Amplified strength gain and neural plasticity can be induced by activating interlimb neural networks.
Key Words: stroke, neural plasticity, strength training, locomotion, rehabilitation
Abstract
Following stroke, sensorimotor brain networks and descending regulation are compromised but spinal interlimb neural connections remain morphologically intact. After cross-education strength and locomotion training, amplified neural plasticity and functional responses are observed in chronic stroke compared with neurologically intact participants. We hypothesize that poststroke neuroplasticity is amplified because of the involvement of interlimb neural connections that persist from our quadrupedal ancestry.
Key Points
Training-induced neural plasticity in the untrained, more affected limb is amplified after training the less affected limb in chronic stroke.
Interlimb neural networks conserved from our quadrupedal ancestry play important roles in mediating neural adaptation and cross-education training-induced strength gains.
Community-based stroke populations should always continue seeking rehabilitation training opportunities.
INTRODUCTION
Following stroke, neuronal damage leads to loss of supraspinal inputs to spinal motor neurons. The excitability in spinal and supraspinal pathways also are affected. Functionally, stroke-induced alterations in the nervous system result in inadequate motor unit recruitment, impaired coordination, and weakened muscle contractions. Strength and motor impairments are found bilaterally and are large on the contralesional side producing neurophysiologically more affected (so-called paretic) and less affected (so-called nonparetic) sides (1).
Training-induced neuroplastic corticospinal, propriospinal, and spinal adaptations play important roles in regulating strength gain (2–4). When translating observations from strength training in the general population to those with neurotraumatic injury, there can be reduced efficacy because of alterations in the neurological integrity of pathways subserving the adaptations themselves (5). Strength and functional training focusing on the more affected side may be hard to initiate because of muscle weakness and spasticity. This has contributed to the idea that the nervous system after injury may represent “fallow ground” with temporally limited and blunted adaptive responses (6).
Previous observations of human locomotor movements suggest spinal and propriospinal interlimb quadrupedal neural linkages are conserved in humans (7). These linkages remain intact and accessible after stroke (8). Therefore, it is possible that neural excitability and muscle strength on the more affected side can be modulated by training the less affected limbs and activating the intact neural pathways below the lesion site. Strength-based cross-education rehabilitation interventions (9,10) show large gains and normalized modulation of reflexes in chronic stroke participants after training with the less affected side. Spinal neural plasticity with improved performances in Timed Up-and-Go, 10-meter walking, and 6-min walking tests were found after arm or arm and leg cycling training (11–13). Neural adaptation in the untrained limbs along with larger percentage increases in cross-education strength indicate amplified neural plasticity in chronic stroke participants compared with that found in the neurologically intact.
It seems that interlimb neural networks may, in fact, be more rather than less responsive to training stimuli after injury. We suggest that the nervous system after stroke represents a fertile ground for adaptive training responses. Our hypothesis is that poststroke adaptive neural plasticity is amplified in response to physical training stimuli compared with the intact and uninjured nervous system. By activating interlimb neural connections evolutionarily conserved from our quadrupedal ancestors (14,15), amplified neuroplasticity can be induced and promote the strength and function in the untrained limbs in those with chronic stroke.
The core concept of our hypothesis is contained in Figure 1 where the nervous system is represented as a tree. Damage to the tree caused by a lightning strike is the analog of the effects of stroke on the brain. The lightning strike may damage parts of the tree and result in withered branches (Fig. 1B and C), yet the roots and trunk remain intact. Thus, watering and fertilizing (i.e., training) not only nourish the intact parts but also boost the regrowth of the damaged branches. The entire tree can flourish again and move closer to the state before damage. Returning to the nervous system, by utilizing intact neural network after stroke, continuous training of the less affected limb can promote significant strength gain in the more affected limbs.
Figure 1.
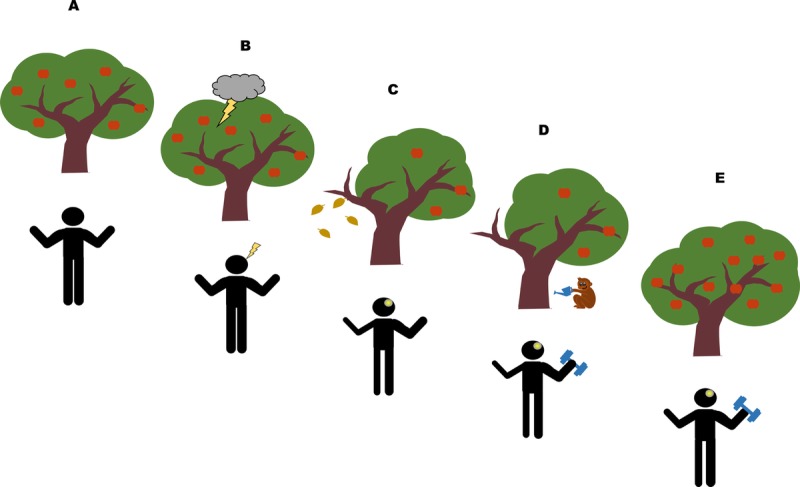
Regrowth of a tree after a lightning strike as a metaphor for recovery of function stroke. The flourishing tree represents the intact nervous system before lesion (A). A lightning strike on the tree results in broken and withered branches (B and C). However, because the trunk and root remain intact, continuous watering and fertilizing the tree help the withered branches to regrow (D and E). In the nervous system, strength in the contralesional limbs is reduced dramatically in the first few months after lesion (C). However, by unitizing the interlimb neural networks conserved from our quadrupedal ancestors (represented by the monkey watering the tree), neural plasticity can be induced across the body even years after lesion (D and E).
Neural Plasticity in Poststroke Strength Training: Bilateral Strength Gain From Unilateral Strength Training
Compared with neurologically intact participants, larger strength improvement and significant neural plasticity are found in people with stroke after various training approaches. Commonly used strength training methods focusing on the more affected side include progressive resistance training (PRT; training with progressively increased resistance, usually 70% or more of maximal strength (16)) and constraint-induced movement therapy (training the more affected side extensively with the movement of the less affected limb constrained through a sling 90% of the waking hours (17)). Although it used to be mistakenly believed that strenuous activity on the more affected side may reinforce spasticity after stroke and must be avoided (18), experiments show that training can actually reduce spasticity and effectively improve muscle strength in the more affected side after stroke (19,20). In addition, compared with the general population, systematic reviews on PRT show large effect sizes (0.98; 95% confidence interval, 0.67 to 1.29) on strength gains for stroke participants (21) and modest effects of PRT on the general elderly population (0.68; 95% confidence interval, 0.52 to 0.84) (22); this suggest that PRT has stronger effects after neurological damage arising from stroke.
Although the benefits of strength training on the more affected side have been confirmed in many studies (20,23), directly training the more affected side is not always possible and is hard to initiate for those with spasticity and muscle weakness. For those with asymmetrical weakness and immobility after stroke, training the less affected side can be used to boost the strength of the more affected side and improve functional symmetry (8). “Cross-education” describes training muscles on one side of the body to improve strength or motor skill in the contralateral untrained limb. Since it was first described by Edward Scripture in 1894 (24), evidence of cross-education has been found after strength training in both upper and lower limb muscles (25). In a meta-analysis with a total of 96 cross-education training studies included, Green and Gabriel (26) found 29% of strength gain on the untrained side in those with stroke, neuromuscular disorder, and osteoarthrosis, which was higher than the 18% increase in able-bodied young adults and 15% increase in older adults. In chronic stroke participants, Dragert and Zehr (9) found 6 weeks of dorsiflexion training with the less affected side improved strength by 34% and 31% in the trained and untrained legs and 15 of 19 participants showed significant strength gains on the untrained side after training. In contrast, with the same training protocol in neurologically intact participants, 5 weeks of unilateral ankle dorsiflexion training increased dorsiflexion strength only by 14.7% and 8.4% on the trained and untrained ankle respectively (27). Sun et al. (10) found 5 weeks of training in chronic stroke improved wrist extension force by 42% and 35% in the trained and untrained side, respectively, and significant improvements found in the more affected arm were maintained for at least 5 weeks after training. The average cross-education strength increase in neurologically intact participants is 17% (26). A comparison of these strength gains in stroke and neurologically intact participants after cross-education training is summarized in Figure 2, where larger strength gains are observed bilaterally in arms and legs in stroke participants.
Figure 2.
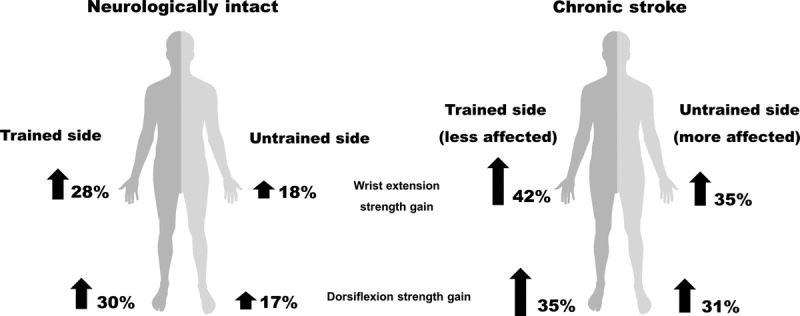
Unilateral wrist extension and ankle dorsiflexion training produce amplified increases in strength after stroke. Compared with neurologically intact, chronic stroke participants show larger strength gains on both trained (less affected) and untrained (more affected) sides. Percentages of the strength gain in chronic stroke participants are obtained from the studies of Dragert and Zehr (9) and Sun et al. (10). Data from neurologically intact participants are from the meta-analysis by Green and Gabriel (26).
By measuring force change during unilateral handgrip training, Barss et al. (28) found significant strength gain on the untrained side occurred around the 15th session of training in neurologically intact participants. In the studies from Dragert and Zehr (9) and Sun et al. (10), a total of 15 and 18 sessions of training were performed, respectively. There is also evidence showing that 12 sessions of elbow extension can significantly improve joint torque in the untrained arm in stroke participants (29). Although there are only a few studies currently showing strength cross-education in stroke participants, larger strength gain after similar doses of training compared with neurologically intact participants suggest poststroke neural network is actually more responsive to the training stimuli.
Along with strength improvement, unilateral training altered neural excitability in both spinal and corticospinal pathways on the untrained side after stroke. Dragert and Zehr (30) found the size of reciprocal inhibition was greater with the same background muscle activities in both legs, which suggests reduced hyperexcitability after training. Following wrist extension training, the slope of the regression line between early latency cutaneous reflexes and background EMG was steeper on the more affected side indicating cutaneous pathway excitability was normalized to that found on the less affected side. Modulation in the cortical and corticospinal pathways was observed as reduced transcallosal inhibition and reduced cortical silent period from the contralesional side (10) (Fig. 3).
Figure 3.
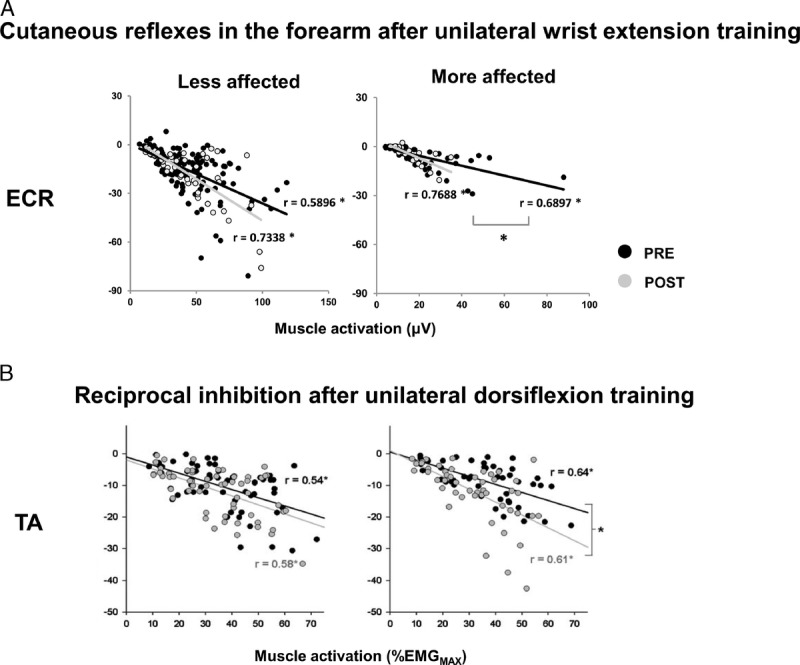
Normalization of spinal interneuronal excitability after cross-education training. A. Normalized cutaneous reflexes in extensor carpi radials (ECR) evoked from superficial radial nerve stimulation (SR) at different background muscle activation levels. Black and gray dots represent reflex amplitude before (PRE) and after (POST) training, respectively. B. Normalized reciprocal inhibition in tibialis anterior (TA) muscle. Black and gray dots represent reflex amplitudes before (PRE) and after (POST) training. Data from less affected and more affected arms are shown at left and right, respectively. Linear regression analyses were performed and Pearson r values were calculated for each best-fit line. * indicates significant linear correlation. # indicates significant difference between linear regression slopes. X-axis represents background muscle activation (%EMGMAX). Y-axis represents reflexes amplitudes. Data adapted from (9,10).
Neural plasticity induced by cross-education training has been found at different regions of the nervous system in neurologically intact participants. A meta-analysis from Manca et al. (31) found reduced short interval cortical inhibition and cortical silent period in the pooled results suggesting unilateral strength training affects intracortical inhibition and GABAAergic excitability in the motor cortex of the untrained hemisphere. In chronic stroke participants, neural plasticity was not seen in these two corticospinal pathways, but changes in spinally mediated reflexes and transcallosal inhibition pathways suggest bilateral strength gain and plastic adaption in the remaining intact neural pathways can be induced (9,10). Spinal neural plasticity was observed after strength training using ankle and wrist muscles in stroke participants (9,10). Thus, it is likely that interlimb neural connections at the spinal level are involved in mediating cross-education of strength after supraspinal lesion.
With all the benefits of unilateral strength training in bilateral strength gain and neural plasticity, we suggest that cross-education strength training should be considered complementary to traditional training approaches focusing on functional improvement of the more affected side. The concept of training the less affected side could be used to “boost” the more affected side strength when the muscle weakness prevents training directly. After the more affected side gained enough strength to initiate the movement, targeted strength or functional training should be focused on the more affected side.
Neural Plasticity in Poststroke Locomotor Training: Arms Can Give Legs a Helping Hand
Reduced descending input affect walking function and poststroke quality of life. Previous studies suggest that both arms and legs share common neural control elements during rhythmic movements such as walking and cycling (32). By utilizing this common interlimb neural connection, walking functions can be facilitated by other rhythmic movements such as arm cycling or arm and leg cycling after lesion.
Strong neural and mechanical linkages between the arms and legs were observed in human during locomotion, although our arms play less direct roles in propulsion during walking. Besides stabilizing the torso from rotating (33), rhythmic arm movements also regulate lumbar spinal cord excitability and muscle activation in the legs. Huang and Ferris investigated the neural coupling between the upper and lower limbs when participants exercised on a recumbent stepper where arm and leg movements were mechanically coupled. Arm movements during recumbent stepping enhanced muscle activity in the leg, and these facilitatory effects increased when arms moved against higher resistance (34) or at a higher frequency (35). Interlimb neural coupling between the arms and legs also was found in other rhythmic tasks, such as walking and arm and leg cycling, with significant phase-dependent reflexes in the leg muscles evoked from sensory stimulation to the arm (36).
After stroke, neural interlimb connections between the arms and legs are preserved. A single session of arm cycling exercise can alter the spinal excitability for the legs (5,37). Such neural connections can be applied in locomotion rehabilitation by incorporating arm and leg rhythmic movements. Klarner et al. (12,13) found 6 weeks of moderate arm and leg cycling training with chronic stroke participants result in increased strength and range of motion in the more affected ankle. In addition, phase-dependent modulation of lower limb muscle activation and cutaneous reflexes were observed during walking indicating that interlimb neural network regulation is normalized to the neurologically intact state. Because the exercise intensity was moderate (participants reported their rate of perceived exertion between 3 and 5), training-induced neural plasticity and functional gain are mainly due to the active rhythmic interlimb movement. Although walking was not directly trained and participants were seated during all the training sessions, arm-leg cycling training can transfer to improvement in untrained walking performance. This supports the hypothesis that rhythmic movements are regulated by common core neural networks (32).
The power of rhythmic movements in amplifying leg muscle activation and walking ability is further confirmed by a study from Kaupp et al. (11). By performing just arm cycling exercise for 5 weeks, 3 times per week for 30 min, significant improvements were found in chronic stroke participants’ Timed Up-and-Go, 10-meter walk, and 6-min walk tests. In addition, arm cycling increased tibialis anterior muscle activation on the more affected side and normalized cutaneous reflexes in both legs (Fig. 4). Such neural adaption is important in assisting with weak dorsiflexion to help improve efficiency and prevent stumbling or falling after stroke (5). Related outcomes have recently been shown after arm cycling training in incomplete spinal cord injury (38).
Figure 4.
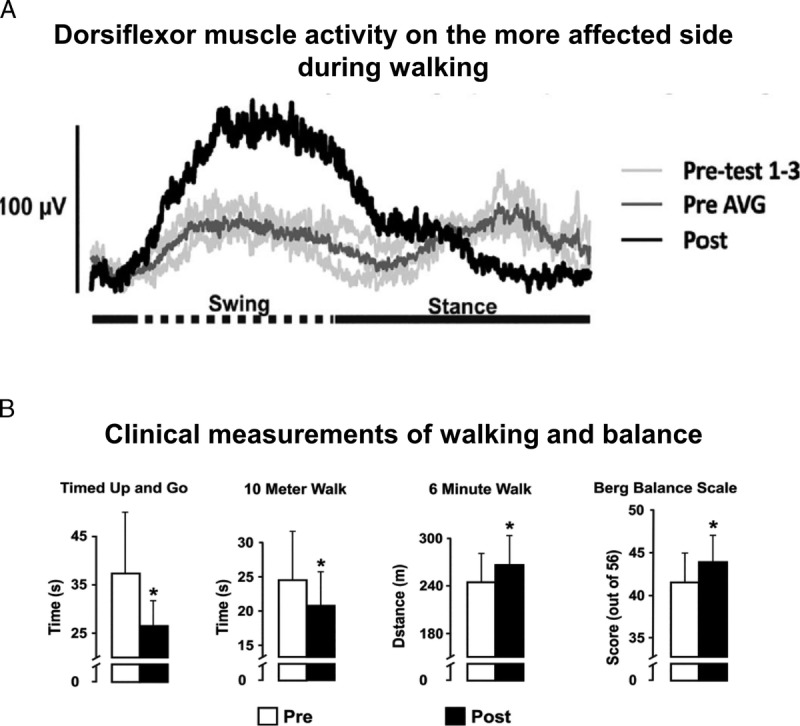
Normalized tibialis anterior muscle activity and walking performance after arm cycling training in stroke. A. An EMG recordings of tibialis anterior (TA) muscle on the more affected side from one participant. Light gray traces are before with the dark gray trace indicating the untrained average, and the black trace is the result after training. B. Improved performance in walking and balance tests. Before (open bars) and after (filled bars) training data from the group for the Timed Up and Go, 10-Meter Walk, 6-Minute Walk, and Berg Balance Scale. [Adapted from (11). Copyright © 2018 The American Physiological Society.]
A more responsive neural network also was observed after spinal cord injury. Thompson and colleagues found down-conditioning soleus H-reflex can modulate plantarflexor muscle activation and improve walking symmetry in spinal cord–injured participants (39) but not in neurologically intact (40). These results suggest guiding the plasticity has more profound effects on the functions and reflex modulation of the other muscles after neurotrauma (39).
In neurologically intact participants, the effects of rhythmic arm movement training on walking function have not been recorded. The modulatory role of arm cycling on the leg muscle activities is largely dependent on the functional state of the leg. Without active cyclic movement in the leg, arm cycling does produce subtle effects in cutaneous reflex modulation in the legs (41). By comparison, the findings from Kaupp et al. (11) present amplified neural plasticity in chronic stroke participants and arm cycling alone can activate the neural networks that regulate leg movements and significantly improve performance in walking tests.
Quadrupedal Neural Pathways Mediating Amplified Neural Plasticity?
Both cross-education strength training and arm cycling training alter reflex modulation at different sites of the central nervous system suggesting widespread adaptive mechanisms in the nervous system. We speculate that amplified strength gain and neural plasticity in the untrained limbs are achieved by activating evolutionarily conserved interlimb neural networks (18). In quadrupedal locomotion, all four limbs are directly engaged in locomotor patterning and the production of propulsive force. Injury to one limb usually compromises but does not prevent locomotion. In such cases, the remaining three intact limbs are subjected to training stresses of increased loading and forced use. Strong neural and biomechanical interlimb coupling would enable quadrupedal animals to continue behaviors such as hunting for food or running away from predators while healing the injured limb. Actively using the noninjured limb may tap into the circuitry underlying cross-education effects to result in more rapid recovery of function in the injured limb.
Without any injury or neural damage, activity of interlimb neural networks between all four limbs in bipedal humans is subliminal and seems less obvious because we can easily perform independent uni- or bimanual motor tasks. However, the human nervous system shares many similarities with quadrupedal animals in regulating interlimb movements (15). Considering interlimb neuromechanical interaction during locomotion from an evolutionary perspective (15), substantial evidence shows that neural control mechanisms in rhythmic movement such as walking, running, and cycling in human are similar to those in quadrupedal animals. Coordinated and smooth interlimb movements during locomotion are regulated by the same spinal neural networks (central pattern generator) for both human and other quadrupedal animals (14,15). It should be noted that the human interlimb neural modulation is not only preserved between the legs to regulate locomotion but also exists between the upper and lower limbs, as well as the ipsilateral and contralateral side during nonlocomotor tasks. For example, sensory stimulation to the arm during walking (42) or rhythmic arm movement (43) affects spinal excitability for leg muscles as shown by altered H-reflex and cutaneous reflex amplitudes. Movement or strength training on one side of the body also can change the H-reflex and reciprocal inhibition amplitudes on the contralateral side (27,44).
Following stroke, neural damage affects the excitabilities in the cortical and corticospinal pathways associated with the lesion. However, neural circuits below the lesion site, including reticulospinal, propriospinal, and other spinal neural pathways, are usually morphologically intact. Studies on spinal cord–injured participants and decerebrate animals provided compelling evidence that interlimb movement can be modulated by the neural network resides in the spinal cord with minimal supraspinal descending input (45,46). With reduced descending input after stroke, the remaining morphologically intact spinal neural pathways play a more critical role in regulating movements as well as facilitating neural rehabilitation. Evidence from animal models show reticulospinal (47) and propriospinal (48) pathways subserving some of the functional recoveries and regulating interlimb coordination after corticospinal lesion.
After stroke, both cross-education strength training and arm cycling training tap into the remaining interlimb neural networks by actively training with the less affected limbs. The fact that spinal excitability can be altered through these training methods in chronic stroke participants (9–13) confirms the contribution of interlimb neural network in mediating training-induced strength and functional improvement. We suggest that, in a manner similar to a quadruped coping with the injured limb by relying more on the unaffected limbs, ongoing activation on the less affected limb in stroke serves as the training stimulus leading to enhanced restoration of function after injury without directly involving the target muscles. It is tempting to speculate on underlying mechanisms of adaptation here, and our hypothesis would be best explained by strengthening of weakened connections and unmasking of underlying and previously silent connections (17).
A compelling, recent, and relevant experiment using a murine model involved mapping the area of cervical gray matter reinnervation by sprouting corticospinal axons contralesional to a stroke lesion created by photothrombosis (49). RNA profiles of the reinnervated area using whole-genome sequencing revealed differentially expressed genes involved in tissue repair related to outgrowth of neurites. The conclusion from this work was that spinal gray matter deneravated as a result of cortical lesion “represents a growth-promoting environment for sprouting corticospinal fibers originating from the contralesional motor cortex” (49). Should such processes remain operational in the human, this could explain a large portion of the amplified neuroplastic training response seen after stroke.
Jon Wolpaw (50) proposed a “negotiated equilibrium” model to explain the activity-induced spinal plasticity found in reduced animal and human studies. This model emphasizes that plasticity in the brain induces and maintains that in the spinal cord. The brain and spinal cord plasticity combine to produce and preserve satisfactory performance by defining a set of key features for each behavior. One prediction of this model is “if the spinal plasticity that produces a new behavior also improves an old behavior, the magnitude of this plasticity is likely to be enhanced because it will be driven by two of the participants in the negotiation, the new behavior and the old.” This approach and framework may be directly related to our hypothesis that neural plasticity is amplified after stroke. Future studies should approach research questions from efforts grounded in these perspectives.
SUMMARY
We hypothesize that neural plasticity is amplified, not diminished, after stroke. Greater strength gains and neural plasticity can be induced by activating the preserved interlimb neural network without directly involving the target muscles. For those with chronic stroke and have difficulties to access and receive conventional strength or locomotion training due to lack of muscle strength and coordination in the more affected side, cross-education, arm cycling, and arm and leg cycling exercise provide an easily applied training paradigm to boost the strength and function of the more affected limbs. Taken together with other rehabilitation training modalities, strengthening and training methods engaging as many limbs as possible may help to achieve continuous strength and functional improvement can be achieved by the community-based chronic stroke population.
Acknowledgments
This study was supported by funding to Dr. E. Paul Zehr from the Heart and Stroke Foundation (British Columbia and Yukon) and the Natural Sciences and Engineering Research Council (NSERC) of Canada.
Footnotes
Editor: Roger M. Enoka, Ph.D.
References
- 1.Zehr EP. Evidence-based risk assessment and recommendations for physical activity clearance: stroke and spinal cord injury. Appl. Physiol. Nutr. Metab. 2011; 36(Suppl. 1):S214–31. [DOI] [PubMed] [Google Scholar]
- 2.Carroll TJ, Riek S, Carson RG. The sites of neural adaptation induced by resistance training in humans. J. Physiol. 2002; 544(2):641–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selvanayagam VS, Riek S, Carroll TJ. Early neural responses to strength training. J. Appl. Physiol. 2011; 111(2):367–75. [DOI] [PubMed] [Google Scholar]
- 4.Adkins DL, Boychuk J, Remple MS, Kleim JA. Motor training induces experience-specific patterns of plasticity across motor cortex and spinal cord. J. Appl. Physiol. 2006; 101(6):1776–82. [DOI] [PubMed] [Google Scholar]
- 5.Zehr EP, Loadman PM. Persistence of locomotor-related interlimb reflex networks during walking after stroke. Clin. Neurophysiol. 2012; 123(4):796–807. [DOI] [PubMed] [Google Scholar]
- 6.Sun Y, Boots J, Zehr EP. The lingering effects of a busted myth—false time limits in stroke rehabilitation. Appl. Physiol. Nutr. Metab. 2015; 861:858–61. [DOI] [PubMed] [Google Scholar]
- 7.Zehr EP, Hundza SR, Vasudevan EV. The quadrupedal nature of human bipedal locomotion. Exerc. Sport Sci. Rev. 2009; 37(2):102–8. [DOI] [PubMed] [Google Scholar]
- 8.Farthing JP, Zehr EP. Restoring symmetry: clinical applications of cross-education. Exerc. Sport Sci. Rev. 2014; 42(2):70–5. [DOI] [PubMed] [Google Scholar]
- 9.Dragert K, Zehr EP. High-intensity unilateral dorsiflexor resistance training results in bilateral neuromuscular plasticity after stroke. Exp. Brain Res. 2013; 225:93–104. [DOI] [PubMed] [Google Scholar]
- 10.Sun Y, Ledwell NMH, Boyd LA, Zehr EP. Unilateral wrist extension training after stroke improves strength and neural plasticity in both arms. Exp. Brain Res. 2018; 236(7):1–13. [DOI] [PubMed] [Google Scholar]
- 11.Kaupp C, Pearcey GEP, Klarner T, et al. Rhythmic arm cycling training improves walking and neurophysiological integrity in chronic stroke: the arms can give legs a helping hand in rehabilitation. J. Neurophysiol. 2018; 119(3):1095–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klarner T, Barss TS, Sun Y, Kaupp C, Loadman PM, Zehr EP. Exploiting interlimb arm and leg connections for walking rehabilitation: a training intervention in stroke. Neural Plast. 2016; 2016 doi: 10.1155/2016/1517968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klarner T, Barss TS, Sun Y, Kaupp C, Loadman PM, Zehr EP. Long-term plasticity in reflex excitability induced by five weeks of arm and leg cycling training after stroke. Brain Sci. 2016; 6(54). doi:10.3390/brainsci6040054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klarner T, Zehr EP. Sherlock Holmes and the curious case of the human locomotor central pattern generator. J. Neurophysiol. 2018; 120:53–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zehr EP, Barss TS, Dragert K, et al. Neuromechanical interactions between the limbs during human locomotion: an evolutionary perspective with translation to rehabilitation. Exp. Brain Res. 2016; 234(11):3059–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hakkennes S, Keating JL. Constraint-induced movement therapy following stroke: a systematic review of randomised controlled trials. Aust. J. Physiother. 2005; 51:221–31. [DOI] [PubMed] [Google Scholar]
- 17.Taub E, Uswatte G, Elbert T. New treatments in neurorehabiliation founded on basic research. Nature. 2002; 3:228–36. [DOI] [PubMed] [Google Scholar]
- 18.Bobath B. Adult Hemiplegia Evaluation and Treatment. 3rd ed Oxford, UK: Butterworth-Heinemann; 1990. [Google Scholar]
- 19.Morris SL, Dodd KJ, Morris ME. Outcomes of progressive resistance strength training following stroke: a systematic review. Clin. Rehabil. 2004; 18:27–39. [DOI] [PubMed] [Google Scholar]
- 20.Patten C, Lexell J, Brown HE. Weakness and strength training in persons with poststroke hemiplegia: rationale, method, and efficacy. J. Rehabil. Res. Dev. 2004; 41(3):293–312. [DOI] [PubMed] [Google Scholar]
- 21.Dorsch S, Ada L, Alloggia D. Progressive resistance training increases strength after stroke but this may not carry over to activity: a systematic review. J. Physiother. 2018; 64(2):84–90. [DOI] [PubMed] [Google Scholar]
- 22.Latham NK, Bennett DA, Stretton CM, Anderson CS. Systematic review of progressive resistance strength training in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2004; 59(1):48–61. [DOI] [PubMed] [Google Scholar]
- 23.Ada L, Dorsch S, Canning CG. Strengthening interventions increase strength and improve activity after stroke: a systematic review. Aust. J. Physiother. 2006; 52(4):241–8. [DOI] [PubMed] [Google Scholar]
- 24.Scripture EW, Theodate L, Smith EMB. On the education of muscular control and power. Yale Psychol. Lab. 1894; 1(2):114–9. [Google Scholar]
- 25.Munn J, Herbert RD, Gandevia SC. Contralateral effects of unilateral resistance training: a meta-analysis. J. Appl. Physiol. 2004; 96(5):1861–6. [DOI] [PubMed] [Google Scholar]
- 26.Green LA, Gabriel DA. The effect of unilateral training on contralateral limb strength in young, older, and patient populations: a meta-analysis of cross education older, and patient populations: a meta-analysis of cross education. Phys. Ther. Rev. 2018; 1–12. [Google Scholar]
- 27.Dragert K, Zehr EP. Bilateral neuromuscular plasticity from unilateral training of the ankle dorsiflexors. Exp. Brain Res. 2011; 208(2):217–27. [DOI] [PubMed] [Google Scholar]
- 28.Barss TS, Klarner T, Pearcey GEP, Sun Y, Zehr EP. Time course of interlimb strength transfer after unilateral handgrip training. J. Appl. Physiol. 2018; 125(5):1594–608. [DOI] [PubMed] [Google Scholar]
- 29.Ehrensberger M, Simpson D, Broderick P, et al. Unilateral strength training and mirror therapy in chronic stroke patients: a pilot randomised trial [published online Feb 13, 2019]. Am. J. Phys. Med. Rehabil. 2019. doi: 10.1097/PHM.0000000000001162. [DOI] [PubMed] [Google Scholar]
- 30.Dragert K, Zehr EP. Differential modulation of reciprocal inhibition in ankle muscles during rhythmic arm cycling. Neurosci. Lett. 2013; 534:269–73. [DOI] [PubMed] [Google Scholar]
- 31.Manca A, Hortobagyi T, Rothwell J, Deriu F. Neurophysiological adaptations in the untrained side in conjunction with cross-education of the muscle strength: a systematic review and meta-analysis. J. Appl. Physiol. 2018; 2(1):86–93. [DOI] [PubMed] [Google Scholar]
- 32.Zehr EP. Neural control of rhythmic human movement: the common core hypothesis. Exerc. Sport Sci. Rev. 2005; 33(1):54–60. [PubMed] [Google Scholar]
- 33.Elftman H. The function of the arms in walking. Hum. Biol. 1939; 11(4):529–35. [Google Scholar]
- 34.Huang HJ, Ferris DP. Neural coupling between upper and lower limbs during recumbent stepping. J. Appl. Physiol. 2004; 97(4):1299–308. [DOI] [PubMed] [Google Scholar]
- 35.Kao PC, Ferris DP. The effect of movement frequency on interlimb coupling during recumbent stepping. Motor Control. 2005; 9:144–63. [DOI] [PubMed] [Google Scholar]
- 36.Zehr EP, Balter JE, Ferris DP, Hundza SR, Loadman PM, Stoloff RH. Neural regulation of rhythmic arm and leg movement is conserved across human locomotor tasks. J. Physiol. 2007; 582(1):209–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barzi Y, Zehr EP. Rhythmic arm cycling suppresses hyperactive soleus H-reflex amplitude after stroke. Clin. Neurophysiol. 2008; 119(6):1443–52. [DOI] [PubMed] [Google Scholar]
- 38.Zhou R, Parhizi B, Assh J, et al. Effect of cervicolumbar coupling on spinal reflexes during cycling after incomplete spinal cord injury. J. Neurophysiol. 2018; 120:3172–86. [DOI] [PubMed] [Google Scholar]
- 39.Thompson AK, Wolpaw JR. Restoring walking after spinal cord injury: operant conditioning of spinal reflexes can help. Neuroscientist. 2015; 21(2):203–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Makihara Y, Segal RL, Wolpaw JR, Thompson AK. Operant conditioning of the soleus H-reflex does not induce long-term changes in the gastrocnemius H-reflexes and does not disturb normal locomotion in humans. J. Neurophysiol. 2014; 112(6):1439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balter JE, Zehr EP. Neural coupling between the arms and legs during rhythmic locomotor-like cycling movement. J. Neurophysiol. 2006; 97(2):1809–18. [DOI] [PubMed] [Google Scholar]
- 42.Haridas C, Zehr EP. Coordinated interlimb compensatory responses to electrical stimulation of cutaneous nerves in the hand and foot during walking. J. Neurophysiol. 2003; 90(5):2850–61. [DOI] [PubMed] [Google Scholar]
- 43.Frigon A. Effect of rhythmic arm movement on reflexes in the legs: modulation of soleus H-reflexes and somatosensory conditioning. J. Neurophysiol. 2004; 91(4):1516–23. [DOI] [PubMed] [Google Scholar]
- 44.Delwaide PJ, Sabatino M, Pepin JL, La Grutta V. Reinforcement of reciprocal inhibition by contralateral movements in man. Exp. Neurol. 1988; 99:10–6. [DOI] [PubMed] [Google Scholar]
- 45.Sherrington CS. Observation on the scratch-reflex in the spinal dog. Proc. Physiol. Soc. 1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang JF, Stephens MJ, Vishram R. Infant stepping: a method to study the sensory control of human walking. J. Physiol. 1998; 507(3):927–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zaaimi B, Edgley SA, Soteropoulos DS, Baker SN. Changes in descending motor pathway connectivity after corticospinal tract lesion in macaque monkey. Brain. 2012; (134):2277–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Juvin L, Le Gal J, Simmers J, Morin D. Cervicolumbar coordination in mammalian quadrupedal locomotion: role of spinal thoracic circuitry and limb sensory inputs. 2012; 32(3):953–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaiser J, Maibach M, Salpeter I, et al. The spinal transcriptome after cortical stroke—in search of molecular factors regulating spontaneous recovery in the spinal cord. J. Neurosci. 2019; 39(24):2571–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wolpaw JR. The negotiated equilibrium model of spinal cord function. J. Physiol. 2018; 596(16):3469–91. [DOI] [PMC free article] [PubMed] [Google Scholar]


