Abstract
Classic psychedelic drugs (serotonin 2A, or 5HT2A, receptor agonists) have notable effects on music listening. In the current report, blood oxygen level-dependent (BOLD) signal was collected during music listening in 25 healthy adults after administration of placebo, lysergic acid diethylamide (LSD), and LSD pretreated with the 5HT2A antagonist ketanserin, to investigate the role of 5HT2A receptor signaling in the neural response to the time-varying tonal structure of music. Tonality-tracking analysis of BOLD data revealed that 5HT2A receptor signaling alters the neural response to music in brain regions supporting basic and higher-level musical and auditory processing, and areas involved in memory, emotion, and self-referential processing. This suggests a critical role of 5HT2A receptor signaling in supporting the neural tracking of dynamic tonal structure in music, as well as in supporting the associated increases in emotionality, connectedness, and meaningfulness in response to music that are commonly observed after the administration of LSD and other psychedelics. Together, these findings inform the neuropsychopharmacology of music perception and cognition, meaningful music listening experiences, and altered perception of music during psychedelic experiences.
Keywords: functional magnetic resonance imaging (fMRI), music information retrieval, psychedelics, tonality
Introduction
Classic psychedelics, including psilocybin, lysergic acid diethylamide (LSD), and dimethyltryptamine (DMT), are potent compounds that have their primary receptor mechanism of action at serotonin 2A (5HT2A) receptor sites (Nichols 2016). 5HT2A receptors are widely distributed throughout the neocortex (Andree et al. 1998). Accordingly, psychedelic drugs, including psilocybin (Griffiths et al. 2011; Studerus et al. 2011), DMT (Strassman et al. 1994; Riba et al. 2003), and LSD (Schmid et al. 2015; Carhart-Harris et al. 2016a), have substantial effects on perception, cognition, and emotional experience (reviewed in Preller and Vollenweider 2016). Psychedelic drugs also have notable effects on the perception of music. This is not surprising, as 5HT2A signaling has been shown to alter neuronal responses to auditory stimuli from the cochlear nucleus (Tang and Trussell 2015) along the precortical primary auditory sensory pathway (Hurley 2006; Hurley and Sullivan 2012) through to primary auditory cortex (Luo et al. 2016; Riga et al. 2016).
Psychedelics, however, do not simply alter the perception of sensory stimuli such as music. LSD has been shown to increase positive mood during music listening (Kaelen et al. 2015) as well as music-induced imagery and communication of related brain regions (Kaelen et al. 2016). LSD has also been shown to increase the personal relevance of both meaningless and meaningful music, and alter functioning of brain areas involved in processing the meaningfulness of stimuli (Preller et al. 2017). This follows from the neurobiology, as the neural response to music involves both the primary auditory pathway and a wide range of domain-general brain networks including those involved in memory, emotions, self-referential processing, and visualization (Peretz and Zatorre 2005; Janata 2009; Koelsch 2014). Many of these brain regions densely express 5HT2A receptors and show marked alterations in activity and/or connectivity during the acute effects of psychedelics (Carhart-Harris et al. 2016b). Thus, there is extensive neurobiological overlap in the brain regions that are impacted by psychedelics and the brain regions that may be recruited during music listening.
Research and clinical methods have taken advantage of altered experience of music during psychedelic experiences. Music has played a key role in the conduct of psychedelic therapy and research for many decades, with the expectation that supportive music may facilitate a meaningful experience (Chwelos et al. 1959; Bonny and Pahnke 1972). Current best practices for safe conduct of a psychedelic session include the use of music, with the goal of providing psychological support (Johnson, Richards, Griffiths 2008).
Neurochemical effects of music listening on stress, immunity, and social affiliation have been demonstrated (reviewed by Chanda and Levitin 2013), and music listening has specifically been shown to lead to dopamine release and drive reward circuitry (Salimpoor et al. 2011). While these effects of music on neurochemical processes are important, there are very few empirical studies (Preller et al. 2017) that have investigated effects in the opposite direction; namely, the more general role of neuropharmacology in supporting or altering music perception and cognition. There is evidence suggesting commonalities among pieces of music that are optimally supportive during peak experiences with psychedelics, supporting the notion that there are structural principles to the relationship between music listening and psychedelic experiences (Barrett et al. 2017). These principles may have emerged from a shared neuropharmacological basis of psychedelic experience and music perception and cognition.
Music is a complex stimulus that varies in time in a number of dimensions that range from lower-level acoustic features (such as loudness, frequency spectra, or simple tonal features such as pitch height) to higher-level cognitive schema that represent relationships between events that define rhythm, meter, and tonality. Tonality refers to the system in Western tonal music of major and minor keys, in which notes and chords change over time and fulfill or violate expectancies to create a sense of tension and resolution. Tonality has been well-defined as an important cognitive schema for shaping expectations during music listening (Toiviainen and Krumhansl 2003; Huron 2006; Collins et al. 2014).
Change over time in the tonal center of a musical selection (i.e., which key is implied by the music at a given time) can be collectively described and computationally modeled as changes in the pattern of activation on a toroidal surface (Krumhansl and Kessler 1982; Janata et al. 2002; Janata 2005; Collins et al. 2014; Toiviainen and Krumhansl 2003). The toroidal surface model was initially derived from multidimensional scaling analysis of subjective ratings of the perceptual “fit” of probe tones after a tonal center was established in each of 24 major and minor keys, where distance between tonal centers on the 4-dimensional surface of a torus directly reflects perceptual distance (the inverse of perceptual “fit”) between notes, chords, and keys (Krumhansl and Kessler 1982). The model was shown to reflect not only the perception of tonal distance when listening to musical stimuli, but also the organization of tonal structure as understood in music theory (Krumhansl 1990). Thus, modeling tonal space on a toroidal surface simultaneously represents concepts in music theory, cognitive psychology, and the pitch statistics of western music (Janata 2005).
The tonal center of a piece of music at any given point in time can be computationally derived by integrating the tonal information in that piece of music over a window of time preceding the point of interest, and this tonal center can subsequently be reflected on a torus (Janata et al. 2002; Janata 2005). As the melodies and harmonies of a piece of music unfold in time, the sense of tonal center of that piece of music also unfolds in time. Change over time in tonal center can be calculated using a sliding window, and across a piece of music, a timecourse of change in tonal center can be calculated and reflected on a torus. This time-varying pattern of information on the torus thus represents the dynamic tonal structure of a piece of music. The rate of change in that timecourse is determined by both the stability of the distributions of tonal information in the music as well as the duration of the sliding window over which one integrates the tonal information (Janata 2007; Collins et al. 2014). Using spherical harmonic analysis, this timecourse of toroidal (4-dimensional) representation can be decomposed into a series of 34 spatially orthogonal patterns with associated weight vectors, and these weight vectors can be entered into a design matrix that can be used to regress fMRI blood oxygen level-dependent (BOLD) activity that was measured while an individual was listening to a piece of music (Janata 2005). This analysis approach has been applied previously, and spherical harmonic regressors describing the change in tonal center over time were shown to explain variance in brain activity measured while volunteers listened to melodies that moved systematically through all 24 major and minor keys in tonal space (Janata et al. 2002). Change in tonal center (and toroidal space) has been associated with variance in BOLD signal in different domain-general brain regions depending on the psychological context of the music listening experience, such as the experience of music-evoked nostalgia (Barrett and Janata 2016) and autobiographical memories (Janata 2009). This type of stimulus/brain coupling has been labeled “tonality-tracking” (TT) (Janata et al. 2002; Barrett and Janata 2016; Janata 2009).
The current report applies TT analysis of BOLD signal collected while participants listened to both personally meaningful and nonmeaningful music after the administration of placebo, LSD, and LSD pretreated with the 5HT2A antagonist ketanserin, to investigate the role of 5HT2A receptor signaling in the neural response to the time-varying tonal structure of music.
Methods
The following is a secondary analysis of data published elsewhere (Preller et al. 2017). The same stimuli and primary fMRI data that were reported previously (Preller et al. 2017) are used in the current report. Participants in this study completed additional ratings and questionnaires, which are reported in the primary publication. The description below refers only to those measures and procedures investigated in the current report. The study was registered at ClinicalTrials.gov (NCT02451072).
Participants
Twenty-five participants were recruited through advertisements placed in local universities in Zürich, Switzerland. Interested persons attended a screening visit before inclusion in the study. Participants were screened with a short hearing test, medical history, physical examination, blood analysis, and electrocardiography, and had normal or corrected-to-normal vision. The Mini-International Neuropsychiatric Interview (Sheehan et al. 1998), the DSM-IV self-rating questionnaire for Axis-II personality disorders (Fydrich et al. 1997), and the Hopkins Symptom Checklist (Franke 1995) were used to exclude individuals with present or previous psychiatric disorders or a history of major psychiatric disorders in first-degree relatives. Left-handedness, poor knowledge of the German language, cardiovascular disease, history of head injury or neurological disorder, history of alcohol or illicit drug dependence, a previous significant adverse reaction to a hallucinogenic drug, and any contraindication for magnetic resonance imaging, including claustrophobia were further exclusion criteria. Participants were asked to abstain from the use of any prescription or illicit drugs for a minimum of 2 weeks prior to the first test day and for the duration of the entire study, and to abstain from drinking alcohol for at least 24 h prior to each test day. Participants were required to abstain from smoking for at least 60 min before MRI assessment and from drinking caffeine at any point during the test day. Urine tests and self-report questionnaires were used to verify the absence of drug and alcohol use on the screening visit and each experimental visit before drug administration. Urine tests were also used to exclude pregnancy. One participant was excluded from final analysis due to head motion during scanning (see MR Data Acquisition and Preprocessing below). Of the remaining 24 participants, 8 reported previous experience with hallucinogens. The Swiss Federal Office of Public Health, Bern, Switzerland, authorized the use of LSD in humans, and the Cantonal Ethics Committee of Zurich approved the study. All participants provided written informed consent statements in accordance with the declaration of Helsinki before participation in the study.
Study Design
In a double-blind, randomized, full cross-over design, participants received either: (1) placebo+placebo (Pla) condition: treatment with placebo (179 mg Mannitol and 1 mg Aerosil po) after pretreatment with placebo (179 mg Mannitol and 1 mg Aerosil po); (2) placebo + LSD (LSD) condition: treatment with LSD (100 μg po) after pretreatment with placebo (179 mg Mannitol and 1 mg Aerosil po); or (3) ketanserin+LSD (Ket + LSD) condition: treatment with LSD (100 μg po) after pretreatment with the 5-HT2A antagonist ketanserin (40 mg po). These 3 conditions were performed on 3 different occasions each separated by 2 weeks. Pretreatment with placebo or ketanserin was administered at 8:30 AM and occurred 60 min before treatment with placebo or LSD. The fMRI music paradigm was conducted 100 min after treatment with placebo or LSD, during the expected peak of subjective effects of LSD (Passie et al. 2008; Schmid et al. 2015).
Music Paradigm
During the music paradigm, participants listened to an equal number of personally meaningful songs (songs provided by the participant), neutral songs (matched to the meaningful songs), and personally meaningless songs while BOLD fMRI signal was acquired. For personally meaningful songs, participants were asked to provide 6 songs that were personally meaningful to them, and identify the most meaningful 20 s of each song. Participants then completed a pre-task questionnaire (PTQ) after listening to each identified 20 s musical excerpt. The PTQ consisted of the following questions for each song: “How personally meaningful is this song for you?” (1: not at all, 4: very much); “How strongly does this song give you the chills?” (1: not at all, 4: very strongly); and “How do you feel when hearing this song?” (1: sad, 9: happy). Participants also answered the question “How meaningful is music to you in general?” (1: not at all meaningful, 9: very meaningful). The song least meaningful to the participant was identified using the response to the first item of the PTQ (“How personally meaningful is this song for you?”) and used for the practice session conducted at each testing session before substance administration. If more than 1 song scored lowest on this item, the mean score of the other 2 items was used to identify the least meaningful song.
To select the meaningless music played during fMRI acquisition, participants were presented with 4 music excerpts of 20-s duration. Two excerpts were classified as free jazz music and 2 as traditional folk music. In each category, 1 excerpt included vocals and 1 was instrumental. Categories were presented in alternating order. For each excerpt, the participants answered the following 4 questions: 1: “How personally meaningful is this song for you?, 2: “How emotionally touched do you feel by this song?, 3: “How pleasant do you consider this song?, and 4: “How connected do you feel to this song? All questions were answered on a 4-point scale ranging from 1 (not at all) to 4 (very much). Music excerpts were presented and responses registered using Presentation (version 17.0; Neurobehavioral Systems). Stimuli from the category with the lowest average mean rating on question 1 was presented during practice trials and fMRI acquisitions on test days. If both categories were rated equally, the category with the lowest mean rating across all 4 questions was presented. Folk music was presented for 3 participants and free jazz music for 21 participants. The music excerpts presented during practice trials and fMRI acquisitions on test days were different from those used at the screening visit.
Six neutral music excerpts were matched to the 6 songs provided by the participant using the “search for similar music” function of music aggregator website, Last.fm (www.last.fm). Results were sorted according to the number of listeners and the song with the lowest number of listeners was chosen to reduce the chance that participants were familiar with the song. A list showing the artists and song titles was presented to the participants on the first test day before substance administration to make sure they did not know the songs and the songs had no special meaning or personal relevance to them. The music excerpts were created by selecting a random 20 s period for the neutral and meaningless songs, and the identified most meaningful 20 s period for the provided songs, using Audacity 2.1.2 (www.audacityteam.org). All music excerpts were normalized to a maximum amplitude of −1 dB.
On each test day participants performed 3 practice trials before drug administration to familiarize themselves with the task. The 3 trials consisted of the least meaningful music excerpt from the participant’s provided songs, the respective matched neutral music excerpt, and one meaningless song from the participant’s meaningless music category. The songs presented during the practice session were not presented during fMRI data acquisition.
During fMRI data acquisition and practice sessions, a trial block consisted of music presentation for 20 s, followed by a period of 6 s in which participants provided a meaningfulness rating. A fixation cross was presented between blocks with a jittered duration of 7–11 s (mean, 9 s). The musical stimuli were presented in pseudo-randomized order using Presentation (version 17.0; Neurobehavioral Systems). Five different music excerpts were chosen for each condition and each musical excerpt was presented twice, for a total of 10 musical excerpt presentations for each condition. There were 30 blocks in total (3 conditions × 10 blocks per condition). The total duration of the paradigm was 17.5 min. Participants listened to the excerpts of music through MR-compatible in-ear headphones (MR Confon http://www.mr-confon.de) additionally shielded by soundproof circumaural headphones. Responses (from the right hand) were collected using a 4-button response box (Current Designs, http://www.curdes.com/). Visual cues were presented with binoculars (NordicNeuroLab VisualSystem, Bergen, Norway, http://www.nordicneurolab.com/). Participants were asked to close their eyes during music presentation. Compliance to this instruction was monitored online using eye tracking (NordicNeuroLab VisualSystem, http://www.nordicneurolab.com/). All participants followed the instructions. A black screen was presented during music presentation. Subsequent to the music, participants heard the spoken word “rating”, indicating that they should open their eyes and rate the meaningfulness of the music excerpt on a 4-point scale ranging from one (not at all meaningful) to 4 (very meaningful). During the rating, the text “Meaningful?” was displayed at the top of the screen and the 4 response options (“Not at all”, “A little”, “Moderate”, and “Very”) were displayed at the bottom of the screen.
MR Data Acquisition and Preprocessing
MR data were acquired on a Philips Achieva 3.0-T whole-body scanner (Best, The Netherlands). The sequence was specifically designed to produce quiet and constant scanner noise using the “SofTone” parameter. A 32-channel receive head coil and MultiTransmit parallel radio frequency transmission was used. Images were acquired using a whole-brain gradient-echo planar imaging sequence (repetition time, 2500 ms; echo time, 25 ms; slice thickness, 3 mm; 39 axial slices; no slice gap; field of view, 220 × 220 mm2; in-plane resolution, 2.75 × 2.75 mm; sensitivity-encoding reduction factor, 2.0). High-resolution anatomical images (voxel size, 0.7 × 0.7 × 0.7 mm) were also acquired using a standard T1-weighted 3D magnetization-prepared rapid gradient-echo (MP-RAGE) sequence. Images were analyzed using SPM12 (www.fil.ion.ucl.ac.uk). Preprocessing consisted of slice time correction, realignment, spatial normalization to the standard echo planar imaging template of the Montreal Neurological Institute (MNI), and spatial smoothing using a Gaussian kernel of 8-mm full-width half-maximum to meet the statistical requirements of the general linear model. Head motion was assessed using the output of the realignment step of preprocessing. One participant was excluded due to having gross motion (>3 mm) in more than 20% of measured volumes. Two additional participants were identified who exhibited gross motion in <10% of measured volumes, and for these individuals, the ArtRepair toolbox (Mazaika et al. 2009) was used to interpolate volumes exhibiting gross motion.
TT Analysis
Custom MATLAB scripts (the Janata Lab music toolbox [http://atonal.ucdavis.edu/resources/software/jlmt]) were used to generate 34 toroidal surface basis functions that describe the time-varying tonal structure of each musical stimulus. These surface basis functions were used as TT regressors in subsequent TT analyses, following previously described methods (Janata et al. 2002; Janata 2005, 2009; Barrett and Janata 2016). TT models were fit to the residuals of a general linear model (the “base” model) that described the experimental design of the study, the results of which have been reported elsewhere (Preller et al. 2017). The base model consisted of a set of block regressors indicating when self-relevant, neutral, and meaningless stimuli were presented, an event-related regressor that indicated when in-scanner behavioral responses were made, and motion parameters calculated from the realignment stage of preprocessing.
Two models were fit to the residuals of the base model: 1 for analysis of music conditions, and 1 for analysis of drug effects. For analysis of music conditions, the residuals of the base model were regressed on a design matrix consisting of a set of 34 TT regressors that were derived from musical stimuli that were personally meaningful, or self-relevant (“self”), and a second set of 34 TT regressors that were derived from musical stimuli that were either personally meaningless or neutral (“other”; 68 regressors total). F-contrasts were calculated to estimate the variance explained by each set of regressors during each scanning session. For analysis of drug effects, the residuals of the base model were regressed on 34 TT regressors that were derived from all musical stimuli that were presented in a given scanning session for each drug condition (102 TT regressors total). F-contrasts were then calculated to estimate the variance explained by each set of regressors for each scanning session (after placebo, LSD, or Ket + LSD). An additional model was fit including separate TT regressors for each combination of drug and stimulus condition, yielding a design matrix with 204 regressors. This model was fit in order to directly test the interaction of drug condition and stimulus condition on TT within the brain, however this model yielded no significant results, likely due to exceeding the empirical degrees of freedom within the dataset. Thus, this model is not reported further.
Monte Carlo simulation was used at the subject-level for each model to identify TT voxels, which are defined as voxels in which a significant amount of variance (nonparametric simulation thresholded at P < 0.05) was predicted by the entire set of TT regressors for a given scanning session (Janata 2009; Barrett and Janata 2016). Preference (bias) of a voxel for TT during a given condition was assessed by calculating the ratio of the F-statistics describing the variance explained by tonality regressors in each given condition in each subject-level model, adjusted for the number of musical selections in each given experimental condition. TT bias was calculated separately for each pair of drug conditions (placebo vs. LSD, Ket + LSD vs. LSD, and placebo vs. Ket + LSD) and for musical condition (self vs. other). Cluster mass thresholding (Bullmore et al. 1999; Hayasaka and Nichols 2004) was used as previously described (Janata 2009; Barrett and Janata 2016) to identify brain areas that showed TT at the group level for each pair of drug conditions (placebo vs. LSD, placebo vs. Ket + LSD, LSD vs. Ket + LSD) and for musical stimulus condition (self vs. other), thresholding at P < 0.05 and a minimum cluster extent of 20 voxels, correcting for multiple comparisons and family-wise error rate. Average TT bias across participants was calculated for each experimental condition in each significant group-level TT cluster. Anatomical labels for TT clusters were identified using the SPM Anatomy Toolbox (Amunts et al. 2005; Eickhoff et al. 2005) and the Duvernoy Atlas (Duvernoy 1999). Brodmann areas that were overlapping with TT clusters were identified using the WFU PickAtlas (http://fmri.wfubmc.edu/software/pickatlas).
Results
Comparing TT Between Meaningful and Nonmeaningful Music Listening Conditions, Across all Drug Conditions
TT is determined by assessing the amount of variance in a given voxel or brain region that is explained by set of regressors that describe the change in tonal toroidal space over time for a piece of music. TT bias, or greater TT in one condition compared with another, is determined by assessing the ratio of variance explained in a given brain region by TT regressors in one condition (for instance, personally meaningful music) compared with the variance explained by TT regressors in another condition (for instance, nonmeaningful, or “other”, music). TT bias has been shown in a number of brain regions involved in memory, emotion, and language while individuals listened to personally meaningful music that was autobiographically salient (Janata 2009) or evoked nostalgia (Barrett and Janata 2016), compared to when individuals were listening to stimuli that was not autobiographically relevant or nostalgic, respectively.
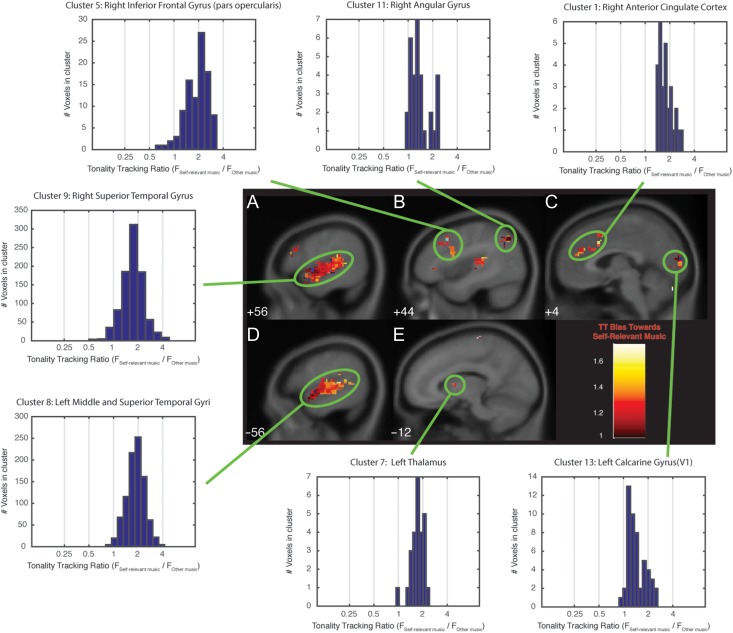
In the current data, brain activity in a series of clusters in the prefrontal, cingulate, insula, temporal, occipital, and cerebellar cortex, as well as in the thalamus, was significantly associated with the time-varying tonal structure of music (Supplementary Table 1). Bias for TT (i.e., stronger TT) during personally meaningful music was observed in bilateral superior temporal cortices (Fig. 1A/D), anterior insula, and anterior cingulate (Fig. 1C), as well as right inferior frontal and angular gyri (Fig. 1B), and left cerebellum, calcarine gyrus (Fig. 1C), and thalamus (Fig. 1E). TT bias was greatest in the left anterior insula, right inferior frontal gyrus, and bilateral superior temporal gyri (in Brodmann areas 41 and 42), with approximately twice the amount of variance explained in each region by TT regressors for personally meaningful music than by TT regressors for nonmeaningful music. No regions demonstrated TT bias toward nonmeaningful music.
Figure 1.
Brain areas tracking the tonal structure of personally meaningful and other musical selections. Each labeled panel (A–E) depicts a sagittal brain slice containing a TT cluster. Shading within each cluster indicates the TT bias for the voxels in the cluster. TT bias is the ratio of variance explained by TT regressors for personally meaningful stimuli to variance explained by the TT regressors for other stimuli (corrected for the number of stimuli in each category). A TT bias value of 1 indicates voxels that tracked personally meaningful and other stimuli equally well; greater than 1 indicates voxels that tracked personally meaningful stimuli more strongly than other stimuli. TT clusters are circled in green, and the distribution of TT bias for all voxels in that cluster is indicated in the associated histogram. Each histogram refers to a cluster listed in Supplementary Table 1. The number in the lower-left-hand corner of each sagittal slice indicates the Montreal Neurologic Institute template coordinate (in mm) of that slice in the x dimension.
Comparing TT Between Drug Conditions
In order to test the hypothesis that LSD and 5HT2A receptor signaling would alter the neural response to the time-varying tonal structure of music, the ratio of variance explained by TT regressors during each drug condition was compared in a pair-wise fashion (placebo vs. LSD, LSD vs. Ket + LSD, and placebo vs. Ket + LSD).
Placebo vs. LSD: Brain activity in temporal (Fig. 2A/D), frontal (Fig. 2B/C/E), cingulate (Fig. 2C), insular, parietal (Fig. 2B), occipital (Fig. 2C), and cerebellar cortex, as well as the amygdala and thalamus (Fig. 2E), was significantly associated with TT regressors during placebo and LSD conditions (Supplementary Table 2). A significant TT bias toward stronger TT during LSD than during placebo was identified in superior, middle, and inferior frontal cortex (including Brodmann areas 10 and 11, and inferior frontal gyrus pars orbitalis), temporal pole, right superior temporal gyrus, angular gyrus, amygdala, and cerebellum. TT bias towards LSD was greatest in regions of the cerebellum (Supplementary Table 2, Clusters 19 and 22), inferior frontal gyrus (Supplementary Table 2, Clusters 7 and 8), temporal pole (Supplementary Table 2, Cluster 11), amygdala (Supplementary Table 2, Cluster 15), and angular gyrus (Supplementary Table 2, Cluster 20) with up to 7 times the amount of variance explained during LSD than during placebo. A significant TT bias toward stronger TT during placebo than LSD was identified in left anterior insula, right inferior frontal gyrus (pars opercularis), precentral gyrus, posterior superior temporal gyrus, and right calcarine gyrus (area V1). TT bias toward placebo was greatest in the left anterior insula (Supplementary Table 2, Cluster 9), where more than 3 times the amount of variance was explained during placebo than during LSD.
Figure 2.
Brain areas tracking the tonal structure of music during placebo compared to LSD. Panels and histograms as in Figure 1. Each labeled panel (A–E) depicts a sagittal brain slice containing a TT cluster. The number in the lower-left-hand corner of each sagittal slice indicates the Montreal Neurologic Institute template coordinate (in mm) of that slice in the x dimension. Shading on the warm gradient within each cluster indicates voxels that are biased toward TT during placebo, and shading on the cool gradient indicates voxels that are biased toward TT during LSD. A TT bias value of 1 indicates voxels that tracked stimuli equally well during placebo and LSD; greater than 1 (plotted on the warm gradient) indicates voxels that tracked stimuli more strongly during placebo than during LSD; less than 1 (plotted on the cool gradient) indicates voxels that tracked stimuli more strongly during LSD than during placebo. Each histogram refers to a cluster listed in Supplementary Table 2.
LSD vs. Ket + LSD: Brain activity in temporal (Fig. 3A/D), frontal (Fig. 3B/C/E), cingulate (Fig. 3C), insular, parietal (Fig. 3B), occipital (Fig. 3C), and cerebellar cortex, as well as the amygdala and thalamus (Fig. 3E), was significantly associated with TT regressors during LSD and Ket + LSD (Supplementary Table 3). Similar to the comparison of placebo and LSD drug conditions, significant TT bias toward stronger TT during LSD than during Ket + LSD was identified in superior, middle, and inferior frontal cortex (including Brodmann areas 9, 10, and 11, and inferior frontal gyrus pars orbitalis), temporal pole, right superior temporal gyrus, angular gyrus, amygdala, and cerebellum. TT bias toward LSD was also observed in the right anterior cingulate and thalamus. TT bias toward LSD was greatest in regions of the cerebellum (Supplementary Table 3, Cluster 24), inferior frontal gyrus (Supplementary Table 3, Cluster 7), and temporal pole (Supplementary Table 3, Cluster 11), with approximately 3–7 times the amount of variance was explained during LSD than during Ket + LSD. A significant TT bias towards stronger TT during Ket + LSD than LSD was identified in left mid and posterior-cingulate cortex (Supplementary Table 3, Cluster 19) and left superior occipital gyrus (Supplementary Table 3, Cluster 23), where up to twice the amount of variance was explained during Ket + LSD that during LSD.
Figure 3.
Brain areas tracking the tonal structure of music during LSD compared to Ket + LSD. Panels and histograms as in Figure 1. Each labeled panel (A–E) depicts a sagittal brain slice containing a TT cluster. The number in the lower-left-hand corner of each sagittal slice indicates the Montreal Neurologic Institute template coordinate (in mm) of that slice in the x dimension. Shading on the cool gradient within each cluster indicates voxels that are biased toward TT during LSD, and shading on the green gradient indicates voxels that are biased toward TT during Ket + LSD. A TT bias value of 1 indicates voxels that tracked stimuli equally well during LSD and Ket + LSD; greater than 1 (plotted on the cool gradient) indicates voxels that tracked stimuli more strongly during LSD than during Ket + LSD; less than 1 (plotted on the green gradient) indicates voxels that tracked stimuli more strongly during Ket + LSD than during LSD. Each histogram refers to a cluster listed in Supplementary Table 3.
Placebo vs. Ket + LSD: Brain activity in temporal (Fig. 4A/D), frontal (Fig. 4B), cingulate (Fig. 4C), insular, and occipital (Fig. 4C) cortex regions as well as left thalamus (Fig. 4E) was significantly associated with TT regressors during placebo and Ket + LSD (Supplementary Table 4). TT bias toward Ket + LSD was observed in bilateral right inferior frontal gyrus (Supplementary Table 4, Cluster 1), right paracentral lobule (Supplementary Table 4, Cluster 9), left mid- and posterior-cingulate cortex (Supplementary Table 4, Cluster 10), and cuneus and precuneus regions (Supplementary Table 4, Cluster 11), where up to 4.5 times the amount of variance was explained by TT regressors during Ket + LSD than during placebo. TT bias towards placebo rather than Ket + LSD was observed in the left insula (Supplementary Table 4, Cluster 3), left inferior frontal gyrus (pars triangularis; Supplementary Table 4, Cluster 4), right inferior frontal gyrus (pars opercularis; Supplementary Table 4, Cluster 5), and left superior temporal gyrus (Supplementary Table 4, Cluster 7), where up to twice the amount of variance was explained by TT regressors during placebo than during Ket + LSD.
Figure 4.
Brain areas tracking the tonal structure of music during placebo compared with Ket + LSD. Panels and histograms as in Figure 1. Each labeled panel (A–E) depicts a sagittal brain slice containing a TT cluster. The number in the lower-left-hand corner of each sagittal slice indicates the Montreal Neurologic Institute template coordinate (in mm) of that slice in the x dimension. Shading on the warm gradient within each cluster indicates voxels that are biased toward TT during placebo, and shading on the green gradient indicates voxels that are biased toward TT during Ket + LSD. A value of 1 indicates voxels that tracked stimuli equally well during placebo and Ket + LSD; greater than 1 (plotted on the warm gradient) indicates voxels that tracked stimuli more strongly during placebo than during Ket + LSD; less than 1 (plotted on the green gradient) indicates voxels that tracked stimuli more strongly during Ket + LSD than during placebo. Each histogram refers to a cluster listed in Supplementary Table 4.
Discussion
The present study utilized music, pharmacological intervention, and a computational model of the time-varying tonal structure of music to investigate the role of LSD in altering the neural response while listening to personally meaningful and nonmeaningful music. Overall, LSD alters the neural response to music in a number of brain regions that have been shown to support varying aspects of subjective experience during music listening. A role for 5HT2A receptor signaling in biasing the neural response to music was shown by comparing effects of placebo to effects of LSD alone and after pretreatment with ketanserin (a 5HT2A antagonist).
LSD, Auditory Processing, Self-Relevance, and the Neural Response to Music
Greater TT bias for LSD compared with placebo and compared with Ket + LSD was expressed in a wide range of brain regions, including a subregion of the right superior temporal gyrus (Brodmann area 21) that was previously shown to be activated by a pleasant melody that meandered systematically through all of tonal space (Janata et al. 2002). This brain region has been shown to preferentially respond to lyrical content in music over melodic content (Sammler et al. 2010) and sentence content more strongly than white noise and musical instrument sounds (Specht, Osnes, Hugdahl 2009), or melody content (Rogalsky et al. 2011). This brain region was also shown to respond to phonetic elements of speech (Turkeltaub and Coslett 2010), and responded to spectral more strongly than temporal changes in speech content (Zaehle et al. 2008). Phonetic and spectral changes in speech are important for vocalization, or singing of lyrics, in music. While a number of other auditory processing regions on the bilateral superior temporal gyri (including Brodmann areas 22, 41, and 42) also displayed TT in the current report, TT within these regions was not biased by drug condition. This suggests a specific effect of LSD in a brain region responsive to lyrical content in music. Altered response to lyrical content may be either a mechanism or a product of the effects of LSD on increased meaning while listening to music.
Brain regions responsive to language and tonality that have previously been shown to exhibit TT (Janata et al. 2002), including bilateral inferior frontal gyrus pars orbitalis, also demonstrated TT bias for LSD compared with placebo and compared to Ket + LSD. Inferior frontal gyrus activity has been linked to processing structured sequences along an rostral-caudal abstraction gradient, where the anterior regions including pars orbitalis are involved in processing more abstract sequences (rather than the more concrete sequences processed by caudal regions) (Udden and Bahlmann 2012). This is consistent with literature that implicates the pars orbitalis structure in predictive semantic processing of both language and music (Levitin and Menon 2003; Matchin, Hammerly, Lau 2017). Left inferior frontal gyrus has typically been shown to respond to speech (Ardila, Bernal, Rosselli 2016), while bilateral inferior frontal gyrus has been shown to respond to music (Levitin and Menon 2003; Tillmann, Janata, Bharucha 2003). TT within the inferior frontal gyrus has shown bias toward greater TT during music-evoked nostalgia (Barrett and Janata 2016) and music-evoked autobiographical memories (Janata 2009). Thus, LSD seems to particularly influence neural function in higher-order association cortices that respond to pitch, semantics, and memory. This might explain the increased salience of music that is anecdotally reported after the administration of classic psychedelics, including LSD and psilocybin.
Medial prefrontal brain regions that have been implicated in core autobiographical memory networks (Svoboda, McKinnon, Levine 2006) and specifically shown to support music-evoked autobiographical memory (Ford, Addis, Giovanello 2011) also demonstrated TT bias toward LSD compared to both placebo and Ket + LSD. These brain regions have been shown to closely track tonal structure (Janata et al. 2002) and exhibit TT bias toward music that evoked autobiographical memories (Janata 2009). This is consistent with previous a report that psychedelics enhance autobiographical recollection, and supports a potential benefit of LSD during psychotherapy either to support the recall of memories or to reverse negative cognitive biases (Carhart-Harris et al. 2012).
The angular gyrus also demonstrated TT bias both for LSD compared with placebo and Ket + LSD, and for personally meaningful stimuli compared with other stimuli. While the precise functional role of the angular gyrus in a given context may depend on the regions that are co-active with it at a given time, a domain-general role of the angular gyrus may be to act as a cross-modal hub that integrates sensory information with top-down predictions in order to direct attention (Seghier 2013). The angular gyrus, while not previously shown to exhibit TT, has previously shown increased activity when participants listened to musical stimuli that were pleasing, familiar, and autobiographically salient (Janata 2009). Increased involvement of the angular gyrus in tracking the tonal structure of music during both personally meaningful stimuli and LSD, along with increased TT in brain regions supporting auditory processing and autobiographical memory recall, may account for the increased salience and emotional impact of musical stimuli (Kaelen et al. 2015) as well as the increased self-relevance of musical stimuli (Preller et al. 2017) during the effects of LSD.
TT bias has been observed in the current report in brain areas that respond to both music and speech (including superior temporal gyrus and inferior frontal gyrus) and also in higher-level cognitive brain regions associated with processing tonality (e.g., inferior frontal gyrus) and memory and emotion (e.g., medial prefrontal cortex and angular gyrus). Enhanced TT in the amygdala is also notable, and may be consistent with an overall increase in sensitivity to auditory stimuli produced by LSD, in line with Silverman (1971) who reported lower thresholds to auditory stimuli after the intake of LSD. Overall, these findings suggest that LSD serves to support a deeper or more integrated experience of music, which could explain the wide range of emotional and cognitive effects that are encountered, especially those experienced in response to music, after the administration of LSD and other serotonergic hallucinogens (Kaelen et al. 2015, 2016; Preller et al. 2017).
Though trending toward TT bias during LSD compared with Ket + LSD, the calcarine sulcus exhibited TT bias toward placebo compared with LSD, and for personally meaningful compared to other musical stimuli. This is consistent with TT bias in extrastriate visual areas that was previously demonstrated for autobiographically salient musical stimuli, notably during eyes-closed music listening conditions (Janata 2009). While there is evidence for increased visual cortex connectivity during the effects of LSD (Kaelen et al. 2016), and clear evidence for a specific auditory-to-visual synesthesic effect of classic psychedelics (Luke and Terhune 2013), this effect was not observed with TT. Given that synesthesia is not necessarily encountered during every psychedelic experience, and given that synesthesia can be a very idiosyncratic experience, our failure to find TT in primary visual brain regions could be due to insufficient frequency or homogeneity of synesthetic experience in our sample. It may also suggest that, rather than being dependent upon the content of the physical properties of an auditory stimulus, the induction of synesthesia may depend on aspects of psychedelic experience that were not well-controlled or characterized within the current study.
The Role of 5HT2A Signaling in the Neural Response to the Time-Varying Tonal Structure of Music
The primary receptor mechanism of action of classic psychedelics, including LSD, is the 5HT2A receptor (Nichols 2016). Within the current study, LSD was given alone after pretreatment with placebo (LSD) or pretreatment with the 5HT2A receptor antagonist ketanserin (Ket + LSD). TT was biased toward LSD in superior, middle, and inferior frontal gyri, temporal pole and superior temporal gyrus, angular gyrus, and the amygdala when compared to both placebo and Ket + LSD. Given that these effects were observed in LSD when comparing to both the absence of 5HT2A receptor agonism (in the placebo condition) and after blockade of the 5HT2A receptor with ketanserin (in the Ket + LSD condition), these findings indicate a role of 5HT2A signaling in the neural response to the time-varying tonal structure of music at multiple neural levels, including mid-brain and primary auditory brain regions up through higher-level cognitive brain regions.
The Unknown Role of Other Receptor Signaling Mechanisms in the Neural Response to the Time-Varying Tonal Structure of Music
While ketanserin and LSD manipulations can help us to understand the role of 5HT2A receptor signaling in TT, some curious effects were found in the current data that cannot be attributed simply to 5HT2A receptor signaling. TT clusters in the right insula exhibited TT towards personally meaningful stimuli and also exhibited TT bias toward LSD when compared to placebo, but exhibited TT bias toward Ket + LSD when compared with LSD. TT in the mid-cingulate and posterior-cingulate cortex was also biased toward Ket + LSD when compared both to placebo and LSD. A TT cluster in the left superior temporal gyrus (Supplementary Table 2, Cluster 18; Brodmann area 41) exhibited greater TT during placebo compared to LSD, but did not display bias in TT between LSD and Ket + LSD conditions. This brain region is primarily within the transverse temporal gyrus/Heschl’s gyrus, and is thought to code basic auditory sensory information, including frequency content (tonotopically mapped from the cochlear nerve), intensity, and duration of sounds (Herdener et al. 2013). Finally, the left calcarine gyrus, which exhibited TT bias towards personally meaningful stimuli, exhibited TT bias towards placebo compared to LSD, and exhibited TT bias toward LSD when compared with Ket + LSD. Given the complex pharmacology of LSD (with affinity for D2, D3/4, alpha 2, and a series of 5HT receptors), these findings suggest the potential involvement of receptors other than 5HT2A in the neural response to music in the insula, the cingulate cortex, in visual processing regions, and in early auditory cortex regions.
Due to the design of the study, the effect of ketanserin alone could not be investigated independently. Ketanserin alone has been shown to reduce neural response to fearful emotional stimuli in the medial orbitofrontal cortex and amygdala (Hornboll et al. 2013). Ketanserin was also shown to reduce the neural response in frontopolar cortex to negative outcomes associated with low-risk choices and associated with large missed rewards, and increase the response to low-risk negative outcomes in risk-taking individuals (Macoveanu et al. 2013). While medial and lateral frontal regions have been previously implicated in TT (Janata et al. 2002; Janata 2009), TT in these regions was not shown to be biased toward Ketanserin + LSD in the current findings. Thus, evidence would suggest that the findings observed in the current report in the Ketanserin + LSD condition are not likely due to effects expected from ketanserin alone.
Conclusion
The current study sheds light on the neuropharmacology and biology of music listening. LSD and ketanserin were used to demonstrate that 5HT2A signaling may alter the coupling of activity in brain areas that support an overall musical experience with patterns of change in the tonal structure of that music. Alteration of the neural response to the tonal structure of music in a number of domain-general brain regions, primarily through 5HT2A receptor signaling, could explain the increases in emotionality, connectedness, and meaningfulness that are observed in response to music after the administration of LSD and other serotonergic hallucinogens (Kaelen et al. 2015, 2016; Preller et al. 2017). This is particularly important since music listening is used to provide psychological support during research and therapy sessions with psychedelics (Bonny and Pahnke 1972; Johnson, Richards, Griffiths 2008). The current findings increase our knowledge about the neurochemical underpinnings of music listening—an experience with many health-relevant implications such as stress reduction and social bonding (Chanda and Levitin 2013) that we seek and encounter without fail in our every-day lives.
Supplementary Material
Notes
Conflict of Interest: All authors declare no conflict of interest.
Authors’ Contributions
K.H.P., M.H., and F.X.V. designed the study. F.S.B. and P.J. conceptualized the analysis. K.H.P. carried out the experiment. F.S.B. analyzed the data. All authors wrote and approved the manuscript.
Funding
The Heffter Research Institute (1-190 413), the Swiss Neuromatrix Foundation (2015-0103), the Usona Institute (2015-2056) to F.X.V., and the Swiss National Science Foundation (SNSF, P2ZHP1_161626) to K.H.P. F.S.B. was partially supported by NIH grant R03DA042336.
References
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, Zilles K. 2005. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: Intersubject variability and probability maps. Anat Embryol (Berl). 210(5–6):343–352. [DOI] [PubMed] [Google Scholar]
- Andree B, Nyberg S, Ito H, Ginovart N, Brunner F, Jaquet F, Halldin C, Farde L. 1998. Positron emission tomographic analysis of dose-dependent MDL 100,907 binding to 5-hydroxytryptamine-2A receptors in the human brain. J Clin Psychopharmacol. 18(4):317–323. [DOI] [PubMed] [Google Scholar]
- Ardila A, Bernal B, Rosselli M. 2016. How localized are language brain areas? A review of brodmann areas involvement in oral language. Arch Clin Neuropsychol. 31(1):112–122. [DOI] [PubMed] [Google Scholar]
- Barrett FS, Janata P. 2016. Neural responses to nostalgia-evoking music modeled by elements of dynamic musical structure and individual differences in affective traits. Neuropsychologia. 91:234–246. [DOI] [PubMed] [Google Scholar]
- Barrett FS, Robbins H, Smooke D, Brown JL, Griffiths RR. 2017. Qualitative and quantitative features of music reported to support peak mystical experiences during psychedelic therapy sessions. Frontiers in Psychol. 8:1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonny HL, Pahnke WN. 1972. The use of music in psychedelic (LSD) psychotherapy. J Music Ther. 9(2):87. [Google Scholar]
- Bullmore ET, Suckling J, Overmeyer S, Rabe-Hesketh S, Taylor E, Brammer MJ. 1999. Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans Med Imaging. 18(1):32–42. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, Kaelen M, Bolstridge M, Williams TM, Williams LT, Underwood R, Feilding A, Nutt DJ. 2016. a. The paradoxical psychological effects of lysergic acid diethylamide (LSD). Psychol Med. 46(7):1379–1390. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, Leech R, Williams TM, Erritzoe D, Abbasi N, Bargiotas T, Hobden P, Sharp DJ, Evans J, Feilding A, et al. 2012. Implications for psychedelic-assisted psychotherapy: functional magnetic resonance imaging study with psilocybin. Br J Psychiatry. 200(3):238–244. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, Muthukumaraswamy S, Roseman L, Kaelen M, Droog W, Murphy K, Tagliazucchi E, Schenberg EE, Nest T, Orban C, et al. 2016. b. Neural correlates of the LSD experience revealed by multimodal neuroimaging. Proc Natl Acad Sci USA. 113(17):4853–4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanda ML, Levitin DJ. 2013. The neurochemistry of music. Trends Cogn Sci. 17(4):179–193. [DOI] [PubMed] [Google Scholar]
- Chwelos N, Blewett DB, Smith CM, Hoffer A. 1959. Use of d-lysergic acid diethylamide in the treatment of alcoholism. Q J Stud Alcohol. 20:577–590. [PubMed] [Google Scholar]
- Collins T, Tillmann B, Barrett FS, Delbe C, Janata P. 2014. A combined model of sensory and cognitive representations underlying tonal expectations in music: from audio signals to behavior. Psychol Rev. 121(1):33–65. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM. 1999. The human brain: surface, three-dimensional sectional anatomy with MRI, and blood supply. Wien: Springer-Verlag. [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. 2005. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 25(4):1325–1335. [DOI] [PubMed] [Google Scholar]
- Ford JH, Addis DR, Giovanello KS. 2011. Differential neural activity during search of specific and general autobiographical memories elicited by musical cues. Neuropsychologia. 49(9):2514–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke G. 1995. Die symptom-check-liste von derogatis - deutsche version. Goettingen, Germany: Beltz Test Gesellschaft. [Google Scholar]
- Fydrich TB, Renneberg B, Schmitz B, Wittchen HU. 1997. SKID-II strukturiertes klinisches interview für DSM-IV, achse II: Persönlichkeitsstörungen. [SCID-II structured cinical interview for DSM-IV, axis II: Personality disorders.]. Goettingen: Hogrefe. [Google Scholar]
- Griffiths RR, Johnson MW, Richards WA, Richards BD, McCann U, Jesse R. 2011. Psilocybin occasioned mystical-type experiences: Immediate and persisting dose-related effects. Psychopharmacology (Berl). 218(4):649–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayasaka S, Nichols TE. 2004. Combining voxel intensity and cluster extent with permutation test framework. Neuroimage. 23(1):54–63. [DOI] [PubMed] [Google Scholar]
- Herdener M, Esposito F, Scheffler K, Schneider P, Logothetis NK, Uludag K, Kayser C. 2013. Spatial representations of temporal and spectral sound cues in human auditory cortex. Cortex. 49(10):2822–2833. [DOI] [PubMed] [Google Scholar]
- Hornboll B, Macoveanu J, Rowe J, Elliott R, Paulson OB, Siebner HR, Knudsen GM. 2013. Acute serotonin 2A receptor blocking alters the processing of fearful faces in the orbitofrontal cortex and amygdala. J Psychopharmacol (Oxford). 27(10):903–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM. 2006. Different serotonin receptor agonists have distinct effects on sound-evoked responses in inferior colliculus. J Neurophysiol. 96(5):2177–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM, Sullivan MR. 2012. From behavioral context to receptors: Serotonergic modulatory pathways in the IC. Front Neural Circuits. 6:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huron D. 2006. Sweet anticipation: music and the psychology of expectation. Cambridge: The MIT Press. [Google Scholar]
- Janata P. 2009. The neural architecture of music-evoked autobiographical memories. Cereb Cortex. 19(11):2579–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janata P. 2005. Brain networks that track musical structure. Ann N Y Acad Sci. 1060:111–124. [DOI] [PubMed] [Google Scholar]
- Janata P, Birk JL, Van Horn JD, Leman M, Tillmann B, Bharucha JJ. 2002. The cortical topography of tonal structures underlying western music. Science. 298(5601):2167–2170. [DOI] [PubMed] [Google Scholar]
- Janata P. 2007. Navigating tonal space In: Hewlett WB, Selfridge-Field E, Correia E, editors. Tonal theory for the digital age. Stanford: Center for Computer Assisted Research in the Humanities; p. 39. [Google Scholar]
- Johnson MW, Richards W, Griffiths RR. 2008. Human hallucinogen research: guidelines for safety. J Psychopharmacol. 22(6):603–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelen M, Barrett FS, Roseman L, Lorenz R, Family N, Bolstridge M, Curran HV, Feilding A, Nutt DJ, Carhart-Harris RL. 2015. LSD enhances the emotional response to music. Psychopharmacology (Berl). 232(19):3607–3614. [DOI] [PubMed] [Google Scholar]
- Kaelen M, Roseman L, Kahan J, Santos-Ribeiro A, Orban C, Lorenz R, Barrett FS, Bolstridge M, Williams T, Williams L, et al. 2016. LSD modulates music-induced imagery via changes in parahippocampal connectivity. Eur Neuropsychopharmacol. 26(7):1099–1109. [DOI] [PubMed] [Google Scholar]
- Koelsch S. 2014. Brain correlates of music-evoked emotions. Nat Rev Neurosci. 15(3):170–180. [DOI] [PubMed] [Google Scholar]
- Krumhansl CL. 1990. Tonal hierarchies and rare intervals in music cognition. Music Perception. 7:309. [Google Scholar]
- Krumhansl CL, Kessler EJ. 1982. Tracing the dynamic changes in perceived tonal organization in a spatial representation of musical keys. Psychol Rev. 89(4):334–368. [PubMed] [Google Scholar]
- Levitin DJ, Menon V. 2003. Musical structure is processed in “language” areas of the brain: a possible role for brodmann area 47 in temporal coherence. Neuroimage. 20(4):2142–2152. [DOI] [PubMed] [Google Scholar]
- Luke DP, Terhune DB. 2013. The induction of synaesthesia with chemical agents: a systematic review. Front Psychol. 4:753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo B, Hu L, Liu C, Guo Y, Wang H. 2016. Activation of 5-HT2A/C receptor reduces glycine receptor-mediated currents in cultured auditory cortical neurons. Amino Acids. 48(2):349–356. [DOI] [PubMed] [Google Scholar]
- Macoveanu J, Rowe JB, Hornboll B, Elliott R, Paulson OB, Knudsen GM, Siebner HR. 2013. Serotonin 2A receptors contribute to the regulation of risk-averse decisions. Neuroimage. 83:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matchin W, Hammerly C, Lau E. 2017. The role of the IFG and pSTS in syntactic prediction: Evidence from a parametric study of hierarchical structure in fMRI. Cortex. 88:123. [DOI] [PubMed] [Google Scholar]
- Mazaika P, Hoeft F, Glover GH, Reissig AL. 2009. Methods and software for fMRI analysis for clinical subjects. Hum Brain Mapp. 47(suppl 1):S58. [Google Scholar]
- Nichols DE. 2016. Psychedelics. Pharmacol Rev. 68(2):264–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passie T, Halpern JH, Stichtenoth DO, Emrich HM, Hintzen A. 2008. The pharmacology of lysergic acid diethylamide: a review. CNS Neurosci Ther. 14(4):295–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz I, Zatorre RJ. 2005. Brain organization for music processing. Annu Rev Psychol. 56:89–114. [DOI] [PubMed] [Google Scholar]
- Preller KH, Vollenweider FX. 2016. Phenomenology, structure, and dynamic of psychedelic states. Curr Top Behav Neurosci. doi: 10.1007/7854_2016_459. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Preller KH, Herdener M, Pokorny T, Planzer A, Kraehenmann R, Stampfli P, Liechti ME, Seifritz E, Vollenweider FX. 2017. The fabric of meaning and subjective effects in LSD-induced states depend on serotonin 2A receptor activation. Curr Biol. 27(3):451–457. [DOI] [PubMed] [Google Scholar]
- Riba J, Valle M, Urbano G, Yritia M, Morte A, Barbanoj MJ. 2003. Human pharmacology of ayahuasca: subjective and cardiovascular effects, monoamine metabolite excretion, and pharmacokinetics. J Pharmacol Exp Ther. 306(1):73–83. [DOI] [PubMed] [Google Scholar]
- Riga MS, Bortolozzi A, Campa L, Artigas F, Celada P. 2016. The serotonergic hallucinogen 5-methoxy-N,N-dimethyltryptamine disrupts cortical activity in a regionally-selective manner via 5-HT(1A) and 5-HT(2A) receptors. Neuropharmacology. 101:370–378. [DOI] [PubMed] [Google Scholar]
- Rogalsky C, Rong F, Saberi K, Hickok G. 2011. Functional anatomy of language and music perception: temporal and structural factors investigated using functional magnetic resonance imaging. J Neurosci. 31(10):3843–3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimpoor VN, Benovoy M, Larcher K, Dagher A, Zatorre RJ. 2011. Anatomically distinct dopamine release during anticipation and experience of peak emotion to music. Nat Neurosci. 14(2):257–262. [DOI] [PubMed] [Google Scholar]
- Sammler D, Baird A, Valabregue R, Clement S, Dupont S, Belin P, Samson S. 2010. The relationship of lyrics and tunes in the processing of unfamiliar songs: a functional magnetic resonance adaptation study. J Neurosci. 30(10):3572–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid Y, Enzler F, Gasser P, Grouzmann E, Preller KH, Vollenweider FX, Brenneisen R, Muller F, Borgwardt S, Liechti ME. 2015. Acute effects of lysergic acid diethylamide in healthy subjects. Biol Psychiatry. 78(8):544–553. [DOI] [PubMed] [Google Scholar]
- Seghier ML. 2013. The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist. 19(1):43–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. 1998. The mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 59(Suppl 20):57. [PubMed] [Google Scholar]
- Silverman J. 1971. Research with psychedelics. some biopsychological concepts and possible clinical applications. Arch Gen Psychiatry. 25(6):498–510. [DOI] [PubMed] [Google Scholar]
- Specht K, Osnes B, Hugdahl K. 2009. Detection of differential speech-specific processes in the temporal lobe using fMRI and a dynamic “sound morphing” technique. Hum Brain Mapp. 30(10):3436–3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassman RJ, Qualls CR, Uhlenhuth EH, Kellner R. 1994. Dose-response study of N,N-dimethyltryptamine in humans. II. subjective effects and preliminary results of a new rating scale. Arch Gen Psychiatry. 51(2):98–108. [DOI] [PubMed] [Google Scholar]
- Studerus E, Kometer M, Hasler F, Vollenweider FX. 2011. Acute, subacute and long-term subjective effects of psilocybin in healthy humans: a pooled analysis of experimental studies. J Psychopharmacol. 25(11):1434–1452. [DOI] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B. 2006. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia. 44(12):2189–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang ZQ, Trussell LO. 2015. Serotonergic regulation of excitability of principal cells of the dorsal cochlear nucleus. J Neurosci. 35(11):4540–4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillmann B, Janata P, Bharucha JJ. 2003. Activation of the inferior frontal cortex in musical priming. Brain Res Cogn Brain Res. 16(2):145–161. [DOI] [PubMed] [Google Scholar]
- Toiviainen P, Krumhansl CL. 2003. Measuring and modeling real-time responses to music: The dynamics of tonality induction. Perception. 32(6):741–766. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Coslett HB. 2010. Localization of sublexical speech perception components. Brain Lang. 114(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udden J, Bahlmann J. 2012. A rostro-caudal gradient of structured sequence processing in the left inferior frontal gyrus. Philos Trans R Soc Lond B Biol Sci. 367(1598):2023–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaehle T, Geiser E, Alter K, Jancke L, Meyer M. 2008. Segmental processing in the human auditory dorsal stream. Brain Res. 1220:179–190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.