Abstract
Background
Several mechanisms underlying the depression-to-cardiovascular disease (CVD) relationship have been proposed; however, few studies have examined whether depression promotes CVD through potentiating traditional cardiovascular risk factors.
Purpose
To test the combined influence of three cardiovascular risk factors and lifetime depressive disorder on incident CVD in a large, diverse, and nationally representative sample of U.S. adults.
Methods
Respondents were 26,840 adults without baseline CVD who participated in Waves 1 (2001–2002) and 2 (2004–2005) of the National Epidemiologic Survey on Alcohol and Related Conditions. Lifetime depressive disorder, tobacco use, hypertension, and incident CVD were determined from structured interviews, and body mass index (BMI) was computed from self-reported height and weight.
Results
Logistic regression models predicting incident CVD (1,046 cases) revealed evidence of moderation, as the interactions between lifetime depressive disorder and current tobacco use (p = .002), hypertension (p < .001), and BMI (p = .031) were significant. The Former Tobacco Use × Lifetime Depressive Disorder interaction was not significant (p = .85). In models stratified by lifetime depressive disorder, current tobacco use (OR = 1.78, 95% CI = 1.36–2.32, p < .001 vs. OR = 1.41, 95% CI = 1.24–1.60, p < .001), hypertension (OR = 2.46, 95% CI = 1.98–3.07, p < .001 vs. OR = 1.39, 95% CI = 1.28–1.51, p < .001), and BMI (OR = 1.10, 95% CI = 1.01–1.20, p = .031 vs. OR = 1.03, 95% CI = 0.99–1.07, p = .16) were stronger predictors of incident CVD in adults with versus without a lifetime depressive disorder.
Conclusions
Our findings suggest that amplifying the atherogenic effects of traditional cardiovascular risk factors may be yet another candidate mechanism that helps to explain the excess CVD risk of people with depression.
Keywords: Depressive disorder, Tobacco use, Hypertension, Body mass index, Cardiovascular disease, Prospective studies
Tobacco use, hypertension, and elevated body mass index are more strongly associated with future cardiovascular disease in adults with a lifetime history of depression than in those without such a history.
Introduction
Over 30 years of research indicates that depression is an independent risk factor for atherosclerotic cardiovascular disease (CVD). To illustrate, recent meta-analyses revealed that adults with a depressive disorder (meeting diagnostic criteria) or elevated depressive symptoms (achieving a higher questionnaire score) have a 30% greater risk of coronary heart disease [1], a 30% greater risk of myocardial infarction (MI) [1, 2], and a 36% greater risk of CVD mortality than those without depression [2], even after adjustment for traditional cardiovascular risk factors. Several candidate mechanisms underlying the depression-to-CVD relationship have been proposed, including the biological mechanisms of systemic inflammation [3], autonomic nervous system dysregulation [4, 5], altered platelet function [6], and impaired endothelial function [7], and the behavioral mechanisms of poor CVD medication adherence [8–10], reduced physical activity [11, 12], poor sleep quality [13], and smoking [11, 14].
A less-studied mechanism by which depression may promote the development of CVD is through potentiating the atherogenic effect of traditional cardiovascular risk factors, possibly through proinflammatory and/or treatment nonadherence pathways. Multiple cardiovascular risk factors are thought to promote CVD through inflammatory pathways. For instance, hypertension, smoking, and obesity have been associated with higher levels of inflammatory markers [15–17] predictive of future CVD events [18, 19]. The presence of depression may negatively affect the body’s ability to mobilize an anti-inflammatory response, as depression has been linked with dysregulation in three systems that typically exert anti-inflammatory effects: the hypothalamic–pituitary–adrenal (HPA) axis [20], the parasympathetic nervous system (PNS) [4, 21], and interleukin (IL)-10 immunoregulation [22–24]. In addition, the presence of depression may have a negative impact on a person’s ability to engage in CVD primary prevention strategies, given that depression has been linked to poor adherence to antihypertensive and lipid-lowering medications [8–10] and to lifestyle recommendations, such as smoking cessation [25], exercise [11, 12], and weight management [12]. Therefore, depression could potentiate the atherogenic effects of cardiovascular risk factors by amplifying or prolonging the inflammatory response to these risk factors and/or by interfering with primary prevention efforts designed to reduce or control these risk factors.
To date, however, few studies have examined the joint effect of cardiovascular risk factors and depression on CVD outcomes, and the available results are mixed. Rutledge and colleagues [26] detected cardiovascular risk factor × depressive symptom interactions for waist-to-hip ratio (WHR), smoking, and diabetes but not for hypertension, dyslipidemia, and physical inactivity in women with suspected myocardial ischemia. While WHR was indeed a stronger predictor of incident CVD events in women with higher versus lower depressive symptoms, smoking and diabetes were stronger predictors in women with lower depressive symptoms, which were unexpected findings. Carroll et al. [27] also detected a smoking × depressive symptom interaction, with the association between smoking exposure over 25 years and coronary artery calcification strengthening as depressive symptoms increased. Other studies did not observe a synergistic effect between depression and cardiovascular risk factors. For instance, Ferketich and colleagues [28] did not detect a smoking × depressive symptom interaction in models predicting incident coronary heart disease in either sex, despite observing the highest relative risk among women with both elevated depressive symptoms and a smoking history. Pan et al. [29] reported a similar additive, but not interactive, effect for diabetes × depression in predicting CVD-related mortality. Gonzales and colleagues [30] did not observe an interaction between the examined CVD risk factors (hypercholesterolemia, smoking, diabetes, and alcohol use) and a summary score for psychological distress/depression in predicting incident MI in either sex. These inconsistent findings highlight the need for continued examination of this novel candidate mechanism underlying the depression-to-CVD relationship in prospective cohort studies.
To our knowledge, no study has simultaneously tested the joint effects of multiple cardiovascular risk factors and depression on incident CVD in a large, diverse, and nationally representative cohort of U.S. adults. Furthermore, no investigation in this literature has examined depressive disorders, which could have stronger moderating effects due to greater severity and duration than elevated depressive symptoms. Thus, the purpose of our study was to test the combined influence of three cardiovascular risk factors and lifetime depressive disorder on incident CVD in the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC), a large prospective cohort study of adults representative of the U.S. population. We hypothesized that depression would potentiate associations between traditional cardiovascular risk factors and incident CVD, such that tobacco use, hypertension, and elevated body mass index (BMI) would be significantly stronger predictors of incident CVD in people with versus without a depressive disorder history.
Methods
Study Design and Sample
NESARC is a prospective cohort study of the U.S. civilian noninstitutionalized population ≥18 years that was designed by the National Institute on Alcohol Abuse and Alcoholism to determine the prevalence of alcohol use disorders and associated disabilities. The NESARC methods are described in detail elsewhere [31–33]. NESARC received ethical approval from the U.S. Census Bureau and the U.S. Office of Management and Budget. At Wave 1 (2001–2002), 43,093 respondents (81.0% response rate) underwent face-to-face, computer-assisted home interviews assessing substance use, psychiatric disorders, medical conditions, and other factors. At Wave 2 (2004–2005) 3 years later (M = 36.6 months), 34,653 (86.7%) of the eligible Wave 1 respondents underwent a second home interview. Some Wave 1 respondents (n = 3,134) were not eligible for Wave 2 due to being deceased, deported, mentally or physically impaired, or on active duty in the armed forces.
From the Wave 2 cohort, we excluded women who reported being pregnant within the 12 months preceding the Wave 1 or 2 assessments (n = 2,035) to rule out pregnancy as a cause of hypertension and elevated BMI. Because of our focus on predicting new-onset CVD, we then excluded respondents whose CVD status at Wave 1 was positive (n = 1,625) or missing (n = 1,760). Next, we excluded respondents with missing data for the Wave 1 predictor variables (tobacco use, hypertension, BMI, and lifetime depressive disorder; n = 970), Wave 2 outcome variable (incident CVD; n = 1,000), and Wave 1 and 2 covariates (n = 423).
The characteristics of our final sample of 26,840 adults are shown in Table 1. As can be seen, we found that respondents with a lifetime depressive disorder (17% of the sample) were significantly younger, more likely to be female and non-Hispanic White, more likely to have higher education, more likely to have a lifetime anxiety disorder, and more likely to have a lifetime alcohol use disorder than those without a lifetime depressive disorder. Regarding the cardiovascular risk factors, respondents with versus without a lifetime depressive disorder were significantly more likely to be current tobacco users and had a higher mean BMI. No other differences in Table 1 characteristics were detected between the depressive disorder groups.
Table 1.
Characteristics of NESARC respondents
| Full sample (N = 26,840) | No lifetime depressive disorder (n = 22,288) | Lifetime depressive disorder (n = 4,552) | |
|---|---|---|---|
| Demographic factors (Wave 1) | |||
| Age, mean (SD), years | 46.0 (16.9) | 46.6 (17.3) | 43.1 (14.7)a |
| Sex, % female | 54.6 | 51.8 | 68.3a |
| Race/ethnicity, % | |||
| Non-Hispanic White | 58.8 | 57.1 | 67.3a |
| Non-Hispanic Black | 18.6 | 19.7 | 13.2a |
| Hispanic/ Latino | 18.1 | 18.7 | 15.2a |
| Other | 4.5 | 4.5 | 4.3 |
| Education level, % | |||
| Less than high school diploma | 15.2 | 15.7 | 12.8a |
| High school or equivalent | 28.6 | 29.0 | 26.6a |
| Some college or associate’s degree | 30.7 | 29.9 | 34.4a |
| Bachelor’s degree or higher | 25.6 | 25.4 | 26.2 |
| Other covariates | |||
| Wave 2 diabetes, % | 8.3 | 8.4 | 7.7 |
| Wave 2 hypercholesterolemia, % | 20.2 | 20.0 | 21.3 |
| Wave 1 lifetime anxiety disorder, % | 10.5 | 5.9 | 33.1a |
| Wave 1 alcohol use disorder, % | 29.1 | 26.3 | 42.6a |
| Cardiovascular risk factors (Wave 1) | |||
| Tobacco use, % | |||
| Lifetime nonuse | 55.1 | 56.7 | 47.2a |
| Current use | 25.9 | 24.2 | 34.1a |
| Former use | 19.1 | 19.1 | 18.8 |
| Hypertension, % | 19.1 | 19.1 | 19.0 |
| Body mass index, mean (SD), kg/m2 | 27.1 (5.6) | 27.0 (5.4) | 27.6 (6.4)a |
| Lifetime depressive disorder | 17.0 | – | – |
| Incident cardiovascular disease (Wave 2) | 1046 (3.9) | 852 (3.8) | 194 (4.3) |
NESARC National Epidemiologic Survey on Alcohol and Related Conditions; SD standard deviation.
aSignificantly different from no lifetime depressive disorder group (p < .05).
Measures and Procedures
Cardiovascular risk factors
During the Wave 1 interview, three cardiovascular risk factors—tobacco use, hypertension, and elevated BMI—were assessed by self-report. No other cardiovascular risk factors were assessed at Wave 1. Lifetime tobacco use was defined using NESARC’s three-level tobacco use variable (current use [in the past 12 months], former use [prior to the past 12 months], and lifetime nonuse), which we recoded into two dummy variables with lifetime nonuse as the reference category. This variable assessed tobacco use broadly (use of cigarettes, cigars, pipe, snuff, and chewing tobacco) and has been found to possess excellent reliability (0.74–0.84) [34]. Other studies have observed high agreement between self-reported smoking and urinary continine [35, 36]. Self-reported physician diagnosis of hypertension was assessed by NESARC’s Medical Conditions and Practices Interview using a two-part question. In Part A, respondents were asked, “In the past 12 months, have you had high blood pressure or hypertension?” If the answer to Part A was “yes,” in Part B, respondents were asked, “Did a doctor or other health professional tell you that you had high blood pressure or hypertension?” We coded respondents as positive for hypertension if they answered “yes” to Parts A and B, and as negative if they answered “no” to Part A. Those who were coded as “unknown” for Part A or B or who did not answer “yes” to both Parts A and B were coded as missing and were excluded. While a hypertension diagnosis based on blood pressure readings is the gold standard, the hypertension awareness rate was found to be as high as 74% in a large, nationally representative sample of U.S. adults [37]. Current BMI (kg/m2) was calculated from self-reported height and weight and was converted to a z score. Although measured BMI is more precise, a National Health and Nutrition Examination Survey III study supports the use of self-reported BMI in epidemiologic studies, as high correlations were observed between measured and self-reported BMI in non-Hispanic Whites (0.95), non-Hispanic Blacks (0.93), and Mexican Americans (0.90) [38].
Lifetime depressive disorder
Lifetime major depressive disorder (MDD) and lifetime dysthymic disorder were determined at Wave 1 by the Alcohol Use Disorder and Associated Disabilities Interview Schedule–IV (AUDADIS-IV), a fully structured diagnostic interview administered by lay interviewers assessing mental disorders using the Diagnostic and Statistical Manual of Mental Disorders (4th ed.; DSM-IV) criteria [39]. We utilized the NESARC variables that excluded illness-induced and substance-induced depressive disorders and ruled out bereavement [32]. NESARC personnel coded two diagnostic variables for both depressive disorders: during the past 12 months and prior to the past 12 months. We combined these four variables to create one composite lifetime depressive disorder variable (yes: positive for MDD or dysthymic disorder for any period; no: negative for MDD and dysthymic disorder for all periods). The AUDADIS-IV assessment of depressive disorders has demonstrated good test–retest reliability [34] and generally good agreement with clinician evaluations [33]. We decided to create a composite lifetime depressive disorder variable instead of separate variables for each disorder type (e.g., single episode MDD, recurrent MDD, and dysthymic disorder) (a) to maximize statistical power and (b) because adults with any clinical depression history (vs. those without) likely have greater lifetime exposure to elevated depressive symptoms and their potential influence on the atherogenic effects of cardiovascular risk factors (see Discussion section for details).
Incident CVD
From the Wave 2 NESARC Medical Conditions and Practices questionnaire data, we computed an incident CVD variable consisting of new-onset arteriosclerosis, angina, or MI based on self-reported physician diagnoses. In Part A, respondents were asked, “In the last 12 months, did you have: (1) hardening of the arteries or arteriosclerosis? (2) chest pain or angina pectoris? (3) a heart attack or myocardial infarction?” If the answer to Part A was “yes,” in Part B, respondents were asked, “Did a doctor or other health professional tell you that you had (name of condition)?” We coded respondents as positive for incident CVD if they answered “yes” to Parts A and B for at least one CVD question, and as negative if they answered “no” to all three Part A questions. Those who were coded as “unknown” for Part A or B for one or more questions and who did not answer “yes” to both Parts A and B for at least one question were coded as missing for incident CVD and were excluded. We also computed a corresponding baseline CVD variable by applying the same coding scheme to the identical Wave 1 CVD questions. Because our focus is predicting new-onset CVD, we included only respondents coded negative for baseline CVD in our cohort. Of note, new-onset stroke could not be included in our incident CVD variable, as stroke was assessed at Wave 2 but not at Wave 1. Coronary revascularization procedures could also not be included because they were not assessed at Wave 1 or 2. Agreement between self-reported and medical record–ascertained clinical CVD has been found to be acceptable to good [40–45].
Covariates
During the Wave 1 interview, demographic factors were assessed by self-report and included age (years), sex (0 = male, 1 = female), race/ethnicity, and education level. Due to low counts in some racial/ethnic categories, we recoded race/ethnicity into a four-level variable (non-Hispanic White, non-Hispanic Black, Hispanic/Latino, other) and created three dummy variables with non-Hispanic White as the reference category. Education level was assessed by the question, “Highest grade or year of school completed?” We computed a four-level variable (less than high school, high school or equivalent, some college or associate’s degree, bachelor’s degree or higher), from which we created three dummy variables with less than high school as the reference category.
Other possible confounders that were included as covariates in preliminary and primary models were diabetes and hypercholesterolemia. While diabetes and hypercholesterolemia are traditional cardiovascular risk factors [46, 47], these variables were assessed only at Wave 2. Given that any observed associations of diabetes and hypercholesterolemia with incident CVD would be cross-sectional, we conceptualized these factors as covariates rather than predictor variables. We coded respondents as positive for hypercholesterolemia and diabetes, respectively, if they answered “yes” to “In the past 12 months, have you had: (1) high cholesterol? (2) diabetes or sugar diabetes?” and “yes” to “Did a doctor or other health professional tell you that you had (name of condition)?” We coded respondents as negative for each condition if they answered “no” to the first question. Those coded by NESARC personnel as “unknown” for either question were coded as missing for that condition and were excluded.
Additional potential confounders included in supplemental models were lifetime anxiety disorder and lifetime alcohol use disorder. Given the evidence suggesting that anxiety disorders also contribute to CVD risk [48], we computed a lifetime anxiety disorder variable using AUDADIS-IV data collected at Wave 1 [39]. Respondents who were coded by NESARC personnel as meeting diagnostic criteria for panic disorder, agoraphobia, generalized anxiety disorder, or social phobia in the past year or prior to the past year (illness- and substance-induced disorders excluded) were coded as positive for lifetime anxiety disorder. Those not meeting criteria for any of these disorders were coded as negative. We also computed a lifetime alcohol use disorder variable using Wave 1 AUDADIS-IV data [39]. Respondents who were coded by NESARC personnel as meeting diagnostic criteria for alcohol abuse or alcohol dependent were coded as positive for lifetime alcohol use disorder. Those not meeting criteria for any of these disorders were coded as negative.
Data Analysis
We initially ran two logistic regression models—a preliminary model and a primary model—with incident CVD as the outcome. Our preliminary model simultaneously tested the main effects of the three cardiovascular risk factors and lifetime depressive disorder in the presence of demographic (age, sex, race/ethnicity [three dummy variables], education level [three dummy variables]) and other (Wave 2 diabetes and Wave 2 hypercholesterolemia) covariates. Our primary model was identical to the preliminary model except that we also included four cross-product interaction terms between each cardiovascular risk factor (current tobacco use vs. lifetime nonuse, former tobacco use vs. lifetime nonuse, hypertension, BMI) and lifetime depressive disorder. To probe significant interactions, we reran the primary model, after removing the lifetime depressive disorder main effect and the interaction terms, stratified by lifetime depressive disorder status.
Next, we ran three supplemental logistic regression models to determine whether any observed cardiovascular risk factor × lifetime depressive disorder interaction effects remained significant in the presence of other potentially confounding interactions. In Supplemental Model 1, we ran three versions of the primary model—one for each cardiovascular risk factor—to which we added interactions between the selected cardiovascular risk factor and age, sex, race/ethnicity, and education level. In Supplemental Model 2, we ran three versions of the primary model to which we added lifetime anxiety disorder and the interactions between the selected cardiovascular risk factor and lifetime anxiety disorder. In Supplemental Model 3, we ran three versions of the primary model to which we added lifetime alcohol use disorder and the interactions between the selected cardiovascular risk factor and lifetime alcohol use disorder. These models are important because depression could act as a proxy for demographic factors, anxiety disorders, or alcohol use disorders in the interaction terms, given that the prevalence of depressive disorders varies by age, sex, race/ethnicity, and education level [49, 50] and that depressive disorders are highly comorbid with anxiety disorders [51, 52] and alcohol use disorders [53].
Analyses were conducted with SAS statistical software 9.3. Models were weighted to account for oversampling, probabilities of selection, and nonresponse. Weighted analyses provide estimates for the U.S. civilian noninstitutionalized population based on the 2000 Decennial Census [33].
Results
Preliminary Model
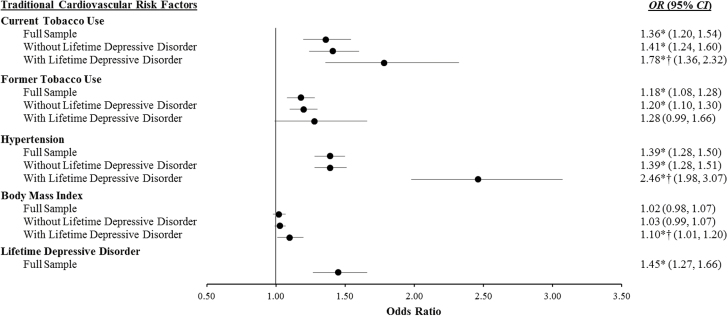
We identified 1,046 cases of incident CVD—250 with arteriosclerosis only, 580 with angina only, 66 with MI only, 53 with arteriosclerosis and angina, 10 with arteriosclerosis and MI, 57 with angina and MI, and 30 with all three outcomes. In our preliminary model that simultaneously tested the main effects of the predictor variables of interest, tobacco use, hypertension, and lifetime depressive disorder were independent predictors of incident CVD (see full sample results in Fig. 1). To illustrate, respondents with current tobacco use had a 36% greater odds (p < .001), those with former tobacco use had an 18% greater odds (p < .001), those with hypertension had a 39% greater odds (p < .001), and those with lifetime MDD had a 45% greater odds (p < .001) of incident CVD than respondents without these factors. Also of note, BMI did not predict incident CVD (p = .275).
Fig. 1.
Logistic regression models examining traditional cardiovascular risk factors (Wave 1: 2001–2002) as predictors of 3 year incidence of cardiovascular disease (Wave 2: 2004–2005) in our full NESARC sample (N = 26,840) and in respondents with (n = 4,552) and without (n = 22,288) a lifetime depressive disorder. All models are adjusted for age, sex, race/ethnicity, education level, Wave 2 diabetes, Wave 2 hypercholesterolemia, and the NESARC sampling design. Lifetime nonuse is the reference category for current and former tobacco use, and no hypertension is the reference category for hypertension. For body mass index, the odds ratios reflect the change in odds per 1 SD increase. OR odds ratio; CI confidence interval. Asterisk indicates significant main effect (p < .05). Dagger indicates significantly different from no lifetime depressive disorder group (p < .01).
Primary Model
In the primary model that simultaneously tested interactions between each cardiovascular risk factor and lifetime depressive disorder, the current tobacco use × lifetime depressive disorder (p = .002), hypertension × lifetime depressive disorder (p < .001), and BMI × lifetime depressive disorder (p = .031) interactions were significant. The former tobacco use × lifetime depressive disorder (p = .85) interaction was not significant. Given this evidence of moderation, we reran the model stratified by lifetime depressive disorder status (see Fig. 1). In respondents without a lifetime depressive disorder, current tobacco use (p < .001), former tobacco use (p < .001), and hypertension (p < .001) were independent predictors of incident CVD, while BMI was not (p = .16). In respondents with a lifetime depressive disorder, current tobacco use (p < .001), hypertension (p < .001), and BMI (p = .031) were independent predictors of incident CVD. Former tobacco use fell just short of significance (p = .062) but had an odds ratio comparable with respondents without a lifetime depressive disorder. Most importantly here, the interaction results indicate that current tobacco use (78% vs. 41% greater odds), hypertension (146% vs. 39% greater odds), and BMI (10% vs. 3% greater odds for every 1 SD increase) were significantly stronger predictors of incident CVD in respondents with a lifetime depressive disorder than in those without such a history.
Supplemental Models
In all three supplemental models adjusting for potentially confounding interactions, the pattern of results remained consistent with those from our primary models, with the exception of BMI in supplemental models 2–3. In supplemental model 1, after adjustment for cardiovascular risk factor x demographic factor interactions, the current tobacco use × lifetime depressive disorder (p = .001), hypertension × lifetime depressive disorder (p = .004), and BMI × lifetime depressive disorder (p = .048) interactions remained significant. In supplemental model 2, after adjustment for cardiovascular risk factor × lifetime anxiety disorder interactions, the current tobacco use × lifetime depressive disorder (p = .001) and hypertension × lifetime depressive disorder (p < .001) interactions remained significant; however, the BMI × lifetime depressive disorder fell short of significance (p = .093). Of note, the current tobacco use × lifetime anxiety disorder (p = .278), hypertension × lifetime anxiety disorder (p = .319), and BMI × lifetime anxiety disorder (p = .605) interactions were not significant; however, the former tobacco use × lifetime anxiety disorder (p < .001) interaction was significant. In models stratified by lifetime anxiety disorder status, former tobacco use was a predictor of CVD in respondents without (OR = 1.22, 95% CI: 1.12–1.33, p < .001) but not with (OR = 0.88, 95% CI: 0.70–1.12, p = .312) a lifetime anxiety disorder. In supplemental model 3, after adjustment for cardiovascular risk factor × lifetime alcohol use disorder interactions, the current tobacco use × lifetime depressive disorder (p = .004) and hypertension × lifetime depressive disorder (p < .001) interactions remained significant; however, the BMI × lifetime depressive disorder was no longer significant (p = .128). In general, these results suggest that lifetime depressive disorder was not operating as a proxy for age, sex, race/ethnicity, education level, lifetime anxiety disorder, or lifetime alcohol use disorder in our primary models.
Discussion
The present results support the hypothesis that depression potentiates prospective associations between traditional cardiovascular risk factors and incident CVD. In a large, diverse, and nationally representative cohort, we found that current tobacco use (78% vs. 41% greater odds), hypertension (146% vs. 39% greater odds), and BMI (10% vs. 3% greater odds for every 1 SD increase) were significantly stronger predictors of new-onset CVD in U.S. adults with a lifetime history of a depressive disorder than in those without such a history. The pattern of results remained similar for current tobacco use and hypertension models adjusting for potentially confounding interactions, increasing our confidence that lifetime depressive disorder is the true moderator variable. Altogether, our findings suggest that amplifying the atherogenic effects of traditional cardiovascular risk factors may be yet another candidate mechanism that helps to explain the excess CVD risk of people with depression.
Our study extends prior investigations in this literature by being the first to examine the joint effects of traditional cardiovascular risk factors and depressive disorders on incident CVD in a nationally representative cohort. The findings we report both complement and contradict the results of past studies. Our findings complement past studies observing that the presence of depression increased the magnitude of relationships between cardiovascular risk factors and CVD outcomes [26, 27]. However, they conflict with past studies that did not detect cardiovascular risk factor × depression interactions [26, 28–30] or found that depressed decreased cardiovascular risk factors–CVD outcome associations [26]. Although the factors contributing to these mixed results are unclear, methodological differences may be playing a role, including differences in sample characteristics, depression assessments, and CVD outcomes. To illustrate, we examined a diverse sample of U.S. adults likely free of baseline CVD, while others examined more select samples, such as women with suspected myocardial ischemia [26] and older adults [30]. In addition, we utilized data from a validated structured diagnostic interview to determine depression status, whereas others used self-report assessments of depressive symptoms [26, 28, 30]. Given the current mixed state of this literature, future analyses of prospective cohort studies are needed to clarify the existence and nature of the combined effect of traditional cardiovascular risk factors and depression on CVD outcomes.
How might depression potentiate the atherogenic effects of traditional cardiovascular risk factors? Plausible mechanisms involving systemic inflammation and treatment nonadherence exist. Several cardiovascular risk factors—including smoking, hypertension, and obesity—are thought to promote CVD through inflammatory pathways [16, 17, 54]. Specifically, adults with these risk factors exhibit higher circulating levels of the proinflammatory cytokine IL-6 and the acute-phase reactant C-reactive protein, both of which predict future CVD events [18, 19]. Depression has been linked with dysregulation in three systems that normally exert anti-inflammatory effects: the HPA axis, the PNS, and IL-10 immunoregulation. Regarding the HPA axis, depression is often accompanied by chronic release of glucocorticoids, which can lead to a blunted response to their typically anti-inflammatory effects over time [20]. Depression is also associated with diminished parasympathetic activation as indicated by reduced heart rate variability [4, 21], and parasympathetic activation has been shown to curb inflammation [55]. Finally, depression may lead to overproduction of the anti-inflammatory cytokine IL-10 in response to proinflammatory cytokine elevations that often occur with depression, which could result in exhaustion of this anti-inflammatory mechanism [22–24]. Thus, depression-related dysregulation in these three systems regulating inflammation could amplify or prolong the inflammatory response to, and ultimately the atherogenic effects of, cardiovascular risk factors.
In addition to proinflammatory pathways, treatment nonadherence pathways may also be involved. Evidence suggests that depression is associated with poorer adherence to medical recommendations intended to prevent CVD. Specifically, depression has been linked with reduced adherence to antihypertensive and lipid-lowering medications [8–10] and lifestyle recommendations, such as smoking cessation [25], exercise [11, 12], and weight management [12]. Thus, depression could amplify the atherogenic effects of cardiovascular risk factors by interfering with primary prevention efforts designed to reduce or control these risk factors. When considering candidate mechanisms, it is important to note that the course of depression is often chronic. The depression recurrence rate after 15 years is 85% in mental health settings and 35% in the general population [56], and residual symptoms frequently persist after remission of a depressive episode [57]. Therefore, adults with a history of clinical depression, versus those without such a history, likely have greater lifetime exposure to elevated depressive symptoms and their possible influence on the atherogenic effects of cardiovascular risk factors.
There are limitations of this study that warrant consideration. First, epidemiological surveys often rely on self-reported physician diagnosis of CVD. Such an approach is supported by evidence indicating acceptable to good agreement between self-reported and medical record–ascertained CVD [40–45]. While a recent study did report low agreement between self-reported and Medicare claims–identified MIs, the authors note that their older sample (mean age = 77 years) and narrow MI definition may have contributed to this result [58]. Second, due to NESARC’s methodology, we could not capture fatal CVD events. Respondents who died between Waves 1 and 2 of NESARC were excluded from the Wave 2 cohort, and cause of death information is not available. Thus, it is unclear whether the present findings extend to incident fatal CVD events. Third, some incident nonfatal MIs may not have been captured because the NESARC Wave 2 questions inquired about CVD diagnoses in the past 12 months only. This is less of a concern for arteriosclerosis and angina because these are chronic conditions and not discrete events. In addition, we used a composite incident CVD outcome, which should reduce the potential for misclassification [40]. For instance, respondents who suffered nonfatal MIs between Waves 1 and 2, but prior to the past 12 months, may have also been diagnosed with one of the other CVD conditions during follow-up. Fourth, our study was limited to the CVD risk factors assessed at both waves of NESARC data collection. Other CVD risk factors (e.g., high cholesterol, diabetes) may also be important in the joint effect of cardiovascular risk factors and depression on CVD outcomes. These four limitations highlight the need for future prospective studies in this area with comprehensive capture of fatal and nonfatal CVD events adjudicated by medical record review. Despite these limitations, our study leverages a prospective design, a large sample of adults representative of the U.S. population, and structured interview assessments of psychiatric disorders.
In conclusion, we found that the traditional cardiovascular risk factors of current tobacco use, hypertension, and elevated BMI were stronger predictors of future CVD in U.S. adults with versus without a history of clinical depression. The present results have potential theoretical, research, and clinical implications. Regarding theory, potentiating the atherogenic effect of traditional cardiovascular risk factors appears to be another candidate mechanism by which depression may promote the development of CVD and, thus, should be added to conceptual frameworks depicting the potential pathways through which depression may increase CVD risk. Future research is needed to (a) identify the mechanisms through which depression potentiates the effect of traditional cardiovascular risk factors on CVD risk and (b) determine whether successful depression treatment neutralizes this potentiating effect. Concerning clinical practice, our findings underscore the importance of systematic screening for depression in settings in which CVD primary prevention frequently occurs, such as primary care. In this setting, knowledge of a patient’s depression status may be of use for risk stratification purposes and for determining the aggressiveness of CVD primary prevention efforts. Overall, our findings raise the possibility that early detection and aggressive management of traditional cardiovascular risk factors is one approach to reducing the excess CVD risk of depressed people that is worthy of further evaluation.
Acknowledgments
NESARC is funded by the National Institute on Alcohol Abuse and Alcoholism with supplemental support from the National Institute on Drug Abuse. A portion of J.C.S.’s time was supported by the National Heart, Lung, and Blood Institute under Award Number R01HL122245. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Compliance with Ethical Standards
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards: None of the authors has any conflicts of interest to declare.
Ethical Approval: The institutional review board at IUPUI has approved this study, and the study was performed in full compliance with ethical standards.
References
- 1. Gan Y, Gong Y, Tong X et al. Depression and the risk of coronary heart disease: a meta-analysis of prospective cohort studies. bmc Psychiatry. 2014;14:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu Q, Kling JM. Depression and the risk of myocardial infarction and coronary death: a meta-analysis of prospective cohort studies. Medicine (Baltimore). 2016;95(6):e2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27(1):24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brunoni AR, Kemp AH, Dantas EM et al. Heart rate variability is a trait marker of major depressive disorder: evidence from the sertraline vs. electric current therapy to treat depression clinical study. Int J Neuropsychopharmacol. 2013;16(9):1937–1949. [DOI] [PubMed] [Google Scholar]
- 5. Kop WJ, Stein PK, Tracy RP, Barzilay JI, Schulz R, Gottdiener JS. Autonomic nervous system dysfunction and inflammation contribute to the increased cardiovascular mortality risk associated with depression. Psychosom Med. 2010;72(7):626–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. von Känel R. Platelet hyperactivity in clinical depression and the beneficial effect of antidepressant drug treatment: how strong is the evidence?Acta Psychiatr Scand. 2004;110(3):163–177. [DOI] [PubMed] [Google Scholar]
- 7. Cooper DC, Tomfohr LM, Milic MS et al. Depressed mood and flow-mediated dilation: a systematic review and meta-analysis. Psychosom Med. 2011;73(5):360–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bautista LE, Vera-Cala LM, Colombo C, Smith P. Symptoms of depression and anxiety and adherence to antihypertensive medication. Am J Hypertens. 2012;25(4):505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. May HT, Sheng X, Catinella AP, Horne BD, Carlquist JF, Joy E. Antilipidemic adherence post-coronary artery disease diagnosis among those with and without an ICD-9 diagnosis of depression. J Psychosom Res. 2010;69(2):169–174. [DOI] [PubMed] [Google Scholar]
- 10. Grenard JL, Munjas BA, Adams JL et al. Depression and medication adherence in the treatment of chronic diseases in the United States: a meta-analysis. J Gen Intern Med. 2011;26(10):1175–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ye S, Muntner P, Shimbo D et al. Behavioral mechanisms, elevated depressive symptoms, and the risk for myocardial infarction or death in individuals with coronary heart disease: the REGARDS (reason for geographic and racial differences in stroke) study. J Am Coll Cardiol. 2013;61(6):622–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berntson J, Stewart KR, Vrany E, Khambaty T, Stewart JC. Depressive symptoms and self-reported adherence to medical recommendations to prevent cardiovascular disease: NHANES 2005-2010. Soc Sci Med. 2015;138:74–81. [DOI] [PubMed] [Google Scholar]
- 13. Sin NL, Kumar AD, Gehi AK, Whooley MA. Direction of association between depressive symptoms and lifestyle behaviors in patients with coronary heart disease: the heart and soul study. Ann Behav Med. 2016;50(4):523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fluharty M, Taylor AE, Grabski M, Munafò MR. The association of cigarette smoking with depression and anxiety: a systematic review. Nicotine Tob Res. 2017;19(1):3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dinh QN, Drummond GR, Sobey CG, Chrissobolis S. Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. Biomed Res Int. 2014;2014:406960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McEvoy JW, Nasir K, DeFilippis AP et al. Relationship of cigarette smoking with inflammation and subclinical vascular disease: the multi-ethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35(4):1002–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mathieu P, Lemieux I, Després JP. Obesity, inflammation, and cardiovascular risk. Clin Pharmacol Ther. 2010;87(4):407–416. [DOI] [PubMed] [Google Scholar]
- 18. Buckley DI, Fu R, Freeman M, Rogers K, Helfand M. C-reactive protein as a risk factor for coronary heart disease: a systematic review and meta-analyses for the U.S. preventive services task force. Ann Intern Med. 2009;151(7):483–495. [DOI] [PubMed] [Google Scholar]
- 19. Kaptoge S, Seshasai SR, Gao P et al. Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis. Eur Heart j. 2014;35(9):578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zunszain PA, Anacker C, Cattaneo A, Carvalho LA, Pariante CM. Glucocorticoids, cytokines and brain abnormalities in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(3):722–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blume J, Douglas SD, Evans DL. Immune suppression and immune activation in depression. Brain Behav Immun. 2011;25(2):221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gazal M, Jansen K, Souza LD et al. Association of interleukin-10 levels with age of onset and duration of illness in patients with major depressive disorder. Rev Bras Psiquiatr. 2015;37(4):296–302. [DOI] [PubMed] [Google Scholar]
- 23. Dhabhar FS, Burke HM, Epel ES et al. Low serum IL-10 concentrations and loss of regulatory association between IL-6 and IL-10 in adults with major depression. J Psychiatr Res. 2009;43(11):962–969. [DOI] [PubMed] [Google Scholar]
- 24. Voorhees JL, Tarr AJ, Wohleb ES et al. Prolonged restraint stress increases IL-6, reduces IL-10, and causes persistent depressive-like behavior that is reversed by recombinant IL-10. PloS One. 2013;8(3):e58488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weinberger AH, Mazure CM, Morlett A, McKee SA. Two decades of smoking cessation treatment research on smokers with depression: 1990-2010. Nicotine Tob Res. 2013;15(6):1014–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rutledge T, Linke SE, Johnson BD et al. Relationships between cardiovascular disease risk factors and depressive symptoms as predictors of cardiovascular disease events in women. j Womens Health (Larchmt). 2012;21(2):133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carroll AJ, Carnethon MR, Liu K et al. Interaction between smoking and depressive symptoms with subclinical heart disease in the coronary artery risk development in young adults (CARDIA) study. Health Psychol. 2017;36(2):101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ferketich AK, Schwartzbaum JA, Frid DJ, Moeschberger ML. Depression as an antecedent to heart disease among women and men in the NHANES I study. national health and nutrition examination survey. Arch Intern Med. 2000;160(9):1261–1268. [DOI] [PubMed] [Google Scholar]
- 29. Pan A, Lucas M, Sun Q et al. Increased mortality risk in women with depression and diabetes mellitus. Arch Gen Psychiatry. 2011;68(1):42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gonzales TK, Yonker JA, Chang V, Roan CL, Herd P, Atwood CS. Myocardial infarction in the Wisconsin longitudinal study: the interaction among environmental, health, social, behavioural and genetic factors. bmj Open. 2017;7(1):e011529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grant BF, Goldstein RB, Chou SP et al. Sociodemographic and psychopathologic predictors of first incidence of DSM-IV substance use, mood and anxiety disorders: results from the wave 2 national epidemiologic survey on alcohol and related conditions. Mol Psychiatry. 2009;14(11):1051–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grant BF, Stinson FS, Hasin DS et al. Prevalence, correlates, and comorbidity of bipolar I disorder and axis I and II disorders: results from the national epidemiologic survey on alcohol and related conditions. J Clin Psychiatry. 2005;66(10):1205–1215. [DOI] [PubMed] [Google Scholar]
- 33. Hasin DS, Grant BF. The national epidemiologic survey on alcohol and related conditions (NESARC) waves 1 and 2: review and summary of findings. Soc Psychiatry Psychiatr Epidemiol. 2015;50(11):1609–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grant BF, Dawson DA, Stinson FS, Chou PS, Kay W, Pickering R. The alcohol use disorder and associated disabilities interview schedule-IV (AUDADIS-IV): reliability of alcohol consumption, tobacco use, family history of depression and psychiatric diagnostic modules in a general population sample. Drug Alcohol Depend. 2003;71(1):7–16. [DOI] [PubMed] [Google Scholar]
- 35. Wong SL, Shields M, Leatherdale S, Malaison E, Hammond D. Assessment of validity of self-reported smoking status. Health Rep. 2012;23(1):47–53. [PubMed] [Google Scholar]
- 36. West R, Zatonski W, Przewozniak K, Jarvis MJ. Can we trust national smoking prevalence figures? Discrepancies between biochemically assessed and self-reported smoking rates in three countries. Cancer Epidemiol Biomarkers Prev. 2007;16(4):820–822. [DOI] [PubMed] [Google Scholar]
- 37. Guo F, He D, Zhang W, Walton RG. Trends in prevalence, awareness, management, and control of hypertension among United States adults, 1999 to 2010. J Am Coll Cardiol. 2012;60(7):599–606. [DOI] [PubMed] [Google Scholar]
- 38. McAdams MA, Van Dam RM, Hu FB. Comparison of self-reported and measured BMI as correlates of disease markers in US adults. Obesity (Silver Spring). 2007;15(1):188–196. [DOI] [PubMed] [Google Scholar]
- 39. Ruan WJ, Goldstein RB, Chou SP et al. The alcohol use disorder and associated disabilities interview schedule-IV (AUDADIS-IV): reliability of new psychiatric diagnostic modules and risk factors in a general population sample. Drug Alcohol Depend. 2008;92(1–3):27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Barr EL, Tonkin AM, Welborn TA, Shaw JE. Validity of self-reported cardiovascular disease events in comparison to medical record adjudication and a statewide hospital morbidity database: the AusDiab study. Intern Med J. 2009;39(1):49–53. [DOI] [PubMed] [Google Scholar]
- 41. Bergmann MM, Byers T, Freedman DS, Mokdad A. Validity of self-reported diagnoses leading to hospitalization: a comparison of self-reports with hospital records in a prospective study of American adults. Am J Epidemiol. 1998;147(10):969–977. [DOI] [PubMed] [Google Scholar]
- 42. Heckbert SR, Kooperberg C, Safford MM et al. Comparison of self-report, hospital discharge codes, and adjudication of cardiovascular events in the women’s health initiative. Am J Epidemiol. 2004;160(12):1152–1158. [DOI] [PubMed] [Google Scholar]
- 43. Lampe FC, Walker M, Lennon LT, Whincup PH, Ebrahim S. Validity of a self-reported history of doctor-diagnosed angina. J Clin Epidemiol. 1999;52(1):73–81. [DOI] [PubMed] [Google Scholar]
- 44. Machón M, Arriola L, Larrañaga N et al. Validity of self-reported prevalent cases of stroke and acute myocardial infarction in the Spanish cohort of the EPIC study. J Epidemiol Community Health. 2013;67(1):71–75. [DOI] [PubMed] [Google Scholar]
- 45. Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol. 2004;57(10):1096–1103. [DOI] [PubMed] [Google Scholar]
- 46. Sattar N. Revisiting the links between glycaemia, diabetes and cardiovascular disease. Diabetologia. 2013;56(4):686–695. [DOI] [PubMed] [Google Scholar]
- 47. Navar-Boggan AM, Peterson ED, D’Agostino RB et al. Hyperlipidemia in early adulthood increases long-term risk of coronary heart disease. Circulation. 2015;131(5):451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Batelaan NM, Seldenrijk A, Bot M, van Balkom AJ, Penninx BW. Anxiety and new onset of cardiovascular disease: critical review and meta-analysis. Br J Psychiatry. 2016;208(3):223–231. [DOI] [PubMed] [Google Scholar]
- 49. Blazer DG, Kessler RC, McGonagle KA, Swartz MS. The prevalence and distribution of major depression in a national community sample: the national comorbidity survey. Am J Psychiatry. 1994;151(7):979–986. [DOI] [PubMed] [Google Scholar]
- 50. Riolo SA, Nguyen TA, Greden JF, King CA. Prevalence of depression by race/ethnicity: findings from the national health and nutrition examination survey III. Am J Public Health. 2005;95(6):998–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kessler RC, Berglund P, Demler O et al. ; National Comorbidity Survey Replication The epidemiology of major depressive disorder: results from the national comorbidity survey replication (NCS-R). JAMA. 2003;289(23):3095–3105. [DOI] [PubMed] [Google Scholar]
- 52. Hasin DS, Goodwin RD, Stinson FS, Grant BF. Epidemiology of major depressive disorder: results from the national epidemiologic survey on alcoholism and related conditions. Arch Gen Psychiatry. 2005;62(10):1097–1106. [DOI] [PubMed] [Google Scholar]
- 53. Sullivan LE, Fiellin DA, O’Connor PG. The prevalence and impact of alcohol problems in major depression: a systematic review. Am J Med. 2005;118(4):330–341. [DOI] [PubMed] [Google Scholar]
- 54. Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340(2):115–126. [DOI] [PubMed] [Google Scholar]
- 55. Pavlov VA, Tracey KJ. The vagus nerve and the inflammatory reflex – linking immunity and metabolism. Nat Rev Endocrinol. 2012;8(12):743–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hardeveld F, Spijker J, De Graaf R, Nolen WA, Beekman AT. Prevalence and predictors of recurrence of major depressive disorder in the adult population. Acta Psychiatr Scand. 2010;122(3):184–191. [DOI] [PubMed] [Google Scholar]
- 57. Nierenberg AA, Husain MM, Trivedi MH et al. Residual symptoms after remission of major depressive disorder with citalopram and risk of relapse: a STAR*D report. Psychol Med. 2010;40(1):41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yasaitis LC, Berkman LF, Chandra A. Comparison of self-reported and Medicare claims-identified acute myocardial infarction. Circulation. 2015;131(17):1477–1485; discussion 1485. [DOI] [PMC free article] [PubMed] [Google Scholar]