Abstract
Background
Bavituximab is a monoclonal antibody that targets phosphatidylserine in the presence of β2 glycoprotein 1 (β2GP1) to exert an antitumor immune response. This phase III trial determined the efficacy of bavituximab combined with docetaxel in patients with previously treated advanced non-small-cell lung cancer (NSCLC).
Patients and methods
Key eligibility criteria included advanced non-squamous NSCLC with disease progression after treatment with platinum-based doublet chemotherapy, evidence of disease control after at least two cycles of first-line therapy, presence of measurable disease, ECOG performance status 0 or 1, adequate bone marrow and organ function, and no recent history of clinically significant bleeding. Eligible patients were randomized 1 : 1 to receive up to six 21-day cycles of docetaxel plus either weekly bavituximab 3 mg/kg or placebo until progression or toxicity. The primary end point was overall survival (OS).
Results
A total of 597 patients were enrolled. Median OS was 10.5 months in the docetaxel + bavituximab arm and was 10.9 months in the docetaxel + placebo arm (HR 1.06; 95% CI 0.88–1.29; P = 0.533). There was no difference in progression-free survival (HR 1.00; 95% CI 0.82–1.22; P = 0.990). Toxicities were manageable and similar between arms. In subset analysis, among patients with high baseline serum β2GP1 levels ≥200 µg/ml, a nonsignificant OS trend favored the bavituximab arm (HR 0.82; 95% CI 0.63–1.06; P = 0.134). Among patients who received post-study immune checkpoint inhibitor therapy, OS favored the bavituximab arm (HR 0.46; 95% CI 0.26–0.81; P = 0.006).
Conclusions
The combination of bavituximab plus docetaxel is not superior to docetaxel in patients with previously treated advanced NSCLC. The addition of bavituximab to docetaxel does not meaningfully increase toxicity. The potential benefit of bavituximab observed in patients with high β2GP1 levels and in patients subsequently treated with immune checkpoint inhibitors requires further investigation.
Clinical trial number
Keywords: antibody, chemotherapy, immunotherapy, phosphatidylserine, vascular
Key Message
Bavituximab is a monoclonal antibody that targets phosphatidylserine in the presence of β2 glycoprotein 1 (β2GP1) to exert antitumor immune effects. In this phase III trial, the addition of bavituximab to docetaxel did not improve OS in previously treated advanced NSCLC. Subset analyses suggested possible benefit in high β2GP1 cases and those subsequently receiving immune checkpoint inhibitors.
Introduction
Bavituximab is an unconjugated, chimeric IgG1 monoclonal antibody that localizes to anionic phospholipids expressed on vascular endothelium. In most tissues, the principal target of bavituximab, the immunosuppressive molecule phosphatidylserine (PS), is restricted to the internal surface of the cell membrane [1, 2]. Various pathophysiologic processes can disrupt this asymmetry, resulting in exposure of PS on the outer membrane leaflet, where it is available for targeting by bavituximab, which forms a complex with PS and β2 glycoprotein 1 (β2GP1) [3].Within solid tumors, hypoxia and other physiologic stresses induce PS exposure, rendering bavituximab targeting tumor-specific [4].
Within the increasingly crowded field of cancer immunotherapeutic agents, bavituximab has distinct physiologic effects. Bavituximab repolarizes myeloid derived suppressor cells and M2 macrophages to M1, resulting in production of pro-inflammatory cytokines, dendritic cell maturation, and induction of tumor-specific cytotoxic T-lymphocyte immunity [5]. These effects appear largely limited to the tumor microenvironment, reflecting bavituximab’s unique mechanism of action and potentially accounting for its favorable safety profile.
In preclinical models, bavituximab inhibits tumor growth, prolongs survival, and enhances efficacy of chemotherapy and radiation [6–8]. This synergy may reflect increased intratumoral PS exposure following administration of cytotoxic therapies, thereby enhancing bavituximab targeting [6, 7]. PS-targeting antibodies have also demonstrated enhanced immune activation and down-regulation of pro-oncogenic factors induced by T-cell checkpoint inhibition, thereby augmenting the activity of antiprogrammed death-1 (PD-1) therapy [9]. In a phase I trial, bavituximab 3 mg/kg weekly was determined to be the optimal biologic dose [10]. In single-arm combination studies with cytotoxic chemotherapy, the addition of bavituximab did not increase the risk of adverse events [11, 12]. In a randomized phase II trial in previously treated advanced non-small-cell lung cancer (NSCLC), the addition of bavituximab to docetaxel demonstrated a trend toward improved survival (HR 0.66; 95% CI 0.40–1.1; P = 0.11) [13]. We therefore conducted Stimulating an Immune Response Through Bavituximab in a Phase III Lung Cancer Study (SUNRISE), an international phase III randomized study of docetaxel with or without bavituximab.
Methods
Study design
SUNRISE (NCT01999673) was a prospective, randomized, double-blind, placebo-controlled, multicenter, phase III study. The primary end point was overall survival (OS). Secondary end points included progression-free survival (PFS), objective response rate (ORR), and safety. Patients were randomized 1 : 1 to receive docetaxel plus bavituximab or docetaxel plus placebo. Stratification factors included geographic region, disease stage (IIIb or IV), and previous maintenance/targeted therapy. The study was conducted according to the Declaration of Helsinki and with approval from Institutional Review Boards of all participating sites. All participants provided written informed consent before any study-related procedures.
Participants
Eligible subjects had histologically or cytologically confirmed advanced stage non-squamous NSCLC with disease progression after treatment with platinum-based doublet chemotherapy. Additionally, patients needed to have evidence of disease control after at least two cycles of first-line therapy. This requirement was based on the earlier observation that potential benefit of docetaxel combinations may be heightened in patients who previously achieved some period of disease control [14]. Prior docetaxel was permitted if completed ≥6 months before study treatment initiation. Prior bevacizumab was allowed. Patients with known EGFR mutations or ALK rearrangements must have progressed after (or not tolerated) appropriate targeted therapy, as well as platinum-based chemotherapy. Additional eligibility criteria included the presence of measurable disease by RECIST 1.1; ECOG performance status 0 or 1; and adequate bone marrow, renal and hepatic function, and coagulation parameters. Patients were excluded if they had history of bleeding diathesis or coagulopathy, cavitary tumors or tumors abutting large blood vessels, clinically significant bleeding or symptomatic cardiac or cerebrovascular disease within 6 months, ≥grade 2 peripheral neuropathy, or unstable brain metastases.
Procedures
Patients received docetaxel 75 mg/m2 i.v. on day 1 of a 21-day cycle plus blinded study treatment (either placebo or bavituximab 3 mg/kg i.v. weekly) for up to 6 cycles. The duration of docetaxel chemotherapy was limited to 6 cycles based on the following: (i) the time-course of chemotherapy-induced PS induction in preclinical models [6]; (ii) observed cases of disease control with bavituximab monotherapy [10]; and (iii) comparable duration of disease control between docetaxel trials with and without cycle limits [8, 13, 15]. With docetaxel, patients received steroids per institutional practice (recommended regimen dexamethasone 8 mg twice daily on day before, day of, and day after). Before blinded study treatment, patients received steroid and antihistamine premedication (recommended regimen hydrocortisone 250 mg i.v. and diphenhydramine 50 mg i.v.). Patients who completed six cycles of combination therapy without disease progression or unacceptable toxicity continued to receive either placebo or bavituximab 3 mg/kg weekly until progression or toxicity. Docetaxel dose reductions were carried out for grade 4 neutropenia or thrombocytopenia, and grade ≥3 non-hematologic toxicities. There were no dose reductions of blinded treatment. Patients who discontinued either docetaxel or blinded study treatment of toxicities attributed to a specific agent could continue the other treatment.
Outcomes
Adverse events were classified using the Common Terminology Criteria for Adverse Events Version 4.02. Tumor response was assessed radiographically every two cycles during combination treatment and every three cycles during the maintenance monotherapy period. All patients were followed for response until progression and for OS.
Based on bavituximab mechanism of action, subgroup analysis by patient β2GP1 level was conducted. β2GP1 levels were determined from pretreatment blood samples using enzyme-linked immunosorbent assay (ELISA). For the ELISA, PS was coated on a plate, a solution containing horseradish peroxidase-conjugated bavituximab was added, followed by the addition of the clinical sample. After a 90-min incubation at 37°C, the plate was washed and 3,3′,5,5′-tetramethylbenzidine peroxidase substrate was added. The response was stopped by the addition of an acid solution resulting in yellow color that absorbs at 450 nm wavelength. Color intensity was proportional to the amount of functional β2GP1 present in the sample. Patient β2GP1 levels were categorized as above or below the study population median value.
Statistical analysis
The primary end point of OS was quantified using Kaplan–Meier estimates of the survival functions within each treatment group. Cox proportional hazard models were fit overall and to subsets of survival data as sensitivity analyses. Sample size was derived using SAS Proc SEQDESIGN (SAS Corporation, Cary, NC). For a one-sided log-rank test at an overall α = 0.025 significance level, 473 OS events provided 80% power to detect a 23% difference in survival (median 9.1 months in docetaxel plus bavituximab arm versus 7.0 months in the docetaxel plus placebo arm; HR 0.77). Interim analyses for futility and superiority at 33% and 50% of targeted OS events were planned using Lan–DeMets spending function boundaries with O’Brien Fleming shape. Assuming patient accrual over 24 months, a study duration of 36 months, and a 10% dropout rate, the total sample size was 582 patients.
Results
A total of 597 patients were enrolled and randomized between 30 December 2013 and 29 January 2016. Of these, 596 received study treatment (see supplementary Figure S1, available at Annals of Oncology online). At the first planned interim analysis, the independent data monitoring committee recommended that recruitment be stopped due to futility, which coincided with completion of planned enrollment. Patients enrolled on to the study were unblinded and permitted to continue docetaxel. Placebo was discontinued. In cases where investigators and patients felt that it was in the best interest of the patient, continuation of bavituximab was permitted.
Baseline characteristics were well balanced across arms and are listed in Table 1. Treatment exposure was similar between both groups. Mean number of docetaxel doses was 4.1 (range 1–6) in the docetaxel + bavituximab arm, and 4.2 (range 1–6) in the docetaxel + placebo arm. Mean number of blinded study treatment doses was 16.1 (range 1–130) in the docetaxel + bavituximab arm, and 15.3 (range 1–77) in the docetaxel + placebo arm.
Table 1.
Baseline characteristics
| Placebo + docetaxel | Bavituximab + docetaxel | |
|---|---|---|
| n = 300 | n = 297 | |
| N (%) or median (range) | N (%) or median (range) | |
| Age, years | 62 (30–82) | 63 (37–85) |
| Sex | ||
| Male | 182 (61) | 177 (60) |
| Female | 118 (39) | 120 (40) |
| Race | ||
| White | 240 (80) | 244 (82) |
| Black or African American | 3 (1) | 5 (2) |
| Asian | 49 (16) | 43 (14) |
| Not reported | 8 (3) | 5 (2) |
| Disease stage | ||
| Stage IIIb | 16 (5) | 16 (5) |
| Stage IV | 284 (95) | 281 (95) |
| Smoking history | ||
| Current/former | 226 (75) | 234 (79) |
| Never | 68 (23) | 56 (19) |
| Missing | 6 (2) | 7 (2) |
| Genomic alteration | ||
| EGFR | 24 (8) | 35 (12) |
| ALK | 6 (2) | 5 (2) |
| Neither | 191 (64) | 174 (58) |
| Unknown | 79 (26) | 83 (28) |
| Performance status | ||
| 0 | 86 (29) | 95 (32) |
| 1 | 209 (70) | 197 (66) |
| ≥2 | 4 (1) | 3 (1) |
| Missing | 1 (0) | 2 (1) |
| Prior therapy | ||
| Maintenance and/or targeted therapy | 170 (57) | 168 (57) |
| Immunotherapy | 12 (4) | 5 (2) |
Post-study treatment was received by 53% of patients in the docetaxel + bavituximab arm and by 58% of patients in the docetaxel + placebo arm. Immediate post-study treatment in the docetaxel + bavituximab arm included chemotherapy (24%), targeted therapy (12%), and immunotherapy (16%). In the docetaxel + placebo arm, immediate post-study treatment included chemotherapy (28%), targeted therapy (13%), and immunotherapy (16%).
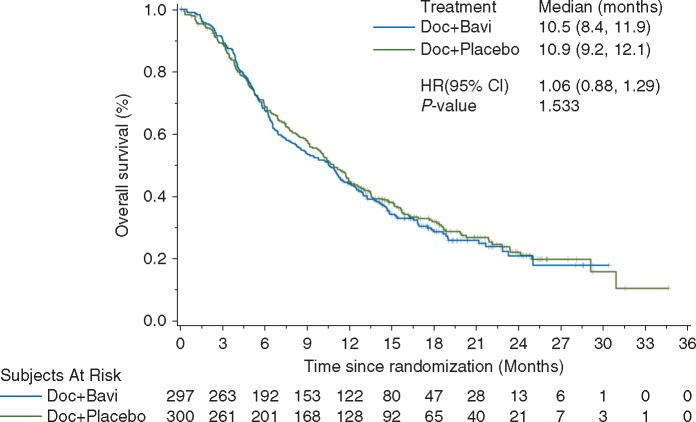
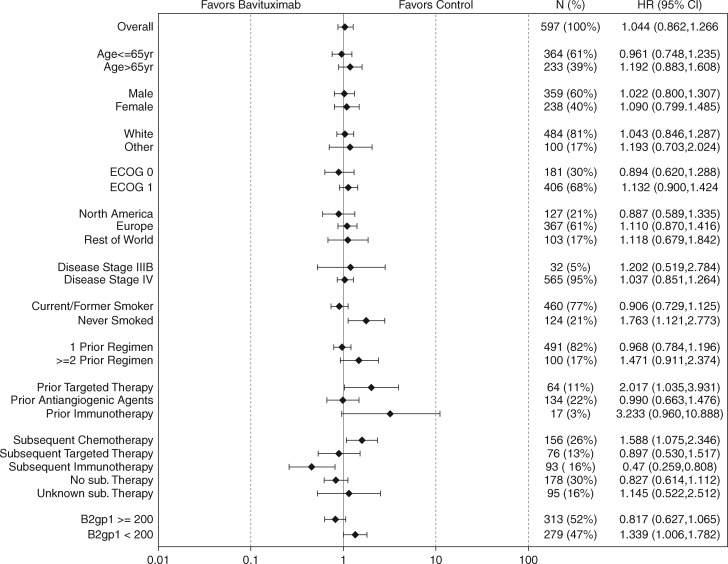
Median OS was 10.5 months in the docetaxel + bavituximab arm and 10.9 months in the docetaxel + placebo arm (HR 1.06; 95% CI 0.88–1.29; P = 0.533; Figure 1). There was no difference in PFS (HR 1.00; 95% CI 0.82–1.22; P = 0.990). ORR was 14% in the docetaxel + bavituximab arm and 11% in the docetaxel + placebo arm (P = 0.18). In the subset of patients with pretreatment serum β2GP1 levels ≥200 µg/ml (the median value in the study population), OS trend favored the bavituximab arm (HR 0.82; 95% CI 0.63–1.06; P = 0.134; supplementary Figure S2, available at Annals of Oncology online). Efficacy according to patient subgroups is shown in Figure 2. In a post hoc analysis, OS favored the bavituximab arm among patients who received immune checkpoint inhibitor therapy after study therapy (n = 93, 16%): median NR versus 12.6 months; HR 0.46; 95% CI 0.26–0.81; P = 0.006; supplementary Figure S3, available at Annals of Oncology online).
Figure 1.
Overall survival in the intent-to-treat population.
Figure 2.
Overall survival according to subgroup analyses.
In the docetaxel + bavituximab arm, 97% of patients experienced adverse events (68% grade ≥3). In the docetaxel + placebo arm, 94% of patients experienced adverse events (60% grade ≥3). Treatment-emergent adverse events occurring in ≥15% of patients are listed in Table 2. Adverse events leading to docetaxel discontinuation occurred in 6% of patients in the docetaxel + bavituximab arm and in 7% of patients in the docetaxel + placebo arm. Adverse events leading to blinded study treatment discontinuation occurred in 13% of patients in the docetaxel + bavituximab arm and in 8% of patients in the docetaxel + placebo arm. Vascular-related toxicities, which represent a hypothetical concern with a PS-targeting therapy, occurred at low and similar rates in both arms: 19% (4% grade ≥3) of patients in the docetaxel + bavituximab arm and 21% (6% grade ≥3) of patients in the docetaxel + placebo arm. There was no apparent difference in rates of characteristic immune-related adverse events: 5% (1% grade ≥3) in the docetaxel + bavituximab arm and 4% (1% grade ≥3) in the docetaxel + placebo arm.
Table 2.
Adverse events reported in ≥15% of patients
| Placebo + docetaxel | Bavituximab + docetaxel | Total | |||
|---|---|---|---|---|---|
|
n = 299 |
n = 297 |
n = 596 | |||
| Any grade | Grade 3–4 | Any Grade | Grade 3–4 | Any grade | |
| Total patients with an event | 94 | 55 | 97 | 64 | 96 |
| Fatigue | 89 (30) | 12 (4) | 107 (36) | 15 (5) | 196 (33) |
| Alopecia | 92 (31) | 0 (0) | 96 (32) | 0 (0) | 188 (32) |
| Diarrhea | 85 (28) | 6 (2) | 98 (33) | 9 (3) | 183 (31) |
| Nausea | 70 (23) | 0 (0) | 95 (32) | 7 (2) | 165 (28) |
| Neutropenia | 78 (26) | 66 (22) | 79 (27) | 68 (23) | 157 (26) |
| Decreased appetite | 66 (22) | 1 (0) | 85 (29) | 6 (2) | 151 (25) |
| Anemia | 62 (21) | 10 (3) | 79 (27) | 14 (5) | 141 (24) |
| Asthenia | 72 (24) | 7 (2) | 64 (22) | 16 (5) | 136 (23) |
| Dyspnea | 61 (20) | 11 (4) | 72 (24) | 11 (4) | 133 (22) |
| Cough | 60 (20) | 1 (0) | 57 (19) | 2 (1) | 117 (20) |
| Constipation | 44 (15) | 0 (0) | 51 (17) | 0 (0) | 95 (16) |
| Vomiting | 32 (11) | 0 (0) | 57 (19) | 6 (2) | 89 (15) |
Discussion
This randomized phase III trial evaluated the efficacy of combining the immune-modulating monoclonal antibody bavituximab with docetaxel in previously treated advanced non-squamous NSCLC. The trial met criteria for early stopping due to futility. In the overall population, the addition of bavituximab to docetaxel did not improve OS, PFS, or RR. Notably, the median survival of ∼11 months in both arms exceeded that of docetaxel monotherapy in earlier trials [15, 16], which may reflect the clinical benefits of recent therapeutic advances such as immune checkpoint inhibitors. Alternatively, this observation could be due to the eligibility requirement for stable disease or better as best response to first-line platinum-doublet therapy.
Post hoc subset analysis according to β2GP1 levels demonstrated that the addition of bavituximab to docetaxel may provide benefit to those patients with higher β2GP1 levels, suggesting that this novel antibody may have efficacy in cases achieving greatest target inhibition. β2GP1, which is required for bavituximab binding to PS, is a plasma protein that functions in normal physiology as an inhibitor of contact activation of the intrinsic coagulation pathway. Although anti-β2GP1 antibodies represent a serologic criterion for antiphospholipid syndrome (an autoimmune coagulation disorder featuring arterial and venous thrombosis), anti-β2GP1 antibodies are also detected in a substantial proportion of the healthy population [17]. Unfortunately, PS characterization by standard immunohistochemical techniques—which do not adequately distinguish between protein expression on the inner versus outer cell membrane leaflet—does not serve as a meaningful predictive biomarker for bavituximab. With elevated serum β2GP1 levels among the most promising bavituximab biomarkers identified to date, further studies in this population may be warranted.
Among the subgroup of patients who received immune checkpoint inhibitor therapy post-study, receipt of bavituximab was associated with improved OS (HR 0.46). Although mechanism of action and preclinical data suggest that bavituximab may enhance the efficacy of checkpoint inhibitors, this post hoc clinical observation in a relatively small subset is subject to selection bias and can only be considered hypothesis-generating for future trials.
Conversely, certain post hoc sub-group analyses suggested the possibility of inferior outcomes when bavituximab is added to docetaxel. Both never smokers (21% of study population) and patients previously treated with molecularly targeted therapies (11% of study population) had worse survival in the docetaxel + bavituximab arm. Although there is no clear explanation for this finding, it is consistent with earlier observations that immunotherapy has either minimal efficacy or the potential for causing hyper-progression in patients with tumors harboring druggable genomic alterations such as EGFR mutations [18, 19]. Similarly, the trend toward worse outcomes in more heavily pre-treated patients lacks an apparent explanation but mirrors trends seen in other immunotherapy trials [18].
The co-administration of docetaxel and bavituximab was tolerated, with a safety profile similar to that expected with docetaxel monotherapy [8, 15]. Despite hypothetical concerns, the addition of bavituximab to docetaxel did not increase rates of vascular or characteristic immune-related toxicities. One potential explanation for this differential safety profile is distribution of therapeutic targets. While anti-CTLA4 and anti-PD1/PDL1 therapies have molecular targets within the host immune system, bavituximab has an intratumoral molecular target. Given the low rate of infusion reactions in this study and experience from earlier clinical trials in which bavituximab monotherapy was administered without premedication [10], it may be possible to eliminate steroids in future bavituximab trials. Theoretically, such a modification might enhance the antitumor immune effects of the drug.
In conclusion, the addition of bavituximab to docetaxel chemotherapy does not improve OS or other efficacy end points in an unselected patient population with previously treated non-squamous NSCLC. The combination was well tolerated. Serum levels of β2GP1, a glycoprotein required for bavituximab targeting of PS, may identify a subset of patients most likely to benefit from the addition of bavituximab to docetaxel. Additionally, the potential benefit of prior bavituximab exposure among patients subsequently treated with immune checkpoint inhibitors, along with the absence of overt immune-related adverse events observed in this trial, provide a rationale for considering future studies combining bavituximab with checkpoint inhibitor immunotherapy. Ideally, the impact of bavituximab on tumor microenvironment observed in preclinical models (repolarization of myeloid derived suppressor cells and M2 macrophages [5]) will be incorporated into trial design and biomarker selection.
Supplementary Material
Acknowledgements
The authors thank Ms Dru Gray for assistance with manuscript preparation.
Funding
This study was funded by Peregrine Pharmaceuticals. This work was also supported by a National Cancer Institute (NCI) Midcareer Investigator Award in Patient-Oriented Research (K24CA201543-01 to DEG).
Disclosure
DEG receives research funding from Peregrine Pharmaceuticals. JSS, MT, JL and NLK were paid employees of Peregrine Pharmaceuticals.
References
- 1. Fadok VA, Bratton DL, Konowal A. et al. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest 1998; 101(4): 890–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Daleke DL. Regulation of transbilayer plasma membrane phospholipid asymmetry. J Lipid Res 2003; 44(2): 233–242. [DOI] [PubMed] [Google Scholar]
- 3. Ran S, Downes A, Thorpe PE.. Increased exposure of anionic phospholipids on the surface of tumor blood vessels. Cancer Res 2002; 62(21): 6132–6140. [PubMed] [Google Scholar]
- 4. Gerber DE, Hao G, Watkins L.. Tumor-specific targeting by bavituximab, a phosphatidylserine-targeting monoclonal antibody with vascular targeting and immune modulating properties, in lung cancer xenografts. Am J Nucl Med Mol Imaging 2015; 5: 493–503. [PMC free article] [PubMed] [Google Scholar]
- 5. He J, Yin Y, Luster TA. et al. Antiphosphatidylserine antibody combined with irradiation damages tumor blood vessels and induces tumor immunity in a rat model of glioblastoma. Clin Cancer Res 2009; 15(22): 6871–6880. [DOI] [PubMed] [Google Scholar]
- 6. Huang X, Bennett M, Thorpe PE.. A monoclonal antibody that binds anionic phospholipids on tumor blood vessels enhances the antitumor effect of docetaxel on human breast tumors in mice. Cancer Res 2005; 65(10): 4408–4416. [DOI] [PubMed] [Google Scholar]
- 7. He J, Luster TA, Thorpe PE.. Radiation-enhanced vascular targeting of human lung cancers in mice with a monoclonal antibody that binds anionic phospholipids. Clin Cancer Res 2007; 13(17): 5211–5218. [DOI] [PubMed] [Google Scholar]
- 8. Fossella FV, DeVore R, Kerr RN. et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol 2000; 18(12): 2354–2362. [DOI] [PubMed] [Google Scholar]
- 9. Gray MJ, Gong J, Hatch MMS. et al. Phosphatidylserine-targeting antibodies augment the anti-tumorigenic activity of anti-PD-1 therapy by enhanging immune activation and downregulating pro-oncogenic factors induced by T-cell checkpoint inhibition in murine triple-negative breast cancers. Breast Cancer Res 2016; 18(1): 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gerber DE, Stopeck AT, Wong L. et al. Phase I safety and pharmacokinetic study of bavituximab, a chimeric phosphatidylserine-targeting monoclonal antibody, in patients with advanced solid tumors. Clin Cancer Res 2011; 17(21): 6888–6896. [DOI] [PubMed] [Google Scholar]
- 11. Digumarti R, Bapsy PP, Shan JS.. A phase Ib safety and pharmacokinetic study of bavituximab plus chemotherapy in patients with refractory advanced solid tumor malignancies. J Clin Oncol 2008; 26(Suppl 15): Abstr 3038. [Google Scholar]
- 12. Digumarti R, Suresh AV, Bhattacharyya GS. et al. Phase II study of bavituximab plus paclitaxel and carboplatin in untreated locally advanced or metastatic non-small cell lung cancer: interim results. J Clin Oncol 2010; 28(Suppl 15): Abstr 7589. [Google Scholar]
- 13. Gerber DE, Spigel DR, Giorgadze D. et al. Docetaxel combined with bavituximab in previously treated, advanced nonsquamous non-small-cell lung cancer. Clin Lung Cancer 2016; 17(3): 169–176. [DOI] [PubMed] [Google Scholar]
- 14. Ramalingam S, Goss G, Rosell R. et al. A randomized phase II study of ganetespib, a heat shock protein 90 inhibitor, in combination with docetaxel in second-line therapy of advanced non-small cell lung cancer (GALAXY-1). Ann Oncol 2015; 26(8): 1741. [DOI] [PubMed] [Google Scholar]
- 15. Hanna N, Shepherd FA, Fossella FV. et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004; 22: 1589–1597. [DOI] [PubMed] [Google Scholar]
- 16. Garassino MC, Martelli O, Broggini M. et al. Erlotinib versus docetaxel as second-line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (TAILOR): a randomised controlled trial. Lancet Oncol 2013; 14(10): 981–988. [DOI] [PubMed] [Google Scholar]
- 17. de Groot PG, Urbanus RT.. The significance of autoantibodies against beta2-glycoprotein I. Blood 2012; 120(2): 266–274. [DOI] [PubMed] [Google Scholar]
- 18. Borghaei H, Paz-Ares L, Horn L. et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015; 373(17): 1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kato S, Goodman A, Walavalkar V. et al. Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res 2017; 23(15): 4242–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.