Abstract
Considering the retina as an extension of the brain provides a platform from which to study diseases of the nervous system. Taking advantage of the clear optical media of the eye and ever-increasing resolution of modern imaging techniques, retinal morphology can now be visualized at a cellular level in vivo. This has provided a multitude of possible biomarkers and investigative surrogates that may be used to identify, monitor and study diseases until now limited to the brain. In many neurodegenerative conditions, early diagnosis is often very challenging due to the lack of tests with high sensitivity and specificity, but, once made, opens the door to patients accessing the correct treatment that can potentially improve functional outcomes. Using retinal biomarkers in vivo as an additional diagnostic tool may help overcome the need for invasive tests and histological specimens, and offers the opportunity to longitudinally monitor individuals over time. This review aims to summarise retinal biomarkers associated with a range of neurological conditions including Alzheimer’s disease (AD), Parkinson’s disease (PD), multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS) and prion diseases from a clinical perspective. By comparing their similarities and differences according to primary pathological processes, we hope to show how retinal correlates can aid clinical decisions, and accelerate the study of this rapidly developing area of research.
Keywords: Alzheimer’s disease, amyotrophic lateral sclerosis, biomarker, multiple sclerosis, neurodegeneration, optical coherence tomography, parkinson’s disease, prion disease, retina
Introduction
The eye provides a unique opportunity for direct observation of an extension of the central nervous system (CNS) through the clear optical media of the eye, consisting of the cornea, aqueous humour, lens and vitreous body. This has earned its title of ‘window to the brain’. The ability to study the retina in vivo with noninvasive techniques brings with it the possibility of finding novel biomarkers and surrogate endpoints with which to diagnose and monitor neurological conditions. There are clear benefits of this approach over pathological diagnosis, not requiring the risk of neurosurgery, the removal of potentially important neurones, or postmortem diagnoses. In comparison to brain imaging, retinal imaging holds two key advantages: the lack of ionising radiation and the direct visualization and cellular resolution that is achievable,1 with or without the use of contrast agents. However, the biological plausibility between correlates and disease must also be explored if the retina is to have a use as a surrogate marker in diseases of the CNS.2,3
Discovering biomarkers or ‘surrogate markers’ that predict future clinical outcomes in neurodegenerative conditions may provide a window of opportunity in which to start treatment early prior to secondary degenerative processes taking hold in response to a variety of initial triggers.4 Given the poor ability of the human CNS to regenerate, minimising cell loss is likely to equate to better long-term outcomes.5
What is common to many neurodegenerative CNS disorders is the difficulty in early detection and a delay in diagnosis.6 As well as the lack of specificity of symptoms, cognitive function testing has inherent issues of repeatability,7 with day-to-day fluctuation sometimes being a key disease characteristic.8 In comparison, objective retinal biomarkers offer good repeatability and reproducibility.9,10 The ease of retinal imaging coupled with its ability to monitor specific cell populations through time in the same individual are attractive prospects.
This review provides an update on retinal findings, correlates, and staging of patients with neurodegenerative conditions. A variety of studies are covered, including those attempting to show an association with retinal biomarkers and disease severity, duration and functional loss. Validation of these biomarkers is crucial to enable their use as surrogates in research trials and clinical practice.
Alzheimer’s disease
Alzheimer’s disease (AD) is the most common cause of dementia (60–70%) in which there is a long ‘preclinical’ phase,11 with pathological features thought to appear years prior to symptoms.12 Diagnostic delay of AD has detrimental effects on efficacy of pharmacological treatments as well as wider ranging social and economic effects.13–15 Often manifesting in mild, nonspecific cognitive and psychological disturbances, early disease is often challenging to differentiate from the ‘normal’ ageing process. Similarly, concomitant ocular and systemic neurodegenerative and cardiovascular conditions that may contribute to retinal nerve fibre layer (RNFL) thinning such as glaucoma and microvascular ischaemia become more prevalent as age increases. These confounders are challenging to identify and rarely controlled for.
Only 8% of patients with mild cognitive impairment (MCI) have been shown to progress to AD.16,17 Biomarkers have been introduced into the clinical criteria for AD,18 including detection of amyloid-beta and tau deposition using positron emission tomography (PET) imaging and cerebrospinal fluid (CSF) sampling,19–23 as well as detection of neuronal injury using fluorodeoxyglucose (FDG)-PET and single photon emission computed tomography (SPECT) imaging.19 However, methods to image individual cells and distinct populations of cells are still lacking.
Pathological studies
AD is macroscopically characterised by loss of brain volume, with heterogenous patterns of atrophy seen in the medial temporal lobes, paralimbic, temporal and parietal cortices on structural mag-netic resonance imaging (MRI).24,25 Microscopically, characteristic amyloid-beta plaques in the brain parenchyma, and hyperphosphorylation of tau proteins forming neurofibrillary tangles lead to neuronal and synaptic degeneration, most commonly attributed to abnormal processing of the amyloid precursor protein (APP).6,26
Visual abnormalities in AD are often detectable early in the disease, and therefore provide the rationale behind retinal biomarkers in early AD. Deficits can be found in visual acuity,27 colour and motion perception,28 contrast sensitivity and visual fields.29–31 Pathological studies attempt to explain this, demonstrating diffuse axonal degeneration with retinal ganglion cell (RGC) and RNFL loss post mortem.32–35 Disruption of circadian rhythm has been proposed to be due to the loss of melanopsin RGCs, with reduced numbers and abnormal morphology in AD.36 Despite multiple transgenic models of AD demonstrating amyloid-beta plaques and tau accumulations,37,38 human evidence for these in the retina remain controversial, with amyloid-beta plaques reported to be present and absent when compared with age-matched controls,37–40 with explanations offered including different assays or methods of tissue preparation.40 Although hyperphosphorylated tau protein has been detected in the retinas of patients with AD,41 the characteristic aggregates have not.32,40 Evidently, it is still unclear as to whether AD changes seen in the eye are as a primary or secondary consequence of amyloid-beta.
In an AD mouse model (APPSWE/PS1ΔE9) intravenous curcumin was used to demonstrate retinal amyloid-beta plaques, verified with immunohistochemistry, and their accumulation with disease progression.38 Curcumin is a polyphenol found in turmeric (Curcuma longa) and is an attractive, inherently fluorescent, diagnostic agent as it is also a novel neuroprotective treatment under investigation.42,43 Supporting amyloid-beta accumulation in the AD retina, proof-of-concept data has demonstrated retinal amyloid curcumin visualisation, with a 2.1-fold increase in AD patients versus age-matched controls (p = 0.0031).44 Hyperspectral imaging (HSI) microscopy is a novel imaging technique that has shown promise by analysing the ‘spectral signature’ reflected from the retina, reporting amyloid-beta deposition in postmortem retinal samples and in vivo animal studies.45 This latter study also claimed to have detected amyloid-beta plaques in mice retinas prior to plaque development in the brain, critical for early diagnosis.
OCT in AD
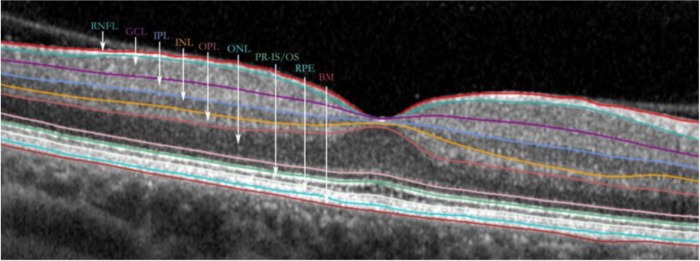
Optical coherence tomography (OCT) is a rapid, noninvasive, reliable and reproducible imaging tool using near-infrared light to form cross-sectional images of the retina and optic disc.46,47 Axial resolutions of up to 5–7 microns enable individual retinal layers to be studied using the principles of interferometry (Figure 1).48 AD findings of atrophy in the anterior visual pathways post mortem have been associated with OCT evidence of RNFL thinning around the optic nerve (peripapillary RNFL, pRNFL) (Figure 2).32 This effect in AD has been noted around the optic disc globally,49–72 but also to have a preponderance in superior and inferiorperipapillary sectors,49,50,52–55,57–59,61–69,71–75 and, to a lesser extent, in the nasal and temporal sectors also (Table 1).52,55,56,58,62,64–66,71,72,74,75 In contrast, a smaller proportion of studies deny finding this association.76–79 Many of these studies have been included in meta-analyses supporting RNFL thinning in AD.80,81 Rate of RNFL thinning also appears greater in AD compared with ‘normal’ ageing over a 12-month period, especially in the inferior quadrant, and in parallel with cognitive decline,54 thus suggesting the rate of pRNFL thinning could function as a potential surrogate marker in itself, as has been proposed in glaucoma.82 Furthermore, the inferior pRNFL sector has been proposed as the most sensitive for detecting cognitive decline in AD.68,83 These patterns have also been functionally correlated to pattern electroretinogram (ERG) abnormalities72,75,84 and visual function tests.50 The challenge will be to separate pathological progression from RNFL thinning due to ageing alone.85 RNFL thinning has been reported to correlate with scores of cognitive function53–55,86; however, this not supported by other studies,64 possibly affected by mental fluctuation and disease heterogeneity. Retinal correlates and patient-related endpoints such as quality-of-life (QoL) scores are still to be assessed in a holistic manner.
Figure 1.
A spectral domain OCT cross-sectional macula scan of the retinal layers.
BM, Bruch’s membrane; GCL, ganglion cell layer; INL, inner nuclear layer; IPL, inner plexiform layer; OCT, optical coherence tomography; ONL, outer nuclear layer; OPL, outer plexiform layer; PR IS/OS, photoreceptor inner segment/outer segment; RNFL, retinal nerve fibre layer; RPE, retinal pigment epithelium.
Figure 2.
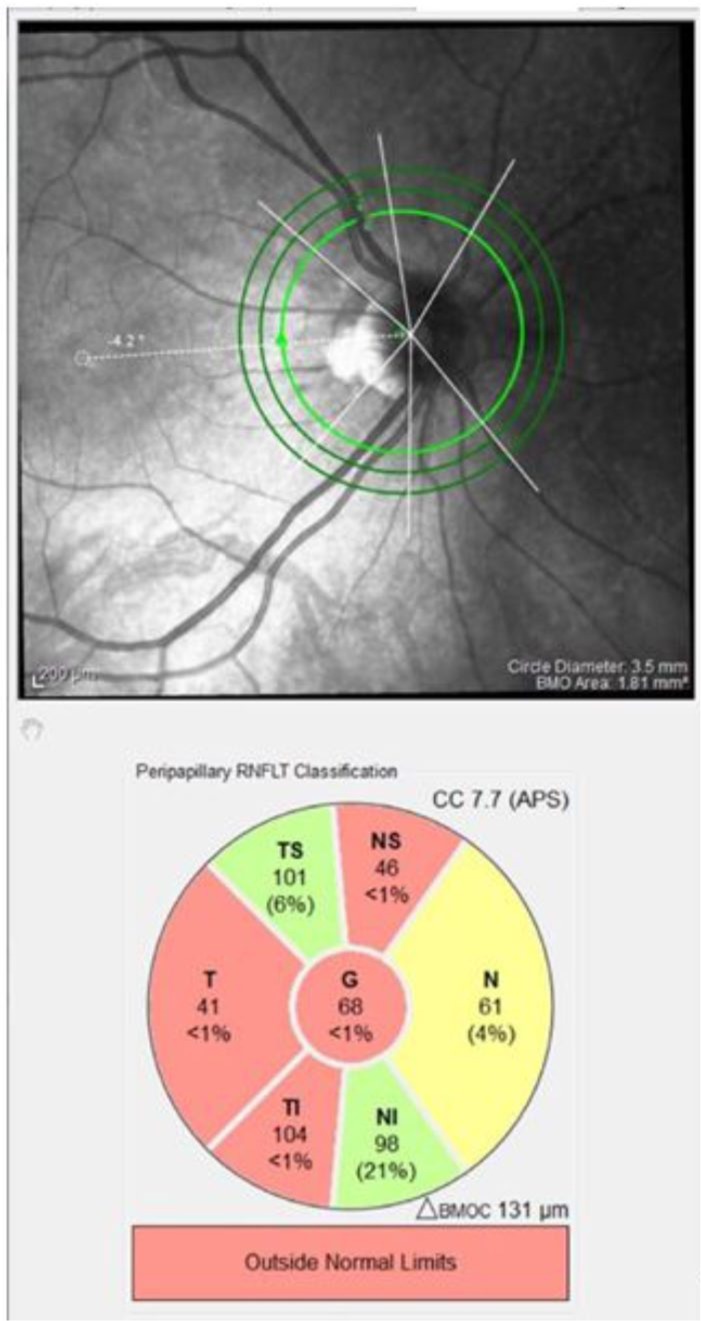
An SD-OCT pRNFL scan of the right eye of a patient who has had ON, demonstrating temporal pRNFL thinning. Red sectors indicate those outside normal limits.
pRNFL, peripapillary retinal nerve fibre layer; ON, optic neuritis; SD-OCT, spectral domain optical coherence tomography.
Table 1.
A summary of studies reporting sector analysis of peripapillary RNFL changes in Alzheimer’s disease, mild cognitive impairment (MCI) and Parkinson’s disease. Effect size has been calculated as mean percentage difference in average RNFL thickness per sector in disease verus healthy controls.
| Alzheimer’s disease |
OCT type | Global | Superior |
Temporal | Inferior |
Nasal | ||
|---|---|---|---|---|---|---|---|---|
| Study | ST | SN | IT | IN | ||||
| Ascaso65,b | TD | 45.4% | 49.8% | 28.6% | 44.3% | 53.1% | ||
| Bambo62 | SD | 12.1% | 70.1% | 5.3% | 10.4% | 15.8% | ||
| Cheung63 | SD | 4.0% | 7.1% | 0.6% | 5.5% | –0.6% | ||
| Choi55 | SD | 7.5% | 11.2% | 3.3% | 5.3% | 12.5% | ||
| Cunha53 | SD | 9.4% | 9.6% | 8.3% | 14.6% | 4.5% | ||
| Cunha52 | SD | 11.8% | 18.0% | 10.5% | 10.6% | 12.4% | 10.1% | 8.4% |
| Eraslan61 | SD | 8.3% | 12.3% | 8.4% | a | 5.5% | 5.9% | a |
| Gao58 | SD | 13.7% | 14.1% | 16.6% | 16.0% | 5.2% | ||
| Garcia-Martin56 | SD | 3.7% | 3.2% | 5.5% | 6.1% | 5.7% | 1.6% | 0.7% |
| Gharbiya79 | SD | –0.9% | 1.2% | –3.9% | –2.3% | –1.6% | ||
| Gunes64 | SD | 24.2% | 19.5% | 18.5% | 29.2% | 23.1% | ||
| Iseri71 | TD | 25.6% | 19.6% | 10.8% | 31.4% | 40.6% | ||
| Kesler68 | TD | 10.7% | 10.5% | 9.4% | 14.3% | 13.4% | ||
| Kirbas67 | SD | 14.3% | 32.0% | 4.0% | 1.9% | 1.3% | ||
| Kromer74 | SD | a | a | a | a | a | a | a |
| Kwon73 | SD | 4.7% | 7.7% | 3.9% | –0.1% | 2.9% | ||
| La Morgia57 | SD | 7.6% | 11.5% | 3.1% | 4.9% | 10.9% | ||
| Larrossa66 | SD | 3.0% | 4.0% | 5.1% | 5.6% | 2.6% | ||
| Liu59 | SD | 8.8% | 9.0% | 10.8% | 10.4% | 21.6% | ||
| Moschos75,b | TD | a | 10.5% | 12.9% | 16.0% | 11.4%e | ||
| Parisi72 | TD | 50.7% | 36.8% | 77.2% | 39.5% | 59.8% | ||
| Pillai77 | SD | –4.1% | –1.1% | –4.5% | –5.1% | –8.0% | ||
| Polo50 | SD | 7.0% | 8.0% | 5.9% | 8.8% | 3.6% | ||
| Trebbastoni54 | SD | 0.9% | 3.3% | –1.4% | –1.0% | 2.9% | ||
| MCI - Study | OCT type | Global | Superior | Temporal | Inferior | Nasal | ||
| Ascaso 2014 | TD | 17.3% | 23.8% | 11.2%d | 19.2% | 12% | ||
| Cheung 2015 | SD | 1.3% | 2.3% | 0.6% | 1.9% | –1.2% | ||
| Gao 2015 | SD | 6.5% | 8.6% | 11.4% | 3.0% | 1.7% | ||
| Kesler 2011 | TD | 9.4% | 8.2% | 5.5% | 12.6% | 14.8% | ||
| Liu 2015 | TD | 4.9% | 3.4% | 5.4% | 4.4% | 6.7% | ||
| Pillai 2016 | SD | –5.3% | –3.1% | –7.0% | –7.4% | –4.2% | ||
| Parkinson’s Disease |
OCT type | Global | Superior | Temporal | Inferior |
Nasal | ||
| Study | ST | SN | IT | IN | ||||
| Aaker87 | SD | 0.0% | 3.0% | –3.8% | 4.1% | 0.0% | 0.0% | –2.7% |
| Albrecht88 | SD | 1.8% | 3.7% | 1.1% | –0.4% | –2.1% | ||
| Altintaş89 | TD | 14.8% | 10.1% | 1.4% | 12.2% | 26.8% | ||
| Archibald90,b | TD | –4.6% | –9.4% | 1.5% | 1.6% | –13.4% | ||
| Aydin91 | SD | 6.9% | 7.4% | 8.4% | 2.7% | 2.7% | 8.1% | 5.6% |
| Bittersohl92 | SD | 0.4% | 1.3% | –1.1% | 1.5% | 6.1% | –1.1% | –0.1% |
| Garcia-Martin93 | SD | 2.2% | 2.2% | 6.9% | 1.5% | 0.9% | ||
| Inzelberg94 | TD | a | –3.8% | 22.0% | 26.0% | 16.7% | ||
| Jiménez95 | TD | 17.4% | 7.7% | 6.4% | 6.9% | 10.7% | ||
| Kirbas96 | SD | 14.5% | 2.3% | 12.8% | 1.8% | 0.0% | ||
| La Morgia97 | TD | 5.2% | 4.4% | 10.6% | 3.0% | 5.3% | ||
| Matlach98 | SD | 5.4% | 8.3% | 2.5% | 3.4% | 2.8% | ||
| Moschos99 | TD | 7.5% | 3.0% | 12.2% | 10.2% | 5.5% | ||
| Moschos100 | SD | 8.8% | 4.8% | 14.6% | 2.5% | 5.2% | ||
| Pillai77 | SD | –3.7% | –3.8% | –0.2% | –2.9% | –8.6% | ||
| Rohani101 | SD | 11.8% | 14.6% | 9.2% | 13.9% | 8.4% | ||
| Satue102 | SD | 7.1% | 7.0% | 5.9% | 7.0% | 0.3% | ||
| Sen103 | SD | 8.0% | 3.2% | a | 11.0% | a | ||
| Sengupta104 | SD | 19.5% | 26.1% | 5.9% | 28.9% | 32.4% | 8.9% | 11.2% |
| Tsironi105 | TD | –0.1% | 4.6% | –2.2% | –1.2% | –2.8% | ||
| Visser106 | SD | 1.1% | –0.9% | 11.4% | 7.6% | –1.5% | ||
Data not available.
Mean of right and left eyes.
Significance data missing
OD significant only
OS significant only
Grey shading indicates statistical significance as chosen by each study investigator.
IT, inferotemporal; IN, inferonasal; SD, spectral domain; TD, time domain; ST, superotemporal, SN, superonasal.
OCT imaging of the macula and posterior pole also allows for further examination of retinal cell populations (Figure 3), of which the inner layers, including the RNFL, ganglion cell layer (GCL) and inner plexiform layer (IPL), are of most current interest in neurodegeneration. GCIPL (ganglion cells and inner plexiform layers combined) thinning has been observed by multiple studies in AD,49,51,53,55,56,61,63,66,76,107 with some studies showing diagnostic superiority when compared with pRNFL changes.63 Changes in both macular volume and GCIPL thickness have been found to correlate with mini mental state examination (MMSE) scores (average GCIPL r = 0.487, p = 0.003).51,71 Extended-depth imaging (EDI) using OCT has been used to consistently report choroidal thinning to be associated with AD, further hypothesising that cerebrovascular disease and hypoperfusion may play a common role in CNS and chorioretinal atrophy.79,108–110 Further-more, these findings have also been also associated with MMSE scores.109
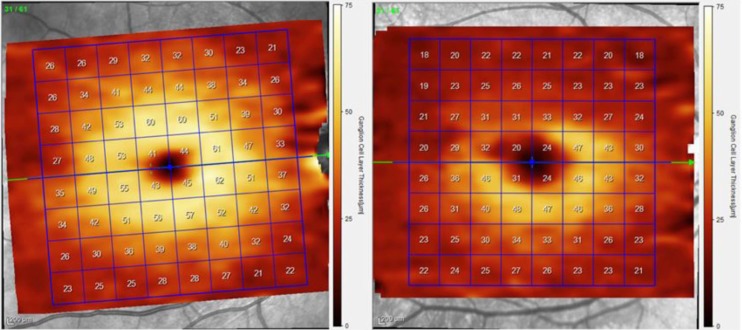
Figure 3.
Ganglion cell layer thickness maps from a segmented SD-OCT scan of the macula from the right eye of a healthy (left) and ON (right) patient. The segmented GCL is selected in this example, demonstrating superior loss of ganglion cells and corresponding thinning.
GCL, ganglion cell layer; ON, optic neuritis; SD-OCT, spectral domain optical coherence tomography.
The risk of AD rises from 1 to 2% per year in the normal population to 10–15% in those classified with MCI.111 Therefore, RNFL has been assessed as a correlate with long-term prognosis. Global differences in pRNFL in mCI versus healthy controls have been reported in several studies (Table 1),49,51,59,60,65,68,70 and specifically inferior, superior, and even temporal sectors.49,58,59,68 To differentiate stable MCI and those progressing to AD, several peripapillary and macular parameters correlate with risk of progression to AD including temporal RNFL thickness and global GCIPL thickness.55 Rate of decay in RNFL thickness has also been reported to be greater in AD than MCI.49 Evidence suggesting retinal correlates can predict future AD in ‘healthy’ individuals, similar to CSF amyloid-beta levels is limited,112–114 with confirmatory studies required. Clearly, differentiating AD and non-AD cognitive decline using OCT parameters will present challenges given the correlations with cognitive decline in general.115
Other retinal imaging in AD
Retinal oximetry is a metabolic imaging technique that estimates the oxygen saturation of haemoglobin in retinal arterioles and venules. Most widely studied in ischaemic diabetic retinopathy and vascular occlusions, some studies have shown a potential role in AD. Higher arteriolar and venular oxygen saturations in moderate AD compared with healthy individuals116 has been reported; however, the absence in early disease limits early diagnosis potential. Uptake of this technique into clinical practice has been limited thus far due to poor disease specificity and the unclear mechanism behind this correlate. Arteriolar and venous pulsations have also been shown to correlate with amyloid-beta imaging in the brain, with increased arteriolar amplitudes and decreased venous amplitudes correlating with neocortical 18F-florbetaben (FBB)-positron emission tomographic amyloid imaging.76 Optic disc colour has been proposed as a biomarker in AD, with pallor suggested as an indicator of axonal loss. Although significantly correlated with AD (p < 0.003), the diagnostic prowess and dynamic range of this technique are still to be fully revealed.62 The likely poor disease specificity may limit its potential in diagnosis.
The role of OCT angiography in CNS conditions is still being explored,117 with the foveal avascular zone (FAZ), retinal vascular density and inner foveal thickness shown to differ in AD,118 while other parameters such as outer retinal and choroidal flow-rates have not.119 This requires further studies to corroborate these results.
An emerging imaging biomarker for clinical trials is DARC (detection of apoptosing retinal cells).120 Using confocal scanning light microscopy, this technique utilizes intravenous fluorescent annexin-A5 binding to phosphatidylserine on the cell membrane in early apoptosis. The Phase I trial primarily confirmed safety and tolerability, and also observed differences between glaucomatous and healthy eyes.121 Given the similar loss of retinal ganglion cells in AD, there is potential for the technique in early diagnosis of AD. A phase II trial has been completed with the results yet to be published, including a cohort of people with Down syndrome, known to display similar amyloid-beta deposition to that in AD.122 The lack of consistent findings of human retinal plaque deposition leaves open the hypotheses of secondary neurodegeneration in AD. Many other genetic risk factors identified in AD may also be contributing, distinct from amyloid precursor protein and apolipoprotein E.123
Parkinson’s disease
Parkinson’s disease (PD) is a neurodegenerative condition causing rigidity, tremor and bradykinesia.124 In addition, cognitive, autonomic and visual functioning can be affected.125 Its pathological hallmark is intraneuronal aggregation of α-synuclein, which in the substantia nigra leads to loss of dopaminergic neurones and the extra pyramidal symptoms.126 Postmortem evidence has similar features in the retinas of PD patients, including reduced dopamine content and α-synuclein accumulation,127 specifically the inner nuclear layer (INL), inner plexiform layer and the ganglion cell layer.128 Combining biomarkers is the current approach to diagnosing PD, integrating brain imaging (SPECT), PET and MRI with genetic and biochemical biomarkers.129 Retinal imaging could emerge as a possible biomarker that has the potential to add another dimension to this matrix. In its investigation, careful consideration is yet to be given to controlling confounding ocular and systemic conditions more commonly found in older people, that may also affect RNFL thickness.
OCT tomography in PD
RNFL thinning in PD has been studied comprehensively by many researchers (Table 1), with many reporting pRNFL thinning. The frequency of this finding is noticeably less common than in AD (see Table 1), with a different peripapillary distribution. Significant thinning in global, superior, inferior, nasal and temporal pRNFL have been observed. 89,91,93–97,99–104,106,119 In contrast, several studies have reported no significant differences.77,87,88,90,92,105 A total of 13 case-control studies were included in a meta-analysis, supporting pRNFL thinning in all four quadrants (WMD = 25.76, 95% CI: 28.99–22.53, p = 0.0005).130 Reports concerning laterality have been conflicting, with one study observing a post hoc association between superior quadrant pRNFL thinning in eyes ipsilateral to the side most affected by bradykinesia.98 However previous work has shown the contrary97. Foveal contour has also been studied, characterising the breadth and depth of the foveal pit. Superior/inferior sloping was found to be the most shallowed in PD, with an error rate of around 34% at detecting PD from healthy controls.131 Interocular asymmetry of foveal contour has also been proposed as a feature of PD.132
As with pRNFL findings, results of macular OCT segmentation have been inconsistent at demonstrating differences between PD patients and healthy age-matched controls, concluding both significant and nonsignificant differences between groups.87,89,98,133–137 To our knowledge, all studies examining both pRNFL and mRNFL thickness in PD have had a similar conclusion with both parameters, be this either no difference or a significant difference.87,89,134–137 This may indicate similar sensitivity of pRNFL and mRNFL OCT scanning as a biomarker in PD, or indicate a correlation of these two parameters with disease severity. Conflicting results are likely due to variability in disease severity between study populations, and the challenges in its quantification. Given that studies examining RNFL thinning in different stages of disease indicate progression towards global thinning across all sectors, any disease specificity may be found only in the order or rate at which they are affected (Table 1).
Establishing the role of OCT in assessing the rate of progression associated with functional decline is important. This may provide prognostic information for patients and surrogacy for therapies in clinical trials. A 5-year prospective longitudinal trial has been completed using OCT imaging and functional testing in order to investigate several aspects of this paradigm.134 OCT demonstrated increased rates of RNFL thinning over superotemporal and temporal peripapillary sectors, and in seven of the nine macular zones in PD. Furthermore, moderate correlations were found between low contrast visual acuity, superotemporal and inferotemporal pRNFL sector thinning and symptom severity.138 Mild association between superotemporal pRNFL thinning and progression of Parkinsonian symptoms was seen in the PD group (r = –0.389, p = 0.028), suggesting OCT progression could play a role in predicting functional and visual outcomes, and possibly even benefits gained from treatment in a clinical trial setting. However logistic regression revealed no parameters of visual function that were able to predict symptomatic decline. Confounding these studies are the difficulties encountered with testing visual function in patients with advanced dementia.
RNFL thinning on OCT imaging has also been correlated with retinal function and dopaminergic activity in the substantia nigra using, microperimetry and PET/MRI imaging.139 RNFL thinning in the temporal and inferior foveal zones was observed in PD when compared with healthy age-matched controls. Retinal thinning in certain sectors was also found to correlate with macular sensitivity and dopaminergic loss in the substantia nigra. The hypothesis that a pathologic connection exists between retinal and nigral dopaminergic cells could raise useful biomarkers in trials of neuroprotective treatment strategies. Similarly, there is a partial treatment effect with l-DOPA on recovering contrast sensitivity in PD patients.140 A further study has suggested dopaminergic treatment effect is visible in retinal changes; however, this is only in the absence of a significant difference between untreated, and treated patients who have more severe disease.103
Disease-specific OCT biomarkers still remain to be found, with some studies failing to show any differences between RNFL characteristics in different dementia types.77 The predilection for temporal RGC loss in PD correlates with the papillomacular bundle and parvocellular pathway, which is distinct from that reported in AD, where the RGCs in the magnocellular pathways are commonly seen to be preferentially affected.141 Selective thinning of the photoreceptor layer in vivo has also been reported in PD as has generalised retinal thinning,133,136,142 to accompany similar postmortem evidence in humans and animal models.127,143 Considering the dopaminergic input to photoreceptors in the retina, trans-synaptic degeneration may explain this observation, preferentially noted to affect blue cones.144,145 Less dramatic RNFL thinning in PD may be explained by the reduced number of RGCs with dopaminergic inputs.146 The more varied OCT findings in PD compared with AD reflect the wider variety of phenotypes contained in the PD spectrum, in which various diseases display Parkinsonian-like traits.147 The observation that mutations in genes such as LRRK2 and those encoding α-synuclein and tau can cause varying clinical syndromes does not support a purely pathogenetic disease process or primary retinal pathology, but implicates environmental factors as supported by associations seen with heavy metal exposure and other environmental toxins.148,149
Combining structural and functional biomarkers is likely to increase diagnostic yield in PD using the retina. For example, parafoveal thickness and contrast sensitivity provided an area under the receiver-operator characteristic (AUROC) curve of 0.784, improving to 0.844 with the addition of visual evoked potentials.150 The reliance on non-imaging parameters may also become necessary in advanced disease due to tremor precluding the acquisition of high-quality images.
Multiple sclerosis
Multiple sclerosis (MS) is an autoimmune demyelinating disorder of the CNS.151 Approximately 2 million people are affected worldwide, making it one of the most common causes of nontraumatic neurological disability affecting young adults.152 Demyelination leads to neuro-axonal loss causing progressive neurological disability and functional decline.153
Multiple clinical features affecting motor, sensory, visual or autonomic pathways exist, commonly presenting in the third or fourth decades with a female predominance. MS may be categorised according to the timing and pattern of disease.154 The commonest type is relapsing-remitting (RRMS, 85%), in which 18% evolve to secondary-progressive disease (SPMS); 15% exhibit progressive disease at onset, with or without relapses.155,156
Ophthalmic involvement is a frequent cause of disability encompassing optic neuritis (ON), uveitis, internuclear ophthalmoplegia (INO) and nystagmus. Half of patients will experience ON over the disease course.157,158 It is the presenting feature in 30% of cases, manifested as retrobulbar pain on eye movement, proceeding to visual loss and dyschromatopsia. Recovery is often incomplete due to demyelination and axonal loss.159,160 Timely recognition of atypical causes of ON can prevent irreversible visual loss or side effects by inappropriate usage of drug therapy.161 OCT has proven useful for diagnosis and monitoring of ON longitudinally.162 Functional connectivity by adaptive reorganisation in the visual system occurs following ON.163
In combination with clinical evaluation, several clinical and paraclinical biomarkers inform the MS diagnostic process. Oligoclonal bands in the CSF are an indicator of CNS immune activity, and MRI detects inflammation and atrophy within the brain and spinal cord.160 The revised McDonald diagnostic criteria have a high degree of sensitivity and specificity in early disease.164–167
OCT in multiple sclerosis
Numerous studies have shown that MS sufferers display neurodegenerative and inflammatory signs in the retina, mirroring those of the CNS seen on MRI.164 OCT quantification of RNFL thickness in MS was first described in 1999.168 Subsequent reports have been made of RNFL thickness and other OCT biomarkers with and without ON versus healthy controls.168–171 Unmyelinated RGC axons within the RNFL provide an objective indicator of axonal loss in the retina, and potentially the CNS globally. The presence of blood vessels contributes to 13% of RNFL thickness, altering the reliability of measurements, especially in severe thinning.172
RNFL thickness is reduced in ON and MS patients compared with healthy controls (Figure 2). This is seen using OCT in eyes with prior ON,171,173–187 particularly temporally and inferiorly.176,177,188 The majority of studies also identified reduced pRNFL thickness in MS patients without prior history of ON (MS-NON), although to a lesser extent.189 Temporal preponderance may be explained by the presence of the large papillo-macula bundle containing a high density of parvocellular axons appearing to be most susceptible to oxidative stress.190 Increased RNFL thinning in ON is associated with incomplete visual recovery.191 Few studies, however, report RNFL thinning after ON to be similar in affected and unaffected eyes.192 RNFL thickness is also a biomarker for MRI-estimated whole brain atrophy in MS patients.193,194
RNFL thinning in the absence of ON suggests underlying CNS disease activity. RNFL thickness was inversely related to optic radiation lesion volume in patients with MS, and CIS patients including those without previous ON.195 RNFL thinning may therefore be related to ON-related retrograde degeneration due to damage propagating backwards from the site of injury.196 It may be either trans-synaptic via the lateral geniculate nucleus for posterior visual pathway lesions, or direct retrograde degeneration, beginning at the ganglion cell axons (RNFL), progressing to GCL cell bodies and finally IPL dendrites.186 Wallerian or anterograde degeneration has also been proposed, due to pathology in the retinal layers leading to RNFL thinning.197 Animal studies have suggested this may not play a major role in MS pathogenesis however.198
The GCIPL thickness is often measured together due to low contrast differences of OCT images. GCIPL comprises RGC cell bodies and retinal astrocytes. As the highest density of RGCs is at the macula, GCIPL is regularly measured as perifoveal volume in cubic millimetres.199 Thinning of the IPL and GCL has been reported in isolated episodes of ON in clinically isolated syndrome183 (Figure 3).
Macula volume measures the size of axons and their associated ganglion cell bodies, with studies demonstrating reduction compared with age-matched controls in both MS-NON and MS-ON eyes.182,199,200 However, other studies have reported conflicting results,201 suggesting inconsistencies in disease duration and severity amongst study cohorts.
Whilst pRNFL and macula volume are the most common OCT markers used in MS, there has been increasing interest in the INL and, less so, in outer retinal layers. The INL contains the cell bodies of the horizontal, bipolar and amacrine cells. Thinning of the INL has been reported in PPMS.202 INL thickening is associated with a range of disorders including inflammatory disease activity in MS.203–205 It may also be characterised by microcystic macular oedema (MME),205–207 the presence of INL cysts occurring with RNFL thinning.207,208 MME was previously thought to reflect blood–retinal barrier breakdown with or without microglial inflammation; however, the absence of leakage on angiography refutes this.209 Impaired water or potassium homeostasis have also been proposed as contributing factors.207 The most likely cause is of retrograde degeneration of the inner retinal layers causing impaired macula fluid resorption.210 INL thickness may prove useful for monitoring response to immunotherapy in inflammatory states.211
Thickening of the outer nuclear layer (ONL) (containing photoreceptor cell bodies) and photoreceptor layer followed by thinning has been reported in ON. Outer retinal dysfunction measured by ERG has also been reported despite normal sublayer thickness measurements.212–214 Transient ONL thickening has also been reported during the active phase of ON, alongside reduced RNFL and total macula volume.213 ONL thickening was observed 4 months following ON, and correlates well with GCIPL thinning.212 From 4 to 12 months ONL thickness returned to baseline. Reports have also identified no ONL differences between MS patients and healthy controls, supporting presence of thickening in active inflammation only.186,202
Retinal vascular changes in MS
OCT angiography (OCTA) detects the motion of red blood cells, providing noninvasive angiography and flow rates of the deep and superficial retinal plexuses, with potential in the management of MS and other neurological disorders.215 There is parafoveal vessel reduction in superficial and deep vascular plexuses in MS independent of ON history.216,217 This correlates with pRNFL and GCIPL layer thinning and also with general disability level, as assessed by the Extended Disability Status Scale (EDSS).216 A longitudinal study, however, showed an increase in parafoveal vessel density over a median follow-up period of 1 year, particularly in patients who demonstrated disease stability.218 There is evidence of reduced flow velocity in parafoveal and optic nerve head vessels in eyes with prior ON, compared with MS-NON eyes and healthy controls.219,220 This may reflect the reduced metabolic demand of the retina due to the retinal thinning, decreased perfusion due to inflammation-induced endotheliopathy and a retinal increase of hypoxia-inducing factors during neurodegeneration.156 However, image artefacts in OCTA have been reported to occur frequently.221 Further studies are needed to improve acquisition and analysis of OCTA images and to assess the course of MS longitudinally to identify useful disease correlates.
OCT to predict visual prognosis and MS subtypes
The use of OCT retinal correlates to measure the severity or duration of MS could prove inexpensive and rapid compared with repeated MRI studies, and more precise than clinical evaluation. Active MS, as identified clinically or radiologically, is associated with accelerated RGC and IPL thinning, fastest in those with clinical relapse, gadolinium-enhancing lesions, and new T2 lesions simultaneously.222 The most severe subtypes of MS showed more marked decreases in RNFL and macula volume than RRMS cases.170,223 Longitudinal studies show progressive RNFL and macula volume reductions in MS patients within 1 year, in particular affecting the mean RNFL thickness even in the absence of ON.224–226 Herrero’s study, which followed patients up over 3 years, identified increased RNFL loss in the superior and inferior regions.226 After 2 years, macular GCIPL layer thinning was also identified. These changes were most apparent in the early phase of the disease, perhaps indicating a later plateau.227 Contradictory results using time-domain OCT to monitor RNFL thickness have also been reported; however, this was using inferior resolution technology.228 In patients with clinically isolated syndrome, OCT assessment of GCIPL and pRNFL thinning are valuable predictors of future MS diagnosis.229 This subset of patients appear to behave similarly to patients with radiologically isolated syndrome (RIS), in which there is an incidental finding of demyelination on MRI. These patients have reduced RNFL and increased INL and ONL volumes, correlating with prospective disease activity and progression in MS.230
Studies have suggested that OCT may predict levels of disability associated with MS and QoL outcomes.231,232 RNFL thickness correlates with visual acuity and EDSS,200,233 with GCIPL thinning also associated with low-contrast visual acuity.212,213 Other studies have demonstrated thinner GCIPL and RNFL layers in MS patients are associated with worse visual functional and QoL assessments.186,234
Visual evoked potentials (VEP) and pattern electroretinograms (PERG) are altered in MS200,235–237 showing prolonged P(100) latencies and normal amplitudes. However, studies are contradictory as to whether VEP findings correlate with RNFL thickness, perhaps suggesting that RFNL loss detected by OCT in some cases is insufficient to cause functional deterioration.
A study evaluating VEPs, in particular P(100) latency, amplitude and waveform morphology, demonstrated a correlation with MS as assessed by the Kurtzke EDSS and whole brain atrophy (BPF).236 A moderate correlation between RFNL thickness, EDSS score and QoL (based on the 54-item Multiple Sclerosis Quality of Life Scale score) was identified.177 The use of functional tools such as VEPs and PERG may be important for identifying subclinical axonal damage in the early stages of MS.
OCT in paediatric patients has been shown to be sensitive to retinal changes in clinical ON, whereas VEPs are useful to detect disseminated lesions in the visual pathway. One study demonstrated RNFL thinning in those with prior ON and also prolonged VEP latency (>109 ms) regardless of ON history.238
A growing body of evidence supports the value of OCT in monitoring MS disease progression, in particular, the use of RNFL thinning to predict QoL. Due to the lack of disease specificity, OCT must be used in combination with MRI and clinical assessment. Combining these with VEPs and PERG may prove useful to link structural and functional change as biomarkers for axonal degeneration.
Neuromyelitis optica spectrum disorders
OCT has been valuable in differentiating ON and MS from differential diagnoses, including neuromyelitis optica spectrum disorders (NMOSD), MOG-IgG seropositive autoimmune inflammatory disorders, and Susac syndrome. NMOSD are rare, chronic autoimmune inflammatory CNS diseases.239 Autoantibodies against the aquaporin-4 (AQP4) water channel located on astrocyte foot processes lead to recurrent attacks of ON, myelitis and brainstem syndromes.240–242 IgG and complement deposition, reduced AQP4 expression, astrocytopathy, neutrophil accumulation and demyelination with axonal loss are the underlying pathogenic processes.243 NMOSD-ON is commonly more severe, bilateral and recurrent than MS-ON. Despite treatment, recovery is often incomplete and remission rarely occurs. Relapsing NMOSD accounts for approximately 85% of cases, wherein neurologic defects frequently accumulate.
Whilst it is most commonly initially misdiagnosed as MS, clinical, immunological and imaging studies establish NMOSD as firmly separate from MS, with treatment strategies for MS ineffective and even harmful in NMOSD.244,245 Treatment strategies for acute relapses include high dose intravenous or oral steroid therapy and apheresis therapies, including plasma exchange and immunoabsorption.246 For long-term treatment, immunosuppressive drugs are used in a stepwise approach.247
In NMOSD, ON often occurs bilaterally, followed by severe structural retinal damage without segmental predominance, and profound visual impairment,205,248,249 with diffuse RNFL thinning seen by OCT in NMOSD patients, when compared with idiopathic disease.250 This is in comparison to MS ON, where temporal RNFL is preferentially thinned.205,251 Nasal thinning of the pRNFL persists in NMOSD in eyes, both with and without prior ON. Temporal thinning of the mRNFL also persisted in NMOSD-ON compared with MS-ON.251
A recent study demonstrated severe bidirectional degeneration in the visual pathway following NMOSD-ON, in particular the lateral geniculate nucleus volume, primary visual cortex volume and decreased integrity of optic radiations, compared with NMOSD without ON.252 Furthermore, there is increased functional connectivity in the primary and secondary visual networks in NMOSD, which correlates with increased visual impairment and reduced structural retinal damage on OCT.253
There is no consensus yet as to whether retinal changes occur in NMOSD without ON. Studies have demonstrated alterations of the optic radiation, GCIPL loss, and altered foveal shape, suggesting astrocytopathy in NMOSD patients.254–256 There is evidence of reduced temporal and superotemporal pRNFL loss in NMOSD-NON (NMOSD eyes without prior ON history) eyes compared with MS-NON.257 It has also been reported that GCIPL, pRNFL, foveal thickness and macula volume are all reduced in NMOSD regardless of ON status.258 In NMOSD, GCIPL thinning occurs longitudinally, independent of ON attacks.258 This finding was, however, contradicted by a small longitudinal observational study showing no change compared with in MNOSD-ON eyes.259
Clinical and histopathological studies indicate attack-independent lesions in the brain and spinal cord in NMOSD.260 Unlike in MS, a clear pattern of VEPs has not yet been defined in NMOSD patients due to conflicting results.261 These may be attributed to difficulties ascertaining a prior history of ON, differences in OCT devices, or segmentation techniques. Further results are required to use OCT as a valuable diagnostic tool in NMOSD eyes without prior ON history.
Macula microcysts also occur more frequently in NMOSD (20–26% of patients) than in MS (5% of patients) and appear to occur exclusively in eyes with previous history of ON.250,262,263 MME is associated with RNFL and INL thinning, and reduced visual acuity.264
OCTA studies showed peripapillary and parafoveal vessel density significantly decreased in NMOSD eyes independent of previous ON status.265 There is reduced microvascular density in superficial and deep plexuses surrounding the macula with increasing prior episodes of ON.266 This density correlated with both RNFL and GCIPL thickness, and also with visual acuity. Reduced microvascular density could occur prior to ON and RNFL atrophy, and it correlated well with visual function studies, suggesting its potential use as a predictor of visual outcomes. Further studies might consider differences between seropositive and seronegative NMOSD.267
Anti-MOG disease
In the 20–40% of patients with clinical signs of NMOSD but no AQP-4 antibodies,245 a subset will have antibodies against myelin-oligodendrocyte-glycoprotein (MOG). MOG is a glycoprotein located on myelin sheath on the cell bodies and processes of oligodendrocytes. These syndromes are referred to as MOG-encephalomyelitis and is a different clinical entity to NMOSD. Here, single ON episodes appear to cause less severe damage, although the high frequency of ON may result in greater structural damage and visual impairment.268 Similar to MS, there may be a temporal preponderance of pRNFL thinning in MOG-IgG seropositive patients.269 Additionally, in paediatric patients, significant RNFL reductions were seen in most retinal segments compared with MOG-negative eyes, and despite this, visual acuities were well preserved.270
Susac syndrome
Susac syndrome (SuS) is a rare condition of presumed autoimmune etiology that features a triad of encephalopathy, hearing loss and visual defects due to microangiopathic occlusions. The combination of visual deficits with neurological disease in predominantly young female patients can make it a masquerade for MS. Fluorescein angiography typically reveals peripheral branch retinal arterial occlusions.271 In SuS, there is hypoxic retinal destruction of the pRNFL, GCIPL, INL and outer plexiform layer, particularly in the temporal quadrant, compared with corresponding segments in RRMS retinae.272 The ONL and photoreceptor layer are unaffected. Susac patients appear to show distinct sectoral patterns of retinal destruction, rather than the diffuse signs seen in RRMS.273,274
Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disorder of upper and lower motor neurons in the cerebral cortex, brainstem and spinal cord, predictably leading to fatal paralysis through respiratory failure usually within 3–5 years of diagnosis.275,276 90% of cases are sporadic and 10% are familial, of which approximately 60% of associated genes have been identified.276 The exact pathogenesis is unclear, but an interplay of genetic and environmental factors is suspected, inducing oxidative stress, mitochondrial damage, neurofilament defects and defects in RNA processing.277 Clinical presentation is typically limited to motor dysfunction leading to limb weakness and difficulties with speech, swallowing and breathing.
Early diagnosis is critical in ALS due to rapid disease progression. Timely introduction of treatment can extend the length and QoL of patients. However, diagnosis is challenging due to disease mimics and variability in initial presentation. The Awaji electrodiagnostic criteria have enabled earlier diagnosis in comparison to the previous gold standard revised El Escorial diagnostic criteria by evaluating the evidence of progressive lower and upper motor neuron dysfunction in the absence of other potential explanatory disease processes and placing equal importance on clinical and electrophysiological findings.278,279
Sensory abnormalities in ALS include aberrations in thermal perception, presence of numbness, neuropathic pain and tingling.275,280 Although visual dysfunction is not predominant in ALS, extramotor features include reduced visual acuity and contrast sensitivity.281,282 Furthermore, structural and functional alterations in the visual system have been identified through VEPs and MRI in ALS patients.283,284 Specifically, reduced grey matter volume in the extramotor cortex, including the occipital cortex, has been identified.280
OCT in ALS
Given the genetic associations between primary open angle glaucoma and familial ALS,285–291 the eye has been investigated as a course of biomarkers. However, OCT has been limited and have produced contradictory results (Table 2). A cross-sectional study of sporadic ALS patients identified reduced total macula thickness compared with controls.292 Further manual segmentation also revealed thinning of the RNFL and INL but the corresponding GCIPL was unaffected.
Table 2.
A summary of studies reporting sector analysis of peripapillary RNFL changes in previous optic neuritis (MSON), multiple sclerosis without optic neuritis (MSNON), and amyotrophic lateral sclerosis (ALS). Effect size has been calculated as mean percentage reduction in disease versus healthy controls.
| MSON - study | OCT type | Global | Superior | Temporal | Inferior | Nasal | ||
|---|---|---|---|---|---|---|---|---|
| Feng176 | SD | 34.8% | 33.1% | 37.5% | 43.6% | 37.4% | ||
| Garcia-Martin177 | SD | 10.1% | 14.2% | 20.4% | 11.3% | 19.7% | ||
| Khalil181 | SD | 33.4% | 25.3% | 25.4% | 21.6% | 29.6% | ||
| Lange182 | SD | 27.7% | 22.2% | 36.2% | 27.0% | 53.0% | ||
| Park184 | SD | 35.3% | 32.4% | 18.0% | 36.3% | 65.0% | ||
| Schneider205 | SD | 16.0% | 13.2%b | 31.6%b | 12.7%b | 21.3%b | ||
| MSNON - study | OCT type | Global | Superior | Temporal | Inferior | Nasal | ||
| Feng176 | SD | 10.4% | 5.6% | 10.2% | 14.7% | 10.7% | ||
| Lange182 | SD | 7.3% | 4.6% | 11.0% | 8.4% | 5.8% | ||
| Garcia-Martin177 | SD | 7.0% | 11.7% | 15.7% | 10.1% | 9.2% | ||
| ALS |
OCT type | Global | Superior |
Temporal | Inferior |
Nasal | ||
| Study | ST | SN | IT | IN | ||||
| Mukherjee293,a | SD | 7.51 μm | 11.27 μm | 10.68 μm | 8.17 μm | –1.45 μm | 2.13 μm | 6.34 μm |
| Rohani294 | SD | 5.0% | 9.0% | –1.0% | –1.0% | 11.0% | ||
| Roth295 | SD | –2.0% | –3.0% | 1.0% | –4.0% | 0.0% | ||
Mean of right and left eyes
Significance data missing
Grey shading indicates statistical significance as chosen by each study investigator.
IN, inferonasal; IT inferotemporal; SD, spectral domain; ST, superotemporal; SN, superonasal; TD, time domain.
A further study also demonstrated thinning of the RNFL and INL in ALS patients compared with healthy controls.296 Significant correlation was further demonstrated between retinal thickness and fractional anisotropy, a measure of axonal density in the corticospinal tract. More recent studies have confirmed these findings of reduced RNFL thickness.293,296,297 Reduced ONL thickness in ALS is reported in a single study.298 However, earlier research contradicts the retinal changes seen in ALS. A study of 76 ALS patients failed to identify any significant difference in OCT measurements of retinal sublayers compared with healthy controls, nor any association with disease severity.295 This negative finding may be explained by the selection of ALS sufferers, with only 19.7% having ‘clinically definite’ ALS, compared with 83.3% in Ringelstein’s study.295 A study examining symmetry in ALS eyes demonstrated significant interocular differences in retinal thickness.296 This finding suggests that characteristic asymmetrical CNS involvement is not limited to the motor system. This may weaken the promise of retinal thinning as a robust biomarker for disease.
Macula thinning has also been a recognized feature in ALS, with total macular volume reduced in ALS patients compared with controls.297 Another study however, showed total macula volume and other sublayers were unaffected, but did demonstrate selective RNFL thinning in ALS patients compared with healthy controls.299 The contradictory results may be explained by cohort heterogeneity, including differences in diagnostic criteria used, and the subtype (familial versus sporadic) and duration of ALS. Further work should aim to allow comparison and consistency between studies.
The observed finding of RNFL thinning in ALS is likely to reflect extramotor axonal degeneration. INL thinning, however, may be explained by inhibitory pathophysiological mechanisms of neurodegeneration in ALS.300 Bipolar, horizontal and amacrine cell bodies in the INL mainly mediate inhibitory circuits and therefore may explain the thinning and degeneration of this layer.296
Retinal vessel pathology
Changes in skin, muscle and CNS microvasculature have been reported in patients with ALS.301 The superior or inferior temporal branch of the retinal artery was assessed in ALS patients with increased outer wall thickness found compared with healthy controls,298 with no differences in inner wall thickness or lumen diameter.
Intraretinal inclusions
Inclusions are the pathological hallmark of ALS, present in the spinal cord as well as certain regions of the brain including the frontal and temporal cortices, hippocampus and cerebellum.302 In ALS, these are predominantly ubiquinated randomly orientated filaments, covered with fine granules.302 Histopathological studies have identified the presence of intraretinal inclusions in one patient and within the inner plexiform layers of an ALS/dementia transgenic UBQLN2(P497H) mouse.297 Further histopathological studies in two, related ALS patients with the C9orf72 mutation identified p62-positive, pTDP43-negative perinuclear inclusions in the inner nuclear layer of the retina and CNS.303 These inclusions are of the type commonly found in the hippocampus and cerebellum in this form of ALS. Further colocalization suggested that the majority of retinal p62-positive inclusions were found within cone bipolar cells as well as some amacrine and horizontal cells. One of the patients had been noted to have contrast sensitivity impairment, which may be explained by cone bipolar cell involvement.
Association with disease severity and duration
There have been contrasting results supporting the presence and absence of correlation between retinal thickness (pRNFL) and disease severity as assessed with the ALS functional rating scale (ALSFRS).294–296,298 Pulmonary function tests are used to monitor neuromuscular involvement in ALS, and correlate well with survival time.304,305 Simonett’s study showed macula RNFL thickness correlated with forced vital capacity (% predicted) and forced expiratory volume in 1 s (% predicted).299 Retinal thinning did not correlate with disease duration or function; however, many other factors are likely to affect this relationship. Disease duration has also been shown to correlate inversely with total macular thickness297; patients with disease duration longer than 40 months had significantly decreased total macular and pRNFL thicknesses compared with those with shorter disease durations. Further work may help address the lack of a widely established method to grade disease progression in ALS. In the future, OCT could be incorporated alongside other clinical, imaging and electrodiagnostic tools to form a prognostic scoring system.
The underlying pathophysiology of possible retinal changes in ALS remains elusive, as does the timeline of their onset. A primary retinal degenerative process involving neuronal and subsequent axonal degeneration causing retinal sublayer thinning is plausible. Given the relatively small OCT differences identified, larger scale studies are required to further confirm RNFL and macula thinning, as well as examining ALS subgroups such as familial, sporadic, juvenile or adult-onset subtypes to identify the specific pattern of visual dysfunction and retinal correlates in these patients.
Creutzfeld-Jakob disease and prion diseases
Prion diseases are rare, fatal neurodegenerative diseases marked by long incubation periods and fast progression.306 Caused by PrPSc (proteinase-resistant prion proteins), a misfolded, infectious and abnormal form of a cell-surface protein, prion diseases are marked by accumulation in the CNS. The term ‘prion disease’ will be used to describe diseases caused by PrPSc.307 The disease is characterised by neuronal loss with rapid increase in glial cells, lack of inflammatory response and formation of small vacuoles in the neuropil creating a ‘spongiform’ appearance throughout the CNS. Patients can present with rapid (weeks to months) cognitive decline including personality changes, visual impairment, memory loss and neurological deficits correlating to affected areas in the CNS.308 Five types of human prion disease have been identified: Gerstmann-Sträussler-Scheinker syndrome (GSS), fatal familial insomnia (FFI), kuru, and Creutzfeldt-Jakob disease (CJD).
CJD is the most common of the human prion diseases, accounting for over 90% of cases worldwide,306 including sporadic (sCJD), genetic/familial (gCJD), iatrogenic (iCJD) and variant CJD (vCJD) subtypes. Although the exact cause is unclear, it is thought that normal brain protein spontaneously misfolds, developing into prions,309 with only 5–15% of cases due to gCJD caused by an inherited mutation (prion protein gene).310 Less than 1% are acquired from iCJD or vCJD.311,312 iCJD is spread through infected medical or surgical equipment, and was historically associated with growth hormone treatment; however, the advent of synthetic human growth hormones and heightened awareness have made iCJD very rare, reported as 1.44 cases per 1 million people worldwide.313 vCJD results from infection through infected meat from cattle causing bovine spongiform encephalopathy (BSE).314
Clinical diagnosis tends to rely on evaluation of rapidly progressing dementia, myoclonus and ataxia. Changes on electroencephalogram, MRI, a positive 14-3-3 protein in the CSF, and tonsil and brain biopsies can make a ‘probable’ diagnosis, yet the latter procedures come with severe risks including brain damage, seizures and bleeding (CDC criteria). Several diagnostic criteria have been developed with the hope of allowing for earlier diagnosis,311,315,316 but exclude atypical features such as unaffected cognitive profiles.317 Visual disturbances are seen in up to 30–50% of sCJD cases at some point during the illness.318,319 Cortical blindness, an early feature first described by Heidenhain in 1928, can occur in up to 25–50% of vCJD, reflecting the extent of pathology in the visual cortex.319 Blurred or dimmed vision is the most commonly reported visual symptom in sCJD (9%).320–328 Several case reports have described presentations of insidious and solitary visual impairments removed from any other cognitive or neurophysiological impairment.329,330
Histopathology
Histological studies in CJD have described spongiform abnormalities in the RNFL coupled with loss of bipolar and ganglion cells, and photoreceptor changes.331–334 Occasionally, optic nerve abnormalities have been reported,331,332,335 as have normal optic nerves.336 Prions themselves have been discovered in the retina,307,337 specifically the central retina sequestered to the plexiform layers in sCJD at low levels.337 More recently researchers observed prion seeding in the retina of sCJD eyes. Using immunohistochemistry, Orrù and colleagues detected prions in the majority of OPL and some IPL eyes of 11 patients, which coincided with accumulation of synaptic prions. Real-time quaking-induced conversion (RT-QuIC) assay detected prion seeds in the optic nerve, choroid, lens, vitreous, extraocular muscle, sclera, all corneas and a general trend towards increased prion seeds the closer tissue was to the brain.307
Ophthalmic exam and ERG
Optic disc pallor335,338–341 and atrophy331,332,335 are the most common findings, although are not always visible until after death.332 RGC degeneration has also been described and may contribute to optic atrophy.333 Electroretinogram (ERG) examination has found b-waves to be significantly decreased in late stages, correlated significantly to abnormalities in the outer plexiform layer of the retina post mortem.334,342 However, Ishikawa and colleagues reported one patient with visual disturbances and normal ERG even after CJD progressed, suggesting visual problems may occur without retinal involvement.343 In an animal study of BSE, cows inoculated with classical BSE showed no notable change in b-wave amplitude. Those inoculated with atypical high-type BSE (BSE-H) showed a decrease in b-wave amplitude but this was not statistically significant. However, significant changes in b-wave implicit times were noted during the incubation period, and significantly prolonged in clinically ill animals compared with baseline.344
OCT in prion disease
OCT studies of prion diseases are limited to case reports due to their low incidence and high mortality. In a 63-year-old female with Heidenhain vCJD, Chin and colleagues observed paracentral retinal thinning in both eyes 2.5 months after cognitive decline and neurophysiological symptoms began. This finding is consistent with thinning in the RNFL, ganglion cell and bipolar cell loss and demyelination of the optic nerve previously reported in histologic findings.330–332,345,346 A study by Greenlee and colleagues of BSE and BSE-H inoculated cattle described antemortem changes in the dorsocentral retina visible up to 11 months before appearance of any other signs or symptoms of disease. Retinal thickness of inoculated cows were markedly decreased at 12 and 15 months post inoculation compared with baseline and prior to signs of disease,344 a replication of findings from a prior study by the same group.347
Given the infectious nature of the disease, lack of treatment and rapid declines in cognition, early diagnosis could prevent unnecessary interventions, and prevent transmission especially in atypical clinical presentations.330 The retina holds potential for studying the pathology of prion disease. Furthermore, because the retina is isolated, the accumulation of PRPSc may be easier to quantify more accurately compared with the rest of the CNS where dissection affects quantification of accumulation regionally.344 It remains to be seen as to whether the use of retinal correlates can add value to the care of these patients, especially hampered by the difficulty in acquiring historic data prior to the clinical onset of such a rare group of diseases.
Conclusion
Retinal correlates have been observed in a range of some of the most common neurological disorders, some of which are not commonly associated with visual symptoms. Further to those conditions discussed, retinal correlates have been found in other neurological diseases such as idiopathic intracranial hypertension (IIH),348 chronic migraines,349–351 and a range of psychiatric conditions.352,353 The majority of evidence exists in the form of cross-sectional studies comparing disease groups and healthy controls; however, further work must be done to take advantage of the unique opportunity the eye provides to serially image subjects through time. This will enable a better understanding of the natural history of retinal changes in neurological disorders and improve our knowledge of how to apply these to clinical scenarios.
Although this review is not intended to be a meta-analysis, from observing the results of meta-analyses and studies relevant to this work (Tables 1–3), the literature does suggest particular characteristics of RNFL loss in a disease-specific manner. AD appears to preferentially cause peripapillary loss of the superior and inferior fibres, in contrast to PD, which appears to more commonly affect temporal RNFL than in AD. Inconsistent findings in the studies examined are likely due to the variation in concomitant medical and ophthalmic conditions within and between study cohorts. Additionally, confounds are likely to include age, duration of disease and treatment effect. Whilst the results from published studies demonstrate predilections for certain peripapillary zones and layers of the macula, these differences are unlikely to provide the specificity required in order to significantly contribute to specific diagnoses. However, with the multiple correlations seen between disease severity, treatment response and functional prognosis, there is sufficient evidence to suggest these correlates are useful in the setting of disease monitoring, and potentially as auxiliary surrogate markers in clinical trials.
Table 3.
Summary of the magnitude of peripapillary RNFL thinning reported in a range of neurodegenerative diseases, as calculated by mean effect size.
| Summary | Global | Superior | Temporal | Inferior | Nasal |
|---|---|---|---|---|---|
| Study | |||||
| Alzheimer’s disease | ++ | ++ | + | ++ | ++ |
| Mild cognitive impairment | + | + | + | + | + |
| Parkinson’s disease | + | + | + | + | + |
| Optic Neuritis | +++ | +++ | +++ | +++ | ++++ |
| Multiple sclerosis | + | + | ++ | + | + |
| Amyotrophic lateral sclerosis | + | + | – | – | + |
1–10%, ++ 11–20%, +++ 21–30%, ++++ 31–40%.
ALS, amyotrophic lateral sclerosis.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and publication of this article.
Conflict of interest statement: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: M.F.C. is a named co-inventor on granted patent EP 2231199B1 and published patent WO 2011055121 A1 owned by UCL and related to DARC technology. The other authors declare no conflicts of interests.
ORCID iD: M. Francesca Cordeiro  https://orcid.org/0000-0001-8663-6525
https://orcid.org/0000-0001-8663-6525
Contributor Information
Timothy E. Yap, The Western Eye Hospital, Imperial College Healthcare NHS Trust (ICHNT), London, UK The Imperial College Ophthalmic Research Group (ICORG), Imperial College London, UK.
Shiama I. Balendra, Glaucoma and Retinal Neurodegeneration Group, Department of Visual Neuroscience, UCL Institute of Ophthalmology, London, UK
Melanie T. Almonte, The Imperial College Ophthalmic Research Group (ICORG), Imperial College London, UK
M. Francesca Cordeiro, The Western Eye Hospital, Imperial College Healthcare NHS Trust (ICHNT), London, NW1 5QH, UK; The Imperial College Ophthalmic Research Group (ICORG), Imperial College, London, NW1 5QH, UK; Glaucoma and Retinal Neurodegeneration Group, Department of Visual Neuroscience, UCL Institute of Ophthalmology, 11–43 Bath Street, London, EC1V 9EL UK.
References
- 1. Pircher M, Zawadzki RJ. Review of adaptive optics OCT (AO-OCT): principles and applications for retinal imaging [Invited]. Biomed Opt Express 2017; 8: 2536–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. MacCormick IJ, Czanner G, Faragher B. Developing retinal biomarkers of neurological disease: an analytical perspective. Biomark Med 2015; 9: 691–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Prentice RL. Surrogate endpoints in clinical trials: definition and operational criteria. Stat Med 1989; 8: 431–440. [DOI] [PubMed] [Google Scholar]
- 4. Glass CK, Saijo K, Winner B, et al. Mechanisms underlying inflammation in neurodegeneration. Cell 2010; 140: 918–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Williams A. Central nervous system regeneration – where are we? QJM 2014; 107: 335–339. [DOI] [PubMed] [Google Scholar]
- 6. Kumar A, Singh A Ekavali. A review on Alzheimer’s disease pathophysiology and its management: an update. Pharmacol Rep 2015; 67: 195–203. [DOI] [PubMed] [Google Scholar]
- 7. Salthouse TA, Nesselroade JR, Berish DE. Short-term variability in cognitive performance and the calibration of longitudinal change. J Gerontol B Psychol Sci Soc Sci 2006; 61: P144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bradshaw J, Saling M, Hopwood M, et al. Fluctuating cognition in dementia with Lewy bodies and Alzheimer’s disease is qualitatively distinct. J Neurol Neurosurg Psychiatry 2004; 75: 382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu H, de Boer JF, Chen TC. Reproducibility of retinal nerve fiber layer thickness measurements using spectral domain optical coherence tomography. J Glaucoma 2011; 20: 470–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carpineto P, Nubile M, Agnifili L, et al. Reproducibility and repeatability of Cirrus HD-OCT peripapillary retinal nerve fibre layer thickness measurements in young normal subjects. Ophthalmologica 2012; 227: 139–145. [DOI] [PubMed] [Google Scholar]
- 11. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011; 7: 280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Morris JC. Early-stage and preclinical Alzheimer disease. Alzheimer Dis Assoc Disord 2005; 19: 163–165. [DOI] [PubMed] [Google Scholar]
- 13. Gauthier SG. Alzheimer’s disease: the benefits of early treatment. Eur J Neurol 2005; 12(Suppl. 3): 11–16. [DOI] [PubMed] [Google Scholar]
- 14. Leifer BP. Early diagnosis of Alzheimer’s disease: clinical and economic benefits. J Am Geriatr Soc 2003; 51: S281–S288. [DOI] [PubMed] [Google Scholar]
- 15. Ising C, Stanley M, Holtzman DM. Current thinking on the mechanistic basis of Alzheimer’s and implications for drug development. Clin Pharmacol Ther 2015; 98: 469–471. [DOI] [PubMed] [Google Scholar]
- 16. Forstl H, Kurz A. Clinical features of Alzheimer’s disease. Eur Arch Psychiatry Clin Neurosci 1999; 249: 288–290. [DOI] [PubMed] [Google Scholar]
- 17. Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia—meta-analysis of 41 robust inception cohort studies. Acta Psychiatr Scand 2009; 119: 252–265. [DOI] [PubMed] [Google Scholar]
- 18. Dubois B, Feldman HH, Jacova C, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol 2014; 13: 614–629. [DOI] [PubMed] [Google Scholar]
- 19. Marcus C, Mena E, Subramaniam RM. Brain PET in the diagnosis of Alzheimer’s disease. Clin Nucl Med 2014; 39: e413–e422; quiz e423–e426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saint-Aubert L, Lemoine L, Chiotis K, et al. Tau PET imaging: present and future directions. Mol Neurodegener 2017; 12: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hansson O, Zetterberg H, Buchhave P, et al. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol 2006; 5: 228–234. [DOI] [PubMed] [Google Scholar]
- 22. Mattsson N, Zetterberg H, Hansson O, et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA 2009; 302: 385–393. [DOI] [PubMed] [Google Scholar]
- 23. Vos SJ, Verhey F, Frolich L, et al. Prevalence and prognosis of Alzheimer’s disease at the mild cognitive impairment stage. Brain 2015; 138: 1327–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Poulakis K, Pereira JB, Mecocci P, et al. Heterogeneous patterns of brain atrophy in Alzheimer’s disease. Neurobiol Aging 2018; 65: 98–108. [DOI] [PubMed] [Google Scholar]
- 25. Vlassenko AG, Benzinger TL, Morris JC. PET amyloid-beta imaging in preclinical Alzheimer’s disease. Biochim Biophys Acta 2012; 1822: 370–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kametani F, Hasegawa M. Reconsideration of amyloid hypothesis and tau hypothesis in Alzheimer’s disease. Front Neurosci 2018; 12: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cogan DG. Visual disturbances with focal progressive dementing disease. Am J Ophthalmol 1985; 100: 68–72. [DOI] [PubMed] [Google Scholar]
- 28. Pache M, Smeets CH, Gasio PF, et al. Colour vision deficiencies in Alzheimer’s disease. Age Ageing 2003; 32: 422–426. [DOI] [PubMed] [Google Scholar]
- 29. Cronin-Golomb A, Corkin S, Rizzo JF, et al. Visual dysfunction in Alzheimer’s disease: relation to normal aging. Ann Neurol 1991; 29: 41–52. [DOI] [PubMed] [Google Scholar]
- 30. Trick GL, Trick LR, Morris P, et al. Visual field loss in senile dementia of the Alzheimer’s type. Neurology 1995; 45: 68–74. [DOI] [PubMed] [Google Scholar]
- 31. Kusne Y, Wolf AB, Townley K, et al. Visual system manifestations of Alzheimer’s disease. Acta Ophthalmol 2017; 95: e668–e676. [DOI] [PubMed] [Google Scholar]
- 32. Hinton DR, Sadun AA, Blanks JC, et al. Optic-nerve degeneration in Alzheimer’s disease. N Engl J Med 1986; 315: 485–487. [DOI] [PubMed] [Google Scholar]
- 33. Blanks JC, Torigoe Y, Hinton DR, et al. Retinal pathology in Alzheimer’s disease. I. Ganglion cell loss in foveal/parafoveal retina. Neurobiol Aging 1996; 17: 377–384. [DOI] [PubMed] [Google Scholar]
- 34. Blanks JC, Schmidt SY, Torigoe Y, et al. Retinal pathology in Alzheimer’s disease. II. Regional neuron loss and glial changes in GCL. Neurobiol Aging 1996; 17: 385–395. [DOI] [PubMed] [Google Scholar]
- 35. Hart NJ, Koronyo Y, Black KL, et al. Ocular indicators of Alzheimer’s: exploring disease in the retina. Acta Neuropathol 2016; 132: 767–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Feng R, Li L, Yu H, et al. Melanopsin retinal ganglion cell loss and circadian dysfunction in Alzheimer’s disease (Review). Mol Med Rep 2016; 13: 3397–3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tsai Y, Lu B, Ljubimov AV, et al. Ocular changes in TgF344-AD rat model of Alzheimer’s disease. Invest Ophthalmol Vis Sci 2014; 55: 523–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Koronyo-Hamaoui M, Koronyo Y, Ljubimov AV, et al. Identification of amyloid plaques in retinas from Alzheimer’s patients and noninvasive in vivo optical imaging of retinal plaques in a mouse model. Neuroimage 2011; 54(Suppl. 1): S204–S217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Williams EA, McGuone D, Frosch MP, et al. Absence of Alzheimer disease neuropathologic changes in eyes of subjects with Alzheimer disease. J Neuropathol Exp Neurol 2017; 76: 376–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ho CY, Troncoso JC, Knox D, et al. Beta-amyloid, phospho-tau and alpha-synuclein deposits similar to those in the brain are not identified in the eyes of Alzheimer’s and Parkinson’s disease patients. Brain Pathol 2014; 24: 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schon C, Hoffmann NA, Ochs SM, et al. Long-term in vivo imaging of fibrillar tau in the retina of P301S transgenic mice. PLoS One 2012; 7: e53547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brondino N, Re S, Boldrini A, et al. Curcumin as a therapeutic agent in dementia: a mini systematic review of human studies. ScientificWorldJournal 2014; 2014: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Davis BM, Pahlitzsch M, Guo L, et al. Topical curcumin nanocarriers are neuroprotective in eye disease. Sci Rep 2018; 8: 11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Koronyo Y, Biggs D, Barron E, et al. Retinal amyloid pathology and proof-of-concept imaging trial in Alzheimer’s disease. JCI Insight 2017; 2 pii: 93621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. More SS, Vince R. Hyperspectral imaging signatures detect amyloidopathy in Alzheimer’s mouse retina well before onset of cognitive decline. ACS Chem Neurosci 2015; 6: 306–315. [DOI] [PubMed] [Google Scholar]
- 46. Doustar J, Torbati T, Black KL, et al. Optical Coherence Tomography in Alzheimer’s Disease and Other Neurodegenerative Diseases. Front Neurol 2017; 8: 701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bussel II, Wollstein G, Schuman JS. OCT for glaucoma diagnosis, screening and detection of glaucoma progression. Br J Ophthalmol 2014; 98(Suppl. 2): ii15–ii19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sharma S, Sayanagi K, Kaiser PK. Pathology detection rate of spectral domain optical coherence tomography devices. Br J Ophthalmol 2014; 98(Suppl. 1): i3–i6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Santangelo R, Ferrari L, Huang SC, et al. The potential role of optical coherence tomography as a marker of disease severity in Alzheimer’s dementia (P4.183). Neurology 2018; 90(Suppl. 15). [Google Scholar]
- 50. Polo V, Rodrigo MJ, Garcia-Martin E, et al. Visual dysfunction and its correlation with retinal changes in patients with Alzheimer’s disease. Eye (Lond) 2017; 31: 1034–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ferrari L, Huang SC, Magnani G, et al. Optical coherence tomography reveals retinal neuroaxonal thinning in frontotemporal dementia as in Alzheimer’s disease. J Alzheimers Dis 2017; 56: 1101–1107. [DOI] [PubMed] [Google Scholar]
- 52. Cunha JP, Proenca R, Dias-Santos A, et al. OCT in Alzheimer’s disease: thinning of the RNFL and superior hemiretina. Graefes Arch Clin Exp Ophthalmol 2017; 255: 1827–1835. [DOI] [PubMed] [Google Scholar]
- 53. Cunha LP, Lopes LC, Costa-Cunha LV, et al. Macular thickness measurements with frequency domain-oct for quantification of retinal neural loss and its correlation with cognitive impairment in Alzheimer’s disease. PLoS One 2016; 11: e0153830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Trebbastoni A, D’Antonio F, Bruscolini A, et al. Retinal nerve fibre layer thickness changes in Alzheimer’s disease: Results from a 12-month prospective case series. Neurosci Lett 2016; 629: 165–170. [DOI] [PubMed] [Google Scholar]
- 55. Choi SH, Park SJ, Kim NR. Macular ganglion cell -inner plexiform layer thickness is associated with clinical progression in mild cognitive impairment and Alzheimers disease. PLoS One 2016; 11: e0162202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Garcia-Martin E, Bambo MP, Marques ML, et al. Ganglion cell layer measurements correlate with disease severity in patients with Alzheimer’s disease. Acta Ophthalmol 2016; 94: e454–e459. [DOI] [PubMed] [Google Scholar]
- 57. La Morgia C, Ross-Cisneros FN, Koronyo Y, et al. Melanopsin retinal ganglion cell loss in Alzheimer disease. Ann Neurol 2016; 79: 90–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gao L, Liu Y, Li X, et al. Abnormal retinal nerve fiber layer thickness and macula lutea in patients with mild cognitive impairment and Alzheimer’s disease. Arch Gerontol Geriatr 2015; 60: 162–167. [DOI] [PubMed] [Google Scholar]
- 59. Liu D, Zhang L, Li Z, et al. Thinner changes of the retinal nerve fiber layer in patients with mild cognitive impairment and Alzheimer’s disease. BMC Neurol 2015; 15: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Oktem EO, Derle E, Kibaroglu S, et al. The relationship between the degree of cognitive impairment and retinal nerve fiber layer thickness. Neurol Sci 2015; 36: 1141–1146. [DOI] [PubMed] [Google Scholar]
- 61. Eraslan M, Cerman E, Cekic O, et al. Neurodegeneration in ocular and central nervous systems: optical coherence tomography study in normal-tension glaucoma and Alzheimer disease. Turk J Med Sci 2015; 45: 1106–1114. [DOI] [PubMed] [Google Scholar]
- 62. Bambo MP, Garcia-Martin E, Gutierrez-Ruiz F, et al. Analysis of optic disk color changes in Alzheimer’s disease: a potential new biomarker. Clin Neurol Neurosurg 2015; 132: 68–73. [DOI] [PubMed] [Google Scholar]
- 63. Cheung CY, Ong YT, Hilal S, et al. Retinal ganglion cell analysis using high-definition optical coherence tomography in patients with mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis 2015; 45: 45–56. [DOI] [PubMed] [Google Scholar]
- 64. Gunes A, Demirci S, Tok L, et al. Evaluation of retinal nerve fiber layer thickness in Alzheimer disease using spectral-domain optical coherence tomography. Turk J Med Sci 2015; 45: 1094–1097. [PubMed] [Google Scholar]
- 65. Ascaso FJ, Cruz N, Modrego PJ, et al. Retinal alterations in mild cognitive impairment and Alzheimer’s disease: an optical coherence tomography study. J Neurol 2014; 261: 1522–1530. [DOI] [PubMed] [Google Scholar]
- 66. Larrosa JM, Garcia-Martin E, Bambo MP, et al. Potential new diagnostic tool for Alzheimer’s disease using a linear discriminant function for Fourier domain optical coherence tomography. Invest Ophthalmol Vis Sci 2014; 55: 3043–3051. [DOI] [PubMed] [Google Scholar]
- 67. Kirbas S, Turkyilmaz K, Anlar O, et al. Retinal nerve fiber layer thickness in patients with Alzheimer disease. J Neuroophthalmol 2013; 33: 58–61. [DOI] [PubMed] [Google Scholar]
- 68. Kesler A, Vakhapova V, Korczyn AD, et al. Retinal thickness in patients with mild cognitive impairment and Alzheimer’s disease. Clin Neurol Neurosurg 2011; 113: 523–526. [DOI] [PubMed] [Google Scholar]
- 69. Lu Y, Li Z, Zhang X, et al. Retinal nerve fiber layer structure abnormalities in early Alzheimer’s disease: evidence in optical coherence tomography. Neurosci Lett 2010; 480: 69–72. [DOI] [PubMed] [Google Scholar]
- 70. Paquet C, Boissonnot M, Roger F, et al. Abnormal retinal thickness in patients with mild cognitive impairment and Alzheimer’s disease. Neurosci Lett 2007; 420: 97–99. [DOI] [PubMed] [Google Scholar]
- 71. Iseri PK, Altinas O, Tokay T, et al. Relationship between cognitive impairment and retinal morphological and visual functional abnormalities in Alzheimer disease. J Neuroophthalmol 2006; 26: 18–24. [DOI] [PubMed] [Google Scholar]
- 72. Parisi V, Restuccia R, Fattapposta F, et al. Morphological and functional retinal impairment in Alzheimer’s disease patients. Clin Neurophysiol 2001; 112: 1860–1867. [DOI] [PubMed] [Google Scholar]
- 73. Kwon JY, Yang JH, Han JS, et al. Analysis of the retinal nerve fiber layer thickness in Alzheimer disease and mild cognitive impairment. Korean J Ophthalmol 2017; 31: 548–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kromer R, Serbecic N, Hausner L, et al. Detection of retinal nerve fiber layer defects in Alzheimer’s disease using SD-OCT. Front Psychiatry 2014; 5: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Moschos MM, Markopoulos I, Chatziralli I, et al. Structural and functional impairment of the retina and optic nerve in Alzheimer’s disease. Curr Alzheimer Res 2012; 9: 782–788. [DOI] [PubMed] [Google Scholar]
- 76. Golzan SM, Goozee K, Georgevsky D, et al. Retinal vascular and structural changes are associated with amyloid burden in the elderly: ophthalmic biomarkers of preclinical Alzheimer’s disease. Alzheimers Res Ther 2017; 9: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Pillai JA, Bermel R, Bonner-Jackson A, et al. Retinal nerve fiber layer thinning in alzheimer’s disease: a case-control study in comparison to normal aging, Parkinson’s disease, and non-Alzheimer’s dementia. Am J Alzheimers Dis Other Demen 2016; 31: 430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Salobrar-Garcia E, Hoyas I, Leal M, et al. Analysis of retinal peripapillary segmentation in early Alzheimer’s disease patients. Biomed Res Int 2015; 2015: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gharbiya M, Trebbastoni A, Parisi F, et al. Choroidal thinning as a new finding in Alzheimer’s disease: evidence from enhanced depth imaging spectral domain optical coherence tomography. J Alzheimers Dis 2014; 40: 907–917. [DOI] [PubMed] [Google Scholar]
- 80. Chan VTT, Sun Z, Tang S, et al. Spectral-domain OCT measurements in Alzheimer’s disease: a systematic review and meta-analysis. Ophthalmology 2019; 126: 497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Thomson KL, Yeo JM, Waddell B, et al. A systematic review and meta-analysis of retinal nerve fiber layer change in dementia, using optical coherence tomography. Alzheimers Dement (Amst) 2015; 1: 136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yu M, Lin C, Weinreb RN, et al. Risk of visual field progression in glaucoma patients with progressive retinal nerve fiber layer thinning: a 5-year prospective study. Ophthalmology 2016; 123: 1201–1210. [DOI] [PubMed] [Google Scholar]
- 83. Shen Y, Shi Z, Jia R, et al. The attenuation of retinal nerve fiber layer thickness and cognitive deterioration. Front Cell Neurosci 2013; 7: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Parisi V. Correlation between morphological and functional retinal impairment in patients affected by ocular hypertension, glaucoma, demyelinating optic neuritis and Alzheimer’s disease. Semin Ophthalmol 2003; 18: 50–57. [DOI] [PubMed] [Google Scholar]
- 85. Leung CK, Yu M, Weinreb RN, et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: a prospective analysis of age-related loss. Ophthalmology 2012; 119: 731–737. [DOI] [PubMed] [Google Scholar]
- 86. Moreno-Ramos T, Benito-Leon J, Villarejo A, et al. Retinal nerve fiber layer thinning in dementia associated with Parkinson’s disease, dementia with Lewy bodies, and Alzheimer’s disease. J Alzheimers Dis 2013; 34: 659–664. [DOI] [PubMed] [Google Scholar]
- 87. Aaker GD, Myung JS, Ehrlich JR, et al. Detection of retinal changes in Parkinson’s disease with spectral-domain optical coherence tomography. Clin Ophthalmol 2010; 4: 1427–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Albrecht P, Muller AK, Sudmeyer M, et al. Optical coherence tomography in parkinsonian syndromes. PLoS One 2012; 7: e34891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Altintaş O, Işeri P, Ozkan B, et al. Correlation between retinal morphological and functional findings and clinical severity in Parkinson’s disease. Doc Ophthalmol 2008; 116: 137–146. [DOI] [PubMed] [Google Scholar]
- 90. Archibald NK, Clarke MP, Mosimann UP, et al. Retinal thickness in Parkinson’s disease. Parkinsonism Relat Disord 2011; 17: 431–436. [DOI] [PubMed] [Google Scholar]
- 91. Aydin TS, Umit D, Nur OM, et al. Optical coherence tomography findings in Parkinson’s disease. Kaohsiung J Med Sci 2018; 34: 166–171. [DOI] [PubMed] [Google Scholar]
- 92. Bittersohl D, Stemplewitz B, Keseru M, et al. Detection of retinal changes in idiopathic Parkinson’s disease using high-resolution optical coherence tomography and heidelberg retina tomography. Acta Ophthalmol 2015; 93: e578–e584. [DOI] [PubMed] [Google Scholar]
- 93. Garcia-Martin E, Larrosa JM, Polo V, et al. Distribution of retinal layer atrophy in patients with Parkinson disease and association with disease severity and duration. Am J Ophthalmol 2014; 157: 470–478 e472. [DOI] [PubMed] [Google Scholar]
- 94. Inzelberg R, Ramirez JA, Nisipeanu P, et al. Retinal nerve fiber layer thinning in Parkinson disease. Vision Res 2004; 44: 2793–2797. [DOI] [PubMed] [Google Scholar]
- 95. Jiménez B, Ascaso FJ, Cristobal JA, et al. Development of a prediction formula of Parkinson disease severity by optical coherence tomography. Mov Disord 2014; 29: 68–74. [DOI] [PubMed] [Google Scholar]
- 96. Kirbas S, Turkyilmaz K, Tufekci A, et al. Retinal nerve fiber layer thickness in Parkinson disease. J Neuroophthalmol 2013; 33: 62–65. [DOI] [PubMed] [Google Scholar]
- 97. La Morgia C, Barboni P, Rizzo G, et al. Loss of temporal retinal nerve fibers in Parkinson disease: a mitochondrial pattern? Eur J Neurol 2013; 20: 198–201. [DOI] [PubMed] [Google Scholar]
- 98. Matlach J, Wagner M, Malzahn U, et al. Retinal changes in Parkinson’s disease and glaucoma. Parkinsonism Relat Disord 2018; 56: 41–46. [DOI] [PubMed] [Google Scholar]
- 99. Moschos MM, Tagaris G, Markopoulos I, et al. Morphologic changes and functional retinal impairment in patients with Parkinson disease without visual loss. Eur J Ophthalmol 2011; 21: 24–29. [DOI] [PubMed] [Google Scholar]
- 100. Moschos MM, Chatziralli IP. Evaluation of choroidal and retinal thickness changes in Parkinson’s disease using spectral domain optical coherence tomography. Semin Ophthalmol 2018; 33: 494–497. [DOI] [PubMed] [Google Scholar]
- 101. Rohani M, Langroodi AS, Ghourchian S, et al. Retinal nerve changes in patients with tremor dominant and akinetic rigid Parkinson’s disease. Neurol Sci 2013; 34: 689–693. [DOI] [PubMed] [Google Scholar]
- 102. Satue M, Garcia-Martin E, Fuertes I, et al. Use of Fourier-domain OCT to detect retinal nerve fiber layer degeneration in Parkinson’s disease patients. Eye (Lond) 2013; 27: 507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sen A, Tugcu B, Coskun C, et al. Effects of levodopa on retina in Parkinson disease. Eur J Ophthalmol 2014; 24: 114–119. [DOI] [PubMed] [Google Scholar]
- 104. Sengupta P, Dutta K, Ghosh S, et al. Optical coherence tomography findings in patients of Parkinson’s disease: an Indian perspective. Ann Indian Acad Neurol 2018; 21: 150–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Tsironi EE, Dastiridou A, Katsanos A, et al. Perimetric and retinal nerve fiber layer findings in patients with Parkinson’s disease. BMC Ophthalmol 2012; 12: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Visser F, Vermeer KA, Ghafaryasl B, et al. In vivo exploration of retinal nerve fiber layer morphology in Parkinson’s disease patients. J Neural Transm (Vienna) 2018; 125: 931–936. [DOI] [PubMed] [Google Scholar]
- 107. Marziani E, Pomati S, Ramolfo P, et al. Evaluation of retinal nerve fiber layer and ganglion cell layer thickness in Alzheimer’s disease using spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci 2013; 54: 5953–5958. [DOI] [PubMed] [Google Scholar]
- 108. Cunha JP, Proenca R, Dias-Santos A, et al. Choroidal thinning: Alzheimer’s disease and aging. Alzheimers Dement (Amst) 2017; 8: 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Bulut M, Yaman A, Erol MK, et al. Choroidal thickness in patients with mild cognitive impairment and Alzheimer’s type dementia. J Ophthalmol 2016; 2016: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Bayhan HA, Aslan Bayhan S, Celikbilek A, et al. Evaluation of the chorioretinal thickness changes in Alzheimer’s disease using spectral-domain optical coherence tomography. Clin Exp Ophthalmol 2015; 43: 145–151. [DOI] [PubMed] [Google Scholar]
- 111. Grundman M, Petersen RC, Ferris SH, et al. Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Arch Neurol 2004; 61: 59–66. [DOI] [PubMed] [Google Scholar]
- 112. Roe CM, Fagan AM, Grant EA, et al. Amyloid imaging and CSF biomarkers in predicting cognitive impairment up to 7.5 years later. Neurology 2013; 80: 1784–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Gramer E, Dirmeyer M. Optical coherence tomography (OCT) to measure nerve fiber layer thickness in normal eyes. Invest Ophthalmol Vis Sci 1998; 39: S933. [Google Scholar]
- 114. Asanad S, Nassisi M, Ross-Cisneros FN, et al. Retinal nerve fiber layer thinning in preclinical alzheimer’s disease using in vivo optical coherence tomography: an investigation of early detection ocular biomarkers. Alzheimer’s & Dementia 2018; 14: P214–P215. [Google Scholar]
- 115. Ko F, Muthy ZA, Gallacher J, et al. Association of retinal nerve fiber layer thinning with current and future cognitive decline: a study using optical coherence tomography. JAMA Neurol 2018; 75: 1198–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Einarsdottir AB, Hardarson SH, Kristjansdottir JV, et al. Retinal oximetry imaging in Alzheimer’s disease. J Alzheimers Dis 2016; 49: 79–83. [DOI] [PubMed] [Google Scholar]
- 117. Ang M, Tan ACS, Cheung CMG, et al. Optical coherence tomography angiography: a review of current and future clinical applications. Graefes Arch Clin Exp Ophthalmol 2018; 256: 237–245. [DOI] [PubMed] [Google Scholar]
- 118. Van Stavern G, Apte R, Kung N, et al. Optical coherence tomography angiography in pre-clinical Alzheimer’s disease (S16.001). Neurology 2018; 90(Suppl. 15). [Google Scholar]
- 119. Bulut M, Kurtulus F, Gozkaya O, et al. Evaluation of optical coherence tomography angiographic findings in Alzheimer’s type dementia. Br J Ophthalmol 2018; 102: 233–237. [DOI] [PubMed] [Google Scholar]
- 120. Yap TE, Davis BM, Guo L, et al. Annexins in Glaucoma. Int J Mol Sci 2018; 19 pii: E1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Cordeiro MF, Normando EM, Cardoso MJ, et al. Real-time imaging of single neuronal cell apoptosis in patients with glaucoma. Brain 2017; 140: 1757–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Head E, Lott IT. Down syndrome and beta-amyloid deposition. Curr Opin Neurol 2004; 17: 95–100. [DOI] [PubMed] [Google Scholar]
- 123. Van Cauwenberghe C, Van Broeckhoven C, Sleegers K. The genetic landscape of Alzheimer disease: clinical implications and perspectives. Genet Med 2016; 18: 421–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Duyckaerts C, Sazdovitch V, Seilhean D, et al. A brain bank in a neuropathology laboratory (with some emphasis on diagnostic criteria). J Neural Transm Suppl 1993; 39: 107–118. [PubMed] [Google Scholar]
- 125. Chaudhuri KR, Schapira AH. Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol 2009; 8: 464–474. [DOI] [PubMed] [Google Scholar]
- 126. Dickson DW. Parkinson’s disease and parkinsonism: neuropathology. Cold Spring Harb Perspect Med 2012; 2: a009258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Harnois C, Di Paolo T. Decreased dopamine in the retinas of patients with Parkinson’s disease. Invest Ophthalmol Vis Sci 1990; 31: 2473–2475. [PubMed] [Google Scholar]
- 128. Bodis-Wollner I, Kozlowski PB, Glazman S, et al. alpha-synuclein in the inner retina in parkinson disease. Ann Neurol 2014; 75: 964–966. [DOI] [PubMed] [Google Scholar]
- 129. Delenclos M, Jones DR, McLean PJ, et al. Biomarkers in Parkinson’s disease: advances and strategies. Parkinsonism Relat Disord 2016; 22(Suppl. 1): S106–S110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Yu JG, Feng YF, Xiang Y, et al. Retinal nerve fiber layer thickness changes in Parkinson disease: a meta-analysis. PLoS One 2014; 9: e85718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Slotnick S, Ding Y, Glazman S, et al. A novel retinal biomarker for Parkinson’s disease: quantifying the foveal pit with optical coherence tomography. Mov Disord 2015; 30: 1692–1695. [DOI] [PubMed] [Google Scholar]
- 132. Shrier EM, Adam CR, Spund B, et al. Interocular asymmetry of foveal thickness in Parkinson disease. J Ophthalmol 2012; 2012: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Hajee ME, March WF, Lazzaro DR, et al. Inner retinal layer thinning in Parkinson disease. Arch Ophthalmol 2009; 127: 737–741. [DOI] [PubMed] [Google Scholar]
- 134. Satue M, Rodrigo MJ, Obis J, et al. Evaluation of progressive visual dysfunction and retinal degeneration in patients with Parkinson’s disease. Invest Ophthalmol Vis Sci 2017; 58: 1151–1157. [DOI] [PubMed] [Google Scholar]
- 135. Garcia-Martin E, Rodriguez-Mena D, Satue M, et al. Electrophysiology and optical coherence tomography to evaluate Parkinson disease severity. Invest Ophthalmol Vis Sci 2014; 55: 696–705. [DOI] [PubMed] [Google Scholar]
- 136. Roth NM, Saidha S, Zimmermann H, et al. Photoreceptor layer thinning in idiopathic Parkinson’s disease. Mov Disord 2014; 29: 1163–1170. [DOI] [PubMed] [Google Scholar]
- 137. Mailankody P, Battu R, Khanna A, et al. Optical coherence tomography as a tool to evaluate retinal changes in Parkinson’s disease. Parkinsonism Relat Disord 2015; 21: 1164–1169. [DOI] [PubMed] [Google Scholar]
- 138. Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967; 17: 427–442. [DOI] [PubMed] [Google Scholar]
- 139. Ahn J, Lee JY, Kim TW, et al. Retinal thinning associates with nigral dopaminergic loss in de novo Parkinson disease. Neurology 2018; 91: e1003–e1012. [DOI] [PubMed] [Google Scholar]
- 140. Hutton JT, Morris JL, Elias JW. Levodopa improves spatial contrast sensitivity in Parkinson’s disease. Arch Neurol 1993; 50: 721–724. [DOI] [PubMed] [Google Scholar]
- 141. Sadun AA, Bassi CJ. Optic nerve damage in Alzheimer’s disease. Ophthalmology 1990; 97: 9–17. [DOI] [PubMed] [Google Scholar]
- 142. Adam CR, Shrier E, Ding Y, et al. Correlation of inner retinal thickness evaluated by spectral-domain optical coherence tomography and contrast sensitivity in Parkinson disease. J Neuroophthalmol 2013; 33: 137–142. [DOI] [PubMed] [Google Scholar]
- 143. Normando EM, Davis BM, De Groef L, et al. The retina as an early biomarker of neurodegeneration in a rotenone-induced model of Parkinson’s disease: evidence for a neuroprotective effect of rosiglitazone in the eye and brain. Acta Neuropathol Commun 2016; 4: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Haug BA, Kolle RU, Trenkwalder C, et al. Predominant affection of the blue cone pathway in Parkinson’s disease. Brain 1995; 118: 771–778. [DOI] [PubMed] [Google Scholar]
- 145. Sartucci F, Orlandi G, Lucetti C, et al. Changes in pattern electroretinograms to equiluminant red-green and blue-yellow gratings in patients with early Parkinson’s disease. J Clin Neurophysiol 2003; 20: 375–381. [DOI] [PubMed] [Google Scholar]
- 146. Frederick JM, Rayborn ME, Laties AM, et al. Dopaminergic neurons in the human retina. J Comp Neurol 1982; 210: 65–79. [DOI] [PubMed] [Google Scholar]
- 147. Zhang PL, Chen Y, Zhang CH, et al. Genetics of Parkinson’s disease and related disorders. J Med Genet 2018; 55: 73–80. [DOI] [PubMed] [Google Scholar]
- 148. Ascherio A, Schwarzschild MA. The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol 2016; 15: 1257–1272. [DOI] [PubMed] [Google Scholar]
- 149. Ince PG, Codd GA. Return of the cycad hypothesis - does the amyotrophic lateral sclerosis/parkinsonism dementia complex (ALS/PDC) of Guam have new implications for global health? Neuropathol Appl Neurobiol 2005; 31: 345–353. [DOI] [PubMed] [Google Scholar]
- 150. Miri S, Glazman S, Mylin L, et al. A combination of retinal morphology and visual electrophysiology testing increases diagnostic yield in Parkinson’s disease. Parkinsonism Relat Disord 2016; 22(Suppl. 1): S134–S137. [DOI] [PubMed] [Google Scholar]
- 151. Compston A, Coles A. Multiple sclerosis. Lancet 2008; 372: 1502–1517. [DOI] [PubMed] [Google Scholar]
- 152. Kingwell E, Marriott JJ, Jette N, et al. Incidence and prevalence of multiple sclerosis in Europe: a systematic review. BMC Neurol 2013; 13: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Criste G, Trapp B, Dutta R. Axonal loss in multiple sclerosis: causes and mechanisms. Handb Clin Neurol 2014; 122: 101–113. [DOI] [PubMed] [Google Scholar]
- 154. Krieger SC, Cook K, De Nino S, et al. The topographical model of multiple sclerosis: A dynamic visualization of disease course. Neurol Neuroimmunol Neuroinflamm 2016; 3: e279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Goldenberg MM. Multiple sclerosis review. P T 2012; 37: 175–184. [PMC free article] [PubMed] [Google Scholar]
- 156. Oertel FC, Zimmermann HG, Brandt AU, et al. Novel uses of retinal imaging with optical coherence tomography in multiple sclerosis. Expert Rev Neurother 2018: 1–13. [DOI] [PubMed] [Google Scholar]
- 157. Lambe J, Murphy OC, Saidha S. Can optical coherence tomography be used to guide treatment decisions in adult or pediatric multiple sclerosis? Curr Treat Options Neurol 2018; 20: 9. [DOI] [PubMed] [Google Scholar]
- 158. Galetta SL, Villoslada P, Levin N, et al. Acute optic neuritis: unmet clinical needs and model for new therapies. Neurol Neuroimmunol Neuroinflamm 2015; 2: e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Toosy AT, Mason DF, Miller DH. Optic neuritis. Lancet Neurol 2014; 13: 83–99. [DOI] [PubMed] [Google Scholar]
- 160. Siffrin V, Vogt J, Radbruch H, et al. Multiple sclerosis - candidate mechanisms underlying CNS atrophy. Trends Neurosci 2010; 33: 202–210. [DOI] [PubMed] [Google Scholar]
- 161. Petzold A, Wattjes MP, Costello F, et al. The investigation of acute optic neuritis: a review and proposed protocol. Nat Rev Neurol 2014; 10: 447–458. [DOI] [PubMed] [Google Scholar]
- 162. Soelberg K, Specovius S, Zimmermann HG, et al. Optical coherence tomography in acute optic neuritis: a population-based study. Acta Neurol Scand 2018; 138: 566–573. [DOI] [PubMed] [Google Scholar]
- 163. Backner Y, Kuchling J, Massarwa S, et al. Anatomical wiring and functional networking changes in the visual system following optic neuritis. JAMA Neurol 2018; 75: 287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018; 17: 162–173. [DOI] [PubMed] [Google Scholar]
- 166. Carroll WM. 2017 McDonald MS diagnostic criteria: evidence-based revisions. Mult Scler 2018; 24: 92–95. [DOI] [PubMed] [Google Scholar]
- 167. Efendi H. Clinically isolated syndromes: clinical characteristics, differential diagnosis, and management. Noro Psikiyatr Ars 2015; 52: S1–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Parisi V, Manni G, Spadaro M, et al. Correlation between morphological and functional retinal impairment in multiple sclerosis patients. Invest Ophthalmol Vis Sci 1999; 40: 2520–2527. [PubMed] [Google Scholar]
- 169. Zaveri MS, Conger A, Salter A, et al. Retinal imaging by laser polarimetry and optical coherence tomography evidence of axonal degeneration in multiple sclerosis. Arch Neurol 2008; 65: 924–928. [DOI] [PubMed] [Google Scholar]
- 170. Pulicken M, Gordon-Lipkin E, Balcer LJ, et al. Optical coherence tomography and disease subtype in multiple sclerosis. Neurology 2007; 69: 2085–2092. [DOI] [PubMed] [Google Scholar]
- 171. Syc SB, Saidha S, Newsome SD, et al. Optical coherence tomography segmentation reveals ganglion cell layer pathology after optic neuritis. Brain 2012; 135: 521–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Oertel FC, Zimmermann H, Mikolajczak J, et al. Contribution of blood vessels to retinal nerve fiber layer thickness in NMOSD. Neurol Neuroimmunol Neuroinflamm 2017; 4: e338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Balk LJ, Tewarie P, Killestein J, et al. Disease course heterogeneity and OCT in multiple sclerosis. Mult Scler 2014; 20: 1198–1206. [DOI] [PubMed] [Google Scholar]
- 174. Behbehani R, Al-Hassan AA, Al-Khars A, et al. Retinal nerve fiber layer thickness and neurologic disability in relapsing-remitting multiple sclerosis. J Neurol Sci 2015; 359: 305–308. [DOI] [PubMed] [Google Scholar]
- 175. Esen E, Sizmaz S, Balal M, et al. Evaluation of the Innermost Retinal Layers and Visual Evoked Potentials in Patients with Multiple Sclerosis. Curr Eye Res 2016; 41: 1353–1358. [DOI] [PubMed] [Google Scholar]
- 176. Feng L, Shen J, Jin X, et al. The evaluation of the retinal nerve fiber layer in multiple sclerosis with special-domain optical coherence tomography. Ophthalmologica 2013; 230: 116–120. [DOI] [PubMed] [Google Scholar]
- 177. Garcia-Martin E, Ara JR, Martin J, et al. Retinal and optic nerve degeneration in patients with multiple sclerosis followed up for 5 years. Ophthalmology 2017; 124: 688–696. [DOI] [PubMed] [Google Scholar]
- 178. Gelfand JM, Goodin DS, Boscardin WJ, et al. Retinal axonal loss begins early in the course of multiple sclerosis and is similar between progressive phenotypes. PLoS One 2012; 7: e36847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Gonzalez-Lopez JJ, Rebolleda G, Leal M, et al. Comparative diagnostic accuracy of ganglion cell-inner plexiform and retinal nerve fiber layer thickness measures by cirrus and spectralis optical coherence tomography in relapsing-remitting multiple sclerosis. Biomed Res Int 2014; 2014: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Huang-Link YM, Fredrikson M, Link H. Benign multiple sclerosis is associated with reduced thinning of the retinal nerve fiber and ganglion cell layers in non-optic-neuritis eyes. J Clin Neurol 2015; 11: 241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Khalil DH, Said MM, Abdelhakim M, et al. OCT and visual field changes as useful markers for follow-up of axonal loss in multiple sclerosis in Egyptian patients. Ocul Immunol Inflamm 2017; 25: 315–322. [DOI] [PubMed] [Google Scholar]
- 182. Lange AP, Zhu F, Sayao AL, et al. Retinal nerve fiber layer thickness in benign multiple sclerosis. Mult Scler 2013; 19: 1275–1281. [DOI] [PubMed] [Google Scholar]
- 183. Oberwahrenbrock T, Ringelstein M, Jentschke S, et al. Retinal ganglion cell and inner plexiform layer thinning in clinically isolated syndrome. Mult Scler 2013; 19: 1887–1895. [DOI] [PubMed] [Google Scholar]
- 184. Park KA, Kim J, Oh SY. Analysis of spectral domain optical coherence tomography measurements in optic neuritis: differences in neuromyelitis optica, multiple sclerosis, isolated optic neuritis and normal healthy controls. Acta Ophthalmol 2014; 92: e57–e65. [DOI] [PubMed] [Google Scholar]
- 185. Soufi G, AitBenhaddou E, Hajji Z, et al. Evaluation of retinal nerve fiber layer thickness measured by optical coherence tomography in Moroccan patients with multiple sclerosis. J Fr Ophtalmol 2015; 38: 497–503. [DOI] [PubMed] [Google Scholar]
- 186. Walter SD, Ishikawa H, Galetta KM, et al. Ganglion cell loss in relation to visual disability in multiple sclerosis. Ophthalmology 2012; 119: 1250–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Xu LT, Bermel RA, Nowacki AS, et al. Optical coherence tomography for the detection of remote optic neuritis in multiple sclerosis. J Neuroimaging 2016; 26: 283–288. [DOI] [PubMed] [Google Scholar]
- 188. Bock M, Brandt AU, Dorr J, et al. Patterns of retinal nerve fiber layer loss in multiple sclerosis patients with or without optic neuritis and glaucoma patients. Clin Neurol Neurosurg 2010; 112: 647–652. [DOI] [PubMed] [Google Scholar]
- 189. Petzold A, Balcer LJ, Calabresi PA, et al. Retinal layer segmentation in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol 2017; 16: 797–812. [DOI] [PubMed] [Google Scholar]
- 190. Evangelou N, Konz D, Esiri MM, et al. Size-selective neuronal changes in the anterior optic pathways suggest a differential susceptibility to injury in multiple sclerosis. Brain 2001; 124: 1813–1820. [DOI] [PubMed] [Google Scholar]
- 191. Costello F, Coupland S, Hodge W, et al. Quantifying axonal loss after optic neuritis with optical coherence tomography. Ann Neurol 2006; 59: 963–969. [DOI] [PubMed] [Google Scholar]
- 192. Garcia-Martin E, Pueyo V, Ara JR, et al. Effect of optic neuritis on progressive axonal damage in multiple sclerosis patients. Mult Scler 2011; 17: 830–837. [DOI] [PubMed] [Google Scholar]
- 193. Gordon-Lipkin E, Chodkowski B, Reich DS, et al. Retinal nerve fiber layer is associated with brain atrophy in multiple sclerosis. Neurology 2007; 69: 1603–1609. [DOI] [PubMed] [Google Scholar]
- 194. Dorr J, Wernecke KD, Bock M, et al. Association of retinal and macular damage with brain atrophy in multiple sclerosis. PLoS One 2011; 6: e18132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Sinnecker T, Oberwahrenbrock T, Metz I, et al. Optic radiation damage in multiple sclerosis is associated with visual dysfunction and retinal thinning – an ultrahigh-field MR pilot study. Eur Radiol 2015; 25: 122–131. [DOI] [PubMed] [Google Scholar]
- 196. Manogaran P, Hanson JV, Olbert ED, et al. Optical coherence tomography and magnetic resonance imaging in multiple sclerosis and neuromyelitis optica spectrum disorder. Int J Mol Sci 2016; 17: 1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Petzold A, de Boer JF, Schippling S, et al. Optical coherence tomography in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol 2010; 9: 921–932. [DOI] [PubMed] [Google Scholar]
- 198. Singh S, Dallenga T, Winkler A, et al. Relationship of acute axonal damage, Wallerian degeneration, and clinical disability in multiple sclerosis. J Neuroinflammation 2017; 14: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Burkholder BM, Osborne B, Loguidice MJ, et al. Macular volume determined by optical coherence tomography as a measure of neuronal loss in multiple sclerosis. Arch Neurol 2009; 66: 1366–1372. [DOI] [PubMed] [Google Scholar]
- 200. Trip SA, Schlottmann PG, Jones SJ, et al. Retinal nerve fiber layer axonal loss and visual dysfunction in optic neuritis. Ann Neurol 2005; 58: 383–391. [DOI] [PubMed] [Google Scholar]
- 201. Khanifar AA, Parlitsis GJ, Ehrlich JR, et al. Retinal nerve fiber layer evaluation in multiple sclerosis with spectral domain optical coherence tomography. Clin Ophthalmol 2010; 4: 1007–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Albrecht P, Ringelstein M, Muller AK, et al. Degeneration of retinal layers in multiple sclerosis subtypes quantified by optical coherence tomography. Mult Scler 2012; 18: 1422–1429. [DOI] [PubMed] [Google Scholar]
- 203. Saidha S, Al-Louzi O, Ratchford JN, et al. Optical coherence tomography reflects brain atrophy in multiple sclerosis: a four-year study. Ann Neurol 2015; 78: 801–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Green AJ, McQuaid S, Hauser SL, et al. Ocular pathology in multiple sclerosis: retinal atrophy and inflammation irrespective of disease duration. Brain 2010; 133: 1591–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Schneider E, Zimmermann H, Oberwahrenbrock T, et al. Optical coherence tomography reveals distinct patterns of retinal damage in neuromyelitis optica and multiple sclerosis. PLoS One 2013; 8: e66151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Brandt AU, Oberwahrenbrock T, Kadas EM, et al. Dynamic formation of macular microcysts independent of vitreous traction changes. Neurology 2014; 83: 73–77. [DOI] [PubMed] [Google Scholar]
- 207. Burggraaff MC, Trieu J, de Vries-Knoppert WA, et al. The clinical spectrum of microcystic macular edema. Invest Ophthalmol Vis Sci 2014; 55: 952–961. [DOI] [PubMed] [Google Scholar]
- 208. Saidha S, Sotirchos ES, Ibrahim MA, et al. Microcystic macular oedema, thickness of the inner nuclear layer of the retina, and disease characteristics in multiple sclerosis: a retrospective study. Lancet Neurol 2012; 11: 963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Gelfand JM, Nolan R, Schwartz DM, et al. Microcystic macular oedema in multiple sclerosis is associated with disease severity. Brain 2012; 135: 1786–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Rebolleda G, Diez-Alvarez L, Casado A, et al. OCT: new perspectives in neuro-ophthalmology. Saudi J Ophthalmol 2015; 29: 9–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Knier B, Schmidt P, Aly L, et al. Retinal inner nuclear layer volume reflects response to immunotherapy in multiple sclerosis. Brain 2016; 139: 2855–2863. [DOI] [PubMed] [Google Scholar]
- 212. Al-Louzi OA, Bhargava P, Newsome SD, et al. Outer retinal changes following acute optic neuritis. Mult Scler 2016; 22: 362–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Gabilondo I, Martinez-Lapiscina EH, Fraga-Pumar E, et al. Dynamics of retinal injury after acute optic neuritis. Ann Neurol 2015; 77: 517–528. [DOI] [PubMed] [Google Scholar]
- 214. Hanson JVM, Hediger M, Manogaran P, et al. Outer retinal dysfunction in the absence of structural abnormalities in multiple sclerosis. Invest Ophthalmol Vis Sci 2018; 59: 549–560. [DOI] [PubMed] [Google Scholar]
- 215. Chan NCY, Chan CKM. The use of optical coherence tomography in neuro-ophthalmology. Curr Opin Ophthalmol 2017; 28: 552–557. [DOI] [PubMed] [Google Scholar]
- 216. Lanzillo R, Cennamo G, Criscuolo C, et al. Optical coherence tomography angiography retinal vascular network assessment in multiple sclerosis. Mult Scler 2018; 24: 1706–1714. [DOI] [PubMed] [Google Scholar]
- 217. Feucht N, Maier M, Lepennetier G, et al. Optical coherence tomography angiography indicates associations of the retinal vascular network and disease activity in multiple sclerosis. Mult Scler 2019; 25: 224–234. [DOI] [PubMed] [Google Scholar]
- 218. Lanzillo R, Cennamo G, Moccia M, et al. Retinal vascular density in multiple sclerosis: a 1-year follow-up. Eur J Neurol 2019; 26: 198–201. [DOI] [PubMed] [Google Scholar]
- 219. Spain RI, Liu L, Zhang X, et al. Optical coherence tomography angiography enhances the detection of optic nerve damage in multiple sclerosis. Br J Ophthalmol 2018; 102: 520–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Wang X, Jia Y, Spain R, et al. Optical coherence tomography angiography of optic nerve head and parafovea in multiple sclerosis. Br J Ophthalmol 2014; 98: 1368–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Iftikhar M, Zafar S, Gonzalez N, et al. Image artifacts in optical coherence tomography angiography among patients with multiple sclerosis. Curr Eye Res 2019: 1–6. [DOI] [PubMed] [Google Scholar]
- 222. Ratchford JN, Saidha S, Sotirchos ES, et al. Active MS is associated with accelerated retinal ganglion cell/inner plexiform layer thinning. Neurology 2013; 80: 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Oberwahrenbrock T, Schippling S, Ringelstein M, et al. Retinal damage in multiple sclerosis disease subtypes measured by high-resolution optical coherence tomography. Mult Scler Int 2012; 2012: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Garcia-Martin E, Pueyo V, Martin J, et al. Progressive changes in the retinal nerve fiber layer in patients with multiple sclerosis. Eur J Ophthalmol 2010; 20: 167–173. [DOI] [PubMed] [Google Scholar]
- 225. Talman LS, Bisker ER, Sackel DJ, et al. Longitudinal study of vision and retinal nerve fiber layer thickness in multiple sclerosis. Ann Neurol 2010; 67: 749–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Herrero R, Garcia-Martin E, Almarcegui C, et al. Progressive degeneration of the retinal nerve fiber layer in patients with multiple sclerosis. Invest Ophthalmol Vis Sci 2012; 53: 8344–8349. [DOI] [PubMed] [Google Scholar]
- 227. Balk LJ, Cruz-Herranz A, Albrecht P, et al. Timing of retinal neuronal and axonal loss in MS: a longitudinal OCT study. J Neurol 2016; 263: 1323–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Henderson AP, Trip SA, Schlottmann PG, et al. A preliminary longitudinal study of the retinal nerve fiber layer in progressive multiple sclerosis. J Neurol 2010; 257: 1083–1091. [DOI] [PubMed] [Google Scholar]
- 229. Zimmermann HG, Knier B, Oberwahrenbrock T, et al. Association of retinal ganglion cell layer thickness with future disease activity in patients with clinically isolated syndrome. JAMA Neurol 2018; 75: 1071–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Knier B, Berthele A, Buck D, et al. Optical coherence tomography indicates disease activity prior to clinical onset of central nervous system demyelination. Mult Scler 2016; 22: 893–900. [DOI] [PubMed] [Google Scholar]
- 231. Martinez-Lapiscina EH, Arnow S, Wilson JA, et al. Retinal thickness measured with optical coherence tomography and risk of disability worsening in multiple sclerosis: a cohort study. Lancet Neurol 2016; 15: 574–584. [DOI] [PubMed] [Google Scholar]
- 232. Garcia-Martin E, Rodriguez-Mena D, Herrero R, et al. Neuro-ophthalmologic evaluation, quality of life, and functional disability in patients with MS. Neurology 2013; 81: 76–83. [DOI] [PubMed] [Google Scholar]
- 233. Fisher JB, Jacobs DA, Markowitz CE, et al. Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmology 2006; 113: 324–332. [DOI] [PubMed] [Google Scholar]
- 234. Schinzel J, Zimmermann H, Paul F, et al. Relations of low contrast visual acuity, quality of life and multiple sclerosis functional composite: a cross-sectional analysis. BMC Neurol 2014; 14: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Gundogan FC, Demirkaya S, Sobaci G. Is optical coherence tomography really a new biomarker candidate in multiple sclerosis? – A structural and functional evaluation. Invest Ophthalmol Vis Sci 2007; 48: 5773–5781. [DOI] [PubMed] [Google Scholar]
- 236. Weinstock-Guttman B, Baier M, Stockton R, et al. Pattern reversal visual evoked potentials as a measure of visual pathway pathology in multiple sclerosis. Mult Scler 2003; 9: 529–534. [DOI] [PubMed] [Google Scholar]
- 237. Rodriguez-Mena D, Almarcegui C, Dolz I, et al. Electropysiologic evaluation of the visual pathway in patients with multiple sclerosis. J Clin Neurophysiol 2013; 30: 376–381. [DOI] [PubMed] [Google Scholar]
- 238. Waldman AT, Liu GT, Lavery AM, et al. Optical coherence tomography and visual evoked potentials in pediatric MS. Neurol Neuroimmunol Neuroinflamm 2017; 4: e356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Borisow N, Mori M, Kuwabara S, et al. Diagnosis and treatment of NMO spectrum disorder and MOG-encephalomyelitis. Front Neurol 2018; 9: 888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Zekeridou A, Lennon VA. Aquaporin-4 autoimmunity. Neurol Neuroimmunol Neuroinflamm 2015; 2: e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Papadopoulos MC, Verkman AS. Aquaporin 4 and neuromyelitis optica. Lancet Neurol 2012; 11: 535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Felix CM, Levin MH, Verkman AS. Complement-independent retinal pathology produced by intravitreal injection of neuromyelitis optica immunoglobulin G. J Neuroinflammation 2016; 13: 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Bennett JL, de Seze J, Lana-Peixoto M, et al. Neuromyelitis optica and multiple sclerosis: Seeing differences through optical coherence tomography. Mult Scler 2015; 21: 678–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Gahlen A, Trampe AK, Haupeltshofer S, et al. Aquaporin-4 antibodies in patients treated with natalizumab for suspected MS. Neurol Neuroimmunol Neuroinflamm 2017; 4: e363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Metz I, Beissbarth T, Ellenberger D, et al. Serum peptide reactivities may distinguish neuromyelitis optica subgroups and multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 2016; 3: e204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Kleiter I, Gahlen A, Borisow N, et al. Apheresis therapies for NMOSD attacks: a retrospective study of 207 therapeutic interventions. Neurol Neuroimmunol Neuroinflamm 2018; 5: e504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Trebst C, Jarius S, Berthele A, et al. Update on the diagnosis and treatment of neuromyelitis optica: recommendations of the neuromyelitis optica study group (NEMOS). J Neurol 2014; 261: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Schmidt F, Zimmermann H, Mikolajczak J, et al. Severe structural and functional visual system damage leads to profound loss of vision-related quality of life in patients with neuromyelitis optica spectrum disorders. Mult Scler Relat Disord 2017; 11: 45–50. [DOI] [PubMed] [Google Scholar]
- 249. Bichuetti DB, de Camargo AS, Falcao AB, et al. The retinal nerve fiber layer of patients with neuromyelitis optica and chronic relapsing optic neuritis is more severely damaged than patients with multiple sclerosis. J Neuroophthalmol 2013; 33: 220–224. [DOI] [PubMed] [Google Scholar]
- 250. Zhao X, Qiu W, Zhang Y, et al. A prospective case-control study comparing optical coherence tomography characteristics in neuromyelitis optica spectrum disorder- optic neuritis and idiopathic optic neuritis. BMC Ophthalmol 2018; 18: 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Bertsch-Gout M, Loeb R, Finch AK, et al. High resolution retinal scanning reveals regional structural differences between MS and NMOSD optic neuritis regardless of antibody status. J Neurol Sci 2018; 384: 61–66. [DOI] [PubMed] [Google Scholar]
- 252. Tian DC, Su L, Fan M, et al. Bidirectional degeneration in the visual pathway in neuromyelitis optica spectrum disorder (NMOSD). Mult Scler 2018; 24: 1585–1593. [DOI] [PubMed] [Google Scholar]
- 253. Finke C, Zimmermann H, Pache F, et al. Association of visual impairment in neuromyelitis optica spectrum disorder with visual network reorganization. JAMA Neurol 2018; 75: 296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Akaishi T, Kaneko K, Himori N, et al. Subclinical retinal atrophy in the unaffected fellow eyes of multiple sclerosis and neuromyelitis optica. J Neuroimmunol 2017; 313: 10–15. [DOI] [PubMed] [Google Scholar]
- 255. Jeong IH, Kim HJ, Kim NH, et al. Subclinical primary retinal pathology in neuromyelitis optica spectrum disorder. J Neurol 2016; 263: 1343–1348. [DOI] [PubMed] [Google Scholar]
- 256. Oertel FC, Kuchling J, Zimmermann H, et al. Microstructural visual system changes in AQP4-antibody-seropositive NMOSD. Neurol Neuroimmunol Neuroinflamm 2017; 4: e334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Outteryck O, Majed B, Defoort-Dhellemmes S, et al. A comparative optical coherence tomography study in neuromyelitis optica spectrum disorder and multiple sclerosis. Mult Scler 2015; 21: 1781–1793. [DOI] [PubMed] [Google Scholar]
- 258. Oertel FC, Havla J, Roca-Fernandez A, et al. Retinal ganglion cell loss in neuromyelitis optica: a longitudinal study. J Neurol Neurosurg Psychiatry 2018; 89: 1259–1265. [DOI] [PubMed] [Google Scholar]
- 259. Manogaran P, Traboulsee AL, Lange AP. Longitudinal study of retinal nerve fiber layer thickness and macular volume in patients with neuromyelitis optica spectrum disorder. J Neuroophthalmol 2016; 36: 363–368. [DOI] [PubMed] [Google Scholar]
- 260. von Glehn F, Jarius S, Cavalcanti Lira RP, et al. Structural brain abnormalities are related to retinal nerve fiber layer thinning and disease duration in neuromyelitis optica spectrum disorders. Mult Scler 2014; 20: 1189–1197. [DOI] [PubMed] [Google Scholar]
- 261. Ringelstein M, Kleiter I, Ayzenberg I, et al. Visual evoked potentials in neuromyelitis optica and its spectrum disorders. Mult Scler 2014; 20: 617–620. [DOI] [PubMed] [Google Scholar]
- 262. Gelfand JM, Cree BA, Nolan R, et al. Microcystic inner nuclear layer abnormalities and neuromyelitis optica. JAMA Neurol 2013; 70: 629–633. [DOI] [PubMed] [Google Scholar]
- 263. Kaufhold F, Zimmermann H, Schneider E, et al. Optic neuritis is associated with inner nuclear layer thickening and microcystic macular edema independently of multiple sclerosis. PLoS One 2013; 8: e71145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Sotirchos ES, Saidha S, Byraiah G, et al. In vivo identification of morphologic retinal abnormalities in neuromyelitis optica. Neurology 2013; 80: 1406–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Huang Y, Zhou L, ZhangBao J, et al. Peripapillary and parafoveal vascular network assessment by optical coherence tomography angiography in aquaporin-4 antibody-positive neuromyelitis optica spectrum disorders. Br J Ophthalmol. Epub ahead of print 20 July 2018. DOI: 10.1136/bjophthalmol-2018-312231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Kwapong WR, Peng C, He Z, et al. Altered macular microvasculature in neuromyelitis optica spectrum disorders. Am J Ophthalmol 2018; 192: 47–55. [DOI] [PubMed] [Google Scholar]
- 267. Oertel FC, Zimmermann H, Paul F, et al. Optical coherence tomography in neuromyelitis optica spectrum disorders: potential advantages for individualized monitoring of progression and therapy. EPMA J 2018; 9: 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Pache F, Zimmermann H, Mikolajczak J, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 4: Afferent visual system damage after optic neuritis in MOG-IgG-seropositive versus AQP4-IgG-seropositive patients. J Neuroinflammation 2016; 13: 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Havla J, Kumpfel T, Schinner R, et al. Myelin-oligodendrocyte-glycoprotein (MOG) autoantibodies as potential markers of severe optic neuritis and subclinical retinal axonal degeneration. J Neurol 2017; 264: 139–151. [DOI] [PubMed] [Google Scholar]
- 270. Narayan RN, McCreary M, Conger D, et al. Unique characteristics of optical coherence tomography (OCT) results and visual acuity testing in myelin oligodendrocyte glycoprotein (MOG) antibody positive pediatric patients. Mult Scler Relat Disord 2019; 28: 86–90. [DOI] [PubMed] [Google Scholar]
- 271. Dorr J, Krautwald S, Wildemann B, et al. Characteristics of Susac syndrome: a review of all reported cases. Nat Rev Neurol 2013; 9: 307–316. [DOI] [PubMed] [Google Scholar]
- 272. Ringelstein M, Albrecht P, Kleffner I, et al. Retinal pathology in Susac syndrome detected by spectral-domain optical coherence tomography. Neurology 2015; 85: 610–618. [DOI] [PubMed] [Google Scholar]
- 273. Brandt AU, Zimmermann H, Kaufhold F, et al. Patterns of retinal damage facilitate differential diagnosis between Susac syndrome and MS. PLoS One 2012; 7: e38741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Brandt AU, Oberwahrenbrock T, Costello F, et al. Retinal lesion evolution in susac syndrome. Retina 2016; 36: 366–374. [DOI] [PubMed] [Google Scholar]
- 275. Tao QQ, Wei Q, Wu ZY. Sensory nerve disturbance in amyotrophic lateral sclerosis. Life Sci 2018; 203: 242–245. [DOI] [PubMed] [Google Scholar]
- 276. Balendra R, Patani R. Quo vadis motor neuron disease? World J Methodol 2016; 6: 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Turner MR, Swash M. The expanding syndrome of amyotrophic lateral sclerosis: a clinical and molecular odyssey. J Neurol Neurosurg Psychiatry 2015; 86: 667–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Li DW, Liu M, Cui B, et al. The Awaji criteria increases the diagnostic sensitivity of the revised El Escorial criteria for amyotrophic lateral sclerosis diagnosis in a Chinese population. PLoS One 2017; 12: e0171522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Costa J, Swash M, de Carvalho M. Awaji criteria for the diagnosis of amyotrophic lateral sclerosis:a systematic review. Arch Neurol 2012; 69: 1410–1416. [DOI] [PubMed] [Google Scholar]
- 280. Agosta F, Weiler M, Filippi M. Propagation of pathology through brain networks in neurodegenerative diseases: from molecules to clinical phenotypes. CNS Neurosci Ther 2015; 21: 754–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Sharma R, Hicks S, Berna CM, et al. Oculomotor dysfunction in amyotrophic lateral sclerosis: a comprehensive review. Arch Neurol 2011; 68: 857–861. [DOI] [PubMed] [Google Scholar]
- 282. Moss HE, McCluskey L, Elman L, et al. Cross-sectional evaluation of clinical neuro-ophthalmic abnormalities in an amyotrophic lateral sclerosis population. J Neurol Sci 2012; 314: 97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Munte TF, Troger MC, Nusser I, et al. Alteration of early components of the visual evoked potential in amyotrophic lateral sclerosis. J Neurol 1998; 245: 206–210. [DOI] [PubMed] [Google Scholar]
- 284. Bede P, Bokde A, Elamin M, et al. Grey matter correlates of clinical variables in amyotrophic lateral sclerosis (ALS): a neuroimaging study of ALS motor phenotype heterogeneity and cortical focality. J Neurol Neurosurg Psychiatry 2013; 84: 766–773. [DOI] [PubMed] [Google Scholar]
- 285. Maruyama H, Morino H, Ito H, et al. Mutations of optineurin in amyotrophic lateral sclerosis. Nature 2010; 465: 223–226. [DOI] [PubMed] [Google Scholar]
- 286. Cirulli ET, Lasseigne BN, Petrovski S, et al. Exome sequencing in amyotrophic lateral sclerosis identifies risk genes and pathways. Science 2015; 347: 1436–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Elden AC, Kim HJ, Hart MP, et al. Ataxin-2 intermediate-length polyglutamine expansions are associated with increased risk for ALS. Nature 2010; 466: 1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Lee T, Li YR, Ingre C, et al. Ataxin-2 intermediate-length polyglutamine expansions in European ALS patients. Hum Mol Genet 2011; 20: 1697–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Minegishi Y, Iejima D, Kobayashi H, et al. Enhanced optineurin E50K-TBK1 interaction evokes protein insolubility and initiates familial primary open-angle glaucoma. Hum Mol Genet 2013; 22: 3559–3567. [DOI] [PubMed] [Google Scholar]
- 290. Bailey JN, Loomis SJ, Kang JH, et al. Genome-wide association analysis identifies TXNRD2, ATXN2 and FOXC1 as susceptibility loci for primary open-angle glaucoma. Nat Genet 2016; 48: 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Kawase K, Allingham RR, Meguro A, et al. Confirmation of TBK1 duplication in normal tension glaucoma. Exp Eye Res 2012; 96: 178–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Ringelstein M, Albrecht P, Sudmeyer M, et al. Subtle retinal pathology in amyotrophic lateral sclerosis. Ann Clin Transl Neurol 2014; 1: 290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Mukherjee N, McBurney-Lin S, Kuo A, et al. Retinal thinning in amyotrophic lateral sclerosis patients without ophthalmic disease. PLoS One 2017; 12: e0185242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Roth NM, Saidha S, Zimmermann H, et al. Optical coherence tomography does not support optic nerve involvement in amyotrophic lateral sclerosis. Eur J Neurol 2013; 20: 1170–1176. [DOI] [PubMed] [Google Scholar]
- 295. Hubers A, Muller HP, Dreyhaupt J, et al. Retinal involvement in amyotrophic lateral sclerosis: a study with optical coherence tomography and diffusion tensor imaging. J Neural Transm (Vienna) 2016; 123: 281–287. [DOI] [PubMed] [Google Scholar]
- 296. Rohani M, Meysamie A, Zamani B, et al. Reduced retinal nerve fiber layer (RNFL) thickness in ALS patients: a window to disease progression. J Neurol 2018; 265: 1557–1562. [DOI] [PubMed] [Google Scholar]
- 297. Volpe NJ, Simonett J, Fawzi AA, et al. Ophthalmic manifestations of amyotrophic lateral sclerosis (an American ophthalmological society thesis). Trans Am Ophthalmol Soc 2015; 113: T12. [PMC free article] [PubMed] [Google Scholar]
- 298. Abdelhak A, Hubers A, Bohm K, et al. In vivo assessment of retinal vessel pathology in amyotrophic lateral sclerosis. J Neurol 2018; 265: 949–953. [DOI] [PubMed] [Google Scholar]
- 299. Simonett JM, Huang R, Siddique N, et al. Macular sub-layer thinning and association with pulmonary function tests in amyotrophic lateral sclerosis. Sci Rep 2016; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Vucic S, Cheah BC, Kiernan MC. Defining the mechanisms that underlie cortical hyperexcitability in amyotrophic lateral sclerosis. Exp Neurol 2009; 220: 177–182. [DOI] [PubMed] [Google Scholar]
- 301. Kolde G, Bachus R, Ludolph AC. Skin involvement in amyotrophic lateral sclerosis. Lancet 1996; 347: 1226–1227. [DOI] [PubMed] [Google Scholar]
- 302. Al-Chalabi A, Jones A, Troakes C, et al. The genetics and neuropathology of amyotrophic lateral sclerosis. Acta Neuropathol 2012; 124: 339–352. [DOI] [PubMed] [Google Scholar]
- 303. Fawzi AA, Simonett JM, Purta P, et al. Clinicopathologic report of ocular involvement in ALS patients with C9orf72 mutation. Amyotroph Lateral Scler Frontotemporal Degener 2014; 15: 569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Javad Mousavi SA, Zamani B, Shahabi Shahmiri S, et al. Pulmonary function tests in patients with amyotrophic lateral sclerosis and the association between these tests and survival. Iran J Neurol 2014; 13: 131–137. [PMC free article] [PubMed] [Google Scholar]
- 305. Stambler N, Charatan M, Cedarbaum JM. Prognostic indicators of survival in ALS. ALS CNTF Treatment Study Group. Neurology 1998; 50: 66–72. [DOI] [PubMed] [Google Scholar]
- 306. Puoti G, Bizzi A, Forloni G, et al. Sporadic human prion diseases: molecular insights and diagnosis. Lancet Neurol 2012; 11: 618–628. [DOI] [PubMed] [Google Scholar]
- 307. Orru CD, Soldau K, Cordano C, et al. Prion seeds distribute throughout the eyes of sporadic Creutzfeldt-Jakob disease patients. MBio 2018; 9: e02095–e02118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Proulx AA, Strong MJ, Nicolle DA. Creutzfeldt-Jakob disease presenting with visual manifestations. Can J Ophthalmol 2008; 43: 591–595. [DOI] [PubMed] [Google Scholar]
- 309. Safar JG. Molecular pathogenesis of sporadic prion diseases in man. Prion 2012; 6: 108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Qina T, Sanjo N, Hizume M, et al. Clinical features of genetic Creutzfeldt-Jakob disease with V180I mutation in the prion protein gene. BMJ Open 2014; 4: e004968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Masters CL, Harris JO, Gajdusek DC, et al. Creutzfeldt-Jakob disease: patterns of worldwide occurrence and the significance of familial and sporadic clustering. Ann Neurol 1979; 5: 177–188. [DOI] [PubMed] [Google Scholar]
- 312. Ladogana A, Puopolo M, Croes EA, et al. Mortality from Creutzfeldt-Jakob disease and related disorders in Europe, Australia, and Canada. Neurology 2005; 64: 1586–1591. [DOI] [PubMed] [Google Scholar]
- 313. Kobayashi A, Kitamoto T, Mizusawa H. Iatrogenic Creutzfeldt-Jakob disease. Handb Clin Neurol 2018; 153: 207–218. [DOI] [PubMed] [Google Scholar]
- 314. Sikorska B, Knight R, Ironside JW, et al. Creutzfeldt-Jakob disease. Adv Exp Med Biol 2012; 724: 76–90. [DOI] [PubMed] [Google Scholar]
- 315. Cathala F, Brown P, Castaigne P, et al. Creutzfeldt-Jacob disease in continental France. Retrospective study from 1968 to 1977. Rev Neurol (Paris) 1979; 135: 439–443. [PubMed] [Google Scholar]
- 316. Kretzschmar HA, Ironside JW, DeArmond SJ, et al. Diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Arch Neurol 1996; 53: 913–920. [DOI] [PubMed] [Google Scholar]
- 317. Saraceno L, Ricigliano VAG, Cavalli M, et al. Sporadic MM-1 type Creutzfeldt-Jakob disease with hemiballic presentation and no cognitive impairment until death: how new NCJDRSU diagnostic criteria may allow early diagnosis. Front Neurol 2018; 9: 739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Lueck CJ, McIlwaine GG, Zeidler M. Creutzfeldt-Jakob disease and the eye. II. Ophthalmic and neuro-ophthalmic features. Eye (Lond) 2000; 14: 291–301. [DOI] [PubMed] [Google Scholar]
- 319. Armstrong RA. Creutzfeldt-Jakob disease and vision. Clin Exp Optom 2006; 89: 3–9. [DOI] [PubMed] [Google Scholar]
- 320. Brown P, Gibbs CJ, Jr, Rodgers-Johnson P, et al. Human spongiform encephalopathy: the National Institutes of Health series of 300 cases of experimentally transmitted disease. Ann Neurol 1994; 35: 513–529. [DOI] [PubMed] [Google Scholar]
- 321. Roos R, Gajdusek DC, Gibbs CJ., Jr. The clinical characteristics of transmissible Creutzfeldt-Jakob disease. Brain 1973; 96: 1–20. [DOI] [PubMed] [Google Scholar]
- 322. Meyer A, Leigh D, Bagg CE. A rare presenile dementia associated with cortical blindness (Heidenhain’s syndrome). J Neurol Neurosurg Psychiatry 1954; 17: 129–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Fisher CM. The clinical picture in Creutzfeldt-Jakob disease. Trans Am Neurol Assoc 1960; 85: 147–150. [PubMed] [Google Scholar]
- 324. Wolpow ER, Kleinman GM. Case 45–1980. N Engl J Med 1980; 303: 1162–1171.6999350 [Google Scholar]
- 325. Shih WJ, Markesbery WR, Clark DB, et al. Iodine-123 HIPDM brain imaging findings in subacute spongiform encephalopathy (Creutzfeldt-Jakob disease). J Nucl Med 1987; 28: 1484–1487. [PubMed] [Google Scholar]
- 326. Aguglia U, Oliveri RL, Gambardella A, et al. Combined neurophysiological studies in Creutzfeldt-Jakob disease: a case report. Clin Electroencephalogr 1989; 20: 103–110. [DOI] [PubMed] [Google Scholar]
- 327. Aguglia U, Oliveri RL, Gambardella A, et al. Functional integrity of benzodiazepine receptors of the geniculo-striate visual pathways in Creutzfeldt-Jakob disease. A pharmacological evoked potential study. J Neurol 1993; 240: 25–27. [DOI] [PubMed] [Google Scholar]
- 328. Will RG, Matthews WB. A retrospective study of Creutzfeldt-Jakob disease in England and Wales 1970–79. I: clinical features. J Neurol Neurosurg Psychiatry 1984; 47: 134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Vargas ME, Kupersmith MJ, Savino PJ, et al. Homonymous field defect as the first manifestation of Creutzfeldt-Jakob disease. Am J Ophthalmol 1995; 119: 497–504. [DOI] [PubMed] [Google Scholar]
- 330. Foundas M, Donaldson MD, McAllister IL, et al. Vision loss due to coincident ocular and central causes in a patient with Heidenhain variant Creutzfeldt-Jakob disease. Age Ageing 2008; 37: 231–232. [DOI] [PubMed] [Google Scholar]
- 331. Sato Y, Chiba S, Miyagishi T. A case of the panencephalopathic type of Creutzfeldt-Jakob disease with retinal involvement. Hokkaido Igaku Zasshi 1992; 67: 703–711. [PubMed] [Google Scholar]
- 332. Lesser RL, Albert DM, Bobowick AR, et al. Creutzfeldt-Jakob disease and optic atrophy. Am J Ophthalmol 1979; 87: 317–321. [DOI] [PubMed] [Google Scholar]
- 333. Tsutsui J, Kawashima S, Kajikawa I, et al. Electrophysiological and pathological studies on Creutzfeldt-Jakob disease with retinal involvement. Doc Ophthalmol 1986; 63: 13–21. [DOI] [PubMed] [Google Scholar]
- 334. de Seze J, Hache JC, Vermersch P, et al. Creutzfeldt-Jakob disease: neurophysiologic visual impairments. Neurology 1998; 51: 962–967. [DOI] [PubMed] [Google Scholar]
- 335. Kitagawa Y, Gotoh F, Koto A, et al. Creutzfeldt-Jakob disease: a case with extensive white matter degeneration and optic atrophy. J Neurol 1983; 229: 97–101. [DOI] [PubMed] [Google Scholar]
- 336. Jibiki I, Fukushima T, Kobayashi K, et al. Utility of 123I-IMP SPECT brain scans for the early detection of site-specific abnormalities in Creutzfeldt-Jakob disease (Heidenhain type): a case study. Neuropsychobiology 1994; 29: 117–119. [DOI] [PubMed] [Google Scholar]
- 337. Head MW, Peden AH, Yull HM, et al. Abnormal prion protein in the retina of the most commonly occurring subtype of sporadic Creutzfeldt-Jakob disease. Br J Ophthalmol 2005; 89: 1131–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Hudson AJ, Farrell MA, Kalnins R, et al. Gerstmann-Straussler-Scheinker disease with coincidental familial onset. Ann Neurol 1983; 14: 670–678. [DOI] [PubMed] [Google Scholar]
- 339. Lane KL, Brown P, Howell DN, et al. Creutzfeldt-Jakob disease in a pregnant woman with an implanted dura mater graft. Neurosurgery 1994; 34: 737–739; discussion 739–740. [DOI] [PubMed] [Google Scholar]
- 340. Adam J, Crow TJ, Duchen LW, et al. Familial cerebral amyloidosis and spongiform encephalopathy. J Neurol Neurosurg Psychiatry 1982; 45: 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Powell-Jackson J, Weller RO, Kennedy P, et al. Creutzfeldt-Jakob disease after administration of human growth hormone. Lancet 1985; 2: 244–246. [DOI] [PubMed] [Google Scholar]
- 342. Miller RF, Dowling JE. Intracellular responses of the Müller (glial) cells of mudpuppy retina: their relation to b-wave of the electroretinogram. J Neurophysiol 1970; 33: 323–341. [DOI] [PubMed] [Google Scholar]
- 343. Ishikawa A, Tanikawa A, Shimada Y, et al. Electroretinograms in three cases of Creutzfeldt-Jakob disease with visual disturbances. Jpn J Ophthalmol 2009; 53: 31–34. [DOI] [PubMed] [Google Scholar]
- 344. Greenlee MH, Smith JD, Platt EM, et al. Changes in retinal function and morphology are early clinical signs of disease in cattle with bovine spongiform encephalopathy. PLoS One 2015; 10: e0119431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. Masters CL, Richardson EP., Jr. Subacute spongiform encephalopathy (Creutzfeldt-Jakob disease). The nature and progression of spongiform change. Brain 1978; 101: 333–344. [DOI] [PubMed] [Google Scholar]
- 346. Brown DR, Schmidt B, Kretzschmar HA. Role of microglia and host prion protein in neurotoxicity of a prion protein fragment. Nature 1996; 380: 345–347. [DOI] [PubMed] [Google Scholar]
- 347. Smith JD, Greenlee JJ, Hamir AN, et al. Retinal function and morphology are altered in cattle infected with the prion disease transmissible mink encephalopathy. Vet Pathol 2009; 46: 810–818. [DOI] [PubMed] [Google Scholar]
- 348. Huang-Link Y, Eleftheriou A, Yang G, et al. Optical coherence tomography represents a sensitive and reliable tool for routine monitoring of idiopathic intracranial hypertension with and without papilledema. Eur J Neurol 2019; 26: 808–e57. [DOI] [PubMed] [Google Scholar]
- 349. Ascaso FJ, Marco S, Mateo J, et al. Optical coherence tomography in patients with chronic migraine: literature review and update. Front Neurol 2017; 8: 684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Galetta KM, Calabresi PA, Frohman EM, et al. Optical coherence tomography (OCT): imaging the visual pathway as a model for neurodegeneration. Neurotherapeutics 2011; 8: 117–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351. Reggio E, Chisari CG, Ferrigno G, et al. Migraine causes retinal and choroidal structural changes: evaluation with ocular coherence tomography. J Neurol 2017; 264: 494–502. [DOI] [PubMed] [Google Scholar]
- 352. Topcu-Yilmaz P, Aydin M, Cetin Ilhan B. Evaluation of retinal nerve fiber layer, macular, and choroidal thickness in schizophrenia: spectral optic coherence tomography findings. Psychiatry Clin Psychopharmacology 2019; 29: 28–33. [Google Scholar]
- 353. Yildiz M, Alim S, Batmaz S, et al. Duration of the depressive episode is correlated with ganglion cell inner plexifrom layer and nasal retinal fiber layer thicknesses: Optical coherence tomography findings in major depression. Psychiatry Res Neuroimaging 2016; 251: 60–66. [DOI] [PubMed] [Google Scholar]