Abstract
Background
The aim of the present study was to determine the feasibility and safety of performing diagnostic laparoscopy (DLS) routinely in patients with suspicion of colorectal peritoneal metastases (PM) to evaluate suitability for cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (CRS + HIPEC).
Methods
Data for consecutive patients who underwent DLS between 2012 and 2018 were extracted retrospectively from an institutional database. The primary outcome was the degree of visibility of the abdominal cavity during DLS. Good laparoscopic evaluation of the abdominal cavity was defined as visibility of at least the regions of the diaphragm, pelvis and small bowel. Secondary outcomes were reasons for perioperative exclusion for CRS + HIPEC, major postoperative complications (Clavien–Dindo grade III or above) and difference in overall survival (OS) between patients deemed suitable or unsuitable for CRS + HIPEC. Kaplan–Meier analyses were performed.
Results
Some 184 patients were analysed. Good laparoscopic evaluation was possible in 138 patients (75·0 per cent), and 24 (13·0 per cent) had conversion to an open procedure. Ninety‐three patients (50·5 per cent) were excluded for CRS + HIPEC, most commonly because of absence of colorectal PM (34 patients, 37 per cent) or extensive disease (Peritoneal Cancer Index 20 or above) (33 patients, 35 per cent). Major complications occurred in five patients (2·7 per cent), with no postoperative deaths. Median OS was significantly decreased in patients who were excluded due to extensive disease (14 (95 per cent c.i. 10 to 18) months) compared with patients suitable for CRS + HIPEC (36 (27 to 45) months) (P < 0·001).
Conclusion
Routinely performing DLS in patients with suspicion of colorectal PM to evaluate suitability for CRS + HIPEC is feasible and safe, avoiding the morbidity of an unnecessary laparotomy in patients with extensive disease.
Diagnostic laparoscopy in the preoperative workup for cytoreductive surgery with hyperthermic intraperitoneal chemotherapy is feasible and safe in patients with suspicion of colorectal peritoneal metastases (PM). Exploratory laparotomy can be omitted for patients with extensive disease, who should not undergo unnecessary interventions, or for patients with no colorectal PM.

Laparoscopic evaluation is feasible
Antecedentes
El objetivo del presente estudio fue determinar la viabilidad y seguridad de realizar una laparoscopia diagnóstica (diagnostic laparoscopy, DLS) de rutina en pacientes con sospecha de metástasis peritoneal (peritoneal metastasis, PM) de origen colorrectal para evaluar la idoneidad para la cirugía citorreductora con quimioterapia intraperitoneal hipertérmica (cytoreductive surgery + hyperthermic intraperitoneal chemotherapy, CRS+HIPEC).
Métodos
Los datos de los pacientes consecutivos que fueron sometidos a DLS entre 2012 y 2018 se obtuvieron retrospectivamente de una base de datos institucional. La visualización de al menos las regiones de los diafragmas, pelvis e intestino delgado se definió como una correcta evaluación laparoscópica de la cavidad abdominal. Los resultados secundarios fueron las complicaciones postoperatorias mayores (Clavien‐Dindo grado ≥ III), razones para la exclusión perioperatoria para CRS+HIPEC y diferencia en supervivencia global (overall survival, OS) entre pacientes que se consideraron apropiados y no apropiados para CRS+HIPEC. Se realizaron análisis de Kaplan‐Meier y análisis de riesgos proporcionales.
Resultados
Se analizaron 181 pacientes. En 138 pacientes (75,0%) fue posible una adecuada evaluación laparoscópica, mientras que 24 casos (13%) fueron convertidos a un procedimiento abierto. Se excluyeron 93 (50,5%) pacientes para CRS+HIPEC, más comúnmente por la ausencia de PM colorrectales (36,6%) o enfermedad extensa (37,6%). En cinco pacientes aparecieron complicaciones mayores (2,7%), sin mortalidad postoperatoria. La mediana de la OS disminuyó de forma significativa en pacientes que fueron excluidos debido a enfermedad extensa (14 meses, i.c. del 95% 10‐18) en comparación con pacientes idóneos para CRS+HIPEC (35 meses, i.c. del 95% 30‐40, P < 0,0001).
Conclusión
La realización rutinaria de DLS en pacientes con sospecha de PM de origen colorrectal para evaluar la idoneidad de la CRS+HIPEC es viable y segura. La morbilidad de una laparotomía innecesaria puede prevenirse en pacientes con enfermedad extensa o ausencia de PM colorrectales.
Introduction
Patients with resectable peritoneal metastases (PM) from colorectal cancer can be treated with cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy (CRS + HIPEC)1, 2, 3, 4. This abdominal procedure begins with surgical removal of all visible tumour tissue followed by perfusion of the peritoneal cavity with heated chemotherapy to eliminate remaining microscopic disease5. The most powerful prognostic factors for survival after CRS + HIPEC are the extent of peritoneal disease (measured with the Peritoneal Cancer Index (PCI)) and completeness of the performed cytoreduction (measured with the Completeness of Cytoreduction (CC) score))2, 6, 7, 8. CRS + HIPEC can be performed with curative intent only in patients with colorectal PM with a PCI of less than 20 in whom a (nearly) complete cytoreduction can be achieved (for example CC‐0, no visible residual disease, or CC‐1, residual tumour deposits smaller than 2·5 mm)3, 7, 9, 10, 11.
Current preoperative imaging modalities fail to estimate the PCI in order to predict the possibility of achieving a complete cytoreduction12, 13, 14. Direct visualization of the abdominal cavity and its contents, such as the small bowel, seems to be the only reliable method to assess PCI and tumour resectability. Up to 50 per cent of patients with colorectal PM are excluded for CRS + HIPEC directly on exploratory laparotomy15, 16, 17. Identification at an earlier stage in patients for whom CRS + HIPEC is not suitable could spare them the morbidity of an unnecessary laparotomy.
Direct visualization can also be achieved with diagnostic laparoscopy (DLS), to evaluate the presence and resectability of colorectal PM. Some argue that adhesions from the cancer or previous abdominal surgery impede optimal visualization during DLS, which could result in underestimation of the PCI and an increased rate of intraoperative and postoperative complications. In contrast, seven retrospective studies15, 16, 17, 18, 19, 20, 21 concluded that DLS is a safe, feasible and accurate staging tool for assessing tumour burden in patients with PM. Therefore, several institutions worldwide perform DLS routinely in patients with PM to investigate their presence and resectability. However, current publications on this subject have involved small series of patients with PM from a variety of primary tumour types and, most importantly, DLS was used in a mostly selective way and not incorporated into a standard preoperative workup for CRS + HIPEC.
The aim of the present study was to determine the feasibility and safety of performing DLS routinely in all patients with suspicion of colorectal PM to evaluate suitability for CRS + HIPEC, and to investigate reasons for perioperative exclusion for CRS + HIPEC.
Methods
Data for all consecutive patients with suspicion of colorectal PM, based on recent imaging or a surgical procedure, who had DLS to examine the presence and extent of peritoneal disease between January 2012 and August 2018 were extracted retrospectively from a prospectively maintained institutional database. The study was approved by the Institutional Ethics Committee of University Medical Centre Groningen (METc 201800395).
Preoperative evaluation and staging
All patients had a standard preoperative assessment to confirm the presence of colorectal PM and to evaluate eligibility for CRS + HIPEC. All were staged by thoracic, abdominal and pelvic CT. Patients with suspicion of colorectal PM who might be a candidate for CRS + HIPEC routinely underwent DLS to confirm the diagnosis of colorectal PM and to evaluate resectability of the metastases.
A multidisciplinary team consisting of a radiologist, gastroenterologist, medical oncologist and oncological surgeons then determined eligibility for CRS + HIPEC according to the preoperative assessment. Contraindications to CRS + HIPEC included: moderate or severe co‐morbidity (ASA score above III); extra‐abdominal metastases; massive disease involvement of the small bowel or its mesentery; extensive peritoneal disease (PCI 20 or above); unresectable primary tumour; invasive growth into the retroperitoneal space; and Eastern Cooperative Oncology Group performance status greater than 2. Patients with no colorectal PM during DLS were also excluded from CRS + HIPEC.
Laparoscopic evaluation
Under general anaesthesia, a pneumoperitoneum was established using an optical trocar. The site of first port placement during DLS was based on imaging and clinical findings of the patient. The 30° laparoscope was introduced through an umbilical port. One or two additional operative trocars were positioned on the left and right side of the optical trocar under direct vision. Adhesiolysis was performed minimally. All visible areas of the peritoneal cavity were reviewed systematically. In all patients the laparoscopic PCI and possibility of performing a complete cytoreduction were determined and recorded in the operation report. Cytology samples and biopsies were taken as indicated. When the tumour size was unacceptably large or there was unresectable disease at DLS, palliative surgery was performed at the surgeon's discretion. The main reasons for perioperative exclusion in patients deemed unsuitable for CRS + HIPEC were noted in the medical record.
Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy
Each CRS + HIPEC procedure was started with an exploratory laparotomy. CRS was performed only when the colorectal PM were deemed to be completely resectable, whereas HIPEC was performed only when there was complete or nearly complete cytoreduction. CRS + HIPEC was performed according to the standardized Dutch HIPEC protocol22. The CC score was classified at the end of the cytoreduction: CC‐0, no residual tumour visible or palpable in the peritoneal cavity; CC‐1, residual tumour deposits smaller than 2·5 mm; CC‐2, residual tumour between 2·5 mm and 2·5 cm; and CC‐3, residual tumour larger than 2·5 cm or a confluence of nodules23. After cytoreduction, HIPEC was performed in patients with CC‐0 (complete) or CC‐1 (nearly complete) cytoreduction according to the open Coliseum technique with mitomycin C (35 kg/m2) for 90 min at 40–41°C.
Follow‐up
Physical examination and carcinoembryonic antigen (CEA) measurements were performed on a 3‐monthly basis for at least 4 years. When disease recurrence was suspected (for example, clinical symptoms or increase in CEA level), CT of the thorax and abdomen was performed, with tissue biopsies in selected patients.
Data collection
Data on patient characteristics, tumour characteristics, operative details, postoperative morbidity and mortality, and overall survival (OS) were collected prospectively. Data on perioperative reasons for exclusion for CRS + HIPEC were obtained retrospectively by reviewing the digital medical records.
Primary and secondary outcomes
The primary outcome was the degree of visibility of the abdominal cavity during DLS: grade I, visibility of two or fewer abdominopelvic regions; grade II, visibility of three to eight abdominopelvic regions; grade III, visibility of at least the diaphragm, pelvis and small bowel regions; or grade IV, visibility of all 13 abdominopelvic regions. Grade III or IV was deemed necessary for adequate judgement of the extent of disease, and therefore defined as a good laparoscopic evaluation of the abdominal cavity. Secondary outcomes were the proportion of patients excluded for CRS + HIPEC, perioperative reasons for exclusion for CRS + HIPEC, major postoperative complications, and OS in suitable and unsuitable patients. Major postoperative complications were defined as grade III or above according to the Clavien–Dindo classification system24. OS was defined as the time between DLS and death, or date of last follow‐up in censored cases.
Statistical analysis
All statistical analyses were conducted using SPSS® Statistics version 24.0 (IBM, Armonk, New York, USA). Continuous variables with a normal distribution are given as mean(s.d.) values, and those without a normal distribution as median (i.q.r.) values. Categorical variables are reported as numbers with percentages. Patient and tumour characteristics were compared and analysed using the χ 2 test. The Kruskal–Wallis H test was used for continuous variables. Kaplan–Meier survival analyses were performed to describe OS for the different groups of patients. All tests were performed two‐sided and P < 0·050 was considered statistically significant.
Results
Data for all 184 patients with suspicion of colorectal PM who had undergone DLS between January 2012 and August 2018 were analysed. During DLS, 91 patients (49·5 per cent) were deemed suitable for CRS + HIPEC, and 93 patients (50·5 per cent) were rejected for the procedure. The group of 93 patients deemed unsuitable for CRS + HIPEC was very heterogeneous, and for further analyses was subdivided into the following categories: no indication for CRS + HIPEC because of absence of colorectal PM (29 patients); signs of extensive disease (54); and other reason for perioperative exclusion10.
Table 1 provides an overview of patient and tumour characteristics for the entire cohort, and a comparison of these characteristics between patients suitable for CRS + HIPEC and patients who were not suitable. Patients who were unsuitable for CRS + HIPEC owing to signs of extensive disease presented more frequently with signet ring cell histology compared with those who were suitable for CRS + HIPEC (20 versus 9 per cent respectively; P < 0·001). Patients with no indication for CRS + HIPEC were less likely to have an N2 status than those who were suitable for CRS + HIPEC (11 versus 40 per cent; P = 0·034). The median age of patients who were not suitable for CRS + HIPEC for other reasons was greater than that of patients who were suitable for CRS + HIPEC (74 versus 65 years; P = 0·021). Other baseline characteristics were similar between the four groups of patients.
Table 1.
Baseline characteristics according to suitability for cytoreductive surgery with hyperthermic intraperitoneal chemotherapy
| CRS with HIPEC | P † | |||||
|---|---|---|---|---|---|---|
| Not suitable (n = 93) | ||||||
| No. of patients (n = 184) | Suitable (n = 91) | No indication (n = 29) | Extensive disease (n = 54) | Other reason (n = 10) | ||
| Age (years) * | 65 (58–70) | 65 (54–69) | 64 (56–71) | 67 (60–70) | 74 (63–76) | 0·021‡ |
| Sex ratio (F : M) | 83 : 101 | 45 : 46 | 12 : 17 | 20 : 34 | 6 : 4 | 0·368 |
| BMI (kg/m 2 ) * | 26·3 (24·1–29·0) | 26·5 (24·3–30·4) | 27·2 (25·4–28·3) | 26·2 (3·5–28·6) | 25·9 (23·6–31·3) | 0·811‡ |
| ASA fitness grade | 0·079 | |||||
| I | 22 (12·0) | 10 (11) | 4 (19) | 7 (13) | 1 (10) | |
| II | 139 (75·5) | 72 (79) | 22 (76) | 41 (76) | 4 (40) | |
| III | 23 (12·5) | 9 (10) | 3 (10) | 6 (11·1) | 5 (50) | |
| Co‐morbidity | ||||||
| Diabetes | 19 (10·3) | 8 (9) | 2 (7) | 9 (17) | 0 (0) | 0·238 |
| Cardiovascular disease | 28 (15·2) | 12 (13) | 4 (14) | 9 (17) | 3 (30) | 0·336 |
| Pulmonary disease | 19 (10·3) | 9 (10) | 5 (17) | 4 (7) | 1 (10) | 0·536 |
| Previous surgery for colorectal cancer | 142 (77·2) | 84 (92) | 23 (79) | 27 (50) | 8 (80) | 0·006 |
| Primary tumour | 0·557 | |||||
| Appendix | 9 (4·9) | 7 (8) | 1 (3) | 1 (2) | 0 (0) | |
| Right colon | 68 (37·0) | 27 (30) | 10 (34) | 27 (50) | 4 (40) | |
| Transverse colon | 10 (5·4) | 6 (7) | 0 (0) | 3 (6) | 1 (10) | |
| Left colon | 16 (8·7) | 14 (15) | 1 (3) | 0 (0) | 1 (10) | |
| Sigmoid | 53 (28·8) | 26 (29) | 10 (34) | 14 (26) | 3 (30) | |
| Rectum | 23 (12·5) | 11 (12) | 5 (17) | 7 (13) | 0 (0) | |
| Rectosigmoid | 5 (2·7) | 0 (0) | 2 (7) | 2 (4) | 1 (10) | |
| Signet cell histology | 19 (10·3) | 8 (9) | 0 (0) | 11 (20) | 0 (0) | < 0·001 |
| T category of primary tumour | n = 154 | n = 83 | n = 27 | n = 35 | n = 9 | 0·400 |
| ≤ 3 | 70 (45·5) | 38 (46) | 12 (44) | 14 (40) | 6 (67) | |
| 4 | 84 (54·5) | 45 (54) | 15 (56) | 21 (60) | 3 (33) | |
| N category of primary tumour | n = 151 | n = 83 | n = 27 | n = 32 | n = 9 | < 0·001 |
| 0 | 48 (31·8) | 24 (29) | 14 (52) | 7 (22) | 3 (33) | 0·006 |
| 1 | 46 (30·5) | 26 (31) | 10 (37) | 9 (28) | 1 (11) | 0·157 |
| 2 | 57 (37·7) | 33 (40) | 3 (11) | 16 (50) | 5 (56) | 0·034 |
| Preoperative imaging | ||||||
| CT | 171 (92·9) | 79 (87) | 29 (100) | 54 (100) | 9 (90) | 0·035 |
| MRI | 33 (17·9) | 13 (14) | 7 (24) | 11 (20) | 2 (20) | 0·501 |
| PET | 72 (39·1) | 35 (38) | 15 (52) | 19 (35) | 3 (30) | 0·030 |
| Onset of suspicion of PM | n = 183 | n = 90 | 0·143 | |||
| Synchronous | 99 (54·1) | 41 (46) | 15 (52) | 38 (70) | 5 (50) | |
| Metachronous | 84 (45·9) | 49 (54) | 14 (48) | 16 (30) | 5 (50) | |
| Suspicion of PM based on | 0·796 | |||||
| Preoperative imaging | 130 (70·7) | 53 (58) | 24 (83) | 43 (80) | 8 (80) | |
| Recent surgical procedure | 25 (13·6) | 17 (19) | 2 (7) | 5 (9) | 1 (10) | |
| Perforated tumour | 2 (1·1) | 1 (1) | 1 (3) | 0 (0) | 0 (0) | |
| Preoperative imaging + surgical procedure | 26 (14·1) | 19 (21) | 2 (7) | 6 (11) | 1 (10) | |
| Preoperative imaging + perforated tumour | 1 (0·5) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | |
| Liver metastases | 21 (11·4) | 10 (11) | 1 (3) | 9 (17) | 1 (10) | 0·344 |
| Lung metastases | 5 (2·7) | 1 (1) | 0 (0) | 4 (7) | 0 (0) | 0·090 |
Values in parentheses are percentages unless indicated otherwise;
values are median (i.q.r). CRS, cytoreductive surgery; HIPEC, hyperthermic intraperitoneal chemotherapy; PM, peritoneal metastases.
χ2 test, except
Kruskal–Wallis H test.
Perioperative reasons for exclusion for cytoreductive surgery with hyperthermic intraperitoneal chemotherapy
Table 2 presents an overview of the reasons for perioperative exclusion of the 93 patients (50·5 per cent) deemed unsuitable for CRS + HIPEC. The reasons can be divided into five categories: absence of colorectal PM; signs of extensive disease; patient characteristics; severe complications after DLS; and tumour biology. In the majority of the patients (65 per cent) only one reason resulted in exclusion for CRS + HIPEC, whereas for fewer patients two (28 per cent) or three (8 per cent) reasons led to the exclusion. The most common perioperative reasons for exclusion for CRS + HIPEC were: absence of colorectal PM in 34 patients (37 per cent) and extensive peritoneal disease (PCI 20 or above) in 33 patients (35 per cent). Other signs of extensive disease (widespread colorectal PM in the small bowel, unresectable primary tumour, liver or lung metastases, or an indication for neoadjuvant chemotherapy) were present in 35 patients (38 per cent). Patient characteristics were less frequently the perioperative reason for exclusion for CRS + HIPEC: age in three patients (3 per cent), poor patient condition in three (3 per cent) and presence of severe co‐morbidity in five patients (5 per cent).
Table 2.
Reasons for perioperative exclusion for cytoreductive surgery with hyperthermic intraperitoneal chemotherapy during diagnostic laparoscopy
| No. of patients (n = 93) | |
|---|---|
| No. of reasons reported | |
| 1 | 60 (65) |
| 2 | 26 (28) |
| 3 | 7 (8) |
| No signs of colorectal PM | 34 (37) |
| Signs of extensive disease | |
| PCI > 20 | 33 (35) |
| PCI probably too high during open procedure* | 6 (6) |
| Widespread colorectal PM in bowel/mesentery | 7 (8) |
| Rapid progression of disease | 7 (8) |
| Indication for neoadjuvant therapy | 5 (5) |
| Liver metastases | 4 (4) |
| Lung metastases | 5 (5) |
| Unresectable primary tumour | 1 (1) |
| Patient characteristics | |
| Patient preference | 7 (8) |
| Co‐morbidity | 5 (5) |
| Patient condition | 3 (3) |
| Patient age | 3 (3) |
| Severe complications after DLS | 3 (3) |
| Tumour biology (signet cell histology) | 2 (2) |
Values in parentheses are percentages.
Peritoneal Cancer Index (PCI) during diagnostic laparoscopy (DLS) below 20, but estimated as above 20 during exploratory laparotomy. PM, peritoneal metastases.
Visibility of abdominal cavity during diagnostic laparoscopy
Grade III or IV visibility of the abdominal cavity was possible in 138 of the 184 patients (75·0 per cent) (Table 3). In 24 patients (13·0 per cent) DLS was converted to an open procedure because of an inadequate laparoscopic overview. Grade of visibility of the abdominal cavity during DLS was not significantly different between patients who were suitable and those who were not suitable for CRS + HIPEC due to absence of colorectal PM or extensive disease (P = 0·807). In the small group of patients who were not suitable for CRS + HIPEC for other reasons, the grade of visibility of the abdominal cavity was poor overall (7 of 10, 70 per cent; P = 0·008).
Table 3.
Morbidity and visibility of diagnostic laparoscopy according to suitability for cytoreductive surgery with hyperthermic intraperitoneal chemotherapy
| CRS with HIPEC | P † | |||||
|---|---|---|---|---|---|---|
| Not suitable (n = 93) | ||||||
| No. of patients (n = 184) | Suitable (n = 91) | No indication (n = 29) | Extensive disease (n = 54) | Other reason (n = 10) | ||
| Interval from primary surgery to DLS (months) * | 11 (2–23) | 12 (2–23) | 6 (6–33) | 6 (1–20) | 11 (2–25) | 0·397‡ |
| Interval from suspicion of PM to DLS (months) * | 1 (0–2) | 1 (0–2) | 1 (0–2) | 1 (0–2) | 1 (0–3) | 0·158‡ |
| Grade of visibility | n = 180 | n = 89 | n = 52 | 0·008 | ||
| I (very poor) | 25 (13·8) | 13 (15) | 5 (17) | 4 (8) | 3 (30) | 0·220 |
| II (poor) | 17 (9·4) | 6 (7) | 4 (14) | 3 (6) | 4 (40) | 0·003 |
| III (good) | 23 (12·8) | 11 (12) | 2 (7) | 9 (17) | 1 (10) | 0·623 |
| IV (excellent) | 115 (63·9) | 59 (66) | 18 (62) | 36 (69) | 2 (20) | 0·040 |
| Conversion rate | 24 (13·0) | 15 (16) | 3 (10) | 5 (9) | 1 (10) | 0·398 |
| PCI at DLS | n = 162 | n = 78 | n = 28 | n = 51 | n = 5 | < 0·001 |
| 0–5 | 84 (51·9) | 43 (55) | 28 (100) | 10 (20) | 3 (60) | < 0·001 |
| 6–10 | 23 (14·2) | 19 (24) | 0 (0) | 3 (6) | 1 (20) | 0·006 |
| 11–15 | 11 (6·8) | 9 (12) | 0 (0) | 1 (2) | 1 (20) | 0·100 |
| 16–20 | 14 (8·6) | 7 (9) | 0 (0) | 7 (14) | 0 (0) | 0·397 |
| 21–25 | 13 (8·0) | 0 (0) | 0 (0) | 13 (25) | 0 (0) | < 0·001 |
| > 25 | 17 (10·5) | 0 (0) | 0 (0) | 17 (33) | 0 (0) | < 0·001 |
| Length of hospital stay (days) * | 2 (1–4) | 2 (2–3) | 3 (1–5) | 2 (1–4) | 2 (1–14) | 0·839‡ |
| Clavien–Dindo complication grade | 17 (9·2) | 4 (4) | 4 (14) | 6 (11) | 3 (30) | 0·027 |
| I | 3 (1·6) | 0 (0) | 1 (3) | 2 (4) | 0 (0) | 0·293 |
| II | 7 (3·8) | 4 (4) | 0 (0) | 2 (4) | 1 (10) | 0·516 |
| III | 5 (2·7) | 0 (0) | 1 (3) | 3 (6) | 1 (10) | 0·030 |
| Complication | 0·040 | |||||
| Ileus | 4 (2·2) | 0 (0) | 0 (0) | 3 (6) | 1 (10) | 0·038 |
| Wound infection | 3 (1·6) | 1 (1) | 1 (3) | 0 (0) | 1 (10) | 0·211 |
| Gastroparesis | 1 (0·5) | 0 (0) | 0 (0) | 1 (2) | 0 (0) | 0·490 |
| Bowel perforation | 1 (0·5) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 0·146 |
| Intra‐abdominal abscess | 1 (0·5) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0·795 |
| Urinary tract infection | 1 (0·5) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0·795 |
| Pneumonia | 1 (0·5) | 0 (0) | 0 (0) | 0 (0) | 1 (10) | 0·001 |
| Myocardial infarction | 1 (0·5) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 0·146 |
| Decompensated liver cirrhosis | 1 (0·5) | 0 (0) | 0 (0) | 1 (2) | 0 (0) | 0·490 |
| Electrolyte disorder | 1 (0·5) | 0 (0) | 0 (0) | 1 (2) | 0 (0) | 0·490 |
| Bacteraemia (cause unknown) | 1 (0·5) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0·795 |
| Enterocutaneous fistula | 1 (0·5) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 0·146 |
Values in parentheses are percentages unless indicated otherwise;
values are median (i.q.r). CRS, cytoreductive surgery; HIPEC, hyperthermic intraperitoneal chemotherapy; DLS, diagnostic laparoscopy; PM, peritoneal metastases; PCI, Peritoneal Cancer Index.
χ2 test, except
Kruskal–Wallis H test.
Surgical morbidity and mortality
Table 3 presents postoperative morbidity rates after DLS, by type and severity according to the Clavien–Dindo classification system24. Major postoperative complications occurred in five patients (2·7 per cent), who were all deemed not suitable for CRS + HIPEC. Three patients (1·6 per cent) with symptoms of preoperative obstruction received direct palliative surgery during DLS without any subsequent clinical improvement. In one morbidly obese patient, a widespread haematoma of the abdominal wall was infected after DLS and required surgical evacuation at three different time points. In the fifth patient, myocardial infarction was diagnosed immediately after DLS. Following percutaneous coronary intervention, the patient recovered successfully within 7 days.
Treatment strategies after diagnostic laparoscopy
The different treatments that patients received after DLS are presented in Tables 4 and 5 according to suitability for CRS + HIPEC. Only 75 of the 91 patients (82 per cent) deemed suitable for CRS + HIPEC eventually underwent the full procedure. The remaining 16 patients (18 per cent) had an open–close procedure after exploratory laparotomy (non‐therapeutic laparotomy), due to a high PCI (9 patients), excessive involvement of the small bowel (2), unresectable primary tumour (4) or liver metastases (1). In retrospect, good or excellent laparoscopic evaluation of the abdominal cavity during DLS had been possible in 12 of these 16 patients. In the remaining four patients it was not possible to investigate all abdominopelvic regions but it was estimated that the PCI would probably be below 20.
Table 4.
Treatment received after diagnostic laparoscopy in patients deemed suitable for cytoreductive surgery with hyperthermic intraperitoneal chemotherapy
| No. of patients* (n = 91) | |
|---|---|
| Type of HIPEC | |
| Open CRS + HIPEC | 75 (82) |
| Open–close procedure | 16 (18) |
| Reason for open–close procedure | |
| PCI too high | 9 (56) |
| Small bowel involvement | 2 (13) |
| Unresectable primary tumour | 4 (25) |
| Liver metastases | 1 (6) |
| Total anatomical resection | 4 (2–7) |
| PCI during CRS + HIPEC | |
| 0–5 | 16 (20) |
| 6–10 | 22 (28) |
| 11–15 | 19 (24) |
| 16–20 | 12 (15) |
| 21–25 | 7 (9) |
| > 25 | 4 (5) |
| No. of anastomoses | |
| 0 | 36 (40) |
| 1 | 39 (43) |
| ≥ 2 | 16 (18) |
| Stoma after HIPEC | 40 (44) |
| Stoma type | |
| Double‐barrel ileostomy | 2 (5) |
| Ileostomy | 7 (18) |
| Double‐barrel colonostomy | 3 (8) |
| Colonostomy | 28 (70) |
| Blood loss (ml) † | 600 (200–1188) |
| Duration of surgery (min) † | 471 (370–523) |
| CC score | |
| 0 | 74 (81) |
| 1 | 3 (3) |
| ≥ 2 | 14 (15) |
| Length of hospital stay (days) † | 19 (13–27) |
| Clavien–Dindo complication grade | |
| No complication | 29 (32) |
| I–II | 31 (34) |
| ≥ III | 31 (34) |
| Reoperation | 16 (18) |
| Adjuvant chemotherapy | 26 (29) |
With percentages in parentheses unless indicated otherwise;
values are median (i.q.r.). HIPEC, hyperthermic intraperitoneal chemotherapy; CRS, cytoreductive surgery; PCI, Peritoneal Cancer Index; CC, Completeness of Cytoreduction.
Table 5.
Treatment received after diagnostic laparoscopy in patients deemed unsuitable for cytoreductive surgery with hyperthermic intraperitoneal chemotherapy
| No. of patients (n = 93) | |
|---|---|
| No indication for CRS + HIPEC | n = 29 |
| No/palliative treatment | 7 (24) |
| Systemic chemotherapy | 3 (10) |
| Combined treatments | 5 (17) |
| Curative surgery | 14 (48) |
| Not suitable for CRS + HIPEC due to extensive disease | n = 54 |
| No/palliative treatment | 13 (24) |
| Systemic chemotherapy | 20 (37) |
| Radiotherapy | 2 (4) |
| Combined treatments | 9 (17) |
| Unknown | 10 (19) |
| Not suitable for CRS + HIPEC for other reason | n = 10 |
| No/palliative treatment | 4 (40) |
| Palliative surgery | 2 (20) |
| Systemic chemotherapy | 1 (10) |
| Combined treatments | 1 (10) |
| Unknown | 2 (20) |
Values in parentheses are percentages. CRS, cytoreductive surgery; HIPEC, hyperthermic intraperitoneal chemotherapy.
In patients deemed unsuitable for CRS + HIPEC, treatment strategy depended on the perioperative reason(s) for exclusion (Table 5). Fourteen of the 29 patients (48 per cent) who had a primary tumour in situ with no colorectal PM had surgery with curative intent. Most patients with no primary tumour in situ did not receive any additional treatment (7 patients, 24 per cent). During a median follow‐up of 16 (95 per cent c.i. 14 to 28) months, four of these 29 patients (14 per cent) developed additional colorectal PM, diagnosed in only two patients (7 per cent) within 6 months after DLS.
In the 54 patients unsuitable for CRS + HIPEC with signs of extensive disease, palliative treatment strategies consisted of comfort care (24 per cent), palliative chemotherapy (37 per cent), radiotherapy (4 per cent) or a combination of treatments (17 per cent). The majority of patients who were not suitable for CRS + HIPEC for other reasons received only comfort care (40 per cent).
Survival outcomes
Fig. 1 shows the median OS after DLS between patients who were suitable for CRS + HIPEC and those who were not suitable owing to the absence of colorectal PM, signs of extensive disease, or other reasons for perioperative exclusion. Median OS for patients deemed suitable for CRS + HIPEC was 36 (95 per cent c.i. 27 to 45) months, and that the three subgroups of patients deemed unsuitable was 49 (40 to 60), 14 10, 11, 12, 13, 14, 15, 16, 17, 18 and 24 (9 to 38) months respectively (P < 0·001).
Figure 1.
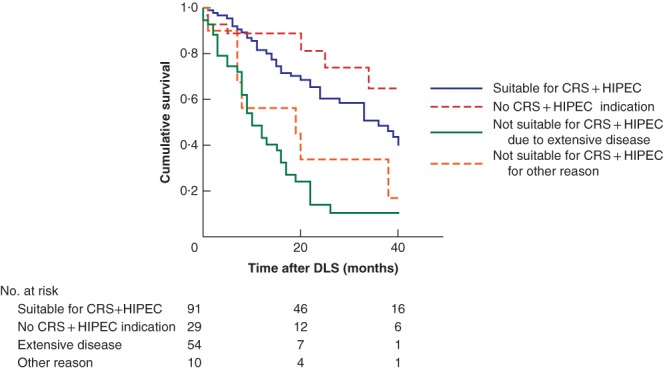
Kaplan–Meier survival curves according to suitability and different reasons for perioperative exclusion for cytoreductive surgery with hyperthermic intraperitoneal chemotherapy CRS, cytoreductive surgery; HIPEC, hyperthermic intraperitoneal chemotherapy; DLS, diagnostic laparoscopy.
Discussion
In this observational study of 184 consecutive patients with suspected colorectal PM, routinely performed laparoscopic evaluation of the abdominal cavity was possible in the majority of patients, with a low risk of major postoperative morbidity. The study demonstrates that patients with extensive disease can be spared an unnecessary laparotomy.
The extent of peritoneal disease (PCI) and the possibility of achieving a complete cytoreduction are the most powerful prognostic factors for survival after CRS + HIPEC, and as current preoperative imaging modalities fail to predict PCI and complete cytoreduction, direct visualization of the abdominal cavity appears to be the only reliable way to assess both prognostic factors. To spare patients the morbidity of a laparotomy, the presence and resectability of colorectal PM could be evaluated by DLS as part of a two‐step approach. In this study, good or excellent laparoscopic evaluation of the abdominal cavity was possible in 75·0 per cent of patients with suspected colorectal PM, despite the fact that 83·7 per cent of these patients had a history of previous abdominal surgery. Major postoperative complications occurred in only five patients (2·7 per cent), with no postoperative deaths.
Comparison of the main results of the present study with those from the seven previously published retrospective studies15, 16, 17, 18, 19, 20, 21 on the value of DLS in the preoperative workup for CRS + HIPEC is challenging. There are striking differences in patient populations, tumour types, definitions of a good laparoscopic evaluation of the abdominal cavity, and the indications for performing DLS or CRS + HIPEC. None of the other studies focused solely on patients with suspicion of PM of colorectal origin; three to nine primary tumour types were included per study. The number of patients with suspected colorectal PM in these studies ranged from 11 to 74. In most studies, it was not possible to subtract the data from patients with colorectal PM from the entire cohort. Only three studies15, 17, 19 made use of DLS as part of a two‐step approach for CRS + HIPEC. In these three studies, complete laparoscopic evaluation according to the PCI scoring system was possible in 73–86 per cent of the patients with PM. DLS resulted in 28–57 per cent of the patients being excluded for CRS + HIPEC. These results are in line with those of the present study. All studies used different definitions of a good laparoscopic evaluation of the abdominal cavity, and three studies gave no definition at all. Only von Breitenbuch and colleagues20 used a definition similar to that used in the present study, resulting in a good laparoscopic evaluation in 88 per cent of patients with no history of previous abdominal surgery and in 70 per cent of those with such a history. Postoperative complication rates from the seven retrospective studies15, 16, 17, 18, 19, 20, 21 ranged between 0 and 2 per cent. These studies included only patients without palliative surgery during DLS, and for this specific group the results of the present study are comparable.
Another important finding of the present study was the unexpectedly high rate (50·5 per cent) of patients who were potential candidates for CRS + HIPEC according to preoperative imaging, but were eventually deemed not suitable for CRS + HIPEC during DLS. On the one hand this reflects the low validity of imaging for colorectal PM to predict the presence and extent of peritoneal disease, and on the other hand it supports the added value of DLS before CRS + HIPEC; almost half of the patients with suspicion of colorectal PM were spared unnecessary laparotomy by performing DLS. Findings in the present study were comparable to those of the other three studies15, 17, 19 that used DLS in a standardized way.
A good laparoscopic evaluation of the abdominal cavity in patients with suspicion of colorectal PM not only allows the exclusion of residual disease and prediction of the likelihood of complete cytoreduction, thereby avoiding an unnecessary laparotomy, but also confers several other advantages. First, DLS allows tissue samples from suspicious lesions to be obtained for analysis or cytological examination. Cytological analysis is gaining in importance, as positive peritoneal cytology seems to be independently associated with a poor median OS compared with negative cytology25. Biopsies from suspicious lesions can confirm the presence or absence of peritoneal disease. For example, in the present cohort, biopsy prevented an unnecessary laparotomy in 34 patients without colorectal PM (37 per cent). Furthermore, biopsies can provide additional information for future systemic therapy or identify a previously unknown primary tumour. Patients who are deemed unsuitable for CRS + HIPEC because of extensive disease can undergo additional systemic or palliative chemotherapy at an earlier stage than patients who are still recovering from a non‐therapeutic laparotomy. In patients who seem suitable for CRS + HIPEC, DLS can provide more detailed information on the burden and location of disease before CRS + HIPEC. This can result in a better informed consent at the outpatient clinic, and may reduce patient anxiety regarding the exact extent of the procedure. Finally, it is also possible during DLS to identify patients who are not fit enough for major surgery.
The present study has some limitations owing to its retrospective design and the fact that all patients came from a single centre. It is possible that the positive results regarding the visibility of the abdominal cavity during DLS were due to extensive experience of the HIPEC surgeons in performing DLS in patients with a history of previous abdominal surgery, and may therefore not be extrapolated to all centres. No patient deemed unsuitable for CRS + HIPEC during DLS underwent an exploratory laparotomy to confirm this assumption. The authors suspect that DLS would understage rather than overstage the extent of peritoneal disease in patients with signs of extensive disease. Therefore, the assumption that a patient is deemed unsuitable for CRS + HIPEC due to extensive disease would probably not change during exploratory laparotomy. However, small peritoneal lesions might be missed during DLS, leading to a false‐negative conclusion. In the present cohort, the likelihood of this appeared to be low, as only two of 29 patients (7 per cent) developed colorectal PM within 6 months after a negative DLS.
Acknowledgements
This study was registered before conducting the research in the authors' institutional research register (UTOPIA, number 201800395). The preregistration adheres to the disclosure requirements of the institutional research registry.
Disclosure: The authors declare no conflict of interest.
Funding information
No funding
References
- 1. Kok NF, de Hingh IHJT. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal metastases of colorectal origin. Br J Surg 2017; 104: 313–315. [DOI] [PubMed] [Google Scholar]
- 2. Verwaal VJ, van Ruth S, Witkamp A, Boot H, van Slooten G, Zoetmulder FA. Long‐term survival of peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol 2005; 12: 65–71. [DOI] [PubMed] [Google Scholar]
- 3. Verwaal VJ, Bruin S, Boot H, van Slooten G, van Tinteren H. 8‐year follow‐up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol 2008; 15: 2426–2432. [DOI] [PubMed] [Google Scholar]
- 4. Baratti D, Kusamura S, Pietrantonio F, Guaglio M, Niger M, Deraco M. Progress in treatments for colorectal cancer peritoneal metastases during the years 2010–2015. A systematic review. Crit Rev Oncol Hematol 2016; 100: 209–222. [DOI] [PubMed] [Google Scholar]
- 5. Sugarbaker PH. Intraperitoneal chemotherapy and cytoreductive surgery for the prevention and treatment of peritoneal carcinomatosis and sarcomatosis. Semin Surg Oncol 1998; 14: 254–261. [DOI] [PubMed] [Google Scholar]
- 6. Glehen O, Kwiatkowski F, Sugarbaker PH, Elias D, Levine EA, De Simone M et al Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi‐institutional study. J Clin Oncol 2004; 22: 3284–3292. [DOI] [PubMed] [Google Scholar]
- 7. Kwakman R, Schrama AM, van Olmen JP, Otten RH, de Lange‐de Klerk ES , de Cuba EM et al Clinicopathological parameters in patient selection for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal cancer metastases: a meta‐analysis. Ann Surg 2016; 263: 1102–1111. [DOI] [PubMed] [Google Scholar]
- 8. Sugarbaker PH. Cytoreductive surgery plus hyperthermic perioperative chemotherapy for selected patients with peritoneal metastases from colorectal cancer: a new standard of care or an experimental approach? Gastroenterol Res Pract 2012; 2012: 309417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. da Silva RG, Sugarbaker PH. Analysis of prognostic factors in seventy patients having a complete cytoreduction plus perioperative intraperitoneal chemotherapy for carcinomatosis from colorectal cancer. J Am Coll Surg 2006; 203: 878–886. [DOI] [PubMed] [Google Scholar]
- 10. Elias D, Gilly F, Boutitie F, Quenet F, Bereder JM, Mansvelt B et al Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol 2010; 28: 63–68. [DOI] [PubMed] [Google Scholar]
- 11. Cavaliere F, De Simone M, Virzì S, Deraco M, Rossi CR, Garofalo A et al Prognostic factors and oncologic outcome in 146 patients with colorectal peritoneal carcinomatosis treated with cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy: Italian multicenter study S.I.T.I.L.O. Eur J Surg Oncol 2011; 37: 148–154. [DOI] [PubMed] [Google Scholar]
- 12. Koh JL, Yan TD, Glenn D, Morris DL. Evaluation of preoperative computed tomography in estimating peritoneal cancer index in colorectal peritoneal carcinomatosis. Ann Surg Oncol 2009; 16: 327–333. [DOI] [PubMed] [Google Scholar]
- 13. Puranik AD, Purandare NC, Agrawal A, Shah S, Rangarajan V. Imaging spectrum of peritoneal carcinomatosis on FDG PET/CT. Jpn J Radiol 2014; 32: 571–578. [DOI] [PubMed] [Google Scholar]
- 14. Esquivel J, Chua TC, Stojadinovic A, Melero JT, Levine EA, Gutman M et al Accuracy and clinical relevance of computed tomography scan interpretation of peritoneal cancer index in colorectal cancer peritoneal carcinomatosis: a multi‐institutional study. J Surg Oncol 2010; 102: 565–570. [DOI] [PubMed] [Google Scholar]
- 15. Iversen LH, Rasmussen PC, Laurberg S. Value of laparoscopy before cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis. Br J Surg 2013; 100: 285–292. [DOI] [PubMed] [Google Scholar]
- 16. Pomel C, Appleyard TL, Gouy S, Rouzier R, Elias D. The role of laparoscopy to evaluate candidates for complete cytoreduction of peritoneal carcinomatosis and hyperthermic intraperitoneal chemotherapy. Eur J Surg Oncol 2005; 31: 540–543. [DOI] [PubMed] [Google Scholar]
- 17. Marmor RA, Kelly KJ, Lowy AM, Baumgartner JM. Laparoscopy is safe and accurate to evaluate peritoneal surface metastasis prior to cytoreductive surgery. Ann Surg Oncol 2016; 23: 1461–1467. [DOI] [PubMed] [Google Scholar]
- 18. Garofalo A, Valle M. Laparoscopy in the management of peritoneal carcinomatosis. Cancer J 2009; 15: 190–195. [DOI] [PubMed] [Google Scholar]
- 19. Jayakrishnan TT, Zacharias AJ, Sharma A, Pappas SG, Gamblin TC, Turaga KK. Role of laparoscopy in patients with peritoneal metastases considered for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC). World J Surg Oncol 2014; 12: 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. von Breitenbuch P, Boerner T, Jeiter T, Piso P, Schlitt HJ. Laparoscopy as a useful selection tool for patients with prior surgery and peritoneal metastases suitable for multimodality treatment strategies. Surg Endosc 2018; 32: 2288–2294. [DOI] [PubMed] [Google Scholar]
- 21. Tabrizian P, Jayakrishnan TT, Zacharias A, Aycart S, Johnston FM, Sarpel U et al Incorporation of diagnostic laparoscopy in the management algorithm for patients with peritoneal metastases: a multi‐institutional analysis. J Surg Oncol 2015; 111: 1035–1040. [DOI] [PubMed] [Google Scholar]
- 22. Kuijpers AM, Mirck B, Aalbers AG, Nienhuijs SW, de Hingh IHJT, Wiezer MJ et al Cytoreduction and HIPEC in the Netherlands: nationwide long‐term outcome following the Dutch protocol. Ann Surg Oncol 2013; 20: 4224–4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sugarbaker PH, Averbach AM, Jacquet P, Stuart OA, Stephens AD. Hyperthermic intraoperative intraperitoneal chemotherapy (HIIC) with mitomycin C. Surg Technol Int 1996; 5: 245–249. [PubMed] [Google Scholar]
- 24. Dindo D, Desmartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240: 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nishikawa T, Sunami E, Tanaka T, Tanaka J, Kiyomatsu T, Kawai K et al Incidence and prognostic significance of positive peritoneal lavage in colorectal cancer. Surg Today 2015; 45: 1073–1081. [DOI] [PubMed] [Google Scholar]


