ABSTRACT
Background
Brain iron deposition is a feature of Alzheimer disease and may contribute to its development. However, the relative contribution of dietary iron remains unclear.
Objectives
We investigated the impact of high dietary iron on brain pathological changes and cognitive function in adult wild-type (WT) mice and amyloid precursor protein/presenilin 1 (APP/PS1) double transgenic mice.
Methods
Male WT mice and APP/PS1 mice aged 10 wk were fed either a control diet (66 mg Fe/kg) (WT-Ctrl and APP/PS1-Ctrl) or a high iron diet (14 g Fe/kg) (WT-High Fe and APP/PS1-High Fe) for 20 wk. Iron concentrations in brain regions were measured by atomic absorption spectrophotometry. Brain iron staining and amyloid-β (Aβ) immunostaining were performed. Protein expressions in the hippocampus were determined by immunoblotting. Superoxide dismutase (SOD) activity and malondialdehyde concentration were examined. Cognitive functions were tested with the Morris water maze system.
Results
In the hippocampus, APP/PS1-High Fe mice had significantly higher iron concentration (2.5-fold) and ferritin (2.0-fold) than APP/PS1-Ctrl mice (P < 0.001), and WT-High Fe mice had significantly higher ferritin (2.0-fold) than WT-Ctrl mice (P < 0.001). Interestingly, APP/PS1 mice had significantly higher iron concentration (2–3-fold) and ferritin (2–2.5-fold) than WT mice fed either diet (P < 0.001). Histological analysis indicated that iron accumulated in the hippocampal dentate gyrus region in APP/PS1 mice, consistent with the pattern of Aβ deposition. For both mouse strains, iron treatment induced Aβ and phospho-τ expression (1.5–3-fold) in the hippocampus, but had little impact on oxidative stress and cognitive function. Furthermore, APP/PS1 mice had significantly lower SOD activity and higher malondialdehyde concentration than WT mice in the hippocampus (P < 0.0001), paralleled by apparent cognitive dysfunction.
Conclusions
Dietary iron overload induces iron disorder and Aβ and phospho-τ expression in the hippocampus of adult WT and APP/PS1 transgenic mice.
Keywords: Alzheimer disease, amyloid-β, APP/PS1 mice, cognitive function, dietary iron overload, hippocampus, oxidative stress
Introduction
Iron is an essential trace element, which plays an important role in the normal functioning of the central nervous system (1). However, excessive iron is toxic as it can catalyze the formation of reactive oxygen species (2). At the systemic level, iron homeostasis is maintained by regulating dietary iron intake (3), so the amount of iron in the diet is particularly important. However, brain cells are unable to take up iron directly from the systemic circulation because of the isolating effect of the blood–brain barrier (BBB).
Despite the effect of the BBB, brain iron accumulation occurs in both the normal ageing process and a range of neurodegenerative diseases, including Alzheimer disease (AD) (1). MRI studies have suggested that iron deposition in specific brain regions such as the hippocampus could reflect the neuropathologic progression of AD (4). Mechanistically, it is assumed that iron contributes to the generation and aggregation of amyloid-β (Aβ) and the aggregation and hyperphosphorylation of τ, thereby facilitating the formation of senile plaques and neurofibrillary tangles (5, 6). Iron may also accelerate the clinical progression of AD by enhancing oxidative stress and acting synergistically with Aβ (7). Recently, we and others reported that combined mutations in the genes encoding 2 multicopper ferroxidases, hephaestin and ceruloplasmin, in mice caused brain iron accumulation, and that this was paralleled by oxidative damage and behavioral abnormalities (i.e., motor disorder and learning and memory defects) (8, 9). Treatment with the oral iron chelator deferiprone, which crosses the BBB, however, diminished the amount of iron in the brain and protected against the neurodegeneration (9). These studies support the notion that brain iron overload contributes to cognitive impairment. However, the question remains of whether dietary iron uptake plays a fundamental role in development of AD, given that dietary iron is absorbed by the intestine into the systemic circulation while the brain is relatively isolated from the circulation by the BBB.
In the present study, we used double transgenic mice expressing both mutant human amyloid precursor protein (APP) and presenilin 1 (PS1) (APP/PS1 mice) as a model for AD (10). These mice show significant Aβ deposition and neuropathology in the brain and spatial learning defects as early as age 6–7 mo (11). We hypothesized that dietary iron overload might have some impacts on their brain iron contents, pathological changes, and cognitive function.
Methods
Mouse models and dietary treatments
Six-wk-old male APPswe/PSEN1ΔE9 (APP/PS1) double transgenic mice were purchased from the Mutant Mouse Regional Resource Center (Stock no. 03,4829) (12), and age-matched wild-type (WT) male littermates were used as controls. Mice were maintained at the Medical School of Nanjing University and were allowed unlimited access to AIN-93 M control diet for another 4 wk (13). At age 10 wk, APP/PS1 and WT control mice were separated into 4 groups containing 40 mice each. Mice were fed with either a control diet (WT-Ctrl and APP/PS1-Ctrl) or a high iron diet (WT-High Fe and APP/PS1-High Fe) for 20 wk. The AIN-93 M–based diet (control) is expected to contain 50 mg Fe/kg, whereas the iron-loaded diet (also based on AIN-93 M) supplemented with 2% carbonyl iron is expected to contain 20,000 mg Fe/kg (13). However, according to the measurement by atomic absorption spectrophotometry, the iron concentrations of the control and iron-loaded diets were 1.18 ± 0.01 μmol/g (∼66 mg/kg) and 251 ± 13.36 μmol/g (∼14 g/kg), respectively. The mice consumed the diets and distilled water ad libitum. All studies were carried out in accordance with NIH guidelines and approved by the Institutional Animal Care and Use Committee of Nanjing University.
Tissue preparation
The mice were killed at age 30 wk. Blood was collected by cardiac puncture and the body was perfused with phosphate-buffered saline via the heart. Liver and brain tissue were isolated, and the cerebral cortex, cerebellum, and hippocampus were quickly separated on ice. Whole blood was centrifuged to provide plasma. All samples were snap-frozen in liquid nitrogen and stored at −80°C until required for protein and iron concentration analyses. In addition, 4 mice from each group were perfused via the heart, first with phosphate-buffered saline and then with 4% paraformaldehyde. The collected brain tissue was fixed in 4% paraformaldehyde solution for later histological analysis.
Measurement of iron concentrations
Iron concentrations of mouse tissues and diets were measured using an atomic absorption spectrometer (model 180–80; Hitachi; Japan) at the Modern Instrumental Analysis Center of Nanjing University after nitric acid digestion (14).
Histology
Diaminobenzidine-enhanced Perls’ Prussian blue staining (for iron detection) was carried out on deparaffinized brain sections as previously described (15). Aβ staining was performed according to the histology protocol we previously used (16). The antibodies used were anti-Aβ antibody (1:100; catalogue no. ab2539, Abcam) and anti-rabbit secondary antibody (1:200; catalogue no. GB23303, Servicebio).
Immunoblot analysis
Hippocampus samples collected from 30-wk-old WT and APP/PS1 mice (n = 7 per group) were lysed as previously described (17). For phospho-τ (p-τ) immunoblots, the lysis buffer contained 2% alkaline phosphatase inhibitor (catalogue no. P1081, Beyotime Biotechnology). The total protein concentration was determined by the bicinchoninic acid method (Bioworld Technology Inc.). Immunoblot was then performed according to the protocol we previously used (17). The following antibodies were used: anti-ferritin light chain (1:1000; catalogue no. sc-74,513, Santa Cruz Biotechnology), anti-p-τ (Ser404) (1:1000; catalogue no. 44–758 G, Invitrogen), anti-Aβ1–42 (1:1000; catalogue no. ab10148, Abcam), anti-β-tubulin (1:5000; catalogue no. M20005, Abmart), anti-mouse secondary antibody (1:2000; catalogue no. sc-2031, Santa Cruz Biotechnology), and anti-rabbit secondary antibody (1:40,000; catalogue no. sc-2030, Santa Cruz Biotechnology). The intensity of individual protein bands was assessed using ImageJ software (NIH) after densitometry.
Superoxide dismutase and malondialdehyde concentration assay
To assess oxidative stress, hippocampus samples collected from 30-wk-old WT and APP/PS1 mice (n = 7 per group) were used to examine superoxide dismutase (SOD) activity and malondialdehyde concentrations with SOD and malondialdehyde assay kits (catalogue nos. S0101 and S0131, Beyotime Biotechnology) as previously described (8).
Morris water maze
Learning and memory of mice at age 28 wk were tested with the Morris water maze system, which fits into a 1.2-m diameter circular pool with opaque water (∼22–24°C). The Morris water maze task was performed in a similar manner to that described by Konopka et al. (18) with minor modifications. Briefly, the experiment was divided into 2 phases. For the first 4 d, mice were trained to locate the platform submerged 1 cm below the water surface 3 times/d. The mice were placed in a random starting position, with their heads facing the pool wall. Time recording started after releasing the animal and stopped after the mouse found the platform. Any mice that failed to locate the platform in 90 s were guided to it, with the escape latency recorded as 90 s. On day 5, a probe test that lasted for 90 s was conducted with the platform removed. Mice were released from the point opposite to the target quadrant, and time to reach the center of the target quadrant where the platform had previously been located was recorded as the escape latency. Swimming velocity, the number of times that each mouse crossed the center of the target quadrant, and the time that each mouse spent in the target quadrant were monitored by a video camera linked to a MiniSee computerized tracking system.
Statistical analysis
All values are presented as means ± SDs. Two-factor ANOVA was used to compare tissue iron concentrations, ferritin, p-τ(Ser404), and Aβ1–42 protein, SOD activity and malondialdehyde concentration, and most of the data obtained from the behavioral study. Body weight and escape latency data were analyzed by 3-factor repeated-measures ANOVA. The P values for main effects or interactions were presented within the relative figures. Two-factor ANOVA tests with a significant interaction between the 2 factors were further analyzed by Bonferroni's post-hoc test. Differences were considered significant at P < 0.05. All statistical analyses were performed using GraphPad Prism 8 Software (GraphPad Software).
Results
Growth and iron status in WT and APP/PS1 mice fed either a control diet or a high iron diet
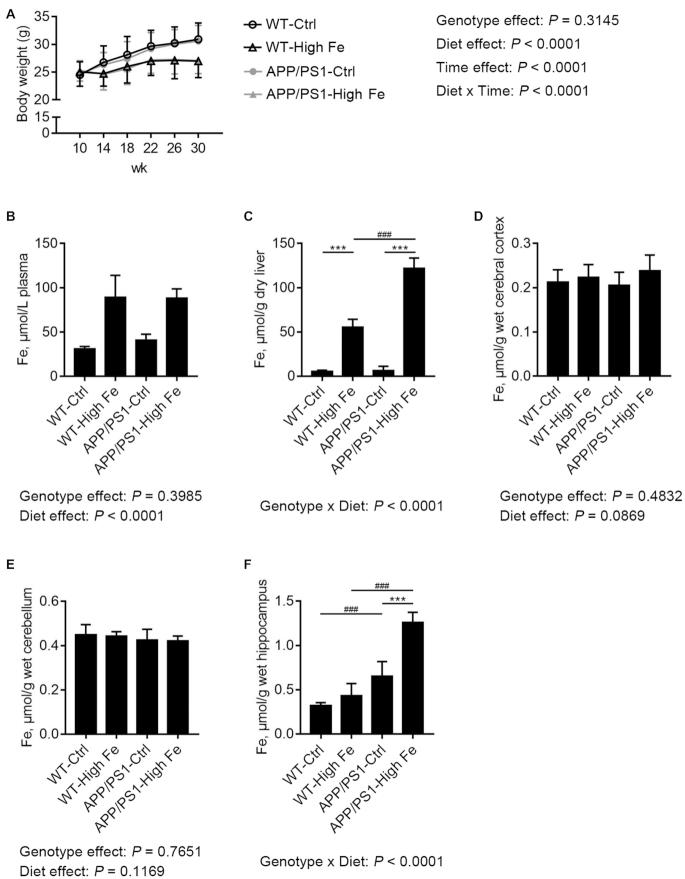
Both WT and APP/PS1 mice grew significantly more slowly when fed a high iron diet (P < 0.0001) (Figure 1A). APP/PS1 mutations did not affect body weight. At age 30 wk, mice fed with a high iron diet displayed significantly higher iron concentrations in their plasma (2–3-fold, P < 0.0001), while there was no significant main effect of genotype (Figure 1B). Interestingly, there was an interaction between dietary iron and genotype in liver iron concentration (P < 0.0001). Further analysis indicated that WT-High Fe mice had a significantly higher liver iron concentration than WT-Ctrl mice (10.4-fold, P < 0.001), and this was also the case in APP/PS1-High Fe mice relative to APP/PS1-Ctrl mice (19.2-fold, P < 0.001). In addition, APP/PS1-High Fe mice had a significantly higher liver iron concentration than WT-High Fe mice (2.2-fold, P < 0.001) (Figure 1C).
FIGURE 1.
Body weight (A) and iron concentrations in the plasma (B), liver (C), cerebral cortex (D), cerebellum (E), and hippocampus (F) of male WT and APP/PS1 mice fed a control or high iron diet at age 30 wk. Values are means ± SDs, n = 7 (B–F) or n = 20 (A). The P value of the interaction is presented when it reaches significance, and further analysis of diet simple effects and genotype simple effects were then performed. ***P < 0.001, different because of diet; ###P < 0.001, different because of genotype (C and F). APP/PS1, amyloid precursor protein/presenilin 1; APP/PS1-Ctrl, APP/PS1 mice fed a control diet; APP/PS1-High Fe, APP/PS1 mice fed a high iron diet; WT, wild-type; WT-Ctrl, wild-type mice fed a control diet; WT-High Fe, wild-type mice fed a high iron diet.
Iron concentrations in various brain regions were also examined. No significant differences were found in iron concentrations in the cerebral cortex or cerebellum, irrespective of the mouse strain or dietary iron content (Figure 1D, E). In hippocampus, there was an interaction between dietary iron and genotype (P < 0.0001). Further analysis indicated that APP/PS1-Ctrl mice had a significantly higher hippocampal iron concentration than WT-Ctrl mice (2.0-fold, P < 0.001), and this effect was exacerbated in APP/PS1-High Fe mice relative to WT-High Fe mice (3.0-fold, P < 0.001). Feeding the mutant mice with a high iron diet significantly increased the iron concentration in their hippocampus (2.0-fold, P < 0.001) (Figure 1F).
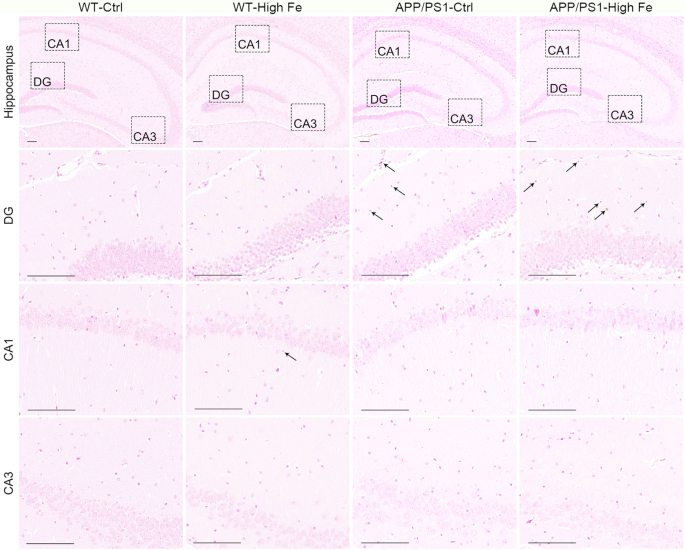
Iron accumulates in the hippocampal dentate gyrus region of APP/PS1 mice
Iron distribution in the hippocampus was then assessed. Although no visible iron accumulation was detected in WT-Ctrl mice, a small amount of stainable iron was observed in the hippocampal CA1 region of WT-High Fe mice. In contrast, APP/PS1 mice showed multiple iron deposits in the hippocampal dentate gyrus (DG), but not in the CA1 or CA3 regions. The pattern of iron deposits was similar in APP/PS1-Ctrl mice and APP/PS1-High Fe mice (Figure 2 and Supplemental Figure 1).
FIGURE 2.
Representative images of diaminobenzidine-enhanced Perls’ staining for iron (arrows) in the hippocampus of male WT and APP/PS1 mice fed a control or high iron diet at age 30 wk, n = 4. The images in the lower 3 rows are magnified images of the corresponding hippocampal DG, CA1, and CA3 regions indicated in the images in the top row. Bar = 100 μm. See Supplemental Figure 1 for magnified images of positive iron staining. APP/PS1, amyloid precursor protein/presenilin 1; APP/PS1-Ctrl, APP/PS1 mice fed a control diet; APP/PS1-High Fe, APP/PS1 mice fed a high iron diet; DG, dentate gyrus; WT, wild-type; WT-Ctrl, wild-type mice fed a control diet; WT-High Fe, wild-type mice fed a high iron diet.
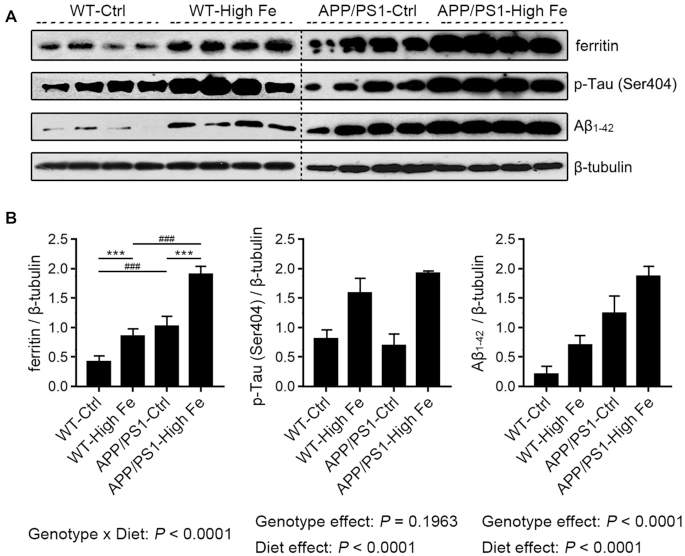
Iron treatment induces ferritin, p-τ and Aβ1–42 protein in the hippocampus in both WT and APP/PS1 mice
Amounts of ferritin, p-τ, and Aβ1–42 protein in the hippocampus were examined. There was an interaction effect between dietary iron and genotype on ferritin (P < 0.0001). APP/PS1-Ctrl mice had 2.4-fold higher ferritin than did WT-Ctrl mice (P < 0.001) (Figure 3). Iron-loading approximately doubled the hippocampal ferritin of both strains (P < 0.001). Amounts of p-τ (phosphorylated at Ser404) were not affected by genotype, whereas they were significantly increased 2–3-fold by iron-loading (P < 0.001) (Figure 3). Amounts of Aβ1–42 protein were significantly increased by both APP/PS1 mutations and iron treatment (P < 0.001 and P < 0.001, respectively), but there was no interaction between the 2 factors (Figure 3). These data showed that although iron treatment stimulated the expression of all 3 tested proteins, APP/PS1 mutations only induced ferritin and Aβ1–42 protein without affecting p-τ.
FIGURE 3.
Amounts of ferritin, p-τ (Ser404), and Aβ1–42 protein in the hippocampus of male WT and APP/PS1 mice fed a control or high iron diet at age 30 wk (A). The immunoblot signals were quantified, and values were normalized using β-tubulin expression (B). Values are means ± SDs, n = 7. The P value of the interaction is presented when it reaches significance, and further analysis of diet simple effects and genotype simple effects were then performed. ***P < 0.001, different because of diet; ###P < 0.001, different because of genotype (B). Aβ1–42, β amyloid 1–42; APP/PS1, amyloid precursor protein/presenilin 1; APP/PS1-Ctrl, APP/PS1 mice fed a control diet; APP/PS1-High Fe, APP/PS1 mice fed a high iron diet; p-τ, phospho-τ; WT, wild-type; WT-Ctrl, wild-type mice fed a control diet; WT-High Fe, wild-type mice fed a high iron diet.
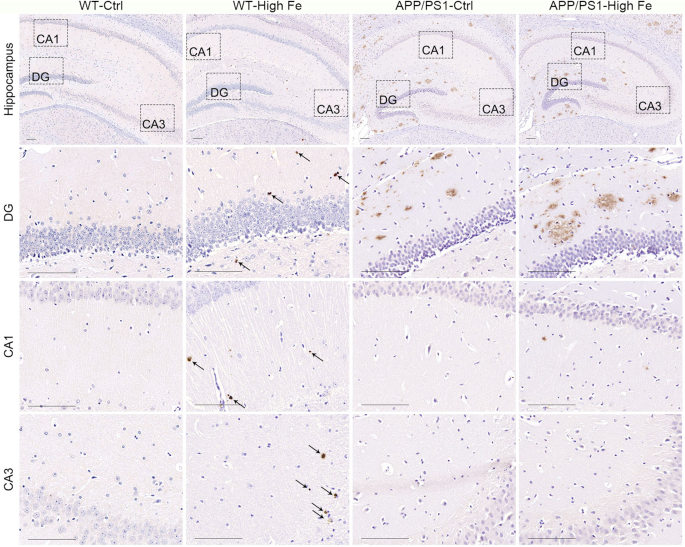
Aβ is differentially distributed in the hippocampus of iron-loaded WT and APP/PS1 mice
WT-Ctrl mice at age 30 wk showed no Aβ deposits in the hippocampus. In contrast, numerous Aβ plaques were observed in the hippocampus of APP/PS1-Ctrl mice (Figure 4). The plaques were largely restricted to the DG region, surrounding the dentate gyrus. Iron-loading of this strain appeared to increase the extent of the staining. WT-High Fe mice also showed a number of Aβ spots in the hippocampal DG region (albeit fewer than in APP/PS1 mice), but in this case Aβ was diffusely distributed throughout the whole hippocampus, including the CA1 and CA3 regions (Figure 4 and Supplemental Figure 2).
FIGURE 4.
Representative images of Aβ staining in the hippocampus of male WT and APP/PS1 mice fed a control or high iron diet at 30 wk of age, n = 4. The images in the lower 3 rows are magnified images of the corresponding hippocampal DG, CA1, and CA3 regions indicated in the images in the top row. Aβ spots in the hippocampus of iron-loaded WT mice are indicated with arrows. Bar = 100 μm. See Supplemental Figure 2 for magnified images of positive Aβ staining in the hippocampus of WT-High Fe mice. Aβ, amyloid-β; APP/PS1, amyloid precursor protein/presenilin 1; APP/PS1-Ctrl, APP/PS1 mice fed a control diet; APP/PS1-High Fe, APP/PS1 mice fed a high iron diet; DG, dentate gyrus; WT, wild-type; WT-Ctrl, wild-type mice fed a control diet; WT-High Fe, wild-type mice fed a high iron diet.
Iron treatment did not induce oxidative damage in the hippocampus
SOD activity and malondialdehyde in the hippocampus were significantly affected by APP/PS1 mutations (P < 0.001 and P < 0.001, respectively), consistent with enhanced oxidative stress, whereas there was no significant main effect of diet (Figure 5). These data indicated that dietary iron-loading did not induce oxidative damage in hippocampus (Figure 5).
FIGURE 5.
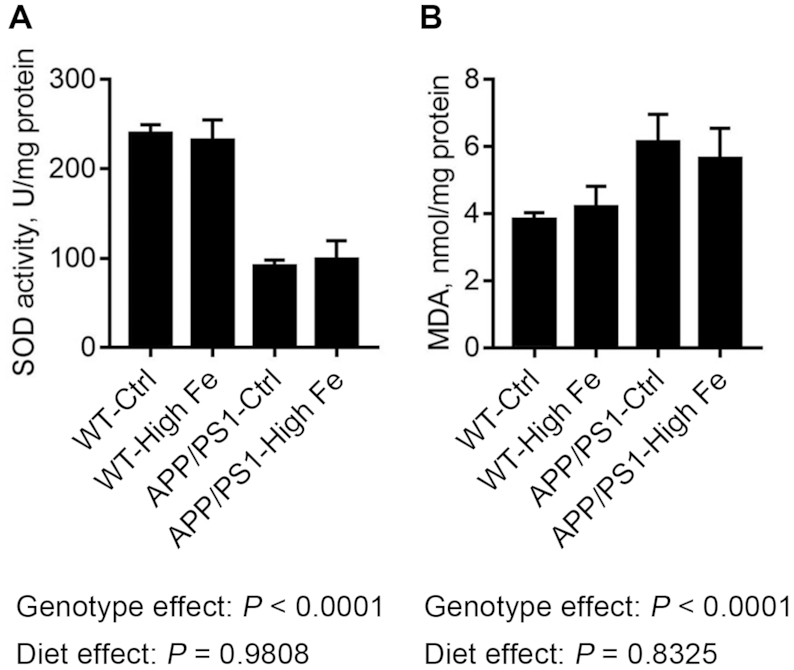
SOD activity (A) and MDA concentration (B) in the hippocampus of male WT and APP/PS1 mice fed a control or high iron diet at age 30 wk. Values are means ± SDs, n = 7. APP/PS1, amyloid precursor protein/presenilin 1; APP/PS1-Ctrl, APP/PS1 mice fed a control diet; APP/PS1-High Fe, APP/PS1 mice fed a high iron diet; MDA, malondialdehyde; SOD, superoxide dismutase; WT, wild-type; WT-Ctrl, wild-type mice fed a control diet; WT-High Fe, wild-type mice fed a high iron diet.
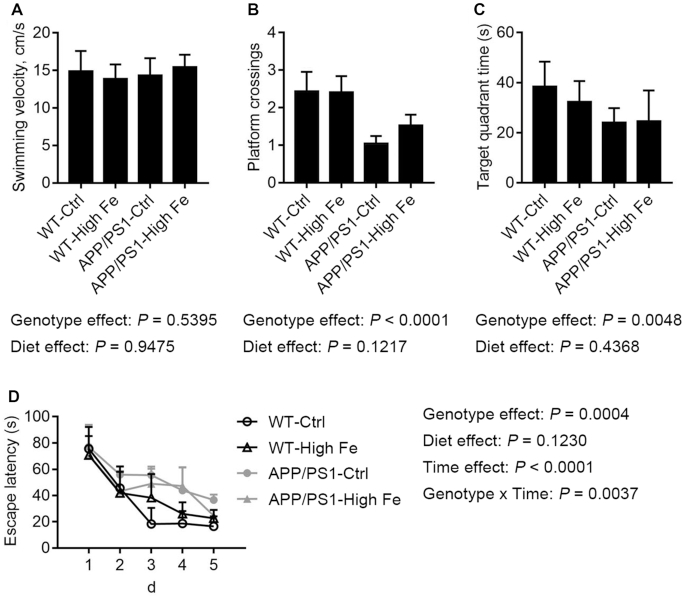
Dietary iron-loading did not alter learning and memory in WT or APP/PS1 mice
The hippocampal function of the 4 groups of mice was tested when the animals were aged 28 wk. The swimming velocity of those mice was not significantly affected by genotype or iron status (Figure 6A). After training for 4 d, mice with APP/PS1 mutations showed deficits of spatial learning and memory, as they crossed the center of the target quadrant fewer times (P < 0.0001) and spent less time in that quadrant (P = 0.0048) compared with WT mice. However, a high iron diet had no significant effect on the behavior of mice (Figure 6B, C). During the 5-d experiment, we noted that all mice improved in their ability to find the hidden platform. However, APP/PS1 mutations in mice induced significantly longer escape latency (P = 0.0004), whereas dietary iron did not have a significant impact (P = 0.1230) (Figure 6D).
FIGURE 6.
Swimming velocity (A), platform crossings (B), and time spent in the target quadrant (C) were recorded on day 5, the day a probe test was conducted, and escape latency was recorded from day 1 to day 5 (D) in male WT and APP/PS1 mice fed a control or high iron diet at age 28 wk. Values are means ± SDs, n = 7. APP/PS1, amyloid precursor protein/presenilin 1; APP/PS1-Ctrl, APP/PS1 mice fed a control diet; APP/PS1-High Fe, APP/PS1 mice fed a high iron diet; WT, wild-type; WT-Ctrl, wild-type mice fed a control diet; WT-High Fe, wild-type mice fed a high iron diet.
Discussion
Consistent with previous reports, we found that mice fed a high iron diet had significantly lower body weight and higher plasma and liver iron concentrations than mice fed a control diet (19–21). However, iron concentrations in cerebral cortex and cerebellum were not significantly affected by dietary iron, in agreement with our previous observations that 12-mo-old WT mice fed an iron-overload diet for 6 mo had significant iron accumulation in a range of organs, but not the brain (21). This may reflect the tight regulation of iron entry into the brain iron by the BBB, which varies brain iron uptake by modulating expression of the transferrin receptor on the luminal surface of vascular endothelial cells (22). Nevertheless, the iron concentration in the hippocampus was significantly higher in APP/PS1-High Fe mice than in APP/PS1-Ctrl mice, and ferritin protein expression in hippocampus was significantly increased in both strains by iron-loading. These data indicated that the high dietary iron did affect the brain iron homeostasis in mice, particularly in APP/PS1 mice. In addition, APP/PS1-Ctrl mice had significantly higher iron concentration and ferritin protein expression in hippocampus than did WT-Ctrl mice, and both iron deposition and Aβ plaques aggregated in the DG region of the hippocampus in APP/PS1 mice. These data are consistent with the previous proposal that iron is recruited by Aβ plaques in APP/PS1 mice (23, 24).
Although APP/PS1-High Fe mice had significantly higher iron concentrations in the hippocampus than APP/PS1-Ctrl mice, there was no significant main effect of diet on oxidative stress, as indicated by SOD activity and malondialdehyde concentration measurements. These results suggested that although high dietary iron could disrupt brain iron homeostasis, the extra iron might be stored in ferritin and hence did not induce oxidative damage. In contrast, the iron sequestered by the Aβ plaque is labile and can have detrimental effects (25, 26).
Consistent with the oxidative stress data, we showed that dietary iron overload did not lead to cognitive dysfunction. One previous study found that weanling rats fed a diet containing 3.5 g/kg iron for 12 wk showed a normal amount of iron in the brain and had few behavioral problems, whereas rats fed an extremely high iron diet (20 g/kg) showed brain iron overload and impaired brain function (27). This study implies that there might be a safe limit for the amount of iron in the diet. Below a certain threshold, no or few adverse events occur, but once the threshold is exceeded, the BBB will be damaged, brain iron homeostasis will be disrupted, and brain function will be impaired. In our study, it seems likely that 14 g/kg of dietary iron is still under the threshold, and thus WT-High Fe mice did not present with significant brain iron alterations, hippocampal oxidative stress, or behavioral changes. A further study showed that weanling rats fed a high iron diet (10 g/kg) for 1 mo had a better memory (28). Mechanistically, it is possible that iron improves long-term potentiation in the hippocampus by regulating the cholinergic and glutamatergic neurotransmission pathways (28, 29). These studies may help to explain why iron treatment did not exacerbate the cognitive impairment in APP/PS1 mice. However, because most APP/PS1 mice succumb to the Aβ toxicity before age 12 mo, it remains unknown whether the high dietary iron would have a negative effect on cognitive function at an older age.
Increased iron is known to stimulate APP expression via the iron-responsive protein/iron-regulatory element system and to enhance the amyloidogenic processing of APP (30, 31). Consistent with this, in our study, mice fed a high iron diet had greater Aβ1–42 protein expression in the hippocampus relative to those fed a control diet. Iron treatment also led to significantly greater p-τ protein expression in both WT and APP/PS1 mice in our studies, and this is in agreement with earlier work (32, 33). These data show that although high dietary iron promotes the expression of Aβ and p-τ, these changes are not sufficient to lead to cognitive impairment in mice at age 30 wk.
In conclusion, our work provides a comprehensive comparison on the impact of dietary iron overload on adult WT and APP/PS1 mice. We show that mice fed an iron-overload diet exhibited higher amounts of Aβ and τ hyperphosphorylation in the hippocampus. However, they did not present increased oxidative stress or impaired cognitive function before age 30 wk. It should be noted that the dose of iron in the iron-loaded diet is 200-fold higher than that of the control diet, and humans could by no means be supplemented with iron of such a high dose. Therefore, this study does not suggest that adults with, or at risk of AD, should avoid iron supplements. Instead, this study implies that iron supplements may be relatively safe to humans, as mice supplemented with iron of such a high dose did not present behavioral changes. Nevertheless, considering iron overload may have long-term effects [ceruloplasmin null mice presented motor deficits at 16 mo (34)], future studies should investigate the impact of dietary iron overload on brain function at an older age (e.g., 24-mo-old mice).
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—HC: designed the research, wrote, and edited the manuscript; MC and JZ: performed the experiments, analyzed the data, prepared the figures, and wrote the manuscript; GL, CZ, EX, and WZ: performed the experiments; GJA: wrote and edited the manuscript; and all authors: read and approved the final manuscript.
Notes
MC and JZ contributed equally to the paper.
Supported by the National Natural Science Foundation of China grants (81773417 and 81273068) to H. Chen.
Author disclosures: MC, JZ, GL, CZ, EX, WZ, GJA, and HC, no conflicts of interest.
Supplemental Figures 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: Aβ, amyloid-β; AD, Alzheimer disease; APP, amyloid precursor protein; APP/PS1-Ctrl, APP/PS1 mice fed a control diet; APP/PS1-High Fe, APP/PS1 mice fed a high iron diet; BBB, blood-brain barrier; DG, dentate gyrus; PS1, presenilin 1; p-τ, phospho-τ; SOD, superoxide dismutase; WT, wild-type; WT-Ctrl, wild-type mice fed a control diet; WT-High Fe, wild-type mice fed a high iron diet.
References
- 1. Rouault TA. Iron metabolism in the CNS: implications for neurodegenerative diseases. Nat Rev Neurosci. 2013;14:551–64. [DOI] [PubMed] [Google Scholar]
- 2. Anderson GJ. Mechanisms of iron loading and toxicity. Am J Hematol. 2007;82:1128–31. [DOI] [PubMed] [Google Scholar]
- 3. Frazer DM, Anderson GJ. Iron imports. I. Intestinal iron absorption and its regulation. Am J Physiol Gastrointest Liver Physiol. 2005;289:G631–5. [DOI] [PubMed] [Google Scholar]
- 4. Zhu WZ, Zhong WD, Wang W, Zhan CJ, Wang CY, Qi JP, Wang JZ, Lei T. Quantitative MR phase-corrected imaging to investigate increased brain iron deposition of patients with Alzheimer disease. Radiology. 2009;253:497–504. [DOI] [PubMed] [Google Scholar]
- 5. Mandel S, Amit T, Bar-Am O, Youdim MB. Iron dysregulation in Alzheimer's disease: multimodal brain permeable iron chelating drugs, possessing neuroprotective-neurorescue and amyloid precursor protein-processing regulatory activities as therapeutic agents. Prog Neurobiol. 2007;82:348–60. [DOI] [PubMed] [Google Scholar]
- 6. Rao SS, Adlard PA. Untangling tau and iron: exploring the interaction between iron and tau in neurodegeneration. Front Mol Neurosci. 2018;11:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ayton S, Fazlollahi A, Bourgeat P, Raniga P, Ng A, Lim YY, Diouf I, Farquharson S, Fripp J, Ames D et al.. Cerebral quantitative susceptibility mapping predicts amyloid-beta-related cognitive decline. Brain. 2017;140:2112–9. [DOI] [PubMed] [Google Scholar]
- 8. Zheng J, Jiang R, Chen M, Maimaitiming Z, Wang J, Anderson GJ, Vulpe CD, Dunaief JL, Chen H. Multi-copper ferroxidase-deficient mice have increased brain iron concentrations and learning and memory deficits. J Nutr. 2018;148:643–9. [DOI] [PubMed] [Google Scholar]
- 9. Zhao L, Hadziahmetovic M, Wang C, Xu X, Song Y, Jinnah HA, Wodzinska J, Iacovelli J, Wolkow N, Krajacic P et al.. Cp/Heph mutant mice have iron-induced neurodegeneration diminished by deferiprone. J Neurochem. 2015;135:958–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Holcomb L, Gordon MN, McGowan E, Yu X, Benkovic S, Jantzen P, Wright K, Saad I, Mueller R, Morgan D et al.. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med. 1998;4:97–100. [DOI] [PubMed] [Google Scholar]
- 11. Reiserer RS, Harrison FE, Syverud DC, McDonald MP. Impaired spatial learning in the APPSwe + PSEN1DeltaE9 bigenic mouse model of Alzheimer's disease. Genes Brain Behav. 2007;6:54–65. [DOI] [PubMed] [Google Scholar]
- 12. Jankowsky JL, Slunt HH, Ratovitski T, Jenkins NA, Copeland NG, Borchelt DR. Co-expression of multiple transgenes in mouse CNS: a comparison of strategies. Biomol Eng. 2001;17:157–65. [DOI] [PubMed] [Google Scholar]
- 13. Reeves PG, Nielsen FH, Fahey GC Jr.. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–51. [DOI] [PubMed] [Google Scholar]
- 14. Chen H, Su T, Attieh ZK, Fox TC, McKie AT, Anderson GJ, Vulpe CD. Systemic regulation of Hephaestin and Ireg1 revealed in studies of genetic and nutritional iron deficiency. Blood. 2003;102:1893–9. [DOI] [PubMed] [Google Scholar]
- 15. Fuqua BK, Lu Y, Darshan D, Frazer DM, Wilkins SJ, Wolkow N, Bell AG, Hsu J, Yu CC, Chen H et al.. The multicopper ferroxidase hephaestin enhances intestinal iron absorption in mice. PLoS One. 2014;9:e98792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen M, Zheng J, Liu G, Xu E, Wang J, Fuqua BK, Vulpe CD, Anderson GJ, Chen H. Ceruloplasmin and hephaestin jointly protect the exocrine pancreas against oxidative damage by facilitating iron efflux. Redox Biol. 2018;17:432–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jiang R, Hua C, Wan Y, Jiang B, Hu H, Zheng J, Fuqua BK, Dunaief JL, Anderson GJ, David S et al.. Hephaestin and ceruloplasmin play distinct but interrelated roles in iron homeostasis in mouse brain. J Nutr. 2015;145:1003–9. [DOI] [PubMed] [Google Scholar]
- 18. Konopka W, Kiryk A, Novak M, Herwerth M, Parkitna JR, Wawrzyniak M, Kowarsch A, Michaluk P, Dzwonek J, Arnsperger T et al.. MicroRNA loss enhances learning and memory in mice. J Neurosci. 2010;30:14835–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sripetchwandee J, Pipatpiboon N, Chattipakorn N, Chattipakorn S. Combined therapy of iron chelator and antioxidant completely restores brain dysfunction induced by iron toxicity. PLoS One. 2014;9:e85115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guo C, Wang T, Zheng W, Shan ZY, Teng WP, Wang ZY. Intranasal deferoxamine reverses iron-induced memory deficits and inhibits amyloidogenic APP processing in a transgenic mouse model of Alzheimer's disease. Neurobiol Aging. 2013;34:562–75. [DOI] [PubMed] [Google Scholar]
- 21. Chen H, Attieh ZK, Gao H, Huang G, Su T, Ke W, Vulpe CD. Age-related changes in iron homeostasis in mouse ferroxidase mutants. Biometals. 2009;22:827–34. [DOI] [PubMed] [Google Scholar]
- 22. Rouault TA, Cooperman S. Brain iron metabolism. Semin Pediatr Neurol. 2006;13:142–8. [DOI] [PubMed] [Google Scholar]
- 23. El Tannir El Tayara N, Delatour B, Le Cudennec C, Guegan M, Volk A, Dhenain M. Age-related evolution of amyloid burden, iron load, and MR relaxation times in a transgenic mouse model of Alzheimer's disease. Neurobiol Dis. 2006;22:199–208. [DOI] [PubMed] [Google Scholar]
- 24. Falangola MF, Lee SP, Nixon RA, Duff K, Helpern JA. Histological co-localization of iron in Abeta plaques of PS/APP transgenic mice. Neurochem Res. 2005;30:201–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jiang D, Li X, Williams R, Patel S, Men L, Wang Y, Zhou F. Ternary complexes of iron, amyloid-beta, and nitrilotriacetic acid: binding affinities, redox properties, and relevance to iron-induced oxidative stress in Alzheimer's disease. Biochemistry. 2009;48:7939–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Khan A, Dobson JP, Exley C. Redox cycling of iron by Abeta42. Free Radic Biol Med. 2006;40:557–69. [DOI] [PubMed] [Google Scholar]
- 27. Sobotka TJ, Whittaker P, Sobotka JM, Brodie RE, Quander DY, Robl M, Bryant M, Barton CN. Neurobehavioral dysfunctions associated with dietary iron overload. Physiol Behav. 1996;59:213–9. [DOI] [PubMed] [Google Scholar]
- 28. Han M, Kim J. Effect of dietary iron loading on recognition memory in growing rats. PLoS One. 2015;10:e0120609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Munoz P, Humeres A, Elgueta C, Kirkwood A, Hidalgo C, Nunez MT. Iron mediates N-methyl-d-aspartate receptor-dependent stimulation of calcium-induced pathways and hippocampal synaptic plasticity. J Biol Chem. 2011;286:13382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wong BX, Duce JA. The iron regulatory capability of the major protein participants in prevalent neurodegenerative disorders. Front Pharmacol. 2014;5:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rogers JT, Randall JD, Cahill CM, Eder PS, Huang X, Gunshin H, Leiter L, McPhee J, Sarang SS, Utsuki T et al.. An iron-responsive element type II in the 5′-untranslated region of the Alzheimer's amyloid precursor protein transcript. J Biol Chem. 2002;277:45518–28. [DOI] [PubMed] [Google Scholar]
- 32. Sripetchwandee J, Wongjaikam S, Krintratun W, Chattipakorn N, Chattipakorn SC. A combination of an iron chelator with an antioxidant effectively diminishes the dendritic loss, tau-hyperphosphorylation, amyloids-beta accumulation and brain mitochondrial dynamic disruption in rats with chronic iron-overload. Neuroscience. 2016;332:191–202. [DOI] [PubMed] [Google Scholar]
- 33. Guo C, Wang P, Zhong ML, Wang T, Huang XS, Li JY, Wang ZY. Deferoxamine inhibits iron induced hippocampal tau phosphorylation in the Alzheimer transgenic mouse brain. Neurochem Int. 2013;62:165–72. [DOI] [PubMed] [Google Scholar]
- 34. Patel BN, Dunn RJ, Jeong SY, Zhu Q, Julien JP, David S. Ceruloplasmin regulates iron levels in the CNS and prevents free radical injury. J Neurosci. 2002;22:6578–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.