Abstract
Xylan, a prominent component of cellulosic biomass, has a high potential for degradation into reducing sugars, and subsequent conversion into bioethanol. This process requires a range of xylanolytic enzymes. Among them, β-xylosidases are crucial, because they hydrolyze more glycosidic bonds than any of the other xylanolytic enzymes. They also enhance the efficiency of the process by degrading xylooligosaccharides, which are potent inhibitors of other hemicellulose-/xylan-converting enzymes. On the other hand, the β-xylosidase itself is also inhibited by monosaccharides that may be generated in high concentrations during the saccharification process. Structurally, β-xylosidases are diverse enzymes with different substrate specificities and enzyme mechanisms. Here, we review the structural diversity and catalytic mechanisms of β-xylosidases, and discuss their inhibition by monosaccharides.
Keywords: biomass, hemicellulose, bioethanol, xylanolytic enzyme, hemicellulase, glycoside hydrolase
1. Introduction
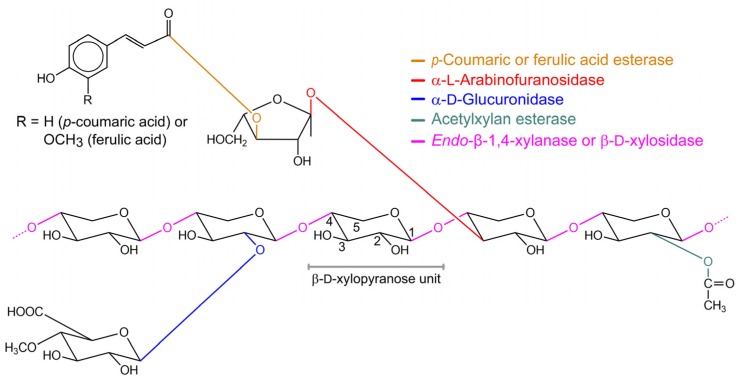
Xylan is a prominent component of cellulosic biomass, a heterogeneous complex of carbohydrate polymers (cellulose and hemicellulose) and lignin, a complex polymer of phenylpropane units [1,2,3]. Hemicellulose, including xylan, makes up approximately one-third of the carbohydrate content of common agricultural and forestry waste [1,2,3]. It is among the most inexpensive non-food biomass that is sustainably available in nature in large quantities, and that can be converted into biofuel or other value-added products, such as low-calorie sweeteners, prebiotics, surfactants and various specialty chemicals [1,4,5,6]. Structurally, xylan is a complex heteropolysaccharide with a glycosidic β-(1,4)-linked d-xylose backbone that is frequently substituted with side chains of arabinose, glucuronic acid and other groups. In turn, these side chains may be further esterified with acetic, ferulic, and p-coumaric acids (Figure 1). The type and frequency of the side chains and their substituents vary with the source of xylans [2,7,8,9,10,11].
Figure 1.
Example of the structure of a plant xylan with the cleavage sites of various xylanolytic enzymes indicated. A β-d-xylopyranose unit with numbered carbon atoms is shown in the middle. Glycosidic bonds and xylanolytic enzymes that hydrolyze them are depicted in the same color [7,9].
As a complex heteropolysaccharide, full degradation of xylan into its monosaccharide constituents requires the concerted action of various hydrolytic xylan-degrading enzymes with different specificities (Figure 1). These enzymes include α-l-arabinofuranosidase (EC 3.2.1.55), α-d-glucuronidase (EC 3.2.1.139), acetylxylan esterase (EC 3.1.1.72), and p-coumaric acid and ferulic acid esterases (EC 3.1.1.73), which release the side chain substituents from the xylan backbone, and endo-β-1,4-xylanase (EC 3.2.1.8), which works synergistically with β-xylosidase (EC 3.2.1.37) to break down the xylan backbone. Endo-β-1,4-xylanase hydrolyses the internal β-(1,4) linkages of the xylan backbone producing short xylooligosaccharides, while β-xylosidase removes xylose units from the non-reducing termini of these xylooligosaccharides [2,7,8,10]. In nature, xylanolytic enzymes are mainly found in numerous saprophytic microorganisms, such as fungi, actinomycetes and other bacteria, as well as in the rumen biota of higher animals. The microorganisms secrete the enzymes, for example, as a strategy for expanding their versatility to use primary carbon sources [7,11,12,13,14].
Xylan-degrading enzymes have found application as environmentally friendly agents in a wide range of industrial processes, such as bleaching of paper pulp, deinking of recycled paper, enhancing the digestibility and nutritional properties of animal feed, degumming of plant fiber sources, manufacturing of beer and wine, clarification of fruit juices and maceration of fruits and vegetables, preparation of high-fiber baked goods, and the extraction of coffee [2,7,15,16]. Furthermore, the enzymes are applied during the saccharification of pretreated agricultural and forestry cellulosic biomass into fermentable sugars [2,15], e.g., for producing biofuel.
In xylan saccharification, β-xylosidase is a crucial enzyme since, of all the xylanolytic enzymes, it cleaves the greatest number of glycosidic bonds. [17,18,19]. In addition, because xylooligosaccharides are potent inhibitors of endo-β-1,4-xylanases and cellulases, the activity of β-xylosidase can improve the efficiency of the saccharification process by degrading the xylooligosaccharides and thus alleviating inhibition of those enzymes [7,11,20,21,22,23]. However, most of the characterized β-xylosidases are, to some extent, also inhibited themselves by xylose, arabinose, glucose, and/or other monosaccharides [2,11,24,25,26,27]. This is an important problem, since in industrial cellulosic biomass saccharification, the monosaccharides may accumulate to high enough concentrations to significantly reduce the activity of β-xylosidase, even in simultaneous saccharification and fermentation processes, where monosaccharides are directly consumed by the fermenting organisms [26,27]. This adverse property may severely reduce the efficiency of β-xylosidases in the saccharification process.
In this review, the structural diversity, catalytic mechanisms and inhibition by monosaccharides of β-xylosidases are discussed.
2. Structural Diversity of β-xylosidases
β-Xylosidases are a group of structurally diverse enzymes with varying specificities, in line with the diversity of the organisms that produce them and the heterogeneity of their substrates [28]. However, as commonly observed in other glycoside hydrolases (GHs), they hydrolyze the glycosidic bond via one of two routes, either with overall retention or with overall inversion of the anomeric carbon configuration [29].
GHs are classified in the Carbohydrate-Active Enzymes database (CAZy; http://www.cazy.org/), which groups the enzymes into families based on their amino acid sequence similarities [30,31]. As there is a direct relationship between amino acid sequence similarity and similarity of folding, the classification also represents the structural features and commonality of the catalytic mechanism of the enzymes. Thus, enzymes in a particular family display highly similar three-dimensional structures and catalytic mechanisms [29,32]. At present, 161 GH families (GH1 to GH161) are represented on the CAZy server. Nevertheless, despite divergent amino acid sequences, several different GH families show significantly similar protein folding and active site architecture. Such GH families are considered to have a common ancestor and, therefore, have been grouped together into a clan [33]. To date, 18 GH clans (GH-A to GH-R) have been assigned in the database.
A search using the enzyme classification number for β-xylosidase (EC 3.2.1.37) in the CAZy database [31] revealed that enzymes with this number are presently found in 11 different GH families (Table 1). Nevertheless, a further literature examination suggests that 3 families, i.e., GH1, GH54 and GH116, may not contain enzymes with β-xylosidase activity on natural substrates. The enzymes from Reticulitermes flavipes (RfBGluc-1; GenPept accession No. ADK12988) [34] and R. santonensis De Feytaud (GenPept ADT62000) [35] in GH1, and Trichoderma koningii G-39 (TkAbf; GenPept AAA81024) [36] in GH54 were classified as β-xylosidases because they hydrolyze artificial nitrophenyl-β-d-xylopyranoside derivatives. However, to our knowledge there is no evidence that these enzymes are able to release xylose from natural substrates. Similarly, a bifunctional aryl β-glucosidase/β-xylosidase from the hyperthermophilic archaeon Saccharolobus solfataricus P2 (formerly Sulfolobus solfataricus; SSO1353; GenPept AAK41589) in GH116 is called so based on its activity on aryl β-glucosides and β-xylosides, but the enzyme likely does not hydrolyze xylooligosaccharides [37]. All in all, this suggests that enzymes with β-xylosidase activity on natural substrates currently occur in only 8 GH families in the CAZy database, i.e., in GH families 3, 5, 30, 39, 43, 51, 52 and 120.
Table 1.
Distribution of the current β-xylosidases in the CAZy database, their catalytic domain fold, their type of catalytic mechanism, and their catalytic residues.
| Family (GH) | Total Number of β-xylosidase Sequences | Clan | Overall Fold of the Catalytic Domain | Catalytic Mechanism † | Nucleophile | General Acid/Base |
|---|---|---|---|---|---|---|
| ‡ 1 | 2 | A | (β/α)8 TIM-barrel | Retention | Glu | Glu |
| 3 | 103 | n.a. # | (β/α)8 TIM-barrel | Retention | Asp | Glu |
| 5 | 1 | A | (β/α)8 TIM-barrel | Retention | Glu | Glu |
| 30 | 4 | A | (β/α)8 TIM-barrel | Retention | Glu | Glu |
| 39 | 24 | A | (β/α)8 TIM-barrel | Retention | Glu | Glu |
| 43 | 96 | F | 5-bladed β-propeller | Inversion | Asp § | Glu |
| 51 | 2 | A | (β/α)8 TIM-barrel | Retention | Glu | Glu |
| 52 | 11 | O | (α/α)6-barrel | Retention | Glu | Asp |
| ‡ 54 | 2 | n.a. # | β-sandwich % | Retention | Glu % | Asp % |
| ‡ 116 | 1 | O | (α/α)6-barrel | Retention | Glu | Asp |
| 120 | 2 | n.a. # | right-handed parallel β-helix | Retention | Asp | Glu |
†: Catalysis by GHs commonly proceeds with either retention or inversion of the substrate’s anomeric carbon configuration. See main text for further information. ‡: It is unknown whether the enzymes from GH1, GH54, and GH116 have β-xylosidase activity on natural substrates. #: Not part of a clan. §: General base; General acid. %: Not assigned in the CAZy database. Data are from the crystal structure of the α-l-arabinofuranosidase from Aspergillus kawachii IFO4308 [38].
2.1. Glycoside Hydrolase Clan A (GH-A)
GH families 1, 5, 30, 39 and 51 are part of clan GH-A, the largest clan in the CAZy database with currently 23 GH families. Enzymes in this clan all have a (β/α)8 catalytic domain, also known as triose-phosphate isomerase (TIM) barrel domain [39].
Of clan GH-A, structural data for β-xylosidases are currently only available for GH39, i.e., β-xylosidases from Thermoanaerobacterium saccharolyticum B6A-RI (TsXynB; Protein Data Bank code 1px8; Figure 2e) [40], Geobacillus stearothermophilus T-6 (GsXynB1; PDB 2BS9) [41] and Caulobacter crescentus NA1000 (CcXynB2; PDB 4EKJ) [42]. These enzymes fold into a three-domain structure, consisting of an N-terminal (β/α)8-barrel catalytic domain, sequentially followed by a β-sandwich and an α-helical accessory domain. Their structures are very similar. Superposition of the structures of isolated proteins gave an overall root mean squared deviation (RMSD) of 1.6 Å for 462 amino acid residues. However, while CcXynB2 exists as a monomer in solution [42], TsXynB and GsXynB1 are present as tetramers [40,41]. The absence of a short amino acid sequence at the C-terminus of CcXynB2, compared to the other two enzymes, has been suggested to prevent the formation of a stable tetramer [42]. Additionally, it has been proposed that subtle structural differences in the accessory domains of these β-xylosidases slightly alter their overall structure and the accessibility of their catalytic region [42].
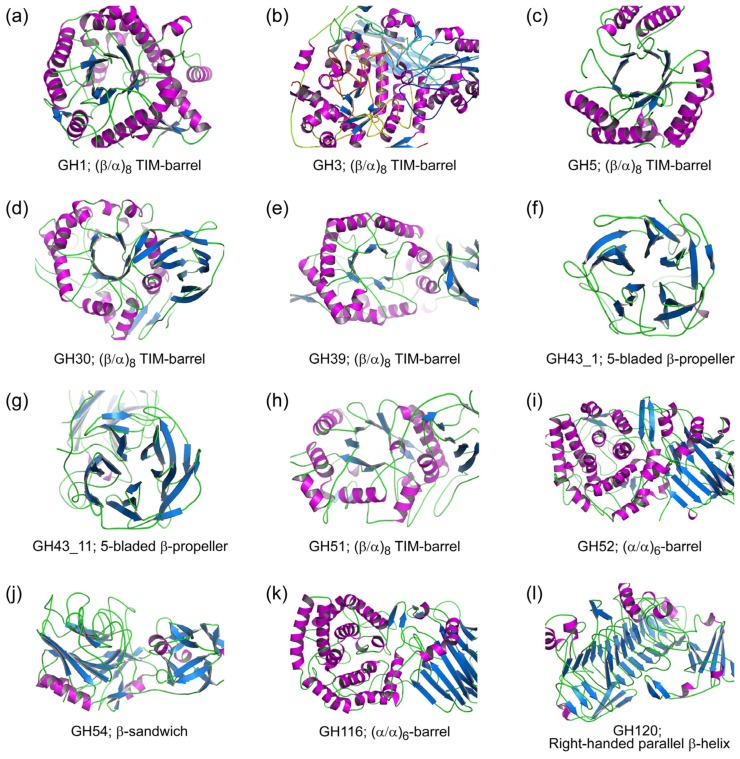
Figure 2.
Three-dimensional (3D) structures of β-xylosidases from various GH families. Helix, strand, and loop structures are colored in magenta, blue, and green, respectively. GH family numbers and fold type of their catalytic domains are shown. The structures represented are (a) RfBGluc-1 from Reticulitermes flavipes (GenPept ADK12988); (b) GlyA1 from metagenomic cow rumen fluid (PDB 5K6L); (c) PcXyl5 from Phanerochaete chrysosporium BKM-F-1767 (GenPept AHL69750) (d) PiBGX1 from Phytophthora infestans (GenPept AAK19754); (e) TsXynB from Thermoanaerobacterium saccharolyticum B6A-RI (PDB 1PX8); (f) RS223-BX from an uncultured organism (PDB 4MLG); (g) GsXynB3 from Geobacillus stearothermophilus T-6 (PDB 2EXH); (h) AtAraf from Arabidopsis thaliana (GenPept AAF19575); (i) GT2_24_00240 from Geobacillus thermoglucosidasius TM242 (PDB 4C1O); (j) TkAbf from Trichoderma koningii G-39 (GenPept AAA81024); (k) SSO1353 from Saccharolobus solfataricus P2 (GenPept AAK41589); and (l) TsXylC from Thermoanaerobacterium saccharolyticum JW/SL-YS485 (PDB 3VST). The structures of (a), (c), (d), (h), (j), and (k) were modeled using PDB entries 3VIK, 1EQP, 2XWE, 2C8N, 1WD3, and 5BVU, respectively, which belong to the same GH family but do not have β-xylosidase activity. Structure modeling was performed using the Swiss-Model server [46]. Figure 2, Figure 3, and Figure 6 were produced using the program PyMol (The PyMOL Molecular Graphics System, v. 0.99, Schrödinger, LLC, http://www.pymol.org).
In the absence of structural data for β-xylosidases from families GH1, GH5, GH30, and GH51 we generated homology models to compare the 3D structures of β-xylosidases from the different families of the GH-A clan. Models were built of the β-glucosidase/β-xylosidase RfBGluc-1 (GH1; Figure 2a) [34], a β-xylosidase from Phanerochaete chrysosporium BKM-F-1767 (PcXyl5; GenPept AHL69750; GH5; Figure 2c) [43], a β-glucosidase/β-xylosidase from Phytophthora infestans (PiBGX1; GenPept AAK19754; GH30; Figure 2d) [44], and an α-l-arabinofuranosidase/β-xylosidase from Arabidopsis thaliana (AtAraf; GenPept AAF19575; GH51; Figure 2h) [45], using 3D structures of their nearest homologs as templates. All resulting models display a (β/α)8-barrel catalytic domain that is highly similar to the catalytic domain of GH39 β-xylosidases (e.g., Figure 2e) and that shows that the catalytic residues of GH39 are present at the equivalent positions in the GH1, GH5, GH30, and GH51 β-xylosidase families. A multiple structural alignment of the catalytic domains of these models and GH39 β-xylosidases gave an overall RMSD of 3.4 Å for 168 amino acid residues, with PcXyl being the most divergent from the other structures. In contrast, the structures of their accessory domains varied with the family. The accessory domains of GH39 β-xylosidases are absent in RfBGluc-1 and PcXyl5, but they are retained at a comparable position in PiBGX1 and AtAraf albeit with some modifications. The major differences are observed for the third domain, in which the GH39 α-helical domain is replaced by a β-sheet and a loop structure in PiBGX1 and AtAraf, respectively.
2.2. Glycoside Hydrolase Family 3 (GH3)
While GH families 1, 30, 39 and 51 are part of clan GH-A, other β-xylosidases belong to other families that are not part of this clan. GH3 is one of the largest and most diverse GH families in the CAZy database [28,47]. It contains more than 23400 entries with various enzyme activities, including β-xylosidase, β-glucosidase, β-glucosylceramidase, β-N-acetylhexosaminidase, and α-l-arabinofuranosidase activities. A number of GH3 enzymes are reported to be bi/multifunctional, particularly toward synthetic substrates.
Enzymes in family GH3 vary considerably in the lengths of their peptide chains [48,49] and, consequently, in the number of tertiary structure domains [48,50]. The basic structure of GH3 members is a single (β/α)8 TIM-barrel domain [48,50], similar to the domain that is observed in clan GH-A. In most members, the domain is followed by an (α/β)-sandwich domain that varies in size [48], e.g., (α/β)6 in Kluyveromyces marxianus NBRC1777 β-glucosidase [51], (α/β)5 in Thermotoga neapolitana β-glucosidase [49], or even only an αβα motif in Bacillus subtilis 168 β-N-acetylglucosaminidases [52]. Sometimes the order of the domains in the primary structure is reversed [48]. Although these two domains are generally sufficient to organize the active site of GH3 enzymes, frequently GH3 members are extended with a fibronectin type III (FnIII) domain of unknown function at the C-terminus of the (α/β)-sandwich domain [48,49]. Moreover, in some GH3 members, the (α/β)-sandwich domain is interrupted by a PA14 domain. This domain appeared to be important for the substrate specificity of the Kluyveromyces marxianus NBRC1777 β-glucosidase [51].
A total of 103 enzymes with β-xylosidase annotation are currently found in GH3, making it the largest β-xylosidase-containing GH family. A protein domain search using the program InterProScan 5 [53] revealed that the majority of the GH3 β-xylosidases are composed of three domains (TIM-barrel, (α/β)-sandwich and FnIII). However, a bifunctional β-xylosidase/β-glucosidase from Erwinia chrysanthemi D1 (EcBgxA; GenPept AAA80156) [54] has two domains (TIM-barrel and (α/β)-sandwich) and a β-xylosidase from an environmental sample (G06-24; GenPept ACY24766) [55] has four domains (TIM-barrel, (α/β)-sandwich, FnIII, and PA14). A phylogenetic analysis clustered these two enzymes divergently from the other GH3 β-xylosidases [56].
3D Structures of GH3 β-xylosidases are available for a β-xylosidase from the fungus Trichoderma reesei RutC-30 (TrBxl1; PDB 5A7M; GenPept CAA93248) [57] and a β-glucosidase/β-xylosidase from metagenomic cow rumen fluid (GlyA1; PDB 5K6L; Figure 2b) [58]. Both structures have a (β/α)8 TIM-barrel, a (α/β)6-sandwich, and a FnIII domain, but at different positions in the primary structure. As observed for the majority of GH3 structures [48], TrBxl1 has its TIM-barrel domain at the N-terminus, followed sequentially by the (α/β)-sandwich and FnIII domains. This order is reversed in GlyA1, where the (α/β)-sandwich domain is at the N-terminus, followed by the FnIII and TIM-barrel domains. In addition, GlyA1 has an additional domain with unknown structure at its C-terminus [58]. Despite this, the 3D structures of TrBxl1 and GlyA1 are conserved, with the TIM-barrel and (α/β)-sandwich domains, as well as the catalytic residues superimposing reasonably well when the domains are structurally aligned.
2.3. Glycoside Hydrolase Family 43 (GH43)
GH43 is the second largest β-xylosidase-containing GH family with currently 96 members annotated as β-xylosidase. In addition to β-xylosidases, this family also contains enzymes with (putative) α-l-arabinofuranosidase, arabinanase, xylanase, galactan 1,3-β-galactosidase, α-1,2-l-arabinofuranosidase, exo-α-1,5-l-arabinofuranosidase, exo-α-1,5-l-arabinanase, or β-1,3-xylosidase activities. As observed for the GH3 members, several enzymes in this family are bi/multifunctional.
Together with GH62, GH43 is grouped into clan GH-F in the CAZy database with a structural characteristic of a 5-bladed β-propeller catalytic domain [59]. Some of its members contain only this single catalytic domain, and, based on their domain architecture, were classified as type I [60]. In other members, the catalytic domain is extended with a family 6 carbohydrate-binding module (type II), or a unique β-sandwich domain that is designated as X19 [61] (type III), or contain an even more complex domain composition and organization (type IV). The extensions are commonly fused at the C-terminus of the catalytic domain [60,62], although in a β-xylosidase from G. thermoleovorans IT-08 (GbtXyl43B), for example, the extension is at the N-terminus [63]. Thus, GH43 contains enzymes that vary both in the lengths of their primary structure and in their number of structure domains.
For detailed characterization, enzymes in GH43 have been divided into 37 subfamilies, GH41_1 to GH43_37 [61]. In this classification, β-xylosidases are currently found in 16 different subfamilies, with the majority belonging to subfamilies GH43_1 and GH43_11. Two GH43_1 β-xylosidase crystal structures are currently present in the PDB database, i.e., from an uncultured organism (RS223-BX; PDB 4MLG; Figure 2f) [64] and from a compost metagenome (CoXyl43; PDB 5GLK) [65]. The most structurally characterized β-xylosidases are from GH43_11, with crystal structures available of seven different β-xylosidases, i.e., the β-xylosidases from B. subtilis (PDB 1YIF) (Patskovsky et al., unpublished work), Clostridium acetobutylicum ATCC 824 (CaXyl43_11; PDB 1YI7) (Teplyakov et al., unpublished work), B. halodurans (PDB 1YRZ) (Fedorov et al., unpublished work), G. stearothermophilus T-6 (PDB 2EXH; Figure 2g) [66], Selenomonas ruminantium GA192 (PDB 3C2U) [67], B. pumilus IPO (PDB 5ZQJ) [68], and Bacillus sp. HJ14 (PDB 6IFE) [69]. Additionally, GH43 β-xylosidase 3D structures are also found in subfamilies GH43_12 and GH43_26, i.e., the β-xylosidases from G. thermoleovorans IT-08 (PDB 5Z5D) [70] and C. acetobutylicum ATCC 824 (CaXyl43_26; PDB 3K1U) (Osipiuk et al., unpublished work), respectively.
While β-xylosidases from GH43_1 and GH43_26 have only a single 5-bladed β-propeller catalytic domain [64,65] and belong to type I GH43 [60], those from GH43_11 and GH43_12 possess an additional X19 domain at the C-terminus [66,67,68,70] and belong to type III GH43 [60]. Although the architecture of the 5-bladed β-propeller is highly conserved among the GH43 β-xylosidases [64], structural superposition of the type I and type III catalytic domains gave a high RMSD. This is because the catalytic domains of the type I enzymes have several significantly longer loops than those of type III. The single catalytic domain of the type I GH43 β-xylosidases is sufficient for activity, but the enzymes are strongly activated by divalent metal ions, particularly calcium. Indeed, those metal-containing enzymes contain a metal-binding site close to the enzymes’ active site [64,65]. In contrast, the type III GH43 β-xylosidases have no such metal-binding site [66,67,68,70]. The X19 domain, which is only found in a subset of GH43 subfamilies [61], appeared to be crucial for catalytic activity of the type III GH43 β-xylosidases, since removing this domain abolished the activity of the GH43_11 β-xylosidases from Thermobifida fusca YX [71] and Enterobacter sp. [72]. In fact, a loop from the X19 domain contributes a Phe residue to the active site of the type III β-xylosidases [66,67,68,70], which is spatially conserved among all GH43 β-xylosidase structures. Only in CaXyl43_26 this Phe is missing. Unfortunately, no biochemical evidence is available on the enzyme’s substrate preferences and catalytic activity, but given that all other enzymes in GH43_26 are α-l-arabinofuranosidases [61], some doubt that CaXyl43_26 is a genuine β-xylosidase seems justified. These observations suggest that although GH43 β-xylosidases adopt different overall folds, the enzymes have a common active site organization and use a conserved Phe to interact with the substrate in subsite −1 (see below; Figure 4b).
2.4. Glycoside Hydrolase Family 52 (GH52)
Currently, GH52 contains 112 entries, of which 11 enzymes are annotated as β-xylosidases. These β-xylosidases have comparable amino acid sequence lengths of about 700 amino acid residues, with the exception of a β-xylosidase from G. stearothermophilus 236 (GsXylA, GenPept AAA50863), which is composed of only 618 amino acid residues. The GH52 β-xylosidases are very similar to each other with amino acid sequence identities of around 41%–90%. In this family, crystal structures are available for β-xylosidases from Parageobacillus thermoglucosidasius TM242 (GT2_24_00240; PDB 4C1O; Figure 2i) [73] and G. stearothermophilus T-6 (Xyn52B2; PDB 4RHH) (Dann et al., unpublished work). With a sequence identity of 86%, the two proteins fold into almost the same structures; they display two distinct domains, an N-terminal β-sandwich domain and a C-terminal (α/α)6-barrel domain. The catalytic residues of the GH52 enzymes, which are Glu-357 and Asp-517 in GT2_24_00240 [73], are located in the (α/α)6-barrel domain. Protein homology modeling based on the structure of GT2_24_00240 suggested that the domains are conserved among the GH52 β-xylosidases, except for the C-terminal domain of GsXylA. Compared to other GH52 β-xylosidases, this latter domain lacks five α-helices of the C-terminal domain, such that it displays an open half-barrel structure.
2.5. Glycoside Hydrolase Family 54 (GH54)
Most of the characterized enzymes in GH54 are annotated as α-l-arabinofuranosidases. However, two sequences in this family are annotated as β-xylosidase. TkAbf from T. koningii G-39 was characterized as a bifunctional α-l-arabinofuranosidase/β-xylosidase due to its activity on synthetic nitrophenyl derivatives of α-l-arabinofuranoside and β-d-xylopyranoside with comparable kcat/Km values [36]. A three-dimensional structure of a GH54 enzyme is currently only available for an α-l-arabinofuranosidase from A. kawachii IFO4308 (AkAbfB; PDB 1WD3) [38]. The primary structures of TkAbf (500 residues) and AkAbfB (499 residues) are very similar with an amino acid sequence identity of 73%. Therefore, the three-dimensional structure of TkAbf was predicted by homology modeling using the crystal structure of AkAbfB as a template. The predicted model (Figure 2j) consists of two domains that correspond to the N-terminal catalytic domain and the C-terminal arabinose-binding domain of the AkAbfB structure [38]. The catalytic domain folds into a β-sandwich similar to that of clan GH-B enzymes, while the arabinose-binding domain has a β-trefoil structure that belongs to the family 42 carbohydrate-binding module [28,38].
2.6. Glycoside Hydrolase Family 116 (GH116)
In GH116, SSO1353 is the only enzyme that exhibits β-xylosidase activity. As mentioned above, this enzyme does not hydrolyze xylooligosaccharides, but it is active on artificial substrates such as p-nitrophenyl- and methylumbelliferyl-linked β-d-xylopyranosides [37]. Currently, a three-dimensional structure of a GH116 member is only available for the β-glucosidase from the thermophilic bacterium T. xylanolyticum LX-11 (TxGH116; PDB 5BVU). This protein folds into an N-terminal β-sandwich domain and a C-terminal (α/α)6 solenoid catalytic domain [74]. The primary structure similarity of SSO1353 and TxGH116 is rather low with an amino acid sequence identity of only ~20%. However, homology modeling of SSO1353 based on the structure of TxGH116 using the Swiss-Model server [46] produced a relatively good quality model with a Global Model Quality Estimation (GMQE) value of 0.63 (on a scale of 0–1). As expected, the model displays a two-domain fold, i.e., an N-terminal β-sandwich domain and a C-terminal (α/α)6-barrel domain (Figure 2k), very much like the domain organization of the GH52 proteins (see above). Importantly, the modeling placed the catalytic nucleophile and acid/base residues of SSO1353 (Glu-335 and Asp-462, respectively) [37] at about the same positions as those of the GH52 β-xylosidase GT2_24_00240 (Glu-357 and Asp-517, respectively) [73]. In view of this structural similarity and the conservation of the catalytic residues, GH families 52 and 116 were recently grouped into clan GH-O [74].
2.7. Glycoside Hydrolase Family 120 (GH120)
Of the 176 sequences that are currently available in the CAZy database for the GH120 family, two enzymes were characterized and identified as β-xylosidases, i.e., enzymes from Thermoanaerobacterium saccharolyticum JW/SL-YS485 (TsXylC; GenPept ABM68042) [75] and Bifidobacterium adolescentis LMG10502 (BaXylB; GenPept BAF39080) [56]. While TsXylC was shown to be active on xylobiose and xylotriose [75], BaXylB prefers xylotriose or longer xylooligosaccharides as its substrate [56]. The three-dimensional structure of TsXylC has been reported to fold into a core domain of a right-handed parallel β-helix, a common fold observed in several GHs, polysaccharide lyases, and carbohydrate esterases. This core domain is intervened by an Ig-like β-sandwich domain (PDB 3VST, Figure 2l). Both domains are important to organize the active site of the enzyme [25]. BaXylB shares 47% amino acid sequence identity with TsXylC. A homology model of BaXylB based on the structure of TsXylC suggested that the active site residues and their positions are conserved in the enzymes, except for Trp-362 in BaXylB, which is a histidine (His-352) in TsXylC.
3. Active Site of β-Xylosidases
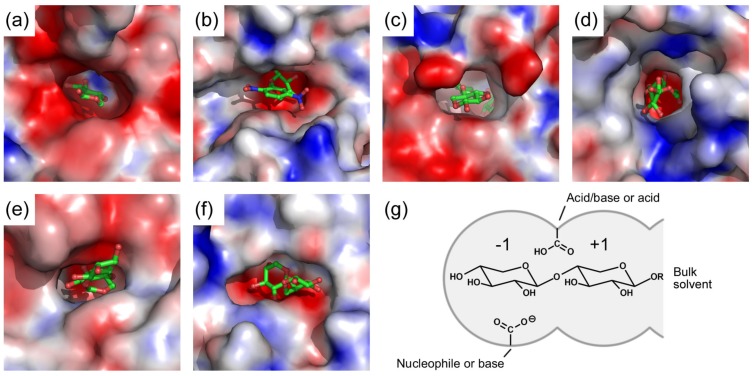
Despite the diversity of their three-dimensional folds, all structurally characterized β-xylosidases display a typical pocket-shaped active site (Figure 3) that is very suitable for exo-acting enzymes [29]. The pocket is negatively charged due to the presence of several acidic residues, but contains also hydrophobic patches of aromatic residues (Figure 3a–f). It has only a single route for substrates to enter and products to exit. The active site pocket can be virtually divided into two subsites with each of them able to accommodate a monosaccharide residue (Figure 3g). One subsite is buried, and, in several enzyme-xylobiose complexes (e.g., PDBs 2EXJ, 4C1P and 3VSU), interacts with the –1 non-reducing-end xylose (subsite –1), while the other is more open and binds the +1 xylose (subsite +1). Substrates with more than two xylose residues must have the additional residues beyond +1 exposed in the bulk solvent [25,67]. The active site architecture seems to be both necessary and sufficient for β-xylosidase activity of the enzymes.
Figure 3.
β-Xylosidase active site. Molecular surface drawing of active sites of β-xylosidases colored according to their electrostatic potential (negative, red; neutral, white; positive, blue). Complexed ligands are depicted in ball and stick representation with carbon atoms in green. The active sites are of (a) GlyA1 from metagenomic cow rumen fluid in complex with xylose (PDB 5K6N; GH3); (b) GsXynB1 from Geobacillus stearothermophilus T-6 in complex with 2,5-dinitrophenyl-β-d-xyloside (PDB 2BFG; GH39); (c) CoXyl43 from a compost metagenome in complex with xylose and xylobiose (PDB 5GLN; GH43_1); (d) GsXynB3 from G. stearothermophilus T-6 in complex with xylobiose (PDB 2EXJ; GH43_11); (e) GT2_24_00240 from Parageobacillus thermoglucosidasius TM242 in complex with xylobiose (PDB 4C1P; GH52); and (f) TsXylC from Thermoanaerobacterium saccharolyticum JW/SL-YS485 in complex with xylobiose (PDB 3VSU; GH120). The electrostatic potential was calculated using the APBS (Adaptive Poisson–Boltzmann Solver) implemented in the program PyMol [76]. (g) Generalized schematic diagram of a β-xylosidase active site with a ligand bound at subsites –1 and +1. Catalytic residues (see below) are represented by carboxylate groups and their catalytic roles are indicated. The exact positions of the catalytic residues vary with enzymes (see Figure 6).
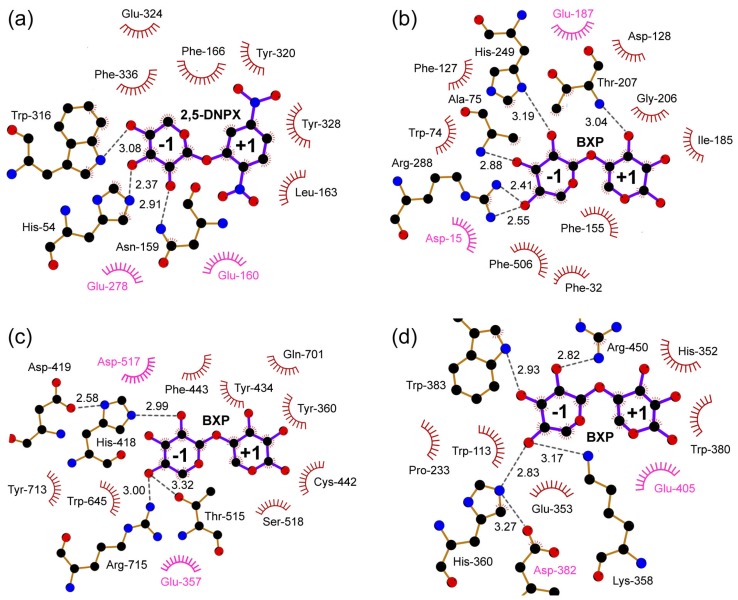
Furthermore, comparison of active site structures of several β-xylosidase-ligand complexes suggests that there are similar interactions between the enzymes and their ligands (Figure 4). The ligand in subsite –1 is strongly bound to the enzyme by a large number of hydrogen bonds and a few hydrophobic stacking interactions. In contrast, the ligand substrate in subsite +1 interacts less strongly with the enzyme with less hydrogen bonds but more hydrophobic stacking interactions.
Figure 4.
Interactions between active site residues of β-xylosidases and their ligands. The ligands 2,5-DNPX (2,5-dinitrophenyl-β-d-xyloside) and BXP (β-d-xylobiopyranose) are represented with purple bonds and their binding subsites -1 and +1 are indicated. Catalytic residues are labeled in magenta. Hydrogen bonds are shown as dashed lines and their distances are indicated in Å, while hydrophobic interactions are rendered with arcs. The active sites are of (a) GsXynB1 (PDB 2bfg); (b) GsXynB3 (PDB 2exj); (c) GT2_24_00240 (PDB 4c1p); and (d) TsXylC (PDB 3vsu), which represent β-xylosidases from GH families 39, 43, 52, and 120, respectively (see caption of Figure 3 for further details of the enzymes). Interaction analysis and figure preparation were performed using LigPlot+ [77].
4. Catalytic Mechanism of β-Xylosidases
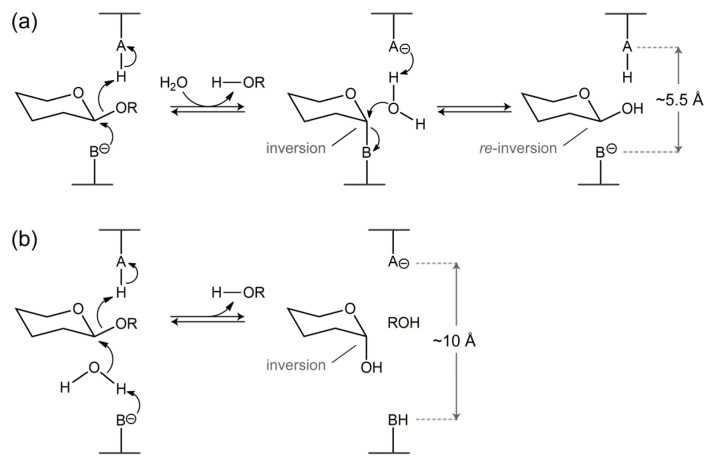
With respect to their catalytic mechanism most GHs can generally be classified into retaining and inverting enzymes [29,78]. The retaining GHs hydrolyze their substrates with overall retention of the stereochemistry of the anomeric carbon atom of the hydrolyzed glycosidic bond, while the inverting GHs yield a product with an inverted stereochemistry of the anomeric carbon atom [29,78]. In both mechanisms, the enzymes rely on two catalytic carboxylate groups that function as a nucleophile and a general acid/base in the retaining enzymes, or as a general base and a general acid in the inverting enzymes, respectively (Figure 5).
Figure 5.
The two common types of catalysis by glycoside hydrolases as adapted from Davies and Henrissat [29]. (a) The retaining mechanism. The nucleophile and the general acid/base are represented as B- and AH, respectively. (b) The inverting mechanism. The general base and the general acid are represented as B- and AH, respectively. The typical distances of the catalytic residues in both mechanisms are indicated in Å. In most GHs, A and B are either Asp or Glu. See main text for further details.
The retaining enzymes use a two-step double-displacement mechanism, in which enzyme glycosylation is followed by deglycosylation. In the glycosylation step, the nucleophile attacks the anomeric carbon to form a glycosyl–enzyme intermediate with the inverted configuration at the anomeric carbon. Concomitantly, the (protonated) acid/base residue transfers its proton to the glycosidic oxygen atom, to cleave the scissile glycosidic bond. Departure of the aglycone creates space allowing a catalytic water molecule to come closer to the anomeric center. In the deglycosylation step, the incoming catalytic water molecule, which is activated by the now negatively charged acid/base, attacks the anomeric carbon to release the glycone product from the intermediate. The attack re-inverts the inverted configuration of the anomeric carbon and hence the released glycone has the same stereochemistry as it had in the substrate. In contrast, the inverting enzymes follow a single-displacement mechanism to hydrolyze the glycosidic bond. A catalytic water molecule, which is deprotonated by the general base, does a nucleophilic attack on the anomeric carbon in concert with the general acid protonating the glycosidic oxygen. This cleaves the scissile glycosidic bond and frees the glycone with the inverted stereochemistry of its anomeric carbon.
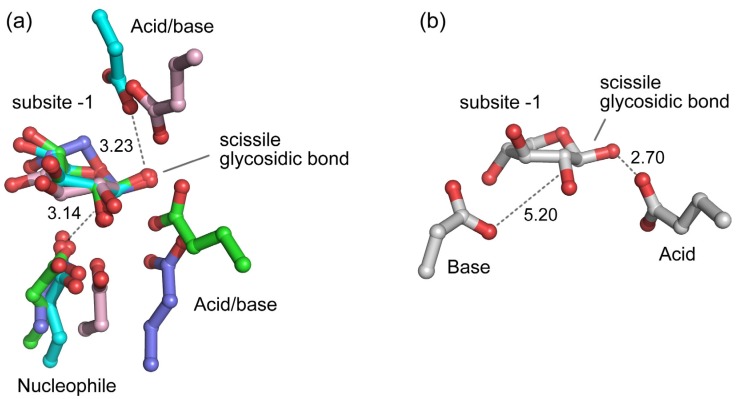
Except for the enzymes from family GH43, all β-xylosidases in the CAZy database are predicted to have a retaining mechanism (Table 1). Among these retaining β-xylosidases, structural data with bound ligand are available for enzymes from GH3 [58], GH39 [40,41,42], GH52 [73], and GH120 [25]. A structural alignment of these β-xylosidases on the basis of their bound ligand revealed that the carboxylate group of their catalytic nucleophiles, which are Glu in GH39 and GH52, and Asp in GH3 and GH120, are spatially conserved relative to the bound ligand (Figure 6a). They are within good distance (~3.1 Å) and right position for reaction with the anomeric carbon of the scissile glycosidic bond. On the other hand, the carboxylate group of their catalytic acid/base, which is Glu in GH3, GH39, and GH120, and Asp in GH52, are spatially less conserved, although they are at productive hydrogen-bonding positions (~3.2 Å on average) to the corresponding glycosidic oxygen atom.
Figure 6.
Positions of the catalytic residues relative to the xylosyl moiety bound in subsite -1 of the active sites of (a) retaining and (b) inverting β-xylosidases. The structures are of GlyA1 (PDB 5K6N; GH3; carbon atoms in pink), GsXynB1 (PDB 2BFG; GH39; green), GT2_24_00240 (PDB 4C1P; GH52; cyan), and TsXylC (PDB 3VSU; GH120; blue), which are retaining β-xylosidases, and GsXynB3 (PDB 2EXJ; GH43; white), which is an inverting β-xylosidase (see caption of Figure 3 for further details of the enzymes). Important distances (in Å) are shown next to dashed lines.
β-Xylosidases from GH43 are inverting enzymes [79]. They use Asp and Glu as the general base and general acid, respectively [70,80,81]. Similar to the catalytic acid/base of the retaining β-xylosidases, their catalytic acid is within hydrogen-bonding distance (~2.7 Å) to the glycosidic oxygen atom of the scissile bond (Figure 6b). However, compared to the catalytic nucleophile of the retaining β-xylosidases, their catalytic base is located further away from the anomeric carbon atom of the scissile glycosidic bond with a distance of ~5.2 Å [65,66,70]. This distance provides sufficient space for accommodating a catalytic water molecule that can be activated by the catalytic base to attack the anomeric carbon [66]. It has been observed generally for GHs that the distance between the carboxylate groups of the catalytic base and acid of retaining enzymes is shorter (~5 Å) than the distance between the carboxylates of the catalytic nucleophile and acid/base of inverting enzymes (~8–10 Å) [29,82]. This is also the case for the GH43 β-xylosidases. Indeed, in the inverting GH43 β-xylosidase from G. stearothermophilus T-6, for example, a distance of ~7.9 Å between the carboxylate groups of its catalytic residues has been observed [66].
5. Inhibition of β-Xylosidases by Monosaccharides
5.1. Inhibition by d-xylose
Many β-xylosidases are inhibited to varying degrees by their main product d-xylose (Table 2). For example, the β-xylosidase from the fungus Trichoderma harzianum C is very sensitive to d-xylose inhibition; its activity is completely inhibited by the presence of only 2 mM d-xylose [83]. In contrast, the GH39 β-xylosidase from the extreme thermophilic bacterium Dictyoglomus thermophilum DSM 3960 is very resistant to d-xylose, with only 40% inhibition in the presence of 3 M of the sugar [84].
Table 2.
Examples of microbial β-xylosidase inhibition by d-xylose.
| Organism | GH Family | d-xylose Concentration (mM) | Inhibition (%) | Reference |
|---|---|---|---|---|
| Bacteria: | ||||
| Bacillus halodurans C-125 | GH39 | 200 | 0 | [85] |
| Bacillus subtilis M015 | GH43_11 | 20 | 45 | [86] |
| Corynebacterium alkanolyticum ATCC 21511 | GH3 | 200 | 70 | [87] |
| Dictyoglomus thermophilum DSM 3960 | GH39 | 3000 | 40 | [84] |
| Geobacillus sp. WSUCF1 | GH39 | 300 | 50 | [88] |
| Geobacillus thermodenitrificans NG80-2 | GH39 | 400 | 50 | [89] |
| Geobacillus thermodenitrificans NG80-2 | GH43 | 300 | 50 | [89] |
| Geobacillus thermodenitrificans NG80-2 | GH52 | 600 | 50 | [89] |
| Lactobacillus brevis ATCC 14869 | GH43_11 | 100 | 20 | [90] |
| Lactobacillus brevis ATCC 14869 | GH43_12 | 100 | 66 | [90] |
| Massilia sp. RBM26 | GH43_11 | 500 | 50 | [91] |
| Paenibacillus woosongensis KCTC 3953 | GH43_35 | 100 | 25 | [92] |
| Selenomonas ruminantium GA192 | GH43_11 | 40 | 57 | [93] |
| Sphingobacterium sp. HP455 | GH43_1 | 247 | 50 | [94] |
| Thermoanaerobacterium saccharolyticum JW/SL-YS485 | GH120 | 200 | 30 | [75] |
| Thermotoga petrophila DSM 13995 | GH3 | 150 | 50 | [95] |
| Thermotoga thermarum DSM 5069 | GH3 | 1000 | 50 | [96] |
| Fungi: | ||||
| Aspergillus nidulans CECT2544 | n.a. # | 25 | 44 | [97] |
| Aspergillus niger 11 | n.a. # | 10 | 50 | [98] |
| Aspergillus niger ADH-11 | GH3 | 12 | 50 | [99] |
| Aureobasidium pullulans CBS 58475 | n.a. # | 6,6 | 42 | [100] |
| Candida utilis IFO 0639 | n.a. # | 300 | 0 | [101] |
| Humicola grisea var. thermoidea | GH43_1 | 603 | 50 | [102] |
| Humicola insolens Y1 | GH43_1 | 79 | 50 | [103] |
| Humicola insolens Y1 | GH43_11 | 292 | 50 | [103] |
| Paecilomyces thermophila J18 | n.a. # | 139 | 50 | [104] |
| Phanerochaete chrysosporium BKM-F-1767 | GH43_14 | 50 | 70 | [43] |
| Pseudozyma hubeiensis NCIM 3574 | n.a. # | 75 | 50 | [105] |
| Rhizophlyctis rosea Fischer NBRC 105426 | GH43_1 | 100 | 49 | [106] |
| Scytalidium thermophilum 77.7.8 | n.a. # | 200 | 0 | [107] |
| Trichoderma harzianum C | n.a. # | 2 | 100 | [83] |
| Trichoderma reesei QM 9414 | GH3 | 53 | 80 | [108] |
| Metagenomes: | ||||
| Synthetic metagenome | GH43_1 | 20 | 44 | [109] |
| Uncultured rumen metagenome | GH3 | 5 | 27 | [110] |
| Yak rumen metagenome (RuBg3A §) | GH3 | 5 | 18 | [111] |
| Yak rumen metagenome (RuBg3B §) | GH3 | 5 | 3 | [111] |
#: GH family is not assigned in the CAZy database; §: Protein symbol.
Most characterized β-xylosidases have significant affinity for d-xylose, with reported inhibition constants (Ki) of less than 10 mM (Table 3). To our knowledge, the β-xylosidase from Talaromyces emersonii has the highest affinity for d-xylose with a Ki value as low as 1.3 mM [112], suggesting that the enzyme is very sensitive to inhibition by this monosaccharide. Other β-xylosidases are much less prone to d-xylose inhibition, such as those from B. pumilus 12 [113], G. thermoleovorans IT-08 [114], and uncultured bacterium [115] with Ki’s of 26.2, 76, and 145 mM, respectively. So far, the β-xylosidase from the bacterium Cellulomonas uda [116] is the β-xylosidase with the highest reported Ki value for d-xylose, i.e., 650 mM, with the caveat that this Ki was determined using crude enzyme. Thus, in general, and as summarized in Table 3, β-xylosidases have relatively high affinity (low Ki) for d-xylose and, therefore, they are susceptible to product inhibition by this sugar.
Table 3.
Examples of inhibition constants for d-xylose of β-xylosidases.
| Organism | GH Family | Inhibition Constant (Ki, mM) | Reference |
|---|---|---|---|
| Bacteria: | |||
| Alkaliphilus metalliredigens QYMF | GH43_11 | 16.2 | [18] |
| Anoxybacillus sp. 3M | GH52 | 21.3 | [117] |
| Bacillus halodurans C-125 | GH43_11 | 62.3 | [118] |
| Bacillus pumilus 12 | n.a. # | 26.2 | [113] |
| Bacillus pumilus IPO | GH43_11 | 70 | [18] |
| Bacillus subtilis subsp. subtilis str. 168 | GH43_11 | 15.6 | [18] |
| Bacteroides ovatus V975 | GH43_1 | 6.6 | [119] |
| Caldocellum saccharolyticum Tp8T6.3.3.1 | n.a. # | 40.0 | [120] |
| Cellulomonas uda | n.a. # | 650.0 | [116] |
| Enterobacter sp. | GH43_11 | 79.9 | [72] |
| Geobacillus thermoleovorans IT-08 | GH43_12 | 76.0 | [114] |
| Lactobacillus brevis ATCC 367 | GH43_11 | 30.1 | [18] |
| Selenomonas ruminantium GA192 | GH43_11 | 6.24 | [121] |
| Streptomyces sp. CH7 | GH3 | 40.0 | [122] |
| Thermoanaerobacterium saccharolyticum B6A-RI | GH39 | 20 | [123] |
| Thermobifida fusca TM51 | GH43_11 | 67.0 | [124] |
| Thermobifida halotolerans YIM 90462T | GH43_11 | 43.8 | [125] |
| Thermomonospora | n.a. # | 35-100 | [126] |
| Thermomonospora fusca BD21 | n.a. # | 19 | [127] |
| Fungi: | |||
| Arxula adeninivorans SBUG 724 | n.a. # | 5.8 | [128] |
| Aspergillus awamori X-100 | GH3 | 7.7 | [129] |
| Aspergillus carbonarius KLU-93 | n.a. # | 1.9 | [130] |
| Aspergillus fumigatus | n.a. # | 4.5 | [131] |
| Aspergillus japonicus | GH3 | 2.9 | [132] |
| Aspergillus niger 15 | n.a. # | 2.9 | [133] |
| Aspergillus niger 90196 | GH3 | 8.3 | [134] |
| Aspergillus niger ATCC 10864 | GH3 | 3.3 | [135] |
| Aspergillus niger NW147 (xlnD I §) | GH3 | 9.8 | [136] |
| Aspergillus niger NW147 (xlnD II §) | GH3 | 13.2 | [136] |
| Aspergillus niger van Tieghem (DSM 22593) | GH3 | 7.5 | [137] |
| Aspergillus oryzae KBN616 | GH3 | 2.7 | [138] |
| Aspergillus terreus IJIRA 6.2 | n.a. # | 10.5 | [139] |
| Aspergillus versicolor (xylose-induced) | n.a. # | 5.3 | [140] |
| Aspergillus versicolor (xylan-induced) | n.a. # | 2.0 | [140] |
| Aureobasidium pullulans CBS 135684 | n.a. # | 18.0 | [141] |
| Colletotrichum graminicola | GH3 | 3.3 | [142] |
| Fusarium proliferatum NRRL 26517 | n.a. # | 5.0 | [143] |
| Fusarium verticillioides NRRL 26518 | n.a. # | 6.0 | [144] |
| Humicola insolens Y1 | GH3 | 29.0 | [145] |
| Neocallimastix frontalis RK 21 | n.a. # | 4.0 | [146] |
| Neurospora crassa ST A (74 A) | GH3 | 1.7 | [147] |
| Penicillium janczewskii CRM 1348 | n.a. # | 6 | [148] |
| Penicillium oxalicum 114-2 | GH43 | 28.1 | [149] |
| Penicillium sclerotiorum | n.a. # | 28.7 | [150] |
| Talaromyces amestolkiae | GH3 | 1.7 | [151] |
| Talaromyces emersonii | GH3 | 1.3 | [112] |
| Thermomyces lanuginosus CAU44 | GH43_1 | 63.0 | [152] |
| Trichoderma koningii G-39 | n.a. # | 5.0 | [153] |
| Trichoderma reesei (βXTR §) | GH3 | 2.4 | [112] |
| Trichoderma reesei | GH3 | 1.4 | [132] |
| Trichoderma reesei QM 9414 | n.a. # | 11.0 | [154] |
| Trichoderma reesei RUT C30 | n.a. # | 2.3 | [155] |
| Trichoderma reesei RUT C30 | n.a. # | 2.4 | [24] |
| Plant: | |||
| Saccharum officinarum L. | n.a. # | 8.0 | [156] |
| Metagenomes: | |||
| Compost starter | GH43 | 145.0 | [115] |
| Mixed microorganism (RS223-BX §) | GH43_1 | 3.4 | [19] |
| Uncultured rumen bacterium | GH30_2 | 10.6 | [157] |
| Uncultured rumen bacterium | GH43_1 | 76.0 | [157] |
#: GH family is not assigned in the CAZy database; §: Protein symbol.
From Table 2 and Table 3 it appears that no direct relationship exists between the inhibition of β-xylosidases by d-xylose and their organismal origin or GH family. Many β-xylosidases from different bacteria and fungi suffer from such product inhibition. Likewise, product inhibition is observed for β-xylosidases belonging to different GH families. This is reasonable because all β-xylosidases bind the same substrate (d-xylose oligomers), necessitating affinity for xylosyl residues and some commonality in their active site structure (see above).
Interestingly, the activity of several β-xylosidases is stimulated by d-xylose, particularly at low concentration. The β-xylosidase from Thermotoga thermarum DSM 5069 was stimulated by d-xylose concentrations of up to 500 mM; its maximum activity was observed in the presence of 200 mM d-xylose, which was ~20% higher than in the absence of the sugar [96]. A similar stimulatory effect has been reported for the bifunctional β-xylosidase/α-l-arabinofuranosidase from Phanerochaete chrysosporium BKM-F-1767 [43] and the β-xylosidase from Dictyoglomus thermophilum DSM 3960 [84,158]. However, the mechanism of such stimulation is currently not known.
5.2. Inhibition by l-arabinose
l-arabinose is one of the monosaccharides produced during enzymatic saccharification of cellulosic biomass [7,8,28]. It is liberated by the action of α-l-arabinofuranosidases, and, during the saccharification process, its concentration may sufficiently increase to inhibit the activity of hemicellulolytic enzymes [115]. Indeed, l-arabinose has been identified as an inhibitor of various β-xylosidases (Table 4). For instance, 50 mM l-arabinose reduces the activity of the aryl β-xylosidase from Caldocellum saccharolyticum Tp8T6.3.3.1 by 15% [120], the β-xylosidase from B. pumilus 12 by 21% [113], and the bifunctional β-xylosidase/α-l-arabinofuranosidase from P. chrysosporium BKM-F-1767 by ~70% [43]. This is not surprising, since the stereochemistry of l-arabinose near the glycosidic bond is similar to that of d-xylose [61], explaining its binding in the active site of a β-xylosidase.
Table 4.
Examples of microbial β-xylosidase inhibition by l-arabinose.
| Organism | GH Family | l-arabinose Concentration (mM) | Inhibition (%) | Reference |
|---|---|---|---|---|
| Bacteria: | ||||
| Bacillus pumilus 12 | n.a. # | 50 | 21 | [113] |
| Caldocellum saccharolyticum Tp8T6.3.3.1 | n.a. # | 50 | 15 | [120] |
| Corynebacterium alkanolyticum ATCC 21511 | GH3 | 200 | 40 | [87] |
| Lactobacillus brevis ATCC 14869 | GH43_11 | 100 | 39 | [90] |
| Lactobacillus brevis ATCC 14869 | GH43_12 | 100 | 38 | [90] |
| Paenibacillus woosongensis KCTC 3953 | GH43_35 | 100 | 40 | [92] |
| Selenomonas ruminantium GA192 | GH43_11 | 80 | 61 | [93] |
| Fungi: | ||||
| Aspergillus niger 11 | n.a. # | 25 | 10 | [98] |
| Aspergillus niger van Tieghem (DSM 22593) | GH3 | 200 | 30 | [137] |
| Colletotrichum graminicola | GH3 | 50 | 15 | [142] |
| Penicillium oxalicum 114-2 | GH43 | 20 | 11 | [149] |
| Phanerochaete chrysosporium BKM-F-1767 | GH43_14 | 50 | 70 | [43] |
#: GH family is not assigned in the CAZy database.
In general, l-arabinose is a weaker inhibitor of β-xylosidase activity than d-xylose. For example, the β-xylosidase from the fungus Trichoderma reesei RUT C30 is strongly inhibited by d-xylose with a Ki of 2.4 mM, but it is not inhibited by l-arabinose, even at a concentration of 500 mM [24]. Several other β-xylosidases can also withstand high concentrations of l-arabinose [95,122,141]. Finally, the β-xylosidase from Enterobacter sp. is competitively inhibited by l-arabinose, but with a quite high Ki value of 102 mM [72], indicating that the enzyme has only low affinity for l-arabinose.
Intriguingly, at low concentration (~5 mM) l-arabinose stimulates rather than inhibits the β-xylosidase activity of the bifunctional β-xylosidase/α-l-arabinofuranosidase from P. chrysosporium BKM-F-1767, as also noticed for d-glucose [43]. Activation by l-arabinose has also been observed for a furan aldehyde-tolerant β-xylosidase/α-l-arabinofuranosidase procured from a metagenomic sample, which showed 65% higher β-xylosidase activity compared with the control without l-arabinose [109]. Although there are many data describing the effects of l-arabinose on inhibition/activation of β-xylosidase activity, the molecular basis of the effects on activity still needs further investigation.
5.3. Inhibition by Other Monosaccharides
Apart from d-xylose and l-arabinose, d-glucose is another monosaccharide that has been frequently reported to affect β-xylosidase activity. d-glucose inhibits the β-xylosidase activity of the β-xylosidases from S. ruminantium GA192 (Ki 44 mM) [27], B. pumilus 12 (9% inhibition at 50 mM) [113], T. harzianum (3% inhibition at 5 mM) [83], and the bifunctional β-glucosidase/β-xylosidases RuBG3A and RuBG3B from the metagenome of yak rumen microorganisms (97.5% and 45.6% inhibition, respectively, at 5 mM) [111]. On the other hand, the sugar did not inhibit β-xylosidases from Thermomonospora fusca [159], A. niger 90196 [134], A. oryzae [138], and N. crassa ST A [147] at concentrations of up to 90, 20, 20, and 10 mM, respectively. The Mg2+-activated β-xylosidase RS223-BX could even withstand much higher d-glucose concentrations displaying a Ki value of 1270 mM on the substrate p-nitrophenyl-α-l-arabinofuranoside (pNPA) [19]. Apparently, inhibition by d-glucose varies considerably among β-xylosidases.
Finally, besides d-xylose, l-arabinose, and d-glucose, also other monosaccharides have been reported to inhibit β-xylosidases, including -d-arabinose [26,113], -d--erythrose [26], -d-fructose [111,138], d-galactose [26,113,120], -d-ribose, and -l-xylose [26,113]. Again, the molecular details of the interactions of these sugars with the enzymes are not known.
5.4. Inhibition Kinetics
The inhibition of β-xylosidases by monosaccharides follows competitive, non-competitive, or un-competitive inhibition kinetics. For most β-xylosidases, d-xylose acts as a competitive inhibitor when using p-nitrophenyl-β-d-xyloside (pNPX) as substrate [113,114,120,134]. However, the β-xylosidase from N. crassa ST A showed non-competitive inhibition by this sugar [147]. Furthermore, d-xylose inhibition of the β-xylosidase from S. ruminantium GA192, which also displays α-l-arabinofuranosidase activity, followed non-competitive kinetics for its β-xylosidase activity on pNPX as substrate, but competitive kinetics for its α-l-arabinofuranosidase activity on pNPA as substrate [26]. This differs slightly from A. carbonarius KLU-93 β-xylosidase, for which d-xylose was a competitive inhibitor for the conversion of both substrates [130]. Similarly, l-arabinose acts as a competitive inhibitor for the hydrolysis of pNPA by the RS223-BX β-xylosidase [19], but it is a non-competitive inhibitor of S. ruminantium GA192 β-xylosidase hydrolyzing pNPX or pNPA [26]. With respect to these substrates, -d-arabinose, d-glucose, and -d-ribose are competitive inhibitors of S. ruminantium GA192 β-xylosidase, whereas -d--erythrose and -l-xylose are non-competitive [26]. As also observed for S. ruminantium GA192 β-xylosidase, d-glucose inhibition of RS223-BX was competitive when using pNPA as substrate [19]. Uniquely, un-competitive inhibition was displayed by -d-fructose for the activity of A. oryzae β-xylosidase on pNPX [138]. Thus, commonly, competitive inhibition by d-xylose is observed. Other monosaccharides can display both competitive and non-competitive inhibition, and in one case, -d-fructose, un-competitive inhibition takes place. The exact mechanism and the structural details of non-competitive and un-competitive inhibition remain unknown.
5.5. Structural Details of Inhibitor Binding in the Active Site of β-Xylosidases
As discussed above, the active site pockets of β-xylosidases contain two substrate-binding subsites, subsites –1 and +1, on either side of the scissile bond. In the active site of S. ruminantium GA192 β-xylosidase, the monosaccharides -d-arabinose, l-arabinose, -d--erythrose, and -d-ribose can bind in both subsites –1 and +1, but d-xylose and -l-xylose bind only in subsite –1 [26]. Similarly, d-glucose binds in one subsite only. Its binding position was speculated to be in subsite –1, with partial occupancy of subsite +1, because glucose is too large to fit in subsite –1 only. Alternatively, it could bind in such a way that it excludes binding of a second sugar [26].
In contrast, G. thermoleovorans IT-08 β-xylosidase shows rather dissimilar binding properties for l-arabinose and d-xylose compared to S. ruminantium GA192 β-xylosidase. Crystal structures of G. thermoleovorans IT-08 β-xylosidase revealed that l-arabinose binds exclusively in subsite –1, while d-xylose prefers subsite +1 [70]. Thus, depending on the enzyme, the –1 and +1 subsites differ in preference for different monosaccharides, which could also contribute to the differences in inhibition kinetics observed for the different enzymes.
5.6. Engineering to Reduce β-Xylosidase Inhibition by Monosaccharides
During saccharification of cellulosic biomass, monosaccharides such as d-xylose, l-arabinose, and d-glucose, may reach concentrations that are high enough to inhibit β-xylosidase activity [26,27]. Therefore, β-xylosidases that are not affected by high monosaccharide concentrations are highly desirable for the efficiency of the saccharification process. To develop such β-xylosidase variants, the W145G mutation was introduced into S. ruminantium GA192 β-xylosidase, resulting in a variant with a 3-fold lower affinity for d-xylose and a 2-fold lower affinity for d-glucose [121]. Subjecting this variant to saturation mutagenesis of residue 145 yielded variants with even lower affinity for monosaccharides and higher catalytic activity than wild-type enzyme [27]. Mutation of Trp-145 alters the affinity of subsite +1 for d-xylose, but not that of subsite -1, where catalysis occurs, suggesting a strategy for reducing inhibition by monosaccharides by mutating residues of subsite +1 [27]. In the structure of G. thermoleovorans IT-08 β-xylosidase, d-xylose binds in subsite +1 interacting, among others, with Asp-198, which is not present in most other β-xylosidases [70]. Therefore, this residue may be a good target for mutation to obtain G. thermoleovorans IT-08 β-xylosidase variants with lower affinity for d-xylose.
6. Concluding Remarks
β-Xylosidases are highly diverse in their amino acid sequence. Currently, enzymes with β-xylosidase activity can be found in 11 different glycoside hydrolase families in the CAZy database, i.e., in GH families 1, 3, 5, 30, 39, 43, 51, 52, 54, 116, and 120. They fold into several distinct three-dimensional structures. While the enzymes from GH families 1, 3, 5, 30, 39, and 51 all show a (β/α)8 TIM-barrel structure, those from families 43, 52 and 116, 54, and 120 have as their main structural feature a 5-bladed β-propeller, a (α/α)6-barrel, a β-sandwich, and a right-handed parallel β-helix, respectively. Likewise, the catalytic mechanism of β-xylosidases is also varied. Although generally β-xylosidases hydrolyze their substrates with retention of the substrate’s anomeric carbon configuration, the enzymes from GH43 invert the anomeric configuration. However, despite their diversity in overall folds, all structurally characterized β-xylosidases have a typical pocket-shaped active site with two carbohydrate-binding subsites, which bind a xylobiosyl moiety of the xylooligosaccharide substrates. Unfortunately, the active sites of many β-xylosidases also possess a relatively high affinity for monosaccharides, such as xylose, arabinose, and erythrose, that competitively inhibit the enzymes’ activity. Moreover, some β-xylosidases are also non-competitively or un-competitively inhibited by monosaccharides. Such product inhibition limits the application of β-xylosidases in xylan saccharification, since high monosaccharide concentrations may easily be generated during the process. Therefore, β-xylosidases with low monosaccharide affinity are highly desirable for various applications. A random mutagenesis approach has already shown success in reducing the affinity of a β-xylosidase for d-xylose. Furthermore, with 3D structures available for a variety of β-xylosidases, rational site-directed mutagenesis may also be a good approach to render the enzymes less prone to product inhibition.
Author Contributions
Conceptualization, A.R., B.W.D., and N.N.T.P.; software, A.R.; validation, A.R., B.W.D., and N.N.T.P.; data curation, A.R.; writing—original draft preparation, A.R.; writing—review and editing, B.W.D., and N.N.T.P.; visualization, A.R.; supervision, B.W.D., and N.N.T.P.; funding acquisition, A.R. and N.N.T.P.
Funding
This work was funded by Universitas Airlangga to A.R. and N.N.T.P. (Hibah Riset Mandat No. 624/UN3.14/LT/2017). N.N.T.P is a receiver of a Tahir Professorship of Universitas Airlangga (SK Rektor Unair No. 1149/UN3/2018).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Gray K.A., Zhao L., Emptage M. Bioethanol. Curr. Opin. Chem. Biol. 2006;10:141–146. doi: 10.1016/j.cbpa.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 2.Saha B.C. Hemicellulose bioconversion. J. Ind. Microbiol. Biotechnol. 2003;30:279–291. doi: 10.1007/s10295-003-0049-x. [DOI] [PubMed] [Google Scholar]
- 3.Scheller H.V., Ulvskov P. Hemicelluloses. Annu. Rev. Plant. Biol. 2010;61:263–289. doi: 10.1146/annurev-arplant-042809-112315. [DOI] [PubMed] [Google Scholar]
- 4.Deutschmann R., Dekker R.F.H. From plant biomass to bio-based chemicals: Latest developments in xylan research. Biotechnol. Adv. 2012;30:1627–1640. doi: 10.1016/j.biotechadv.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Hahn-Hagerdal B., Galbe M., Gorwa-Grauslund M.F., Lidén G., Zacchi G. Bio-ethanol—The fuel of tomorrow from the residues of today. Trends Biotechnol. 2006;24:549–556. doi: 10.1016/j.tibtech.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Menon V., Rao M. Trends in bioconversion of lignocellulose: Biofuels, platform chemicals & biorefinery concept. Prog. Energy Combust. Sci. 2012;38:522–550. doi: 10.1016/j.pecs.2012.02.002. [DOI] [Google Scholar]
- 7.Beg Q.K., Kapoor M., Mahajan L., Hoondal G.S. Microbial xylanases and their industrial applications: A review. Appl. Microbiol. Biotechnol. 2001;56:326–338. doi: 10.1007/s002530100704. [DOI] [PubMed] [Google Scholar]
- 8.Biely P. Microbial xylanolytic systems. Trends Biotechnol. 1985;3:286–290. doi: 10.1016/0167-7799(85)90004-6. [DOI] [Google Scholar]
- 9.Collins T., Gerday C., Feller G. Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiol. Rev. 2005;29:3–23. doi: 10.1016/j.femsre.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Polizeli M.L.T.M., Rizzatti A.C.S., Monti R., Terenzi H.F., Jorge J.A., Amorim D.S. Xylanases from fungi: Properties and industrial applications. Appl. Microbiol. Biotechnol. 2005;67:577–591. doi: 10.1007/s00253-005-1904-7. [DOI] [PubMed] [Google Scholar]
- 11.Sunna A., Antranikian G. Xylanolytic enzymes from fungi and bacteria. Crit. Rev. Biotechnol. 1997;17:39–67. doi: 10.3109/07388559709146606. [DOI] [PubMed] [Google Scholar]
- 12.Chávez R., Bull P., Eyzaguirre J. The xylanolytic enzyme system from the genus Penicillium. J. Biotechnol. 2006;123:413–433. doi: 10.1016/j.jbiotec.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 13.Kulkarni N., Shendye A., Rao M. Molecular and biotechnological aspects of xylanases. FEMS Microbiol. Rev. 1999;23:411–456. doi: 10.1111/j.1574-6976.1999.tb00407.x. [DOI] [PubMed] [Google Scholar]
- 14.Prade R.A. Xylanases: From biology to biotechnology. Biotechnol. Genet. Eng. Rev. 1996;13:101–132. doi: 10.1080/02648725.1996.10647925. [DOI] [PubMed] [Google Scholar]
- 15.Subramaniyan S., Prema P. Biotechnology of microbial xylanases: Enzymology, molecular biology, and application. Crit. Rev. Biotechnol. 2002;22:33–64. doi: 10.1080/07388550290789450. [DOI] [PubMed] [Google Scholar]
- 16.Viikari L., Kantelinen A., Sundquist J., Linko M. Xylanases in bleaching: From an idea to the industry. FEMS Microbiol. Rev. 1994;13:335–350. doi: 10.1111/j.1574-6976.1994.tb00053.x. [DOI] [Google Scholar]
- 17.Jordan D.B., Wagschal K. Properties and applications of microbial β-d-xylosidases featuring the catalytically efficient enzyme from Selenomonas ruminantium. Appl. Microbiol. Biotechnol. 2010;86:1647–1658. doi: 10.1007/s00253-010-2538-y. [DOI] [PubMed] [Google Scholar]
- 18.Jordan D.B., Wagschal K., Grigorescu A.A., Braker J.D. Highly active β-xylosidases of glycoside hydrolase family 43 operating on natural and artificial substrates. Appl. Microbiol. Biotechnol. 2013;97:4415–4428. doi: 10.1007/s00253-012-4475-4. [DOI] [PubMed] [Google Scholar]
- 19.Lee C.C., Braker J.D., Grigorescu A.A., Wagschal K., Jordan D.B. Divalent metal activation of a GH43 β-xylosidase. Enzym. Microb. Technol. 2013;52:84–90. doi: 10.1016/j.enzmictec.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 20.Qing Q., Wyman C.E. Supplementation with xylanase and β-xylosidase to reduce xylo-oligomer and xylan inhibition of enzymatic hydrolysis of cellulose and pretreated corn stover. Biotechnol. Biofuels. 2011;4:18. doi: 10.1186/1754-6834-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qing Q., Yang B., Wyman C.E. Xylooligomers are strong inhibitors of cellulose hydrolysis by enzymes. Bioresour. Technol. 2010;101:9624–9630. doi: 10.1016/j.biortech.2010.06.137. [DOI] [PubMed] [Google Scholar]
- 22.Royer J.C., Nakas J.P. Purification and characterization of two xylanases from Trichoderma longibrachiatum. Eur. J. Biochem. 1991;202:521–529. doi: 10.1111/j.1432-1033.1991.tb16404.x. [DOI] [PubMed] [Google Scholar]
- 23.Williams S.J., Hoos R., Withers S.G. Nanomolar versus millimolar inhibition by xylobiose-derived azasugars: Significant differences between two structurally distinct xylanases. J. Am. Chem. Soc. 2000;122:2223–2235. doi: 10.1021/ja993805j. [DOI] [Google Scholar]
- 24.Herrmann M.C., Vrsanska M., Jurickova M., Hirsch J., Biely P., Kubicek C.P. The β-d-xylosidase of Trichoderma reesei is a multifunctional β-d-xylan xylohydrolase. Biochem. J. 1997;321:375–381. doi: 10.1042/bj3210375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang C.H., Sun Y., Ko T.P., Chen C.C., Zheng Y., Chan H.C., Pang X., Wiegel J., Shao W., Guo R.T. The substrate/product-binding modes of a novel GH120 β-xylosidase (XylC) from Thermoanaerobacterium saccharolyticum JW/SL-YS485. Biochem. J. 2012;448:401–407. doi: 10.1042/BJ20121359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jordan D.B., Braker J.D. Inhibition of the two-subsite β-d-xylosidase from Selenomonas ruminantium by sugars: Competitive, noncompetitive, double binding, and slow binding modes. Arch. Biochem. Biophys. 2007;465:231–246. doi: 10.1016/j.abb.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 27.Jordan D.B., Wagschal K., Fan Z., Yuan L., Braker J.D., Heng C. Engineering lower inhibitor affinities in β-d-xylosidase of Selenomonas ruminantium by site-directed mutagenesis of Trp145. J. Ind. Microbiol. Biotechnol. 2011;38:1821–1835. doi: 10.1007/s10295-011-0971-2. [DOI] [PubMed] [Google Scholar]
- 28.Lagaert S., Pollet A., Courtin C.M., Volckaert G. β-Xylosidases and α-l-arabinofuranosidases: Accessory enzymes for arabinoxylan degradation. Biotechnol. Adv. 2014;32:316–332. doi: 10.1016/j.biotechadv.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Davies G., Henrissat B. Structures and mechanisms of glycosyl hydrolases. Structure. 1995;3:853–859. doi: 10.1016/S0969-2126(01)00220-9. [DOI] [PubMed] [Google Scholar]
- 30.Henrissat B. A classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem. J. 1991;280:309–316. doi: 10.1042/bj2800309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lombard V., Golaconda Ramulu H., Drula E., Coutinho P.M., Henrissat B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014;42:D490–D495. doi: 10.1093/nar/gkt1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Claeyssens M., Henrissat B. Specificity mapping of cellulolytic enzymes: Classification into families of structurally related proteins confirmed by biochemical analysis. Protein Sci. 1992;1:1293–1297. doi: 10.1002/pro.5560011008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henrissat B., Bairoch A. Updating the sequence-based classification of glycosyl hydrolases. Biochem. J. 1996;316:695–696. doi: 10.1042/bj3160695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scharf M.E., Kovaleva E.S., Jadhao S., Campbell J.H., Buchman G.W., Boucias D.G. Functional and translational analyses of a β-glucosidase gene (glycosyl hydrolase family 1) isolated from the gut of the lower termite Reticulitermes flavipes. Insect Biochem. Mol. Biol. 2010;40:611–620. doi: 10.1016/j.ibmb.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 35.Mattéotti C., Haubruge E., Thonart P., Francis F., De Pauw E., Portetelle D., Vandenbol M. Characterization of a new β-glucosidase/β-xylosidase from the gut microbiota of the termite (Reticulitermes santonensis) FEMS Microbiol. Lett. 2011;314:147–157. doi: 10.1111/j.1574-6968.2010.02161.x. [DOI] [PubMed] [Google Scholar]
- 36.Wan C.-F., Chen C.-T., Huang L., Li Y.-K. Expression, purification and characterization of a bifunctional α-l-arabinofuranosidase/β-d-xylosidase from Trichoderma koningii G-39. J. Chin. Chem. Soc. 2007;54:109–116. doi: 10.1002/jccs.200700018. [DOI] [Google Scholar]
- 37.Cobucci-Ponzano B., Aurilia V., Riccio G., Henrissat B., Coutinho P.M., Strazzulli A., Padula A., Corsaro M.M., Pieretti G., Pocsfalvi G., et al. A new archaeal β-glycosidase from Sulfolobus solfataricus: Seeding a novel retaining β-glycan-specific glycoside hydrolase family along with the human non-lysosomal glucosylceramidase GBA2. J. Biol. Chem. 2010;285:20691–20703. doi: 10.1074/jbc.M109.086470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyanaga A., Koseki T., Matsuzawa H., Wakagi T., Shoun H., Fushinobu S. Crystal structure of a family 54 α-l-arabinofuranosidase reveals a novel carbohydrate-binding module that can bind arabinose. J. Biol. Chem. 2004;279:44907–44914. doi: 10.1074/jbc.M405390200. [DOI] [PubMed] [Google Scholar]
- 39.Naumoff D.G. Hierarchical classification of glycoside hydrolases. Biochemistry. 2011;76:764–780. doi: 10.1134/S0006297911060022. [DOI] [PubMed] [Google Scholar]
- 40.Yang J.K., Yoon H.J., Ahn H.J., Lee B.I., Pedelacq J.-D., Liong E.C., Berendzen J., Laivenieks M., Vieille C., Zeikus G.J., et al. Crystal structure of β-d-xylosidase from Thermoanaerobacterium saccharolyticum, a family 39 glycoside hydrolase. J. Mol. Biol. 2004;335:155–165. doi: 10.1016/j.jmb.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 41.Czjzek M., Ben-David A., Bravman T., Shoham G., Henrissat B., Shoham Y. Enzyme-substrate complex structures of a GH39 β-xylosidase from Geobacillus stearothermophilus. J. Mol. Biol. 2005;353:838–846. doi: 10.1016/j.jmb.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 42.Santos C.R., Polo C.C., Corrêa J.M., Simão R.C.G., Seixas F.A.V., Murakami M.T. The accessory domain changes the accessibility and molecular topography of the catalytic interface in monomeric GH39 β-xylosidases. Acta Crystallogr. D Biol. Crystallogr. 2012;68:1339–1345. doi: 10.1107/S0907444912028491. [DOI] [PubMed] [Google Scholar]
- 43.Huy N.D., Thayumanavan P., Kwon T.-H., Park S.-M. Characterization of a recombinant bifunctional xylosidase/arabinofuranosidase from Phanerochaete chrysosporium. J. Biosci. Bioeng. 2013;116:152–159. doi: 10.1016/j.jbiosc.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 44.Brunner F., Wirtz W., Rose J.K.C., Darvill A.G., Govers F., Scheel D., Nürnberger T. A β-glucosidase/xylosidase from the phytopathogenic oomycete, Phytophthora infestans. Phytochemistry. 2002;59:689–696. doi: 10.1016/S0031-9422(02)00045-6. [DOI] [PubMed] [Google Scholar]
- 45.Minic Z., Rihouey C., Do C.T., Lerouge P., Jouanin L. Purification and characterization of enzymes exhibiting β-d-xylosidase activities in stem tissues of Arabidopsis. Plant. Physiol. 2004;135:867–878. doi: 10.1104/pp.104.041269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Biasini M., Bienert S., Waterhouse A., Arnold K., Studer G., Schmidt T., Kiefer F., Cassarino T.G., Bertoni M., Bordoli L., et al. SWISS-MODEL: Modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014;42:W252–W258. doi: 10.1093/nar/gku340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suzuki K., Sumitani J.-i., Nam Y.-W., Nishimaki T., Tani S., Wakagi T., Kawaguchi T., Fushinobu S. Crystal structures of glycoside hydrolase family 3 β-glucosidase 1 from Aspergillus aculeatus. Biochem. J. 2013;452:211–221. doi: 10.1042/BJ20130054. [DOI] [PubMed] [Google Scholar]
- 48.Harvey A.J., Hrmova M., De Gori R., Varghese J.N., Fincher G.B. Comparative modeling of the three-dimensional structures of family 3 glycoside hydrolases. Proteins. 2000;41:257–269. doi: 10.1002/1097-0134(20001101)41:2<257::AID-PROT100>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 49.Pozzo T., Pasten J.L., Karlsson E.N., Logan D.T. Structural and functional analyses of β-glucosidase 3B from Thermotoga neapolitana: A thermostable three-domain representative of glycoside hydrolase 3. J. Mol. Biol. 2010;397:724–739. doi: 10.1016/j.jmb.2010.01.072. [DOI] [PubMed] [Google Scholar]
- 50.Karkehabadi S., Helmich K.E., Kaper T., Hansson H., Mikkelsen N.-E., Gudmundsson M., Piens K., Fujdala M., Banerjee G., Scott-Craig J.S., et al. Biochemical characterization and crystal structures of a fungal family 3 β-glucosidase, Cel3A from Hypocrea jecorina. J. Biol. Chem. 2014;289:31624–31637. doi: 10.1074/jbc.M114.587766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoshida E., Hidaka M., Fushinobu S., Koyanagi T., Minami H., Tamaki H., Kitaoka M., Katayama T., Kumagai H. Role of a PA14 domain in determining substrate specificity of a glycoside hydrolase family 3 β-glucosidase from Kluyveromyces marxianus. Biochem. J. 2010;431:39–49. doi: 10.1042/BJ20100351. [DOI] [PubMed] [Google Scholar]
- 52.Litzinger S., Fischer S., Polzer P., Diederichs K., Welte W., Mayer C. Structural and kinetic analysis of Bacillus subtilis N-acetylglucosaminidase reveals a unique Asp-His dyad mechanism. J. Biol. Chem. 2010;285:35675–35684. doi: 10.1074/jbc.M110.131037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jones P., Binns D., Chang H.Y., Fraser M., Li W., McAnulla C., McWilliam H., Maslen J., Mitchell A., Nuka G., et al. InterProScan 5: Genome-scale protein function classification. Bioinformatics. 2014;30:1236–1240. doi: 10.1093/bioinformatics/btu031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vroemen S., Heldens J., Boyd C., Henrissat B., Keen N.T. Cloning and characterization of the bgxA gene from Erwinia chrysanthemi D1 which encodes a β-glucosidase/xylosidase enzyme. Mol. Gen. Genet. 1995;246:465–477. doi: 10.1007/BF00290450. [DOI] [PubMed] [Google Scholar]
- 55.Beloqui A., Nechitaylo T.Y., López-Cortés N., Ghazi A., Guazzaroni M.-E., Polaina J., Strittmatter A.W., Reva O., Waliczek A., Yakimov M.M., et al. Diversity of glycosyl hydrolases from cellulose-depleting communities enriched from casts of two earthworm species. Appl. Environ. Microbiol. 2010;76:5934–5946. doi: 10.1128/AEM.00902-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lagaert S., Pollet A., Delcour J.A., Lavigne R., Courtin C.M., Volckaert G. Characterization of two β-xylosidases from Bifidobacterium adolescentis and their contribution to the hydrolysis of prebiotic xylooligosaccharides. Appl. Microbiol. Biotechnol. 2011;92:1179–1185. doi: 10.1007/s00253-011-3396-y. [DOI] [PubMed] [Google Scholar]
- 57.Margolles-Clark E., Tenkanen M., Nakari-Setälä T., Penttilä M. Cloning of genes encoding α-l-arabinofuranosidase and β-xylosidase from Trichoderma reesei by expression in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 1996;62:3840–3846. doi: 10.1128/aem.62.10.3840-3846.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramírez-Escudero M., del Pozo M.V., Marín-Navarro J., González B., Golyshin P.N., Polaina J., Ferrer M., Sanz-Aparicio J. Structural and functional characterization of a ruminal β-glycosidase defines a novel subfamily of glycoside hydrolase family 3 with permuted domain topology. J. Biol. Chem. 2016;291:24200–24214. doi: 10.1074/jbc.M116.747527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nurizzo D., Turkenburg J.P., Charnock S.J., Roberts S.M., Dodson E.J., McKie V.A., Taylor E.J., Gilbert H.J., Davies G.J. Cellvibrio japonicus α-l-arabinanase 43A has a novel five-blade β-propeller fold. Nat. Struct. Biol. 2002;9:665–668. doi: 10.1038/nsb835. [DOI] [PubMed] [Google Scholar]
- 60.Yoshida S., Hespen C.W., Beverly R.L., Mackie R.I., Cann I.K.O. Domain analysis of a modular α-l-arabinofuranosidase with a unique carbohydrate binding strategy from the fiber-degrading bacterium Fibrobacter succinogenes S85. J. Bacteriol. 2010;192:5424–5436. doi: 10.1128/JB.00503-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mewis K., Lenfant N., Lombard V., Henrissat B. Dividing the large glycoside hydrolase family 43 into subfamilies: A Motivation for detailed enzyme characterization. Appl. Environ. Microbiol. 2016;82:1686–1692. doi: 10.1128/AEM.03453-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ferrer M., Ghazi A., Beloqui A., Vieites J.M., López-Cortés N., Marín-Navarro J., Nechitaylo T.Y., Guazzaroni M.-E., Polaina J., Waliczek A., et al. Functional metagenomics unveils a multifunctional glycosyl hydrolase from the family 43 catalysing the breakdown of plant polymers in the calf rumen. PLoS ONE. 2012;7:e38134. doi: 10.1371/journal.pone.0038134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ratnadewi A.A.I., Fanani M., Kurniasih S.D., Sakka M., Wasito E.B., Sakka K., Nurachman Z., Puspaningsih N.N.T. β-d-Xylosidase from Geobacillus thermoleovorans IT-08: Biochemical characterization and bioinformatics of the enzyme. Appl. Biochem. Biotechnol. 2013;170:1950–1964. doi: 10.1007/s12010-013-0329-5. [DOI] [PubMed] [Google Scholar]
- 64.Jordan D.B., Braker J.D., Wagschal K., Lee C.C., Chan V.J., Dubrovska I., Anderson S., Wawrzak Z. X-ray crystal structure of divalent metal-activated β-xylosidase, RS223BX. Appl. Biochem. Biotechnol. 2015;177:637–648. doi: 10.1007/s12010-015-1767-z. [DOI] [PubMed] [Google Scholar]
- 65.Matsuzawa T., Kaneko S., Kishine N., Fujimoto Z., Yaoi K. Crystal structure of metagenomic β-xylosidase/α-l-arabinofuranosidase activated by calcium. J. Biochem. 2017;162:173–181. doi: 10.1093/jb/mvx012. [DOI] [PubMed] [Google Scholar]
- 66.Brüx C., Ben-David A., Shallom-Shezifi D., Leon M., Niefind K., Shoham G., Shoham Y., Schomburg D. The structure of an inverting GH43 β-xylosidase from Geobacillus stearothermophilus with its substrate reveals the role of the three catalytic residues. J. Mol. Biol. 2006;359:97–109. doi: 10.1016/j.jmb.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 67.Brunzelle J.S., Jordan D.B., McCaslin D.R., Olczak A., Wawrzak Z. Structure of the two-subsite β-d-xylosidase from Selenomonas ruminantium in complex with 1,3-bis[tris(hydroxymethyl)methylamino]propane. Arch. Biochem. Biophys. 2008;474:157–166. doi: 10.1016/j.abb.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 68.Hong S., Kyung M., Jo I., Kim Y.-R., Ha N.-C. Structure-based protein engineering of bacterial β-xylosidase to increase the production yield of xylobiose from xylose. Biochem. Biophys. Res. Commun. 2018;501:703–710. doi: 10.1016/j.bbrc.2018.05.051. [DOI] [PubMed] [Google Scholar]
- 69.Zhang R., Li N., Liu Y., Han X., Tu T., Shen J., Xu S., Wu Q., Zhou J., Huang Z. Biochemical and structural properties of a low-temperature-active glycoside hydrolase family 43 β-xylosidase: Activity and instability at high neutral salt concentrations. Food Chem. 2019;301:125266. doi: 10.1016/j.foodchem.2019.125266. [DOI] [PubMed] [Google Scholar]
- 70.Rohman A., van Oosterwijk N., Puspaningsih N.N.T., Dijkstra B.W. Structural basis of product inhibition by arabinose and xylose of the thermostable GH43 β-1,4-xylosidase from Geobacillus thermoleovorans IT-08. PLoS ONE. 2018;13:e0196358. doi: 10.1371/journal.pone.0196358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morais S., Salama-Alber O., Barak Y., Hadar Y., Wilson D.B., Lamed R., Shoham Y., Bayer E.A. Functional association of catalytic and ancillary modules dictates enzymatic activity in glycoside hydrolase family 43 β-xylosidase. J. Biol. Chem. 2012;287:9213–9221. doi: 10.1074/jbc.M111.314286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ontañon O.M., Ghio S., Marrero Díaz de Villegas R., Piccinni F.E., Talia P.M., Cerutti M.L., Campos E. EcXyl43 β-xylosidase: Molecular modeling, activity on natural and artificial substrates, and synergism with endoxylanases for lignocellulose deconstruction. Appl. Microbiol. Biotechnol. 2018;102:6959–6971. doi: 10.1007/s00253-018-9138-7. [DOI] [PubMed] [Google Scholar]
- 73.Espina G., Eley K., Pompidor G., Schneider T.R., Crennell S.J., Danson M.J. A novel β-xylosidase structure from Geobacillus thermoglucosidasius: The first crystal structure of a glycoside hydrolase family GH52 enzyme reveals unpredicted similarity to other glycoside hydrolase folds. Acta Crystallogr. D Biol. Crystallogr. 2014;70:1366–1374. doi: 10.1107/S1399004714002788. [DOI] [PubMed] [Google Scholar]
- 74.Charoenwattanasatien R., Pengthaisong S., Breen I., Mutoh R., Sansenya S., Hua Y., Tankrathok A., Wu L., Songsiriritthigul C., Tanaka H., et al. Bacterial β-glucosidase reveals the structural and functional basis of genetic defects in human glucocerebrosidase 2 (GBA2) ACS Chem. Biol. 2016;11:1891–1900. doi: 10.1021/acschembio.6b00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shao W., Xue Y., Wu A., Kataeva I., Pei J., Wu H., Wiegel J. Characterization of a novel β-xylosidase, XylC, from Thermoanaerobacterium saccharolyticum JW/SL-YS485. Appl. Environ. Microbiol. 2011;77:719–726. doi: 10.1128/AEM.01511-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baker N.A., Sept D., Joseph S., Holst M.J., McCammon J.A. Electrostatics of nanosystems: Application to microtubules and the ribosome. Proc. Natl. Acad. Sci. USA. 2001;98:10037–10041. doi: 10.1073/pnas.181342398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Laskowski R.A., Swindells M.B. LigPlot{+}: Multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 2011;51:2778–2786. doi: 10.1021/ci200227u. [DOI] [PubMed] [Google Scholar]
- 78.McCarter J.D., Withers S.G. Mechanisms of enzymatic glycoside hydrolysis. Curr. Opin. Struct. Biol. 1994;4:885–892. doi: 10.1016/0959-440X(94)90271-2. [DOI] [PubMed] [Google Scholar]
- 79.Braun C., Meinke A., Ziser L., Withers S.G. Simultaneous high-performance liquid chromatographic determination of both the cleavage pattern and the stereochemical outcome of the hydrolysis reactions catalyzed by various glycosidases. Anal. Biochem. 1993;212:259–262. doi: 10.1006/abio.1993.1320. [DOI] [PubMed] [Google Scholar]
- 80.Jordan D.B., Li X.-L., Dunlap C.A., Whitehead T.R., Cotta M.A. Structure-function relationships of a catalytically efficient β-d-xylosidase. Appl. Biochem. Biotechnol. 2007;141:51–76. doi: 10.1007/s12010-007-9210-8. [DOI] [PubMed] [Google Scholar]
- 81.Shallom D., Leon M., Bravman T., Ben-David A., Zaide G., Belakhov V., Shoham G., Schomburg D., Baasov T., Shoham Y. Biochemical characterization and identification of the catalytic residues of a family 43 β-d-xylosidase from Geobacillus stearothermophilus T-6. Biochemistry. 2005;44:387–397. doi: 10.1021/bi048059w. [DOI] [PubMed] [Google Scholar]
- 82.Rye C.S., Withers S.G. Glycosidase mechanisms. Curr. Opin. Chem. Biol. 2000;4:573–580. doi: 10.1016/S1367-5931(00)00135-6. [DOI] [PubMed] [Google Scholar]
- 83.Ximenes F.D.A., de Paula Silveira F.Q., FFilho E.X. Production of β-xylosidase activity by Trichoderma harzianum strains. Curr. Microbiol. 1996;33:71–77. doi: 10.1007/s002849900077. [DOI] [PubMed] [Google Scholar]
- 84.Li Q., Wu T., Qi Z., Zhao L., Pei J., Tang F. Characterization of a novel thermostable and xylose-tolerant GH 39 β-xylosidase from Dictyoglomus thermophilum. BMC Biotechnol. 2018;18:29. doi: 10.1186/s12896-018-0440-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wagschal K., Franqui-Espiet D., Lee C.C., Robertson G.H., Wong D.W.S. Cloning, expression and characterization of a glycoside hydrolase family 39 xylosidase from Bacillus halodurans C-125. Appl. Biochem. Biotechnol. 2008;146:69–78. doi: 10.1007/s12010-007-8055-5. [DOI] [PubMed] [Google Scholar]
- 86.Banka A.L., Guralp S.A., Gulari E. Secretory expression and characterization of two hemicellulases, xylanase, and β-xylosidase, isolated from Bacillus subtilis M015. Appl. Biochem. Biotechnol. 2014;174:2702–2710. doi: 10.1007/s12010-014-1219-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Watanabe A., Hiraga K., Suda M., Yukawa H., Inui M. Functional characterization of Corynebacterium alkanolyticum β-xylosidase and xyloside ABC transporter in Corynebacterium glutamicum. Appl. Environ. Microbiol. 2015;81:4173–4183. doi: 10.1128/AEM.00792-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bhalla A., Bischoff K.M., Sani R.K. Highly thermostable GH39 β-xylosidase from a Geobacillus sp. strain WSUCF1. BMC Biotechnol. 2014;14:963. doi: 10.1186/s12896-014-0106-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huang D., Liu J., Qi Y., Yang K., Xu Y., Feng L. Synergistic hydrolysis of xylan using novel xylanases, β-xylosidases, and an α-l-arabinofuranosidase from Geobacillus thermodenitrificans NG80-2. Appl. Microbiol. Biotechnol. 2017;101:6023–6037. doi: 10.1007/s00253-017-8341-2. [DOI] [PubMed] [Google Scholar]
- 90.Michlmayr H., Hell J., Lorenz C., Böhmdorfer S., Rosenau T., Kneifel W. Arabinoxylan oligosaccharide hydrolysis by family 43 and 51 glycosidases from Lactobacillus brevis DSM 20054. Appl. Environ. Microbiol. 2013;79:6747–6754. doi: 10.1128/AEM.02130-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu B., Dai L., Zhang W., Yang Y., Wu Q., Li J., Tang X., Zhou J., Ding J., Han N., et al. Characterization of a novel salt-, xylose- and alkali-tolerant GH43 bifunctional β-xylosidase/α-l-arabinofuranosidase from the gut bacterial genome. J. Biosci. Bioeng. 2019;128:429–437. doi: 10.1016/j.jbiosc.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 92.Kim Y.A., Yoon K.-H. Characterization of a Paenibacillus woosongensis β-xylosidase/α-arabinofuranosidase produced by recombinant Escherichia coli. J. Microbiol. Biotechnol. 2010;20:1711–1716. [PubMed] [Google Scholar]
- 93.Whitehead T.R., Cotta M.A. Identification of a broad-specificity xylosidase/arabinosidase important for xylooligosaccharide fermentation by the ruminal anaerobe Selenomonas ruminantium GA192. Curr. Microbiol. 2001;43:293–298. doi: 10.1007/s002840010304. [DOI] [PubMed] [Google Scholar]
- 94.Sheng P., Xu J., Saccone G., Li K., Zhang H. Discovery and characterization of endo-xylanase and β-xylosidase from a highly xylanolytic bacterium in the hindgut of Holotrichia parallela larvae. J. Mol. Catal. B Enzym. 2014;105:33–40. doi: 10.1016/j.molcatb.2014.03.019. [DOI] [Google Scholar]
- 95.Zhang S., Xie J., Zhao L., Pei J., Su E., Xiao W., Wang Z. Cloning, overexpression and characterization of a thermostable β-xylosidase from Thermotoga petrophila and cooperated transformation of ginsenoside extract to ginsenoside 20(S)-Rg3 with a β-glucosidase. Bioorg. Chem. 2019;85:159–167. doi: 10.1016/j.bioorg.2018.12.026. [DOI] [PubMed] [Google Scholar]
- 96.Shi H., Li X., Gu H., Zhang Y., Huang Y., Wang L., Wang F. Biochemical properties of a novel thermostable and highly xylose-tolerant β-xylosidase/α-arabinosidase from Thermotoga. thermarum. Biotechnol. Biofuels. 2013;6:27. doi: 10.1186/1754-6834-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kumar S., Ramón D. Purification and regulation of the synthesis of a β-xylosidase from Aspergillus nidulans. FEMS Microbiol. Lett. 1996;135:287–293. doi: 10.1016/0378-1097(95)00468-8. [DOI] [Google Scholar]
- 98.John M., Schmidt B., Schmidt J. Purification and some properties of five endo-1,4-β-d-xylanases and a β-d-xylosidase produced by a strain of Aspergillus niger. Can. J. Biochem. 1979;57:125–134. doi: 10.1139/o79-016. [DOI] [PubMed] [Google Scholar]
- 99.Patel H., Kumar A.K., Shah A. Purification and characterization of novel bi-functional GH3 family β-xylosidase/β-glucosidase from Aspergillus niger ADH-11. Int. J. Biol. Macromol. 2018;109:1260–1269. doi: 10.1016/j.ijbiomac.2017.11.132. [DOI] [PubMed] [Google Scholar]
- 100.Dobberstein J., Emeis C.C. Purification and characterization of β-xylosidase from Aureobasidium pullulans. Appl. Microbiol. Biotechnol. 1991;35:210–215. doi: 10.1007/BF00184688. [DOI] [Google Scholar]
- 101.Yanai T., Sato M. Purification and characterization of an β-d-xylosidase from Candida utilis IFO 0639. Biosci. Biotechnol. Biochem. 2001;65:527–533. doi: 10.1271/bbb.65.527. [DOI] [PubMed] [Google Scholar]
- 102.Cintra L.C., Fernandes A.G., Oliveira I.C.M.d., Siqueira S.J.L., Costa I.G.O., Colussi F., Jesuíno R.S.A., Ulhoa C.J., Faria F.P.d. Characterization of a recombinant xylose tolerant β-xylosidase from Humicola grisea var. thermoidea and its use in sugarcane bagasse hydrolysis. Int. J. Biol. Macromol. 2017;105:262–271. doi: 10.1016/j.ijbiomac.2017.07.039. [DOI] [PubMed] [Google Scholar]
- 103.Yang X., Shi P., Huang H., Luo H., Wang Y., Zhang W., Yao B. Two xylose-tolerant GH43 bifunctional β-xylosidase/α-arabinosidases and one GH11 xylanase from Humicola insolens and their synergy in the degradation of xylan. Food Chem. 2014;148:381–387. doi: 10.1016/j.foodchem.2013.10.062. [DOI] [PubMed] [Google Scholar]
- 104.Yan Q.J., Wang L., Jiang Z.Q., Yang S.Q., Zhu H.F., Li L.T. A xylose-tolerant β-xylosidase from Paecilomyces thermophila: Characterization and its co-action with the endogenous xylanase. Bioresour. Technol. 2008;99:5402–5410. doi: 10.1016/j.biortech.2007.11.033. [DOI] [PubMed] [Google Scholar]
- 105.Mhetras N., Liddell S., Gokhale D. Purification and characterization of an extracellular β-xylosidase from Pseudozyma hubeiensis NCIM 3574 (PhXyl), an unexplored yeast. AMB Express. 2016;6:73. doi: 10.1186/s13568-016-0243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huang Y., Zheng X., Pilgaard B., Holck J., Muschiol J., Li S., Lange L. Identification and characterization of GH11 xylanase and GH43 xylosidase from the chytridiomycetous fungus, Rhizophlyctis rosea. Appl. Microbiol. Biotechnol. 2019;103:777–791. doi: 10.1007/s00253-018-9431-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zanoelo F.F., Polizeli Md M.d.L.T.d.M., Terenzi H.F., Jorge J.A. Purification and biochemical properties of a thermostable xylose-tolerant β-d-xylosidase from Scytalidium thermophilum. J. Ind. Microbiol. Biotechnol. 2004;31:170–176. doi: 10.1007/s10295-004-0129-6. [DOI] [PubMed] [Google Scholar]
- 108.Fujii T., Yu G., Matsushika A., Kurita A., Yano S., Murakami K., Sawayama S. Ethanol production from xylo-oligosaccharides by xylose-fermenting Saccharomyces cerevisiae expressing β-xylosidase. Biosci. Biotechnol. Biochem. 2011;75:1140–1146. doi: 10.1271/bbb.110043. [DOI] [PubMed] [Google Scholar]
- 109.Maruthamuthu M., Jiménez D.J., van Elsas J.D. Characterization of a furan aldehyde-tolerant β-xylosidase/α-arabinosidase obtained through a synthetic metagenomics approach. J. Appl. Microbiol. 2017;123:145–158. doi: 10.1111/jam.13484. [DOI] [PubMed] [Google Scholar]
- 110.Gruninger R.J., Gong X., Forster R.J., McAllister T.A. Biochemical and kinetic characterization of the multifunctional β-glucosidase/β-xylosidase/α-arabinosidase, Bgxa1. Appl. Microbiol. Biotechnol. 2014;98:3003–3012. doi: 10.1007/s00253-013-5191-4. [DOI] [PubMed] [Google Scholar]
- 111.Bao L., Huang Q., Chang L., Sun Q., Zhou J., Lu H. Cloning and characterization of two β-glucosidase/xylosidase enzymes from yak rumen metagenome. Appl. Biochem. Biotechnol. 2012;166:72–86. doi: 10.1007/s12010-011-9405-x. [DOI] [PubMed] [Google Scholar]
- 112.Rasmussen L.E., Sorensen H.R., Vind J., Viksø-Nielsen A. Mode of action and properties of the β-xylosidases from Talaromyces emersonii and Trichoderma reesei. Biotechnol. Bioeng. 2006;94:869–876. doi: 10.1002/bit.20908. [DOI] [PubMed] [Google Scholar]
- 113.Kersters-Hilderson H., Loontiens F.G., Claeyssens M., De Bruyne C.K. Partial purification and properties of an induced β-d-xylosidase of Bacillus pumilus 12. Eur. J. Biochem. 1969;7:434–441. doi: 10.1111/j.1432-1033.1969.tb19628.x. [DOI] [PubMed] [Google Scholar]
- 114.Wagschal K., Heng C., Lee C.C., Robertson G.H., Orts W.J., Wong D.W.S. Purification and characterization of a glycoside hydrolase family 43 β-xylosidase from Geobacillus thermoleovorans IT-08. Appl. Biochem. Biotechnol. 2009;155:304–313. doi: 10.1007/s12010-008-8362-5. [DOI] [PubMed] [Google Scholar]
- 115.Wagschal K., Heng C., Lee C.C., Wong D.W.S. Biochemical characterization of a novel dual-function arabinofuranosidase/xylosidase isolated from a compost starter mixture. Appl. Microbiol. Biotechnol. 2009;81:855–863. doi: 10.1007/s00253-008-1662-4. [DOI] [PubMed] [Google Scholar]
- 116.Rapp P., Wagner F. Production and properties of xylan-degrading enzymes from Cellulomonas uda. Appl. Environ. Microbiol. 1986;51:746–752. doi: 10.1128/aem.51.4.746-752.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Marcolongo L., La Cara F., Del Monaco G., Paixão S.M., Alves L., Marques I.P., Ionata E. A novel β-xylosidase from Anoxybacillus sp. 3M towards an improved agro-industrial residues saccharification. Int. J. Biol. Macromol. 2019;122:1224–1234. doi: 10.1016/j.ijbiomac.2018.09.075. [DOI] [PubMed] [Google Scholar]
- 118.Wagschal K., Jordan D.B., Braker J.D. Catalytic properties of β-d-xylosidase XylBH43 from Bacillus halodurans C-125 and mutant XylBH43-W147G. Process. Biochem. 2012;47:366–372. doi: 10.1016/j.procbio.2011.07.009. [DOI] [Google Scholar]
- 119.Jordan D.B., Stoller J.R., Lee C.C., Chan V.J., Wagschal K. Biochemical characterization of a GH43 β-Xylosidase from Bacteroides ovatus. Appl. Biochem. Biotechnol. 2017;182:250–260. doi: 10.1007/s12010-016-2324-0. [DOI] [PubMed] [Google Scholar]
- 120.Hudson R.C., Schofield L.R., Coolbear T., Daniel R.M., Morgan H.W. Purification and properties of an aryl β-xylosidase from a cellulolytic extreme thermophile expressed in Escherichia coli. Biochem. J. 1991;273:645–650. doi: 10.1042/bj2730645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fan Z., Yuan L., Jordan D.B., Wagschal K., Heng C., Braker J.D. Engineering lower inhibitor affinities in β-d-xylosidase. Appl. Microbiol. Biotechnol. 2010;86:1099–1113. doi: 10.1007/s00253-009-2335-7. [DOI] [PubMed] [Google Scholar]
- 122.Pinphanichakarn P., Tangsakul T., Thongnumwon T., Talawanich Y., Thamchaipenet A. Purification and characterization of β-xylosidase from Streptomyces sp. CH7 and its gene sequence analysis. World J. Microbiol. Biotechnol. 2004;20:727–733. doi: 10.1007/s11274-004-4513-1. [DOI] [Google Scholar]
- 123.Vocadlo D.J., Wicki J., Rupitz K., Withers S.G. A case for reverse protonation: Identification of Glu160 as an acid/base catalyst in Thermoanaerobacterium saccharolyticum β-xylosidase and detailed kinetic analysis of a site-directed mutant. Biochemistry. 2002;41:9736–9746. doi: 10.1021/bi020078n. [DOI] [PubMed] [Google Scholar]
- 124.Fekete C.A., Kiss L. Purification and characterization of a recombinant β-d-xylosidase from Thermobifida fusca TM51. Protein J. 2012;31:641–650. doi: 10.1007/s10930-012-9440-7. [DOI] [PubMed] [Google Scholar]
- 125.Yin Y.-R., Xian W.-D., Han M.-X., Zhou E.-M., Liu L., Alkhalifah D.H.M., Hozzein W.N., Xiao M., Li W.-J. Expression and characterisation of a pH and salt tolerant, thermostable and xylose tolerant recombinant GH43 β-xylosidase from Thermobifida halotolerans YIM 90462T for promoting hemicellulose degradation. Antonie Leeuwenhoek. 2019;112:339–350. doi: 10.1007/s10482-018-1161-2. [DOI] [PubMed] [Google Scholar]
- 126.Ristroph D.L., Humphrey A.E. The β-xylosidase of Thermomonospora. Biotechnol. Bioeng. 1985;27:909–913. doi: 10.1002/bit.260270626. [DOI] [PubMed] [Google Scholar]
- 127.Bachmann S.L., McCarthy A.J. Purification and characterization of a thermostable β-xylosidase from Thermomonospora fusca. J. Gen. Microbiol. 1989;135:293–299. doi: 10.1099/00221287-135-2-293. [DOI] [Google Scholar]
- 128.Büttner R., Bode R. Purification and characterization of β-xylosidase activities from the yeast Arxula adeninivorans. J. Basic Microbiol. 1992;32:159–166. doi: 10.1002/jobm.3620320304. [DOI] [PubMed] [Google Scholar]
- 129.Eneyskaya E.V., Ivanen D.R., Bobrov K.S., Isaeva-Ivanova L.S., Shabalin K.A., Savel'ev A.N., Golubev A.M., Kulminskaya A.A. Biochemical and kinetic analysis of the GH3 family β-xylosidase from Aspergillus awamori X-100. Arch. Biochem. Biophys. 2007;457:225–234. doi: 10.1016/j.abb.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 130.Kiss T., Kiss L. Purification and characterization of an extracellular β-d-xylosidase from Aspergillus carbonarius. World J. Microbiol. Biotechnol. 2000;16:465–470. doi: 10.1023/A:1008912025896. [DOI] [Google Scholar]
- 131.Kitpreechavanich V., Hayashi M., Nagai S. Purification and characterization of extracellular β-xylosidase and β-glucosidase from Aspergillus fumigatus. Agric. Biol. Chem. 1986;50:1703–1711. doi: 10.1271/bbb1961.50.1703. [DOI] [Google Scholar]
- 132.Semenova M.V., Drachevskaya M.I., Sinitsyna O.A., Gusakov A.V., Sinitsyn A.P. Isolation and properties of extracellular β-xylosidases from fungi Aspergillus japonicus and Trichoderma reesei. Biochemistry. 2009;74:1002–1008. doi: 10.1134/S0006297909090089. [DOI] [PubMed] [Google Scholar]
- 133.Rodionova N.A., Tavobilov I.M., Bezborodov A.M. β-Xylosidase from Aspergillus niger 15: Purification and properties. J. Appl. Biochem. 1983;5:300–312. [PubMed] [Google Scholar]
- 134.La Grange D.C., Pretorius I.S., Claeyssens M., Van Zyl W.H. Degradation of xylan to d-xylose by recombinant Saccharomyces cerevisiae coexpressing the Aspergillus niger β-xylosidase (xlnD) and the Trichoderma reesei xylanase II (xyn2) genes. Appl. Environ. Microbiol. 2001;67:5512–5519. doi: 10.1128/AEM.67.12.5512-5519.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Selig M.J., Knoshaug E.P., Decker S.R., Baker J.O., Himmel M.E., Adney W.S. Heterologous expression of Aspergillus niger β-d-xylosidase (XlnD): Characterization on lignocellulosic substrates. Appl. Biochem. Biotechnol. 2008;146:57–68. doi: 10.1007/s12010-007-8069-z. [DOI] [PubMed] [Google Scholar]
- 136.van Peij N.N., Brinkmann J., Vrsanska M., Visser J., de Graaff L.H. β-Xylosidase activity, encoded by xlnD, is essential for complete hydrolysis of xylan by Aspergillus niger but not for induction of the xylanolytic enzyme spectrum. Eur. J. Biochem. 1997;245:164–173. doi: 10.1111/j.1432-1033.1997.00164.x. [DOI] [PubMed] [Google Scholar]
- 137.Boyce A., Walsh G. Purification and characterisation of a thermostable β-xylosidase from Aspergillus niger van Tieghem of potential application in lignocellulosic bioethanol production. Appl. Biochem. Biotechnol. 2018;186:712–730. doi: 10.1007/s12010-018-2761-z. [DOI] [PubMed] [Google Scholar]
- 138.Kirikyali N., Wood J., Connerton I.F. Characterisation of a recombinant β-xylosidase (xylA) from Aspergillus oryzae expressed in Pichia pastoris. AMB Express. 2014;4:68. doi: 10.1186/s13568-014-0068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chakrabarti S.K., Ranu R.S. Characterization of a β-xylosidase from Aspergillus terreus (IJIRA 6.2) J. Plant Biochem. Biotechnol. 1995;4:117–120. doi: 10.1007/BF03262966. [DOI] [Google Scholar]
- 140.Andrade S.d.V., Polizeli M.d.L.T.d.M., Terenzi H.F., Jorge J.A.l. Effect of carbon source on the biochemical properties of β-xylosidases produced by Aspergillus versicolor. Process Biochem. 2004;39:1931–1938. doi: 10.1016/j.procbio.2003.09.024. [DOI] [Google Scholar]
- 141.Bankeeree W., Akada R., Lotrakul P., Punnapayak H., Prasongsuk S. Enzymatic hydrolysis of black liquor xylan by a novel xylose-tolerant, thermostable β-xylosidase from a tropical strain of Aureobasidium pullulans CBS 135684. Appl. Biochem. Biotechnol. 2018;184:919–934. doi: 10.1007/s12010-017-2598-x. [DOI] [PubMed] [Google Scholar]
- 142.Carvalho D.R.d., Carli S., Meleiro L.P., Rosa J.C., Oliveira A.H.C.d., Jorge J.A., Furriel R.P.M. A halotolerant bifunctional β-xylosidase/α-l-arabinofuranosidase from Colletotrichum graminicola: Purification and biochemical characterization. Int. J. Biol. Macromol. 2018;114:741–750. doi: 10.1016/j.ijbiomac.2018.03.111. [DOI] [PubMed] [Google Scholar]
- 143.Saha B.C. Purification and properties of an extracellular β-xylosidase from a newly isolated Fusarium proliferatum. Bioresour. Technol. 2003;90:33–38. doi: 10.1016/S0960-8524(03)00098-1. [DOI] [PubMed] [Google Scholar]
- 144.Saha B.C. Purification and characterization of an extracellular β-xylosidase from a newly isolated Fusarium verticillioides. J. Ind. Microbiol. Biotechnol. 2001;27:241–245. doi: 10.1038/sj.jim.7000184. [DOI] [PubMed] [Google Scholar]
- 145.Xia W., Shi P., Xu X., Qian L., Cui Y., Xia M., Yao B. High level expression of a novel family 3 neutral β-xylosidase from Humicola insolens Y1 with high tolerance to d-xylose. PLoS ONE. 2015;10:e0117578. doi: 10.1371/journal.pone.0117578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Garcia-Campayo V., Wood T.M. Purification and characterisation of a β-d-xylosidase from the anaerobic rumen fungus Neocallimastix frontalis. Carbohydr. Res. 1993;242:229–245. doi: 10.1016/0008-6215(93)80037-F. [DOI] [PubMed] [Google Scholar]
- 147.Kirikyali N., Connerton I.F. Heterologous expression and kinetic characterisation of Neurospora crassa β-xylosidase in Pichia pastoris. Enzym. Microb. Technol. 2014;57:63–68. doi: 10.1016/j.enzmictec.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 148.Terrasan C.R.F., Guisan J.M., Carmona E.C. Xylanase and β-xylosidase from Penicillium janczewskii: Purification, characterization and hydrolysis of substrates. Electron. J. Biotechnol. 2016;23:54–62. doi: 10.1016/j.ejbt.2016.08.001. [DOI] [Google Scholar]
- 149.Ye Y., Li X., Zhao J. Production and characteristics of a novel xylose- and alkali-tolerant GH 43 β-xylosidase from Penicillium oxalicum for promoting hemicellulose degradation. Sci. Rep. 2017;7:11600. doi: 10.1038/s41598-017-11573-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Knob A., Carmona E.C. Cell-associated acid β-xylosidase production by Penicillium sclerotiorum. N. Biotechnol. 2009;26:60–67. doi: 10.1016/j.nbt.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 151.Nieto-Domínguez M., de Eugenio L.I., Barriuso J., Prieto A., Fernández de Toro B., Canales-Mayordomo Á., Martínez M.J. Novel pH-stable glycoside hydrolase family 3 β-xylosidase from Talaromyces amestolkiae: An Enzyme displaying regioselective transxylosylation. Appl. Environ. Microbiol. 2015;81:6380–6392. doi: 10.1128/AEM.01744-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chen Z., Jia H., Yang Y., Yan Q., Jiang Z., Teng C. Secretory expression of a β-xylosidase gene from Thermomyces lanuginosus in Escherichia coli and characterization of its recombinant enzyme. Lett. Appl. Microbiol. 2012;55:330–337. doi: 10.1111/j.1472-765X.2012.03299.x. [DOI] [PubMed] [Google Scholar]
- 153.Li Y.K., Yao H.J., Cho Y.t. Effective induction, purification and characterization of Trichoderma koningii G-39 β-xylosidase with high transferase activity. Biotechnol. Appl. Biochem. 2000;31:119–125. doi: 10.1042/BA19990072. [DOI] [PubMed] [Google Scholar]
- 154.Dekker R.F. Bioconversion of hemicellulose: Aspects of hemicellulase production by Trichoderma reesei QM 9414 and enzymic saccharification of hemicellulose. Biotechnol. Bioeng. 1983;25:1127–1146. doi: 10.1002/bit.260250419. [DOI] [PubMed] [Google Scholar]
- 155.Poutanen K., Puls J. Characteristics of Trichoderma reesei β-xylosidase and its use in the hydrolysis of solubilized xylans. Appl. Microbiol. Biotechnol. 1988;28:425–432. doi: 10.1007/BF00268208. [DOI] [Google Scholar]
- 156.Chinen I., Oouchi K., Tamaki H., Fukuda N. Purification and properties of thermostable β-xylosidase from immature stalks of Saccharum officinarum L. (sugar cane) J. Biochem. 1982;92:1873–1881. doi: 10.1093/oxfordjournals.jbchem.a134117. [DOI] [PubMed] [Google Scholar]
- 157.Zhou J., Bao L., Chang L., Zhou Y., Lu H. Biochemical and kinetic characterization of GH43 β-d-xylosidase/α-l-arabinofuranosidase and GH30 α-l-arabinofuranosidase/β-d-xylosidase from rumen metagenome. J. Ind. Microbiol. Biotechnol. 2012;39:143–152. doi: 10.1007/s10295-011-1009-5. [DOI] [PubMed] [Google Scholar]
- 158.Li Q., Wu T., Zhao L., Pei J., Wang Z., Xiao W. Highly efficient biotransformation of Astragaloside IV to Cycloastragenol by sugar-stimulated β-glucosidase and β-xylosidase from Dictyoglomus thermophilum. J. Microbiol. Biotechnol. 2018 doi: 10.4014/jmb.1807.07020. [DOI] [PubMed] [Google Scholar]
- 159.Bachmann S.L., McCarthy A.J. Purification and cooperative activity of enzymes constituting the xylan-degrading system of Thermomonospora fusca. Appl. Environ. Microbiol. 1991;57:2121–2130. doi: 10.1128/aem.57.8.2121-2130.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]