Abstract
Failure of the efficacy of antimalarial drugs is recognized in different classes of medicines for treating malaria, which urges the need for new drugs. This study tried to check the in vivo antimalarial activity of the root extracts of Acanthus polystachyus Delile against Plasmodium berghei–infected mice. The study revealed that the methanolic crude extract of the root of Acanthus polystachyus Delile showed significant (P < .01) parasitemia suppressive activities in both models compared with the negative control. Parasitemia suppressive activities were 25.26%, 33.46%, and 51.48% in a 4-day suppressive test and 23.31%, 31.20%, and 43.54% in prophylaxis test at 100, 200, and 400 mg/kg of the extract, respectively, as compared to the negative control. Besides, the extract increases mean survival time significantly in all tested doses in a 4-day suppressive test, but in the prophylaxis model, only mice treated with 200 and 400 mg/kg significantly lived longer. Based on this finding, the root of Acanthus polystachyus Delile has strong antimalarial activity, which may be a good candidate for new antimalarial agents.
Keywords: antimalaria, Acanthus polystachyus Delile, Plasmodium berghei
Malaria is a vector-borne infectious disease caused by a protozoan of the genus Plasmodium. Around 44% of the world’s population is at risk of malaria.1 Most of these illnesses (82%) are in Africa, Asia, and the Eastern Mediterranean.2 Malaria is caused by 4 parasite species: P falciparum, P vivax, P malariae, and P ovale. P falciparum and P vivax are the most common species clinically.3
Malaria is one of the deadliest diseases in the world, particularly in Ethiopia. That is, more than 50 million people are at risk of malaria, and 4 to 5 million people are affected by malaria annually.4,5 According to the World Health Organization, about 80% of the world’s population depends on traditional medicine to treat infections including malaria, though less attention is given to the sector.6,7 However, the increasing problems of drug-resistant parasites are a great problem for health care providers and pharmaceutical companies. This necessitates the need for new antimalarials that are effective and affordable.8–10
The root of Acanthus polystachyus Delile is one among such plants applied to manage malaria, intestinal worms, and vomit.11,12 However, the literature review failed to offer any scientific validation on the antimalarial activity of the root of this plant.
Methodology
Plant Material
The root of Acanthus polystachyus Delile was collected from around Bahir Dar, which is 560 km northwest of Addis Ababa, Central Ethiopia. The plant material was checked by the National Herbarium of Addis Ababa University, and a voucher specimen with a voucher identity of DD002 was deposited.
Reagents and Drugs
Different chemicals and reagents (Giemsa, trisodium citrate, n-hexane, hydrochloric acid, Tween 80, potassium ferrocyanide, lead acetate, chloroform, ferric chloride, absolute methanol, ethyl acetate, acetic anhydride, chloroquine phosphate) were used for the experiment. All the chemicals were analytical grade and most of them were purchased from the Pharmaceutical Fund and Supply Agency, Addis Ababa, Ethiopia.
Experimental Animals
A total of 65 adult albino mice of both sexes (age 6-8 weeks) with a weight range of 22 to 26 g were used for the experiment. They were kept in a regular plastic cage at a controlled room temperature of 21 ± 2°C and a relative humidity of 50%. Care and handling of mice were performed according to the internationally accepted standard guideline for use of laboratory animals.13
Parasite
Plasmodium berghei ANKA strain was purchased from the Aklilu Lemma Institute of Pathobiology. The parasite was kept by serial blood passage weekly. A donor mouse was sacrificed and its blood was collected in a slightly heparinized syringe, which was then diluted with trisodium citrate medium. Each mouse was given about10 million parasites/gram.
Extract Preparation
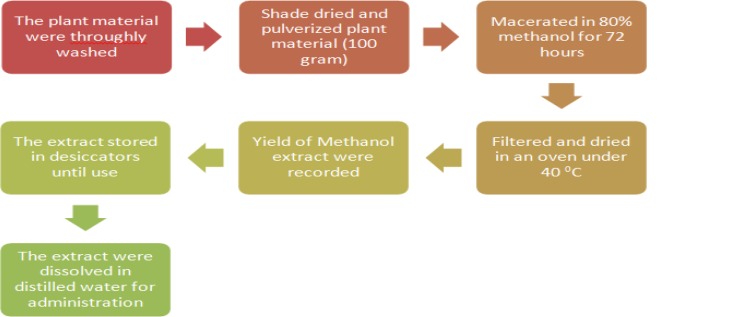
The fresh root of the plant was thoroughly washed with distilled water and dried under a shade with optimal ventilation for 2 weeks. After chopping, roots were powdered by electronic crushers, then macerated using 80% methanol. The plant and solvent mixtures were placed on an orbital shaker (at 160 rpm) for 72 hours at room temperature. After filtration and repeated maceration using a Whatman filter paper, the drying of filtrates was conducted in an oven of about 40°C. Finally, the weight of the extract was measured to decide the percentage yield. All the dry extracts were moved to a vial and kept in a desiccator till applied for the experiment as shown in Figure 1.
Figure 1.
Schematic diagram of plant extraction.
Eighty percent of methanol was used to get a greater yield of extract as methanol is more efficient in the cell wall and seed degradation. Also, alcoholic or hydroalcoholic extracts contain a variety of compounds with different polarity.14–16
Preliminary Phytochemical Screening
The methanolic root extracts of the plant were tested for the presence of alkaloids, flavonoids, polyphenols, tannins, saponins, terpenoids, glycosides, anthraquinones, and steroidal compounds.
Acute Oral Toxicity Test
Based on the limit test advice of OECD 425 guideline, acute oral toxicity testing was done on young female mice.17 Once acclimatized for a week, one mouse was fasted for 3 hours (with constant delivery of water). Then, a single dose of 2000 mg/kg was administered to the mouse orally. After the administration, food was withheld for a further 2 hours. Close observation was done 30 minutes after dosing and regularly for the next 24 hours for any physical and behavioral changes. Daily follow-up of the mouse was also conducted for 14 days. Based on the results from this mouse, the same procedure was applied to 4 additional mice.
Pharmacological Screening
Four-Day Chemo-Suppressive Test
This test was done using the standard 4-day suppressive test against P berghei–infected mice.18 A total of 30 mice were randomly selected and acclimatized for 1 week. Three days before commencing the experiment, all the mice were infected with the parasite (1 × 107 P berghei–infected red blood cell, 0.2 mL). The infected mice were classified into 5 groups each. Group 1 was used as a negative control and administered with distilled water (0.2 mL/100 g/day). Groups 2 (A100), 3 (A200), and 4 (A400) were given 100 mg/kg, 200 mg/kg, and 400 mg/kg of the root extracts of the plant orally, respectively. Mice of group 5 received 10 mg/kg of chloroquine. Treatment was conducted for 4 consecutive days.
Blood smears from the tail of every mouse were prepared on microscopic slides and fixed with absolute methanol on the fifth day. Staining was done using a 10% Giemsa solution at pH 7.2 for 15 minutes. The number of parasitized red blood cells (RBCs) was counted using a microscope. The percentage suppression of parasitemia was calculated; compounds reducing parasitemia by ≥30% were considered as active. Average percent parasitemia and percent parasitemia suppression were computed using the following formula19,20:
Chemo-Prophylactic Activity
Evaluation of prophylactic activity was done as per the methods described in various guidelines.19,21 The grouping and administration were done as described earlier. On the fifth day (D4), a standard inoculum (1 × 107 P berghei–infected red blood cells, 0.2 mL) was given intraperitoneally to each mouse. After 72 hours, the preparation of blood smears was performed from the tail blood. Percentage parasitemia and the percentage of chemo-suppression of parasitemia were computed.
Effect of Extracts on Mean Survival Times
For both models, the mean survival time was established by considering the average number of survival days of the mice in 30 days after administering the parasite. If death occurs before the fifth day in infected and treated mice, the extract was regarded as toxic. The parasitemia level of the animals that survived after 30 days were also established.20–22 The mean survival time of each group was calculated as follows:
Effect of Extracts on Body Weights of Mice
Measurement of body weight of test animals before infection and after the infection was also conducted. Each mouse in a group was measured using a sensitive balance. A comparison of the average bodyweight with control groups was then performed.
Data Analysis
The results obtained during the experiment were articulated as the mean and standard error of the mean. Statistical Package for the Social Sciences (SPSS) Version 20.0 software was used for data analysis. Analysis of variance (ANOVA) was performed, followed by Tukey’s HSD post hoc test to look at the significant differences among and within group on parasitemia suppression, mean survival time, and changes in bodyweight. Data were analyzed at a 95% confidence interval (α = .05), and P value <.05 was taken as statistically significant.
Results
Phytochemical Screening
The phytochemical analysis confirmed the presence of tannins, flavonoids, saponins, polyphenols, terpenoids, glycosides, and anthraquinones, but alkaloids and steroids were not detected (Table 1). The percentage yield of the dried root extracts was 18.82% (w/w).
Table 1.
Results of Phytochemical Screening.
| Tests | Extract |
|---|---|
| Tannins | + |
| Flavonoids | + |
| Saponins | + |
| Polyphenols | + |
| Terpenoids | + |
| Glycosides | + |
| Anthraquinones | + |
| Alkaloids | − |
| Steroids | − |
Abbreviations: +, present; −, absent.
Acute Toxicity Study
Physical and behavioral observations did not show any abnormalities like diarrhea, lacrimation, or any other central nervous system disturbances. Mortality was not observed at a dose of 2000 mg/kg, which indicates that the median lethal dose of the plant extract is greater than 2000 mg/kg.
Four-Day Suppressive Test
This study indicated that the percentages suppression of the methanolic root extracts of the plant was 25.26%, 33.46%, and 51.48% at A100, A200, and A400 of the extract, respectively. The root extracts produced a dose-dependent chemo-suppression activity. The highest suppression of parasitemia was seen at a dose of 400 mg/kg (Table 2).
Table 2.
Chemo-Suppressive Results.
| Group No. | Treatment | Dose (mg/kg/day) | Average % Parasitemia | Average % Parasitemia Suppression |
|---|---|---|---|---|
| Group 1 (negative control) | Vehicle | 0.2 mL | 23.77 ± 2.66 | 0.00 |
| Group 2 (A100) | Extract | 100 mg/kg | 17.55 ± 1.19 | 25.26 ± 4.61a4b4d1e4 |
| Group 3 (A200) | Extract | 200 mg/kg | 15.82 ± 1.06 | 33.46 ± 4.44a4b4c1e4 |
| Group 4 (A400) | Extract | 400 mg/kg | 11.53 ± 1.09 | 51.48 ± 4.61a4b4c4d4 |
| Group 5 (positive control) | Chloroquine | 10 mg/kg | 0.67 ± 0.37 | 97.09 ± 1.59 |
Chemo-Prophylactic Activity
Root extracts also showed a significant (P < .05) chemo-prophylactic activity. The percentages of chemo-prophylaxis at a dose of A100, A200, and A400 of the root extracts were 38.22%, 49.53%, 61.33%, respectively. Dose-dependent chemo-prophylaxis activity was observed as seen in the 4-day suppressive model (Table 3).
Table 3.
Chemo-Prophylactic Results.
| Group No. | Treatment | Dose (mg/kg/day) | Average % Parasitemia | Average % Parasitemia Suppression |
|---|---|---|---|---|
| Group 1 (negative control) | Vehicle | 0.2 mL | 26.96 ± 1.65 | 0.00 |
| Group 2 (A100) | Extract | 100 mg/kg | 20.68 ± 1.16 | 23.31 ± 4.30a4b4d1e4 |
| Group 3 (A200) | Extractact | 200 mg/kg | 18.55 ± 0.79 | 31.20 ± 2.95a4b4c1e3 |
| Group 4 (A400) | Extract | 400 mg/kg | 15.23 ± 1.81 | 43.54 ± 6.70a4b4c4d3 |
| Group 5 (positive control) | Chloroquine | 10 mg/kg | 1.57 ± 0.46 | 94.19 ± 1.69 |
Effect of Extracts on Mean Survival Times of Mice
All mice that received the root extracts significantly live longer than the corresponding negative control groups. However, in the prophylaxis model, only mice that took 200 mg/kg and 400 mg/kg showed a statistically significant rise in mean survival time (Table 4).
Table 4.
Effect on Mean Survival Time.
| Group No. | Treatment | Dose (mg/kg/day) | Mean Survival Time (in Days) | Mean Survival Time (in Days) in the Prophylactic Model |
|---|---|---|---|---|
| Group 1 (negative control) | Vehicle | 0.2 mL | 6.77 ± 1.26 | 6.85 ± 1.03 |
| Group 2 (A100) | Extract | 100 mg/kg | 9.10 ± 0.93a3b4d2e4 | 8.55 ± 0.95a1b4d1e3 |
| Group 3 (A200) | Extract | 200 mg/kg | 10.97 ± 0.81a4b4c2e4 | 8.85 ± 1.36a2b4c1e3 |
| Group 4 (A400) | Extract | 400 mg/kg | 15.90 ± 1.49a4b4c4d4 | 11.43 ± 1.19a4b4c3d3 |
| Group 5 (positive control) | Chloroquine | 10 mg/kg | 28.00 ± 0.00 | 27.28 ± 1.23 |
Effect of Extracts on Body Weight
In both models, the extracts prevented body weight loss as compared to the negative control (Tables 5 and 6).
Table 5.
Result of Root Crude Extract on Body Weight on Suppressive Model.
| Group No. | Treatment | Dose (mg/kg/day) | Weight D0 | Weight D4 | Weight Change % |
|---|---|---|---|---|---|
| Group 1 (negative control) | Vehicle | 0.2 mL | 24.80 ± 0.78 | 22.54 ± 1.27 | −2.43 ± 0.66 |
| Group 2 (A100) | Extract | 100 mg/kg | 24.76 ± 0.98 | 24.52 ± 0.75 | −0.24 ± 0.52a4b1d1e1 |
| Group 3 (A200) | Extract | 200 mg/kg | 24.83 ± 0.85 | 25.05 ± 0.77 | 0.22 ± 0.33a4b1c1e1 |
| Group 4 (A400) | Extract | 400 mg/kg | 24.29 ± 1.19 | 24.41 ± 1.32 | 0.12 ± 0.44a4b1c1d1 |
| Group 5 (positive control) | Chloroquine | 10 mg/kg | 24.29 ± 1.22 | 24.50 ± 1.26 | 0.20 ± 0.45 |
Table 6.
Result of Root Crude Extract on Body Weight on Prophylactic Model*.
| Group No. | Treatment | Dose (mg/kg/day) | Weight D0 | Weight D4 | Weight Change % |
|---|---|---|---|---|---|
| Group 1 (negative control) | Vehicle | 0.2 mL | 24.85 ± 0.81 | 21.93 ± 1.32 | −2.87 ± 1.21 |
| Group 2 (A100) | Extract | 100 mg/kg | 24.69 ± 1.10 | 24.54 ± 0.87 | −0.15 ± 0.63a4b1d1e1 |
| Group 3 (A200) | Extract | 200 mg/kg | 24.27 ± 1.19 | 24.37 ± 0.56 | 0.10 ± 1.03a4b1c1e1 |
| Group 4 (A400) | Extract | 400 mg/kg | 24.28 ± 1.09 | 24.46 ± 0.76 | 0.18 ± 0.93a4b1c1d1 |
| Group 5 (positive control) | Chloroquine | 10 mg/kg | 24.30 ± 1.28 | 24.38 ± 1.05 | 0.18 ± 0.78 |
* a, compared to negative control; b compared to positive control, c to 100 mg/kg, d to 200 mg/kg, e to 400 mg/kg; 1 P > .05 (not significant), 2 P < .05, 3 P < .01, 4 P < .001.
Discussion
There were no observed death and adverse effect at the end of 14 days, after administration of maximum OCED recommended dose 2000 mg/kg body weight. So the median lethal dose of methanolic root extract of this plant is greater than 2000 mg/kg, which agrees with the nontoxic World Health Organization classification of hazardous substances.23
The results of the present study revealed that the root extracts have a statistically significant parasitemia suppression effect in a 4-day suppressive test at all tested doses. A statistically significant difference in antimalarial activity was also observed at different doses of the extract. However, at all tested doses, the root of this plant has much lower parasitemia suppression activity compared to the positive control. The low level of active compound(s) because of the crude nature of the extract is one possible reason. Therefore, based on the in vivo antimalarial activity classification system, the root extracts of the plant has moderate 4-day suppressive antimalarial activity.24 The methanolic root extract of this plant is nontoxic up to the dose of 2000 mg/kg, which agrees with the World Health Organization classification of hazardous substances.23,25
In the prophylaxis model, the hydroalcoholic root extracts exhibited dose-dependent chemo-prophylactic activity against residual infection at all tested doses. Based on the in vivo antimalarial activity classification system, the crude extract also exhibits moderate chemo-prophylactic antimalarial activity.24
The root extracts prolonged the survival time of mice in both 4-day suppressive and chemoprophylaxis test in a dose-dependent way. This might be due to either the parasitemia is suppressed and/or the overall pathologic effect of the parasite is reduced.
At all tested doses, the extracts of the plant significantly prevented weight loss in a dose-dependent way as compared to the negative control. This is because of the suppression of parasitemia level, prevention of anemia, and the overall pathologic effect of the parasite.
The antimalarial activity of the root of the plant may be because of the presence of active secondary metabolites, such as saponins, polyphenols, tannins, anthraquinones, flavonoids, terpenoids, and glycosides. Saponins have proven anti-protozoal activity, possibly because of a deterrent effect on the infected cell membrane of red blood cells and the parasite.26–29 Phenols have good antioxidant activity. Anthraquinones play a role in antimalarial activity by interfering with heme detoxification through free radical hydroxylation and binding with free heme.22,30 Flavonoids inhibit fatty acid synthesis in the parasite.31 The extract may also have various effects on the host cell such as the immunomodulatory, generation of free radicals, changing the selectivity permeable nature of membrane of infected red blood cells, which may affect the entry of essential nutrients like amino acids into the red blood cells in the parasite.
Conclusion
This study indicated that the administration of crude extract of the root of Acanthus polystachyus Delile possesses antimalarial activity as it has promising findings on body weight, survival time, and chemo-suppression. The secondary metabolites identified in the plant may be the possible reasons for the plant’s activity, but further structural and safety studies are essential.
Acknowledgment
The authors would like to thank the laboratory workers of the School of Pharmacy, University of Gondar, for handling and breeding of laboratory animals.
Footnotes
Author Contributions: DD designed the study, performed the statistical analysis, wrote the protocol, and wrote the first draft of the manuscript. MW also participated in the design of the study and wrote the manuscript besides managing the analyses of the study. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Dagninet Derebe  https://orcid.org/0000-0002-2287-6586
https://orcid.org/0000-0002-2287-6586
Muluken Wubetu  https://orcid.org/0000-0003-1727-9231
https://orcid.org/0000-0003-1727-9231
Ethical Approval: This study was ethically approved by research and ethics review board of Gondar University. Experimental animals were handled as per the international animal care and welfare guideline. Ethical clearance was obtained from the Research and Ethics Review Board of Gondar University before commencing the actual research work.
References
- 1. Roll Back Malaria Partnership. Evidence for advocacy: key statistics on the fight against malaria. http://www.makingmalariahistory.org/wp-content/uploads/2015/06/Malaria_Evidence-for-Advocacy_April_2015.pdf. Published March 2015. Accessed November 1, 2019.
- 2. World Health Organization. World Malaria Report. Geneva, Switzerland: World Health Organization; 2014. [Google Scholar]
- 3. Singh S. Current scenario of control of malaria. Trop Parasitol. 2011;1:52–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. The Carter Center’s Malaria Control Program. Summary proceedings: 4th Annual Malaria Control Program Review: Ethiopia and Nigeria. https://www.cartercenter.org/resources/pdfs/news/health_publications/malaria/malaria-summary-proceedings-final-2012.pdf. Published October 2013. Accessed November 1, 2019.
- 5. Federal Democratic Republic of Ethiopia Ministry of Health. National Five-Year Strategic Plan for Malaria Prevention and Control in Ethiopia, 2006-2010 Addis Ababa, Ethiopia: Federal Democratic Republic of Ethiopia Ministry of Health; 2006. [Google Scholar]
- 6. Trivedi PC. Medicinal Plants: Traditional Knowledge. New Delhi, India: IK International; 2006. [Google Scholar]
- 7. Akinjogunla OJ, Adegoke AA, Udokang IP, Adebayo-Tayo BC. Antimicrobial potential of Nymphaea lotus (Nymphaeaceae) against wound pathogens. J Med Plant Res. 2009;3:138–141. [Google Scholar]
- 8. Akinjogunla OJ, Ekoi OH, Odeyemi AT, Odeyemy A. Phytochemical screening and in vitro antibacterial assessment of aqueous leaf extracts of Vernonia amygdalina (Asteraceae) and Ocimum gratissimum (Lamiaceae) on moxifloxacin resistant Escherichia coli isolated from clinical and environmental samples. Nat Sci. 2011;9:12–16. [Google Scholar]
- 9. Qureshi R, Waheed A, Arshad MU, Umbreen T. Medico-ethnobotanical inventory of tehsil Chakwal, Pakistan. Pak J Bot. 2009;41:529–538. [Google Scholar]
- 10. Kassaye KD, Amberbir A, Getachew B, Mussema Y. A historical overview of traditional medicine practices and policy in Ethiopia. Ethiop J Health Dev. 2006;20:127–134. [Google Scholar]
- 11. Asnake S, Teklehaymanot T, Hymete A, Erko B, Giday M. Antimalarial medicinal plants used by Gumuz people of Mandura Woreda, Benishangul-Gumuz Regional State, Ethiopia. Indian J Tradit Knowledge. 2016;15:546–552. [Google Scholar]
- 12. Giday M, Teklehaymanot T, Animut A, Mekonnen Y. Medicinal plants of the Shinasha, Agew-Awi and Amhara peoples in northwest Ethiopia. J Ethnopharmacol. 2007;110:516–525. [DOI] [PubMed] [Google Scholar]
- 13. US National Research Council Institute for Laboratory Animal Research. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academies Press; 1996. [Google Scholar]
- 14. Tiwari P, Kumar B, Kaur M, Kaur G, Kaur H. Phytochemical screening and extraction: a review. Int Pharm Sci. 2011;1:98–106. [Google Scholar]
- 15. Jones WP, Kinghorn AD. Extraction of plant secondary metabolites. Methods Mol Biol. 2012;864:341–366. [DOI] [PubMed] [Google Scholar]
- 16. Otsuka H. Purification by solvent extraction using partition coefficient In: Sarker SD, Latif, Gray AI, eds. Natural Products Isolation. Vol 20 Totowa, NJ: Humana Press; 2005:269–273. [Google Scholar]
- 17. Organization of Economic Cooperation and Development. Guidelines for Testing of Chemicals: Guideline 425: Acute Oral Toxicity. Paris, France: Organization of Economic Cooperation and Development; 2008. [Google Scholar]
- 18. Fidock DA, Rosenthal PJ, Croft SL, Brun R, Nwaka S. Antimalarial drug discovery: efficacy models for compound screening. Nat Rev Drug Discov. 2004;3:509–520. [DOI] [PubMed] [Google Scholar]
- 19. Kalra BS, Chawla S, Gupta P, Valecha N. Screening of antimalarial drugs: an overview. Indian J Pharmacol. 2006;38:5–12. [Google Scholar]
- 20. Mengiste B, Makonnen E, Urga K. In vivo antimalarial activity of Dodonaea angustifolia seed extracts against Plasmodium berghei in mice model. Momona Ethiop J Sci. 2012;4:47–63. [Google Scholar]
- 21. Petros Z, Melaku D. In vivo anti-plasmodial activity of Adhatoda schimperiana leaf extract in mice. Pharmacol Online. 2012;3:95–103. [Google Scholar]
- 22. Geremedhin G, Bisrat D, Asres K. Isolation, characterization and in vivo antimalarial evaluation of anthrones from the leaf latex of Aloe percrassa Todaro. J Nat Remed. 2014;14:119–125. [Google Scholar]
- 23. World Health Organization. The guidebook to the registration of public health pesticides and repellents against vectors: hazard classification-acute LD50 values of formulated products. https://www.nea.gov.sg/docs/default-source/resource/guidebook-for-the-registration-of-public-health-pesticides-and-repellents.pdf. Published July 2015. Accessed November 1, 2019.
- 24. Deharo E, Bourdy G, Quenevo C, Muñoz V, Ruiz G, Sauvain M. A search for natural bioactive compounds in Bolivia through a multidisciplinary approach. Part V. Evaluation of the antimalarial activity of plants used by the Tacana Indians. J Ethnopharmacol. 2001;77:91–98. [DOI] [PubMed] [Google Scholar]
- 25. Nahrevanian H, Milan BS, Kazemi M, et al. Antimalarial effects of Iranian flora Artemisia sieberi on Plasmodium berghei in vivo in mice and phytochemistry analysis of its herbal extracts. Malar Res Treat. 2012;2012:727032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Parekh J, Chanda S. Antibacterial and phytochemical studies on twelve species of Indian medicinal plants. Afr J Biomed Res. 2007;10:175–181. [Google Scholar]
- 27. Bero J, Frédérich M, Quentin-Leclercq J. Antimalarial compounds isolated from plants used in traditional medicine. J Pharm Pharmacol. 2009;61:1401–1433. [DOI] [PubMed] [Google Scholar]
- 28. Onguéné PA, Nite-Kang F, Lifongo LL, Ndom JC, Sippl W, Mbaze LM. The potential of antimalarial compounds derived from African medicinal plants. Part I: a pharmacological evaluation of alkaloids and terpenoids. Malaria J. 2013;12:449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dharani N, Rukunga G, Yenesew A, Jamnadass R. Common Antimalarial Trees and Shrubs of East Africa. A Description of Species and a Guide to Cultivation and Conservation through Use. Nairobi, Kenya: World Agroforestry Centre (ICRAF; ); 2010:73–76. [Google Scholar]
- 30. Newbold CJ, el Hassan SM, Wang J, Ortega ME, Wallace RJ. Influence of foliage from African multipurpose trees on the activity of rumen protozoa and bacteria. Br J Nutr. 1997;78:237–249. [DOI] [PubMed] [Google Scholar]
- 31. Osman CP, Ismail NH. Antiplasmodial anthraquinones from medicinal plants: the chemistry and possible mode of actions. Nat Prod Commun. 2018;13:1591–1597. [Google Scholar]