ABSTRACT
Background
Chronically higher inflammation, which may partly result from diet and lifestyle, is implicated in risk for multiple chronic diseases. The dietary inflammatory index (DII) and empirical dietary inflammatory pattern (EDIP), developed to characterize dietary contributions to systemic inflammation, have several limitations. There are no scores to characterize contributions of lifestyle to inflammation.
Objectives
To reflect dietary/lifestyle contributions to inflammation, we developed novel, inflammation biomarker panel-weighted, dietary (DIS) and lifestyle (LIS) inflammation scores in a subset (n = 639) of the Reasons for Geographic and Racial Differences in Stroke Study (REGARDS) cohort.
Methods
We selected a priori 19 food groups and 4 lifestyle characteristics to comprise the DIS and LIS, respectively. We calculated the components’ weights based on their strengths of association with an inflammation biomarker score [comprising high-sensitivity C-reactive protein (hsCRP), IL-6, IL-8, and IL-10] using multivariable linear regression. The sums of the weighted components constitute the scores, such that higher scores reflect, on balance, more proinflammatory exposures. We calculated the DIS, LIS, DII, and EDIP with cross-sectional data from the remaining REGARDS cohort ( n = 14,210 with hsCRP measurements) and 2 other study populations with hsCRP and/or an 8-component inflammation biomarker panel, and investigated their associations with circulating inflammation biomarker concentrations using multivariable logistic regression.
Results
In REGARDS, those in the highest relative to the lowest DIS, LIS, DII, and EDIP quintiles had statistically significant 1.66-, 4.29-, 1.56-, and 1.32-fold higher odds of a high hsCRP concentration (>3 mg/dL), respectively (all P-trend < 0.001). Those in the highest relative to the lowest joint DIS/LIS quintile had a statistically significant 7.26-fold higher odds of a high hsCRP concentration. Similar findings were noted in the other 2 validation populations.
Conclusion
Our results support that dietary and lifestyle exposures collectively contribute substantially to systemic inflammation, and support the use of our novel DIS and LIS.
Keywords: dietary intake, smoking, obesity, alcohol intake, physical activity, systemic inflammation, humans, inflammation biomarkers, inflammation scores
Introduction
Deregulation of the inflammation response has been implicated repeatedly in the etiology of chronic diseases that are leading causes of death in the United States (1–4). Dietary and lifestyle exposures likely contribute to higher chronic inflammation (5–8). Consequently, reducing inflammation via dietary or lifestyle interventions could help reduce risk for chronic diseases and premature death (9, 10).
The contributions of most dietary/lifestyle exposures to inflammation individually likely are relatively small, but collectively may be substantial. To address this, 2 questionnaire-based scores to represent aggregates of inflammation-related dietary exposures were reported: the dietary inflammatory index (DII) (11) and the empirical dietary inflammatory pattern (EDIP) (12). The DII (11) is a summation of previously reported effects/associations of selected dietary factors (mostly micro- and macronutrients) on/with various inflammation biomarkers. The EDIP (12) was developed through a data-driven approach to identify food groups most associated with plasma inflammation biomarkers in a subset of the Nurse's Health Study cohort. Limitations of these indices include issues with reproducibility, generalizability, assumptions, and for the DII, a heavy focus on nutrients. No reported index addresses the collective contributions of lifestyle characteristics to inflammation.
To address these issues, we developed and validated weighted dietary- and lifestyle-inflammation scores based on FFQ and lifestyle questionnaire responses, by quantifying associations of aggregates of food groups and of lifestyle exposures with a panel of circulating biomarkers of systemic inflammation in a diverse population. Our premise was that composing scores primarily of whole foods (rather than nutrients), which contain thousands of bioactive substances (13), and lifestyle exposures, and use of a hypothesis-driven (rather than an agnostic, data-driven) approach may be a more productive direction for epidemiologic research on the roles of diet and lifestyle in inflammation and the etiology of inflammation-related diseases. We also compared the strengths of associations of our new inflammation scores with biomarkers of inflammation to those for the DII and EDIP in 3 study populations.
Methods
Study population and data collection for developing the dietary inflammation score and lifestyle inflammation score: Reasons for Geographic and Racial Differences in Stroke Study
The Reasons for Geographic and Racial Differences in Stroke Study (REGARDS) is a national, ongoing prospective cohort study that recruited 30,239 participants aged ≥45 y in January 2003–October 2007, with oversampling of black individuals and residents in the southeastern United States. Details on the objectives, study population, recruitment, and exclusion criteria were described previously (14). We developed the dietary inflammation score (DIS) and lifestyle inflammation score (LIS) with use of a case-cohort sample nested in REGARDS that had a panel of plasma inflammation biomarkers measured at baseline (n = 639) (15). Cases were those diagnosed with incident ischemic stroke during follow-up. The cohort comparison sample was randomly sampled from 20 strata to ensure sufficient representation of individuals in each race, sex, and 10-y age group. We incorporated sampling weights and stratum/cluster-specific estimates in all case-cohort analyses described further below.
Dietary and supplemental vitamin/mineral intakes were assessed with a self-administered, 109-food item, Block 98 FFQ (NutritionQuest) that was validated in multiple diverse populations (16, 17). Pictures were provided to assist respondents in identifying standard portion sizes, and 9 possible frequency-of-consumption responses, ranging from “never” to “every day” were given for each food item. Total daily energy and nutrient intakes were calculated by summing energy and nutrients, respectively, from all food sources.
Lifestyle information was obtained via a 30–45-min telephone interview with use of lifestyle questionnaires similar to those used in previous studies of cerebrovascular and cardiovascular disease (18, 19). The lifestyle questionnaire ascertained self-reported frequency of physical activity intense enough to work up a sweat (20), how many alcoholic drinks the respondent usually consumed, and cigarette smoking status. At an in-home visit, height and weight without shoes were measured with a metal tape measure and balance scale, respectively, and fasting venous blood samples were drawn.
Baseline circulating high-sensitivity C-reactive protein (hsCRP) concentrations were measured in the entire cohort. Baseline circulating IL-6, IL-8, and IL-10 concentrations were also measured in the case-cohort. hsCRP was measured via a validated, high-sensitivity, particle-enhanced, immunonephelometric assay in batches with a BNII nephelometer (Dade Behring). The intra-assay CV ranged from 2.3% to 4.4%, and interassay CVs ranged from 2.1% to 5.7%. IL-6 was measured via an ultra-sensitive ELISA (Quantikine HS Human IL-6 Immunoassay; R&D Systems); the interassay CV range was 6.8–7.3%. IL-10 was measured with the Milliplex MAP Human Cardiovascular Disease Panel 3 (Millipore Corporation), run as a single-plex assay; the interassay CV range was 8.3–12.1%. IL-8 was measured with the Human Serum Adipokine Panel B LINCOplex Kit (Linco Research, Inc.); the interassay CV range was 1.4–7.9% (21).
Validation study populations and data collection
We assessed the validity of the DIS and LIS in 3 populations: 2 with hsCRP measurements, including the remaining REGARDS cohort (n = 14,210) and a pooled cross-sectional study (n = 423), and 1 with an 8-component inflammation biomarker panel (n = 173). The latter 2 validation populations are described below.
Pooled Markers of Adenomatous Polyps I and II studies
We pooled data from 2 cross-sectional studies among populations with no history of colorectal neoplasms scheduled for outpatient, elective, colonoscopies in large, community-based gastroenterology practices. These studies, the Markers of Adenomatous Polyps (MAP) studies I and II [MAP I (22) and MAP II (23)], were conducted by the same principal investigator (RMB) through use of virtually identical protocols and questionnaires, and hereinafter are referred to as MAP. MAP I was conducted in 1994–1997 in North Carolina, and MAP II was conducted in 2002 in South Carolina. Details on the study design and implementation were described previously (22, 23).
Before colonoscopy, participants provided detailed demographic, medical history, diet, lifestyle, and anthropometric information. Diet and supplement intakes over the previous 12 mo were assessed with self-administered Willett FFQs (24). A standard portion size and 9 possible frequency-of-consumption responses, ranging from “never, or less than once per month” to “6 or more times per day” were given for each item. Total daily energy and nutrient intakes were calculated by summing energy and nutrients, respectively, from all food sources using the dietary database developed by Willett (24, 25). Physical activity was assessed using a modified Paffenbarger questionnaire (26). Before colonoscopy, fasting peripheral venous blood samples were drawn, and hsCRP measured via latex-enhanced immunonephelometry on a Behring nephelometer II analyzer (interassay CV: 4.0%; Behring Diagnostics).
Calcium and Colorectal Epithelial Cell Proliferation trial
We used baseline questionnaire data and blood samples collected in 1990–1991 from Calcium and Colorectal Epithelial Cell Proliferation (CECP) participants (all sporadic colorectal adenoma patients), on whom a panel of inflammation biomarkers was measured in 2013. The purpose of the original trial was to test the efficacy of supplemental calcium in modulating a biomarker of colorectal epithelial cell proliferation in the normal rectal mucosa. Details on the study design and implementation were described previously (27). Participants provided detailed demographic, medical history, diet, lifestyle, and anthropometric information through questionnaires identical to those in MAP. Circulating inflammation biomarker concentrations were measured at the Emory Multiplexed Immunoassay Core with electrochemiluminescence detection-based immunoassays based on a Meso Scale Discovery Sector 2400 instrument. An individual assay was conducted for hsCRP, and a 10-plex assay was conducted for IL-6, IL-8, IL-10, vascular endothelial growth factor (VEGF), TNF-α, IL-1β, IL-12p40, IL-17, IL-4, and IFN-γ. All biomarkers were measured in duplicate, according to the manufacturer's protocol. The average intra-assay CV for IL-6 was 7.0%, for IL-8 3.5%, for IL-10 5.7%, for hsCRP 4.6%, for TNF-α 4.3%, for VEGF 4.5%, for IL-1β 13.0%, for IL-12p40 6.9%, for IL-17 21.3%, for IL-4 17.6%, and for IFN-γ 16.7%. Biomarkers with CVs ≥ 15% (IFN-γ, IL-17, and IL-4) were excluded from further analyses. Biomarker measurements below the lower limit of detection were set to the lowest limit of detection for each batch (27).
In addition to the original inclusion/exclusion criteria described for each study above, for the present analyses we excluded participants aged ≥ 75 y or with hsCRP concentrations ≥10 mg/dL (28), extreme outlying values for other measured inflammation biomarkers (REGARDS case-cohort and CECP; Supplemental Methods), end-stage renal disease (estimated glomerular filtration rates <15 mL/min), implausible total energy intakes (<500 and >4500 kcal/d for women, and <800 and >5000 kcal/d for men), >15% missing FFQ data, or missing lifestyle questionnaire data (Supplemental Figure 1). In the REGARDS case-cohort, to reduce potential for bias and/or error in estimating the DIS/LIS weights, we used more stringent inclusion/exclusion criteria and excluded those missing > 10% FFQ data and those with ≥2 comorbidities (a history of cancer, heart disease, diabetes mellitus, or chronic kidney disease).
All original studies were approved by the Institutional Review Boards of their respective institutions, and all participants provided written informed consent.
DIS components
Foods, beverages, and micronutrient supplements for the DIS were selected and grouped into 19 score components (Table 1) a priori based on biological plausibility, prior literature, and consideration of easily recreating them with use of a variety of FFQs used in major epidemiologic studies in many Western populations (11, 27, 29–83) . We eliminated food groups if <1% of the REGARDS case-cohort consumed ≥1 serving/wk (soy, shellfish) or if measurement was inadequate using the Block 98 FFQ (whole grains). To account for supplemental micronutrients, we calculated a supplement score by ranking supplemental micronutrient intakes, based on the sex-specific distribution, into tertiles. The tertiles were assigned values of 0–2 and multiplied by +1 or –1 based on their literature-supported anti-inflammatory or proinflammatory contributions, respectively (Table 1 and Supplemental Methods) (11, 27, 34, 40, 46, 49, 55, 65, 74–79); then the values were summed. A higher score indicated a predominance of anti-inflammatory supplemental micronutrient intakes.
TABLE 1.
Components of the DIS and LIS, their descriptions, rationales for inclusion, and assigned weights1
| Components | Rationales for inclusion | General descriptions | Weights2 |
|---|---|---|---|
| DIS components3 | |||
| Leafy greens and cruciferous vegetables | Kale, spinach, lettuce (iceberg, head, romaine, or leaf), broccoli, Brussels sprouts, cabbage, cauliflower, parsley, watercress | Contain a variety of potent antioxidants (e.g., β-carotene, folacin, magnesium, calcium, glucosinolates, isothiocyanates, lutein, and indoles); contain flavonoids and polyphenols, which activate the transcription factor, Nrf2, which plays a key role in cellular protection against oxidative stress and inflammation (29–31, 50, 61, 72, 80–83) | −0.14 |
| Tomatoes | Tomatoes, tomato juice, tomato sauce, salsa | Contain β-carotene, vitamin C, and lycopene, the latter of which is a potent singlet oxygen quencher and one of the most powerful antioxidants among the natural carotenoids (32–35) | −0.78 |
| Apples and berries | Fresh apples, pears, apple juice or cider, strawberries, blueberries, raspberries, cherries | Contain flavonoids (e.g., anthocyanins, quercetin, and phenolic acids) that suppress proinflammatory cytokine production and are powerful antioxidants; potentially increase postprandial plasma antioxidant capacity (36–38) | −0.65 |
| Deep yellow or orange vegetables and fruit | Cantaloupe, peaches, carrots, dark yellow or orange squash, figs | Contain provitamin A carotenoids (e.g., β-carotene and α-carotene), which have a conjugated double-bond structure making them strong antioxidants (40) | −0.57 |
| Other fruits and real fruit juices | Fresh fruits other than those listed above (e.g., pineapples, honeydew, grapes, kiwi, watermelon, lemon, grapefruit, and oranges), orange juice, grapefruit juice, grape juice, and other real fruit juice | Contain antioxidants (e.g., flavonoids, such as hesperidin, naringenin, neohesperidin, limonene, vitamin C, β-cryptoxanthin, plant sterols, salicylates, naringin, nobelitin, and narirutin) with similar mechanisms to those described above (41–48, 72) | −0.16 |
| Other vegetables | Vegetables other than those listed above (e.g., okra, green peppers, onions, zucchini, and eggplant) | Contain antioxidants and polyphenols with similar mechanisms to those described above | −0.16 |
| Legumes | String beans, peas, lima beans, lentils, and other beans (excluding soybeans) | Contain folacin, iron, isoflavones, protein, vitamin B6, and have a high antioxidant capacity; rich in fiber, which is associated with beneficial alterations to the gut microbiota, reducing immune response in the gut (49, 51, 61) | −0.04 |
| Fish | Tuna fish, salmon, other light and dark meat fish, breaded fish cakes or fish sticks | Contain Ω-3 fatty acids, which compete with proinflammatory Ω-6 fatty acids by synthesizing eicosanoids and suppress the capacity of monocytes to synthesize IL-1β and TNF-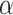 (52–54) (52–54) |
−0.08 |
| Poultry | Chicken or turkey with and without skin | Inversely associated with inflammation (55); contain low amounts of saturated fat (56); contain l-arginine, which improves endothelium-dependent dilation (precursor of the endogenous vasodilator nitric oxide) and decreases platelet aggregation and monocyte adhesion (61) | −0.45 |
| Red and organ meats | Hamburger, beef, pork, lamb, liver, gizzards, other organ meats | Contain heme iron, which increases the bioavailability of iron, which in turn increases oxidative stress; contain Ω-6 fatty acids, which increase oxidative stress through free radical production and are converted to arachidonic acid which stimulates expression of IL-1β and TNF-α in monocytes, and IL-6 and IL-8 in endothelial cells (57–59); contain saturated fats that mimic lipopolysaccharide, a proinflammatory stimulant, in the gut, and increase cytotoxic, pro-oxidant, and proinflammatory bile acids in the colon (57, 60) | 0.02 |
| Processed meats | Bacon, beef or pork hotdogs, chicken or turkey hot dogs, salami, bologna, other processed meats | Contain heme iron, higher saturated fat contents, Ω-6 fatty acids (see above), and additives, such as nitrites, with suspected proinflammatory properties (55,62) | 0.68 |
| Added sugars | Sugar-sweetened soda, punch, lemonade, chocolate candy bars, other mixed candy bars, candy without chocolate, jams, jellies, preserves, syrup or honey, dried or canned fruit | Sparse in nutrients; induce postprandial hyperglycemia, which act as stressful stimuli through subsequent repeated mild postprandial hypoglycemia (55, 62) and reduce nitric oxide availability [plays role in regulation of inflammatory response (63)]; elevate proinflammatory free fatty acid levels (54); produce oxidative stress through oxidation of membrane lipids, proteins, lipoproteins, and DNA (64) | 0.56 |
| High-fat dairy | Whole milk, 2% milk, cream, high-fat ice cream, high-fat yogurt, cream cheese, other high-fat cheeses | Contains calcium, which binds bile acids and free fatty acids, decreasing oxidative damage in the gut; dairy fat contains fatty acids with potential inflammation-reducing properties, such as CLA, cis- and trans-palmitoleic acid, butyric acid, phytanic acid, and α-linolenic acid (65–67, 75) | −0.14 |
| Low-fat dairy | Skim milk, 1% milk, low-fat yogurt, low-fat ice cream, low-fat cottage or ricotta cheese, low-fat cheeses | Similar mechanisms to high-fat dairy (see above), with lower fat content | −0.12 |
| Coffee and tea | Coffee (decaffeinated and regular), herbal and non-herbal tea | Tea contains flavonoids and antioxidants (e.g., epicatechin and quercetin) (68); coffee contains phytochemicals and antioxidants, such as javamide; both coffee and tea contain varying amounts of caffeine, which inhibits secretion of IL-1β induced by adenine and N4-acetylcytidine (49, 69) | −0.25 |
| Nuts | Peanut butter, peanuts, other nuts | Contain Ω-3 fatty acids (52, 54, 70, 71) and l-arginine (61) (mechanisms similar to those described in “Fish” and “Poultry”) | −0.44 |
| Other fats | Mayonnaise, margarine, butter, vegetable oil | Contain Ω-6 fatty acids and saturated fats (see “Red and organ meats”) | 0.31 |
| Refined grains and starchy vegetables | Cold and cooked breakfast cereal, white or dark bread, bagels, English muffins, rolls, corn bread, white rice, pasta, pancakes, waffles, potatoes (French fried, scalloped, baked, boiled or mashed), sweet potato/yams, potato chips, crackers, tortillas, popcorn, pretzels, cookies, brownies, doughnuts, cake, pie, sweet rolls, coffee cakes, granola bars | Some processed grains contain emulsifiers, which potentially break down mucin in the gut leading to inflammation (73); and induce hyperglycemia (mechanisms described similar to those described in “Added sugars”) | 0.72 |
| Supplement score4 | Ranked score of supplemental micronutrients, including: vitamins A, B-12, B-6, C, D, and E; and β-carotene, folate, niacin, riboflavin, calcium, magnesium, selenium, thiamin, zinc, and iron | Comprises micronutrients, minerals, and vitamins solely from supplement intakes, some with similar mechanisms to those described above (e.g., iron as pro-oxidant, vitamins A, C, and E as antioxidants) (11, 27, 34, 40, 46, 49, 55, 65, 74–79) | −0.80 |
| LIS components5 | |||
| Heavy drinker | Heavy [> 1 drink (> 14 g ethanol)/d for women, > 2 drinks (28 g ethanol)/d for men] vs. nondrinker | Heavy alcohol intake results in oxidative stress via oxidation of ethanol to acetaldehyde (86, 87) | 0.30 |
| Moderate drinker | Moderate [1 drink (14 g ethanol)/d for women, 1–2 drinks or (14–28 g ethanol)/d for men] vs. nondrinker | A metabolite of ethanol is acetate, which can acutely lower proinflammatory free fatty acid concentrations; moderate alcohol intake increases serum adiponectin concentrations (an anti-inflammatory inflammation biomarker) (88) and inhibits IL-6 production and activity (89) | −0.66 |
| Moderately physically active6 | Exercises 1–3 times/wk vs. does not exercise | Physical activity improves systemic plasma antioxidant capacity (increases adaptive responses to oxidative stress), increases concentrations of anti-inflammatory cytokines, and lowers vascular wall inflammation (90, 91) | −0.18 |
| Heavily physically active6 | Exercises ≥ 4 times/wk vs. does not exercise | Mechanisms similar to those described above | −0.41 |
| Current smoker | Currently smokes tobacco vs. does not currently smoke tobacco | Toxins injure tissues, upregulating cytokines and acute-phase reactants (92) | 0.50 |
| Overweight BMI | Overweight BMI vs. normal BMI | Adipose tissue synthesizes and releases proinflammatory adipokines, such as PAI-1 and TNF-α (91, 93) | 0.89 |
| Obese BMI | Obese BMI vs. normal BMI | Mechanisms similar to those described above | 1.57 |
CLA, conjugated linoleic acid; DIS, dietary inflammation score; hsCRP, high-sensitivity C-reactive protein; LIS, lifestyle inflammation score; MET, metabolic equivalent of task; Nrf2, nuclear factor-erythroid 2 (NF-E2)-related factor 2; NSAID, nonsteroidal anti-inflammatory drug; PAI-1, plasminogen activator inhibitor-1; REGARDS, Reasons for Geographic and Racial Differences in Stroke Study.
Weights are β-coefficients from multivariable linear regression models conducted in the REGARDS case-cohort sample (n = 639), representing the average change in a summary inflammation biomarker score [sum of z scores for hsCRP, IL-6, IL-8, IL-10 (the latter with a negative sign)] per 1 SD increase in a dietary component or the presence of lifestyle component. A negative weight indicates that a component has an anti-inflammatory effect, a positive weight indicates that a component has a proinflammatory effect. Covariates in the final model included: age, sex, race (black or white), education (high school graduate or less vs. some college or more), region (stroke belt, stroke buckle, or other region in the United States), a comorbidity score (comprises a history of cancer, heart disease, diabetes mellitus, or chronic kidney disease), regular use of aspirin, other NSAIDs, or lipid-lowering medications (≥ twice/wk), hormone replacement therapy (among women), total energy intake (kcal/d), season of baseline interview (spring, summer, fall, or winter); and all the dietary/lifestyle components in the DIS and LIS.
Dietary components were standardized to the case-cohort sample, by sex, to a mean of zero and SD of 1.
All vitamin and mineral supplement intakes measured (from multivitamin/mineral and individual supplements) were ranked into quantiles of intake and assigned a value of 0 (low or no intake), 1, or 2 (highest intake) for hypothesized anti-inflammatory micronutrients (i.e., all listed micronutrients except iron), and 0 (low or no intake), –1, or –2 (highest intake) for hypothesized proinflammatory micronutrients (iron only).
All lifestyle components were dummy variables, coded as “1” for the nonreferent category and “0” for the referent category.
When calculating the LIS using lifestyle behavior measurement instruments where “times physically active per wk” could not be derived, the given variables (e.g., METs/wk) were ranked into quantiles, which were taken to construct dummy variables, and the respective weights were similarly applied.
Mixed dishes (e.g., pizza, spaghetti) in the Block 98 FFQ were disaggregated using the My Pyramid Equivalents Database (84). Briefly, we calculated mean food group equivalents/100 g of each mixed dish, weighted by how often each variation of the mixed dish was consumed over 2-d food records in black and white individuals aged ≥ 45 y in the NHANES 2003–2004 (85), multiplied the equivalent by the gram amount consumed by each individual, converted the equivalent to the appropriate units, and added it to its respective DIS food group (Supplemental Methods).
LIS components
The LIS included 4 components: smoking status, physical activity, alcohol intake, and BMI (86–93). Because the weights were developed based on cross-sectional exposure–biomarker associations, smoking was categorized as “current” or “former/never.” BMI (kg/m2) was categorized as underweight/normal (<25), overweight (25–29.99), or obese (≥30). Heavy alcohol consumption was defined as > 1 or > 2 drinks (>14 or >28 g of ethanol, respectively)/d for women and men, respectively; moderate consumption was defined as consumed alcohol, but in less than these amounts (94). Physical activity in REGARDS was categorized as the frequency of being physically active enough to work up a sweat (0, 1–3, or ≥ 4 times/wk), a previously well-validated measure (95, 96); in MAP and CECP, we ranked participants according to tertiles of weekly metabolic equivalents of task (METs)-min of moderate/vigorous physical activity as assessed from Paffenbarger questionnaire responses (26).
DIS and LIS development
First, to represent systemic inflammation, we created an inflammation biomarker score comprising the 4 available biomarkers in the REGARDS case-cohort: hsCRP, IL-6, IL-8, and IL-10 (the latter considered anti-inflammatory) (97, 98). To do this, we transformed the biomarker values by the natural logarithm (ln), standardized the values to a mean of 0 and SD of 1.0, and then summed the standardized inflammation biomarkers values (IL-10 with a negative sign).
Next, we calculated weights for the DIS and LIS components in the REGARDS case-cohort based on the strengths of the associations of each component with the inflammation biomarker score. To do this, first, for the DIS, we standardized each food group (all continuous), by sex, to a mean of 0 and SD of 1.0. For the LIS, because all components were categorical variables, we created dummy variables. Then, ensuring that linear regression model assumptions were met, and multicollinearity ruled out, we conducted multivariable linear regression to estimate the maximum likelihood estimates for the β-coefficients, which represent the average change in the inflammation biomarker score per 1 SD increase in a dietary component or having a certain lifestyle behavior relative to its referent category. The modeling procedures are described further in the Statistical Analyses subsection. To calculate a DIS and LIS for participants in other populations, each dietary/lifestyle component can be multiplied by the weight (the β-coefficient) calculated above, and the weighted components summed.
DIS and LIS validation
To assess the validity of the DIS and LIS, we calculated both as well as the DII and the EDIP in the remaining REGARDS cohort, MAP, and CECP. We calculated the DII (11) and EDIP (12) according to previous reports (Supplemental Table 1 and Supplemental Methods). In REGARDS, MAP, and CECP, 34, 38, and 37 of the 45 DII components were available, respectively. Briefly, to calculate the DII (11), we first calculated a z score for each component [based on total micronutrient intakes (dietary plus supplemental)] using the published global means and SDs. We then calculated normalized, centered percentiles for each component, and then multiplied each component by its reported respective weight. To calculate the EDIP, we formed dietary groups based on servings of intake as described by Tabung et al. (12), multiplied each component by its reported respective weight, and divided the score by 1000 to scale it. For all inflammation scores, a higher score indicates more proinflammatory relative to anti-inflammatory exposures.
Statistical analyses
Next, we investigated associations of the various scores with the various inflammation biomarkers in the 3 other populations. We first categorized participants in each study into quantiles of each inflammation score, such that higher quantiles represent more proinflammatory scores. The characteristics of the study populations were summarized and compared across quantiles of the DIS and LIS, using chi-square tests for categorical variables and ANOVA for continuous variables (transformed to meet normality assumptions when indicated). We calculated Spearman correlations between the DIS and the DII and EDIP.
We used multivariable unconditional logistic regression to assess associations of the DIS, LIS, DII, and EDIP with high circulating hsCRP concentrations or inflammation biomarker scores. In REGARDS and MAP, we defined a high hsCRP as >3.0 mg/dL, a clinically relevant cutoff (28). In CECP, we calculated an inflammation biomarker score [a sum of z scores for ln-transformed IL-6, IL-8, IL-10 (with a negative sign), hsCRP, VEGF, TNF-α, IL-1β, and IL-12p40], and dichotomized the inflammation biomarker score at the population median. A term for the sex-specific median of each inflammation score quantile was entered into the multivariable regression models as a continuous variable to test for trend.
To assess potential interaction between the DIS and LIS, we conducted a joint/combined (cross-classification) analysis using multivariable logistic regression models in which the reference group was participants in the first quintile of both scores.
Consideration for inclusion of covariates in all the aforementioned multivariable linear and logistic regression models were based on biological plausibility, previous literature, and the magnitude of change in the association of interest when including/excluding the variable from the model (further details in Supplemental Methods). Covariates considered for all models included age, sex, race/ethnicity, income, education, region of the United States, comorbidities, hormone replacement therapy use (for women), total energy intake, season of year the participant completed dietary/lifestyle questionnaires and had inflammation biomarkers measured, and regular aspirin, other nonsteroidal anti-inflammatory drug (NSAID), and lipid-lowering medication use. Models for the dietary inflammation scores additionally included the LIS components [smoking, BMI, alcohol intake (except for the DII and EDIP because alcohol intake is a component), and physical activity]. Models to estimate weights for the DIS and LIS additionally included all dietary and lifestyle components as covariates. Although energy intake is typically a DII component, we explored adding and removing energy intake as a covariate in the multivariable regression models for the DII to ensure adequate control for confounding by energy intake. The final covariates for all models are listed in the table footnotes.
To investigate potential effect modification, separate analyses were conducted for each dietary/lifestyle inflammation score within categories of age (dichotomized at age 65 y), sex, race (black/white), comorbidity status (yes/no), aspirin or other NSAID use (take NSAID ≥ twice/wk or < twice/wk); and for the dietary inflammation scores, within categories of current smoking status (former and never or current), BMI (≤ normal, overweight, obese), alcohol status (current nondrinker, moderate drinker, heavy drinker), and physical activity (none, moderate, heavy). We assessed effect modification by comparing the stratum-specific estimates and by calculating Wald test P values for model interaction terms.
Sensitivity analyses
To assess the associations’ sensitivities to various considerations, we repeated the analyses with these variations: 1) removed the supplemental micronutrients from the DIS; 2) assigned positive or negative equal weights to dietary/lifestyle components hypothesized a priori to be proinflammatory or anti-inflammatory, respectively; 3) calculated and compared adjusted mean ln-transformed hsCRP concentrations (REGARDS and MAP) and inflammation biomarker scores (CECP) by quantile of each inflammation score using multivariable general linear models; and 4) recognizing that the estimated strengths of associations of the DIS and LIS components with inflammation biomarker concentrations contain some uncertainty, simulated a range of DIS and LIS weight estimates over 1,000,000/n iterations (where n = the number of external population participants) using Monte Carlo methods (MCM) (99). For each iteration, the resulting β-coefficients were then applied as weights for the DIS and LIS components, participants were categorized into quantiles based on the iteration-specific DIS or LIS distribution, and the bootstrap technique was used to simulate the error from the DIS and LIS weights and the estimated DIS/LIS-inflammation biomarker associations.
All analyses were conducted using SAS statistical software, version 9.3. All statistical tests were 2-sided, and P values < 0.05 or 95% CIs that excluded 1.0 were considered to be statistically significant.
Results
The weights for the 19-component DIS and the 4-component LIS are presented in Table 1. All β-coefficient weights were in the hypothesized directions, and the range of weights was wide.
Selected characteristics of the REGARDS case-cohort participants according to DIS and LIS quintiles are summarized in Table 2. The population age range was 45–74 y (mean ± SD 61.7 ± 8.0), 48.7% were men, 51.3% were women, 65.0% were white, and 35.0% were black. The DIS and LIS ranges were –1.7 to 1.9 and –1.1 to 2.4, respectively. Those in the highest (most proinflammatory) relative to the lowest (most anti-inflammatory) DIS quintile were more likely to be black, have an income < $20,000/y, have less than a college education, be a current smoker, be overweight or obese, and participate in physical activity ≤ 3 times/wk. On average, they had lower dietary fiber intakes; higher plasma IL-6, IL-8, and hsCRP concentrations; and higher inflammation biomarker scores. Differences in participant characteristics were similar across quintiles of the DII and EDIP (Supplemental Table 2). Those in the highest relative to the lowest LIS quintile were more likely to be female or black, have less than a college education, live in the southeastern United States, have a comorbidity, be a current smoker, be overweight or obese, be a nondrinker, and participate in physical activity ≤ 3 times/wk. On average, they had lower dietary fiber intakes, higher plasma IL-6 and hsCRP concentrations, and higher inflammation biomarker scores. Differences in participant characteristics across DIS and LIS quantiles in the entire REGARDS cohort, MAP, and CECP populations were similar to those in the REGARDS case-cohort (Supplemental Tables 3–5).
TABLE 2.
Selected characteristics of the participants in the REGARDS case-cohort (n = 639) across quintiles of the DIS and LIS1
| DIS quintile | LIS quintile | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | 1 (n = 129) | 3 (n = 127) | 5 (n = 127) | P 2 | 1 (n = 132) | 3 (n = 131) | 5 (n = 113) | P 2 |
| Score range | −1.7 to −0.4 | −0.1 to 0.2 | 0.6 to 1.9 | −1.1 to −0.2 | 0.5 to 0.7 | 1.4 to 2.4 | ||
| Demographics | ||||||||
| Age, y | 62.8 ± 7.8 | 61.6 ± 8.1 | 60.3 ± 8.2 | 0.09 | 62.1 ± 9.1 | 62 ± 7.1 | 61.2 ± 7.9 | 0.83 |
| Men, % | 48.8 | 48.8 | 48.8 | 1.00 | 54.4 | 51.7 | 39.3 | 0.03 |
| White, % | 79.8 | 60.6 | 41.7 | <0.001 | 80.9 | 61.1 | 57.8 | <0.001 |
| Income <$20,000, % | 6.2 | 14.2 | 24.4 | 0.001 | 20.6 | 15.4 | 21.5 | 0.02 |
| College graduate or higher, % | 59.7 | 40.2 | 21.3 | <0.001 | 52.9 | 40.3 | 25.2 | <0.001 |
| Southeastern US resident, % | 47.3 | 52.0 | 63.8 | 0.22 | 48.5 | 61.7 | 63.0 | 0.003 |
| Medical history, % | ||||||||
| Has comorbidity3 | 37.2 | 33.1 | 44.9 | 0.11 | 26.5 | 37.6 | 49.6 | 0.003 |
| Take NSAID/aspirin ≥ twice/wk | 55.8 | 44.9 | 50.4 | 0.50 | 48.2 | 49.0 | 53.3 | 0.89 |
| HRT user (women) | 65.7 | 64.6 | 49.2 | 0.15 | 64.5 | 69.4 | 61.5 | 0.62 |
| Lifestyle, % | ||||||||
| Current smoker | 10.1 | 11.8 | 25.2 | <0.001 | 5.2 | 18.1 | 23.0 | <0.001 |
| Normal BMI | 33.6 | 17.5 | 16.0 | <0.001 | 79.0 | 4.7 | 0.0 | <0.001 |
| Nondrinker4 | 48.8 | 48.0 | 61.4 | 0.46 | 37.5 | 53.7 | 88.2 | <0.001 |
| Exercises ≤3 times/wk5 | 62.8 | 66.9 | 78.7 | <0.001 | 42.7 | 70.5 | 99.3 | <0.001 |
| Dietary intakes | ||||||||
| Total energy, kcal/d | 1717 ± 670 | 1809 ± 713 | 1902 ± 905 | 0.23 | 1728 ± 550 | 1845 ± 798 | 1844 ± 896 | 0.20 |
| Dietary fiber, g/(1000 kcal · d) | 12.5 ± 3.9 | 9.2 ± 3.0 | 6.7 ± 2.5 | <0.001 | 10.2 ± 3.7 | 9.3 ± 3.3 | 9.1 ± 3.9 | 0.003 |
| Total fat, % kcal/d | 37.3 ± 9.0 | 36.9 ± 6.9 | 38.1 ± 8.1 | 0.21 | 36.6 ± 7.8 | 38.0 ± 8.1 | 38.0 ± 7.6 | 0.05 |
| Polyunsaturated fat, g/(1000 kcal · d) | 10.9 ± 3.4 | 10.4 ± 2.8 | 10.5 ± 3.6 | 0.58 | 10.5 ± 3.3 | 11.0 ± 3.3 | 10.6 ± 3.3 | 0.49 |
| Monounsaturated fat, g/(1000 kcal · d) | 16.3 ± 4.6 | 15.4 ± 3.5 | 15.8 ± 3.8 | 0.10 | 15.7 ± 4.0 | 16.1 ± 4.1 | 15.9 ± 3.7 | 0.36 |
| Carbohydrates, % kcal/d | 46.8 ± 10.4 | 47.8 ± 8.5 | 48.2 ± 9.5 | 0.20 | 47.8 ± 9.3 | 47.3 ± 9.0 | 48.4 ± 9.6 | 0.08 |
| Protein, % kcal/d | 16.1 ± 2.9 | 14.9 ± 3.1 | 13.0 ± 2.9 | <0.001 | 14.7 ± 2.9 | 15.0 ± 2.9 | 14.6 ± 3.4 | 0.73 |
| Inflammation markers | ||||||||
| Plasma IL-6, pg/mL | 2.2 ± 1.7 | 2.6 ± 1.7 | 3.2 ± 1.7 | <0.001 | 2.0 ± 1.7 | 2.7 ± 1.7 | 3.2 ± 1.7 | <0.001 |
| Plasma IL-8, pg/mL | 2.1 ± 1.6 | 2.2 ± 1.8 | 2.6 ± 1.5 | 0.003 | 2.2 ± 1.8 | 2.2 ± 1.6 | 2.4 ± 1.7 | 0.10 |
| Plasma IL-10, pg/mL | 7.3 ± 2.1 | 9.0 ± 2.0 | 8.0 ± 1.8 | 0.12 | 8.0 ± 2.1 | 8.3 ± 1.9 | 8.4 ± 2.0 | 0.98 |
| Plasma hsCRP, mg/dL | 1.2 ± 2.7 | 1.7 ± 2.7 | 2.5 ± 2.5 | <0.001 | 0.9 ± 2.6 | 2.0 ± 2.4 | 2.7 ± 2.4 | <0.001 |
| Inflammation biomarker score | −0.6 ± 2.2 | −0.2 ± 2.2 | 1.1 ± 1.8 | <0.001 | −1.1 ± 2.2 | 0.2 ± 2.1 | 1.0 ± 1.9 | <0.001 |
| Plasma hsCRP >3 mg/dL, % | 18.6 | 33.1 | 45.7 | <0.001 | 11.8 | 34.9 | 45.2 | <0.001 |
For construction of scores, see text and Table 1; higher scores (i.e., those in the higher quintiles) indicate more proinflammatory diets or lifestyles. Data are presented as means ± SDs unless otherwise specified. DIS, dietary inflammation score; HRT, hormone replacement therapy; hsCRP, high-sensitivity C-reactive protein; LIS, lifestyle inflammation score; NSAID, nonsteroidal anti-inflammatory drug; REGARDS, Reasons for Racial and Geographic Differences in Stroke Study.
P values calculated using 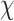 2 test for categorical variables and ANOVA for continuous variables (natural logarithm-transformed, when indicated, to meet normality assumptions).
2 test for categorical variables and ANOVA for continuous variables (natural logarithm-transformed, when indicated, to meet normality assumptions).
Includes a history of cancer, heart disease, diabetes mellitus, or chronic kidney disease.
Zero (0) g of ethanol daily.
Frequency/wk physically active enough to work up a sweat.
Spearman correlations of the DIS with the DII in the 3 validation populations were: ρ = 0.66 in REGARDS, 0.66 in MAP, and 0.59 in CECP, and for the DIS with the EDIP they were 0.43 in REGARDS, 0.17 in MAP, and 0.23 in CECP. Quantile classification agreement was greater between the DIS and DII than between the DIS and EDIP (Supplemental Table 6). For example, in REGARDS, in the first and fifth quintiles, agreement between the DIS and DII and the DIS and EDIP was approximately 54% and 41%, respectively.
Associations of the DIS, LIS, DII, and EDIP with inflammation biomarkers in REGARDS, MAP, and CECP are shown in Table 3. Higher dietary and lifestyle inflammation scores of all types were generally strongly, positively associated with inflammation biomarkers in all 3 studies. In REGARDS, there was a statistically significant trend of increasing odds of high plasma hsCRP concentrations with an increasing DIS, and for those in the highest relative to the lowest DIS quintile, there was a statistically significant 66% higher odds of having a high plasma hsCRP concentration. In MAP, there was a similar pattern, and those in the highest relative to the lowest DIS quartile had 2.1-fold higher odds of having a high hsCRP concentration. Similarly, in CECP, those in the highest relative to the lowest DIS quantile had an estimated 42% higher odds of having a high inflammation biomarker score, although this finding was not statistically significant in this small study. The findings for the LIS were stronger than those for the DIS in all 3 study populations. There were statistically significant trends of increasing odds of having a high hsCRP concentration with an increasing LIS in REGARDS and MAP, with a statistically significant 4.3-fold and 6.8-fold higher odds for those in the upper LIS quantile in REGARDS and MAP, respectively. In CECP, those in the highest relative to the lowest LIS quantile had an estimated 56% higher odds of having a high inflammation biomarker score, although this finding was not statistically significant.
TABLE 3.
Cross-sectional associations of dietary and lifestyle inflammation scores with plasma inflammation biomarker concentrations in the remaining REGARDS (n = 14,210), MAP (n = 423), and CECP (n = 173) study populations 1
| Inflammation scores2 | ||||||||
|---|---|---|---|---|---|---|---|---|
| DIS3 | LIS4 | DII5 | EDIP5 | |||||
| n 6 | Adjusted OR (95% CI) | n 6 | Adjusted OR (95% CI) | n 6 | Adjusted OR (95% CI) | n 6 | Adjusted OR (95% CI) | |
| REGARDS, quintiles | ||||||||
| Q1 | 680/2843 | 1.00 | 526/3149 | 1.00 | 761/2843 | 1.00 | 764/2843 | 1.00 |
| Q2 | 834/2842 | 1.25 (1.10, 1.41) | 555/2226 | 1.58 (1.38, 1.82) | 919/2842 | 1.32 (1.17, 1.50) | 872/2842 | 1.10 (0.97, 1.24) |
| Q3 | 976/2842 | 1.38 (1.22, 1.56) | 1088/3263 | 2.31 (2.05, 2.61) | 961/2842 | 1.31 (1.15, 1.48) | 958/2842 | 1.19 (1.05, 1.35) |
| Q4 | 1058/2842 | 1.50 (1.32, 1.70) | 1005/2582 | 2.74 (2.42, 3.12) | 1016/2842 | 1.42 (1.25, 1.62) | 999/2842 | 1.18 (1.04, 1.34) |
| Q5 | 1201/2841 | 1.66 (1.46, 1.90) | 1575/2990 | 4.29 (3.79, 4.87) | 1092/2841 | 1.56 (1.35, 1.81) | 1156/2841 | 1.32 (1.17, 1.49) |
| P-trend | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| MAP, quartiles | ||||||||
| Q1 | 35/108 | 1.00 | 26/114 | 1.00 | 38/107 | 1.00 | 38/109 | 1.00 |
| Q2 | 43/104 | 1.90 (0.99, 3.65) | 39/103 | 2.43 (1.29, 4.60) | 40/108 | 0.97 (0.50, 1.85) | 47/106 | 1.37 (0.73, 2.54) |
| Q3 | 42/107 | 1.47 (0.76, 2.83) | 43/112 | 2.52 (1.34, 4.73) | 50/104 | 1.69 (0.86, 3.32) | 40/108 | 0.75 (0.39, 1.46) |
| Q4 | 48/104 | 2.05 (1.03, 4.08) | 60/94 | 6.79 (3.45, 13.35) | 40/104 | 1.36 (0.64, 2.91) | 43/100 | 1.20 (0.62, 2.32) |
| P-trend | 0.09 | <0.001 | 0.31 | 0.91 | ||||
| CECP, quantiles | ||||||||
| Q1 | 48/87 | 1.00 | 36/85 | 1.00 | 43/87 | 1.00 | 42/87 | 1.00 |
| Q2 | 38/86 | 1.42 (0.71, 2.82) | 50/88 | 1.56 (0.82, 2.97) | 43/86 | 0.94 (0.44, 2.01) | 44/86 | 1.72 (0.87, 3.42) |
In the REGARDS and MAP studies, the outcome was hsCRP concentrations categorized as ≤/>3 mg/dL, and in the CECP trial, the outcome was the inflammation biomarker score [comprising IL-1β, IL-6, IL-8, IL-12p40, TNF-α, VEGF, and IL-10 (the latter with a negative sign)] dichotomized as 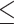 /
/ 0 (based on the study population median); all associations assessed using multivariable logistic regression. CECP, Calcium and Colorectal Epithelial Cell Proliferation trial; DII, dietary inflammatory index; DIS, dietary inflammation score; EDIP, empirical dietary inflammatory pattern; hsCRP, high-sensitivity C-reactive protein; LIS, lifestyle inflammation score; MAP, Markers of Adenomatous Polyps; NSAID, nonsteroidal anti-inflammatory drug; REGARDS, Reasons for Racial and Geographic Differences in Stroke Study; VEGF, vascular endothelial growth factor.
0 (based on the study population median); all associations assessed using multivariable logistic regression. CECP, Calcium and Colorectal Epithelial Cell Proliferation trial; DII, dietary inflammatory index; DIS, dietary inflammation score; EDIP, empirical dietary inflammatory pattern; hsCRP, high-sensitivity C-reactive protein; LIS, lifestyle inflammation score; MAP, Markers of Adenomatous Polyps; NSAID, nonsteroidal anti-inflammatory drug; REGARDS, Reasons for Racial and Geographic Differences in Stroke Study; VEGF, vascular endothelial growth factor.
For construction of scores, see text; higher scores indicate more proinflammatory diets or lifestyles. Weights for all dietary and lifestyle components in the DIS and LIS are equal to the maximum likelihood for the β-coefficients obtained from multivariable linear regression models (dependent variable: summary inflammation biomarker score) in the REGARDS case-cohort sample; DII and EDIP: weights and components derived from Shivappa et al. (11) and Tabung et al. (12), respectively.
For each study, covariates in the DIS logistic regression models were: REGARDS: age, sex, race (black or white), education (less than high school and high school graduate or some college or more), region (Belt, Buckle, Other), comorbidity score (comprises a history of cancer, heart disease, diabetes mellitus, or chronic kidney disease), current hormone replacement therapy use (among women), smoking (current or former and never), BMI (kg/m2), alcohol intake (nondrinker, moderate drinker, or heavy drinker), physical activity level (exercises 0, 1–3, or ≥ 4 times/wk), total energy intake (kcal/d), season of baseline interview (spring, summer, fall, or winter), and regular use of aspirin, other NSAIDs, or lipid-lowering medications (≥ twice/wk); MAP: age, sex, education (less than high school and high school graduate or some college or more), current hormone replacement use (among women), smoking (current or former and never), BMI category (based on WHO BMI classifications), alcohol intake (nondrinker, moderate drinker, or heavy drinker), physical activity level (tertiles based on the distribution of weekly metabolic equivalents of task-min/wk expenditure in the study population), total energy intake (kcal/d), study (MAP I or MAP II), and regular (≥ once/wk) aspirin or other NSAID use; CECP: age, sex, a comorbidity score (comprising diabetes mellitus or heart disease), smoking (current or former and never), BMI (kg/m2), alcohol intake (nondrinker, moderate drinker, or heavy drinker), physical activity level (tertiles based on the distribution of weekly minutes of physical activity in the study population), and total energy intake (kcal/d), and regular (≥ once/wk) aspirin or other NSAID use.
For each study, covariates in the LIS logistic regression models were: REGARDS: age, sex, race (black or white), education (less than high school and high school graduate or some college or more), region (Belt, Buckle, Other), comorbidity score (comprises a history of cancer, heart disease, diabetes mellitus, or chronic kidney disease), current hormone replacement therapy use (among women), energy intake (kcal/d), season of baseline interview (spring, summer, fall, or winter), the DIS, and regular use of aspirin, other NSAIDs, or lipid-lowering medications (≥ twice/wk); MAP: age, sex, education (less than high school and high school graduate or some college or more), current hormone replacement use (among women), total energy intake (kcal/d), study (MAP I or MAP II), the DIS, and regular aspirin or other NSAID use (≥ once/wk); CECP: age, sex, a comorbidity score (comprising diabetes mellitus or heart disease), total energy intake (kcal/d), the DIS, and regular aspirin or other NSAID use (≥ once/wk).
For each study, covariates in DII and EDIP logistic regression models included those listed in footnote 3, except for alcohol intake.
Number of participants with hsCRP concentrations >3 mg/dL/total participants in quantile.
Also shown in Table 3, in REGARDS, the DIS and DII findings were similar, but the strengths of the associations for the EDIP were much weaker, although still statistically significant. However, in MAP, the estimated positive associations involving the DIS were larger than those for the DII and EDIP, whereas in CECP, these associations were larger than for those for the DII (which were close to the null), but smaller than those for the EDIP, although none of the findings for the CECP study was statistically significant and the CIs around the estimated associations were wide.
The joint/combined (cross-classification) associations of the DIS and LIS with high plasma hsCRP concentrations in REGARDS are presented in Table 4. Being in the highest relative to the lowest joint quintile of the DIS and LIS was associated with the highest odds (OR: 7.3, 95% CI: 6.1, 8.6) of a high hsCRP concentration. Among those in the lowest LIS quintile, there was increasing odds of a high hsCRP concentration with a higher DIS, culminating in a statistically significant 69% higher odds for those in the highest DIS quintile. Among those in the lowest DIS quintile, there was increasing odds of a higher hsCRP concentration with a higher LIS, culminating in statistically significant 4.3-fold higher odds for those in the highest LIS quintile.
TABLE 4.
Joint/combined associations of the DIS and LIS with plasma hsCRP concentrations in the remaining REGARDS cohort (n = 14,210)1
| LIS quintiles2,3 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||||||
| n 4 | OR (95% CI) | n 4 | OR (95% CI) | n 4 | OR (95% CI) | n 4 | OR (95% CI) | n 4 | OR (95% CI) | P-interaction5 | |
| DIS quintiles2,3 | |||||||||||
| 1 | 110/938 | 1.00 (ref) | 91/461 | 1.58 (1.38, 1.82) | 169/649 | 2.31 (2.04, 2.61) | 143/410 | 2.74 (2.41, 3.11) | 167/385 | 4.30 (3.80, 4.87) | |
| 2 | 124/782 | 1.25 (1.11, 1.42) | 101/464 | 1.98 (1.65, 2.38) | 223/680 | 2.89 (2.43, 3.43) | 160/465 | 3.43 (2.88, 4.09) | 226/451 | 5.38 (4.52, 6.41) | |
| 3 | 102/573 | 1.41 (1.24, 1.59) | 121/469 | 2.23 (1.86, 2.67) | 224/664 | 3.24 (2.74, 3.85) | 208/512 | 3.85 (3.24, 4.58) | 321/624 | 6.05 (5.10, 7.16) | |
| 4 | 102/497 | 1.53 (1.35, 1.73) | 117/423 | 2.42 (2.02, 2.89) | 236/653 | 3.52 (2.97, 4.17) | 231/572 | 4.18 (3.52, 4.97) | 372/697 | 6.56 (5.54, 7.77) | |
| 5 | 88/359 | 1.69 (1.49, 1.92) | 125/409 | 2.68 (2.23, 3.21) | 236/617 | 3.90 (3.28, 4.63) | 263/623 | 4.63 (3.90, 5.50) | 489/833 | 7.26 (6.13, 8.60) | 0.03 |
The outcome was hsCRP concentrations categorized as ≤/> 3 mg/dL; all associations assessed using multivariable logistic regression. DIS, dietary inflammation score; hsCRP, high-sensitivity C-reactive protein; LIS, lifestyle inflammation score; NSAID, nonsteroidal anti-inflammatory drug; REGARDS, Reasons for Racial and Geographic Differences in Stroke Study.
For construction of scores, see text and Table 1; higher scores indicate more proinflammatory diets or lifestyles; weights for all dietary and lifestyle components in the DIS and LIS are equal to the maximum likelihood for the β-coefficients obtained from multivariable linear regression models [dependent variable: inflammation biomarker score (sum of z scores for hsCRP, IL-6, IL-8, IL-10, the latter with a negative sign)] in the REGARDS case-cohort sample.
Covariates in logistic regression model: age, sex, race (black or white), education (less than high school and high school graduate or some college or more), region (Belt, Buckle, Other), comorbidity score (comprises a history of cancer, heart disease, diabetes mellitus, or chronic kidney disease), current hormone replacement therapy use (among women), energy intake (kcal/d), season of baseline interview (spring, summer, fall, or winter), and self-reported regular use of aspirin, other NSAIDs, or lipid-lowering medications (≥ twice/wk).
Number of participants with hsCRP concentrations > 3 mg/dL/total participants in DIS and LIS quintile combination.
From DIS × LIS interaction term in the full logistic regression model, calculated using the Wald test.
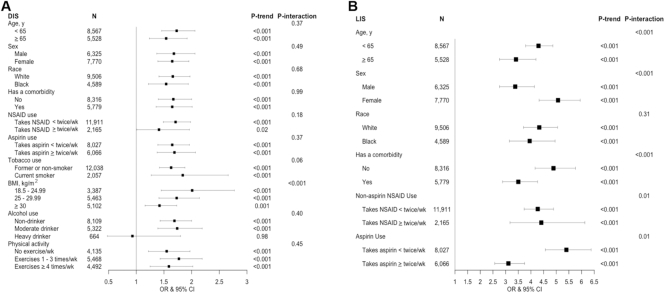
Associations of the DIS and LIS with hsCRP concentrations in REGARDS according to selected participant characteristics are shown in Figure 1 and Supplemental Table 7. The pattern of findings across participants with different characteristics was similar, although the DIS–hsCRP association tended to be stronger among those who were not obese (similar findings for the DII/EDIP–hsCRP associations shown in Supplemental Table 8) and not a heavy drinker, and the LIS–hsCRP association tended to be somewhat stronger among those who were younger, female, had no comorbidity, and did not regularly take aspirin.
FIGURE 1.
ORs (95% CIs) for comparisons of participants in the fifth relative to first quintile of the DIS (A) and LIS (B), by selected participant characteristics in the remaining REGARDS cohort (n = 14,210). The outcome was hsCRP concentrations categorized as ≤/>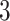 mg/dL in multivariable logistic regression models. For construction of scores, see text and Table 1; higher scores indicate more proinflammatory diets or lifestyles; for covariates for models, see Table 3 footnotes 3 and 4. DIS, dietary inflammation score; hsCRP, high-sensitivity C-reactive protein; LIS, lifestyle inflammation score; NSAID, nonsteroidal anti-inflammatory drug; REGARDS, Reasons for Racial and Geographic Differences in Stroke Study.
mg/dL in multivariable logistic regression models. For construction of scores, see text and Table 1; higher scores indicate more proinflammatory diets or lifestyles; for covariates for models, see Table 3 footnotes 3 and 4. DIS, dietary inflammation score; hsCRP, high-sensitivity C-reactive protein; LIS, lifestyle inflammation score; NSAID, nonsteroidal anti-inflammatory drug; REGARDS, Reasons for Racial and Geographic Differences in Stroke Study.
In sensitivity analyses, the DIS–inflammation biomarker associations without supplemental micronutrients were somewhat weaker in the 3 validation populations (Supplemental Table 9). The associations of the equal-weight DIS (Supplemental Table 10) were similar to those for the DIS in REGARDS, somewhat weaker in MAP, and stronger in the smaller CECP. The associations of the equal-weight LIS with inflammation biomarkers (Supplemental Table 11) were weaker than those for the LIS in REGARDS and MAP, but stronger in CECP. The findings from the analyses of multivariable-adjusted mean inflammation biomarker values and their proportional differences across the quantiles of each dietary and lifestyle inflammation score (Supplemental Tables 12 and 13) closely paralleled those in Tables 3 and 4. Applying the MCM/bootstrap technique (Supplemental Table 14) resulted in slight attenuation of the estimated DIS–inflammation biomarker associations in REGARDS and MAP, but not in CECP. The estimated associations of the LIS with inflammation biomarkers were somewhat stronger when applying the MCM/bootstrap technique in CECP, but not in REGARDS and MAP. In REGARDS, the joint/combined associations of the MCM/bootstrap technique DIS and LIS (Supplemental Table 15) and their associations according to selected characteristics (Supplemental Table 16) followed similar patterns. The CIs using the MCM/bootstrap technique were wider, reflecting the additional random error incorporated into the estimated associations.
Discussion
Our results support that 1) individual dietary and lifestyle components contribute modestly to systemic inflammation, and 2) diet and lifestyle in aggregate both contribute substantially—lifestyle more than diet—but especially in interaction with one another. Our results also support the use of our predominately whole foods-based DIS and novel LIS. As discussed below, the DIS has theoretical advantages over the more nutrient-based DII and data-driven EDIP, is applicable to multiple FFQs applied in many Western study populations, and may be more easily translated into clinical and public health dietary recommendations for inflammation reduction.
As summarized in Table 1, there is considerable biological plausibility/basic science support for the contributions of our dietary and lifestyle inflammation score components to inflammation. Many studies investigated associations of individual diet constituents (e.g., nutrients) with inflammation; however, these constituents are not consumed in isolation, but rather are contained within a matrix of thousands of other known and unknown substances that may be acting and interacting along the same and complementary pathways (13, 100). There is even more substantial evidence that individual lifestyle characteristics may be strongly associated with, or strongly affect, inflammation (86–93). Our findings of possible particularly strong aggregate contributions of lifestyle to inflammation, and even stronger synergistic contributions of diet and lifestyle to inflammation, support further investigation of dietary and lifestyle contributions to inflammation.
The DIS was more strongly directly associated with circulating inflammation biomarkers than was the DII in REGARDS, MAP, and CECP—findings that were robust to variations in sensitivity analyses. The DII was previously positively associated with biomarkers of inflammation in a range of populations (12, 101–105). The DII was also associated with inflammation-mediated diseases, such as cardiovascular diseases and colorectal cancer, and with premature mortality (106–110). However, the DII has several limitations. First, the DII is primarily based on classically measured nutrients and does not account for the myriad nonclassical, unmeasured, natural, anti-inflammatory or proinflammatory compounds found in whole foods and beverages. Also, although the DII weights were drawn from findings of many studies, the weighting scheme for the contributions of the findings from those studies was somewhat arbitrary, and the developers keep some methods and data underlying the weights proprietary.
The DIS was also more strongly directly associated with circulating inflammation biomarkers than was the EDIP in the larger REGARDS and MAP study populations, but not in the small CECP study in which the results were unstable. The EDIP was previously moderately to strongly positively associated with a panel of inflammation biomarkers in 3 studies (12, 101, 111). The more attenuated associations of the EDIP with hsCRP in REGARDS and MAP may, in part, be because the EDIP was developed in a relatively homogeneous population using a population-dependent, a posteriori, data-driven (compared with biological plausibility-driven) reduced rank regression approach. Dietary patterns and weights derived using reduced rank regression can be specific to the data in the population from which they are derived, making them less reproducible in other populations.
The DIS and LIS have several strengths, many of which address DII and EDIP limitations, including that: 1) both were developed in a clear, straightforward fashion, making them easy to calculate using data from many FFQs and lifestyle questionnaires commonly used in many Western study populations; 2) the directions of the weights are biologically plausible (Table 1); 3) the use of both accounts for the contributions of both diet and lifestyle to systemic inflammation; 4) composing the DIS mostly of whole foods facilitates clinical and public health applications; and 5) we addressed limitations in studying associations of mixed dishes with inflammation biomarker concentrations by disaggregating mixed dishes into their component parts using the My Pyramid Equivalents Database.
The DIS and LIS also have limitations. First, we developed the DIS and LIS weights in a US population enriched with future stroke cases and with a sample size that limited stratified analyses. However, the cohort comparison sample was selected randomly, all individuals were disease-free at the time of biomarker measurement and dietary/lifestyle questionnaire completion, and adjustment for future case status did not meaningfully affect the DIS and LIS weights. It is possible that a more comprehensive inflammation biomarker panel with which to assess the strengths of associations of the diet/lifestyle factors with systemic inflammation would yield more accurate associations, and in the entire REGARDS and MAP validation populations, only hsCRP was available. However, the REGARDS case-cohort biomarker panel was reliably measured in a heterogeneous population, and the validation results were similar across 3 validation populations, including 1 with a larger inflammation biomarker panel. Inherent to studying dietary data are known limitations of FFQs (e.g., respondent error, limited food options, and unmeasured food preparation methods). The DIS and LIS components’ weights are based on cross-sectional associations, so it is possible that if diet and biomarkers had been assessed at intervals over, say, a year, and averaged, the associations may have been somewhat different. However, FFQs are designed to capture dietary patterns over an extended period, and were found to do so reasonably well (24).
Taken together with previous literature, our findings support that individual diet and lifestyle components may contribute modestly to systemic inflammation, but that diet in aggregate and lifestyle in aggregate, contribute substantially—lifestyle more so than diet—and especially in interaction with one another. Our results also support the use of our hypothesis-driven, predominantly whole foods-based dietary inflammation score and our novel LIS as epidemiological tools to quantify the collective contributions of dietary and lifestyle exposures to systemic inflammation. The DIS and LIS address some limitations of previous dietary inflammation scores, and may be more useful for formulating clinical and public health dietary recommendations for inflammation reduction for disease prevention, pending further validation and application in relation to chronic disease incidence and premature mortality.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—DAB and RMB: designed the research and wrote the paper; DAB: performed the statistical analysis; SEJ, WDF, TJH, and VF: contributed to the data analysis plan and manuscript preparation; RMB: provided study oversight and had primary responsibility for the final content; and all authors: read and approved the final manuscript.
Notes
Supported by the National Institute of Neurological Disorders and Stroke, National Institutes of Health (grant U01 NS041588); National Cancer Institute, National Institutes of Health (grants R01 CA66539 and R01 CA51932); The Fullerton Foundation; and The Franklin Foundation.
Author disclosures: DAB, SEJ, WDF, TJH, VF, and RMB, no conflicts of interest.
Supplemental Methods, Supplemental Figure 1, and Supplemental Tables 1–16 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: CECP, Calcium and Colorectal Epithelial Cell Proliferation trial; DII, dietary inflammatory index; DIS, dietary inflammation score; EDIP, empirical dietary inflammatory pattern; hsCRP, high-sensitivity C-reactive protein; LIS, lifestyle inflammation score; MAP, Markers of Adenomatous Polyps; MCM, Monte Carlo Methods; NSAID, nonsteroidal anti-inflammatory drug; REGARDS, Reasons for Racial and Geographic Differences in Stroke Study; VEGF, vascular endothelial growth factor.
References
- 1. Wu Y, Antony S, Meitzler JL, Doroshow JH. Molecular mechanisms underlying chronic inflammation-associated cancers. Cancer Lett. 2014;345(2):164–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–35. [DOI] [PubMed] [Google Scholar]
- 3. CDC. National Vital Statistics System. Leading Causes of Death, 1900–1998. CDC, National Center for Health Statistics. Available from: https://www.cdc.gov/nchs/nvss/mortality_historical_data.htm. [Google Scholar]
- 4. American Cancer Society. Cancer Facts & Figures 2018. Atlanta American Cancer Society; 2018. [Google Scholar]
- 5. Barbaresko J, Koch M, Schulze MB, Nöthlings U. Dietary pattern analysis and biomarkers of low-grade inflammation: a systematic literature review. Nutr Rev. 2013;71(8):511–27. [DOI] [PubMed] [Google Scholar]
- 6. Kantor ED, Lampe JW, Kratz M, White E. Lifestyle factors and inflammation: associations by body mass index. PLoS ONE. 2013;8(7):e67833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cavicchia PP, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, Hébert JR. A new dietary inflammatory index predicts interval changes in serum high-sensitivity C-reactive protein. J Nutr. 2009;139:2365–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bruce WR, Giacca A, Medline A. Possible mechanisms relating diet and risk of colon cancer. Cancer Epidemiol Biomarkers Prev. 2000;9(12):1271–9. [PubMed] [Google Scholar]
- 9. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. [DOI] [PubMed] [Google Scholar]
- 10. National Center for Health Statistics (US). Health, United States, 2015: With Special Feature on Racial and Ethnic Health Disparities, [Internet], Report No. 2016-1232, Hyattsville (MD): National Center for Health Statistics (US) Available from: http://www.ncbi.nlm.nih.gov/pubmed/27308685 [PubMed] [Google Scholar]
- 11. Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17(8):1689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tabung FK, Smith-Warner SA, Chavarro JE, Wu K, Fuchs CS, Hu FB, Chan AT, Willett WC, Giovannucci EL. Development and validation of an empirical dietary inflammatory index. J Nutr. 2016;146(8):1560–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jacobs DR, Tapsell LC. Food, not nutrients, is the fundamental unit in nutrition. Nutr Rev. 2007;65(10):439–50. [DOI] [PubMed] [Google Scholar]
- 14. Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The Reasons for Geographic and Racial Differences in Stroke Study: objectives and design. Neuroepidemiology. 2005;25:135–43. [DOI] [PubMed] [Google Scholar]
- 15. Cushman M, Judd SE, Howard VJ, Kissela B, Gutiérrez OM, Jenny NS, Ahmed A, Thacker EL, Zakai NA. N-terminal pro-B-type natriuretic peptide and stroke risk: the reasons for geographic and racial differences in stroke cohort. Stroke. 2014;45(6):1646–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boucher B, Cotterchio M, Kreiger N, Nadalin V, Block T, Block G. Validity and reliability of the Block98 food-frequency questionnaire in a sample of Canadian women. Public Health Nutr. 2005;9(1):84–93. [DOI] [PubMed] [Google Scholar]
- 17. Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–87. [DOI] [PubMed] [Google Scholar]
- 18. Kannel WB. The Framingham Study: its 50-year legacy and future promise. J Atheroscler Thromb. 2000;6(2):60–6. [DOI] [PubMed] [Google Scholar]
- 19. The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 20. McDonnell MN, Hillier SL, Hooker SP, Le A, Judd SE, Howard VJ. Physical activity frequency and risk of incident stroke in a National US study of blacks and whites. Stroke. 2013;44(9):2519–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Olson NC, Cushman M, Lutsey PL, Mcclure LA, Judd S, Tracy RP, Folsom AR, Zakai NA. Inflammation markers and incident venous thromboembolism: the REasons for Geographic And Racial Differences in Stroke (REGARDS) Cohort. J Thromb Haemost. 2014;12(12):1993–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gong Z, Xie D, Deng Z, Bostick RM, Muga SJ, Hurley TG, Hebert JR. The PPARg Pro12Ala polymorphism and risk for incident sporadic colorectal adenomas. Carcinogenesis. 2005;26(3):579–85. [DOI] [PubMed] [Google Scholar]
- 23. Daniel CR, Bostick RM, Flanders WD, Long Q, Fedirko V, Sidelnikov E, Seabrook ME. TGF-α expression as a potential biomarker of risk within the normal-appearing colorectal mucosa of patients with and without incident sporadic adenoma. Cancer Epidemiol Biomarkers Prev. 2009;18(1):65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51–65. [DOI] [PubMed] [Google Scholar]
- 25. MacIntosh D, Williams PL, Hunter D, Sampson L, Morris S, Willett W, Rimm EB. Evaluation approach of a food frequency for estimating dietary intake of inorganic and composition arsenic. Cancer Epidemiol Biomarkers Prev. 1997;6(December):1043–50. [PubMed] [Google Scholar]
- 26. Pereira MA, FitzerGerald SJ, Gregg EW, Joswiak ML, Ryan WJ, Suminski RR, Utter AC, Zmuda JM. A collection of physical activity questionnaires for health-related research. Med Sci Sports Exerc. 1997;29(6 Suppl):S1–205. [PubMed] [Google Scholar]
- 27. Yang B, Gross MD, Fedirko V, Mccullough ML, Bostick RM. Effects of calcium supplementation on biomarkers of inflammation and oxidative stress in colorectal adenoma patients: a randomized controlled trial. Cancer Prev Res. 2015;8(11):1069–75. [DOI] [PubMed] [Google Scholar]
- 28. Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL et al.. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511. [DOI] [PubMed] [Google Scholar]
- 29. Johnson EJ. Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutr Rev. 2014;72(9):605–12. [DOI] [PubMed] [Google Scholar]
- 30. Nidhi B, Sharavana G, Ramaprasad TR, Vallikannan B. Lutein derived fragments exhibit higher antioxidant and anti-inflammatory properties than lutein in lipopolysaccharide induced inflammation in rats. Food Funct. 2015;6(2):450–60. [DOI] [PubMed] [Google Scholar]
- 31. Sommerburg O, Keunen JEE, Bird AC, van Kuijk FJGM. Fruits and vegetables that are sources for lutein and zeaxanthin: the macular pigment in human eyes. Br J Ophthalmol. 1998;82:907–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rao AV. Lycopene, tomatoes, and the prevention of coronary heart disease. Exp Biol Med. 2002;227(10):908–13. [DOI] [PubMed] [Google Scholar]
- 33. Burton-Freeman BM, Sesso HD. Whole food versus supplement: comparing the clinical evidence of tomato intake and lycopene supplementation on cardiovascular risk factors. Adv Nutr. 2014;5:457–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jacob K, Periago MJ, Böhm V, Berruezo GR. Influence of lycopene and vitamin C from tomato juice on biomarkers of oxidative stress and inflammation. Br J Nutr. 2017;99(01):137–46. [DOI] [PubMed] [Google Scholar]
- 35. Markovits N, Amotz A, Levy Y. The effect of tomato-derived lycopene on low carotenoids and enhanced systemic inflammation and oxidation in severe obesity. Isr Med Assoc J. 2009;11:598–601. [PubMed] [Google Scholar]
- 36. Prior R, Gu L, Wu X. Plasma antioxidant capacity changes following a meal as a measure of the ability of a food to alter in vivo antioxidant status. J Am Coll Nutr. 2013;26(2):170–81. [DOI] [PubMed] [Google Scholar]
- 37. Espley RV, Butts CA, Laing WA, Martell S, Smith H, Mcghie TK, Zhang J, Paturi G, Hedderley D, Bovy A et al.. Dietary flavonoids from modified apple reduce inflammation markers and modulate gut microbiota in mice. J Nutr. 2014;144:146–54. [DOI] [PubMed] [Google Scholar]
- 38. Codoñer-Franch P, Betoret E, Betoret N, López-Jaén AB, Valls-Belles V, Fito P. Dried apples enriched with mandarin juice by vacuum impregnation improve antioxidant capacity and decrease inflammation in obese children. Nutr Hosp. 2013;28(3):1177–83. [DOI] [PubMed] [Google Scholar]
- 39. Hussain T, Tan B, Liu G, Murtaza G, Rahu N, Saleem M, Yin Y. Modulatory mechanism of polyphenols and Nrf2 signaling pathway in LPS challenged pregnancy disorders. Oxid Med Cell Longev. 2017;2017:8254289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kaulmann A, Bohn T. Carotenoids, inflammation, and oxidative stress—implications of cellular signaling pathways and relation to chronic disease prevention. Nutr Res. 2014;34:907–29. [DOI] [PubMed] [Google Scholar]
- 41. Ghanim H, Mohanty P, Pathak R, Chaudhuri A, Chang LS, Dandona P. Orange juice or fructose intake does not induce oxidative and inflammatory response. Diabetes Care. 2007;30(6):1406–11. [DOI] [PubMed] [Google Scholar]
- 42. Knekt P, Kumpulainen J, Järvinen R, Rissanen H, Heliövaara M, Reunanen A, Hakulinen T, Aromaa A. Flavonoid intake and risk of chronic diseases. Am J Clin Nutr. 2002;76:560–8. [DOI] [PubMed] [Google Scholar]
- 43. Fortis-Barrera A, Alarcon-Aguilar FJ, Banderas-Dorantes T, Diaz-Flores M, Roman-Ramos R, Cruz M, Garcia-Macedo R. Cucurbita ficifoliaBouche (Cucurbitaceae) and d-chiro-inositol modulate the redox state and inflammation in 3T3-L1 adipocytes. J Pharm Pharmacol. 2013;65(10):1563–76. [DOI] [PubMed] [Google Scholar]
- 44. Sharma D, Rawat I, Goel HC. Anticancer and anti-inflammatory activities of some dietary cucurbits. Indian J Exp Biol. 2015;53(4):216–21. [PubMed] [Google Scholar]
- 45. Hale LP, Chichlowski M, Trinh CT, Greer PK. Dietary supplementation with fresh pineapple juice decreases inflammation and colonic neoplasia in IL-10-deficient mice with colitis. Inflamm Bowel Dis. 2010;16(12):2012–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Böhm F, Settergren M, Pernow J. Vitamin C blocks vascular dysfunction and release of interleukin-6 induced by endothelin-1 in humans in vivo. Atherosclerosis. 2007;190:408–15. [DOI] [PubMed] [Google Scholar]
- 47. Alam MA, Subhan N, Rahman MM, Uddin SJ, Reza HM, Sarker SD. Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv Nutr. 2014;5:404–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jia Q, Cheng W, Yue Y, Hu Y, Zhang J, Pan X, Xu Z, Zhang P. Cucurbitacin e inhibits TNF-α-induced inflammatory cytokine production in human synoviocyte MH7A cells via suppression of PI3K/Akt/NF-κB pathways. Int Immunopharmacol. 2015;29(2):884–90. [DOI] [PubMed] [Google Scholar]
- 49. Zitvogel L, Pietrocola F, Kroemer G. Nutrition, inflammation and cancer. Nat Immunol. 2017;18(8):843–50. [DOI] [PubMed] [Google Scholar]
- 50. Johnson M, Pace RD, Mcelhenney WH. Green leafy vegetables in diets with a 25:1 omega-6/omega-3 fatty acid ratio modify the erythrocyte fatty acid profile of spontaneously hypertensive rats. Lipids Health Dis. 2018;17(1)140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hartman TJ, Albert PS, Zhang Z, Bagshaw D, Kris-Etherton PM, Ulbrecht J, Miller CK, Bobe G, Colburn NH, Lanza E. Consumption of a legume-enriched, low-glycemic index diet is associated with biomarkers of insulin resistance and inflammation among men at risk for colorectal cancer. J Nutr. 2010;140(1):60–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Simopoulos AP. Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr. 2002;21(6):495–505. [DOI] [PubMed] [Google Scholar]
- 53. Calder PC. Omega-3 fatty acids and inflammatory processes. Nutrients. 2010;2:355–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation. Emphasis on the metabolic syndrome. J Am Coll Cardiol. 2006;48(4):677–85. [DOI] [PubMed] [Google Scholar]
- 55. Van Woudenbergh GJ, Kuijsten A, Tigcheler B, Sijbrands EJG, Van Rooij FJA, Hofman A, Witteman JCM, Feskens EJM. Meat consumption and its association with C-reactive protein and incident type 2 diabetes: the Rotterdam Study. Diabetes Care. 2012;35:1499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Van Bussel BC, Henry RM, Ferreira I, Van Greevenbroek M, Van Der Kallen CJ, Twisk JW, Feskens EJ, Schalkwijk CG, Da Stehouwer C. A healthy diet is associated with less endothelial dysfunction and less low-grade inflammation over a 7-year period in adults at risk of cardiovascular disease. J Nutr. 2015;145(3):532–40. [DOI] [PubMed] [Google Scholar]
- 57. Song M, Garrett WS, Chan AT. Nutrients, foods, and colorectal cancer prevention. Gastroenterology. 2015;148(6):1244–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Minihane AM, Vinoy S, Russell WR, Baka A, Roche HM, Tuohy KM, Teeling JL, Blaak EE, Fenech M, Vauzour D et al.. Low-grade inflammation, diet composition and health: current research evidence and its translation. Br J Nutr. 2015;114:999–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lopez-Garcia E, Schulze MB, Meigs JB, Manson JE, Rifai N, Stampfer MJ, Willett WC, Hu FB. Consumption of trans fatty acids is related to plasma biomarkers of inflammation and endothelial dysfunction. J Nutr. 2005;135(3):562–6. [DOI] [PubMed] [Google Scholar]
- 60. Ley SH, Sun Q, Willett WC, Eliassen AH, Wu K, Pan A, Grodstein F, Hu FB. Associations between red meat intake and biomarkers of inflammation and glucose metabolism in women. Am J Clin Nutr. 2014;99(2):352–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Brown A, Frank Hu. Dietary modulation of endothelial function: implications for cardiovascular disease. Am J Clin Nutr. 2001;73:673–86. [DOI] [PubMed] [Google Scholar]
- 62. White DL, Collinson A. Red meat, dietary heme iron, and risk of type 2 diabetes: the involvement of advanced lipoxidation endproducts. Adv Nutr. 2013;4:403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Guzik T, Korbut R, Adamek-Guzik T. Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmacol. 2003;54(4):469–87. [PubMed] [Google Scholar]
- 64. Ludwig D. The glycemic index physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. J Am Med Assoc. 2002;287(18):2414–23. [DOI] [PubMed] [Google Scholar]
- 65. Govers M, Termont D, Lapre J. Calcium in milk products precipitates intestinal fatty acids and secondary bile acids and thus inhibits colonic cytotoxicity in humans. Cancer Res. 1996;56:3270–5. [PubMed] [Google Scholar]
- 66. Dash C, Goodman M, Dana Flanders W, Mink PJ, McCullough ML, Bostick RM. Using pathway-specific comprehensive exposure scores in epidemiology: application to oxidative balance in a pooled case-control study of incident, sporadic colorectal adenomas. Am J Epidemiol. 2013;178(4):610–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kratz M, Baars T, Guyenet S. The relationship between high-fat dairy consumption and obesity, cardiovascular, and metabolic disease. Eur J Nutr. 2013;52:1–24. [DOI] [PubMed] [Google Scholar]
- 68. Dower JI, Geleijnse JM, Gijsbers L, Schalkwijk C, Kromhout D, Hollman PC. Supplementation of the pure flavonoids epicatechin and quercetin affects some biomarkers of endothelial dysfunction and inflammation in (pre)hypertensive adults: a randomized double-blind, placebo-controlled, crossover trial. J Nutr. 2015;145(7):1459–63. [DOI] [PubMed] [Google Scholar]
- 69. Park JB. Javamide-II found in coffee is better than caffeine at suppressing TNF-α production in PMA/PHA-treated lymphocytic Jurkat cells. J Agric Food Chem. 2018;66(26):6782–9. [DOI] [PubMed] [Google Scholar]
- 70. Wall R, Ross RP, Fitzgerald GF, Stanton C. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev. 2010;68(5):280–9. [DOI] [PubMed] [Google Scholar]
- 71. Casas-Agustench P, Bulló M, Salas-Salvadó J. Nuts, inflammation and insulin resistance. Asia Pac J Clin Nutr. 2010;19(1):124–30. [PubMed] [Google Scholar]
- 72. Guardia T, Rotelli AE, Osvaldo Juarez A, Pelzer LE. Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Farm. 2001;56:683–7. [DOI] [PubMed] [Google Scholar]
- 73. Chassaing B, Koren O, Goodrich J, Poole A, Srinivasan S, Ley RE, Gewirtz AT. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015;519(7541):92–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hopkins MH, Fedirko V, Jones DP, Terry PD, Bostick RM. Antioxidant micronutrients and biomarkers of oxidative stress and inflammation in colorectal adenoma patients: results from a randomized, controlled clinical trial. Cancer Epidemiol Biomarkers Prev. 2010;19(3):850–8. [DOI] [PubMed] [Google Scholar]
- 75. Hopkins MH, Owen J, Ahearn T, Fedirko V, Flanders WD, Jones DP, Bostick RM. Effects of supplemental vitamin D and calcium on biomarkers of inflammation in colorectal adenoma patients: a randomized, controlled clinical trial. Cancer Prev Res. 2011;4(10):1645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cai X, Wang S, Yu W, Wang F, Shen N, Fan W, Wu P, Wang C, Li X, Cai X. Selenium exposure and cancer risk: an updated meta-analysis and meta-regression. Sci Rep. 2016;6(1):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ko HJ, Youn CH, Kim HM, Cho YJ, Lee GH, Lee WK. Dietary magnesium intake and risk of cancer: a meta-analysis of epidemiologic studies. Nutr Cancer. 2014;66(6):915–23. [DOI] [PubMed] [Google Scholar]
- 78. Tappel A. Heme of consumed red meat can act as a catalyst of oxidative damage and could initiate colon, breast and prostate cancers, heart disease and other diseases. Med Hypotheses. 2007;68(3):562–4. [DOI] [PubMed] [Google Scholar]
- 79. Glei M, Latunde-Dada GO, Klinder A, Becker TW, Hermann U, Voigt K, Pool-Zobel BL. Iron-overload induces oxidative DNA damage in the human colon carcinoma cell line HT29 clone 19A. Mutat Res - Genet Toxicol Environ Mutagen. 2002;519(1–2):151–61. [DOI] [PubMed] [Google Scholar]
- 80. Obeid R, Kirsch SH, Kasoha M, Eckert R, Herrmann W. Concentrations of unmetabolized folic acid and primary folate forms in plasma after folic acid treatment in older adults. Metabolism. 2011;60(5):673–80. [DOI] [PubMed] [Google Scholar]
- 81. Kelly KB, Kennelly JP, Ordonez M, Nelson R, Leonard K, Stabler S, Gomez-Muñoz A, Field CJ, Jacobs RL. Excess folic acid increases lipid storage, weight gain, and adipose tissue inflammation in high fat diet-fed rats. Nutrients. 2016;8(10):E594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Du S-Y, Zhang Y-L, Bai R-X, Ai Z-L, Xie B-S, Yang H-Y. Lutein prevents alcohol-induced liver disease in rats by modulating oxidative stress and inflammation. Int J Clin Exp Med. 2015;8(6):8785–93. [PMC free article] [PubMed] [Google Scholar]
- 83. Wang M-X, Jiao J-H, Li Z-Y, Liu R-R, Shi Q, Ma L. Lutein supplementation reduces plasma lipid peroxidation and C-reactive protein in healthy nonsmokers. Atherosclerosis. 2013;227(2):380–5. [DOI] [PubMed] [Google Scholar]
- 84. Bowman SA, Friday JE, Moshfegh AJ. MyPyramid Equivalents Database, 2.0 for USDA Survey Foods, 2003–2004: Documentation and User Guide. 2008. [Google Scholar]
- 85. CDC. NHANES 2003–2004: Data Documentation, Codebook, and Frequencies [Internet]. 2007. Available from: https://wwwn-cdc-gov.proxy.library.emory.edu/Nchs/Nhanes/2003-2004/AUX_C.htm. [Google Scholar]
- 86. Wu D, Zhai Q, Shi X. Alcohol-induced oxidative stress and cell responses. J Gastroenterol Hepatol. 2006;21:26–9. [DOI] [PubMed] [Google Scholar]
- 87. Kumar Das S, Vasudevan DM. Alcohol-induced oxidative stress. Life Sci. 2007;81(3):177–87. [DOI] [PubMed] [Google Scholar]
- 88. Mathews MJ, Liebenberg L, Mathews EH. The mechanism by which moderate alcohol consumption influences coronary heart disease. Nutr J. 2015;14:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. McCarty M. Interleukin-6 as a central mediator of cardiovascular risk associated with chronic inflammation, smoking, diabetes, and visceral obesity: down-regulation with essential fatty acids, ethanol and pentoxifylline. Med Hypotheses. 1999;52(5):465–77. [DOI] [PubMed] [Google Scholar]
- 90. Gomez-Cabrera M-C, Domenech E, Vina J. Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic Biol Med. 2008;44(2):126–31. [DOI] [PubMed] [Google Scholar]
- 91. Calder PC, Ahluwalia N, Brouns F, Buetler T, Clement K, Cunningham K, Esposito K, Jönsson LS, Kolb H, Lansink M et al.. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br J Nutr. 2011;106(S3):S5–78. [DOI] [PubMed] [Google Scholar]
- 92. van der Vaart H, Postma D, Timens W, Ten Hacken N. Acute effects of cigarette smoke on inflammation and oxidative stress: a review. Thorax. 2004;59:713–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114(12):1752–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Centers for Disease Control and Prevention. CDC—Fact Sheets-Alcohol Use And Health—Alcohol [Internet]. [cited 2019 Mar 7]. Available from: https://www.cdc.gov/alcohol/fact-sheets/alcohol-use.htm. [Google Scholar]
- 95. Washburn R, Goldfield S, Smith K, McKinlay J. The validity of self-reported exercise-induced sweating as a measure of physical activity. Am J Epidemiol. 1990;132(1):107–13. [DOI] [PubMed] [Google Scholar]
- 96. Washburn RA, Adams LL, Haile GT. Physical activity assessment for epidemiologic research: the utility of two simplified approaches. Prev Med (Baltim). 1987;16(5):636–46. [DOI] [PubMed] [Google Scholar]
- 97. Couper KN, Blount DG, Riley EM. IL-10: The master regulator of immunity to infection. J Immunol. 2017;180:5771–7. [DOI] [PubMed] [Google Scholar]
- 98. Moore KW, De Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. [DOI] [PubMed] [Google Scholar]
- 99. Greenland S. Sensitivity analysis, Monte Carlo risk analysis, and Bayesian uncertainty assessment. Risk Anal. 2001;21(4):579–83. [DOI] [PubMed] [Google Scholar]
- 100. Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13(1):3–9. [DOI] [PubMed] [Google Scholar]
- 101. Tabung FK, Smith-Warner SA, Chavarro JE, Fung TT, Hu FB, Willett WC, Giovannucci EL. An empirical dietary inflammatory pattern score enhances prediction of circulating inflammatory biomarkers in adults. J Nutr. 2017;147(8):1567–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Shivappa N, Bonaccio M, Hebert ScD JR, Di Castelnuovo A, Costanzo S, Ruggiero E, Pounis G, Benedetta Donati M, de Gaetano G, Iacoviello L. Association of proinflammatory diet with low-grade inflammation: results from the Moli-sani study. Nutrition. 2018;54:182–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Shivappa N, Wirth MD, Hurley TG, Ebert JRH. Association between the dietary inflammatory index (DII) and telomere length and C-reactive protein from the National Health and Nutrition Examination. Mol Nutr Food Res. 2017;61(7):1600630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Tabung FK, Steck SE, Zhang J, Ma Y, Liese AD, Agalliu I, Hingle M, Hou L, Hurley TG, Jiao L et al.. Construct validation of the dietary inflammatory index among postmenopausal women. Ann Epidemiol. 2015;25(6):398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Wirth MD, Shivappa N, Davis L, Hurley TG, Ortaglia A, Drayton R, Blair SN, Hebert JR. Construct validation of the dietary inflammatory index among African Americans. J Nutr Heal Aging. 2017;21(5):487–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ramallal R, Toledo E, Martínez-González MA, Hernández-Hernández A, García-Arellano A, Shivappa N, Hébert JR, Ruiz-Canela M. Dietary inflammatory index and incidence of cardiovascular disease in the SUN cohort. PLoS ONE. 2015;10(9):e0135221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Tabung FK, Steck SE, Ma Y, Liese AD, Zhang J, Caan B, Hou L, Johnson KC, Mossavar-Rahmani Y, Shivappa N et al.. The association between dietary inflammatory index and risk of colorectal cancer among postmenopausal women: results from the Women's Health Initiative. Cancer Causes Control. 2015;26(3):399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Shivappa N, Blair C, Prizment A, Jacobs D Jr., Steck SE, Hebert JR. Association between inflammatory potential of diet and mortality in the Iowa Women's Health study. Eur J Nutr. 2016;55(4):1491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Shivappa N, Zucchetto A, Montella M, Serraino D, Steck SE, La Vecchia C, Hebert JR. Inflammatory potential of diet and risk of colorectal cancer: a case-control study from Italy. Br J Nutr. 2015;114(1):152–8. [DOI] [PubMed] [Google Scholar]
- 110. Deng EF, Shivappa N, Tang Y, Mann JR, Hebert JR. Association between diet-related inflammation, all-cause, all-cancer, and cardiovascular disease mortality, with special focus on prediabetics: findings from NHANES III. Eur J Nutr. 2017;56:1085–93. [DOI] [PubMed] [Google Scholar]
- 111. Tabung FK, Giovannucci EL, Giulianini F, Liang L, Chandler PD, Balasubramanian R, Manson JE, Feliciano EMC, Hayden KM, Van Horn L et al.. An empirical dietary inflammatory pattern score is associated with circulating inflammatory biomarkers in a multi-ethnic population of postmenopausal women in the United States. J Nutr. 2018;148(5):771–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.