Abstract
Background:
The humanized anti-α4 integrin monoclonal antibody natalizumab has proven to be very effective in patients with highly active relapsing-remitting multiple sclerosis (MS), but harbors the risk of progressive multifocal leukoencephalopathy (PML). Recently, new therapeutic options have become available for patients with high risk of developing PML while on natalizumab treatment. One of these new therapeutics is the oral synthetic purine analogue cladribine. In order to determine whether therapy with cladribine is effective and safe in patients with MS who previously had been treated with natalizumab, we analyzed clinical, radiological, and laboratory data of 17 patients whose disease modifying treatment (DMT) was switched from natalizumab to cladribine.
Methods:
A total of 17 patients with prior natalizumab treatment were switched to a DMT with cladribine because of a John Cunningham virus (JCV) antibody index above 1.5 (N = 13), ongoing disease activity (N = 6), magnetic resonance imaging (MRI) disease activity (N = 4), or patients preference (N = 2). A chart review and follow up of those patients was performed. In addition to MRI and laboratory data, clinical data regarding MS relapses and disease progression or possible adverse events were analyzed.
Results:
The median duration of cladribine treatment between February 2018 and April 2019 amounted to 9.7 months (range: 1.5–15 months). None of our 17 patients presented with a clinical relapse. Only two patients showed a new T2 lesion on brain MRI, but without any signs of PML. As expected, reduction of lymphocyte count was frequent in cladribine-treated patients, but only four patients exhibited lymphopenia grade 2 (500–800/µl).
Conclusions:
In our cohort the switch from natalizumab to cladribine treatment was effective and safe. So far, no serious adverse events other than lymphopenia have been observed, especially no cases of PML.
Keywords: relapsing-remitting multiple sclerosis, natalizumab, cladribine, progressive multifocal leukoencephalopathy, lymphopenia
Introduction
In recent years major progress has been made in the treatment of multiple sclerosis (MS) with disease-modifying therapies (DMTs) and several new therapeutic agents have been approved. In 2004 natalizumab was granted approval by the US Food and Drug Administration (FDA), and in 2006 it was also released on to the European market. Natalizumab is a humanized anti-α4 integrin monoclonal antibody, which inhibits lymphocyte migration and finally attenuates central nervous system (CNS) inflammation.1 In February 2005 the first cases of progressive multifocal leukoencephalopathy (PML) during natalizumab administration were reported.2 PML is now a well-known, serious adverse event in natalizumab-treated patients. As of 7 March 2019, 814 confirmed cases of PML in natalizumab-treated patients have been reported.3 The individual risk of every patient for developing PML can be classified on the basis of John Cunningham virus (JCV) antibody index, treatment duration, and exposure to immunosuppressive therapies prior to natalizumab administration. It is recommended that natalizumab treatment should be ended after 2 years of therapy and a JCV antibody index above 1.5.4,5
Recently, new therapeutic options have become available for patients with highly active relapsing-remitting MS. These drugs may constitute an alternative for those patients with high risk of developing PML while on natalizumab treatment. One of these new therapeutics is the oral, synthetic purine analogue cladribine which mainly depletes lymphocytes and consecutively induces a dose-dependent reduction of T cells and B cells. It was approved for the therapy of highly active relapsing-remitting MS in Europe in 20176,7 and approved in March 2019 by the FDA. As patients with prior immunomodulating therapies like natalizumab were excluded from clinical trials no data on the safety and efficiency of cladribine treatment in those patients exist so far. With our study we aimed to close this gap by means of a special cohort of patients.
Patients and methods
At the Neurocenter in Barsinghausen, Germany, a total of 17 patients with prior natalizumab treatment were switched to a DMT with cladribine between February 2018 and April 2019. We performed a retrospective follow up of this special cohort to determine how effective and safe the new therapy was.
Treatment change was due to a high JCV antibody index (N = 13), and/or ongoing disease activity (N = 6), and/or magnetic resonance imaging (MRI) disease activity (N = 4), or patients preference (N = 2). Laboratory data, brain MRI, and clinical data including assessment of adverse events and clinical disease activity during cladribine therapy were analyzed. Clinical data were based on the patient’s report during their regular visits. They were interviewed with regard to new symptoms that may have occurred (MS related or not) and the Expanded Disability Status Scale (EDSS) was performed. Laboratory data included measurement of liver and kidney function, blood count, and analysis of the lymphocyte subgroups (CD3+-, CD4+-, CD8+ T-cells, and CD19+ B-cells). All measurements were performed according to the standards of the local laboratory.
Brain MRI examinations were performed using 1.5 Tesla machines and included T1-weighted, T2-weighted, fluid-attenuation inversion recovery, diffusion-weighted sequences, and additionally contrast-enhanced T1-weighted sequences. The last observation of patients took place between 5 March 2019 and 30 April 2019.
Study approval
All patients gave written informed consent for publication and approval of the local ethical standards committee was given (18/2019).
Data availability
MS and EV had full access to all datasets and take full responsibility for the integrity of the data and accuracy of the data analysis. Data will be shared on request from any qualified investigator.
Results
Patient characteristics
The clinical characteristics of all study patients are summarized in Table 1. The cohort consisted of 11 women and 6 men with MS aged between 37 years and 67 years (median age: 53 years). All patients were diagnosed with highly active relapsing-remitting MS, which means at least two or more serious clinical relapses within 1 year or at least two new T2 lesions on brain MRI. Serious clinical relapses were defined as an EDSS value increase of at least 0.5 points with a significant and functional relevant deterioration of pre-existing neurological symptoms or the occurrence of new neurological symptoms with functional relevance. The median duration of illness amounted to 15.3 years (range: 5.5–28 years). On average, they had been treated with two other disease-modifying drugs before natalizumab therapy was initiated. Median duration of natalizumab treatment was 5.6 years (range: 0.96–12.25 years) with a median of 50.4 infusions. A total of 13 out of the 17 patients (76.4%) had a JCV antibody index over 1.5. Two (Clad3 and Clad6) of the four patients exhibiting a JCV antibody index below 1.5 showed disease activity (either a new MRI lesion or new relapse) during natalizumab treatment. The other two patients (Clad1 and Clad15) asked for an oral treatment, so in these two cases the individual patient’s preference rather than increased PML risk or ongoing disease activity was the rationale to switch DMT. While on natalizumab treatment six patients (35.3%) presented with at least one relapse, and four subjects (23.5%) showed MRI activity. In 15 patients (Clad1–12, –15, –16, and Clad17) neutralizing antibodies against natalizumab were tested via enzyme-linked immunosorbent assay (ELISA). None of the tested patients exhibited drug antibodies. In patients Clad13 and Clad14, who had no disease activity during treatment with natalizumab, neutralizing antibodies were not assessed. The average interval between termination of natalizumab treatment and the initiation of cladribine was 16 weeks (range: 9–23 weeks). In most cases the long latency period was not a conscious decision but resulted from different delays, for example due to necessary vaccinations, unscheduled surgery, or infections requiring antibiotic treatment. The cohort’s median EDSS at the start of cladribine was 3.34, ranging from 1.5 points to 6.5 points. Secondary disease progression was excluded via clinical examination including EDSS during quarterly follow-up visits. No patient showed gradual deterioration of symptoms indicating secondary progressive MS. Severe comorbidities were not common within our cohort. The most frequent accompanying diseases were depression (17.6%), degenerative spine alterations (17.6%), and hypothyroidism (11.8%). One woman presented with a positive QuantiFERON(R)-Test but had no clinical or radiological evidence of tuberculosis.
Table 1.
Clinical data and MS history.
| Age (years) | Sex | Years since MS diagnosis | Start date Clad | Duration Nat therapy years (cycles) | Latency Nat-Clad (weeks) | MRI activity during Nat | Number of new T2 (Gd+) lesions (years prior Nat) | Number of relapses last years prior Nat | Annual relapse rate before Nat | DMTs before Nat | Azathioprine yes/ no | JCV antibody index | Comorbidity | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clad1 | 55 | m | 16.5 | Jan 2019 | 5.2 (53) | 14 | No | 1 (0) | 2 | 1.0 | 2 | No | 1.13 | Degenerative LWS syndrome |
| Clad2 | 48 | f | 10 | Apr 2018 | 3.9 (41) | 16 | No | 1 (0) | 2 | 0.83 | 2 | No | 4.55 | Meningioma frontal left |
| Clad3 | 44 | m | 5.5 | Mar 2018 | 3 (30) | 23 | No | NA | 2 | 0.8 | 1 | No | 0.25 | Recurrent depression |
| Clad4 | 67 | f | 18 | Feb 2019 | 12.25 (93) | 17 | No | 0 (0) | 3 | 1.74 | 4 | Yes | 2.58 | Hysterectomy |
| Clad5 | 50 | f | 13 | Mar 2018 | 1.35 (19) | 17 | No | 0 (1) | 1 | 0.17 | 1 | No | 1.58 | |
| Clad6 | 50 | m | 20 | Mar 2018 | 9.38 (74) | 23 | Yes | 0 (0) | 1 | 0.28 | 3 | No | 0.58 | |
| Clad7 | 59 | f | 11 | May 2018 | 4.85 (53) | 17 | Yes | 2 (0) | 2 | 0.65 | 2 | Yes | 2.9 | RLS, recurrent depression, degenerative cervical spine alterations |
| Clad8 | 52 | f | 18 | Jan 2019 | 6.58 (65) | 13 | No | 0 (3) | 0 | 0.26 | 1 | No | 2.56 | |
| Clad9 | 57 | m | 28 | Oct 2018 | 6.75 (65) | 12 | No | NA | 3 | 0.47 | 2 | No | 2.7 | IDDM, hypercholesterolemia |
| Clad10 | 56 | m | 20 | Apr 2018 | 5.33 (54) | 9 | No | NA | 2 | 0.41 | 2 | No | 3.69 | |
| Clad11 | 37 | m | 12 | Apr 2018 | 0.96 (12) | 18 | Yes | 1 (0) | 1 | 0.36 | 4 | No | 1.68 | |
| Clad12 | 48 | f | 22 | Feb 2018 | 10.67 (97) | 9 | Yes | NA | 3 | 1.06 | 2 | Yes | 3.26 | Depression |
| Clad13 | 45 | f | 14 | Jun 2018 | 10 (41) | 12 | No | NA | 3 | 1.25 | 2 | No | 1.79 | Hypothyroidism |
| Clad14 | 47 | f | 11 | Apr 2018 | 1.08 (11) | 20 | No | 0 (1) | 1 | 0.71 | 1 | No | 2.9 | |
| Clad15 | 66 | f | 12 | Jan 2018 | 4.54 (48) | 14 | No | 0 (0) | 1 | 0.54 | 1 | No | 1.03 | |
| Clad16 | 57 | f | 8 | Oct 2018 | 2.83 (28) | 20 | No | 0 (0) | 1 | 1.16 | 1 | No | 2.21 | QuantiFERON test positive (no evidence for tuberculosis), spinal osteochondrosis, sulcus ulnaris syndrome |
| Clad17 | 65 | f | 21 | Mar 2019 | 6.75 (73) | 17 | No | NA | 2 | 0.63 | 2 | Yes | 1.74 | Trigeminal neuralgia, hypothyroidism |
Clinical data for all patients including disease duration, MS disease activity prior to cladribine treatment, JCV antibody index, comorbidities, and latency between natalizumab and cladribine. Clad, cladribine; DMTs, disease modifying therapies; Gd, gadolinium; IDDM, insulin dependent diabetes mellitus; JCV, John Cunningham virus; LWS, MRI, magnetic resonance imaging; MS, multiple sclerosis; NA, not available; Nat, natalizumab; RLS.
Cladribine is effective in patients with prior natalizumab treatment
The first patient whose medication was switched from natalizumab to cladribine started the new treatment 15.5 months ago. So far, six patients have completed at least the first month of the second treatment year and four patients have completely finished the cladribine therapy. The last patient who was included in our cohort received the first cladribine tablet 1.5 months before this analysis. Given this range of 1.5–15.5 months the median follow-up period amounted to 9.7 months (Table 2). During this period, no patient reported symptoms indicating MS disease activity. In one woman and one man (11.8%) a new T2 brain lesion was detected on MRI, while none of the patients exhibited signs of PML (Table 2). In both patients the new lesions occurred within 1 month and 3 months of initiation of cladribine, respectively. Four patients described mild transient complaints after cladribine administration such as headache, vertigo, or hypertension. Until now, no patient has experienced deterioration in EDSS.
Table 2.
Adverse events and disease activity during cladribine treatment.
| Start date Clad | Duration Clad (months) | Adverse events Clad (duration) | MRI activity during Clad (date) | Evidence of PML | Relapse during Clad | EDSS start Clad | Current EDSS | |
|---|---|---|---|---|---|---|---|---|
| Clad1 | 23.01.2019 | 3.25 | None | 1 new lesion; right nucleus lentiformis (08.04.2019) | No | No | 3 | 3 |
| Clad2 | 30.04.2018 | 12 | None | No | No | No | 3 | 3 |
| Clad3 | 23.03.2018 | 13.25 | None | No | No | No | 6 | 6 |
| Clad4 | 11.02.2019 | 2.5 | Vertigo, hypertension (1 week) | No | No | No | 4 | 4 |
| Clad5 | 28.03.2018 | 13 | None | No | No | No | NA | NA |
| Clad6 | 08.03.2018 | 13.75 | Flatulence (1 week) | No | No | No | 2 | 2 |
| Clad7 | 28.05.2018 | 11 | None | No | No | No | 2 | 2 |
| Clad8 | 08.01.2019 | 3.75 | None | 1 new lesion; cortical temporal on the right (28.01.2019) | No | No | 2.5 | 2.5 |
| Clad9 | 01.10.2018 | 7 | None | No | No | No | 4 | 4 |
| Clad10 | 09.04.2018 | 12.75 | Headache (5 days) | No | No | No | 2.5 | 2.5 |
| Clad11 | 28.04.2018 | 12 | None | No | No | No | 1.5 | 1.5 |
| Clad12 | 21.02.2018 | 14.25 | None | No | No | No | 6.5 | 6.5 |
| Clad13 | 11.06.2018 | 10.5 | None | No | No | No | 5 | 4.5 |
| Clad14 | 20.04.2018 | 12.25 | None | No | No | No | 4 | 4 |
| Clad15 | 16.01.2018 | 15.5 | None | No | No | No | 2.5 | 2.5 |
| Clad16 | 08.10.2018 | 6.75 | Acid indigestion (a few days) | No | No | No | 2 | 2 |
| Clad17 | 15.03.2019 | 1.5 | None | No | No | No | 3 | 3 |
Clinical data for all patients with regard to adverse events (including evidence of PML) and disease activity (radiological, clinical) during cladribine therapy. Cut-off date: 30.4.2019. Clad, cladribine; EDSS, Expanded Disability Status Scale; MRI, magnetic resonance imaging; PML, progressive multifocal leukoencephalopathy.
Cladribine proved safe in patients with prior natalizumab treatment
Besides clinical data, results of regular laboratory tests were used to evaluate the safety of cladribine treatment. Figure 1 shows the average lymphocyte count of all individuals at different points in time. Blood counts were assessed before cladribine treatment was started. The follow-up analyses one to four (fu1–4) were performed at 1–3 months (fu1), 4–6 months (fu2), 7–9 months (fu3), or more than 9 months (fu4) after the initiation of cladribine therapy, respectively. The cohort showed the lowest lymphocyte counts at 3–5 months after the start of cladribine. The lowest average value was seen in fu2 (median: 1029/µl), which is a reduction of ‒58% from baseline pretreatment levels (median: 2458/µl). Overall, we did not observe a dramatic decrease in lymphocyte count. One patient exhibited a lymphocyte count of 500/µl 2 months after cladribine treatment was initiated. This was the lowest overall value among all patients. In addition to blood count, transaminases were monitored on a regular basis. No patient exhibited a significant elevation of alanine aminotransferase or aspartate aminotransferase during the follow-up period (data not shown). Except for the transient and mild symptoms briefly after the drug intake such as vertigo or headache (Table 1), no other adverse events have been reported so far. In the context of safety issues, the fact that no patient exhibited radiological signs of PML is of great importance. The timing of brain MRI was slightly variable within our cohort. The majority of patients received a brain MRI every 3–6 months. In Clad3 MRI was performed every 12 months and Clad14 only had sporadic MRI examinations. In comparison with previous years and according to current recommendations since 2018 most MRI examinations have been performed without gadolinium-enhanced scans in case of inconspicuous non-contrast MRI.
Figure 1.
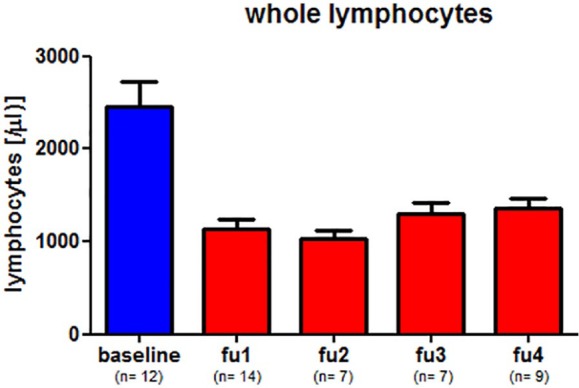
Lymphocyte measurement during cladribine therapy.
Measurement of lymphocyte count in all 17 patients at different points of time. Baseline was defined as lymphocyte count not more than 1.5 months prior to cladribine treatment. The follow-up periods were defined as follows: fu1: 1–3 months after cladribine; fu2: 4–6 months after cladribine; fu3: 7–9 months after cladribine; fu4: > 9 months after cladribine.
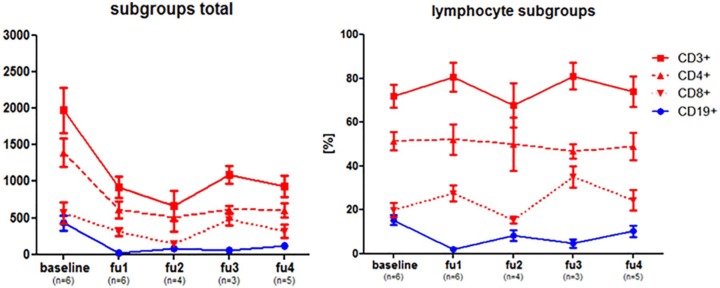
To further investigate cladribine’s mode of action, an analysis of lymphocyte subgroups was performed. Figure 2 shows an exemplary course of five patients (Clad6, Clad9, Clad10, Clad11, Clad12). These five patients were selected as they had the most frequent and most representative laboratory data. Other patients had fewer values, which, however, did not change the general implication of Figure 2. In absolute numbers, CD3+-, CD4+-, CD8+, and CD19+-cells all decreased after baseline. In most cases, T cells (CD3+, as well as CD4+ and CD8+) reached their minimum at fu2, whereas CD19+ cells showed an earlier decrease (Figure 2(a)). Thus, at fu1 the proportion of subgroups was shifted to a higher percentage of T cells (Figure 2(b)). During the course of investigation the proportion of CD19+ cells rose again.
Figure 2.
Analysis of lymphocyte subgroups during cladribine therapy.
Measurement of lymphocyte subgroups of selected patients (Clad3, Clad6, Clad9, Clad10, Clad11, Clad12). In (a) absolute numbers are presented while (b) shows the percentage of CD3+, CD19+, CD4+, and CD8+ cells. Baseline and follow-up periods are as defined in Figure 1.
Discussion
Finding alternative treatments to natalizumab for patients with highly active relapsing-remitting MS and a high JCV antibody index is a topic of great importance. The oral, synthetic purine analogue cladribine is a promising candidate drug. Cladribine is activated by intracellular phosphorylation with deoxycytidine kinase (DCK).8 Since lymphocytes contain a higher ratio of DCK compared with other cell types, they are particularly vulnerable to accumulation of the active metabolite of cladribine and thus for cell death.9 The fact that nonhematologic cells are less susceptible to cladribine might explain the low rate of side effects during treatment with cladribine.10 Within our cohort only four patients presented with transient, nonspecific symptoms after the first cladribine cycle. Serious adverse events did not occur (Table 2).
In line with our findings, cladribine showed a favorable safety profile across all studies.11 As a reflection of cladribine’s mechanism of action10 lymphopenia was more frequent in the cladribine groups (combined cladribine group 27% versus 1.8% in placebo) in the CLARITY trial.6 Among our patients a clear reduction of lymphocytes was observed after cladribine treatment (Figure 1). Lowest levels were reached 3–4 months after start of therapy. Only two patients exhibited lymphopenia of 800/µl and lower, so cladribine proved to be safe regarding this expected side effect. B cells as well as T cells are reduced by cladribine. It is known that lymphocyte reductions following cladribine administration are relatively gradual compared with the rapid decrease after treatment with monoclonal antibodies.12 Additionally, immunophenotyping studies have been performed in 309 CLARITY participants.12 In contrast to our cohort, the mean number of lymphocytes within this study reached its nadir at the end of the second week of treatment (about 5 weeks after initiation of therapy). Our patients who had received previous natalizumab treatment reached their lymphocytes nadir at weeks 12–16 after cladribine start. Baker and colleagues demonstrated that the magnitude and kinetics of depletion are clearly different between T cells and B cells. They observed that the B cell population is depleted to the greatest extent, but with a faster recovery compared with T cells.12 We confirmed these findings with reference to the lymphocyte subpopulation analysis in our patients (Figure 2). CD19 + cells almost completely disappeared at fu1. Afterwards their relative proportion constantly increased. In contrast, CD3+ T cells reached their lowest value at fu2. Magnitude and kinetics of the overall T cell reduction was mirrored by CD4+ T cells. In summary, except for the time of maximum reduction of lymphocytes, the characteristics of the lymphocyte subgroup analysis in our cohort are comparable with data published previously. Thus, prior natalizumab treatment does not seem to affect the impact of cladribine on lymphocytes. It should be noted that latency between the end of natalizumab treatment and induction of cladribine was rather long (average of 16 weeks) (Table 1). Recently, Jacobs and colleagues postulated that especially memory B cells (CD19+ CD27+) are susceptible to cladribine and that cladribine might reduce disease activity in MS by depleting these circulating memory B cells.13 We did not investigate the impact of cladribine on memory B cells in our study, but for subsequent examinations it could serve as a useful tool to discriminate between patients with ongoing disease activity after cladribine administration and patients who remain free of relapse.
Although several weeks elapsed between the end of natalizumab and start of cladribine, none of our patients presented with a clinical relapse in the interval. In general, we recommend a latency period no longer than 8–9 weeks. It is remarkable that among all patients whose treatment latency between natalizumab and cladribine exceeded 12 months (n = 14) none suffered a clinical relapse. One could assume that our cohort consists of patients with very low overall disease activity, however, clinical data before natalizumab treatment do not confirm this assumption (Table 1). Within our cohort not all patients had a stable disease under natalizumab treatment. Four subjects exhibited MRI activity and six patients presented with relapses (Table 1). Except for one woman (Clad8) and one man (Clad1) none of these patients so far has shown disease activity after cladribine induction. In both MRI activity occurred within the first 3 months of the start of cladribine. As lymphocytes reach their lowest level at fu2 (4–6 months after cladribine initiation), it might be assumed that cladribine had not unfolded its full potential when the new MRI lesions emerged. Patient Clad8 had also presented with high MRI activity while on other DMTs. Six patients suffered clinical relapses during natalizumab therapy (Table 1). Thus, cladribine treatment turned out to be effective not only in stable patients, but also in patients with ongoing disease activity during natalizumab therapy. In addition to its efficacy, the absence of indications for a (carry-over) PML in our patients, some of which have been treated with natalizumab for many years, makes cladribine an attractive alternative for patients with highly active relapsing-remitting MS and high JCV antibody index. However, those patients need to be examined conscientiously and their MRI scans must be carefully reviewed prior to the start of cladribine to minimize the risk of a potential carry-over PML.
As an alternative to replacing natalizumab, the administration of natalizumab with extended interval dosing (EID) has been proposed as a strategy potentially to reduce the incidence of PML while maintaining its therapeutic efficacy. Individual retrospective studies14–16 have postulated that therapeutic efficacy of natalizumab is not affected by EID. The fact that EID leads to a reduction of PML incidence has not yet been proven. In our region we have experienced individual cases of patients treated with natalizumab who developed PML despite EID. This is the reason why we strived for a complete change in DMT.
Our study has several limitations. Even though our cohort is unique up to now, the number of patients is limited. However, we think that relevant results concerning a newly approved drug should be published early. As this is a retrospective examination, not all participants have been monitored at exactly the same time points. Moreover, the latency between first cladribine administration and the following blood analyses as well as the number of overall blood tests varied from patient to patient. Our study has an observational character, therefore no control patients were included. This clinical experience demonstrates that a favorable tolerability and convenient handling raises patients’ adherence for taking cladribine tablets, but clinical monitoring is impeded as patients often do not keep their regular appointments.
Conclusion
Patients suffering from highly active relapsing-remitting MS with prior natalizumab treatment and high JCV antibody index need a safe and effective therapeutic alternative. On the basis of a retrospective follow-up study of 17 patients whose DMT was switched from natalizumab to cladribine, we showed that cladribine is not only highly effective, but also does not lead to severe adverse events.
Acknowledgments
EV and MS, the senior authors, contributed equally to the manuscript.
Footnotes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: TS received honoraria for lecturing and travel expenses for attending meetings from Bayer Vital, Biogen, Merck Serono, Novartis, Roche, and Sanofi. KWS received speaker’s fees and/or travel grants from Merck Serono and UCB. SM received honoraria for lecturing from Bayer, Biogen, Celgene, Merck Serono, Novartis, Roche, Teva, and Sanofi-Genzyme and financial research support from Novartis and Roche. EV received honoraria for lecturing from Bayer, Biogen, Celgene, Merck Serono, Novartis, Roche, and Sanofi-Genzyme and financial research support from Novartis and Roche. MS received honoraria for lecturing or consultancy from Alexion Pharmaceuticals, Bayer Healthcare, Biogen, CSL Behring, Grifols, Merck Serono, Novartis, Roche, Sanofi-Genzyme, Takeda, and Teva and research support from Merck Serono and Sanofi-Genzyme.
ORCID iD: Martin Stangel  https://orcid.org/0000-0003-2504-5398
https://orcid.org/0000-0003-2504-5398
Contributor Information
Nora Möhn, Department of Neurology, Hannover Medical School, Hannover, Germany.
Thomas Skripuletz, Department of Neurology, Hannover Medical School, Hannover, Germany.
Kurt-Wolfram Sühs, Department of Neurology, Hannover Medical School, Hannover, Germany.
Sylvia Menck, Neurocenter Barsinghausen, Barsinghausen, Germany.
Elke Voß, Neurocenter Barsinghausen, Barsinghausen, Germany.
Martin Stangel, Clinical Neuroimmunology and Neurochemistry, Department of Neurology, Hannover Medical School, Carl-Neuberg-Str. 1, 30625 Hannover, Germany.
References
- 1. Yednock TA, Steinmant L, Karin Nannon C, et al. Prevention of experimental autoimmune encephalomyelitis by antibodies against α4β1 integrin. Nature 1992; 356: 63–66. [DOI] [PubMed] [Google Scholar]
- 2. Langer-Gould A, Atlas SW, Green AJ, et al. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med 2005; 353: 375–381. [DOI] [PubMed] [Google Scholar]
- 3. Biogen Medinfo. Tysabri safety-management. www.tysabri.de (accessed 28 May 2019).
- 4. Bloomgren G, Richman S, Hotermans C, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med 2012; 366: 1870–1880. [DOI] [PubMed] [Google Scholar]
- 5. Sorensen PS, Bertolotto A, Edan G, et al. Risk stratification for progressive multifocal leukoencephalopathy in patients treated with natalizumab. Mult Scler 2012; 18: 143–152. [DOI] [PubMed] [Google Scholar]
- 6. Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med 2010; 362: 416–426. [DOI] [PubMed] [Google Scholar]
- 7. Giovannoni G. Cladribine to treat relapsing forms of multiple sclerosis. Neurother 2017; 14: 874–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sipe JC. Cladribine for multiple sclerosis: review and current status. Exp Rev Neurother 2005; 5: 721–727. [DOI] [PubMed] [Google Scholar]
- 9. Kawasaki H, Carrera CJ, Piro LD, et al. Relationship of deoxycytidine kinase and cytoplasmic 5′-nucleotidase to chemotherapeutive efficacy of 2-chlorodeoxyadenosine. Blood 1993; 81: 597–601. [PubMed] [Google Scholar]
- 10. Leist TP, Weissert R. Cladribine: mode of action and implications for treatment of multiple sclerosis. Clin Neuropharmacol 2011; 34: 28–35. [DOI] [PubMed] [Google Scholar]
- 11. Sorensen PS, Sellebjerg F. Pulsed immune reconstitution therapy in multiple sclerosis. Ther Adv Neurol Disord. Epub ahead of print 28 March 2019. DOI: 10.1177/1756286419836913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baker D, Herrod SS, Alvarez-Gonzalez C, et al. Both cladribine and alemtuzumab may effect MS via B-cell depletion. Neurol Neuroimmunol Neuroinflamm. Epub ahead of print 5 June 2017. DOI: 10.1212/NXI.0000000000000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jacobs BM, Ammoscato F, Giovannoni G, et al. Cladribine: mechanisms and mysteries in multiple sclerosis. J Neurol Neurosurg Psychiatry 2018; 89: 1266–1271. [DOI] [PubMed] [Google Scholar]
- 14. Zhovtis Ryerson L, Frohman TC, Foley J, et al. Extended interval dosing of natalizumab in multiple sclerosis. J Neurol Neurosurg Psychiatry 2016; 87: 885–889. [DOI] [PubMed] [Google Scholar]
- 15. Bomprezzi R, Pawate S. Extended interval dosing of natalizumab: a two-center, 7-year experience. Ther Adv Neurol Disord 2014; 7: 227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yamout BI, Sahraian MA, et al. Efficacy and safety of natalizumab extended interval dosing. Mult Scler Relat Disord 2018; 24: 113–116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
MS and EV had full access to all datasets and take full responsibility for the integrity of the data and accuracy of the data analysis. Data will be shared on request from any qualified investigator.