Abstract
Background
Peritoneal dialysis (PD)-related infections lead to significant morbidity. The International Society for Peritoneal Dialysis (ISPD) guidelines for the prevention and treatment of PD-related infections are based on variable evidence. We describe practice patterns across facilities participating in the Peritoneal Dialysis Outcomes and Practice Patterns Study (PDOPPS).
Methods
PDOPPS, a prospective cohort study, enrolled nationally representative samples of PD patients in Australia/New Zealand (ANZ), Canada, Thailand, Japan, the UK and the USA. Data on PD-related infection prevention and treatment practices across facilities were obtained from a survey of medical directors’.
Results
A total of 170 centers, caring for >11 000 patients, were included. The proportion of facilities reporting antibiotic administration at the time of PD catheter insertion was lowest in the USA (63%) and highest in Canada and the UK (100%). Exit-site antimicrobial prophylaxis was variably used across countries, with Japan (4%) and Thailand (28%) having the lowest proportions. Exit-site mupirocin was the predominant exit-site prophylactic strategy in ANZ (56%), Canada (50%) and the UK (47%), while exit-site aminoglycosides were more common in the USA (72%). Empiric Gram-positive peritonitis treatment with vancomycin was most common in the UK (88%) and USA (83%) compared with 10–45% elsewhere. Empiric Gram-negative peritonitis treatment with aminoglycoside therapy was highest in ANZ (72%) and the UK (77%) compared with 10–45% elsewhere.
Conclusions
Variation in PD-related infection prevention and treatment strategies exist across countries with limited uptake of ISPD guideline recommendations. Further work will aim to understand the impact these differences have on the wide variation in infection risk between facilities and other clinically relevant PD outcomes.
Keywords: infection, peritoneal dialysis, peritonitis
INTRODUCTION
Peritoneal dialysis (PD)-related infections, including peritonitis, are the leading cause of technique failure and PD-related hospitalization episodes for patients receiving PD [1, 2]. Peritonitis is associated with long-term alterations to the peritoneal membrane and an increased risk of death [3–8]. Variation in peritonitis risk has been reported between centers, both within and between countries [1]. Moreover, center-related factors are increasingly recognized as important determinants of the variabilities in the incidence and outcomes of peritonitis between facilities [9–12].
National and international consensus guidelines, such as those produced by the International Society for Peritoneal Dialysis (ISPD), continue to focus on identifying best practices associated with the prevention and treatment of PD-related infections [9, 11]. While such guidelines provide several strong (level 1A or 1B) recommendations, many remain supported by a limited evidence base.
The Peritoneal Dialysis Outcomes and Practice Patterns Study (PDOPPS) is conducted in collaboration with the ISPD and is based on the hypothesis that variations in local practice patterns may account for an appreciable proportion of the observed variation in PD outcomes across centers [13]. Identification of optimal practices could help critically inform strategies to reduce the incidence of PD-related infections and improve outcomes following PD-related infections, thereby reducing unwanted variation in clinical outcomes. As a preliminary step in PDOPPS, our objective in the present report was to describe variations in local practices associated with the prevention and treatment of PD-related infections, with a specific emphasis on adherence to those endorsed by the ISPD guidelines.
MATERIALS AND METHODS
PDOPPS is an international prospective cohort study of PD patients ≥18 years of age. Patients in the PDOPPS are enrolled randomly from a representative sample of PD facilities within Australia, Canada, Japan, New Zealand, Thailand, the UK and the USA, in collaboration with the ISPD as described previously [13]. Study approval was obtained by a central institutional review board. Additional study approval and patient consent were obtained as required by national and local ethics committee regulations [13].
Facility practices regarding the prevention and treatment of PD-related infections were collected via a survey of medical directors in each facility at PDOPPS enrollment. Facility characteristics, including facility size, mean patient age, percentage of patients with diabetes and percentage of patients prescribed automated PD (APD) were derived from patient-level data collected at study commencement. Comparison between countries was made with respect to ISPD guidelines related to monitoring of peritonitis incidence, use of prophylactic antimicrobials to prevent PD-related infections and the initial (empiric) treatment of suspected peritonitis. Other facility characteristics were obtained from a survey of the nurse manager in each facility. Data quality was ensured through standardized protocols, uniform data collection forms, training and monitoring of study sites.
Data are presented from Canada and the USA (commenced recruitment in January 2014), Japan (commenced recruitment in June 2014), Australia and New Zealand (commenced recruitment in January 2015), the UK (commenced recruitment in August 2015) and Thailand (commenced recruitment in May 2016). Australia and New Zealand were analyzed together. Data collected as of July 2017 were included.
Data are summarized descriptively as mean ± standard deviation (or medians and interquartile ranges) for continuous data and proportions for categorical data. All analyses used SAS software, version 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
Demographics
Medical director surveys were returned from 170 (83%) of the 206 participating PD units from six countries: 65 from the USA, 28 from Japan, 20 from Canada, 18 from Australia/New Zealand (ANZ), 22 from Thailand and 17 from the UK (see Table 1). The range of facility sizes differed appreciably between countries, with Thailand having the largest proportion of PD units with >100 patients (55%) compared with none in the UK.
Table 1.
PD facility characteristics in PDOPPS
| Facility characteristics | ANZ | Canada | Japan | Thailand | UK | USA |
|---|---|---|---|---|---|---|
| Number of facilitiesa | 18 | 20 | 28 | 22 | 17 | 65 |
| Facility size, median (IQR) | 56.0 (47.0–82.0) | 51.5 (40.0–92.5) | 29.0 (22.0–34.5) | 104 (49–215) | 51.0 (38.0–64.0) | 38.0 (25.0–57.0) |
| Facility aggregated patient characteristics | ||||||
| Patient age (years), median (IQR) | 62.5 (61.4–63.6) | 62.7 (60.3–65.4) | 63.8 (61.6–66.3) | 56.0 (54.2–57.9) | 62.4 (60.8–64.4) | 56.4 (55.0–59.8) |
| Patients with diabetes (%), median (IQR) | 38 (33–47) | 43 (40–50) | 31 (23–53) | 46 (41–51) | 31 (23–35) | 56 (55–60) |
| Patients prescribed APD (%), median (IQR) | 65 (52–86) | 74 (54–86) | 35 (18–52) | 0 (0–4) | 60 (50–89) | 88 (76–95) |
| Facility location within a hospital, % | 27 | 69 | 100 | 95 | 93 | 2 |
| Facility affiliated with a university, % | 44 | 37 | 36 | 9 | 53 | 18 |
| PD facility age (years), mean (SD) | 25.8 (8.5) | 26.3 (13.4) | 26.7 (5.1) | 11.5 (8.3) | 30.5 (6.0) | 16.8 (10.1) |
| Physician:patient ratio, median (IQR) | 1:10 (1:22–1:5) | 1:15 (1:22–1:9) | 1:7 (1:11–1:4) | 1:64 (1:100–1:19) | 1:26 (1:48–1:18) | 1:8 (1:15–1:5) |
| Nurse:patient ratio, median (IQR) | 1:12 (1:16–1:10) | 1:15 (1:17–1:11) | 1:6 (1:8–1:3) | 1:39 (1:51–1:19) | 1:8 (1:10–1:6) | 1:14 (1:17–1:9) |
| In-center HD provided on-site, % | 44 | 95 | 92 | 91 | 88 | 71 |
| Routine home HD care provided on-site, % | 88 | 70 | 32 | 5 | 82 | 64 |
| Percentage of nurses who care for PD patients and also provide care for in-center HD patients | ||||||
| None | 56 | 70 | 8 | 23 | 65 | 74 |
| <10% | 19 | 10 | 4 | 23 | 29 | 18 |
| 10–60% | 6 | 15 | 28 | 23 | 6 | 5 |
| >60% | 19 | 5 | 60 | 32 | 0 | 3 |
| Routine multidisciplinary review, % | 44 | 40 | 68 | 46 | 82 | 78 |
| Laboratory program on-site, % | 44 | 45 | 91 | 100 | 81 | 5 |
Facilities that completed the medical director survey by 12 July 2017 were included in this analysis.
IQR, interquartile range; SD, standard deviation.
The participating units included data on 11 389 patients. The proportion of male patients ranged from 49% in Thailand to 66% in Japan. The most common age group in all countries was 60–74 years. The mean body mass index ranged from 22.5 kg/m2 in Thailand to 28.5 kg/m2 in the USA. The causes of end-stage kidney disease (ESKD) also varied between countries, although most had a preponderance of diabetic nephropathy and chronic glomerulonephritis.
ISPD guideline: we recommend that every program should monitor, at least on a yearly basis, the incidence of catheter-related infections (evidence level 1C)
As part of routine clinical practice, the majority of facilities in all countries performed recording of peritonitis episodes, although only 61% of facilities did so in Japan (Table 2). Calculation of peritonitis rates was performed at least annually in 99% of facilities in the USA, 95% in Canada, 94% in ANZ and the UK, 86% in Thailand and 14% in Japan (Table 2). Target peritonitis rates varied between facilities within the same country, with the median facility’s target for countries ranging from 0.55 episodes per patient-year in the UK to 0.21 episodes per patient-year in Japan. Self-reported peritonitis rates (Supplementary data, Figure S1) above target ranged from 12% in Thailand to 50% in Japan and the USA. Similar patterns were observed for the recording of exit-site infections.
Table 2.
PD-related infection audit practices among facilities in PDOPPS (percentage within country)
| Facility characteristics | ANZ | Canada | Japan | Thailand | UK | USA |
|---|---|---|---|---|---|---|
| Number of facilities | 18 | 20 | 28 | 22 | 17 | 65 |
| Record and track peritonitis episodes, % | 100 | 100 | 61 | 96 | 100 | 100 |
| Record and track exit-site infection episodes, % | 100 | 95 | 41 | 91 | 88 | 98 |
| Frequency of calculating peritonitis rates, % | ||||||
| At least annually | 94 | 95 | 14 | 86 | 94 | 99 |
| Less often than annually | 6 | 5 | 43 | 14 | 6 | 0 |
| Never | 0 | 0 | 43 | 0 | 0 | 2 |
| Frequency of calculating exit-site infection rates, % | ||||||
| At least annually | 89 | 90 | 7 | 91 | 82 | 97 |
| Less often than annually | 11 | 5 | 29 | 5 | 6 | 0 |
| Never | 0 | 5 | 64 | 5 | 12 | 3 |
| Target peritonitis rate, median (IQR) episode per patient yeara, median (IQR) | 0.33 (0.25–0.50) | 0.39 (0.25–0.50) | 0.21 (0.20–0.30) | 0.50 (0.40–0.67) | 0.55 (0.41–0.67) | 0.33 (0.13–0.40) |
| Self-reported peritonitis rate above targeta, % | 46 | 22 | 50 | 12 | 36 | 50 |
Among facilities that routinely record and track peritonitis episodes. IQR, Interquartile range.
ISPD guideline: we recommend daily topical application of antibiotic cream or ointment to the catheter exit site (evidence level 1A)
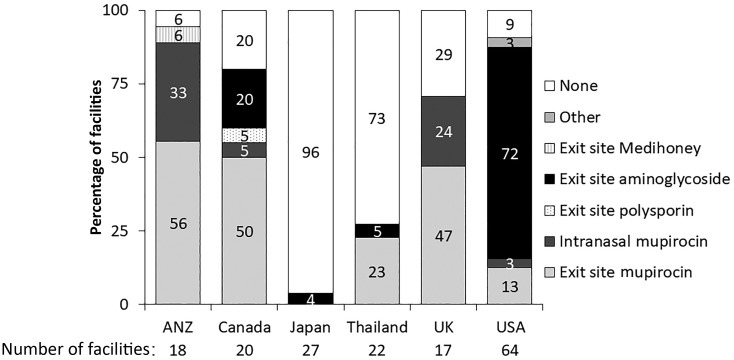
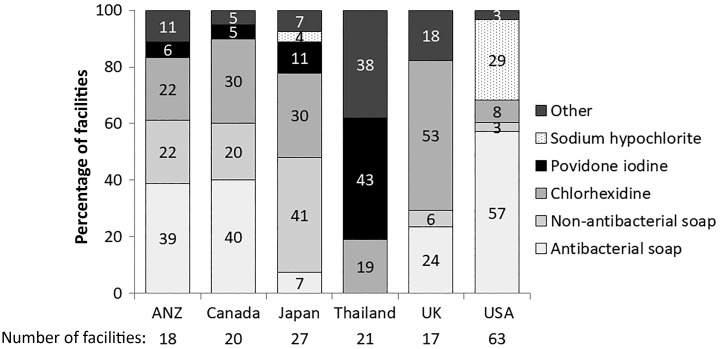
Topical antimicrobial prophylaxis varied between countries, with ANZ using mupirocin extensively (89%: 56% on the exit site and 33% intranasal application), compared with Japan, which only used exit-site prophylaxis in 4% of units (Figure 1). Cleaning strategies for exit sites varied between countries—antibacterial soap was used in 39% of ANZ units, 40% of Canadian units and 57% of US units (Figure 2). In contrast, the most common strategy employed in Japanese units was non-antibacterial soap (41%). When we examined facility characteristics, we noted that those facilities that followed this guideline tended to be larger, have more incident patients commencing PD on APD, were less likely to be located within a hospital, were less likely to be colocated with an in-center hemodialysis (HD) unit, had more routine home HD care provided on-site, had PD nurses that were less likely to provide care for in-center HD patients and were less likely to have a laboratory program on-site (Supplementary data, Table S1).
FIGURE 1.
Facility exit-site antimicrobial prophylaxis, by country involved in PDOPPS. One facility each in Japan and the USA did not answer the exit-site antimicrobial prophylaxis question.
FIGURE 2.
Exit-site cleaning strategies, by country involved in PDOPPS. 14% of facilities in Thailand, 13% of facilities in the USA, 10% of facilities in Japan and none in other countries routinely alternate between various topical antibiotic preparations within patients. One facility each in Japan and Thailand and two facilities in the USA did not answer the exit-site cleaning strategies question.
When we examined for differences within individual countries, the facilities that followed this guideline were larger in ANZ, Canada and the USA, smaller in Japan and Thailand and no difference among facilities in the UK. For physician:patient ratio, the facilities that followed this guideline had a larger ratio of patients in ANZ, smaller in Japan and Thailand and the same in the rest of the countries. For nurse:patient ratio, the facilities that followed this guideline had a larger ratio of patients in the USA, lower in Thailand and the UK and no difference among facilities in ANZ, Canada and Japan.
ISPD guideline: we recommend that prophylactic antibiotics be administered immediately before catheter insertion (evidence level 1A) and we recommend antifungal prophylaxis when PD patients receive antibiotic courses to prevent fungal peritonitis (evidence level 1B)
Antibiotic prophylaxis at the time of PD catheter insertion was universal only in Canada and the UK (Table 3). In other countries, administration of prophylactic antibiotics prior to PD catheter insertion varied from 63% in the USA to 89% in Japan (Table 3). Similarly, antibiotic prophylaxis was heterogeneously given for procedures that potentially carried a risk of subsequent peritonitis (ISPD guideline with evidence level 2C and 2D) [9]. Routine use of antifungal prophylaxis during antibiotic therapy also varied considerably between countries (Figure 3). Facilities that did prescribe antibiotics at the time of PD catheter insertion were larger, had fewer new PD patients starting on APD, were more likely to be located within a hospital, were more likely to be affiliated with a university, were established for longer period and were more likely to have a laboratory program on-site (Supplementary data, Table S1).
Table 3.
Use of antibiotic prophylaxis and screening for S. aureus among facilities in PDOPPS (percentage within country)
| Facility characteristics | ANZ | Canada | Japan | Thailand | UK | USA |
|---|---|---|---|---|---|---|
| Number of facilities | 18 | 20 | 28 | 22 | 17 | 65 |
| Procedures/situations where antibiotic prophylaxis is used or recommended at your center: | ||||||
| 1. PD catheter insertion, % | 83 | 100 | 89 | 86 | 100 | 63 |
| 2. Nonsurgical PD catheter manipulation, % | 47 | 60 | 29 | 32 | 62 | 33 |
| 3. Routine dental procedures, % | 22 | 35 | 7 | 29 | 0 | 51 |
| 4. Complicated dental procedures, % | 61 | 70 | 68 | 43 | 24 | 83 |
| 5. Gynecological procedures, % | 53 | 40 | 32 | 48 | 53 | 65 |
| 6. Genitourinary procedures, % | 47 | 35 | 41 | 45 | 47 | 53 |
| 7. Upper gastrointestinal endoscopy, % | 17 | 25 | 7 | 36 | 0 | 31 |
| 8. Lower gastrointestinal endoscopy, % | 61 | 65 | 36 | 40 | 77 | 66 |
| 9. Wet contaminationa, % | 94 | 90 | 68 | 68 | 88 | 91 |
| No antibiotic use for procedures 3–8, % | 35 | 25 | 12 | 42 | 12 | 10 |
| Routinely screen patients for nasal carriage of S. aureus, % | ||||||
| Yes, only once | 22 | 10 | 18 | 5 | 12 | 6 |
| Yes, on a recurrent basis in patients previously identified as carriers | 22 | 5 | 4 | 0 | 6 | 6 |
| Yes, on recurrent basis in all patients | 28 | 25 | 0 | 0 | 59 | 6 |
| Never | 28 | 60 | 79 | 96 | 24 | 81 |
| Eradicate S. aureus carriage with intranasal mupirocin, % | 92 | 63 | 33 | 0 | 92 | 80 |
Wet contamination includes disconnection between the transfer set and the catheter at the connector, a hole in the transfer set or the catheter or accidental opening of the transfer set and escape of PD fluid.
FIGURE 3.
Antifungal prophylaxis use, by country involved in PDOPPS.
When we examined for differences within individual countries, the facilities that followed antibiotic prophylaxis at the time of catheter insertion were larger in Thailand, smaller in ANZ and Japan and no difference among facilities in the USA. For physician:patient ratio, the facilities that followed this guideline had a larger ratio of patients in Thailand, smaller in ANZ and Japan and the same in USA. For nurse:patient ratio, the facilities that followed this guideline had a larger ratio of patients in Thailand, lower in ANZ and no difference among facilities in Japan and the USA.
Empiric antibiotics for peritonitis treatment
The choice of empiric antibiotics also varied between countries, with the most commonly prescribed being vancomycin (83%) and cephalosporin (second generation or higher; 60%) in the USA; vancomycin (88%) and aminoglycoside (77%) in the UK; cephalosporin (first generation; 72%) and aminoglycoside (72%) in ANZ and dual cephalosporin use in Canada (70% first generation and 50% second generation or higher) and Japan (63% for first generation and second generation or higher) (Table 4). Notable variation was seen in the self-administration of empiric antibiotics prior to presentation to a health professional, with this occurring for all patients in 40% of units in the USA compared with none of the units in Japan, Thailand and the UK. In addition, the initial route of administration of antibiotics for the treatment of exit-site infections varied between countries, with topical administration varying from 0% in ANZ to 25% in Canada and Japan.
Table 4.
Antibiotic prescription for prevention and treatment of peritonitis among facilities in PDOPPS (percentage within country)
| Facility characteristics | ANZ | Canada | Japan | Thailand | UK | USA |
|---|---|---|---|---|---|---|
| Number of facilities | 18 | 20 | 28 | 22 | 17 | 65 |
| Antibiotics at home prior to seeking medical attention, % | ||||||
| Yes, all patients | 6 | 10 | 0 | 0 | 0 | 40 |
| Yes, some patients | 0 | 20 | 15 | 5 | 6 | 11 |
| No patients | 94 | 70 | 85 | 96 | 94 | 49 |
| After-hours nurse provides care at PD facility, % | 7 | 25 | 33 | 55 | 12 | 55 |
| Number of antibiotics against Pseudomonas aeruginosa, % | ||||||
| 1 only | 11 | 5 | 56 | 27 | 29 | 15 |
| 2 | 89 | 95 | 44 | 68 | 65 | 83 |
| >2 | 0 | 0 | 0 | 5 | 6 | 2 |
| Initial antibiotic administration for treatment of an exit-site infection, % | ||||||
| Oral | 83 | 75 | 68 | 82 | 71 | 73 |
| Intraperitoneal | 17 | 0 | 7 | 0 | 18 | 19 |
| Topical | 0 | 25 | 25 | 18 | 12 | 8 |
| Initial antibiotic administration for PD catheter tunnel infection, % | ||||||
| Oral | 28 | 50 | 86 | 57 | 53 | 43 |
| Intraperitoneal | 61 | 45 | 4 | 10 | 18 | 56 |
| Other | 11 | 5 | 11 | 33 | 29 | 2 |
| Empiric antibiotic treatment for peritonitisa, % | ||||||
| First-generation cephalosporin | 72 | 70 | 63 | 91 | 0 | 40 |
| Second- or third-generation cephalosporin | 33 | 50 | 63 | 91 | 12 | 60 |
| Vancomycin | 33 | 45 | 15 | 10 | 88 | 83 |
| Aminoglycoside | 72 | 45 | 26 | 10 | 77 | 32 |
| Other | 11 | 35 | 37 | 10 | 41 | 17 |
Medical directors could choose more than one answer so column percentages may not add up to 100%.
DISCUSSION
In this multicenter, observational, international study of PD patients, we found that significant practice differences exist between facilities as summarized here by participating country. Differences were most notable in the domains of frequency and monitoring of PD-related infection rates, use of periprocedural antibiotic prophylaxis, type of exit-site cleaning strategy, use and choice of exit-site antimicrobial prophylaxis strategies and the use of antifungal therapy during a course of antibiotic therapy. With regard to peritonitis treatment practices, significant differences existed with initial patient self-administration of antibiotics, route of antibiotic administration preferred for the treatment of exit-site and tunnel infections, as well as the choice of empiric antibiotic therapy in the initial treatment of suspected PD peritonitis.
Our findings are highly concerning given that deviation from ISPD guidelines was demonstrated to be associated with suboptimal PD outcomes in a recent report from Australia [8]. In our study, a key finding was the lack of adherence at some facilities to those practices for which a strong evidence base exists and supported by level 1A or 1B ISPD guidelines [9, 11]. In this regard, it was disappointing to note that routine monitoring of PD infection rates was not universal across all participating PD facilities. Moreover, four randomized controlled trials (RCTs) and a systematic review have demonstrated the benefit of antibiotic prophylaxis at the time of PD catheter insertion (level 1A ISPD guideline) [9, 14–17]. While this practice was universally applied in all surveyed facilities in Canada and the UK, only 63% of facilities in the USA reported using prophylactic antibiotics at the time of PD catheter insertion and between 82% and 89% of facilities across other countries. Similar practice deviation has been found in other local survey studies both from Canada and Australia [8, 18]. We have identified some overall facility characteristics that differ between PD units that adhered to grade 1A guidelines and those that did not. However, some of the characteristics seem to be inconsistent across guidelines. A more detailed exploration is warranted. Within individual countries, there was some consistency in the differences between facilities that followed versus those that did not follow guidelines, but the patterns varied across countries.
An alternative explanation for the above findings is that antibiotics were in fact administered outside of the PD unit during routine surgical perioperative care without medical directors being aware of what occurred, which would in and of itself be concerning. This may be particularly relevant in the USA, where PD catheter insertion typically occurs at a location distant to the PD facility, unlike in Canada, the UK and ANZ, where both the PD care and catheter insertion typically take place at the same site. Alternatively, it is possible that limited knowledge of existing practice guidelines may have led to a lack of universal adoption of this practice across all facilities. This may be particularly salient in the USA (but not isolated to just this country), where education regarding management of patients on PD has been previously cited as a major gap in many nephrology training programs [19, 20].
We observed significant variation between facilities in the use of antifungal prophylaxis during antibiotic therapy among centers in the PDOPPS, with the highest routine use in ANZ compared with minimal use in facilities in Japan, Thailand and the UK (Figure 3). The development of fungal peritonitis carries with it significant morbidity and mortality [21]. Moreover, a significant proportion of fungal peritonitis episodes occur following a course of antibiotics [22]. Based on these observations coupled with the findings of two RCTs and a systematic review, the ISPD guidelines recommend antifungal prophylaxis with either oral nystatin or fluconazole when PD patients receive antibiotic courses (level 1B evidence) [9, 14, 23]. The particularly high uptake in ANZ may reflect ongoing continuous quality improvement initiatives, including a recent call to action in which the use of antifungal prophylaxis was strongly and locally endorsed [8, 24, 25]. In contrast, it is possible that the low rates observed across other countries may reflect the cost and availability of antifungal agents or inherently lower baseline rates of fungal peritonitis where antifungal prophylaxis may be perceived to be less beneficial. This will require confirmation via further enquiry into peritonitis events within PDOPPS.
Also notable is the significant variation in the use of peri-procedural antibiotic prophylaxis among procedures, including dental procedures, lower endoscopy and gynecologic procedures (Table 3). The use of periprocedural antibiotics is predicated on the observation that a number of procedures have been associated with an increased risk of peritonitis [26, 27]. For example, there have been several reports of peritonitis due to enteric organisms following a lower gastrointestinal endoscopy procedure [26, 28]. The significant variation that we observed in the use of periprocedural antibiotics may relate to the limited evidence that exists to support these recommendations. Taken together, these findings may suggest the need for a stronger evidence base to support these practices.
ISPD guidelines (level 1B) recommend daily topical application of antibiotic (mupirocin or gentamicin) cream or ointment to the catheter exit site. This guideline was based on several studies demonstrating the beneficial effect of topical mupirocin in reducing the risk of Staphylococcus aureus and overall catheter infections and a reduction in the risk of peritonitis [14, 29]. The recommendation for gentamicin was based on a single RCT comparing exit-site mupirocin versus gentamicin [30]. In this study, among the 133 PD patients randomized, patients in the gentamicin group had a reduction in Gram-negative catheter infections and an overall reduction in peritonitis episodes [30].
In Japan, Thailand and the UK, 96%, 73% and 29% of facilities, respectively, reported using no antimicrobial exit-site strategy, while some form of prophylaxis was used in 94% of facilities in ANZ, 91% of facilities in the USA and 80% of facilities in Canada. Possible reasons for the considerable variation in exit-site antimicrobial strategies may include variable regional access to guidelines (including guideline translation), physician and treatment team bias and economic constraints that may limit medication availability, particularly in Thailand. Indeed, in Japan, the use of mupirocin is limited only to the eradication of nasal methicillin-resistant S. aureus (MRSA) carriage and is not permitted for exit-site application. Among centers that reported an exit-site antimicrobial strategy, mupirocin was the most common strategy in ANZ, Canada, the UK and Thailand while exit-site aminoglycoside was used in 72% of facilities in the USA. It is interesting to note that the RCT demonstrating the superiority of gentamicin was conducted in the USA, which may have led to its overwhelming application there [30]. In addition, concern about the potential development of mupirocin resistance may be influencing prescribing habits [31, 32].
Given that two large dialysis organizations participate in PDOPPS in the USA, policies and protocols across these organizations’ sites may have dictated a global preference for the use of an exit-site aminoglycoside. Although exit-site aminoglycoside use may provide greater benefit, particularly for Gram-negative catheter infection and peritonitis, two observational studies have shown no difference in overall infection and peritonitis rates comparing exit-site mupirocin and gentamicin [33, 34]. Local profiles of pathogens associated with exit-site infections and their susceptibilities need to be considered however, with the prescription of the most appropriate agent made [35]. Future PDOPPS research will evaluate the risk and outcomes of exit-site infections and peritonitis based on exit-site antimicrobial strategy and will also evaluate organism-specific rates.
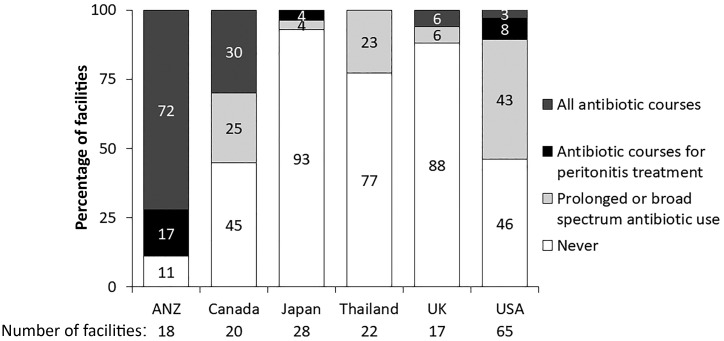
It is worth noting that a number of practices varied with respect to the treatment of PD-related infections. Interestingly, self-administration of antibiotics at home by patients was rarely endorsed, with the exception of USA, where it was endorsed by 40% of facilities. It has been previously shown that PD patients in the USA who reside in rural locations have a higher risk of adverse outcomes [36]. It is tempting to speculate that this strategy may be particularly useful for these patients to circumvent a delay in presentation to a health care facility for antibiotics after suspected peritonitis. However, such a practice may increase the risk of culture-negative peritonitis due to potential antibiotic treatment prior to proper PD effluent sampling. A recent study from Australia demonstrated the benefit of earlier administration of antibiotics in suspected peritonitis with respect to achieving successful treatment [37]. Taken together, further study is necessary to evaluate the impact of initial administration of antibiotics at home by patients for suspected peritonitis.
We also observed that empiric peritonitis treatment, which included vancomycin, was most common in the UK (88%) and USA (83%) versus 10–45% elsewhere. Although a recent Cochrane systematic review demonstrated that a vancomycin-based regimen appears optimal for the complete cure of peritonitis, the evidence for this finding was assessed as low quality [38]. The basis for the wide variability in vancomycin use is unclear but may relate in part to ease of administration of vancomycin, which does not require daily administration. In addition, it may be driven by local patterns of antimicrobial resistance, particularly with regard to a high rate of methicillin-resistant organisms, which will be the subject of future investigation in PDOPPS. Concerns about higher levels of Clostridium difficile infection may also be driving the preferential use of vancomycin over other agents, such as cephalosporins [39].
The strengths of this study include its widespread coverage, including national samples of PD facilities across several countries with a high rate of survey completion among sites. However, there are several limitations. As with all surveys, a potential limitation is responder bias, such as those due to acquiescence and social desirability (although this would tend to overestimate adherence to guidelines). Moreover, the views and practices collected by the survey were those of the medical director survey respondents. The responses, therefore, may reflect unit policy or the respondent’s biases. It is possible that, even within a given facility, significant practice pattern variation exists among treating clinicians for those practices under study. To partially circumvent these limitations, several key practices, such as exit-site antimicrobial agent used and choice of antibiotic at initial peritonitis presentation, are being captured with prospective collection of patient-level data in the PDOPPS and will be topics of future analyses.
Notwithstanding these limitations, this study demonstrated that across an international representative sample of PD facilities there were considerable differences in PD-related infection prevention and treatment strategies. A number of reported practices in different countries deviated substantially from ISPD guideline recommendations, some of which were underpinned by a stronger level of evidence.
Future efforts are under way by PDOPPS in collaboration with the ISPD and a recently awarded ancillary study by the Agency for Healthcare Research and Quality in the USA. This proposal will examine factors associated with a lower risk of peritonitis within PDOPPS on several fronts: (i) to better understand the reasons for these deviations by facilities, (ii) to address existing gaps in guideline dissemination and adoption while identifying novel opportunities for knowledge translation and (iii) to determine the impact that variations in guideline adherence have on clinically relevant outcomes, including the risk of infection. It is highly conceivable that the notable differences in peritonitis rates between jurisdictions may be related to differences in practice patterns and that standardization to the optimal practices may lead to reductions in the variability between units and an improvement in patient outcomes.
SUPPLEMENTARY DATA
Supplementary data are available at ndt online.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank participating center medical directors, nurses, adminstrators, research coordinators and patients who allowed for data collection for the PDOPPS.
FUNDING
Funding for PDOPPS has been provided by the National Health and Medical Research Council (Australia); National Institute for Health Research (UK); National Institute of Diabetes and Digestive and Kidney Diseases (USA); Patient-Centered Outcomes Research Institute (USA); Japanese Society of Peritoneal Dialysis; Canadian Institute for Health Research (Canada); Baxter International (USA); National Research Council of Thailand (2558-113); Rachadaphiseksompot Endorsement Fund (GCURS_59_12_30_03), Chulalongkorn University, Thailand and the Thailand Research Foundation (IR65780017), Thailand.
AUTHORS’ CONTRIBUTIONS
N.B., J.Z., B.A.B., R.L.P., D.W.J., B.P., J.B., S.J.N., Y.I., G.W., F.B., J.C., T.K., C-C.S. and J.P. were responsible for conception or design and/or analysis and interpretation of data. N.B., J.Z., B.A.B., R.L.P., D.W.J., B.P., J.B., S.J.N., Y.I., G.W., F.B., J.C., T.K., C-C.S. and J.P. were responsible for drafting and/or revising the article. N.B., J.Z., B.A.B., R.L.P., D.W.J., B.P., J.B., S.J.N., Y.I., G.W., F.B., J.C., T.K., C-C.S. and J.P. were responsible for providing intellectual content of critical importance to the work described. N.B., J.Z., B.A.B., R.L.P., D.W.J., B.P., J.B., S.J.N., Y.I., G.W., F.B., J.C., T.K., C-C.S. and J.P. provided final approval of the version to be published.
CONFLICT OF INTEREST STATEMENT
N.B. has received unrestricted educational grants, research grants, consultancy fees and speakers’ honoraria from Roche Pharmaceuticals, Baxter Healthcare and AstraZeneca. Y.I. has received lecture fees and is supported as an endowment from Baxter and has received lecture fees from Terumo and JMS. D.W.J. has received consulting/advisory board fees from AstraZeneca and lecture fees from Baxter Healthcare and Fresenius Medical Care and is supported by grants from Baxter. D.W.J. is a current recipient of a National Health and Medical Research Practitioner Fellowship. S.J.N. has received consulting/advisory board and lecture fees from Baxter Healthcare, consulting fees from Fresenius and advisory board fees from Boehringer Ingelheim. F.B. has received consulting and lecture fees from Baxter Healthcare. T.K. has received lecture fees and travel support from Baxter Pharmaceutical and Fresenius Medical Care. C.-C.S. has received lecture fees and is supported by a grant from Baxter Healthcare. J.P. has received speaking honoraria from Baxter Healthcare and has received consulting fees from Baxter Healthcare, Fresenius Medical Care, Otsuka, Janssen Ortho Shire, Takeda and Boehringer Ingelheim as well as research support from Baxter Healthcare and salary support from Arbor Research Collaborative for Health.
REFERENCES
- 1.ANZDATA Registry. 39th Report, Chapter 1: Incidence of End Stage Kidney Disease. Australia and New Zealand Dialysis and Transplant Registry, Adelaide, Australia, 2017. Available at: http://www.anzdata.org.au (25 June 2018, date last accessed).
- 2. Ghali JR, Bannister KM, Brown FG.. Microbiology and outcomes of peritonitis in australian peritoneal dialysis patients. Perit Dial Int 2011; 31: 651–662 [DOI] [PubMed] [Google Scholar]
- 3. Mujais S. Microbiology and outcomes of peritonitis in North America. Kidney Int 2006; 70: S55–S62 [DOI] [PubMed] [Google Scholar]
- 4. Boudville N, Kemp A, Clayton P. et al. Recent peritonitis associates with mortality among patients treated with peritoneal dialysis. J Am Soc Nephrol 2012; 23: 1398–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fried LF, Bernardini J, Johnston JR. et al. Peritonitis influences mortality in peritoneal dialysis patients. J Am Soc Nephrol 1996; 7: 2176–2182 [DOI] [PubMed] [Google Scholar]
- 6. Fried L, Abidi S, Bernardini J. et al. Hospitalization in peritoneal dialysis patients. Am J Kidney Dis 1999; 33: 927–933 [DOI] [PubMed] [Google Scholar]
- 7. Guo A, Mujais S.. Patient and technique survival on peritoneal dialysis in the United States: evaluation in large incident cohorts. Kidney Int 2003; 64(Suppl 88): S3–S12 [DOI] [PubMed] [Google Scholar]
- 8. Jose MD, Johnson DW, Mudge DW. et al. Peritoneal dialysis practice in Australia and New Zealand: a call to action. Nephrology 2011; 16: 19–29 [DOI] [PubMed] [Google Scholar]
- 9. Li PK, Szeto CC, Piraino B. et al. ISPD peritonitis recommendations: 2016 update on prevention and treatment. Perit Dial Int 2016; 36: 481–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nadeau-Fredette A-C, Johnson DW, Hawley CM. et al. Centre-specific factors associated with peritonitis risk—a multi-center registry analysis. Perit Dial Int 2016; 36: 509–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Szeto CC, Li PK, Johnson DW. et al. ISPD catheter-related infection recommendations: 2017 update. Perit Dial Int 2017; 37: 141–154 [DOI] [PubMed] [Google Scholar]
- 12. Htay H, Cho Y, Pascoe EM. et al. Multicenter registry analysis of center characteristics associated with technique failure in patients on incident peritoneal dialysis. Clin J Am Soc Nephrol 2017; 12: 1090–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Perl J, Davies SJ, Lambie M. et al. The Peritoneal Dialysis Outcomes and Practice Patterns Study (PDOPPS): unifying efforts to inform practice and improve global outcomes in peritoneal dialysis. Perit Dial Int 2016; 36: 297–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Campbell D, Mudge DW, Craig JC. et al. Antimicrobial agents for preventing peritonitis in peritoneal dialysis patients. Cochrane Database Syst Rev 2017; 4: CD004679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wikdahl AM, Engman U, Stegmayr BG. et al. One-dose cefuroxime i.v. and i.p. reduces microbial growth in PD patients after catheter insertion. Nephrol Dial Transplant 1997; 12: 157–160 [DOI] [PubMed] [Google Scholar]
- 16. Gadallah MF, Ramdeen G, Mignone J. et al. Role of preoperative antibiotic prophylaxis in preventing postoperative peritonitis in newly placed peritoneal dialysis catheters. Am J Kidney Dis 2000; 36: 1014–1019 [DOI] [PubMed] [Google Scholar]
- 17. Lye WC, Lee EJ, Tan CC.. Prophylactic antibiotics in the insertion of Tenckhoff catheters. Scand J Urol Nephrol 1992; 26: 177–180 [DOI] [PubMed] [Google Scholar]
- 18. Shiff B, Pierrato A, Oliver MJ. et al. Knowledge, attitudes, and practices with regard to PD access: a report from the Peritoneal Dialysis Access Subcommittee of the Ontario Renal Network Committee on Independent Dialysis. Perit Dial Int 2014; 34: 791–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mehrotra R, Blake P, Berman N. et al. An analysis of dialysis training in the United States and Canada. Am J Kidney Dis 2002; 40: 152–160 [DOI] [PubMed] [Google Scholar]
- 20. Yadlapalli NG, Ghose RP, Schreiber MJ.. Peritoneal dialysis training in U.S. nephrology training programs. J Am Soc Nephrol 2001; 12: A1806 [Google Scholar]
- 21. Miles R, Hawley CM, McDonald SP. et al. Predictors and outcomes of fungal peritonitis in peritoneal dialysis patients. Kidney Int 2009; 76: 622–628 [DOI] [PubMed] [Google Scholar]
- 22. Restrepo C, Chacon J, Manjarres G.. Fungal peritonitis in peritoneal dialysis patients: successful prophylaxis with fluconazole, as demonstrated by prospective randomized control trial. Perit Dial Int 2010; 30: 619–625 [DOI] [PubMed] [Google Scholar]
- 23. Lo WK, Chan CY, Cheng SW. et al. A prospective randomized control study of oral nystatin prophylaxis for Candida peritonitis complicating continuous ambulatory peritoneal dialysis. Am J Kidney Dis 1996; 28: 549–552 [DOI] [PubMed] [Google Scholar]
- 24. Mudge DW, Boudville N, Brown F. et al. Peritoneal dialysis practice in Australia and New Zealand: a call to sustain the action. Nephrology (Carlton) 2016; 21: 535–546 [DOI] [PubMed] [Google Scholar]
- 25. Cho Y, Johnson DW.. Peritoneal dialysis-related peritonitis: towards improving evidence, practices, and outcomes. Am J Kidney Dis 2014; 64: 278–289 [DOI] [PubMed] [Google Scholar]
- 26. Yip T, Tse KC, Lam MF. et al. Risks and outcomes of peritonitis after flexible colonoscopy in CAPD patients. Perit Dial Int 2007; 27: 560–564 [PubMed] [Google Scholar]
- 27. Li PK, Leung CB, Leung AK. et al. Posthysteroscopy fungal peritonitis in a patient on continuous ambulatory peritoneal dialysis. Am. J Kidney Dis 1993; 21: 446–448 [DOI] [PubMed] [Google Scholar]
- 28. Wu HH, Li IJ, Weng CH. et al. Prophylactic antibiotics for endoscopy-associated peritonitis in peritoneal dialysis patients. PLoS One 2013; 8: e71532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu G, Tu W, Xu C.. Mupirocin for preventing exit-site infection and peritonitis in patients undergoing peritoneal dialysis. Nephrol Dial Transplant 2010; 25: 587–592 [DOI] [PubMed] [Google Scholar]
- 30. Bernardini J, Bender F, Florio T. et al. Randomized, double-blind trial of antibiotic exit site cream for prevention of exit site infection in peritoneal dialysis patients. J Am Soc Nephrol 2005; 16: 539–545 [DOI] [PubMed] [Google Scholar]
- 31. Perez-Fontan M, Rosales M, Rodriguez-Carmona A. et al. Mupirocin resistance after long-term use for Staphylococcus aureus colonization in patients undergoing chronic peritoneal dialysis. Am J Kidney Dis 2002; 39: 337–341 [DOI] [PubMed] [Google Scholar]
- 32. Cavdar C, Saglam F, Sifil A. et al. Effect of once-a-week vs thrice-a-week application of mupirocin on methicillin and mupirocin resistance in peritoneal dialysis patients: three years of experience. Ren Fail 2008; 30: 417–422 [DOI] [PubMed] [Google Scholar]
- 33. Mahaldar A, Weisz M, Kathuria P.. Comparison of gentamicin and mupirocin in the prevention of exit-site infection and peritonitis in peritoneal dialysis. Adv Perit Dial 2009; 25: 56–59 [PubMed] [Google Scholar]
- 34. Chu KH, Choy WY, Cheung CCW. et al. A prospective study of the efficacy of local application of gentamicin versus mupirocin in the prevention of peritoneal dialysis catheter-related infections. Perit Dial Int 2008; 28: 505–508 [PubMed] [Google Scholar]
- 35. Pierce DA, Williamson JC, Mauck VS. et al. The effect on peritoneal dialysis pathogens of changing topical antibiotic prophylaxis. Perit Dial Int 2012; 32: 525–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mehrotra R, Story K, Guest S. et al. Neighborhood location, rurality, geography, and outcomes of peritoneal dialysis patients in the United States. Perit Dial Int 2012; 32: 322–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Muthucumarana K, Howson P, Crawford D. et al. The relationship between presentation and the time of initial administration of antibiotics with outcomes of peritonitis in peritoneal dialysis patients: the PROMPT study. Kidney Int Rep 2016; 1: 65–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ballinger AE, Palmer SC, Wiggins KJ. et al. Treatment for peritoneal dialysis-associated peritonitis. Cochrane Database Syst Rev 2014; 4: CD005284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Burke KE, Lamont JT.. Clostridium difficile infection: a worldwide disease. Gut Liver 2014; 8: 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.