Abstract
Nonalcoholic fatty liver disease (NAFLD) is common in children and may progress to nonalcoholic steatohepatitis (NASH), advanced fibrosis, and even cirrhosis in childhood or early adulthood, indicating the need for pharmacologic treatment in this age group. Multiple trials are evaluating different therapeutic targets for NASH with fibrosis in adults, and the U.S. Food and Drug Administration has recently provided clear guidance to the pharmaceutical industry on developing drugs for the treatment of noncirrhotic NASH with liver fibrosis. Pediatric NAFLD has several unique aspects that distinguish it from the adult disease in terms of histology, our understanding of the natural history, and the utility of noninvasive tests. These differences have the potential to impact the design of clinical trials to test different drugs in the pediatric population. The aim of this article is to provide a review of common misconceptions regarding pediatric NAFLD and key differences from adult NAFLD. We have provided our recommendations on the design of early proof‐of‐concept and late phase 2 trials based on lessons learned from previous clinical trials. We believe that clinical drug development for children with NAFLD should happen in parallel with ongoing adult trials.
Pediatric NAFLD has several unique aspects that distinguishes it from the adult disease in terms of histology, natural history, and the utility of noninvasive tests. These differences have the potential to impact the design of clinical trials to test different drugs in the pediatric population. The aim of this article is to provide guidance on the design of early proof‐of‐concept and late phase 2 trials in children with NAFLD.

Abbreviations
- ALT
alanine aminotransferase
- CBDR
cysteamine bitartrate delayed‐release
- CyNCh
Cysteamine Bitartrate Delayed‐Release for the Treatment of NAFLD in Children
- FDA
U.S. Food and Drug Administration
- MRE
magnetic resonance elastography
- MRI‐PDFF
magnetic resonance imaging–proton density fat fraction
- NAFLD
nonalcoholic fatty liver disease
- NAS
nonalcoholic fatty liver disease activity score
- NASH
nonalcoholic steatohepatitis
- POC
proof‐of‐concept
Due to the epidemics of obesity and type 2 diabetes, nonalcoholic fatty liver disease (NAFLD) has rapidly become the most common cause of abnormal liver function tests and chronic liver disease in both adults and children in the United States.1, 2 NAFLD includes a spectrum of diseases, ranging from the relatively benign nonalcoholic fatty liver (NAFL) to the aggressive nonalcoholic steatohepatitis (NASH) to fibrosis and eventually cirrhosis, in the absence of excessive alcohol consumption.3 NAFL is defined as the presence of steatosis in at least 5% of the hepatocytes without evidence of significant inflammation or hepatocellular injury. NASH is defined as the presence of steatosis with inflammation and hepatocyte injury, such as ballooning, with or without liver fibrosis.4 In 2016, NASH became the most common indication for liver transplantation in female adults in the United States5 and is anticipated to become the most common indication overall by the year 2020.6 Despite its significant health care burden, there are no U.S. Food and Drug Administration (FDA)‐approved medications for NASH. Several trials are evaluating different therapeutic targets for NASH with fibrosis in adults.7, 8 The FDA has provided a draft guidance to the pharmaceutical industry on developing drugs for the treatment of noncirrhotic NASH with liver fibrosis (https://www.fda.gov/media/119044/download). For proof‐of‐concept (POC) early phase 2 trials in adults, the FDA accepts noninvasive biomarkers, such as alanine aminotransferase (ALT), and imaging modalities that assess liver stiffness or hepatic fat content, such as magnetic resonance elastography (MRE) or magnetic resonance imaging–proton density fat fraction (MRI‐PDFF). For late phase 2 and phase 3 registration trials, the FDA recommends that sponsors should provide evidence of efficacy on histologic endpoints, and this is defined as either resolution of NASH with no worsening of fibrosis or improvement in fibrosis of at least one stage with no worsening of NASH. If a NASH drug is approved using histologic endpoints under the accelerated approval pathway, further randomized clinical trials will be needed to demonstrate efficacy on clinical benefit through a composite endpoint, including progression to cirrhosis, reduction in hepatic decompensation events, change in Model for End‐Stage Liver Disease score from less than or equal to 12 to more than 15, liver transplant, and all‐cause mortality. Unfortunately, the picture is less clear for pediatric NAFLD in terms of the appropriate patient population, treatment endpoints, and FDA approval requirements.
In this article, we discuss the unique aspects of pediatric NAFLD and their potential impact on the design of clinical trials to test different drugs for the treatment of this ongoing epidemic. We believe that now is the time to address the unmet need for treatment of the pediatric population with NAFLD and that clinical drug development in this space should happen in parallel with ongoing adult trials.
Misconceptions Regarding Pediatric NAFLD
NAFLD and NASH are Not Common in Children
Estimating the true prevalence of NAFLD in children and adolescents is challenging. The best evidence comes from the Study of Child and Adolescent Liver Epidemiology (SCALE), an autopsy‐based study that showed that after adjusting for age, sex, race, and ethnicity, the prevalence of NAFLD in the U.S. pediatric population was 9.6%, increasing with age and obesity (17.3% in adolescents and 38% in children with obesity).9 A more recent study that was conducted in a diverse population from New York City using a similar design showed a lower prevalence of NAFLD at 4.5%.10 There were no cases with advanced fibrosis or cirrhosis. The lowest prevalence was noted in black children at 1%, while white and Hispanic children had the highest percentages of NAFLD at 8.3% and 7.9%, respectively. Another study estimated the changes in the prevalence of suspected NAFLD, defined as above‐normal weight or obesity in addition to elevated serum ALT and the absence of other known chronic liver diseases, of adolescents aged 12 to 19 years. By using different periods of the National Health and Nutrition Examination Survey (NHANES) database, Welsh et al.11 showed that the prevalence of NAFLD more than doubled among American adolescents over the previous 3 decades, rising from 3.9% in 1988‐1994 to 10.7% in 2007‐2010. Taken together, these studies show that NAFLD affects approximately 10% of American children, with a higher prevalence in adolescents and those with obesity.
NAFLD is Neither Progressive Nor Severe in Children
A number of cross‐sectional studies have shown that the entire spectrum of NAFLD may occur during childhood, from hepatic steatosis to NASH to advanced fibrosis. In fact, in the SCALE study, NASH was observed in 23% of children with NAFLD, of which 9% had advanced fibrosis or cirrhosis, indicating that the disease can be progressive even during childhood. In a retrospective review of 67 children with biopsy‐proven NAFLD, significant fibrosis (stages 2‐3) was found in approximately 15% of the children.12 In a multicenter retrospective study that included 108 children with biopsy‐proven NAFLD from five medical centers in the United States, stage 1 fibrosis was seen in 43% of patients, stage 2 fibrosis in 24%, and stage 3 fibrosis in 20%.13 Overall, progression of liver disease to significant fibrosis is present in approximately 10% to 25% of children with NAFLD.
Differences Between Pediatric and Adult NAFLD
Difference in Histology
The NAFLD activity score (NAS) was developed by the National Institute of Diabetes and Digestive and Kidney Diseases NASH Clinical Research Network (CRN) and consists of the unweighted sum of scores for each of the following histologic lesions: steatosis, hepatocyte ballooning, and lobular inflammation.14 This semiquantitative score was specifically developed to assess response to therapeutic intervention. Since the development of NAS, it has become evident that some children with NASH may present with a distinct histopathologic pattern characterized mainly by the presence of portal‐based disease, including portal inflammation and fibrosis,15, 16 and the absence of hepatocyte ballooning; this is often termed type 2 NASH.
In a large study from the NASH CRN, Africa et al.17 showed that zone 1 steatosis was found in 18% of children with biopsy‐proven NAFLD. Compared to children with zone 3 steatosis (perisinusoidal [or adult type]), those with zone 1 steatosis (periportal [or pediatric type]) were younger (mean age zone 1, 10.8 years vs. mean age zone 3, 14.7 years); more likely to be Hispanic (zone 1, 75% vs. zone 3, 57.6%), have any fibrosis (zone 1, 81.4% vs. zone 3, 50.1%), and have bridging fibrosis (zone 1, 13.1% vs. zone 3, 4.5%); and less likely to have ballooned hepatocytes (zone 1, none in 71.2% vs. zone 3, 52.9%) and definitive NASH (zone 1, 6.2% vs. zone 3, 30.3%). Importantly, the only independent risk factor for having the pediatric type (or zone 1 steatosis) was age, with the transition to the adult type (or zone 3 steatosis) occurring around the age of 12 to 13 years. This may have significant implications for designing clinical trials in children given our better understanding of the prognostic values of having ballooned hepatocytes or definitive NASH and less clear understanding of the significance of periportal disease.
Differences in Our Understanding of the Natural History
The natural history of adult NAFLD is relatively well established, with NASH being considered the aggressive form of the disease and fibrosis stage being the strongest predictor of long‐term overall mortality and liver disease complications.18 NASH cirrhosis is currently the most common etiology of liver disease among women awaiting or receiving liver transplantation in the United States and the second most common etiology in men6, 19 and is associated with a significant risk of developing hepatocellular carcinoma.20 Studies evaluating the natural history of NAFLD in children are limited, but progression to advanced fibrosis and cirrhosis is well documented during childhood. A landmark study examined the long‐term prognosis of children with NAFLD and compared their survival with the expected survival of the general population.21 A total of 66 children with NAFLD with a mean age of 14 years were followed for up to 20 years, with a total of 409.6 person‐years of follow‐up. Metabolic syndrome was present in 19 (29%) children at the time of NAFLD diagnosis, with 55 (83%) presenting with at least one feature, including obesity, hypertension, dyslipidemia, and/or hyperglycemia. Four children with baseline normal fasting glucose developed type 2 diabetes 4 to 11 years after NAFLD diagnosis. A total of 13 liver biopsies were obtained from 5 patients over a mean of approximately 5 years showing progression of fibrosis stage in 4 children. During follow‐up, 2 children died and 2 underwent liver transplantation for decompensated cirrhosis. NAFLD recurred in the allograft in the two cases of transplantation, with one case progressing to cirrhosis and requiring retransplantation.
It is important to highlight the fact that the natural history of type 2 (or pediatric) NASH is less well understood. For example, in the study by Africa et al.,16 bridging fibrosis was found in 13% of young children with pediatric NASH; however, whether these children develop cirrhosis in early adulthood or regress through a fibrolytic response remains unknown at this point.
Noninvasive Diagnostic Tests
Several noninvasive tests to identify patients with significant or advanced fibrosis have been developed and validated in the adult population. Fibrosis scores comprising routinely measured clinical and laboratory variables, including the NAFLD fibrosis score and the fibrosis‐4 index, are commonly used to rule out the presence of advanced fibrosis in adults.22 However, recent data from our group and others have suggested that these adult scores may not be accurate in predicting advanced fibrosis in children.23, 24
The assessment of liver fibrosis by imaging the propagation characteristics of shear waves in the liver to determine liver stiffness has gained wide acceptance among hepatologists who treat adult patients with NAFLD. This can be done by different technologies that include vibration‐controlled transient elastography (VCTE) and MRE. These same imaging modalities are being studied in children. Nobili et al.25 used VCTE to measure liver stiffness in 52 children with NAFLD and showed excellent accuracy for predicting advanced fibrosis. MRE was studied in a pilot project that included 35 children with different liver diseases, including NAFLD, with promising results.26 There is an urgent need to establish specific cut‐off values for children with NAFLD before the wide use of these imaging techniques can be recommended.
Several studies have validated the use of MRI‐PDFF to quantify hepatic fat against both histology‐determined steatosis grade and MRI spectroscopy fat quantification.27, 28, 29, 30 A recent study assessed the performance of MRI‐PDFF in 110 children with biopsy‐proven NASH and demonstrated excellent accuracy in classifying mild steatosis versus moderate/severe steatosis with an area under the receiving operator characteristic curve of 0.87.31
Lessons Learned From Previous Pediatric NASH Trials
Major Pediatric NASH Trials With Histologic Endpoints
The Treatment of NAFLD in Children (TONIC) trial was a multicenter, randomized, placebo‐controlled trial that evaluated the efficacy of vitamin E and metformin for 96 weeks in pediatric NAFLD.32 The primary endpoint was the sustainability of reduction in ALT levels, defined as 50% or less of the baseline level or 40 U/L or less at visits every 12 weeks from 48 to 96 weeks of treatment. This endpoint was chosen as there was insufficient information on the histology of NAFLD in children at the time of study design for calculation of an appropriate sample size.
The trial randomized a total of 173 children, and surprisingly, data on improvement in histology based on paired liver biopsies were reported for 147 patients, indicating that the acceptance of biopsy was higher than initially expected.
Unfortunately, the primary outcome of sustained ALT reduction was not achieved by either metformin or vitamin E.32 However, patients on vitamin E had greater resolution of NASH when compared to the other groups (58% vs. 28%, respectively; P = 0.006), with significant improvement in their histologic activity score (P = 0.02). The relative odds of resolution of NASH with every 10 U/L decrease in ALT over 96 weeks were 1.37 (95% confidence interval, 1.19‐1.58; P < 0.001).
Based on the success of TONIC in obtaining paired liver biopsy in children with NAFLD, the Cysteamine Bitartrate Delayed‐Release for the Treatment of NAFLD in Children (CyNCh) trial used a histologic endpoint as the primary endpoint, defined as a decrease in NAS of 2 or more points and no worsening of liver fibrosis. This was a multicenter, placebo‐controlled, double‐blind trial of 169 children aged 8 to 17 years with biopsy‐proven NASH, defined as NAS of 4 or greater.33 Patients were randomized to receive cysteamine bitartrate delayed‐release (CBDR) at 600, 750, or 900 mg/day or placebo for 52 weeks. Despite significant improvement in ALT and lobular inflammation, there was no significant difference between the CBDR and placebo groups in achieving the primary outcome (28% and 22%, respectively; P = 0.34). Moreover, there were no significant differences in improvements in ballooning, portal inflammation, steatosis, or fibrosis between treatment groups. Interestingly, in a post‐hoc analysis of children weighing less than 65 kg who ended up receiving a substantially higher per kilogram dose, those in the CBDR arm reached the primary outcome of histologic improvement in 50% compared to only 13% in the placebo arm (4‐fold increase in response rate; P = 0.005). The CyNCh trial provided significant insights on the need to study the pharmacokinetics/pharmacodynamics (PK/PD) of new therapeutic agents and the effect of body weight on drug exposure. This was clearly relevant in CyNCh, where children with body weight less than 65 kg received a significantly higher dose of CBDR compared to those weighing more than 65 kg, leading to significant improvements in histology.
MRI‐PDFF can be Used for POC Trials
MRI‐PDFF‐estimated hepatic fat is sensitive in detecting longitudinal fat changes in the context of adult NASH trials.34 Based on a small study that included 35 subjects with biopsy‐proven NASH who underwent paired biopsy and MRI‐PDFF at baseline and end of treatment after receiving ezetimibe or placebo for 24 weeks, Patel et al.35 demonstrated that an absolute reduction in MRI‐PDFF of 4% or a relative reduction of 30% was associated with histologic response, defined as a reduction of 2 or more points in the NAS without worsening of fibrosis.
A recent randomized trial evaluated the efficacy of a low free‐sugar diet compared to a usual diet for 8 weeks in 40 adolescent boys aged 11 to 16 years, using an innovative approach in the pediatric population.36 To identify subjects with active disease, they used the combination of hepatic fat greater than 10%, as determined by MRI‐PDFF and ALT 45 U/L or greater. The primary outcome was change in hepatic fat estimated by MRI‐PDFF, with a difference of 4% being the minimal clinically significant change based on the clinical experience of the authors and previous adult data. At the end of the 8‐week trial, the mean hepatic fat decrease was significantly greater in the low free‐sugar diet (25% to 17%) compared with the usual diet group (21% to 20%), with an adjusted mean difference of −6.23% (P < 0.001). Furthermore, the levels of ALT, aspartate aminotransferase, and gamma‐glutamyltransferase were significantly lower in the low free‐sugar intervention group.
Designing a Successful NASH Trial in Children
The FDA has provided preliminary guidance for industry on designing clinical trials for pediatric NASH (https://www.fda.gov/media/119044/download) that can be summarized as follows:
Applying the adult NAS to children with NASH is challenging (less ballooning, lobular inflammation, and classic zone 3 fibrosis).
Extrapolation of efficacy from adult to pediatric patients solely on PK/PD information is not appropriate.
Longitudinal natural history data in pediatric patients are needed.
The risk/benefit of each drug will determine the timing of pediatric studies in relation to adult trials.
Designing POC Trials
Based on the previous discussion, one option would be to target adolescents age 12‐17 years for early POC trials, given the limited information on disease progression and natural history in younger children (Fig. 1). In the opinion of the authors, the target disease within the spectrum of NAFLD should be those with active disease, defined as having significant intrahepatic triglyceride accumulation of 10% or higher and elevated ALT of 45 U/L or higher. The duration can vary from 8 to 12 weeks given the fact that the recent trial by Schwimmer et al.36 was able to clearly demonstrate the treatment effect based on a reduction in MRI‐PDFF liver fat fraction and ALT with 8 weeks of lifestyle intervention. The primary endpoint could be either reduction in liver fat fraction (by 4% for absolute reduction or 30% for relative reduction) or ALT (by 10 U/L) from baseline. A more rigorous approach will be to have a coprimary endpoint of achieving both reduction in liver fat fraction and ALT, although this approach may lead to an increase in the required sample size and negative trial outcomes with drugs that would have had potential. This proposed design based on improvement in liver fat fraction and/or ALT might be reasonable for drugs with metabolic targets that significantly decrease steatosis but may not be ideal for drugs with antifibrotic effects.
Figure 1.
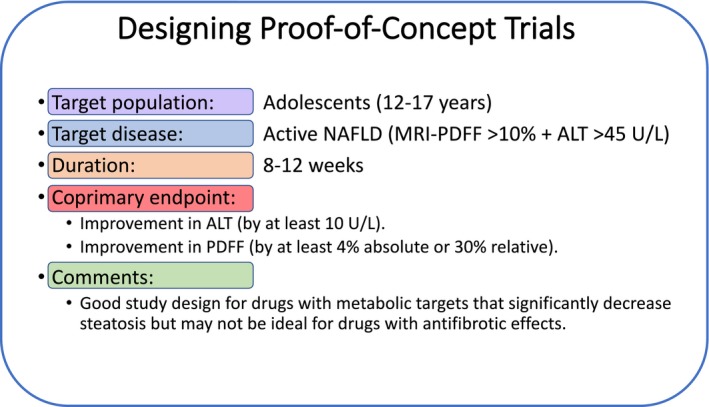
Recommendations for designing POC trials in pediatric NAFLD. Specific designs for each therapeutic target will vary based on the mechanism of action and should be adapted as new information becomes available.
Designing Late Phase 2 Trials
Similar to POC trials, we recommend that the target population for late phase 2 trials should be adolescents aged 12 to 17 years (Fig. 2). In the authors’ opinion, the target disease should be that in patients with biopsy‐proven NASH (based on pathologist diagnosis) and liver fibrosis (F1‐F3) to ensure the inclusion of children with significant disease burden who are at higher risk for progression. In the future, therapeutic targets with excellent safety profiles and positive impact on the metabolic syndrome may be considered in children with borderline NASH or those with no fibrosis (F0). Given the very low prevalence of NASH cirrhosis in this age group and the difficulties we are facing in drug development for cirrhotic NASH in adults, we recommend not including adolescents with cirrhosis at this point. The primary endpoint should be histologic improvement, defined similar to adult trials as NASH resolution without worsening in fibrosis or fibrosis regression by one stage without worsening in NASH after 12 months of treatment with the investigational drug. This makes the target population even more relevant given the known histologic differences in younger children who lack ballooning degeneration and have mainly portal‐based injury, making the accepted definition of NASH resolution problematic. Stratifying patients according to the presence of certain genetic polymorphisms, such as patatin‐like phospholipase domain‐containing protein 3 (PNPLA3) rs738409, that are associated with more aggressive disease progression should be considered in the future.37 We cannot overemphasize the importance of having a biomarker strategy in conjunction with histologic assessment to help develop noninvasive tests that can predict baseline histologic severity and response to treatment. This will likely eliminate the need for liver biopsy to decide on treatment initiation and monitoring disease progression/response in the future. It is reasonable to use an adaptive trial design to transition from phase 2 to phase 3 trials to eliminate the need for repeat histologic assessment in some patients given the limited number of adolescents/families who are willing to consent to several liver biopsies. Furthermore, due to the fact that long‐term treatment with a specific drug is needed to assess the effects on clinical outcomes, an adaptive design may help decrease the overall sample size while allowing patients to continue treatment from one phase to the next.38 According to the FDA, pediatric phase 3 efficacy trials are not necessarily required for drug approval as long as pediatric safety and dosing information are available. Therefore, it is possible that some NASH drugs that show efficacy on surrogate endpoints in children may not need to show efficacy on reducing the occurrence of hard clinical outcomes, such as progression to cirrhosis or hepatic decompensation, as long as these data can be extrapolated from adequate and well‐controlled adult trials.
Figure 2.
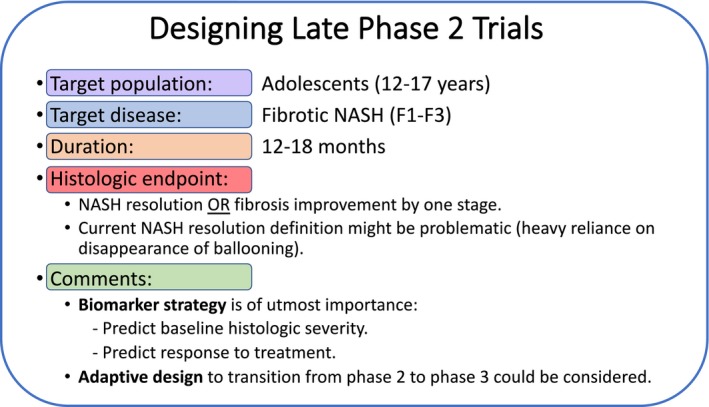
Recommendations for designing late phase 2 trials in children with NAFLD. Specific designs for each therapeutic target will vary based on the mechanism of action and should be adapted as new information becomes available.
Pediatric NAFLD is a common and potentially serious condition that may progress to advanced fibrosis and even cirrhosis during adolescence and early adulthood. Importantly, pediatric NASH has several unique aspects that will make designing trials in this population challenging, albeit feasible, including the different histology (less ballooning, more portal‐based injury), the limited natural history data, and the lack of reliable noninvasive tests to determine disease severity. POC studies should be started now, ideally with medications with high efficacy on metabolic targets and a good safety profile. The FDA is expected to provide future guidance addressing drug development for pediatric NASH.
Potential conflict of interest: Nothing to report.
References
- 1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease‐Meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73‐84. [DOI] [PubMed] [Google Scholar]
- 2. Yu EL, Golshan S, Harlow KE, Angeles JE, Durelle J, Goyal NP, et al. Prevalence of nonalcoholic fatty liver disease in children with obesity. J Pediatr 2019;207:64‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anstee QM, McPherson S, Day CP. How big a problem is non‐alcoholic fatty liver disease? BMJ 2011;343:d3897. [DOI] [PubMed] [Google Scholar]
- 4. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328‐357. [DOI] [PubMed] [Google Scholar]
- 5. Noureddin M, Vipani A, Bresee C, Todo T, Kim IK, Alkhouri N, et al. NASH leading cause of liver transplant in women: updated analysis of indications for liver transplant and ethnic and gender variances. Am J Gastroenterol 2018;113:1649‐1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology 2011;141:1249‐1253. [DOI] [PubMed] [Google Scholar]
- 7. Alkhouri N, Lawitz E, Noureddin M. Looking into the crystal ball: predicting the future challenges of fibrotic NASH treatment. Hepatol Commun 2019;3:605‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Konerman MA, Jones JC, Harrison SA. Pharmacotherapy for NASH: current and emerging. J Hepatol 2018;68:362‐375. Erratum in: J Hepatol 2018;68:1337. [DOI] [PubMed] [Google Scholar]
- 9. Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics 2006;118:1388‐1393. [DOI] [PubMed] [Google Scholar]
- 10. Fernandes DM, Pantangi V, Azam M, Salomao M, Iuga AC, Lefkowitch JH, et al. Pediatric nonalcoholic fatty liver disease in New York City: an autopsy study. J Pediatr 2018;200:174‐180. [DOI] [PubMed] [Google Scholar]
- 11. Welsh JA, Karpen S, Vos MB. Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988‐1994 to 2007‐2010. J Pediatr 2013;162:496‐500.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alkhouri N, Sedki E, Alisi A, Lopez R, Pinzani M, Feldstein AE, et al. Combined paediatric NAFLD fibrosis index and transient elastography to predict clinically significant fibrosis in children with fatty liver disease. Liver Int 2013;33:79‐85. [DOI] [PubMed] [Google Scholar]
- 13. Carter‐Kent C, Brunt EM, Yerian LM, Alkhouri N, Angulo P, Kohli R, et al. Relations of steatosis type, grade, and zonality to histological features in pediatric nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr 2011;52:190‐197. [DOI] [PubMed] [Google Scholar]
- 14. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al.; Nonalcoholic Steatohepatitis Clinical Research Network . Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313‐1321. [DOI] [PubMed] [Google Scholar]
- 15. Carter‐Kent C, Yerian LM, Brunt EM, Angulo P, Kohli R, Ling SC, et al. Nonalcoholic steatohepatitis in children: a multicenter clinicopathological study. Hepatology 2009;50:1113‐1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schwimmer JB, Behling C, Newbury R, Deutsch R, Nievergelt C, Schork NJ, et al. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology 2005;42:641‐649. [DOI] [PubMed] [Google Scholar]
- 17. Africa JA, Behling CA, Brunt EM, Zhang N, Luo Y, Wells A, et al.; Nonalcoholic Steatohepatitis Clinical Research Network . In children with nonalcoholic fatty liver disease, zone 1 steatosis is associated with advanced fibrosis. Clin Gastroenterol Hepatol 2018;16:438‐446.e431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Angulo P, Kleiner DE, Dam‐Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long‐term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015;149:389‐397.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology 2014;59:2188‐2195. [DOI] [PubMed] [Google Scholar]
- 20. Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 2010;51:1972‐1978. [DOI] [PubMed] [Google Scholar]
- 21. Feldstein AE, Charatcharoenwitthaya P, Treeprasertsuk S, Benson JT, Enders FB, Angulo P. The natural history of non‐alcoholic fatty liver disease in children: a follow‐up study for up to 20 years. Gut 2009;58:1538‐1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alkhouri N, McCullough A. Noninvasive diagnosis of NASH and liver fibrosis within the spectrum of NAFLD. Gastroenterol Hepatol (N Y) 2012;8:661‐668. [PMC free article] [PubMed] [Google Scholar]
- 23. Yang HR, Kim HR, Kim MJ, Ko JS, Seo JK. Noninvasive parameters and hepatic fibrosis scores in children with nonalcoholic fatty liver disease. World J Gastroenterol 2012;18:1525‐1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mansoor S, Yerian L, Lopez R, Kohli R, Xanthakos S, Angulo P, et al. The evaluation of hepatic fibrosis scores in children with nonalcoholic fatty liver disease. Dig Dis Sci 2015;60:1440‐1447. [DOI] [PubMed] [Google Scholar]
- 25. Nobili V, Vizzutti F, Arena U, Abraldes JG, Marra F, Pietrobattista A, et al. Accuracy and reproducibility of transient elastography for the diagnosis of fibrosis in pediatric nonalcoholic steatohepatitis. Hepatology 2008;48:442‐448. [DOI] [PubMed] [Google Scholar]
- 26. Xanthakos SA, Podberesky DJ, Serai SD, Miles L, King EC, Balistreri WF, et al. Use of magnetic resonance elastography to assess hepatic fibrosis in children with chronic liver disease. J Pediatr 2014;164:186‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Meisamy S, Hines CD, Hamilton G, Sirlin CB, McKenzie CA, Yu H, et al. Quantification of hepatic steatosis with T1‐independent, T2‐corrected MR imaging with spectral modeling of fat: blinded comparison with MR spectroscopy. Radiology 2011;258:767‐775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Permutt Z, Le TA, Peterson MR, Seki E, Brenner DA, Sirlin C, et al. Correlation between liver histology and novel magnetic resonance imaging in adult patients with non‐alcoholic fatty liver disease ‐ MRI accurately quantifies hepatic steatosis in NAFLD. Aliment Pharmacol Ther 2012;36:22‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tang A, Desai A, Hamilton G, Wolfson T, Gamst A, Lam J, et al. Accuracy of MR imaging‐estimated proton density fat fraction for classification of dichotomized histologic steatosis grades in nonalcoholic fatty liver disease. Radiology 2015;274:416‐425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tang A, Tan J, Sun M, Hamilton G, Bydder M, Wolfson T, et al. Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology 2013;267:422‐431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Middleton MS, Van Natta ML, Heba ER, Alazraki A, Trout AT, Masand P, et al.; NASH Clinical Research Network . Diagnostic accuracy of magnetic resonance imaging hepatic proton density fat fraction in pediatric nonalcoholic fatty liver disease. Hepatology 2018;67:858‐872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P, et al.; Nonalcoholic Steatohepatitis Clinical Research Network . Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA 2011;305:1659‐1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schwimmer JB, Lavine JE, Wilson LA, Neuschwander‐Tetri BA, Xanthakos SA, Kohli R, et al.; NASH CRN . In children with nonalcoholic fatty liver disease, cysteamine bitartrate delayed release improves liver enzymes but does not reduce disease activity scores. Gastroenterology 2016;151:1141‐1154.e1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Noureddin M, Lam J, Peterson MR, Middleton M, Hamilton G, Le TA, et al. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology 2013;58:1930‐1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Patel J, Bettencourt R, Cui J, Salotti J, Hooker J, Bhatt A, et al. Association of noninvasive quantitative decline in liver fat content on MRI with histologic response in nonalcoholic steatohepatitis. Therap Adv Gastroenterol 2016;9:692‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schwimmer JB, Ugalde‐Nicalo P, Welsh JA, Angeles JE, Cordero M, Harlow KE, et al. Effect of a low free sugar diet vs usual diet on nonalcoholic fatty liver disease in adolescent boys: a randomized clinical trial. JAMA 2019;321:256‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Valenti L, Alisi A, Galmozzi E, Bartuli A, Del Menico B, Alterio A, et al. I148M patatin‐like phospholipase domain‐containing 3 gene variant and severity of pediatric nonalcoholic fatty liver disease. Hepatology 2010;52:1274‐1280. [DOI] [PubMed] [Google Scholar]
- 38. Filozof C, Chow SC, Dimick‐Santos L, Chen YF, Williams RN, Goldstein BJ, et al. Clinical endpoints and adaptive clinical trials in precirrhotic nonalcoholic steatohepatitis: facilitating development approaches for an emerging epidemic. Hepatol Commun 2017;1:577‐585. [DOI] [PMC free article] [PubMed] [Google Scholar]


