Supplemental Digital Content is available in the text.
Keywords: imaging, MRI, off-resonance, silicone oil, ultrasound, uveal melanoma, vitrectomy
Abstract
Uveal melanoma (UM), the most common primary intraocular tumour, is often complicated by exudative retinal detachment (RD). Sometimes, this exudative RD is mistaken for a rhegmatogenous detachment and is subsequently treated with vitrectomy with silicone oil (SiOil) tamponade. As SiOil prevents ultrasound imaging, the diagnosis, treatment planning and/or follow-up of UM underlying the detachment are often severely hindered by the SiOil. We aim to develop and evaluate new MRI methods to image UM patients with a SiOil tamponade and evaluate this in vivo. A dedicated MRI protocol for 3 and 7 T was developed and subsequently evaluated in three patients. The MRI protocol developed was evaluated in three patients. In the first patient, SiOil hindered follow-up and therefore MRI was indicated. No tumour recurrence was found after two follow-up scans. The second and third patient underwent vitrectomy with SiOil for assumed rhegmatogenous RD in another hospital, during which a mass was found. In these cases, MRI was used to determine whether the lesion was UM and perform measurements to plan brachytherapy treatment. In general, the proposed workflow is more complicated on 7 T than on 3 T as the off-resonance effects scale linearly with field strength. For example, the shimming procedure needed modifications at 7 T, whereas at 3 T, the automatic shimming sufficed. However, at 7 T, higher resolution images were obtained compared with 3 T (0.6 vs. 0.8 mm3). A dedicated MRI protocol enables high-resolution imaging of vitrectomized eyes with SiOil tamponade, enabling treatment planning or follow-up in UM patients.
Introduction
Uveal melanoma (UM) is the most common primary intraocular tumour [1,2]. Exudative retinal detachment (RD) is a common complication of UM, possibly the result of a reduced venous return, caused by the tumour, leading to diffuse choroidal leakage [3]. Exudative RD can be mistaken for a rhegmatogenous RD, in which case the RD is treated by vitrectomy, that is, the vitreous body may be replaced with a silicone oil (SiOil) tamponade. Ultrasound (US), an important imaging modality in the diagnosis, treatment planning and follow-up of UM (Fig. 1a), is severely hindered by the SiOil as the sound waves reflect at the SiOil–water interface (Fig. 1b). As the lack of an accurate imaging modality makes treatment planning or adequate follow-up impossible, alternative imaging options need to be developed to save the eye and vision of these patients.
Fig. 1.
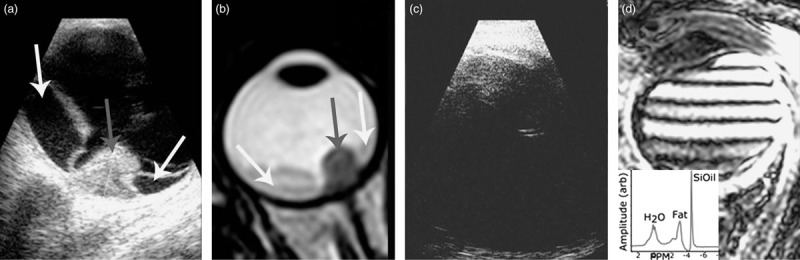
(a) Imaging using US reveals UM (grey arrow) and RD (white arrows). (b) MRI allows for a 3D evaluation of the tumour and surrounding structures. A T2-weighted image on which the tumour appears as a hypointense mass (grey arrow) with adjacent less hypointense RD (white arrows). (c) As US waves are reflected at the SiOil–water interface, US imaging is not possible in UM patients after vitrectomy, preventing accurate diagnosis or follow-up. (d) Although MRI should be possible in eyes with a SiOil tamponade, the off-resonance of the SiOil causes the conventional protocols to fail, resulting in strong artefacts. The MR spectrum of an UM patient with SiOil tamponade, showing the strong 4.5 ppm off-resonance of SiOil compared with water. The peak at 3.2 ppm is the fat signal from the orbital fat. RD, retinal detachment; SiOil, silicone oil; UM, uveal melanoma; US, ultrasound.
MRI, shown in Fig. 1c, is, in principle, not hindered by the presence of SiOil, and could potentially provide the imaging data for these patients [4–7]. However, the strong off-resonance effects of SiOil prevent clinical interpretation of MRI acquired with conventional protocols (Fig. 1d). We therefore developed and evaluated a dedicated protocol to image UM patients with SiOil.
Methods
An MRI protocol was developed at 7T (Achieva; Philips Healthcare, Best, The Netherlands) and subsequently translated to 3 T (Ingenia; Philips Healthcare). Preliminary experiments on the MR characteristics of the SiOil (RS-OIL ECS 1000cS, AL.CHI.MI.A. SRL, Ponte S. Nicolo, Italy) (Supplementary Information, Supplemental digital content 1, http://links.lww.com/MR/A99) showed a T1 relaxation time of 0.9 and 1.3 s at 3 and 7 T, respectively, with a 4.5 parts-per-million (ppm) frequency offset from water.
For optimal image quality, a surface coil should be used for MRI of the orbit [5,6,8]. On 7 T, a house build dedicated eye coil [5] was used in combination with the Nova Medical transmit coil (Nova Medical Inc., Wilmington, Massachusetts, USA), whereas at 3 T, a 47 mm surface receive coil (Philips Healthcare) in combination with the body transmit coil was used. As these surface coils have a very local sensitivity, they mainly receive a signal from the SiOil, which has a 4.5 ppm offset compared with water.
This off-resonance of the SiOil severely hinders the automatic calibration procedure of the MRI; thus, a manual calibration strategy was developed. First, a fast, low resolution, scan of the complete head was performed with the automatic calibration for an initial determination of the water resonance frequency, F0, and RF-power settings. Subsequently, the correct settings for imaging the eye are determined by performing single voxel MR-spectroscopy (MRS) on the eye as MRS allows for a direct inspection of the resulting calibration (Fig. 1d). These parameters are then fixed and used for subsequent scans.
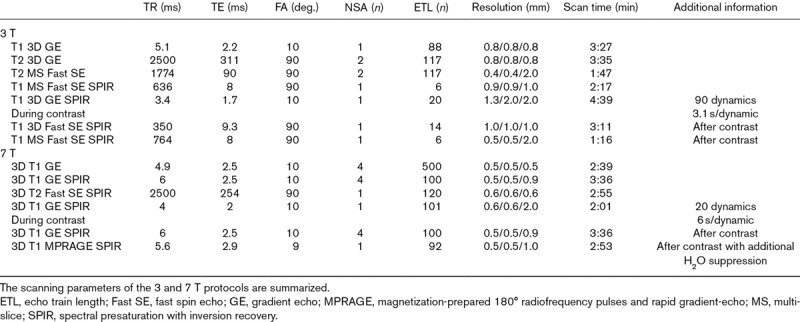
The clinical, 7 T, imaging protocol consisted of T1-weighted scans with and without SPIR SiOil suppression. As some residual liquid remains in the vitreous, an inversion-recovery MPRAGE scan (T-inv: 1280 ms) with SPIR SiOil suppression was used to supress both the remaining vitreous liquid and SiOil. The protocol furthermore included a T2-weighted scan with SPIR SiOil suppression. During contrast administration of 0.1 mmol/kg gadoterate meglumine (gd-DOTA, DOTAREM; Guerbet, Roissy CdG Cedex, France), a dynamic contrast enhancement scan was performed. Finally, the T1-weighted scans with SiOil suppression with and without water suppression were performed after gadolinium.
On 3 T, the imaging protocol consisted of T1-weighted and T2-weighted scans without any suppression and with SPIR fat suppression. During contrast administration of 0.1 mmol/kg gd-DOTA, a dynamic contrast enhancement scan was performed. Finally, after contrast, the T1-weighted scans with fat suppression were performed again.
The total MRI protocol took less than 50 min. An overview of the scan parameters can be found in Table 1. This protocol was evaluated at 7 T in one patient after informed consent. Later, two additional patients were referred from Ophthalmology and scanned on clinical indication. Ethical approval was obtained to access the data of these patients. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or the national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Table 1.
Scanning parameters of the 3T and 7T protocols.
Results
Determination of the correct MR settings did not always succeed after the first MRS scan. Often, there was still too large an F0 offset to produce good shimming. This was, however, easily recognized in the resulting spectrum, and after manually correcting the initial F0, on the basis of the offset of the first MRS-spectrum, a second MRS scan produced the correct settings (Fig. 1d inset).
The first patient received a SiOil tamponade after developing RD after UM resection and brachytherapy and was scanned at 7 T. As the SiOil prevented US-based measurements needed for adequate screening for tumour recurrence, an MRI was requested. The MRI showed multiple lesions in the eye with a maximum basal diameter of 3 mm (Fig. 2a–c; Supplementary Figs 2 and 3, Supplemental digital content 1, http://links.lww.com/MR/A99). The lesions located near the RD did not enhance after contrast administration, whereas the lesion located at the position of the original tumour did enhance. As this enhancement could be the result of either new tumour activity or radiotherapeutic damage, the patient received a second MRI ~6 months later. As the enhancing lesion did not show any significant changes or growth compared with the first scan, it was classified as scarred residue or damage after radiotherapy treatment and thus enucleation could be avoided.
Fig. 2.
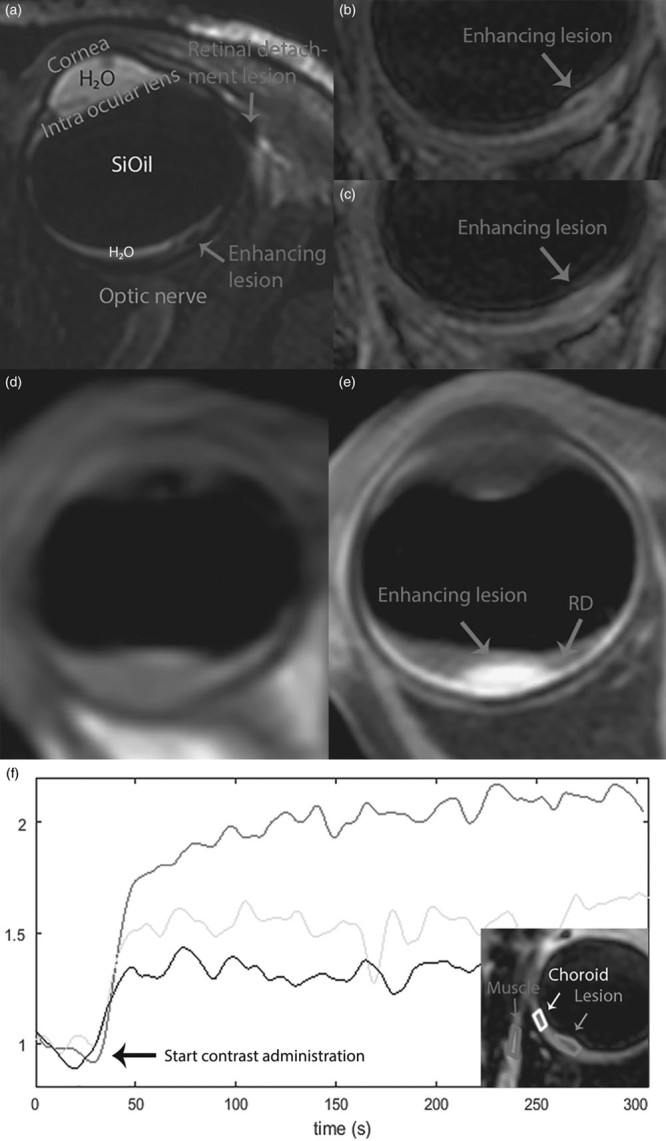
(a–c) 7 T MRI of the first patient. (a) Annotated T2 image with spectral presaturation with inversion recovery silicone oil (SiOil) suppression on the basis of all images of Supplementary Fig. 2 (Supplemental digital content 1, http://links.lww.com/MR/A99). (b) The T1-weighted image after contrast with SiOil and free-water suppression showed an enhancing lesion (arrow), which did not significantly change after a rescan 6 months later (c). (d) Precontrast and (e) postcontrast T1-weighted image of the second patient, scanned at 3 T. (f) Dynamic imaging during the contrast administration allows for an analysis of the perfusion of the different anatomies of the eye. The enhancement curves of muscle, lesion and choroid show that the lesion enhances, but only slightly more than the normal retinal tissue. RD, retinal detachment.
The second patient was referred after UM was suspected during vitrectomy for RD. On fundoscopy, the diagnosis of UM was confirmed and ruthenium plaque brachytherapy was considered optimal treatment. MRI obtained at 3 T showed a slight residual RD and a lesion with a maximum prominence of 1.5 mm (without choroid) and a basal diameter of 6 mm (Fig. 2e; Supplementary Fig. 4, Supplemental digital content 1, http://links.lww.com/MR/A99). On the basis of these measurements, the optimal ruthenium brachytherapy applicator was selected and dose calculations for further treatment planning were performed.
In the third patient, a small mass was discovered during vitrectomy. On fundoscopy, a UM was suspected. As US imaging was not possible because of the SiOil, an MRI was requested to confirm diagnosis and to assess the tumour dimensions. The images, acquired at 3 T, showed a slight focal thickening of the inner aspect of the choroid located superior and medial to the optic nerve, which appears slightly hyperintense (Fig. 2f; Supplementary Fig. 5, Supplemental digital content 1, http://links.lww.com/MR/A99): because of the small size of the lesion, it was difficult to differentiate between physiologic enhancement, caused by the high vascularity of the uvea, and pathologic enhancement of the tumour. Overall, the MRI showed a lesion with characteristics corresponding with UM, which was later confirmed by a biopsy.
Discussion
In 1998, Herrick et al. [9] showed that 1.5 T MRI can be used to image the eye after vitrectomy, but at that time, MRI had insufficient resolution to evaluate UM characteristics and size. Currently, surface coils [8] and higher field strengths enable high-resolution imaging, but both these advances make the MRI more susceptible to the off-resonance effects of SiOil. We have shown that through a modified set-up of the MRI, the eyes of vitrectomized patients can be evaluated successfully, which opens the route to eye-preserving therapy.
In general, the proposed workflow is more complicated at 7 T than at 3 T as the off-resonance effects scale linearly with field strength. As a result, at 3 T, these patients can be scanned without major modifications to the scanner software. Some off-resonance effects are present in the images (Supplementary Fig. 4A, Supplemental digital content 1, http://links.lww.com/MR/A99), but these do not limit clinical evaluation as at 7 T, some minor adjustments to the scanner software were needed to scan these patients. However, at 7 T, higher resolution images were obtained compared with 3 T (0.6 vs. 0.8 mm3). At both field strengths, eye motion is a problem, but several techniques have been proposed, such as cued blinking [5,10], that can limit these effects.
As the appearance of UM on MRI is significantly different from that on US, it is often difficult to relate the US images to the MRI. We therefore recommend that if a known UM patient develops RD, both an MRI and US are performed before the vitrectomy to correlate the MRI after vitrectomy to the US images taken before vitrectomy.
Conclusion
Although RD is a common complication of UM, the UM is often missed when these patients present in a peripheral hospital. If UM is discovered during the vitrectomy, the patient is referred to a specialized centre, but the SiOil tamponade hinders subsequent diagnosis and therapy. We have shown that a dedicated MRI protocol enables high-resolution imaging of vitrectomized eyes with UM, enabling eye-preserving treatment and follow-up of these patients.
Acknowledgements
The authors thank Maarten Versluis (Philips Healtcare, Best, The Netherlands) for stimulating discussion of the design of the MRI protocol and Berit Verbist (Radiology, LUMC) for assistance with the interpretation of the MR images. This work is part of the research programme protons4vision with project number 14654, which is financed by the Netherlands Organisation for Scientific Research (NWO).
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Footnotes
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website, www.melanomaresearch.com.
References
- 1.Singh AD, Turell ME, Topham AK. Uveal melanoma: trends in incidence, treatment, and survival. Ophthalmology. 2011; 118: 1881–1885 [DOI] [PubMed] [Google Scholar]
- 2.Chang AE, Karnell LH, Menck HR. The national cancer data base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. Cancer. 1998; 83: 1664–1678 [DOI] [PubMed] [Google Scholar]
- 3.Kivelä T, Eskelin S, Mäkitie T, Summanen P. Exudative retinal detachment from malignant uveal melanoma: predictors and prognostic significance. Invest Ophthalmol Vis Sci. 2001; 42: 2085–2093 [PubMed] [Google Scholar]
- 4.Wong T, Lo LW, Fung PY, Lai HY, She HL, Ng WK, et al. Magnetic resonance imaging of breast augmentation: a pictorial review. Insights Imaging. 2016; ; 7: 399–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beenakker JWM, Ferreira TA, Soemarwoto KP, Genders SW, Teeuwisse WM, Webb AG, Luyten GP. Clinical evaluation of ultra-high-field MRI for three-dimensional visualisation of tumour size in uveal melanoma patients, with direct relevance to treatment planning. MAGMA. 2016; 29: 571–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Graaf P, Göricke S, Rodjan F, Galluzzi P, Maeder P, Castelijns JA, Brisse HJ; European Retinoblastoma Imaging Collaboration (ERIC). Guidelines for imaging retinoblastoma: imaging principles and MRI standardization. Pediatr Radiol. 2012; 42: 2–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamrava M, Sepahdari AR, Leu K, Wang PC, Roberts K, Demanes DJ, et al. Quantitative multiparametric MRI in uveal melanoma: increased tumor permeability may predict monosomy 3 Neuroradiology. 2015; 57: 833–840 [DOI] [PubMed] [Google Scholar]
- 8.Vokurka EA, Watson NA, Watson Y, Thacker NA, Jackson A. Improved high resolution MR imaging for surface coils using automated intensity non-uniformity correction: feasibility study in the orbit. J Magn Reson Imaging. 2001; 14: 540–546 [DOI] [PubMed] [Google Scholar]
- 9.Herrick RC, Hayman LA, Maturi RK, Diaz-Marchan PJ, Tang RA, Lambert HM. Optimal imaging protocol after intraocular silicone oil tamponade. Am J Neuroradiol. 1998; 19: 101–108 [PMC free article] [PubMed] [Google Scholar]
- 10.Berkowitz BA, McDonald C, Ito Y, Tofts PS, Latif Z, Gross J. Measuring the human retinal oxygenation response to a hyperoxic challenge using MRI: eliminating blinking artifacts and demonstrating proof of concept. Magn Reson Med. 2001; 46: 412–416 [DOI] [PubMed] [Google Scholar]


