Abstract
Microglia are resident immune cells that play multiple roles in central nervous system (CNS) development and disease. Although the classical concept of microglia/macrophage activation is based on a biphasic beneficial‐versus‐deleterious polarization, growing evidence now suggests a much more heterogenous profile of microglial activation that underlie their complex roles in the CNS. To date, the majority of data are focused on microglia in gray matter. However, demyelination is a prominent pathologic finding in a wide range of diseases including multiple sclerosis, Alzheimer's disease, and vascular cognitive impairment and dementia. In this mini‐review, we discuss newly discovered functional subsets of microglia that contribute to white matter response in CNS disease onset and progression. Microglia show different molecular patterns and morphologies depending on disease type and brain region, especially in white matter. Moreover, in later stages of disease, microglia demonstrate unconventional immuno‐regulatory activities such as increased phagocytosis of myelin debris and secretion of trophic factors that stimulate oligodendrocyte lineage cells to facilitate remyelination and disease resolution. Further investigations of these multiple microglia subsets may lead to novel therapeutic approaches to treat white matter pathology in CNS injury and disease.
Keywords: demyelination, microglia, oligodendrocyte, white matter damage, white matter repair
1. INTRODUCTION
White matter primarily comprises myelinated axons that connect neurons in various regions of the brain. Filling in nearly half of the human brain,1 growing evidence shows that appropriate myelination in the white matter is required for the development of cognition, memory, motor function, and complex skills.2, 3, 4, 5, 6 Thus damage or abnormalities in white matter can result in various neuronal diseases, such as multiple sclerosis (MS),7, 8, 9 Alzheimer's disease (AD),10, 11, 12 traumatic brain injury (TBI)13, 14, 15 and vascular cognitive impairment and dementia (VCID) including subcortical ischemic vascular dementia (SIVD).12, 16, 17 Moreover, structural changes in white matter or abnormalities in myelin genes are reported to be the risk factors of psychiatric disorders such as depression,18, 19, 20 schizophrenia,21, 22, 23 and obsessive‐compulsive disorder.24, 25, 26 Therefore, appropriate myelination by oligodendrocytes in the white matter is critical for proper development and maintenance of various brain functions.
Clinical observations of these white matter–related diseases have commonly reported activation of microglia. PET studies in MS and postmortem brain section studies from AD patients showed that the increased population of activated microglial cells correlates with their clinical disability.27, 28, 29, 30 Genome‐wide meta‐analysis identified functional pathways influencing AD risk, which included genes related to activation of microglia.31, 32 Experiments on experimental autoimmune encephalomyelitis (EAE), an animal model of MS, have also revealed correlations between microglial and macrophage activation and the disease as their depletion or inactivation resulted in a delay of the disease onset along with decreased severity of clinical symptoms.33, 34, 35 These observations indicate that the change in microglial activation plays a pivotal role in pathophysiology of various white matter–related diseases.
Microglia are resident immune cells that constitute up to 10 ~ 15% of the cells in CNS. These cells contribute to brain homeostasis by surveying their surrounding microenvironment for indicators of injury or infection known as damage‐associated molecular patterns (DAMPs) or pathogen‐associated molecular patterns (PAMPs).36, 37, 38 Upon encountering these signs microglia quickly become activated to clear cellular debris and dead neurons by phagocytosis. In various neurodegenerative diseases, however, they serve as powerful source of pro‐inflammatory molecules cytotoxic to other components of CNS, including neurons and oligodendrocytes, thus contributing to disease initiation and progression.39, 40, 41, 42, 43 Interestingly, unlike the conventional detrimental roles of activated microglia, recent studies have also reported their protective effects including regeneration of myelin in the white matter.44, 45 Therefore, the existence of different microglial subsets and their functional diversity depending on spatiotemporal status of neuronal disorders has risen to be investigated.
In this mini‐review, we survey the diverse microglial changes in white matter during CNS injury and disease, with an emphasis on understanding how these responses affect disease progression and resolution, and the development of potential therapeutic opportunities.
2. DIVERSE ACTIVATION OF MICROGLIA IN WHITE MATTER–RELATED DISEASES
As previously mentioned, microglia are found to be “activated” in various white matter–related diseases in a classical way. As competent presenters of antigen, highly activated microglia express molecules for antigen presentation such as major histocompatibility complex II (MHC II) and its costimulatory factors CD40 and CD86 (also known as B7‐2), classical markers of microglia activation, in MS and AD patients.46, 47, 48, 49 This was also observed in animal model as microglia in EAE mice underwent proliferation and showed increased expression of CD45, MHCII, CD40, and CD86 as well.50 These activated microglia also synthesize a wide range of cytokines, chemokines, cell adhesion glycoproteins, and reactive oxygen radicals, which could be damaging to axons, myelin, and oligodendrocytes.42, 51, 52 Both clinical and animal model studies also revealed that the high proliferation of microglia along with their activation mainly occurred at early stages of the disease particularly at the active sites of demyelination, which was not observed in the later recovery stage.50, 53, 54 These studies demonstrate that classical microglia activation plays critical roles during early stages of MS and AD pathology.
Similar phenomena were observed in ischemic dementia models as well. Inducing white matter lesions in rats by clipping the bilateral carotid arteries to mimic ischemic dementia revealed that microglia and macrophages in white matter showed elevated expression of MHC‐I/II or matrix metalloprotease‐2 (MMP‐2) at 3 days hypoperfusion, indicating their early activation after the onset of cerebral hypoperfusion.55, 56, 57 In mice treated with right unilateral common carotid arteries occlusion (rUCCAO), an animal model for SVID, microglia in the corpus callosum were also found to be activated as the number of ionizing calcium‐binding adaptor molecule 1 (Iba‐1) positive microglia was greatly increased.58 Treatment of carnosine (β‐alanyl‐L‐histidine), a natural dipeptide highly expressed in CNS, resulted in microglial deactivation and ameliorated cognitive impairment and white matter lesion in these mice. These results also support that early classical activation of microglia contributes to demyelination at the early stages of white matter diseases.
Interestingly, recent studies have revealed that microglia activation is not a singular phenotypical change but rather a dynamic response that involves spatially and transcriptionally distinct subpopulations. Mrdjen et al performed high‐dimensional single‐cell mapping in EAE mouse CNS and discovered a unique activation profile of microglia.59 Although they expressed universal microglia activation markers such as CD44, CD86, and programmed death‐ligand 1 (PDL1), activated microglia from EAE mice showed decreased CD14 expression and increased MHC II and stem cell antigen‐1 (Sca‐1) expressions which were unobserved in those from aged or AD mice, suggesting their unique activation status in EAE. More strikingly, multiple subsets of activated microglia were observed even within the same disease model. Hammond et al carried out single‐cell RNA sequencing of microglia from lysolecithin (LPC) injected mice, which triggers focal demyelination of the subcortical white matter to mimic MS.60 This revealed multiple clusters of activated microglia subpopulations with different gene expression profiles in demyelinated lesions. These subsets shared some common gene expressions such as apoE (apolipoprotein E) and yet showed unique expressions as well such as Ccl4 or Cxcl10 depending on the clusters, indicating that microglia can have multiple forms of activation where both generalized and selective transcriptional programs are delicately orchestrated. These findings were able to be translated to human disease as at least one of the subsets shared the similar gene expression profile to the previously discovered microglia group in human MS lesions,61, 62 suggesting that these unique markers of activated microglia could potentially be used as biomarkers or therapeutic targets.
Evidence of different activation status of microglia was also observed in ischemic dementia as well depending on the region within the white matter. Simpson et al investigated the microglia of white matter lesions of aged human brains and discovered that periventricular lesions contained significantly more activated microglia as they expressed MHC II, CD40, and B7‐2 than either control white matter or deep subcortical lesions.63 Although both microglia found in periventricular and subcortical lesions were activated as they both showed high proliferation rate, the morphological difference existed as microglia in periventricular lesion showed ramified and activated shape, whereas those within subcortical lesions had more ameboid and phagocytic phenotype. This may reflect the different types of “activated” microglia subsets depending on the lesion site as previously observed in the mouse MS model.60
Moreover, recent studies have revealed that microglial subtype can be altered depending on the disease pathology. Locatelli et al utilized a real‐time in vivo imaging in EAE mice and observed the evolution of microglia and mononuclear macrophages in a spatiotemporal manner.64 Interestingly, their molecular expression patterns were switched from pro‐inflammatory markers such as inducible nitric oxide synthase (iNOS) to immuno‐regulatory ones including arginase1, suggesting that their subtypes have changed. The highest rates of these conversions were observed in initial lesions and increased over time, peaking during lesion resolution. This finding demonstrates that microglial subtype can be regulated depending on spatial and temporal status of EAE and this transition may affect disease progression and resolution. Another study done in TBI model reported a transition in microglial subtype as well. When TBI was induced in mice by a controlled cortical impact (CCI), microglia and macrophages expressed early immuno‐regulatory phenotype after the impact but was gradually replaced by the pro‐inflammatory phenotype at the site of injury.13 Notably, the severity of white matter injury was strongly correlated with the number of pro‐inflammatory microglia and macrophages, suggesting that this subset could be a possible therapeutic target.
Collectively, these findings suggest that multiple subpopulations of activated microglia exist depending on the disease type or spatiotemporal status of the disease and they contribute to progression and resolution of various white matter–related diseases.
3. MICROGLIAL ACTIVITY IN WHITE VS GRAY MATTER
After white matter damage, clearance of myelin debris from demyelination is critical for oligodendrocyte precursor cell (OPC) recruitment and their maturation into oligodendrocytes.65, 66, 67 Therefore, the phagocytic activity of microglia is important not only for regional clearance but also for efficient remyelination.67, 68, 69 Could this phagocytic activity of microglia be also differentially regulated like its activation status? In normal human brain, white matter contained significantly higher number of microglia and macrophage than gray matter,70 which was also observed in adult rat brain as well.71 Moreover, these microglia and macrophages in the normal human white matter expressed more phagocytosis‐related proteins such as CD68 and CD86 compared to the gray matter,72 indicating that the basal level of microglial phagocytosis is higher in the white matter. After ischemic damage, the microglia within the lesion were all highly activated in both white and gray matter, but during the late scaring phase less population of white matter microglia expressed purinergic receptor P2Y12 (P2RY12), a “resting” marker than the gray matter, suggesting a differential regulation of microglial activity depending on the region of the brain.
Similar results were observed in animal models as well. In TgAPP21 transgenic rats, an AD model, microglial activation and accumulation was significantly stronger in white matter tract including supraventricular corpus callosum (SVCC) than gray matter.73 Notably, this SVCC microglial activation was found to be positively correlated to impaired executive functioning, suggesting that this may contribute to the underlying mechanism of the impairment. Moreover, when mice were fed with cuprizone to induce demyelination especially in the corpus callosum area, microglial cell response was found to be stronger in the white matter than in gray matter as the accumulation of CD107b (Mac3) positive‐activated microglia was significantly higher in corpus callosum than the cortex.74 Interestingly, the cortex layer closer to the corpus callosum contained more activated microglia, suggesting that the high content of myelinated fibers or proximity to white matter may affect the activation and proliferation of microglia.
To investigate how white and gray matter may contribute to this difference, Poel et al performed a transcriptional profiling of microglia from white and gray matter using postmortem tissues of both control and MS donors.75 In both groups, a clear region‐specific profile of microglia was observed, as microglia in gray matter showed higher expression of type‐1 interferon genes, whereas those in white matter showed higher expression of NF‐kB pathway‐related genes. In MS lesions, the increased expression of lipid metabolism‐related genes was observed in white matter microglia, while in gray matter the expression of glycolysis and iron homeostasis‐associated genes were found to be elevated. These differential gene profiles support the difference in microglial subsets between the white and gray matter.
The differential profile of microglia may be due to its surrounding environment. Huizinga et al conditioned mouse macrophage cell line J774.2 by incubating with either gray or white matter from humans and measured their phagocytic activity and found that the number of cells containing myelin or neuronal antigens was significantly higher in cells incubated with white matter.76 Although there may be different mechanisms involved between macrophages and microglia, the finding suggests the existence of white matter‐specific factors that can boost the phagocytic activity. One of the factors could be the OPCs in the white matter, as Liu et al recently discovered that selective OPC depletion using small‐molecule inhibitors of platelet‐derived growth factor (PDGF) signaling abolished the homeostatic microglia signature but did not change their disease‐associated profiles in cultured brain slices.77 Similar findings were also observed in vivo by inducing conditional depletion of OPCs in adult mouse brain, suggesting that OPC has a crucial influence onto cellular states of microglia which are relevant to neurodegenerative diseases. Although a small population of OPCs exists in the gray matter as well,78 they are reported to be mostly quiescent or slowly proliferating,79 whereas OPCs in white matter continuously generate mature and myelinating oligodendrocytes in response to PDGF signaling,79, 80 showing that OPCs have different characteristics depending on its region. These all suggest that white matter OPCs may exhibit specific effects on white matter residing microglia, differentiating them from gray matter residing ones.
Overall, the above findings demonstrate that activities of microglia in white matter could be differentially regulated from gray matter, hence, may result in different outcome in terms of remyelination and disease resolution (Table 1).
Table 1.
Comparison of microglial traits between white and gray matter
| CNS status | Traits | White matter | Gray matter | Reference |
|---|---|---|---|---|
| Basal or normal | Microglial cell number | Higher | Lower | 70, 71 |
| Expression of phagocytic markers | Higher | Lower | 72 | |
| Expression of type‐I interferon genes | Lower | Higher | 75 | |
| Expression of NF‐κB pathway genes | Higher | Lower | 75 | |
| Disease or disease model | Expression of resting markers after ischemic damage | Lower | Higher | 72 |
| Accumulation of activated microglia in AD mouse model | Dramatic increase | Mild increase | 73 | |
| Accumulation of activated microglia after demyelination | Dramatic increase | Mild increase | 74 | |
| Expression of lipid metabolism genes in MS | Elevated | No significant changes | 75 | |
| Expression of genes associated with glycolysis and iron homeostasis in MS | No significant changes | Elevated | 75 | |
| Phagocytic activity when treated to macrophage cell line J774.2 | Dramatic increase | Mild increase | 76 |
Abbreviations: AD, Alzheimer's disease; CNS, central nervous system; MS, multiple sclerosis; NF‐kB, nuclear factor kappa‐light‐chain‐enhancer of activated B cells.
4. MICROGLIA‐DERIVED FACTORS AND OLIGODENDROCYTES LINEAGE CELLS
Aside from its phagocytic activities, multiple studies have shown that activated microglia secrete various factors that directly affect the fate of OPCs and oligodendrocytes, key component cells of white matter homeostasis and repair. Traditionally, activated microglia were considered to secrete pro‐inflammatory factors involved in the initiation and propagation of inflammatory cascade thus promoting demyelination in neural disorders. LPS‐activated microglia, polarized to pro‐inflammatory status, secreted tumor necrosis factor alpha (TNFα) and interleukin‐1beta (IL‐1β), both known to be cytotoxic to oligodendrocytes.81, 82, 83 Indeed, in rat primary oligodendrocyte cultures, co‐treatment of TNFα and cuprizone resulted in a significant decrease in their cell viability.84 When microglial activation was blocked by treatment of minocycline in cuprizone administered mice, the demyelination was prevented suggesting the detrimental roles of microglia‐derived factors on oligodendrocytes. In addition, a positive correlation between nitric oxide (NO2) production by microglia and death of oligodendrocytes was observed in rat cells, suggesting NO2 ‐induced damage as the cytotoxic mechanism for oligodendrocytes.85 In another study, LPS‐activated microglia secreted heat shock protein 60 (HSP60), a stress chaperone protein that has dual functions in cell apoptosis depending on specific conditions. These secreted HSP60 were found to bind to Toll‐like receptor (TLR) 4 of OPCs and initiated its apoptotic mechanism.86
On the other hand, recent studies have revealed protective roles of microglia during the remyelination phase. After myelin damage in MS model, a genome‐wide gene expression analysis was performed for the microglia in corpus callosum during remyelination. A repertoire of cytokines and chemokines such as Cxcl10, Tgfb1, Pdgfa, and Pdgfb were found to be expressed which are known to recruit OPCs to the lesion site and promote their differentiation, thus possibly facilitating the remyelination process.44 Transforming growth factor α (TGFα) was also found to be secreted by microglia after ischemic damage and showed protective roles on OPCs and oligodendrocytes in vitro and contributed to white matter integrity in vivo.87 Moreover, multiple factors including activin A, galectin‐3, and insulin‐like growth factor 1 (IGF1) were produced by microglia which supported oligodendrocyte differentiation in vivo.88, 89, 90 Interestingly, TNFα and IL‐1β, mentioned earlier as oligodendrocyte‐damaging factors secreted by microglia, were found to play beneficial roles in remyelination as well along with other pro‐inflammatory factors such as CXCL13 and endothelin‐2.91, 92, 93 These contrasting effects were driven by time‐dependent expression change of their different receptor subtypes or their promotion of secondary proremyelinating factor secretion in the later phase of disease onset, demonstrating complex roles of microglia in regulating the fate of oligodendrocyte lineage cells. Thus, delicate orchestration of these microglia‐derived factors could play critical roles in white matter disease onset and resolution.
This regulation of secretion factors could be influenced by different activation states of microglia. Miron et al discovered that the microglial and macrophage switch from pro‐inflammatory to immuno‐regulatory occurred over time after inducing focal demyelination by LPC in the mouse corpus callosum. These regulatory populations secreted higher levels of IGF1 and activin A than those of pro‐inflammatory phenotypes and promoted oligodendrocyte differentiation.45 Yu et al94 also reported similar findings as when microglia was polarized to regulatory state by inducing homeobox gene msh‐like homeobox‐3 (Msx3), levels of activin A, and IGF1 mRNAs were increased and facilitated remyelination in mice MS models. Moreover, Wang et al discovered that treatment of histone deacetylase (HDAC) inhibitor scriptaid also triggered the beneficial switch as it resulted in less production of TNFα and NO by microglia and macrophages with increased preservation of co‐cultured oligodendrocytes in vitro and prevented white matter damage in vivo.95 Notably, some microglial subset can be induced by neighboring microglia. When signal regulatory protein α (SIRPα), a membrane protein, was genetically ablated, the number of CD11c + microglia was significantly increased in white matter of the brain.96 Microglia‐specific ablation of SIRPα alleviated cuprizone‐induced demyelination, suggesting that microglial SIRPα suppresses the induction of CD11c + microglia and demyelination damage in white matter, possibly involving indirect roles on oligodendrocyte lineage cells. These studies indicate that depending on the subset of microglia the expression of derived factors can be altered, thus causing contrasting effects on oligodendrocytes.
These findings above demonstrate the untraditional subset of microglia that secretes trophic factors for OPC generation, migration and maturation, revealing the complex nature of microglia population. Altogether, depending on the pathological stage of white matter damage, different subsets of microglia could affect the fate of OPCs and oligodendrocytes by secreting factors of various effects (Figure 1).
Figure 1.
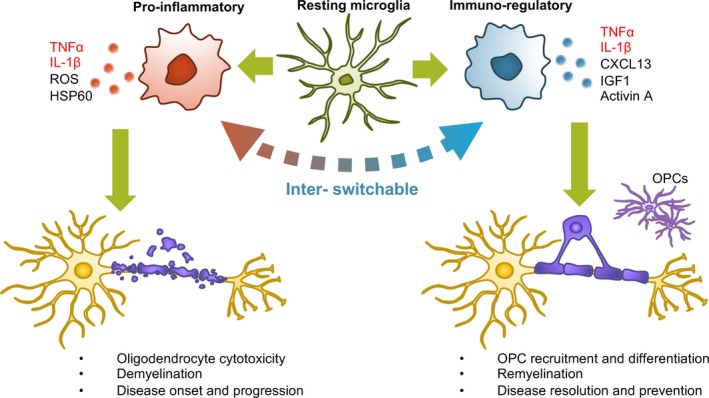
Effects of microglia‐derived factors on oligodendrocyte lineage depending on microglial subsets. A pro‐inflammatory microglial subset secretes cytokines and reactive oxygen species (ROS) that can directly damage oligodendrocytes thus resulting in demyelination. Immuno‐regulatory subsets secrete trophic factors that promote OPC migration and differentiation thus resulting in remyelination. Switching the pro‐inflammatory subset into an immuno‐regulatory subset may represent a potential therapeutic approach for rescuing white matter in CNS injury and disease. Note that microglia‐derived TNFα and IL‐1β can both be cytotoxic or beneficial to oligodendrocyte lineage cells (marked in red). These complex effects are likely to be dependent on signals from neighboring cells and surrounding environmental conditions. Future studies are warranted to investigate how and why identical factors from microglia can show opposite effects on oligodendrocyte damage/recovery
5. POTENTIAL THERAPEUTIC OPPORTUNITIES
Currently, no therapies designed to improve remyelination are approved, especially none targeted directly to microglia.97 However, several drugs undergoing clinical trials to directly enhance OPC differentiation have shown off‐target effects that reduced neuroinflammatory activity of microglia,98 raising the possibility that microglia could be directly targeted as well. In this section, we introduce recent studies that reported potential therapeutics associated with modulation of microglia phenotype that can improve white matter integrity in various injury models.
In mice induced with ischemic stroke model, hypothermia decreased the inflammatory phenotype and increased the immuno‐regulatory type of microglia along with decrease in their total number compared to the control group, which promoted the long‐term integrity of the white matter.99 Similarly, treatment of VK‐28 (5‐[4‐(2‐hydroxyethyl) piperazine‐1‐ylmethyl]‐quinoline‐8‐ol), a brain‐permeable iron chelator to intracerebral hemorrhage model of mice, polarized microglia to immuno‐regulatory subtype and deceased white matter injury.100 Treatment of dimethyl fumarate, a drug known to suppress microglial inflammation, to mice with severe brain hypoperfusion resulted in modest decrease in the number of inflammatory microglia and macrophages and improved functional impairment in the white matter.101 Moreover, in rat TBI model, rats co‐treated with minocycline and N‐acetylcysteine showed altered ratio of pro‐inflammatory vs immuno‐regulatory population of microglia along with increased remyelination and improved cognition and memory.14 Overall, these studies support the idea that directly targeting and converting microglial subtypes could be a novel and efficient therapy for various white matter diseases.
For the microglial phenotype switching strategy to be developed successfully, fully understanding the difference between various microglial subtypes will be beneficial. For instance, if unique biomarkers of specific subset of microglia in certain disease could be validated, specifically ameliorating this subset and maintaining other beneficial population could facilitate the resolution of the disease. As recent single‐cell sequencing studies have already discovered genes that can potentially be used as biomarkers such as Ccl4 or Cxcl10 in MS‐related microglia,60 targeted drug designing could prove to be an efficient strategy minimizing the side effects. Moreover, if the molecular difference between the demyelination and remyelination promoting microglial subsets could fully be understood, finding a way to switch to the beneficial population will also be a novel therapeutic approach. As overexpressing MSX3 polarized microglia into regulatory state and promoted remyelination in mice,94 discovering and validating these “switching factors” is expected to be a first step to develop the microglial phenotype switchig strategy for white matter‐related diseases.
Another potential strategy is the targeted drug delivery to specific brain regions. As activated microglial subpopulation is also diverse depending on their location within the brain, bioengineering of lesion‐specific drug delivery platforms should be considered as well. As several studies have reported that white matter residing microglia tend to have different activation status compared to those of the gray matter, specific delivery of microglia‐targeted drug to white matter could prove to be beneficial to the disease resolution. Moreover, as studies have revealed not only inter‐regional but also intra‐regional differences exist between the microglial subsets, uncovering the region‐specific markers and mechanisms and region‐specific drug delivery could prove to be a novel and efficient strategy. Of course, there is always a risk that phenomena observed in experimental models can often be different from actual human disease pathology. Therefore, the thorough identification of microglia subtypes in cell or animal experimental models must be carefully investigated with clinical samples first to confirm whether they could actually be translated in human disease in order to develop these strategies.
6. CONCLUSIONS AND FUTURE OPPORTUNITIES
Microglia were traditionally considered as deleterious cells in CNS disease. In white matter, excessive microglial activation damages neuronal axons and myelin sheaths. However, an emerging literature now suggests a more nuanced scenario with both damaging and beneficial microglia phenotypes. As discussed in this mini‐review, recent advances in molecular technologies now allow one to detect multiple subsets of microglia within the broadly defined “activated” states. After white matter damage, microglia are activated in multiple forms depending on various conditions such as their proximity to the lesion, location within the brain, and the type of disease. During the acute stage, deleterious microglia promote an inflammatory microenvironment involving antigen presentation and secretion of pro‐inflammatory molecules that trigger demyelination and neuronal damage. During the later recovery period, however, excessive microglial activation is toned down and their phagocytic activity contributes to clearance of myelin debris and dead cells. Immuno‐regulatory microglia also secrete factors that promote OPC migration and their maturation into oligodendrocytes, thus facilitating the remyelination process. Although accumulating evidence of these multiple damaging vs beneficial roles has been reported,102, 103 the mechanisms involved in these contrasting phenomena remain to be fully understood. How can one quantitatively distinguish and map the various microglia phenotypes, and is it possible to manipulate these microglia subsets for therapeutic benefit? After CNS injury and disease, heterogenous populations of activated microglia significantly influence white matter response. Understanding the molecular regulation and functional properties of microglia subsets should contribute to the development of novel therapies for white matter treatments in CNS injury and disease.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
Supported in part by National Institutes of Health. The authors thank Drs. Ryo Ohtomo and Hajime Takase for many helpful discussions.
Lee J, Hamanaka G, Lo EH, Arai K. Heterogeneity of microglia and their differential roles in white matter pathology. CNS Neurosci Ther. 2019;25:1290–1298. 10.1111/cns.13266
REFERENCES
- 1. Fields RD. White matter matters. Sci Am. 2008;298(3):42‐49. [PubMed] [Google Scholar]
- 2. Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. J Cogn Neurosci. 2004;16(7):1227‐1233. [DOI] [PubMed] [Google Scholar]
- 3. Mabbott DJ, Noseworthy M, Bouffet E, et al. White matter growth as a mechanism of cognitive development in children. NeuroImage. 2006;33(3):936‐946. [DOI] [PubMed] [Google Scholar]
- 4. Klingberg T, Vaidya CJ, Gabrieli JD, et al. Myelination and organization of the frontal white matter in children: a diffusion tensor MRI study. NeuroReport. 1999;10(13):2817‐2821. [DOI] [PubMed] [Google Scholar]
- 5. Bengtsson SL, Nagy Z, Skare S, et al. Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci. 2005;8(9):1148‐1150. [DOI] [PubMed] [Google Scholar]
- 6. Barnea‐Goraly N, Menon V, Eckert M, et al. White matter development during childhood and adolescence: a cross‐sectional diffusion tensor imaging study. Cereb Cortex. 2005;15(12):1848‐1854. [DOI] [PubMed] [Google Scholar]
- 7. Steinman L. Multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system. Cell. 1996;85(3):299‐302. [DOI] [PubMed] [Google Scholar]
- 8. Kutzelnigg A, Lucchinetti CF, Stadelmann C, et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain. 2005;128(Pt 11):2705‐2712. [DOI] [PubMed] [Google Scholar]
- 9. Bodini B, Khaleeli Z, Cercignani M, et al. Exploring the relationship between white matter and gray matter damage in early primary progressive multiple sclerosis: an in vivo study with TBSS and VBM. Hum Brain Mapp. 2009;30(9):2852‐2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bartzokis G, Cummings JL, Sultzer D, et al. White matter structural integrity in healthy aging adults and patients with Alzheimer disease: a magnetic resonance imaging study. Arch Neurol. 2003;60(3):393‐398. [DOI] [PubMed] [Google Scholar]
- 11. Roher AE, Weiss N, Kokjohn TA, et al. Increased A beta peptides and reduced cholesterol and myelin proteins characterize white matter degeneration in Alzheimer's disease. Biochemistry. 2002;41(37):11080‐11090. [DOI] [PubMed] [Google Scholar]
- 12. Jang H, Kwon H, Yang JJ, et al. Correlations between gray matter and white matter degeneration in pure alzheimer's disease, pure subcortical vascular dementia, and mixed dementia. Sci Rep. 2017;7(1):9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang G, Zhang J, Hu X, et al. Microglia/macrophage polarization dynamics in white matter after traumatic brain injury. J Cereb Blood Flow Metab. 2013;33(12):1864‐1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haber M, James J, Kim J, et al. Minocycline plus N‐acteylcysteine induces remyelination, synergistically protects oligodendrocytes and modifies neuroinflammation in a rat model of mild traumatic brain injury. J Cereb Blood Flow Metab. 2017;38(8):1312‐1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kinnunen KM, Greenwood R, Powell JH, et al. White matter damage and cognitive impairment after traumatic brain injury. Brain. 2011;134(Pt 2):449‐463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Prins ND, van Dijk EJ, den Heijer T, et al. Cerebral white matter lesions and the risk of dementia. Arch Neurol. 2004;61(10):1531‐1534. [DOI] [PubMed] [Google Scholar]
- 17. Liu CK, Miller BL, Cummings JL, et al. A quantitative MRI study of vascular dementia. Neurology. 1992;42(1):138‐143. [DOI] [PubMed] [Google Scholar]
- 18. Tkachev D, Mimmack ML, Ryan MM, et al. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362(9386):798‐805. [DOI] [PubMed] [Google Scholar]
- 19. Thomas AJ, O'Brien JT, Davis S, et al. Ischemic basis for deep white matter hyperintensities in major depression: a neuropathological study. Arch Gen Psychiatry. 2002;59(9):785‐792. [DOI] [PubMed] [Google Scholar]
- 20. Herrmann LL, Le Masurier M, Ebmeier KP. White matter hyperintensities in late life depression: a systematic review. J Neurol Neurosurg Psychiatry. 2008;79(6):619‐624. [DOI] [PubMed] [Google Scholar]
- 21. Hakak Y, Walker JR, Li C, et al. Genome‐wide expression analysis reveals dysregulation of myelination‐related genes in chronic schizophrenia. Proc Natl Acad Sci USA. 2001;98(8):4746‐4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Davis KL, Stewart DG, Friedman JI, et al. White matter changes in schizophrenia: evidence for myelin‐related dysfunction. Arch Gen Psychiatry. 2003;60(5):443‐456. [DOI] [PubMed] [Google Scholar]
- 23. Douaud G, Smith S, Jenkinson M, et al. Anatomically related grey and white matter abnormalities in adolescent‐onset schizophrenia. Brain. 2007;130(Pt 9):2375‐2386. [DOI] [PubMed] [Google Scholar]
- 24. Stewart SE, Platko J, Fagerness J, et al. A genetic family‐based association study of OLIG2 in obsessive‐compulsive disorder. Arch Gen Psychiatry. 2007;64(2):209‐214. [DOI] [PubMed] [Google Scholar]
- 25. Jenike MA, Breiter HC, Baer L, et al. Cerebral structural abnormalities in obsessive‐compulsive disorder. A quantitative morphometric magnetic resonance imaging study. Arch Gen Psychiatry. 1996;53(7):625‐632. [DOI] [PubMed] [Google Scholar]
- 26. Szeszko PR, Ardekani BA, Ashtari M, et al. White matter abnormalities in obsessive‐compulsive disorder: a diffusion tensor imaging study. Arch Gen Psychiatry. 2005;62(7):782‐790. [DOI] [PubMed] [Google Scholar]
- 27. Allen IV, McQuaid S, Mirakhur M, et al. Pathological abnormalities in the normal‐appearing white matter in multiple sclerosis. Neurol Sci. 2001;22(2):141‐144. [DOI] [PubMed] [Google Scholar]
- 28. Xiang Z, Haroutunian V, Ho L, et al. Microglia activation in the brain as inflammatory biomarker of Alzheimer's disease neuropathology and clinical dementia. Dis Markers. 2006;22(1‐2):95‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Frischer JM, Weigand SD, Guo Y, et al. Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque. Ann Neurol. 2015;78(5):710‐721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hofman FM, von Hanwehr RI, Dinarello CA, et al. Immunoregulatory molecules and IL 2 receptors identified in multiple sclerosis brain. J Immunol. 1986;136(9):3239‐3245. [PubMed] [Google Scholar]
- 31. Jansen IE, Savage JE, Watanabe K, et al. Genome‐wide meta‐analysis identifies new loci and functional pathways influencing Alzheimer's disease risk. Nat Genet. 2019;51(3):404‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Malik M, Parikh I, Vasquez JB, et al. Genetics ignite focus on microglial inflammation in Alzheimer's disease. Mol Neurodegener. 2015;10:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bogie JF, Stinissen P, Hendriks JJ. Macrophage subsets and microglia in multiple sclerosis. Acta Neuropathol. 2014;128(2):191‐213. [DOI] [PubMed] [Google Scholar]
- 34. Chen X, Ma X, Jiang Y, et al. The prospects of minocycline in multiple sclerosis. J Neuroimmunol. 2011;235(1‐2):1‐8. [DOI] [PubMed] [Google Scholar]
- 35. Murphy AC, Lalor SJ, Lynch MA, et al. Infiltration of Th1 and Th17 cells and activation of microglia in the CNS during the course of experimental autoimmune encephalomyelitis. Brain Behav Immun. 2010;24(4):641‐651. [DOI] [PubMed] [Google Scholar]
- 36. Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10(11):1387‐1394. [DOI] [PubMed] [Google Scholar]
- 37. Kettenmann H, Hanisch UK, Noda M, et al. Physiology of microglia. Physiol Rev. 2011;91(2):461‐553. [DOI] [PubMed] [Google Scholar]
- 38. Wake H, Moorhouse AJ, Miyamoto A, et al. Microglia: actively surveying and shaping neuronal circuit structure and function. Trends Neurosci. 2013;36(4):209‐217. [DOI] [PubMed] [Google Scholar]
- 39. Block ML, Zecca L, Hong JS. Microglia‐mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8(1):57‐69. [DOI] [PubMed] [Google Scholar]
- 40. Torreilles F, Salman‐Tabcheh S, Guerin M, et al. Neurodegenerative disorders: the role of peroxynitrite. Brain Res Brain Res Rev. 1999;30(2):153‐163. [DOI] [PubMed] [Google Scholar]
- 41. Liu B, Gao HM, Wang JY, et al. Role of nitric oxide in inflammation‐mediated neurodegeneration. Ann NY Acad Sci. 2002;962:318‐331. [DOI] [PubMed] [Google Scholar]
- 42. Banati RB, Gehrmann J, Schubert P, et al. Cytotoxicity of microglia. Glia. 1993;7(1):111‐118. [DOI] [PubMed] [Google Scholar]
- 43. Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nat Rev Neurol. 2010;6(4):193‐201. [DOI] [PubMed] [Google Scholar]
- 44. Olah M, Amor S, Brouwer N, et al. Identification of a microglia phenotype supportive of remyelination. Glia. 2012;60(2):306‐321. [DOI] [PubMed] [Google Scholar]
- 45. Miron VE, Boyd A, Zhao JW, et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16(9):1211‐1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gobin SJ, Montagne L, Van Zutphen M, et al. Upregulation of transcription factors controlling MHC expression in multiple sclerosis lesions. Glia. 2001;36(1):68‐77. [DOI] [PubMed] [Google Scholar]
- 47. Hoftberger R, Aboul‐Enein F, Brueck W, et al. Expression of major histocompatibility complex class I molecules on the different cell types in multiple sclerosis lesions. Brain Pathol. 2004;14(1):43‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Szpak GM, Lechowicz W, Lewandowska E, et al. Neurones and microglia in central nervous system immune response to degenerative processes. Part 1: Alzheimer's disease and Lewy body variant of Alzheimer's disease. Quantitative study. Folia Neuropathol. 2001;39(3):181‐192. [PubMed] [Google Scholar]
- 49. Lehmann DJ, Wiebusch H, Marshall SE, et al. HLA class I, II & III genes in confirmed late‐onset Alzheimer's disease. Neurobiol Aging. 2001;22(1):71‐77. [DOI] [PubMed] [Google Scholar]
- 50. Ponomarev ED, Shriver LP, Maresz K, et al. Microglial cell activation and proliferation precedes the onset of CNS autoimmunity. J Neurosci Res. 2005;81(3):374‐389. [DOI] [PubMed] [Google Scholar]
- 51. Raivich G, Banati R. Brain microglia and blood‐derived macrophages: molecular profiles and functional roles in multiple sclerosis and animal models of autoimmune demyelinating disease. Brain Res Brain Res Rev. 2004;46(3):261‐281. [DOI] [PubMed] [Google Scholar]
- 52. Minghetti L, Levi G. Microglia as effector cells in brain damage and repair: focus on prostanoids and nitric oxide. Prog Neurogibol. 1998;54(1):99‐125. [DOI] [PubMed] [Google Scholar]
- 53. Matsumoto Y, Ohmori K, Fujiwara M. Microglial and astroglial reactions to inflammatory lesions of experimental autoimmune encephalomyelitis in the rat central nervous system. J Neuroimmunol. 1992;37(1‐2):23‐33. [DOI] [PubMed] [Google Scholar]
- 54. Schonrock LM, Kuhlmann T, Adler S, et al. Identification of glial cell proliferation in early multiple sclerosis lesions. Neuropathol Appl Neurobiol. 1998;24(4):320‐330. [DOI] [PubMed] [Google Scholar]
- 55. Wakita H, Tomimoto H, Akiguchi I, et al. Glial activation and white matter changes in the rat brain induced by chronic cerebral hypoperfusion: an immunohistochemical study. Acta Neuropathol. 1994;87(5):484‐492. [DOI] [PubMed] [Google Scholar]
- 56. Ihara M, Tomimoto H, Kinoshita M, et al. Chronic cerebral hypoperfusion induces MMP‐2 but not MMP‐9 expression in the microglia and vascular endothelium of white matter. J Cereb Blood Flow Metab. 2001;21(7):828‐834. [DOI] [PubMed] [Google Scholar]
- 57. Rosenberg GA, Sullivan N, Esiri MM. White matter damage is associated with matrix metalloproteinases in vascular dementia. Stroke. 2001;32(5):1162‐1168. [DOI] [PubMed] [Google Scholar]
- 58. Ma J, Xiong JY, Hou WW, et al. Protective effect of carnosine on subcortical ischemic vascular dementia in mice. CNS Neurosci Ther. 2012;18(9):745‐753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mrdjen D, Pavlovic A, Hartmann FJ, et al. High‐dimensional single‐cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity. 2018;48(3):599. [DOI] [PubMed] [Google Scholar]
- 60. Hammond TR, Dufort C, Dissing‐Olesen L, et al. Single‐cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell‐state changes. Immunity. 2019;50(1):253‐271.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Szczucinski A, Losy J. Chemokines and chemokine receptors in multiple sclerosis. Potential targets for new therapies. Acta Neurol Scand. 2007;115(3):137‐146. [DOI] [PubMed] [Google Scholar]
- 62. Zrzavy T, Hametner S, Wimmer I, et al. Loss of 'homeostatic' microglia and patterns of their activation in active multiple sclerosis. Brain. 2017;140(7):1900‐1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Simpson JE, Fernando MS, Clark L, et al. White matter lesions in an unselected cohort of the elderly: astrocytic, microglial and oligodendrocyte precursor cell responses. Neuropathol Appl Neurobiol. 2007;33(4):410‐419. [DOI] [PubMed] [Google Scholar]
- 64. Locatelli G, Theodorou D, Kendirli A, et al. Mononuclear phagocytes locally specify and adapt their phenotype in a multiple sclerosis model. Nat Neurosci. 2018;21(9):1196‐1208. [DOI] [PubMed] [Google Scholar]
- 65. Kotter MR, Li WW, Zhao C, et al. Myelin impairs CNS remyelination by inhibiting oligodendrocyte precursor cell differentiation. J Neurosci. 2006;26(1):328‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Syed YA, Baer AS, Lubec G, et al. Inhibition of oligodendrocyte precursor cell differentiation by myelin‐associated proteins. Neurosurg Focus. 2008;24(3‐4):3‐4. [DOI] [PubMed] [Google Scholar]
- 67. Franklin RJ, Ffrench‐Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9(11):839‐855. [DOI] [PubMed] [Google Scholar]
- 68. Kotter MR, Zhao C, van Rooijen N, et al. Macrophage‐depletion induced impairment of experimental CNS remyelination is associated with a reduced oligodendrocyte progenitor cell response and altered growth factor expression. Neurobiol Dis. 2005;18(1):166‐175. [DOI] [PubMed] [Google Scholar]
- 69. Lampron A, Larochelle A, Laflamme N, et al. Inefficient clearance of myelin debris by microglia impairs remyelinating processes. J Exp Med. 2015;212(4):481‐495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Carlson SL, Parrish ME, Springer JE, et al. Acute inflammatory response in spinal cord following impact injury. Exp Neurol. 1998;151(1):77‐88. [DOI] [PubMed] [Google Scholar]
- 71. Savchenko VL, McKanna JA, Nikonenko IR, et al. Microglia and astrocytes in the adult rat brain: comparative immunocytochemical analysis demonstrates the efficacy of lipocortin 1 immunoreactivity. Neuroscience. 2000;96(1):195‐203. [DOI] [PubMed] [Google Scholar]
- 72. Zrzavy T, Machado‐Santos J, Christine S, et al. Dominant role of microglial and macrophage innate immune responses in human ischemic infarcts. Brain Pathol. 2018;28(6):791‐805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Levit A, Regis AM, Gibson A, et al. Impaired behavioural flexibility related to white matter microgliosis in the TgAPP21 rat model of Alzheimer disease. Brain Behav Immun. 2019;80:25‐34. [DOI] [PubMed] [Google Scholar]
- 74. Gudi V, Moharregh‐Khiabani D, Skripuletz T, et al. Regional differences between grey and white matter in cuprizone induced demyelination. Brain Res. 2009;1283:127‐138. [DOI] [PubMed] [Google Scholar]
- 75. van der Poel M, Ulas T, Mizee MR, et al. Transcriptional profiling of human microglia reveals grey‐white matter heterogeneity and multiple sclerosis‐associated changes. Nat Commun. 2019;10(1):1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Huizinga R, van der Star BJ, Kipp M, et al. Phagocytosis of neuronal debris by microglia is associated with neuronal damage in multiple sclerosis. Glia. 2012;60(3):422‐431. [DOI] [PubMed] [Google Scholar]
- 77. Liu Y, Aguzzi A. NG2 glia are required for maintaining microglia homeostatic state. Glia. 2019. 1–11. 10.1002/glia.23721 [DOI] [PubMed] [Google Scholar]
- 78. Dawson MR, Polito A, Levine JM, et al. NG2‐expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci. 2003;24(2):476‐488. [DOI] [PubMed] [Google Scholar]
- 79. Dimou L, Simon C, Kirchhoff F, et al. Progeny of Olig2‐expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J Neurosci. 2008;28(41):10434‐10442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hill RA, Patel KD, Medved J, et al. NG2 cells in white matter but not gray matter proliferate in response to PDGF. J Neurosci. 2013;33(36):14558‐14566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Nakajima K, Kohsaka S, Tohyama Y, et al. Activation of microglia with lipopolysaccharide leads to the prolonged decrease of conventional protein kinase C activity. Brain Res Mol Brain Res. 2003;110(1):92‐99. [DOI] [PubMed] [Google Scholar]
- 82. Merrill JE. Effects of interleukin‐1 and tumor necrosis factor‐alpha on astrocytes, microglia, oligodendrocytes, and glial precursors in vitro. Dev Neurosci. 1991;13(3):130‐137. [DOI] [PubMed] [Google Scholar]
- 83. Selmaj KW, Raine CS. Tumor necrosis factor mediates myelin and oligodendrocyte damage in vitro. Ann Neurol. 1988;23(4):339‐346. [DOI] [PubMed] [Google Scholar]
- 84. Pasquini LA, Calatayud CA, Bertone Una AL, et al. The neurotoxic effect of cuprizone on oligodendrocytes depends on the presence of pro‐inflammatory cytokines secreted by microglia. Neurochem Res. 2007;32(2):279‐292. [DOI] [PubMed] [Google Scholar]
- 85. Merrill JE, Ignarro LJ, Sherman MP, et al. Microglial cell cytotoxicity of oligodendrocytes is mediated through nitric oxide. J Immunol. 1993;151(4):2132‐2141. [PubMed] [Google Scholar]
- 86. Li Y, Zhang R, Hou X, et al. Microglia activation triggers oligodendrocyte precursor cells apoptosis via HSP60. Mol Med Rep. 2017;16(1):603‐608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Dai X, Chen J, Xu F, et al. TGFalpha preserves oligodendrocyte lineage cells and improves white matter integrity after cerebral ischemia. J Cereb Blood Flow Metab. 2019. 1–17. 10.1177/0271678X19830791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Dillenburg A, Ireland G, Holloway RK, et al. Activin receptors regulate the oligodendrocyte lineage in health and disease. Acta Neuropathol. 2018;135(6):887‐906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pasquini LA, Millet V, Hoyos HC, et al. Galectin‐3 drives oligodendrocyte differentiation to control myelin integrity and function. Cell Death Differ. 2011;18(11):1746‐1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hlavica M, Delparente A, Good A, et al. Intrathecal insulin‐like growth factor 1 but not insulin enhances myelin repair in young and aged rats. Neurosci Lett. 2017;648:41‐46. [DOI] [PubMed] [Google Scholar]
- 91. Arnett HA, Mason J, Marino M, et al. TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci. 2001;4(11):1116‐1122. [DOI] [PubMed] [Google Scholar]
- 92. Mason JL, Suzuki K, Chaplin DD, et al. Interleukin‐1beta promotes repair of the CNS. J Neurosci. 2001;21(18):7046‐7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Yuen TJ, Johnson KR, Miron VE, et al. Identification of endothelin 2 as an inflammatory factor that promotes central nervous system remyelination. Brain. 2013;136(Pt 4):1035‐1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Yu Z, Sun D, Feng J, et al. MSX3 Switches microglia polarization and protects from inflammation‐induced demyelination. J Neurosci. 2015;35(16):6350‐6365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wang G, Shi Y, Jiang X, et al. HDAC inhibition prevents white matter injury by modulating microglia/macrophage polarization through the GSK3beta/PTEN/Akt axis. Proc Natl Acad Sci USA. 2015;112(9):2853‐2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Sato‐Hashimoto M, Nozu T, Toriba R, et al. Microglial SIRPalpha regulates the emergence of CD11c(+) microglia and demyelination damage in white matter. Elife. 2019;8:e42025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lloyd AF, Miron VE. The pro‐remyelination properties of microglia in the central nervous system. Nat Rev Neurol. 2019;15(8):447‐458. [DOI] [PubMed] [Google Scholar]
- 98. Samanani S, Mishra M, Silva C, et al. Screening for inhibitors of microglia to reduce neuroinflammation. CNS Neurol Disord Drug Targets. 2013;12(6):741‐749. [DOI] [PubMed] [Google Scholar]
- 99. Liu LQ, Liu XR, Zhao JY, et al. Brain‐selective mild hypothermia promotes long‐term white matter integrity after ischemic stroke in mice. CNS Neurosci Ther. 2018;24(12):1275‐1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Li Q, Wan J, Lan X, et al. Neuroprotection of brain‐permeable iron chelator VK‐28 against intracerebral hemorrhage in mice. J Cereb Blood Flow Metab. 2017;37(9):3110‐3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Fowler JH, McQueen J, Holland PR, et al. Dimethyl fumarate improves white matter function following severe hypoperfusion: Involvement of microglia/macrophages and inflammatory mediators. J Cereb Blood Flow Metab. 2018;38(8):1354‐1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Napoli I, Neumann H. Protective effects of microglia in multiple sclerosis. Exp Neurol. 2010;225(1):24‐28. [DOI] [PubMed] [Google Scholar]
- 103. Czeh M, Gressens P, Kaindl AM. The yin and yang of microglia. Dev Neurosci. 2011;33(3‐4):199‐209. [DOI] [PubMed] [Google Scholar]


