Abstract
Background
There is a lack of large studies focusing on the prognostic significance of lateral lymph node (LLN) metastasis following LLN dissection (LLND) in rectal cancer. The aim of this study was to evaluate the prognostic impact of LLN metastases on survival of patients with advanced low rectal cancer.
Methods
Consecutive patients with locally advanced, but not metastatic, extraperitoneal rectal cancer treated with neoadjuvant (chemo)radiotherapy plus total mesorectal excision between 2004 and 2015 were included in the study. LLND was performed when pretreatment imaging documented enlarged LLNs (7 mm or greater in size). Localization of nodal metastases and long‐term outcomes were analysed. Kaplan–Meier analysis was used to compare the survival of patients with ypN0 disease with that of patients with mesorectal ypN+/LLN− status and patients with positive LLNs. The Cox proportional hazards model was used to evaluate predictors of disease‐free survival (DFS) and local recurrence.
Results
A total of 613 patients were included in the study; LLND was performed in 212 patients (34·6 per cent) and 57 (9·3 per cent) had LLN metastasis. Patients with LLN metastasis had improved DFS and local recurrence cumulative incidence rates compared with patients with mesorectal ypN2+/LLN− disease (DFS: P = 0·014; local recurrence: P = 0·006). Although the DFS rate of patients with LLN metastasis was worse than that of patients with ypN0 disease (P < 0·001), the cumulative incidence of local recurrence was similar (P = 0·491). In multivariable analysis, residual LLN metastasis was not an independent predictor of worse DFS or local recurrence.
Conclusion
LLN metastasis is not an independent predictor of local recurrence or survival. Survival of patients presenting with LLN metastasis after (chemo)radiotherapy was intermediate between that of patients with ypN0 status and those with mesorectal ypN2 positivity.
The prognostic significance of residual lateral lymph node metastasis following neoadjuvant (chemo)radiotherapy and lateral lymph node dissection in patients with advanced low rectal cancer remains unknown. This study shows that residual lateral lymph node metastasis after (chemo)radiotherapy can be considered an intermediate‐risk disease, similar to residual mesorectal ypN1 disease.
Lateral nod dissection and rectal cancer
Antecedentes
No existen en la literatura grandes estudios dirigidos a investigar la importancia pronóstica de las metástasis en los ganglios linfáticos laterales (lateral lymph nodes, LLN) después de la disección de los mismos (LLN dissection, LLND) en pacientes con cáncer de recto. El objetivo de este estudio fue evaluar el impacto pronóstico de las metástasis en los LLN sobre la supervivencia de los pacientes con cáncer de recto.
Métodos
Se analizaron 613 pacientes consecutivos con cáncer de recto localmente avanzado extraperitoneal y no metastásico tratados con (quimio)radioterapia neoadyuvante seguida de resección total del mesorrecto (total mesorectal excision, TME) entre 2004 y 2015. Se realizó una LLND cuando el estudio mediante pruebas de imagen previo el tratamiento mostró LLN aumentados de tamaño ≥ 7 mm. Se analizó la localización de las metástasis ganglionares y los resultados a largo plazo. El análisis de supervivencia se realizó mediante el método de Kaplan‐Meier para comparar las supervivencias de los pacientes ypN0 frente a los pacientes ypN con positividad mesorrectal/LLN negativos y frente a los pacientes LLN positivos. Se utilizó el modelo de riesgo proporcional de Cox para evaluar los factores predictivos de supervivencia libre de enfermedad y de recidiva local.
Resultados
Se realizó una LLND en 212 (34,6%) pacientes, y 57 (9,3%) pacientes presentaban metástasis en los LLN. Los pacientes con metástasis en los LLN presentaron mejores curvas de incidencia acumulada de recidiva local y de supervivencia libre de enfermedad en comparación con los pacientes con ganglios mesorrectales ypN2 positivos/LLN negativos (respectivamente, P = 0,0135 y P = 0,0060). Aunque la curva de la supervivencia libre de enfermedad de los pacientes con metástasis en los LLN fue peor que la de los pacientes ypN0 (P < 0,0001), la incidencia acumulada de recidiva local fue similar (P = 0,4905). En el análisis multivariable, la metástasis residual en los LLN no fue un factor predictivo independiente de peor supervivencia libre de enfermedad ni de recidiva local.
Conclusión
Las metástasis en los LLN no es un factor predictivo independiente de recidiva local o supervivencia. Los pacientes que presentaron metástasis en los LLN después de (quimio)radioterapia mostraron características de supervivencia intermedias entre ypN0 y pacientes con ganglios mesorrectales ypN2 positivos.
Introduction
Neoadjuvant (chemo)radiotherapy ((C)RT) followed by total mesorectal excision (TME) is currently the standard treatment for locally advanced rectal cancer1. However, previous studies from Eastern countries2, 3, 4 have reported that lateral lymph node (LLN) metastases outside the field of TME occurred in 10–20 per cent of patients with stage II–III extraperitoneal rectal cancer.
A recent prospective multicentre RCT (JCOG0212)5 compared TME with TME plus ‘prophylactic’ LLN dissection (LLND) in patients without enlargement of LLNs undergoing upfront surgery, and found that TME with LLND resulted in significantly lower local recurrence rates compared with TME alone (7·4 versus 12·6 per cent respectively). In contrast, in Western countries, neoadjuvant (C)RT protocols are mostly employed, resulting in local recurrence rates of less than 10 per cent6, with minimal indication for LLND7. Recently, however, Eastern8, 9, 10 and Western11, 12, 13, 14, 15 studies have suggested that (C)RT and TME without LLND may not be sufficient in patients with enlarged LLNs, with a lateral pelvic recurrence rate of 19·5 per cent in patients with LLNs 7 mm or more in size15.
However, large‐scale studies investigating the prognostic impact of pathologically residual LLN metastasis after (C)RT are sparse. Most previous studies focusing on LLN metastasis did not employ neoadjuvant (C)RT protocols, and poor disease‐free survival (DFS) and high local recurrence rates were also found after LLND in patients with pathological LLN metastasis2, 3, 4. Accordingly, residual LLN metastases are considered a feature of high‐risk tumours16, 17.
The aim of this study was to investigate the prognostic impact of LLN metastasis in patients with rectal cancer treated with neoadjuvant (C)RT and TME.
Methods
All consecutive patients with locally advanced extraperitoneal rectal cancer without distant metastasis, treated by (C)RT and TME at the Cancer Institute Hospital (between July 2004 and December 2015) or Toranomon Hospital (between April 2010 and December 2015), were reviewed retrospectively for inclusion in the study. Exclusion criteria included the presence of inguinal metastases, owing to their rarity and the different route of lymphatic spread18.
In these hospitals, neoadjuvant (C)RT followed by TME is the standard treatment for locally advanced (cT3–4 or cN+) extraperitoneal rectal cancer. LLND is performed only when pretreatment imaging shows enlarged LLNs (7 mm or more in long‐axis diameter), regardless of the size of the LLNs after neoadjuvant treatment (selective LLND)19.
Clinical and pathological variables reviewed included: patient demographics (age, sex), tumour features (distance from anal verge, clinical stage, histological type), neoadjuvant and adjuvant protocols, surgical procedures and pathological data (ypT and ypN category according to the seventh edition of the AJCC Cancer Staging Manual20, nodal harvest, R status, lymphovascular invasion and grading).
Ethical approval for the study was obtained at each institution (Cancer Institute Hospital: approval number 2018‐1045; Toranomon Hospital: approval number 1711).
Neoadjuvant (chemo)radiotherapy
Pretreatment clinical stage was assessed by CT and/or MRI in all patients. All patients received fluorouracil‐based long‐course (C)RT (total dose 45–50·4 Gy) or short‐course radiotherapy (5 × 5 Gy) with or without induction chemotherapy (mFOLFOX6 (modified folinic acid–fluorouracil–oxaliplatin regimen) or CAPOX (capecitabine–oxaliplatin) with or without bevacizumab) following evaluation by a multidisciplinary team.
The radiation field generally included all gross disease as well as the internal iliac and obturator nodes, and the perirectal and presacral spaces with the upper border at the sacral promontory. The external iliac and common iliac areas were generally not included in the radiation field. Radiotherapy was administered using the three‐field technique with the patient in the prone position.
Lateral lymph node metastasis
Lymph nodes (LNs) were classified and numbered according to the second English edition of the Japanese Classification of Colorectal Carcinoma21. Mesorectal LNs included classification numbers 251 (perirectal nodes), 252 (inferior mesenteric trunk nodes) and 253 (inferior mesenteric nodes). LLNs included 263D (distal internal iliac nodes), 263P (proximal internal iliac nodes), 283 (obturator nodes), 293 (external iliac nodes), 273 (common iliac nodes) and 280 (aortic bifurcation nodes). The location of LNs was recorded by the surgeon, according to guidelines set by the Japanese Society for Cancer of the Colon and Rectum21.
Lateral lymph node dissection
The LLND procedure (open or laparoscopic) has been standardized in both hospitals, and was introduced into Toranomon Hospital in April 201022. LLND is performed primarily by laparoscopy (Video S1 and Appendix S1 , supporting information). Briefly, both internal iliac and obturator areas are dissected en bloc, and common iliac and aortic bifurcation areas are dissected only when metastasis is suspected, as determined by pretreatment imaging.
Follow‐up
The follow‐up protocol included: measurement of serum carcinoembryonic antigen every 3 months for the first 3 years and every 6 months thereafter, and CT (chest to pelvis) every 6 months for 5 years. Patients who experienced no events were censored at the final follow‐up.
Outcome measures
Localization of nodal metastases and long‐term outcomes were analysed. DFS was defined as the date of first surgery to the date on which disease recurrence (local or distant) was first detected, or death from any cause, as described previously23, 24. Local recurrence was defined as any anastomotic, pelvic or perineal tumour recurrence, diagnosed radiologically, histologically or clinically. Lateral pelvic recurrence was defined as recurrence in the lateral pelvic region.
Statistical analysis
Continuous variables are expressed as median (i.q.r.) values, and compared with the Mann–Whitney U test. Survival analysis was performed using the Kaplan–Meier method, with the log rank test to compare survival of patients with ypN0 status versus mesorectal ypN1+/LLN− patients versus mesorectal ypN2+/LLN− patients versus LLN+ patients. Predictors of DFS and local recurrence were evaluated using the Cox proportional hazards model and included the following co‐variables: age, sex, distance from anal verge, grading, ypT category, LLN metastasis, mesorectal nodal metastasis, lymphovascular invasion and adjuvant chemotherapy. Results were reported using hazard ratios (HRs) with 95 per cent confidence intervals. Analyses were performed using GraphPad Prism® 7 software (GraphPad, San Diego, California, USA) or JMP® software V10.0.2 (SAS Institute, Cary, North Carolina, USA).
Results
A total of 615 patients were reviewed; two patients were excluded as they had inguinal LN metastasis, leaving 613 patients eligible for data analysis (Fig. 1). Table 1 shows clinical and pathological features of the selected cohort. LLND was performed in 212 patients (34·6 per cent).
Figure 1.
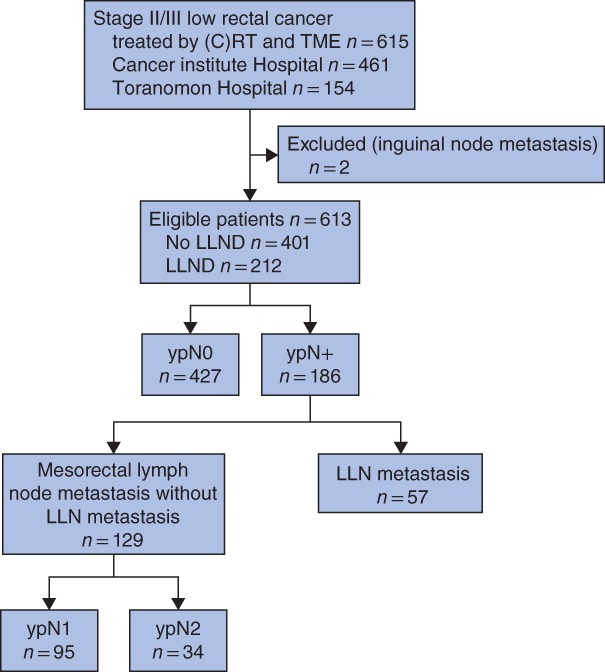
Flow diagram for the study (C)RT, (chemo)radiotherapy; TME, total mesorectal excision; LLND, lateral lymph node dissection; LLN, lateral lymph node.
Table 1.
Patient and tumour characteristics
| No. of patients* (n = 613) | |
|---|---|
| Age (years) † | 61 (52–68) |
| Sex ratio (M : F) | 415 : 198 |
| Distance from anal verge (mm) † | 40 (30–50) |
| Clinical stage | |
| I | 3 (0·5) |
| II | 213 (34·7) |
| III | 397 (64·8) |
| Histological type | |
| Well/moderately differentiated | 568 (92·7) |
| Other | 45 (7·3) |
| (Chemo)radiotherapy regimen | |
| Long course | 520 (84·8) |
| Short course | 93 (15·2) |
| Induction systemic chemotherapy | 94 (15·3) |
| Interval from completion of long‐course CRT to surgery (days) † | 48 (44–53) |
| Interval from completion of short‐course RT to surgery (days) † | 11 (9–17) |
| Operative procedure | |
| Sphincter preserving | 442 (72·1) |
| Sphincter non‐preserving | 171 (27·9) |
| LLND | |
| Overall | 212 (34·6) |
| Unilateral | 176 (83·0) |
| Bilateral | 36 (17·0) |
| Laparoscopic procedure | 575 (93·8) |
| ypT status ‡ | |
| ypT0 | 95 (15·5) |
| ypT1 | 36 (5·9) |
| ypT2 | 179 (29·2) |
| ypT3 | 279 (45·5) |
| ypT4 | 24 (3·9) |
| No. of harvested lymph nodes † | 18 (14–24) |
| R status | |
| R0 | 606 (98·9) |
| R1 | 7 (1·1) |
| Lymphovascular invasion | 333 (54·3) |
| Adjuvant chemotherapy | 292 (47·6) |
With percentages in parentheses unless indicated otherwise;
values are median (i.q.r.).
Residual tumor depth, according to the AJCC Cancer Staging Manual20. CRT, chemoradiotherapy; RT, radiotherapy; LLND, lateral lymph node dissection.
Of the 613 patients, 129 (21·0 per cent) had exclusively mesorectal LN metastasis (ypN1, 95; ypN2, 34) and 57 patients (9·3 per cent) had LLN metastasis (Fig. 1). Of the 57 patients with LLN metastasis, 26 (46 per cent) had exclusively LLN metastasis and 31 (54 per cent) had both mesorectal and LLN metastasis. Fig. 2 shows the locations of the LN metastases. The most common site of LLN metastasis was the distal internal iliac nodes (263D). The rate of LLN metastasis was higher than that of inferior mesenteric trunk node metastasis (site 252: 4·2 per cent).
Figure 2.
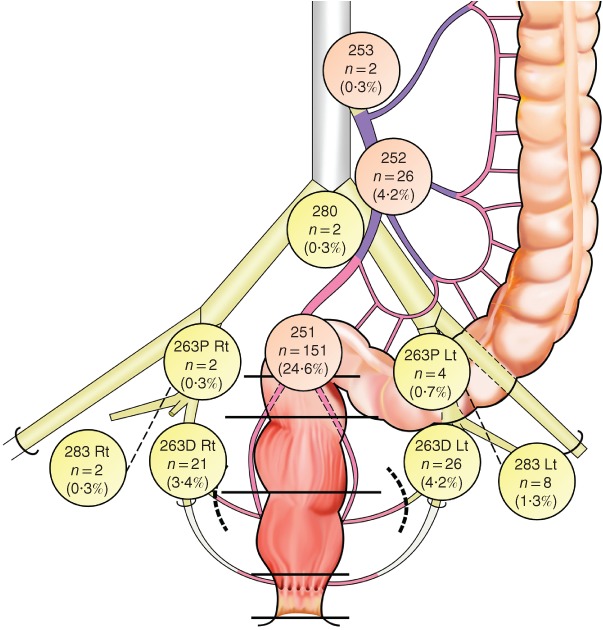
Distribution of residual lymph node metastasis after (chemo)radiotherapy in patients with advanced low rectal cancer Five patients had residual lateral lymph node metastasis in at least two different lateral areas, and three had bilateral residual lateral lymph node metastasis. Rt, right; Lt, left.
Nodal metastases
The median number of LLN metastases per patient was 1 (range 1–4). Of the 57 patients with LLN metastasis, 41 patients (72 per cent) had one metastatic node, ten (18 per cent) had two metastatic nodes, and the remaining six had three or more nodal metastases. In contrast, among the 160 patients with mesorectal LN metastasis, the median number of mesorectal LN metastases per patient was 2 (range 1–13) (P < 0·001). Sixty‐two patients (39 per cent) had one mesorectal LN metastasis, 36 (22 per cent) had two mesorectal LN metastases and 62 (39 per cent) had three or more mesorectal LN metastases. Among the 57 patients with residual LLN metastasis, 39 (68 per cent) had three or fewer total (mesorectal and lateral) LN metastases, whereas 18 (32 per cent) had four or more.
Local recurrence and survival
Median follow‐up was 51·4 (i.q.r. 36·8–66·4) months. DFS and the cumulative incidence of local recurrence according to the pathological nodal status (ypN) are summarized in Fig. 3. Twenty‐six patients had local recurrence, of whom 20 (77 per cent) had lateral pelvic recurrence. Of these 20 patients, five (25 per cent) underwent unilateral LLND and 15 (75 per cent) did not have LLND (ypN0, 4; ypN1, 5; ypN2, 6) in relation to: the absence of enlarged LLNs (less than 7 mm in long‐axis diameter) on pretreatment imaging (13 patients); refusal to undergo LLND despite the presence of enlarged LLNs (9 × 8 mm) (1 patient); and undetected enlarged LLNs (9 × 9 mm) (1 patient), noted on a second imaging revision. Of the five patients who underwent LLND, four (80 per cent) without LLN metastasis had ipsilateral lateral pelvic recurrence and one patient (20 per cent) with LLN metastasis had contralateral lateral pelvic recurrence.
Figure 3.
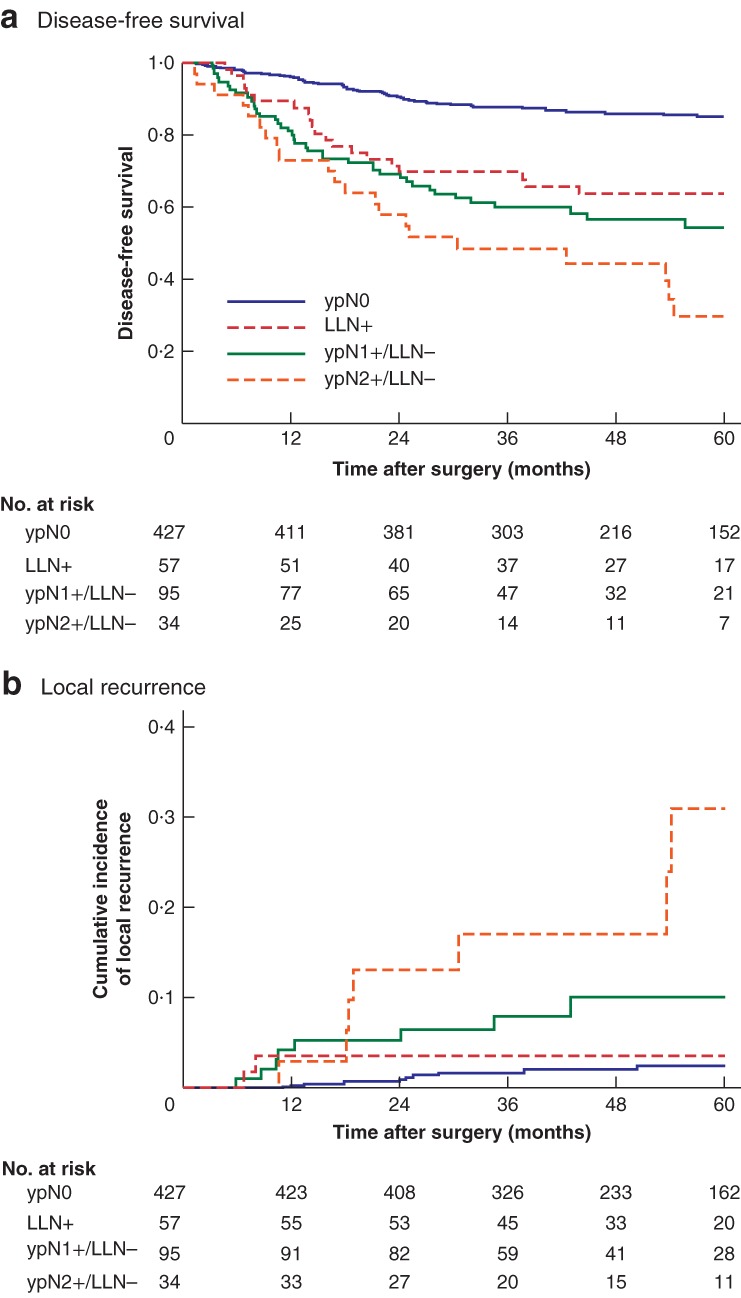
Kaplan–Meier analysis of disease‐free survival and local recurrence in patients with advanced low rectal cancer, showing the long‐term prognostic significance of ypN status after neoadjuvant (chemo)radiotherapy a Disease‐free survival and b cumulative incidence of local recurrence according to ypN metastasis status. LLN, lateral lymph node. a P < 0·001 (all groups), P < 0·001 (ypN0 versus LLN+), P = 0·014 (LLN+ versus ypN2+/LLN−); b P < 0·001 (all groups), P = 0·006 (LLN+ versus ypN2+/LLN−) (all log rank test).
DFS was significantly worse in patients with residual LLN metastasis than in those with ypN0 status (3‐year DFS rate: 70 versus 88 per cent respectively; P < 0·001), but improved in comparison with that in mesorectal ypN2+/LLN− patients (70 versus 48 per cent respectively; P = 0·014). The cumulative incidence of local recurrence in patients with LLN metastasis was significantly improved compared with that in mesorectal ypN2+/LLN− patients (3‐year local recurrence: 3·6 versus 17 per cent respectively; P = 0·006). No significant difference in the cumulative incidence of local recurrence was found for patients with LLN metastasis versus patients with ypN0 disease (3·6 versus 1·7 per cent respectively; P = 0·491) or those with mesorectal ypN1+/LLN− status (3·6 versus 8·0 per cent; P = 0·224).
In multivariable analysis, local recurrence was independently associated with distance from the anal verge of 40 mm or less (HR 2·76, 95 per cent c.i. 1·18 to 7·22; P = 0·019), ypT3–4 status (HR 3·33, 1·05 to 13·07; P = 0·040) and mesorectal LN metastasis (1–3 LNs: HR 4·35, 1·06 to 12·99; 4 or more LNs: HR 7·67, 2·35 to 25·17; P = 0·001), whereas worse DFS was independently associated with distance from the anal verge of 40 mm or less (HR 1·50, 1·07 to 2·14; P = 0·020), ypT3–4 status (HR 2·78, 1·79 to 4·41; P < 0·001), mesorectal LN metastasis (1–3 LNs: HR 3·36, 2·17 to 5·20; 4 or more LNs: HR 4·10, 2·37 to 6·98; P < 0·001) and no adjuvant chemotherapy (HR 1·72, 1·16 to 2·55; P = 0·007) (Table 2). The presence of residual LLN metastasis was not independently associated with DFS or local recurrence.
Table 2.
Univariable and multivariable Cox proportional hazards analysis of clinicopathological factors for disease‐free survival and local recurrence
| Disease‐free survival | Local recurrence | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariable analysis | Multivariable analysis | Univariable analysis | Multivariable analysis | |||||
| HR | P | HR | P | HR | P | HR | P | |
| Age (years) | 0·061 | 0·547 | 0·581 | 0·942 | ||||
| < 70 | 1·00 (reference) | 1·00 (reference) | 1·00 (reference) | 1·00 (reference) | ||||
| ≥ 70 | 1·45 (0·98, 2·10) | 1·13 (0·75, 1·68) | 1·30 (0·48, 3·06) | 1·04 (0·35, 2·68) | ||||
| Sex | 0·521 | 0·376 | 0·748 | 0·969 | ||||
| M | 1·00 (reference) | 1·00 (reference) | 1·00 (reference) | 1·00 (reference) | ||||
| F | 0·89 (0·61, 1·27) | 0·85 (0·58, 1·22) | 1·14 (0·49, 2·06) | 1·02 (0·42, 2·31) | ||||
| Distance from anal verge (mm) | 0·037 | 0·020 | 0·033 | 0·019 | ||||
| > 40 | 1·00 (reference) | 1·00 (reference) | 1·00 (reference) | 1·00 (reference) | ||||
| ≤ 40 | 1·423 (1·02, 2·01) | 1·50 (1·07, 2·14) | 2·44 (1·07, 6·26) | 2·76 (1·18, 7·22) | ||||
| Grading | 0·005 | 0·220 | 0·040 | 0·378 | ||||
| Well/moderate (G1–2) | 1·00 (reference) | 1·00 (reference) | 1·00 (reference) | 1·00 (reference) | ||||
| Other (G3–4) | 2·15 (1·28, 3·42) | 1·39 (0·81, 2·27) | 3·18 (1·06, 7·81) | 1·65 (0·51, 4·42) | ||||
| ypT status | < 0·001 | < 0·001 | < 0·001 | 0·040 | ||||
| ≤ ypT2 | 1·00 (reference) | 1·00 (reference) | 1·00 (reference) | 1·00 (reference) | ||||
| ypT3–4 | 4·08 (2·80, 6·11) | 2·78 (1·79, 4·41) | 5·99 (2·29, 20·47) | 3·33 (1·05, 13·07) | ||||
| Lateral LN metastasis | 0·042 | 0·992 | 0·766 | 0·132 | ||||
| No | 1·00 (reference) | 1·00 (reference) | 1·00 (reference) | 1·00 (reference) | ||||
| Yes | 1·69 (1·02, 2·64) | 1·00 (0·59, 1·61) | 0·81 (0·13, 2·72) | 0·37 (0·06, 1·30) | ||||
| Mesorectal LN metastasis | < 0·001 | < 0·001 | < 0·001 | 0·001 | ||||
| No | 1·00 (reference) | 1·00 (reference) | 1·00 (reference) | 1·00 (reference) | ||||
| 1–3 | 3·49 (2·41, 5·03) | 3·36 (2·17, 5·20) | 4·85 (1·95, 12·20) | 4·35 (1·06, 12·99) | ||||
| ≥ 4 | 5·13 (3·18, 8·00) | 4·10 (2·37, 6·98) | 8·96 (3·20, 24·06) | 7·67 (2·35, 25·17) | ||||
| Lymphovascular invasion | < 0·001 | 0·337 | 0·004 | 0·826 | ||||
| No | 1·00 (reference) | 1·00 (reference) | 1·00 (reference) | 1·00 (reference) | ||||
| Yes | 3·02 (2·08, 4·50) | 1·25 (0·80, 1·99) | 3·61 (1·47, 10·80) | 1·14 (0·38, 4·01) | ||||
| Adjuvant chemotherapy | 0·376 | 0·007 | 0·352 | 0·468 | ||||
| No | 1·00 (reference) | 1·00 (reference) | 1·00 (reference) | 1·00 (reference) | ||||
| Yes | 1·16 (0·84, 1·61) | 0·58 (0·39, 0·86) | 1·44 (0·67, 3·22) | 0·70 (0·28, 1·84) | ||||
Values in parentheses are 95 per cent confidence intervals. HR, hazard ratio; LN, lymph node.
Discussion
Previous studies8, 9, 10 have documented high rates of lateral pelvic recurrence in patients with enlarged LLNs not treated by LLND after (C)RT. One study8 of 116 patients with ypN+ disease reported lateral pelvic recurrence rates of 35·7 and 87·5 per cent respectively when enlarged LLNs had a short‐axis diameter of 5–9·9 mm and 10 mm or more. Similar data have been reported from the West, with a recent study11 finding a lateral local recurrence rate of 33·3 per cent at 4 years when LLNs had a short‐axis diameter of 10 mm or above. A recent multi‐institutional international retrospective study15 from 12 institutions reported that patients with LLNs and a short‐axis diameter of at least 7 mm had a significantly higher risk of lateral local recurrence than patients with LLNs of less than 7 mm. In addition, a recent survey25 showed that most radiation oncologists in the USA treat involved LLNs with curative intent and recommend treatment intensification, in the form of LLND, radiotherapy boost, or both. Collectively, these studies indicate the need to consider a standardized treatment approach.
Although patients with LLNs had a better outcome than those with mesorectal ypN2+ disease, a previous multi‐institutional study2 from Japan showed that the survival rate of patients with LLN metastasis was similar to that of patients with pN2 status when neoadjuvant (C)RT was not performed. Importantly, LLND was highly standardized in the present series with en bloc lymphadenectomy; a previous study15 in this field showed little benefit from limiting resection to the affected LLNs, as more than half of the patients later developed local recurrence in the same compartment.
LLND was probably unnecessary in 73·1 per cent of the patients. Furthermore, six patients with ypN2 status without LLN metastasis had lateral pelvic recurrence even though they showed no enlarged LLNs on imaging. Therefore, further studies are necessary to determine the optimal indication for LLND after (C)RT.
LLND is a technically challenging procedure in obese patients, and quality control, formal training with structured courses or proctoring13 might be necessary for international uptake of LLND.
Limitations of this study include the relatively shorter interval from (C)RT to surgery and the lower rate of induction chemotherapy compared with modern Western standards, which may have affected the rate of LLN metastasis. Second, patients were treated in high‐volume centres and most of the Japanese institutions do not use neoadjuvant (C)RT routinely.
A prospective randomized trial comparing TME alone versus TME with LLND after (C)RT for patients with enlarged LLNs could be difficult to design, considering the low percentage of patients with residual LLN metastasis.
Supporting information
Video S1. Lateral lymph node dissection
Appendix S1. Procedure for lateral lymph node dissection
Acknowledgements
This work was supported by Japan Society for the Promotion of Science, grant numbers 18K08664 and 18K08635. The authors thank R. Jackson for editorial support.
Disclosure: The authors declare no conflict of interest.
Funding information
Japan Society for the Promotion of Science, 18K08664, 18K08635
References
- 1. Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1986; 1: 1479–1482. [DOI] [PubMed] [Google Scholar]
- 2. Akiyoshi T, Watanabe T, Miyata S, Kotake K, Muto T, Sugihara K; Japanese Society for Cancer of the Colon and Rectum . Results of a Japanese nationwide multi‐institutional study on lateral pelvic lymph node metastasis in low rectal cancer: is it regional or distant disease? Ann Surg 2012; 255: 1129–1134. [DOI] [PubMed] [Google Scholar]
- 3. Sugihara K, Kobayashi H, Kato T, Mori T, Mochizuki H, Kameoka S et al Indication and benefit of pelvic sidewall dissection for rectal cancer. Dis Colon Rectum 2006; 49: 1663–1672. [DOI] [PubMed] [Google Scholar]
- 4. Kanemitsu Y, Komori K, Shida D, Ochiai H, Tsukamoto S, Kinoshita T et al Potential impact of lateral lymph node dissection (LLND) for low rectal cancer on prognoses and local control: a comparison of 2 high‐volume centers in Japan that employ different policies concerning LLND. Surgery 2017; 162: 303–314. [DOI] [PubMed] [Google Scholar]
- 5. Fujita S, Mizusawa J, Kanemitsu Y, Ito M, Kinugasa Y, Komori K et al; Colorectal Cancer Study Group of Japan Clinical Oncology Group . Mesorectal excision with or without lateral lymph node dissection for clinical stage II/III lower rectal cancer (JCOG0212): a multicenter, randomized controlled, noninferiority trial. Ann Surg 2017; 266: 201–207. [DOI] [PubMed] [Google Scholar]
- 6. Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R et al; German Rectal Cancer Study Group . Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004; 351: 1731–1740. [DOI] [PubMed] [Google Scholar]
- 7. Georgiou P, Tan E, Gouvas N, Antoniou A, Brown G, Nicholls RJ et al Extended lymphadenectomy versus conventional surgery for rectal cancer: a meta‐analysis. Lancet Oncol 2009; 10: 1053–1062. [DOI] [PubMed] [Google Scholar]
- 8. Kim TH, Jeong SY, Choi DH, Kim DY, Jung KH, Moon SH et al Lateral lymph node metastasis is a major cause of locoregional recurrence in rectal cancer treated with preoperative chemoradiotherapy and curative resection. Ann Surg Oncol 2008; 15: 729–737. [DOI] [PubMed] [Google Scholar]
- 9. Kim TG, Park W, Choi DH, Park HC, Kim SH, Cho YB et al Factors associated with lateral pelvic recurrence after curative resection following neoadjuvant chemoradiotherapy in rectal cancer patients. Int J Colorectal Dis 2014; 29: 193–200. [DOI] [PubMed] [Google Scholar]
- 10. Kim MJ, Kim TH, Kim DY, Kim SY, Baek JY, Chang HJ et al Can chemoradiation allow for omission of lateral pelvic node dissection for locally advanced rectal cancer? J Surg Oncol 2015; 111: 459–464. [DOI] [PubMed] [Google Scholar]
- 11. Kusters M, Slater A, Muirhead R, Hompes R, Guy RJ, Jones OM et al What to do with lateral nodal disease in low locally advanced rectal cancer? A call for further reflection and research. Dis Colon Rectum 2017; 60: 577–585. [DOI] [PubMed] [Google Scholar]
- 12. Schaap DP, Ogura A, Nederend J, Maas M, Cnossen JS, Creemers GJ et al Prognostic implications of MRI‐detected lateral nodal disease and extramural vascular invasion in rectal cancer. Br J Surg 2018; 105: 1844–1852. [DOI] [PubMed] [Google Scholar]
- 13. Perez RO, São Julião GP, Vailati BB, Fernandez LM, Mattacheo AE, Konishi T. Lateral node dissection in rectal cancer in the era of minimally invasive surgery: a step‐by‐step description for the surgeon unacquainted with this complex procedure with the use of the laparoscopic approach. Dis Colon Rectum 2018; 61: 1237–1240. [DOI] [PubMed] [Google Scholar]
- 14. Malakorn S, Ouchi A, Sammour T, Bednarski BK, Chang GJ. Robotic lateral pelvic lymph node dissection after neoadjuvant chemoradiation: view from the West. Dis Colon Rectum 2018; 61: 1119–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ogura A, Konishi T, Cunningham C, Garcia‐Aguilar J, Iversen H, Toda S et al; Lateral Node Study Consortium . Neoadjuvant (chemo)radiotherapy with total mesorectal excision only is not sufficient to prevent lateral local recurrence in enlarged nodes: results of the multicenter lateral node study of patients with low cT3/4 rectal cancer. J Clin Oncol 2019; 37: 33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Glynne‐Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A et al; ESMO Guidelines Committee . Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2017; 28(Suppl 4):iv22–iv40. [DOI] [PubMed] [Google Scholar]
- 17. São Julião GP, Habr‐Gama A, Vailati BB, Perez RO. The good, the bad and the ugly: rectal cancers in the twenty‐first century. Tech Coloproctol 2017; 21: 573–575. [DOI] [PubMed] [Google Scholar]
- 18. Hagemans JAW, Rothbarth J, van Bogerijen GHW, van Meerten E, Nuyttens JJME, Verhoef C et al Treatment of inguinal lymph node metastases in patients with rectal adenocarcinoma. Ann Surg Oncol 2019; 26: 1134–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Akiyoshi T, Ueno M, Matsueda K, Konishi T, Fujimoto Y, Nagayama S et al Selective lateral pelvic lymph node dissection in patients with advanced low rectal cancer treated with preoperative chemoradiotherapy based on pretreatment imaging. Ann Surg Oncol 2014; 21: 189–196. [DOI] [PubMed] [Google Scholar]
- 20. Edge S, Byrd DR, Compton CC, Fritz AG, Greene F, Trotti A. AJCC Cancer Staging Manual (7th edn). Springer: New York, 2010. [Google Scholar]
- 21. Japanese Society for Cancer of the Colon and Rectum . Japanese Classification of Colorectal Carcinoma (2nd edn). Kanehara: Tokyo, 2009. [Google Scholar]
- 22. Ogura A, Akiyoshi T, Nagasaki T, Konishi T, Fujimoto Y, Nagayama S et al Feasibility of laparoscopic total mesorectal excision with extended lateral pelvic lymph node dissection for advanced lower rectal cancer after preoperative chemoradiotherapy. World J Surg 2017; 41: 868–875. [DOI] [PubMed] [Google Scholar]
- 23. Twelves C, Wong A, Nowacki MP, Abt M, Burris H 3rd, Carrato A et al Capecitabine as adjuvant treatment for stage III colon cancer. New Engl J Med 2005; 352: 2696–2704. [DOI] [PubMed] [Google Scholar]
- 24. Chua YJ, Sargent D, Cunningham D. Definition of disease‐free survival: this is my truth – show me yours. Ann Oncol 2005; 16: 1719–1721. [DOI] [PubMed] [Google Scholar]
- 25. Yahya JB, Herzig DO, Farrell MJ, Degnin CR, Chen Y, Holland J et al Does a fine line exist between regional and metastatic pelvic lymph nodes in rectal cancer‐striking discordance between national guidelines and treatment recommendations by US radiation oncologists. J Gastrointest Oncol 2018; 9: 441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. Lateral lymph node dissection
Appendix S1. Procedure for lateral lymph node dissection


