Abstract
Prior to the advent of the monoclonal antibody trastuzumab, human epidermal growth-factor receptor 2 (HER2)-positive (HER2+) breast cancer (BC) was associated with an aggressive clinical course and poor survival outcomes. In the era of effective HER2-directed therapies, median survival rates for patients with metastatic HER2+ BC now approach 5 years. Despite these improvements, the majority of affected patients unfortunately die from disease. Therapies to overcome treatment resistance are being actively pursued. One strategy has been to target the cyclin-dependent kinases 4/6 (CDK4/6), as they are downstream of HER2 and many of the cellular pathways driving resistance to HER2-targeted therapies, and play a key role in proliferation by controlling transition through the G1 restriction point to the S phase of the cell cycle. In this article, we review the published literature with regard to the rationale for CDK4/6-directed therapies in HER2+ BC and discuss ongoing clinical research and new challenges in the field.
Keywords: breast cancer, CDK4/6 inhibitor, HER2+ breast cancer, HER2-targeted therapy, metastasis
Introduction
Breast cancer (BC) is the most frequently diagnosed malignancy in women, accounting for approximately 25% of cancer diagnoses and 15% of cancer-related deaths in women, globally.1 Approximately 15–20 % of BCs are classified as human epidermal growth-factor receptor 2 (HER2) positive (HER2+).2 Prior to the development of HER2-directed therapies, a diagnosis of HER2+ BC was associated with a poor prognosis. In the late 1990s, the addition of trastuzumab, a monoclonal antibody targeting HER2, to standard chemotherapy regimens resulted in marked improvements in disease-free survival (DFS) and overall survival (OS) in both the adjuvant and metastatic settings.3 This has led to the development of other effective HER2-directed therapies, including lapatinib (a dual tyrosine-kinase inhibitor) pertuzumab (an HER2/HER3-dimerization inhibitor), ado-trastuzumab emtansine (T-DM1, an antibody–drug conjugate) and neratinib (an irreversible pan-HER inhibitor).4,5
Although much progress has been made for patients with HER2+ BC, approximately 15–20% of patients receiving trastuzumab in the adjuvant setting will eventually relapse.6 Approximately 70% of patients with HER2+ metastatic BC (MBC) develop resistance to trastuzumab within 1 year (secondary resistance), and over a third of patients never respond (de novo resistance).7 Further, approximately 30–55% of patients with advanced HER2+ BC eventually develop brain metastases,8 a devastating diagnosis associated with considerable morbidity and mortality. Most patients with HER2+ MBC will succumb to their disease.9
One approach to improving the long-term outcome of women with HER2+ BC has been targeting of the cyclin-dependent kinases (CDKs), CDK4/6. CDKs regulate cell-cycle transitions. In particular, CDK4/6 plays a central role in cell proliferation by controlling the transition through the G1 restriction point to the S phase. They interact with the D-type cyclins by inactivating the retinoblastoma (Rb) tumor-suppressor protein (pRb), and promoting transition from G1 to S phase.10 Deregulation of the CDK4/6-D-type-Rb pathway occurs in many tumors, including BC, and has spurred the development of specific CDK4/6 inhibitors to induce G1 arrest and apoptosis.11,12 Targeting of CDK4/6 in HER2+ BC is attractive as it is downstream of HER2 and many of the processes driving resistance to HER2-targeted therapies.13 The focus of this review is the role of CDK4/6 inhibitors (CDK4/6is) in HER2+ BC.
CDK4/6is in HER2-positive breast cancer
The importance of the CDK4/6-D-type-Rb pathway was first demonstrated in HER2+ cell lines almost 2 decades ago. Lee and colleagues found that mammary tumors had elevated levels of cyclin D1 protein, due to amplification of wild-type or activating mutations of Neu in transgenic mice and in MCF7 cells, which overexpressed transforming Neu.14 They also demonstrated that HER2-mutated MCF7 cells exhibited specific C-terminal autophosphorylation sites and the extracellular domain had fundamental roles in cyclin D1 promoter activation. The authors concluded that an HER2-signaling cascade to cyclin D1 was promoted by transcription factor E2F1, and that cyclin D1 was a key downstream target of Neu-induced transformation. Roberts and colleagues subsequently demonstrated that palbociclib monotherapy was associated with antineoplastic activity in MMTV-c-Neu mice.15 However, the combination of palbociclib and carboplatin decreased antineoplastic activity compared with carboplatin monotherapy in Rb-competent mice, which inferred that DNA-damaging agents and CDK4/6is should not be coadministered in the treatment of tumors reliant on CDK4/6 activity for proliferation. Furthermore, Nikolai and colleagues16 noted that HER2-signaling promotes BC growth via regulation of E2F1-driven deoxyribonucleic acid (DNA) metabolism and replication genes, along with phosphorylation and activation of SRC-3, a transcriptional coactivator. They also identified a CDK-signaling node and determined that the combination of palbociclib and the dual epithelial growth-factor receptor (EGFR)/HER2 tyrosine kinase, lapatinib, inhibits de novo DNA synthesis, largely via disruption of E2F1 and its target genes. Collectively, preclinical data supported clinical investigation of CDK4/6is in HER2+ BC, predominantly in persons with hormone receptor (HR)+, HER2+ disease.
The cyclin D1/CDK4/6/pRb axis and resistance to HER2-directed therapies
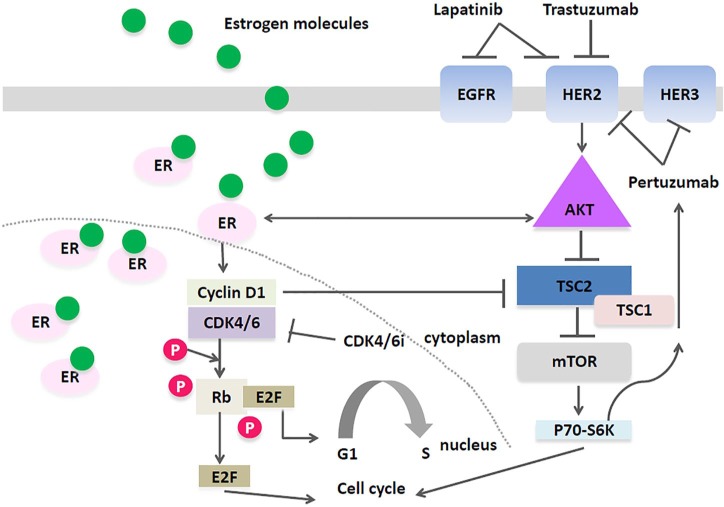
A number of mechanisms drive primary and secondary resistance to HER2-directed therapies, including alterations in the phosphoinositide-3 kinase (PI3K-Akt) and phosphatase and tensin homolog (PTEN) pathways;17 increases in EGFR and insulin-like growth factor, as well as crosstalk between mammalian target of rapamycin (mTOR), PI3K and mitogen-activated protein-kinase/extracellular signal-regulated-kinase signaling pathways.18 The cyclin D1/CDK4/6/pRb axis is also an important pathway involved in resistance to HER2-directed treatments (Figure 1).17 The cyclin D1/CDK4/6/pRb axis is activated by HER2 ligand interaction with the PI3K-Akt pathway and downstream activation of cyclin D1 can induce resistance to trastuzumab and other HER2-targeted treatments. Goel and colleagues studied secondary resistance mechanisms to HER2-directed therapies in a transgenic mouse model of HER2+ BC.19 Tumor cells resistant to HER2- directed therapy expressed high levels of nuclear cyclin D1 and CDK4, and the combined inhibition of cyclin D1/CDK4 was synergistic with respect to antineoplastic effect, suggesting that both cyclin D1 and CDK4 play a fundamental role in the development of resistance to HER2-directed therapies. Further, cell lines resistant to HER2-directed treatments demonstrated restoration of sensitivity to these agents when a CDK4/6i was added.19 The antineoplastic activity of abemaciclib in these models was attributed to its ability to restore signaling via the EGFR kinase family by downstream activation of Akt kinase. Furthermore, by activation of TSC2 (tuberin), an mTOR inhibitor, abemaciclib was able to supersede the inhibitory feedback exerted by mTOR on the EGFR kinase family and their downstream effectors.
Figure 1.
TSC2 mediated crosstalk between the Akt/mTOR pathway and the cyclin D1-CDK4/6 axis.
The cyclinD1/CDK4/6 axis is responsible for increased phosphorylation TSC2 and TSC1, and promotes the phosphorylation of the mTOR substrates, 4E-BP1 and S6K1. Additionally, EGFR/HER2 (via Akt) triggers TSC2 phosphorylation, and the combination of CDK4/6is and HER2 inhibitors may suppress mTORC1 activity more than either drug alone; Additionally, greater suppression of mTORC1 activity may relive feedback inhibition of EGFR family kinases. This may render HER2+ breast cancer cells more sensitive to the effects of EGFR/HER2 inhibitors.
Akt, protein-kinase B; CDK4/6, cyclin-dependent kinase 4/6; CDK4/6is, CDK4/6 inhibitors; EGFR, epithelial growth-factor receptor; ER, estrogen receptor; E2F, a transcription factor; HER2, human epidermal growth-factor receptor 2; mTOR, mammalian target of rapamycin; P, phosphate; Rb, retinoblastoma protein; TSC1/2, tuberous sclerosis complex 1/2.
Observation of antitumor activity of CDK 4/6 inhibitors in luminal HER2+ BC has been confirmed by others. Finn and colleagues noted that cell lines representing the luminal ER+ subtype (including those that overexpressed HER2) were sensitive to palbociclib while nonluminal/basal subtypes were resistant. Additionally, palbociclib was synergistic with tamoxifen and trastuzumab in ER+ and HER2+ cell lines, respectively.20 Dean and colleagues used an ex vivo model of breast tumor tissue to characterize the cytostatic response to palbociclib;21 it was noted that the CDK4/6i was very effective at inhibiting cellular proliferation in approximately 85% of cases, irrespective of ER or HER2-status. El-Chaarani and colleagues performed preclinical studies in HER2+ cell lines and noted that palbociclib had an inhibitory effect in resistant and nonresistant HER2+ cell lines.22
There are three selective CDK4/6is in clinical development: palbociclib, ribociclib and abemaciclib. These agents have CDK4 and CDK6 as their primary target and have been shown to disrupt the CDK4/6-D-type-Rb pathway leading to G1 cell-cycle arrest.20 CDK4/6is are most effective in combination with endocrine therapy in patients with HR+ BC, although abemaciclib has activity as monotherapy. The reason for the higher abemaciclib monotherapy response rates compared with the other CDK4/6is is uncertain, but may be due to more potent CDK4 inhibition. Further, the different toxicity profile of abemaciclib (less neutropenia; more nausea and diarrhea) may also be attributed to the greater selectivity of this compound for CDK4 than for CDK6.10 All three compounds are US Food and Drug Administration (FDA) approved in combination with endocrine therapy for the first- and second-line treatment of ER+, HER2− MBC; abemaciclib is also approved for use as monotherapy in more heavily pretreated ER+, HER2− MBC.23–28 Given the encouraging preclinical findings outlined in this article, clinical development in the HER2+ BC space has ensued, predominantly in the ER+/HER2+ setting. In patients with HER2+ BC, combining CDK4/6is with endocrine therapy and HER2-directed therapy is warranted, based on evidence that continued HER2 targeting after initial progression on trastuzumab-based therapy can be associated with significant clinical benefit.29
Predictive biomarkers of response to CDK4/6is
As discussed above, in HR+, HER2− MBC, the combination of CDK4/6is and endocrine therapy has resulted in clinically significant improvements in progression-free survival (PFS) and OS compared with endocrine therapy alone. However, resistance to CDK4/6is is generally inevitable.30 There are numerous mechanisms of resistance to CDK4/6is; efforts to identify reliable biomarkers of early resistance or response to treatment are underway and reflect a major unmet need. The majority of work in this area has been in HR+, HER2− BC. In this setting, there are numerous reports implicating the Rb tumor-suppressor gene (Rb1) function, cyclin E1, and the PIK3CA pathway as potential biomarkers of CDK 4/6is.31–34 Loss of Rb has been implicated as a characteristic of BCs that are chemosensitive but resistant to CDK4/6is. Condorelli and colleagues recently noted that patients with HR+ HER2− MBC develop somatic Rb1 mutations after exposure to palbociclib or ribociclib; further study is warranted to validate these findings and to develop appropriate therapeutic strategies.35 Herrera-Abreu and colleagues noted that combined inhibition of CDK4/6 and PI3K induced cancer-cell apoptosis in vitro and in patient-derived tumor xenograft (PDX) models, resulting in tumor shrinkage and improved disease control.36 Further, a triplet combination of CDK4/6, PI3K inhibition, and endocrine therapy had superior efficacy compared with paired combinations, triggering rapid tumor regressions in a PDX model. Studies investigating the feasibility and utility of combining CDK4/6 and PI3K inhibition are ongoing [ClinicalTrials.gov identifier: NCT02088684], and as the PI3K-Akt pathway is also implicated in resistance to HER2-targeted therapies, trials of CDK4/6is, PI3Ki and HER2-targeted therapy could also be conducted in persons with HER2+ MBC.37 In TRINITI-1 [ClinicalTrials.gov identifier: NCT02732119], a phase I/II trial of ribociclib, everolimus, and exemestane in men or postmenopausal women with HR+, HER2− advanced BC that progressed on prior CDK4/6i, PFS outcomes were inferior in participants with a baseline mutation in PIK3CA or estrogen receptor 1, which infers that specific tumor molecular alterations are associated with resistance to treatment and possibly a worse prognosis; further study is needed to validate these findings in other biomarker-focused clinical trials.38 Notably, Arteaga and colleagues found that fibroblast growth-factor receptor 1 (FGFR1)-amplified/ER+ BC cells and MCF7 cells transduced with FGFR1 were resistant to treatment with fulvestrant ± ribociclib or palbociclib;39 therefore, clinical trials incorporating endocrine therapy, CDK4/6 and FGFR antagonists are underway in this patient subgroup [ClinicalTrials.gov identifier: NCT03238196].
Appropriate de-escalation of systemic therapy in selected persons with early-stage HER2+ BC is also a research priority, and biomarker discovery is ongoing in this patient population. The goal is to avoid the toxicities of chemotherapy in patients who may have equally favorable outcomes with dual HER2-directed therapy ± endocrine therapy. For example, Prat and colleagues have noted that a ribonucleic acid (RNA)-based assay combining HER2-enriched BC subtype and Erb-B2 receptor tyrosine-kinase 2 messenger RNA can identify tumors that are highly sensitive to HER2-targeted therapy.40 This biomarker could assist with chemotherapy de-escalation in ∼40% of patients with HER2+ BC. Further, Veeraraghavan and colleagues have demonstrated that there is a clinical BC subtype (e.g. with high HER2 amplification and an intact PI3K pathway) that is particularly sensitive to HER2-targeted therapies alone.41 Finally, Risi and colleagues performed a retrospective analysis (n = 514) to determine whether Rbsig, a gene signature of Rb loss, could be used as a predictive biomarker of response to neoadjuvant chemotherapy in persons with ER+/HER2+ BC.42 In this patient population, it was noted that low expression of Rbsig was associated with low pathologic complete response (pCR) rates after neoadjuvant chemotherapy ± HER2-targeted therapy. In summary, these studies suggest that future clinical trials should not only focus on de-escalation of chemotherapy for patients with HER2+ BC with a high probability of pCR, but additionally, in patients with ER+/HER2+ BC and a low probability of pCR, to prospectively study whether chemotherapy may be omitted in favor of a combination regimen of dual HER2-directed therapy, endocrine therapy, and CDK4/6is.
CDK4/6is in HER2-positive breast cancer metastatic trials
One of the earliest observations that CDK4/6is could exert single-agent activity in ER+, HER2+ BC was reported in a phase I study of abemaciclib in patients with advanced solid tumors.43 Among the 11 women with HR+ HER2+ MBC enrolled on this trial, there were 4 (36%) partial responses (PRs).Common adverse events noted in the 36 patients with HR+ BC included grade 1 or 2 diarrhea and grade 3 diarrhea [one patient (5%)]; no patients discontinued due to diarrhea. Grade 3 neutropenia occurred in six patients (32%); however, febrile neutropenia was not observed. These findings fueled the further evaluation of CDK4/6is in HER2+ BC.
PATRICIA [ClinicalTrials.gov identifier: NCT02448420], three concurrent two-stage phase II clinical trials in postmenopausal females with advanced HER2+ BC who had received prior chemotherapy and trastuzumab, examined the antitumor activity of trastuzumab and palbociclib ± letrozole. The results of the first stage of each trial have been recently reported. The proportion of patients who remained progression free at least 6 months was 33% (5/15) among patients with ER− HER2+ BC treated with trastuzumab and palbociclib; 40% (6/15) among patients with ER+ HER2+ BC treated with trastuzumab and palbociclib; and 53% (8/15) among patients with ER+HER2+ BC treated with trastuzumab, palbociclib, and letrozole. Preliminary results indicate the clinical benefit rate was 72% (8/11) among the patients with ER+HER2+ BC with a luminal subtype, and 72% (25%; 4/16) among the patients with ER+HER2+ BC with a nonluminal subtype. We await the results from the second stages of these trials to see if these findings are consistent.
PATINA [ClinicalTrials.gov identifier: NCT02947685] is a randomized, open-label, phase III FDA-registered study, designed to assess whether PFS is prolonged with the combination of palbociclib, HER2-directed therapy, and endocrine therapy relative to HER2-directed therapy and endocrine therapy alone in persons with HR+ HER2+ advanced or MBC. Participants who have completed standard first-line treatment with dual HER2-directed therapy and a taxane, are randomized to receive continued dual HER2-targeted treatment and endocrine therapy ± palbociclib. A limitation of the study design is that it does not allow for a conclusive determination of the additional benefit of palbociclib in this patient population. Results of this trial are expected in early 2021.
Another trial [ClinicalTrials.gov identifier: NCT02657343], is an ongoing single-arm phase Ib/II study of ribociclib added to trastuzumab (or T-DM1) in HER2+ advanced BC, irrespective of HR status. This trial includes correlatives to evaluate the potential immune-based mechanisms of CDK4/6i action first observed by Goel and colleagues.44 In preclinical studies, Goel noted that CDK4/6is activated tumor-cell expression of endogenous retroviral elements, thus increasing intracellular levels of double-stranded RNA, production of type III interferons and tumor antigen presentation. Further, CDK4/6is suppress regulatory T-cell proliferation. The effects of CDK4/6is both on tumor cells and on regulatory T cells result in reduced activity of DNA methyltransferase 1, a target of E2F. These events ultimately promote cytotoxic T-cell-mediated clearance of tumor cells, which is further enhanced by the addition of immune-checkpoint blockade.
A phase II study of T-DM1 ± abemaciclib in advanced HER2+ BC is expected to activate in late 2019 through the Academic and Community Cancer Research United (ACCRU); (O’Sullivan, Principal Investigator). In this study, patients will be randomized 1:1 to receive T-DM1 or T-DM1 plus abemaciclib. Two parallel phase II studies will be conducted; one for ER+ HER2+ MBC and one for ER− HER2+ MBC. The primary endpoint will be to compare PFS in both treatment arms. Secondary objectives will include an assessment of toxicity, overall response rate, and OS. For blood biomarkers, CTCs, ctDNA and serum thymidine kinase (TK1) will be collected at baseline, 6 months, and disease progression, to determine whether there is an association with clinical benefit. Additional correlative studies will evaluate tumor-infiltrating lymphocyte levels and CD8/FOXP3 expression, as well as expression of the epithelial–mesenchymal transition (EMT) marker, vimentin, with PFS. The latter correlative studies are based upon data from Liu and colleagues; these investigators demonstrated that CDK4/6is target SNAIL1 through the deubiquitinase DUB3, which mediates the post-translational stabilization of SNAIL. The potential importance of this finding was demonstrated in an Rb-deficient triple-negative PDX, wherein treatment with palbociclib had no effect on the growth of the primary tumor but inhibited development of lung and liver metastases.45
Several other clinical studies evaluating the combination of HER2-directed therapy, CDK4/6is, and endocrine therapy are in progress in various clinical settings, and the results of these trials are eagerly awaited (Table 1).
Table 1.
Ongoing or planned trials of CDK4/6is in HER2+ breast cancer.
| Setting | CDK4/6i | Trial | Phase | Primary endpoint(s) ± results | ClinicalTrials.gov identifier |
|---|---|---|---|---|---|
| Metastatic | Palbociclib | Tucatinib, palbociclib and letrozole in metastatic HR+ and HER2+ breast cancer | 1/2 | Phase I: RP2D Phase II: PFS | NCT03054363 |
| Metastatic | Palbociclib | Study of palbociclib and trastuzumab with or without letrozole in HER2+ metastatic breast cancer (PATRICIA) | 2 | PFS at 6 months pCR rate: 27% (8/30 patients) |
NCT02448420 |
| Metastatic (Brain metastases) |
Palbociclib | Palbociclib in treating patients with metastatic HER2+ breast cancer with brain metastasis | 2 | Radiographic RR in CNS | NCT02774681 |
| Metastatic | Palbociclib | T-DM1 and palbociclib for metastatic HER2 breast cancer (T-DM1) | 2 | PFS | NCT03530696 |
| Metastatic | Palbociclib | Anastrozole, palbociclib, trastuzumab and pertuzumab in HER2+ metastatic breast cancer | 1/2 | DLT MTD CBR |
NCT03304080 |
| Metastatic | Palbociclib | Randomized, open-label clinical study of the targeted therapy, palbociclib, to treat metastatic breast cancer (PATINA) | 3 | PFS | NCT02947685 |
| Metastatic | Palbociclib | Phase Ib study of PD-0332991 in combination with T-DM1 | 1 | DLT, MTD, others | NCT01976169 |
| Metastatic | Palbociclib | Study of the pan-ERBB inhibitor neratinib given in combination with everolimus, palbociclib or trametinib in advanced cancer subjects with EGFR mutation/amplification, HER2 mutation/amplification or HER3/4 mutation | 1 | MTD | NCT03065387 |
| Metastatic | Palbociclib | Palbociclib in ER+, HER2+, metastatic breast cancer (combined with letrozole and T-DM1) | 1/2 | MTD with or without R2PD | NCT03709082 |
| Metastatic | Ribociclib | An open-label, phase Ib/II clinical trial of CDK4/6 inhibitor, ribociclib (Lee011), in combination with trastuzumab or T-DM1 for advanced/metastatic HER2+ breast cancer | 1/2 | MTD with or without R2PD CBR |
NCT02657343 |
| Metastatic | Abemaciclib | A study of abemaciclib (LY2835219) in women with HR+, HER2+ locally advanced or metastatic breast cancer (monarcHER) | 2 | PFS | NCT02675231 |
| Neoadjuvant | Palbociclib | ‘Neoadjuvant treatment with palbociclib: effect on Ki67 and apoptosis before, during and after treatment’ (NA-PHER2) | 2 | Serial measures of Ki67 Serial measures of apoptosis |
NCT02530424 |
| Neoadjuvant | Palbociclib | To reduce the use of chemotherapy in elderly patients with ER+ and HER2+ breast cancer (TOUCH) (paclitaxel and trastuzumab plus pertuzumab versus ET, palbociclib, pertuzumab and trastuzumab) |
2 | pCR | NCT03644186 |
CBR, clinical benefit rate; CDK4/6i, cyclin-dependent kinase 4/6 inhibitor; CNS, central nervous system; DLT, dose-limiting toxicity; EGFR, epithelial growth-factor receptor; ER, estrogen receptor; ERBB, receptor tyrosine kinases; ET, endocrine therapy; PFS, progression-free survival; HER2, human epidermal growth-factor receptor 2; HR, hormone receptor; MTD, maximum tolerated dose; pCR, pathologic complete response rate; RP2D, recommended dose for phase II evaluation; RR, response rate; T-DM1, trastuzumab-DM1.
Neoadjuvant setting
NA-PHER2 [ClinicalTrials.gov identifier: NCT02530424] was a phase II clinical trial of palbociclib, trastuzumab, pertuzumab, and fulvestrant as neoadjuvant treatment for patients with HR+, HER2+ BC. The coprimary endpoints were change from baseline in Ki67 expression at 2 weeks of treatment and at surgery and changes in apoptosis from baseline to surgery. Secondary endpoints were clinical objective response and pathological complete response (pCR). Significant reductions were seen after 2 weeks on treatment (in Ki67 expression), and at surgery (in both Ki67 expression and apoptosis). Further, 8 of the 30 evaluable patients (27%) had a pCR in breast and axillary nodes. PALTAN [ClinicalTrials.gov identifier: NCT02907918] is an ongoing phase II neoadjuvant trial of palbociclib in combination with letrozole and trastuzumab in women with stage II–III ER+ HER2+ BC; results are expected sometime in 2021.
Adjuvant/postneoadjuvant setting
At the time of writing, the ClinicalTrials.gov website was queried; no adjuvant or postneoadjuvant studies of CDK4/6i combined with HER2-directed therapy were planned or enrolling participants.
Breast cancer brain metastases
Approximately one half of patients with advanced HER2+ BC ultimately develop breast cancer brain metastases (BCBM), a devastating diagnosis which adversely affects prognostic outcomes.8 The relationship between the incidence of BCBM and receipt of HER2-directed therapies (e.g. trastuzumab) in persons with HER2+ MBC is unclear. Although higher rates of central nervous system (CNS) events have been reported, this is likely related to improved patient survival outcomes, in addition to the inability of trastuzumab to penetrate the blood–brain barrier (BBB). It is unlikely that treatment with HER2-directed agents ‘increases’ this risk, given the improved DFS and OS outcomes when trastuzumab is added to standard treatment regimens in the adjuvant and metastatic settings.46 The management of patients with BCBM is fraught with complexities and typically requires multidisciplinary input. The BBB poses a unique challenge, given that the majority of chemotherapies and targeted therapies penetrate the BBB incompletely, or not at all.47 Although there are increasing systemic therapy options available for patients with BCBM, both surgery and radiotherapy are often an integral part of the management plan. However, neurocognitive impairment is a particular concern for patients with BCBM expecting long-term disease control, and postponing whole-brain radiotherapy may be an option for selected candidates. Therefore, identifying novel systemic therapies that can penetrate the BBB effectively and concomitantly target extracranial disease is an attractive prospect. The utility of other HER2-targeted therapies, including neratinib, T-DM1, and high-dose trastuzumab, have been, or are currently being, evaluated for patients with HER2+ MBC and BCBM. While all three CDK4/6is penetrate the BBB,48 preclinical and clinical studies showed that abemaciclib CNS concentrations are reached more efficiently at potentially lower doses than palbociclib and are potentially on target for a longer duration.49 Therefore, strong scientific rationale exists to continue study of CDK4/6is as monotherapy or in combination with other targeted therapies in patients with HER2+ BCBM.
Responses to abemaciclib in patients with BCBM have been noted.50 I3Y-MC-JPBO [ClinicalTrials.gov identifier: NCT02308020] is an open-label, phase II trial, evaluating the safety and efficacy of abemaciclib 200 mg twice daily in patients with new or progressive brain metastases secondary to HR+ MBC, non-small-cell lung cancer, or melanoma. For stage I efficacy, in patients with HR+, HER2− MBC, futility was met. However, for HR+, HER2− patients, two confirmed, durable PRs were observed and enrollment to stage II is ongoing. Further, there is a single-arm pilot study of palbociclib monotherapy currently enrolling patients with HER2+ BCBM [ClinicalTrials.gov identifier: NCT02774681]; the primary objective is to determine overall radiographic response rate in the CNS by using modified RANO-BM criteria. Concurrent treatment with trastuzumab is permitted. Secondary objectives include PFS, OS, systemic response rates, safety, and tolerability.
Summary and outstanding questions
The role of CDK4/6is in patients with advanced HER2+ BC is now emerging, and further clinical studies in patients with advanced HER2+ BC are eagerly anticipated. Given the ability of the CDK4/6is to cross the BBB and the significant clinical problem of brain metastases in patients with HER2+ BC, a major clinical question is whether CDK4/6is will provide utility in the prevention and treatment of HER2+ BCBM. Going forward, studies evaluating combination therapy with CDK4/6is and HER2-directed therapy (e.g. with T-DM1 and neratinib) could be considered. Further, a provocative question is whether the CDK4/6is can prevent the incidence of BCBM in high-risk patients. Taken together, the utility of the CDK4/6is is likely much broader than initially envisioned, and results from future and ongoing trials will clarify whether these agents will become part of the treatment armamentarium for patients with HER2+ advanced BC.
Footnotes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article. Publication fees were charged to Dr. O’Sullivan’s ASCO Career Development Award.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Ciara C. O’Sullivan, Department of Oncology, Mayo Clinic, 200 First Street SW, Rochester, MN, 55905-0002, USA.
Vera J. Suman, Department of Biostatistics, Mayo Clinic, Rochester, MN, USA
Matthew P. Goetz, Department of Oncology, Mayo Clinic, MN, USA Department of Molecular Pharmacology and Experimental Therapeutics and Mayo Clinic, Rochester, MN, USA.
References
- 1. Torre LA, Islami F, Siegel RL, et al. Global cancer in women: burden and trends. Cancer Epidemiol Biomarkers Prev 2017; 26: 444–457. [DOI] [PubMed] [Google Scholar]
- 2. Wolff AC, Hammond MEH, Allison KH, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol 2018: Jco2018778738. [DOI] [PubMed] [Google Scholar]
- 3. Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001; 344: 783–792. [DOI] [PubMed] [Google Scholar]
- 4. O’Sullivan CC, Smith KL. Therapeutic considerations in treating HER2-positive metastatic breast cancer. Curr Breast Cancer Rep 2014; 6: 169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chan A, Delaloge S, Holmes FA, et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2016; 17: 367–377. [DOI] [PubMed] [Google Scholar]
- 6. Martin M, Lopez-Tarruella S. Emerging therapeutic options for HER2-positive breast cancer. Am Soc Clin Oncol Educ Book 2016; 35: e64–e70. [DOI] [PubMed] [Google Scholar]
- 7. Labidi S, Mejri N, Lagha A, et al. Targeted therapies in HER2-overexpressing metastatic breast cancer. Breast Care 2016; 11: 418–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lin NU, Amiri-Kordestani L, Palmieri D, et al. CNS metastases in breast cancer: old challenge, new frontiers. Clin Cancer Res 2013; 19: 6404–6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Veeraraghavan J, De Angelis C, Reis-Filho JS, et al. De-escalation of treatment in HER2-positive breast cancer: determinants of response and mechanisms of resistance. Breast 2017; 34(Suppl. 1): S19–S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barroso-Sousa R, Shapiro GI, Tolaney SM. Clinical development of the CDK4/6 inhibitors ribociclib and abemaciclib in breast cancer. Breast Care 2016; 11: 167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Law ME, Corsino PE, Narayan S, et al. Cyclin-dependent kinase inhibitors as anticancer therapeutics. Mol Pharmacol 2015; 88: 846–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sanchez-Martinez C, Gelbert LM, Lallena MJ, et al. Cyclin dependent kinase (CDK) inhibitors as anticancer drugs. Bioorg Med Chem Lett 2015; 25: 3420–3435. [DOI] [PubMed] [Google Scholar]
- 13. Witkiewicz AK, Cox D, Knudsen ES. CDK4/6 inhibition provides a potent adjunct to Her2-targeted therapies in preclinical breast cancer models. Genes Cancer 2014; 5: 261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee RJ, Albanese C, Fu M, et al. Cyclin D1 is required for transformation by activated Neu and is induced through an E2F-dependent signaling pathway. Mol Cell Biol 2000; 20: 672–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roberts PJ, Bisi JE, Strum JC, et al. Multiple roles of cyclin-dependent kinase 4/6 inhibitors in cancer therapy. J Natl Cancer Inst 2012; 104: 476–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nikolai BC, Lanz RB, York B, et al. HER2 signaling drives DNA anabolism and proliferation through SRC-3 phosphorylation and E2F1-regulated genes. Cancer Res 2016; 76: 1463–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Corona SP, Ravelli A, Cretella D, et al. CDK4/6 inhibitors in HER2-positive breast cancer. Crit Rev Oncol Hematol 2017; 112: 208–214. [DOI] [PubMed] [Google Scholar]
- 18. Axelrod MJ, Gordon V, Mendez RE, et al. p70S6 kinase is a critical node that integrates HER-family and PI3 kinase signaling networks. Cell Signal 2014; 26: 1627–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garrido-Castro AC, Goel S. CDK4/6 inhibition in breast cancer: mechanisms of response and treatment failure. Curr Breast Cancer Rep 2017; 9: 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Finn RS, Dering J, Conklin D, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res 2009; 11: R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dean JL, McClendon AK, Hickey TE, et al. Therapeutic response to CDK4/6 inhibition in breast cancer defined by ex vivo analyses of human tumors. Cell Cycle 2012; 11: 2756–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. ElChaarani B, Stires H, Pohlmann PR, et al. Pre-clinical analysis of the CDK4/6 inhibitor palbociclib in HER2-positive breast cancer. J Clin Oncol 2017; 35(15_Suppl.): e12520. [Google Scholar]
- 23. Food and Drug Administration. FDA approval of palbociclib and letrozole for first line treatment in postmenopausal women with HR-positive, HER2-negative advanced breast cancer. https://www.ascopost.com/News/22637 (2015, accessed 30 October 2019).
- 24. Food and Drug Administration. FDA Expands Palbociclib Approval for Breast Cancer. https://www.cancernetwork.com/breast-cancer/fda-expands-palbociclib-approval-breast-cancer (2016, accessed 30 October 2019).
- 25. Food and Drug Administration. FDA approves abemaciclib for HR-positive, HER2-negative breast cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-abemaciclib-hr-positive-her2-negative-breast-cancer (accessed 30 October 2019).
- 26. Food and Drug Administration. FDA approves abemaciclib and fulvestrant for HR-positive, HER2-negative breast cancer with disease progression following endocrine therapy. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-abemaciclib-hr-positive-her2-negative-breast-cancer (accessed 30 October 2019).
- 27. Food and Drug Administration. FDA approves abemaciclib as initial therapy for HR-positive, HER2-negative metastatic breast cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-abemaciclib-hr-positive-her2-negative-breast-cancer (accessed 30 October 2019).
- 28. Shah A, Bloomquist E, Tang S, et al. FDA approval: ribociclib for the treatment of postmenopausal women with hormone receptor-positive, HER2-negative advanced or metastatic breast cancer. Clin Cancer Res 2018; 24: 2999–3004. [DOI] [PubMed] [Google Scholar]
- 29. Von Minckwitz G, Schwedler K, Schmidt M, et al. Trastuzumab beyond progression: overall survival analysis of the GBG 26/BIG 3-05 phase III study in HER2-positive breast cancer. Eur J Cancer 2011; 47: 2273–2281. [DOI] [PubMed] [Google Scholar]
- 30. McCartney A, Migliaccio I, Bonechi M, et al. Mechanisms of resistance to CDK4/6 inhibitors: potential implications and biomarkers for clinical practice. Front Oncol 2019; 9: 666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med 2016; 375: 1925–1936. [DOI] [PubMed] [Google Scholar]
- 32. Spring L, Bardia A, Modi S. Targeting the cyclin D-cyclin-dependent kinase (CDK) 4/6-retinoblastoma pathway with selective CDK 4/6 inhibitors in hormone receptor-positive breast cancer: rationale, current status, and future directions. Discov Med 2016; 21: 65–74. [PMC free article] [PubMed] [Google Scholar]
- 33. DeMichele A, Clark AS, Tan KS, et al. CDK 4/6 inhibitor palbociclib (PD0332991) in Rb+ advanced breast cancer: phase II activity, safety, and predictive biomarker assessment. Clin Cancer Res 2015; 21: 995–1001. [DOI] [PubMed] [Google Scholar]
- 34. Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 2016; 17: 425–439. [DOI] [PubMed] [Google Scholar]
- 35. Condorelli R, Spring L, O’Shaughnessy J, et al. Polyclonal RB1 mutations and acquired resistance to CDK 4/6 inhibitors in patients with metastatic breast cancer. Ann Oncol 2018; 29: 640–645. [DOI] [PubMed] [Google Scholar]
- 36. Herrera-Abreu MT, Palafox M, Asghar U, et al. Early adaptation and acquired resistance to CDK4/6 inhibition in estrogen receptor–positive breast cancer. Cancer Res 2016; 76: 2301–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kwapisz D. Cyclin-dependent kinase 4/6 inhibitors in breast cancer: palbociclib, ribociclib, and abemaciclib. Breast Cancer Res Treat 2017; 166: 41–54. [DOI] [PubMed] [Google Scholar]
- 38. Bardia A, Hurvitz SA, DeMichele A, et al. Triplet therapy (continuous ribociclib, everolimus, exemestane) in HR+/HER2− advanced breast cancer postprogression on a CDK4/6 inhibitor (TRINITI-1): efficacy, safety, and biomarker results. J Clin Oncol 2019; 37(15_Suppl.): 1016. [Google Scholar]
- 39. Formisano L, Lu Y, Servetto A, et al. Aberrant FGFR signaling mediates resistance to CDK4/6 inhibitors in ER+ breast cancer. Nat Commun 2019; 10: 1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Prat A, Pascual T, De Angelis C, et al. HER2-enriched subtype and ERBB2 expression in HER2-positive breast cancer treated with dual HER2 blockade. Journal of the National Cancer Institute. Epub ahead of print 30 April 2019. DOI: 10.1093/jnci/djz042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Veeraraghavan J, De Angelis C, Mao R, et al. A combinatorial biomarker predicts pathologic complete response to neoadjuvant lapatinib and trastuzumab without chemotherapy in patients with HER2+ breast cancer. Ann Oncol. Epub ahead of print 23 March 2019. DOI: 10.1093/annonc/mdz076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Risi E, Grilli A, Migliaccio I, et al. A gene expression signature of retinoblastoma loss-of-function predicts resistance to neoadjuvant chemotherapy in ER-positive/HER2-positive breast cancer patients. Breast Cancer Res Treat 2018; 170: 329–341. [DOI] [PubMed] [Google Scholar]
- 43. Patnaik A, Rosen LS, Tolaney SM, et al. Efficacy and safety of abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non-small cell lung cancer, and other solid tumors. Cancer Discov 2016; 6: 740–753. [DOI] [PubMed] [Google Scholar]
- 44. Goel S, DeCristo MJ, Watt AC, et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature 2017; 548: 471–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu T, Yu J, Deng M, et al. CDK4/6-dependent activation of DUB3 regulates cancer metastasis through SNAIL1. Nat Commun 2017; 8: 13923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lin NU, Winer EP. Brain metastases: the HER2 paradigm. Clin Cancer Res 2007; 13: 1648–1655. [DOI] [PubMed] [Google Scholar]
- 47. Bates SE. Central nervous system metastasis from breast cancer. Oncologist 2015; 20: 3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yin L, Li H, Liu W, et al. A highly potent CDK4/6 inhibitor was rationally designed to overcome blood brain barrier in glioblastoma therapy. Eur J Med Chem 2017; 144: 1–28. [DOI] [PubMed] [Google Scholar]
- 49. Raub TJ, Wishart GN, Kulanthaivel P, et al. Brain exposure of two selective dual CDK4 and CDK6 inhibitors and the antitumor activity of CDK4 and CDK6 inhibition in combination with temozolomide in an intracranial glioblastoma xenograft. Drug Metab Dispos 2015; 43: 1360–1371. [DOI] [PubMed] [Google Scholar]
- 50. Tolaney SM, Lin NU, Thornton D, et al. Abemaciclib for the treatment of brain metastases (BM) secondary to hormone receptor positive (HR+), HER2 negative breast cancer. J Clin Oncol 2017; 35(15_Suppl.): 1019. [Google Scholar]