Abstract
The field of mitochondrial ion channels has undergone a rapid development during the last three decades, due to the molecular identification of some of the channels residing in the outer and inner membranes. Relevant information about the function of these channels in physiological and pathological settings was gained thanks to genetic models for a few, mitochondria‐specific channels. However, many ion channels have multiple localizations within the cell, hampering a clear‐cut determination of their function by pharmacological means. The present review summarizes our current knowledge about the ins and outs of mitochondrial ion channels, with special focus on the channels that have received much attention in recent years, namely, the voltage‐dependent anion channels, the permeability transition pore (also called mitochondrial megachannel), the mitochondrial calcium uniporter and some of the inner membrane‐located potassium channels. In addition, possible strategies to overcome the difficulties of specifically targeting mitochondrial channels versus their counterparts active in other membranes are discussed, as well as the possibilities of modulating channel function by small peptides that compete for binding with protein interacting partners. Altogether, these promising tools along with large‐scale chemical screenings set up to identify new, specific channel modulators will hopefully allow us to pinpoint the actual function of most mitochondrial ion channels in the near future and to pharmacologically affect important pathologies in which they are involved, such as neurodegeneration, ischaemic damage and cancer.
Linked Articles
This article is part of a themed section on Mitochondrial Pharmacology: Featured Mechanisms and Approaches for Therapy Translation. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v176.22/issuetoc
Abbreviations
- CGS7184
Ethyl1‐[[(4‐chlorophenyl)amino]oxo]‐2‐hydroxy‐6‐trifluoromethyl‐1H‐indole‐3‐carboxy‐late
- CIC
intracellular chloride channel
- CPP
cell penetrating peptide
- CSA
cyclosporin A
- DPC
diphenyl carbonate
- EMRE
essential MCU regulator
- GSK‐3
glycogen synthase kinase 3
- G3139
phosphorothioate oligodeoxyribonucleotide
- Hsp
heat shock protein
- IMAC
inner membrane anion channel
- IMM
inner mitochondrial membrane
- HK
hexokinase
- MCU
mitochondrial calcium uniporter
- MMC
mitochondrial mega‐channel
- NS1619
(1‐[2‐hydroxy‐5‐(trifluoromethyl)phenyl]‐5‐(trifluoromethyl)‐1,3‐dihydro‐2H‐benzimidazol‐2‐one
- OMM
outer mitochondrial membrane
- OSCP
oligomycin sensitivity conferral protein
- PAP‐1
5‐(4‐phenoxybutoxy)psoralen
- PDAC
pancreatic ductal adenocarcinoma
- PM
plasma membrane
- PTP
permeability transition pore
- ROMK
renal outer medullary potassium channel
- RR
ruthenium red
- RyR1
ryanodine receptor
- SITS
stilbene isothiocyanate sulfonic acid
- TPP
triphenylphosphonium
- TRPC
transient receptor potential cation channel
- UCP
uncoupling protein
- VDAC
voltage‐dependent anion channel
Introduction
A relatively recent and certainly noteworthy development in the health sciences is the emergence of ‘mitochondrial medicine', based on the recognition of the key role mitochondria play in many physiological processes whose alteration contributes to disease, such as metabolism, Ca2+ handling, redox signalling and cell death (see recent, excellent reviews on this topic, e.g. Heller et al., 2012; Smith et al., 2012; Edeas and Weissig, 2013; Szeto et al., 2014; Milane et al., 2015; Guzman‐Villanueva and Weissig, 2017; Masgras et al., 2017).
An important opportunity for intervention to modulate these processes is offered by mitochondrial ion channels (reviews on mitochondrial channels; Brini et al., 2017; Citi et al., 2017; Krabbendam et al., 2018; Mammucari et al., 2018; O'Rourke, 2007; Ponnalagu and Singh, 2017; Szabo and Zoratti, 2014; Szewczyk et al., 2009). These channels are especially important, at least in the contexts of cancer (Figure 1) (for reviews, see Leanza et al., 2013a, 2014b; Madamba et al., 2015; Peruzzo et al., 2016; Mazure, 2017; Szabo and Leanza, 2017; Shoshan‐Barmatz et al., 2017b), neurodegeneration (Rodriguez et al., 2013; Rao et al., 2014; Kalani et al., 2018) and ischaemia (Balderas et al., 2015; Testai et al., 2015b; Smith et al., 2017). In the present review, we discuss the recent developments in the field of mitochondrial ion channels, especially from the point of view of their pharmacological targeting. Specifically modulating members of this varied population may well be a rewarding approach, in particular in the case of cancer due to the major roles mitochondria play in cancer cell metabolism and in cell death. Pharmacological manipulation of mitochondrial channels may lead to cell death independently of upstream elements of the apoptotic cascade, such as http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=910 expression or p53 status, and alterations in signalling pathways (Fulda et al., 2010; Leanza et al., 2013a, 2014a, 2017, 2014b). Expression levels of ion channels in general and of mitochondrial channels in particular may vary from one cell type to another, and between healthy and diseased or activated cells. Examples of drastic variations of this type are provided by the http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=520 (Mu et al., 2003) and intracellular anion‐selective http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=128 channels, which also change localization and function in malignant cells (e.g. Peretti et al., 2015; Tasiopoulou et al., 2015). Silencing K2P9.1 channels affects mitochondrial parameters and induces apoptosis in melanoma cells (Nagy et al., 2014). In the case of the potassium‐selective http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=540 channel, a higher expression correlated with greater sensitivity to chemotherapeutic treatment (Leanza et al., 2014b).
Figure 1.
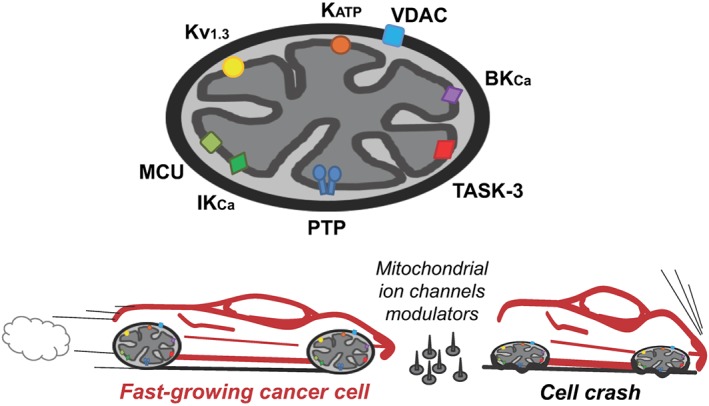
A cartoon illustrating the main mitochondrial ion channels discussed in this review and their impact on cancer.
Multiple localizations of some of the ion channels found in mitochondria
When taking into account the possible pharmacological modulation of mitochondrial ion channels, one important distinction must be immediately made between mitochondria‐specific and multiple‐location channels. In fact, a few channels, such as the mitochondrial calcium uniporter (MCU), the inner membrane anion channel (IMAC), the magnesium‐transporting Mrs2 and the uncoupling protein (UCP), are specific to mitochondria. However, most of the other known ones reside in the plasma membrane as well, and possibly also elsewhere, as detailed in Table 1. These channels include the voltage‐dependent anion channel (VDAC), the big‐, intermediate‐ and small‐conductance calcium‐dependent potassium channels (http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=380 (BKCa), http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=384(http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=384) and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=381 (SKCa)] as well as the voltage‐dependent K+ channels Kv1.3, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=542 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=563, the two‐pore channel K2P9.1, the ATP‐dependent K+ channel (KATP; http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=437) and the intracellular chloride channels http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=128 and http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=128 (for summarizing review, e.g. Szabo and Zoratti, 2014). The mechanisms underlying multiple targeting are being investigated (Singh et al., 2013; Kilisch et al., 2015; von Charpuis et al., 2015; Sole et al., 2016) but are still unclear in most cases. The mechanisms accounting for different localizations of proteins in general vary and may include differences among isoforms (e.g. due to alternative splicing), ‘piggybacking' other proteins, the formation of different heteromers [e.g. in the case of KCa1.1 (BKCa ) channels; Chen et al., 2009], post‐translational modifications such as, for example, phosphorylation or myristoylation, interactions with structures such as, for example, ‘scaffolds', the cytoskeleton or, in membranes, ‘lipid rafts'.
Table 1.
Mitochondrial channels in non‐mitochondrial locations
| Channel | Locationa | Proposed functionsb | Referencesb |
|---|---|---|---|
| VDAC | Plasma membrane |
Cell volume regulation Apoptosis ATP release |
Jakob et al. (1995) |
| VDAC | Endosomes | – | Reymann et al. (1998) |
| VDAC | Endo/sarcoplasmic reticulum |
ATP transport SR/ER‐Mito Ca2+ transport/signalling |
Jurgens et al. (1995) Shoshan‐Barmatz et al. (1996); Shoshan‐Barmatz and Israelson (2005) |
|
BKCa (SLO1, KCa1.1) |
Plasma membrane | Integration of cell signalling | Singh et al. (2012); Yu et al. (2016); Latorre et al. (2017) |
|
BKCa (SLO1, KCa1.1) |
ER membrane | – | Ma et al. (2007); Cox et al. (2014) |
|
BKCa (SLO1, KCa1.1) |
Nuclear envelope | Control of nuclear Ca2+ signalling | Singh et al. (2012); Li et al. (2014) |
|
BKCa (SLO1, KCa1.1) |
Lysosomal membrane | Control of lysosomal Ca2+ | Wang et al. (2017a, 2017b); Sterea et al. (2018) |
|
IKCa (KCa3.1, KCNN4) |
Plasma membrane |
Cell proliferation, differentiation, migration Control of NO synthesis (in vascular epithelia) |
Sforna et al. (2018); Zhan et al. (2018) Grgic et al. (2005); Sheng and Braun (2007); Ruggieri et al. (2012); Ohya and Kito (2018) |
|
SKCa (KCa2.x) |
Plasma membrane | Control of electrical excitability | Yang et al. (2015); Kshatri et al. (2018) |
|
SKCa (KCa2.x) |
ER membrane | Control of ER Ca2+ homeostasis | Kuum et al. (2012, 2015); Richter et al. (2016); Honrath et al. (2017a) |
|
Kv7.4 |
Plasma membrane |
Control of electrical excitability Smooth muscle function Regulation of vascular tone |
Chang et al. (2018) Miceli et al. (2008) Martelli et al. (2013) |
|
SLO2.1 (KNa1.2) |
Plasma membrane |
Control of electrical excitability and of synaptic transmission Anaesthetic preconditioning |
Kameyama et al. (1984) Wojtovich et al. (2016) |
| Kv1.3 | Plasma membrane | Control of voltage and Ca2+ fluxes | Cahalan and Chandy (2009); Zhao et al. (2015); Perez‐Verdaguer et al. (2016); Chandy and Norton (2017); Serrano‐Albarras et al. (2018) |
| Kv1.3 | ER and Golgi | – | Sole et al. (2009); Zhu et al. (2014) |
| Kv1.3 | Nuclear envelope | Regulation of nuclear transmembrane potential | Jang et al. (2015) |
| Kv1.5 | Plasma membrane | O2 sensing | Comes et al. (2013) |
|
TASK‐3 (K2P9.1) |
Plasma membrane |
Cell excitability (background current) |
Zuzarte et al. (2007); Kilisch et al. (2015) |
|
ROMK2 (Kir1.1b) |
Plasma membrane (renal epithelia) | Ion homeostasis | Zhou et al. (1994); Yoo et al. (2005); Angsanakul and Sitprija (2013); Frindt et al. (2013); Welling (2016) |
| KATPc | Plasma membrane | Control of transmembrane potential | Noma (1983) |
| KATPc | Nuclear envelope | Control of nuclear Ca2+ ‘waves' | Quesada et al. (2002) |
| KATPc | ER | Control of Ca2+ load and dynamics | Ashrafpour et al. (2008); Salari et al. (2015) |
| TRPC3 | Plasma membrane |
Mechanosensitive Ca2+ signalling Cellular Ca2+ homeostasis |
Mizoguchi and Monji (2017); Yamaguchi et al. (2017); Tiapko and Groschner (2018) |
| RyR | Sarcoplasmic reticulum |
Ca2+ dynamics Excitation/contraction coupling |
Allard (2018) Treves et al. (2017) |
| RyR | Nuclear envelope | – | Gerasimenko and Gerasimenko (2004); Marius et al. (2006); Zheng et al. (2012) |
| IP3R | Endoplasmic reticulum |
Ca2+ signalling Signal integration ER‐mitochondria communication |
Foskett et al. (2007); Bustos et al. (2017); Kania et al. (2017) |
| IP3R | Nuclear envelope | Ca2+ signalling | Mak and Foskett (1994); Stehno‐Bittel et al. (1995); Gerasimenko and Gerasimenko (2004); Mak et al. (2013) |
| IP3R |
Plasma membrane (low levels) |
Ca2+ entry | Mayrleitner et al. (1995); Dellis et al. (2006) |
| CIC‐4 | Plasma membrane | – | Peretti et al. (2015) |
| CIC‐4 | Nuclear envelope | – | Domingo‐Fernandez et al. (2017) |
| CIC‐1 | Plasma membrane |
Volume regulation Cell migration Metastasis |
Valenzuela et al. (2000) Novarino et al. (2004) Wang et al. (2012); Setti et al. (2013); Peretti et al. (2015) |
| CIC‐1 | Nuclear envelope | – | Valenzuela et al. (1997) |
Refers to subcellular compartments. Organ‐, tissue‐ or cell type‐specific or signalling‐dependent expression is not addressed. Due to cellular membrane traffic and protein flux, integral PM proteins are expected to be found also in the ER, Golgi (anterograde transport) and endosomes, lysosomes (degradative retrograde transport). Thus, for example, IKCa, which has a rapid turnover, is abundant in lysosomes (Balut et al., 2010). The presence in these compartments is therefore not highlighted here, except if evidence exists of a functional presence in the organellar membrane and in the case of VDAC, a largely mitochondrial channel for which it constitutes confirmation of its presence also in the PM.
Examples are given.
Kir/SUR assemblies.
The channel populations residing in different compartments in general have different tasks, roles and/or substrates, and it may therefore be useful to pharmacologically target them specifically in mitochondria or elsewhere. Unfortunately, the art of subcellular targeting is still in its infancy – mitochondrial targeting is by far the most developed – so that little information is available comparing the effects of inhibiting a given channel in one subcellular compartment or another. A partial exception is provided by the plasma membrane, since some of the ion channels in it are inhibited by membrane‐impermeant toxins. The clearest example may be that of potassium‐selective channel Kv1.3: selective block of the plasma membrane population by peptide toxins impairs cell proliferation and migration (e.g. Chhabra et al., 2014; Aissaoui et al., 2018), while selective inhibition of the mitochondrial population by permeant, mitochondriotropic psoralenic compounds precipitates cell death (Leanza et al., 2017). It ought also to be mentioned that mitochondria themselves may differ in structure, properties, functions and proteic composition depending on their location within the cell (e.g. see Diaz et al., 1999, 2000; Riva et al., 2005; Boengler et al., 2009; Baseler et al., 2011; Asemu et al., 2013; Kasumov et al., 2013; Hollander et al., 2014; Fedorovich et al., 2017), not to mention cell type and age. It is also important to mention that not all mitochondrial ion channels have been described/found in all tissues, but they are certainly observable in healthy tissues, not only in pathological cells (cell lines). In the present review, we illustrate our current knowledge on the drugs identified so far that act on mitochondrial ion channels and comment on some of the strategies that can be useful to target specifically the mitochondria‐located channels.
Mitochondrial outer membrane (OMM) channels
VDAC
The VDAC, or ‘mitochondrial porin', is the most extensively studied mitochondrial channel (rev.s: Szabo and Zoratti, 2014; Magri et al., 2018; Shoshan‐Barmatz et al., 2018). The most abundant and most investigated of the three mammalian isoforms (VDAC1, VDAC2 and VDAC3) (for recent review, see, e.g. Messina et al., 2012) is VDAC1, which is present both in the OMM and in the PM (Bathori et al., 2000; De Pinto et al., 2010), in endosomes (Reymann et al., 1998) and in the sarco/endoplasmic reticulum (Jurgens et al., 1995; Shoshan‐Barmatz et al., 1996) (Table 1). The effects of the various modulators have nearly always been automatically attributed to their action on mitochondrial porin(s). The possibility of a contribution by populations located elsewhere has rarely been adequately explored.
VDAC1 is a β‐barrel composed of 19 β‐strands and of an N‐terminal mobile α‐helical segment (for recent review, see, e.g. Zeth and Zachariae, 2018). The latter contains the major binding sites for ions and other proteins (Caterino et al., 2017; Magri and Messina, 2017). As the functions of the N‐terminal peptide and the network of interactions were progressively characterized, VDAC1 has progressed from a more or less inert ‘molecular sieve' in the OMM to a dynamic participant in a host of cellular processes. One is that of allowing and regulating the passage of Ca2+ ions through the OMM (Gincel et al., 2001; Bathori et al., 2006) and at ER/mitochondria contact sites (Hajnoczky et al., 2002; Rapizzi et al., 2002). The binding of Bcl‐xL to the N‐terminal segment may modulate this flux. Mitochondrial Ca2+ overload may induce cell death, in which VDAC1 is implicated in various other ways as well. In particular, VDAC can oligomerize in a Ca2+‐driven process, with the involvement of the N‐terminus, and oligomers are proposed to be implicated in the release of pro‐apoptotic factors such as cytochrome c and apoptosis‐inducing factor (AIF) (for recent review, see Shoshan‐Barmatz et al., 2017a). Furthermore, VDAC1 regulates cell death due to its interactions with anti‐apoptotic members of the Bcl‐2 family (i.e. Bcl2 and Bcl‐xL) and with http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=890 (HK) (Shimizu et al., 2000; Arbel and Shoshan‐Barmatz, 2010).
Various chemical compounds interact with and alter the activity of VDAC1 and may eventually be exploited for therapy in the context of VDAC‐related diseases (Figure 2). The first VDAC blocker to be identified was Konig's polyanion, a 1:2:3 copolymer of methacrylate, maleate and styrene that acts at rather low concentrations (27 μg·mL−1 polyanion) (Colombini et al., 1987). The general anion channel inhibitors http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4177, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4314, H(2) DIDS, 4,4′‐dinitrostilbene‐2,2′‐disulfonic acid (DNDS) and diphenyl carbonate (DPC) were also shown to interact with VDAC1 and to decrease its conductance in electrophysiological experiments with bilayer‐reconstituted VDAC1 (Thinnes et al., 1994; Ben‐Hail and Shoshan‐Barmatz, 2016). Furthermore, ubiquinone specifically binds to VDAC1 through its quinone‐head ring (Murai et al., 2017).
Figure 2.
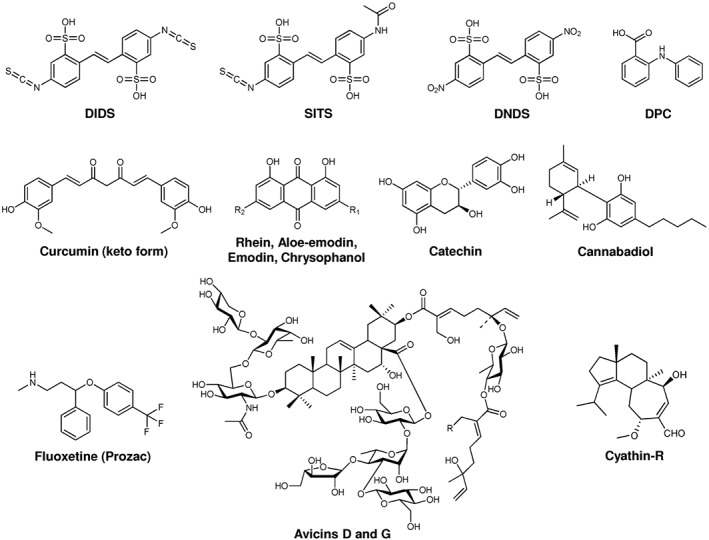
Chemical structure of some VDAC modulators. For further information, see text.
The phosphorothioate oligodeoxyribonucleotide G3139 (http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=8269) was shown to induce rapid flickering of the VDAC1 channel activity and even its closure at 1 μM concentration without affecting the respiration complexes, the adenine nucleotide translocator or the ATP synthase (Tan et al., 2007; Stein and Colombini, 2008). G3139 interferes with metabolite flow through VDAC1 (for review, see, e.g. Klasa et al., 2002). G3139 targets the first six codons of Bcl‐2 mRNA and is successfully used in anticancer treatments in combination with other therapeutic agents (including chemotherapy and radiotherapy), in tumour types ranging from chronic lymphocytic leukaemia beta cell lymphoma to breast cancer and many solid tumours (Kamal et al., 2014). The relevance of the block of VDAC1 activity for the anticancer effect of G3139 is not clear. In general, closure of VDAC would lead to an overall lowering of the cell energy metabolism, subsequently triggering apoptosis.
Other compounds that decrease the conductance of VDAC are avicins, a class of plant stress‐induced metabolites of triterpenoids displaying selective pro‐apoptotic and cytotoxic activity in cancer cells, as well as antioxidant and anti‐inflammatory properties (see, e.g. Haridas et al., 2007). Avicins however stimulate tumour cell death via multiple mechanisms, as they target various cellular components in addition to VDAC, such as tubulin and topoisomerase. They increase the OMM permeability to cytochrome c by inhibiting respiration and by reducing the efficiency of antioxidant systems, leading to a hypersensitivity of mitochondria to oxidative stress. In particular, mitochondria are the main targets of avicin D and avicin G, which can induce apoptotic cell death via intrinsic and extrinsic pathways but also autophagic programmed cell death due to depletion of cell energy. Avicins could thus be of value as chemical agents for the treatment and/or prevention of malignancy (Wang et al., 2010).
The activity of VDAC can also be manipulated by other plant‐derived molecules. http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4150, from the Cannabis species, has strong in vitro anti‐tumour effects in numerous cancer cells types (Massi et al., 2013), and was shown to act on VDAC (Rimmerman et al., 2013). http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7000, a component of Curcuma longa with anti‐inflammatory and anti‐tumour activity, has also been shown to affect VDAC function by interacting with its N‐terminal domain, at least in vitro (Tewari et al., 2015). Chrysophanol, emodin, rhein, aloe‐emodin and catechin, identified as the bioactive components of the herb Rheum officinale Baill and able to induce apoptosis in many human cancer cell lines, were shown to target VDAC‐1 through Thr207 and the N‐terminal region of the protein (Li et al., 2017). However, a direct effect on channel conductance and behaviour has still not been proven.
The list of chemicals that directly modify VDAC1 activity by decreasing channel conductance also includes http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=203 (Prozac) (Nahon et al., 2005; Thinnes, 2005), a clinically used antidepressant drug, that also inhibits the opening of the mitochondrial permeability transition pore, the release of cytochrome c, and protects against http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=346‐induced apoptotic cell death. Lastly, cyathin‐R, a cyathane diterpenoid, was shown to decrease both channel conductance and cancer growth, by promoting apoptosis even in Bax/Bak‐deficient cells (Huang et al., 2015). Cyathin‐R also induced VDAC1 oligomerization, and apoptosis was found to be inhibited by VDAC1‐interacting molecules, such as DIDS and DIDS analogues (H2DIDS, SITS and DPC). These drugs decrease the channel conductance (Thinnes et al., 1994) and counteract apoptosis at the early stage, putatively by diminishing VDAC1 oligomerization and thus preventing cytochrome c release to the cytosol (Keinan et al., 2010).
The above examples point to an important role for VDAC in the regulation of apoptosis; however in some cases, inhibition/decrease of its activity by pharmacological means leads to apoptosis (e.g. G3139), while in other cases, the same modulation of VDAC activity results in protection against cell death (e.g. Prozac). In fact, the role of VDAC activity/oligomerization in OMM permeabilization (MOMP)‐induced apoptosis seems to be rather complex. Bax and t‐Bid have been proposed to take part in the formation of OMM pores in apoptotic cells by assembling heteromeric complexes with VDAC (Tajeddine et al., 2008). Other studies however indicate a cooperative role of Bak and Bax and VDAC2, rather than of VDAC1, in MOMP (see, e.g. Cheng et al., 2003 and for review, Lauterwasser et al., 2016), and also the involvement of other outer membrane ‘receptors' for pro‐apoptotic proteins, including cardiolipin (for recent review, see, e.g. Veresov and Davidovskii, 2014). This may explain why cells lacking all VDAC isoforms were found to be still capable of undergoing apoptosis (Baines et al., 2007). In addition, the interaction of VDAC with other partners such as with the glycolytic enzyme hexokinase is also important, as it gives a proliferative advantage to cancer cells (see below).
Unfortunately, one drawback in this field is the absence of VDAC isoform‐specific pharmacological agents. VDAC2 (Gattin et al., 2015) and VDAC3 (Checchetto et al., 2014) also form ion channels, although the latter one with small conductance. It is worthwhile to mention that a recent work reported the synthesis of an arsenate‐based fluorescent probe, which can preferentially target the mitochondrial VDAC2, this channel being a member of the membrane‐bound vicinal dithiol protein class (Yang et al., 2018). Even if isoform‐specific small molecule inhibitors are not available, as an alternative strategy for specific targeting of VDAC isoforms, peptides copying specific VDAC sequences that can modulate the interaction with various pro‐ and anti‐apoptotic proteins (see below) can be designed and can be therefore useful to modulate a specific VDAC‐related function. Of course this approach would require an extension of our knowledge on the isoform‐specific interaction partners (if there are specific ones) for all three isoforms.
Other mitochondrial outer membrane channels
Beside VDAC isoforms, the OMM also harbours a few other channels such as an unidentified inward rectifying potassium channel (http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=74) (Fieni et al., 2010) and CIC‐4, a member of the intracellular chloride channel family (Ponnalagu et al., 2016a; Ponnalagu and Singh, 2017). CICs are http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4217 (IAA‐94)‐sensitive, but several mitochondrial CIC family members are present also in the endoplasmic reticulum (Ponnalagu et al., 2016b) making it difficult to delineate the function of mitochondrial CICs by pharmacological means. The existence of CICs and Kir channels, both small‐conductance channels, in the OMM challenges the traditional view that the outer membrane forms an unspecific size‐exclusion filter for the flux of small hydrophilic molecules (Becker and Wagner, 2018). In yeast mitochondria, the recent identification of three novel ion channels in the OMM with unknown substrate specificity (Kruger et al., 2017) points to the same conclusion (Checchetto and Szabo, 2018). Whether homologues of the yeast OMM channels also operate in mammalian OMM is still an open question as is the pharmacology of these channels.
Inner membrane ion channels
The permeability transition pore
The permeability transition is a well‐known process that occurs in mitochondria: an increased permeability to solutes of the inner mitochondrial membrane (IMM) caused by the opening of the permeability transition pore (PTP) (Zoratti and Szabo, 1995; Bernardi et al., 2015). PTP is a 1.3 nS conductance unspecific channel located in the IMM whose opening induces depolarization, loss of ionic homeostasis and block of respiratory chain activity and ATP synthesis. The expression ‘permeability transition' to describe these phenomena (considered as an aspecific, lipid‐perturbation effect) was used for the first time in the late seventies by Haworth and Hunter (1979). The discovery of the ability of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1024 (CsA) to inhibit PTP in classical bioenergetics experiments (Crompton et al., 1988) and the observation of a CsA‐sensitive 1.3 nS ion channel in patch clamp experiments on mitoplasts, that is, mitochondria devoid of OMM (Szabo and Zoratti, 1991), completely changed the perspectives concerning the permeability transition (PT). The observed channel, the so‐called mitochondrial mega‐channel (MMC), reproduced all known pharmacological features of the PTP: inhibition by CsA, adenine nucleotides, Mg2+, acidic pH and reducing agents, induction by Ca2+ (Szabo and Zoratti, 2014). PT, that thanks to these studies could be ascribed to a pore (PTP), changed its ‘status' from being a supposed in vitro artefact or lipid perturbation to an important physio‐pathological process involved in cell death, apoptosis resistance in cancer cells, oxidative stress, anoxia and ischaemia followed by reperfusion, just to name a few (for reviews, see, e.g. Di Lisa and Bernardi, 2006; Brenner and Moulin, 2012; Rasola and Bernardi, 2014; Leanza et al., 2014b).
Despite the fact that the role of PTP in mitochondrial physiology was intensively studied in the last decades in different pathophysiological contexts, the structure and molecular identity of PTP is still highly debated (Biasutto et al., 2016; Giorgio et al., 2018), making difficult the ‘smart design' of a structure‐based specific channel modulator. As to date, the scientific community is converging towards the idea that PTP is formed by dimers of the FOF1 ATP synthase (Alavian et al., 2014; Beutner et al., 2017; Bonora et al., 2013; Carraro et al., 2014; Giorgio et al., 2013; He et al., 2017), even though the exact mechanism and the site and mode of the actual pore formation is still unclear. In this respect, as well as regarding the possibility of designing specific drugs, the strategies undertaken, involving site‐directed mutagenesis combined with functional assays (Giorgio et al., 2017; Antoniel et al., 2018), might turn out to be extremely useful. Indeed, strong support in favour of the identification of the ATP synthase with PTP/MMC is the finding that modulation of MMC activity directly recorded by patch clamp in the native mitochondrial inner membrane by protons was altered in a single point mutant of the oligomycin sensitivity conferral protein (OSCP) subunit of the ATP synthase complex (Antoniel et al., 2018). In addition, subunits e, g and the first transmembrane segment of subunit b have recently been shown to contribute to channel formation in yeast, by combining multiple gene deletion with electrophysiology (Carraro et al., 2018).
Given the central role of the PTP in pathophysiological contexts, considerable effort was made in the field to discover new, direct modulators to treat disorders, even in the absence of a clear molecular identity. Pharmacological targeting of PTP is not straightforward, since no bona fide pore blockers are available. In fact, even the action of the relatively specific CsA, which is effective at sub‐μM concentrations, is indirect, as it is mediated by cyclophilin D, whose expression level varies among different cells. Furthermore, not only is cyclophilin D expression important but also its ratio with F‐ATPase OSCP subunits: considering that normally there are more F‐ATP synthase complexes than cyclophylin D molecules in the cells, most of the PTP complexes present are expected to be insensitive to CsA treatment (Bernardi et al., 2015). CsA inhibits calcineurin and thus acts as an immunosuppressant (Griffiths and Halestrap, 1995). Therefore, several new, calcineurin‐indifferent cyclophilin D inhibitors have been developed over the past 20 years. The most promising are sanglifehrin A (Clarke et al., 2002), 9‐(N‐methyl‐L‐isoleucine)‐cyclosporin A (Zulian et al., 2014) and MeAla(3)EtVal(4)‐cyclosporin (Tiepolo et al., 2009). Recently, other factors have also been shown to modulate PTP by targeting cyclophilin D, such as http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=621 (Burstein et al., 2018), http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7527 (Roy et al., 2015) and n‐phenylbenzamides (Roy et al., 2016). A virtual chemical screening identified several compounds that were able to bind to cyclophilin D with a K D of <100 nM (Park et al., 2017). Synthetic compounds from the diarylquinoline and organotin families instead target the c‐ring of the F‐ATPase and strongly inhibit the enzyme (Pagliarani et al., 2016). A member of these families, tributyltin, induces mitochondrial swelling, which has long been associated with the PT, but is CsA‐insensitive, at least according to some authors (see, e.g. Gogvadze et al., 2002).
Several different classes of compounds acting at sites that do not involve the PTP pore itself have been characterized for their ability to antagonize the mitochondrial permeability transition in an indirect way (Figure 3). Among these substances are gasotransmitters (NO‐donors, H2S‐donors and Co‐donors) (for review, see Andreadou et al., 2015) and antioxidants (Javadov and Karmazyn, 2007), just to name a few classes. Altogether, all the above‐mentioned inhibitors, even though by an indirect effect, were proven to affect PTP activity in various systems. Some of them might represent good starting points for the design of more specific pharmacological agents able to counteract those degenerative diseases where PTP opening plays a crucial role.
Figure 3.
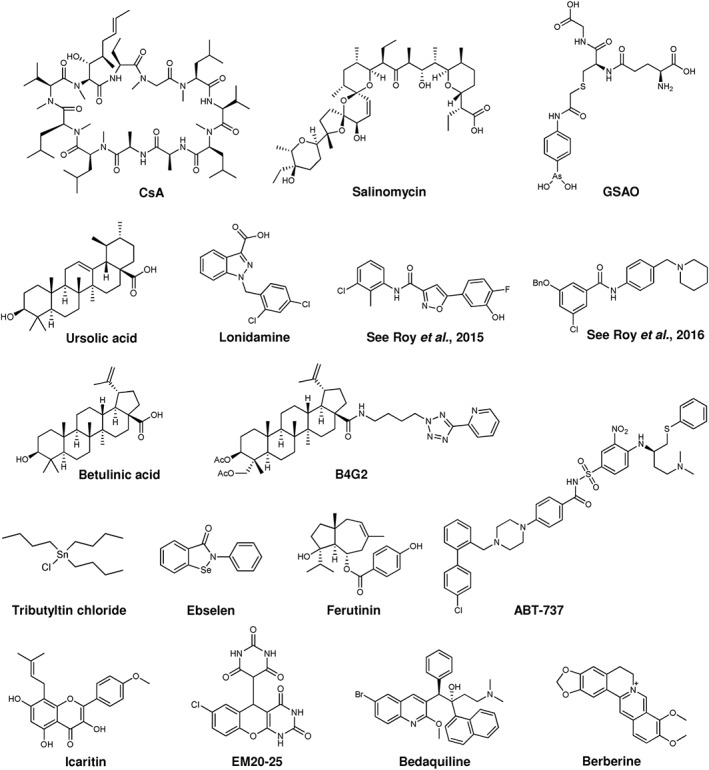
Chemical structure of some modulators of the permeability transition pore. For further information, see text.
Conversely, several PTP inducers with different chemical structures and displaying distinct mechanisms of PTP activation are promising agents for the treatment of cancer. Indeed, some of these compounds are already in clinical trials; others have been tested in vitro in tumour cell lines or in vivo in preclinical models for different neoplasms. Some of these molecules are mastoparan‐like peptides (Jones et al., 2008), 4‐(N‐(Sglutathionylacetyl)amino)phenylarsonous acid (Elliott et al., 2012), ursolic acid (Lu et al., 2014), http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=3945 (Fulda et al., 1999; Potze et al., 2015), B4G2 (an hydroxybetulinic acid derivative) (Yao et al., 2015), triterpenoid derivatives (Laszczyk, 2009; Serafim et al., 2014), curcumin (Qiu et al., 2014), lonidamine (Lena et al., 2009), http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=8320 (a BH3 mimetic) (Yu et al., 2015), the K+/H+ antiporter salinomycin (Manago et al., 2015; Qin et al., 2015), berberine (Shen et al., 2014), ebselen (Pavon et al., 2015), the gold complex AUL‐12 (Chiara et al., 2012), icaritin (Zhou et al., 2015), EM20‐25 (Milanesi et al., 2006) and ferutinin (Ilyich et al., 2018). It has to be stressed that in most cases, possible side effects can be envisioned as opening of the PTP might lead to loss of mitochondrial function in healthy cells as well. Specific targeting of these molecules exclusively to tumour tissues employing various strategies (for a recent review, see Ruoslahti, 2017) is a challenge but might be a worthwhile goal to pursue.
As in the case of VDAC, one might envision not only the direct modulation of channel activity by small molecules but also the possibility to fine‐tune the activity of the PTP (proposed to correspond to the ATP synthase) or of its partners/modulators (for review, see Miura and Tanno, 2012), by post‐translational modifications by kinases, such as hexokinase II (HK II) (Rasola et al., 2010a) or the mitochondrial http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2030 (mGSK‐3) (Rasola et al., 2010a). PTP can be indirectly and negatively modulated in cancer cells by mGSK‐3 phosphorylation of cyclophilin D, and mGSK‐3 can in turn be inhibited by a mitochondria‐located http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1494 fraction, which is normally under the control of the often dysregulated http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=897. An active ERK in mitochondria of cancer cells was shown to lead to mGSK‐3 phosphorylation, thus preventing cyclophilin D phosphorylation and in turn ‘desensitizing' PTP (Rasola et al., 2010a). Similarly, the PTP is inhibited when cyclophilin D is nitrosylated on specific cysteine residues (Nguyen et al., 2011) or interacts with the transcription factor STAT‐3 (Boengler et al., 2010). Furthermore, chaperones such as heat shock protein (Hsp) 60, Hsp90 and tumour necrosis factor receptor‐associated protein‐1 have been demonstrated to modulate PTP activity, and these chaperones themselves can be regulated by post‐translational modification (Masgras et al., 2017; Rasola, 2017). Opening of the PTP can be induced also by HK II detachment from the OMM: this event can be controlled by signalling pathways that involve either the Ser/Thr kinase Akt or GSK‐3, even if the mechanism accounting for this regulation is still debated (Miyamoto et al., 2008; Rasola et al., 2010b). Finally, some direct sites of post‐translational modifications, for example, phosphorylation, acetylation and S‐nitrosylation, have also been identified in the F‐ATPase subunits (Bernardi et al., 2015), although they may hardly offer a more specific way of intervention.
Mitochondrial calcium uniporter and other calcium channels
Mitochondria play a crucial role in intracellular Ca2+ regulation by shaping, remodelling, relaying and decoding Ca2+ signals, due to their ability to rapidly and transiently accumulate Ca2+ (Drago et al., 2011). Ca2+ uptake into mammalian mitochondria is efficiently blocked by low concentrations of ruthenium red (RR) and Ru360, thanks to the fact that they lead to a direct inhibition of the uniporter (Moore, 1971; Vasington et al., 1972; Reed and Bygrave, 1974). The molecular identity of the protein(s) giving rise to uniporter activity was not known until the last decade. The finding that a highly Ca2+ selective ion channel replicated all key characteristics of the mammalian mitochondrial uniporter represented a milestone towards its molecular identification (Kirichok et al., 2004). Another important step was the publication of the MitoCarta database (Pagliarini et al., 2008), which then provided the basis for the identification of several mitochondrial calcium uniporter complex (MCUC) components in mammals, including the central pore forming protein MCU (mitochondrial calcium uniporter) (Baughman, 2011; De Stefani et al., 2011). At the current stage, in mammals, the MCUC appears to include at least the pore‐forming protein MCU, an MCU paralogue (MCUb), the essential MCU regulator (EMRE), the regulatory MICU proteins and, possibly, the mitochondrial calcium uniport regulator 1 (MCUR1). In other systems, such as plants, MCUC seems simpler and consists primarily of MCU and MICU (Wagner et al., 2016). Mammalian MCU activity can be regulated through its paralogue MCUb (Raffaello et al., 2013), through interaction with MICU1, the first uniporter component identified (Perocchi et al., 2010), by two other MICU1 isoforms, namely, MICU2 and MICU3 (Plovanich et al., 2013) and by post‐translational modifications (Jhun et al., 2016). MICU2 forms a heterodimer with MICU1 through an intermolecular disulphide bond and closes the channel at low extramitochondrial Ca2+ concentrations (Patron et al., 2014; Petrungaro et al., 2015). Currently, two models coexist that find MICU1 to act exclusively as a uniporter activator at high cytosolic Ca2+ concentrations (Patron et al., 2014) or to gradually disinhibit the uniporter with increasing Ca2+ concentrations in the cytosol (Csordas et al., 2013). Studies resolving for the first time the 3D structure of proposed pentameric MCU by a combination of NMR and electron microscopy allowed the proposal of the hypothesis to explain the requirement of the presence of EMRE in the native membrane to observe MCU activity: the outer and inner juxtamembrane helices as well as a loop region in MCU seem to be unstable regions, which may undergo conformational changes upon activation by EMRE in order to create the lateral exit path for Ca2+ in the MCU channel (Oxenoid et al., 2016). Recently, a more in‐depth insight into the structure of the tetrameric MCU has been obtained, thanks to the resolution of the structure of MCU by X‐ray and cryo‐EM simultaneously by different groups and from different organisms (Baradaran et al., 2018; Fan et al., 2018; Nguyen et al., 2018; Yoo et al., 2018). EMRE is a metazoan‐specific protein able to mediate MCU‐MICU1/MICU2 dimer interaction and, as shown recently, able to regulate MCU channel activity depending on the matrix Ca2+ concentration (Sancak et al., 2013; Vais et al., 2016). Furthermore, the activity of MCU can be also indirectly regulated, for example, by the uncoupling protein UCP2, as mitochondria isolated from UCP2−/− mice showed decreased RR‐sensitive calcium uptake (Motloch et al., 2016).
The importance of the correct regulation of MCU activity is underlined by the finding that patients carrying a loss‐of‐function mutation of MICU1 are characterized by myopathy, cognitive impairment and an extrapyramidal movement disorder (Logan et al., 2014). MICU1 has recently been shown to play a role in Ca2+ overload‐induced mitochondrial PTP opening and also to be a decisive factor for adaptation to postnatal life and for tissue repair after injury of liver (Antony et al., 2016). Other studies underline the importance of MCU expression/activity in the context of the regulation of metabolism in various organisms ranging from Trypanosoma to zebrafish and mice (e.g. Duchen, 2000; Huang et al., 2013; Prudent et al., 2013; Gorlach et al., 2015; Mammucari et al., 2018). The manipulation of MCU expression changed muscle function, through a novel Ca2+‐dependent mitochondria‐to‐nucleus signalling pathway (Mammucari et al., 2015). MCU function was also shown to affect oxidative phosphorylation and to ensure correct pacemaker cell function (Wu et al., 2015). MCU is overexpressed in several type of cancer cells (e.g. Davis et al., 2013; Peruzzo et al., 2016; Bustos et al., 2017) and has been implicated in cancer‐related processes, in particular in the control of migration and metastasis (Hall et al., 2014; Tang et al., 2015; Tosatto et al., 2016; Szabo and Leanza, 2017).
Given the vast literature demonstrating the fundamental impact of calcium uptake into mitochondria for many physiological and pathological processes, it became clear that either activation (to induce calcium overload and PTP opening) or inhibition (to decrease ATP production and/or to prevent calcium overload) of MCU might be useful in pathological settings. However, regarding pharmacological modulation of MCU, the direct inhibitors identified to date are only RR and Ru‐360 (Kirichok et al., 2004; De Stefani et al., 2011; Wu et al., 2018), which also affect other cationic channels. No specific activators are known to our knowledge. It has been proposed that a small heterocyclic molecule, 5‐[(4‐methylphenyl)thio]‐9H‐pyrimido[4,5‐b]indole‐2,4‐diamine (AG311), retards tumour growth by activating MCU, but a direct proof is still missing (Bastian et al., 2015). Likewise, the protective effect of Necrox‐5 ([5‐(1,1‐dioxo‐thiomorpholin‐4‐ylmethyl)‐2‐phenyl‐1H‐indol‐7‐yl]‐(tetrahydro‐pyran‐4‐ylmethyl)‐amine) on myocardial damage in ischaemic heart disease has been ascribed to its ability to inhibit MCU (Thu et al., 2012) but also in this case, a direct electrophysiological proof is missing. A recent work identified http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7242 as an MCU modulator by systematic chemical screening (Arduino et al., 2017). Likewise, a high‐throughput screening of 120 000 small‐molecule compounds identified the membrane‐permeant drug DS1657, an indolebutyric acid derivative (Sawada et al., 2001), that dose‐dependently inhibited serum‐induced mitochondrial Ca2+ influx in HEK293A cells with an IC50 of 7 μM (Kon et al., 2017). Formal proof and information about the mechanism of inhibition is lacking in the latter case. In this respect, electrophysiological lipid bilayer experiments using recombinant proteins might be useful. MCU produced in a cell‐free system as well as that purified from Escherichia coli using His‐tag, either added to the recording chamber as the purified protein or following purification and incorporation into liposomes, showed channel activity in a reproducible manner in more than 200 experiments performed in different settings (De Stefani et al., 2011; Raffaello et al., 2013; Patron et al., 2014; Teardo et al., 2017). However, a recent work using human MCU expressed in mammalian cells with FLAG‐tag concluded, on the basis of only three experiments, that this protein does not form channels by itself (Wu et al., 2018). Further work will be required to clarify the reasons for this discrepancy. It may be due to different conditions used for protein solubilization and purification and/or the presence of cholesterol in the latter system that is known to affect the activity of several channels (but is largely absent from the IMM) (Brini et al., 2017).
Other mitochondrial Ca2+ channels might contribute to the mediation of Ca2+ fluxes into/out of this organelle, particularly in specialized tissues where the MCU may play a secondary role. Potential candidates mediating calcium flux include the transient receptor potential cation http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=488 (Feng et al., 2013; Wang et al., 2017a, 2015, 2017b) and the mitochondrial ryanodine receptor (mhttp://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=747). A low level of RyR1 is detectable in heart mitochondria and provides rapid transport of Ca2+ that is insensitive to ruthenium red (Beutner et al., 2001, 2005). The TRPC3 channel, whose structure has been recently resolved (Tang et al., 2018), is specifically inhibited by ethyl‐1‐(4‐(2,3,3‐trichloroacrylamide)phenyl)‐5‐(trifluoromethyl)‐1H‐pyrazole‐4‐carboxylate (Pyr3) that was shown to alter mitochondrial membrane potential and ROS production when applied together with dexamethasone (Abdoul‐Azize et al., 2016). However, TRPC3 and RyR1 show multiple localizations within the cell, making it difficult to ascribe the observed physiological effects to the regulation of these channels only in mitochondria.
Mitochondrial inner membrane potassium channels
IMM K+ channels studied by electrophysiology are the calcium‐dependent channels – big‐conductance potassium channel (mtBKCa) (Singh et al., 2012), intermediate‐conductance K+ channel (mtIKCa) (De Marchi et al., 2009) and small‐conductance K+ channel (mtSKCa) (Dolga et al., 2013), voltage‐gated shaker type K+ channel Kv1.3 (Szabo et al., 2005) and the ATP‐dependent potassium channel (mtKATP) (Inoue et al., 1991). An interesting new development in the field is the discovery that mtBKCa behaves as a mechanosensitive channel in electrophysiological experiments (Walewska et al., 2018). Biochemical/genetic evidence indicates the presence of the pH‐sensitive two‐pore potassium channel K2P9.1 (TASK‐3) (Pocsai et al., 2006; Toczylowska‐Maminska et al., 2014), of Kv1.5 (Leanza et al., 2012b), of http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=429 (Foster et al., 2012), of the http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=385 (Wojtovich et al., 2011; Smith et al., 2018) and of Kv7.4 (Testai et al., 2015a, 2015b) and another pH sensitive K+ channel (Kajma and Szewczyk, 2012) (for recent reviews, see, e.g. Szabo and Zoratti, 2014; Laskowski et al., 2016; Augustynek et al., 2017). Almost all of the above‐mentioned IMM K+ channels identified thus far are considered to be the mitochondrial counterparts of well‐known plasma membrane (PM) channels and many of them even display multiple subcellular localizations in the ER, nucleus, Golgi and PM membranes in addition to mitochondria (Table 1) (for review, see, e.g. Laskowski et al., 2016; Brini et al., 2017). Nevertheless, mtKATP seems to have a different composition from its PM counterpart: a splicing variant of the renal outer medullary potassium channel (ROMK) has been proposed to form the ion‐conducting subunit (Foster et al., 2012) and indeed, the ROMK protein is present in the IMM (Bednarczyk et al., 2018). Since ROMK apparently does not have a wide tissue‐expression while mtKATP activity has been observed in various tissues, a contribution of other pore‐forming proteins to mtKATP channel activity cannot, as yet, be fully excluded.
IMM K+ channels have been proposed to contribute to the regulation of matrix volume, in addition to influencing the mitochondrial transmembrane potential (ΔΨm) and ΔpH, calcium transport, production of ROS and mitochondrial dynamics. Activation of a mitochondrial calcium‐dependent K+ channel modulates K+ uptake and matrix volume and has been proposed to increase bioenergetic efficiency (Aon et al., 2010). Alternatively, a protective mechanism involves activation of different IMM K+ channels that induces a slight uncoupling effect that would in turn decrease energetic efficiency (Cardoso et al., 2010). Activity of the evolutionarily conserved ATP‐dependent K+ channel mitoKATP (Garlid et al., 2009; Lefer et al., 2009), of BKCa (Frankenreiter et al., 2017), of the voltage‐gated channel Kv7.4 (Testai et al., 2015a, 2015b) as well as of the SK channels (Dolga et al., 2013) has been linked to ischaemic preconditioning, ischaemic postconditioning and cytoprotection in general. For this latter channel, a recent work reveals that a neuroprotective mechanism involves attenuation of [Ca2+]m uptake upon SK channel activation and that respiration and complex I activity upon pharmacological activation or overexpression of mitochondrial KCa2.2 (SK2) channels resulted in reduced mitochondrial ROS formation (Honrath et al., 2017a, 2017b). However, in general, the exact basis of K+ channel openers' cytoprotective effects still remains to be elucidated (for recent reviews, see, e.g. Honrath et al., 2017a; Krabbendam et al., 2018). Importantly, in the case of mtBKCa, a cGMP‐dependent protective effect in the cases of acute cardiac damage and adverse long‐term events that occur after myocardial infarction has been demonstrated also in vivo, thanks to tissue‐dependent knockout of BKCa in the cardiac tissue in mice (Frankenreiter et al., 2017).
Interestingly, many of the K+ channels found in the IMM (in addition to the PM and other membranes) are highly overexpressed in cancer cells/tissues. For example, the two‐pore leak channel K2P9.1(TASK‐3) is overexpressed five‐ to 100‐fold in 44% of breast tumours and is also highly expressed in lung, colon and ovarian cancers (Peruzzo et al., 2016). Its overexpression promotes tumour formation and confers resistance to hypoxia in vitro, suggesting that its up‐regulation plays a pathologically important role in human breast cancer (Mu et al., 2003). IKCa (also called KCa3.1) is expressed in almost all migrating cells and various studies point to its important role in the control of proliferation in human breast cancer, in hepatocellular carcinoma, in chronic lymphocytic leukaemia (B‐CLL) and in lung cancer (for review, see Schwab et al., 2012). mtIKCa is functional in HCT116 colon carcinoma and HeLa cells (Sassi et al., 2010), is inhibited by http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2336 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2330 (De Marchi et al., 2009) and regulates oxidative phosphorylation in pancreatic ductal adenocarcinoma cells (Kovalenko et al., 2016). In vitro data suggest that inhibition of IKCa and likely of mtIKCa by membrane‐permeant inhibitors sensitizes melanoma cells to B‐Raf inhibitors, such as http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5893, and induces release of mitochondrial ROS (Bauer et al., 2017).
More information is available about mtKv1.3: it has been demonstrated to have a crucial role in apoptosis in various cancer cells (Leanza et al., 2012b; Szabo et al., 2008). The Kv1.3 channel is overexpressed in various cancer tissues/cells (Pardo and Stuhmer, 2014) and is important for anti‐tumour immunity (Eil et al., 2016). Membrane‐permeant inhibitors of the Kv1.3 channel such as http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?tab=summary&ligandId=9184 and psoralen derivatives act by inducing intrinsic apoptosis via the following chain of events: stopping the depolarizing K+ influx causes IMM hyperpolarization, with ensuing increased ROS level, PTP activation, swelling, loss of Δψm, loss of cytochrome c and further ROS release (Leanza et al., 2015; Checchetto et al., 2018). These drugs also killed pathological cells in the absence of Bax and Bak, independently of p53 status and of Bcl‐2 overexpression thanks to the direct targeting of mt function and permeability at the level of the inner membrane. In vivo experiments in melanoma and pancreatic ductal adenocarcinoma orthotopic models corroborated the effectiveness of Kv1.3 inhibitors and showed their selective action only on pathological cells. Specificity depends on the synergy between different factors such as high Kv1.3 expression and altered basal redox state in cancer cells (Leanza et al., 2017).
Most of the evidence supporting the involvement of the above K+ channels in protection against ischaemic/reperfusion damage (Szewczyk et al., 2010; Laskowski et al., 2016) or induction of apoptosis (Szabo et al., 2012) is pharmacological (for a review summarizing the pharmacology of these channels, see, e.g. Szabo and Zoratti, 2014) (Figure 4). Unfortunately, other cellular targets possibly accounting for the observed effects have been identified (Augustynek et al., 2017). Activators of BKCa, such as Ca2+, diCl‐DHAA (12,14‐dichlorodehydroabietic acid), http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4272, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=8802 and hypoxia, or inhibitors of this channel like http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2328, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4218 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2309 do not only seem to act on the mitochondrial mtBKCa channel (Szewczyk et al., 2009). CGS7184 (ethyl1‐[[(4‐chlorophenyl)amino]oxo]‐2‐hydroxy‐6‐trifluoromethyl‐1H‐indole‐3‐carboxy‐late), a synthetic BKCa channel opener, directly activates the ryanodine receptor calcium release (http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=748) channel in the sarcoplasmic reticulum (Wrzosek et al., 2012). CGS7184 and CGS7181, in contrast to NS1619 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4271, were also able to induce cell death in neurons (Augustynek et al., 2018). Furthermore, SERCA, complex I of the respiratory chain and ATP‐synthase inhibition are involved in pleiotropic effects of the BKCa channel activator NS1619 on endothelial cells (Lukasiak et al., 2016). One of the most specific BKCa activators identified so far is NS11021 (N′‐[3,5‐bis(trifluoromethyl)phenyl]‐N‐[4‐bromo‐2‐(2H‐tetrazol‐5‐yl‐phenyl]thiourea; however, possible intracellular off‐target effects of this drug have not been studied in detail to our knowledge. KATP channel activators instead might act as uncouplers (Szewczyk et al., 2010) and KCa3.1 (IKCa) as well as KCa2.1 (SKCa) modulators may well have off‐target effects, similarly to those affecting BKCa channels. In the case of mtK2P9.1 (TASK‐3) channels, no highly specific modulators are available but dihydropyrrolo[2,1‐α]isoquinolines compounds that are able to inhibit these K2P (TASK) channels could become possible candidates for developing new, selective inhibitors (Noriega‐Navarro et al., 2014).
Figure 4.
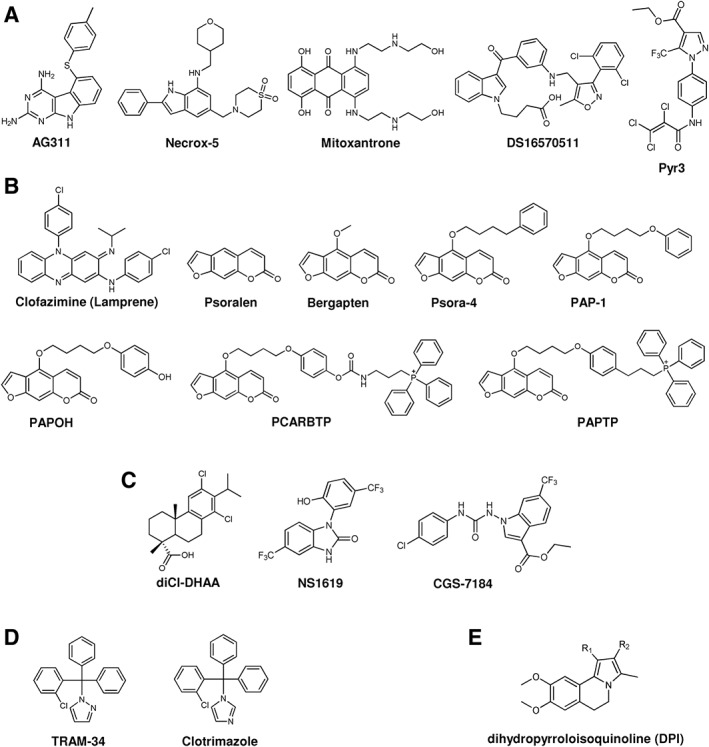
Chemical structure of some modulators of other mitochondrial ion channels: (A) MCU; (B) Kv1.3; (C) KCa1.1 (BKCa); (D) KCa3.1(IKCa); (E) K2P9.1 (TASK‐3). For further information see text and, for example, Augustynek et al. (2017).
One possible solution to the issue of pleotropic effects of the drugs due to multiple localization of the channels within the cells is chemical targeting of the most specific modulators of these channels to mitochondria by virtue of addition of a functional lipophilic cation (e.g. triphenylphosphonium group) that drives the accumulation of a certain drug into mitochondria (Murphy and Smith, 2007). This strategy has been successfully applied also in vivo in the case of mtKv1.3 (see below). In summary, the relationship between mitochondrial potassium transport and diseases linked to altered mitochondrial function is still only partially explored, but the data available so far point to IMM K+ channels as possible targets for therapeutic applications against various pathologies, including ischaemia and cancer, and possibly neurodegenerative diseases (see, e.g. Matschke et al., 2018).
Inner mitochondrial membrane chloride channels
In addition to CIC‐4 in the OMM, CIC‐1 (Yang et al., 2009) and CIC‐5 (Ponnalagu et al., 2016a, 2016b) have been located to the IMM where the latter plays a role in regulating the release of mitochondrial ROS from respiratory chain complexes. Indeed, the emerging view is that mitochondrial chloride channels also have a role in cardiac function and cardioprotection from ischaemia–reperfusion (IR) injury. Chloride intracellular channels (CIC) are non‐classical ion channels lacking a signal sequence for membrane targeting. Interestingly, CIC‐like proteins exist even in bacteria (Gururaja Rao et al., 2017; Ponnalagu and Singh, 2017). Among CICs in mammals, CIC‐1, CIC‐4 and CIC‐5 are abundant in the heart. In contrast to CIC‐1 and CIC‐4 (found in the OMM, see above), CIC‐5 does not show co‐localization to the endoplasmic reticulum (Ponnalagu et al., 2016b). Again, as yet, no isoform‐specific modulators of CICs are available (for recent review, see Ponnalagu and Singh, 2017). As to the long‐studied IMAC, due to the uncertainties regarding its molecular identity, only general modulators have been identified (for review, see Ponnalagu and Singh, 2017).
Successfully applied strategies for in vivo subcellular targeting of small molecule modulators of mitochondrial ion channels
Thanks to the highly negative matrix side membrane potential across the IMM, mitochondria can be massively ‘loaded' with positively charged, membrane‐permeant compounds, achieving concentration gradients of up to 1000‐fold. This concept has been used in many studies since its inception 60 years ago (Liberman et al., 1969) (for reviews, see, e.g. Ross et al., 2005; Horobin et al., 2007; Hoye et al., 2008; Murphy, 2008; Smith et al., 2008, 2011; Zielonka et al., 2017). Molecules directed to mitochondria must obviously be capable of entering the cell and be at least moderately lipophilic. If mitochondrial targeting is associated with the presence of a positive charge, as is generally the case, the charge is therefore distributed by resonance over a lipophilic structure. Since the seminal study of Liberman, Skulachev and colleagues (1969), this has most often been the triphenylphosphonium group, connected to the rest of the molecule by a linker, commonly an alkyl chain. Other moderately lipophilic positively charged groups that have been used for this purpose have been mainly heteroaromatic systems, including rhodamines (e.g. Chernyak et al., 2013), berberine and palmatine (Chernyak et al., 2013), pyridinium (Beckham et al., 2013; Hou et al., 2014), indolium (Huang et al., 2017) and cyanines. Constructs comprising a number of positively charged guanidinium groups have also been found to be effective mitochondria‐targeting devices (Fernandez‐Carneado et al., 2005; Maiti et al., 2007). These constructs may be considered as non‐peptidic penetrating agents.
The dication dequalinium – more generally a family of compounds comprising two quinolinium moieties linked by an aliphatic chain spacer –was used early on to obtain mitochondria‐targeted nanocarriers (DQAsomes), intended principally as a tool for mitochondrial transfection (e.g. Weissig, 2015). Interaction with the mitochondria was proposed to be mediated by cardiolipin (Weissig and Torchilin, 2000; Weissig et al., 2001). Dequaliniums are known inhibitors of K+ channels (Castle et al., 1993; Galanakis et al., 1996; Malik‐Hall et al., 2000), and – unsurprisingly at this point – they are toxic for leukaemia cells (Galeano et al., 2005; Sancho et al., 2007; Garcia‐Perez et al., 2011). Toxicity has been linked to redox unbalance (Sancho et al., 2007; Garcia‐Perez et al., 2011). DQAsomes are at any rate only one example of the several nanostructured devices that have been devised to selectively transport cargo to mitochondria (reviewed by: Marrache et al., 2015; Ma et al., 2016; Paleos et al., 2016; Sato et al., 2016). The underlying principle generally is the use of positive charges to promote delivery to mitochondria.
To minimize the possibility of interference with the pharmacological activity, one may resort to prodrugs in which the mitochondria‐targeting portion is shed, hopefully after performing its task. In some such studies, the strategy has relied on the incorporation, into the linker portion of the molecule, of a hydrolyzable bond system such as a carbamate group ( Leanza et al., 2017; Peruzzo et al., 2017; Teixeira et al., 2017; Venturini et al., 2017; Mattarei et al., 2018). Insurance that the regeneration of the active principle takes place only, or mostly, at mitochondria may be a desirable feature. This may be achieved by exploiting a mitochondrial enzyme, such as nitroreductase (Chevalier et al., 2016) or aldehyde dehydrogenase ALDH‐2 (Ripcke et al., 2009), or a reactive compound plentiful in mitochondria, such as H2O2 (McQuaker et al., 2013).
The lipophilic or amphipathic character of mitochondria‐targeted constructs, necessary to allow permeation of cell membranes, also confers affinity for the membranes themselves, leading to extensive binding, in particular of triphenylphosphonium (TPP)‐comprising compounds (James et al., 2007; Ross et al., 2008). Association with membranes may pose difficulties but also be a bonus if the targeted proteins, like ion channels, reside in mitochondrial membranes. This strategy has been successfully applied recently in the case of the mitochondrial potassium channel Kv1.3. MtKv1.3 mediates an inward potassium flux to the mitochondrial matrix and likely modulates coupling between ATP synthesis and mitochondrial respiration. In vivo evidence has been obtained suggesting that inhibition of mtKv1.3 by pharmacological means represents an unconventional but promising strategy to selectively eliminate cancer cells. The Kv1.3 (and mtKv.3) channel is overexpressed in various cancer tissues/cells, and mtKv1.3 was identified as a novel target of Bax (Szabo et al., 2008, 2011). http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=9583, PAP‐1 (5‐(4‐phenoxybutoxy)psoralen) and clofazimine, three distinct membrane‐permeant inhibitors of Kv1.3 channels (Cahalan and Chandy, 2009), induced death in multiple human and mouse cancer cell lines by triggering a series of events involving hyperpolarization, ROS release, activation of PTP and apoptosis (Szabo and Zoratti, 2014). In contrast, membrane‐impermeant, selective and high‐affinity Kv1.3 inhibitors http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2549 or http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2547 did not trigger cell death, suggesting a crucial role for the mitochondrial Kv1.3 versus PM Kv1.3 channel. In preclinical mouse models for melanoma (Leanza et al., 2012a) and for pancreatic ductal adenocarcinoma (PDAC) (Zaccagnino et al., 2016), an i.p. injection of clofazimine significantly reduced tumour size while no adverse effects were observed in several healthy tissues. Clofazimine is a clinically used drug with anti‐inflammatory and immunosuppressive activities (Cholo et al., 2012). This drug also inhibits the transporter http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=768 (Van Rensburg et al., 1994) and may activate the tumour suppressor p53 (Casey et al., 2009; Patil, 2013). Therefore, a more specific drug, acting exclusively on the mitochondrial counterpart of Kv1.3, was required to more efficiently and specifically target this channel. Two mitochondriotropic derivatives of PAP‐1, the most specific membrane‐permeant inhibitor, were designed and synthesized and shown to drastically reduce tumour growth at a low concentration in animal models for melanoma and PDAC, without causing in vivo side effects such as cardiotoxicity and immune depletion (Leanza et al., 2017). These mitochondria‐targeted mtKv1.3 inhibitors, therefore, can be considered especially promising molecules for the treatment of several types of cancer, provided the tumour cells express high levels of mtKv1.3. A recent study identified some TPP‐related compounds that might even eradicate cancer stem cells (Ozsvari et al., 2018).
An alternative strategy to target mitochondria may still exploit transmembrane potential differences but make use of peptides rather than of lipophilic charged groups like TPP (reviewed by: Yousif et al., 2009b; Frantz and Wipf, 2010; Zielonka et al., 2017). This approach may be considered to be related to the use of so‐called ‘cell‐penetrating peptides' (CPPs) to overcome the barrier posed by the plasma membrane (reviewed by: Borrelli et al., 2018; Guidotti et al., 2017; Kalafatovic and Giralt, 2017). CPPs are short sequences of amino acids, typically comprising basic residues along with hydrophobic ones, which are taken up by cells mostly via endocytotic pathways (clathrin‐ or caveolin‐mediated, macropinocytosis) – entailing a subsequent escape from the endosome – or via processes involving a degree of membrane disorganization, for example, the formation of transient pores, which can result in toxicity (reviewed by: Cleal et al., 2013; LeCher et al., 2017; Walrant et al., 2017).
Reaching the cytosol in principle allows a second‐stage delivery to other subcellular compartments (Cerrato et al., 2017; Ngwa et al., 2017). This has been achieved in a mitochondria‐specific manner with the so‐called Szeto–Schiller peptides, in particular SS‐31 (e.g. Szeto, 2006, 2014) (for other examples, see Cerrato et al., 2017; Zielonka et al., 2017). These devices, which interact with cardiolipin, have antioxidant effects. Peptides can in principle serve to transport small‐molecule cargos linked via peptide bonds (Yousif et al., 2009a; Jean et al., 2016). A variation on the theme involves gramicidin S, a natural peptidic antibiotic with a marked affinity for mitochondrial membranes (Kanai et al., 2007; Frantz and Wipf, 2010).
Mitochondrial leader peptide sequences in principle offer a specific approach, as they are recognized and accepted by the mitochondrial protein import system (MPTS), which, incidentally, needs a transmembrane potential to operate (Wiedemann and Pfanner, 2017). The attempts to deliver cargo to mitochondria in this manner have been few (Vestweber and Schatz, 1988, 1989; Flierl et al., 2003), but the idea has been adapted to favour interactions of multifunctional nanocarriers with mitochondria (Kawamura et al., 2013).
With regard to mitochondrial ion channels, peptides have been used to functionally modulate VDAC. The most relevant case may be that of its interaction with hexokinase, which results in inhibition of apoptosis, promotion of glycolysis and cancer cell survival (Nakashima, 1989; Zaid et al., 2005; Galluzzi et al., 2008; Krasnov et al., 2013). The interaction was mapped to the N‐terminal portion of VDAC, and detachment of hexokinase was accomplished by methyljasmonate, a plant hormone (Goldin et al., 2008). More efficient are specific peptides based on the relevant sequences and made into membrane‐penetrating peptide that antagonized the interaction and had pro‐apoptotic effects in vitro (Arzoine et al., 2009; Prezma et al., 2013). A recent paper reports that two VDAC1 N‐terminal sequences fused to a cell‐penetrating sequence from the Drosophila antennapedia‐homeodomain or to a human transferrin receptor (hTfR)‐recognition sequence, respectively, were able to attack glioblastoma, a notoriously elusive target, also in an in vivo murine xenograft model. At least the transferrin‐based construct was able to cross the blood brain barrier and was thus effective in an orthotopic model upon i.v. administration as the free compound or as an inclusion in PLGA nanoparticles (Shteinfer‐Kuzmine et al., 2017). The same group provided evidence that a cell‐penetrating peptide derived from VDAC1 is also highly effective against hepatocellular carcinoma in a preclinical model (Pittala et al., 2018).
It should also be mentioned that the pH difference between the mitochondrial matrix and cytoplasm (more alkaline in the matrix) may be exploited to drive the accumulation of permeant weak acids into mitochondria. Normally, however, this difference is in the order of only 0.5 units, permitting a concentration difference of about three. This approach therefore has not been much used, but it should be kept in mind.
Conclusions
The field of mitochondrial ion channels can be likened to a vast stretch of territory comprising some freshly ploughed, fertile, already productive land and a dense thicket waiting to be cleared to put the ground to further good use. To do so will involve imaginative research to design specific drugs or specifically targeted drugs and to reach a more detailed understanding of their distinct localization‐dependent functions, of the partnerships – with proteins and lipids – involved in the execution of these functions, of the signalling pathways affected, of the mechanisms regulating ion channel localization and of their possible modulation, of possible species and/or tissue specificities for each of these aspects. Subcellular targeting of drugs is clearly a new frontier of pharmacological science and the practical exploitation of mitochondrial channels for the improvement of public health is one of the goals the metaphorical pioneers/researchers have set for themselves. As in many other cases, the effectiveness and specificity of drug delivery is a major hurdle.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017a,b,c,d,e,f).
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors' work is supported by the Italian Association for Cancer Research (AIRC IG grant 15544 to I.S.), by the Italian Ministry of University and Education (PRONAT project to M.Z. and L.B.; PRIN 2015795S5W to I.S.) and by the Consiglio Nazionale delle Ricerche (Project of Special Interest on Aging and InterOmics to M.Z. and L.B.). L.L. is the recipient of a young researcher grant by the University of Padova (n. GRIC12NN5G) and a PRID 2017 (n. BIRD162511) from the University of Padova.
Leanza, L. , Checchetto, V. , Biasutto, L. , Rossa, A. , Costa, R. , Bachmann, M. , Zoratti, M. , and Szabo, I. (2019) Pharmacological modulation of mitochondrial ion channels. British Journal of Pharmacology, 176, 4258–4283. 10.1111/bph.14544.
References
- Abdoul‐Azize S, Buquet C, Vannier JP, Dubus I (2016). Pyr3, a TRPC3 channel blocker, potentiates dexamethasone sensitivity and apoptosis in acute lymphoblastic leukemia cells by disturbing Ca(2+) signaling, mitochondrial membrane potential changes and reactive oxygen species production. Eur J Pharmacol 784: 90–98. [DOI] [PubMed] [Google Scholar]
- Aissaoui D, Mlayah‐Bellalouna S, Jebali J, Abdelkafi‐Koubaa Z, Souid S, Moslah W et al (2018). Functional role of Kv1.1 and Kv1.3 channels in the neoplastic progression steps of three cancer cell lines, elucidated by scorpion peptides. Int J Biol Macromol 111: 1146–1155. [DOI] [PubMed] [Google Scholar]
- Alavian KN, Beutner G, Lazrove E, Sacchetti S, Park HA, Licznerski P et al (2014). An uncoupling channel within the c‐subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore. Proc Natl Acad Sci U S A 111: 10580–10585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Cidlowski JA, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017a). The Concise Guide to PHARMACOLOGY 2017/18: Nuclear hormone receptors. Br J Pharmacol 174: S208–S224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD et al (2017b). The Concise Guide to PHARMACOLOGY 2017/18: Transporters. Br J Pharmacol 174: S360–S446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Striessnig J, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017c). The Concise Guide to PHARMACOLOGY 2017/18: Voltage‐gated ion channels. Br J Pharmacol 174: S160–S194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017d). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD et al (2017e). The Concise Guide to PHARMACOLOGY 2017/18: Overview. Br J Pharmacol 174: S1–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD et al (2017f). The Concise Guide to PHARMACOLOGY 2017/18: Other ion channels. Br J Pharmacol 174: S195–S207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard B (2018). From excitation to intracellular Ca(2+) movements in skeletal muscle: basic aspects and related clinical disorders. Neuromuscul Disord: NMD 28: 394–401. [DOI] [PubMed] [Google Scholar]
- Andreadou I, Iliodromitis EK, Rassaf T, Schulz R, Papapetropoulos A, Ferdinandy P (2015). The role of gasotransmitters NO, H2S and CO in myocardial ischaemia/reperfusion injury and cardioprotection by preconditioning, postconditioning and remote conditioning. Br J Pharmacol 172: 1587–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angsanakul J, Sitprija V (2013). Scorpion venoms, kidney and potassium. Toxicon 73: 81–87. [DOI] [PubMed] [Google Scholar]
- Antoniel M, Jones K, Antonucci S, Spolaore B, Fogolari F, Petronilli V et al (2018). The unique histidine in OSCP subunit of F‐ATP synthase mediates inhibition of the permeability transition pore by acidic pH. EMBO Rep 19: 257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony AN, Paillard M, Moffat C, Juskeviciute E, Correnti J, Bolon B et al (2016). MICU1 regulation of mitochondrial Ca(2+) uptake dictates survival and tissue regeneration. Nat Commun 7: 10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aon MA, Cortassa S, Wei AC, Grunnet M, O'Rourke B (2010). Energetic performance is improved by specific activation of K+ fluxes through K (Ca) channels in heart mitochondria. Biochim Biophys Acta 1797: 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbel N, Shoshan‐Barmatz V (2010). Voltage‐dependent anion channel 1‐based peptides interact with Bcl‐2 to prevent antiapoptotic activity. J Biol Chem 285: 6053–6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arduino DM, Wettmarshausen J, Vais H, Navas‐Navarro P, Cheng Y, Leimpek A et al (2017). Systematic identification of MCU modulators by orthogonal interspecies chemical screening. Mol Cell 67: 711–723.e717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzoine L, Zilberberg N, Ben‐Romano R, Shoshan‐Barmatz V (2009). Voltage‐dependent anion channel 1‐based peptides interact with hexokinase to prevent its anti‐apoptotic activity. J Biol Chem 284: 3946–3955. [DOI] [PubMed] [Google Scholar]
- Asemu G, O'Connell KA, Cox JW, Dabkowski ER, Xu W, Ribeiro RF Jr et al (2013). Enhanced resistance to permeability transition in interfibrillar cardiac mitochondria in dogs: effects of aging and long‐term aldosterone infusion. Am J Physiol Heart Circ Physiol 304: H514–H528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafpour M, Eliassi A, Sauve R, Sepehri H, Saghiri R (2008). ATP regulation of a large conductance voltage‐gated cation channel in rough endoplasmic reticulum of rat hepatocytes. Arch Biochem Biophys 471: 50–56. [DOI] [PubMed] [Google Scholar]
- Augustynek B, Koprowski P, Rotko D, Kunz WS, Szewczyk A, Kulawiak B (2018). Mitochondrial BK channel openers CGS7181 and CGS7184 exhibit cytotoxic properties. Int J Mol Sci 19 pii: E353. 10.3390/ijms19020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustynek B, Kunz WS, Szewczyk A (2017). Guide to the pharmacology of mitochondrial potassium channels. Handb Exp Pharmacol 240: 103–127. [DOI] [PubMed] [Google Scholar]
- Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD (2007). Voltage‐dependent anion channels are dispensable for mitochondrial‐dependent cell death. Nat Cell Biol 9: 550–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balderas E, Zhang J, Stefani E, Toro L (2015). Mitochondrial BKCa channel. Front Physiol 6: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balut CM, Gao Y, Murray SA, Thibodeau PH, Devor DC (2010). ESCRT‐dependent targeting of plasma membrane localized KCa3.1 to the lysosomes. Am J Physiol Cell Physiol 299: C1015–C1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baradaran R, Wang C, Siliciano AF, Long SB (2018). Cryo‐EM structures of fungal and metazoan mitochondrial calcium uniporters. Nature 559: 580–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseler WA, Dabkowski ER, Williamson CL, Croston TL, Thapa D, Powell MJ et al (2011). Proteomic alterations of distinct mitochondrial subpopulations in the type 1 diabetic heart: contribution of protein import dysfunction. Am J Physiol Regul Integr Comp Physiol 300: R186–R200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian A, Thorpe JE, Disch BC, Bailey‐Downs LC, Gangjee A, Devambatla RK et al (2015). A small molecule with anticancer and antimetastatic activities induces rapid mitochondrial‐associated necrosis in breast cancer. J Pharmacol Exp Ther 353: 392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathori G, Csordas G, Garcia‐Perez C, Davies E, Hajnoczky G (2006). Ca2+‐dependent control of the permeability properties of the mitochondrial outer membrane and voltage‐dependent anion‐selective channel (VDAC). J Biol Chem 281: 17347–17358. [DOI] [PubMed] [Google Scholar]
- Bathori G, Parolini I, Szabo I, Tombola F, Messina A, Oliva M et al (2000). Extramitochondrial porin: facts and hypotheses. J Bioenerg Biomembr 32: 79–89. [DOI] [PubMed] [Google Scholar]
- Bathori G, Parolini I, Tombola F, Szabo I, Messina A, Oliva M et al (1999). Porin is present in the plasma membrane where it is concentrated in caveolae and caveolae‐related domains. J Biol Chem 274: 29607–29612. [DOI] [PubMed] [Google Scholar]
- Bauer D, Werth F, Nguyen HA, Kiecker F, Eberle J (2017). Critical role of reactive oxygen species (ROS) for synergistic enhancement of apoptosis by vemurafenib and the potassium channel inhibitor TRAM‐34 in melanoma cells. Cell Death Dis 8: e2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughman JM (2011). Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476: 341–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckham TH, Lu P, Jones EE, Marrison T, Lewis CS, Cheng JC et al (2013). LCL124, a cationic analog of ceramide, selectively induces pancreatic cancer cell death by accumulating in mitochondria. J Pharmacol Exp Ther 344: 167–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker T, Wagner R (2018). Mitochondrial outer membrane channels: emerging diversity in transport processes. Bioessays 40: e1800013. [DOI] [PubMed] [Google Scholar]
- Bednarczyk P, Kicinska A, Laskowski M, Kulawiak B, Kampa R, Walewska A et al (2018). Evidence for a mitochondrial ATP‐regulated potassium channel in human dermal fibroblasts. Biochim Biophys Acta 1859: 309–318. [DOI] [PubMed] [Google Scholar]
- Ben‐Hail D, Shoshan‐Barmatz V (2016). VDAC1‐interacting anion transport inhibitors inhibit VDAC1 oligomerization and apoptosis. Biochim Biophys Acta 1863: 1612–1623. [DOI] [PubMed] [Google Scholar]
- Bernardi P, Rasola A, Forte M, Lippe G (2015). The mitochondrial permeability transition pore: channel formation by F‐ATP synthase, integration in signal transduction, and role in pathophysiology. Physiol Rev 95: 1111–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutner G, Alavian KN, Jonas EA, Porter GA Jr (2017). The mitochondrial permeability transition pore and ATP synthase. Handb Exp Pharmacol 240: 21–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutner G, Sharma VK, Giovannucci DR, Yule DI, Sheu SS (2001). Identification of a ryanodine receptor in rat heart mitochondria. J Biol Chem 276: 21482–21488. [DOI] [PubMed] [Google Scholar]
- Beutner G, Sharma VK, Lin L, Ryu SY, Dirksen RT, Sheu SS (2005). Type 1 ryanodine receptor in cardiac mitochondria: transducer of excitation‐metabolism coupling. Biochim Biophys Acta 1717: 1–10. [DOI] [PubMed] [Google Scholar]
- Biasutto L, Azzolini M, Szabo I, Zoratti M (2016). The mitochondrial permeability transition pore in AD 2016: an update. Biochim Biophys Acta 1863: 2515–2530. [DOI] [PubMed] [Google Scholar]
- Boengler K, Stahlhofen S, van de Sand A, Gres P, Ruiz‐Meana M, Garcia‐Dorado D et al (2009). Presence of connexin 43 in subsarcolemmal, but not in interfibrillar cardiomyocyte mitochondria. Basic Res Cardiol 104: 141–147. [DOI] [PubMed] [Google Scholar]
- Boengler K, Hilfiker‐Kleiner D, Heusch G, Schulz R (2010). Inhibition of permeability transition pore opening by mitochondrial STAT3 and its role in myocardial ischemia/reperfusion. Basic Res Cardiol 105: 771–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonora M, Bononi A, De Marchi E, Giorgi C, Lebiedzinska M, Marchi S et al (2013). Role of the c subunit of the FO ATP synthase in mitochondrial permeability transition. Cell Cycle 12: 674–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelli A, Tornesello AL, Tornesello ML, Buonaguro FM (2018). Cell penetrating peptides as molecular carriers for anti‐cancer agents. Molecules (Basel, Switzerland) 23: pii: E295. 10.3390/molecules23020295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner C, Moulin M (2012). Physiological roles of the permeability transition pore. Circ Res 111: 1237–1247. [DOI] [PubMed] [Google Scholar]
- Brini M, Leanza L, Szabo I (2017). Lipid‐mediated modulation of intracellular ion channels and redox state: physiopathological implications. Antioxid Redox Signal 10.1089/ars.2017.7215. [DOI] [PubMed] [Google Scholar]
- Burstein SR, Kim HJ, Fels JA, Qian L, Zhang S, Zhou P et al (2018). Estrogen receptor β modulates permeability transition in brain mitochondria. Biochim Biophys Acta 1859: 423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustos G, Cruz P, Lovy A, Cardenas C (2017). Endoplasmic reticulum‐mitochondria calcium communication and the regulation of mitochondrial metabolism in cancer: a novel potential target. Front Oncol 7: 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan MD, Chandy KG (2009). The functional network of ion channels in T lymphocytes. Immunol Rev 231: 59–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso AR, Queliconi BB, Kowaltowski AJ (2010). Mitochondrial ion transport pathways: role in metabolic diseases. Biochim Biophys Acta 1797: 832–838. [DOI] [PubMed] [Google Scholar]
- Carraro M, Giorgio V, Sileikyte J, Sartori G, Forte M, Lippe G et al (2014). Channel formation by yeast F‐ATP synthase and the role of dimerization in the mitochondrial permeability transition. J Biol Chem 289: 15980–15985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraro M, Checchetto V, Sartori G, Kucharczyk R, di Rago JP, Minervini G et al (2018). High‐conductance channel formation in yeast mitochondria is mediated by F‐ATP synthase e and g subunits. Cell Physiol Biochem 50: 1840–1855. 10.1159/000494864. [DOI] [PubMed] [Google Scholar]
- Casey FP, Pihan E, Shields DC (2009). Discovery of small molecule inhibitors of protein‐protein interactions using combined ligand and target score normalization. J Chem Inf Model 49: 2708–2717. [DOI] [PubMed] [Google Scholar]
- Castle NA, Haylett DG, Morgan JM, Jenkinson DH (1993). Dequalinium: a potent inhibitor of apamin‐sensitive K+ channels in hepatocytes and of nicotinic responses in skeletal muscle. Eur J Pharmacol 236: 201–207. [DOI] [PubMed] [Google Scholar]
- Caterino M, Ruoppolo M, Mandola A, Costanzo M, Orru S, Imperlini E (2017). Protein‐protein interaction networks as a new perspective to evaluate distinct functional roles of voltage‐dependent anion channel isoforms. Mol Biosyst 13: 2466–2476. [DOI] [PubMed] [Google Scholar]
- Cerrato CP, Kunnapuu K, Langel U (2017). Cell‐penetrating peptides with intracellular organelle targeting. Expert Opin Drug Deliv 14: 245–255. [DOI] [PubMed] [Google Scholar]
- Chandy KG, Norton RS (2017). Peptide blockers of Kv1.3 channels in T cells as therapeutics for autoimmune disease. Curr Opin Chem Biol 38: 97–107. [DOI] [PubMed] [Google Scholar]
- Chang A, Abderemane‐Ali F, Hura GL, Rossen ND, Gate RE, Minor DL Jr (2018). A calmodulin C‐lobe Ca(2+)‐dependent switch governs Kv7 channel function. Neuron 97: 836–852.e836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checchetto V, Azzolini M, Peruzzo R, Capitanio P, Leanza L (2018). Mitochondrial potassium channels in cell death. Biochem Biophys Res Commun 500: 51–58. [DOI] [PubMed] [Google Scholar]
- Checchetto V, Reina S, Magri A, Szabo I, De Pinto V (2014). Recombinant human voltage dependent anion selective channel isoform 3 (hVDAC3) forms pores with a very small conductance. Cell Physiol Biochem 34: 842–853. [DOI] [PubMed] [Google Scholar]
- Checchetto V, Szabo I (2018). Novel channels of the outer membrane of mitochondria: recent discoveries change our view. Bioessays 40: e1700232. [DOI] [PubMed] [Google Scholar]
- Chen H, Kronengold J, Yan Y, Gazula VR, Brown MR, Ma L et al (2009). The N‐terminal domain of Slack determines the formation and trafficking of Slick/Slack heteromeric sodium‐activated potassium channels. J Neurosci 29: 5654–5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng EH, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ (2003). VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science (New York, NY) 301: 513–517. [DOI] [PubMed] [Google Scholar]
- Chernyak BV, Antonenko YN, Domnina LV, Ivanova OY, Lyamzaev KG, Pustovidko AV et al (2013). Novel penetrating cations for targeting mitochondria. Curr Pharm Des 19: 2795–2806. [DOI] [PubMed] [Google Scholar]
- Chevalier A, Zhang Y, Khdour OM, Kaye JB, Hecht SM (2016). Mitochondrial nitroreductase activity enables selective imaging and therapeutic targeting. J Am Chem Soc 138: 12009–12012. [DOI] [PubMed] [Google Scholar]
- Chhabra S, Chang SC, Nguyen HM, Huq R, Tanner MR, Londono LM et al (2014). Kv1.3 channel‐blocking immunomodulatory peptides from parasitic worms: implications for autoimmune diseases. FASEB J 28: 3952–3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiara F, Gambalunga A, Sciacovelli M, Nicolli A, Ronconi L, Fregona D et al (2012). Chemotherapeutic induction of mitochondrial oxidative stress activates GSK‐3α/β and Bax, leading to permeability transition pore opening and tumor cell death. Cell Death Dis 3: e444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholo MC, Steel HC, Fourie PB, Germishuizen WA, Anderson R (2012). Clofazimine: current status and future prospects. J Antimicrob Chemother 67: 290–298. [DOI] [PubMed] [Google Scholar]
- Citi V, Calderone V, Martelli A, Breschi MC, Testai L (2017). Pathophysiological role of mitochondrial potassium channels and their modulation by drugs. Curr Med Chem 10.2174/0929867324666171012115300. [DOI] [PubMed] [Google Scholar]
- Clarke SJ, McStay GP, Halestrap AP (2002). Sanglifehrin A acts as a potent inhibitor of the mitochondrial permeability transition and reperfusion injury of the heart by binding to cyclophilin‐D at a different site from cyclosporin A. J Biol Chem 277: 34793–34799. [DOI] [PubMed] [Google Scholar]
- Cleal K, He L, Watson PD, Jones AT (2013). Endocytosis, intracellular traffic and fate of cell penetrating peptide based conjugates and nanoparticles. Curr Pharm Des 19: 2878–2894. [DOI] [PubMed] [Google Scholar]
- Colombini M, Yeung CL, Tung J, Konig T (1987). The mitochondrial outer membrane channel, VDAC, is regulated by a synthetic polyanion. Biochim Biophys Acta 905: 279–286. [DOI] [PubMed] [Google Scholar]
- Comes N, Bielanska J, Vallejo‐Gracia A, Serrano‐Albarras A, Marruecos L, Gomez D et al (2013). The voltage‐dependent K(+) channels Kv1.3 and Kv1.5 in human cancer. Front Physiol 4: 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox N, Toro B, Pacheco‐Otalora LF, Garrido‐Sanabria ER, Zarei MM (2014). An endoplasmic reticulum trafficking signal regulates surface expression of β4 subunit of a voltage‐ and Ca(2)(+)‐activated K(+) channel. Brain Res 1553: 12–23. [DOI] [PubMed] [Google Scholar]
- Crompton M, Ellinger H, Costi A (1988). Inhibition by cyclosporin A of a Ca2+‐dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem J 255: 357–360. [PMC free article] [PubMed] [Google Scholar]
- Csordas G, Golenar T, Seifert EL, Kamer KJ, Sancak Y, Perocchi F et al (2013). MICU1 controls both the threshold and cooperative activation of the mitochondrial Ca(2)(+) uniporter. Cell Metab 17: 976–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis FM, Parsonage MT, Cabot PJ, Parat MO, Thompson EW, Roberts‐Thomson SJ et al (2013). Assessment of gene expression of intracellular calcium channels, pumps and exchangers with epidermal growth factor‐induced epithelial‐mesenchymal transition in a breast cancer cell line. Cancer Cell Int 13: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marchi U, Sassi N, Fioretti B, Catacuzzeno L, Cereghetti GM, Szabo I et al (2009). Intermediate conductance Ca2+‐activated potassium channel (KCa3.1) in the inner mitochondrial membrane of human colon cancer cells. Cell Calcium 45: 509–516. [DOI] [PubMed] [Google Scholar]
- De Pinto V, Messina A, Lane DJ, Lawen A (2010). Voltage‐dependent anion‐selective channel (VDAC) in the plasma membrane. FEBS Lett 584: 1793–1799. [DOI] [PubMed] [Google Scholar]
- De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R (2011). A forty‐kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476: 336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellis O, Dedos SG, Tovey SC, Taufiq Ur R, Dubel SJ, Taylor CW (2006). Ca2+ entry through plasma membrane IP3 receptors. Science (New York, NY) 313: 229–233. [DOI] [PubMed] [Google Scholar]
- Di Lisa F, Bernardi P (2006). Mitochondria and ischemia‐reperfusion injury of the heart: fixing a hole. Cardiovasc Res 70: 191–199. [DOI] [PubMed] [Google Scholar]
- Diaz G, Setzu MD, Zucca A, Isola R, Diana A, Murru R et al (1999). Subcellular heterogeneity of mitochondrial membrane potential: relationship with organelle distribution and intercellular contacts in normal, hypoxic and apoptotic cells. J Cell Sci 112 (Pt 7): 1077–1084. [DOI] [PubMed] [Google Scholar]
- Diaz G, Falchi AM, Gremo F, Isola R, Diana A (2000). Homogeneous longitudinal profiles and synchronous fluctuations of mitochondrial transmembrane potential. FEBS Lett 475: 218–224. [DOI] [PubMed] [Google Scholar]
- Dolga AM, Netter MF, Perocchi F, Doti N, Meissner L, Tobaben S et al (2013). Mitochondrial small conductance SK2 channels prevent glutamate‐induced oxytosis and mitochondrial dysfunction. J Biol Chem 288: 10792–10804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo‐Fernandez R, Coll RC, Kearney J, Breit S, O'Neill LAJ (2017). The intracellular chloride channel proteins CLIC1 and CLIC4 induce IL‐1β transcription and activate the NLRP3 inflammasome. J Biol Chem 292: 12077–12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drago I, Pizzo P, Pozzan T (2011). After half a century mitochondrial calcium in‐ and efflux machineries reveal themselves. EMBO J 30: 4119–4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen MR (2000). Mitochondria and Ca(2+)in cell physiology and pathophysiology. Cell Calcium 28: 339–348. [DOI] [PubMed] [Google Scholar]
- Edeas M, Weissig V (2013). Targeting mitochondria: strategies, innovations and challenges: the future of medicine will come through mitochondria. Mitochondrion 13: 389–390. [DOI] [PubMed] [Google Scholar]
- Eil R, Vodnala SK, Clever D, Klebanoff CA, Sukumar M, Pan JH et al (2016). Ionic immune suppression within the tumour microenvironment limits T cell effector function. Nature 537: 539–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott MA, Ford SJ, Prasad E, Dick LJ, Farmer H, Hogg PJ et al (2012). Pharmaceutical development of the novel arsenical based cancer therapeutic GSAO for Phase I clinical trial. Int J Pharm 426: 67–75. [DOI] [PubMed] [Google Scholar]
- Fan C, Fan M, Orlando BJ, Fastman NM, Zhang J, Xu Y et al (2018). X‐ray and cryo‐EM structures of the mitochondrial calcium uniporter. Nature 559: 575–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorovich SV, Waseem TV, Puchkova LV (2017). Biogenetic and morphofunctional heterogeneity of mitochondria: the case of synaptic mitochondria. Rev Neurosci 28: 363–373. [DOI] [PubMed] [Google Scholar]
- Feng S, Li H, Tai Y, Huang J, Su Y, Abramowitz J et al (2013). Canonical transient receptor potential 3 channels regulate mitochondrial calcium uptake. Proc Natl Acad Sci U S A 110: 11011–11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez‐Carneado J, Van Gool M, Martos V, Castel S, Prados P, de Mendoza J et al (2005). Highly efficient, nonpeptidic oligoguanidinium vectors that selectively internalize into mitochondria. J Am Chem Soc 127: 869–874. [DOI] [PubMed] [Google Scholar]
- Fieni F, Parkar A, Misgeld T, Kerschensteiner M, Lichtman JW, Pasinelli P et al (2010). Voltage‐dependent inwardly rectifying potassium conductance in the outer membrane of neuronal mitochondria. J Biol Chem 285: 27411–27417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flierl A, Jackson C, Cottrell B, Murdock D, Seibel P, Wallace DC (2003). Targeted delivery of DNA to the mitochondrial compartment via import sequence‐conjugated peptide nucleic acid. Mol Ther 7: 550–557. [DOI] [PubMed] [Google Scholar]
- Foskett JK, White C, Cheung KH, Mak DO (2007). Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev 87: 593–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster DB, Ho AS, Rucker J, Garlid AO, Chen L, Sidor A et al (2012). Mitochondrial ROMK channel is a molecular component of mitoK (ATP). Circ Res 111: 446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenreiter S, Bednarczyk P, Kniess A, Bork NI, Straubinger J, Koprowski P et al (2017). cGMP‐elevating compounds and ischemic conditioning provide cardioprotection against ischemia and reperfusion injury via cardiomyocyte‐specific BK channels. Circulation 136: 2337–2355. [DOI] [PubMed] [Google Scholar]
- Frantz MC, Wipf P (2010). Mitochondria as a target in treatment. Environ Mol Mutagen 51: 462–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frindt G, Li H, Sackin H, Palmer LG (2013). Inhibition of ROMK channels by low extracellular K+ and oxidative stress. Am J Physiol Renal Physiol 305: F208–F215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulda S, Jeremias I, Steiner HH, Pietsch T, Debatin KM (1999). Betulinic acid: a new cytotoxic agent against malignant brain‐tumor cells. Int J Cancer 82: 435–441. [DOI] [PubMed] [Google Scholar]
- Fulda S, Galluzzi L, Kroemer G (2010). Targeting mitochondria for cancer therapy. Nat Rev Drug Discov 9: 447–464. [DOI] [PubMed] [Google Scholar]
- Galanakis D, Davis CA, Ganellin CR, Dunn PM (1996). Synthesis and quantitative structure‐activity relationship of a novel series of small conductance Ca(2+)‐activated K+ channel blockers related to dequalinium. J Med Chem 39: 359–370. [DOI] [PubMed] [Google Scholar]
- Galeano E, Nieto E, Garcia‐Perez AI, Delgado MD, Pinilla M, Sancho P (2005). Effects of the antitumoural dequalinium on NB4 and K562 human leukemia cell lines. Mitochondrial implication in cell death. Leuk Res 29: 1201–1211. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Kepp O, Tajeddine N, Kroemer G (2008). Disruption of the hexokinase‐VDAC complex for tumor therapy. Oncogene 27: 4633–4635. [DOI] [PubMed] [Google Scholar]
- Garlid KD, Costa AD, Quinlan CL, Pierre SV, Dos Santos P (2009). Cardioprotective signaling to mitochondria. J Mol Cell Cardiol 46: 858–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Perez AI, Galeano E, Nieto E, Sancho P (2011). Dequalinium induces human leukemia cell death by affecting the redox balance. Leuk Res 35: 1395–1401. [DOI] [PubMed] [Google Scholar]
- Gattin Z, Schneider R, Laukat Y, Giller K, Maier E, Zweckstetter M et al (2015). Solid‐state NMR, electrophysiology and molecular dynamics characterization of human VDAC2. J Biomol NMR 61: 311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko O, Gerasimenko J (2004). New aspects of nuclear calcium signalling. J Cell Sci 117: 3087–3094. [DOI] [PubMed] [Google Scholar]
- Gincel D, Zaid H, Shoshan‐Barmatz V (2001). Calcium binding and translocation by the voltage‐dependent anion channel: a possible regulatory mechanism in mitochondrial function. Biochem J 358: 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio V, Burchell V, Schiavone M, Bassot C, Minervini G, Petronilli V et al (2017). Ca(2+) binding to F‐ATP synthase β subunit triggers the mitochondrial permeability transition. EMBO Rep 18: 1065–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio V, Guo L, Bassot C, Petronilli V, Bernardi P (2018). Calcium and regulation of the mitochondrial permeability transition. Cell Calcium 70: 56–63. [DOI] [PubMed] [Google Scholar]
- Giorgio V, von Stockum S, Antoniel M, Fabbro A, Fogolari F, Forte M et al (2013). Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc Natl Acad Sci U S A 110: 5887–5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogvadze V, Stridh H, Orrenius S, Cotgreave I (2002). Tributyltin causes cytochrome C release from isolated mitochondria by two discrete mechanisms. Biochem Biophys Res Commun 292: 904–908. [DOI] [PubMed] [Google Scholar]
- Goldin N, Arzoine L, Heyfets A, Israelson A, Zaslavsky Z, Bravman T et al (2008). Methyl jasmonate binds to and detaches mitochondria‐bound hexokinase. Oncogene 27: 4636–4643. [DOI] [PubMed] [Google Scholar]
- Gorlach A, Bertram K, Hudecova S, Krizanova O (2015). Calcium and ROS: a mutual interplay. Redox Biol 6: 260–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grgic I, Eichler I, Heinau P, Si H, Brakemeier S, Hoyer J et al (2005). Selective blockade of the intermediate‐conductance Ca2+‐activated K+ channel suppresses proliferation of microvascular and macrovascular endothelial cells and angiogenesis in vivo. Arterioscler Thromb Vasc Biol 25: 704–709. [DOI] [PubMed] [Google Scholar]
- Griffiths EJ, Halestrap AP (1995). Mitochondrial non‐specific pores remain closed during cardiac ischaemia, but open upon reperfusion. Biochem J 307 (Pt 1): 93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti G, Brambilla L, Rossi D (2017). Cell‐penetrating peptides: from basic research to clinics. Trends Pharmacol Sci 38: 406–424. [DOI] [PubMed] [Google Scholar]
- Gururaja Rao S, Ponnalagu D, Sukur S, Singh H, Sanghvi S, Mei Y et al (2017). Identification and characterization of a bacterial homolog of chloride intracellular channel (CLIC) protein. Sci Rep 7: 8500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman‐Villanueva D, Weissig V (2017). Mitochondria‐targeted agents: mitochondriotropics, mitochondriotoxics, and mitocans. Handb Exp Pharmacol 240: 423–438. [DOI] [PubMed] [Google Scholar]
- Hajnoczky G, Csordas G, Yi M (2002). Old players in a new role: mitochondria‐associated membranes, VDAC, and ryanodine receptors as contributors to calcium signal propagation from endoplasmic reticulum to the mitochondria. Cell Calcium 32: 363–377. [DOI] [PubMed] [Google Scholar]
- Hall DD, Wu Y, Domann FE, Spitz DR, Anderson ME (2014). Mitochondrial calcium uniporter activity is dispensable for MDA‐MB‐231 breast carcinoma cell survival. PloS one 9: e96866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al (2018). The IUPHAR/BPS guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haridas V, Li X, Mizumachi T, Higuchi M, Lemeshko VV, Colombini M et al (2007). Avicins, a novel plant‐derived metabolite lowers energy metabolism in tumor cells by targeting the outer mitochondrial membrane. Mitochondrion 7: 234–240. [DOI] [PubMed] [Google Scholar]
- Haworth RA, Hunter DR (1979). The Ca2+‐induced membrane transition in mitochondria. II. Nature of the Ca2+ trigger site. Arch Biochem Biophys 195: 460–467. [DOI] [PubMed] [Google Scholar]
- He J, Ford HC, Carroll J, Ding S, Fearnley IM, Walker JE (2017). Persistence of the mitochondrial permeability transition in the absence of subunit c of human ATP synthase. Proc Natl Acad Sci U S A 114: 3409–3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller A, Brockhoff G, Goepferich A (2012). Targeting drugs to mitochondria. Eur J Pharm Biopharm 82: 1–18. [DOI] [PubMed] [Google Scholar]
- Hollander JM, Thapa D, Shepherd DL (2014). Physiological and structural differences in spatially distinct subpopulations of cardiac mitochondria: influence of cardiac pathologies. Am J Physiol Heart Circ Physiol 307: H1–H14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honrath B, Krabbendam IE, Culmsee C, Dolga AM (2017a). Small conductance Ca(2+)‐activated K(+) channels in the plasma membrane, mitochondria and the ER: Pharmacology and implications in neuronal diseases. Neurochem Int 109: 13–23. [DOI] [PubMed] [Google Scholar]
- Honrath B, Matschke L, Meyer T, Magerhans L, Perocchi F, Ganjam GK et al (2017b). SK2 channels regulate mitochondrial respiration and mitochondrial Ca(2+) uptake. Cell Death Differ 24: 761–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horobin RW, Trapp S, Weissig V (2007). Mitochondriotropics: a review of their mode of action, and their applications for drug and DNA delivery to mammalian mitochondria. J Control Release 121: 125–136. [DOI] [PubMed] [Google Scholar]
- Hou JT, Yang J, Li K, Liao YX, Yu KK, Xie YM et al (2014). A highly selective water‐soluble optical probe for endogenous peroxynitrite. Chem Comm (Cambridge, England) 50: 9947–9950. [DOI] [PubMed] [Google Scholar]
- Hoye AT, Davoren JE, Wipf P, Fink MP, Kagan VE (2008). Targeting mitochondria. Acc Chem Res 41: 87–97. [DOI] [PubMed] [Google Scholar]
- Huang G, Vercesi AE, Docampo R (2013). Essential regulation of cell bioenergetics in Trypanosoma brucei by the mitochondrial calcium uniporter. Nat Commun 4: 2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Han J, Ben‐Hail D, He L, Li B, Chen Z et al (2015). A new fungal diterpene induces VDAC1‐dependent apoptosis in Bax/Bak‐deficient cells. J Biol Chem 290: 23563–23578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Chen W, Kuang YQ, Liu W, Liu XJ, Tang LJ et al (2017). A novel off‐on fluorescent probe for sensitive imaging of mitochondria‐specific nitroreductase activity in living tumor cells. Org Biomol Chem 15: 4383–4389. [DOI] [PubMed] [Google Scholar]
- Ilyich T, Charishnikova O, Sekowski S, Zamaraeva M, Cheshchevik V, Dremza I et al (2018). Ferutinin induces membrane depolarization, permeability transition pore formation, and respiration uncoupling in isolated rat liver mitochondria by stimulation of Ca(2+)‐permeability. J Membr Biol; 10.1007/s00232-018-0032-0. [DOI] [PubMed] [Google Scholar]
- Inoue I, Nagase H, Kishi K, Higuti T (1991). ATP‐sensitive K+ channel in the mitochondrial inner membrane. Nature 352: 244–247. [DOI] [PubMed] [Google Scholar]
- Jakob C, Gotz H, Hellmann T, Hellmann KP, Reymann S, Florke H et al (1995). Studies on human porin: XIII. The type‐1 VDAC ‘porin 31HL' biotinylated at the plasmalemma of trypan blue excluding human B lymphocytes. FEBS Lett 368: 5–9. [DOI] [PubMed] [Google Scholar]
- James AM, Sharpley MS, Manas AR, Frerman FE, Hirst J, Smith RA et al (2007). Interaction of the mitochondria‐targeted antioxidant MitoQ with phospholipid bilayers and ubiquinone oxidoreductases. J Biol Chem 282: 14708–14718. [DOI] [PubMed] [Google Scholar]
- Jang SH, Byun JK, Jeon WI, Choi SY, Park J, Lee BH et al (2015). Nuclear localization and functional characteristics of voltage‐gated potassium channel Kv1.3. J Biol Chem 290: 12547–12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javadov S, Karmazyn M (2007). Mitochondrial permeability transition pore opening as an endpoint to initiate cell death and as a putative target for cardioprotection. Cell Physiol Biochem 20: 1–22. [DOI] [PubMed] [Google Scholar]
- Jean SR, Ahmed M, Lei EK, Wisnovsky SP, Kelley SO (2016). Peptide‐mediated delivery of chemical probes and therapeutics to mitochondria. Acc Chem Res 49: 1893–1902. [DOI] [PubMed] [Google Scholar]
- Jhun BS, Mishra J, Monaco S, Fu D, Jiang W, Sheu SS et al (2016). The mitochondrial Ca2+ uniporter: regulation by auxiliary subunits and signal transduction pathways. Am J Physiol Cell Physiol 311: C67–C80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Martel C, Belzacq‐Casagrande AS, Brenner C, Howl J (2008). Mitoparan and target‐selective chimeric analogues: membrane translocation and intracellular redistribution induces mitochondrial apoptosis. Biochim Biophys Acta 1783: 849–863. [DOI] [PubMed] [Google Scholar]
- Jurgens L, Kleineke J, Brdiczka D, Thinnes FP, Hilschmann N (1995). Localization of type‐1 porin channel (VDAC) in the sarcoplasmatic reticulum. Biol Chem Hoppe Seyler 376: 685–689. [PubMed] [Google Scholar]
- Kajma A, Szewczyk A (2012). A new pH‐sensitive rectifying potassium channel in mitochondria from the embryonic rat hippocampus. Biochim Biophys Acta 1817: 1867–1878. [DOI] [PubMed] [Google Scholar]
- Kalafatovic D, Giralt E (2017). Cell‐penetrating peptides: design strategies beyond primary structure and amphipathicity. Molecules 22: pii: E1929. 10.3390/molecules22111929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalani K, Yan SF, Yan SS ( 2018). Mitochondrial permeability transition pore: a potential drug target for neurodegeneration. Drug Discov Today 23: 1983–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal A, Faazil S, Malik MS (2014). Apoptosis‐inducing agents: a patent review (2010‐2013). Expert Opin Ther Pat 24: 339–354. [DOI] [PubMed] [Google Scholar]
- Kameyama M, Kakei M, Sato R, Shibasaki T, Matsuda H, Irisawa H (1984). Intracellular Na+ activates a K+ channel in mammalian cardiac cells. Nature 309: 354–356. [DOI] [PubMed] [Google Scholar]
- Kanai A, Zabbarova I, Amoscato A, Epperly M, Xiao J, Wipf P (2007). Mitochondrial targeting of radioprotectants using peptidyl conjugates. Org Biomol Chem 5: 307–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania E, Roest G, Vervliet T, Parys JB, Bultynck G (2017). IP3 receptor‐mediated calcium signaling and its role in autophagy in cancer. Front Oncol 7: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasumov T, Dabkowski ER, Shekar KC, Li L, Ribeiro RF Jr, Walsh K et al (2013). Assessment of cardiac proteome dynamics with heavy water: slower protein synthesis rates in interfibrillar than subsarcolemmal mitochondria. Am J Physiol Heart Circ Physiol 304: H1201–H1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura E, Yamada Y, Yasuzaki Y, Hyodo M, Harashima H (2013). Intracellular observation of nanocarriers modified with a mitochondrial targeting signal peptide. J Biosci Bioeng 116: 634–637. [DOI] [PubMed] [Google Scholar]
- Keinan N, Tyomkin D, Shoshan‐Barmatz V (2010). Oligomerization of the mitochondrial protein voltage‐dependent anion channel is coupled to the induction of apoptosis. Mol Cell Biol 30: 5698–5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilisch M, Lytovchenko O, Schwappach B, Renigunta V, Daut J (2015). The role of protein‐protein interactions in the intracellular traffic of the potassium channels TASK‐1 and TASK‐3. Pflugers Arch 467: 1105–1120. [DOI] [PubMed] [Google Scholar]
- Kirichok Y, Krapivinsky G, Clapham DE (2004). The mitochondrial calcium uniporter is a highly selective ion channel. Nature 427: 360–364. [DOI] [PubMed] [Google Scholar]
- Klasa RJ, Gillum AM, Klem RE, Frankel SR (2002). Oblimersen Bcl‐2 antisense: facilitating apoptosis in anticancer treatment. Antisense Nucleic Acid Drug Dev 12: 193–213. [DOI] [PubMed] [Google Scholar]
- Kon N, Murakoshi M, Isobe A, Kagechika K, Miyoshi N, Nagayama T (2017). DS16570511 is a small‐molecule inhibitor of the mitochondrial calcium uniporter. Cell death Discov 3: 17045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalenko I, Glasauer A, Schockel L, Sauter DR, Ehrmann A, Sohler F et al (2016). Identification of KCa3.1 channel as a novel regulator of oxidative phosphorylation in a subset of pancreatic carcinoma cell lines. PloS one 11: e0160658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krabbendam IE, Honrath B, Culmsee C, Dolga AM (2018). Mitochondrial Ca(2+)‐activated K(+) channels and their role in cell life and death pathways. Cell Calcium 69: 101–111. [DOI] [PubMed] [Google Scholar]
- Krasnov GS, Dmitriev AA, Lakunina VA, Kirpiy AA, Kudryavtseva AV (2013). Targeting VDAC‐bound hexokinase II: a promising approach for concomitant anti‐cancer therapy. Expert Opin Ther Targets 17: 1221–1233. [DOI] [PubMed] [Google Scholar]
- Kruger V, Becker T, Becker L, Montilla‐Martinez M, Ellenrieder L, Vogtle FN et al (2017). Identification of new channels by systematic analysis of the mitochondrial outer membrane. J Cell Biol 216: 3485–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kshatri AS, Gonzalez‐Hernandez A, Giraldez T (2018). Physiological roles and therapeutic potential of Ca(2+) activated potassium channels in the nervous system. Front Mol Neurosci 11: 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuum M, Veksler V, Kaasik A (2015). Potassium fluxes across the endoplasmic reticulum and their role in endoplasmic reticulum calcium homeostasis. Cell Calcium 58: 79–85. [DOI] [PubMed] [Google Scholar]
- Kuum M, Veksler V, Liiv J, Ventura‐Clapier R, Kaasik A (2012). Endoplasmic reticulum potassium‐hydrogen exchanger and small conductance calcium‐activated potassium channel activities are essential for ER calcium uptake in neurons and cardiomyocytes. J Cell Sci 125: 625–633. [DOI] [PubMed] [Google Scholar]
- Laskowski M, Augustynek B, Kulawiak B, Koprowski P, Bednarczyk P, Jarmuszkiewicz W et al (2016). What do we not know about mitochondrial potassium channels? Biochim Biophys Acta 1857: 1247–1257. [DOI] [PubMed] [Google Scholar]
- Laszczyk MN (2009). Pentacyclic triterpenes of the lupane, oleanane and ursane group as tools in cancer therapy. Planta Med 75: 1549–1560. [DOI] [PubMed] [Google Scholar]
- Latorre R, Castillo K, Carrasquel‐Ursulaez W, Sepulveda RV, Gonzalez‐Nilo F, Gonzalez C et al (2017). Molecular determinants of BK channel functional diversity and functioning. Physiol Rev 97: 39–87. [DOI] [PubMed] [Google Scholar]
- Lauterwasser J, Todt F, Zerbes RM, Nguyen TN, Craigen W, Lazarou M et al (2016). The porin VDAC2 is the mitochondrial platform for Bax retrotranslocation. Sci Rep 6: 32994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leanza L, Biasutto L, Manago A, Gulbins E, Zoratti M, Szabo I (2013a). Intracellular ion channels and cancer. Front Physiol 4: 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leanza L, Henry B, Sassi N, Zoratti M, Chandy KG, Gulbins E et al (2012a). Inhibitors of mitochondrial Kv1.3 channels induce Bax/Bak‐independent death of cancer cells. EMBO Mol Med 4: 577–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leanza L, Manago A, Zoratti M, Gulbins E, Szabo I (2015). Pharmacological targeting of ion channels for cancer therapy: in vivo evidences. Biochim Biophys Acta 1863: 1385–1397. [DOI] [PubMed] [Google Scholar]
- Leanza L, O'Reilly P, Doyle A, Venturini E, Zoratti M, Szegezdi E et al (2014a). Correlation between potassium channel expression and sensitivity to drug‐induced cell death in tumor cell lines. Curr Pharm Des 20: 189–200. [DOI] [PubMed] [Google Scholar]
- Leanza L, Romio M, Becker KA, Azzolini M, Trentin L, Manago A et al (2017). Direct pharmacological targeting of a mitochondrial ion channel selectively kills tumor cells in vivo. Cancer Cell 31: 516–531.e510. [DOI] [PubMed] [Google Scholar]
- Leanza L, Trentin L, Becker KA, Frezzato F, Zoratti M, Semenzato G et al (2013b). Clofazimine, Psora‐4 and PAP‐1, inhibitors of the potassium channel Kv1.3, as a new and selective therapeutic strategy in chronic lymphocytic leukemia. Leukemia 27: 1782–1785. [DOI] [PubMed] [Google Scholar]
- Leanza L, Zoratti M, Gulbins E, Szabo I (2012b). Induction of apoptosis in macrophages via Kv1.3 and Kv1.5 potassium channels. Curr Med Chem 19: 5394–5404. [DOI] [PubMed] [Google Scholar]
- Leanza L, Zoratti M, Gulbins E, Szabo I (2014b). Mitochondrial ion channels as oncological targets. Oncogene 33: 5569–5581. [DOI] [PubMed] [Google Scholar]
- LeCher JC, Nowak SJ, McMurry JL (2017). Breaking in and busting out: cell‐penetrating peptides and the endosomal escape problem. Biomol Concepts 8: 131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefer DJ, Nichols CG, Coetzee WA (2009). Sulfonylurea receptor 1 subunits of ATP‐sensitive potassium channels and myocardial ischemia/reperfusion injury. Trends Cardiovasc Med 19: 61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lena A, Rechichi M, Salvetti A, Bartoli B, Vecchio D, Scarcelli V et al (2009). Drugs targeting the mitochondrial pore act as cytotoxic and cytostatic agents in temozolomide‐resistant glioma cells. J Transl Med 7: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Jie W, Huang L, Wei P, Li S, Luo Z et al (2014). Nuclear BK channels regulate gene expression via the control of nuclear calcium signaling. Nat Neurosci 17: 1055–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Qiao P, Chen X, Wang J, Bian L, Zheng X (2017). Affinity chromatographic methodologies based on immobilized voltage dependent anion channel isoform 1 and application in protein‐ligand interaction analysis and bioactive compounds screening from traditional medicine. J Chromatogr A 1495: 31–45. [DOI] [PubMed] [Google Scholar]
- Liberman EA, Topaly VP, Tsofina LM, Jasaitis AA, Skulachev VP (1969). Mechanism of coupling of oxidative phosphorylation and the membrane potential of mitochondria. Nature 222: 1076–1078. [DOI] [PubMed] [Google Scholar]
- Logan CV, Szabadkai G, Sharpe JA, Parry DA, Torelli S, Childs AM et al (2014). Loss‐of‐function mutations in MICU1 cause a brain and muscle disorder linked to primary alterations in mitochondrial calcium signaling. Nat Genet 46: 188–193. [DOI] [PubMed] [Google Scholar]
- Lu CC, Huang BR, Liao PJ, Yen GC (2014). Ursolic acid triggers nonprogrammed death (necrosis) in human glioblastoma multiforme DBTRG‐05MG cells through MPT pore opening and ATP decline. Mol Nutr Food Res 58: 2146–2156. [DOI] [PubMed] [Google Scholar]
- Lukasiak A, Skup A, Chlopicki S, Lomnicka M, Kaczara P, Proniewski B et al (2016). SERCA, complex I of the respiratory chain and ATP‐synthase inhibition are involved in pleiotropic effects of NS1619 on endothelial cells. Eur J Pharmacol 786: 137–147. [DOI] [PubMed] [Google Scholar]
- Ma X, Gong N, Zhong L, Sun J, Liang XJ (2016). Future of nanotherapeutics: targeting the cellular sub‐organelles. Biomaterials 97: 10–21. [DOI] [PubMed] [Google Scholar]
- Ma D, Nakata T, Zhang G, Hoshi T, Li M, Shikano S (2007). Differential trafficking of carboxyl isoforms of Ca2+‐gated (Slo1) potassium channels. FEBS Lett 581: 1000–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madamba SM, Damri KN, Dejean LM, Peixoto PM (2015). Mitochondrial ion channels in cancer transformation. Front Oncol 5: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magri A, Messina A (2017). Interactions of VDAC with proteins involved in neurodegenerative aggregation: an opportunity for advancement on therapeutic molecules. Curr Med Chem 24: 4470–4487. [DOI] [PubMed] [Google Scholar]
- Magri A, Reina S, De Pinto V (2018). VDAC1 as pharmacological target in cancer and neurodegeneration: focus on its role in apoptosis. Front Chem 6: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiti KK, Lee WS, Takeuchi T, Watkins C, Fretz M, Kim DC et al (2007). Guanidine‐containing molecular transporters: sorbitol‐based transporters show high intracellular selectivity toward mitochondria. Angew Chem Int Ed Engl 46: 5880–5884. [DOI] [PubMed] [Google Scholar]
- Mak DO, Foskett JK (1994). Single‐channel inositol 1,4,5‐trisphosphate receptor currents revealed by patch clamp of isolated Xenopus oocyte nuclei. J Biol Chem 269: 29375–29378. [PubMed] [Google Scholar]
- Mak DO, Vais H, Cheung KH, Foskett JK (2013). Patch‐clamp electrophysiology of intracellular Ca2+ channels. Cold Spring Harb Protoc 2013: 787–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik‐Hall M, Ganellin CR, Galanakis D, Jenkinson DH (2000). Compounds that block both intermediate‐conductance (IK (Ca)) and small‐conductance (SK (Ca)) calcium‐activated potassium channels. Br J Pharmacol 129: 1431–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammucari C, Gherardi G, Zamparo I, Raffaello A, Boncompagni S, Chemello F et al (2015). The mitochondrial calcium uniporter controls skeletal muscle trophism in vivo. Cell Rep 10: 1269–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammucari C, Raffaello A, Vecellio Reane D, Gherardi G, De Mario A, Rizzuto R (2018). Mitochondrial calcium uptake in organ physiology: from molecular mechanism to animal models. Pflugers Archiv: European journal of physiology; 10.1007/s00424-018-2123-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manago A, Leanza L, Carraretto L, Sassi N, Grancara S, Quintana‐Cabrera R et al (2015). Early effects of the antineoplastic agent salinomycin on mitochondrial function. Cell Death Dis 6: e1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marius P, Guerra MT, Nathanson MH, Ehrlich BE, Leite MF (2006). Calcium release from ryanodine receptors in the nucleoplasmic reticulum. Cell Calcium 39: 65–73. [DOI] [PubMed] [Google Scholar]
- Marrache S, Pathak RK, & Dhar S (2015). Formulation and optimization of mitochondria‐targeted polymeric nanoparticles. Methods in molecular biology (Clifton, NJ) 1265: 103‐112. [DOI] [PubMed]
- Martelli A, Testai L, Breschi MC, Lawson K, McKay NG, Miceli F et al (2013). Vasorelaxation by hydrogen sulphide involves activation of Kv7 potassium channels. Pharmacol Res 70: 27–34. [DOI] [PubMed] [Google Scholar]
- Masgras I, Sanchez‐Martin C, Colombo G, Rasola A (2017). The chaperone TRAP1 as a modulator of the mitochondrial adaptations in cancer cells. Front Oncol 7: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massi P, Solinas M, Cinquina V, Parolaro D (2013). Cannabidiol as potential anticancer drug. Br J Clin Pharmacol 75: 303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matschke LA, Rinne S, Snutch TP, Oertel WH, Dolga AM, Decher N (2018). Calcium‐activated SK potassium channels are key modulators of the pacemaker frequency in locus coeruleus neurons. Mol Cell Neurosci 88: 330–341. [DOI] [PubMed] [Google Scholar]
- Mattarei A, Romio M, Manago A, Zoratti M, Paradisi C, Szabo I et al (2018). Novel mitochondria‐targeted furocoumarin derivatives as possible anti‐cancer agents. Front Oncol 8: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrleitner M, Schafer R, Fleischer S (1995). IP3 receptor purified from liver plasma membrane is an (1,4,5)IP3 activated and (1,3,4,5)IP4 inhibited calcium permeable ion channel. Cell Calcium 17: 141–153. [DOI] [PubMed] [Google Scholar]
- Mazure NM (2017). VDAC in cancer. Biochim Biophys Acta 1858: 665–673. [DOI] [PubMed] [Google Scholar]
- McQuaker SJ, Quinlan CL, Caldwell ST, Brand MD, Hartley RC (2013). A prototypical small‐molecule modulator uncouples mitochondria in response to endogenous hydrogen peroxide production. Chembiochem: a European journal of chemical biology 14: 993–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina A, Reina S, Guarino F, De Pinto V (2012). VDAC isoforms in mammals. Biochim Biophys Acta 1818: 1466–1476. [DOI] [PubMed] [Google Scholar]
- Miceli F, Soldovieri MV, Martire M, Taglialatela M (2008). Molecular pharmacology and therapeutic potential of neuronal Kv7‐modulating drugs. Curr Opin Pharmacol 8: 65–74. [DOI] [PubMed] [Google Scholar]
- Milane L, Trivedi M, Singh A, Talekar M, Amiji M (2015). Mitochondrial biology, targets, and drug delivery. J Control Release 207: 40–58. [DOI] [PubMed] [Google Scholar]
- Milanesi E, Costantini P, Gambalunga A, Colonna R, Petronilli V, Cabrelle A et al (2006). The mitochondrial effects of small organic ligands of BCL‐2: sensitization of BCL‐2‐overexpressing cells to apoptosis by a pyrimidine‐2,4,6‐trione derivative. J Biol Chem 281: 10066–10072. [DOI] [PubMed] [Google Scholar]
- Miura T, Tanno M (2012). The mPTP and its regulatory proteins: final common targets of signalling pathways for protection against necrosis. Cardiovasc Res 94: 181–189. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Murphy AN, Brown JH (2008). Akt mediates mitochondrial protection in cardiomyocytes through phosphorylation of mitochondrial hexokinase‐II. Cell Death Differ 15: 521–529. [DOI] [PubMed] [Google Scholar]
- Mizoguchi Y, Monji A (2017). Microglial intracellular Ca(2+) signaling in synaptic development and its alterations in neurodevelopmental disorders. Front Cell Neurosci 11: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CL (1971). Specific inhibition of mitochondrial Ca++ transport by ruthenium red. Biochem Biophys Res Commun 42: 298–305. [DOI] [PubMed] [Google Scholar]
- Motloch LJ, Larbig R, Gebing T, Reda S, Schwaiger A, Leitner J et al (2016). By regulating mitochondrial Ca2+‐uptake UCP2 modulates intracellular Ca2+. PloS one 11: e0148359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu D, Chen L, Zhang X, See LH, Koch CM, Yen C et al (2003). Genomic amplification and oncogenic properties of the KCNK9 potassium channel gene. Cancer Cell 3: 297–302. [DOI] [PubMed] [Google Scholar]
- Murai M, Okuda A, Yamamoto T, Shinohara Y, Miyoshi H (2017). Synthetic ubiquinones specifically bind to mitochondrial voltage‐dependent anion channel 1 (VDAC1) in saccharomyces cerevisiae mitochondria. Biochemistry 56: 570–581. [DOI] [PubMed] [Google Scholar]
- Murphy MP (2008). Targeting lipophilic cations to mitochondria. Biochim Biophys Acta 1777: 1028–1031. [DOI] [PubMed] [Google Scholar]
- Murphy MP, Smith RA (2007). Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu Rev Pharmacol Toxicol 47: 629–656. [DOI] [PubMed] [Google Scholar]
- Nagy D, Gonczi M, Dienes B, Szoor A, Fodor J, Nagy Z et al (2014). Silencing the KCNK9 potassium channel (TASK‐3) gene disturbs mitochondrial function, causes mitochondrial depolarization, and induces apoptosis of human melanoma cells. Arch Dermatol Res 306: 885–902. [DOI] [PubMed] [Google Scholar]
- Nahon E, Israelson A, Abu‐Hamad S, Varda SB (2005). Fluoxetine (Prozac) interaction with the mitochondrial voltage‐dependent anion channel and protection against apoptotic cell death. FEBS Lett 579: 5105–5110. [DOI] [PubMed] [Google Scholar]
- Nakashima RA (1989). Hexokinase‐binding properties of the mitochondrial VDAC protein: inhibition by DCCD and location of putative DCCD‐binding sites. J Bioenerg Biomembr 21: 461–470. [DOI] [PubMed] [Google Scholar]
- Nguyen TT, Stevens MV, Kohr M, Steenbergen C, Sack MN, Murphy E (2011). Cysteine 203 of cyclophilin D is critical for cyclophilin D activation of the mitochondrial permeability transition pore. J Biol Chem 286: 40184–40192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen NX, Armache JP, Lee C, Yang Y, Zeng W, Mootha VK et al (2018). Cryo‐EM structure of a fungal mitochondrial calcium uniporter. Nature 559: 570–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngwa VM, Axford DS, Healey AN, Nowak SJ, Chrestensen CA, McMurry JL (2017). A versatile cell‐penetrating peptide‐adaptor system for efficient delivery of molecular cargos to subcellular destinations. PLoS One 12: e0178648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma A (1983). ATP‐regulated K+ channels in cardiac muscle. Nature 305: 147–148. [DOI] [PubMed] [Google Scholar]
- Noriega‐Navarro R, Lopez‐Charcas O, Hernandez‐Enriquez B, Reyes‐Gutierrez PE, Martinez R, Landa A et al (2014). Novel TASK channels inhibitors derived from dihydropyrrolo[2,1‐a]isoquinoline. Neuropharmacology 79: 28–36. [DOI] [PubMed] [Google Scholar]
- Novarino G, Fabrizi C, Tonini R, Denti MA, Malchiodi‐Albedi F, Lauro GM et al (2004). Involvement of the intracellular ion channel CLIC1 in microglia‐mediated β‐amyloid‐induced neurotoxicity. The Journal of neuroscience: the official journal of the Society for Neuroscience 24: 5322–5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rourke B (2007). Mitochondrial ion channels. Annu Rev Physiol 69: 19–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohya S, Kito H (2018). Ca(2+)‐activated K(+) channel KCa3.1 as a therapeutic target for immune disorders. Biol Pharm Bull 41: 1158–1163. [DOI] [PubMed] [Google Scholar]
- Oxenoid K, Dong Y, Cao C, Cui T, Sancak Y, Markhard AL et al (2016). Architecture of the mitochondrial calcium uniporter. Nature 533: 269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozsvari B, Sotgia F, Lisanti MP (2018). Exploiting mitochondrial targeting signal(s), TPP and bis‐TPP, for eradicating cancer stem cells (CSCs). Aging (Albany NY) 10: 229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliarani A, Nesci S, Ventrella V (2016). Novel drugs targeting the c‐ring of the F1FO‐ATP synthase. Mini Rev Med Chem 16: 815–824. [DOI] [PubMed] [Google Scholar]
- Pagliarini DJ, Calvo SE, Chang B, Sheth SA, Vafai SB, Ong S‐E et al (2008). A mitochondrial protein compendium elucidates complex I disease biology. Cell 134: 112–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paleos CM, Tsiourvas D, Sideratou Z (2016). Triphenylphosphonium decorated liposomes and dendritic polymers: prospective second generation drug delivery systems for targeting mitochondria. Mol Pharm 13: 2233–2241. [DOI] [PubMed] [Google Scholar]
- Pardo LA, Stuhmer W (2014). The roles of K(+) channels in cancer. Nat Rev Cancer 14: 39–48. [DOI] [PubMed] [Google Scholar]
- Park I, Londhe AM, Lim JW, Park BG, Jung SY, Lee JY et al (2017). Discovery of non‐peptidic small molecule inhibitors of cyclophilin D as neuroprotective agents in Aβ‐induced mitochondrial dysfunction. J Comput Aided Mol Des 31: 929–941. [DOI] [PubMed] [Google Scholar]
- Patil SP (2013). FOLICation: engineering approved drugs as potential p53‐MDM2 interaction inhibitors for cancer therapy. Med Hypotheses 81: 1104–1107. [DOI] [PubMed] [Google Scholar]
- Patron M, Checchetto V, Raffaello A, Teardo E, Vecellio Reane D, Mantoan M et al (2014). MICU1 and MICU2 finely tune the mitochondrial Ca2+ uniporter by exerting opposite effects on MCU activity. Mol Cell 53: 726–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavon N, Correa F, Buelna‐Chontal M, Hernandez‐Esquivel L, Chavez E (2015). Ebselen induces mitochondrial permeability transition because of its interaction with adenine nucleotide translocase. Life Sci 139: 108–113. [DOI] [PubMed] [Google Scholar]
- Peretti M, Angelini M, Savalli N, Florio T, Yuspa SH, Mazzanti M (2015). Chloride channels in cancer: focus on chloride intracellular channel 1 and 4 (CLIC1 AND CLIC4) proteins in tumor development and as novel therapeutic targets. Biochim Biophys Acta 1848: 2523–2531. [DOI] [PubMed] [Google Scholar]
- Perez‐Verdaguer M, Capera J, Serrano‐Novillo C, Estadella I, Sastre D, Felipe A (2016). The voltage‐gated potassium channel Kv1.3 is a promising multitherapeutic target against human pathologies. Expert Opin Ther Targets 20: 577–591. [DOI] [PubMed] [Google Scholar]
- Perocchi F, Gohil VM, Girgis HS, Bao XR, McCombs JE, Palmer AE et al (2010). MICU1 encodes a mitochondrial EF hand protein required for Ca(2+) uptake. Nature 467: 291–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peruzzo R, Biasutto L, Szabo I, Leanza L (2016). Impact of intracellular ion channels on cancer development and progression. Eur Biophy J: EBJ 45: 685–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peruzzo R, Mattarei A, Romio M, Paradisi C, Zoratti M, Szabo I et al (2017). Regulation of proliferation by a mitochondrial potassium channel in pancreatic ductal adenocarcinoma cells. Front Oncol 7: 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrungaro C, Zimmermann KM, Kuttner V, Fischer M, Dengjel J, Bogeski I et al (2015). The Ca(2+)‐dependent release of the Mia40‐induced MICU1‐MICU2 dimer from MCU regulates mitochondrial Ca(2+) uptake. Cell Metab 22: 721–733. [DOI] [PubMed] [Google Scholar]
- Pittala S, Krelin Y, Shoshan‐Barmatz V (2018). Targeting liver cancer and associated pathologies in mice with a mitochondrial VDAC1‐based peptide. Neoplasia (New York, NY) 20: 594–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plovanich M, Bogorad RL, Sancak Y, Kamer KJ, Strittmatter L, Li AA et al (2013). MICU2, a paralog of MICU1, resides within the mitochondrial uniporter complex to regulate calcium handling. PloS one 8: e55785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocsai K, Kosztka L, Bakondi G, Gonczi M, Fodor J, Dienes B et al (2006). Melanoma cells exhibit strong intracellular TASK‐3‐specific immunopositivity in both tissue sections and cell culture. Cell Mol Life Sci 63: 2364–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnalagu D, Gururaja Rao S, Farber J, Xin W, Hussain AT, Shah K et al (2016a). Molecular identity of cardiac mitochondrial chloride intracellular channel proteins. Mitochondrion 27: 6–14. [DOI] [PubMed] [Google Scholar]
- Ponnalagu D, Rao SG, Farber J, Xin W, Hussain AT, Shah K et al (2016b). Data supporting characterization of CLIC1, CLIC4, CLIC5 and DmCLIC antibodies and localization of CLICs in endoplasmic reticulum of cardiomyocytes. Data Brief 7: 1038–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnalagu D, Singh H (2017). Anion channels of mitochondria. Handb Exp Pharmacol 240: 71–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potze L, Di Franco S, Grandela C, Pras‐Raves ML, Picavet DI, van Veen HA et al (2015). Betulinic acid induces a novel cell death pathway that depends on cardiolipin modification. Oncogene 35: 427–437. [DOI] [PubMed] [Google Scholar]
- Prezma T, Shteinfer A, Admoni L, Raviv Z, Sela I, Levi I et al (2013). VDAC1‐based peptides: novel pro‐apoptotic agents and potential therapeutics for B‐cell chronic lymphocytic leukemia. Cell Death Dis 4: e809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudent J, Popgeorgiev N, Bonneau B, Thibaut J, Gadet R, Lopez J et al (2013). Bcl‐wav and the mitochondrial calcium uniporter drive gastrula morphogenesis in zebrafish. Nat Commun 4: 2330. [DOI] [PubMed] [Google Scholar]
- Qin LS, Jia PF, Zhang ZQ, Zhang SM (2015). ROS‐p53‐cyclophilin‐D signaling mediates salinomycin‐induced glioma cell necrosis. J Exp Clin Cancer Res: CR 34: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Yu T, Wang W, Pan K, Shi D, Sun H (2014). Curcumin‐induced melanoma cell death is associated with mitochondrial permeability transition pore (mPTP) opening. Biochem Biophys Res Commun 448: 15–21. [DOI] [PubMed] [Google Scholar]
- Quesada I, Rovira JM, Martin F, Roche E, Nadal A, Soria B (2002). Nuclear KATP channels trigger nuclear Ca(2+) transients that modulate nuclear function. Proc Natl Acad Sci U S A 99: 9544–9549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaello A, De Stefani D, Sabbadin D, Teardo E, Merli G, Picard A et al (2013). The mitochondrial calcium uniporter is a multimer that can include a dominant‐negative pore‐forming subunit. EMBO J 32: 2362–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao VK, Carlson EA, Yan SS (2014). Mitochondrial permeability transition pore is a potential drug target for neurodegeneration. Biochim Biophys Acta 1842: 1267–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapizzi E, Pinton P, Szabadkai G, Wieckowski MR, Vandecasteele G, Baird G et al (2002). Recombinant expression of the voltage‐dependent anion channel enhances the transfer of Ca2+ microdomains to mitochondria. J Cell Biol 159: 613–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasola A (2017). HSP90 proteins in the scenario of tumor complexity. Oncotarget 8: 20521–20522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasola A, Bernardi P (2014). The mitochondrial permeability transition pore and its adaptive responses in tumor cells. Cell Calcium 56: 437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasola A, Sciacovelli M, Chiara F, Pantic B, Brusilow WS, Bernardi P (2010a). Activation of mitochondrial ERK protects cancer cells from death through inhibition of the permeability transition. Proc Natl Acad Sci U S A 107: 726–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasola A, Sciacovelli M, Pantic B, Bernardi P (2010b). Signal transduction to the permeability transition pore. FEBS Lett 584: 1989–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed KC, Bygrave FL (1974). The inhibition of mitochondrial calcium transport by lanthanides and ruthenium red. Biochem J 140: 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymann S, Haase W, Krick W, Burckhardt G, Thinnes FP (1998). Endosomes: another extra‐mitochondrial location of type‐1 porin/voltage‐dependent anion‐selective channels. Pflugers Arch 436: 478–480. [DOI] [PubMed] [Google Scholar]
- Richter M, Vidovic N, Honrath B, Mahavadi P, Dodel R, Dolga AM et al (2016). Activation of SK2 channels preserves ER Ca(2)(+) homeostasis and protects against ER stress‐induced cell death. Cell Death Differ 23: 814–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimmerman N, Ben‐Hail D, Porat Z, Juknat A, Kozela E, Daniels MP et al (2013). Direct modulation of the outer mitochondrial membrane channel, voltage‐dependent anion channel 1 (VDAC1) by cannabidiol: a novel mechanism for cannabinoid‐induced cell death. Cell Death Dis 4: e949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripcke J, Zarse K, Ristow M, Birringer M (2009). Small‐molecule targeting of the mitochondrial compartment with an endogenously cleaved reversible tag. Chembiochem 10: 1689–1696. [DOI] [PubMed] [Google Scholar]
- Riva A, Tandler B, Loffredo F, Vazquez E, Hoppel C (2005). Structural differences in two biochemically defined populations of cardiac mitochondria. Am J Physiol Heart Circ Physiol 289: H868–H872. [DOI] [PubMed] [Google Scholar]
- Rodriguez MJ, Martinez‐Moreno M, Ortega FJ, Mahy N (2013). Targeting microglial K (ATP) channels to treat neurodegenerative diseases: a mitochondrial issue. Oxid Med Cell Longev 2013: 194546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross MF, Kelso GF, Blaikie FH, James AM, Cocheme HM, Filipovska A et al (2005). Lipophilic triphenylphosphonium cations as tools in mitochondrial bioenergetics and free radical biology. Biochemistry Biokhimiia 70: 222–230. [DOI] [PubMed] [Google Scholar]
- Ross MF, Prime TA, Abakumova I, James AM, Porteous CM, Smith RA et al (2008). Rapid and extensive uptake and activation of hydrophobic triphenylphosphonium cations within cells. Biochem J 411: 633–645. [DOI] [PubMed] [Google Scholar]
- Roy S, Sileikyte J, Neuenswander B, Hedrick MP, Chung TD, Aube J et al (2016). N‐phenylbenzamides as potent inhibitors of the mitochondrial permeability transition pore. ChemMedChem 11: 283–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Sileikyte J, Schiavone M, Neuenswander B, Argenton F, Aube J et al (2015). Discovery, synthesis, and optimization of diarylisoxazole‐3‐carboxamides as potent inhibitors of the mitochondrial permeability transition pore. ChemMedChem 10: 1655–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggieri P, Mangino G, Fioretti B, Catacuzzeno L, Puca R, Ponti D et al (2012). The inhibition of KCa3.1 channels activity reduces cell motility in glioblastoma derived cancer stem cells. PLoS One 7: e47825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E (2017). Tumor penetrating peptides for improved drug delivery. Adv Drug Deliv Rev 110‐111: 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salari S, Ghasemi M, Fahanik‐Babaei J, Saghiri R, Sauve R, Eliassi A (2015). Evidence for a KATP channel in rough endoplasmic reticulum (rerKATP channel) of rat hepatocytes. PLoS One 10: e0125798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancak Y, Markhard AL, Kitami T, Kovacs‐Bogdan E, Kamer KJ, Udeshi ND et al (2013). EMRE is an essential component of the mitochondrial calcium uniporter complex. Science 342: 1379–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho P, Galeano E, Nieto E, Delgado MD, Garcia‐Perez AI (2007). Dequalinium induces cell death in human leukemia cells by early mitochondrial alterations which enhance ROS production. Leuk Res 31: 969–978. [DOI] [PubMed] [Google Scholar]
- Sassi N, De Marchi U, Fioretti B, Biasutto L, Gulbins E, Franciolini F et al (2010). An investigation of the occurrence and properties of the mitochondrial intermediate‐conductance Ca2+‐activated K+ channel mtKCa3.1. Biochim Biophys Acta 1797: 1260–1267. [DOI] [PubMed] [Google Scholar]
- Sato Y, Nakamura T, Yamada Y, Harashima H (2016). Development of a multifunctional envelope‐type nano device and its application to nanomedicine. J Control Release 244: 194–204. [DOI] [PubMed] [Google Scholar]
- Sawada K, Okada S, Kuroda A, Watanabe S, Sawada Y, Tanaka H (2001). 4‐(Benzoylindolizinyl) butyric acids; novel nonsteroidal inhibitors of steroid 5α‐reductase. III. Chem Pharm Bull 49: 799–813. [DOI] [PubMed] [Google Scholar]
- Schwab A, Fabian A, Hanley PJ, Stock C (2012). Role of ion channels and transporters in cell migration. Physiol Rev 92: 1865–1913. [DOI] [PubMed] [Google Scholar]
- Serafim TL, Carvalho FS, Bernardo TC, Pereira GC, Perkins E, Holy J et al (2014). New derivatives of lupane triterpenoids disturb breast cancer mitochondria and induce cell death. Bioorg Med Chem 22: 6270–6287. [DOI] [PubMed] [Google Scholar]
- Serrano‐Albarras A, Estadella I, Cirera‐Rocosa S, Navarro‐Perez M, Felipe A (2018). Kv1.3: a multifunctional channel with many pathological implications. Expert Opin Ther Targets 22: 101–105. [DOI] [PubMed] [Google Scholar]
- Setti M, Savalli N, Osti D, Richichi C, Angelini M, Brescia P et al (2013). Functional role of CLIC1 ion channel in glioblastoma‐derived stem/progenitor cells. J Natl Cancer Inst 105: 1644–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sforna L, Megaro A, Pessia M, Franciolini F, Catacuzzeno L (2018). Structure, gating and basic functions of the Ca2+‐activated K channel of intermediate conductance. Curr Neuropharmacol 16: 608–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Du T, Wang X, Duan C, Gao G, Zhang J et al (2014). α‐Synuclein amino terminus regulates mitochondrial membrane permeability. Brain Res 1591: 14–26. [DOI] [PubMed] [Google Scholar]
- Sheng JZ, Braun AP (2007). Small‐ and intermediate‐conductance Ca2+‐activated K+ channels directly control agonist‐evoked nitric oxide synthesis in human vascular endothelial cells. Am J Physiol Cell Physiol 293: C458–C467. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Shinohara Y, Tsujimoto Y (2000). Bax and Bcl‐xL independently regulate apoptotic changes of yeast mitochondria that require VDAC but not adenine nucleotide translocator. Oncogene 19: 4309–4318. [DOI] [PubMed] [Google Scholar]
- Shoshan‐Barmatz V, De S, Meir A (2017a). The mitochondrial voltage‐dependent anion channel 1, Ca(2+) transport, apoptosis, and their regulation. Front Oncol 7: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoshan‐Barmatz V, Hadad N, Feng W, Shafir I, Orr I, Varsanyi M et al (1996). VDAC/porin is present in sarcoplasmic reticulum from skeletal muscle. FEBS Lett 386: 205–210. [DOI] [PubMed] [Google Scholar]
- Shoshan‐Barmatz V, Israelson A (2005). The voltage‐dependent anion channel in endoplasmic/sarcoplasmic reticulum: characterization, modulation and possible function. J Membr Biol 204: 57–66. [DOI] [PubMed] [Google Scholar]
- Shoshan‐Barmatz V, Krelin Y, Shteinfer‐Kuzmine A (2018). VDAC1 functions in Ca(2+) homeostasis and cell life and death in health and disease. Cell Calcium 69: 81–100. [DOI] [PubMed] [Google Scholar]
- Shoshan‐Barmatz V, Krelin Y, Shteinfer‐Kuzmine A, Arif T (2017b). Voltage‐dependent anion channel 1 as an emerging drug target for novel anti‐cancer therapeutics. Front Oncol 7: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shteinfer‐Kuzmine A, Arif T, Krelin Y, Tripathi SS, Paul A, Shoshan‐Barmatz V (2017). Mitochondrial VDAC1‐based peptides: attacking oncogenic properties in glioblastoma. Oncotarget 8: 31329–31346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H, Lu R, Bopassa JC, Meredith AL, Stefani E, Toro L (2013). MitoBK (Ca) is encoded by the Kcnma1 gene, and a splicing sequence defines its mitochondrial location. Proc Natl Acad Sci U S A 110: 10836–10841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H, Stefani E, Toro L (2012). Intracellular BK (Ca) (iBK (Ca)) channels. J Physiol 590: 5937–5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CO, Nehrke K, Brookes PS (2017). The Slo(w) path to identifying the mitochondrial channels responsible for ischemic protection. Biochem J 474: 2067–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CO, Wang YT, Nadtochiy SM, Miller JH, Jonas EA, Dirksen RT , et al (2018). Cardiac metabolic effects of KNa1.2 channel deletion and evidence for its mitochondrial localization. FASEB journal: official publication of the Federation of American Societies for Experimental Biology: fj201800139R. [DOI] [PMC free article] [PubMed]
- Smith RA, Adlam VJ, Blaikie FH, Manas AR, Porteous CM, James AM et al (2008). Mitochondria‐targeted antioxidants in the treatment of disease. Ann N Y Acad Sci 1147: 105–111. [DOI] [PubMed] [Google Scholar]
- Smith RA, Hartley RC, Cocheme HM, Murphy MP (2012). Mitochondrial pharmacology. Trends Pharmacol Sci 33: 341–352. [DOI] [PubMed] [Google Scholar]
- Smith RA, Hartley RC, Murphy MP (2011). Mitochondria‐targeted small molecule therapeutics and probes. Antioxid Redox Signal 15: 3021–3038. [DOI] [PubMed] [Google Scholar]
- Sole L, Roig SR, Vallejo‐Gracia A, Serrano‐Albarras A, Martinez‐Marmol R, Tamkun MM et al (2016). The C‐terminal domain of Kv1.3 regulates functional interactions with the KCNE4 subunit. J Cell Sci 129: 4265–4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sole L, Roura‐Ferrer M, Perez‐Verdaguer M, Oliveras A, Calvo M, Fernandez‐Fernandez JM et al (2009). KCNE4 suppresses Kv1.3 currents by modulating trafficking, surface expression and channel gating. J Cell Sci 122: 3738–3748. [DOI] [PubMed] [Google Scholar]
- Stehno‐Bittel L, Luckhoff A, Clapham DE (1995). Calcium release from the nucleus by InsP3 receptor channels. Neuron 14: 163–167. [DOI] [PubMed] [Google Scholar]
- Stein CA, Colombini M (2008). Specific VDAC inhibitors: phosphorothioate oligonucleotides. J Bioenerg Biomembr 40: 157–162. [DOI] [PubMed] [Google Scholar]
- Sterea AM, Almasi S, El Hiani Y (2018). The hidden potential of lysosomal ion channels: a new era of oncogenes. Cell Calcium 72: 91–103. [DOI] [PubMed] [Google Scholar]
- Szabo I, Bock J, Grassme H, Soddemann M, Wilker B, Lang F et al (2008). Mitochondrial potassium channel Kv1.3 mediates Bax‐induced apoptosis in lymphocytes. Proc Natl Acad Sci U S A 105: 14861–14866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo I, Bock J, Jekle A, Soddemann M, Adams C, Lang F et al (2005). A novel potassium channel in lymphocyte mitochondria. J Biol Chem 280: 12790–12798. [DOI] [PubMed] [Google Scholar]
- Szabo I, Leanza L (2017). The roles of mitochondrial cation channels under physiological conditions and in cancer. Handb Exp Pharmacol 240: 47–69. [DOI] [PubMed] [Google Scholar]
- Szabo I, Leanza L, Gulbins E, Zoratti M (2012). Physiology of potassium channels in the inner membrane of mitochondria. Pflugers Arch 463: 231–246. [DOI] [PubMed] [Google Scholar]
- Szabo I, Soddemann M, Leanza L, Zoratti M, Gulbins E (2011). Single‐point mutations of a lysine residue change function of Bax and Bcl‐xL expressed in Bax‐ and Bak‐less mouse embryonic fibroblasts: novel insights into the molecular mechanisms of Bax‐induced apoptosis. Cell Death Differ 18: 427–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo I, Zoratti M (1991). The giant channel of the inner mitochondrial membrane is inhibited by cyclosporin A. J Biol Chem 266: 3376–3379. [PubMed] [Google Scholar]
- Szabo I, Zoratti M (2014). Mitochondrial channels: ion fluxes and more. Physiol Rev 94: 519–608. [DOI] [PubMed] [Google Scholar]
- Szeto HH (2006). Cell‐permeable, mitochondrial‐targeted, peptide antioxidants. AAPS J 8: E277–E283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto HH (2014). First‐in‐class cardiolipin‐protective compound as a therapeutic agent to restore mitochondrial bioenergetics. Br J Pharmacol 171: 2029–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto HH, James LP, Atkinson AJ (2014). Mitochondrial pharmacology: its future is now. Clin Pharmacol Ther 96: 629–633. [DOI] [PubMed] [Google Scholar]
- Szewczyk A, Jarmuszkiewicz W, Kunz WS (2009). Mitochondrial potassium channels. IUBMB Life 61: 134–143. [DOI] [PubMed] [Google Scholar]
- Szewczyk A, Kajma A, Malinska D, Wrzosek A, Bednarczyk P, Zablocka B et al (2010). Pharmacology of mitochondrial potassium channels: dark side of the field. FEBS Lett 584: 2063–2069. [DOI] [PubMed] [Google Scholar]
- Tajeddine N, Galluzzi L, Kepp O, Hangen E, Morselli E, Senovilla L et al (2008). Hierarchical involvement of Bak, VDAC1 and Bax in cisplatin‐induced cell death. Oncogene 27: 4221–4232. [DOI] [PubMed] [Google Scholar]
- Tan W, Loke YH, Stein CA, Miller P, Colombini M (2007). Phosphorothioate oligonucleotides block the VDAC channel. Biophys J 93: 1184–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q, Guo W, Zheng L, Wu JX, Liu M, Zhou X et al (2018). Structure of the receptor‐activated human TRPC6 and TRPC3 ion channels. Cell Res In press, 10.1038/s41422-018-0038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S, Wang X, Shen Q, Yang X, Yu C, Cai C et al (2015). Mitochondrial Ca(2)(+) uniporter is critical for store‐operated Ca(2)(+) entry‐dependent breast cancer cell migration. Biochem Biophys Res Commun 458: 186–193. [DOI] [PubMed] [Google Scholar]
- Tasiopoulou V, Magouliotis D, Solenov EI, Vavougios G, Molyvdas PA, Gourgoulianis KI et al (2015). Transcriptional over‐expression of chloride intracellular channels 3 and 4 in malignant pleural mesothelioma. Comput Biol Chem 59 (Pt A): 111–116. [DOI] [PubMed] [Google Scholar]
- Teardo E, Carraretto L, Wagner S, Formentin E, Behera S, De Bortoli S et al (2017). Physiological characterization of a plant mitochondrial calcium uniporter in vitro and in vivo. Plant Physiol 173: 1355–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira J, Cagide F, Benfeito S, Soares P, Garrido J, Baldeiras I et al (2017). Development of a mitochondriotropic antioxidant based on caffeic acid: proof of concept on cellular and mitochondrial oxidative stress models. J Med Chem 60: 7084–7098. [DOI] [PubMed] [Google Scholar]
- Testai L, Barrese V, Soldovieri MV, Ambrosino P, Martelli A, Vinciguerra I et al (2015a). Expression and function of Kv7.4 channels in Rat cardiac mitochondria: possible targets for cardioprotection. Cardiovasc Res 110: 40–50. [DOI] [PubMed] [Google Scholar]
- Testai L, Rapposelli S, Martelli A, Breschi MC, Calderone V (2015b). Mitochondrial potassium channels as pharmacological target for cardioprotective drugs. Med Res Rev 35: 520–553. [DOI] [PubMed] [Google Scholar]
- Tewari D, Ahmed T, Chirasani VR, Singh PK, Maji SK, Senapati S et al (2015). Modulation of the mitochondrial voltage dependent anion channel (VDAC) by curcumin. Biochim Biophys Acta 1848: 151–158. [DOI] [PubMed] [Google Scholar]
- Thinnes FP (1992). Evidence for extra‐mitochondrial localization of the VDAC/porin channel in eucaryotic cells. J Bioenerg Biomembr 24: 71–75. [DOI] [PubMed] [Google Scholar]
- Thinnes FP (2005). Does fluoxetine (Prozak) block mitochondrial permeability transition by blocking VDAC as part of permeability transition pores? Mol Genet Metab 84: 378. [DOI] [PubMed] [Google Scholar]
- Thinnes FP (2014). Opening up of plasmalemma type‐1 VDAC to form apoptotic “find me signal” pathways is essential in early apoptosis – evidence from the pathogenesis of cystic fibrosis resulting from failure of apoptotic cell clearance followed by sterile inflammation. Mol Genet Metab 111: 439–444. [DOI] [PubMed] [Google Scholar]
- Thinnes FP (2015). After all, plasmalemmal expression of type‐1 VDAC can be understood. Phosphorylation, nitrosylation, and channel modulators work together in vertebrate cell volume regulation and either apoptotic pathwayin. Front Physiol 6: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thinnes FP, Florke H, Winkelbach H, Stadtmuller U, Heiden M, Karabinos A et al (1994). Channel active mammalian porin, purified from crude membrane fractions of human B lymphocytes or bovine skeletal muscle, reversibly binds the stilbene‐disulfonate group of the chloride channel blocker DIDS. Biol Chem Hoppe Seyler 375: 315–322. [DOI] [PubMed] [Google Scholar]
- Thu VT, Kim HK, Long le T, Lee SR, Hanh TM, Ko TH et al (2012). NecroX‐5 prevents hypoxia/reoxygenation injury by inhibiting the mitochondrial calcium uniporter. Cardiovasc Res 94: 342–350. [DOI] [PubMed] [Google Scholar]
- Tiapko O, Groschner K (2018). TRPC3 as a target of novel therapeutic interventions. Cell 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiepolo T, Angelin A, Palma E, Sabatelli P, Merlini L, Nicolosi L et al (2009). The cyclophilin inhibitor Debio 025 normalizes mitochondrial function, muscle apoptosis and ultrastructural defects in Col6a1−/− myopathic mice. Br J Pharmacol 157: 1045–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toczylowska‐Maminska R, Olszewska A, Laskowski M, Bednarczyk P, Skowronek K, Szewczyk A (2014). Potassium channel in the mitochondria of human keratinocytes. J Invest Dermatol 134: 764–772. [DOI] [PubMed] [Google Scholar]
- Tosatto A, Sommaggio R, Kummerow C, Bentham RB, Blacker TS, Berecz T et al (2016). The mitochondrial calcium uniporter regulates breast cancer progression via HIF‐1α. EMBO Mol Med 8: 569–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treves S, Jungbluth H, Voermans N, Muntoni F, Zorzato F (2017). Ca(2+) handling abnormalities in early‐onset muscle diseases: novel concepts and perspectives. Semin Cell Dev Biol 64: 201–212. [DOI] [PubMed] [Google Scholar]
- Vais H, Mallilankaraman K, Mak DO, Hoff H, Payne R, Tanis JE et al (2016). EMRE is a matrix Ca(2+) sensor that governs gatekeeping of the mitochondrial Ca(2+) uniporter. Cell Rep 14: 403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela SM, Martin DK, Por SB, Robbins JM, Warton K, Bootcov MR et al (1997). Molecular cloning and expression of a chloride ion channel of cell nuclei. J Biol Chem 272: 12575–12582. [DOI] [PubMed] [Google Scholar]
- Valenzuela SM, Mazzanti M, Tonini R, Qiu MR, Warton K, Musgrove EA et al (2000). The nuclear chloride ion channel NCC27 is involved in regulation of the cell cycle. J Physiol 529 (Pt 3): 541–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rensburg CE, Anderson R, Myer MS, Joone GK, O'Sullivan JF (1994). The riminophenazine agents clofazimine and B669 reverse acquired multidrug resistance in a human lung cancer cell line. Cancer Lett 85: 59–63. [DOI] [PubMed] [Google Scholar]
- Vasington FD, Gazzotti P, Tiozzo R, Carafoli E (1972). The effect of ruthenium red on Ca 2+ transport and respiration in rat liver mitochondria. Biochim Biophys Acta 256: 43–54. [DOI] [PubMed] [Google Scholar]
- Venturini E, Leanza L, Azzolini M, Kadow S, Mattarei A, Weller M et al (2017). Targeting the potassium channel Kv1.3 kills glioblastoma cells. Neurosignals 25: 26–38. [DOI] [PubMed] [Google Scholar]
- Veresov VG, Davidovskii AI (2014). Structural insights into proapoptotic signaling mediated by MTCH2, VDAC2, TOM40 and TOM22. Cell Signal 26: 370–382. [DOI] [PubMed] [Google Scholar]
- Vestweber D, Schatz G (1988). Mitochondria can import artificial precursor proteins containing a branched polypeptide chain or a carboxy‐terminal stilbene disulfonate. J Cell Biol 107: 2045–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestweber D, Schatz G (1989). DNA‐protein conjugates can enter mitochondria via the protein import pathway. Nature 338: 170–172. [DOI] [PubMed] [Google Scholar]
- von Charpuis C, Meckel T, Moroni A, Thiel G (2015). The sorting of a small potassium channel in mammalian cells can be shifted between mitochondria and plasma membrane. Cell Calcium 58: 114–121. [DOI] [PubMed] [Google Scholar]
- Wagner S, De Bortoli S, Schwarzlander M, Szabo I (2016). Regulation of mitochondrial calcium in plants versus animals. J Exp Bot 67: 3809–3829. [DOI] [PubMed] [Google Scholar]
- Walewska A, Kulawiak B, Szewczyk A, & Koprowski P (2018). Mechanosensitivity of mitochondr ial large‐conductance calcium‐activated potassium channels. Biochimica et biophysica acta. pii: S0005‐2728(18)30121‐X. 10.1016/j.bbabio.2018.05.006 [DOI] [PubMed]
- Walrant A, Cardon S, Burlina F, Sagan S (2017). Membrane crossing and membranotropic activity of cell‐penetrating peptides: dangerous liaisons? Acc Chem Res 50: 2968–2975. [DOI] [PubMed] [Google Scholar]
- Wang H, Haridas V, Gutterman JU, Xu ZX (2010). Natural triterpenoid avicins selectively induce tumor cell death. Commun Integr Biol 3: 205–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Xiong S, Lin S, Xia W, Li Q, Zhao Z et al (2017a). Enhanced mitochondrial transient receptor potential channel, canonical type 3‐mediated calcium handling in the vasculature from hypertensive rats. J American Heart Assoc 6: pii: e005812. 10.1161/JAHA.117.005812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Yang X, Shen Y (2015). Molecular mechanism of mitochondrial calcium uptake. Cell Mol Life Sci 72: 1489–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Zhang C, Yu P, Tang B, Liu T, Cui H et al (2012). Regulation of colon cancer cell migration and invasion by CLIC1‐mediated RVD. Mol Cell Biochem 365: 313–321. [DOI] [PubMed] [Google Scholar]
- Wang W, Zhang X, Gao Q, Lawas M, Yu L, Cheng X et al (2017b). A voltage‐dependent K(+) channel in the lysosome is required for refilling lysosomal Ca(2+) stores. J Cell Biol 216: 1715–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissig V (2015). DQAsomes as the prototype of mitochondria‐targeted pharmaceutical nanocarriers: preparation, characterization, and use. Methods Mol Biol (Clifton, NJ) 1265: 1–11. [DOI] [PubMed] [Google Scholar]
- Weissig V, D'Souza GG, Torchilin VP (2001). DQAsome/DNA complexes release DNA upon contact with isolated mouse liver mitochondria. J Control Release 75: 401–408. [DOI] [PubMed] [Google Scholar]
- Weissig V, Torchilin VP (2000). Mitochondriotropic cationic vesicles: a strategy towards mitochondrial gene therapy. Curr Pharm Biotechnol 1: 325–346. [DOI] [PubMed] [Google Scholar]
- Welling PA (2016). Roles and regulation of renal K channels. Annu Rev Physiol 78: 415–435. [DOI] [PubMed] [Google Scholar]
- Wiedemann N, Pfanner N (2017). Mitochondrial machineries for protein import and assembly. Annu Rev Biochem 86: 685–714. [DOI] [PubMed] [Google Scholar]
- Wojtovich AP, Sherman TA, Nadtochiy SM, Urciuoli WR, Brookes PS, Nehrke K (2011). SLO‐2 is cytoprotective and contributes to mitochondrial potassium transport. PloS one 6: e28287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtovich AP, Smith CO, Urciuoli WR, Wang YT, Xia XM, Brookes PS et al (2016). Cardiac Slo2.1 is required for volatile anesthetic stimulation of K+ transport and anesthetic preconditioning. Anesthesiology 124: 1065–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrzosek A, Tomaskova Z, Ondrias K, Lukasiak A, Szewczyk A (2012). The potassium channel opener CGS7184 activates Ca(2)(+) release from the endoplasmic reticulum. Eur J Pharmacol 690: 60–67. [DOI] [PubMed] [Google Scholar]
- Wu G, Li S, Zong G, Liu X, Fei S, Shen L et al (2018). Single channel recording of a mitochondrial calcium uniporter. Biochem Biophys Res Commun 496: 127–132. [DOI] [PubMed] [Google Scholar]
- Wu Y, Rasmussen TP, Koval OM, Joiner ML, Hall DD, Chen B et al (2015). The mitochondrial uniporter controls fight or flight heart rate increases. Nat Commun 6: 6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Iribe G, Nishida M, Naruse K (2017). Role of TRPC3 and TRPC6 channels in the myocardial response to stretch: linking physiology and pathophysiology. Prog Biophys Mol Biol 130: 264–272. [DOI] [PubMed] [Google Scholar]
- Yang F, Chen ZY, Wu YL (2015). Unique interactions between scorpion toxins and small conductance Ca(2+)‐activated potassium channels. Sheng li xue bao: [Acta physiologica Sinica] 67: 255–260. [PubMed] [Google Scholar]
- Yang JY, Jung JY, Cho SW, Choi HJ, Kim SW, Kim SY et al (2009). Chloride intracellular channel 1 regulates osteoblast differentiation. Bone 45: 1175–1185. [DOI] [PubMed] [Google Scholar]
- Yang Z, Kang DH, Lee H, Shin J, Yan W, Rathore B et al (2018). A fluorescent probe for stimulated emission depletion super‐resolution imaging of vicinal‐dithiol‐proteins on mitochondrial membrane. Bioconjug Chem 29: 1446–1453. [DOI] [PubMed] [Google Scholar]
- Yao N, Li YJ, Zhang DM, Liu DL, Tang MK, Yiu A et al (2015). B4G2 induces mitochondrial apoptosis by the ROS‐mediated opening of Ca2+‐dependent permeability transition pores. Cell Physiol Biochem 37: 838–852. [DOI] [PubMed] [Google Scholar]
- Yoo D, Fang L, Mason A, Kim BY, Welling PA (2005). A phosphorylation‐dependent export structure in ROMK (Kir 1.1) channel overrides an endoplasmic reticulum localization signal. J Biol Chem 280: 35281–35289. [DOI] [PubMed] [Google Scholar]
- Yoo J, Wu M, Yin Y, Herzik MA Jr, Lander GC, Lee SY (2018). Cryo‐EM structure of a mitochondrial calcium uniporter. Science 361: 506–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousif LF, Stewart KM, Horton KL, Kelley SO (2009a). Mitochondria‐penetrating peptides: sequence effects and model cargo transport. Chembiochem 10: 2081–2088. [DOI] [PubMed] [Google Scholar]
- Yousif LF, Stewart KM, Kelley SO (2009b). Targeting mitochondria with organelle‐specific compounds: strategies and applications. Chembiochem 10: 1939–1950. [DOI] [PubMed] [Google Scholar]
- Yu T, Chen C, Sun Y, Sun H, Li TH, Meng J et al (2015). ABT‐737 sensitizes curcumin‐induced anti‐melanoma cell activity through facilitating mPTP death pathway. Biochem Biophys Res Commun 464: 286–291. [DOI] [PubMed] [Google Scholar]
- Yu M, Liu SL, Sun PB, Pan H, Tian CL, Zhang LH (2016). Peptide toxins and small‐molecule blockers of BK channels. Acta Pharmacol Sin 37: 56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccagnino A, Manago A, Leanza L, Gontarewitz A, Linder B, Azzolini M et al (2016). Tumor‐reducing effect of the clinically used drug clofazimine in a SCID mouse model of pancreatic ductal adenocarcinoma. Oncotarget 8: 38276–38293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaid H, Abu‐Hamad S, Israelson A, Nathan I, Shoshan‐Barmatz V (2005). The voltage‐dependent anion channel‐1 modulates apoptotic cell death. Cell Death Differ 12: 751–760. [DOI] [PubMed] [Google Scholar]
- Zeth K, Zachariae U (2018). Ten years of high resolution structural research on the voltage dependent anion channel (VDAC)‐recent developments and future directions. Front Physiol 9: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan P, Liu L, Nie D, Liu Y, Mao X (2018). Effect of inhibition of intermediate‐conductance‐Ca(2+)‐activated K(+) channels on HeLa cell proliferation. J Cancer Res Ther 14: S41–s45. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Huang J, Yuan X, Peng B, Liu W, Han S et al (2015). Toxins targeting the Kv1.3 channel: potential immunomodulators for autoimmune diseases. Toxins 7: 1749–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Chen Z, Yin W, Miao L, Zhou Z, Ji G (2012). Ryanodine receptors are involved in nuclear calcium oscillation in primary pancreatic beta‐cells. Biochem Biophys Res Commun 423: 207–211. [DOI] [PubMed] [Google Scholar]
- Zhou C, Chen Z, Lu X, Wu H, Yang Q, Xu D (2015). Icaritin activates JNK‐dependent mPTP necrosis pathway in colorectal cancer cells. Tumour Biol 37: 3135–3144. [DOI] [PubMed] [Google Scholar]
- Zhou H, Tate SS, Palmer LG (1994). Primary structure and functional properties of an epithelial K channel. Am J Physiol 266: C809–C824. [DOI] [PubMed] [Google Scholar]
- Zhu J, Yan J, Thornhill WB (2014). The Kv1.3 potassium channel is localized to the cis‐Golgi and Kv1.6 is localized to the endoplasmic reticulum in rat astrocytes. FEBS J 281: 3433–3445. [DOI] [PubMed] [Google Scholar]
- Zielonka J, Joseph J, Sikora A, Hardy M, Ouari O, Vasquez‐Vivar J et al (2017). Mitochondria‐targeted triphenylphosphonium‐based compounds: syntheses, mechanisms of action, and therapeutic and diagnostic applications. Chem Rev 117: 10043–10120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoratti M, Szabo I (1995). The mitochondrial permeability transition. Biochim Biophys Acta 1241: 139–176. [DOI] [PubMed] [Google Scholar]
- Zulian A, Rizzo E, Schiavone M, Palma E, Tagliavini F, Blaauw B et al (2014). NIM811, a cyclophilin inhibitor without immunosuppressive activity, is beneficial in collagen VI congenital muscular dystrophy models. Hum Mol Genet 23: 5353–5363. [DOI] [PubMed] [Google Scholar]
- Zuzarte M, Rinne S, Schlichthorl G, Schubert A, Daut J, Preisig‐Muller R (2007). A di‐acidic sequence motif enhances the surface expression of the potassium channel TASK‐3. Traffic (Copenhagen, Denmark) 8: 1093–1100. [DOI] [PubMed] [Google Scholar]


