Abstract
While some epidemiologic studies support the hypothesis that stress can adversely affect fertility, few prospective studies have assessed the association in couples from the general population. We used data from Pregnancy Study Online, a web-based preconception cohort study of pregnancy planners from the United States and Canada (2013–2018), to examine the association between women’s and men’s perceived stress levels prior to conception and fecundability. Women (aged 21–45 years) and their male partners (aged ≥21 years) who were attempting conception without fertility treatment were eligible. We measured perceived stress using the 10-item Perceived Stress Scale (PSS). We ascertained pregnancy information using bimonthly follow-up questionnaires of female participants. We followed 4,769 couples until self-reported pregnancy, initiation of fertility treatment, loss to follow-up, or 12 menstrual cycles of attempt time, whichever came first. We used proportional probabilities regression models to estimate fecundability ratios and 95% confidence intervals, adjusting for potential confounders. Higher PSS scores among the women were associated with slight reductions in fecundability (comparing PSS scores of ≥25 vs. <10, fecundability ratio = 0.87, 95% confidence interval: 0.74, 1.02). PSS scores among the men were not substantially associated with fecundability.
Keywords: fecundability, perceived stress, preconception cohort, time to pregnancy
In North America, 20%–25% of women and 18%–21% of men of reproductive age report daily psychological stress (1, 2). Stress adversely affects quality of life and is associated with cardiovascular and mental illness, accelerated disease progression, and premature mortality (3–7). The physiology of the human stress response is well-established. A stressful stimulus results in signaling to the hypothalamus, which activates the sympathetic-adrenomedullary axis and increases blood norepinephrine and salivary α-amylase. If the stress becomes chronic, the sympathetic-adrenomedullary axis remains hyperactive, while the hypothalamic-pituitary-adrenal axis is also activated, increasing blood and salivary cortisol. Suppression of the hypothalamic-pituitary-gonadal axis by hypothalamic-pituitary-adrenal axis activation represents a physiological link between the stress response and the female reproductive system (8).
Reports among infertile couples of spontaneous conception following adoption of a child (9, 10) and positive associations between psychiatric treatments and improved in vitro fertilization outcomes (11–13) indicate that stress affects fertility. Among women, stress could influence fertility by delaying or inhibiting the luteinizing hormone surge of the menstrual cycle (14), affecting gamete transport (15), increasing risk of anovulation (16), or creating an unfavorable environment for implantation (17).
Epidemiologic studies of the association between stress among women and fertility report conflicting results (16). Two preconception cohort studies have found inverse associations between salivary levels of α-amylase, but not cortisol, and fecundability (18, 19), while studies of questionnaire-based stress measures and fecundability have reported an inverse association with luteal-phase psychological distress (20), an inverse association with follicular-phase stress but a positive association with luteal phase stress (21), or little association (22). Inconsistencies across studies could stem from differing methods and timing of stress measurements.
Among men, stress might affect fertility through decreased testosterone levels, altered spermatogenesis (23), and erectile dysfunction and ejaculatory problems (24, 25). Studies of male medical students during examinations (26, 27) and men experiencing war-time conditions (28–30), work-related stress (31), and recent bereavement (32) suggest that psychological stress adversely affects sperm concentration and semen quality. The only epidemiologic study, to our knowledge, to examine the association between male stress and fertility found that psychological stress (33), but not job strain (high demand and low control) (34), was associated with reduced fecundability.
In the present study, we examined the association between perceived stress among men and women and fecundability in a North American cohort of couples planning a pregnancy.
METHODS
Study population
Pregnancy Study Online (PRESTO) is an ongoing web-based preconception cohort study of pregnancy planners from the United States and Canada (35). Eligible women are 21–45 years old and attempting to conceive without fertility treatment. Women’s participation involves completion of a baseline questionnaire on demographic, behavioral, medical, and reproductive factors and bimonthly follow-up questionnaires to update pregnancy status and exposure information. Women may also invite their male partners aged ≥21 years to participate. Men’s participation involves completion of a baseline questionnaire. Ten days after baseline, participants complete a validated food frequency questionnaire (36).
From June 2013 through January 2018, 6,813 women completed the baseline questionnaire. We excluded women with implausible last menstrual period dates (n = 123), history of infertility (n = 623), or who had been attempting conception for >6 cycles at study entry (n = 1,298). The final study population comprised 4,769 women and 1,272 men.
The institutional review board at Boston University Medical Center approved this study. All participants provided informed consent.
Exposure assessment
We measured perceived stress using the 10-item version of the Perceived Stress Scale (PSS), designed to assess how unpredictable, uncontrollable, and overwhelming individuals find their life circumstances (37). Reliability studies demonstrate that the PSS effectively captures stress over the previous 4–8 weeks in community-based samples with at least an 8th grade education (37–39). Given that stress-associated pathology results from cognitively mediated responses to a stressful event, rather than from the event itself, the PSS is considered a better measure of relevant stress than objective measures of stressful events (37).
Participants completed the PSS at baseline (both partners) and at each follow-up (women only). The items referred to the past month, with 5 response choices ranging from 0 (never) to 4 (very often). We summed the responses of the 10 items to compute a total score (range, 0–40), with higher scores indicating higher perceived stress.
Outcome assessment
On follow-up questionnaires, women reported whether they were currently pregnant and, if not, whether they had experienced any pregnancy losses since their last questionnaire, were still attempting conception, or had initiated fertility treatment. For nonresponders, we sought pregnancy information via telephone contact, linking with birth registry data, and searching for baby registries and announcements online.
At baseline, women reported the number of menstrual periods they had had since they began trying to conceive. They also reported whether their menstrual cycles were regular and, if yes, their usual cycle length. For women with irregular cycles, we estimated usual cycle length using last menstrual period dates at baseline and follow-up. We calculated pregnancy attempt time, rounded to the nearest whole cycle, as follows: (menstrual cycles of attempt time at baseline) + ([last menstrual period date from most recent follow-up questionnaire − date of baseline questionnaire] ÷ cycle length) + 1.
Covariate assessment
We collected covariate information on the baseline questionnaires, including age, race/ethnicity, education, household income, height, weight, sleep duration, employment status, hours per week of work, smoking history, alcohol and caffeine consumption, intake of sugar-sweetened beverages, physical activity, intercourse frequency, use of methods to improve chances of conception, multivitamin and folic acid use, depressive symptoms (Major Depression Inventory score (40)), and reproductive history. From the food frequency questionnaire, we calculated Healthy Eating Index scores (41).
Statistical analysis
A factor analysis using orthogonal rotation to identify domains within the PSS revealed 2 factor loadings, confirming results from studies assessing the psychometric properties of the PSS (38, 42, 43). The first factor included the 6 negatively worded items on perceived stress and had factor loadings from 0.61 to 0.78. The second factor included the 4 positively worded items on coping skills and had factor loadings from 0.64 to 0.77. Cronbach’s α values for the 6 stress items and 4 coping items were 0.85 and 0.76, respectively, indicating high internal consistency.
We generated 5 imputation data sets using a Markov chain Monte Carlo method to impute missing exposure, covariate, and outcome data. For women not completing any follow-up questionnaires (n = 542, 11.4%), we assigned 1 menstrual cycle of follow-up and imputed their pregnancy status. PSS score was missing for <0.1% of women and 40.6% of men (administering the PSS to men was added to the baseline questionnaire in January 2015). Missing covariate data ranged from 0% (age) to 3% (income).
We used the Anderson-Gill data structure (44, 45) with 1 observation per menstrual cycle to update covariates over time and account for left truncation (46, 47). Women contributed observed menstrual cycles to the analysis from study entry until pregnancy, fertility treatment, cessation of pregnancy attempt, loss to follow-up, or 12 cycles, whichever came first. We used proportional probabilities regression to estimate fecundability ratios (FRs), the per-cycle probability of conception comparing exposed with unexposed individuals, and 95% confidence intervals.
For women, we examined PSS score in 3 ways: at baseline, as a time-varying variable (for each cycle, using the PSS score from the most recently completed follow-up questionnaire), and as a cumulative average variable (for each cycle, the average PSS score from baseline through that cycle). We administered the PSS to men at baseline only. Given the lack of clinical cutpoints for the PSS, we categorized PSS scores based on their distributions in the cohort: <10 (referent), 10–14, 15–19, 20–24, and ≥25. We assessed the shape of the curve relating PSS score to fecundability using restricted cubic splines (48, 49). We conducted a couples-based analysis comparing fecundability within joint categories of PSS scores from each partner.
We used a directed acyclic graph (Web Figure 1, available at https://academic.oup.com/aje) to identify confounders. With the exception of irregular cycles, the directed acyclic graphs for women and men were identical. These included age (in years: <25, 25–29, 30–34, ≥35), body mass index (calculated as weight (kg)/height (m)2: <25, 25–29, 30–34, ≥35), race/ethnicity (non-Hispanic white vs. not), education (up to high school education, some college, college degree, graduate school), annual household income (less than $50,000, $50,000–$99,999, $100,000–$149,999, at least $150,000), and employment (in hours per week: <20, 20–39, 40–49, ≥50 or unemployed).
To assess potential for reverse causation, we stratified models by attempt time at study entry (<3 vs. 3–6 cycles). We also examined the association between PSS score and fecundability in the first observed cycle only. We stratified models by women’s age (<35 years vs. ≥35 years) to determine whether associations were stronger in older women.
Baseline PSS scores were positively associated with attrition: Among women with PSS scores of ≥25 and <25, attrition was 32.4% and 22.3%, respectively. We corrected for differential attrition using inverse probability weights (50–52). First, we developed pooled logistic regression models for the probability of study continuation at each observed questionnaire cycle, conditional on remaining uncensored in the previous cycle. We included variables, some of which were time-varying, hypothesized to predict attrition (Web Table 1). We fitted a separate pooled logistic regression model including only baseline variables. From these models, we calculated the predicted probability of continuation for each observation and computed stabilized weights inversely proportional to the probability of continuation at each questionnaire cycle. Participants with a low probability of continuation received larger weights, compensating for underrepresentation in the data. We applied stabilized weights to the regression models and reported weighted FRs and 95% confidence intervals.
High levels of stress can cause sleep disturbances, depressive symptoms, uptake of unhealthy behaviors (e.g., consumption of tobacco, alcohol, and caffeine), lower intercourse frequency, and abnormal menstrual function (38, 53–56). We examined whether any observed association between perceived stress and fecundability was partially or completely mediated through these variables using a causal mediation analysis (57). We estimated the direct effects of women’s baseline PSS scores (≥25 vs. <10) on fecundability (natural direct effect) and effects that operate through the mediating variables (natural indirect effect). We calculated the proportion mediated as (FRNDE × [FRNIE − 1])/(FRNDE × FRNIE − 1).
RESULTS
We observed 19,130 cycles among 4,769 women. During follow-up, we identified 2,851 (59.8%) pregnancies: 2,327 self-reported on a follow-up questionnaire, 364 identified using other methods, and 160 imputed to women with no follow-up. Of the remaining 1,918 women, 334 initiated fertility treatment, 46 stopped trying to conceive, 704 were censored at 12 cycles, 744 were lost to follow-up, and 90 were still actively participating in the study.
Women had higher baseline PSS scores than men (mean = 15.8 (standard deviation, 5.7) and 14.7 (standard deviation, 6.0), respectively). Among the women followed for 12 menstrual cycles, mean PSS score was 15.4 at baseline, 14.7 at 6 months, and 15.3 at 12 months, indicating little individual change in PSS scores over time. Higher PSS scores among women were associated with longer attempt times at study entry, higher body mass index, current smoking, alcohol use, caffeine intake, hours/week of work, Major Depression Inventory score, history of physician-diagnosed depression and anxiety, gravidity, irregular cycles, and short cycles, and they were inversely associated with age, white non-Hispanic race/ethnicity, education, income, physical activity, sleep duration, regular multivitamin intake, and intercourse frequency (Table 1). Patterns were similar for men, although men’s PSS scores were not substantially associated with age, alcohol use, or physical activity (Table 2). Women’s and men’s PSS scores were weakly positively correlated (Pearson correlation coefficient = 0.18).
Table 1.
Baseline Characteristics of 4,769 Female Pregnancy Planners According to Score on the 10-Item Perceived Stress Scale, Pregnancy Study Online, United States and Canada, 2013–2018
| Characteristica | Perceived Stress Scale Score | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <10 | 10–14 | 15–19 | 20–24 | ≥25 | ||||||
| (n = 627) | (n = 1,443) | (n = 1,522) | (n = 837) | (n = 340) | ||||||
| Mean | % | Mean | % | Mean | % | Mean | % | Mean | % | |
| Age, years | 30.6 | 30.2 | 29.9 | 29.3 | 29.3 | |||||
| Partner’s age, years | 32.2 | 31.7 | 31.9 | 31.7 | 31.6 | |||||
| Cycles of attempt time at study entry | 1.7 | 2.0 | 2.1 | 2.2 | 2.3 | |||||
| Partner’s PSS score | 12.5 | 14.6 | 15.0 | 15.6 | 16.8 | |||||
| Non-Hispanic white | 86.3 | 85.8 | 84.5 | 84.7 | 79.6 | |||||
| Less than a college degree | 17.9 | 20.0 | 22.8 | 26.1 | 33.5 | |||||
| Married | 96.1 | 94.6 | 92.7 | 91.1 | 90.5 | |||||
| Annual household income of <$50,000 | 9.7 | 15.3 | 18.0 | 20.1 | 28.7 | |||||
| Body mass indexb | 25.7 | 26.6 | 27.3 | 28.2 | 28.2 | |||||
| Physical activity (MET-hours/week) | 39.7 | 37.0 | 34.2 | 32.7 | 30.7 | |||||
| Stress-reduction activities (i.e., yoga) of ≥1 hour/week | 36.9 | 33.3 | 33.2 | 35.5 | 33.0 | |||||
| Current regular smoker | 4.2 | 5.2 | 5.0 | 6.3 | 9.7 | |||||
| Current alcohol consumption, no. of drinks/week | 3.4 | 3.3 | 3.4 | 3.3 | 4.4 | |||||
| Current caffeine intake, mg/day | 107.8 | 116.7 | 122.9 | 120.9 | 143.7 | |||||
| Sleep duration of <7 hours/night | 15.1 | 19.8 | 21.0 | 27.4 | 39.3 | |||||
| Unemployed | 1.9 | 2.7 | 3.4 | 3.7 | 7.8 | |||||
| Work duration of ≥50 hours/weekc | 9.6 | 12.2 | 12.3 | 12.2 | 15.4 | |||||
| Regular multivitamin intake | 87.3 | 81.3 | 81.5 | 76.1 | 76.4 | |||||
| Major Depression Inventory score | 4.4 | 6.7 | 9.8 | 15.1 | 23.0 | |||||
| History of physician-diagnosed depression | 14.1 | 15.1 | 22.5 | 35.0 | 44.4 | |||||
| History of physician-diagnosed anxiety | 12.6 | 12.6 | 20.8 | 35.1 | 40.5 | |||||
| Parous | 27.0 | 25.9 | 25.0 | 28.5 | 33.1 | |||||
| History of spontaneous abortion | 16.9 | 19.9 | 21.6 | 24.5 | 27.4 | |||||
| Intercourse frequency of <1 time/week | 16.4 | 18.5 | 21.0 | 22.9 | 23.6 | |||||
| Doing something to improve chances of conception | 73.4 | 74.3 | 76.3 | 77.2 | 73.7 | |||||
| Last method of contraception was hormonal | 36.8 | 39.5 | 39.5 | 38.4 | 36.4 | |||||
| Irregular cycles | 10.7 | 12.4 | 14.0 | 17.5 | 22.0 | |||||
| Cycle length of <25 days | 2.0 | 3.3 | 3.0 | 3.9 | 4.5 | |||||
| Cycle length of ≥32 days | 20.2 | 21.2 | 20.8 | 23.2 | 24.7 | |||||
Abbreviations: MET, metabolic equivalent; PSS, Perceived Stress Scale.
a All characteristics except for age are standardized to the cohort age at baseline.
b Weight (kg)/height (m)2.
c Among employed individuals.
Table 2.
Baseline Characteristics of 1,272 Male Pregnancy Planners According to Score on the 10-Item Perceived Stress Scale, Pregnancy Study Online, United States and Canada, 2013–2018
| Characteristica | Perceived Stress Scale Score | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <10 | 10–14 | 15–19 | 20–24 | ≥25 | ||||||
| (n = 235) | (n = 416) | (n = 331) | (n = 213) | (n = 77) | ||||||
| Mean | % | Mean | % | Mean | % | Mean | % | Mean | % | |
| Age, years | 31.4 | 31.5 | 31.7 | 31.9 | 31.1 | |||||
| Partner’s age, years | 29.8 | 29.9 | 29.8 | 29.8 | 29.7 | |||||
| Cycles of attempt time at study entry | 1.9 | 1.8 | 1.8 | 1.9 | 2.3 | |||||
| Partner’s PSS score | 13.8 | 14.9 | 15.9 | 16.1 | 17.5 | |||||
| Non-Hispanic white | 86.3 | 88.2 | 86.5 | 84.9 | 88.9 | |||||
| Less than a college degree | 26.0 | 21.8 | 33.5 | 36.2 | 34.7 | |||||
| Married | 95.8 | 96.6 | 95.8 | 95.4 | 87.1 | |||||
| Annual household income of <$50,000 | 14.2 | 14.7 | 14.4 | 20.2 | 26.8 | |||||
| Body mass indexb | 27.7 | 27.0 | 27.7 | 28.9 | 28.9 | |||||
| Physical activity (MET-hours/week) | 35.2 | 33.9 | 32.3 | 30.6 | 34.0 | |||||
| Stress-reduction activities (i.e., yoga) of ≥1 hour/week | 14.4 | 14.5 | 13.8 | 13.2 | 8.8 | |||||
| Current regular smoker | 3.8 | 3.6 | 9.1 | 5.2 | 9.3 | |||||
| Current alcohol consumption, no. of drinks/week | 5.8 | 5.8 | 6.1 | 7.3 | 7.4 | |||||
| Current caffeine intake, mg/day | 162.1 | 168.1 | 184.4 | 184.4 | 215.1 | |||||
| Sleep duration of <7 hours/night | 23.7 | 32.9 | 35.8 | 39.3 | 45.6 | |||||
| Unemployed | 0.8 | 2.4 | 1.9 | 3.4 | 13.1 | |||||
| Work duration of ≥50 hours/weekc | 25.6 | 28.0 | 27.9 | 32.9 | 35.2 | |||||
| Regular multivitamin intake | 32.5 | 35.2 | 32.0 | 32.8 | 24.3 | |||||
| Major Depression Inventory score | 4.4 | 6.8 | 9.8 | 14.3 | 21.3 | |||||
| History of physician-diagnosed depression | 1.7 | 7.2 | 10.3 | 17.2 | 30.9 | |||||
| History of physician-diagnosed anxiety | 2.8 | 4.8 | 7.4 | 14.4 | 25.7 | |||||
| Intercourse frequency of <1 time/week | 12.4 | 18.2 | 25.6 | 20.3 | 23.0 | |||||
| Doing something to improve chances of conception | 70.1 | 78.1 | 79.3 | 77.6 | 72.6 | |||||
| Last method of contraception was hormonal | 37.7 | 33.1 | 33.3 | 38.1 | 36.0 | |||||
Abbreviations: MET, metabolic equivalent; PSS, Perceived Stress Scale.
a All characteristics except for age are standardized to the cohort age at baseline.
b Weight (kg)/height (m)2.
c Among employed individuals.
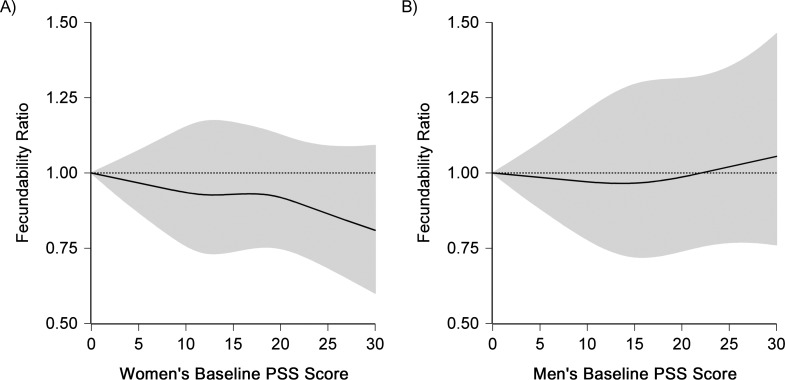
After adjusting for confounders, we observed an inverse association between perceived stress and fecundability (Table 3). Compared with baseline PSS scores <10, women with baseline PSS scores of 10–14, 15–19, 20–24, and ≥25 had FRs of 0.96 (95% confidence interval (CI): 0.87, 1.07), 0.99 (95% CI: 0.89, 1.09), 0.95 (95% CI: 0.84, 1.07), and 0.87 (95% CI: 0.74, 1.02). Results for time-varying and cumulative average PSS scores were similar to the baseline-only results. Consistent with the categorical results, restricted cubic spline analyses showed an approximate linear decline in fecundability with increasing PSS scores (Figure 1A).
Table 3.
Women’s and Men’s Scores on the Perceived Stress Scale and Fecundability, Pregnancy Study Online, United States and Canada, 2013–2018
| Exposure | No. of Pregnancies | No. of Cycles | Unadjusted | Adjusteda | ||
|---|---|---|---|---|---|---|
| FR | 95% CI | FR | 95% CI | |||
| Women’s baseline PSS scoreb | ||||||
| <10 | 420 | 2,525 | 1.00 | Referent | 1.00 | Referent |
| 10–14 | 898 | 5,932 | 0.94 | 0.85, 1.05 | 0.96 | 0.87, 1.07 |
| 15–19 | 901 | 5,955 | 0.96 | 0.86, 1.06 | 0.99 | 0.89, 1.09 |
| 20–24 | 459 | 3,336 | 0.90 | 0.80, 1.02 | 0.95 | 0.84, 1.07 |
| ≥25 | 173 | 1,382 | 0.80 | 0.68, 0.94 | 0.87 | 0.74, 1.02 |
| Women’s time-varying PSS scoreb | ||||||
| <10 | 446 | 2,762 | 1.00 | Referent | 1.00 | Referent |
| 10–14 | 875 | 5,717 | 0.94 | 0.85, 1.05 | 0.96 | 0.87, 1.07 |
| 15–19 | 902 | 5,839 | 0.97 | 0.87, 1.08 | 0.99 | 0.90, 1.10 |
| 20–24 | 450 | 3,387 | 0.88 | 0.78, 0.99 | 0.91 | 0.81, 1.03 |
| ≥25 | 178 | 1,425 | 0.81 | 0.69, 0.95 | 0.87 | 0.74, 1.02 |
| Women’s cumulative average PSS scoreb | ||||||
| <10 | 436 | 2,621 | 1.00 | Referent | 1.00 | Referent |
| 10–14 | 897 | 6,031 | 0.92 | 0.83, 1.02 | 0.94 | 0.85, 1.05 |
| 15–19 | 916 | 5,905 | 0.97 | 0.87, 1.07 | 0.99 | 0.90, 1.10 |
| 20–24 | 447 | 3,330 | 0.87 | 0.77, 0.98 | 0.91 | 0.81, 1.03 |
| ≥25 | 155 | 1,243 | 0.76 | 0.64, 0.90 | 0.83 | 0.70, 0.98 |
| Men’s baseline PSS score | ||||||
| <10 | 155 | 970 | 1.00 | Referent | 1.00 | Referent |
| 10–14 | 265 | 1,724 | 1.01 | 0.89, 1.14 | 0.95 | 0.79, 1.15 |
| 15–19 | 230 | 1,348 | 1.12 | 0.96, 1.31 | 1.07 | 0.86, 1.33 |
| 20–24 | 126 | 923 | 1.02 | 0.82, 1.27 | 1.02 | 0.76, 1.36 |
| ≥25 | 47 | 331 | 1.04 | 0.73, 1.48 | 1.03 | 0.69, 1.54 |
Abbreviations: CI, confidence interval; FR, fecundability ratio; PSS, Perceived Stress Scale.
a Adjusted for age, body mass index, race/ethnicity, education, income, employment status, work duration, and physical activity.
b Final models for women were inverse probability weighted.
Figure 1.
Association between baseline women’s and men’s scores on the Perceived Stress Scale (PSS) and fecundability, fitted using restricted cubic splines, Pregnancy Study Online, United States and Canada, 2013–2018. The reference level for the fecundability ratio (FR) is a PSS score of 0. The curves were adjusted for age, body mass index, race/ethnicity, education, income, employment status, work duration, and physical activity. The solid line represents the FR, and the shaded area represents the 95% confidence band. The splines are trimmed at the 99th percentile and have 4 knot points each at the 10th, 25th, 75th, and 90th percentiles.
The association between women’s baseline PSS scores and fecundability was stronger among couples attempting conception for 0–2 cycles at enrollment (n = 3,158; comparing baseline PSS score ≥25 with <10, FR = 0.83, 95% CI: 0.68, 1.00) than for couples attempting conception for 3–6 cycles at enrollment (n = 1,611; FR = 1.01, 95% CI: 0.73, 1.41). When we restricted the analysis to the first observed cycle (n = 4,769), FRs for women’s baseline PSS scores 10–14, 15–19, 20–24, and ≥25 compared with <10 were 0.91 (95% CI: 0.78, 1.07), 1.01 (95% CI: 0.86, 1.19), 0.91 (95% CI: 0.75, 1.10), and 0.72 (95% CI: 0.55, 0.95), respectively. The association was stronger among younger women (for PSS score ≥25 vs. <10 and age <35 years (n = 4,138), FR = 0.83, 95% CI: 0.70, 0.99; for women aged ≥35 years (n = 631), FR = 1.18, 95% CI: 0.76, 1.84) (data not shown).
Men’s PSS scores were not substantially associated with fecundability (Table 3); adjustment for women’s PSS score made little difference in FRs (data not shown). Results were similar among men with complete data on PSS score. In the restricted cubic spline analysis, we also observed little relationship between men’s PSS score and fecundability (Figure 1B). Results were similar across strata of attempt time at study entry and female partner’s age, and when restricted to the first observed cycle of follow-up.
We observed lower fecundability in couples where the man had a PSS score <10 and the woman had a PSS score of ≥20 (FR = 0.74, 95% CI: 0.42, 1.30), compared with couples where both partners had PSS scores <10 (Table 4). Conversely, couples where both partners had PSS scores ≥20 did not have reduced fecundability, although results were imprecise (FR = 1.22, 95% CI: 0.74, 2.03).
Table 4.
Women’s and Men’s Baseline Scores on the Perceived Stress Scale and Fecundability, Pregnancy Study Online, United States and Canada, 2013–2018
| Couple’s PSS Score Category | No. of Pregnancies | No. of Cycles | Unadjusted | Adjusteda | ||
|---|---|---|---|---|---|---|
| FR | 95% CI | FR | 95% CI | |||
| Both partners <10 | 41 | 263 | 1.00 | Referent | 1.00 | Referent |
| Woman’s 10–19, man’s <10 | 96 | 528 | 1.11 | 0.73, 1.66 | 1.04 | 0.69, 1.57 |
| Woman’s ≥20, man’s <10 | 18 | 179 | 0.78 | 0.44, 1.37 | 0.74 | 0.42, 1.30 |
| Woman’s <10, man’s 10–19 | 82 | 426 | 1.21 | 0.79, 1.85 | 1.12 | 0.73, 1.70 |
| Both partners 10–19 | 309 | 1,959 | 1.04 | 0.74, 1.45 | 0.98 | 0.70, 1.36 |
| Woman’s ≥20, man’s 10–19 | 104 | 687 | 0.98 | 0.69, 1.39 | 0.95 | 0.67, 1.35 |
| man’s | 20 | 95 | 1.40 | 0.80, 2.47 | 1.12 | 0.62, 2.03 |
| Woman’s 10–19, man’s ≥20 | 106 | 815 | 0.93 | 0.65, 1.33 | 0.92 | 0.64, 1.34 |
| Both partners ≥20 | 47 | 344 | 1.15 | 0.70, 1.88 | 1.22 | 0.74, 2.03 |
Abbreviations: CI, confidence interval; FR, fecundability ratio; PSS, Perceived Stress Scale.
a Adjusted for age, body mass index, race/ethnicity, education, income, employment status, work duration, and physical activity.
In mediation analyses (Table 5), natural indirect effects were small (range, FR = 0.98–1.00), whereas natural direct effects were similar to the overall association. The proportions of the association mediated by intercourse frequency and irregular cycles were 10.0% and 9.7%, respectively; the proportion mediated for other variables was small (<5%).
Table 5.
Natural Direct and Indirect Effects of Women’s Baseline Scores on the Perceived Stress Scale (≥25 vs. <10) on Fecundability, Pregnancy Study Online, United States and Canada, 2013–2018
| Mediator | Natural Indirect Effect | Natural Direct Effect | Proportion Mediated, % | ||
|---|---|---|---|---|---|
| FRa | 95% CI | FRa | 95% CI | ||
| Sleep duration (<7 vs. ≥7 hours/night) | 0.99 | 0.96, 1.04 | 0.86 | 0.70, 1.02 | 1.9 |
| Current smoker (yes vs. no) | 1.00 | 0.99, 1.01 | 0.85 | 0.70, 1.00 | −0.7 |
| Alcohol intake (≥14 vs. <14 drinks/week) | 1.00 | 0.99, 1.01 | 0.85 | 0.70, 1.02 | 0.3 |
| Caffeine intake (≥300 vs.<300 mg/day) | 1.00 | 0.99, 1.01 | 0.85 | 0.70, 1.01 | −0.5 |
| Major Depression Inventory score (≥30 vs. <30) | 1.00 | 0.94, 1.06 | 0.86 | 0.70, 1.03 | 1.0 |
| Intercourse frequency (<1 vs. ≥1 times/week) | 0.98 | 0.97, 1.00 | 0.87 | 0.72, 1.04 | 10.0 |
| Irregular cycles (yes vs. no) | 0.98 | 0.97, 1.00 | 0.86 | 0.71, 1.02 | 9.7 |
Abbreviations: CI, confidence interval; FR, fecundability ratio.
a Adjusted for age, body mass index, race/ethnicity, education, income, employment status, work duration, and physical activity.
DISCUSSION
In this preconception cohort study, we found that greater perceived stress among women, but not men, was associated with lower couple fecundability. Results were similar regardless of how women’s stress was analyzed (fixed baseline, time-varying, or cumulative average) but were stronger among women attempting conception for 0–2 cycles at enrollment and for younger women.
We measured stress using the PSS, a validated scale that reflects perceived stress in a relatively short time frame (38). Our results diverge from the one prior study of the relationship between PSS score and fecundability. Among 339 female pregnancy planners from the United Kingdom, PSS scores measured on day 6 of the menstrual cycle showed little association with fecundability (for PSS scores 10–14, 15–19, and ≥20 vs. <10, FRs were 0.81 (95% CI: 0.52, 1.25), 0.98 (95% CI: 0.61, 1.56), and 0.94 (95% CI: 0.59, 1.50), respectively) (22). Our strongest findings were for PSS scores ≥25; if few women in the UK study had PSS scores ≥25, this could explain the discrepant findings. Our results are consistent with a study of 430 Danish pregnancy planners in which short-term psychological distress on day 21 of the menstrual cycle was associated with reduced fecundability (20).
Other studies have measured stress at a more granular level, with either daily stress diaries or measurement of salivary biomarkers of stress. In the Mount Sinai Study of Women Office Workers, a 1-unit increase in self-reported daily stress levels during the ovulatory and preovulatory windows was associated with 46% and 27% reductions in fecundability, respectively (21). Among 274 female pregnancy planners from the United Kingdom, a 1-unit increase in the natural logarithm of salivary α-amylase measured on the sixth day of each menstrual cycle was associated with a 10% reduction in first-cycle fecundability (95% CI: 29, −13) (18). Likewise, pregnancy planners from Michigan and Texas (n = 373) in the highest tertile of salivary α-amylase (measured on first day of the first observed menstrual cycle) had a fecundability odds ratio of 0.71 (95% CI: 0.51, 1.00) compared with the lowest tertile (19). We did not measure stress during specific windows of susceptibility, for example, during ovulation or implantation.
We ascertained PSS score every 8 weeks, but we asked the questions in reference to the previous 4 weeks. When we restricted the analysis to the first observed cycle of pregnancy attempt, our results were stronger, indicating possible attenuation from nondifferential exposure misclassification.
Consistency in results across baseline, time-varying, and cumulative average analyses could relate to the strong correlations among the 3 measures: correlation coefficients ranged from 0.88 to 0.97, and over 75% of women remained in the same PSS score category throughout follow-up. Neither our study nor others studying fecundability have measured stress over longer periods of time.
Ours is, to our knowledge, the first study to report that a male partner’s stress might modify the association between the female partner’s stress and fecundability, with partner-stress discordance resulting in stronger associations. Given the small number of couples in each category when cross-classified by stress, these findings might be due to chance. However, concordance of stress levels between partners could be a marker for relationship quality. The “buffering model” of stress hypothesizes that positive quality relationships can buffer the health effects of stress (58). If relationship quality modifies the association between stress and fecundability, and if partner stress discordance is a marker of relationship quality, this could explain the association among women whose partners report low levels of perceived stress.
Mediation analysis results indicated that intercourse frequency and irregular cycles, but not other factors, each explained a meaningful proportion (approximately 10%) of the association between PSS and fecundability. This observation indicates that if perceived stress exerts a causal effect on fertility, it is likely through decreased intercourse frequency, increased irregular cycles, and/or mechanisms not measured in our study.
Difficulty conceiving can cause stress (59); thus this association is particularly susceptible to reverse causation. However, associations were stronger among women who had been attempting conception for 0–2 cycles at enrollment, indicating that reverse causation was unlikely.
We found that the inverse association between PSS score and fecundability was present only among women <35 years of age. The absence of an association among older women might reflect chance variation, the higher baseline risk for infertility among older women, or biological differences related to age.
The lack of repeated measures of men’s perceived stress precluded an examination of the relationship between men’s time-varying PSS scores and fecundability. Baseline and time-varying results among women, however, were similar, implying that either the timing of stress was not an important factor or we had insufficient variability in stress over time to assess the extent to which acute versus chronic stress is important for fecundability. To the extent that our measure of stress was outside of a relevant time window, nondifferential misclassification would have attenuated our findings.
Women with lower income, less education, or higher body mass index are less likely to recognize their pregnancies early in gestation (60). These factors are also positively associated with stress. If stress delays pregnancy recognition, then outcome misclassification could have biased our results because nonstressed women would identify pregnancy losses earlier than stressed women. However, we found that women with PSS scores of <10 and ≥25 had similar mean gestational ages at positive pregnancy test (4.2 and 4.4 weeks, respectively), providing little evidence for pregnancy recognition bias.
Stressed women might be at higher risk of unplanned pregnancy (61), and unplanned pregnancies likely occur among more fecund women. Therefore, studies restricted to pregnancy planners might be susceptible to selection bias. However, many Pregnancy Study Online participants enroll at the beginning of their pregnancy attempt time. These women represent the full fertility spectrum, including those who conceive right away and those who take longer. Among women attempting pregnancy for 0 or 1 cycles at study entry, results were stronger than in the main analysis, indicating that a pregnancy planning bias is unlikely to explain our findings.
Adjustment for a wide range of demographic, behavioral, reproductive, and dietary variables did not substantially alter the association between PSS and fecundability, indicating little confounding by measured variables. Nevertheless, unmeasured confounding could have affected our results.
Women with higher baseline PSS scores were more likely to be lost to follow-up than those with lower scores. Accounting for potential differential attrition using inverse probability weights made little difference in our results.
Internet-based recruitment should not affect the internal validity of the study unless the relationship between perceived stress and fecundability differs according to internet access, which seems unlikely. Further, we have demonstrated in an internet-based cohort of Danish pregnancy planners that even when enrollment is related to factors such as age or parity, the measures of association are not biased (62).
There are several biological mechanisms through which women’s stress could directly affect fecundability. Stress is associated with higher levels of corticotropin-releasing hormone and glucocorticoids, which suppress the function of gonadotropin-releasing hormone and could delay or inhibit the luteinizing hormone surge of the menstrual cycle (14). Glucocorticoids could also suppress the uterine cytokine NF-κB and local inflammation, which are essential for implantation (17, 63). Stress might also reduce ovarian reserve, given that salivary α-amylase has been related to lower levels of anti-Müllerian hormone (64). Moreover, a study of couples undergoing in vitro fertilization found an association between nonfertility stressors and reduced conception, and the association was partially mediated by a lower number of oocytes harvested during oocyte retrieval (65).
In summary, in this large prospective cohort study, high levels of perceived stress among women were associated with reduced fecundability. Part of the association might be mediated through decreased intercourse frequency and increased risk of irregular cycles.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Boston University School of Public Health, Boston, Massachusetts (Amelia K. Wesselink, Elizabeth E. Hatch, Kenneth J. Rothman, Jennifer L. Weuve, Ann Aschengrau, Rebecca J. Song, Lauren A. Wise); and RTI International, Research Triangle Park, North Carolina (Kenneth J. Rothman).
This work was supported by the Eunice Kennedy Shriver National Institute for Child Health and Human Development (grants R01-HD086742, R21-HD072326, R03-HD090315, and T32-HD052458).
We thank Michael Bairos for developing and maintaining the web-based infrastructure of Pregnancy Study Online and Dr. Anne Marie Jukic for her thorough review of this manuscript.
Conflict of interest: none declared.
Abbreviations
- CI
confidence interval
- FR
fecundability ratio
- PSS
Perceived Stress Scale
REFERENCES
- 1. Witt WP, Cheng ER, Wisk LE, et al. Maternal stressful life events prior to conception and the impact on infant birth weight in the United States. Am J Public Health. 2014;104(suppl 1):S81–S89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Statistics Canada Stress and well-being. Health Reports. 2001;12(3):21–32. [Google Scholar]
- 3. Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007;298(14):1685–1687. [DOI] [PubMed] [Google Scholar]
- 4. Figueredo VM. The time has come for physicians to take notice: the impact of psychosocial stressors on the heart. Am J Med. 2009;122(8):704–712. [DOI] [PubMed] [Google Scholar]
- 5. Pennebaker JW. The Psychology of Physical Symptoms. New York, NY: Springer-Verlag; 1982. [Google Scholar]
- 6. McEwen B, Norton Lasley E. The End of Stress as We Know It. New York, NY: Dana Press; 2002. [Google Scholar]
- 7. Rao K. Recent research in stress, coping and women’s health. Curr Opin Psychiatry. 2009;22(2):188–193. [DOI] [PubMed] [Google Scholar]
- 8. Chrousos GP, Torpy DJ, Gold PW. Interactions between the hypothalamic-pituitary-adrenal axis and the female reproductive system: clinical implications. Ann Intern Med. 1998;129(3):229–240. [DOI] [PubMed] [Google Scholar]
- 9. Mai FM. Conception after adoption: an open question. Psychosom Med. 1971;33(6):509–514. [DOI] [PubMed] [Google Scholar]
- 10. Rock J, Tietze C, McLaughlin HB. Effect of adoption on infertility. Fertil Steril. 1965;16:305–312. [DOI] [PubMed] [Google Scholar]
- 11. Domar AD, Clapp D, Slawsby EA, et al. Impact of group psychological interventions on pregnancy rates in infertile women. Fertil Steril. 2000;73(4):805–811. [DOI] [PubMed] [Google Scholar]
- 12. Domar AD, Rooney KL, Wiegand B, et al. Impact of a group mind/body intervention on pregnancy rates in IVF patients. Fertil Steril. 2011;95(7):2269–2273. [DOI] [PubMed] [Google Scholar]
- 13. Klonoff-Cohen H, Chu E, Natarajan L, et al. A prospective study of stress among women undergoing in vitro fertilization or gamete intrafallopian transfer. Fertil Steril. 2001;76(4):675–687. [DOI] [PubMed] [Google Scholar]
- 14. Ferin M. Clinical review 105: stress and the reproductive cycle. J Clin Endocrinol Metab. 1999;84(6):1768–1774. [DOI] [PubMed] [Google Scholar]
- 15. Schenker JG, Meirow D, Schenker E. Stress and human reproduction. Eur J Obstet Gynecol Reprod Biol. 1992;45(1):1–8. [DOI] [PubMed] [Google Scholar]
- 16. Schliep KC, Mumford SL, Vladutiu CJ, et al. Perceived stress, reproductive hormones, and ovulatory function: a prospective cohort study. Epidemiology. 2015;26(2):177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Makrigiannakis A, Zoumakis E, Kalantaridou S, et al. Corticotropin-releasing hormone promotes blastocyst implantation and early maternal tolerance. Nat Immunol. 2001;2(11):1018–1024. [DOI] [PubMed] [Google Scholar]
- 18. Louis GM, Lum KJ, Sundaram R, et al. Stress reduces conception probabilities across the fertile window: evidence in support of relaxation. Fertil Steril. 2011;95(7):2184–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lynch CD, Sundaram R, Maisog JM, et al. Preconception stress increases the risk of infertility: results from a couple-based prospective cohort study—the LIFE study. Hum Reprod. 2014;29(5):1067–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hjollund NH, Jensen TK, Bonde JP, et al. Distress and reduced fertility: a follow-up study of first-pregnancy planners. Fertil Steril. 1999;72(1):47–53. [DOI] [PubMed] [Google Scholar]
- 21. Akhter S, Marcus M, Kerber RA, et al. The impact of periconceptional maternal stress on fecundability. Ann Epidemiol. 2016;26(10):710–716.e7. [DOI] [PubMed] [Google Scholar]
- 22. Lynch CD, Sundaram R, Buck Louis GM, et al. Are increased levels of self-reported psychosocial stress, anxiety, and depression associated with fecundity? Fertil Steril. 2012;98(2):453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nargund VH. Effects of psychological stress on male fertility. Nat Rev Urol. 2015;12(7):373–382. [DOI] [PubMed] [Google Scholar]
- 24. Althof SE, Needle RB. Psychological factors associated with male sexual dysfunction: screening and treatment for the urologist. Urol Clin North Am. 2011;38(2):141–146. [DOI] [PubMed] [Google Scholar]
- 25. Byun JS, Lyu SW, Seok HH, et al. Sexual dysfunctions induced by stress of timed intercourse and medical treatment. BJU Int. 2013;111(4 Pt B):E227–E234. [DOI] [PubMed] [Google Scholar]
- 26. Eskiocak S, Gozen AS, Taskiran A, et al. Effect of psychological stress on the L-arginine-nitric oxide pathway and semen quality. Braz J Med Biol Res. 2006;39(5):581–588. [DOI] [PubMed] [Google Scholar]
- 27. Lampiao F. Variation of semen parameters in healthy medical students due to exam stress. Malawi Med J. 2009;21(4):166–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Abu-Musa AA, Nassar AH, Hannoun AB, et al. Effect of the Lebanese civil war on sperm parameters. Fertil Steril. 2007;88(6):1579–1582. [DOI] [PubMed] [Google Scholar]
- 29. Zorn B, Sucur V, Stare J, et al. Decline in sex ratio at birth after 10-day war in Slovenia: brief communication. Hum Reprod. 2002;17(12):3173–3177. [DOI] [PubMed] [Google Scholar]
- 30. DeStefano F, Annest JL, Kresnow MJ, et al. Semen characteristics of Vietnam veterans. Reprod Toxicol. 1989;3(3):165–173. [DOI] [PubMed] [Google Scholar]
- 31. Jurewicz J, Hanke W, Sobala W, et al. The effect of stress on the semen quality [in Polish]. Med Pr. 2010;61(6):607–613. [PubMed] [Google Scholar]
- 32. Fenster L, Katz DF, Wyrobek AJ, et al. Effects of psychological stress on human semen quality. J Androl. 1997;18(2):194–202. [PubMed] [Google Scholar]
- 33. Hjollund NH, Bonde JP, Henriksen TB, et al. Reproductive effects of male psychologic stress. Epidemiology. 2004;15(1):21–27. [DOI] [PubMed] [Google Scholar]
- 34. Hjollund NH, Bonde JP, Henriksen TB, et al. Job strain and male fertility. Epidemiology. 2004;15(1):114–117. [DOI] [PubMed] [Google Scholar]
- 35. Wise LA, Rothman KJ, Mikkelsen EM, et al. Design and conduct of an internet-based preconception cohort study in North America: Pregnancy Study Online. Paediatr Perinat Epidemiol. 2015;29(4):360–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Subar AF, Thompson FE, Kipnis V, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America’s Table Study. Am J Epidemiol. 2001;154(12):1089–1099. [DOI] [PubMed] [Google Scholar]
- 37. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 38. Cohen S, Williamson GM. Perceived stress in a probability sample of the United States In: Spacapan S, Oskamp S, eds. The Social Psychology of Health. Thousand Oaks, CA: Sage Publications; 1988:31–67. [Google Scholar]
- 39. Lee EH. Review of the psychometric evidence of the Perceived Stress Scale. Asian Nurs Res (Korean Soc Nurs Sci). 2012;6(4):121–127. [DOI] [PubMed] [Google Scholar]
- 40. Bech P. Quality of life instruments in depression. Eur Psychiatry. 1997;12(4):194–198. [DOI] [PubMed] [Google Scholar]
- 41. Guenther PM, Casavale KO, Reedy J, et al. Update of the Healthy Eating Index: HEI-2010. J Acad Nutr Diet. 2013;113(4):569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hewitt PL, Flett GL, Mosher SW. The Perceived Stress Scale: factor structure and relation to depression symptoms in a psychiatric sample. J Psychopathol Behav Assess. 1992;14(3):247–257. [Google Scholar]
- 43. Perera MJ, Brintz CE, Birnbaum-Weitzman O, et al. Factor structure of the Perceived Stress Scale-10 (PSS) across English and Spanish language responders in the HCHS/SOL Sociocultural Ancillary Study. Psychol Assess. 2017;29(3):320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Therneau TM. Extending the Cox model In: Lin DY, Fleming TR, eds. Proceedings of the First Seattle Symposium in Biostatistics: Survival Analysis. New York, NY: Springer-Verlag; 1997:51–84. [Google Scholar]
- 45. Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York, NY: Springer-Verlag; 2000. [Google Scholar]
- 46. Schisterman EF, Cole SR, Ye A, et al. Accuracy loss due to selection bias in cohort studies with left truncation. Paediatr Perinat Epidemiol. 2013;27(5):491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Howards PP, Hertz-Picciotto I, Poole C. Conditions for bias from differential left truncation. Am J Epidemiol. 2007;165(4):444–452. [DOI] [PubMed] [Google Scholar]
- 48. Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551–561. [DOI] [PubMed] [Google Scholar]
- 49. Li R, Hertzmark E, Spiegelman D. The SAS GLMCURV9 Macro. Boston, MA: Channing Laboratory, 2008. [Google Scholar]
- 50. Howe CJ, Cole SR, Lau B, et al. Selection bias due to loss to follow up in cohort studies. Epidemiology. 2016;27(1):91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Weuve J, Tchetgen Tchetgen EJ, Glymour MM, et al. Accounting for bias due to selective attrition: the example of smoking and cognitive decline. Epidemiology. 2012;23(1):119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hernán MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11(5):561–570. [DOI] [PubMed] [Google Scholar]
- 53. Hall KS, Kusunoki Y, Gatny H, et al. Stress symptoms and frequency of sexual intercourse among young women. J Sex Med. 2014;11(8):1982–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kashani M, Eliasson A, Vernalis M. Perceived stress correlates with disturbed sleep: a link connecting stress and cardiovascular disease. Stress. 2012;15(1):45–51. [DOI] [PubMed] [Google Scholar]
- 55. Yamamoto K, Okazaki A, Sakamoto Y, et al. The relationship between premenstrual symptoms, menstrual pain, irregular menstrual cycles, and psychosocial stress among Japanese college students. J Physiol Anthropol. 2009;28(3):129–136. [DOI] [PubMed] [Google Scholar]
- 56. Barsom SH, Mansfield PK, Koch PB, et al. Association between psychological stress and menstrual cycle characteristics in perimenopausal women. Womens Health Issues. 2004;14(6):235–241. [DOI] [PubMed] [Google Scholar]
- 57. Valeri L, VanderWeele TJ. SAS macro for causal mediation analysis with survival data. Epidemiology. 2015;26(2):e23–e24. [DOI] [PubMed] [Google Scholar]
- 58. Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychol Bull. 1985;98(2):310–357. [PubMed] [Google Scholar]
- 59. Seibel MM, Taymor ML. Emotional aspects of infertility. Fertil Steril. 1982;37(2):137–145. [DOI] [PubMed] [Google Scholar]
- 60. Ayoola AB, Stommel M, Nettleman MD. Late recognition of pregnancy as a predictor of adverse birth outcomes. Am J Obstet Gynecol. 2009;201(2):156.e1–156.e6. [DOI] [PubMed] [Google Scholar]
- 61. Hall KS, Kusunoki Y, Gatny H, et al. The risk of unintended pregnancy among young women with mental health symptoms. Soc Sci Med. 2014;100:62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hatch EE, Hahn KA, Wise LA, et al. Evaluation of selection bias in an internet-based study of pregnancy planners. Epidemiology. 2016;27(1):98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Parker VJ, Douglas AJ. Stress in early pregnancy: maternal neuro-endocrine-immune responses and effects. J Reprod Immunol. 2010;85(1):86–92. [DOI] [PubMed] [Google Scholar]
- 64. Dong YZ, Zhou FJ, Sun YP. Psychological stress is related to a decrease of serum anti-mullerian hormone level in infertile women. Reprod Biol Endocrinol. 2017;15:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ebbesen SM, Zachariae R, Mehlsen MY, et al. Stressful life events are associated with a poor in-vitro fertilization (IVF) outcome: a prospective study. Hum Reprod. 2009;24(9):2173–2182. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.