interaction hinders CGI-58 to stimulate ATGL activity. The PKA phosphorylation of Plin5 releases CGI-58 to act as ATGL co-activator thereby increasing TAG hydrolysis [94]. The in vivo role of Plin5 in the regulation of hepatic lipolysis via Plin5 interaction is particularly evident in mice lacking Plin5, which show reduced neutral lipid staining and TAG levels in hepatic tissues of mice on chow or HFD and upon fasting [95]. The increase in lipolysis and FAO in Plin5-deficient primary hepatocytes implicates that Plin5-deficient mice show enhanced TAG catabolism most likely due to augmented availability of non-bound CGI-58 to stimulate ATGL activity. Increased lipolysis in the liver of Plin5-deficient mice additionally stimulates PPARα-induced expression of genes from the FAO machinery, thereby increasing FAO rates and possibly levels of reactive oxygen species, which may trigger oxidative stress, inflammation and ER stress in these mice [95]. Besides the consequences of Plin5-deficiency on hepatic lipolysis, this study also showed increased hepatic Plin5 expression in patients presenting liver steatosis and in ob/ob mice exhibiting benign liver steatosis. These findings implicate that Plin5 may exert a protective role towards the progression of NAFLD to NASH via decelerating lipolysis thereby lowering FFA levels to counteract lipotoxicity. In line with such an assumption, transient adenovirus-mediated hepatic Plin5 overexpression further increased hepatic steatosis in mice on HFD without the induction of liver injury or insulin resistance [96]. Together, mice lacking Plin5 are an interesting model to study the impact of increased hepatic lipolysis on NAFLD development.
Targeting the inhibitory protein G0S2
The lipolytic activity of ATGL is also under negative regulation by a small protein designated as G0/G1 switch gene 2 (G0S2). G0S2 is reciprocally expressed in adipose tissue and liver thereby coordinating adipose TAG catabolism and FA supply to hepatic energy catabolism [97]. Similar to the function of CGI-58 as an ATGL co-activator, G0S2-mediated inhibition of ATGL lipolysis depends on the physical interaction with ATGL [98]. The critical role of G0S2 in hepatic TAG catabolism is evident in mice globally lacking G0S2, which show a strong decline in hepatic TAG levels on chow diet [99]. Particularly interesting, these mice were resistant towards HDF-induced hepatic steatosis, paralleled by reduced plasma alanine-aminotransferase and aspartate-aminotransferase levels compared to controls indicating less hepatic injury in the absence of G0S2. The siRNA-mediated knockdown of G0s2 exclusively in the liver enhanced hepatic lipolysis and reduced hepatic TAG disposition implicating that the decline in hepatic TAG levels of mice globally lacking G0S2 originates from the absence of G0S2 in the liver. In contrast, and in line with this assumption, adenovirus-mediated hepatic overexpression of G0S2 increased hepatic TAG content and reduced hepatic lipolysis via inhibition of ATGL-mediated TAG catabolism. Notably, hepatic steatosis due to increased hepatic G0S2 expression improves whole body glucose tolerance and enhances hepatic glucose uptake [100]. Benign hepatic steatosis via increased G0S2 expression in the liver and hence inhibition of hepatic ATGL activity is in accordance with the more moderate NAFLD caused by hepatic ATGL deletion and in contrast to hepatic CGI-58 deficiency which provokes NASH.
In summary, ATGL plays a pivotal role in hepatic TAG and energy catabolism. Remarkably, reduced or increased expression of all currently known ATGL co-regulators including CGI-58, Plin5 and G0S2 profoundly interferes with hepatic TAG homeostasis. Both, liver-specific deletion of either ATGL or the ATGL co-activator CGI-58 provoke severe hepatic steatosis. Particularly interesting, exclusively CGI-58 deficiency in the liver provokes NASH demonstrating an additional and ATGL-independent role of CGI-58 in liver lipid metabolism, which triggers the transition from mild NAFLD to NASH. In depth characterization of these mouse models will ultimately lead to a better understanding on the pathways and factors causing NAFLD and the eventual progression to NASH.
Targeting HSL and MGL
Although HSL catalyzes all three steps of TAG hydrolysis, HSL is essential for the second step of the lipolytic cascade, the hydrolysis of DAG to MAG (Figure 1) [101]. Mice global lacking HSL are characterized by DAG accumulation in many tissues, but exhibit reduced hepatic TAG levels (and increased insulin sensitivity), indicating that HSL is not essential in liver TAG catabolism [102]. This finding is challenged in humans carrying mutated Hsl alleles, where the lack of HSL protein provokes systemic insulin resistance and hepatic steatosis [103]. This discrepancy was resolved by a very recent study where the impact of adipose- versus liver-specific Hsl deletion on hepatic TAG homeostasis was investigated: Global or adipose-specific deletion of Hsl caused age-dependent hepatic fat accumulation, whereas the lack of HSL specifically in the liver was compatible with normal hepatic TAG homeostasis [104]. Moreover, adipose (or systemic) HSL-deficiency caused progressive lipodystrophy, macrophage infiltration and systemic insulin resistance in aged mice [104]. These phenotypic changes were accompanied by decreased hepatic lipolysis, FAO and VLDL-TG production, which altogether affect hepatic TAG homeostasis. Humans lacking HSL are skinny in appearance and it is conceivable that the metabolic changes presented in HSL-deficient mice play also a role in the development of hepatic steatosis and insulin resistance in humans [103]. Similar to hepatic ATGL ablation, NAFLD caused by the lack of adipose HSL does not trigger hepatic fibrosis and inflammation, which may indicate that reduced ATGL lipolysis may play a role in NAFLD development in HSL mutant mice. Together, these studies implicate that hepatic steatosis in patients carrying mutated Hsl alleles rather originates from lipodystrophy, which then interferes with hepatic lipid and energy metabolism. Besides the hydrolysis of TAG and DAG, HSL also acts as a neutral CE hydrolase. As aforementioned, hepatic HSL-deficiency in mice had no impact on hepatic TAG homeostasis [104]. In contrast, global HSL-deficient mice exhibited CE accumulation on a high cholesterol diet or on the Leptin-deficient background, paralleled by reduced CE hydrolase activities in liver preparations indicative for impaired CE catabolism in the absence of HSL [55]. Moreover, primary hepatocytes from HSL-deficient mice already exhibited increased CE levels and incubation with cholesterol rich low-density lipoprotein further increased CE levels [55] suggesting that HSL participates in hepatic CE catabolism. Another study showed increased CE levels in the liver of HSL-deficient mice even on chow and HFD together with increased expression of the cholesterol transporter ABCA1 in liver tissue, further corroborating a role for HSL in hepatic CE catabolism [105]. The overall increase in plasma cholesterol levels was due to an increase in HDL and VLDL cholesterol levels implicating that global HSL-deficiency interferes with whole body cholesterol homeostasis. The raise in plasma cholesterol of HSL-deficient mice may be an adaption to impaired hepatic CE catabolism and an increase in liver CE content, leading to elevated hepatic ABCA1 expression, which could enhance cholesterol efflux. Whether HSL-deficiency in humans [103] plays a role in hepatic steatosis development and insulin resistance via impaired liver CE catabolism is actually unknown.
As aforementioned (see Figure 1), MGL performs the third and final step in TAG hydrolysis. Global MGL deficiency leads to MAG accumulation in many tissues, including the liver [44]. One of the MAGs, 2-arachidonoyl glycerol, is the most abundant endogenous ligand of cannabinoid receptors, which is known to promote appetite and to decrease energy expenditure [106]. However, MGL deficient mice to not show hyperphagia and decreased locomotor activity since the cannabinoid receptors are desensitized [44]. Yet, global deficiency of MGL in mice significantly reduced hepatic VLDL-TAG secretion and TAG deposition [44], most likely via reduced glycerol shuttling from adipose tissue to the liver, where glycerol can be re-utilized for FA-esterification and incorporation into VLDL-TAG. Additionally, Douglass et al. [107] demonstrated reduced intestinal TAG secretion in MGL deficient mice which may also impact hepatic TAG. Together, the beneficial effect of MGL-deficiency on hepatic TAG homeostasis and insulin sensitivity even on a HFD elucidates MGL as a prominent pharmacological target to combat NAFLD development and progression.
In summary, every component of the lipolysis machinery, encompassing neutral lipid hydrolases and co-factors, may directly, via hepatic or indirectly via adipose lipolysis and hepatic FA supply, impact hepatic lipid metabolism and the development of NAFLD. Several mouse models are available to study the effect of changes in adipose tissue lipolysis on NAFLD development, which may mimic changes in hepatic FA flux of obese patients. Studying the pathway(s) leading to the progression from benign NAFLD to NASH in mice with hepatic CGI-58 deficiency compared to hepatic ATGL deficiency (despite massive hepatic fat accumulation in both mouse models) may increase our understanding of the factors that trigger the transition to pathological NAFLD. Finally, various mouse models with impaired lipolysis are available to test novel therapeutic strategies to counteract NAFLD/NASH development caused by changes in liver and whole body lipid and energy catabolism.
Targeting PNPLA3
A non-synonymous single nucleotide polymorphism in Pnpla3 (rs738409 C/G, a coding variant that encodes the amino acid substitution I148M) was found in a genome-wide association study to strongly correlate with hepatic fat accumulation, susceptibility of a more aggressive liver disease and a higher risk of developing liver fibrosis [108]. This notion brought much attention to PNPLA3, the third member of the mammalian patatin domain containing protein family, the closest homologue of the TAG hydrolase ATGL (PNPLA2), and also termed Calcium-independent phospholipase A2 epsilon (iPLA2ε) or Adiponutrin [109]. PNPLA3’s expression profile would rather suggest a predominant role of PNPLA3 in adipose tissue metabolism because of high mRNA expression in adipose tissue and much lower expression in the liver [109]. Pnpla3 mRNA expression in white adipose tissue and liver is upregulated upon food intake, while low upon fasting [109]. For comparison, ATGL/PNPLA2, the rate-limiting TAG hydrolase in white adipose tissue is inversely regulated to PNPLA3 and thus down-regulated in white adipose tissue and liver upon feeding but upregulated upon fasting [109]. Furthermore, PNPLA3 has been shown to localize to LDs [63]. Thus, it appears plausible that PNPLA3 may function as neutral lipid modifying enzyme on LDs, likely remodeling LD composition by hydrolase or acyltransferase activity. In fact, different groups have demonstrated in in vitro activity assays that PNPLA3 exhibit lipase activity for artificial TAG substrates [109], for TAG (triolein) [48,110], for RE [63] and acyltransferase activity for LPA (LPAAT activity) [111]. However, these activities attributed to PNPLA3 have been questioned by the inconsistent outcome of the aforementioned studies including lack of RE hydrolase activity [110], insignificant TAG (triolein) hydrolase activity [111], and no LPAAT activity [110]. Interestingly, the I148M mutant of human PNPLA3 was reported to represent a loss-of-function mutation for TAG and RE hydrolytic activities [48,63], while it was reported to represent a gain-of-function mutation for LPAAT activity [111].
Insights into this conflicting observations around PNPLA3’s enzymatic activities, whether PNPLA3 exhibits hydrolytic or acyltransferase activity and whether I148M represents a loss- or gain-of-function mutation, can be obtained from cell experiments and animal models. Expression of murine or human PNPLA3, led to increased cellular TAG content in mammalian cell lines, while this increase in TAG content was even higher upon expression of the I148M variant (Kumari et al., 2012). Liver-specific transgenic overexpression of the PNPLA3 mutant I148M, but not of wild-type PNPLA3, led to increased hepatic TAG content, recapitulating the liver phenotype of individuals carrying the rs738409 C/G polymorphism [113]. The TAG liver content of liver-specific transgenic mice expressing the I148M variant of PNPLA3 was further increased when mice were fed a high sucrose but not a high fat diet. Furthermore, in-depth analysis of livers of I148M Pnpla3 liver-specific transgenic mice revealed that LDs were increased in number and size, and hepatic FA and TAG biosynthesis were elevated [113]. Furthermore, the hepatic TAG pool of PNPLA3 transgenic mice comprised of increased amounts (% of total), of the FA oleate (C18:1), while levels of poly-unsaturated FAs, such as FA C20:4 and C22:4/5/6, were decreased [113]. These changes in FA species composition suggests increased TAG biosynthesis and remodeling, since FA C18:1 is typically very abundant in the diet. Furthermore, primary hepatocytes of liver-specific transgenic mice expressing the PNPLA3 mutant I148M exhibited reduced glycerol release, indicative for defective TAG hydrolysis. Interestingly, in these transgenic I148M mutant Pnpla3 mice, the hepatic levels of LPA was tentatively decreased (~30%) [113], which would support the gain-of-function hypothesis that mutant I148M PNPLA3 exhibits increased LPAAT activity [111]. Interestingly, also the “physiological expression” of the I148M PNPLA3 mutant using a I148M mutant Pnpla3 knock-in mouse model showed increased liver fat content, but only when mice were fed a high sucrose diet and not when fed chow diet [114]. Another study, using Pnpla3 knockout mice, showed decreased LD-associated LPAAT activity in livers, accompanied by decreased LD-associated PA content [111]. However, two independent studies on Pnpla3 knockout mice (i.e. loss-of-function model) concluded that PNPLA3-deficiency does not affect overall body composition, energy homeostasis, glucose homeostasis, insulin sensitivity, and does not provoke increased hepatic fat content [115,116], indicating that the I148M mutation of PNPLA3 is not “simply” a dead mutation, solely inactivating PNPLA3 function. Thus, the genetic deletion and loss of PNPLA3 function is not the main mechanism by which human I148M variant of PNPLA3 provokes fatty liver disease or metabolic disorders. Given the results from the I148M Pnpla3 transgenic mice that the overexpression, at least in combination with a high sucrose diet, provokes fatty liver [113] suggests that the I148M polymorphism represent a gain-of-function mutation. Alterations in TAG species composition of transgenic I148M Pnpla3 mutant mice in conjunction with the reported gain of LPAAT activity of the I148M Pnpla3 mutant would suggest a physiological role of PNPLA3 in LD synthesis and remodeling. This hypothesis is seemingly supported by the observation in affected human subjects, who exhibit in addition to increased liver fat content, smaller adipocytes in their adipose tissue [117].
The observation that PNPLA3, in addition to its potential role as LPAAT and TAG lipase, may also act as RE hydrolase was made after the generation of the knockout, transgenic and knock in mouse models. Thus, in any of these mouse models the mobilization of RE stores has not been addressed. Yet, two studies [63,112] using primary human hepatic stellate cells and the human hepatic stellate cell line LX-2 demonstrated that expression of wild-type PNPLA3 but not of the I148M mutant PNPLA3 affects RE homeostasis in HSCs, concluding that the I148M variant represents a loss-of-function mutation. In line with such an assumption, humans carrying the I148M PNPLA3 mutation exhibit decreased plasma retinol levels and increased hepatic RE content [118,119].
Targeting Ces3/TGH
VLDLs are fundamental for the distribution of endogenous TAG from the liver to peripheral tissues. In liver, VLDLs are synthesized in hepatocytes and are stuffed with neutral lipids, a large portion being TAGs [120]. Storages sites of TAGs molecules are LDs, which may reside in the cytosol, in close proximity to the ER membranes or in the lumen of the ER [121]. Interestingly, expression of ATGL and in particular of HSL, the classical lipases of TAG catabolism, is very low in liver [45,55,122]. Expression of HSL is higher in the parenchymal than non-parenchymal cell fractions [55]. Furthermore, overexpression or ablation of HSL or ATGL in the liver does not affect hepatic VLDL secretion [69,123], suggesting that other lipases, which may reside in the lumen of the ER, are crucial for VLDL synthesis. One of the best-investigated luminal carboxylesterases in murine liver is Ces3/TGH (the human homologue is annotated as CES1 and is also termed neutral cholesteryl ester hydrolase, NCEH or CEH). Ces3 was initially isolated from porcine liver microsomes [124]. It was named TGH because of its in vitro lipase activity against medium- and long-chain TAG [124], although Ces3 also hydrolyzes CEs [125]. In the mouse, Ces3 is highly expressed in the liver and to a lesser extent in adipose tissue, heart, kidney, and small intestine [126]. Already initial studies [127] in the rat hepatoma cell line McA-RH7777 stably expressing rat Ces3 revealed that Ces3 promotes VLDL secretion while glycerol release was not changed. This was indicating that Ces3 rather plays a role in mobilizing hepatic TAG stores via VLDL secretion than via lipolysis, i.e. the catabolism of TAG from cytosolic LDs. This prominent role of Ces3 in VLDL secretion was further corroborated by inhibitor studies, where treatment of rat primary hepatocytes with a Ces3 specific inhibitor almost completely blunted apolipoprotein B-100 and TAG secretion into the supernatant [128]. Similar to cell experiments also the inducible transgenic expression of Ces3 in mice led to increased plasma TAG and apolipoprotein B-100 levels upon fasting [129]. Conversely, ablation of Ces3 in mice resulted in decreased plasma TAG and apolipoprotein B-100 and FFA levels in both fasted and fed state [130]. Authors argued that the decreased plasma FA levels in Ces3 knockout mice may be a result of decreased lipolysis in the adipose tissue, since Ces3 is also expressed in adipose tissue and participates in adipose tissue basal lipolysis [130–132]. However, it was unexpected that despite decreased VLDL secretion of Ces3 knockout mice, their livers were not steatotic but hepatic TAG content was even reduced [130]. Furthermore, Ces3 knockout mice exhibited increased respiratory quotient and increased glucose tolerance and insulin sensitivity, indicative for elevated glucose utilization because of decreased FA availability [130].
To address the specific role of Ces3 in hepatic TAG and CE homeostasis, two liver specific Ces3 knockout mouse models, one on wild-type background and the other on low-density lipoprotein receptor (Ldlr) knockout background, respectively, were generated [56,58]. Similarly, as observed in the global Ces3 knockout mouse model, also the liver specific Ces3 knockout mouse model on wild-type background exhibited decreased VLDL secretion [58]. Interestingly, in this same study it was found that male (but not female) mice exhibit increased hepatic TAG and CE contents, suggesting that TAG and CE may be the physiological substrates of Ces3. This increase in hepatic TAG and CE contents was accompanied by increased number of smaller cytosolic LDs [58], indicating that Ces3-deficiency counteracts FA supply for VLDL-TAG synthesis and thereby promotes the formation of nascent LDs in the cytosol. In contrast to global Ces3 knockout mice, liver specific Ces3 knockout mice showed unchanged plasma FA levels upon feeding or fasting as well as unaltered glucose tolerance and in females deteriorated insulin sensitivity [58]. Since decreased plasma FA levels as well as improved glucose tolerance and insulin sensitivity were solely observed in global Ces3 knockout mice [130], it is conceivable that reduced adipose TAG catabolism and consequently lower FA release provokes increased glucose utilization and not impaired VLDL secretion. The second mouse model, Ces3-deficiency on a Ldlr knockout background, showed unaltered hepatic TAG as well as CE contents, increased atherosclerotic lesions, and decreased fecal sterol content, indicating that hepatic Ces3 activity is required for efficient sterol elimination [56].
The beneficial impact of global Ces3 deficiency on hepatic TAG homeostasis was further tested on the Phosphatidylethanolamine N-methyltransferase (Pemt) knockout and the Ldlr knockout background, which are established mouse models resembling NASH and NAFLD. Notably, Ces3/Pemt as well as Ces3/Ldlr deficient mice on a HFD feeding exhibited decreased liver inflammation, oxidative stress and fibrosis as well as decreased NASH, respectively, as compared to littermates [133]. These ameliorations of NAFLD and NASH development and progression cannot simply be attributed to decreased VLDL secretion and decreased plasma FFA levels but obviously involve a complex network of metabolic consequences, which are entailed by Ces3-deficiency. Together, the improvement of NAFLD and NASH in Ces3 knockout mice renders the human orthologue CES1 as a therapeutic target for the treatment of hypertriglyceridemia, insulin resistance, and of various stages of fatty liver disease.
Targeting Ces1/Es-x
Another member of the large carboxylesterase family is murine Ces1/Es-x (recently annotated as Ces1g) [134]. The human homologue of murine Ces1/Es-x is thought to be CES1, consisting of three variants (CES1A1 to CES1A3). Murine Ces1 shares around 76% protein sequence identity with murine Ces3/TGH and also contains an N-terminal hydrophobic signal peptide, which directs the protein into the microsomal lumen and a C-terminal HVEL consensus sequence for retaining the protein in the lumen of the ER [135]. Ces1/Es-x is mainly expressed in liver and to a much lower degree in kidney and lung [136]. Purified Ces1/Es-x was demonstrated to hydrolyzes the artificial ester substrate 4-methyl umbelliferyl heptanoate (=hydrophobic short chain monoester) [51]. Adenoviral overexpression of Ces1/Es-x led to increased TAG hydrolase activity in cell and liver lysates [137]. Further clues on the physiological role of Ces1/Es-x in liver derived from expression experiments in rat McArdle-RH7777 hepatocytes [51]. That said, Ces1/Es-x stably expressing cells contained less TAG but produced increased amounts of acid soluble metabolites, indicating a repartitioning of FAs from TAG storage to beta-oxidation [51].
Further insights into the physiological role of Ces1/Es-x in liver lipid metabolism were obtained from the Ces1/Es-x knockout mouse model [138]. Ces1/Es-x knockout mice exhibited elevated plasma lipid levels (including TAG, cholesterol, CE, phospholipids, and FAs), which was most likely due to increased VLDL secretion [138]. Hepatic TAG deposition (3-fold increased) led to steatosis, which was accompanied by obesity. In liver of Ces1/Es-x knockout mice, the expression of the transcription factors sterol-regulatory element binding protein-1c and 2 was upregulated, which is known to induce lipogenesis and hepatic TAG deposition [139,140]. Authors speculated that polyunsaturated FA species such as FA22:6 of the TAG pool might be the preferred substrate of Ces1/Es-x and thus these polyunsaturated FAs accumulate in TAGs of Ces1/Es-x deficient mice. This defect in the release of certain polyunsaturated FAs might be directly linked to increased sterol-regulatory element binding protein expression and lipogenesis, since polyunsaturated FAs have been shown to suppress sterol-regulatory element binding protein activity and to enhance its proteosomal degradation [141,142]. Because of the obese phenotype of Ces1/Es-x knockout mice, it was not surprising that these mice were hyper-insulinemic and accordingly less insulin sensitive. Interestingly, the plasma and liver phenotype (lipid accumulation and defective insulin signaling) of Ces1/Es-x deficient mice could be reversed by transgenic expression of Ces1/Es-x exclusively in the liver, pinpointing that Ces1/Es-x is an important lipid-hydrolyzing enzyme in the liver, thereby affecting hepatic lipid and energy metabolism.
From the transgenic and knockout mouse models it is obvious that Ces1/Es-x exerts different functions in hepatic lipid catabolism as compared to Ces3/TGH, albeit both proteins share high sequence homology and both reside in the lumen of the ER and apparently hydrolyze TAG. Similar to CES3, also Ces1/Es-x is an important hepatic enzyme playing a dual role in hepatic lipid homeostasis by interfering with both, VLDL formation and lipogenesis, by mechanisms involving polyunsaturated FA derived signaling lipids and sterol-regulatory element binding protein activation. Furthermore, it has been shown that Ces1/Es-x is a target gene of the farnesoid X receptor [137]. Accordingly, oxysterols induce the expression of CES1/Es-x, which in turn releases specific polyunsaturated FAs from TAG stores and/or cholesterol from CEs. Polyunsaturated FAs and free cholesterol are known to activate PPAR-alpha and beta-oxidation and to inhibit hepatic sterol-regulatory element binding protein processing and function, respectively [137,143]. However, to translate the important role of Ces1/Es-x to human liver lipid homeostasis, a mouse model examining human CES1 variants would be required. Since the murine Ces1 family comprises of eight members, while the human CES1 family comprises of three members [134], it is unclear if the physiological role of Ces1/Es-x is conserved in humans.
Targeting LAL
Neutral lipid esters of two different sources may undergo acid hydrolysis: Neutral lipid esters, which derive from the endocytic pathway via the endocytosis of neutral lipid ester-rich lipoprotein particles (e.g. chylomicron remnants), or neutral lipid esters, which derive from cytosolic LDs and are engulfed from autophagosomes and fuse with lysosomes, undergo lysosomal degradation. The latter process is termed lipophagy (for recent reviews see [144–146]). Either way, the importance of acid hydrolysis of neutral lipid esters in lysosomes is evident from mutations in the LAL gene. Mutations with functional disruption of LAL activity are causative for autosomal recessive lysosomal storage disorders. Patients presenting complete disruption of LAL activity develop the more severe form of lysosomal storage disease, known as Wolman disease (OMIM #278000), and usually die within the first year [147]. Patients with mutations in LAL, which retain residual LAL activity (1-12%, [148]), develop cholesteryl ester storage disease (OMIM #278000) and life until adulthood [149]. General symptoms include CE and TAG accumulation in many tissues encompassing severe hepatic CE and TAG accumulation together with a yellowish and greasy appearance of the liver, hepatosplenomegaly, dyslipidemia/hyperlipidemia, and infiltration of lipid-filled Kupffer cells [147,150]. Fasting plasma dyslipidemia is characterized by increased TAG and LDL-cholesterol content, and accompanied by increased apolipoprotein-B levels, while HDL-cholesterol content is decreased, indicative for increased VLDL secretion [59,151]. In Wolman disease, secondary abnormalities due to chronic diarrhea and malabsorption may lead to a decline of fat in subcutaneous tissue [152,153].
Similar to affected humans, also Lal knockout mice exhibit massive CE and TAG accumulation in the liver and many other tissues, hepatosplenomegaly, proliferation of hepatocytes and Kupffer cells, and reduced survival [57,154,155]. Massive accumulation of CE in the liver leads to the formation of cholesterol crystals [57], which may disturb cellular integrity and induce an inflammatory response. Similar to patients, Lal knockout mice show increased plasma free FAs and LDL cholesterol levels, while HDL cholesterol level are decreased and total cholesterol and TAG levels are normal [57]. More recently, LAL has been shown not only to degrade CE and TAG in lysosomes but also REs [53]. However, LAL-deficient mice exhibit reduced and not increased hepatic RE content. This is likely a consequence of defective intestinal absorption/decreased nutritional availability of vitamin A, since LAL-deficient mice exhibit lower postprandial circulating RE levels. Furthermore, prolonged, 12 hour-fasting of LAL-deficient mice led to reduced VLDL secretion and lowered plasma TAG levels [156]. Reduced VLDL secretion after such prolonged fasting may be a result of diminished FA supply via the circulation because LAL-deficient mice exhibit a lipodystrophy-like phenotype, largely devoid of adipose tissue depots [57]. On the other hand, reduced VLDL secretion upon fasting may also indicate defective lipophagy in these animals, since in the fasted state, liver lipophagy is thought to significantly contribute to FA supply as building blocks and as energy substrate [54]. This view is supported by observations that inhibition of lysosomal activity upon chloroquine treatment lowers VLDL secretion of hepatocytes and murine liver [157,158] and that Atg7 knockout mice develop hepatosteatosis [54]. Thus, the obvious defect in CE and TAG mobilization in liver and other tissues of LAL-deficient mice may suggest defective autophagy as cause for hepatic steatosis. However, it is difficult to dissociate between the contribution of i) lipoprotein-particle associated lipid uptake, ii) lipophagy of cytosolic LDs, and iii) impaired whole-body lipid supply as a consequence of progressive loss of adipose tissue in these mice [57,156]. Additionally, more recent studies have indicated that the lipophagy and lipopolytic pathway are apparently linked together via a process termed chaperon-mediated autophagy [159]: LD-associated proteins, such as perilipins or ATGL, carry a canonical KFERQ motif, which allows the direct interaction with the protein 1A/1B-light chain 3 (LC3), which initiates the formation of autolysosomes and finally lipophagy [160,161]. Thus, in view of such interrelation between lipophagy and lipolysis, both processes obviously go hand-in-hand and, thus, their relative contributions is hard to assess.
Overall, global deletion of LAL in mice results in a phenotype that mirrors more closely cholesteryl ester storage disease in humans. To date it is unclear, why in mice the phenotype of LAL-deficiency is more moderate. Possible explanations could be that additional lysosomal activities for neutral lipid ester hydrolysis in mice exist, which can partially compensate for the loss of LAL activity or differences in lipoprotein metabolism when considering the different lipoprotein profile of mice compared to humans with regard to LDL cholesterol levels. Furthermore, the clearance of TAG and RE of endocytosed particles may occur rather in the endosome at neutral pH than in the lysosome [162]. That’s why the relative accumulation of CE vs TAG and RE is much higher (40-fold vs 2-fold accumulation of CEs and TAGs, respectively), while the amount of REs were actually decreased [53]). These differences in the degree of hepatic CE vs TAG accumulation is also evident from patients suffering Wolman and cholesteryl ester storage disease, who show around 50-100-fold increase in CE, while that of TAG is merely 7 to 10-fold increased [59]. Apart from this discrepancy of very different magnitude of accumulation of different neutral lipid ester classes and the overall contribution of lipophagy to whole liver neutral lipid mobilization, it is also unclear, why large amounts of cholesterol (crystals) actually accumulate in hepatocytes and Kupffer cells and both cell types massively proliferate [155]. From textbook knowledge, one would assume rather a major contribution of hepatocytes to the uptake of chylomicron remnants, the mobilization of neutral lipids of cytosolic LDs, and the secretion of neutral lipids via VLDL. One would expect a defect in lysosomal activity would lead to very pronounced accumulation in hepatocytes rather than in Kupffer cells. Since Kupffer cells are obviously largely affected, it suggests that at least under such pathological conditions, Kupffer cells significantly participate in the uptake and turnover of exogenous neutral lipids. Furthermore, it has been suggested that upon HSC activation, LAL activity (via lipophagy) drives the loss of cytosolic LD-associated neutral lipid stores [65,163], a hallmark associated with the development of liver fibrosis. Moreover, inhibition of LAL activity has been shown to attenuate HSC activation in cell culture [64]. Yet, the phenotypes of mice and humans, deficient in LAL activity argue against this view, since LAL-deficient mice exhibit reduced hepatic RE stores [53] and more than 50% of cholesteryl ester storage disease patients have documented evidence of fibrosis [164]. Thus, it suggests that LAL activity is not essential for hepatic RE mobilization and HSC activation.
Table 1 summarizes the different approaches and studies to investigate the physiological impact of modulating hepatic neutral lipid ester mobilization.
Table 1. Summary of genetic mouse models to study hepatic lipid catabolism*.
| Genetic manipulation | Function of gene | Changes in serum | Changes in liver | Global effect/conclusion | Ref. |
|---|---|---|---|---|---|
| global ATGL ko | TAG, RE hydrolase |
↓ fasting FFA ↓ fasting TAG ↓ fed insulin = ROH |
↑ TAG = RE ↑ p-HSL |
ATGL rate-limiting TAG hydrolase; ATGL ko improves GTT and ITT; ATGL not rate-limiting for RE hydrolysis | Haemmerle et al. Science 2006 [42] Taschler et al. BBA 2015 [62] |
| global ATGL ko challenged with tunica-mycin | TAG hydrolase | ↓ ApoB-100 | ↑ TAG, ↓ iNos ↓ TNFα ↓ Chop, ↓ ErdJ4 |
ATGL-ko protects against hepatic ER stress and inflammation |
Fuchs et al. Hepatology 2012 [72] |
| global ATGL ko challenged with methionine-choline-deficient diet | TAG hydrolase | ↑ TNFalpa ↑ IL6 ↓ FGF21 |
↑ TAG, ↑ TNFα ↑ iNos, ↑ IL6 ↑ MCP-1 |
ATGL-ko increases susceptibility to MCD- and LPS-induced NASH | Jha et al. 2014 [74] |
| liver specific ATGL ko | TAG hydrolase | = lipids ↑ ALT ↑ ALT/AST |
↑TAG | ATGL rate-limiting TAG hydrolase in liver, ATGL-ko in liver induces progressive hepatic steatosis | Wu et al. Hepatology 2011 [70] |
| adipose specific CGI-58 ko | ATGL co-activator | ↓ fasting FFA ↓ fasting glycerol ↓ fasting insulin ↓ FGF21 |
↓ fasting TAG ↓ PPARα signaling ↑ CREBH |
hepatic FGF21 and PPARα-regulated gene expression depends on exogenous FA supply | Jaeger et al. J Hepatol 2015 [76] |
| global CGI-58 ko | ATGL co-activator | ↑ TAG | severe skin defect: specific role of CGI-58 in skin, independent of ATGL | Radner et al. JBC 2010 [84] | |
| ASO mediated CGI-58 knockdown in WAT and liver on HFD | ATGL co-activator | ↑ ALT, ↑ AST ↓ fed TAG, ↓ NEFA ↓ VLDL secretion ↓ keton b. ↓ glucose |
↑ TAG, ↑ DAG ↓ NEFA, ↑ PG ↑ ceramide |
CGI-58 ko prevents HFD-induced obesity, hepatic insulin resistance, more prone to hepatic inflammation, via mechanism distinct of ATGL | Brown et al. JLR 2010 [85] Cantley et al. PNAS 2013 [86] Lord et al. Diabetes 2012 [89] Lord et al Cell Rep. 2016 [90] |
| liver-specific Cgi-58 ko | ATGL co-activator | = lipids ↓ keton b. |
↑ TAG, ↓ PL ↑ CE, ↑ Chol hepatomegaly |
CGI-58 ko induces steatohepatitis and fibrosis | Guo et al. JLR 2013 [88] |
| liver-specific Plin5 ko | LD shielding protein | ↑ ALT ↑ AST |
↓ TAG, ↑ NEFA ↑ MDA, ↑ 4HNE |
Plin5 ko induces hepatic lipotoxicity by increased lipolysis | Wang et al. Hepatology 2014 [95] |
| adenoviral overexpression of Plin5 in liver | LD shielding protein | = lipids = keton b. |
↑ TAG, ↑ DAG ↑ CE, liver injury |
Plin5 overexpression induces hepatosteatosis but not insulin resistance | Trevino et al. Mol Endocrinol 2015 [96] |
| global or liver specific G0/S2 ko | ATGL inhibitor | ↑ NEFA, ↓ ALT ↓ AST ↑ keton b. |
↓ TAG ↑ glycogen |
G0/S2 ko induces resistance to HFD-induced hepatic steatosis, improves insulin sensitivity | Zhang et al. Diabetes 2014 [99] |
| hepatic G0/S2 overexpression | ATGL inhibitor | ↑ TAG ↓ keton b. ↑ LDL/VLDL Chol |
↑ TAG, ↑ NEFA ↓ glycerol ↑ glucose uptake hepatomegaly |
improved whole body glucose tolerance; increased hepatic glucose uptake | Wang et al. PLoS One 2013 [100] |
| global HSL ko | DAG, TAG, MAG, CE, RE hydrolase | ↓ fasted TAG ↓ fasted NEFA ↓ fasted insulin ↓ adiponectin |
↓ TAG, ↑ CE ↓ CE hydrolase activity |
HSL ko increases hepatic insulin sensitivity | Voshol et al. Endocrinology 2003 [102] Sekiya et al. JLR 2008 [55] |
| (global, liver-) or adipose-specific Hsl ko | DAG, TAG, MAG, CE, RE hydrolase | ↓ FFA, ↓ TAG ↓ adiponectin ↓ leptin ↓ VLDL secretion |
↑ weight ↑ TAG ↓ FAO steatosis |
adipose-specific HSL ko induces lipodystrophy and fatty liver | Xia et al. PLoS Genet 2017 [104] |
| liver specific HSL ko on chow and HFD |
DAG, TAG, MAG, CE, RE hydrolase | ↑ Chol ↑ HDL-Chol ↑ VLDL-Chol ↑ VLDL-TAG ↑ IDL/LDL-TAG |
on HFD: ↑ CE ↑ ABCA1 ↓ LDL-receptor |
HSL is critical for cholesterol homeostasis in liver | Fernandez et al. Am J Physiol Endocrinol Metab 2018 [105] |
| global MGL ko | MAG hydrolase | ↓ glycerol ↓ fasting TAG ↓ VLDL secretion |
↑ MAG ↑ 2-AG ↓ TAG |
MGL ko improves insulin sensitivity and glucose tolerance | Taschler et al. JBC 2011 [44] |
| liver specific PNPLA3I148M expression | TAG hydrolase LPAAT | = TAG = CE = VLDL secretion |
↑TAG ↑ CE |
PNPLA3I148M expression in liver recapitulates hepatic steatosis in humans | Li et al. JCI 2012 [113] |
| PNPLA3I148M knock-in on high sucrose vs S47A PNPLA3 | TAG hydrolase LPAAT | = ALT = AST |
↑TAG | inactive S47A variant of PNPLA3 similar as PNPLA3I148M induces NAFLD | Smagris et al. Hepatology 2015 [114] |
| global PNPLA3 ko | TAG hydrolase LPAAT | ↓ PA activity ↓ PA/LPA ratio |
PNPLA3 function as LPAAT and PNPLA3I148M is a gain of function mutation | Kumari et al. Cell Metab 2012 [111] | |
| inducible transgenic expression of Ces3 | TAG hydrolase | ↑TAG, ↑ Chol ↑ apo B-100 |
Ces3 induces VLDL secretion, proatherogenic lipid profile | Wei et al. JLR 2007 [129] | |
| global Ces3 ko | TAG hydrolase | ↓ Chol, ↓ CE ↓ TAG, ↓ FFA ↓ apo B-100 |
↓ TAG | improved glucose tolerance and insulin sensitivity because of decreased WAT lipolysis | Wei et al. Cell Metab 2010 [130] |
| liver-specific Ces3 ko | TAG hydrolase | ↓ Chol ↓ CE ↓ PL ↓ TAG ↓ lipoproteins |
↑ TAG ↑ CE in males ↑ number of smaller LDs ↓ p-HSL |
Ces3 ko decreases plasma lipids and induces hepatic steatosis | Lian et al. Hepatology 2012 [58] |
| global Ces3/Pemt and Ces3/Ldlr ko vs Pemt ko on HFD/WTD | TAG hydrolase | ↓ VLDL secretion ↓ ALT ↓ inflammatory cytokines |
↓ TAG ↓ Chol ↓ CE |
Ces3 ko attenuates inflammation, fibrosis, NAFLD, NASH in Pemt ko mice | Lian et al. Sci Rep 2016 [133] |
| global Ces1/Es-x ko | PUFA specific TAG hydrolase | ↑ TAG, ↑ Chol ↑ CE, ↑ PL ↑ FA, ↑ AST ↑ ALT ↑ VLDL secretion |
↑ TAG ↑ Chol ↑ CE ↑ lipogenesis steatosis |
Ces1/Es-x ko induces hyperlipidemia, obesity, hyperinsulinemia | Quiroga et al. Hepatology 2012 [138] |
| global Lal ko | acid CE, TAG hydrolase | ↑ FA = TAG = total Chol ↑ ALT ↓ glucose |
↑ CE, ↑ TAG ↑ Chol, CE crytsals ↑ CE biosynthesis hepato-splenomegaly |
Lal ko induces sever liver CE accumulation, hepatomegaly, lipodystrophy and reduced shortened lifespan | Du et al. JLR 2001 [57] Aqul et al. Liver Physiol 2014 [154] Du et al. Hum Mol Genet 1998 [155] |
| global Lal ko | acid CE, TAG, RE hydrolase | ↑ RBP4, ↑ ROH ↓ postprandial RE |
↓ RE, ↓ ROH = LRAT |
Lal ko induces decreased nutritional availability of vitamin A | Grumet et al. J Biol Chem 2016 [53] |
Symbols : ↑ .. increased; ↓ .. decreased; = .. unchanged
Conclusions
The emerging picture of the genetic mouse models targeting hepatic lipid mobilization reveals that lipases of different organelles are embedded in a specific metabolic network and if absent it strongly interferes with hepatic lipid homeostasis and lipid-related liver pathologies. Apparently, the sites of uptake, synthesis and storage of neutral lipid esters are interconnected, but if defective, the metabolic derangement can only be partially compensated by shifting the lipid overload (by mean of e.g. FAs) to other sites for processing.
Genetic mouse models lacking or overexpressing ATGL or ATGL co-regulators in the adipose tissue have clearly demonstrated the high impact of adipose lipolysis and adipose tissue derived FAs on hepatic FA homeostasis. Thus, future studies will clarify whether the pharmaceutical inhibition of adipose tissue lipolysis constitutes a strategy for the treatment of fatty liver diseases.
Lipases of cytosolic LDs apparently do not directly affect TAG incorporation into VLDL, but deliver FAs for energy production and as ligands for the activation of nuclear receptors (such as PPARs). Particularly interesting, even severe hepatic steatosis as evident in mice with hepatic ATGL-deficiency does not detrimentally affect liver function and is compatible with normal hepatic insulin sensitivity. In strong contrast, hepatic steatosis caused by hepatic deficiency of the ATGL co-activator CGI-58 provokes NASH, implicating that additional changes in hepatic lipid catabolism may involving lipid signaling pathways that act as a trigger in the progression from benign NAFLD to NASH. To identify and characterize the molecular pathway behind these divergences will definitely increase our knowledge in understanding the mechanism(s) in the transition from benign steatosis to hepatic inflammation, ER stress and fibrosis as an instigator of severe liver diseases. Conversely, the disruption or overexpression of lipid hydrolases at the ER profoundly affect VLDL lipid synthesis. The lack of ER-related hydrolases may thereby promote FA channeling for storage within cytosolic LDs and for energy production by mitochondrial FAO. Interestingly, the substrates for the lipid hydrolases at the ER, i.e. Ces3/TGH and Ces1/Es-x, may not be long-chain TAGs and the elucidation of the nature of the preferred lipid substrates will be of particular interest. In any case, the Ces3/TGH and Ces1/Es-x mouse models clearly demonstrate the important role of ER residing esterases/lipases in determining VLDL secretion rate. Thus, inhibition of ER residing proteins will interfere with hepatic TAG homeostasis and of therapeutic use for ameliorating hyperlipidemia.
The molecular mechanisms how PNPLA3 determines hepatic TAG homeostasis and more detrimental NAFLD in humans (and mice) either via acting on lipid synthesis or lipid hydrolysis in the liver (and likely in adipose tissue) is still incompletely understood and a challenge for future studies. The lysosomal clearance of lipoprotein associated neutral lipid esters is, no doubts, the sole gateway for certain neutral lipid esters classes. CE breakdown apparently and largely depends on lysosomal activity, while this pathway is much less important for the catabolism of TAG and REs. Whether these neutral lipid esters are preferentially cleared already in the endosome or alternative lipases play a role in addition to LAL awaits further investigation. Moreover, and at least in murine liver, even after prolonged fasting, acid hydrolysis of cytosolic LDs via lipophagy does not significantly contribute to FA supply and in particular to VLDL synthesis and secretion, asking whether lipophagy participates in VLDL production in humans and in the context of NAFLD development.
From the phenotype of the described mouse models, it is evident that the rate-limiting enzyme in liver RE mobilization is unknown or, alternatively, the mobilization of REs might be a highly redundant system and may involve several enzymes that can compensate the lack of each other. Similarly, the principal enzyme in neutral CE breakdown in the liver awaits to be identified or exerts similar redundancy as indicated for RE mobilization.
The specific role of parenchymal and non-parenchymal cells in neutral lipid metabolism is incompletely understood. Apparently, at least under pathological conditions, non-parenchymal cells including Kupffer cells and HSCs may play a very prominent role in liver disease progression and resolution.
Acknowledgements
We thank Mag. Dr. Birgit Stueckler for the careful reading of the manuscript and the valuable comments and suggestions.
Funding: This work was supported by the grants I 3535 (A. L.), P 29253 (G.H.), and the DK Metabolic and Cardiovascular Disease W 1226 (G.H.), which are all funded by the Austrian Science Fund (FWF).
Abbreviations
- ATGL
adipose triglyceride lipase
- CE
cholesteryl ester
- CES
carboxylesterase
- CGI-58
comparative gene identification-58
- DAG
diacylglycerol
- ER
endoplasmic reticulum
- ES
esterase
- FA
fatty acid
- FFA
free fatty acid
- FAO
fatty acid oxidation
- G0S2
G0/G1 switch gene 2
- HDL
high-density lipoprotein
- HFD
high fat diet
- HSC
hepatic stellate cell
- HSL
hormone-sensitive lipase
- LAL
lysosomal acid lipase
- LD
lipid droplet
- LDL
low-density lipoprotein
- LDLR
low-density lipoprotein receptor
- LRAT
lecithin:retinol acyltransferase
- MAG
monoacylglycerol
- MCD
methionine-choline-deficient
- MGL
monoglyceride lipase
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steato hepatitis
- PEMT
phosphatidylethanolamine N-methyltransferase
- PLIN
perilipin
- PNPLA
patatin-like phospholipase domain containing
- PPAR
peroxisome proliferator-activated receptor
- RE
retinyl ester
- ROH
retinol
- TAG
triacylglycerol
- TGH
triglyceride hydrolase
- VLDL
very low-density lipoprotein
References
- [1].Blouin A, Bolender RP, Weibel ER. Distribution of organelles and membranes between hepatocytes and nonhepatocytes in the rat liver parenchyma. A stereological study. J Cell Biol. 1977;72 doi: 10.1083/jcb.72.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kmieć Z. Cooperation of liver cells in health and disease. Springer Berlin Heidelberg; Berlin, Heidelberg: 2001. [DOI] [PubMed] [Google Scholar]
- [3].Dijk MCM, Ziere GJ, Berkel TJC. Characterization of the chylomicron-remnant-recognition sites on parenchymal and Kupffer cells of rat liver Selective inhibition of parenchymal cell recognition by lactoferrin. Eur J Biochem. 1992;205:775–784. doi: 10.1111/j.1432-1033.1992.tb16842.x. [DOI] [PubMed] [Google Scholar]
- [4].Sahini N, Borlak J. Recent insights into the molecular pathophysiology of lipid droplet formation in hepatocytes. Prog Lipid Res. 2014;54:86–112. doi: 10.1016/J.PLIPRES.2014.02.002. [DOI] [PubMed] [Google Scholar]
- [5].Lippiello PM, Sisson PJ, Waite M. The uptake and metabolism of chylomicron-remnant lipids by rat liver parenchymal and non-parenchymal cells in vitro. [accessed February 12, 2018];Biochem J. 1985 232:395. doi: 10.1042/bj2320395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Groot PHE, Van Berkel TJC, Van Tol A. Relative contributions of parenchymal and non-parenchymal (sinusoidal) liver cells in the uptake of chylomicron remnants. Metabolism. 1981;30:792–797. doi: 10.1016/0026-0495(81)90025-1. [DOI] [PubMed] [Google Scholar]
- [7].Dixon LJ, Barnes M, Tang H, Pritchard MT, Nagy LE. Compr Physiol. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2013. Kupffer Cells in the Liver; pp. 785–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hendriks HFJ, Brekelmans PJAM, Buytenhek R, Brouwer A, de Leeuw A Margreet, Knook DL. Liver parenchymal cells differ from the fat-storing cells in their lipid composition. Lipids. 1987;22:266–273. doi: 10.1007/BF02533990. [DOI] [PubMed] [Google Scholar]
- [9].Blaner WS, Li Y, Brun P-J, Yuen JJ, Lee S-A, Clugston RD. Vitamin A Absorption, Storage and Mobilization. Subcell Biochem. 2016;81:95–125. doi: 10.1007/978-94-024-0945-1_4. [DOI] [PubMed] [Google Scholar]
- [10].Giampieri MP, Jezequel AM, Orlandi F. The lipocytes in normal human liver. A quantitative study. Digestion. 1981;22:165–169. doi: 10.1159/000198640. [DOI] [PubMed] [Google Scholar]
- [11].Eichmann TO, Grumet L, Taschler U, Hartler J, Heier C, Woblistin A, Pajed L, Kollroser M, Rechberger G, Thallinger GGGG, Zechner R, et al. ATGL and CGI-58 are lipid droplet proteins of the hepatic stellate cell line HSC-T6. J Lipid Res. 2015;56:1972–1984. doi: 10.1194/jlr.M062372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yamada M, Blaner WS, Soprano DR, Dixon JL, Kjeldbye HM, Goodman DS. Biochemical characteristics of isolated rat liver stellate cells. Hepatology. 1987;7:1224–9. doi: 10.1002/hep.1840070609. [DOI] [PubMed] [Google Scholar]
- [13].Moriwaki H, Blaner WS, Piantedosi R, Goodman DS. Effects of dietary retinoid and triglyceride on the lipid composition of rat liver stellate cells and stellate cell lipid droplets. [accessed July 24, 2014];J Lipid Res. 1988 29:1523–34. [PubMed] [Google Scholar]
- [14].Senoo H, Kojima N, Sato M. Vitamin A-storing cells (stellate cells) Vitam Horm. 2007;75:131–59. doi: 10.1016/S0083-6729(06)75006-3. [DOI] [PubMed] [Google Scholar]
- [15].Green MH, Green JB, Berg T, Norum KR, Blomhoff R. Changes in hepatic parenchymal and nonparenchymal cell vitamin A content during vitamin A depletion in the rat. J Nutr. 1988;118:1331–5. doi: 10.1093/jn/118.11.1331. [DOI] [PubMed] [Google Scholar]
- [16].Tanumihardjo SA. Vitamin A: biomarkers of nutrition for development. Am J Clin Nutr. 2011;94:658S–65S. doi: 10.3945/ajcn.110.005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kluwe J, Wongsiriroj N, Troeger JS, Gwak GY, Dapito DH, Pradere JP, Jiang H, Siddiqi M, Piantedosi R, O’Byrne SM, Blaner WS, et al. Absence of hepatic stellate cell retinoid lipid droplets does not enhance hepatic fibrosis but decreases hepatic carcinogenesis. Gut. 2011;60:1260–1268. doi: 10.1136/gut.2010.209551. [DOI] [PubMed] [Google Scholar]
- [18].Liu L, Gudas LJ. Disruption of the lecithin:retinol acyltransferase gene makes mice more susceptible to vitamin A deficiency. J Biol Chem. 2005;280:40226–34. doi: 10.1074/jbc.M509643200. [DOI] [PubMed] [Google Scholar]
- [19].Ghyselinck NB, Vernet N, Dennefeld C, Giese N, Nau H, Chambon P, Viville S, Mark M. Retinoids and spermatogenesis: Lessons from mutant mice lacking the plasma retinol binding protein. Dev Dyn. 2006;235:1608–1622. doi: 10.1002/dvdy.20795. [DOI] [PubMed] [Google Scholar]
- [20].Quadro L, Blaner WS, Salchow DJ, Vogel S, Piantedosi R, Gouras P, Freeman S, Cosma MP, Colantuoni V, Gottesman ME. Impaired retinal function and vitamin A availability in mice lacking retinol-binding protein. EMBO J. 1999;18:4633–44. doi: 10.1093/emboj/18.17.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Vogel S, Piantedosi R, O’Byrne SM, Kako Y, Quadro L, Gottesman ME, Goldberg IJ, Blaner WS. Retinol-binding protein-deficient mice: Biochemical basis for impaired vision. Biochemistry. 2002;41:15360–15368. doi: 10.1021/bi0268551. [DOI] [PubMed] [Google Scholar]
- [22].Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–69. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tacke F, Weiskirchen R. Update on hepatic stellate cells: pathogenic role in liver fibrosis and novel isolation techniques. Expert Rev Gastroenterol Hepatol. 2012;6:67–80. doi: 10.1586/egh.11.92. [DOI] [PubMed] [Google Scholar]
- [24].Malins DC, Mangold HK. Analysis of complex lipid mixtures by thin-layer chromatography and complementary methods. J Am Oil Chem Soc. 1960;37:576–578. doi: 10.1007/BF02631604. [DOI] [Google Scholar]
- [25].Stahl E. Thin-Layer Chromatography - A Laboratory Handbook. Springer Int. Student Ed.; 1969. p. 1023. [DOI] [Google Scholar]
- [26].Skipski VP, Smolowe AF, Sullivan RC, Barclay M. Separation of lipid classes by thin-layer chromatography. Biochim Biophys Acta - Lipids Lipid Metab. 1965;106:386–396. doi: 10.1016/0005-2760(65)90047-0. [DOI] [PubMed] [Google Scholar]
- [27].Olkkonen VM, Li S. Oxysterol-binding proteins: Sterol and phosphoinositide sensors coordinating transport, signaling and metabolism. Prog Lipid Res. 2013;52:529–538. doi: 10.1016/j.plipres.2013.06.004. [DOI] [PubMed] [Google Scholar]
- [28].Thumser AE, Moore JB, Plant NJ. Fatty acid binding proteins. Curr Opin Clin Nutr Metab Care. 2014;17:124–129. doi: 10.1097/MCO.0000000000000031. [DOI] [PubMed] [Google Scholar]
- [29].Wang H, Quiroga AD, Lehner R. Analysis of lipid droplets in hepatocytes. Methods Cell Biol. 2013;116:107–127. doi: 10.1016/B978-0-12-408051-5.00007-3. [DOI] [PubMed] [Google Scholar]
- [30].Farese RV, Walther TC. Lipid Droplets Finally Get a Little R-E-S-P-E-C-T. Cell. 2009;139:855–860. doi: 10.1016/j.cell.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yao Z, Zhou H, Figeys D, Wang Y, Sundaram M. Microsome-associated lumenal lipid droplets in the regulation of lipoprotein secretion. Curr Opin Lipidol. 2013;24:160–70. doi: 10.1097/MOL.0b013e32835aebe7. [DOI] [PubMed] [Google Scholar]
- [32].Bickel PE, Tansey JT, Welte MA. PAT proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores. Biochim Biophys Acta. 2009;1791:419–40. doi: 10.1016/j.bbalip.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Harris CA, Haas JT, Streeper RS, Stone SJ, Kumari M, Yang K, Han X, Brownell N, Gross RW, Zechner R, Farese RV. DGAT enzymes are required for triacylglycerol synthesis and lipid droplets in adipocytes. J Lipid Res. 2011;52:657–667. doi: 10.1194/jlr.M013003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].McFie PJ, Banman SL, Kary S, Stone SJ. Murine Diacylglycerol Acyltransferase-2 (DGAT2) Can Catalyze Triacylglycerol Synthesis and Promote Lipid Droplet Formation Independent of Its Localization to the Endoplasmic Reticulum. J Biol Chem. 2011;286:28235–28246. doi: 10.1074/jbc.M111.256008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Yen C-LE, Monetti M, Burri BJ, Farese RV. The triacylglycerol synthesis enzyme DGAT1 also catalyzes the synthesis of diacylglycerols, waxes, and retinyl esters. J Lipid Res. 2005;46:1502–11. doi: 10.1194/jlr.M500036-JLR200. [DOI] [PubMed] [Google Scholar]
- [36].Blaner WS, Prystowsky JH, Smith JE, Goodman DWS. Rat liver retinyl palmitate hydrolase activity. Relationship to cholesteryl oleate and triolein hydrolase activities. Biochim Biophys Acta (BBA)/Lipids Lipid Metab. 1984;794:419–427. doi: 10.1016/0005-2760(84)90008-0. [DOI] [PubMed] [Google Scholar]
- [37].Nielsen TS, Jessen N, Jorgensen JOL, Moller N, Lund S. Dissecting adipose tissue lipolysis: molecular regulation and implications for metabolic disease. J Mol Endocrinol. 2014;52:R199–R222. doi: 10.1530/JME-13-0277. [DOI] [PubMed] [Google Scholar]
- [38].Bolsoni-Lopes A, Alonso-Vale MIC. Lipolysis and lipases in white adipose tissue – An update. Arch Endocrinol Metab. 2015;59:335–342. doi: 10.1590/2359-3997000000067. [DOI] [PubMed] [Google Scholar]
- [39].Lass A, Zimmermann R, Oberer M, Zechner R. Lipolysis – A highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog Lipid Res. 2011;50:14–27. doi: 10.1016/j.plipres.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Villena JA, Roy S, Sarkadi-Nagy E, Kim K-H, Sul HS. Desnutrin, an adipocyte gene encoding a novel patatin domain-containing protein, is induced by fasting and glucocorticoids: ectopic expression of desnutrin increases triglyceride hydrolysis. J Biol Chem. 2004;279:47066–75. doi: 10.1074/jbc.M403855200. [DOI] [PubMed] [Google Scholar]
- [41].Jenkins CM, Mancuso DJ, Yan W, Sims HF, Gibson B, Gross RW. Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J Biol Chem. 2004;279:48968–75. doi: 10.1074/jbc.M407841200. [DOI] [PubMed] [Google Scholar]
- [42].Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, Heldmaier G, Maier R, Theussl C, Eder S, Kratky D, et al. Defective Lipolysis and Altered Energy Metabolism in Mice Lacking Adipose Triglyceride Lipase. Science (80-. ) 2006;312:734–737. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- [43].Haemmerle G, Zimmermann R, Hayn M, Theussl C, Waeg G, Wagner E, Sattler W, Magin TM, Zechner R. Hormone-sensitive lipase deficiency in mice causes diglyceride accumulation in adipose tissue, muscle, and testis. J Biol Chem. 2002;277:4806–4815. doi: 10.1074/jbc.M110355200. [DOI] [PubMed] [Google Scholar]
- [44].Taschler U, Radner FPW, Heier C, Schreiber R, Schweiger M, Schoiswohl G, Preiss-Landl K, Jaeger D, Reiter B, Koefeler HC, Wojciechowski J, et al. Monoglyceride lipase deficiency in mice impairs lipolysis and attenuates diet-induced insulin resistance. J Biol Chem. 2011;286:17467–17477. doi: 10.1074/jbc.M110.215434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zimmermann R, Strauss JGJG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A, Zechner R. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science (80-. ) 2004;306:1383–1386. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]
- [46].Fredrikson G, Tornqvist H, Belfrage P. Hormone-sensitive lipase and monoacylglycerol lipase are both required for complete degradation of adipocyte triacylglycerol. Biochim Biophys Acta - Lipids Lipid Metab. 1986;876:288–293. doi: 10.1016/0005-2760(86)90286-9. [DOI] [PubMed] [Google Scholar]
- [47].Fredrikson G, Stralfors P, Nilsson NO, Belfrage P. Hormone-sensitive lipase of rat adipose tissue. Purification and some properties. J Biol Chem. 1981;256:6311–6320. [PubMed] [Google Scholar]
- [48].He S, McPhaul C, Li JZ, Garuti R, Kinch L, Grishin NV, Cohen JC, Hobbs HH. A sequence variation (I148M) in PNPLA3 associated with nonalcoholic fatty liver disease disrupts triglyceride hydrolysis. J Biol Chem. 2010;285:6706–15. doi: 10.1074/jbc.M109.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Chamoun Z, Vacca F, Parton RG, Gruenberg J. PNPLA3/adiponutrin functions in lipid droplet formation. Biol Cell. 2013;105:219–233. doi: 10.1111/boc.201200036. [DOI] [PubMed] [Google Scholar]
- [50].Lehner R, Cui Z, Vance DE. Subcellullar localization, developmental expression and characterization of a liver triacylglycerol hydrolase. Biochem J. 1999;338(Pt 3):761–8. doi: 10.1042/bj3380761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ko KWS, Erickson B, Lehner R. Es-x/Ces1 prevents triacylglycerol accumulation in McArdle-RH7777 hepatocytes. Biochim Biophys Acta - Mol Cell Biol Lipids. 2009;1791:1133–1143. doi: 10.1016/J.BBALIP.2009.07.006. [DOI] [PubMed] [Google Scholar]
- [52].Robbi M, Beaufay H. The COOH terminus of several liver carboxylesterases targets these enzymes to the lumen of the endoplasmic reticulum. [accessed February 13, 2018];J Biol Chem. 1991 266:20498–20503. [PubMed] [Google Scholar]
- [53].Grumet L, Eichmann TO, Taschler U, Zierler KAKA, Leopold C, Moustafa T, Radovic B, Romauch M, Yan C, Du H, Haemmerle G, et al. Lysosomal acid lipase hydrolyzes retinyl ester and affects retinoid turnover. J Biol Chem. 2016;291:17977–17987. doi: 10.1074/jbc.M116.724054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Sekiya M, Osuga J-I, Yahagi N, Okazaki H, Tamura Y, Igarashi M, Takase S, Harada K, Okazaki S, Iizuka Y, Ohashi K, et al. Hormone-sensitive lipase is involved in hepatic cholesteryl ester hydrolysis. J Lipid Res. 2008;49:1829–38. doi: 10.1194/jlr.M800198-JLR200. [DOI] [PubMed] [Google Scholar]
- [56].Bie J, Wang J, Marqueen KE, Osborne R, Kakiyama G, Korzun W, Ghosh SS, Ghosh S. Liver-specific cholesteryl ester hydrolase deficiency attenuates sterol elimination in the feces and increases atherosclerosis in ldlr-/- mice. Arterioscler Thromb Vasc Biol. 2013;33:1795–802. doi: 10.1161/ATVBAHA.113.301634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Du H, Heur M, Duanmu M, Grabowski Ga, Hui DY, Witte DP, Mishra J. Lysosomal acid lipase-deficient mice: depletion of white and brown fat, severe hepatosplenomegaly, and shortened life span. [accessed September 16, 2014];J Lipid Res. 2001 42:489–500. [PubMed] [Google Scholar]
- [58].Lian J, Wei E, Wang SP, Quiroga AD, Li L, Di Pardo A, van der Veen J, Sipione S, Mitchell GA, Lehner R. Liver specific inactivation of carboxylesterase 3/triacylglycerol hydrolase decreases blood lipids without causing severe steatosis in mice. Hepatology. 2012;56:2154–2162. doi: 10.1002/hep.25881. [DOI] [PubMed] [Google Scholar]
- [59].Hoeg JM, Demosky SJ, Pescovitz OH, Brewer HB. Cholesteryl ester storage disease and Wolman disease: phenotypic variants of lysosomal acid cholesteryl ester hydrolase deficiency. [accessed September 22, 2015];Am J Hum Genet. 1984 36:1190–203. [PMC free article] [PubMed] [Google Scholar]
- [60].Tylki-Szymańska A, Jurecka A. Lysosomal acid lipase deficiency: wolman disease and cholesteryl ester storage disease. [accessed July 21, 2016];Pril (Makedonska Akad Na Nauk i Umet Oddelenie Za Med Nauk. 2014 35:99–106. [PubMed] [Google Scholar]
- [61].Todoroki T, Matsumoto K, Watanabe K, Tashiro Y, Shimizu M, Okuyama T, Imai K. Accumulated lipids, aberrant fatty acid composition and defective cholesterol ester hydrolase activity in cholesterol ester storage disease. Ann Clin Biochem. 2000;37:187–193. doi: 10.1258/0004563001899195. [DOI] [PubMed] [Google Scholar]
- [62].Taschler U, Schreiber R, Chitraju C, Grabner GF, Romauch M, Wolinski H, Haemmerle G, Breinbauer R, Zechner R, Lass A, Zimmermann R. Adipose triglyceride lipase is involved in the mobilization of triglyceride and retinoid stores of hepatic stellate cells. Biochim Biophys Acta - Mol Cell Biol Lipids. 2015;1851:937–945. doi: 10.1016/j.bbalip.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Pirazzi C, Valenti L, Motta BM, Pingitore P, Hedfalk K, Mancina RM, Burza MA, Indiveri C, Ferro Y, Montalcini T, Maglio C, et al. PNPLA3 has retinyl-palmitate lipase activity in human hepatic stellate cells. Hum Mol Genet. 2014;23:4077–85. doi: 10.1093/hmg/ddu121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Tuohetahuntila M, Molenaar MR, Spee B, Brouwers JF, Wubbolts R, Houweling M, Yan C, Du H, VanderVen BC, Vaandrager AB, Helms JB. Lysosome-mediated degradation of a distinct pool of lipid droplets during hepatic stellate cell activation. J Biol Chem. 2017;292:12436–12448. doi: 10.1074/jbc.M117.778472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Hernandez-Gea V, Ghiassinejad Z, Rozenfeld R, Gordon R, Fiel MI, Yue Z, Czaja MJ, Friedman SL, Hernndezgea V, Ghiassinejad Z, Rozenfeld R, et al. Autophagy releases lipid that promotes fibrogenesis by activated hepatic stellate cells in mice and in human tissues. Gastroenterology. 2012;142:938–946. doi: 10.1053/j.gastro.2011.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Grumet L, Taschler U, Lass A. Hepatic retinyl ester hydrolases and the mobilization of retinyl ester stores. Nutrients. 2017;9:1–21. doi: 10.3390/nu9010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Schreiber R, Taschler U, Wolinski H, Seper A, Tamegger SNSN, Graf M, Kohlwein SDSD, Haemmerle G, Zimmermann R, Zechner R, Lass A. Esterase 22 and beta-glucuronidase hydrolyze retinoids in mouse liver. J Lipid Res. 2009;50:2514–23. doi: 10.1194/jlr.M000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Turpin SM, Hoy AJ, Brown RD, Rudaz C Garcia, Honeyman J, Matzaris M, Watt MJ. Adipose triacylglycerol lipase is a major regulator of hepatic lipid metabolism but not insulin sensitivity in mice. Diabetologia. 2011;54:146–156. doi: 10.1007/s00125-010-1895-5. [DOI] [PubMed] [Google Scholar]
- [69].Ong KT, Mashek MT, Bu SY, Greenberg AS, Mashek DG. Adipose triglyceride lipase is a major hepatic lipase that regulates triacylglycerol turnover and fatty acid signaling and partitioning. Hepatology. 2011;53:116–26. doi: 10.1002/hep.24006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Wu JW, Wang SP, Alvarez F, Casavant S, Gauthier N, Abed L, Soni KG, Yang G, Mitchell GA. Deficiency of liver adipose triglyceride lipase in mice causes progressive hepatic steatosis. Hepatology. 2011;54:122–32. doi: 10.1002/hep.24338. [DOI] [PubMed] [Google Scholar]
- [71].Schaffer JE. Lipotoxicity: when tissues overeat. Curr Opin Lipidol. 2003;14:281–7. doi: 10.1097/01.mol.0000073508.41685.7f. [DOI] [PubMed] [Google Scholar]
- [72].Fuchs CD, Claudel T, Kumari P, Haemmerle G, Pollheimer MJ, Stojakovic T, Scharnagl H, Halilbasic E, Gumhold J, Silbert D, Koefeler H, et al. Absence of adipose triglyceride lipase protects from hepatic endoplasmic reticulum stress in mice. Hepatology. 2012;56:270–280. doi: 10.1002/hep.25601. [DOI] [PubMed] [Google Scholar]
- [73].Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am J Physiol Endocrinol Metab. 2006;291:E275–81. doi: 10.1152/ajpendo.00644.2005. [DOI] [PubMed] [Google Scholar]
- [74].Jha P, Claudel T, Baghdasaryan A, Mueller M, Halilbasic E, Das SK, Lass A, Zimmermann R, Zechner R, Hoefler G, Trauner M. Role of adipose triglyceride lipase (PNPLA2) in protection from hepatic inflammation in mouse models of steatohepatitis and endotoxemia. Hepatology. 2014;59:858–869. doi: 10.1002/hep.26732. [DOI] [PubMed] [Google Scholar]
- [75].Ip E, Farrell GC, Robertson G, Hall P, Kirsch R, Leclercq I. Central role of PPARα-dependent hepatic lipid turnover in dietary steatohepatitis in mice. Hepatology. 2003;38:123–132. doi: 10.1053/jhep.2003.50307. [DOI] [PubMed] [Google Scholar]
- [76].Jaeger D, Schoiswohl G, Hofer P, Schreiber R, Schweiger M, Eichmann TO, Pollak NM, Poecher N, Grabner GF, Zierler KA, Eder S, et al. Fasting-induced G0/G1 switch gene 2 and FGF21 expression in the liver are under regulation of adipose tissue derived fatty acids. J Hepatol. 2015;63:437–445. doi: 10.1016/j.jhep.2015.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Mayer N, Schweiger M, Romauch M, Grabner GF, Eichmann TO, Fuchs E, Ivkovic J, Heier C, Mrak I, Lass A, Höfler G, et al. Development of small-molecule inhibitors targeting adipose triglyceride lipase. Nat Chem Biol. 2013;9:785–787. doi: 10.1038/nchembio.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Schweiger M, Romauch M, Schreiber R, Grabner GF, Hütter S, Kotzbeck P, Benedikt P, Eichmann TO, Yamada S, Knittelfelder O, Diwoky C, et al. Pharmacological inhibition of adipose triglyceride lipase corrects high-fat diet-induced insulin resistance and hepatosteatosis in mice. Nat Commun. 2017;8 doi: 10.1038/ncomms14859. 14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Lai C-H. Identification of Novel Human Genes Evolutionarily Conserved in Caenorhabditis elegans by Comparative Proteomics. Genome Res. 2000;10:703–713. doi: 10.1101/gr.10.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Lefèvre C, Jobard F, Caux F, Bouadjar B, Karaduman a, Heilig R, Lakhdar H, Wollenberg a, Verret LJ, Weissenbach J, Ozgüc M, et al. Mutations in CGI-58, the gene encoding a new protein of the esterase/lipase/thioesterase subfamily, in Chanarin-Dorfman syndrome. Am J Hum Genet. 2001;69:1002–12. doi: 10.1086/324121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Lass A, Zimmermann R, Haemmerle G, Riederer M, Schoiswohl G, Schweiger M, Kienesberger P, Strauss JG, Gorkiewicz G, Zechner R. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab. 2006;3:309–319. doi: 10.1016/j.cmet.2006.03.005. [DOI] [PubMed] [Google Scholar]
- [82].Schweiger M, Lass A, Zimmermann R, Eichmann TO, Zechner R. Neutral lipid storage disease: genetic disorders caused by mutations in adipose triglyceride lipase/PNPLA2 or CGI-58/ABHD5. Am J Physiol Endocrinol Metab. 2009;297:E289–96. doi: 10.1152/ajpendo.00099.2009. [DOI] [PubMed] [Google Scholar]
- [83].Fischer J, Lefèvre C, Morava E, Mussini J-M, Laforêt P, Negre-Salvayre A, Lathrop M, Salvayre R. The gene encoding adipose triglyceride lipase (PNPLA2) is mutated in neutral lipid storage disease with myopathy. Nat Genet. 2007;39:28–30. doi: 10.1038/ng1951. [DOI] [PubMed] [Google Scholar]
- [84].Radner FPW, Streith IE, Schoiswohl G, Schweiger M, Kumari M, Eichmann TO, Rechberger G, Koefeler HC, Eder S, Schauer S, Theussl HC, et al. Growth retardation, impaired triacylglycerol catabolism, hepatic steatosis, and lethal skin barrier defect in mice lacking comparative gene identification-58 (CGI-58) J Biol Chem. 2010;285:7300–11. doi: 10.1074/jbc.M109.081877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Brown JM, Betters JL, Lord C, Ma Y, Han X, Yang K, Alger HM, Melchior J, Sawyer J, Shah R, Wilson MD, et al. CGI-58 knockdown in mice causes hepatic steatosis but prevents diet-induced obesity and glucose intolerance. J Lipid Res. 2010;51:3306–15. doi: 10.1194/jlr.M010256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Cantley JL, Yoshimura T, Camporez JPG, Zhang D, Jornayvaz FR, Kumashiro N, Guebre-Egziabher F, Jurczak MJ, Kahn M, Guigni BA, Serr J, et al. CGI-58 knockdown sequesters diacylglycerols in lipid droplets/ER-preventing diacylglycerol-mediated hepatic insulin resistance. Proc Natl Acad Sci. 2013;110:1869–1874. doi: 10.1073/pnas.1219456110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Schreiber R, Hofer P, Taschler U, Voshol PJ, Rechberger GN, Kotzbeck P, Jaeger D, Preiss-Landl K, Lord CC, Brown JM, Haemmerle G, et al. Hypophagia and metabolic adaptations in mice with defective ATGL-mediated lipolysis cause resistance to HFD-induced obesity. Proc Natl Acad Sci USA. 2015;112:13850–5. doi: 10.1073/pnas.1516004112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Guo F, Ma Y, Kadegowda AKG, Betters JL, Xie P, Liu G, Liu X, Miao H, Ou J, Su X, Zheng Z, et al. Deficiency of liver Comparative Gene Identification-58 causes steatohepatitis and fibrosis in mice. J Lipid Res. 2013;54:2109–2120. doi: 10.1194/jlr.M035519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Lord CC, Betters JL, Ivanova PT, Milne SB, Myers DS, Madenspacher J, Thomas G, Chung S, Liu M, Davis MA, Lee RG, et al. CGI-58/ABHD5-Derived Signaling Lipids Regulate Systemic Inflammation and Insulin Action. Diabetes. 2012;61:355–363. doi: 10.2337/db11-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Lord CC, Ferguson D, Thomas G, Brown AL, Schugar RC, Burrows A, Gromovsky AD, Betters J, Neumann C, Sacks J, Marshall S, et al. Regulation of Hepatic Triacylglycerol Metabolism by CGI-58 Does Not Require ATGL Co-activation. Cell Rep. 2016;16:939–949. doi: 10.1016/j.celrep.2016.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Postic C, Magnuson MA. DNA excision in liver by an albumin-Cre transgene occurs progressively with age. [accessed February 23, 2018];Genesis. 2000 26:149–50. doi: 10.1002/(sici)1526-968x(200002)26:2<149::aid-gene16>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- [92].Pruniau VPEG, Louagie E, Brouwers B, Declercq J, Creemers JWM. The AlfpCre mouse revisited: evidence for liver steatosis related to growth hormone deficiency. Hepatology. 2013;58:2209–10. doi: 10.1002/hep.26483. [DOI] [PubMed] [Google Scholar]
- [93].Kimmel AR, Sztalryd C. Perilipin 5, a lipid droplet protein adapted to mitochondrial energy utilization. Curr Opin Lipidol. 2014;25:110–7. doi: 10.1097/MOL.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Mason RR, Watt MJ. Unraveling the roles of PLIN5: linking cell biology to physiology. Trends Endocrinol Metab. 2015;26:144–52. doi: 10.1016/j.tem.2015.01.005. [DOI] [PubMed] [Google Scholar]
- [95].Wang C, Zhao Y, Gao X, Li L, Yuan Y, Liu F, Zhang L, Wu J, Hu P, Zhang X, Gu Y, et al. Perilipin 5 improves hepatic lipotoxicity by inhibiting lipolysis. Hepatology. 2014 doi: 10.1002/hep.27409. [DOI] [PubMed] [Google Scholar]
- [96].Trevino MB, Mazur-Hart D, Machida Y, King T, Nadler J, Galkina EV, Poddar A, Dutta S, Imai Y. Liver Perilipin 5 Expression Worsens Hepatosteatosis But Not Insulin Resistance in High Fat-Fed Mice. Mol Endocrinol. 2015;29:1414–25. doi: 10.1210/me.2015-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Zhang X, Heckmann BL, Campbell LE, Liu J. G0S2: A small giant controller of lipolysis and adipose-liver fatty acid flux. Biochim Biophys Acta - Mol Cell Biol Lipids. 2017;1862:1146–1154. doi: 10.1016/j.bbalip.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Yang X, Lu X, Lombès M, Rha GB, Chi Y-I, Guerin TM, Smart EJ, Liu J. The G(0)/G(1) switch gene 2 regulates adipose lipolysis through association with adipose triglyceride lipase. Cell Metab. 2010;11:194–205. doi: 10.1016/j.cmet.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Zhang X, Xie X, Heckmann BL, Saarinen AM, Czyzyk TA, Liu J. Targeted disruption of G0/G1 switch gene 2 enhances adipose lipolysis, alters hepatic energy balance, and alleviates high-fat diet-induced liver steatosis. Diabetes. 2014;63:934–46. doi: 10.2337/db13-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Wang Y, Zhang Y, Qian H, Lu J, Zhang Z, Min X, Lang M, Yang H, Wang N, Zhang P. The g0/g1 switch gene 2 is an important regulator of hepatic triglyceride metabolism. PLoS One. 2013;8:e72315. doi: 10.1371/journal.pone.0072315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Zechner R, Zimmermann R, Eichmann TO, Kohlwein SD, Haemmerle G, Lass A, Madeo F. FAT SIGNALS - Lipases and Lipolysis in Lipid Metabolism and Signaling. Cell Metab. 2012;15:279–291. doi: 10.1016/j.cmet.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Voshol PJ, Haemmerle G, Ouwens DM, Zimmermann R, Zechner R, Teusink B, Maassen JA, Havekes LM, Romijn JA. Increased hepatic insulin sensitivity together with decreased hepatic triglyceride stores in hormone-sensitive lipase-deficient mice. Endocrinology. 2003;144 doi: 10.1210/en.2002-0036. [DOI] [PubMed] [Google Scholar]
- [103].Albert JS, Yerges-Armstrong LM, Horenstein RB, Pollin TI, Sreenivasan UT, Chai S, Blaner WS, Snitker S, O’Connell JR, Gong D-W, Breyer RJ, et al. Null Mutation in Hormone-Sensitive Lipase Gene and Risk of Type 2 Diabetes. N Engl J Med. 2014;370:2307–2315. doi: 10.1056/NEJMoa1315496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Xia B, Cai GH, Yang H, Wang SP, Mitchell GA, Wu JW. Adipose Tissue Deficiency of Hormone- Sensitive Lipase Causes Fatty Liver in Mice. PLoS Genet. 2017;13:1–17. doi: 10.1371/journal.pgen.1007110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Fernandez C, Lindholm M, Krogh M, Lucas S, Larsson S, Osmark P, Berger K, Borén J, Fielding B, Frayn K, Holm C. Disturbed cholesterol homeostasis in hormone-sensitive lipase-null mice. Am J Physiol Endocrinol Metab. 2008;295:E820–E831. doi: 10.1152/ajpendo.90206.2008. [DOI] [PubMed] [Google Scholar]
- [106].Grabner GF, Zimmermann R, Schicho R, Taschler U. Monoglyceride lipase as a drug target: At the crossroads of arachidonic acid metabolism and endocannabinoid signaling. Pharmacol Ther. 2017;175:35–46. doi: 10.1016/j.pharmthera.2017.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Douglass JD, Zhou YX, Wu A, Zadrogra JA, Gajda AM, Lackey AI, Lang W, Chevalier KM, Sutton SW, Zhang S-P, Flores CM, et al. Global deletion of MGL in mice delays lipid absorption and alters energy homeostasis and diet-induced obesity. J Lipid Res. 2015;56:1153–1171. doi: 10.1194/jlr.M058586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Sookoian S, Pirola CJ. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology. 2011;53:1883–1894. doi: 10.1002/hep.24283. [DOI] [PubMed] [Google Scholar]
- [109].Lake AC, Sun Y, Li J-L, Kim JE, Johnson JW, Li D, Revett T, Shih HH, Liu W, Paulsen JE, Gimeno RE. Expression, regulation, and triglyceride hydrolase activity of Adiponutrin family members. J Lipid Res. 2005;46:2477–87. doi: 10.1194/jlr.M500290-JLR200. [DOI] [PubMed] [Google Scholar]
- [110].Huang Y, Cohen JC, Hobbs HH. Expression and characterization of a PNPLA3 protein isoform (I148M) associated with nonalcoholic fatty liver disease. J Biol Chem. 2011;286:37085–93. doi: 10.1074/jbc.M111.290114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Kumari M, Schoiswohl G, Chitraju C, Paar M, Cornaciu I, Rangrez AYAYY, Wongsiriroj N, Nagy HMMHM, Ivanova PTPTT, Scott SASAA, Knittelfelder O, et al. Adiponutrin functions as a nutritionally regulated lysophosphatidic acid acyltransferase. Cell Metab. 2012;15:691–702. doi: 10.1016/j.cmet.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Pingitore P, Dongiovanni P, Motta BM, Meroni M, Lepore SM, Mancina RM, Pelusi S, Russo C, Caddeo A, Rossi G, Montalcini T, et al. PNPLA3 overexpression results in reduction of proteins predisposing to fibrosis. Hum Mol Genet. 2016;25 doi: 10.1093/hmg/ddw341. ddw341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Li JZ, Huang Y, Karaman R, Ivanova PT, Brown HA, Roddy T, Castro-Perez J, Cohen JC, Hobbs HH. Chronic overexpression of PNPLA3I148M in mouse liver causes hepatic steatosis. J Clin Invest. 2012;122:4130–4144. doi: 10.1172/JCI65179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Smagris E, BasuRay S, Li J, Huang Y, Lai KV, Gromada J, Cohen JC, Hobbs HH. I restriction site so digestion of the PCR fragment from the KI mice generated two fragments upon digestion with. Hepatology. 2015;148:108–18. doi: 10.1002/hep.27242. [DOI] [Google Scholar]
- [115].Chen W, Chang B, Li L, Chan L. Patatin-like phospholipase domain-containing 3/adiponutrin deficiency in mice is not associated with fatty liver disease. Hepatology. 2010;52:1134–42. doi: 10.1002/hep.23812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Basantani MK, Sitnick MT, Cai L, Brenner DS, Gardner NP, Li JZ, Schoiswohl G, Yang K, Kumari M, Gross RW, Zechner R, et al. Pnpla3/Adiponutrin deficiency in mice does not contribute to fatty liver disease or metabolic syndrome. J Lipid Res. 2011;52:318–29. doi: 10.1194/jlr.M011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Santoro N, Kursawe R, D’Adamo E, Dykas DJ, Zhang CK, Bale AE, Calí AM, Narayan D, Shaw MM, Pierpont B, Savoye M, et al. A common variant in the patatin-like phospholipase 3 gene (PNPLA3) is associated with fatty liver disease in obese children and adolescents. Hepatology. 2010;52:1281–90. doi: 10.1002/hep.23832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Mondul A, Mancina RM, Merlo A, Dongiovanni P, Rametta R, Montalcini T, Valenti L, Albanes D, Romeo S. PNPLA3 I148M Variant Influences Circulating Retinol in Adults with Nonalcoholic Fatty Liver Disease or Obesity. J Nutr. 2015;145:1687–1691. doi: 10.3945/jn.115.210633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Kovarova M, Königsrainer I, Königsrainer A, Machicao F, Häring H-U, Schleicher E, Peter A. The Genetic Variant I148M in PNPLA3 Is Associated With Increased Hepatic Retinyl-Palmitate Storage in Humans. J Clin Endocrinol Metab. 2015;100:E1568–E1574. doi: 10.1210/jc.2015-2978. [DOI] [PubMed] [Google Scholar]
- [120].Kuchinskiene Z, Carlson La. Composition, concentration, and size of low density lipoproteins and of subfractions of very low density lipoproteins from serum of normal men and women. [accessed February 21, 2018];J Lipid Res. 1982 23:762–9. [PubMed] [Google Scholar]
- [121].Alexander CA, Hamilton RL, Havel RJ. Subcellular localization of B apoprotein of plasma lipoproteins in rat liver. [accessed February 19, 2018];J Cell Biol. 1976 69:241–63. doi: 10.1083/jcb.69.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Holm C, Belfrage P, Fredrikson G. Immunological evidence for the presence of hormone-sensitive lipase in rat tissues other than adipose tissue. Biochem Biophys Res Commun. 1987;148:99–105. doi: 10.1016/0006-291X(87)91081-3. [DOI] [PubMed] [Google Scholar]
- [123].Reid BN, Ables GP, Otlivanchik OA, Schoiswohl G, Zechner R, Blaner WS, Goldberg IJ, Schwabe RF, Chua SC, Huang L-S, Huang L-S. Hepatic overexpression of hormone-sensitive lipase and adipose triglyceride lipase promotes fatty acid oxidation, stimulates direct release of free fatty acids, and ameliorates steatosis. J Biol Chem. 2008;283:13087–99. doi: 10.1074/jbc.M800533200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Lehner R, Verger R. Purification and Characterization of a Porcine Liver Microsomal Triacylglycerol Hydrolase †. Biochemistry. 1997;36:1861–1868. doi: 10.1021/bi962186d. [DOI] [PubMed] [Google Scholar]
- [125].Ghosh S, Mallonee DH, Hylemon PB, Grogan WM. Molecular cloning and expression of rat hepatic neutral cholesteryl ester hydrolase. Biochim Biophys Acta - Lipids Lipid Metab. 1995;1259:305–312. doi: 10.1016/0005-2760(95)00184-0. [DOI] [PubMed] [Google Scholar]
- [126].Dolinsky VW, Sipione S, Lehner R, Vance DE. The cloning and expression of a murine triacylglycerol hydrolase cDNA and the structure of its corresponding gene. [accessed February 10, 2015];Biochim Biophys Acta. 2001 1532:162–72. doi: 10.1016/s1388-1981(01)00133-0. [DOI] [PubMed] [Google Scholar]
- [127].Lehner R, Vance DE. Cloning and expression of a cDNA encoding a hepatic microsomal lipase that mobilizes stored triacylglycerol. Biochem J. 1999;343(Pt 1):1–10. doi: 10.1042/0264-6021:3430001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Gilham D, Ho S, Rasouli M, Martres P, Vance DE, Lehner R. Inhibitors of hepatic microsomal triacylglycerol hydrolase decrease very low density lipoprotein secretion. FASEB J. 2003;17:1685–1687. doi: 10.1096/fj.02-0728fje. [DOI] [PubMed] [Google Scholar]
- [129].Wei E, Alam M, Sun F, Agellon LB, Vance DE, Lehner R. Apolipoprotein B and triacylglycerol secretion in human triacylglycerol hydrolase transgenic mice. J Lipid Res. 2007;48:2597–2606. doi: 10.1194/jlr.M700320-JLR200. [DOI] [PubMed] [Google Scholar]
- [130].Wei E, Ben Ali Y, Lyon J, Wang H, Nelson R, Dolinsky VW, Dyck JRB, Mitchell G, Korbutt GS, Lehner R. Loss of TGH/Ces3 in Mice Decreases Blood Lipids, Improves Glucose Tolerance, and Increases Energy Expenditure. Cell Metab. 2010;11:183–193. doi: 10.1016/j.cmet.2010.02.005. [DOI] [PubMed] [Google Scholar]
- [131].Wei E, Gao W, Lehner R. Attenuation of adipocyte triacylglycerol hydrolase activity decreases basal fatty acid efflux. J Biol Chem. 2007;282:8027–8035. doi: 10.1074/jbc.M605789200. [DOI] [PubMed] [Google Scholar]
- [132].Soni KG, Lehner R, Metalnikov P, O’Donnell P, Semache M, Gao W, Ashman K, Pshezhetsky AV, Mitchell GA. Carboxylesterase 3 (EC 3.1.1.1) is a major adipocyte lipase. J Biol Chem. 2004;279:40683–9. doi: 10.1074/jbc.M400541200. [DOI] [PubMed] [Google Scholar]
- [133].Lian J, Wei E, Groenendyk J, Das SK, Hermansson M, Li L, Watts R, Thiesen A, Oudit GY, Michalak M, Lehner R. Ces3/TGH deficiency attenuates steatohepatitis. Sci Rep. 2016;6 doi: 10.1038/srep25747. 25747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Holmes RS, Wright MW, Laulederkind SJF, Cox LA, Hosokawa M, Imai T, Ishibashi S, Lehner R, Miyazaki M, Perkins EJ, Potter PM, et al. Recommended nomenclature for five mammalian carboxylesterase gene families: human, mouse, and rat genes and proteins. Mamm Genome. 2010;21:427–441. doi: 10.1007/s00335-010-9284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Dolinsky VW, Gilham D, Alam M, Vance DE, Lehner R. Review Triacylglycerol hydrolase: role in intracellular lipid metabolism. C Cell Mol Life Sci. 2004;61:1633–1651. doi: 10.1007/s00018-004-3426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Ellinghaus P, Seedorf U, Assmann G. Cloning and sequencing of a novel murine liver carboxylesterase cDNA. Biochim Biophys Acta - Gene Struct Expr. 1998;1397:175–179. doi: 10.1016/S0167-4781(98)00023-2. [DOI] [PubMed] [Google Scholar]
- [137].Xu J, Li Y, Chen W-D, Xu Y, Yin L, Ge X, Jadhav K, Adorini L, Zhang Y. Hepatic carboxylesterase 1 is essential for both normal and farnesoid X receptor-controlled lipid homeostasis. Hepatology. 2014;59:1761–1771. doi: 10.1002/hep.26714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Quiroga AD, Li L, Trötzmüller M, Nelson R, Proctor SD, Köfeler H, Lehner R. Deficiency of carboxylesterase 1/esterase-x results in obesity, hepatic steatosis, and hyperlipidemia. Hepatology. 2012;56:2188–2198. doi: 10.1002/hep.25961. [DOI] [PubMed] [Google Scholar]
- [139].Shimomura I, Bashmakov Y, Horton JD. Increased levels of nuclear SREBP-1c associated with fatty livers in two mouse models of diabetes mellitus. [accessed February 26, 2018];J Biol Chem. 1999 274:30028–32. doi: 10.1074/jbc.274.42.30028. [DOI] [PubMed] [Google Scholar]
- [140].Moon Y-A. The SCAP/SREBP Pathway: A Mediator of Hepatic Steatosis. Endocrinol Metab. 2017;32:6. doi: 10.3803/EnM.2017.32.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Deng X, Cagen LM, Wilcox HG, Park EA, Raghow R, Elam MB. Regulation of the Rat SREBP-1c Promoter in Primary Rat Hepatocytes. Biochem Biophys Res Commun. 2002;290:256–262. doi: 10.1006/bbrc.2001.6148. [DOI] [PubMed] [Google Scholar]
- [142].Jump DB, Botolin D, Wang Y, Xu J, Christian B, Demeure O. Fatty acid regulation of hepatic gene transcription. J Nutr. 2005;135:2503–6. doi: 10.1080/10408360490278341. [DOI] [PubMed] [Google Scholar]
- [143].Nohturfft A, Yabe D, Goldstein JL, Brown MS, Espenshade PJ. Regulated Step in Cholesterol Feedback Localized to Budding of SCAP from ER Membranes. Cell. 2000;102:315–323. doi: 10.1016/S0092-8674(00)00037-4. [DOI] [PubMed] [Google Scholar]
- [144].Zhang Z, Yao Z, Chen Y, Qian L, Jiang S, Zhou J, Shao J, Chen A, Zhang F, Zheng S. Lipophagy and liver disease: New perspectives to better understanding and therapy. Biomed Pharmacother. 2018;97:339–348. doi: 10.1016/j.biopha.2017.07.168. [DOI] [PubMed] [Google Scholar]
- [145].Jaishy B, Abel ED. Lipids, lysosomes, and autophagy. J Lipid Res. 2016;57:1619–1635. doi: 10.1194/jlr.R067520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Schulze RJ, Sathyanarayan A, Mashek DG. Breaking fat: The regulation and mechanisms of lipophagy. Biochim Biophys Acta - Mol Cell Biol Lipids. 2017;1862:1178–1187. doi: 10.1016/J.BBALIP.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Boldrini R, Devito R, Biselli R, Filocamo M, Bosman C. Wolman disease and cholesteryl ester storage disease diagnosed by histological and ultrastructural examination of intestinal and liver biopsy. Pathol - Res Pract. 2004;200:231–240. doi: 10.1016/J.PRP.2003.11.001. [DOI] [PubMed] [Google Scholar]
- [148].Porto AF. Lysosomal acid lipase deficiency: Diagnosis and treatment of Wolman and Cholesteryl Ester Storage Diseases. [accessed February 26, 2018];Pediatr Endocrinol Rev. 2014 12:125–132. [PubMed] [Google Scholar]
- [149].Bernstein DL, Hülkova H, Bialer MG, Desnick RJ. Cholesteryl ester storage disease: review of the findings in 135 reported patients with an underdiagnosed disease. J Hepatol. 2013;58:1230–43. doi: 10.1016/j.jhep.2013.02.014. [DOI] [PubMed] [Google Scholar]
- [150].Hůlková H, Elleder M. Distinctive histopathological features that support a diagnosis of cholesterol ester storage disease in liver biopsy specimens. Histopathology. 2012;60:1107–1113. doi: 10.1111/j.1365-2559.2011.04164.x. [DOI] [PubMed] [Google Scholar]
- [151].Cummings MH, Watts GF, Umpleby AM, Hennessy TR, Naoumova R, Slavin BM, Thompson GR, Sönksen PH. Increased hepatic secretion of very-low-density lipoprotein apolipoprotein B-100 in NIDDM. Diabetologia. 1995;38:959–967. doi: 10.1007/BF00400586. [DOI] [PubMed] [Google Scholar]
- [152].Aguisanda F, Thorne N, Zheng W. Targeting Wolman Disease and Cholesteryl Ester Storage Disease: Disease Pathogenesis and Therapeutic Development. Curr Chem Genomics Transl Med. 2017;11:1–18. doi: 10.2174/2213988501711010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Bay L, Canero Velasco C, Ciocca M, Cotti A, Cuarterolo M, Fainboim A, Fassio E, Galoppo M, Pinero F, Rozenfeld P. Liver disease and dyslipidemia as a manifestation of lysosomal acid lipase deficiency (LAL-D). Clinical and diagnostic aspects, and a new treatment. An update. Arch Argent Pediatr. 2017;115:287–293. doi: 10.5546/aap.2017.eng.287. [DOI] [PubMed] [Google Scholar]
- [154].Aqul A, Lopez AM, Posey KS, Taylor AM, Repa JJ, Burns DK, Turley SD. Hepatic entrapment of esterified cholesterol drives continual expansion of whole body sterol pool in lysosomal acid lipase-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2014;307:G836–G847. doi: 10.1152/ajpgi.00243.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Du H, Duanmu M, Witte D, Grabowski GA. Targeted disruption of the mouse lysosomal acid lipase gene: Long-term survival with massive cholesteryl ester and triglyceride storage. Hum Mol Genet. 1998;7:1347–1354. doi: 10.1093/hmg/7.9.1347. [DOI] [PubMed] [Google Scholar]
- [156].Radović B, Vujić N, Leopold C, Schlager S, Goeritzer M, Patankar JV, Korbelius M, Kolb D, Reindl J, Wegscheider M, Tomin T, et al. Lysosomal acid lipase regulates VLDL synthesis and insulin sensitivity in mice. Diabetologia. 2016 doi: 10.1007/s00125-016-3968-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Škop V, Cahová M, Papácková Z, Pálenícková E, Danková H, Baranowski M, Zabielski P, Ždychová J, Zídková J, Kazdová L. Autophagy-lysosomal pathway is involved in lipid degradation in rat liver. [accessed February 26, 2018];Physiol Res. 2012 61:287–297. doi: 10.33549/physiolres.932285. [DOI] [PubMed] [Google Scholar]
- [158].Nossen JO, Rustan AC, Barnard T, Drevon CA. Inhibition by chloroquine of the secretion of very low density lipoproteins by cultured rat hepatocytes. [accessed February 26, 2018];Biochim Biophys Acta. 1984 803:11–20. doi: 10.1016/0167-4889(84)90049-1. [DOI] [PubMed] [Google Scholar]
- [159].Schulze RJ, Dri Zyt_ K, Casey CA, Mcniven MA. Hepatic Lipophagy: New Insights Into Autophagic Catabolism of Lipid Droplets in the Liver. Hepatol Commun. 2017;1:359–369. doi: 10.1002/hep4.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Kaushik S, Cuervo AM. Degradation of lipid droplet-associated proteins by chaperone-mediated autophagy facilitates lipolysis. Nat Cell Biol. 2015;17:759–70. doi: 10.1038/ncb3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Martinez-Lopez N, Garcia-Macia M, Sahu S, Athonvarangkul D, Liebling E, Merlo P, Cecconi F, Schwartz GJ, Singh R. Autophagy in the CNS and Periphery Coordinate Lipophagy and Lipolysis in the Brown Adipose Tissue and Liver. Cell Metab. 2016;23:113–27. doi: 10.1016/j.cmet.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Blomhoff R, Eskild W, Kindberg GM, Prydz K, Berg T. Intracellular transport of endocytosed chylomicron [3H]retinyl ester in rat liver parenchymal cells. Evidence for translocation of a [3H]retinoid from endosomes to endoplasmic reticulum. [accessed July 22, 2016];J Biol Chem. 1985 260:13566–13570. [PubMed] [Google Scholar]
- [163].Hernández-Gea V, Friedman SL. Autophagy fuels tissue fibrogenesis. Autophagy. 2012;8:849–50. doi: 10.4161/auto.19947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Burton BK, Deegan PB, Enns GM, Guardamagna O, Horslen S, Hovingh GK, Lobritto SJ, Malinova V, McLin VA, Raiman J, Di Rocco M, et al. Clinical Features of Lysosomal Acid Lipase Deficiency. J Pediatr Gastroenterol Nutr. 2015;61:619–25. doi: 10.1097/MPG.0000000000000935. [DOI] [PMC free article] [PubMed] [Google Scholar]