Abstract
The permeability transition pore (PTP) is a latent, high‐conductance channel of the inner mitochondrial membrane. When activated, it plays a key role in cell death and therefore in several diseases. The investigation of the PTP took an unexpected turn after the discovery that cyclophilin D (the target of the PTP inhibitory effect of cyclosporin A) binds to FOF1 (F)‐ATP synthase, thus inhibiting its catalytic activity by about 30%. This observation was followed by the demonstration that binding occurs at a particular subunit of the enzyme, the oligomycin sensitivity conferral protein (OSCP), and that F‐ATP synthase can form Ca2+‐activated, high‐conductance channels with features matching those of the PTP, suggesting that the latter originates from a conformational change in F‐ATP synthase. This review is specifically focused on the OSCP subunit of F‐ATP synthase, whose unique features make it a potential pharmacological target both for modulation of F‐ATP synthase and its transition to a pore.
Linked Articles
This article is part of a themed section on Mitochondrial Pharmacology: Featured Mechanisms and Approaches for Therapy Translation. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v176.22/issuetoc
Abbreviations
- Abeta
beta amyloid protein
- AD
Alzheimer disease
- ANT
adenine nucleotide translocator
- Bz 423
benzodiazepine 423
- cryo‐EM
cryo‐electron microscopy
- CsA
cyclosporin A
- CyPD
cyclophilin D
- DAPIT
diabetes‐associated protein in insulin‐sensitive tissue
- FRET
fluorescence resonance energy transfer
- IF1
inhibitor factor 1
- Lrpprc
leucine-rich pentatricopeptide repeat containing protein
- MnSOD
Manganese superoxide dismutase
- mtDNA
mitochondrial DNA
- NMR
nuclear magnetic resonance
- OSCP
oligomycin sensitivity conferral protein
- pdx‐1
pancreatic duodenal homeobox 1
- PT
permeability transition
- PTP
permeability transition pore
- Sirt3
Sirtuin 3
- Strap
stress responsive activator p300
- TSPO
translocator protein
- VDAC
voltage‐dependent anion channel
The FOF1 ATP synthase
The http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=156 is a ubiquitous enzyme located in energy‐converting membranes that conform to essentially the same ‘building plan' across all kingdoms of life (Kühlbrandt and Davies, 2016). This enzyme comprises two distinct parts, that is, the globular, catalytic F1 sector and the membrane‐embedded FO subcomplex, which are connected by central and peripheral stalks (Figure 1). The http://www.guidetopharmacology.org/GRAC/DatabaseSearchForward?searchString=oligomycin+sensitivity-conferring+protein&searchCategories=all&species=none&type=all&comments=includeComments&order=rank&submit=Search+Database) in mitochondria (and its orthologous http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=156#799 in bacteria) is located in the upper part of the peripheral stalk which, along with the central stalk, ensures efficient energy interconversion (Devenish et al., 2000; Antoniel et al., 2014). Indeed, FO and F1 are molecular motors exerting rotational torque against one another. When the electrochemical proton gradient is sufficient to impose a large Fo torque, the enzyme catalyses the synthesis of ATP from ADP and Pi. When the phosphate potential predominates, F1 reverses the rotation of FO to build up a membrane potential (Noji et al., 2017). The OSCP (or δ) subunit located on top of F1 ensures the structural coupling between FO and F1. This coupling is disrupted by the antibiotic oligomycin, which inhibits both ATP synthesis and hydrolysis.
Figure 1.
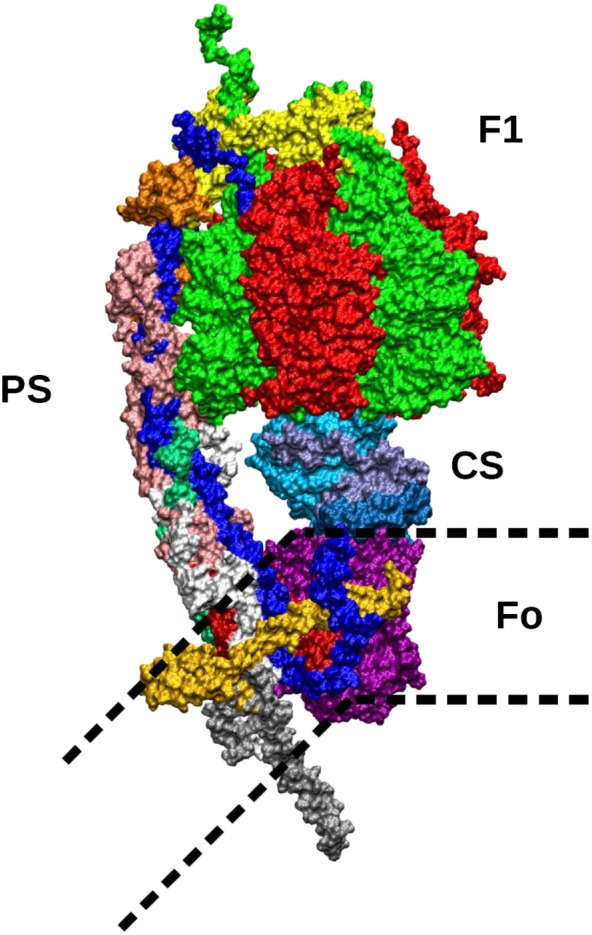
Molecular structure of the mitochondrial F‐ATP synthase. Subunits are shown in colours as follows. In the upper part of the figure, F1 is shown with the alternating α (green) and β subunits (red). On the left, the peripheral stalk (PS), connecting the top of F1 with the membrane subunits, includes the OSCP subunit (yellow) on top of F1; the b subunit (dark blue) which can be followed from the top of F1 along the peripheral stalk down to the membrane region; the F6 subunit (orange) and the d subunit (pink). In the lower part of the PS, reaching the Fo region in the membrane, subunits f (white) and A6L (emerald, mostly covered by other subunits) can be seen. The central stalk (CS) connecting the α/β subunits to the c‐ring in the membrane includes the γ (cyan), δ (blue) and ε subunits (ice blue). The membrane‐embedded c‐ring interacts with the CS and is composed of eight identical subunits c (purple). The Fo membrane subunits include the a subunit in contact with the c‐ring (dark red, mostly covered in the picture by other subunits), the g subunit (light orange) disposed in parallel with the membrane surface and the e subunit (silver) whose helix is protruding outside the membrane on the opposite side of F1.
Bacterial FO has the simplest subunit composition with (i) the ring of 10–15 http://www.guidetopharmacology.org/GRAC/DatabaseSearchForward?searchString=ATP5G&searchCategories=all&species=none&type=all&comments=includeComments&order=rank&submit=Search+Database, (ii) the membrane‐embedded subcomplex ab2 and (iii) the peripheral stalk comprising the extrinsic part of the http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=156#802 and the F1 subunit δ (Noji et al., 2017). In the mitochondrial enzyme, FO mediates the formation of http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=157 (Hahn et al., 2016; Guo et al., 2017), which are not found in bacteria, and has a far more complex structure. Besides the c‐ring (formed by 8–10 subunits), the membrane sector consists of single copies of subunits http://www.guidetopharmacology.org/GRAC/DatabaseSearchForward?searchString=ATP6&searchCategories=all&species=none&type=all&comments=includeComments&order=rank&submit=Search+Database and http://www.guidetopharmacology.org/GRAC/DatabaseSearchForward?searchString=ATP5F1&searchCategories=all&species=none&type=all&comments=includeComments&order=rank&submit=Search+Database, which contact each other, of other four conserved proteins (http://www.guidetopharmacology.org/GRAC/DatabaseSearchForward?searchString=ATP8&searchCategories=all&species=none&type=all&comments=includeComments&order=rank&submit=Search+Database, http://www.guidetopharmacology.org/GRAC/DatabaseSearchForward?searchString=ATP5k&searchCategories=all&species=none&type=all&comments=includeComments&order=rank&submit=Search+Database, http://www.guidetopharmacology.org/GRAC/DatabaseSearchForward?searchString=ATP5J2&searchCategories=all&species=none&type=all&comments=includeComments&order=rank&submit=Search+Database, http://www.guidetopharmacology.org/GRAC/DatabaseSearchForward?searchString=ATP5L2&searchCategories=all&species=none&type=all&comments=includeComments&order=rank&submit=Search+Database) and of two or three additional subunits, diabetes‐associated protein in insulin‐sensitive tissues (DAPIT) and 6.8PL in vertebrates (Lee et al., 2015). Besides OSCP, the peripheral stalk was originally proposed to be formed by the membrane‐distal part of subunit b and by subunits http://www.guidetopharmacology.org/GRAC/DatabaseSearchForward?searchString=ATP5J&searchCategories=all&species=none&type=all&comments=includeComments&order=rank&submit=Search+Database and http://www.guidetopharmacology.org/GRAC/DatabaseSearchForward?searchString=ATP5H&searchCategories=all&species=none&type=all&comments=includeComments&order=rank&submit=Search+Database (Rees et al., 2009). More recently, the C‐terminal region of subunit A6L of the yeast and bovine complex (Lee et al., 2015; Guo et al., 2017), the N‐terminal domain of subunit f (Guo et al., 2017) and subunit i/j (Srivastava et al., 2018) of the yeast species have been found at the base of the peripheral stalk. In all F‐ATP synthases, proton translocation through the two half‐channels of subunit a drives the rotation of the c‐ring (Kühlbrandt and Davies, 2016).
The F1 sector is always made up of 3http://www.guidetopharmacology.org/GRAC/DatabaseSearchForward?searchString=ATP5A1&searchCategories=all&species=none&type=all&comments=includeComments&order=rank&submit=Search+Database and 3http://www.guidetopharmacology.org/GRAC/DatabaseSearchForward?searchString=ATP5B&searchCategories=all&species=none&type=all&comments=includeComments&order=rank&submit=Search+Database subunits that alternate around a central stalk, composed by subunits γ and ε in bacteria and by subunits http://www.guidetopharmacology.org/GRAC/DatabaseSearchForward?searchString=ATP5C&searchCategories=all&species=none&type=all&comments=includeComments&order=rank&submit=Search+Database, http://www.guidetopharmacology.org/GRAC/DatabaseSearchForward?searchString=ATP5D&searchCategories=all&species=none&type=all&comments=includeComments&order=rank&submit=Search+Database and http://www.guidetopharmacology.org/GRAC/DatabaseSearchForward?searchString=ATP5E&searchCategories=all&species=none&type=all&comments=includeComments&order=rank&submit=Search+Database in mitochondria. The central stalk is also bound to the c‐ring, thus being able to transmit the FO torque to the catalytic β subunits. Together, the c‐ring and the F1 central stalk constitute the rotor, while the rest of the complex acts as the stator of the molecular engine. Rotation of γ sequentially takes each of the three β subunits through the three major functional states βDP, βTP and βE or vice versa, thereby synthesizing or hydrolysing three Mg2+‐ATP molecules during each 360° rotation (Walker, 2013). In bacteria, ATPase activity can be inhibited by the ε subunit (Nakanishi‐Matsui et al., 2016) and in mitochondria by the endogenous inhibitor IF1, which helps prevent 6dissipation of cellular ATP under conditions of respiratory impairment (Faccenda et al., 2017).
As the two stepping motors FO and F1 differ in the number of steps during the catalytic cycle, their cooperation is smoothed by elastic power transmission rather than by fine tuning of the respective reactions, thereby increasing kinetic efficiency (Junge et al., 2009). Fluctuation analysis of FOF1 in Escherichia coli established that the main flexibility is associated with the globular portions of subunits γ and ε in contact with the c‐ring (Junge et al., 2009), while the peripheral stalk is rather stiff (Sielaff et al., 2008). Conversely, the observation that deletion of multiple residues between bA50 and bI75 was without effect on enzyme catalysis led to the proposal that the stator in E. coli rather resembles a flexible rope (Dunn et al., 2000).
The stiffness of the peripheral stalk is consistent with its role of helping α3β3 resist against the mechanical torsional stress of the rotor. A major structural role is played by the high binding strength of the N‐terminal domain of OSCP (δ) to the α subunits and of the C‐terminal domain of OSCP (δ) to the peripheral stalk (Figure 2). The latter interaction was documented by FRET in yeast FOF1, showing that native OSCP conformation remains almost unaltered relative to subunit b during catalysis (Gavin et al., 2003). In contrast, the enzyme complexes containing a G166 N mutation (corresponding to G161 in Bos taurus, a residue that is conserved in virtually all phyla) exhibited large changes in FRET, highlighting the stress that develops between F1 and OSCP during ATP hydrolysis (Gavin et al., 2003). These complexes were more susceptible to dissociation than complexes containing native OSCP and were insensitive to inhibition by oligomycin (Boyle et al., 2000). As oligomycin binds to the c subunit (Symersky et al., 2012), this result demonstrates that OSCP is capable of modulating the proton conductance within FO. Interestingly, binding of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1013 to OSCP also promotes an uncoupled state of F‐ATP synthase in rat liver mitochondria (Moreno et al., 2013). These findings suggest the presence of flexible regions in OSCP that could impair the stator integrity. It is of note that electron cryo‐EM did reveal combined conformational changes between the OSCP C‐terminus and subunit b at its point of entrance into the membrane (Zhou et al., 2015).
Figure 2.
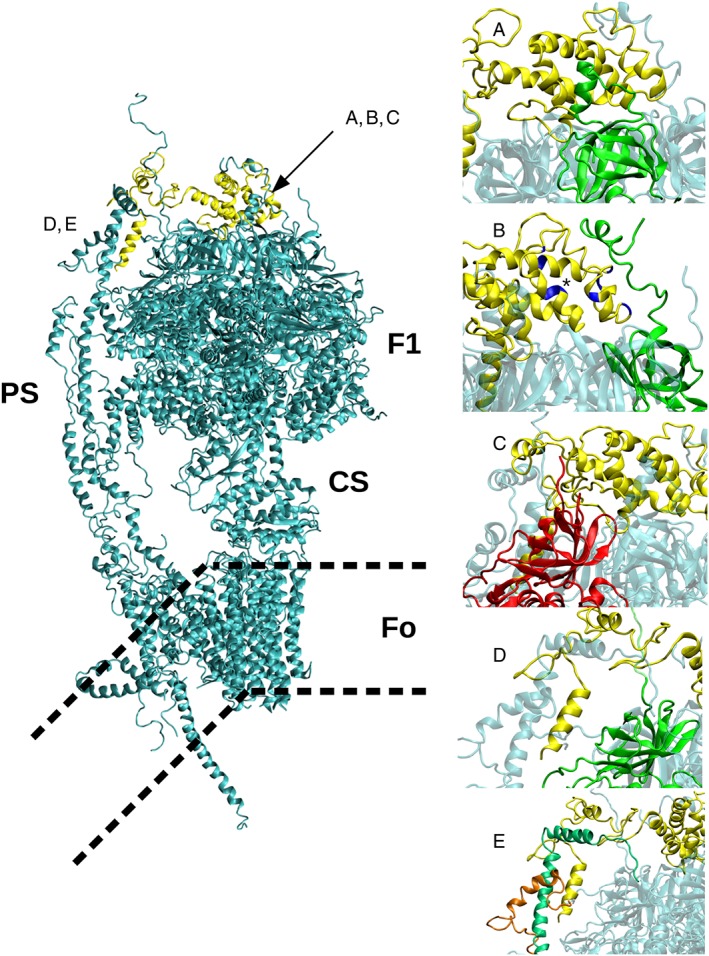
Structure of F‐ATP synthase and its interactions with OSCP. The F1 and FO subunits are shown in the central panel. PS, peripheral stalk; CS, central stalk. The position of the membrane (thick dotted lines) is purely indicative because the view is not along the line of bending to better show the overall structure of the complex. Interactions between OSCP and the N‐terminal region of subunits αE (A), αTP (B), βDP (C), αDP (D) and with subunit b and F6 (E) are reported in the right panels. In panel (B) the residues encompassing the ‘shoulder' region of the Bz 423 binding site (asterisk) are in blue. Highlighted subunits are shown in colour as follows: OSCP in yellow, α subunit in green, β subunit in red, F6 in orange and b in emerald. The image has been built from available crystal structures. Missing parts that could not be modelled by homology to experimental data have been built using the software Modeller (Webb and Sali, 2016).
Structure, interactions and assembly of the OSCP subunit
Rees et al. (2009) were the first to define the crystal structure of the OSCP by analysing an F1‐peripheral stalk subcomplex from bovine heart. The N‐terminal domain consists of six α‐helices (OH1–OH6), as recently found in the crystal structure of the F‐ATP synthase from Paraccocus denitrificans (Morales‐Rios et al., 2015) and in the cryo‐EM structure of the F‐ATP synthases from Pichia angusta (Vinothkumar et al., 2016), Yarrowia lipolytica (Hahn et al., 2016) and Saccharomyces cerevisiae (Srivastava et al., 2018). Helices 1 (residues 18–26, bovine numbering) and 5 (residues 83–92) together with adjacent residues provide the binding site for residues 2–17 of subunit αE, which occurs both through hydrophobic contacts and through electrostatic interactions between α R15/OSCP E91 and α E7/OSCP R94 (Rees et al., 2009) (Figure 2A). This first interaction is present in all published structures, where the states E, TP and DP of α and β subunits may be different. For example, in the most recent published structure, this interaction involves a random coil of αDP (Srivastava et al., 2018). Further points of contact between the N‐terminus of OSCP and F1 have been defined. Indeed, a second interaction occurs at the N‐terminus of αTP around residue 50 (Rees et al., 2009) (Figure 2B). Similar contacts with αTP are found in other available structures, where the N‐terminus of the α subunit forms a helix and interacts with the region corresponding to helices OH2, OH3 and OH4 of OSCP in the bovine complex. Based on sequence similarity, we think that this type of contact is conserved also in B. taurus. A third interaction occurs between the N‐terminal β‐barrel domain of the βDP‐subunit with residues 1–14 of OSCP (Rees et al., 2009) (Figure 2C). Finally, the N‐terminus of αDP has been seen to contact the C‐terminal domain of OSCP (Figure 2D).
The structure of the C‐terminal domain of bovine heart OSCP consists of a β‐hairpin, which could represent a point of flexion in the peripheral stalk followed by two α‐helices, OH7 and OH8 (Rees et al., 2009). A similar structure is found in the P. angusta (Vinothkumar et al., 2016) and Y. lipolytica enzymes (Hahn et al., 2016). A flexible stretch of about 30 amino acids, not resolved in all available structures, is found between helices OH7 and OH8. In Srivastava et al. (2018), OH8 is mapped to a sequence region closer to OH7, thus reducing the length of the link region. In the bovine enzyme, this stretch forms a five‐helix bundle with the N‐terminal α‐helix of F6 and a segment of subunit b, resulting in an extensive interface between the three subunits (Figure 2E). A similar arrangement is found in Y. lipolytica, P. angusta and S. cerevisiae, where the C‐terminus of OSCP forms a helix bundle with subunit b, subunit h (the fungal orthologue of bovine F6) and the N‐terminus of αDP/TP (not resolved in the bovine enzyme), which positions the F1 sector towards the peripheral stalk. This latter interface is largely established via hydrophobic interactions, except for those mediated by the conserved residues αE33 and αR41 (Hahn et al., 2016). It is worth mentioning that the N‐ and C‐terminal domains of OSCP are almost independent, with only a very short region (encompassing residues V111 to V116) showing contacts with both domains. This region appears unique in its ability to control the relative position of the C‐ and N‐terminal domains, thereby influencing the position of the peripheral stalk relative to the rest of the F‐ATP synthase (the connecting region is highlighted in green in Figure 3, and detailed as sticks in Figure 3A).
Figure 3.
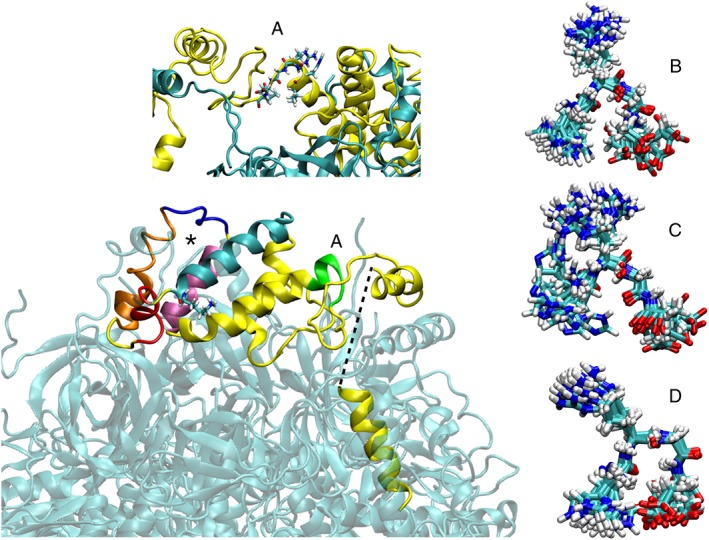
OSCP structure and features. OSCP is shown in the context of F‐ATP synthase (transparent). The missing region joining residues 146 and 174 of OSCP and encompassing G161 is indicated by the dashed thick line. Helices OH3 (red), OH4 (orange) and OH5 (mauve) encompassing the Bz 423 binding region (asterisk) are highlighted on the structure. Helices OH2 (cyan) and OH3 (red) and the loop between OH4 and OH5 (blue) that fluctuates most in the presence of Ca2+ and the location of K47 (shown as sticks) possibly involved in CyPD binding are also shown. Residues V111 to V116 encompassing H112 and connecting the C‐terminal to the N‐terminal domains are shown in panel (A). The superposition of snapshots from molecular dynamics simulations of the OSCP subunit (residues H112–E115) is reported in the three right panels corresponding to the two neutral forms [proton on Nε (panel B) or Nδ (panel C)] and to the protonated form (panel D).
OSCP can also undergo phosphorylation at the C‐terminal domain (Covian and Balaban, 2012), and glycoforms of OSCP and d subunit have been detected in bovine heart (Burnham‐Marusich and Berninsone, 2012), but the functional implications of these posttranslational modifications are still unknown. On the other hand, the metabolic significance of OSCP (de)acetylation has been elucidated and will be discussed further later.
OSCP is encoded by the nuclear ATP5PO gene and plays a key role in F‐ATP synthase assembly, which occurs in a modular fashion preventing formation of intermediates that could depolarize the membrane or waste ATP (Rühle and Leister, 2015). At variance from other subunits, in rat tissues, OSCP is a low‐transcript gene (Sangawa et al., 1997). Pulse‐labelling experiments in yeast indicate that OSCP binding is the last step in FOF1 assembly (Rak et al., 2011). Although this pathway has been questioned, based on disruption of individual human genes for subunits e, f, g, DAPIT and 6.8PL (He et al., 2018), it is consistent with the observation that a decreased OSCP level in human cells did not alter the expression of other subunits (Johnson et al., 2005; Giorgio et al., 2013) unlike suppression of subunit ε, which in HEK293 cells caused a marked decrease of assembly intermediates (Havlickova et al., 2010).
In summary, OSCP ensures the structural and functional coupling between FO and F1 (which is essential for enzyme catalysis) through its contacts with both the α3β3 hexamer and with the other subunits of the peripheral stalk. OSCP also undergoes regulatory posttranslational modifications. The possible presence of flexible regions in OSCP is intriguing, because such regions could influence the position of the peripheral stalk and thus potentially modulate the membrane‐embedded Fo sector where, as we will discuss more in detail in the following paragraphs, the permeability transition pore (PTP) may form (Antoniel et al., 2018; Giorgio et al., 2018).
OSCP and the permeability transition pore
The PTP is a high‐conductance channel located in the inner mitochondrial membrane that is modulated by a series of effectors, among which matrix Ca2+ is an essential permissive factor (Giorgio et al., 2018). As the PTP plays a key role in cell death and in Ca2+ signalling, it is a viable target for therapy in a variety of diseases, most notably heart ischaemia/reperfusion injury, stroke, muscular dystrophies, neurodegenerative diseases and cancer (Bernardi et al., 2015). The molecular composition of the PTP is still a matter of debate. Putative components such as the http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=206 (ANT), http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=131#709 and http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=919#2879 have been ruled out by genetic ablation studies (Kokoszka et al., 2004; Krauskopf et al., 2006; Baines et al., 2007; Šileikyte et al., 2014). The most recent hypothesis is that the channel forms from a specific, Ca2+‐dependent rearrangement of F‐ATP synthase, which is favoured by cyclophilin (CyP) D binding (Giorgio et al., 2009, 2013). Channel formation has been observed in F‐ATP synthase from beef heart (Giorgio et al., 2013), human cells (Alavian et al., 2014), yeast (Carraro et al., 2014) and Drosophila (von Stockum et al., 2015), but the mechanism of formation and whether this channel coincides with the PTP are still matters of debate (Bernardi et al., 2015; Bernardi and Lippe, 2018).
A potential role of OSCP in modulation of the PTP is suggested by the observation that CyPD (the receptor for the PTP inhibitory effects of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1024) binds the peripheral stalk of F‐ATP synthase at OSCP (Giorgio et al., 2009, 2013; Lee et al., 2016; Burstein et al., 2018). CyPD is the only mitochondrial isoform of this family of peptidyl prolyl cis‐trans isomerases and sensitizes the PTP to matrix Ca2+ as revealed by studies on CyPD‐null mice (Baines et al., 2005; Basso et al., 2005; Nakagawa et al., 2005; Schinzel et al., 2005). The nature of the OSCP‐CyPD interactions appears to be mainly electrostatic, and surface potential studies have identified two putative regions on the OSCP N‐terminal domain at residues E48, D71, E76 and F78 located between helices OH3, OH4 and OH5 and at residues H112, E115, V116, E128 and E133 (Antoniel et al., 2014) (Figure 3). CyPD is displaced by cyclosporin (Cs) A, a well‐characterized inhibitor of the permeability transition (Giorgio et al., 2010).
OSCP is also the binding site of the immunomodulatory benzodiazepine (Bz) 423 (Johnson et al., 2005; Cleary et al., 2007; Stelzer et al., 2010), which like CyPD acts as an inducer of the PTP (Giorgio et al., 2013). In striking analogy with CyPD, this molecule also mildly inhibits both ATP synthesis and hydrolysis (Johnson et al., 2005) and can cause cytochrome c release and cell death (Blatt et al., 2002). Bz 423 binding to OSCP displaces CyPD in a concentration‐dependent manner, suggesting competition for a common binding site (Giorgio et al., 2013). Consistent with a PTP modulatory role, Bz 423 promotes channel formation in electrophysiological studies performed on preparations lacking CyPD (Giorgio et al., 2013). NMR studies demonstrated that Bz 423 binding to OSCP (asterisk, Figure 3) causes conformational changes that affect protein regions that are not directly involved in interactions with the drug (Stelzer et al., 2010). This finding further supports the notion that OSCP is a highly flexible protein potentially prone to ligand‐induced conformational changes. Indeed, the chemical shift perturbations caused by Bz 423 fall in three general regions of OSCP: (i) the binding region, which is located between helices OH3, OH4 and OH5 (the ‘shoulder'), includes residues M51, L56, K65, V66, K75, K77, T82, S83 and N92 and covers the first potential CyPD binding site (Figures 2B and 3); (ii) the region comprising the C‐terminal tail of helices OH1 and OH6 (the ‘tail'), which includes residues V111, R113, V116, C118 and T119 and partially covers a second hypothetical binding site of CyPD (Figure 3); (iii) a third region located between the tail and the shoulder.
As already proposed (Stelzer et al., 2010), the contacts of the OSCP shoulder region with the N terminus of αTP (Rees et al., 2009) (Figure 2B) can potentially transmit conformational changes to the F1 part of the enzyme and thus affect catalysis. On the other hand, the tail region involving residues V111–V116 may control the position of the peripheral stalk relative to the rest of F‐ATP synthase. It is tempting to speculate that the conformational changes occurring in this region, due to CyPD or Bz 423 binding to OSCP, may be transmitted through the peripheral stalk to the intramembrane portion of Fo, where the PTP should form. Molecular dynamics simulations highlighted a possible role of OSCP in this mechanical transmission. Trajectory inspection in the presence of Ca2+ indeed revealed that the larger van der Waals radius of this cation (compared to that of Mg2+) in the catalytic sites may induce spatial rearrangements increasing overall F1 rigidity, causing in turn fluctuations in the OSCP helices (Giorgio et al., 2017). Peaks of movements included OH2 and OH3 (residues 26–55) and the loop between OH4 and OH5 (residues 74–80), that is, the same overlapping helices perturbed by Bz 423 binding (Figure 3). The mechanical energy transmitted to OSCP could then be transferred to the inner membrane through the peripheral stalk (Figure 2). Our working hypothesis is that the conformational changes of OSCP induced by CyPD or Bz 423 binding could also affect Ca2+ binding to the catalytic site, thus increasing the probability of PTP opening.
The relevance of the region encompassing residues V111–V116 (sticks in Figure 3A) in modulation of the PTP was confirmed by the recent finding that H112 (position in the human mature protein) is responsible for the inhibitory effect of acidic matrix pH on the pore (Antoniel et al., 2018), a long‐standing observation that awaited molecular clarification (Bernardi et al., 1992; Nicolli et al., 1993). Point mutations of H112 abolished pH‐dependent inhibition of PTP‐dependent swelling in permeabilized HEK293 cells and prevented H+‐induced channel block of the mega‐channel studied by patch‐clamp experiments (Antoniel et al., 2018). Molecular dynamics simulations indicate that protonation of H112 may cause a shift in conformational preference within OSCP from more open (Figure 3B,C) to a more tight form (Figure 3D). Simulation results suggest that, upon protonation, (i) a polar interaction occurs between side chains of H112 and E115 of OSCP and (ii) the HRGE stretch 112–115 interacts with region 130–135 (as shown in the crystal structure), which in turn contacts subunit b). Both contacts are more frequent for the protonated form of H112 (Figure 3D). Interestingly, HRGE residues 112–115 in OSCP are conserved in all Vertebrata (from Homo sapiens to Xenopus laevis, excluding Sauria and most divergent sequences). The HRGE motif also shows a conformational preference for tight turn or 3–10‐helix or α‐helix conformations, often with a hydrogen bond or salt bridge between H and E (Wang and Dunbrack Jr., 2003). These data suggest that a direct interaction between the side chains of H112 and E115 in OSCP is likely and could establish pH‐dependent interactions with the flexible C‐terminal region influencing the position of the peripheral stalk relative to the rest of F‐ATP synthase, as the N‐terminus of OSCP is rather rigidly linked to the top of F1 (Figure 2).
The role of OSCP in the modulation of the PTP has apparently been questioned by the results of a recent study where OSCP was genetically ablated in HAP1 haploid clones (He et al., 2017). The authors found that a CsA‐sensitive PT could still occur, yet PTP‐dependent swelling in KCl‐based media was significantly decreased, suggesting a major effect of OSCP ablation on pore size (Bernardi and Lippe, 2018). The persistence of the inhibitory effect of CsA is unexpected because several laboratories have reported the occurrence of PTP‐regulatory and CsA‐sensitive interactions of CyPD with OSCP (Giorgio et al., 2013; Lee et al., 2016; Burstein et al., 2018). We suspect that inhibition by CsA in OSCP‐null cells may be due to insofar undetected interactions of CyPD with additional subunits of F‐ATP synthase. Subunit b appears to be a good candidate, as first suggested by Kühlbrandt and co‐workers based on high‐resolution structures of the Podospora anserina F‐ATP synthase (Daum et al., 2013). This putative interaction would be consistent with a faint positivity for subunit b in our immunoprecipitation experiments (Giorgio et al., 2013), which we may have overlooked. Indeed, the interaction between OSCP and CyPD could have been weakened by dodecylsulfate, which was added at low concentrations to allow dissociation of the OSCP, b and d subunits (Giorgio et al., 2013).
OSCP interactors
OSCP is strategically located on top of F‐ATP synthase, where it is easily accessible to protein interactors (such as CyPD) and drugs. Decrease of OSCP expression with matching increase of CyPD expression and PTP activation were described in brain mitochondria from aging mice, where CyPD depletion preserved OSCP expression and mitochondrial function (Gauba et al., 2017). Intriguingly, physical interaction of OSCP with http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4865 (Aβ) and selective loss of OSCP was also observed in the brain of individuals with Alzheimer's disease (AD) and in an AD mouse model (Beck et al., 2016). The predicted Aβ binding region encompasses residues 107–122 of OSCP, leading to a reduced ability to bind the F1 sector and potentially mediating the mitochondrial impairment characteristic of AD brains. Indeed, in cultured neurons, the OSCP‐Aβ interaction sensitized PTP opening and caused synaptic injury despite unaltered CyPD expression levels while OSCP overexpression in mouse and human neurons ameliorated Aβ‐mediated mitochondrial and synaptic impairment (Beck et al., 2016).
It is remarkable that earlier studies had already suggested an involvement of CyPD and the PTP in the pathogenesis of Aβ‐dependent brain diseases. Thus, in cortical mitochondria of AD brains, as well as in the mAPP mouse model overexpressing a mutant form of human Aβ, a direct interaction of Aβ with CyPD has been described (Du et al., 2008). Cortical mAPP mitochondria showed an increased ROS generation and PTP activation, which were attenuated in Ppif −/− mice (which lack CyPD). CyPD deficiency also protected neurons from synaptic impairment and improved learning and memory (Du et al., 2008) also during aging (Du et al., 2011). Based on the crystal structure of the CyPD‐CsA complex, 45 compounds have been recently identified in the ChemDiv database, 15 of which inhibited CyPD and showed excellent protective effects against Aβ‐induced mitochondrial dysfunction in neuronal cells (Park et al., 2017). Although mechanistic details of how changes of expression of OSCP may affect assembly and/or stability of the F‐ATP synthase complex have not been addressed in these models, these findings point to a possible PTP dysregulation mediated by OSCP and CyPD in Aβ‐dependent diseases that deserves further attention.
Another condition where OSCP alterations led to F‐ATP synthase dysfunction and PTP dysregulation is the French Canadian variant of Leigh Syndrome, which is characterized by low amounts of the leucine‐rich pentatricopeptide repeat containing protein (Lrpprc), an RNA‐binding protein involved in the stabilization of most mtDNA‐encoded mRNAs. In mice with a heart conditional knockout of Lrpprc, impairment of ATP production was matched by lack of assembly of F‐ATP synthase and presence of F‐ATP synthase subcomplexes, which lacked subunits OSCP and A6L and catalysed oligomycin‐insensitive ATP hydrolysis (Mourier et al., 2014). Mice with hepatocyte‐specific conditional inactivation of Lrpprc showed assembly defects of F‐ATP synthase (whose activity was largely insensitive to oligomycin) with decreased formation of dimers and a remarkable inactivation of the PTP (Cuillerier et al., 2017). Interestingly, in spite of increased CyPD expression, the PTP was insensitive to CsA, suggesting that alterations in the peripheral stalk could have prevented CyPD binding (Cuillerier et al., 2017).
It has been reported that, unexpectedly, OSCP is a client protein of the abundant chaperone http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=784. In COLO 205 colon cancer cells, Hsp90 inhibitors stabilized OSCP levels and elicited reversal of transformation and differentiation. In these cells, OSCP levels were decreased and Hsp90 inhibition increased OSCP levels more markedly than those of other F‐ATP synthase subunits and promoted apoptosis via the mitochondrial pathway (Margineantu et al., 2007), a possible PTP‐dependent event that awaits to be explored and hopefully exploited therapeutically.
OSCP gene expression appears to be altered in diabetes. Up‐regulation of OSCP mRNA was found in a rat beta cell line overexpressing the transcription factor pdx‐1 (encoded by pancreatic duodenal homeobox gene‐1) and exposed to IL‐1β for 24 h (Bergholdt et al., 2007) while decreased levels of OSCP‐encoding ATP5PO transcripts were observed in skeletal muscle from type 2 diabetic patients (Rönn et al., 2009). Modulation of OSCP transcription may be related to DAPIT, which was originally identified in skeletal muscle of rats made diabetic by treatment with streptozotocin (Paivarinne and Kainulainen, 2001) and later shown to be a subunit of F‐ATP synthase (Chen et al., 2007; Meyer et al., 2007) essential for its assembly and oligomerization (Ohsakaya et al., 2011; He et al., 2018). The above findings suggest that F‐ATP synthase is involved in diabetes, possibly through PTP formation. This hypothesis is supported by the demonstration that mutations of the Pdx1 gene, which cause diabetes in both mice and humans, also cause increased death of beta cells through CyPD‐dependent PTP opening (Fujimoto et al., 2010).
p53 is an oncosuppressor regulating several target genes with diverse biological functions (Sullivan et al., 2018). Although p53 is not necessary for PTP formation (Karch and Molkentin, 2012), it has been reported to trigger PTP opening by physical interaction with CyPD after import into the matrix (Vaseva et al., 2012). The p53‐CyPD complex appears to form during brain ischaemia/reperfusion injury, and pretreatment of mice with CsA prevented complex formation resulting in brain protection (Vaseva et al., 2012). Intriguingly, a mitochondrially targeted p53 was also found to interact with OSCP and suggested to take part in the assembly or stabilization of the mature FOF1 complex (Bergeaud et al., 2013). Mitochondrial p53 also increased oxygen consumption and decreased ROS levels, suggesting that it may play a role in mitochondrial physiology (Bergeaud et al., 2013). Finally, the newly characterized protein stress responsive activator p300 (Strap), which is known to increase p53 transcripts, has been shown to interact with β subunit of F‐ATP synthase decreasing ATP production (Maniam et al., 2015). As cancer cells are sensitized to apoptosis by mitochondrial Strap under glucose‐limiting conditions, the interaction of Strap with F‐ATP synthase might contribute to the pro‐apoptotic effects of p53.
OSCP interacts with the NAD+‐dependent deacetylase http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=848#2709 (Sirt3) (Yang et al., 2016). Remarkably, decreased activity of Sirt3 in complex I deficiency favours CyPD binding through increased acetylation of OSCP K47 (Lee et al., 2016) (Figure 3), providing a solid mechanistic explanation for the observation that mitochondria from hearts of complex I deficient mice are sensitized to PTP opening (Karamanlidis et al., 2013). The relevance of OSCP acetylation was also studied in human cells with the 4977 bp deletion of mtDNA that causes chronic progressive external ophthalmoplegia, where ROS production induced a reduction of Sirt3 expression. Suppression of Sirt3 reduced F‐ATP synthase activity, decreased ATP levels and respiratory capacity and prevented galactose utilization (Wu et al., 2013). Binding of Sirt3 to F‐ATP synthase critically involves the unique His of OSCP (Yang et al., 2016), which is the same residue mediating inhibition of the PTP by acidic pH (Antoniel et al., 2018). When matrix pH decreases, this pool of Sirt3 is released leading to rapid deacetylation of matrix proteins and to mitochondrial depolarization (Yang et al., 2016). These results indicate that the interaction of Sirt3 with OSCP has a regulatory role in mitochondrial bioenergetics and possibly in regulation of the PTP.
Oestrogens affect both ATP synthase and PTP activity. It was shown that 17β‐oestradiol binds OSCP (Zheng and Ramirez, 1999). This interaction provides a mechanistic basis for the reduced efficiency of ATP synthesis by 17β‐oestradiol that could be reversed by oligomycin but not resveratrol and was attributed to increased intrinsic uncoupling of the enzyme (Moreno et al., 2013). In a recent development, it has been suggested that sex differences can exist in susceptibility to PTP induction, as shown by the higher sensitivity to Ca2+ of mitochondria from female mouse forebrain (Burstein et al., 2018). This higher sensitivity was blunted by an oestrogen receptor β antagonist, which also decreased sensitivity to inhibition by CsA. The effects appear to be mediated by the F‐ATP synthase and to involve OSCP because genetic ablation of the oestrogen receptor decreased the interaction between OSCP and CyPD (Burstein et al., 2018).
Honokiol, a low MW polyphenolic compound isolated from Magnolia, has been proposed as an anti‐cancer compound. It blocks signalling in tumours with defective p53 function and promotes PTP‐dependent cell death by up‐regulating CyPD levels and affecting channel formation (Li et al., 2007). More recently, and in line with its effect on the pore, honokiol has been found to induce apoptosis in lung cancer cells by promoting ROS production in mitochondria and by inhibiting oligomycin‐sensitive respiration (Pan et al., 2014) and to inhibit the mTOR signalling pathway (Tian et al., 2016). Honokiol treatment also enhanced Sirt3 expression nearly twofold and further increased its deacetylase activity with a direct effect on OSCP and MnSOD (Pillai et al., 2015). These effects were associated with amelioration of the Sirt3‐dependent cardiac hypertrophic responses in mice, which is consistent with the reported inverse effect of Sirt3 inactivation on the CyPD‐OSCP interaction (Lee et al., 2016).
It has already been mentioned that OSCP is the binding site of the immunomodulatory compound Bz 423. Bz 423 was originally characterized in the search for apoptosis‐inducing agents able to selectively kill autoreactive B lymphocytes (Blatt et al., 2002). OSCP was identified as its target by screening a phage display library, and it was then shown that Bz 423 is an inhibitor of the F‐ATP synthase (Johnson et al., 2005, 2006; Stelzer et al., 2010). Bz 423 competes for CyPD binding on OSCP. Like CyPD, it sensitizes the PTP to Ca2+ and pore opening remains sensitive to inhibition by CsA (Giorgio et al., 2013). Bz 423 is thus a chemical mimic of CyPD that can be used to stimulate PTP opening in the absence of CyPD (Giorgio et al., 2013).
The OSCP interactors that affect F‐ATP synthase function and/or PTP formation are summarized in Table 1.
Table 1.
OSCP interactors affecting F‐ATP synthase function and/or PTP formation
| Compound/Interactor | F‐ATP synthase function | PTP modulation |
|---|---|---|
| 17β‐oestradiol | Zheng and Ramirez (1999) | Burstein et al. (2018) |
| Moreno et al. (2013) | ||
| Bz 423 | Johnson et al. (2005) | Giorgio et al. (2013) |
| Stelzer et al. (2010) | ||
| Honokiol | Pan et al. (2014) | Li et al. (2007) |
| Pillai et al. (2015) | Tian et al. (2016) | |
| Cyclophilin D | Giorgio et al. (2009) | Giorgio et al. (2013) |
| Lee et al. (2016) | ||
| Gauba et al. (2017) | ||
| Burstein et al. (2018) | ||
| β amyloid protein | Beck et al. (2016) | Du et al. (2008) |
| Du et al. (2011) | ||
| Beck et al. (2016) | ||
| p53 | Bergeaud et al. (2013) | Vaseva et al. (2012) |
| Sirtuin 3 | Wu et al. (2013) | Lee et al. (2016) |
| Yang et al. (2016) |
Conclusions
OSCP is a key site for the interaction of regulatory proteins and drugs with F‐ATP synthase. The recent discovery that F‐ATP synthase is also critically involved in the PT has placed OSCP in the spotlight as a potential site of regulation of the transition of the complex into a pore, with a postulated role in the transmission of PTP‐regulatory signals to the inner membrane. Molecular definition of the interactions of OSCP with these regulators will provide novel insights into the mechanism through which the energy‐conserving enzyme is transformed into an energy‐dissipating structure.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017a, 2017b, 2017c).
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
Research in our laboratories is funded by grants from Associazione Italiana per la Ricerca sul Cancro (MFAG20316 to V.G. and IG17067 to P.B.), Fondation Leducq (16CVD04) and Fondazione Telethon (GGP17092).
Giorgio, V. , Fogolari, F. , Lippe, G. , and Bernardi, P. (2019) OSCP subunit of mitochondrial ATP synthase: role in regulation of enzyme function and of its transition to a pore. British Journal of Pharmacology, 176, 4247–4257. 10.1111/bph.14513.
References
- Alavian KN, Beutner G, Lazrove E, Sacchetti S, Park HA, Licznerski P et al (2014). An uncoupling channel within the c‐subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore. Proc Natl Acad Sci U S A 111: 10580–10585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD et al (2017a). The Concise Guide to PHARMACOLOGY 2017/18: Transporters. Br J Pharmacol 174: S360–S446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD et al (2017b). The Concise Guide to PHARMACOLOGY 2017/18: Other proteins. Br J Pharmacol 174: S1–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017c). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 174 (Suppl. 1): S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniel M, Giorgio V, Fogolari F, Glick GD, Bernardi P, Lippe G (2014). The oligomycin‐sensitivity conferring protein of mitochondrial ATP synthase: emerging new roles in mitochondrial pathophysiology. Int J Mol Sci 15: 7513–7536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniel M, Jones K, Antonucci S, Spolaore B, Fogolari F, Petronilli V et al (2018). The unique histidine in OSCP subunit of F‐ATP synthase mediates inhibition of the permeability transition pore by acidic pH. EMBO Rep 19: 257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA et al (2005). Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434: 658–662. [DOI] [PubMed] [Google Scholar]
- Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD (2007). Voltage‐dependent anion channels are dispensable for mitochondrial‐dependent cell death. Nat Cell Biol 9: 550–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P (2005). Properties of the permeability transition pore in mitochondria devoid of cyclophilin D. J Biol Chem 280: 18558–18561. [DOI] [PubMed] [Google Scholar]
- Beck SJ, Guo L, Phensy A, Tian J, Wang L, Tandon N et al (2016). Deregulation of mitochondrial F1FO‐ATP synthase via OSCP in Alzheimer/'s disease. Nat Commun 7: 11483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeaud M, Mathieu L, Guillaume A, Moll U, Mignotte B, Le Floch N et al (2013). Mitochondrial p53 mediates a transcription‐independent regulation of cell respiration and interacts with the mitochondrial F1F0‐ATP synthase. Cell Cycle 12: 2781–2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergholdt R, Karlsen AE, Hagedorn PH, Aalund M, Nielsen JH, Kruhoffer M et al (2007). Transcriptional profiling of type 1 diabetes genes on chromosome 21 in a rat beta‐cell line and human pancreatic islets. Genes Immun 8: 232–238. [DOI] [PubMed] [Google Scholar]
- Bernardi P, Lippe G (2018). Channel formation by F‐ATP synthase and the permeability transition pore: an update. Curr Opin Physiol 3: 1–5. [Google Scholar]
- Bernardi P, Vassanelli S, Veronese P, Colonna R, Szabó I, Zoratti M (1992). Modulation of the mitochondrial permeability transition pore. Effect of protons and divalent cations. J Biol Chem 267: 2934–2939. [PubMed] [Google Scholar]
- Bernardi P, Rasola A, Forte M, Lippe G (2015). The mitochondrial permeability transition pore: channel formation by F‐ATP synthase, integration in signal transduction, and role in pathophysiology. Physiol Rev 95: 1111–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt NB, Bednarski JJ, Warner RE, Leonetti F, Johnson KM, Boitano A et al (2002). Benzodiazepine‐induced superoxide signals B cell apoptosis: mechanistic insight and potential therapeutic utility. J Clin Invest 110: 1123–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle GM, Roucou X, Nagley P, Devenish RJ, Prescott M (2000). Modulation at a distance of proton conductance through the Saccharomyces cerevisiae mitochondrial F1F0‐ATP synthase by variants of the oligomycin sensitivity‐conferring protein containing substitutions near the C‐terminus. J Bioenerg Biomembr 32: 595–607. [DOI] [PubMed] [Google Scholar]
- Burnham‐Marusich AR, Berninsone PM (2012). Multiple proteins with essential mitochondrial functions have glycosylated isoforms. Mitochondrion 12: 423–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein SR, Kim HJ, Fels JA, Qian L, Zhang S, Zhou P et al (2018). Estrogen receptor beta modulates permeability transition in brain mitochondria. Biochim Biophys Acta 1859: 423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraro M, Giorgio V, Šileikyte J, Sartori G, Forte M, Lippe G et al (2014). Channel formation by yeast F‐ATP synthase and the role of dimerization in the mitochondrial permeability transition. J Biol Chem 289: 15980–15985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Runswick MJ, Carroll J, Fearnley IM, Walker JE (2007). Association of two proteolipids of unknown function with ATP synthase from bovine heart mitochondria. FEBS Lett 581: 3145–3148. [DOI] [PubMed] [Google Scholar]
- Cleary J, Johnson KM, Opipari AW Jr, Glick GD (2007). Inhibition of the mitochondrial F1F0‐ATPase by ligands of the peripheral benzodiazepine receptor. Bioorg Med Chem Lett 17: 1667–1670. [DOI] [PubMed] [Google Scholar]
- Covian R, Balaban RS (2012). Cardiac mitochondrial matrix and respiratory complex protein phosphorylation. Am J Physiol Heart Circ Physiol 303: H940–H966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuillerier A, Honarmand S, Cadete VJJ, Ruiz M, Forest A, Deschenes S et al (2017). Loss of hepatic LRPPRC alters mitochondrial bioenergetics, regulation of permeability transition and trans‐membrane ROS diffusion. Hum Mol Genet 26: 3186–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum B, Walter A, Horst A, Osiewacz HD, Kühlbrandt W (2013). Age‐dependent dissociation of ATP synthase dimers and loss of inner‐membrane cristae in mitochondria. Proc Natl Acad Sci U S A 110: 15301–15306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devenish RJ, Prescott M, Boyle GM, Nagley P (2000). The oligomycin axis of mitochondrial ATP synthase: OSCP and the proton channel. J Bioenerg Biomembr 32: 507–515. [DOI] [PubMed] [Google Scholar]
- Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM et al (2008). Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer's disease. Nat Med 14: 1097–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Guo L, Zhang W, Rydzewska M, Yan S (2011). Cyclophilin D deficiency improves mitochondrial function and learning/memory in aging Alzheimer disease mouse model. Neurobiol Aging 32: 398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn SD, McLachlin DT, Revington M (2000). The second stalk of Escherichia coli ATP synthase. Biochim Biophys Acta 1458: 356–363. [DOI] [PubMed] [Google Scholar]
- Faccenda D, Nakamura J, Gorini G, Dhoot GK, Piacentini M, Yoshida M et al (2017). Control of mitochondrial remodeling by the atpase inhibitory factor 1 unveils a pro‐survival relay via OPA1. Cell Rep 18: 1869–1883. [DOI] [PubMed] [Google Scholar]
- Fujimoto K, Chen Y, Polonsky KS, Dorn GW (2010). Targeting cyclophilin D and the mitochondrial permeability transition enhances β‐cell survival and prevents diabetes in Pdx1 deficiency. Proc Natl Acad Sci U S A 107: 10214–10219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauba E, Guo L, Du H (2017). Cyclophilin D promotes brain mitochondrial F1FO ATP synthase dysfunction in aging mice. J Alzheimers Dis 55: 1351–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin PD, Devenish RJ, Prescott M (2003). FRET reveals changes in the F1‐stator stalk interaction during activity of F1F0‐ATP synthase. Biochim Biophys Acta 1607: 167–179. [DOI] [PubMed] [Google Scholar]
- Giorgio V, Bisetto E, Soriano ME, Dabbeni‐Sala F, Basso E, Petronilli V et al (2009). Cyclophilin D modulates mitochondrial F0F1‐ATP synthase by interacting with the lateral stalk of the complex. J Biol Chem 284: 33982–33988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio V, Burchell V, Schiavone M, Bassot C, Minervini G, Petronilli V et al (2017). Ca2+ binding to F‐ATP synthase β subunit triggers the mitochondrial permeability transition. EMBO Rep 18: 1065–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio V, Guo L, Bassot C, Petronilli V, Bernardi P (2018). Calcium and regulation of the mitochondrial permeability transition. Cell Calcium 70: 56–63. [DOI] [PubMed] [Google Scholar]
- Giorgio V, Soriano ME, Basso E, Bisetto E, Lippe G, Forte MA et al (2010). Cyclophilin D in mitochondrial pathophysiology. Biochim Biophys Acta 1797: 1113–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio V, von Stockum S, Antoniel M, Fabbro A, Fogolari F, Forte M et al (2013). Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc Natl Acad Sci U S A 110: 5887–5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Bueler SA, Rubinstein JL (2017). Atomic model for the dimeric FO region of mitochondrial ATP synthase. Science 358: 936–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A, Parey K, Bublitz M, Mills DJ, Zickermann V, Vonck J et al (2016). Structure of a complete ATP synthase dimer reveals the molecular basis of inner mitochondrial membrane morphology. Mol Cell 63: 445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havlickova V, Kaplanova V, Nuskova H, Drahota Z, Houštek J (2010). Knockdown of F1 ε subunit decreases mitochondrial content of ATP synthase and leads to accumulation of subunit c. Biochim Biophys Acta 1797: 1124–1129. [DOI] [PubMed] [Google Scholar]
- He J, Carroll J, Ding S, Fearnley IM, Walker JE (2017). Permeability transition in human mitochondria persists in the absence of peripheral stalk subunits of ATP synthase. Proc Natl Acad Sci U S A 114: 9086–9091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Ford HC, Carroll J, Douglas C, Gonzales E, Ding S et al (2018). Assembly of the membrane domain of ATP synthase in human mitochondria. Proc Natl Acad Sci U S A 115: 2988–2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KM, Chen X, Boitano A, Swenson L, Opipari AW Jr, Glick GD (2005). Identification and validation of the mitochondrial F1F0‐ATPase as the molecular target of the immunomodulatory benzodiazepine Bz‐423. Chem Biol 12: 485–496. [DOI] [PubMed] [Google Scholar]
- Johnson KM, Cleary J, Fierke CA, Opipari AW Jr, Glick GD (2006). Mechanistic basis for therapeutic targeting of the mitochondrial F1F0‐ATPase. ACS Chem Biol 1: 304–308. [DOI] [PubMed] [Google Scholar]
- Junge W, Sielaff H, Engelbrecht S (2009). Torque generation and elastic power transmission in the rotary FOF1‐ATPase. Nature 459: 364–370. [DOI] [PubMed] [Google Scholar]
- Karamanlidis G, Lee CF, Garcia‐Menendez L, Kolwicz SC Jr, Suthammarak W, Gong G et al (2013). Mitochondrial complex i deficiency increases protein acetylation and accelerates heart failure. Cell Metab 18: 239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch J, Molkentin JD (2012). Is p53 the long‐sought molecular trigger for cyclophilin D‐regulated mitochondrial permeability transition pore formation and necrosis? Circ Res 111: 1258–1260. [DOI] [PubMed] [Google Scholar]
- Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP et al (2004). The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature 427: 461–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauskopf A, Eriksson O, Craigen WJ, Forte MA, Bernardi P (2006). Properties of the permeability transition in VDAC1 −/− mitochondria. Biochim Biophys Acta 1757: 590–595. [DOI] [PubMed] [Google Scholar]
- Kühlbrandt W, Davies KM (2016). Rotary ATPases: a new twist to an ancient machine. Trends Biochem Sci 41: 106–116. [DOI] [PubMed] [Google Scholar]
- Lee CF, Chavez JD, Garcia‐Menendez L, Choi YS, Roe ND, Chiao YA et al (2016). Normalization of NAD+ redox balance as a therapy for heart failure. Circulation 134: 883–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Ding S, Walpole TB, Holding AN, Montgomery MG, Fearnley IM et al (2015). Organisation of subunits in the membrane domain of the bovine F‐ATPase revealed by covalent cross‐linking. J Biol Chem 290: 13308–13320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Han W, Gu Y, Qiu S, Lu Q, Jin J et al (2007). Honokiol induces a necrotic cell death through the mitochondrial permeability transition pore. Cancer Res 67: 4894–4903. [DOI] [PubMed] [Google Scholar]
- Maniam S, Coutts AS, Stratford MR, McGouran J, Kessler B, La Thangue NB (2015). Cofactor Strap regulates oxidative phosphorylation and mitochondrial p53 activity through ATP synthase. Cell Death Differ 22: 156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margineantu DH, Emerson CB, Diaz D, Hockenbery DM (2007). Hsp90 inhibition decreases mitochondrial protein turnover. PLoS One 2: e1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer B, Wittig I, Trifilieff E, Karas M, Schägger H (2007). Identification of two proteins associated with mammalian ATP synthase. Mol Cell Proteomics 6: 1690–1699. [DOI] [PubMed] [Google Scholar]
- Morales‐Rios E, Montgomery MG, Leslie AG, Walker JE (2015). Structure of ATP synthase from Paracoccus denitrificans determined by X‐ray crystallography at 4.0 Å resolution. Proc Natl Acad Sci U S A 112: 13231–13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno AJ, Moreira PI, Custodio JB, Santos MS (2013). Mechanism of inhibition of mitochondrial ATP synthase by 17β‐estradiol. J Bioenerg Biomembr 45: 261–270. [DOI] [PubMed] [Google Scholar]
- Mourier A, Ruzzenente B, Brandt T, Kühlbrandt W, Larsson NG (2014). Loss of LRPPRC causes ATP synthase deficiency. Hum Mol Genet 23: 2580–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H et al (2005). Cyclophilin D‐dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 434: 652–658. [DOI] [PubMed] [Google Scholar]
- Nakanishi‐Matsui M, Sekiya M, Futai M (2016). ATP synthase from Escherichia coli: mechanism of rotational catalysis, and inhibition with the ε subunit and phytopolyphenols. Biochim Biophys Acta 1857: 129–140. [DOI] [PubMed] [Google Scholar]
- Nicolli A, Petronilli V, Bernardi P (1993). Modulation of the mitochondrial cyclosporin A‐sensitive permeability transition pore by matrix pH. Evidence that the pore open‐closed probability is regulated by reversible histidine protonation. Biochemistry 32: 4461–4465. [DOI] [PubMed] [Google Scholar]
- Noji H, Ueno H, McMillan DGG (2017). Catalytic robustness and torque generation of the F1‐ATPase. Biophys Rev 9: 103–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsakaya S, Fujikawa M, Hisabori T, Yoshida M (2011). Knockdown of DAPIT (diabetes‐associated protein in insulin‐sensitive tissue) results in loss of ATP synthase in mitochondria. J Biol Chem 286: 20292–20296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paivarinne H, Kainulainen H (2001). DAPIT, a novel protein down‐regulated in insulin‐sensitive tissues in streptozotocin‐induced diabetes. Acta Diabetol 38: 83–86. [DOI] [PubMed] [Google Scholar]
- Pan J, Zhang Q, Liu Q, Komas SM, Kalyanaraman B, Lubet RA et al (2014). Honokiol inhibits lung tumorigenesis through inhibition of mitochondrial function. Cancer Prev Res 7: 1149–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park I, Londhe AM, Lim JW, Park BG, Jung SY, Lee JY et al (2017). Discovery of non‐peptidic small molecule inhibitors of cyclophilin D as neuroprotective agents in Aβ‐induced mitochondrial dysfunction. J Comput Aided Mol Des 31: 929–941. [DOI] [PubMed] [Google Scholar]
- Pillai VB, Samant S, Sundaresan NR, Raghuraman H, Kim G, Bonner MY et al (2015). Honokiol blocks and reverses cardiac hypertrophy in mice by activating mitochondrial Sirt3. Nat Commun 6: 6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rak M, Gokova S, Tzagoloff A (2011). Modular assembly of yeast mitochondrial ATP synthase. EMBO J 30: 920–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees DM, Leslie AG, Walker JE (2009). The structure of the membrane extrinsic region of bovine ATP synthase. Proc Natl Acad Sci U S A 106: 21597–21601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rönn T, Poulsen P, Tuomi T, Isomaa B, Groop L, Vaag A et al (2009). Genetic variation in ATP5O is associated with skeletal muscle ATP50 mRNA expression and glucose uptake in young twins. PLoS One 4: e4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rühle T, Leister D (2015). Assembly of F1F0‐ATP synthases. Biochim Biophys Acta 1847: 849–860. [DOI] [PubMed] [Google Scholar]
- Sangawa H, Himeda T, Shibata H, Higuti T (1997). Gene expression of subunit c(P1), subunit c(P2), and oligomycin sensitivity‐conferring protein may play a key role in biogenesis of H+‐ATP synthase in various rat tissues. J Biol Chem 272: 6034–6037. [DOI] [PubMed] [Google Scholar]
- Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J et al (2005). Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc Natl Acad Sci U S A 102: 12005–12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sielaff H, Rennekamp H, Wachter A, Xie H, Hilbers F, Feldbauer K et al (2008). Domain compliance and elastic power transmission in rotary FOF1‐ATPase. Proc Natl Acad Sci U S A 105: 17760–17765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šileikyte J, Blachly‐Dyson E, Sewell R, Carpi A, Menabò R, Di Lisa F et al (2014). Regulation of the mitochondrial permeability transition pore by the outer membrane does not involve the peripheral benzodiazepine receptor (translocator protein of 18 kDa (TSPO)). J Biol Chem 289: 13769–13781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava AP, Luo M, Zhou W, Symersky J, Bai D, Chambers MG et al (2018). High‐resolution cryo‐EM analysis of the yeast ATP synthase in a lipid membrane. Science 360 eaas9969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzer AC, Frazee RW, Van Huis C, Cleary J, Opipari AW Jr, Glick GD et al (2010). NMR studies of an immunomodulatory benzodiazepine binding to its molecular target on the mitochondrial F1F0‐ATPase. Biopolymers 93: 85–92. [DOI] [PubMed] [Google Scholar]
- Sullivan KD, Galbraith MD, Andrysik Z, Espinosa JM (2018). Mechanisms of transcriptional regulation by p53. Cell Death Differ 25: 133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symersky J, Osowski D, Walters DE, Mueller DM (2012). Oligomycin frames a common drug‐binding site in the ATP synthase. Proc Natl Acad Sci U S A 109: 13961–13965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian W, Xiong J, Zhu S, Xu D, Shen H, Deng Y (2016). mTOR signaling pathway is inhibited downstream of the cyclophilin D‐mediated mitochondrial permeability transition in honokiol‐triggered regulated necrosis. Mol Med Rep 13: 3227–3235. [DOI] [PubMed] [Google Scholar]
- Vaseva AV, Marchenko ND, Ji K, Tsirka SE, Holzmann S, Moll UM (2012). p53 opens the mitochondrial permeability transition pore to trigger necrosis. Cell 149: 1536–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinothkumar KR, Montgomery MG, Liu S, Walker JE (2016). Structure of the mitochondrial ATP synthase from Pichia angusta determined by electron cryo‐microscopy. Proc Natl Acad Sci U S A 113: 12709–12714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Stockum S, Giorgio V, Trevisan E, Lippe G, Glick GD, Forte MA et al (2015). F‐ATPase of D. melanogaster forms 53 picosiemen (53‐pS) channels responsible for mitochondrial Ca2+‐induced Ca2+ release. J Biol Chem 290: 4537–4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JE (2013). The ATP synthase: the understood, the uncertain and the unknown. Biochem Soc Trans 41: 1–16. [DOI] [PubMed] [Google Scholar]
- Wang G, Dunbrack RL Jr (2003). PISCES: a protein sequence culling server. Bioinformatics 19: 1589–1591. [DOI] [PubMed] [Google Scholar]
- Webb B, Sali A (2016). Comparative protein structure modeling using MODELLER. Curr Protoc Protein Sci 86: 2. [DOI] [PubMed] [Google Scholar]
- Wu YT, Lee HC, Liao CC, Wei YH (2013). Regulation of mitochondrial FoF1ATPase activity by Sirt3‐catalyzed deacetylation and its deficiency in human cells harboring 4977 bp deletion of mitochondrial DNA. Biochim Biophys Acta 1832: 216–227. [DOI] [PubMed] [Google Scholar]
- Yang W, Nagasawa K, Münch C, Xu Y, Satterstrom K, Jeong S et al (2016). Mitochondrial sirtuin network reveals dynamic SIRT3‐dependent deacetylation in response to membrane depolarization. Cell 167: 985–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Ramirez VD (1999). Purification and identification of an estrogen binding protein from rat brain: oligomycin sensitivity‐conferring protein (OSCP), a subunit of mitochondrial F0F1‐ATP synthase/ATPase. J Steroid Biochem Mol Biol 68: 65–75. [DOI] [PubMed] [Google Scholar]
- Zhou A, Rohou A, Schep DG, Bason JV, Montgomery MG, Walker JE et al (2015). Structure and conformational states of the bovine mitochondrial ATP synthase by cryo‐EM. eLife Sciences 4: e10180. [DOI] [PMC free article] [PubMed] [Google Scholar]


