Abstract
The pharmacological targeting of cholesterol levels continues to generate interest due to the undoubted success of therapeutic agents, such as statins, in extending life expectancy by modifying the prognosis of diseases associated with the impairment of lipid metabolism. Advances in our understanding of mitochondrial dysfunction in chronic age‐related diseases of the brain have disclosed an emerging role for mitochondrial cholesterol in their pathophysiology, thus delineating an opportunity to provide mechanistic insights and explore strategies of intervention. This review draws attention to novel signalling mechanisms in conditions linked with impaired metabolism associated with impaired handling of cholesterol and its oxidized forms (oxysterols) by mitochondria. By emphasizing the role of mitochondrial cholesterol in neurological diseases, we here call for novel approaches and new means of assessment.
Linked Articles
This article is part of a themed section on Mitochondrial Pharmacology: Featured Mechanisms and Approaches for Therapy Translation. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v176.22/issuetoc
Abbreviations
- 24‐OHC
24‐hydroxycholesterol
- 25‐OHC
25‐hydroxycholesterol
- 27‐OHC
27‐hydroxycholesterol
- 4α‐OHC
4α‐hydroxycholesterol
- 4β‐OHC
4β‐hydroxycholesterol
- 7‐KC
7‐ketocholesterol
- 7α‐OHC
7α‐hydroxycholesterol
- 7β‐OHC
7β‐hydroxycholesterol
- ACBD1/3
acyl‐CoA‐binding domain‐containing 3
- AD
Alzheimer's disease
- ALS
amyotrophic lateral sclerosis
- APOE4
apolipoprotein isoform 4
- Aβ
amyloid β peptide
- AβPP
amyloid β precursor protein
- ATAD3
ATPase family AAA domain‐containing protein 3
- CoQ10
coenzyme Q10
- CYP11A1
cytochrome P450 family 11 subfamily A member 1
- ER
endoplasmic reticulum
- ER‐MAM
endoplasmic reticulum–mitochondria associated membrane
- IMM
inner mitochondrial membrane
- mChol
mitochondrial cholesterol
- MitoQ
10‐(4,5‐dimethoxy‐2‐methyl‐3,6‐dioxo‐1,4‐cyclohexadien‐1‐yl)decyl triphenylphosphonium methane sulfonate
- MPP+
1‐methyl‐4‐phenylpyridinium
- mtDNA
mitochondrial DNA
- PD
Parkinson's disease
- SkQ1
10‐(6′‐plastoquinonyl)decyltriphenylphosphonium
- StAR
steroidogenic acute regulatory protein
- TSPO
translocator protein
- VDAC1
voltage‐dependent anion selective channel 1
- α‐epoxy C
α‐epoxy cholesterol
- β‐epoxy C
β‐epoxy cholesterol
1. INTRODUCTION
http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2718 has emerged as a keystone lipid in mammalian cellular physiology and pathology since it was identified as a bile solid in gallstones in 1769 by François Poulletier de la Salle. Cholesterol promotes an increase in lipid conformational order thus providing protection to animal cells (Simons & Ikonen, 1997) besides being a precursor for steroid hormones, bile acids, and vitamin D. The insertion of cholesterol into various organellar membranes lends rigidity to these and offers protein‐tethering platforms, such as synaptic lipid rafts or endoplasmic reticulum–mitochondria associated membrane (ER‐MAM) structures (Fujimoto, Hayashi, & Su, 2012; Lajoie, Goetz, Dennis, & Nabi, 2009).
Cholesterol is loaded differently between the diffrent organelles and intracellular compartments. For instance, the plasma membrane contains cholesterol 40‐fold higher than the ER and mitochondria (Horvath & Daum, 2013). In the mitochondria, cholesterol is (a) a structural component of the inner and outer mitochondrial membranes, (b) a precursor of steroidogenesis (of which the first steps are conducted in the mitochondrial lumen), (c) a core to a platform of interaction with ER, lysosomes, and other compartments, and (d) a tethering element for mitochondrial DNA (mtDNA). As a consequence, alterations in mitochondrial cholesterol (mChol) occur in several diseases, including Alzheimer's disease (AD) and other neurodegenerative conditions (Desai et al., 2017; Elustondo, Martin, & Karten, 2017).
The juxtaposition of mChol with the respiratory chain complex where ROS are produced creates ideal conditions for the production of the auto‐oxidative products of cholesterols: the oxysterols (Zerbinati & Iuliano, 2017) which are also implicated in CNS diseases. Some oxysterols are intermediates of cholesterol metabolism, enzymatically transformed into bile acids, steroid hormones, and vitamin D. Auto‐oxidation of cholesterol by ROS also results in the formation of oxysterols implying a pro‐pathological positive feedback which amplifies mitochondrial dysfunction and hence severity of the condition.
http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4352 (7β‐OHC), 7‐ketocholesterol (7‐KC), 5,6‐epoxides, and the secosterols are all produced under oxidative stress. In keeping with this, the levels of several species of oxysterols reflect the degree of pathology in chronic CNS conditions (Zerbinati & Iuliano, 2017). It remains unclear, though, how these processes can be pharmacologically modulated to inform therapeutic protocols or re‐purposing existing cholesterol‐targeting chemicals. Here, we overview our current knowledge of mChol homeostasis and its link to neurodegeneration to generate interest and encourage further exploitation of this lipid in mitochondrial physiopathology.
2. ROLE OF CHOLESTEROL IN MITOCHONDRIAL DNA MAINTENANCE
mtDNA is associated with nucleic acid‐binding proteins forming complexes known as nucleoids (Spelbrink, 2010). Mutations in mtDNA and in nuclear‐encoded mitochondrial genes cause primary mitochondrial diseases (Gorman et al., 2016). Cholesterol‐rich patches in the mitochondrial inner membrane tether the mtDNA to the inner mitochondrial membrane (IMM) via nucleoprotein complexes called nucleoids. These patches and their components enable mtDNA processing, protein synthesis, and replication (Gerhold et al., 2015; He et al., 2007). While the lipid composition of mitochondria has been described (Fleischer, Rouser, Fleischer, Casu, & Kritchevsky, 1967), dynamics of distribution and regulation remain fairly unexplored. Mitochondria are “cholesterol‐poor” organelles with a cholesterol to phospholipid ratio as low as 0.1 (van Meer, Voelker, & Feigenson, 2008). This low level of cholesterol is unlikely to form classical lipid rafts with close association with sphingolipids (Zheng, Berg, & Foster, 2009). However, from what is known about the behaviour of bilayer membranes and lipid movement, it can be inferred that cholesterol is restricted to nanodomains in the tight curvatures of the IMM (Rukmini, Rawat, Biswas, & Chattopadhyay, 2001). This implies that although mitochondria are cholesterol‐poor, that cholesterol is vital to the function of the organelles. The majority of primary mitochondrial diseases caused by mutations in the mtDNA are associated with neurological deficits, ranging from mild ataxia to severe early onset of neurodegeneration (Carelli & La Morgia, 2018). Explorations into the mChol‐modulating proteins suggest that they directly affect mtDNA, and hence, associated mutations cause severe primary mitochondrial disorders.
One of the components of the nucleoprotein complex associated with mtDNA is the protein, ATPase family AAA domain‐containing protein 3 (ATAD3), which has mtDNA‐binding properties (He et al., 2007; He et al., 2012). ATAD3 affects the rate of steroidogenesis by facilitating cholesterol transport from the ER to mitochondria (He et al., 2007; He et al., 2012; Issop et al., 2015). Mutations in the ATAD3 family of proteins, which alter cholesterol metabolism, cause severe neurodegeneration, mitochondrial cristae defects, and impaired mtDNA segregation (Desai et al., 2017; Peralta et al., 2018). Pharmacologically, interfering with cholesterol shuttling in ATAD3 deficient human fibroblasts via U18666A or by altering cholesterol biosynthesis via statins (e.g., http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2953) results in exacerbated mtDNA de‐segregation. Aggregation and disorganization imbalance are also observed in the Niemann–Pick type C disorder, further supporting the critical role of cholesterol inserts in mitochondria by controlling the tuned segregation of the organelle DNA (Desai et al., 2017).
More recently, it has been also shown that deficiency in ATAD3 affects the formation of mitochondrial cristae (Peralta et al., 2018) suggesting that the optimum level of cholesterol inserts into the IMM is equally crucial in maintaining membrane structure and mtDNA integrity. Dysregulation of mChol may therefore result in primary mitochondrial dysfunction perturbing mtDNA homeostasis leading to deficits in the energy balance (Figure 1).
Figure 1.
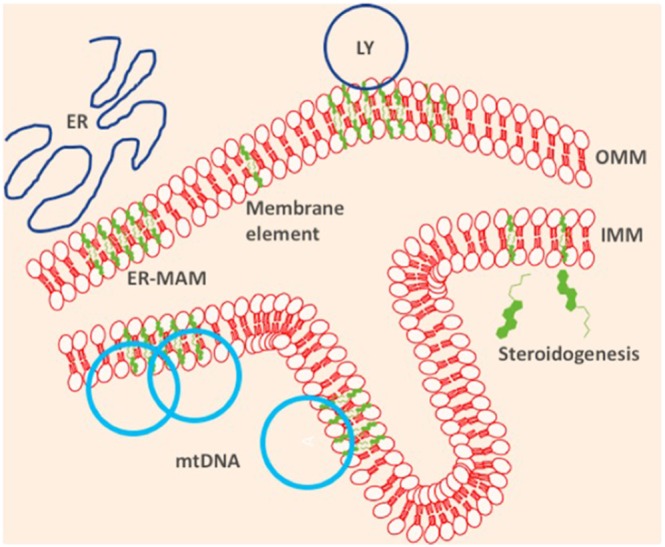
Cholesterol (green) serves many purposes in the mitochondria (a) as a structural component of the inner and outer mitochondrial membrane (OMM, IMM); (b) as a precursor of steroidogenesis, of which the first steps are conducted in the mitochondrial lumen; (c) as providing the platform for inter‐organellar interaction with endoplasmic reticulum (ER), lysosomes (LY), and other intracellular compartments, and (d) as the tether for mitochondrial DNA (mtDNA)
3. MITOCHONDRIAL CHOLESTEROL AND OXYSTEROLS IN NEURODEGENERATION
Several studies have reported dysregulated cholesterol metabolism in AD, and the E4 variant of cholesterol gene apolipoprotein E (APOE) is a common risk factor for familiar AD (as comprehensively reviewed in Arenas, Garcia‐Ruiz, & Fernandez‐Checa, 2017). However, less is understood about mChol in the disease. In a model of hypercholesterolaemia, where mice deficient in the LDL receptor ((LDLr−/−) are fed a high cholesterol diet, the mice develop cholesterol loading in the mitochondria and subsequent cognitive deficiencies and AD‐mimicking neurodegeneration. The cerebral cortex of LDLr−/− mice fed with cholesterol‐enriched diet showed (a) a decrease in the activities of mitochondrial complexes I and II and glutathione levels and (b) an imbalance between the peroxide‐removing‐related enzymes, GSH peroxidase and GSH reductase (de Oliveira et al., 2011).
Del Prete et al. studied a mutant form of amyloid β precursor protein (AβPP) and found that there was an increased incidence of the endoplasmic reticulum–mitochondria associated membrane (ER‐MAM) structures, which in turn captured more of the secretase‐processed metabolites of the mutant AβPP in this micro‐region, thus interfering with MAM functions (Del Prete et al., 2017). This adds to the growing evidence that ER‐MAM interactions are key platforms of AD aetiology (Area‐Gomez et al., 2018). The AD peptide http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4865 (Aβ), when targeted to mitochondria, is thought to be crucially involved in associated toxicity. Aβ induces ER stress leading to the increased synthesis of cholesterol and loading into the mitochondria via ER‐MAM structures. Additionally, enrichment of cholesterol in mitochondrial membranes is reported in AD pathology.
Mitochondria from a mouse model of cholesterol overload exhibit increased susceptibility to Aβ‐induced oxidative stress and consequent cytochrome c release (Fernandez, Llacuna, Fernandez‐Checa, & Colell, 2009). Coupled with this observation, loading of mChol is increased in a mouse model of AD (Fernandez, Llacuna, Fernandez‐Checa, & Colell, 2009), accompanied by an overexpression of the steroidogenic acute regulatory (StAR) protein (Barbero‐Camps et al., 2014; Hashimoto et al., 2018). StAR is a lipoprotein that transports cholesterol from the ER to mitochondria, regulating the intra‐organelle distribution of the lipid.
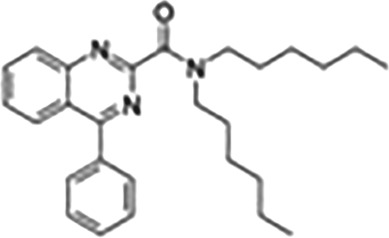
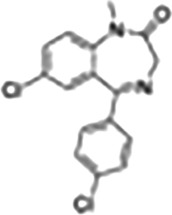
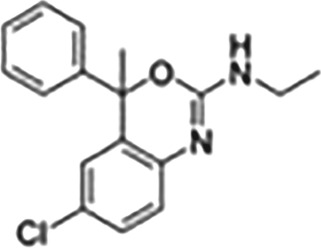
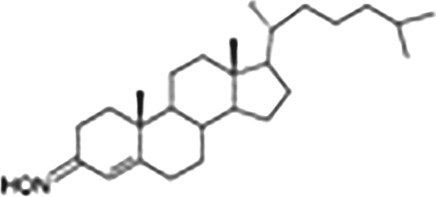
Components of the “transduceome” are known to affect cholesterol processing in the mitochondria and steroidogenesis—including neurosteroidogenesis—making them an attractive target for pharmacological regulation of these important biological processes (Rone et al., 2012; Rone, Fan, & Papadopoulos, 2009; Strobbe & Campanella, 2018). The transduceome, which has been studied more extensively in non‐neuronal cells, is a complex of cholesterol‐binding proteins that orchestrate movement of cholesterol into the mitochondria. It comprises the voltage‐dependent anion channel 1 along with interacting partners, ACBD1/3, and, under steroidogenic conditions, http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=919#2879 and StAR on the outer mitochondrial membrane. They connect with ATAD3 on the IMM via http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=206#1063 to deliver cholesterol to the processing enzyme http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1358 (CYP11A1) to generate http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2376—the first step of steroidogenesis (Rone et al., 2012; Rone, Fan, & Papadopoulos, 2009). Under conditions of stress, such as neuroinflammation and neurodegeneration, the 18‐kDa TSPO is overexpressed to fuel the cholesterol‐processing machinery (Figure 1). Although functions beyond the trafficking of cholesterol have been attributed to TSPO such as the inhibition of mitophagy (Gatliff et al., 2014), the protein presents two validated cholesterol‐binding sites (Fantini, Di Scala, Evans, Williamson, & Barrantes, 2016; Jaipuria et al., 2017; Jaremko, Jaremko, Giller, Becker, & Zweckstetter, 2014). When bound to cholesterol, TSPO changes confirmation (Jaipuria et al., 2017) implying that it has a key function in regulating intra‐organellar distribution of cholesterol. As a result of a druggable structure (Jaremko, Jaremko, Giller, Becker & Zweckstetter, 2014) and temporally regulated expression (Gavish & Veenman, 2018), TSPO has been a target of several generations of chemical PET tracers and pharmaceutical ligands (Veenman, Vainshtein, Yasin, Azrad, & Gavish, 2016; see Table 1).
Table 1.
Synthetic ligands of the translocator protein (TSPO)
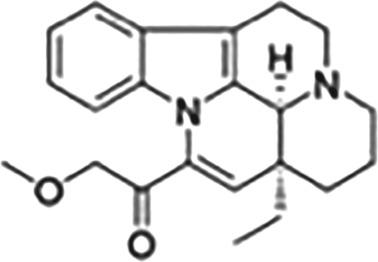
| Class | Compound | Structure | Properties |
|---|---|---|---|
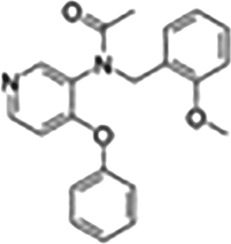
| 4‐Phenylquinazoline‐2‐carboxamides | ER176 |
|
Aza‐isosteres of PK11195. In particular, PET radioligands with sensitivity to robustly image all three TSPO genotypes in human brain |
| Benzodiazepines | Ro‐5‐4864 |
|
Sedative, neuroprotective, agonist, or partial agonist of TSPO with nanomolar binding affinity |
| Benzoxazines | Etifoxine |
|
Anxiolytic effects, anti‐neurodegenerative effects mediated by TSPO, PET ligand |
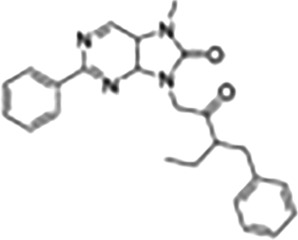
| Cholest‐4‐en‐3‐one | TRO40303 |
|
Agonist, used in cardioprotection, ALS, putatively interrupts the formation of mitochondrial transition pore |
| Imidazopyridine acetamides | DPA |
|
Ligand used for in vivo imaging of TSPO |
| Indoleacetamides | FGIN‐1‐27 |
|
TSPO ligand characterized by steroidogenic and pro‐apoptotic activities |
| Isoquinoline carboxamides | PK11195 |
|
TSPO antagonist with nanomolar affinity, widely used for characterizing expression and function in various tissues and cells, widely used PET ligand |
| N,N‐Dialkyl‐2‐phenylindol‐3‐ylglyoxylamide (PIGA) | PIGA 1128 |
|
Used to modify steroidogenic activity of TSPO, specifically in relation to neurosteroids; developed for anxiolytic activity |
| Phenoxyphenyl acetamides | PBR28 |
|
Developed as PET ligands for TSPO, specifically used for neuroinflammation; brain penetrant |
| Phenylpurines | Emapunil |
|
Ligand with rapid anxiolytic effects |
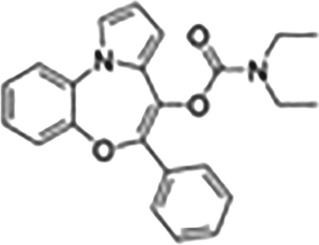
| Pyrrolobenzoxazepines | OXA‐17 |
|
Developed as anti‐cancer therapies, some activity for cannabinoid receptors |
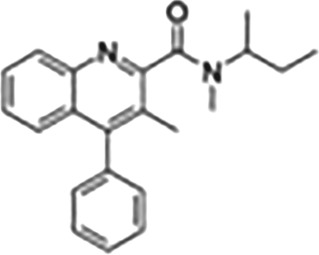
| Quinoline carboxamides | VCM198M |
|
Used as radioligand for TSPO imaging |
| Vinca alkaloids | Vinpocetine |
|
Ligand with neuroprotective activity that binds TSPO and other receptors such as adrenoceptors |
This Table also shows the chemical structures of the TSPO ligands along with their pharmacological effects and clinical or preclinical use.
In our opinion, this represents an opportunity for exploration of potential protective effects by disrupting the transduceome with TSPO regulators (Gatliff & Campanella, 2016). A better understanding of TSPO function and its role of the steroidogenic transduceome in neuronal dysfunction is therefore necessary to lay a foundation for foreseeable therapeutic interventions. While cholesterol synthesis inhibitors such as the “blockbuster” statins (Cholesterol Treatment Trialists' Collaboration, 2015) occupy an elite place in cholesterol modulation, it is perhaps time to look beyond this strategy and turn to more subtle and selective mechanisms of cholesterol shuttling. The TSPO ligands are indeed exploited for anti‐inflammatory and neuroprotective effects (Qiu et al., 2016; Scholz et al., 2015).
In a rat model of hypercholesterolaemia, ischaemia–reperfusion injury results in mitochondrial sterol (both cholesterol and oxysterol) accumulation in mitochondria (Paradis, Leoni, Caccia, Berdeaux, & Morin, 2013), which can be ablated by TSPO ligands such as SSR180575 (a benzodiazepine), 4′‐chlorodiazepam, or TRO40303. This methodology may prove useful in diseases of the CNS where mitochondrial sterol levels are altered and the range of TSPO ligands (Table 1) can be a useful toolkit to explore this.
Within the context of mitochondrial dysfunction, classically, the research on Parkinson's disease (PD) has focussed on the deficiencies in quality control regulation of mitochondria by autophagy (Larsen, Hanss, & Kruger, 2018). However, disrupted cholesterol dynamics are also associated with established molecular features of PD (see Arenas, Garcia‐Ruiz, & Fernandez‐Checa, 2017). One of the earliest pieces of evidence was from fibroblasts derived from PD patients which showed 50% reduction in cholesterol biosynthesis (Musanti, Parati, Lamperti, & Ghiselli, 1993).
Lim et al. found that a cholesterol precursor http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2746 was 50% lower in a neurotoxin‐induced mouse model (1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine) of PD. This evidence was reproduced in vitro, as a redistribution of http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2434 from the ER to mitochondria in dopaminergic neurons exposed to http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4568 (MPP+) thus implying a survival effect via mitochondria (Lim et al., 2012). Similarly, in neuroblastoma cells treated with MPP+, there was a marked accumulation of cholesterol in lysosomes (Eriksson, Nath, Bornefall, Giraldo, & Ollinger, 2017). When this effect was mimicked by the cholesterol blocking agent U18666A, cell death was reduced,suggesting that lysosomal cholesterol accumulation may be an adaptive stress response. Furthermore, the cholesterol synthesis inhibitor http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2739 reduced MPP+‐induced cell death by lowering ROS production without preventing the accumulation of cholesterol into lysosomes (Eriksson, Nath, Bornefall, Giraldo, & Ollinger, 2017). This argues for further functions for mtChol. Like the ER‐MAM communication, that between lysosomes and mitochondria is cholesterol dependent and the delicate balance of membrane lipid composition critical for a healthy CNS.
A recent publication by Lin et al. (2018) has elucidated the transcriptomic changes accompanying the isoform change of apolipoprotein (APOE3 ➔ APOE4) which has for long been the largest genetic risk factor acknowledged for late onset sporadic AD. The authors dissected the transcriptomic changes in different types of derived CNS cells, showing that astrocytes, neurons, and microglia regulate different pathways to compensate for the loss of APOE4. Most notably, APOE4 astrocytes have altered cholesterol metabolism, a change of particular interest because astrocytes are known to supply cholesterol to neurons. The gene‐edited CNS cells exhibit other features of AD such as compromised Aβ clearance, altered synaptic formation, immune activation, increased Aβ production, and hyperphosphorylated Tau protein.
Line et al. present in dish modelling of AD a compelling scientific validation for the exploitation of cholesterol shuttling as therapeutic target. Perhaps, this balance of cholesterol at the level of inter‐organellar interactions, is key to the mitochondrial network dynamics that ultimately define cellular health, especially in neurons, which are highly dependent on oxidative phosphorylation. In the case of familiar PD, in which deficiency leads to mitochondrial quality control, by autophagy, to the extent of dysfunction and ultimately death of the dopaminergic neurons, the ability to modulate mitophagy can prove critical. Relevantly, the protein TSPO (see above) has an anti‐mitophagy effect when overexpressed (Gatliff et al., 2014; Gatliff & Campanella, 2015). By disrupting the activity of TSPO via its ligands or changing its residence time on the mitochondria, efficient mitophagy could be restored and, consequently, cellular health. Intriguingly, modulation of mChol may allow the same beneficial outcome. This is supported by observations in preclinical and clinical studies, in which TSPO expression correlates with longitudinal progression of neurological conditions (Cumming & Borghammer, 2012; Maia et al., 2012).
Unlike AD, other neurodegenerative diseases do not have such clear evidence of mChol involvement in their pathophysiology. Nonetheless, in Huntington's disease, the http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5199 protein‐induced mitochondrial fluidity, can be rescued by http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=8542, a cholesterol‐like product (Eckmann et al., 2014) which also exerts neuroprotective effects in amyotrophic lateral sclerosis (ALS; Martin, 2010).
4. OXYSTEROLS–SCHRODINGER'S CAT OF MITOCHONDRIAL CHOLESTEROL‐RELATED DYSFUNCTION
Cholesterol is present at the site of mitochondrial ROS production and susceptible to auto‐oxidation into oxysterols. Heightened oxysterol levels are being used as biomarkers for neurodegenerative diseases and lysosomal storage disorder progression (Griffiths et al., 2017; Testa et al., 2016). Cholesterol can be enzymatically broken down into http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2750 (24‐OHC) and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2752 (27‐OHC) or auto‐oxidized by ROS to products like 7‐KC, 4α‐hydroxycholesterol (4α‐OHC), 4β‐hydroxycholesterol (4β‐OHC), http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4352, α‐epoxy cholesterol (α‐epoxy C), and β‐epoxy cholesterol (β‐epoxy C), while http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2885 (25‐OHC) and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4351 (7α‐OHC) can be formed by either pathway. When tested in AD patients, all of the oxysterols listed above were raised in the late stage of the condition except for 24‐OHC, which was decreased as the disease progressed (Testa et al., 2016).
ALS is a primary target of exploited oxysterol signalling. Since the discovery that mutation in the mitochondrial antioxidant enzyme SOD1 can cause ALS (Ince, Shaw, Slade, Jones, & Hudgson, 1996), mitochondrial oxidative stress in motor neurons has become a key research interest in the field. Recent studies have revealed that http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=602 α and β (LXR‐α and LXR‐β) are key players in ALS aetiology. LXRs are nuclear receptors for oxysterols, which regulate cholesterol synthesis among other cellular processes (dependent on the cellular type). A recent study identified two single nucleotide polymorphisms of LXR‐α, rs2279238 and rs7120118, associated with delayed age of ALS onset amongst a cohort of 330 ALS patients (Mouzat et al., 2016). Moreover, male LXR‐β−/− mice develop severe motor impairment closely resembling ALS at 7 months which later progresses to hind‐limb paralysis (Andersson, Gustafsson, Warner, & Gustafsson, 2005).
If auto‐oxidative forms of oxysterols produced in the mitochondria are acknowledged to be contributing factors in ALS, any antioxidant protocols adopted will be beneficial to the condition. Miquel et al. assessed the therapeutic benefit of a mitochondrially targeted antioxidant MitoQ (10‐(4,5‐dimethoxy‐2‐methyl‐3,6‐dioxo‐1,4‐cyclohexadien‐1‐yl)decyl triphenylphosphonium methane sulfonate) in a mouse model of familial ALS in which the decline of mitochondrial function was slowed down, in both the spinal cord and the quadriceps muscle (Miquel et al., 2014).
While the preclinical data are substantive, no antioxidant therapy has, hitherto, proved to modify ALS or any other neurodegenerative condition. Intriguingly though, there are some reports of long‐term statins usage causing ALS, which is attributed to the parallel reduction of mitochondrial antioxidant coenzyme Q10 (CoQ10) by the inhibition of HMG‐CoA reductase enzyme (Edwards, Star, & Kiuru, 2007).
Testing other antioxidants, such as 10‐(6′‐plastoquinonyl)decyltriphenylphosphonium (SkQ1), Mitohttp://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5346, Mitohttp://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7000, Mitohttp://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=8741, MitoHonokiol, Mitoapocynin, AntiOxCIN4, AntiOxBEN2, could anyway prove beneficial in ALS, more importantly in the models where the antioxidant system is disrupted (Teixeira, Deus, Borges, & Oliveira, 2018).
After almost two decades since the approval of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2326 for ALS, another agent, edaravone, was only recently approved by the FDA (May 2017), after showing benefit in a randomized double‐blind clinical trial (Rothstein, 2017; Writing & Edaravone, 2017). While edaravone has other effects, such as reducing inflammation, its main activity is antioxidant. Although this compound is not mitochondrially targeted, there is a possibility that by quenching ROS, it facilitates the modulation of oxysterols and mChol for therapeutic benefit. This strategy has not been tested in neurological conditions but warrants further investigation.
The interest in LXRs as therapeutic targets has steadily increased for several diseases ranging from vascular to metabolic and neurological conditions. Potent and selective LXR ligands continue to emerge from screening of small molecule libraries, rational design, and empirical medicinal chemistry approaches. In spite of this, challenges remain in minimizing the undesirable effects of LXR activation on lipid metabolism (Komati et al., 2017) for which a mitochondrial health assay approach may prove useful to implement drug screening (Figure 2).
Figure 2.
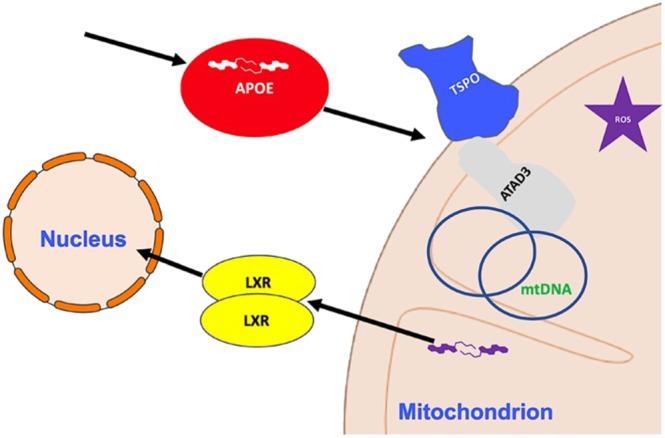
Cholesterol interplay between mitochondria and cytosol. The Figure shows the major mechanisms of cholesterol influx (e.g., TSPO) into the mitochondria, exploited by the intracellular accumulation of APOE. The two isoforms of the transcription factor liver X receptor (LXR‐α and LXR‐β) are activated and hence translocated to the nucleus by the oxidized derivatives of cholesterol (oxysterols), which are formed by the high redox stress produced by malfunctioning mitochondria are also highlighted in the scheme
5. CONCLUSIONS AND PERSPECTIVES
All these results indicate that mChol has been well studied in the context of steroidogenesis but largely ignored in neurodegeneration. While there are the known functions of precursor to (a) steroids, (b) ER‐MAM, (c) mitochondrial–lysosomal interaction, and (d) mtDNA tethering, there are other biochemical or mechanobiological processes that involve homeostasis of cholesterol in the mitochondria, which remain ill‐defined.
With the recognition that ER‐MAMs are important in AD , the mitochondria–lysosomal interaction is compromised in PD, and the disruption of oxysterol signalling can lead to ALS, mChol appears as a logical target to inform and treat these conditions. Furthermore, a dissection of oxysterol signalling could, per se, lead to identification of potential therapeutic avenues in neurodegeneration. The genetic evidence in ALS supporting this is as strong as that from studies with SOD1 mutants and LXR‐β−/− mice: Lack of ROS neutralization and dysregulated oxysterol signalling leads to motor neuron degeneration (Abdel‐Khalik et al., 2017; Mouzat et al., 2016; Mouzat et al., 2018). Most notably, increasing antioxidant levels improve the tone of cholesterol signalling via LXR‐β leading to a beneficial outcome in neurodegeneration (Bond et al., 2018; Sandoval‐Hernandez, Restrepo, Cardona‐Gomez, & Arboleda, 2016; Stachel et al., 2016). Along with the need to continue gathering evidence on the beneficial effect of cholesterol‐modulating agents on neurodegeneration, it is crucial to devise novel means of measuring neuroprotection, such as the handling of lipids by mitochondria.
5.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander, Cidlowski et al., 2017; Alexander, Fabbro et al., 2017; Alexander, Kelly et al., 2017a,b).
CONFLICT OF INTEREST
The authors declare no financial competing interests with the matter of this publication.
ACKNOWLEDGEMENTS
The authors are grateful to Miss A. Singh for critical reading and editing of the manuscript and Miss L. Hardy for assistance. The research activities led by M.C. are supported by the following funders, which are gratefully acknowledged: the Biotechnology and Biological Sciences Research Council (BBSRC) Project Grant (BB/N007042/ 1), LiDo and Case Studentships; The Petplan Charitable Trust (PPCT); The Rotary Foundation and the European Research Council (ERC) (Project acronym FIRM, grant number 819600).
Desai R, Campanella M. Exploring mitochondrial cholesterol signalling for therapeutic intervention in neurological conditions. Br J Pharmacol. 2019;176:4284–4292. 10.1111/bph.14697
REFERENCES
- Abdel‐Khalik, J. , Yutuc, E. , Crick, P. J. , Gustafsson, J. A. , Warner, M. , Roman, G. , … Wang, Y. (2017). Defective cholesterol metabolism in amyotrophic lateral sclerosis. Journal of Lipid Research, 58, 267–278. 10.1194/jlr.P071639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Cidlowski, J. A. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators . (2017). The Concise Guide to PHARMACOLOGY 2017/18: Nuclear hormone receptors. British Journal of Pharmacology, 174, S208–S224. 10.1111/bph.13880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators . (2017). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. British Journal of Pharmacology, 174, S272–S359. 10.1111/bph.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , Harding, S. D. , … CGTP Collaborators . (2017a). The Concise Guide to PHARMACOLOGY 2017/18: Other proteins. British Journal of Pharmacology, 174, S1–S16. 10.1111/bph.13882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , Harding, S. D. , … CGTP Collaborators . (2017b). The Concise Guide to PHARMACOLOGY 2017/18: Transporters. British Journal of Pharmacology, 174, S360–S446. 10.1111/bph.13883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson, S. , Gustafsson, N. , Warner, M. , & Gustafsson, J. A. (2005). Inactivation of liver X receptor β leads to adult‐onset motor neuron degeneration in male mice. Proceedings of the National Academy of Sciences of the United States of America, 102, 3857–3862. 10.1073/pnas.0500634102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Area‐Gomez, E. , de Groof, A. , Bonilla, E. , Montesinos, J. , Tanji, K. , Boldogh, I. , … Schon, E. A. (2018). A key role for MAM in mediating mitochondrial dysfunction in Alzheimer disease. Cell Death & Disease, 9, 335 10.1038/s41419-017-0215-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenas, F. , Garcia‐Ruiz, C. , & Fernandez‐Checa, J. C. (2017). Intracellular cholesterol trafficking and impact in neurodegeneration. Frontiers in Molecular Neuroscience, 10, 382 10.3389/fnmol.2017.00382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbero‐Camps, E. , Fernandez, A. , Baulies, A. , Martinez, L. , Fernandez‐Checa, J. C. , & Colell, A. (2014). Endoplasmic reticulum stress mediates amyloid β neurotoxicity via mitochondrial cholesterol trafficking. The American Journal of Pathology, 184, 2066–2081. 10.1016/j.ajpath.2014.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond, L. , Bernhardt, K. , Madria, P. , Sorrentino, K. , Scelsi, H. , & Mitchell, C. S. (2018). A metadata analysis of oxidative stress etiology in preclinical amyotrophic lateral sclerosis: Benefits of antioxidant therapy. Frontiers in Neuroscience, 12, 10 10.3389/fnins.2018.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli, V. , & La Morgia, C. (2018). Clinical syndromes associated with mtDNA mutations: Where we stand after 30 years. Essays in Biochemistry, 62, 235–254. 10.1042/EBC20170097 [DOI] [PubMed] [Google Scholar]
- Cholesterol Treatment Trialists' Collaboration , Fulcher, J. , O'Connell, R. , Voysey, M. , Emberson, J. , Blackwell, L. , … Keech, A. (2015). Efficacy and safety of LDL‐lowering therapy among men and women: Meta‐analysis of individual data from 174,000 participants in 27 randomised trials. Lancet, 385, 1397–1405. [DOI] [PubMed] [Google Scholar]
- Cumming, P. , & Borghammer, P. (2012). Molecular imaging and the neuropathologies of Parkinson's disease. Current Topics in Behavioral Neurosciences, 11, 117–148. 10.1007/7854_2011_165 [DOI] [PubMed] [Google Scholar]
- de Oliveira, J. , Hort, M. A. , Moreira, E. L. , Glaser, V. , Ribeiro‐do‐Valle, R. M. , Prediger, R. D. , … de Bem, A. F. (2011). Positive correlation between elevated plasma cholesterol levels and cognitive impairments in LDL receptor knockout mice: Relevance of cortico‐cerebral mitochondrial dysfunction and oxidative stress. Neuroscience, 197, 99–106. 10.1016/j.neuroscience.2011.09.009 [DOI] [PubMed] [Google Scholar]
- Del Prete, D. , Suski, J. M. , Oules, B. , Debayle, D. , Gay, A. S. , Lacas‐Gervais, S. , … Chami, M. (2017). Localization and processing of the amyloid‐β protein precursor in mitochondria‐associated membranes. Journal of Alzheimer's Disease, 55, 1549–1570. 10.3233/JAD-160953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai, R. , Frazier, A. E. , Durigon, R. , Patel, H. , Jones, A. W. , Dalla Rosa, I. , … Spinazzola, A. (2017). ATAD3 gene cluster deletions cause cerebellar dysfunction associated with altered mitochondrial DNA and cholesterol metabolism. Brain, 140, 1595–1610. 10.1093/brain/awx094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckmann, J. , Clemens, L. E. , Eckert, S. H. , Hagl, S. , Yu‐Taeger, L. , Bordet, T. , … Eckert, G. P. (2014). Mitochondrial membrane fluidity is consistently increased in different models of Huntington disease: Restorative effects of olesoxime. Molecular Neurobiology, 50, 107–118. 10.1007/s12035-014-8663-3 [DOI] [PubMed] [Google Scholar]
- Edwards, I. R. , Star, K. , & Kiuru, A. (2007). Statins, neuromuscular degenerative disease and an amyotrophic lateral sclerosis‐like syndrome: An analysis of individual case safety reports from vigibase. Drug Safety, 30, 515–525. 10.2165/00002018-200730060-00005 [DOI] [PubMed] [Google Scholar]
- Elustondo, P. , Martin, L. A. , & Karten, B. (2017). Mitochondrial cholesterol import. Biochimica et Biophysica Acta, 1862, 90–101. 10.1016/j.bbalip.2016.08.012 [DOI] [PubMed] [Google Scholar]
- Eriksson, I. , Nath, S. , Bornefall, P. , Giraldo, A. M. , & Ollinger, K. (2017). Impact of high cholesterol in a Parkinson's disease model: Prevention of lysosomal leakage versus stimulation of α‐synuclein aggregation. European Journal of Cell Biology, 96, 99–109. 10.1016/j.ejcb.2017.01.002 [DOI] [PubMed] [Google Scholar]
- Fantini, J. , Di Scala, C. , Evans, L. S. , Williamson, P. T. , & Barrantes, F. J. (2016). A mirror code for protein‐cholesterol interactions in the two leaflets of biological membranes. Scientific Reports, 6, 21907 10.1038/srep21907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez, A. , Llacuna, L. , Fernandez‐Checa, J. C. , & Colell, A. (2009). Mitochondrial cholesterol loading exacerbates amyloid β peptide‐induced inflammation and neurotoxicity. The Journal of Neuroscience, 29, 6394–6405. 10.1523/JNEUROSCI.4909-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer, S. , Rouser, G. , Fleischer, B. , Casu, A. , & Kritchevsky, G. (1967). Lipid composition of mitochondria from bovine heart, liver, and kidney. Journal of Lipid Research, 8, 170–180. [PubMed] [Google Scholar]
- Fujimoto, M. , Hayashi, T. , & Su, T. P. (2012). The role of cholesterol in the association of endoplasmic reticulum membranes with mitochondria. Biochemical and Biophysical Research Communications, 417, 635–639. 10.1016/j.bbrc.2011.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatliff, J. , & Campanella, M. (2015). TSPO is a REDOX regulator of cell mitophagy. Biochemical Society Transactions, 43, 543–552. 10.1042/BST20150037 [DOI] [PubMed] [Google Scholar]
- Gatliff, J. , & Campanella, M. (2016). TSPO: Kaleidoscopic 18‐kDa amid biochemical pharmacology, control and targeting of mitochondria. The Biochemical Journal, 473, 107–121. 10.1042/BJ20150899 [DOI] [PubMed] [Google Scholar]
- Gatliff, J. , East, D. , Crosby, J. , Abeti, R. , Harvey, R. , Craigen, W. , … Campanella, M. (2014). TSPO interacts with VDAC1 and triggers a ROS‐mediated inhibition of mitochondrial quality control. Autophagy, 10, 2279–2296. 10.4161/15548627.2014.991665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavish, M. , & Veenman, L. (2018). Regulation of mitochondrial, cellular, and organismal functions by TSPO. Advances in Pharmacology, 82, 103–136. 10.1016/bs.apha.2017.09.004 [DOI] [PubMed] [Google Scholar]
- Gerhold, J. M. , Cansiz‐Arda, S. , Lohmus, M. , Engberg, O. , Reyes, A. , van Rennes, H. , … Spelbrink, J. N. (2015). Human mitochondrial DNA‐protein complexes attach to a cholesterol‐rich membrane structure. Scientific Reports, 5, 15292 10.1038/srep15292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman, G. S. , Chinnery, P. F. , DiMauro, S. , Hirano, M. , Koga, Y. , McFarland, R. , … Turnbull, D. M. (2016). Mitochondrial diseases. Nature Reviews. Disease Primers, 2, 16080 10.1038/nrdp.2016.80 [DOI] [PubMed] [Google Scholar]
- Griffiths, W. J. , Abdel‐Khalik, J. , Yutuc, E. , Morgan, A. H. , Gilmore, I. , Hearn, T. , & Wang, Y. (2017). Cholesterolomics: An update. Analytical Biochemistry, 524, 56–67. 10.1016/j.ab.2017.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR . (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto, S. , Ishii, A. , Kamano, N. , Watamura, N. , Saito, T. , Ohshima, T. , … Saido, T. C. (2018). Endoplasmic reticulum stress responses in mouse models of Alzheimer's disease: Overexpression paradigm versus knockin paradigm. The Journal of Biological Chemistry, 293, 3118–3125. 10.1074/jbc.M117.811315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, J. , Cooper, H. M. , Reyes, A. , Di Re, M. , Sembongi, H. , Litwin, T. R. , … Holt, I. J. (2012). Mitochondrial nucleoid interacting proteins support mitochondrial protein synthesis. Nucleic Acids Research, 40, 6109–6121. 10.1093/nar/gks266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, J. , Mao, C. C. , Reyes, A. , Sembongi, H. , Di Re, M. , Granycome, C. , … Holt, I. J. (2007). The AAA+ protein ATAD3 has displacement loop binding properties and is involved in mitochondrial nucleoid organization. The Journal of Cell Biology, 176, 141–146. 10.1083/jcb.200609158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath, S. E. , & Daum, G. (2013). Lipids of mitochondria. Progress in Lipid Research, 52, 590–614. 10.1016/j.plipres.2013.07.002 [DOI] [PubMed] [Google Scholar]
- Ince, P. G. , Shaw, P. J. , Slade, J. Y. , Jones, C. , & Hudgson, P. (1996). Familial amyotrophic lateral sclerosis with a mutation in exon 4 of the Cu/Zn superoxide dismutase gene: Pathological and immunocytochemical changes. Acta Neuropathologica, 92, 395–403. 10.1007/s004010050535 [DOI] [PubMed] [Google Scholar]
- Issop, L. , Fan, J. , Lee, S. , Rone, M. B. , Basu, K. , Mui, J. , & Papadopoulos, V. (2015). Mitochondria‐associated membrane formation in hormone‐stimulated Leydig cell steroidogenesis: Role of ATAD3. Endocrinology, 156, 334–345. 10.1210/en.2014-1503 [DOI] [PubMed] [Google Scholar]
- Jaipuria, G. , Leonov, A. , Giller, K. , Vasa, S. K. , Jaremko, L. , Jaremko, M. , … Zweckstetter, M. (2017). Cholesterol‐mediated allosteric regulation of the mitochondrial translocator protein structure. Nature Communications, 8, 14893 10.1038/ncomms14893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaremko, L. , Jaremko, M. , Giller, K. , Becker, S. , & Zweckstetter, M. (2014). Structure of the mitochondrial translocator protein in complex with a diagnostic ligand. Science, 343, 1363–1366. 10.1126/science.1248725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komati, R. , Spadoni, D. , Zheng, S. , Sridhar, J. , Riley, K. E. , & Wang, G. (2017). Ligands of therapeutic utility for the liver X receptors. Molecules, 22 10.3390/molecules22010088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajoie, P. , Goetz, J. G. , Dennis, J. W. , & Nabi, I. R. (2009). Lattices, rafts, and scaffolds: Domain regulation of receptor signaling at the plasma membrane. The Journal of Cell Biology, 185, 381–385. 10.1083/jcb.200811059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen, S. B. , Hanss, Z. , & Kruger, R. (2018). The genetic architecture of mitochondrial dysfunction in Parkinson's disease. Cell and Tissue Research, 373, 21–37. 10.1007/s00441-017-2768-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, L. , Jackson‐Lewis, V. , Wong, L. C. , Shui, G. H. , Goh, A. X. , Kesavapany, S. , … Wenk, M. R. (2012). Lanosterol induces mitochondrial uncoupling and protects dopaminergic neurons from cell death in a model for Parkinson's disease. Cell Death and Differentiation, 19, 416–427. 10.1038/cdd.2011.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Y. T. , Seo, J. , Gao, F. , Feldman, H. M. , Wen, H. L. , Penney, J. , … Tsai, L. H. (2018). APOE4 causes widespread molecular and cellular alterations associated with Alzheimer's disease phenotypes in human iPSC‐derived brain cell types. Neuron, 98, 1294 10.1016/j.neuron.2018.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia, S. , Arlicot, N. , Vierron, E. , Bodard, S. , Vergote, J. , Guilloteau, D. , & Chalon, S. (2012). Longitudinal and parallel monitoring of neuroinflammation and neurodegeneration in a 6‐hydroxydopamine rat model of Parkinson's disease. Synapse, 66, 573–583. 10.1002/syn.21543 [DOI] [PubMed] [Google Scholar]
- Martin, L. J. (2010). Olesoxime, a cholesterol‐like neuroprotectant for the potential treatment of amyotrophic lateral sclerosis. IDrugs, 13, 568–580. [PMC free article] [PubMed] [Google Scholar]
- Miquel, E. , Cassina, A. , Martinez‐Palma, L. , Souza, J. M. , Bolatto, C. , Rodriguez‐Bottero, S. , … Cassina, P. (2014). Neuroprotective effects of the mitochondria‐targeted antioxidant MitoQ in a model of inherited amyotrophic lateral sclerosis. Free Radical Biology & Medicine, 70, 204–213. 10.1016/j.freeradbiomed.2014.02.019 [DOI] [PubMed] [Google Scholar]
- Mouzat, K. , Molinari, N. , Kantar, J. , Polge, A. , Corcia, P. , Couratier, P. , … Camu, W. (2018). Liver X receptor genes variants modulate ALS phenotype. Molecular Neurobiology, 55, 1959–1965. 10.1007/s12035-017-0453-2 [DOI] [PubMed] [Google Scholar]
- Mouzat, K. , Raoul, C. , Polge, A. , Kantar, J. , Camu, W. , & Lumbroso, S. (2016). Liver X receptors: From cholesterol regulation to neuroprotection‐a new barrier against neurodegeneration in amyotrophic lateral sclerosis? Cellular and Molecular Life Sciences, 73, 3801–3808. 10.1007/s00018-016-2330-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musanti, R. , Parati, E. , Lamperti, E. , & Ghiselli, G. (1993). Decreased cholesterol biosynthesis in fibroblasts from patients with Parkinson disease. Biochemical Medicine and Metabolic Biology, 49, 133–142. 10.1006/bmmb.1993.1016 [DOI] [PubMed] [Google Scholar]
- Paradis, S. , Leoni, V. , Caccia, C. , Berdeaux, A. , & Morin, D. (2013). Cardioprotection by the TSPO ligand 4′‐chlorodiazepam is associated with inhibition of mitochondrial accumulation of cholesterol at reperfusion. Cardiovascular Research, 98, 420–427. 10.1093/cvr/cvt079 [DOI] [PubMed] [Google Scholar]
- Peralta, S. , Goffart, S. , Williams, S. L. , Diaz, F. , Garcia, S. , Nissanka, N. , … Moraes, C. T. (2018). ATAD3 controls mitochondrial cristae structure in mouse muscle, influencing mtDNA replication and cholesterol levels. Journal of Cell Science, 131, jcs217075 10.1242/jcs.217075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, Z. K. , He, J. L. , Liu, X. , Zhang, G. H. , Zeng, J. , Nie, H. , … Chen, J. S. (2016). The antidepressant‐like activity of AC‐5216, a ligand for 18KDa translocator protein (TSPO), in an animal model of diabetes mellitus. Scientific Reports, 6, 37345 10.1038/srep37345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rone, M. B. , Fan, J. , & Papadopoulos, V. (2009). Cholesterol transport in steroid biosynthesis: Role of protein‐protein interactions and implications in disease states. Biochimica et Biophysica Acta, 1791, 646–658. 10.1016/j.bbalip.2009.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rone, M. B. , Midzak, A. S. , Issop, L. , Rammouz, G. , Jagannathan, S. , Fan, J. , … Papadopoulos, V. (2012). Identification of a dynamic mitochondrial protein complex driving cholesterol import, trafficking, and metabolism to steroid hormones. Molecular Endocrinology, 26, 1868–1882. 10.1210/me.2012-1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein, J. D. (2017). Edaravone: A new drug approved for ALS. Cell, 171, 725 10.1016/j.cell.2017.10.011 [DOI] [PubMed] [Google Scholar]
- Rukmini, R. , Rawat, S. S. , Biswas, S. C. , & Chattopadhyay, A. (2001). Cholesterol organization in membranes at low concentrations: Effects of curvature stress and membrane thickness. Biophysical Journal, 81, 2122–2134. 10.1016/S0006-3495(01)75860-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval‐Hernandez, A. G. , Restrepo, A. , Cardona‐Gomez, G. P. , & Arboleda, G. (2016). LXR activation protects hippocampal microvasculature in very old triple transgenic mouse model of Alzheimer's disease. Neuroscience Letters, 621, 15–21. 10.1016/j.neulet.2016.04.007 [DOI] [PubMed] [Google Scholar]
- Scholz, R. , Caramoy, A. , Bhuckory, M. B. , Rashid, K. , Chen, M. , Xu, H. , … Langmann, T. (2015). Targeting translocator protein (18 kDa) (TSPO) dampens pro‐inflammatory microglia reactivity in the retina and protects from degeneration. Journal of Neuroinflammation, 12, 201 10.1186/s12974-015-0422-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons, K. , & Ikonen, E. (1997). Functional rafts in cell membranes. Nature, 387, 569–572. 10.1038/42408 [DOI] [PubMed] [Google Scholar]
- Spelbrink, J. N. (2010). Functional organization of mammalian mitochondrial DNA in nucleoids: History, recent developments, and future challenges. IUBMB Life, 62, 19–32. 10.1002/iub.282 [DOI] [PubMed] [Google Scholar]
- Stachel, S. J. , Zerbinatti, C. , Rudd, M. T. , Cosden, M. , Suon, S. , Nanda, K. K. , … Renger, J. (2016). Identification and in vivo evaluation of liver X receptor β‐selective agonists for the potential treatment of Alzheimer's disease. Journal of Medicinal Chemistry, 59, 3489–3498. 10.1021/acs.jmedchem.6b00176 [DOI] [PubMed] [Google Scholar]
- Strobbe, D. , & Campanella, M. (2018). Anxiolytic therapy: A paradigm of successful mitochondrial pharmacology. Trends in Pharmacological Sciences, 39, 437–439. 10.1016/j.tips.2018.02.008 [DOI] [PubMed] [Google Scholar]
- Teixeira, J. , Deus, C. M. , Borges, F. , & Oliveira, P. J. (2018). Mitochondria: Targeting mitochondrial reactive oxygen species with mitochondriotropic polyphenolic‐based antioxidants. The International Journal of Biochemistry & Cell Biology, 97, 98–103. 10.1016/j.biocel.2018.02.007 [DOI] [PubMed] [Google Scholar]
- Testa, G. , Staurenghi, E. , Zerbinati, C. , Gargiulo, S. , Iuliano, L. , Giaccone, G. , … Gamba, P. (2016). Changes in brain oxysterols at different stages of Alzheimer's disease: Their involvement in neuroinflammation. Redox Biology, 10, 24–33. 10.1016/j.redox.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer, G. , Voelker, D. R. , & Feigenson, G. W. (2008). Membrane lipids: Where they are and how they behave. Nature Reviews. Molecular Cell Biology, 9, 112–124. 10.1038/nrm2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenman, L. , Vainshtein, A. , Yasin, N. , Azrad, M. , & Gavish, M. (2016). Tetrapyrroles as endogenous TSPO ligands in eukaryotes and prokaryotes: Comparisons with synthetic ligands. International Journal of Molecular Sciences, 17 10.3390/ijms17060880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Writing, G. , & Edaravone, A. L. S. S. G. (2017). Safety and efficacy of Edaravone in well defined patients with amyotrophic lateral sclerosis: A randomised, double‐blind, placebo‐controlled trial. Lancet Neurology, 16, 505–512. [DOI] [PubMed] [Google Scholar]
- Zerbinati, C. , & Iuliano, L. (2017). Cholesterol and related sterols autoxidation. Free Radical Biology & Medicine, 111, 151–155. 10.1016/j.freeradbiomed.2017.04.013 [DOI] [PubMed] [Google Scholar]
- Zheng, Y. Z. , Berg, K. B. , & Foster, L. J. (2009). Mitochondria do not contain lipid rafts, and lipid rafts do not contain mitochondrial proteins. Journal of Lipid Research, 50, 988–998. 10.1194/jlr.M800658-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]