To the Editor,
Myeloid/lymphoid neoplasm with FGFR1 rearrangement [1] is a rare aggressive disease characterized by myeloid hyperplasia, marked eosinophilia, tendency to rapidly progress to an acute leukemia (AML) and high refractoriness to chemotherapy [2].
The molecular pathogenesis results from a chromosomal translocation involving the FGFR1 gene at the 8p11 locus with various partner genes causing a constitutive activation of the FGFR1 tyrosine kinase, impacting cell proliferation and survival [3].
Recently, a phase I/II study evaluating a novel, highly selective FGFR kinase inhibitor, INCB054828, in patients with refractory advanced malignancies (ClinicalTrials.gov: NCT02393248) has been initiated [4]. Herein, we present a patient who achieved a complete remission on this highly selective inhibitor.
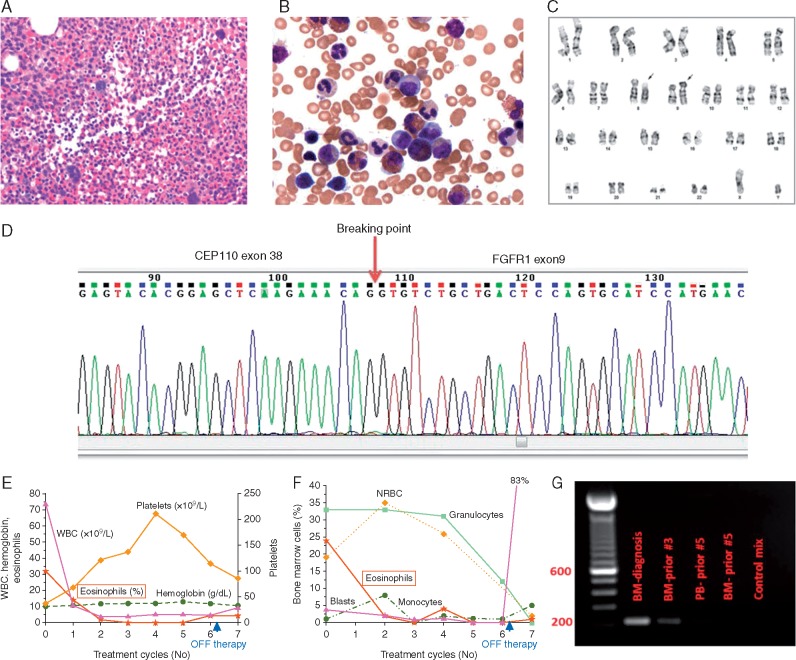
A 50-year-old male presented with a 5-month history of leukocytosis, fatigue and anorexia. He was found to have leukocytosis with an absolute eosinophilia, anemia, and thrombocytopenia, and bone marrow biopsy revealed hypercellular marrow with eosinophilia consistent with a myeloproliferative neoplasm. Further studies involving conventional cytogenetics, fluorescent in situ hybridization, reverse transcriptase polymerase chain reaction, and Sanger sequencing confirmed t(8; 9)(p11.2; q33) with an FGFR1 rearrangement leading to an abnormal CEP110-FGFR1 fusion transcript (breakpoints at exon 38 and 9, respectively) (Figure 1).
Figure 1.
Diagnostic findings. (A) Hypercellular bone marrow with granulocytic hyperplasia (H&E, ×400). (B) Bone marrow with granulocytic hyperplasia and eosinophilia (Wright Giemsa, ×500). (C) Chromosomal analysis showing t(8; 9) (p11; q33) (black arrows). (D) Sanger sequencing of the CEP110-FGFR1 fused PCR product shows breaking points at exon 38 of the CEP110 gene and at exon 9 of the FGFR1 gene. (E) Peripheral blood counts and (F) bone marrow findings at diagnosis and over time on during therapy with INCB054828 (x-axis = treatment cycles 1–6, cycle 7 was not administered). (G) RT-PCR demonstrating CEP110–FGFR1 fusion at diagnosis and over time: electrophoresis on a 1.5% agarose gel; PCR product of 212 bp was amplified from bone marrow cells (lanes 2, 3, 5—BM) and peripheral blood (lane 4, PB): lane 2 at initial diagnosis; lanes 3–5 during therapy; and control sample (lane 6). Before cycle 5, the patient was in remission, therefore no signal was detected.
After signing informed consent, the patient was enrolled into the clinical trial with INCB054828, 9 mg orally once daily on a 2 weeks-on/1 week-off schedule, in 21 day cycles. The patient achieved rapid response on therapy with complete resolution of eosinophilia, complete hematologic and cytogenetic remission, and complete molecular remission with undetectable CEP110-FGFR1 fusion transcript after the second, third, and fourth cycles, respectively (Figure 1E–G). Overall tolerance was excellent without any treatment interruptions, or requirement of blood product transfusion.
Fourteen days after starting the sixth cycle, the patient had treatment interrupted for an unrelated issue, and his disease progressed rapidly to AML. The patient was subsequently taken off study, and underwent treatment with intensive chemotherapy followed by allogeneic stem-cell transplantation (SCT). Twenty-four months from the original diagnosis, the patient continues to be in complete remission.
To the best of our knowledge, this report marks the first demonstration of a deep complete remission in a patient with myeloid/lymphoid neoplasm with FGFR1 rearrangement achieved with a targeted therapy, a highly selective FGFR kinase inhibitor, INCB054828. Up until now, molecular remissions in this disease have only been attainable with SCT. The use of a targeted inhibitor of a pathway clearly implicated in disease pathogenesis may offer more effective therapy with the possibility of ‘functional cure’, as first noted in chronic myeloid leukemia treated with tyrosine kinase inhibitors. INCB054828 not only represents a ‘bridge’ to SCT, but might also be an important treatment option for patients not eligible for an SCT. Based on this one patient experience, a phase II trial of INCB054828 in patients with myeloid/lymphoid neoplasms with FGFR1 rearrangement (NCT03011372) [5] has been initiated and is recruiting patients.
Funding
This work was supported in part by the National Cancer Institute of Health through the MD Anderson Cancer Center Support Grant CA016672, and Incyte Corporation.
Disclosure
SV and NGD receive research funding from Incyte Corporation. The remaining authors have declared no conflicts of interest.
References
- 1. Arber DA, Orazi A, Hasserjian R. et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016; 127: 2391–2405. [DOI] [PubMed] [Google Scholar]
- 2. Macdonald D, Reiter A, Cross NC.. The 8p11 myeloproliferative syndrome: a distinct clinical entity caused by constitutive activation of FGFR1. Acta Haematol 2002; 107: 101–107. [DOI] [PubMed] [Google Scholar]
- 3. Turner N, Grose R.. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer 2010; 10: 116–129. [DOI] [PubMed] [Google Scholar]
- 4. Liu PCCWL, Koblish H, Wu L. et al. Preclinical characterization of the selective FGFR inhibitor INCB054828. Proceedings of AACR 106th Annual Meeting 2015, 18–22 April 2015; Philadelphia, PA, abstract nr 771.
- 5. Verstovsek SRA, Rambaldi A, Asatiani E. et al. Phase 2, open-label, multicenter study to evaluate the efficacy and safety of INCB054828 in patients with myeloid/lymphoid neoplasms with fibroblast growth factor receptor 1 (FGFR1) rearrangement. Clin Lymphoma Myeloma Leukemia 2017; 17(Suppl 2): S350–S351. [Google Scholar]