Lifetime number of sex partners, marital status, and history of sexually transmitted disease were associated with human papillomavirus (HPV) acquisition among women without prior exposure to HPV infection, with no observed differences in risk determinants patterns for HPV infections by length of follow-up.
Supplemental digital content is available in the text.
Background
Global variation in human papillomavirus (HPV) prevalence and persistence may be explained by differences in risk factors, such as sexual activity, oral contraceptive use, and behavioral factors. We evaluated determinants of acquisition and clearance of HPV infection among young women previously unexposed to HPV.
Methods
Five hundred thirty-four women aged 15 to 25 years who were cytology and HPV DNA negative, and seronegative for anti-HPV-16/18 antibodies, were recruited (July 2000–September 2001) from study centers in Brazil, the United States, and Canada (NCT00689741/NCT00120848). They were followed up for 76 months. Cervical samples were HPV genotyped via polymerase chain reaction. We used multivariable (forward stepwise, P = 0.15) Cox proportional hazards regression to estimate rate ratios (RR) and 95% confidence intervals (CI), separately according to length of follow-up time.
Results
On short-term follow-up (0–27 months), 257 (48%; 8535.80 person-months; incidence rate = 30.11; 95% CI, 26.64–34.02) incident HPV infections were detected. Marital status, lifetime number of sex partners, history of any sexually transmitted disease, and occasional use of oral contraceptives were strongly associated with acquisition of any HPV. Having 2 or more lifetime sex partners (RR, 2.03; 95% CI, 1.37–3.02) and a history of any sexually transmitted disease (RR, 1.98; 95% CI, 1.19–3.29) were the most important determinants of high-risk HPV (hrHPV) incidence. During the entire follow-up (0–76 months), an increased hrHPV clearance was found among women in North America (RR, 1.38; 95% CI, 1.08–1.78) and black women (RR, 1.64; 95% CI, 1.04–2.60). Greater number of lifetime partners was associated with reduced clearance rates for any HPV (RR, 0.65; 95% CI, 0.43–0.98).
Conclusions
We identified variation in risk of HPV acquisition and clearance among women unexposed to HPV at baseline.
Human papillomavirus (HPV) is one of the most common sexually transmitted infections worldwide. Although most infections resolve spontaneously within 3 years,1 persistent infection with high-risk types can lead to oral and anogenital cancers via consecutive premalignant stages.2 Low-risk genotypes (eg, HPVs 6 and 11), also known as non-oncogenic HPV, are associated with benign conditions, such as genital warts and respiratory papillomatosis.3 Knowledge of determinants of HPV acquisition and persistence is essential in understanding its epidemiology and natural history to develop future effective interventions aimed at reducing HPV-related disease burden.4
Epidemiologic studies have identified risk factors associated with HPV infection. There is clear evidence that some patterns of sexual behavior, such as age at sexual debut (ie, commencement of sexual activity at an early age), multiple sexual partners, and partners' prior sexual history are directly associated with an increased risk of genital HPV acquisition.4,5 Age, smoking, co-infection with other sexually transmitted agents, long-term oral contraceptive use and parity are also key cofactors to cervical carcinogenesis in high-risk HPV (hrHPV) positive women.6
Most research on HPV-induced carcinogenesis included women over the age of 30 years, focusing on those attending cervical cancer screening. HPV clearance of prevalent (ie, existing cases) or mixed prevalent and incident infections was mainly studied. In an earlier article by our group that assessed a population with no evidence of prior exposure to HPV infection, the overall detection of incident HPV infections was 20.61% (95% confidence interval [CI], 18.47–22.99) per 1000 person-months during a 6-year follow-up among the placebo group of young women participating in a vaccine clinical trial.7 In the current report, we present results on determinants of acquisition and clearance of HPV infections in the same cohort of women. Because participants had no serological or virological evidence of an HPV infection at study entry, we also stratified the analyses by length of follow-up to identify key determinants according to follow-up time and evaluate the robustness of findings.
MATERIALS AND METHODS
Study Design and Population
A detailed description of the clinical trial methodology including eligibility, sample collection methods, study sites, and recruitment and follow-up dates, has been published previously.8,9 Briefly, a cohort of 1,113 women (age, 15–25 years) from 32 study centers in Brazil, the United States, and Canada were enrolled in a phase II double-blind, randomized controlled trial assessing the HPV-16/18 AS04-adjuvanted vaccine. Recruitment took place between July 2000 and September 2001. Figure 1 provides a schematic of study visits and follow-up phases. Participants followed up until 18 months were asked to participate in an extended follow-up period until 27 months after receiving the first vaccine dose [HPV-001 (NCT00689741, Clinicaltrials.gov)]. Upon completion of short-term follow-up (0–27 months), vaccine and placebo recipients were invited to participate in a long-term follow-up study of ∼3-year duration (up to 76 months) [HPV-007 (NCT00120848, Clinicaltrials.gov)]. Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
Figure 1.
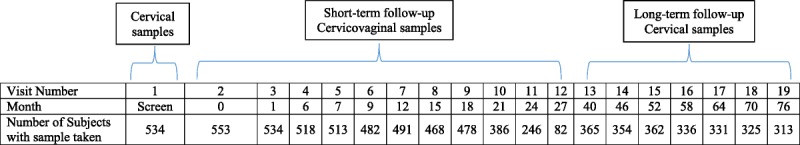
Schematic timeline and specimen collection. Cervicovaginal testing was done at every visit between months 0 and 27 (short-term follow-up), but was optional at month 21 and month 27 (Visits 10 and 12, respectively). Cervical testing was done at every visit between months 40 and 76 (long-term follow-up), but was also done at the screening visit and at month 6, month 12, and month 18 (visits 1, 4, 7, and 9, respectively). Cervicovaginal testing was optional at month 46, month 58, and month 70 (visits 14, 16, and 18, respectively).
The current ad-hoc analysis was restricted to 534 women randomized to the placebo arm (received only aluminum hydroxide adjuvant), who, at the screening visit, were seronegative for HPV16 and HPV18 antibodies and HPV DNA-negative, and had no more than 6 sexual partners and no prior abnormal Pap smear or treatment for cervical disease. Women who participated in the initial phase (0–18 months) and received 3 doses of placebo were eligible to participate in the extended and long-term follow-up phases. This study design provided a unique opportunity to specifically study determinants of incident infections as early events at the beginning of follow-up.
The guidelines of the Declaration of Helsinki, the International Conference on Harmonization-Good Clinical Practices were followed. The study protocol was approved by independent ethics committees or institutional review boards of the respective study centers. Written informed consent was obtained at the screening visit from all participants or from a legally acceptable representative for those younger than the legal age of consent.
Specimen Collection and HPV DNA Testing
As illustrated in Figure 1, cervical samples were collected for cytology and HPV DNA testing at the screening visit; at months 6, 12, and 18 in the initial phase and at 6-month intervals in the long-term follow-up period. Self-collected cervicovaginal samples were obtained at months 0, 6, and then every 3 months until the end of the extended follow-up phase.
HPV DNA testing for 25 genotypes was based on the broad-spectrum polymerase chain reaction (PCR) SPF10 LiPA25 system.10 The genotypes tested included hrHPV (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68) and low-risk HPV (lrHPV; 6, 11, 34, 40, 42, 43, 44, 53, 54, 70, and 74) types. Type-specific PCR was also done for HPVs 16 and 18.11
Risk Determinants
Participants completed a self-administered baseline questionnaire at the enrollment visit. Information on sociodemographic characteristics as well as behavioral, sexual, contraceptive, and other gynecological medical history was collected, covering known risk factors for HPV infection. The questionnaire was translated to the appropriate local language.
Statistical Analysis
Descriptive statistics were used to examine characteristics of the population by study site. Differences in distribution were assessed using the χ2 test. Univariate (adjusted for age and study site) and multivariable Cox proportional hazards models were used to estimate rate ratios (RRs) and 95% CI of HPV acquisition, separately for the short-term (0–27 months) and long-term (40–76 months) follow-up phases as well as for the entire follow-up (0–76 months). A parsimonious multivariable model was constructed using forward selection (stepwise, P < 0.15).
The association with HPV clearance over the entire follow-up was modeled through Cox proportional hazards regression with adjustment for age and region. Clearance was defined in 2 ways: as a single HPV-negative visit after 1 or more HPV-positive visits (liberal clearance) or conditioning on at least 2 consecutively negative visits definition (conservative clearance).
The following covariates were considered a priori as potential correlates of HPV acquisition and clearance: age (<20 years, ≥20 years), country/region (Brazil, North America), marital status (single/divorced, married/partner), ethnicity (White, black, others), education (<primary, primary-high school, ≥college), smoking history (never, former, current), lifetime number of sex partners (0–1, ≥2), age at first sexual intercourse (<15 years, ≥15 years), history of any sexually transmitted disease (no, yes), condom use (never, regularly, sometimes), and oral contraceptive use (never, regularly, sometimes). Analyses were performed for any HPV infection and grouped HPV infections (lrHPV, hrHPV) using combined clinician-collected cervical and self-collected cervicovaginal samples as we have previously shown comparable HPV detection between the 2 in this population.7 Months since infection acquisition and clearance were used as the time metric. Data were analyzed with Stata (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP.).
RESULTS
Table 1 presents baseline characteristics of the population stratified by study site. Mean age of participants was 20.79 years ±2.70 SD (standard deviation); women in Brazil were slightly younger (SD, 20.21 ± 2.93 years) compared with those in North America (21.26 years ±2.40 SD). Overall, the majority were single or divorced (77.3%), 69.1% were white, and 55.4% had beyond high school education. Regarding behavioral characteristics, 15.7% of women were current smokers, 10.7% had 2 or more lifetime sexual partners, 60.5% were 15 years and older at first sexual intercourse, 5.2% had a history of sexually transmitted diseases, 27.7% regularly used condoms, and 53.2% regularly used oral contraceptive. Comparison by study site indicated significant differences in certain characteristics (ie, age group, marital status, ethnicity, education, lifetime number of sex partners and age at first sexual intercourse), but not for smoking, history of any sexually transmitted disease, condom use, and oral contraceptive use.
TABLE 1.
Baseline Characteristics of the Study Population (N = 534) by Study Region, Control Arm of a Phase II HPV-16/18 Vaccine Trial
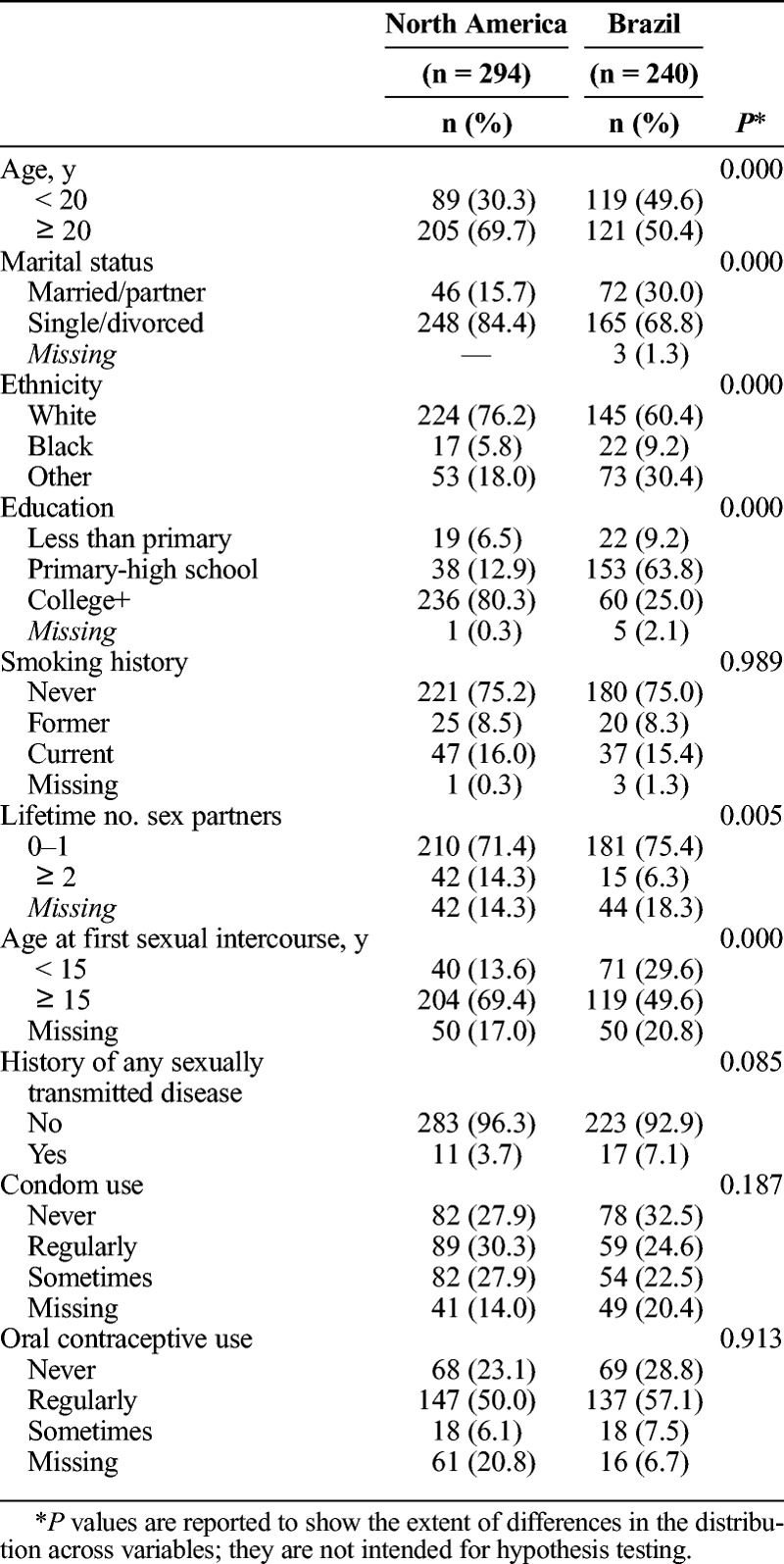
Table 2 presents results from age- and region-adjusted Cox regression models of risk factors associated with new HPV infections within the initial, short-term follow-up. During this period, the cumulative incidence of any HPV infection was 48.1% (8535.80 person-months; incidence rate, 30.11; 95% CI, 26.64–34.02); 19.1% for lrHPV (10783.57 person-months; incidence rate, 9.46; 95% CI, 7.79–11.48) and 45.3% for hrHPV (8926.5 person-months; incidence rate, 27.11, 95% CI: 23.90–30.75) types. Married women had a significantly lower incidence of HPV (any, hrHPV, lrHPV) compared with single/divorced women. Women who reported 2 or more lifetime sex partners had more than twice the risk of incident infections with any type and with hrHPV types relative to those with 1 partner or none. We did not find any association between the number of lifetime sexual partners and lrHPV infection. Having a history of any sexually transmitted disease, regular condom use, and occasional oral contraceptive use were also significantly associated with any HPV infection acquisition. We found no significant associations with age, study site, ethnicity, education, smoking, or age at first sexual intercourse.
TABLE 2.
Age- and Region-Adjusted Associations Between Baseline Sociodemographic and Behavioral Factors and HPV Acquisition, Short-Term Follow-Up (0–27 Months)
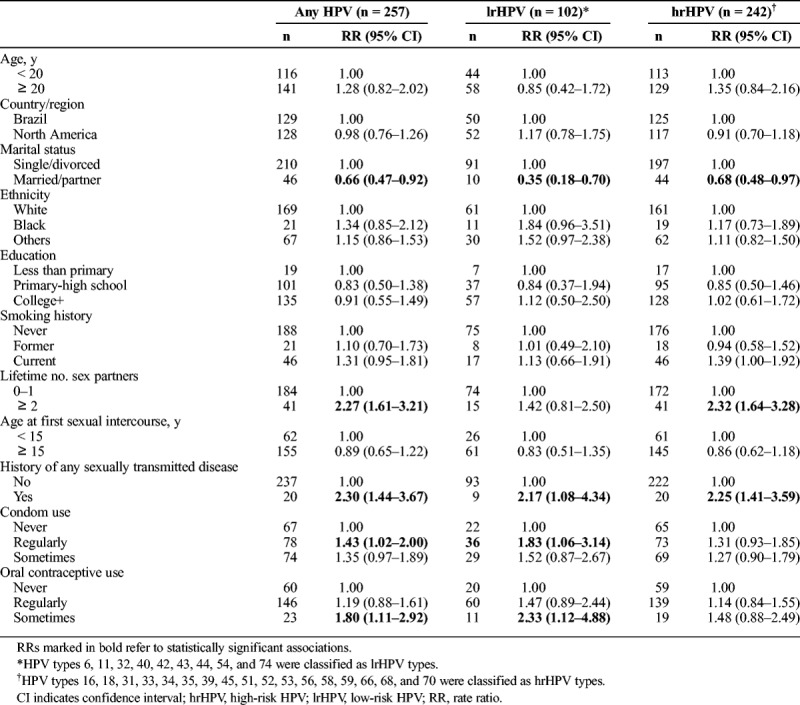
Table 3 shows multivariable analysis results. Marital status, lifetime number of sex partners, history of any sexually transmitted disease, and occasional use of oral contraceptives retained their independent associations with acquisition of any HPV infection, in addition to ethnicity. Having 2 or more lifetime sex partners (adjusted RR, 2.03; 95% CI, 1.37–3.02) and a history of any sexually transmitted disease (adjusted RR, 1.98; 95% CI, 1.19–3.29) were also positively associated with risk of incident hrHPV infections. A protective association was still observed with marital status, whereas age, study site, education, and smoking were generally unrelated to HPV acquisition.
TABLE 3.
Multivariable* Association Between Baseline Sociodemographic and Behavioral Factors and HPV Acquisition, Short-Term Follow-Up (0–27 Months)
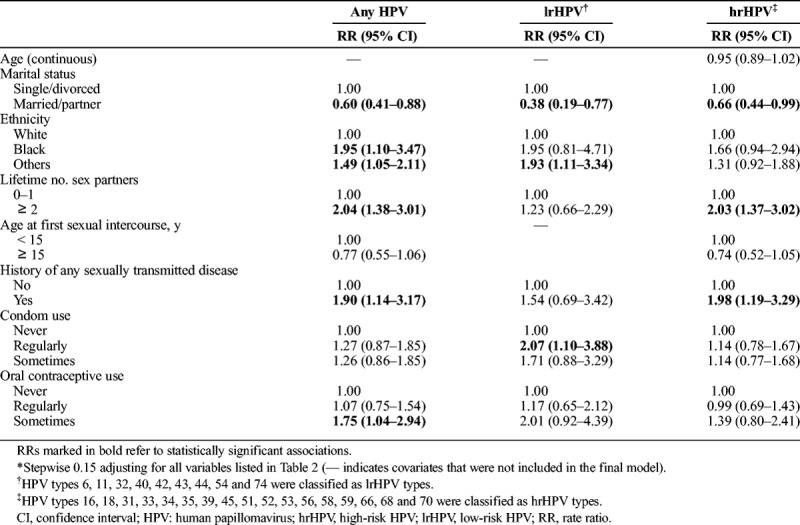
Supplemental Tables 1 http://links.lww.com/OLQ/A399 and 2 http://links.lww.com/OLQ/A400 present, respectively, age- and region- adjusted as well as multivariable analyses considering the entire follow-up (0–76 months), whereas corresponding findings restricted to long-term follow-up (40–76 months, ignoring outcomes prior to 40 months) are presented in Supplemental Tables 3 http://links.lww.com/OLQ/A401 and 4 http://links.lww.com/OLQ/A402. Overall, similar patterns of determinants were found irrespective of length of follow-up.
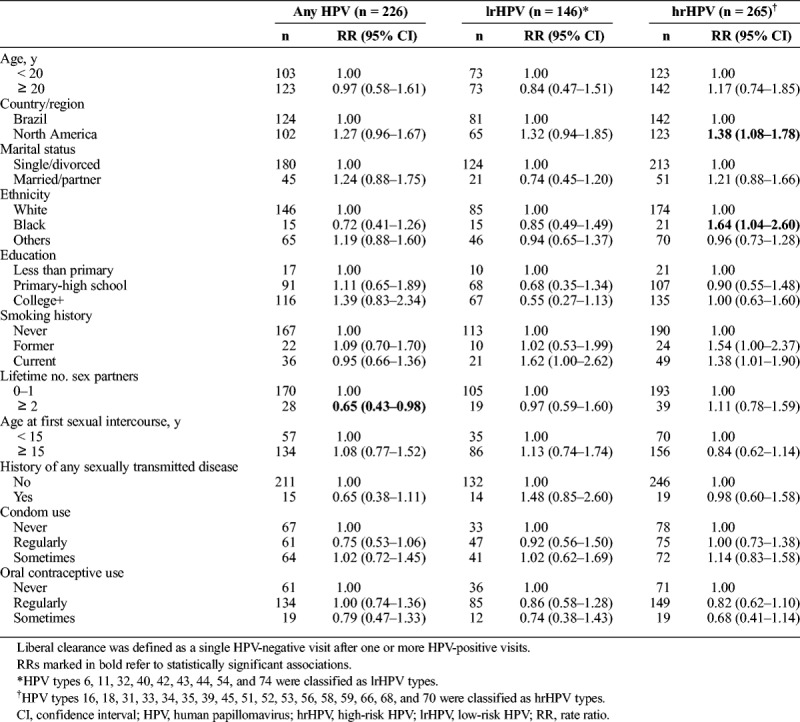
Table 4 presents age- and region-adjusted associations of baseline sociodemographic and behavioral factors with clearance of incident HPV infections. A total of 226 (71.1%) of 318 incident infections during follow-up cleared, including 146 (88.5%) of 165 lrHPV and 265 (86.9%) of 305 hrHPV infections. No significant associations were observed between each of age, marital status, education, smoking, age at first sexual intercourse, history of any sexually transmitted disease, condom use, and oral contraceptive use with HPV clearance (any, lrHPV, and hrHPV types). There was a tendency toward increased hrHPV clearance among women in North America (adjusted RR, 1.38; 95% CI, 1.08–1.78) and among black women (adjusted RR, 1.64; 95% CI, 1.04–2.60). A greater number of lifetime sex partners was significantly associated with reduced clearance rates only for any HPV infection. Only the negative effect of multiple sexual partners remained when defining clearance conservatively as 2 or more consecutive negative visits with the index HPV outcome (Supplemental Table 5 http://links.lww.com/OLQ/A403).
TABLE 4.
Age- and Region-Adjusted Associations Between Baseline Sociodemographic and Behavioral Factors and HPV Clearance (Liberal), Entire Follow-Up (0–76 Months)
DISCUSSION
Understanding the role of risk factors for the acquisition and clearance of HPV infection will support the development of effective prevention and screening strategies. We undertook the present analysis to better understand factors associated with HPV acquisition and clearance in a prospectively followed cohort of young women with no evidence of previous exposure to HPV (based on well-characterized serological and cervical samples results) and the restriction to having 6 or fewer lifetime sexual partners. We also attempted to identify a time frame when these factors were most influential. We found lifetime number of sex partners, marital status, and history of sexually transmitted disease at baseline to be the most important determinants of HPV acquisition. There were no remarkable differences in patterns of risk determinants when stratifying the analyses by length of follow-up.
Sexual behavior and age are well-recognized risk determinants for HPV infections. The number of lifetime sex partners emerged as a determinant of incident HPV infection, in line with previous studies. A high risk of acquiring a new HPV infection was reported in women aged 18–24 years soon after sexual debut with a first male sex partner, and the 1-year cumulative incidence was 28.5% which increased to 39.2% by 2 years and to 49.1% by 3 years.12 In a longitudinal study on 97 virgin women and 105 monogamous women (ie, only had 1 sex partner) at enrollment, HPV acquisition was strongly determined by the accumulation of sexual partners; women with ≥3 partners had a 9-fold increased risk for acquiring HPV compared with women with 1 partner during follow-up.13 Unfortunately, we did not collect information on new sexual partners during follow-up.
In a meta-analysis of 194 studies published between 1995 and 2009, comprising more than one million women with normal cytological findings, the age-specific HPV distribution had a first peak in prevalence at younger ages (<25 years) just after sexual debut, a lower prevalence plateau at middle ages, followed by a varying rebound at older ages (≥45 years).14 Similarly, HPV acquisition predominated at younger ages in a population-based cohort of 7,237 women in Costa Rica.15 Although age in the current analysis was not a strong predictor of HPV acquisition, it is possible that the restricted age range of participants in this cohort (15–25 years) may have been too narrow to allow age-specific comparisons in risk. Various other studies found younger age and higher number of lifetime sex partners to be significant risk factors for new HPV infections,16–19 but the patterns are not always consistent across populations.20
Two early prospective studies on the epidemiology of HPV acquisition reported differences in risk-factor profiles according to HPV oncogenicity.21,22 In a subset of 1425 participants aged 18 to 60 years in the Ludwig-McGill cohort longitudinal investigation of the natural history of HPV infection in Brazil, an association was found between age, age at first intercourse and oral contraceptive use with risk of hrHPV, but not with lrHPV, whereas number of sexual partners was associated with both hrHPV and lrHPV types.21 In a cohort of 1610 women aged 15 to 85 years who were HPV negative and with normal cytological results at enrollment in Colombia, age and having new sex partners during follow-up were consistent predictors of oncogenic, but not non-oncogenic, HPV in multivariable analyses.22 We found a strong protective association between married women (ie, more likely to be in monogamous relationship than divorced/separated women) and incident hrHPV and lrHPV, even after adjustment whereas for having a history of any sexually transmitted disease the observed increased risk remained statistically significant in the multivariable model only for hrHPV types.
We did not find an association between smoking and HPV acquisition, consistent with some18,22,23 but not with all studies.21,24 Interestingly, condom use was associated with an increased risk of HPV (any, lrHPV, hrHPV) in multivariable analyses; statistical significance was attained only for lrHPV among regular condom users. It is also likely that women with an increased number of sex partners were more likely to use condoms.
As for determinants of clearance of grouped HPV infections, these have been less frequently studied. Apart from finding an inverse association between an increased number of lifetime sex partners and clearance of any HPV, and a marginal increased risk of clearance of hrHPV types among black women and those in North America, no associations were found between other sociodemographic and behavioral factors and HPV persistence. Our finding that black women cleared their hrHPV infection more rapidly than whites is in keeping with the results from another study.25 A cohort of 416 Canadian Inuit women also reported no association between sociodemographic, lifestyle or sexual factors with clearance of any incident HPV infections.26 Other studies found that condom and oral contraceptive use conferred varying levels of protection in terms of HPV clearance based on oncogenic potential.25,27
A limitation of our study is the lack of information on other epidemiological risk factors such as parity, genital hygiene, nutritional status, behaviors of sexual partners, and male circumcision.28 The main strength of our study is that our sample had a low risk profile; participants were young healthy women who had a maximum of 6 lifetime sex partners. The fact that participants were young and ascertained to be HPV negative upon entry supports the contention that what we detected as incident infections may be presumed as true new infections. However, given the uncertainty that exists concerning latency, instances of new detection may represent the unmasking of a previously undetectable infection because of better sampling via exfoliation or fluctuations in productivity of infection foci.29
It is plausible to assume that universal vaccination against some of the most clinically relevant HPV types will transform the epidemiology of these infections, a change that is already noticeably strong even in settings with suboptimal vaccination coverage.30 Establishing an epidemiologic baseline, such as our study does, will help in assisting the design of surveillance mechanisms to monitor infection outcomes and behavioral risk factors in vaccinated populations.
Footnotes
Clinical Trial Registration: NCT00689741 and NCT00120848.
A.-B.M. is currently affiliated to the Division of Adolescent and Young Adult Medicine, Department of Pediatrics, University of California, Los Angeles, CA; D.M.H. is currently affiliated to the Departments of Family Medicine, Obstetrics and Gynecology, University of Michigan, Ann Arbor, MI.
Acknowledgments: The authors thank the study participants and their families, the study investigators and their staff members and the central and local teams of GSK Vaccines for their participation in the clinical studies HPV-001 (NCT00689741) and HPV-007 (NCT00120848). The authors would also like to thank Thibaud Andre and Benjamin Lemaire (Business & Decision Life Sciences, on behalf of GSK) for coordinating communications among coauthors during the development of this manuscript and technical proofreading. The authors gratefully acknowledge Mrs Anne Schuind for her involvement in the design and conduct of the parent trial at GlaxoSmithKline LLC, and Dr. Barbara Romanowski for her leadership role in patient recruitment in Canada.
Authors contributions: A.-B.M., D.M.H., and S.K.T. (US centers); P.C.dB., N.S.dC., P.N., C.M.R.-M., and J.C.T. (Brazilian centers); and E.L.F. and B.R. (Canadian centers) were principal investigators of clinical studies HPV-001 (NCT00689741) and HPV-007 (NCT00120848) and participated in the design, recruitment and/or follow-up of participants. At GlaxoSmithKline LLC (USA), G.D. contributed toward study conception/design and supervised the conduct of these studies. M.E.Z. and E.L.F. (McGill University) developed the statistical methodology used in the analysis of these epidemiological data, interpreted the findings, and drafted the manuscript. All other authors reviewed and commented on initial and subsequent versions. All authors had full access to the data and gave final approval before submission.
Conflicts of Interest and Sources of Funding: A.V.R. has received reimbursement from the GSK group of companies for travel expenses, presenting at the International Papillomavirus Conference in 2011 (Berlin) and 2012 (Puerto Rico). P.N., C.M.R.-M., J.C.T., A.-B.M., D.M.H., and S.K.T. have received, either directly or through their institution, research grants from the GSK group of companies to conduct the clinical studies reported here. P.N. has received research grants for other clinical trials sponsored by the GSK group of companies. J.C.T. has received payments from the GSK group of companies for lectures including service on speaker bureau. A.-B.M. and J.C.T. have received payments for participating in advisory board meetings for the GSK group of companies. N.S.dC. has received financial support from the GSK group of companies as clinical investigator for the PATRICIA trial and for lectures. E.L.F. has served as an occasional consultant to companies involved in human papillomavirus (HPV) vaccines (Merck and GSK group of companies) and in HPV diagnostics (Roche, Gen-Probe, BD). His institution has received an unrestricted research grant from Merck. G.D. was employed by the GSK group of companies at the time the study was conducted but is now a full-time employee of Takeda Pharmaceuticals. He holds shares in the GSK group of companies and Takeda Pharmaceuticals, and holds patents in the Human Papillomavirus and in the Herpes Simplex virus vaccine fields. B.R. and P.C.dB. were paid by GSK as part of study. M.E.Z. declares no conflicts of interest. The following studies (NCT00689741 and NCT00120848) were funded by GlaxoSmithKline Biologicals S.A., which also provided support for publication coordination. The McGill University (Quebec, Canada) covered all costs related to statistical analysis and publication development. G.S.K. was not involved in the analysis of data. The authors received no financial support or other form of compensation for the development of the manuscript. G.S.K. had the opportunity to review the manuscript.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (http://www.stdjournal.com).
Contributor Information
Collaborators: for the HPV-007 Study Group
REFERENCES
- 1.Bosch FX, Burchell AN, Schiffman M, et al. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine 2008; (26 Suppl 1):K1–K16. [DOI] [PubMed] [Google Scholar]
- 2.Clifford GM, Smith JS, Plummer M, et al. Human papillomavirus types in invasive cervical cancer worldwide: A meta-analysis. Br J Cancer 2003; 88:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muñoz N, Bosch FX, de Sanjosé S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003; 348:518–527. [DOI] [PubMed] [Google Scholar]
- 4.de Sanjosé S, Brotons M, Pavón MA. The natural history of human papillomavirus infection. Best Pract Res Clin Obstet Gynaecol 2018; 47:2–13. [DOI] [PubMed] [Google Scholar]
- 5.Malagón T, Burchell A, El-Zein M, et al. Assortativity and mixing by sexual behaviors and sociodemographic characteristics in young adult heterosexual dating partnerships. Sex Transm Dis 2017; 44:329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castellsagué X, Bosch FX, Muñoz N. Environmental co-factors in HPV carcinogenesis. Virus Res 2002; 89:191–199. [DOI] [PubMed] [Google Scholar]
- 7.Ramanakumar AV, Naud P, Roteli-Martins CM, et al. Incidence and duration of type-specific human papillomavirus infection in high-risk HPV-naïve women: Results from the control arm of a phase II HPV-16/18 vaccine trial. BMJ Open 2016; 6:e011371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harper DM, Franco EL, Wheeler C, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: A randomised controlled trial. Lancet 2004; 364:1757–1765. [DOI] [PubMed] [Google Scholar]
- 9.Harper DM, Franco EL, Wheeler CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: Follow-up from a randomised control trial. Lancet 2006; 367:1247–1255. [DOI] [PubMed] [Google Scholar]
- 10.Kleter B, Van Doorn LJ, Schrauwen L, et al. Development and clinical evaluation of a highly sensitive PCR-reverse hybridization line probe assay for detection and identification of anogenital human papillomavirus. J Clin Microbiol 1999; 37:2508–2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baay MF, Quint WG, Koudstaal J, et al. Comprehensive study of several general and type-specific primer pairs for detection of human papillomavirus DNA by PCR in paraffin-embedded cervical carcinomas. J Clin Microbiol 1996; 34:745–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winer RL, Feng Q, Hughes JP, et al. Risk of female human papillomavirus acquisition associated with first male sex partner. J Infect Dis 2008; 197:279–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kjaer SK, Chackerian B, Van Den Brule AJ, et al. High-risk human papillomavirus is sexually transmitted: Evidence from a follow-up study of virgins starting sexual activity (intercourse). Cancer Epidemiol Biomarkers Prev 2001; 10:101–106. [PubMed] [Google Scholar]
- 14.Bruni L, Diaz M, Castellsagué X, et al. Cervical human papillomavirus prevalence in 5 continents: Meta-analysis of 1 million women with normal cytological findings. J Infect Dis 2010; 202:1789–1799. [DOI] [PubMed] [Google Scholar]
- 15.Castle PE, Schiffman M, Herrero R, et al. A prospective study of age trends in cervical human papillomavirus acquisition and persistence in Guanacaste, Costa Rica. J Infect Dis 2005; 191:1808–1816. [DOI] [PubMed] [Google Scholar]
- 16.Safaeian M, Kiddugavu M, Gravitt PE, et al. Determinants of incidence and clearance of high-risk human papillomavirus infections in rural Rakai, Uganda. Cancer Epidemiol Biomarkers Prev 2008; 17:1300–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franco E, Villa LL, Sobrinho JP, et al. Epidemiology of acquisition and clearance of cervical human papillomavirus infection in women from a high-risk area for cervical cancer. J Infect Dis 1999; 180:1415–1423. [DOI] [PubMed] [Google Scholar]
- 18.Goodman MT, Shvetsov YB, McDuffie K, et al. Prevalence, acquisition, and clearance of cervical human papillomavirus infection among women with normal cytology: Hawaii human papillomavirus cohort study. Cancer Res 2008; 68:8813–8824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moscicki AB, Hills N, Shiboski S, et al. Risks for incident human papillomavirus infection and low-grade squamous intraepithelial lesion development in young females. JAMA 2001; 285:2995–3002. [DOI] [PubMed] [Google Scholar]
- 20.Banura C, Sandin S, Van Doorn LJ, et al. Type-specific incidence, clearance and predictors of cervical human papillomavirus infections (HPV) among young women: A prospective study in Uganda. Infect Agent Cancer 2010; 5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rousseau MC, Franco EL, Villa LL, et al. A cumulative case-control study of risk factor profiles for oncogenic and nononcogenic cervical human papillomavirus infections. Cancer Epidemiol Biomarkers Prev 2000; 9:469–476. [PubMed] [Google Scholar]
- 22.Muñoz N, Méndez F, Posso H, et al. Incidence, duration, and determinants of cervical human papillomavirus infection in a cohort of Colombian women with normal cytological results. J Infect Dis 2004; 190:2077–2087. [DOI] [PubMed] [Google Scholar]
- 23.Nahar Q, Sultana F, Alam A, et al. Genital human papillomavirus infection among women in Bangladesh: Findings from a population-based survey. PLoS One 2014; 9:e107675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chatzistamatiou K, Moysiadis T, Vryzas D, et al. Cigarette smoking promotes infection of cervical cells by high-risk human papillomaviruses, but not subsequent E7 Oncoprotein expression. Int J Mol Sci 2018; 19:pii: E422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richardson H, Abrahamowicz M, Tellier PP, et al. Modifiable risk factors associated with clearance of type-specific cervical human papillomavirus infections in a cohort of university students. Cancer Epidemiol Biomarkers Prev 2005; 14:1149–1156. [DOI] [PubMed] [Google Scholar]
- 26.Bennett R, Cerigo H, Coutlée F, et al. Incidence, persistence, and determinants of human papillomavirus infection in a population of inuit women in northern Quebec. Sex Transm Dis 2015; 42:272–278. [DOI] [PubMed] [Google Scholar]
- 27.Kjaer SK, van den Brule AJ, Bock JE, et al. Determinants for genital human papillomavirus (HPV) infection in 1000 randomly chosen young Danish women with normal pap smear: Are there different risk profiles for oncogenic and nononcogenic HPV types? Cancer Epidemiol Biomarkers Prev 1997; 6:799–805. [PubMed] [Google Scholar]
- 28.Veldhuijzen NJ, Snijders PJ, Reiss P, et al. Factors affecting transmission of mucosal human papillomavirus. Lancet Infect Dis 2010; 10:862–874. [DOI] [PubMed] [Google Scholar]
- 29.Gravitt PE, Winer RL. Natural history of HPV infection across the lifespan: Role of viral latency. Viruses 2017; 9 pii:E267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Markowitz LE, Gee J, Chesson H, et al. Ten years of human papillomavirus vaccination in the United States. Acad Pediatr 2018; 18:S21–S22. [DOI] [PMC free article] [PubMed] [Google Scholar]


