Abstract
BACKGROUND
To explore how central hemodynamics respond to dietary sodium and potassium interventions, and whether the responses are associated with metabolic traits.
METHODS
We conducted a dietary intervention study including a 7-day low-sodium (51.3 mmol sodium/day) intervention, a 7-day high-sodium (307.8 mmol sodium/day) intervention, and a 7-day high-sodium with potassium supplementation (60.0 mmol potassium/day) intervention among 99 northern Chinese subjects aged 18–60 years. Five metabolic traits included abdominal obesity, high triglycerides, low HDL cholesterol, raised blood pressure (BP), and high glucose. Central hemodynamics were measured at baseline and during each intervention.
RESULTS
Central systolic BP (SBP), diastolic BP (DBP), pulse pressure (PP), and augmentation index (AIx@75) significantly decreased during low-sodium intervention, increased during high-sodium intervention, and then decreased during potassium supplementation. We observed potential linear trends toward significance of central SBP and PP responses to low-sodium intervention, and significant linear trends of responses to high-sodium intervention as the number of metabolic traits grows. For example, among participants with 0 or 1, 2 or 3, and 4 or 5 metabolic traits, central SBP responses to high-sodium intervention were 8.8 [95% confidence interval (5.8, 11.8)], 9.3 (7.1, 11.6), and 14.0 (11.6, 16.3) mmHg, respectively (P for trend = 0.009). Significant linear trends of central SBP and DBP responses to potassium supplementation were also observed.
CONCLUSIONS
Central BP and AIx@75 were lowered by sodium reduction and potassium supplementation, and elevated by sodium-loading. The responses of central BP were pronounced among individuals with metabolic traits clustering.
CLINICAL TRIALS REGISTRATION
Trial Number NCT00721721 (The current study is registered on ClinicalTrials.gov; https://clinicaltrials.gov).
Keywords: blood pressure, central blood pressure, hypertension, metabolic traits, sodium, potassium
Central blood pressure (BP) does not correspond to brachial BP due to pressure pulse amplification from the aorta to the periphery.1 Accumulating studies have suggested that central systolic BP (SBP) and pulse pressure (PP) are more closely related to subclinical organ damage2–4 and cardiovascular diseases4–7 than brachial BP, indicating a better predictive ability of central BP.
Low-sodium and high-potassium diets have been well documented to lower brachial BP.8,9 Currently, several randomized controlled trails have also examined the effects of dietary sodium or potassium intervention on central BP10–14; however, the effects of sodium or potassium on central BP independent of peripheral BP have not been evaluated. Besides, central augmentation index (AIx) response to dietary sodium restriction and potassium supplementation remains controversial.11–18 Thus, clarifying the responses of central hemodynamics to dietary sodium and potassium interventions is needed.
BP responses to sodium intake vary among individuals, and this phenomenon was called “salt sensitivity”.19 Previous studies have uncovered that insulin resistance20 and metabolic syndrome21 (MS) increase salt sensitivity of brachial BP. However, there is no study investigating how metabolic traits influence salt sensitivity of central BP up to present. Moreover, the relationship between metabolic traits and brachial or central BP responses to potassium supplementation has not been evaluated.
Therefore, we conducted this dietary intervention study to investigate the responses of central hemodynamics to dietary sodium and potassium interventions, and whether the responses are associated with metabolic traits. The current study may be helpful to elucidate whether dietary sodium and potassium interventions are more effective among individuals with more metabolic traits.
METHODS
Study participants
The present study was conducted in rural areas of Shandong province in northern China from June to October 2010. The study participants were a part of the Genetic Epidemiology Network of Salt Sensitivity (GenSalt) study. Details of the study population and methods for the GenSalt study have been published elsewhere.22 In brief, a community-based BP screening was conducted among persons aged 18–60 years to identify potential probands. Those with mean brachial SBP of 130–160 mm Hg and/or diastolic BP (DBP) of 85–100 mm Hg were recruited, along with their parents, siblings, spouses, and offspring aged 16 years or older. Individuals with stage-2 hypertension, secondary hypertension, a history of clinical cardiovascular disease, diabetes, or chronic kidney disease, using antihypertensive medications, pregnant, heavy alcohol users, or currently on a low-sodium diet were excluded from the study. A total of 108 participants were recruited at baseline, among whom 7 did not take central hemodynamic measurements during low-sodium intervention, 1 during high-sodium intervention, and another one during high-sodium with potassium supplementation intervention. Thus, 99 participants were eligible and included in the current analysis. This study was approved by the institutional review board of Fuwai Hospital and participating institutions. Written informed consent was obtained from each participant before data collection.
Dietary intervention
After a 3-day baseline examination, study participants received a 7-day low-sodium diet (3 g salt or 51.3 mmol sodium per day), followed by a 7-day high-sodium diet (18 g salt or 307.8 mmol sodium per day). During these 2 intervention periods, potassium intake remained consistent. Although not recorded, the potassium intake was monitored by the 24-hour urinary potassium excretion data. In the final week, participants maintained a high-sodium diet and took a 60 mmol potassium supplement daily. Although dietary sodium intake was the same for all study participants during each intervention, dietary total energy intake varied according to their baseline energy intake level. Participants were categorized into 5 energy intake levels (1,600; 2,100; 2,600; 3,100; and 3,600 kcal/day) based on baseline 24-hour Dietary Recall data. All meals were prepared without salt by full-time chefs in onsite kitchens. Prepackaged salt was added to each participant’s meal by study staff when it was served. All participants were required to have their meals at the study kitchen under the supervision of the study staff during the entire study period, and were instructed to avoid consuming any foods that were not provided by the study. Besides, 3-timed urinary specimens (1 24-hour and 2 overnight) were collected at baseline and in each intervention period to monitor participants’ compliance with dietary interventions. The overnight urinary excretions of sodium and potassium were converted to 24-hour values on the basis of formulas developed from data obtained in the GenSalt study. The results showed good compliance with the interventions. The mean (SD) 24-hour urinary excretions of sodium and potassium were 214.4 (52.0) and 42.0 (10.9) mmol at baseline, 55.3 (18.3) and 35.7 (9.1) mmol during low-sodium intervention, 234.4 (31.6) and 44.2 (9.3) mmol during high-sodium intervention, and 230.3 (24.5) and 77.5 (10.8) mmol during high-sodium intervention with potassium supplement, respectively.
Data collection
A standard questionnaire was administered by trained staff at the baseline examination to collect information on demographic characteristics, personal and family medical history, and lifestyle risk factors. We adapted the Paffenbarger Physical Activity Questionnaire to collect information on the physical activity.23 The physical activity information obtained from the questionnaire was converted to metabolic equivalent hours per day. Body weight and height were measured twice in light indoor clothing without shoes. Body mass index was calculated as kilograms per square meter (kg/m2). Waist circumference was measured 1 cm above the participant’s navel during light breathing. Overnight (≥8 hour) fasting blood specimens were obtained for measurement of glucose and lipid concentrations. Plasma glucose concentration was measured with a modified hexokinase enzymatic method (Hitachi automatic clinical analyser, model 7060, Tokyo, Japan). Concentrations of HDL cholesterol and triglycerides were assessed enzymatically with commercially available reagents.
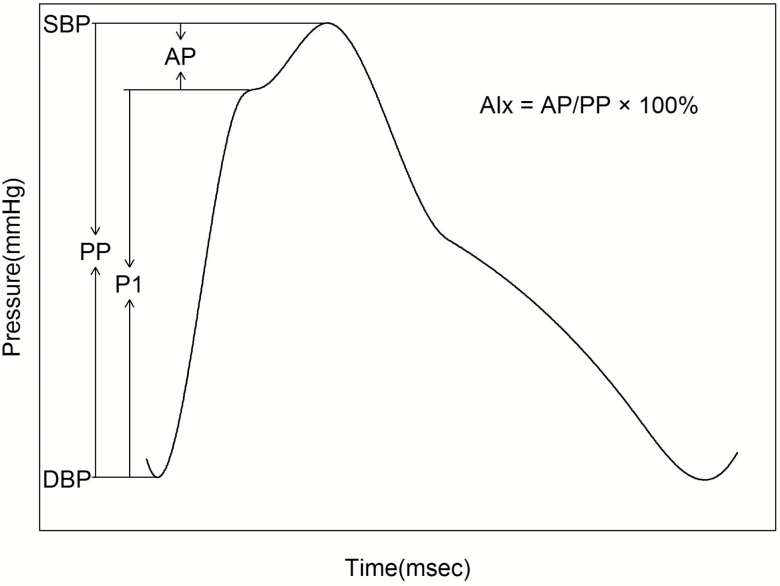
Three brachial BP measurements were obtained in every morning of the 3-day baseline examination by certified technicians using a random-zero sphygmomanometer according to a standard protocol. Pulse wave analysis was performed using the SphygmoCor CP system (AtCor Medical Ltd, Australia) in the morning of baseline examination and day 6 of each intervention. The radial pressure wave was obtained using applanation tonometry at the right radial artery, and then converted to central aortic pressure wave by a validated generalized transfer function.24 Central hemodynamics included central SBP, DBP, PP, forward wave amplitude (P1), and augmentation pressure normalized to a heart rate of 75 bpm (AP@75). Central AIx was calculated as AP/PP×100%, and then normalized to a heart rate of 75 bpm25 (AIx@75) (Figure 1). Peripheral (that is, radial artery) SBP, DBP, PP, and heart rate were also obtained. All participants were measured in sitting position after 5-minute rest. Additionally, participants were advised to avoid smoking, consuming alcohol, coffee or tea, and physical activity for at least 30 minutes before measurement.
Figure 1.
Typical central aortic pressure waveform from a middle-aged subject. Abbreviations: AIx, augmentation index; AP, augmentation pressure; DBP, diastolic blood pressure; PP, pulse pressure; P1, forward wave amplitude; SBP, systolic blood pressure.
Statistical analysis
Five metabolic traits included abdominal obesity (waist circumference ≥90 cm in men and ≥80 cm in women), high triglycerides (serum concentration of triglycerides ≥150 mg/dl), low HDL cholesterol (HDL cholesterol concentration <40 mg/dl in men and <50 mg/dl in women), raised brachial BP (SBP ≥ 130 mm Hg or DBP ≥ 85 mm Hg), and high glucose (plasma glucose concentration ≥ 100 mg/dl).26 Participants were divided into 3 groups according to the number of metabolic traits: 0 or 1, 2 or 3, and 4 or 5. The responses of central hemodynamics were defined as follows: responses to low-sodium intervention = values on low-sodium diet − values at baseline; responses to high-sodium intervention = values on high-sodium diet − values on low-sodium diet; responses to potassium supplementation = values on high-sodium diet with potassium supplement − values on high-sodium diet.
Continuous variables were presented as mean ± SD or median with interquartile range based on normality of data and categorical variables as frequency with percentage. Baseline characteristics among 3 groups were compared by 1-way analysis of variance for normal variables and by Kruskal–Wallis test for nonnormal variables, using Student–Newman–Keuls test for post hoc contrast. Chi-square test was used to compare differences in categorical variables. Central and peripheral hemodynamics between 2 consecutive interventions were compared by paired t test. The responses of central BP independent of corresponding peripheral BP were assessed by linear mixed-effects model. We performed multivariable linear regression analysis to test linear trends of the responses of central hemodynamics as the number of metabolic traits increasing, adjusted for age, gender, body mass index, physical activity, smoking and drinking status, as well as baseline 24-hour urinary excretion of sodium and potassium. We additionally calculated the standardized β coefficients of each metabolic trait with the central hemodynamics responses to interventions, and the false discovery rate was used to adjust the results for multiple comparisons. A 2-tailed P < 0.05 was considered statistically significant. All statistical analyses were performed with SAS software version 9.4 (SAS Institute, Inc., Cary, NC).
RESULTS
A total of 99 participants completed the dietary sodium and potassium interventions, and the majority had 4 or 5 (38.4%) or 2 or 3 (36.4%) metabolic traits. As shown in Table 1, participants with more metabolic traits were more likely to be women. As expected, brachial SBP and DBP, body mass index, waist circumference, fasting concentration of triglycerides and glucose were significantly higher, whereas HDL cholesterol concentration was significantly lower, in participants with more metabolic traits. There were no significant differences in age, physical activity, and 24-hour urinary excretion of sodium and potassium by the number of metabolic traits.
Table 1.
Baseline characteristics of study participants by the number of metabolic traits
| Overall (n = 99) | Number of metabolic traits | P | |||
|---|---|---|---|---|---|
| 0 or 1 (n = 25) | 2 or 3 (n = 36) | 4 or 5 (n = 38) | |||
| Age (years) | 53.4 ± 6.6 | 54.2 ± 5.9 | 52.2 ± 8.1 | 54.0 ± 5.3 | 0.396 |
| Male, n (%) | 60 (60.6%) | 19 (76.0%) | 24 (66.7%) | 17 (44.7%) | 0.030 |
| Body mass index (kg/m2) | 25.1 ± 3.4 | 22.2 ± 2.2b | 25.0 ± 3.2c | 27.0 ± 2.8d | <0.001 |
| Physical activity (METsa per day) | 57.2 ± 19.9 | 62.5 ± 21.4 | 58.5 ± 21.2 | 52.5 ± 16.9 | 0.132 |
| Current smoking, n (%) | 23 (23.2%) | 8 (32.0%) | 11 (30.6%) | 4 (10.5%) | 0.061 |
| Current drinking, n (%) | 31 (31.3%) | 6 (24.0%) | 15 (41.7%) | 10 (26.3%) | 0.240 |
| Brachial SBP (mm Hg) | 134.3 ± 20.4 | 119.7 ± 12.7b | 128.4 ± 14.1c | 136.2 ± 11.6d | <0.001 |
| Brachial DBP (mm Hg) | 83.0 ± 9.0 | 77.2 ± 8.4b | 79.8 ± 8.0 | 84.6 ± 8.0d | 0.002 |
| Waist circumference (cm) | 87.8 ± 10.6 | 78.6 ± 7.4b | 87.5 ± 10.0c | 94.1 ± 8.2d | <0.001 |
| HDL cholesterol (mg/dl) | 42.6 (35.4~50.9) | 49.1 (43.0~52.1)b | 44.5 (40.5~58.1) | 35.0 (31.6~39.6)d | <0.001 |
| Triglyceride (mg/dl) | 157.8 (112.5~255.6) | 93.8 (82.9~119.8)b | 137.8 (119.0~198.6)c | 263.5 (201.8~345.8)d | <0.001 |
| Glucose (mg/dl) | 104.1 (93.9~121.9) | 92.3 (88.0~97.8)b | 102.0 (95.1~110.2)c | 115.4 (105.7~135.9)d | <0.001 |
| Urinary excretion (mmol per 24-hour) | |||||
| Sodium | 214.4 ± 52.0 | 201.9 ± 49.1 | 221.2 ± 48.2 | 216.3 ± 56.9 | 0.351 |
| Potassium | 42.0 ± 10.9 | 37.9 ± 7.3 | 44.0 ± 9.6 | 42.8 ± 13.3 | 0.083 |
Values are mean ± SD or median (IQR) for continuous variables and frequency (%) for categorical variables. Abbreviations: DBP, diastolic blood pressure; HDL, high-density lipoprotein; MET, metabolic equivalent; SBP, systolic blood pressure.
aOne MET is equivalent to a metabolic rate consuming 1 kcal per kg of body weight per hour.
b0 or 1 vs. 4 or 5, P < 0.05.
c0 or 1 vs. 2 or 3, P < 0.05.
d2 or 3 vs. 4 or 5, P < 0.05.
Central and peripheral hemodynamics at baseline and during each intervention are presented in Table 2. Central SBP, DBP, PP, AP@75, and AIx@75 significantly decreased during low-sodium diet, increased during high-sodium diet, and then decreased during high-sodium diet with potassium supplement. Peripheral SBP, DBP, and PP significantly decreased during low-sodium diet and increased during high-sodium diet. However, only peripheral SBP significantly decreased during potassium supplementation. After adjustment for corresponding peripheral BP, we observed a −2.7 [95% confidence interval (−3.9, −1.5)] mm Hg decrease and a 2.0 (1.0, 3.0) mm Hg increase of central SBP, as well as a −2.2 (−3.3, −1.0) mmHg decrease and a 1.7 (0.8, 2.6) mm Hg increase of central PP, during low-sodium and high-sodium intervention, respectively (all P < 0.001) (Supplementary Table S1).
Table 2.
Central and peripheral hemodynamics of study participants at baseline and during dietary interventions
| Baseline | Low sodium | High sodium | High-sodium + potassium supplementation | |
|---|---|---|---|---|
| SBP, mm Hg | ||||
| Central | 123.2 ± 17.2 | 111.6 ± 12.9a | 122.6 ± 14.2b | 120.0 ± 13.4c |
| Peripheral | 134.3 ± 20.4 | 123.1 ± 14.3a | 133.2 ± 15.2b | 131.1 ± 14.8c |
| DBP, mm Hg | ||||
| Central | 84.3 ± 9.1 | 79.4 ± 8.6a | 84.9 ± 8.9b | 83.8 ± 8.3c |
| Peripheral | 83.0 ± 9.0 | 78.1 ± 8.6a | 83.7 ± 8.9b | 82.7 ± 8.2 |
| PP, mm Hg | ||||
| Central | 38.9 ± 14.1 | 32.2 ± 9.4a | 37.7 ± 11.4b | 36.2 ± 10.1c |
| Peripheral | 51.3 ± 17.6 | 45.0 ± 11.4a | 49.6 ± 12.6b | 48.5 ± 11.5 |
| Central P1, mm Hg | 28.7 ± 10.1 | 25.0 ± 6.7a | 27.7 ± 7.3b | 27.4 ± 6.9 |
| Central AP@75, mm Hg | 10.1 ± 5.2 | 7.7 ± 4.4a | 9.9 ± 5.0b | 8.9 ± 4.6c |
| Central AIx@75, % | 25.5 ± 8.4 | 22.6 ± 10.7a | 25.7 ± 9.5b | 23.5 ± 9.3c |
| Heart rate, bpm | 75.9 ± 11.5 | 78.7 ± 10.4a | 76.3 ± 10.1 | 76.7 ± 8.9 |
Values are mean ± SD. Abbreviations: AIx@75, augmentation index normalized to a heart rate of 75 bpm; AP@75, augmentation pressure normalized to a heart rate of 75 bpm; DBP, diastolic blood pressure; PP: pulse pressure; P1, forward wave amplitude; SBP, systolic blood pressure.
a P < 0.05 vs. baseline.
b P < 0.05 vs. low-sodium intervention.
c P < 0.05 vs. high-sodium intervention.
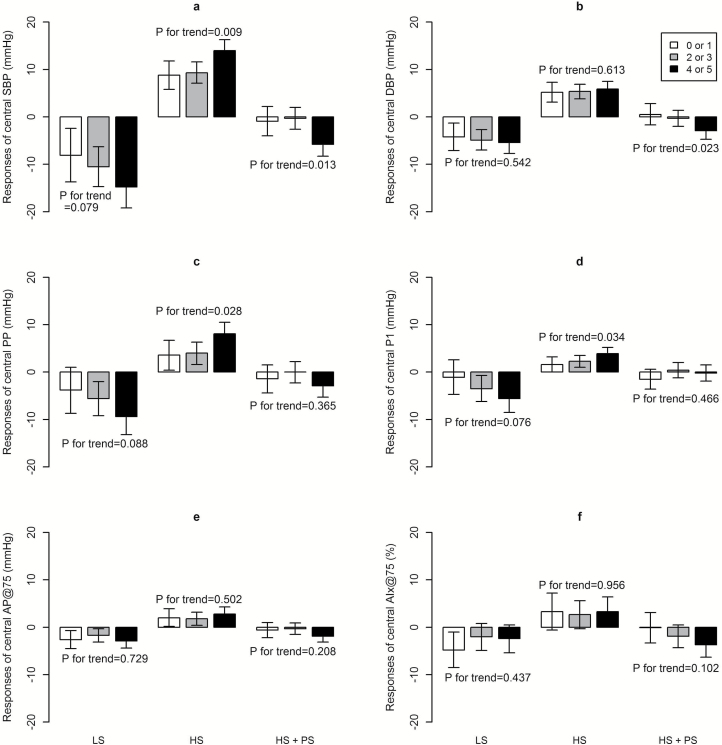
After multivariable adjustment, we observed potential linear trends toward significance of central SBP, PP, and P1 responses to low-sodium intervention, and significant linear trends of the responses to high-sodium intervention with the increment number of metabolic traits (Figure 2). For example, among participants with 0 or 1, 2 or 3, and 4 or 5 metabolic traits, the responses of central SBP were −8.1 (−13.7, −2.4), −10.5 (−14.7, −6.3), and −14.8 (−19.2, −10.3) mm Hg during low-sodium intervention (P for trend = 0.079), and 8.8 (5.8, 11.8), 9.3 (7.1, 11.6), and 14.0 (11.6, 16.3) mm Hg during high-sodium intervention (P for trend = 0.009). Moreover, significant linear trends of central SBP and DBP responses to potassium supplementation were also observed (both P for trend < 0.05) (Figure 2). In addition, we found that glucose was significantly correlated to central SBP, PP and P1 responses to low-sodium intervention, and brachial SBP was significantly associated with central PP response to high-sodium intervention. During potassium supplementation, waist circumference and HDL cholesterol significantly related to the responses of central SBP and DBP, and triglyceride was significantly associated with the response of central SBP. However, only waist circumference remained significantly associated with central DBP response to potassium supplementation after adjustment for multiple comparisons (Supplementary Table S2–S4).
Figure 2.
Multivariable-adjusteda mean responses of central hemodynamics to low-sodium (LS), high-sodium (HS), and high-sodium with potassium supplementation (HS + PS) intervention by the number of metabolic traits for central SBP (a), DBP (b), PP (c), P1(d), AP@75 (e), and AIx@75 (f). aAdjusted for age, gender, body mass index, physical activity, smoking and drinking status, and baseline 24-hour urinary excretion of sodium and potassium. Error bars show 95% confidence intervals. Abbreviations: AP@75 and AIx@75, augmentation pressure and augmentation index normalized to a heart rate of 75 bpm; DBP, diastolic blood pressure; PP: pulse pressure; P1: forward wave amplitude; SBP, systolic blood pressure.
DISCUSSION
The present dietary intervention study in a group of northern Chinese individuals suggested that central BP and AIx@75 could be lowered by sodium reduction and potassium supplementation, and elevated by sodium-loading. Furthermore, we found central SBP and PP responses to low-sodium and high-sodium interventions remained significant even after adjustment for corresponding peripheral BP. Additionally, central BP responses to high-sodium intervention and potassium supplementation significantly increased as the number of metabolic traits growing, and these associations were independent of important covariates.
Previous observational studies have suggested that dietary sodium intake, indicated by 24-hour urinary sodium or sodium/potassium ratio, is positively associated with central SBP and PP in African27 and Asian28 population. Meanwhile, a randomized controlled trial conducted in Dutch adults found that high-sodium diet significantly increased central SBP, DBP, and PP,11 with which our results are consistent. Gates et al.14 also reported that sodium restriction significantly lowered central SBP and DBP. The effect of potassium supplement on central BP remains controversial in western population, with 1 study observed significant decrease of central SBP and DBP,13 however, the other 2 did not.11,12 In addition, Hu et al.10 reported that replacing 35% dietary sodium with 25% potassium and 10% magnesium significantly lowered central SBP and PP but not DBP after 12-month intervention in northern Chinese. In Hu’s trial, participants’ meals were cooked by themselves with salt substitute or usual salt provided by the study, thus the investigators failed to assess the exact consumed amount of the salt substitute or salt. While our study had a better quality control strategy that all meals were prepared by full-time chefs and prepackaged salt was added to each participant’s meal by study staff. The present study suggested that both sodium reduction and potassium supplementation lowered central SBP, DBP, and PP in northern Chinese population.
Although previous studies have suggested a potential decrease of central BP by dietary sodium reduction, whether the effects are independent of corresponding peripheral BP has not been investigated. In our analysis, we observed an approximate 2 mm Hg response of central SBP and PP during dietary sodium intervention after adjustment for corresponding peripheral BP. These findings could provide additional evidence to the opinion that central BP may serve as a better intervention target in clinical practice.1
Despite of the doubt of central AIx being used as an indicator of arterial stiffness, it independently predicts cardiovascular events and mortality.29 Therefore, interventions to lower central AIx could be of significance. Previous studies have evaluated the effects of short-term dietary sodium intervention on central Aix; however, the results differ cross studies.14–16 The inconsistency across studies may lie in the different sample characteristics,14,16 magnitude of sodium reduction,11,14 and length of intervention period.15,16 Besides, most studies in western population11–13,18 and a meta-analysis17 found no significant effect of potassium supplement on central AIx. In the present study, however, we found central AIx@75 significantly decreased during low-sodium intervention and potassium supplementation, indicating that dietary sodium and potassium interventions may be helpful to lower central AIx.
The association between MS and salt sensitivity of brachial BP has been demonstrated in previous studies. In 56 Japanese patients, Uzu et al.30 reported a significant higher prevalence of salt-sensitive hypertension among patients with MS than those without (70.6% vs. 36.0%, P = 0.017). In 301 participants from Venezuela, Hoffmann and Cubeddu31 observed significant greater responses of SBP and DBP to salt restriction among those with more metabolic traits, which was subsequently confirmed by Chen et al. in a large population of China.21 However, no study has investigated the relationship between metabolic traits and central BP responses to dietary sodium intervention so far. We are the first to find central SBP and PP responses to high-sodium intervention increase as the number of metabolic traits grows. Moreover, we for the first time reported that central SBP and DBP responses to potassium supplementation were also greater among those with more metabolic traits. Our findings may have potential clinical relevance, that is, dietary sodium reduction and potassium supplementation are especially effective in lowering central BP among individuals with metabolic traits clustering.
The underlying mechanism of the associations between metabolic traits and salt sensitivity of BP is not fully understood. Insulin resistance and obesity are the most important mechanisms involved in MS.26 Previous studies have suggested that insulin resistance–related hyperinsulinemia, sympathetic overdrive, and rennin–angiotensin system activation contribute to sodium retention, and thereby increasing BP responses to sodium intake.32–34 Besides, the enhanced salt sensitivity of BP in obesity has been noted35 and might be explained by increased renal tubular reabsorption of sodium in obsess individuals.36 The variation in BP responses to potassium supplementation among individuals has been reported by the GenSalt collaborative research group, and the responses are associated with baseline brachial BP levels.37 The present study further demonstrated that central BP responses to potassium supplementation varied according to the number of metabolic traits, which could be partly interpreted by the positive effect of potassium on salt-induced insulin resistance.32
Our study is the first free-living population-based dietary intervention study in which the central hemodynamics responses to dietary sodium and potassium interventions were examined according to metabolic risk status. We excluded patients with chronic kidney disease, who had increased salt sensitivity, and adjusted for important covariates in analysis to minimize study bias. The compliance with dietary interventions was excellent as measured by 24-hour urinary excretion of sodium and potassium. Nevertheless, 3 potential limitations should be addressed. First, the 24-hour sodium measured was lower than the prescribed sodium intake during high-sodium intervention, probably due to the fact that urinary sodium is usually less than dietary sodium intake.38 However, some participants may not have finished their meals, so 24-hour urinary sodium excretion was estimated using a regression coefficient when only an overnight urine was collected. Second, our study might slightly underestimate the associations between metabolic traits clustering and central BP responses to dietary sodium intervention. To avoid potential adverse effects of a high-sodium diet, we excluded patients with stage 2 hypertension. Since hypertensive patients are more likely to have MS and salt sensitivity, the exclusion of stage 2 hypertensive patients is prone to bias the associations toward the null. Third, central and peripheral hemodynamics were measured only once in each intervention period, and the 30-minute abstention from smoking, consuming alcohol, coffee or tea, and physical activity prior to measurements was not long enough. However, the lack of restrictions which could affect the hemodynamic parameters is more likely to bias the results toward the null. Further studies with repeated measurements are needed to validate our findings.
In summary, the current study suggested that central BP and AIx@75 could be lowered by sodium reduction and potassium supplement, and elevated by sodium-loading. Moreover, we for the first time revealed significant effects of dietary sodium intervention on central SBP and PP independent of corresponding peripheral BP, which supports the opinion that central BP is a superior intervention target over peripheral BP. In addition, we found the responses of central BP increased with the number of metabolic traits, indicating that dietary sodium and potassium interventions are especially beneficial among individuals with metabolic traits clustering.
SUPPLEMENTARY MATERIAL
Supplementary data are available at American Journal of Hypertension online.
DISCLOSURE
The authors declared no conflict of interest.
ACKNOWLEDGMENTS
This study is supported by the National Natural Science Foundation of China (grant no. 81570386). The Genetic Epidemiology Network of Salt Sensitivity (GenSalt) is supported by a cooperative agreement project grant (U01HL072507) from the National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, USA.
REFERENCES
- 1. Agabiti-Rosei E, Mancia G, O’Rourke MF, Roman MJ, Safar ME, Smulyan H, Wang JG, Wilkinson IB, Williams B, Vlachopoulos C. Central blood pressure measurements and antihypertensive therapy: a consensus document. Hypertension 2007; 50:154–160. [DOI] [PubMed] [Google Scholar]
- 2. Roman MJ, Okin PM, Kizer JR, Lee ET, Howard BV, Devereux RB. Relations of central and brachial blood pressure to left ventricular hypertrophy and geometry: the Strong Heart Study. J Hypertens 2010; 28:384–388. [DOI] [PubMed] [Google Scholar]
- 3. Jankowski P, Kawecka-Jaszcz K, Czarnecka D, Brzozowska-Kiszka M, Styczkiewicz K, Styczkiewicz M, Pośnik-Urbańska A, Bryniarski L, Dudek D. Ascending aortic, but not brachial blood pressure-derived indices are related to coronary atherosclerosis. Atherosclerosis 2004; 176:151–155. [DOI] [PubMed] [Google Scholar]
- 4. Roman MJ, Devereux RB, Kizer JR, Lee ET, Galloway JM, Ali T, Umans JG, Howard BV. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension 2007; 50:197–203. [DOI] [PubMed] [Google Scholar]
- 5. Roman MJ, Devereux RB, Kizer JR, Okin PM, Lee ET, Wang W, Umans JG, Calhoun D, Howard BV. High central pulse pressure is independently associated with adverse cardiovascular outcome the strong heart study. J Am Coll Cardiol 2009; 54:1730–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pini R, Cavallini MC, Palmieri V, Marchionni N, Di Bari M, Devereux RB, Masotti G, Roman MJ. Central but not brachial blood pressure predicts cardiovascular events in an unselected geriatric population: the ICARe Dicomano Study. J Am Coll Cardiol 2008; 51:2432–2439. [DOI] [PubMed] [Google Scholar]
- 7. Wang KL, Cheng HM, Chuang SY, Spurgeon HA, Ting CT, Lakatta EG, Yin FC, Chou P, Chen CH. Central or peripheral systolic or pulse pressure: which best relates to target organs and future mortality?J Hypertens 2009; 27:461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. He FJ, Li J, Macgregor GA. Effect of longer term modest salt reduction on blood pressure: cochrane systematic review and meta-analysis of randomised trials. BMJ (Clinical Research Ed) 2013; 346:f1325. [DOI] [PubMed] [Google Scholar]
- 9. Aburto NJ, Hanson S, Gutierrez H, Hooper L, Elliott P, Cappuccio FP. Effect of increased potassium intake on cardiovascular risk factors and disease: systematic review and meta-analyses. BMJ (Clinical Research Ed) 2013; 346: f1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hu J, Jiang X, Li N, Yu X, Perkovic V, Chen B, Zhao L, Neal B, Wu Y. Effects of salt substitute on pulse wave analysis among individuals at high cardiovascular risk in rural China: a randomized controlled trial. Hypertens Res 2009; 32:282–288. [DOI] [PubMed] [Google Scholar]
- 11. Gijsbers L, Dower JI, Mensink M, Siebelink E, Bakker SJ, Geleijnse JM. Effects of sodium and potassium supplementation on blood pressure and arterial stiffness: a fully controlled dietary intervention study. J Hum Hypertens 2015; 29:592–598. [DOI] [PubMed] [Google Scholar]
- 12. Matthesen SK, Larsen T, Vase H, Lauridsen TG, Pedersen EB. Effect of potassium supplementation on renal tubular function, ambulatory blood pressure and pulse wave velocity in healthy humans. Scand J Clin Lab Invest 2012; 72:78–86. [DOI] [PubMed] [Google Scholar]
- 13. Graham UM, McCance DR, Young IS, Mullan KR. A randomised controlled trial evaluating the effect of potassium supplementation on vascular function and the renin-angiotensin-aldosterone system. J Hum Hypertens 2014; 28:333–339. [DOI] [PubMed] [Google Scholar]
- 14. Gates PE, Tanaka H, Hiatt WR, Seals DR. Dietary sodium restriction rapidly improves large elastic artery compliance in older adults with systolic hypertension. Hypertension 2004; 44:35–41. [DOI] [PubMed] [Google Scholar]
- 15. Todd AS, Macginley RJ, Schollum JB, Johnson RJ, Williams SM, Sutherland WH, Mann JI, Walker RJ. Dietary salt loading impairs arterial vascular reactivity. Am J Clin Nutr 2010; 91:557–564. [DOI] [PubMed] [Google Scholar]
- 16. Dickinson KM, Clifton PM, Keogh JB. A reduction of 3 g/day from a usual 9 g/day salt diet improves endothelial function and decreases endothelin-1 in a randomised cross_over study in normotensive overweight and obese subjects. Atherosclerosis 2014; 233:32–38. [DOI] [PubMed] [Google Scholar]
- 17. Tang X, Wu B, Luo Y, Peng L, Chen Y, Zhu J, Peng C, Li S, Liu J. Effect of potassium supplementation on vascular function: a meta-analysis of randomized controlled trials. Int J Cardiol 2017; 228:225–232. [DOI] [PubMed] [Google Scholar]
- 18. Blanch N, Clifton PM, Petersen KS, Willoughby SR, Keogh JB. Effect of high potassium diet on endothelial function. Nutr Metab Cardiovasc Dis 2014; 24:983–989. [DOI] [PubMed] [Google Scholar]
- 19. Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension 1996; 27:481–490. [DOI] [PubMed] [Google Scholar]
- 20. Galletti F, Strazzullo P, Ferrara I, Annuzzi G, Rivellese AA, Gatto S, Mancini M. NaCl sensitivity of essential hypertensive patients is related to insulin resistance. J Hypertens 1997; 15:1485–1491. [DOI] [PubMed] [Google Scholar]
- 21. Chen J, Gu D, Huang J, Rao DC, Jaquish CE, Hixson JE, Chen CS, Chen J, Lu F, Hu D, Rice T, Kelly TN, Hamm LL, Whelton PK, He J; GenSalt Collaborative Research Group . Metabolic syndrome and salt sensitivity of blood pressure in non-diabetic people in China: a dietary intervention study. Lancet 2009; 373:829–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. GenSalt Collaborative Research Group. GenSalt: rationale, design, methods and baseline characteristics of study participants. J Hum Hypertens 2007; 21:639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Paffenbarger RS Jr, Blair SN, Lee IM, Hyde RT. Measurement of physical activity to assess health effects in free-living populations. Med Sci Sports Exerc 1993; 25:60–70. [DOI] [PubMed] [Google Scholar]
- 24. Chen CH, Nevo E, Fetics B, Pak PH, Yin FC, Maughan WL, Kass DA. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure. Validation of generalized transfer function. Circulation 1997; 95:1827–1836. [DOI] [PubMed] [Google Scholar]
- 25. Stoner L, Faulkner J, Lowe A, M Lambrick D, M Young J, Love R, S Rowlands D. Should the augmentation index be normalized to heart rate?J Atheroscler Thromb 2014; 21:11–16. [DOI] [PubMed] [Google Scholar]
- 26. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr, Spertus JA, Costa F; American Heart Association; National Heart, Lung, and Blood Institute . Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005; 112:2735–2752. [DOI] [PubMed] [Google Scholar]
- 27. Redelinghuys M, Norton GR, Scott L, Maseko MJ, Brooksbank R, Majane OH, Sareli P, Woodiwiss AJ. Relationship between urinary salt excretion and pulse pressure and central aortic hemodynamics independent of steady state pressure in the general population. Hypertension 2010; 56:584–590. [DOI] [PubMed] [Google Scholar]
- 28. Park S, Park JB, Lakatta EG. Association of central hemodynamics with estimated 24-h urinary sodium in patients with hypertension. J Hypertens 2011; 29:1502–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vlachopoulos C, Aznaouridis K, O’Rourke MF, Safar ME, Baou K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J 2010; 31:1865–1871. [DOI] [PubMed] [Google Scholar]
- 30. Uzu T, Kimura G, Yamauchi A, Kanasaki M, Isshiki K, Araki S, Sugiomoto T, Nishio Y, Maegawa H, Koya D, Haneda M, Kashiwagi A. Enhanced sodium sensitivity and disturbed circadian rhythm of blood pressure in essential hypertension. J Hypertens 2006; 24:1627–1632. [DOI] [PubMed] [Google Scholar]
- 31. Hoffmann IS, Cubeddu LX. Increased blood pressure reactivity to dietary salt in patients with the metabolic syndrome. J Hum Hypertens 2007; 21:438–444. [DOI] [PubMed] [Google Scholar]
- 32. Fujita T. Insulin resistance and salt-sensitive hypertension in metabolic syndrome. Nephrol Dial Transplant 2007; 22:3102–3107. [DOI] [PubMed] [Google Scholar]
- 33. Strazzullo P, Barbato A, Vuotto P, Galletti F. Relationships between salt sensitivity of blood pressure and sympathetic nervous system activity: a short review of evidence. Clin Exp Hypertens 2001; 23:25–33. [DOI] [PubMed] [Google Scholar]
- 34. Fujita T. Spotlight on renin. The renin system, salt-sensitivity and metabolic syndrome. J Renin Angiotensin Aldosterone Syst 2006; 7:181–183. [DOI] [PubMed] [Google Scholar]
- 35. Rocchini AP, Key J, Bondie D, Chico R, Moorehead C, Katch V, Martin M. The effect of weight loss on the sensitivity of blood pressure to sodium in obese adolescents. N Engl J Med 1989; 321:580–585. [DOI] [PubMed] [Google Scholar]
- 36. Hall JE. Mechanisms of abnormal renal sodium handling in obesity hypertension. Am J Hypertens 1997; 10:49S–55S. [PubMed] [Google Scholar]
- 37. He J, Gu D, Chen J, Jaquish CE, Rao DC, Hixson JE, Chen JC, Duan X, Huang JF, Chen CS, Kelly TN, Bazzano LA, Whelton PK; GenSalt Collaborative Research Group . Gender difference in blood pressure responses to dietary sodium intervention in the GenSalt study. J Hypertens 2009; 27:48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Holbrook JT, Patterson KY, Bodner JE, Douglas LW, Veillon C, Kelsay JL, Mertz W, Smith JC Jr. Sodium and potassium intake and balance in adults consuming self-selected diets. Am J Clin Nutr 1984; 40:786–793. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.