Abstract
Pregnancy represents a critical period in fetal development, such that the prenatal environment can, in part, establish a lifelong trajectory of health or disease for the offspring. Poor nutrition (macro- or micronutrient deficiencies) can adversely affect brain development and significantly increase offspring risk for metabolic and neurological disease development. The concentration of dietary methyl-donor nutrients is known to alter DNA methylation in the brain, and alterations in DNA methylation can have long-lasting effects on gene expression and neuronal function. The decreased availability of methyl-donor nutrients to the developing fetus in models of poor maternal nutrition is one mechanism hypothesized to link maternal malnutrition and disease risk in offspring. Animal studies indicate that supplementation of both maternal and postnatal (early- and later-life) diets with methyl-donor nutrients can attenuate disease risk in offspring; however, clinical research is more equivocal. The objective of this review is to summarize how specific methyl-donor nutrient deficiencies and excesses during pre- and postnatal life alter neurodevelopment and cognition. Emphasis is placed on reviewing the current literature, highlighting challenges within nutrient supplementation research, and considering potential strategies to ensure robust findings in future studies.
INTRODUCTION
Pregnancy and early life are critical periods of development, particularly with regard to neurocognitive endpoints, because significant brain maturation continues throughout the postnatal period. Beginning in pregnancy but continuing well into adolescence, neural systems, particularly those in the prefrontal cortex that are essential for cognition and executive function processes such as attention and cognitive flexibility, are established and refined. This significant postnatal development means that the neural systems underlying cognition remain vulnerable to adverse environmental influences, such as poor diet, alcohol or drugs of abuse, stressors, or inflammation, for a prolonged period of time. Early exposure to these environmental challenges can therefore increase the risk for a wide range of adverse neurodevelopmental outcomes, such as cognitive deficits, executive function deficits, and other social and emotional behavioral problems.1,2
Epigenetic modifications and their resultant changes in gene expression have been proposed as one mechanism linking poor early-life environment with long-term changes in physiology and increased disease risk. Epigenetics, meaning “on” or “above” the genome, is the study of modifications to DNA or histones that can regulate gene expression, some of which can be heritable and/or reversible.3 In the context of poor early-life nutrition, the decreased availability of methyl-donor nutrients to the developing offspring is one mechanism hypothesized to link maternal malnutrition and disease risk in offspring. Animal studies indicate that supplementation of both maternal and postnatal (early- and later-life) diets with methyl-donor nutrients can attenuate disease risk in offspring. This review will first examine 1-carbon metabolism and methyl-donor nutrients and will then summarize the literature pertaining to effects of methyl-donor supplementation on cognitive endpoints in both animals and humans. It will conclude with a discussion of some of the caveats and limitations within the field and will consider opportunities for future studies to fill gaps in the current knowledge.
ONE-CARBON METABOLISM AND METHYL-DONOR NUTRIENTS
Methyltransferases receive methyl groups to perform methylation reactions through the conversion of S-adenosylmethionine to S-adenosylhomocysteine. This reaction is one in a cyclical series of biochemical reactions called 1-carbon metabolism (Figure 1). One-carbon metabolism refers to the intersection of folate and methionine metabolism cycles that donate and regenerate 1-carbon units for various reactions in the cell.4–6 Through these reactions, cells synthesize purines, thymidylate, creatine, phosphatidylcholine, and multiple hormones. One-carbon metabolism also provides methyl groups for at least 50 different methylation reactions that occur within the cell, including methylation of RNA, DNA, histones, neurotransmitters, and other proteins. It is also involved in the catabolism of choline and histidine, the interconversion of serine and glycine, and in almost every cellular process; thus, it is important that these reactions occur efficiently.
Figure 1.
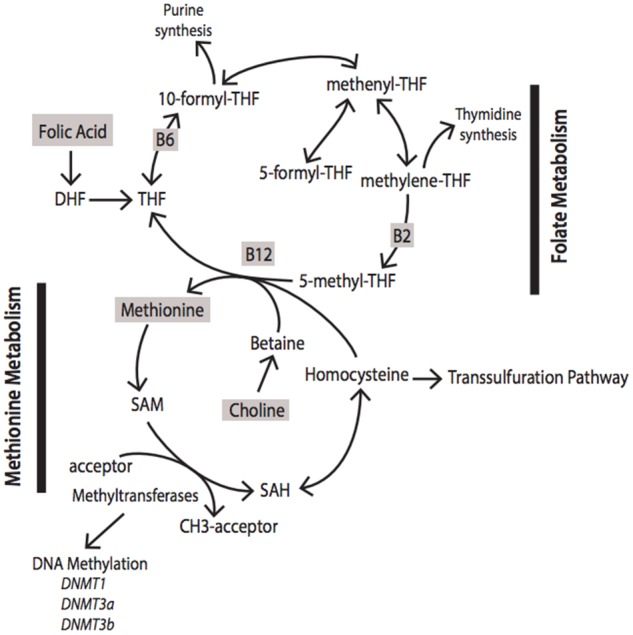
Simplified schematic diagram of the intermediates, enzymes, and nutrients involved in 1-carbon metabolism. Abbreviations: DHF, dihydrofolate; SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine; THF, tetrahydrofolate.
One-carbon metabolism is highly dependent on nutritional status. In Figure 1, highlighted in gray are nutrients essential for 1-carbon metabolism, known as methyl donors. Vitamin B12, vitamin B6, vitamin B9 (folate), methionine, betaine, choline, and zinc are all necessary for 1-carbon metabolism. These methyl-donor nutrients are critical intermediates or cofactors for enzymes involved in 1-carbon metabolism. Specifically within the brain, methyl-donor nutrients are known to be involved in multiple neurotransmitter systems, epigenetic modifications, and cellular membrane structures. For example, choline is a precursor for acetylcholine synthesis, a major neurotransmitter system involved in attention, memory, and motivation.7–9 It is also a precursor for phosphatidylcholine, a class of phospholipids integral to the formation of biological membranes.10 Folate is integral to the donation of methyl groups that are utilized by multiple methyltransferases.11 DNA methyltransferases mediate the addition of methyl groups to DNA, a well-studied epigenetic modification known to mediate gene expression changes and neuronal function. Catechol-O-methyltransferase, another methyltransferase, utilizes methyl groups to aid in the degradation of catecholamines (dopamine, epinephrine, norepinephrine). Imbalances in methyl donors can also negatively affect methyltransferase function, S-adenosylmethionine regeneration, and DNA methylation.12–17 Additionally, deficiencies and excesses of these nutrients also can alter cognition, behavior, and risk for disease.18–24
Folate metabolism serves to create and transfer 1-carbon units for the biosynthetic processes listed above. Natural folates in the body and diet are typically found in reduced form, mainly 5-methyltetrahydrofolate in humans.25 The tetrahydrofolate backbone, which is the active form of folate, is used to carry and transfer 1-carbon units. One-carbon units, also known as methyl groups, are covalently bound to the 5- and 10-positions on the pteridine ring of tetrahydrofolate. Tetrahydrofolate can be maintained in 3 oxidative states, each of which plays a specific role in biosynthesis (recently reviewed by Ducker and Rabinowitz5). Briefly, 5,10-methylenetetrahydrofolate produces thymidine and serine, 5-methyltetrahydrofolate produces methionine, and 10-formyltetrahydrofolate produce purines, formate, and carbon dioxide.
The most abundant form of folate, 5-methyltetrahydrofolate, can be converted to methionine via a vitamin B12–dependent methyltransferase in which the methyl group from 5-methyltetrahydrofolate is transferred to homocysteine, resulting in the formation of methionine and unsubstituted tetrahydrofolate. Tetrahydrofolate can then begin the folate cycle again, and methionine can continue through its unique metabolism. Methionine is the precursor of S-adenosylmethionine, the universal methyl donor, as it is the final carrier of methyl groups before they are donated to the methyltransferases. Donation of a methyl group from S-adenosylmethionine creates S-adenosylhomocysteine, which is reversibly metabolized into homocysteine. Homocysteine can be remethylated to reform methionine via betaine–homocysteine methyltransferase (expressed in liver and kidney, but not found in the brain or other tissues) or methionine synthase, a vitamin B12–dependent methyltransferase, to recycle through methionine metabolism. Alternatively, homocysteine can enter the transsulfuration pathway, which is required to support glutathione synthesis and remove reactive oxygen species from the brain.26
Methyl-donor nutrients are necessary for normal growth and development of the central nervous system (CNS). Deficiencies in methyl donors and imbalanced concentrations of methyl donors have both been linked to abnormal CNS development as well as to neurological diseases. For example, choline deficiency during gestation alters global and gene-specific DNA methylation in the developing mouse hippocampus, a region important for learning and memory.17 Increased homocysteine is a potential risk factor for cognitive impairment and other neurological diseases.27,28 Additionally, low vitamin B12 status has also been associated with poorer memory performance and reduced microstructural integrity of the hippocampus.29 Conversely, higher folate intake has been associated with lower risk for dementia,30 and it has been suggested that using B vitamins to help remethylate homocysteine to methionine could be one mechanism to improve cognition or to slow cognitive decline.31
The efficiency and reactions of 1-carbon metabolism have been shown to affect a number of CNS outcomes, including neural tube closure,32,33 neurological diseases,34,35 and cognition.36 The efficiency of this cycle is highly dependent on nutritional status, and studies in both animals and humans suggest that nutritional interventions can be used for therapeutic benefit. The subsequent sections will summarize animal studies (Table 1)37–50 and human clinical trials (Table 2)23,30,31,51–72 linking methyl-donor supplementation with neurodevelopment and cognition.
Table 1.
Animal studies linking methyl-donor supplementation with neurodevelopment and cognition
| Reference | Behavior | Supplementation period | Nutrient(s) supplemented | Outcomes |
|---|---|---|---|---|
| Thomas et al. (2009)37 | Motor coordination and balance, open field test, spatial learning, working memory | Pregnancy | Choline | Choline supplementation reduced effects of prenatal ethanol exposure; increased brain weight; and normalized righting reflex, motor coordination, and negative geotaxis |
| Thomas et al. (2010)38 | Spontaneous alternation, parallel bar motor coordination, Morris water maze, spatial working memory | Pregnancy | Choline | Choline supplementation may reduce severity of fetal alcohol effects. It increased rates of spontaneous alternation; increased memory retention |
| Naninck et al. (2016)39 | Elevated plus maze, object recognition task, object location task, Morris water maze, and T maze | Pregnancy | Folic acid, vitamin B12, vitamin B6, choline, betaine, zinc, and methionine | Supplementation normalized object recognition but did not change object location or performance on Morris water maze |
| Zhu et al. (2016)40 | Barnes maze test | Pregnancy | Choline | Choline supplementation decreased spatial learning deficits |
| Langley et al. (2015)41 | Open field test, elevated plus maze, marble burying test | Pregnancy + lactation | Choline | Choline supplementation lowered anxiety levels |
| Carlin et al. (2013)42 | Fat and sucrose preference | Pregnancy + lactation | Folic acid, vitamin B12, choline, betaine, zinc, and methionine | Supplementation decreased palatable food preference |
| Bahous et al. (2017)43 | Open field test, ladder beam test, novel object recognition test, Y-maze | Pregnancy + lactation | Folic acid | Excessive folic acid supplementation impaired short-term memory; no difference observed in movement score, anxiety, or spatial memory |
| McKee et al. (2017)44 | 5-choice serial reaction time task | Early life (3–6 wk) | Folic acid, vitamin B12, choline, betaine, zinc, and methionine | Methyl-donor supplementation increased motivation, enhanced operant learning, and shortened reaction time needed to perform correct responses |
| Carletti et al. (2012)45 | Open field test and inhibitory avoidance task | Early life (PD7–30) | Folic acid | Folic acid supplementation reversed neonatal hypoxia-ischemia–induced anxiogenic effects and impaired aversive memory |
| Sittig et al. (2012)46 | Morris water maze | Early life (PD30–60) | Folic acid | Excessive folic acid led to deficits in motivation and spatial memory |
| Paternain et al. (2016)47 | Open field test, forced swim test, novel object recognition task | Adult (8–26 wk) | Folic acid, vitamin B12, betaine, and choline | Methyl-donor supplementation reversed early-life–induced depression in rats |
| Singh et al. (2011)48 | Active avoidance, passive avoidance, and elevated plus maze | Adult (6, 11, 16 mo) | Folic acid | Folic acid supplementation for 8 wk improved memory in behavioral tasks |
| Brocardo et al. (2010)49 | Open field test | Adult (3–4 mo) | Folic acid | Folic acid supplementation decreased hyperlocomotion in a rodent model of bipolar disorder |
| McKee et al. (2017)44 | Progressive ratio task | Adult (32 wk) | Folic acid, vitamin B12, choline, betaine, zinc, and methionine | Methyl-donor supplementation increased motivation |
| Barua et al. (2014)50 | Ultrasonic vocalization (P2, P4, P6), social approach task, trace fear conditioning, open field test (2–8 mo) | Pregnancy, lactation, and postweaning | Folic acid | Folic acid supplementation during both gestational development and postweaning period increased anxiety-like behavior, ultrasonic vocalization, and hyperactivity |
Abbreviation: PD, postnatal day.
Table 2.
Clinical studies in humans linking methyl-donor supplementation with neurodevelopment and cognition
| Reference | Behavioral test | Supplementation period | Nutrient(s) supplemented or analyzed | Outcomes |
|---|---|---|---|---|
| Boeke et al. (2013)23 | Offspring visual memory and intelligence | Pregnancy (1st and 2nd trimesters) | Analyzed: choline, vitamin B12, betaine, and folate (maternal intake questionnaire) | Increased gestational choline intake was associated with better visual memory at age 7; no associations for other methyl donors |
| Wu et al. (2012)51 | Bayley Scales of Infant Development, Third Edition | Pregnancy (2nd trimester) | Analyzed: choline, betaine, dimethylglycine, methionine, homocysteine, cysteine, vitamin B12, holotranscobalamin, and folate (maternal plasma) | Maternal plasma free choline and betaine in the first half of pregnancy associated with increased infant cognitive test scores |
| Valera-Gran et al. (2014)52 | Bayley Scales of Infant Development (1 y) | Pregnancy | Analyzed: folic acid (maternal intake questionnaire) | Children from mothers who consumed over 5000 µg of folic acid daily had lower psychomotor scores and increased risk for delayed psychomotor development |
| Signore et al. (2008)53 | Intelligence quotient (5 y) | Pregnancy | Analyzed: choline (maternal cord blood) | No correlations found |
| Julvez et al. (2009)54 | McCarthy Scale of Children's Abilities (4 y) | Pregnancy (1st trimester) | Analyzed: folic acid supplementation (maternal intake questionnaire) | Reported folic acid supplement use during pregnancy was associated with improved neurodevelopment and decreased inattention symptoms |
| Roth et al. (2011)55 | Children’s language competency (3 y) | Pregnancy (4 wk before conception to 8 wk after conception) | Analyzed: folic acid supplementation (maternal intake questionnaire) | Folic acid supplementation during early pregnancy was associated with reduced risk of severe language delay |
| Veena et al. (2010)56 | Cognitive function assessment—memory, attention and fluid reasoning (9–10 y) | Pregnancy (30 wk) | Analyzed: folic acid, vitamin B12 and homocysteine (maternal plasma) | Increased folic acid concentrations associated with increased cognitive test scores; no associations were seen with vitamin B12 or homocysteine |
| Chatzi et al. (2012)57 | Bayley Scales of Infant Development, Third Edition (18 mo) | Pregnancy (14–18 wk) | Analyzed: folic acid supplementation (maternal intake questionnaire and RBC concentration) | Folic acid supplementation (5 mg/d) was associated with enhanced vocabulary development, communication skills, and verbal comprehension |
| Villamor et al. (2012)58 | Peabody Picture Vocabulary Test III and Wide Range Assessment of Visual Motor Abilities (3 y) | Pregnancy (1st and 2nd trimesters) | Analyzed: folic acid, vitamin B12, choline, betaine, and methionine (maternal intake questionnaire) | Higher folate intake in 1st trimester was associated with higher receptive language; weak inverse association between vitamin B12 intake during 2nd trimester and receptive language; no association with choline, betaine, or methionine |
| Cheatham et al. (2012)59 | Short-term visuospatial memory, long-term episodic memory, language development, and global development | Pregnancy + lactation (18 wk gestation to 90 d post partum) | Supplemented: phosphatidylcholine | No effects seen |
| Prado et al. (2017)60 | General intellectual ability, declarative memory, procedural memory, executive function, academic achievement, fine motor dexterity, and socioemotional health (9–12 y) | Pregnancy + lactation (pregnancy + 3 mo post partum) | Supplemented: multiple micronutrients (iron, folic acid, retinol, vitamins D, E, C, B1, B2, B6, B12, zinc, copper, selenium, and iodine) or iron and folic acid | Supplementation with multiple micronutrients increased child cognitive development more than that with iron and folic acid alone |
| Ross et al. (2016)61 | Child Behavior Checklist (40 mo) | Pregnancy + lactation (2nd trimester to 3 mo) | Supplemented: phosphatidylcholine | Phosphatidylcholine supplementation decreased attention problems and social withdrawal |
| Nguyen et al. (2016)62 | Memory, executive function, attention, and hyperactivity | Early life (5–10 y) | Supplemented: choline (6 wk) | No effects seen |
| Strain et al. (2013)63 | Finger tapping test, Preschool Language Scale–Revised, Woodcock-Johnson Test of Scholastic Achievement, Child Behavior Checklist, and Kaufman Brief Intelligence Test | Early life (5 y) | Analyzed: choline, betaine, dimethylglycine, methionine, and homocysteine (plasma) | No associations found |
| Rauh-Pfeiffer et al. (2014)64 | HAWIVA-III, WPPSI-III, Kaufman Assessment Battery for Children | Early life (4–6 y) | Supplemented: folic acid, riboflavin, pyridoxine, cobalamin, and calcium lactate pentahydrate (3 mo) | Supplementation decreased plasma homocysteine concentration, but no changes were seen in cognitive performance |
| Lefèvra-Arbogast et al. (2016)30 | Dementia | Adult | Analyzed: folic acid, vitamins B6 and B12 (24-h recall) | Higher folate intakes associated with decreased risk of dementia; no associations found for vitamin B6 or B12 |
| Morris et al. (2010)65 | Wechsler Adult Intelligence Scale III | Adult (≥ 60 y) | Analyzed: folic acid, vitamin B12, and 5-MeTHF (serum) | Normal vitamin B12 status together with higher serum 5MeTHF associated with higher cognitive test scores |
| Morris et al. (2007)66 | Wechsler Adult Intelligence Scale III | Adult (≥ 60 y) | Analyzed: folic acid and vitamin B12 (serum) | Low vitamin B12 together with high serum folate associated with cognitive impairment; normal vitamin B12 status together with high serum folate associated with protection against cognitive impairment |
| Wald et al. (2010)67 | Cognitive function test scores (3 y from start of treatment) | Adult (≥45 y) | Analyzed: meta-analysis of 9 placebo-controlled randomized trials, supplementing folic acid with or without other B vitamins | No associations found |
| Durga et al. (2007)68 | Performance for memory, sensorimotor speed, complex speed, information processing speed, and word fluency | Adult (50–70 y) | Supplemented: folic acid (3 y) | 3-y changes in memory, information processing speed, and sensorimotor speed were significantly better in the folic acid–supplemented group |
| Walker et al. (2012)69 | Telephone Interview for Cognitive Status–Modified, the Brief Test of Adult Cognition by Telephone, and the Informant Questionnaire on Cognitive Decline in the Elderly | Adult (60–74 y) | Supplemented: folic acid and vitamin B12 (2 y) | Long-term supplementation of daily oral folic acid and vitamin B12 improved immediate and delayed memory performance; no differences seen in orientation, attention, semantic memory, processing speed, or informant reports |
| de Koning et al. (2016)70 | Geriatric Depression Scale | Adult (≥ 65 y) | Supplemented: folic acid and vitamin B12 (2 y) | No changes in depressive symptoms observed |
| Zhang et al. (2017)31 | MMSE | Adult (≈ 75 y) | Analyzed: meta-analysis of 77 studies, supplementing folic acid, and vitamin B12 and/or vitamin B6 (supplementation length varied from 6 to 18 mo) | No associations found |
| Clarke et al. (2014)71 | Specific cognitive domains and MMSE | Adult (≈ ≥60 y) | Analyzed: meta-analysis of 11 studies, supplementing B vitamins (supplementation length varied from 0.3 to 7 y) | No associations found |
| Porter et al. (2017)72 | MMSE, RBANS, and the Frontal Assessment Battery | Adult (≥ 60 y) | Analyzed: folate, vitamins B12, B6, B2, and homocysteine (at 5-y follow-up) | Lower vitamin B6 status was associated with accelerated rate of cognitive decline as assessed by RBANS and MMSE; lower vitamin B2 status was associated with accelerated rate of cognitive decline as assessed by RBANS, but not MMSE |
Abbreviations: HAWIVA-III, Hannover-Wechsler-Intelligenztest für das Vorschulalter; MMSE, Mini Mental State Examination; RBC, red blood cell; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status; WPPSI-III, Wechsler Preschool and Primary Scale of Intelligence, 3rd edition; 5-MeTHF, 5-methyltetrahydrofolate.
METHYL DONORS, NEURODEVELOPMENT, AND COGNITION
Beginning at conception, methyl-donor nutrients are important for the development of the CNS, specifically for closure of the neural tube. Beyond this, emerging evidence suggests that maternal folate status throughout pregnancy affects offspring neurodevelopment, postnatal behavior, and cognition.34 Folate concentrations may decline during pregnancy because of increased demand, larger blood volume, decreased intestinal absorption, or inadequate intake.73 The demand for energy and nutrients required for continuous fetal development also increases during pregnancy. Fortification of grain products with folic acid, a stable oxidized form of folate, was introduced in the United States in the 1990s to help increase the amount of folate consumed by women of childbearing age to prevent the risk of neural tube and other midline defects. Since the introduction of folic acid fortification, the incidence of neural tube defects has decreased by about 25%.32 The use of supplementation with folic acid and other methyl-donor nutrients (mainly vitamin B12, choline, and methionine) as therapeutic interventions to enhance CNS development, prevent neurological disease, and slow cognitive decline has burgeoned in the last 20 years.
Maternal supplementation during pregnancy or lactation
Maternal choline availability influences offspring brain development and function (reviewed by Zeisel and Niculescu10). In animal models, the positive relationship between maternal choline supplementation and neurodevelopment and cognition is well established. Maternal choline supplementation enhances offspring cognition (fewer working memory errors, fewer responses to reach criterion, fewer reference memory errors, faster behavior acquisition, and shorter escape latency in the Morris water maze), prevents age-related memory decline, enhances hippocampal function,40,74 reduces the effects of fetal alcohol exposure in the offspring,37,38 increases levels of nerve growth factor in the hippocampus and frontal cortex,75 and normalizes choline deficiency–induced DNA hypermethylation.76 Choline supplementation can also reverse both the effect of folate deficiency on neurogenesis and apoptosis at E17 in the fetal forebrain77 and iron deficiency–induced reprogramming within the hippocampus.78 In a mouse model of Down syndrome, maternal supplementation with choline attenuated degeneration of the basal forebrain cholinergic neuron system,79 while in a mouse model of autism, high maternal choline consumption improved anxiety-like behaviors and increased social interaction41 (for review, see Jiang et al80). Because of the beneficial findings in rodent models, effects in humans are being investigated. The current results are mixed. Choline levels in early second-trimester maternal plasma are positively correlated with early cognitive development51 as well as with cognitive development at 7 years.23 However, neither supplementing with phosphatidylcholine59 nor maternal cord blood choline concentrations53 correlated with enhanced infant cognition (for review, see McCann and Ames74 and Jiang et al80).
In contrast to the link between maternal choline supplementation and neurodevelopment, the positive link between maternal folic acid supplementation and neurodevelopment and cognition is stronger in humans than it is in animal models. In humans, maternal folic acid supplementation is associated with improved verbal, motor, and verbal-executive scores in 4-year-olds.54 Maternal use of folic acid supplements is associated with reduced risk of severe language delay in children at 3 years of age.55 Higher plasma folate during week 30 of pregnancy is associated with increases in learning, long-term storage, and memory retrieval and a decrease in inattention at 10 years of age.56 Folic acid intake during the first trimester is associated with reduced language delay, increased communication and verbal skills, and increased cognitive performance.55,57,58 Furthermore, supplementation with folic acid during the second and third trimesters decreased the levels of homocysteine in maternal and cord blood, although outcomes in offspring were not studied.81 In animal models, offspring from rodents deficient in methyl donors during pregnancy but supplemented with folic acid during late gestation had reduced risk for structural and functional defects in the CNS during the prenatal period.82 Additionally, reduced intake of folate in pregnant mice impaired short-term memory in offspring and increased apoptosis in the hippocampus.83 Folic acid supplementation in late gestation restored expression of specific microRNAs in the brain in methyl donor–deficient animals.82 Expression of a number of genes related to neuronal function is altered in the brain of offspring after folic acid supplementation.50
In naturally occurring food sources, a combination of multiple methyl-donor nutrients is typically found within a single food. Additionally, methyl-donor nutrients work in concert with each other within 1-carbon metabolism. Therefore, supplementation with mixtures of methyl-donor nutrients, as opposed to supplementation with single nutrients, has been examined as well. Children of mothers supplemented with a mixture of micronutrients that included multiple methyl-donor nutrients had increased procedural memory and higher general intellectual ability at 10 years of age.60 A study of maternal methyl-donor nutrient consumption found positive associations between folic acid intake and cognition in children at 3 years, but no associations were found for choline, betaine, or methionine consumption.58 In rodents, methyl-donor supplementation during pregnancy and lactation increased DNA methylation in the brain42,84 and normalized the increased preference for palatable foods observed in offspring from high-fat diet-fed dams.42 Postnatal supplementation with multiple methyl-donor nutrients during lactation (postnatal day 2–9) restored methionine levels and reduced early-life-stress–induced cognitive impairments in adult animals.39 Additionally, maternal micronutrient imbalance (excessive folic acid and/or vitamin B12 deficiency) leads to global DNA hypomethylation and reduced expression of neurotrophins in the brain of offspring.14,85
Early postnatal supplementation
Supplementation with methyl-donor nutrients during early life (infancy, childhood, or adolescence) is relatively understudied, as the majority of supplementation studies have focused on supplementation during pregnancy or later life. Infants supplemented with choline were shown to have fewer attention problems and less social withdrawal at 40 months.61 However, if administered at a later time point (to school-aged children), 6 weeks of choline supplementation did not improve cognition.62 Similarly, 3 months of mixed B vitamin supplementation in kindergarten children improved folate and homocysteine status but did not affect cognitive performance.64 However, betaine status, but not choline status, in 5-year-olds was positively associated with total language scores.63 In rodent models, rat pups deficient in folate from birth to 3 weeks of age exhibited deficits in long-term memory, spatial learning, and set-shifting.86 Three weeks of 90% methyl-donor depletion increased depression-like behaviors in the forced swim test, while 3 weeks of methyl-donor supplementation increased DNA methylation in the amygdala and reduced depression-like behaviors.87 Similarly, 3 weeks of folic acid supplementation prevented hypoxia-ischemia–induced anxiogenic and memory impairments, possibly by partial recovery of Na+, K+-ATPase in the frontal cortex.45 Finally, early-life postnatal supplementation with methyl-donor nutrients during weeks 3 to 6 of life in female mice increased motivation and learning in an operant chamber task.44 Collectively, these studies demonstrate that methyl-donor supplementation can affect neurodevelopment and cognition beyond the well-established critical period of pregnancy; however, studies in humans indicate the window of opportunity for intervention may be reduced by the time children have reached school age.
Supplementation in adults
Memory loss, increased neurological disease risk, and increased oxidative stress in the brain are characteristic of aging adults. In aged rats, 8 weeks of folic acid supplementation improved memory and decreased lipid peroxidation, a marker of aging, in multiple brain regions.48 In adult rats, 7 days of folic acid treatment decreased locomotor activity and lipid peroxidation in the hippocampus in a pharmacological model of manic behavior.49 Furthermore, 1 week of methyl-donor supplementation in mice increased motivation to respond in an operant task, although only if animals had been exposed to a high-fat diet during pregnancy/lactation or had been previously exposed to methyl-donor nutrients early in life.44 Lastly, 18 weeks of dietary methyl-donor supplementation normalized depression-like behaviors induced by maternal separation during lactation.47
While studies of methyl-donor supplementation in aged animals are limited, the use of methyl-donor nutrients in clinical trials is extensive. The results, however, are mixed. A meta-analysis examined whether 6 months of folic acid supplementation, with or without other B vitamins, altered cognitive function (ie, memory speed, language, and executive function) in healthy individuals and found no changes in cognition.67 However, a study of 3 years of folic acid supplementation in healthy individuals reported higher scores for memory, information processing speed, and sensorimotor speed.68 In a trial of 2-year supplementation with folic acid and B12, improved cognitive functioning, including improved immediate and delayed memory performance, was observed.69 However, in 2 separate trials, 2 years of B12 and folic acid supplementation in older adults did not reduce depressive symptoms.70,88 A recent meta-analysis identified 77 human trials designed to examine whether B vitamin supplementation reduced homocysteine levels and improved cognition in elderly patients with Alzheimer disease or dementia.31 After filtering and selection, 4 randomized controlled trials were included in the analysis. With the Mini-Mental State Examination score used as a measure of cognition, the meta-analysis found that B vitamins do reduce homocysteine levels; however, there was no overall improvement in cognition in these patients.31 Similarly, another meta-analysis of 11 studies involving 22 000 study participants failed to find an effect of B vitamin supplementation on cognition, even though verified reductions in homocysteine and increases in folate were observed.71 Future longitudinal studies may focus on whether these B vitamins could perhaps slow the progression of cognitive decline or be efficacious in select clinical populations.89 Additionally, a study showed that lower concentrations of vitamin B6 and riboflavin (B2) were associated with an accelerated rate of cognitive decline as assessed by the Repeatable Battery for the Assessment of Neuropsychological Status, but the same association with vitamin B2 was not found when the Mini-Mental State Examination was used to measure cognition.72
Adverse effects of supplementation
The Swedish physician Paracelsus said, “The dose makes the poison,” which is a basic principle of toxicology and applies equally to the study of dietary supplementation. Owing to both supplementation of processed food products and adherence to dietary recommendations for nutrient consumption, 5% of the US adult population and between 30% and 66% of children aged 1 to 13 years consume more than the recommended daily dose of methyl vitamins.90,91 As this subset of the population emerged, studies began showing the detrimental effects of excessive methyl-donor supplementation. The current recommendation for folic acid intake is 400 µg/d for adult males and nonpregnant females. The recommendation increases to 600 µg/d for pregnant females and 500 µg/d for lactating females. Offspring of women who consumed higher-than-recommended amounts of folic acid during pregnancy (5000 µg/d, almost 10 times the recommended daily allowance) had lower psychomotor scores at 1 year of age.52 In mice, offspring of dams fed high levels of folic acid (10 times the control, 20 mg/kg of diet) during gestation displayed altered development of the cortical layers at E17.5 and short-term memory impairment and decreased hippocampal size at 3 weeks.43 High-vitamin diets during pregnancy have been shown to increase the risk of metabolic syndrome in offspring by altering the neural pathways involved in energy homeostasis and feeding,92,93 but this could be prevented by providing a postweaning diet high in multivitamins or folic acid.94 Excessive folic acid intake (4 times the control, 8.0 mg/kg of diet) during adolescence has been correlated with deficits in motivation and spatial memory tasks.46 Another factor that affects response to methyl-donor supplementation is genetic variations in the enzymes that metabolize the nutrients, such as methylenetetrahydrofolate reductase (MTHFR). In dams with a MTHFR deficiency, methyl-donor supplementation was associated with embryonic delay and growth retardation at E10.5 in their offspring, but longer-term outcomes were not studied.95 It is evident that a genetic and environmental interaction exists in which concentrations of methyl-donor nutrients are no longer healthful, but are harmful. Studying these interactions remains critical (reviewed by Shorter et al96).
CONSIDERATIONS FOR FUTURE RESEARCH
It is clear that both excessive and inadequate levels of methyl levels are detrimental to neurodevelopment and cognition. Further research is needed to understand and standardize which nutrients to provide supplementally, which concentrations of each nutrient are appropriate, how long a supplement should be taken, and which populations should receive supplementation as well as to determine whether methyl-donor supplementation can alter CNS development and long-term cognition. Many of the basic research studies point to methyl-donor supplementation as a way to modulate cognition, but the current clinical literature is inconclusive.97 A number of experimental variables need to be thoughtfully considered to ensure the robustness of research in this field. The focus here is on animal research, but similar considerations are applicable to human clinical research as well.
Single-nutrient vs mixed-nutrient supplementation
In reviewing the current supplements used to enhance brain health, many studies promote single-nutrient supplementation while others tout a mixed-nutrient approach. The mixed-nutrient approach may be more relevant to what is found in naturally occurring food sources (which contain multiple methyl donors). Additionally, there is the unique relationship between folate deficiency and vitamin B12 deficiency, wherein the surplus of one may mask a deficiency of the other (ie, excess folate/folic acid may mask a vitamin B12 deficiency). Generally, cellular folate is present in low concentrations; therefore, regenerating tetrahydrofolate from 5-methyltetrahydrofolate is extremely important for 1-carbon metabolism and regulation of cellular homocysteine levels. This regeneration can occur in 2 ways. One is dependent on the availability of vitamin B12 to act as a coenzyme for methionine synthase. When vitamin B12 is not present, the cell has increased concentrations of both 5-methyltetrahydrofolate, which has been shown to impair DNA and RNA synthesis,98 and homocysteine, high levels of which have been observed in many neurological disorders.5,24,35,99 Folic acid supplementation has been shown to decrease the homocysteine concentration, the biomarker routinely used to determine cellular levels of vitamin B12 and folic acid. However, when high folate levels exist together with low vitamin B12 levels, individuals present with anemia, macrocytosis, and cognitive impairment.65,66,100 Therefore, a mixed-nutrient approach to methyl-donor supplementation may mitigate these masking effects.
One-carbon metabolism and the brain
The liver is responsible for the vast majority of metabolic processes in the body. Therefore, 1-carbon metabolism has largely been functionally characterized within the liver. It is widely thought that these methyl-donor nutrients are metabolized by the liver, and then metabolites are transported by the blood to be distributed throughout the body, passing through the blood–brain barrier to reach the brain. However, because enzymes that mediate 1-carbon metabolism are present within the brain, this would suggest that local 1-carbon metabolism can also occur within the brain.
Even though 1-carbon metabolism in the liver is well studied and research to date has provided an understanding of how perturbations such as diet or disease alter this metabolism, 1-carbon metabolism in the brain has not been well characterized. It is thought, on the basis of increased homocysteine concentrations in plasma and cerebrospinal fluid, that 1-carbon metabolism in the brain is disrupted in various neurological diseases such as Alzheimer disease, depression, and schizophrenia.101–104 This relies, however, on the assumption that peripheral metabolite levels are reliable biomarkers of brain levels, which has not yet been demonstrated. Importantly, a few functional differences between 1-carbon metabolism in the brain and 1-carbon metabolism in the liver have been found. As recently as 2006, there remained controversy about whether the brain contained a functional transsulfuration pathway, important for the elimination of reactive oxygen species.26 Because the brain consumes a relatively high level of oxygen, effective strategies for the elimination of high levels of reactive oxygen species are necessary. Possible impairments to the brain transsulfuration pathway are found both in patients with Alzheimer disease and in those with Parkinson disease.26 Furthermore, levels of cystathionine are higher in the brain than in other organs, while γ-cystathionase activity in the brain is 100-fold lower than that in the liver. It is now known that the brain contains a functional transsulfuration pathway, but these 2 functional differences provide evidence that this pathway may function differently in the brain than in the liver.
Additionally, the brain lacks the enzyme betaine-homocysteine methyltranserfase.24 Therefore, it lacks the ability to utilize betaine to resynthesize methionine from homocysteine, a pathway present in the liver. This may indicate that the brain, compared with the liver, has increased reliance on the presence of vitamin B12 to aid in the maintenance of cellular methionine levels. There is evidence that the brain is extremely resilient when nutrient deficiencies are present. One study found that, when rats were fed a folate-free diet, total folate concentrations in the liver and kidney decreased by 60%, but levels of folate in the brain remained the same throughout the study.105 Additionally, a recent study showed that, when folate deficiency was induced, the brain—as compared with plasma, erythrocytes, liver, kidney, and spleen—maintained folate metabolite levels better than the other organs.106 These studies indicate a possible maintenance system or protective mechanism present in the brain but not in other organs. One potential mechanism could involve folate-binding proteins, which are expressed at high levels within the choroid plexus107,108 and may contribute to higher folate levels in the brain at the expense of levels in other tissues. This same phenomenon may also occur in other organs with high expression of folate-binding proteins, such as the placenta,109,110 which would result in folate being provided to the offspring at the expense of the mother. However, it has also been shown that vitamin B12 levels in the brain decreased during dietary folate deficiency.111 Current gaps in the literature include a lack of studies evaluating brain levels of 1-carbon metabolites in response to dietary changes. There is also a need to determine whether plasma levels of metabolites reflect changes in brain levels, since findings of an association could support the potential use of plasma levels as a biomarker for brain dysfunction.
It is well known that the brain is composed of multiple cell types, including neurons, astrocytes, microglia, and endothelial cells, that work coordinately. Functional differences in various cell types could alter 1-carbon metabolism.5 For example, the reliance on glucose to provide serine for 1-carbon metabolism is higher in the brain than in other organs, since serine does not easily pass through the blood–brain barrier.5 Additionally, the lack of betaine-homocysteine methyltransferase in the brain alters 1-carbon metabolism by eliminating the ability to utilize betaine directly to remethylate homocysteine. Neurons, glia, and astrocytes could also conceivably utilize the same nutrients in different ways because of their functional differences. One possible example is the increased reliance of the brain on astrocytes and oligodendrocytes to mediate transsulfuration pathway reactions. The brain consumes a disproportionate amount of the body’s oxygen and therefore must eliminate copious amounts of reactive oxygen species. Synthesis of glutathione is critical to this elimination. In turn, glutathione synthesis is dependent on the cysteine concentration. Excitatory amino acid transporter 3, the main transporter of extracellular cysteine, has been found in greater densities on astrocytes and oligodendrocytes,112 possibly indicating increased participation of these 2 cell types in transsulfuration pathway–mediated elimination of reactive oxygen species. Further characterization of the roles each cell type plays in brain 1-carbon metabolism will be important in developing a full appreciation of the role of 1-carbon metabolism in the brain.
Influence of sex on outcomes
The pervasiveness of sex differences in the brain and neurological disorders warrants a discussion in this context. While investigations of sex differences are increasingly included in preclinical research and are typically included in the human clinical literature, analysis of both sexes remains underrepresented in the current animal literature. Only 4 of the 14 studies shown in Table 1 included a comparison between sexes, while 8 focused on a single sex, 1 studied combined sexes without analysis, and another did not report the sex of the animals. Sex differences within 1-carbon metabolism have not been extensively examined, although some reports suggest that sex is an important variable with regard to the study of 1-carbon metabolism and brain function. Sex was found to influence vitamin B12 and 1-carbon–related enzymes as well as plasma homocysteine levels in the fetal liver, and sex moderated the effect of maternal smoking on these outcomes.113 Plasma levels of homocysteine were found to correlate with some brain metabolite levels (as determined through imaging) in elderly females, but not males.114 Furthermore, in an animal study involving supplementation of high-dose folic acid to pregnant dams, the expression of transcription factors related to neuronal function in whole brain samples was oppositely affected in males (decreased) compared with females (increased).115 In addition, in a mouse model with a deletion of the Mthfr gene, early-life manipulations, either neonatal stress116 or administration of a γ-aminobutyric acid–potentiating drug,117 were found to have sex-specific effects on behavior.
Sex differences in DNA methylation patterns in the brain have also been noted. At postnatal day 1, female rats were found to have significantly higher activity of DNA methyltransferase 1 as well as an increase in DNA methylation118 in the preoptic area of the hypothalamus. Sex differences in the expression of genes that either increase DNA methyltransferases119 or decrease (Gadd45b) DNA methylation120 have been demonstrated in the amygdala, and sex differences in promoter-specific DNA methylation of the bdnf gene have been observed within the prefrontal cortex.121 It has been well characterized that sex alters the prevalence of many neurological disorders, including Parkinson disease, Alzheimer disease, cognitive decline, autism spectrum disorders, mood and anxiety disorders, major depression, trauma-related disorders, depressive disorders, autoimmune disease affecting the nervous system, attention-deficit/hyperactivity disorder, and neurodegenerative disorders.122 Collectively, these findings suggest that understanding sex differences in 1-carbon metabolism in the brain, both at baseline and in response to methyl-donor supplementation, may increase the current understanding of sex differences in neurodevelopmental outcomes.
In mammals, the presence of SRY on the male Y chromosome drives the development of the testis. Gonadal sex hormones (androgens, estrogens, and progestins) then drive the sexual differentiation. During development, the male brain is exposed to a testosterone surge, which is converted to estradiol. At the same time, in females, the estradiol levels remain low.123 Within the brain, these sex hormones are known to influence neuroanatomy, neurochemistry, and neuron structure.122 One interesting difference between the sexes is the growth speed of the cortex. During development, cortical volume increases faster in males than in females.124 This growth difference is hypothesized to drive, in part, the predisposition in males for autism spectrum disorders.125 Additionally, during development, maternal care, estrogen receptor expression, and neonatal hormone exposure all have been shown to correlate with sex-specific differences in DNA methylation, which in turn can influence development and behavior.126
Sex hormones can also have effects during adulthood, when females are exposed to high levels of estradiol and progesterone and males are exposed to high levels of testosterone. To add complexity, the female estrus cycle alters the levels of the female sex hormones, which is known to change behavior.127–131 Additionally, steroid hormone receptors, which can be found in different densities throughout the brain, are known to act as transcription factors and influence epigenetic machinery (coactivators and corepressors).123,126 Interestingly, there is even a sex-specific difference in the expression of these coactivators.132 Monitoring the estrus cycle would inform whether observed sex differences remain throughout the entire estrus cycle or are stronger at one point versus another, while testing at a standard stage of the estrus cycle may decrease the variance that could be observed in female study participants. Lastly, the presence of sex chromosomes, or genetic sex (XX vs XY), can also influence neurodevelopment and sexual differentiation in the brain (reviewed by McCarthy and Arnold133). Thus, genetic sex and the presence of sex hormones during development and throughout adulthood both have the potential to influence the physiological endpoints of interest. The inclusion and analysis of both sexes will prove essential to understanding how supplementation and dietary interventions alter neurodevelopment and cognition.
CONCLUSION
The current literature suggests that dietary methyl-donor supplementation is a promising therapeutic strategy with the potential to improve or protect cognition and neurodevelopment in vulnerable populations. Earlier diagnosis of nutrient deficiencies and excesses, understanding critical periods for intervention, and identification of which populations would most benefit from supplementation could all help to increase the efficacy of supplementation. Ensuring robustness in animal studies through the careful consideration of diet composition, tissue and cellular specificity, and sex differences will significantly advance the understanding of how methyl-donor supplementation affects neurodevelopment on a mechanistic level as well as increase the potential for translation to at-risk human populations.
Acknowledgments
Author contributions. S.E.M. and T.M.R. contributed equally to the planning, writing, and editing of this review.
Funding/support. This work was supported by National Institutes of Health grants MH087978 and MH106330 to T.M.R.
Declaration of interest. The authors have no relevant interests to declare.
References
- 1. Moody L, Chen H, Pan Y-X.. Early-life nutritional programming of cognition—the fundamental role of epigenetic mechanisms in mediating the relation between early-life environment and learning and memory process. Adv Nutr. 2017;8:337–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen Y, Baram TZ.. Toward understanding how early-life stress reprograms cognitive and emotional brain networks. Neuropsychopharmacology. 2016;41:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li E. Chromatin modification and epigenetic reprogramming in mammalian development. Nat Rev Genet. 2002;3:662–673. [DOI] [PubMed] [Google Scholar]
- 4. Fox JT, Stover PJ.. Folate-mediated one-carbon metabolism. Vitam Horm. 2008;79:1–44. [DOI] [PubMed] [Google Scholar]
- 5. Ducker GS, Rabinowitz JD.. One-carbon metabolism in health and disease. Cell Metab. 2016;25:27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tibbetts AS, Appling DR.. Compartmentalization of mammalian folate-mediated one-carbon metabolism. Annu Rev Nutr. 2010;30:57–81. [DOI] [PubMed] [Google Scholar]
- 7. Cohen EL, Wurtman RJ.. Brain acetylcholine: control by dietary choline. Science. 1976;191:561–562. [DOI] [PubMed] [Google Scholar]
- 8. Klinkenberg I, Sambeth A, Blokland A.. Acetylcholine and attention. Behav Brain Res. 2011;221:430–442. [DOI] [PubMed] [Google Scholar]
- 9. Savelkoul PJM, Janickova H, Kuipers AAM, et al. A specific multi-nutrient formulation enhances M1 muscarinic acetylcholine receptor responses in vitro. J Neurochem. 2012;120:631–640. [DOI] [PubMed] [Google Scholar]
- 10. Zeisel SH, Niculescu MD.. Perinatal choline influences brain structure and function. Nutr Rev. 2006;64:197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Scott JM. Folate-vitamin B12 interrelationships in the central nervous system. Proc Nutr Soc. 1992;51:219–224. [DOI] [PubMed] [Google Scholar]
- 12. Anderson OS, Sant KE, Dolinoy DC.. Nutrition and epigenetics: an interplay of dietary methyl donors, one-carbon metabolism and DNA methylation. J Nutr Biochem. 2012;23:853–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Niculescu MD, Zeisel SH.. Diet, methyl donors and DNA methylation: interactions between dietary folate, methionine and choline. J Nutr. 2002;132(8 suppl):2333S–2335S. [DOI] [PubMed] [Google Scholar]
- 14. Sable P, Kale A, Joshi A, et al. Maternal micronutrient imbalance alters gene expression of BDNF, NGF, TrkB and CREB in the offspring brain at an adult age. Int J Dev Neurosci. 2014;34:24–32. [DOI] [PubMed] [Google Scholar]
- 15. Fernàndez-Roig S, Lai S-C, Murphy MM, et al. Vitamin B12 deficiency in the brain leads to DNA hypomethylation in the TCblR/CD320 knockout mouse. Nutr Metab (Lond). 2012;9:41. doi:10.1186/1743-7075-9-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ly A, Hoyt L, Crowell J, et al. Folate and DNA methylation. Antioxid Redox Signal. 2012;17:302–326. [DOI] [PubMed] [Google Scholar]
- 17. Niculescu MD, Craciunescu CN, Zeisel SH.. Dietary choline deficiency alters global and gene-specific DNA methylation in the developing hippocampus of mouse fetal brains. FASEB J. 2006;20:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tomizawa H, Matsuzawa D, Ishii D, et al. Methyl-donor deficiency in adolescence affects memory and epigenetic status in the mouse hippocampus. Genes Brain Behav. 2015;14:301–309. [DOI] [PubMed] [Google Scholar]
- 19. Breimer LH, Nilsson TK.. Has folate a role in the developing nervous system after birth and not just during embryogenesis and gestation?. Scand J Clin Lab Invest. 2012;72:185–191. [DOI] [PubMed] [Google Scholar]
- 20. Araújo JR, Martel F, Borges N, et al. Folates and aging: role in mild cognitive impairment, dementia and depression. Ageing Res Rev. 2015;22:9–19. [DOI] [PubMed] [Google Scholar]
- 21. Zhang Y, Hodgson NW, Trivedi MS, et al. Decreased brain levels of vitamin B12 in aging, autism and schizophrenia. PLoS One. 2016;11:e0146797. doi:10.1371/journal.pone.0146797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grayson DR, Guidotti A.. The dynamics of DNA methylation in schizophrenia and related psychiatric disorders. Neuropsychopharmacology. 2012;38:138–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boeke CE, Gillman MW, Hughes MD, et al. Choline intake during pregnancy and child cognition at age 7 years. Am J Epidemiol. 2013;177:1338–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bottiglieri T. Homocysteine and folate metabolism in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1103–1112. [DOI] [PubMed] [Google Scholar]
- 25. Suh JR, Herbig AK, Stover PJ.. New perspectives on folate catabolism. Annu Rev Nutr. 2001;21:255–282. [DOI] [PubMed] [Google Scholar]
- 26. Vitvitsky V, Thomas M, Ghorpade A, et al. A functional transsulfuration pathway in the brain links to glutathione homeostasis. J Biol Chem. 2006;281:35785–35793. [DOI] [PubMed] [Google Scholar]
- 27. Jiang B, Yao G, Ding C, et al. Serum homocysteine, folic acid and VitB12 in patients with mild cognitive impairment. 2017;10:3731–3736. [Google Scholar]
- 28. Setién-Suero E, Suárez-Pinilla M, Suárez-Pinilla P, et al. Homocysteine and cognition: a systematic review of 111 studies. Neurosci Biobehav Rev. 2016;69:280–298. [DOI] [PubMed] [Google Scholar]
- 29. Köbe T, Witte AV, Schnelle A, et al. Vitamin B-12 concentration, memory performance, and hippocampal structure in patients with mild cognitive impairment. Am J Clin Nutr. 2016;103:1045–1054. [DOI] [PubMed] [Google Scholar]
- 30. Lefèvre-Arbogast S, Féart C, Dartigues J-F, et al. Dietary B vitamins and a 10-year risk of dementia in older persons. Nutrients. 2016;8:761. doi:10.3390/nu8120761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang D-M, Ye J-X, Mu J-S, et al. Efficacy of vitamin B supplementation on cognition in elderly patients with cognitive-related diseases. J Geriatr Psychiatry Neurol. 2017;30:50–59. [DOI] [PubMed] [Google Scholar]
- 32. Blom HJ. Folic acid, methylation and neural tube closure in humans. Birth Defects Res Part A Clin Mol Teratol. 2009;85:295–302. [DOI] [PubMed] [Google Scholar]
- 33. Zeisel SH. Importance of methyl donors during reproduction. Am J Clin Nutr. 2009;89:673S–677S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McGarel C, Pentieva K, Strain JJ, et al. Emerging roles for folate and related B-vitamins in brain health across the lifecycle. Proc Nutr Soc. 2015;74:46–55. [DOI] [PubMed] [Google Scholar]
- 35. Kennedy DO. B vitamins and the brain: mechanisms, dose and efficacy—a review. Nutrients. 2016;8. doi:10.3390/nu8020068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Morris MS. The role of B vitamins in preventing and treating cognitive impairment and decline. Adv Nutr. 2012;3:801–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thomas JD, Abou EJ, Dominguez HD.. Prenatal choline supplementation mitigates the adverse effects of prenatal alcohol exposure on development in rats. Neurotoxicol Teratol. 2009;31:303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Thomas JD, Idrus NM, Monk BR, et al. Prenatal choline supplementation mitigates behavioral alterations associated with prenatal alcohol exposure in rats. Birth Defects Res Part A Clin Mol Teratol. 2010;88:827–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Naninck EFG, Oosterink JE, Yam K-Y, et al. Early micronutrient supplementation protects against early stress-induced cognitive impairments. FASEB J. 2016;9:fj.201600834R. doi:10.1096/fj.201600834R [DOI] [PubMed] [Google Scholar]
- 40. Zhu CH, Wu T, Jin Y, et al. Prenatal choline supplementation attenuates spatial learning deficits of offspring rats exposed to low-protein diet during fetal period. J Nutr Biochem. 2016;32:163–170. [DOI] [PubMed] [Google Scholar]
- 41. Langley EA, Krykbaeva M, Blusztajn JK, et al. High maternal choline consumption during pregnancy and nursing alleviates deficits in social interaction and improves anxiety-like behaviors in the BTBR T+Itpr3tf/J mouse model of autism. Behav Brain Res. 2015;278:210–220. [DOI] [PubMed] [Google Scholar]
- 42. Carlin J, George R, Reyes TM.. Methyl donor supplementation blocks the adverse effects of maternal high fat diet on offspring physiology. PLoS One. 2013;8:e63549..doi:10.1371/journal.pone.0063549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bahous RH, Jadavji NM, Deng L, et al. High dietary folate in pregnant mice leads to pseudo-MTHFR deficiency and altered methyl metabolism, with embryonic growth delay and short-term memory impairment in offspring. Hum Mol Genet. 2017;26:888–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McKee SE, Grissom NM, Herdt CT, et al. Methyl donor supplementation alters cognitive performance and motivation in female offspring from high-fat diet–fed dams. FASEB J. 2017;31:2352–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Carletti JV, Deniz BF, Miguel PM, et al. Folic acid prevents behavioral impairment and Na+, K+-ATPase inhibition caused by neonatal hypoxia–ischemia. Neurochem Res. 2012;37:1624–1630. [DOI] [PubMed] [Google Scholar]
- 46. Sittig LJ, Herzing LBK, Xie H, et al. Excess folate during adolescence suppresses thyroid function with permanent deficits in motivation and spatial memory. Genes Brain Behav. 2012;11:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Paternain L, Martisova E, Campión J, et al. Methyl donor supplementation in rats reverses the deleterious effect of maternal separation on depression-like behaviour. Behav Brain Res. 2016;299:51–58. [DOI] [PubMed] [Google Scholar]
- 48. Singh R, Kanwar SS, Sood PK, et al. Beneficial effects of folic acid on enhancement of memory and antioxidant status in aged rat brain. Cell Mol Neurobiol. 2011;31:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Brocardo PS, Budni J, Pavesi E, et al. Folic acid administration prevents ouabain-induced hyperlocomotion and alterations in oxidative stress markers in the rat brain. Bipolar Disord. 2010;12:414–424. [DOI] [PubMed] [Google Scholar]
- 50. Barua S, Chadman KK, Kuizon S, et al. Increasing maternal or post-weaning folic acid alters gene expression and moderately changes behavior in the offspring. PLoS One. 2014;9:e101674. doi:10.1371/journal.pone.0101674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wu BTF, Dyer RA, King DJ, et al. Early second trimester maternal plasma choline and betaine are related to measures of early cognitive development in term infants. PLoS One. 2012;7:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Valera-Gran D, Garcia de la Hera M, Navarrete-Munoz EM, et al. Folic acid supplements during pregnancy and child psychomotor development after the first year of life. JAMA Pediatr. 2014;168:e142611. doi:10.1001/jamapediatrics.2014.2611 [DOI] [PubMed] [Google Scholar]
- 53. Signore C, Ueland PM, Troendle J, et al. Choline concentrations in human maternal and cord blood and intelligence at 5 y of age. Am J Clin Nutr. 2008;87:896–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Julvez J, Fortuny J, Mendez M, et al. Maternal use of folic acid supplements during pregnancy and four-year-old neurodevelopment in a population-based birth cohort. Paediatr Perinat Epidemiol. 2009;23:199–206. [DOI] [PubMed] [Google Scholar]
- 55. Roth C, Magnus P, Schjølberg S, et al. Folic acid supplements in pregnancy and severe language delay in children. JAMA. 2011;306:1566–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Veena SR, Krishnaveni GV, Srinivasan K, et al. Higher maternal plasma folate but not vitamin B-12 concentrations during pregnancy are associated with better cognitive function scores in 9- to 10-year-old children in South India. J Nutr. 2010;140:1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chatzi L, Papadopoulou E, Koutra K, et al. Effect of high doses of folic acid supplementation in early pregnancy on child neurodevelopment at 18 months of age:the mother–child cohort “Rhea” study in Crete, Greece. Public Health Nutr. 2012;15:1728–1736. [DOI] [PubMed] [Google Scholar]
- 58. Villamor E, Rifas-Shiman SL, Gillman MW, et al. Maternal intake of methyl-donor nutrients and child cognition at 3 years of age. Paediatr Perinat Epidemiol. 2012;26:328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cheatham CL, Goldman BD, Fischer LM, et al. Phosphatidylcholine supplementation in pregnant women consuming moderate-choline diets does not enhance infant cognitive function: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2012;96:1465–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Prado EL, Sebayang SK, Apriatni M, et al. Maternal multiple micronutrient supplementation and other biomedical and socioenvironmental influences on children’s cognition at age 9–12 years in Indonesia: follow-up of the SUMMIT randomised trial. Lancet Glob Health. 2017;5:e217–e228. [DOI] [PubMed] [Google Scholar]
- 61. Ross RG, Hunter SK, Hoffman MC, et al. Perinatal phosphatidylcholine supplementation and early childhood behavior problems: evidence for CHRNA7 moderation. Am J Psychiatry. 2016;173:509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nguyen TT, Risbud RD, Mattson SN, et al. Randomized, double-blind, placebo-controlled clinical trial of choline supplementation in school-aged children with fetal alcohol spectrum disorders. Am J Clin Nutr. 2016;104:1683–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Strain JJ, McSorley EM, van Wijngaarden E, et al. Choline status and neurodevelopmental outcomes at 5 years of age in the Seychelles Child Development Nutrition Study. Br J Nutr. 2013;110:330–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rauh-Pfeiffer A, Handel U, Demmelmair H, et al. Three-month B vitamin supplementation in pre-school children affects folate status and homocysteine, but not cognitive performance. Eur J Nutr. 2014;53:1445–1456. [DOI] [PubMed] [Google Scholar]
- 65. Morris MS, Jacques PF, Rosenberg IH, et al. Circulating unmetabolized folic acid and 5-methyltetrahydrofolate in relation to anemia, macrocytosis, and cognitive test performance in American seniors. Am J Clin Nutr. 2010;91:1733–1744. [DOI] [PubMed] [Google Scholar]
- 66. Morris MS, Jacques PF, Rosenberg IH, et al. Folate and vitamin B-12 status in relation to anemia, macrocytosis, and cognitive impairment in older Americans in the age of folic acid fortification. Am J Clin Nutr. 2007;85:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wald DS, Kasturiratne A, Simmonds M.. Effect of folic acid, with or without other B vitamins, on cognitive decline: meta-analysis of randomized trials. Am J Med. 2010;123:522.e2–527.e2. [DOI] [PubMed] [Google Scholar]
- 68. Durga J, van Boxtel MPJ, Schouten EG, et al. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: a randomised, double blind, controlled trial. Lancet. 2007;369:208–216. [DOI] [PubMed] [Google Scholar]
- 69. Walker JG, Batterham PJ, Mackinnon AJ, et al. Oral folic acid and vitamin B-12 supplementation to prevent cognitive decline in community-dwelling older adults with depressive symptoms—the Beyond Ageing Project: a randomized controlled trial. Am J Clin Nutr. 2012;95:194–203. [DOI] [PubMed] [Google Scholar]
- 70. de Koning E, van der Zwaluw N, van Wijngaarden J, et al. Effects of two-year vitamin B12 and folic acid supplementation on depressive symptoms and quality of life in older adults with elevated homocysteine concentrations: additional results from the B-PROOF Study, an RCT. Nutrients. 2016;8:748. doi:10.3390/nu8110748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Clarke R, Bennett D, Parish S, et al. Effects of homocysteine lowering with B vitamins on cognitive aging: meta-analysis of 11 trials with cognitive data on 22,000 individuals. Am J Clin Nutr. 2014;100:657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Porter K, Hughes CF, Hoey L, et al. Biomarkers of folate and related B-vitamins as predictors of cognitive decline in older Irish adults over a 5 year follow up period. Proc Nutr Soc. 2017;76:E4. doi:.10.1017/S0029665117000040 [Google Scholar]
- 73. Ladipo OA. Nutrition in pregnancy: mineral and vitamin supplements. Am J Clin Nutr. 2000;72(1 suppl):280S–290S. [DOI] [PubMed] [Google Scholar]
- 74. McCann JC, Ames BN.. An overview of evidence for a causal relation between iron deficiency during development and deficits in cognitive or behavioral function. Am J Clin Nutr. 2007;85:931–945. [DOI] [PubMed] [Google Scholar]
- 75. Sandstrom NJ, Loy R, Williams CL.. Prenatal choline supplementation increases NGF levels in the hippocampus and frontal cortex of young and adult rats. Brain Res. 2002;947:9–16. [DOI] [PubMed] [Google Scholar]
- 76. Davison JM, Mellott TJ, Kovacheva VP, et al. Gestational choline supply regulates methylation of histone H3, expression of histone methyltransferases G9a (Kmt1c) and Suv39h1 (Kmt1a), and DNA methylation of their genes in rat fetal liver and brain. J Biol Chem. 2009;284:1982–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Craciunescu CN, Johnson AR, Zeisel SH.. Dietary choline reverses some, but not all, effects of folate deficiency on neurogenesis and apoptosis in fetal mouse brain. J Nutr. 2010;140:1162–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tran PV, Kennedy BC, Pisansky MT, et al. Prenatal choline supplementation diminishes early-life iron deficiency–induced reprogramming of molecular networks associated with behavioral abnormalities in the adult rat hippocampus. J Nutr. 2016;146:484–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kelley CM, Powers BE, Velazquez R, et al. Maternal choline supplementation differentially alters the basal forebrain cholinergic system of young-adult Ts65Dn and disomic mice. J Comp Neurol. 2014;522:1390–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Jiang X, West AA, Caudill MA.. Maternal choline supplementation:a nutritional approach for improving offspring health? Trends Endocrinol Metab. 2014;25:263–273. [DOI] [PubMed] [Google Scholar]
- 81. McNulty B, McNulty H, Marshall B, et al. Impact of continuing folic acid after the first trimester of pregnancy: findings of a randomized trial of folic acid supplementation in the second and third trimesters. Am J Clin Nutr. 2013;98:92–98. [DOI] [PubMed] [Google Scholar]
- 82. Geoffroy A, Kerek R, Pourié G, et al. Late maternal folate supplementation rescues from methyl donor deficiency-associated brain defects by restoring Let-7 and miR-34 pathways. Mol Neurobiol. 2017;54:5017–5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jadavji NM, Deng L, Malysheva O, et al. MTHFR deficiency or reduced intake of folate or choline in pregnant mice results in impaired short-term memory and increased apoptosis in hippocampus of wild-type offspring. Neuroscience. 2015;300:1–9. [DOI] [PubMed] [Google Scholar]
- 84. Cooney CA, Dave AA, Wolff GL.. Maternal methyl supplements in mice affect epigenetic variation and DNA methylation of offspring. J Nutr. 2002;132(8 suppl):2393S–2400S. [DOI] [PubMed] [Google Scholar]
- 85. Sable P, Randhir K, Kale A, et al. Maternal micronutrients and brain global methylation patterns in the offspring. Nutr Neurosci. 2015;18:30–36. [DOI] [PubMed] [Google Scholar]
- 86. Berrocal-Zaragoza MI, Sequeira JM, Murphy MM, et al. Folate deficiency in rat pups during weaning causes learning and memory deficits. Br J Nutr. 2014;112:1323–1332. [DOI] [PubMed] [Google Scholar]
- 87. McCoy CR, Jackson NL, Day J, et al. Genetic predisposition to high anxiety- and depression-like behavior coincides with diminished DNA methylation in the adult rat amygdala. Behav Brain Res. 2017;320:165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Walker JG, Mackinnon AJ, Batterham P, et al. Mental health literacy, folic acid and vitamin B12, and physical activity for the prevention of depression in older adults: randomised controlled trial. Br J Psychiatry. 2010;197:45–54. [DOI] [PubMed] [Google Scholar]
- 89. McCaddon A, Miller JW.. Assessing the association between homocysteine and cognition: reflections on Bradford Hill, meta-analyses, and causality. Nutr Rev. 2015;73:723–735. [DOI] [PubMed] [Google Scholar]
- 90. Bailey RL, Dodd KW, Gahche JJ, et al. Total folate and folic acid intake from foods and dietary supplements in the United States: 2003–2006. Am J Clin Nutr. 2010;91:231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bailey RL, McDowell MA, Dodd KW, et al. Total folate and folic acid intakes from foods and dietary supplements of US children aged 1–13 y. Am J Clin Nutr. 2010;92:353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Cho CE, Sánchez-Hernández D, Reza-López SA, et al. High folate gestational and post-weaning diets alter hypothalamic feeding pathways by DNA methylation in Wistar rat offspring. Epigenetics. 2013;8:710–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Pannia E, Cho CE, Kubant R, et al. A high multivitamin diet fed to Wistar rat dams during pregnancy increases maternal weight gain later in life and alters homeostatic, hedonic and peripheral regulatory systems of energy balance. Behav Brain Res. 2014;278C:1–11. [DOI] [PubMed] [Google Scholar]
- 94. Cho CE, Sánchez-Hernández D, Reza-López SA, et al. Obesogenic phenotype of offspring of dams fed a high multivitamin diet is prevented by a post-weaning high multivitamin or high folate diet. Int J Obes (Lond). 2013;37:1177–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Pickell L, Brown K, Li D, et al. High intake of folic acid disrupts embryonic development in mice. Birth Defects Res Part A Clin Mol Teratol. 2011;91:8–19. [DOI] [PubMed] [Google Scholar]
- 96. Shorter KR, Felder MR, Vrana PB.. Consequences of dietary methyl donor supplements: is more always better? Prog Biophys Mol Biol. 2015;118:14–20. [DOI] [PubMed] [Google Scholar]
- 97. Veena SR, Gale CR, Krishnaveni GV, et al. Association between maternal nutritional status in pregnancy and offspring cognitive function during childhood and adolescence; a systematic review. BMC Pregnancy Childbirth. 2016;16:220. doi:10.1186/s12884-016-1011-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Herbert V, Zalusky R.. Interrelations of vitamin B12 and folic acid metabolism: folic acid clearance studies. J Clin Invest. 1962;41:1263–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Mattson MP, Shea TB.. Folate and homocysteine metabolism in neural plasticity and neurodegenerative disorders. Trends Neurosci. 2003;26:137–146. [DOI] [PubMed] [Google Scholar]
- 100. Selhub J, Paul L.. Folic acid fortification: why not vitamin B12 also? Biofactors. 2011;37:269–271. [DOI] [PubMed] [Google Scholar]
- 101. Dayon L, Guiraud SP, Corthésy J, et al. One-carbon metabolism, cognitive impairment and CSF measures of Alzheimer pathology: homocysteine and beyond. Alzheimers Res Ther. 2017;9:43. doi:10.1186/s13195-017-0270-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Smythies J. The role of abnormalities related to the one carbon cycle in depression and schizophrenia. Neurosci Med. 2012;3:101–106. [Google Scholar]
- 103. Gottfries J, Blennow K, Lehmann MW, et al. One-carbon metabolism and other biochemical correlates of cognitive impairment as visualized by principal component analysis. J Geriatr Psychiatry Neurol. 2001;14:109–114. [DOI] [PubMed] [Google Scholar]
- 104. Coppedè F. One-carbon metabolism and Alzheimer’s disease: focus on epigenetics. Curr Genomics. 2010;11:246–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Varela-Moreiras G, Selhub J.. Long-term folate deficiency alters folate content and distribution differentially in rat tissues. J Nutr. 1992;122:986–991. [DOI] [PubMed] [Google Scholar]
- 106. Kopp M, Morisset R, Rychlik M.. Characterization and interrelations of one-carbon metabolites in tissues, erythrocytes, and plasma in mice with dietary induced folate deficiency. Nutrients. 2017;9:462. doi:10.3390/nu9050462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Wollack JB, Makori B, Ahlawat S, et al. Characterization of folate uptake by choroid plexus epithelial cells in a rat primary culture model. J Neurochem. 2008;104:1494–1503. [DOI] [PubMed] [Google Scholar]
- 108. Holm J, Hansen SI, Høier-Madsen M, et al. High-affinity folate binding in human choroid plexus. Characterization of radioligand binding, immunoreactivity, molecular heterogeneity and hydrophobic domain of the binding protein. Biochem J. 1991;280(pt 1):267–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Ratnam M, Marquardt H, Duhring JL, et al. Homologous membrane folate binding proteins in human placenta: cloning and sequence of a cDNA. Biochemistry. 1989;28:8249–8254. [DOI] [PubMed] [Google Scholar]
- 110. da Costa M, Rothenberg SP.. Purification and characterization of folate binding proteins from rat placenta. Biochim Biophys Acta. 1996;1292:23–30. [DOI] [PubMed] [Google Scholar]
- 111. Birn H, Nexø E, Christensen EI, et al. Diversity in rat tissue accumulation of vitamin B12 supports a distinct role for the kidney in vitamin B12 homeostasis. Nephrol Dial Transplant. 2003;18:1095–1100. [DOI] [PubMed] [Google Scholar]
- 112. Bjørn-Yoshimoto WE, Underhill SM.. The importance of the excitatory amino acid transporter 3 (EAAT3). Neurochem Int. 2016;98:4–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Drake AJ, O’Shaughnessy PJ, Bhattacharya S, et al. In utero exposure to cigarette chemicals induces sex-specific disruption of one-carbon metabolism and DNA methylation in the human fetal liver. BMC Med. 2015;13:18. doi:.10.1186/s12916-014-0251-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Chen C-S, Kuo Y-T, Tsai H-Y, et al. Brain biochemical correlates of the plasma homocysteine level: a proton magnetic resonance spectroscopy study in the elderly subjects. Am J Geriatr Psychiatry. 2011;19:618–626. [DOI] [PubMed] [Google Scholar]
- 115. Barua S, Kuizon S, Ted Brown W, et al. High gestational folic acid supplementation alters expression of imprinted and candidate autism susceptibility genes in a sex-specific manner in mouse offspring. J Mol Neurosci. 2016;58:277–286. [DOI] [PubMed] [Google Scholar]
- 116. Kezurer N, Galron D, Golan HM.. Increased susceptibility to mild neonatal stress in MTHFR deficient mice. Behav Brain Res. 2013;253:240–252. [DOI] [PubMed] [Google Scholar]
- 117. Blumkin E, Levav-Rabkin T, Melamed O, et al. Gender-specific effect of Mthfr genotype and neonatal vigabatrin interaction on synaptic proteins in mouse cortex. Neuropsychopharmacology. 2011;36:1714–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Nugent BM, Wright CL, Shetty AC, et al. Brain feminization requires active repression of masculinization via DNA methylation. Nat Neurosci. 2015;18:690–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Wright EC, Johnson SA, Hao R, et al. Exposure to extrinsic stressors, social defeat or bisphenol A, eliminates sex differences in DNA methyltransferase expression in the amygdala. J Neuroendocrinol. 2017;29:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Kigar SL, Chang L, Hayne MR, et al. Sex differences in Gadd45b expression and methylation in the developing rodent amygdala. Brain Res. 2016;1642:461–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Blaze J, Scheuing L, Roth TL.. Differential methylation of genes in the medial prefrontal cortex of developing and adult rats following exposure to maltreatment or nurturing care during infancy. Dev Neurosci. 2013;35:306–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Zagni E, Simoni L, Colombo D.. Sex and gender differences in central nervous system-related disorders. Neurosci J. 2016;2016:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Lenz KM, Nugent BM, McCarthy MM.. Sexual differentiation of the rodent brain: dogma and beyond. Front Neurosci. 2012;6:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Raznahan A, Shaw P, Lalonde F, et al. How does your cortex grow? J Neurosci. 2011;31:7174–7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Raznahan A, Toro R, Daly E, et al. Cortical anatomy in autism spectrum disorder: an in vivo MRI study on the effect of age. Cereb Cortex. 2010;20:1332–1340. [DOI] [PubMed] [Google Scholar]
- 126. McCarthy MM, Auger AP, Bale TL, et al. The epigenetics of sex differences in the brain. J Neurosci. 2009;29:12815–12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Frye CA. Estrus-associated decrements in a water maze task are limited to acquisition. Physiol Behav. 1995;57:5–14. [DOI] [PubMed] [Google Scholar]
- 128. Korol DL, Malin EL, Borden KA, et al. Shifts in preferred learning strategy across the estrous cycle in female rats. Horm Behav. 2004;45:330–338. [DOI] [PubMed] [Google Scholar]
- 129. Stackman RW, Blasberg ME, Langan CJ, et al. Stability of spatial working memory across the estrous cycle of Long-Evans rats. Neurobiol Learn Mem. 1997;67:167–171. [DOI] [PubMed] [Google Scholar]
- 130. Conrad CD, Jackson JL, Wieczorek L, et al. Acute stress impairs spatial memory in male but not female rats: influence of estrous cycle. Pharmacol Biochem Behav. 2004;78:569–579. [DOI] [PubMed] [Google Scholar]
- 131. Kolb B, Mychasiuk R, Muhammad A, et al. Experience and the developing prefrontal cortex. Proc Natl Acad Sci USA. 2012;109:17186–17193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Auger AP, Tetel MJ, McCarthy MM.. Steroid receptor coactivator-1 (SRC-1) mediates the development of sex-specific brain morphology and behavior. Proc Natl Acad Sci USA. 2000;97:7551–7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. McCarthy MM, Arnold AP.. Reframing sexual differentiation of the brain. Nat Neurosci. 2011;14:677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]


