ABSTRACT
The middle longitudinal fasciculus (MdLF) is a small and somewhat controversial white matter tract of the human cerebrum, confined to the posterior superior temporal region from which it courses posteriorly to connect at the occipital–parietal interface. The tract appears to be involved in language processing as well as auditory organization and localization, while sub-serving other higher level cognitive functions that have yet to be fully elucidated. Little is known about the specific, interparcellation connections that integrate to form the MdLF. Utilizing diffusion spectrum magnetic resonance imaging tractography coupled with the human cortex parcellation data presented earlier in this supplement, we aim to describe the macro-connectome of the MdLF in relation to the linked parcellations present within the human cortex. The purpose of this study is to present this information in an indexed, illustrated, and tractographically aided series of figures and tables for anatomic and clinical reference.
Keywords: Anatomy, Cerebrum, Connectivity, DTI, Functional connectivity, Human, Parcellations
ABBREVIATIONS
- DSI
diffusion spectrum imaging
- DTI
diffusion tensor imaging
- MdLF
middle longitudinal fasciculus
- MR
magnetic resonance
- ROI
region of interest
- SLF/AF
superior longitudinal fasciculus/arcuate fasciculus
The middle longitudinal fasciculus (MdLF) is a small white matter tract located in the posterior aspect of the temporal lobe, coursing posterosuperiorly to the parietal–occipital region. The MdLF is classically one of the most poorly understood tracts within the human cerebrum,1 and as such poses a challenge to the neurosurgeon cutting in close proximity to this white matter pathway.
Although diffusion tensor imaging (DTI) tractography studies have described the controversial structural anatomy of the MdLF,2,3 little is known about its various cortical terminations. Recently, the Human Connectome Project published parcellation data redefining the human cortex.4 This provides a unique opportunity to elucidate the macro-connectome of the human cerebrum, in that high-resolution DTI tractography has been shown to accurately illustrate the anatomy and structure of different white matter tracts in the human brain.2,5,6
In this anatomic study, we utilized high-resolution diffusion spectrum imaging (DSI) tractography in conjunction with the Glasser parcellation scheme to illustrate the macro-connectivity between various associated functionally and anatomically connected areas of the cerebrum within the confines of the MdLF. The purpose of this study is to present the structural connectivity of the MdLF in an indexed, illustrated, and tractographically aided series of figures and tables for anatomic and clinical reference.
METHODS
Identification of Relevant Cortical Regions
The parcellation data entries within the first nine chapters of this supplement were reviewed to determine the specific cortical regions with structural connectivity in the distribution of the MdLF. These data were tabulated, and connections between individual parcellations within the MdLF were recorded. These results served as the basis for constructing a simplified tractography map of the MdLF and performing deterministic tractography.
Deterministic Tractography
Publicly available imaging data from the Human Connectome Project was obtained for this study from the HCP database (http://humanconnectome.org, release Q3). Diffusion imaging with corresponding T1-weighted images from 10 healthy, unrelated controls were analyzed (subjects IDs: 100307, 103414, 105115, 110411, 111312, 113619, 115320, 117112, 118730, 118932). A multishell diffusion scheme was used, and the b-values were 990, 1985, and 1980 s/mm2. Each b-value was sampled in 90 directions. The in-plane resolution was 1.25 mm. The diffusion data were reconstructed using generalized q-sampling imaging with a diffusion sampling length ratio of 1.25.7
We performed brain registration to Montreal neurologic institute space, wherein imaging is warped to fit a standardized brain model comparison between subjects. Tractography was performed in DSI studio using a region of interest approach to initiate fiber tracking from a user-defined seed region. A two region of interest (ROI) approach was used to isolate tracts. Voxels within each ROI were automatically traced with a maximum angular threshold of 45°. When a voxel was approached with no tract direction or a direction change of greater than 45°, the tract was halted. Tractography was stopped after reaching a maximum length of 800 mm. In some instances, exclusion ROIs were placed to exclude obvious spurious tracts that were not involved in the white matter pathway of interest. Tractographic results are shown only for regions of interest within the left cerebral hemisphere.
CONNECTIVITY OVERVIEW
Presented in Figure 1, we demonstrate the functionally relevant and anatomically connected cerebral parcellation data that integrates within the confines of the MdLF. In Table, these connected areas are summarized for reference. In addition, Figures 2 to 4 illustrate key connectivity examples of the MdLF, chosen for the strength and breadth of linked parcellation data. It should be noted that the figures and tables presented in this study do not imply directionality, instead supposed information transit is utilized as a simplified means for connectivity description. In general, the MdLF connects parcellations of the superior temporal gyrus and superior temporal sulcus to visual cortical regions in the cuneus and lingual gyrus as well as regions in the superior parietal lobe and intraparietal sulcus.
FIGURE 1.
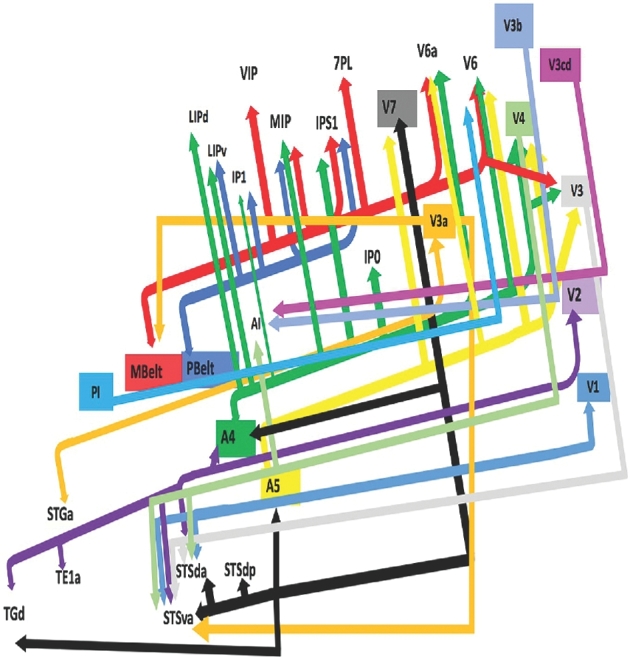
Simplified tract map showing the structural connections that integrate within the MdLF. Connections between cortical areas are color-coded based on the parcellation of origin (eg yellow arrows indicate structural connections from origin A5 to areas V7, V6a, V6, V4, and V3). Note that arrows are not meant to imply the direction of information transmit.
TABLE.
Regions Integrating Within the MdLF
| Original parcellation | Terminations |
|---|---|
| A4 | IP0 |
| IP1 | |
| IPS1 | |
| LIPd | |
| LIPv | |
| MIP | |
| V3 | |
| V4 | |
| V6 | |
| V6a | |
| A5 | V3 |
| V4 | |
| V6 | |
| V6a | |
| V7 | |
| MBelt | 7PL |
| IPS1 | |
| MIP | |
| V3 | |
| V6 | |
| V6a | |
| VIP | |
| PBelt | IP1 |
| IPS1 | |
| LIPv | |
| MIP | |
| PI | V6 |
| POI1 | POI2 |
| V1 | STSda |
| STSva | |
| V2 | A4 |
| STSda | |
| STSva | |
| TE1a | |
| TGd | |
| V3 | STSda |
| STSva | |
| V3a | STGa |
| STSva | |
| MBelt | |
| V3b | A1 |
| V3cd | A1 |
| V4 | STSda |
| STSva | |
| A1 | |
| V7 | A4 |
| STSda | |
| STSdp | |
| STSva | |
| TGd |
FIGURE 2.
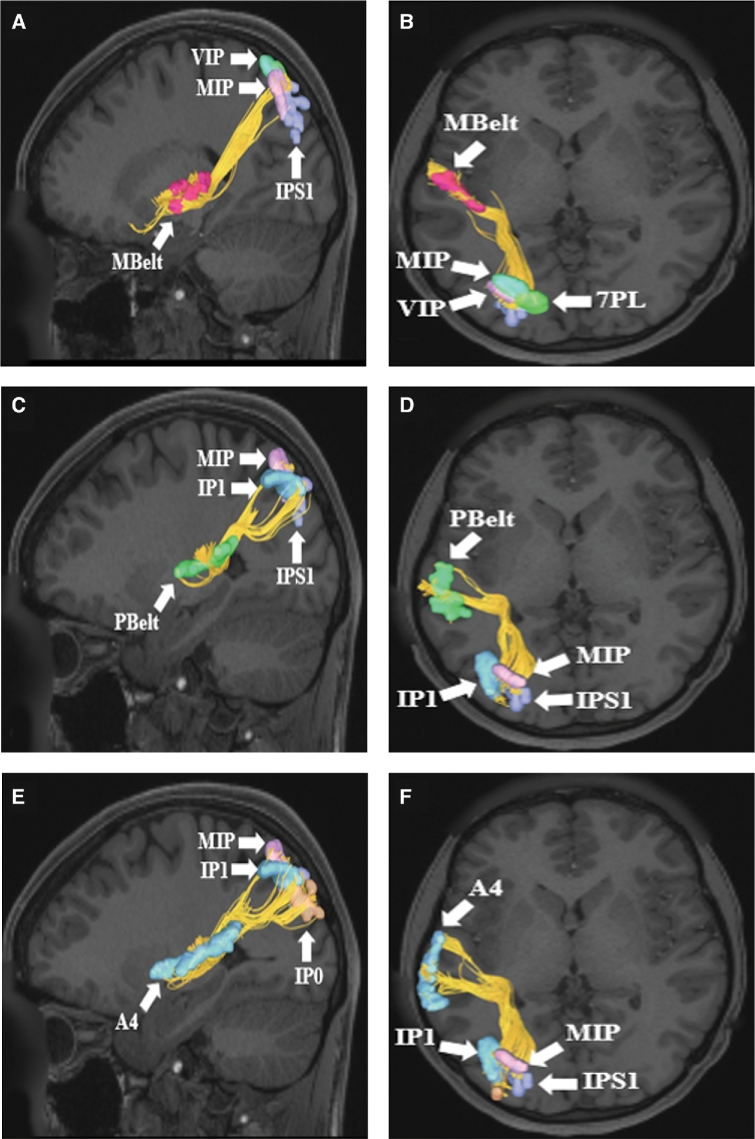
MdLF connections from superior temporal lobe parcellations A and B, Mbelt, C and D, PBelt, E and F, A4 in the left cerebral hemisphere. Connections are shown on T1-weighted magnetic resonance (MR) images in the sagittal and axial planes. All three cortical areas demonstrate structural connections to different parietal lobe parcellations, including IP0, IP1, MIP, VIP, IPS1, and 7PL. The MdLF is readily seen in this figure coursing from the superior temporal lobe posteriorly and superiorly to terminate in the parietal lobe.
FIGURE 4.
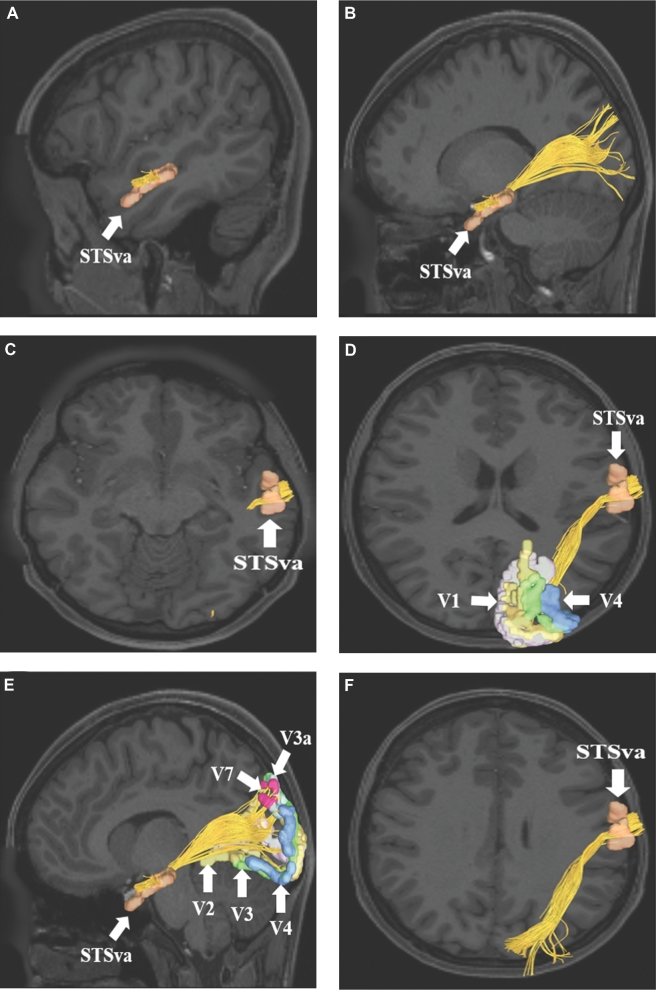
MdLF connections from area STSva to early processing regions V1, V2, V3, V3a, V4, and V7. Connections are shown on T1-weighted MR images in the A, B, and E, sagittal and C, D, and F, axial planes. The MdLF is readily seen coursing from the superior temporal lobe posteriorly and superiorly to terminate in parts of the occipital lobe.
FIGURE 3.
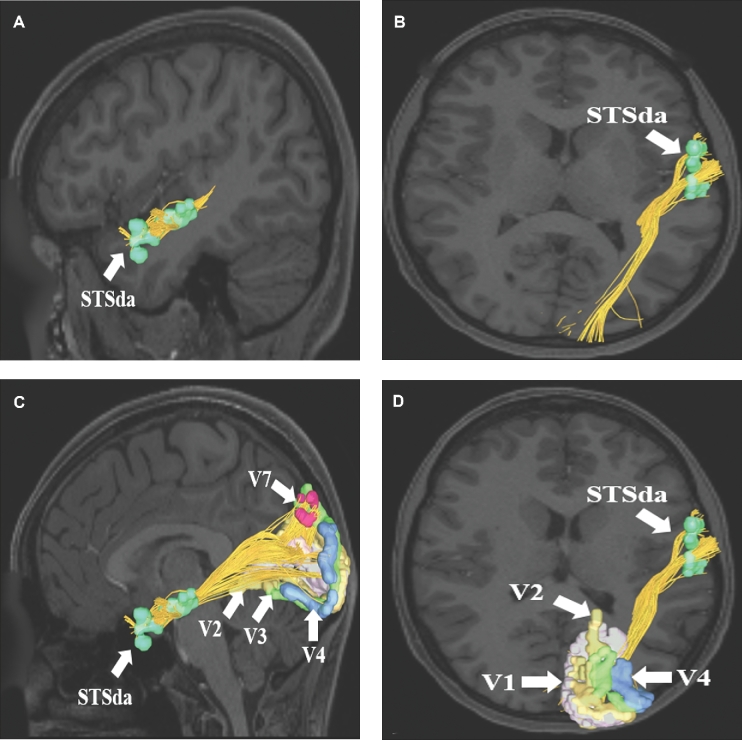
MdLF connections from area STSda to visual processing regions V1, V2, V3, V4, and V7. Connections are shown on T1-weighted MR images in the A and C, sagittal and B and D, axial planes. The MdLF is readily seen coursing from the superior temporal lobe posteriorly and superiorly to terminate in parts of the occipital lobe.
DISCUSSION
The MdLF is absent in historical neuroanatomic studies,8-12 as well as in contemporary atlases of white matter connectivity.13 Although it was first described as a specific tract in the primate brain in the mid-1980s,14 some recent DTI tractography studies failed to define the MdLF as a specific white matter bundle,15-18 even with region of interest labeling within association pathways of the temporal lobe,19 as well as during blunt fiber dissection.19 however more recent work has demonstrated the MdLF, other groups have illustrated the MdLF within the human cerebrum.20-24 One proposed explanation for the contradictory description of the MdLF’s existence lies within an apparent histological misidentification of the MdLF as the temporal stem portion of the superior longitudinal fasciculus/arcuate fasciculus (SLF/AF) complex.20-24
More recent studies utilizing diffusion tractography suggest that the MdLF courses anteriorly (medial to the SLF/AF complex) from its origin in the angular gyrus to its termination in the anterior superior temporal gyrus or temporal pole.2,20-26 There is disagreement regarding where the MdLF actually arises, with some proposing that this white matter tract originates more posteriorly within the superior parietal lobule, at the parieto–occipital interface, or within the occipital lobe itself.27,28 Still others have reported some evidence based on high-angular resolution diffusion imaging (HARDI) that the MdLF is composed of two distinct fiber bundles, one originating near the angular gyrus and the other originating within the superior parietal lobule.25 Overall, it is felt that the MdLF is one of the most poorly understood tracts within the human cerebrum.1
Inconsistent evidence suggests that the MdLF may play a role in language function.21,29 For example, there are some studies implicating the MdLF in sound-to-meaning conversion,22,23 which is consistent with the notion that the MdLF is anchored by the ventral stream.30 Some studies have disputed this finding, though.31,32 Additional studies in human language function implicate this white matter pathway in both phonological and semantic language processing. However, the evidence regarding these functionalities in relation to the MdLF is limited.20,33-35 Finally, this tract may be involved in the repetition-phonological network responsible for carrying information regarding the learning and acquisition of new words.36
At least one study has illustrated a lack of major structural connectivity between the MdLF and the angular gyrus, further complicating our understanding of the MdLF’s role in language processing.27 This is further reinforced by direct subcortical electrical stimulation during awake brain mapping wherein stimulation of the MdLF elicited no observed paraphasias.31 Moreover, operative resection of the anterior portion of the MdLF did not appear to affect picture naming, purportedly eliminating support for the role of this tract in semantic language processing.31 However, it is possible that these resections did not involve the posterior stem of the tract, thus sparing the language network.31 Consequently, the MdLF currently is theorized to be a small white matter tract that does not have an essential role in language function. Instead, it is felt that MdLF likely contributes to language network redundancy and compensatory mechanisms.37
Beyond the language domain of human cognition, there is some support for the role of the MdLF in auditory localization processing.27 It has also been theorized that the MdLF plays a role in other higher level cognitive functions,38 such as spatial organization, memory, and motivation.39 However, the precise role of the MdLF in these functional networks remains poorly defined and needs to be further characterized.40
CONCLUSION
The MdLF is a somewhat controversial white matter tract connecting multiple regions of the human cerebrum, and appears at least to be localized to the posterior temporal–occipital–parietal junction. The MdLF is likely important in language and auditory processing, auditory organization, and possibly visuospatial function and memory. Further, subtract-guided functional and anatomic studies are needed to enhance our understanding of the functional connectivity of this white matter bundle and characterize its role in the human connectome.
Disclosures
Synaptive Medical assisted in the funding of all 18 chapters of this supplement. No other funding sources were utilized in the production or submission of this work.
Acknowledgments
Data were provided [in part] by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University. We would also like to thank Brad Fernald, Haley Harris, and Alicia McNeely of Synaptive Medical for their assistance in constructing the network figures for Chapter 18 and for coordinating the completion and submission of this supplement.
REFERENCES
- 1. Bajada CJ, Haroon HA, Azadbakht H, Parker GJ, Lambon Ralph MA, Cloutman LL. The tract terminations in the temporal lobe: their location and associated functions. Cortex. 2017;97:277-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Menjot de Champfleur N, Lima Maldonado I, Moritz-Gasser Set al. Middle longitudinal fasciculus delineation within language pathways: a diffusion tensor imaging study in human. Eur J Radiol. 2013;82(1):151-157. [DOI] [PubMed] [Google Scholar]
- 3. Burks JD, Boettcher LB, Conner AKet al. White matter connections of the inferior parietal lobule: a study of surgical anatomy. Brain Behav. 2017;7(4):e00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Glasser MF, Coalson TS, Robinson ECet al. A multi-modal parcellation of human cerebral cortex. Nature. 2016;536(7615):171-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kamali A, Flanders AE, Brody J, Hunter JV, Hasan KM. Tracing superior longitudinal fasciculus connectivity in the human brain using high resolution diffusion tensor tractography. Brain Struct Funct. 2014;219(1):269-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lemaire JJ, Cosnard G, Sakka Let al. White matter anatomy of the human deep brain revisited with high resolution DTI fibre tracking. Neurochirurgie. 2011;57(2):52-67. [DOI] [PubMed] [Google Scholar]
- 7. Yeh FC, Wedeen VJ, Tseng WY. Generalized q-sampling imaging. IEEE Trans Med Imaging. 2010;29(9):1626-1635. [DOI] [PubMed] [Google Scholar]
- 8. Burdach K. Vom bau und leben des gehirns und rückenmarks. Leipzig; 1819. [Google Scholar]
- 9. Foville AL. Traité Complet de L’anatomie, de la Physiologie et de la Pathologie du Système Nerveux Cérébro-Spinal. Vol 1: Masson; 1844. [Google Scholar]
- 10. Meynert T. Psychiatry: A Clinical Treatise on Diseases of the Fore-brain…The Anatomy, Physiology, and Chemistry of the Brain; 1885. [Google Scholar]
- 11. Déjérine J. Anatomie des Centres Nerveux. Vol 1. Paris; 1895. [Google Scholar]
- 12. Déjérine J. Anatomie des Centres Nerveux. Vol 2. Paris; 1901. [Google Scholar]
- 13. Oishi K, Faria AV, van Zijl PC, Mori S. MRI Atlas of Human White Matter. Academic Press; 2010. [Google Scholar]
- 14. Seltzer B, Pandya DN. Further observations on parieto-temporal connections in the rhesus monkey. Exp Brain Res. 1984;55(2):301-312. [DOI] [PubMed] [Google Scholar]
- 15. Bürgel U, Amunts K, Hoemke L, Mohlberg H, Gilsbach JM, Zilles K. White matter fiber tracts of the human brain: three-dimensional mapping at microscopic resolution, topography and intersubject variability. Neuroimage. 2006;29(4):1092-1105. [DOI] [PubMed] [Google Scholar]
- 16. Wakana S, Caprihan A, Panzenboeck MMet al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36(3):630-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Catani M, De Schotten MT. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44(8):1105-1132. [DOI] [PubMed] [Google Scholar]
- 18. de Schotten MT, Bizzi A, Dell’Acqua Fet al. Atlasing location, asymmetry and inter-subject variability of white matter tracts in the human brain with MR diffusion tractography. Neuroimage. 2011;54(1):49-59. [DOI] [PubMed] [Google Scholar]
- 19. Holl N, Noblet V, Rodrigo Set al. Temporal lobe association fiber tractography as compared to histology and dissection. Surg Radiol Anat. 2011;33(8):713-722. [DOI] [PubMed] [Google Scholar]
- 20. Makris N, Papadimitriou GM, Kaiser JR, Sorg S, Kennedy DN, Pandya DN. Delineation of the middle longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex. 2009;19(4):777-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Turken AU, Dronkers NF. The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. Front Syst Neurosci. 2011;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wong FC, Chandrasekaran B, Garibaldi K, Wong PC. White matter anisotropy in the ventral language pathway predicts sound-to-word learning success. J Neurosci. 2011;31(24):8780-8785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Saur D, Kreher BW, Schnell Set al. Ventral and dorsal pathways for language. Proc Natl Acad Sci U S A. 2008;105(46):18035-18040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Frey S, Campbell JS, Pike GB, Petrides M. Dissociating the human language pathways with high angular resolution diffusion fiber tractography. J Neurosci. 2008;28(45):11435-11444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Makris N, Preti MG, Wassermann Det al. Human middle longitudinal fascicle: segregation and behavioral-clinical implications of two distinct fiber connections linking temporal pole and superior temporal gyrus with the angular gyrus or superior parietal lobule using multi-tensor tractography. Brain Imaging Behav. 2013;7(3):335-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Makris N, Preti MG, Asami Tet al. Human middle longitudinal fascicle: variations in patterns of anatomical connections. Brain Struct Funct. 2013;218(4):951-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Y, Fernandez-Miranda JC, Verstynen T, Pathak S, Schneider W, Yeh FC. Rethinking the role of the middle longitudinal fascicle in language and auditory pathways. Cereb Cortex. 2013;23(10):2347-2356. [DOI] [PubMed] [Google Scholar]
- 28. Maldonado IL, de Champfleur NM, Velut S, Destrieux C, Zemmoura I, Duffau H. Evidence of a middle longitudinal fasciculus in the human brain from fiber dissection. J Anat. 2013;223(1):38-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dick AS, Tremblay P. Beyond the arcuate fasciculus: consensus and controversy in the connectional anatomy of language. Brain. 2012;135(12):3529-3550. [DOI] [PubMed] [Google Scholar]
- 30. Dick AS, Bernal B, Tremblay P. The Language Connectome. Neuroscientist. 2014;20(5):453-467. [DOI] [PubMed] [Google Scholar]
- 31. De Witt Hamer PC, Moritz-Gasser S, Gatignol P, Duffau H. Is the human left middle longitudinal fascicle essential for language? A brain electrostimulation study. Hum Brain Mapp. 2011;32(6):962-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Duffau H, Herbet G, Moritz-Gasser S. Toward a pluri-component, multimodal, and dynamic organization of the ventral semantic stream in humans: Lessons from stimulation mapping in awake patients. Front Syst Neurosci. 2013;7:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8(5):393-402. [DOI] [PubMed] [Google Scholar]
- 34. Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19(12):2767-2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brownsett SL, Wise RJ. The contribution of the parietal lobes to speaking and writing. Cereb Cortex. 2010;20(3):517-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rauschecker JP, Scott SK. Maps and streams in the auditory cortex: Nonhuman primates illuminate human speech processing. Nat Neurosci. 2009;12(6):718-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fernandez Coello A, Moritz-Gasser S, Martino J, Martinoni M, Matsuda R, Duffau H. Selection of intraoperative tasks for awake mapping based on relationships between tumor location and functional networks. J Neurosurg. 2013;119(6):1380-1394. [DOI] [PubMed] [Google Scholar]
- 38. Vassal F, Schneider F, Boutet C, Jean B, Sontheimer A, Lemaire JJ. Combined DTI tractography and functional MRI study of the language connectome in healthy volunteers: Extensive mapping of white matter fascicles and cortical activations. PLoS One. 2016;11(3):e0152614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schmahmann JD, Smith EE, Eichler FS, Filley CM. Cerebral white matter: neuroanatomy, clinical neurology, and neurobehavioral correlates. Ann N Y Acad Sci. 2008;1142(1):266-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Egger K, Yang S, Reisert Met al. Tractography of association fibers associated with language processing. Clin Neuroradiol. 2015;25(S2):231-236. [DOI] [PubMed] [Google Scholar]