Abstract
Background and study aims TC-325 is a novel mineral hemostatic powder that creates a mechanical barrier by absorbing blood components and promoting clotting. Recently approved for use in humans, it has shown promise for treatment of upper gastrointestinal bleeding (UGIB). However, because there have been no large studies of TC-325, its true efficacy and safety profile remain unknown. We performed a systematic review and meta-analysis to determine the safety and efficacy of TC-325 in treating UGIB, based on rates of initial hemostasis, rebleeding, and adverse events (AEs).
Methods We searched the MEDLINE/PubMed, EMBASE, CENTRAL, Latin-American and Caribbean Health Sciences Literature databases, as well as the gray literature, to identify articles describing use of TC-325 up to October 2018. Primary outcomes were initial hemostasis and rebleeding. AEs were described as a secondary outcome. Risk of bias was assessed with international scores.
Results We identified 2077 records after removal of duplicates. We included 50 studies, involving a collective total of 1445 patients, in the quantitative synthesis. Primary hemostasis and rebleeding rates were 90.7 % and 26.1 %, respectively. Subgroup analyses showed similar results. Only eight AEs were reported.
Conclusions TC-325 appears to be a safe, effective treatment for UGIB. The overall rate of initial hemostasis after TC-325 use is high, regardless of etiology of bleeding or whether TC-325 is used as a primary or rescue therapy. Although it is also associated with high rebleeding rates, rates of AEs and equipment failure after TC-325 use are extremely low.
Introduction
Upper gastrointestinal bleeding (UGIB) is a common condition, with an annual incidence ranging from 37 to 172 cases per 100,000 population. In the United States, UGIB is responsible for more than 300,000 hospital admissions per year, at an annual cost of $ 7.6 billion 1 2 3 4 . The most common etiologies are peptic ulcer (in 31 % – 67 % of cases), erosive disease (in 7% – 31 %), variceal hemorrhage (in 4 %-20 %), esophagitis (in 3% – 12 %), malignancy (in 2 % – 8 %), and Mallory-Weiss tears (in 4 % – 8 %) 4 5 .
Recent advances in clinical and endoscopic treatments have changed outcomes of UGIB 1 6 . The most widely used therapies for non-variceal bleeding are endoscopic clips, injection therapy, thermocoagulation, and argon plasma coagulation, whereas band ligation and sclerotherapy are recommended modalities for variceal bleeding 7 . Despite such advances, some specific situations still pose a challenge when conventional endoscopic methods are employed 8 9 10 As a consequence, mean estimated rates of primary hemostasis failure and rebleeding after endoscopic treatment of UGIB are approximately 15 % and 25 %, respectively, with mortality rates as high as 14 % reported in some studies 4 5 7 11 . Therefore, development of novel devices and products is particularly opportune. For example, three topical hemostatic powders have recently been added to the endoscopic armamentarium against UGIB: Ankaferd Blood Stopper (Ankaferd Health Products Ltd., Istanbul, Turkey); EndoClot (EndoClot Plus Inc., Santa Clara, California, United States); and TC-325 (Hemospray; Cook Medical, Winston-Salem, North Carolina, United States). Ankaferd Blood Stopper is an herbal extract, with an unknown mechanism of action that is widely used in Turkey (where it was developed) but has yet to be approved by the US Food and Drug Administration. EndoClot is an absorbable modified polymer that rapidly absorbs water from blood, thus concentrating red blood cells, platelets, and coagulation factors at the bleeding site, resulting in the rapid formation of a hemostatic layer. Finally, TC-325 is an inorganic, nonabsorbable, biologically inert mineral blend powder that is delivered directly to the bleeding area by a catheter introduced into the working channel of the endoscope with the aid of a pressurized CO 2 canister. When TC-325 comes into contact with moisture on the bleeding surface, it adheres to it, creating a mechanical barrier for hemostasis 6 12 . Some data also suggest that TC-325 reduces coagulation time and promotes clot formation by absorbing blood components and activating the clotting cascade 13 . It has been largely employed worldwide and has become the main hemostatic powder used in daily practice.
Since its first use in humans in 2011, there have been several studies demonstrating that TC-325 shows promise for treatment of UGIB. However, because such studies have involved small patient samples, the precise efficacy and safety profile of TC-325 remains unclear. Therefore, given the large amount of information generated from a variety of studies, each of which, in isolation, provides a low level of evidence, we found it necessary to pool all of the available data, thus creating a more reliable body of evidence.
In this systematic review and meta-analysis, we evaluated the literature to determine the safety and efficacy of TC-325 hemostatic powder for treatment of UGIB. Our analyses were based on the initial hemostasis achieved, as well as the rates of rebleeding and adverse events.
Methods
Protocol and registration
This study was registered in the International Prospective Register of Systematic Reviews database ( www.crd.york.ac.uk/prospero/ ; registration number CRD42018109354). All steps were conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines 14 15 .
Search strategy and study selection
Two reviewers, working independently, conducted thorough searches of the MEDLINE/PubMed, EMBASE, CENTRAL, and Latin-American and Caribbean Health Sciences Literature databases, as well as the gray literature, to identify articles describing initial hemostasis achieved with and rebleeding rates for the use of TC-325 in patients with UGIB. Any disagreement was resolved by consensus with a third reviewer. The search strategies are outlined in ( Table 1 ).
Table 1. Database search strategies.
| Database | Search strategy |
| MEDLINE | ((hemostatics OR powder OR hemospray OR TC 325 OR Endoclot))) AND (((((gastrointestinal OR non-variceal OR gastric OR stomach OR duodenum OR duodenal OR TGI) AND (hemorrhage OR bleeding)))) OR gastrointestinal hemorrhage) |
| EMBASE | ('hemospray'/exp OR hemospray OR 'tc 325' OR 'hemostatic powder' OR 'endoclot':ti,ab,kw) AND [embase]/lim |
| CENTRAL | hemospray or TC 325 or "hemostatic powder" or endoclot in Title, Abstract, Keywords in Trials |
| LILACS | tw:(hemospray)) OR (tw:("TC 325")) OR (tw:("hemostatic powder")) OR (tw:(endoclot)) |
| Gray literature | Annals of recent conferences and bibliographies of the principle studies selected |
LILACS, Literatura Latinoamericana y del Caribe en Ciencias de la Salud (Latin-American and Caribbean Health Sciences Literature).
We selected articles published up to October 2018, in any format, that contained an abstract in English, Spanish, or Portuguese. We included full articles (clinical trials, cohort studies, and case series), as well as conference abstracts if they contained all of the necessary data. Case reports were excluded.
Eligibility
Articles were considered eligible if they described use of TC-325 in patients with UGIB. Articles describing use of a hemostatic powder other than TC-325 were excluded, as were those in which more than 25 % of patients in the sample had bleeding in the lower gastrointestinal tract, those in which the distinction between upper and lower gastrointestinal bleeding was unclear, and those providing insufficient data for analysis.
Qualitative analysis of comparative studies
Data from comparative studies (cohort studies and clinical trials) were collected, and a qualitative analysis was performed for each such study. We assessed risk of bias using the Jadad scale for clinical trials and the Newcastle – Ottawa scale for cohort studies 16 17 .
Data extraction and evaluation
Data were collected by using a customized Excel spreadsheet structured to show population characteristics and outcomes analyzed. For comparative studies, data related to the TC-325 group were selectively extracted.
Primary outcomes were initial hemostasis and rebleeding rates. Adverse events (AEs) were described as secondary outcomes. Initial hemostasis was defined as cessation of bleeding after TC-325 application, regardless of timing of the observation. All author definitions of rebleeding were accepted, regardless of length of the follow-up period and whether follow-up evaluations were performed clinically or endoscopically. If follow-up evaluations were performed at more than one time point, we considered the data for post-procedure Day 7, because it was the most common time point evaluated.
Initially, overall primary outcomes were analyzed. We then performed a subgroup analysis by etiology (peptic ulcer, malignancy, or variceal hemorrhage) and by the category of the treatment (e. g., primary – if it was used as a first-line therapy or monotherapy – or rescue – if standard treatments had failed to achieve hemostasis). Additional analyses were performed for patients undergoing anticoagulant treatment (with oral anticoagulants or antithrombotic agents), in prophylactic or therapeutic doses.
Data analyses and evaluation of biases
We employed the Comprehensive Meta-Analysis software, version 3 (Biostat Inc., Englewood, New Jersey, United States) 18 , and the results are expressed as relative values and ranges. The software imposes certain restrictions for specific subgroup analyses. Therefore, studies with a subgroup comprising only one individual could not be analyzed and were excluded from the subgroup analyses. All AEs were reported individually (i. e., were not pooled). Finally, risk of bias was assessed using the checklists provided in Joanna Briggs Institute Critical Appraisal Tools 19 . A risk of bias was not considered a criterion for exclusion.
Results
Study selection and characteristics
We identified 2077 records after removal of the duplicates ( Fig. 1 ). Of those, 70 articles met the inclusion criteria and were full-text assessed for eligibility. We excluded 20 articles, for one of the following reasons: use of other hemostatic powders; no clear distinction between upper and lower gastrointestinal bleeding; bleeding in the lower gastrointestinal tract in more than 25 % of the patients in the sample; incomplete or illegible data; and use of the same cases in more than one article. Therefore, the final sample comprised 50 studies (20 – 69), all of which were included in the quantitative synthesis.
Fig. 1 .
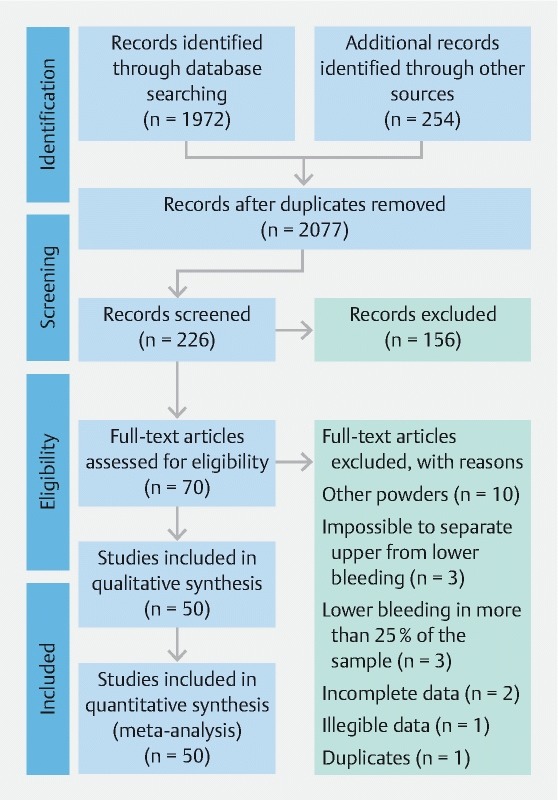
Flow diagram of the article selection process.
Of those 50 studies, 28 (56 %) were conference abstracts and 22 (44 %) were full-text articles. As for the types of studies, 42 (84 %) were case series, four (8 %) were clinical trials, and four (8%) were cohort studies. As detailed in Table 1s in the online-only Supplementary material, the appropriate checklists were applied to all selected articles, and the median score was 7 (range, 2 – 9).
Individual qualitative analysis of the comparative studies
Kweak et al. 45 performed a randomized pilot study comparing TC-325 and the conventional combined technique (CCT) for endoscopic treatment of peptic ulcers. Ten patients were enrolled in each group. Hemostasis success was achieved in 9 of the patients in the TC-325 group and in all 10 of those in the CCT group ( P = 1.0). In a second-look endoscopy performed on the following day, rebleeding was observed in 3 patients in the TC-325 group and in one patient in the CCT group at ( P = 0.582). The Jadad score for the study was 3.
Chen et al. 32 presented, in abstract form, results of a randomized controlled trial comparing use of TC-325 and the standard of care (SoC) – defined as traditional endoscopic, radiological, surgical, and interventional radiology approaches – in managing gastrointestinal bleeding caused by malignancy. Twenty patients were randomized to treatment with TC-325 or SoC. Immediate hemostasis was achieved in 90 % (95 % CI: 59.6 – 98.2) of the patients treated with TC-325 and in 40 % (95 % CI: 16.8 – 68.7) of those treated with the SoC. Of the six patients in which the SoC failed, 5 (83.3 %) were crossed over and submitted to treatment with TC-325. Among those five patients, hemostasis was achieved with TC-325 in four (80 %; 95 % CI: 37.6 – 96.4). None of the TC-325 patients crossed over to SC. Overall, treatment with TC-325 (initially or after SoC failure) resulted in hemostasis in 87.7 % of the patients (95 % CI: 62.1 – 96.3). The rebleeding rate was lower in the TC-325 group than in the SoC group – 22.2 % (95 % CI: 0 – 56.1) versus 60 % (95 % CI: 23.1 – 96.9). Other outcomes were not addressed in the abstract as published. The Jadad score for the abstract was 1.
In a randomized clinical trial, Ibrahim et al. 41 found that early application of TC-325 can change outcomes in patients with cirrhosis and acute variceal bleeding. Eighty-six patients were randomized to one of two groups: a study group, comprising patients submitted to immediate (urgent) endoscopy with TC-325 application within the first 2 hours after admission, plus the standard pharmacotherapy; and a control group, comprising patients treated only with the standard pharmacotherapy. At 12 to 24 hours after admission, patients in both groups were submitted to elective endoscopy. At that time, patients with esophageal varices underwent endoscopic band ligation and those with gastric varices underwent endoscopic cyanoacrylate injection. Whenever necessary, patients were submitted to rescue endoscopy. Five patients in the study group required rescue endoscopy before post-admission Hour 12 – due to uncontrolled spurting bleeding (in four cases) or early bleeding recurrence (in one). Of the 43 control group patients, 13 (30 %) required rescue endoscopic intervention for failure to achieve clinical hemostasis, compared with only five (12 %) of the 43 study group patients ( P = 0.034). Therefore, clinical hemostasis was achieved in 38 of the study group patients and in 30 of the control group patients. However, in the subsequent elective endoscopy, hemostasis was maintained in all 38 patients in the study group, whereas all 30 of the patients in the control group presented either active bleeding or fresh blood in the stomach. Within the first 5 days after elective endoscopy, rebleeding was observed in three control group patients and in none of the study group patients. Within the first 6 weeks, one study group patient and five control group patients died from UGIB. The Jadad score for the study was 3.
An abstract authored by da Costa Martins et al. 21 presented preliminary results of a randomized controlled trial comparing the efficacy of TC-325 with that of optimal clinical treatment in management of UGIB caused by malignant lesions. Eighteen patients were enrolled in each group. Successful initial hemostasis was achieved in all of the TC-325 group patients. Need for additional treatment was similar in both groups. There were no statistical differences between the TC-325 and control groups in terms of the 30-day rebleeding rate (61.4 % vs. 38.9%; P = 0.502) or the 30-day mortality rate (27.8 % vs. 22.2 %; P = 1.00). The Jadad score for the abstract was 1.
Cohort studies
In one of the cohort studies selected, Holster et al. 62 compared outcomes of TC-325 treatment between patients receiving and not receiving antithrombotic therapy (ATT). Initial hemostasis after TC-325 application was achieved in five of the eight patients (63 %) on ATT and in all eight of the patients not on ATT ( P = 0.20). Rates of rebleeding within 7 days were similar between the two groups (37.5 % vs. 25.0 %; P = 1.0).
In another cohort study, Pittayanon et al. 51 evaluated outcomes in 20 patients with active UGIB caused by a tumor: 10 patients in whom hemostasis was achieved with TC-325 treatment; and 10 matched historical controls (patients treated with conventional endoscopic techniques). Outcomes were only slightly better in the TC-325 group than in the control group: additional intervention during first 10 days (0 % vs. 30 % [no P value provided]); 14-day rebleeding rate (10 % vs. 30 %; P = 0.60); and 30-day mortality rate (10 % vs. 30 %; P = 0.70).
Sinha et al. 50 compared outcomes of 20 patients with Forrest IA or IB ulcers who underwent TC-325 treatment as an adjunct to conventional hemostatic measures with those of a previous cohort of 20 patients who had received conventional therapy only. Initial hemostasis was achieved in 19 (95 %) of the TC-325 group patients and in 16 (80 %) of the control group patients. Within the next 7 days, 19 TC-325 group patients and 16 control group patients were reevaluated and rebleeding was observed in three (15.8 %) and four (25.0 %), respectively. The 30-day gastrointestinal bleed-related mortality rate was 5 % in the study group and 15 % in the control group.
In the fourth cohort study selected, Thomson et al. 40 evaluated pediatric patients with non-variceal UGIB, comparing outcomes in a group of 17 patients with ages ranging from 1 day to 16 years who were treated with TC-325 with those reported for a historical cohort of 29 children previously treated with conventional endoscopic techniques. Initial hemostasis was achieved in 100 % of the patients in both groups. Rebleeding within 72 hours was observed in three (18 %) of the 17 TC-325 group patients and in seven (24 %) of the 29 control group patients ( P = 0.69). No AEs were reported.
All of the cohort studies had a low risk of bias, as assessed with the Newcastle – Ottawa Quality Assessment Scale. The results of that assessment are outlined in Table 2 .
Table 2. Newcastle – Ottawa Quality Assessment Scale results for the cohort studies evaluated.
Quantitative synthesis (meta-analysis)
Patient characteristics and sources of bleeding
The collective sample size was 1445 applications of TC-325 in 1437 patients. Although not all articles reported complete demographic data, median patient age was 60 years (range, 6.5 – 77.5 years) and there was a predominance of men. Bleeding scores indicated a high risk in all of the patients, approximately a quarter of whom were using anticoagulant drugs. Patient characteristics are outlined in Table 3 .
Table 3. Demographic characteristics and bleeding risk in the patient samples of the studies selected.
| Characteristic | Articles reporting | Sample size | Result 1 |
| n | n | ||
| Age (years), median | 41 | 1216 | 60 |
| Males/females, n/n | 41 | 1291 | 870/421 |
| Anticoagulant, % | 18 | 708 | 25.9 |
| Risk score, median | |||
|
9 | 223 | 10.8 |
|
6 | 239 | 7.8 |
Medians were calculated as the simple average of the medians reported in all of the articles reporting.
Causes of UGIB were clearly described in almost all of the articles ( Fig. 2 ): malignancy, in 422 cases; post-endoscopic procedures, in 149; ulcers, in 492; esophageal varices or esophageal band ligation, in 117; and benign lesions, in 265. There were also six cases in which the TC-325 application had to be repeated and the source of the bleeding was not specified by the authors.
Fig. 2 .
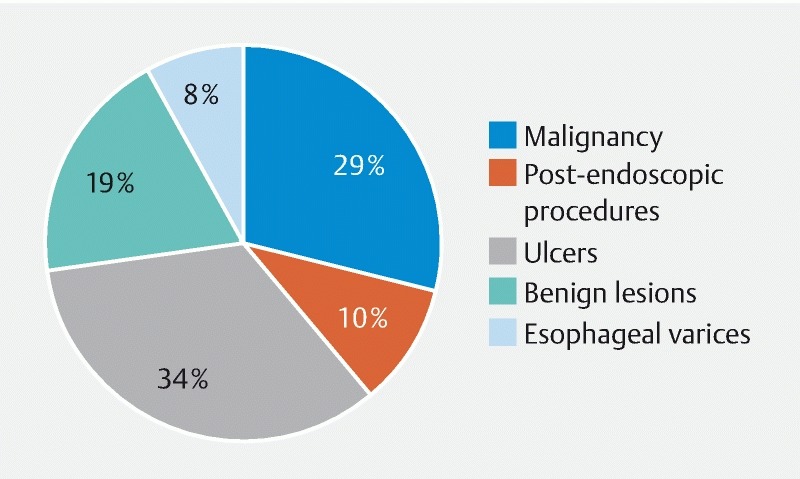
Proportional distribution of causes of upper gastrointestinal bleeding reported among the articles selected.
Hemostasis and rebleeding
Observation time for confirmation of initial hemostasis was noted in 18 articles, ranging from immediately after TC-325 application (no need for additional observation) to 5 minutes after verification of the initial hemostasis (8 articles). Means and timing of the assessment of rebleeding were described in 39 articles. Time from the procedure to the follow-up evaluation ranged from 12 hours to 3 months, most commonly 3 days (in 9 studies), 7 days (in 15 studies), and 30 days (in 13 studies).
Initial hemostasis was analyzed in all 50 articles (in 1445 applications of TC-325), and rebleeding was assessed in 47 articles (in 1275 patients). The overall pooled initial hemostasis rate was 90.7 %, and the pooled rebleeding rate was 26.2 %. The respective forest plots for those meta-analyses are shown in Fig.1s and Fig. 2s in the online-only Supplementary material.
The available data on etiology allowed subgroup analyses for peptic ulcers, neoplasms, and esophageal varices. Twelve articles reported initial hemostasis data on peptic ulcer cases, totaling 239 applications of TC-325. Rebleeding was reported in 11 articles, including a collective total of 208 patients. The overall pooled initial hemostasis rate was 91.5 %, and the pooled rebleeding rate was 33.1 %. The respective forest plots for those meta-analyses are shown in Fig. 3s and Fig. 4s .
For UGIB related to malignancy, the initial hemostasis rate was assessed in 16 articles, collectively including 315 applications of TC-325. Fifteen articles reported data on rebleeding rates, with a collective sample of 301 patients. The overall pooled initial hemostasis rate was 94.9 %, and the pooled rebleeding rate was 30.3 %. The respective forest plots for those meta-analyses are shown in Fig. 5s and Fig. 6s .
Five articles, collectively including 86 applications of TC-325, assessed primary hemostasis for variceal bleeding. Only in two cases band ligation was performed at the same time of the powder application. Four of those articles, with a collective total of 80 patients, reported data on rebleeding rates. The overall pooled initial hemostasis rate was 90.4 %, and the pooled rebleeding rate was 4.2 %. The respective forest plots for those meta-analyses are shown in Fig. 7s and Fig. 8s .
Regarding the category of treatment, two different subgroups were analyzed: primary therapy and rescue therapy. Twenty-six articles, evaluating a collective total of 555 applications of TC-325, described the initial hemostasis rate after use of TC-325 as primary therapy, and 22 of those articles assessed rebleeding rates in a collective total of 471 patients. The overall pooled initial hemostasis rate was 89.6 %, and the pooled rebleeding rate was 24.9 %. Respective forest plots for those meta-analyses are shown in Fig. 9s and Fig. 10s . Thirteen articles, with a collective total of 273 applications of TC-325, assessed initial hemostasis after use of TC-325 as rescue therapy, and 12 of those articles, collectively including 252 patients, reported rebleeding data. The overall pooled initial hemostasis rate was 93.2 %, and the pooled rebleeding rate was 43.6 %. The respective forest plots for those meta-analyses are shown in Fig. 11s and Fig. 12s . The results are outlined in Table 4 .
Table 4. Rates of initial hemostasis and rebleeding after treatment with the hemostatic powder TC-325.
| Usage | Initial hemostasis | Studies included | Rebleeding | Studies included |
| % (range) | n | % (range) | n | |
| General | 90.7 (88.7 – 92.3) | 50 | 26.2 (23.7 – 29.0) | 47 |
| Peptic ulcer | 91.5 (86.6 – 94.7) | 12 | 33.0 (26.6 – 40.2) | 11 |
| Neoplasm | 94.9 (91.7 – 97.0) | 16 | 30.3 (25.2 – 35.9) | 15 |
| Esophageal varices | 90.4 (80.3 – 95.6) | 5 | 4.2 (01.3 – 12.3) | 4 |
| Primary therapy | 89.6 (86.2 – 92.3) | 26 | 24.9 (20.6 – 29.7) | 22 |
| Rescue therapy | 93.2 (88.9 – 96.0) | 13 | 43.6 (37.3 – 50.2) | 12 |
Four articles, involving a collective total of 32 patients, reported data on efficacy of TC-325 in patients on anticoagulation therapy (oral anticoagulants or ATT), in prophylactic or therapeutic doses. Only primary hemostasis was reported. The overall pooled initial hemostasis rate was 92.3 %. Forest plots for that meta-analyses are shown in Fig. 13s .
Adverse events and equipment failure
Among the articles selected, a total of only eight AEs were reported, in 6 studies 25 35 42 47 57 60 : perforation, in one case; adherence of the endoscope to the gastric cardia when TC-325 was applied in retroflexed view, in four cases; and events not directly related to the procedure, in three cases (1 case each of unexpected cardiopulmonary arrest, abdominal distention, and splenic infarction). However, there were 11 articles in which AEs were not evaluated.
Equipment failures were reported in eight articles. Among 427 applications of TC-325, the application catheter became blocked by the powder in 19 cases (4.45 %), the CO 2 propellant cartridge malfunctioned in one case (0.23 %), and the working channel of the endoscope became temporarily occluded in one case (0.23 %). In most of those cases, the problem was resolved by replacing the application catheter.
Discussion
To our knowledge, this is the first systematic review and meta-analysis of use of TC-325 in digestive endoscopy, thus representing the most reliable source of evidence available to date. Although Chen et al. 6 published a similar study in 2015, it included a collective total of only 195 cases and did not include a meta-analysis. In addition, 43 eligible studies have been published since 2015.
Time from procedure to evaluation of the initial hemostasis varied little across the selected studies – from immediately after TC-325 application to 5 minutes thereafter. The overall rate of initial hemostasis was 90.7 % (range, 88.7 – 92.3 %). This outcome seems as impressive as those of other standard endoscopic therapies such as epinephrine injection, thermocoagulation, and hemoclip placement, for which reported rates of initial hemostasis are 95.1 %, 94.5 %, and 98.5 %, respectively 70 . However, the overall rebleeding rate after treatment with TC-325 was 26.2 % (range, 23.7 – 29.0 %), which is higher than the 19.6 %, 13.3 %, and 9.5 % reported for epinephrine injection, thermocoagulation, and hemoclip placement, respectively 70 . One comparative study obtained similar results, for initial hemostasis and for rebleeding 45 . Given the higher rebleeding rates, active surveillance for rebleeding becomes more important than ever when TC-325 is used.
The high rates of initial hemostasis and rebleeding might be explained by the mechanism of action of TC-325, which adheres to the gastrointestinal wall, thus creating a mechanical barrier for hemostasis and inducing clot formation when coming into contact with moisture (i. e., blood). It is usually completely eliminated within 24 hours 13 71 which suggests that it works better as a bridging therapy or damage control modality.
Comparing results obtained with use of TC-325 in treatment of UGIB among and between the articles evaluated, we found that the overall rate of initial hemostasis (90.7 %) was lower than the 95 %, 97 %, and 100 % reported in the earliest, largest, and most recent studies, respectively 20 49 63 whereas the overall rebleeding rate (26.2 %) was higher than that reported in the earliest and largest studies (10.5 % and 14.7 %, respectively), although it was lower than the 40.0 % reported in the most recent study. Such variations underscore the importance of performing a meta-analysis of the outcomes to obtain more precise results.
In the subgroup analyses of initial hemostasis, we found that, even when TC-325 was used as rescue therapy, there was a high rate of success in terms of the initial hemostasis. Application of TC-325 has produced favorable results in cases of uncontrolled bleeding after standard therapies, bleeding in hard-to-reach locations, bleeding caused by large ulcers, bleeding caused by tumors, large bleeding surfaces, and bleeding in other problematic situations 22 33 37 43 44 57 59 62 65 72 73 74 . In additional analyses, we observed a similar (92.3 %) success rate in patients on anticoagulation therapy. Holster et al. 62 reported lackluster results with use of TC-325 in patients on ATT. However, those authors included only patients on ATT, whereas we considered those under treatment with oral anticoagulants or antithrombotic agents, in prophylactic or therapeutic doses, which could explain the discrepant results. Nevertheless, both results are promising and demonstrate that at least part of the mechanism of action of TC-325 is somewhat independent of any underlying hemostatic disorder and can be considered a therapeutic option in patients on anticoagulation therapy.
The analyses of rebleeding rates highlight two quite different results after TC-325 application. The rebleeding rate after use of TC-325 as a primary treatment for esophageal varices was very low (4.2 %; range, 1.3 % – 12.3 %) and was associated with a low (3.6 %) mortality rate in the follow-up period. That could be explained by the short follow-up period after TC-325 application specially in the esophageal varices group. When necessary, standard therapy that is considered definitive (sclerosis or band ligation) was applied within 24 hours after treatment with TC-325. In one comparative study, Ibrahim et al. 41 showed that early application of TC-325 can improve outcomes for patients with acute variceal bleeding when added to standard therapy. In contrast, when TC-325 was applied as rescue therapy, the rebleeding rate was significantly higher (43.6 %; range, 37.3 % – 50.2 %). That is probably related to the greater complexity of these cases, in which the standard therapy was not sufficient to stop the active bleeding.
Safety of the equipment was described in most of the studies selected. Only 11 provided no data on AEs. Among 1367 uses in the remaining 39 studies, only eight AEs were reported 25 35 42 47 57 60 . In one study, the authors associated a case of gastric perforation with the pressure of applying TC-326 to the inflamed gastric wall, requiring immediately surgery. In four other cases, the gastroscope temporarily adhered to the wall of the esophagus during retroflexion when the TC-325 was applied, the problem being resolved in all four cases without serious damage to the mucosa. The three remaining AEs (unexpected cardiopulmonary arrest, abdominal distention, and splenic infarction) are unlikely to have been directly related to use of TC-325. In two experimental studies involving necropsies of animals after TC-325 use 75 76 , there was no macroscopic or histological evidence of systemic embolization, bowel obstruction, or systemic coagulopathy, thus corroborating the hypothesis that the last three AEs described above were unrelated to use of the product. The comparative studies evaluated here found no difference between TC-325 and traditional endoscopic techniques in terms of occurrence of AEs 41 45 50 51 62 .
Of the 50 studies selected, only eight reported instances of equipment failure (n = 19). However, it is not possible to know if there were in fact few equipment failures or if the authors did not consider it a relevant aspect to be reported.
In emergency situations, such as when hemostasis is not achieved with traditional treatment modalities 47 , short-term hemostasis provided by TC-325 might provide the time required for cardiovascular stabilization; transfusion of blood products (packed red blood cells) or replacement of coagulation factors; spontaneous recovery of coagulation parameters in direct oral anticoagulant-treated patients, or further semi-elective radiological or surgical treatment. Therefore, the latest consensus guideline on non-variceal UGIB recommends the use of TC-325 as a rescue or temporary treatment in patients with active non-variceal UGIB that was not controlled by standard endoscopic hemostatic therapies, due to a lack of expertise on the part of the endoscopist or persistent bleeding after attempts with standard methods, as well as in patients with bleeding caused by upper gastrointestinal malignancy. However, the quality of evidence and the grade of recommendation were both low. The high hemostasis rates demonstrated in our study provide evidence to increase the grade of recommendation 77 78 .
Given the promising results obtained with TC-325, some authors have enthusiastically attempted to summarize their findings into a few recommendations. Barkun et al. 6 proposed an algorithm for use of TC-325. In view of the new evidence provided by our study, we reiterate the ideal situations for use of TC-325, including uncontrolled bleeding after failure of conventional treatment modalities, bleeding due to malignancy, and variceal bleeding, adding to traditional modalities 79 . However, due to the paucity of comparative studies and despite the large number of cases of therapeutic success already described, it still seems too early to replace the traditional therapies with TC-325, either as monotherapy in lesions with a low risk of rebleeding after 24 hours or as an adjuvant therapy in those with a high risk of rebleeding after 24 hours, as suggested in the algorithm proposed. Further, it should be borne in mind that, unlike other modalities, use of hemostatic powder may impair immediate application of other methods in cases of continued bleeding because the bleeding site may no longer be visualized after application of the powder.
Our study has some limitations, mainly related to the low quality of information available in the literature. Most of the studies identified were conference abstracts, most were case series, most involved heterogeneous patient samples, and some contained incomplete information. We opted to include all available data in the literature to increase the size of our sample, thus making our results more representative of real outcomes seen in clinical practice. In addition, data related to need for surgery, need for interventional radiology procedures, and mortality were investigated but could not be adequately analyzed due to the limited data provided in most of the studies. Furthermore, there was pronounced variation in the means and timing of follow-up, time from the procedure to the follow-up examination ranging from 12 hours to 3 months. In 12 studies, timing of the follow-up examination was not specified. Such variation was also observed in the systematic review conducted by Chen et al. 6 . It might be explained by the wide variety of bleeding etiologies as well as by the descriptive and noncomparative nature of the studies, most of which had no clear protocols or eligibility criteria. Nevertheless, because our study does not intend to draw direct comparisons, heterogeneity of the follow-up does not seem to compromise our results.
In summary, this systematic review was comprehensive and had a strict methodology. It therefore provides the best source of evidence to date on use of hemostatic powder in UGIB. We found TC-325 to be safe and effective for use in most clinical situations. Although the lack of controlled and comparative studies still limits its use as a replacement for the standard treatment modalities, our results are promising. Future randomized controlled trials might support routine use of hemostatic powder in treatment of UGIB, especially in challenging cases.
Conclusion
The hemostatic powder TC-325 appears to be a safe and effective treatment for UGIB. It is associated with a high overall rate of initial hemostasis, regardless of the etiology and whether it is used as primary or rescue therapy. However, it is also associated with high rates of rebleeding. Nevertheless, rates of AEs and equipment failure are extremely low.
Footnotes
Competing interests None
Supplementary material: Table 1s, Figs. 1s – 13s :
References
- 1.Abougergi M S, Travis A C, Saltzman J R et al. The in-hospital mortality rate for upper GI hemorrhage has decreased over 2 decades in the United States: a nationwide analysis. Gastrointest Endosc. 2015;81:882–8880. doi: 10.1016/j.gie.2014.09.027. [DOI] [PubMed] [Google Scholar]
- 2.Sostres C, Lanas A. Epidemiology and Demographics of Upper Gastrointestinal Bleeding: Prevalence, Incidence, and Mortality. Gastrointest Endosc Clin N Am. 2011;21:567–581. doi: 10.1016/j.giec.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 3.van Leerdam M E. Epidemiology of acute upper gastrointestinal bleeding. Best Pract Res Clin Gastroenterol. 2008;22:209–224. doi: 10.1016/j.bpg.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Rotondano G. Epidemiology and diagnosis of acute nonvariceal upper gastrointestinal bleeding. Gastroenterol Clin North Am. 2014;43:643–663. doi: 10.1016/j.gtc.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Hearnshaw S A, Logan R FA, Lowe D et al. Acute upper gastrointestinal bleeding in the UK: patient characteristics, diagnoses and outcomes in the 2007 UK audit. Gut. 2011;60:1327–1335. doi: 10.1136/gut.2010.228437. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y I, Barkun A N. Hemostatic powders in gastrointestinal bleeding. a systematic review. Gastrointest Endosc Clin N Am. 2015;25:535–552. doi: 10.1016/j.giec.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Baracat F, Moura E, Bernardo W et al. Endoscopic hemostasis for peptic ulcer bleeding: systematic review and meta-analyses of randomized controlled trials. Surg Endosc. 2016;30:2155–2168. doi: 10.1007/s00464-015-4542-x. [DOI] [PubMed] [Google Scholar]
- 8.Ribeiro I B, Rezende D T, Madruga Neto A C et al. Endoscopic dual therapy for giant peptic ulcer hemorrhage. Endoscopy. 2018;50:E316–E317. doi: 10.1055/a-0665-4142. [DOI] [PubMed] [Google Scholar]
- 9.Lera M E, Minata M K, Duarte R B et al. Massive bleeding after plastic stent removal during ERCP: what’s next? Endoscopy. 2017;49:E303–E304. doi: 10.1055/s-0043-119350. [DOI] [PubMed] [Google Scholar]
- 10.Chaves D M, Costa F F, Matuguma S et al. Splenic artery pseudoaneurysm treated with thrombin injection guided by endoscopic ultrasound. Endoscopy. 2012;44 02:E99–100. doi: 10.1055/s-0030-1256740. [DOI] [PubMed] [Google Scholar]
- 11.Lau J YW, Barkun A, Fan D et al. Challenges in the management of acute peptic ulcer bleeding. Lancet (London, England) 2013;381:2033–2043. doi: 10.1016/S0140-6736(13)60596-6. [DOI] [PubMed] [Google Scholar]
- 12.Garber A, Jang S. Novel therapeutic strategies in the management of non-variceal upper gastrointestinal bleeding. Clin Endosc. 2016;49:421–424. doi: 10.5946/ce.2016.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holster I L, Van Beusekom H MM et al. Effects of a hemostatic powder hemospray on coagulation and clot formation. Endoscopy. 2015;98:638–645. doi: 10.1055/s-0034-1391353. [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535–b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liberati A, Altman D G, Tetzlaff J et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jadad A R, Moore R A, Carroll D et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 17.Wells G A, Shea B, OʼConnel Det al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysesAvailable at:http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 18.Borenstein M, Hedges L, Higgins Jet al. Comprehensive meta-analysis [Internet] Englewood, NJ; 2013. Available athttps://www.meta-analysis.com
- 19.Moola S, Munn Z, Tufanaru Cet al. Systematic reviews of etiology and riskIn: Aromataris E, Munn Z (Editors). Joanna Briggs Institute Reviewer’s Manual. The Joanna Brig 2017.Available athttps://reviewersmanual.joannabriggs.org/
- 20.Nova da Costa L S, Marson F P et al. Use of hemostatic powder (tc-325) in patients with upper gastrointestinal bleeding at a tertiary center: a case series. Gastrointest Endosc. 2018;87:AB173. [Google Scholar]
- 21.da Costa Martins B, Scomparin R C, Bento L H et al. Preliminary results of a randomized controlled trial comparing hemostatic powder versus optimal clinical treatment in the management of gastrointestinal bleeding from malignancy. Gastrointest Endosc. 2018;87:AB415–AB416. [Google Scholar]
- 22.Ting M SR, Lim L L, Doshi Bet al. Experience of novel endoscopic hemostasis with hemospray in a tertiary medical centre in patients with acute non-variceal upper gastrointestinal bleeding J Gastroenterol Hepatol 2016317–441.26426426 [Google Scholar]
- 23.Sonthalia N, Jain S S, Surude R G.et al.Use of hemospray for gastrointestinal bleeding: A single tertiary care centre experience from Western India Indian J Gastroenterol 201635 (Suppl 1)A85 [Google Scholar]
- 24.Sokpon M, Tata A, Bernasconi M et al. Expérience de l’utilisation de la poudre Hémospray ® dans la prise en charge des hémorragies digestives sévères dans un centre hospitalier général . J Africain d’Hépato-Gastroentérologie. 2016;10:125–128. [Google Scholar]
- 25.Vivar R M, Valenzuela C, Gonzalez R G et al. Safety and efficacy of Hemospray ® in upper gastrointestinal bleeding: Experience in a chilean academic hospital . Gastrointest Endosc. 2016;83:AB499. [Google Scholar]
- 26.Nasr I, DeMartino S, Borrow D-M et al. Hemospray: When should we plug the gap? A single centre UK experience. Gastrointest Endosc. 2016;83:AB500–AB501. [Google Scholar]
- 27.Dhesi E, Lam V, Tang K et al. Single-centre clinical experience of hemospray endotherapy in patients with acute upper gastrointestinal bleeding. United Eur Gastroenterol J. 2015;3:146–687. [Google Scholar]
- 28.Disney B, Kurup A, Muhammad H et al. Hemospray use for the management of acute bleeding from upper gastrointestinal cancer: the Russells Hall experience. Gut. 2015;64:A71.3–A72. [Google Scholar]
- 29.Widlak M, Wijesinghe H, Siau K et al. Hemospray for acute upper gastrointestinal bleeding – a single centre experience. Gut. 2015;64:A225.1–A225. [Google Scholar]
- 30.Dixon S, Tate D, Przemioslo R et al. Hemospray may not reliably achieve hemostasis beyond 48 hours in acute upper gastrointestinal bleeding. Gut. 2015;64:A420.3–A421. [Google Scholar]
- 31.Malik A, Duane P, Eadala P et al. Use of hemospray for non variceal upper gastrointestinal bleed in a district general hospital. Gut. 2015;64:A65.3–A66. [Google Scholar]
- 32.Chen Y-I, Lu Y, Wyse Jet al. (Hemospray TM ) versus standard of care in managing malignant gastrointestinal bleeding: A pilot randomized clinical trial Am J Gastroenterol 2017112S285–S319.28981029 [Google Scholar]
- 33.Jang S, Parsi M A, Stevens T et al. Use of hemospray in intractable upper GI bleeding: U.S. Single Center Experience. Gastrointest Endosc. 2015;81:AB453. [Google Scholar]
- 34.Minelli Grazioli L, Rando G, Lombardi L et al. Hemospray as first-line treatment for upper gi tumoral bleeding in emergency endoscopy. Report of three cases. Dig Liver Dis. 2015;47:e157. [Google Scholar]
- 35.Mangiavillano B, Arena M, Morandi E et al. Use of hemospray powder in the acute upper GI bleeding. Dig Liver Dis. 2013;45:S203. [Google Scholar]
- 36.Sagar N. Early clinical experience of the effectiveness of hemospray in achieving haemostasis in patients with acute non-variceal bleeding. United Eur Gastroenterol J. 2014;2:A329. [Google Scholar]
- 37.Ali R, Carr-Locke D, Komanduri S et al. Hemospray for refractory gastrointestinal bleeding: Initial United States experience. Am J Gastroenterol. 2014;109:S585. [Google Scholar]
- 38.Masci E, Arena M, Morandi E et al. Upper gastrointestinal active bleeding ulcers: review of literature on the results of endoscopic techniques and our experience with Hemospray. Scand J Gastroenterol. 2014;49:1290–1295. doi: 10.3109/00365521.2014.946080. [DOI] [PubMed] [Google Scholar]
- 39.Disney B, Kurup A K, Ishaq S et al. Initial experience with hemospray in the treatment of acute upper gastrointestinal bleeding. United Eur Gastroenterol J. 2014;2:A252. [Google Scholar]
- 40.Thomson M, Urs A, Narula P et al. The use and safety of a novel haemostatic spray in the endoscopic management of acute nonvariceal upper gastrointestinal bleeding in children. J Pediatr Gastroenterol Nutr. 2018;67:e47–e50. doi: 10.1097/MPG.0000000000001967. [DOI] [PubMed] [Google Scholar]
- 41.Ibrahim M, El-Mikkawy A, Abdel Hamid M et al. Early application of haemostatic powder added to standard management for oesophagogastric variceal bleeding: A randomised trial. Gut. 2018:1–10. doi: 10.1136/gutjnl-2017-314653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pittayanon R, Rerknimitr R, Barkun A. Prognostic factors affecting outcomes in patients with malignant GI bleeding treated with a novel endoscopically delivered hemostatic powder. Gastrointest Endosc. 2018;87:994–1002. doi: 10.1016/j.gie.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 43.Alzoubaidi D, Gulati S, Murino A et al. Outcomes from an international multicentre registry of patients with gastrointestinal bleeding undergoing endoscopic treatment with hemospray. United Eur Gastroenterol J. 2017;5:A161–A836. doi: 10.1111/den.13502. [DOI] [PubMed] [Google Scholar]
- 44.Cahyadi O, Bauder M, Meier B et al. Effectiveness of TC-325 (Hemospray) for treatment of diffuse or refractory upper gastrointestinal bleeding – a single center experience. Endosc Int Open. 2017;05:E1159–E1164. doi: 10.1055/s-0043-118794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kwek B EA, Ang T L, Ong P LJ et al. TC-325 versus the conventional combined technique for endoscopic treatment of peptic ulcers with high-risk bleeding stigmata: A randomized pilot study. J Dig Dis. 2017;18:323–329. doi: 10.1111/1751-2980.12481. [DOI] [PubMed] [Google Scholar]
- 46.Arena M, Masci E, Eusebi L H et al. Hemospray for treatment of acute bleeding due to upper gastrointestinal tumours. Dig Liver Dis. 2017;49:514–517. doi: 10.1016/j.dld.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 47.Hagel A F, Albrecht H, Nägel A et al. The application of Hemospray in gastrointestinal bleeding during emergency endoscopy. Gastroenterol Res Pract. 2017;2017:6–8. doi: 10.1155/2017/3083481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giles H, Lal D, Gerred S et al. Efficacy and safety of TC-325 (HemosprayTM) for non-variceal upper gastrointestinal bleeding at Middlemore Hospital: the early New Zealand experience. N Z Med J. 2016;129:38–43. [PubMed] [Google Scholar]
- 49.Haddara S, Jacques J, Lecleire S et al. A novel hemostatic powder for upper gastrointestinal bleeding: a multicenter study (the “GRAPHE” registry) Endoscopy. 2016;48:1084–1095. doi: 10.1055/s-0042-116148. [DOI] [PubMed] [Google Scholar]
- 50.Sinha R, Lockman K A, Church N I et al. The use of hemostatic spray as an adjunct to conventional hemostatic measures in high-risk nonvariceal upper GI bleeding (with video) Gastrointest Endosc. 2016;84:900–906000. doi: 10.1016/j.gie.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 51.Pittayanon R, Prueksapanich P, Rerknimitr R. The efficacy of Hemospray in patients with upper gastrointestinal bleeding from tumor. Endosc Int Open. 2016;04:E933–E936. doi: 10.1055/s-0042-109863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ibrahim M, El-Mikkawy A, Abdalla H et al. Management of acute variceal bleeding using hemostatic powder. United Eur Gastroenterol J. 2015;3:277–283. doi: 10.1177/2050640615570148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Szalai M, Kullmann T, Durcsán H et al. [Hemospray: a novel therapeutic option in the management of acute upper gastrointestinal bleeding] Orv Hetil. 2015;156:528–531. doi: 10.1556/OH.2015.30112. [DOI] [PubMed] [Google Scholar]
- 54.Appleby R, Hoare J. PTH-022 Hemospray in a large tertiary nhs trust: a descriptive analysis of the first three years of use. Endoscopy. 2017:A216.1–A216. [Google Scholar]
- 55.Chen Y I, Barkun A N, Soulellis C et al. Use of the endoscopically applied hemostatic powder TC-325 in cancer-related upper GI hemorrhage: Preliminary experience (with video) Gastrointest Endosc. 2012;75:1278–1281. doi: 10.1016/j.gie.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 56.Chen Y-I, Barkun A, Nolan S. Hemostatic powder TC-325 in the management of upper and lower gastrointestinal bleeding: a two-year experience at a single institution. Endoscopy. 2015;47:167–171. doi: 10.1055/s-0034-1378098. [DOI] [PubMed] [Google Scholar]
- 57.Smith L A, Stanley A J, Bergman J J et al. Hemospray application in nonvariceal upper gastrointestinal bleeding: results of the Survey to Evaluate the Application of Hemospray in the Luminal Tract. J Clin Gastroenterol. 2014;48:e89–92. doi: 10.1097/MCG.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 58.Barkun A, Adam V, Martel M. TC-325 in the management of upper and lower GI bleeding: A two-year experience at a single institution. Value Heal. 2014;17:A749. doi: 10.1016/j.jval.2014.08.187. [DOI] [PubMed] [Google Scholar]
- 59.Sulz M C, Frei R, Meyenberger C et al. Routine use of Hemospray for gastrointestinal bleeding: Prospective two-center experience in Switzerland. Endoscopy. 2014;46:619–624. doi: 10.1055/s-0034-1365505. [DOI] [PubMed] [Google Scholar]
- 60.Yau A HL, Ou G, Galorport C et al. Safety and efficacy of Hemospray ® in upper gastrointestinal bleeding . Can J Gastroenterol Hepatol. 2014;28:72–76. doi: 10.1155/2014/759436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ibrahim M, El-Mikkawy A, Mostafa I et al. Endoscopic treatment of acute variceal hemorrhage by using hemostatic powder TC-325: A prospective pilot study. Gastrointest Endosc. 2013;78:769–773. doi: 10.1016/j.gie.2013.07.037. [DOI] [PubMed] [Google Scholar]
- 62.Holster I L, Kuipers E J, Tjwa E TTL. Hemospray in the treatment of upper gastrointestinal hemorrhage in patients on antithrombotic therapy. Endoscopy. 2013;45:63–66. doi: 10.1055/s-0032-1325793. [DOI] [PubMed] [Google Scholar]
- 63.Sung J J, Luo D, Wu J C et al. Early clinical experience of the safety and effectiveness of Hemospray in achieving hemostasis in patients with acute peptic ulcer bleeding. Endoscopy. 2011;43:291–295. doi: 10.1055/s-0030-1256311. [DOI] [PubMed] [Google Scholar]
- 64.Hanna M S. Hemospray in acute non-variceal upper gastrointestinal bleeding: First experiences in a uk teaching hospital. United Eur Gastroenterol J. 2015;3:146–687. [Google Scholar]
- 65.Thayalasekaran S, Dixon S, Mundre P et al. PTH-054A Hemospray use in the management of upper gastrointestinal haemorrhage: a 2-year experience across 2 teaching hospitals in the north and south of england. Gut. 2017;66:A232.1–A232. [Google Scholar]
- 66.Alzoubaidi D, Magee C, Gulati S et al. Outcomes from an international multicentre registry of patients with gastrointestinal bleeding undergoing endoscopic treatment with hemospray. Gut. 2017;66:A65. doi: 10.1111/den.13502. [DOI] [PubMed] [Google Scholar]
- 67.Häberle M F, Senler W A, Saenz R et al. Hemostatic spray powder, in critical gastrointestinal bleeding. a highly effective treatment alternative. Gastrointest Endosc. 2017;85:AB522. [Google Scholar]
- 68.Eusebi L H, Arena M, Despott E et al. Acute bleeding due to upper gastrointestinal tumors treated with hemospray: A case series from 4 European endoscopy centers. Gastrointest Endosc. 2017;85:AB457–AB458. [Google Scholar]
- 69.Meng Z W, Marr K J, Mohamed R. JPD. Study, Long-term efficacy and safety of TC-325 for malignancyrelated upper gastrointestinal bleeds a multicenter retrospective. Gastroenterology. 2017;85:S256. doi: 10.1093/jcag/gwy031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sung J JY, Tsoi K KF, Lai L H et al. Endoscopic clipping versus injection and thermo-coagulation in the treatment of non-variceal upper gastrointestinal bleeding: a meta-analysis. Gut. 2007;56:1364–1373. doi: 10.1136/gut.2007.123976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barkun A, Adam V, Martel M. TC-325 in the management of upper and lower GI bleeding: a two-year experience at a single institution. Value Health. 2014;17:A749. doi: 10.1016/j.jval.2014.08.187. [DOI] [PubMed] [Google Scholar]
- 72.Yau A HL, Ou G, Galorport C et al. Safety and efficacy of Hemospray® in upper gastrointestinal bleeding. Can J Gastroenterol Hepatol. 2014;28:72–76. doi: 10.1155/2014/759436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sakai C M, Duarte R B, Baracat F I et al. Endoscopic treatment of upper-GI ulcer bleeding with hemostatic powder spray. VideoGIE an Off video J Am Soc Gastrointest Endosc. 2017;2:12–13. doi: 10.1016/j.vgie.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baracat F I, Tranquillini C V, Brunaldi V O et al. Hemostatic powder: a new ally in the management of postsphincterotomy bleeding. VideoGIE. 2017;2:303–304. doi: 10.1016/j.vgie.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Giday S, Kim Y, Krishnamurty D et al. Long-term randomized controlled trial of a novel nanopowder hemostatic agent (TC-325) for control of severe arterial upper gastrointestinal bleeding in a porcine model. Endoscopy. 2011;43:296–299. doi: 10.1055/s-0030-1256125. [DOI] [PubMed] [Google Scholar]
- 76.Giday S, van Alstine W, van Vleet J et al. Safety analysis of a hemostatic powder in a porcine model of acute severe gastric bleeding. Dig Dis Sci. 2013;58:3422–3428. doi: 10.1007/s10620-013-2846-z. [DOI] [PubMed] [Google Scholar]
- 77.Sung J JY, Chiu P CY, Chan F KL et al. Asia-Pacific working group consensus on non-variceal upper gastrointestinal bleeding: An update 2018. Gut. 2018:1757–1768. doi: 10.1136/gutjnl-2018-316276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gralnek I, Dumonceau J-M, Kuipers E et al. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:a1–a46. doi: 10.1055/s-0034-1393172. [DOI] [PubMed] [Google Scholar]
- 79.Hemospray Label 20181–15.Available athttps://www.cookmedical.com/data/IFU_PDF/12898_0318.PDF
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


