ABSTRACT
Background
Dietary nitrate improves exercise performance by reducing the oxygen cost of exercise, although the mechanisms responsible are not fully understood.
Objectives
We tested the hypothesis that nitrate and nitrite treatment would lower the oxygen cost of exercise by improving mitochondrial function and stimulating changes in the availability of metabolic fuels for energy production.
Methods
We treated 9-mo-old zebrafish with nitrate (sodium nitrate, 606.9 mg/L), nitrite (sodium nitrite, 19.5 mg/L), or control (no treatment) water for 21 d. We measured oxygen consumption during a 2-h, strenuous exercise test; assessed the respiration of skeletal muscle mitochondria; and performed untargeted metabolomics on treated fish, with and without exercise.
Results
Nitrate and nitrite treatment increased blood nitrate and nitrite levels. Nitrate treatment significantly lowered the oxygen cost of exercise, as compared with pretreatment values. In contrast, nitrite treatment significantly increased oxygen consumption with exercise. Nitrate and nitrite treatments did not change mitochondrial function measured ex vivo, but significantly increased the abundances of ATP, ADP, lactate, glycolytic intermediates (e.g., fructose 1,6-bisphosphate), tricarboxylic acid (TCA) cycle intermediates (e.g., succinate), and ketone bodies (e.g., β-hydroxybutyrate) by 1.8- to 3.8-fold, relative to controls. Exercise significantly depleted glycolytic and TCA intermediates in nitrate- and nitrite-treated fish, as compared with their rested counterparts, while exercise did not change, or increased, these metabolites in control fish. There was a significant net depletion of fatty acids, acyl carnitines, and ketone bodies in exercised, nitrite-treated fish (2- to 4-fold), while exercise increased net fatty acids and acyl carnitines in nitrate-treated fish (1.5- to 12-fold), relative to their treated and rested counterparts.
Conclusions
Nitrate and nitrite treatment increased the availability of metabolic fuels (ATP, glycolytic and TCA intermediates, lactate, and ketone bodies) in rested zebrafish. Nitrate treatment may improve exercise performance, in part, by stimulating the preferential use of fuels that require less oxygen for energy production.
Keywords: ATP, fatty acids, ketone bodies, lactate, mitochondria, metabolomics, nitrate, nitrite, nitric oxide
Introduction
Optimal athletic performance requires physical conditioning, sport-specific experience, personalized training, and appropriate nutrition (1). Moderate-to-intense exercise is fueled principally by the oxidation of glycogen in muscle and, to a lesser extent, lipids, while longer-duration exercise stimulates the mobilization of substrates from the liver and gut (e.g., glucose) and adipocytes (e.g., free fatty acids) (2–5). The consumption of dietary inorganic nitrate (NO3−) as leafy greens and root vegetables reduces the oxygen cost of submaximal physical activity (6–8). This is significant, because it improves exercise tolerance and performance in humans and is among a select number of dietary factors that produce exercise benefits (1, 9). Nitrate-containing foods and dietary supplements are increasing in popularity among elite and recreational athletes and the public, even while the mechanisms that are responsible for this exercise benefit are not well understood (10).
The physiological effects of dietary nitrate depend on its reduction into nitrite (NO2−) and NO, termed the nitrate-nitrite-NO pathway (11, 12). Humans concentrate nitrate in the salivary glands before facultative anaerobic bacteria, residing in the crypts of the tongue, reduce salivary nitrate to nitrite. Nitrite is swallowed and absorbed into circulation or reduced to NO in the stomach (13). Absorbed nitrite acts as a bioavailable reservoir for NO, with its reduction favored in acidic and hypoxic conditions, such as during strenuous exercise (14). Importantly, NO homeostasis is also maintained by an endogenous pathway, where the NO synthases produce NO from O2 and L-arginine (15).
NO is a potent signaling molecule that regulates vascular tone and blood pressure by stimulating cyclic guanosine monophosphate–dependent vasodilation in vascular smooth muscle (15, 16). Notably, NO donors facilitate glucose transport in skeletal muscles via the stimulation of glucose transporter type 4 abundance: an important regulatory step for glucose uptake in skeletal muscle, heart, and adipose tissue (17–19). Long-term nitrate deficiency is associated with glucose intolerance, hyperglycemia, and an increased risk of atherosclerosis in mice (20), while the supplementation of nitrate-deficient diets with nitrate improves glucose tolerance, insulin resistance, and dyslipidemia in rodent models of hyperglycemia and diet-induced obesity (21, 22). These findings are consistent with the concept that dietary nitrate may be an essential nutrient (20, 23, 24).
The lower oxygen cost of exercise associated with nitrate consumption is ascribed to several potential mechanisms, including improved muscle hemodynamics, improved contractile efficiency, and fiber-type switching in skeletal muscles (25–28). Likewise, improved mitochondrial efficiency has been associated with nitrate consumption in skeletal muscle mitochondria, as measured by increased oxidative phosphorylation efficiency, as indicated by the ratio of ATP production to O2 consumption (P:O), decreased state 4 respiration, and a reduction of ATP/ADP translocase protein (29, 30). Notably, a subsequent study did not observe nitrate-associated, improved mitochondrial efficiency, highlighting the need for additional research (31). Furthermore, while fuels for energy production (e.g., fatty acids and glucose) are critical for ATP production during exercise, and can directly influence the rate of oxygen consumption, it is not known whether nitrate or nitrite treatment influences the availability of metabolic fuels (32, 33).
To begin addressing these gaps in knowledge, we tested the hypothesis that nitrate and nitrite treatment lower the oxygen cost of exercise by increasing mitochondrial efficiency. We further hypothesized that the treatment stimulated changes in the availability of metabolic fuels for energy production. Exercise requires the intricate coordination of multiple organ systems, and we chose a zebrafish (Danio rerio) model because the zebrafish's small size allows for the assessment of whole-body metabolomic profiling. Zebrafish models are effective for exercise studies, and have high genetic homology to humans (34–38), including a conserved nitrate-nitrite-NO pathway, functional nitric oxide synthase (nos) genes, and the capacity to reduce plasma nitrate through the nitrate-nitrite-NO pathway (39, 40). We treated adult zebrafish with nitrate or nitrite for 21 d; measured oxygen consumption during a 2-h, strenuous, graded exercise test; assessed mitochondrial respiration in ex vivo skeletal muscle isolations; and performed untargeted metabolomics on whole zebrafish (41).
Methods
Experimental design
A total of 192 zebrafish were randomized into 3 treatment groups, designated as: 1) control (no treatment); 2) nitrate (606.9 mg NaNO3/L water); and 3) nitrite (19.5 mg NaNO2/L of water). The 9-mo-old zebrafish were treated for a duration of 21–28 d. These doses were chosen to model nitrate or nitrite levels that could be achieved by regularly consuming high-nitrate foods and supplements, which have been associated with improvements in exercise performance in humans. Nitrite in fish water can be toxic to fish and, thus, limited the exposure range for nitrite treatment more than nitrate. As such, we based our nitrate and nitrite treatments on 2 considerations: 1) the concentrations needed to achieve high blood levels; and 2) the adult zebrafish literature, in order to select a significant but non-toxic nitrate concentration (42). Likewise, the nitrite dose was chosen because it was higher than normal fish water but included a 10-fold safety factor as compared to the lowest dose previously tested (which was only associated with mild growth suppression) (43). The oxygen consumption during an exercise test was assessed before and after treatment, and mitochondria from skeletal muscles were examined. Fish were also collected at rest or directly following the 2-h, graded swim test for metabolomics analyses. A subset of the zebrafish were switched to 15N-nitrate or 15N-nitrite treatment for the final 3 d of exposure. At the study termination (28 d) all remaining zebrafish were euthanized, with a subset sent for pathological examination.
Fish husbandry
Wild-type zebrafish (5D) were raised and maintained at the Sinnhuber Aquatic Research Laboratory at Oregon State University (OSU), in accordance with protocols approved by the OSU Institutional Animal Care and Use Committee. Fish were maintained at 6 fish per tank (3 male and 3 female) in 4-liter enclosures. Fish water was made with reverse-osmosis water and included Instant Ocean at 1.4 g of salt/gallon of water (Instant Ocean, Spectrum Brands) with a conductivity between 500–600 µS. Nitrate and nitrite were dissolved in freshly prepared fish water and, unless otherwise indicated, chemicals were purchased from Sigma-Aldrich. For labeling experiments, a subset of fish were switched to water containing either >99% stable isotopes of Na15NO3 or 100% Na15NO2 (Cambridge Isotope Laboratories) at Day 18 for 3 d of treatment prior to collection. The fish water, which contained the nitrate or nitrite treatment, was replaced every 36–42 h to maintain low ammonia concentrations and consistent nitrate and nitrite treatments. Fish water was monitored for pH (6.8–7), total ammonia concentrations (0–2.0 ppm), and temperature (27–29°C). Fish were fed a standard lab diet (Gemma Micro. Skretting) at a volume of ∼3% body weight/d, as determined by weighing of fish just prior to the beginning of the study. The diet composition was 59% protein, 14% lipids, 14% ash, 1.5% calcium, 1.3% phosphorus, 0.7% sodium, and 0.2% fiber, and 9.3% proprietary ingredients under patent by Skretting. It also contained vitamins A (23,000 IU/kg), D3 (2,800 IU\kg), C (1,000 mg/kg), and E (400 mg/kg). Food was given over 2 feedings per day, which contained 0.32 mg/kg of nitrite and 49.3 mg/kg of nitrate. Fish were treated for up to 28 d. Fish were humanely euthanized with an overdose of the anesthesia drug tricaine mesylate, then dried, weighed, measured for length, and either snap frozen, dissected for muscle, or whole blood–collected, as previously described (44). The pathological examinations of fish tissue and nitrate and nitrite quantifications in whole zebrafish, with and without labels, were as previously published (41) and are described in the Supplemental Methods.
Nitrate and nitrite quantification in water and blood
Water was collected during the first week of the experiment and saved directly after a water change (designated as fresh) or 36–42 h post–water change (used), as previously published (45). Similar nitrate and nitrite contents of fish water were confirmed at day 20 (data not shown). Whole blood collection was as previously published, except fluid volumes were modified to 2 µL blood and 18 µL ice-cold reverse-osmosis water for nitrate quantification or to 16 µL blood and a 4 µL stop solution for nitrite measurements. Nitrate and nitrite concentrations were determined by ozone chemiluminescence, as previously described, on a Sievers Nitric Oxide Analyzer (45, 46).
Oxygen consumption with exercise
Fish exercised in a swim tunnel (Loligo Systems), where oxygen consumption rates were measured using a Fibox fiber optic oxygen probe and an oxy4 sensor, as previously described, with small modifications (47). We weighed 6 fish per group, placed them in a swim tunnel, and measured their oxygen consumption rate (O2 mg · kg body weight−1 · h−1) every 10 min, calculating it using AutoResp automated intermittent respirometry software (Loligo Systems), as previously described (47). Each treatment group had 5 experiments for both pre- and post-treatment (days 21–25 of treatment), with all data points in analysis having an R2 value of 0.8 or higher (48, 49). Zebrafish that were exercised had a 50-min free swim at a water flow of 5 cm/s, followed by stages of increasing water current (10, 20, 30, and 40 cm/s), where each stage was held for a 20 min duration. Fish were then returned to the baseline flow rate of 5 cm/s for 20 minutes and were either euthanized and snap frozen or returned to their tank for later use.
Mitochondrial analysis
We isolated mitochondria from epaxial and hypaxial muscles in rested fish using differential centrifugation on days 21–25 of treatment (50). High-resolution respirometry was performed using Oxygraph-2k (Oroboros Instruments) with 2 mL chambers at 37°C and 750 revolutions per minute stirring of the respiration buffer (0.5 mM EGTA, 3 mM MgCl2–6H2O, 60 mM lactobionic acid, 20 mM taurine, 10 mM KH2PO4, 20 mM HEPES, 110 mM sucrose, and 1 g/l bovine serum albumin). We measured oxygen consumption, normalized to the protein content of mitochondrial isolation, with H2O2 (JH2O2), using 10 μM Amplex red, 5 U/mL superoxide dismutase, and 1 U/mL horseradish peroxidase and calibrated using injections of H2O2. To examine Complex I, the titration sequence began with mitochondrial suspension, followed by 10 mM glutamate + 2 mM malate and 5 mM ADP (NADH-supported oxidative phosphorylation). Complexes I and II were evaluated with a 10 mM succinate addition (NADH + succinate-supported oxidative phosphorylation). 0.5 μM of Complex I inhibitor rotenone generated succinate-supported oxidative phosphorylation (Complex II). For proton leak, 2 μg/mL oligomycin inhibited Complex IV. Sequential additions of 0.05 mM carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone stimulated a succinate-supported electron transfer system (Complex II). Antimycin A (2.5 mM) was applied to measured residual oxygen consumption. The P:O was calculated using a sub-saturating pulse of 114 nmol of ADP during NADH-supported respiration, prior to saturating ADP (5 mM). The P:O assumed a 1:1 ratio of ADP consumption and ATP production (51). We determined mitochondrial protein abundances in skeletal muscle lysates using Western blotting, as previously described (52). Antibodies were anti–citrate synthase (Abcam ab96600), ATP synthase F1 subunit α (ab110273), β-actin (ab8226), goat anti-rabbit, or goat anti-mouse secondary (Santa Cruz Biotechnology).
Metabolomics analysis
Sample preparation
Whole zebrafish were ground in liquid nitrogen with a mortar and pestle, after which the metabolites were extracted (100 mg fish powder/100 µL 80:20 methanol:water), mixed vigorously, chilled at −20°C for 1 h, centrifuged at 13,000 x g for 10 min, freeze dried, and then re-suspended in cold solvent (50:50 methanol:water). Extracts were sonicated for 5 min and centrifuged at 13,000 x g for 10 min, and supernatants were transferred to MS vials. Metabolomics were performed using LC-MS/MS in both positive and negative ion modes, as previously described (53). Briefly, HPLC was performed on a Shimadzu Nexera system (Shimadzu) with a phenyl-3 stationary phase column (Inertsil Phenyl-3, 4.6 × 150 mm; GL Sciences) coupled to a quadrupole time-of-flight MS (AB SCIEX TripleTOF 5600). Samples were randomized, auto-calibration was performed every 2 samples, and a pooled quality control was analyzed every 10 samples. An MS/MS analysis (information dependent acquisition and sequential window acquisition of all theoretical spectra ) was performed on quality control samples.
Metabolomics data processing
Data were analyzed using PeakView with XIC Manager 1.2.0 (ABSciex). Metabolite identities were assigned by matching the accurate mass (error <10 ppm), retention time (error <10%), MS/MS fragmentation (library score >70), and isotope distribution (error <20%) with an in-house library of 650 commercially available standards (including IROA Technology) or querying online databases (METLIN, Human Metabolome Database). The peak list was exported to MultiQuant 3.0.2 (SCIEX) to integrate chromatograms to obtain peak areas.
Metabolomics data visualization
Principal components analysis plots were generated with MetaboAnalyst 3.0 (McGill University) using log-transformed peak intensities of all annotated metabolites detected in positive and negative ion modes. Pathway-specific diagrams were created in PowerPoint 2016 (Microsoft) and graphs were generated with GraphPad Prism 4, showing mean peak area (counts). Log-fold changes shown in pathways were generated with MetaboAnalyst 3.0. Heat maps were generated with all detected acyl carnitines and fatty acids with MetaboAnalyst 3.0 on log-transformed peak intensities, using Euclidian distance measures, auto-scale features, and no clustering algorithms. ChemRICH (University of California, Davis) was used to determine those clusters of metabolites that were significantly altered with exercise.
Statistical analysis
Statistical comparisons were generated, unless otherwise indicated, with GraphPad Prism 4. Statistical tests were done for all endpoints examined and significant differences are indicated by letters in the figures. When no statistically significant differences were observed between treatments, no letters were used in the figures. Comparisons made between the control, nitrate, and nitrite treatments groups were made using a 1-way ANOVA with a Tukey post hoc analysis. We used 2-way ANOVAs with Tukey post hoc analyses when treatment and time or treatment and exercise conditions were examined. A mixed-effects ANOVA with a Tukey post hoc analysis was conducted in R for an analysis of the AutoResp swim tunnel results, to account for treatment, speed, and pre- to post-exposure. A P value < 0.05 was considered significant when comparing pre- versus post-treatment conditions.
A statistical analysis of metabolomics data was conducted with annotated metabolites, and multivariate analyses were generated using MetaboAnalyst 3.0 (54). A 2-factor ANOVA, followed by a Fisher's post hoc analysis and Holm false discovery rate (FDR) correction, was used to calculate significant differences for each annotated metabolite between the treatment groups, where a P value of < 0.05 indicated significance (n = 9). Significant differences in ChemRICH were calculated using a Kolmogorov-Smirnov test with an FDR correction (P < 0.05) (55).
Results
Nitrate and nitrite alter exercise performance
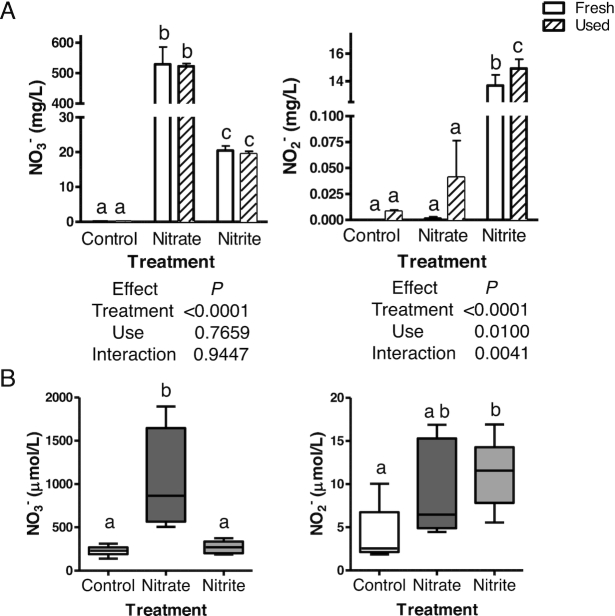
Nitrate and nitrite treatment significantly increased nitrate or nitrite concentrations in the water, as compared to the control water (Figure 1A). Nitrate was present in the water of the nitrite treatment group, but was well below the concentration for nitrate treatment. Nitrite concentration increased with use, likely due to nitrogenous waste excretion, but the nitrite concentrations in the used water of control and nitrate-treated fish were significantly lower than the concentrations in the water from the nitrite treatment groups (Figure 1A and data not shown). Zebrafish treated with nitrate had a significant, 4.5-fold greater blood nitrate concentration, and 2-fold higher blood nitrite concentrations, as compared to blood concentrations collected from control fish (Figure 1B). Nitrite treatment significantly increased blood nitrite by 2.6-fold, as compared to blood nitrite levels in control fish, although it did not significantly alter the amount of nitrate in the blood.
FIGURE 1.
Concentrations of nitrate and nitrite in (A) fish water and (B) zebrafish blood, as determined by Sievers Nitric Oxide Analyzer. (A) Values show means ± SE for n = 5 tanks. (B) Box and whiskers plots from min to max, n = 7–10 fish. (A and B) Labeled means without a common letter differ, P < 0.05. Fresh, newly treated fish water; NO2−, nitrite concentrations; NO3−, nitrate concentrations; Used, water at end of 42 h of use.
There were no significant differences in fish lengths, weights, or ammonia exposure concentrations with nitrate or nitrite treatment, as compared to control fish (Supplemental Figure 1). Pathological examinations did not reveal negative health outcomes in control or nitrate-treated fish. The only adverse pathological finding for nitrite-treated fish was diffusely mildly plump and reactive gill epithelium, noted in 60% of fish examined. This finding is consistent with nitrite acting as a mild toxic irritant at the gill (Supplemental Figure 1). Glutathione and oxidized glutathione, markers of oxidative stress, were not significantly changed by nitrate or nitrite treatment, as compared to the abundances in control fish (Supplemental Figure 1). Together, these results indicate that nitrate or nitrite treatment did not cause broad, adverse health effects.
Nitrate treatment significantly increased whole-body nitrate and nitrite concentrations, by 2.4- and 3.6-fold, respectively, as compared to the control fish (Supplemental Table 1). Likewise, nitrite treatment significantly increased whole-body nitrate (1.4-fold) and nitrite concentrations (2.2-fold). As nitrate and nitrite can also be endogenously produced, 15N labeling was used to determine the proportions of nitrate and nitrite in the fish that were derived from exogenous treatment. The 15N-nitrate treatment caused an 88% enrichment of nitrate and a 44% enrichment of nitrite, which was well above the 4% observed for each chemical in the control fish (Supplemental Table 1). The 15N-nitrite treatment also increased the percentage enrichments of nitrite (61%) and nitrate (57%; Supplemental Table 1). Overall, these results demonstrate the conversion of exogenous nitrate to nitrite in the fish, and that the exogenous treatments were a primary source for the increased nitrate and nitrite in whole fish.
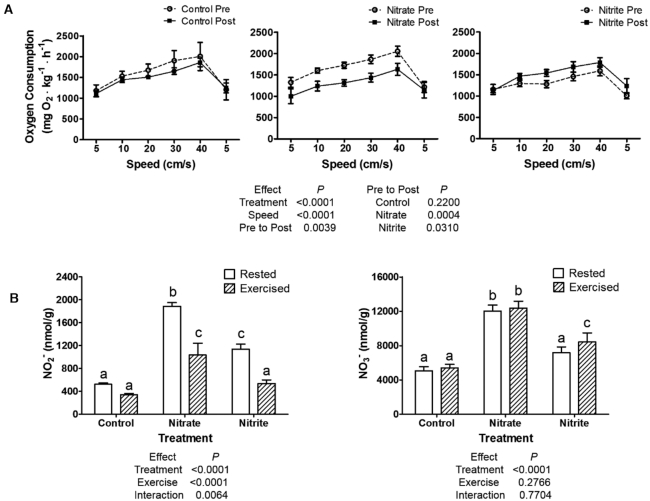
Zebrafish movement and exercise performance were tested, and nitrate and nitrite treatments did not have significant effects on baseline movements (Supplemental Figure 1). Control fish also did not have significant differences in oxygen consumption in a graded exercise test, when results were compared to measurements in the same fish before the treatment period. Nitrate treatment significantly decreased oxygen consumption during exercise, as compared to pre-treatment values in the same fish (Figure 2A). Nitrite treatment caused a significant increase in oxygen consumption with exercise. Furthermore, exercise significantly depleted whole-body nitrite concentrations in the nitrate and nitrite treatment conditions, by ∼2-fold, but did not decrease nitrate levels (Figure 2B).
FIGURE 2.
Zebrafish performed a 2-h graded swim test and (A) oxygen consumption was plotted against swimming speed before and after treatment with control, nitrate, or nitrite in n = 5 groups of fish. (B) Whole-body nitrite and nitrate concentrations were determined by the 2,3-diaminonaphthalene method for n = 9 fish. (A and B) values show means ± SE. (A) Mixed effects ANOVA results are given (left) with Tukey Honest Significant Difference post-hoc test results (right), which directly compared performance pre- to post-treatment. (B) Labeled means without a common letter differ, P < 0.05. NO2−, nitrite concentrations; NO3−, nitrate concentrations; Post, after 21 d of treatment; Pre, before treatment.
Mitochondrial efficiency or abundance
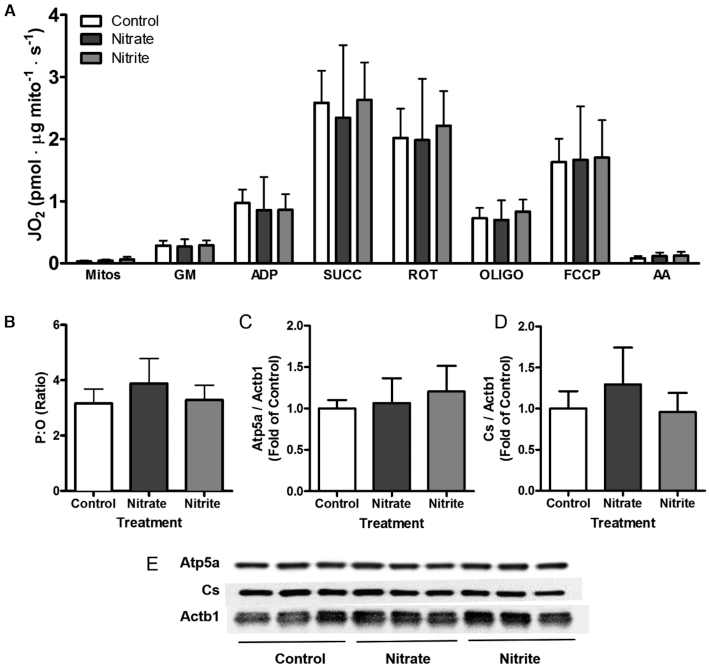
Nitrate and nitrite treatment effects on isolated mitochondria were examined, and no significant differences were found for oxidative phosphorylation, proton leaks, or residual oxygen consumption between mitochondria from control, nitrate-treated, and nitrite-treated zebrafish (Figure 3A). Compared to control conditions, the P:O ratio, an indicator of mitochondrial efficiency, was not significantly altered by nitrate or nitrite treatment (Figure 3B, P = 0.116). Nitrate and nitrite treatment also did not alter the amount of reactive oxygen species produced by the mitochondria (data not shown). Mitochondrial protein abundance markers were examined, because nitrate- or nitrite-induced increases in mitochondrial abundance have been suggested (56, 57), but no significant treatment effect was observed (Figure 3C–E). Overall, nitrate and nitrite treatment did not change mitochondrial abundances or maximal respiratory capacities in zebrafish. We next used metabolomics to investigate alternative mechanisms that may have contributed to whole-body exercise efficiency.
FIGURE 3.
Effect of 21–25 d control, nitrate, or nitrite treatment on respiration and abundance of skeletal muscle mitochondria in zebrafish. Isolated mitochondria were evaluated for (A) oxygen consumption following indicated chemical treatments to examine each respiratory state, or (B) the ratio of ATP production to O2 consumption. (C–E) Representative Western blot images of protein from skeletal muscle, with corresponding densitometry results. (A–B) Values show means. (C and D) Values are means expressed relative to control treatment (A–D) ± SD, n = 6–8 fish. AA, antimycin A; Actb1, β actin; Atp5a, ATP synthase; Cs, citrate synthase; FCCP, carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone; GM, glutamate and malate; JO 2, oxygen consumption; Mitos, mitochondrial suspension; OLIGO, oligomycin; P:O, ratio of ATP production to O2 consumption; ROT, rotenone; SUCC, succinate.
Availability of metabolic fuels in rested and exercised zebrafish
Untargeted metabolomics was performed, and 10,183 unique monoisotopic features were identified. Of these, 386 were significantly changed among at least 1 treatment group, and 197 metabolites were annotated (Supplemental Table 2). Supplemental Table 3 includes P values calculated between all treatment groups for all annotated metabolites. A principal component analysis demonstrated clustering and separation of the metabolome among different treatments (Supplemental Figure 2).
Exercise in control fish significantly increased whole-body amounts of carnitines, tricarboxylic acids (TCA), and ketones, yet significantly decreased purine nucleosides and amino acids, as compared to rested control fish (Supplemental Figure 3). Exercise in nitrate-treated fish had similar responses as exercise in control fish, yet differed notably in ketones (unchanged, absent from plot), gluconates, and the direction of fold changes for amino acids and purines. Exercise in nitrite-treated fish had significant decreases in carnitines, fatty acids, purines, amino acids, and TCA cycle intermediates, as compared to rested, nitrite-treated fish (Supplemental Figure 3). Thus, exercise depleted many metabolites in nitrite-treated zebrafish that increased with exercise in the control and nitrate-treated zebrafish. Overall, we observed significant changes in metabolic fuel sources for exercise, suggesting that fuel abundance and use varied between the treatment groups as a mechanism for nitrate- and nitrite-induced changes in oxygen consumption during exercise. We next analyzed the whole-body metabolomics data in the context of specific metabolic pathways.
As a proof of principle, we examined the metabolomics data set for metabolites related to the endogenous NO production pathway, where Nos enzymes catalyze the conversion of L-arginine to L-citrulline and NO (39, 40). Compared to in rested control fish, L-arginine was higher in the nitrate and nitrite treatment groups and in all exercised conditions (Figure 4). L-citrulline was stable in exercised control fish, yet was significantly lower in the exercised nitrate and exercised nitrite treatment conditions. Furthermore, L-ornithine significantly increased in all exercise treatment conditions and in the rested nitrite condition, as compared to in rested control fish. Xanthine oxidoreductase (Xor, also called Xdh), the final enzyme in purine metabolism in humans, also catalyzed the conversion of nitrate and nitrite into NO. At rest, hypoxanthine significantly accumulated in fish treated with nitrate or nitrite, as compared to rested controls, and this was associated with a significant decrease in urate abundance (Figure 4). Exercise significantly decreased hypoxanthine in both the nitrate and nitrite treatment groups, as compared to all rested conditions, which corresponded with a significant increase in urate concentrations with exercise in all treatment conditions.
FIGURE 4.
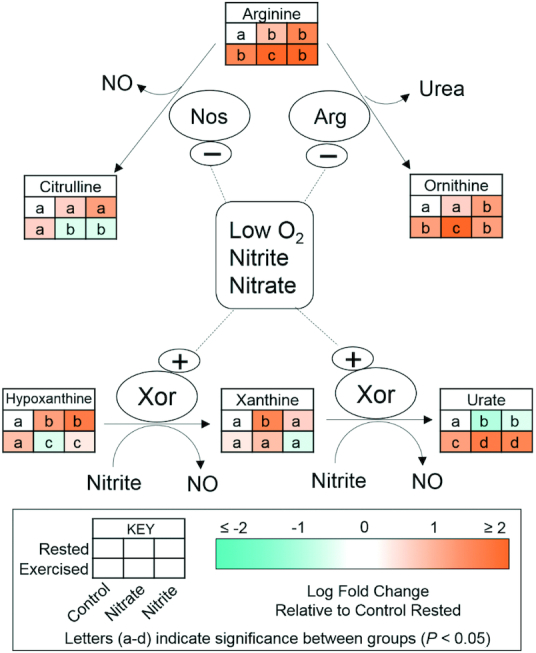
Relative concentrations of metabolites related to NO homeostasis after 21–23 d of control, nitrate, or nitrite treatment and under rested or exercised conditions. Metabolites were measured by LC-MS/MS. Labeled means without a common letter differ, P< 0.05, n = 9 fish. Arg, arginase; Nos, NO synthase; Xor, xanthine oxidoreductase.
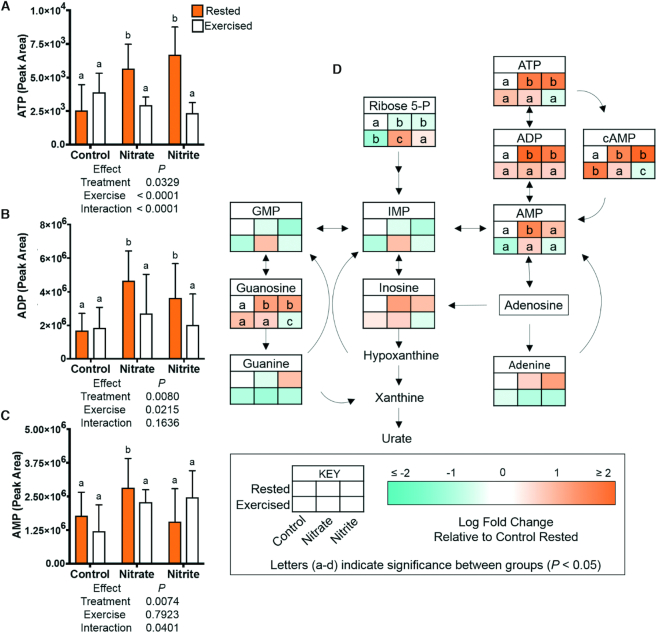
We next addressed the role of ATP as a critical metabolic regulator. Significant 2.1- to 2–8-fold increases in ATP, ADP, and cAMP were observed in the rested nitrate and rested nitrite treatment conditions. Concentrations of these metabolites significantly decreased, by 2- to 3-fold, in the exercised nitrate and exercised nitrite treatment groups, as compared to in their rested counterparts (Figure 5). Most other purines (GMP, inosine monophosphate, guanine, inosine, and adenine) were not significantly changed with treatment (Figure 5). We considered a shift in glycolytic metabolism as a potential mechanism for divergent oxygen costs of exercise with nitrate or nitrite treatment and to elucidate the cause of these significant changes in key energy metabolites.
FIGURE 5.
Relative concentrations of metabolites involved in purine metabolism and nucleotide synthesis, including ATP and ADP, after 21–23 d of control, nitrate, or nitrite treatment and under rested or exercised conditions. Metabolites were measured by LC-MS/MS. Data are (A–C) graphs of mean metabolite values ± SD or (D) metabolic pathway. (A–D) Labeled means without a common letter differ, P < 0.05, n = 9 fish.
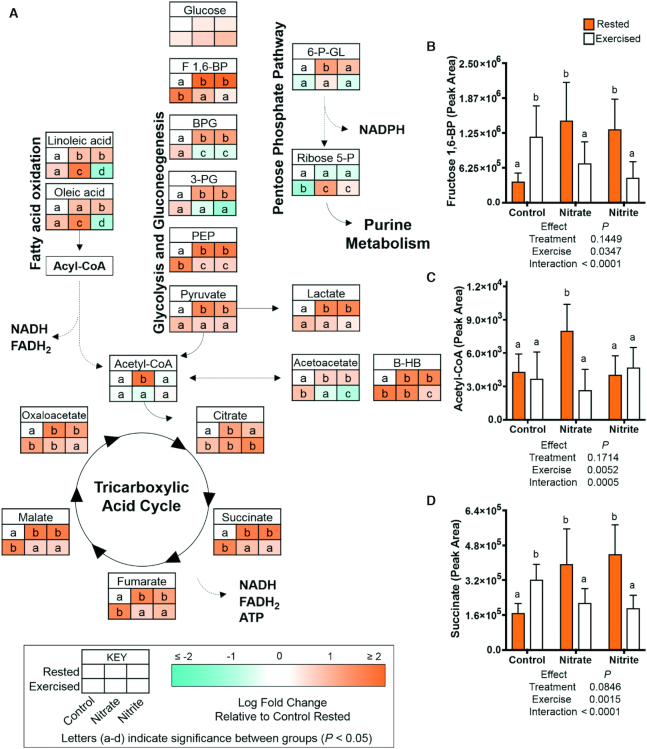
There was no significant difference in glucose concentrations with any treatment. However, nitrate and nitrite treatments in rested fish significantly increased the abundance of glycolytic intermediates (fructose 1,6-bisphosphate, bisphosphoglycerate, 3-phosphoglycerate, phosphoenolpyruvate, pyruvate) and TCA cycle intermediates (succinate, fumarate, malate, oxaloacetate), and also increased ketone bodies (acetoacetate, β-hydroxybutyrate) and lactate by 1.8- to 3.8-fold (Figure 6). Significantly increased glycolytic intermediates (fructose 1,6-bisphosphate, phosphoenolpyruvate), TCA cycle intermediates (citrate, succinate, fumarate, malate, oxaloacetate), and ketone bodies (acetoacetate, β-hydroxybutyrate) were also observed with exercise in the control fish (as compared to rested controls). In contrast, in the exercised nitrate and exercised nitrite treatment groups, there were significantly lowered amounts of glycolytic, several TCA cycle intermediates, and lactate, by 1.3- to 2.9-fold, as compared to the rested nitrate or rested nitrite conditions. In summary, while nitrate and nitrite treatment in fish at rest increased glycolytic and TCA cycle intermediates, the net decreases in these metabolites following exercise suggest that the intermediates were used to produce reducing equivalents for ATP production.
FIGURE 6.
Relative concentrations of energy substrates related to glycolysis, fatty acid oxidation, and the tricarboxylic acid cycle, after 21–23 d of control, nitrate, or nitrite treatment and under rested or exercised conditions. Metabolites were measured by LC-MS/MS. Data are presented as (A) metabolic pathways, n = 7–9 fish, or (B–D) graphs of mean metabolite values ± SD, n = 9 fish. (A–D) Labeled means without a common letter differ, P < 0.05. B-HB, β-hydroxybutyrate; BPG, bisphosphoglycerate; F 1,6-BP, fructose 1,6-bisphosphate; PEP, phosphoenolpyruvate; 3-PG, 3-phosphoglyceric acid; ribose 5-P, ribose 5-phosphate; 6-P-GL, 6-phosphogluconic acid.
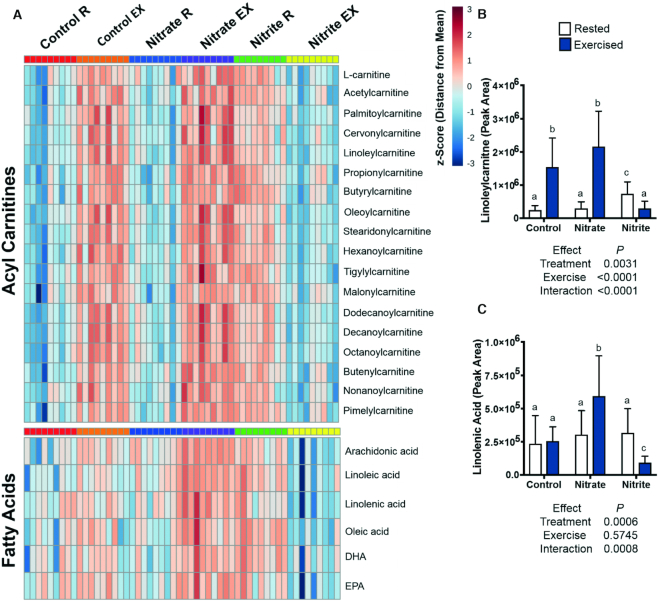
The abundances of fatty acids and acyl carnitine metabolites were examined, as these are important metabolic fuels during exercise. Exercise significantly increased acyl carnitines and some fatty acids in control fish (Figure 7) (4, 5). Exercise significantly increased net fatty acids and acyl carnitines in nitrate-treated fish (1.5- to 12-fold), as compared to in their rested counterparts; however, they generally significantly increased more in exercised, nitrate-treated fish, as compared to exercised control fish (Figures 6 and 7). These changes in fatty acids, acyl carnitines, and glycolytic intermediates correspond to significant, 1.8-fold increases in acetyl-CoA with rested nitrate treatment, as compared to in rested controls. A significant, 3.4-fold decrease in acetyl-CoA was observed in exercised, nitrate-treated fish, as compared to the rested nitrate condition (Figure 6). Strikingly, fatty acids and acyl carnitines were significantly more abundant in rested, nitrite-treated fish, yet significantly decreased by 2- to 4-fold in exercised nitrite fish, relative to their rested counterparts (Figure 7).
FIGURE 7.
Relative concentrations of acyl carnitines and fatty acids after 21–23 d of control, nitrate, or nitrite treatment and under rested or exercised conditions. Metabolites were measured by LC-MS/MS. Data are presented as a (A) heat map with z-scores based on peak intensities of metabolites or (B and C) graphs of mean metabolite values ± SD. (A–C) Labeled means without a common letter differ, P < 0.05, n = 9 fish. EX, exercised; R, rested.
Discussion
We demonstrated that treatment with nitrate, but not nitrite, decreased the oxygen cost of exercise in zebrafish. There were no changes to mitochondrial respiration capacities (ex vivo) or protein abundances following nitrate and nitrite treatment, indicating that maximal mitochondrial characteristics did not explain changes to oxygen consumption. We used untargeted metabolomics in whole fish to identify the potential mechanisms by which nitrate and nitrite treatment altered exercise performance. A primary finding of our study is that nitrate-induced improvements in the oxygen cost of exercise were associated with significant changes in the availability of metabolic fuels. Specifically, at rest, nitrate-treated zebrafish had increased abundances of glycolytic, TCA cycle intermediates, lactate, and ketone bodies (acetoacetate, β-hydroxybutyrate). There were also increased resting acetyl-CoA and ATP concentrations. In nitrate-treated zebrafish, exercise decreased glycolytic and TCA cycle intermediates, relative to their rested counterparts. Furthermore, exercise in nitrate-treated fish increased the net abundances of some fatty acids and acyl carnitines, compared to rested, nitrate-treated fish. Together, these observations suggest potential mechanisms by which nitrate increases the use of fuel sources that require less oxygen to produce ATP and lowers the oxygen cost of exercise (32).
The nitrate-mediated effects on lactate and ketone bodies are also of interest, because these substrates serve as efficient fuel sources that are preferred by most extrahepatic tissues (58–60). Lactate and ketone bodies spare glucose by supporting the maintenance of a high rate of glycolysis (59–61), and share similar monocarboxylic acid transporters across the plasma membrane and mitochondrial inner membranes (62–64). The increased abundances of lactate and ketone bodies from nitrate treatment in rested zebrafish resulted from glucose and lipid oxidation, respectively (60, 61). Lactate links glycolytic and oxidative metabolism during exercise between tissues through the lactate “shuttle,” which is consistent with our observed decrease in lactate in exercised, nitrate-treated fish (61). Similarly, we observed a net decrease in acetoacetate in exercised, nitrate-treated fish, which is of interest because ketone bodies have a higher thermodynamic efficiency as an energy substrate, relative to glucose (65, 66). Ketone bodies had net increases following exercise in control fish, as also observed in humans and rodents (67, 68).
The findings of potentially improved respiratory efficiency through shifts in metabolic fuels are consistent with the ergogenic effects of nitrate. However, the nitrate-induced changes in these metabolites, being associated with decreasing the oxygen cost of exercise, did not provide insights into whether other mechanisms contribute to this effect, including improved hemodynamics and contractile efficiency (25–27). Likewise, nitrate can induce Peroxisome proliferator-activated receptor γ coactivator 1-α and stimulate a switch from Type IIb (glycolytic) to Type I (oxidative) and Type IIa (intermediate) muscle fibers (28). We cannot exclude that hemodynamic or skeletal muscle alterations contributed to the improved exercise performance. Our treatments cannot differentiate between the potential direct or indirect effects of nitrate, through reduction to nitrite and NO, on exercise efficiency (8). Other methodological considerations include the static nature of the metabolomics data, the necessity to make inferences based on relative abundances of metabolite concentrations, and timing of the exercise collection at 20 min following the peak exercise intensity. Likewise, because of the small size of zebrafish, the metabolic profiles reported here are on whole zebrafish, which limits organ-specific inferences on treatment effects. However, this model allowed us to gain a comprehensive overview of the relative abundances of many metabolites related to energy metabolism. These experimental considerations create opportunities in future experiments to explore the treatment effects on specific organs, abundance of energy metabolites at peak exercise intensities, rate of flux of fuel sources during exercise, and conservation of these mechanisms in humans.
These data establish the utility of the zebrafish as a model to examine nitrate- and nitrite-mediated effects on exercise. Even so, it is important to note the routes and periodicities of exposure vary from those of humans that receive nitrate/nitrite from the diet in episodic meals. We found that exogenous nitrate was converted to nitrite (as occurs in humans) and nitrate and nitrite treatment changed the abundances of substrates related to the endogenous pathways that generate NO (69). We cannot comment on the mechanisms of nitrate reduction to nitrite and NO in zebrafish, as we did not interrogate the known mechanisms by which this occurs. Future experiments, using antibiotics to address the role of bacterial nitrate reductases, as well as genetic knockout and/or chemical inhibitors of Nos and Xor, could inform our knowledge of the potential sources of nitrite and NO in this model system. We did, however, observe changes in hypoxanthine and urate abundances with nitrate and nitrite treatment and exercise, which is consistent with increased XOR activity and expression after strenuous exercise in humans (70). Together, these results support the theory that exercise alters NO metabolic homeostasis (71, 72). Further, nitrate and nitrite altered the abundances of substrates for Nos and Xor, and these treatments significantly changed the effect of exercise on some NO-related metabolites. Whole-body nitrite concentrations decreased following strenuous exercise, suggesting that exercise-stimulated NO production may be the result of transient changes in oxygen availability in muscles during strenuous exercise (8, 73). In contrast, whole-body nitrate concentrations did not change in zebrafish: a result that is different from data found in a rodent exercise study, where concentrations in specific tissues were examined (71, 72). Differences in these results may be related to dissimilar exercise regimens and the time points, species, and tissues examined. We also observed no statistically significant differences in the total distances or swimming velocities of nitrate- or nitrite-treated zebrafish; this result stands in contrast to that found in mice that increased physical activity, as assessed by voluntary running, with nitrate treatment (74).
The increase in the oxygen cost of exercise with nitrite treatment is a novel and unexpected finding. Interestingly, while many of the changes in metabolites that occurred with nitrate-treated zebrafish also occurred in nitrite-treated fish, an important consideration between the treatments is the different abundances of fatty acids, acyl carnitines, and ketones. In contrast to nitrate-treated and control fish, nitrite treatment increased the abundance of acyl carnitines in fish at rest, while net depletions of acyl carnitines, fatty acids, and ketone bodies were observed in the exercised, nitrite-treated fish. This suggests that nitrite stimulates the oxidation of fatty acids and ketone bodies for energy production. If fatty acid oxidation constituted a greater proportion of energy substrates during exercise in nitrite-treated fish, then these substrates would have required more oxygen consumption per mole of ATP produced, and contributed to the increased oxygen consumption observed with nitrite. Our data are consistent with a mouse study, where endothelial Nos knockout animals had nitrite-dependent reductions in the utilization of fat to generate energy (75).
Other explanations related to nitrite-associated toxicity may also contribute to decreased exercise performance. Nitrite concentrations are closely regulated in aquatic environments, because nitrite can have toxic effects (76). For this reason, we used a nitrite concentration well below that associated with toxicity (77). Nitrite treatment produced limited evidence of toxicity among the endpoints examined (no changes in survival, body size, basal movement, or reduced glutathione/oxidized glutathione ratios), but higher concentrations of nitrite were present in the blood of nitrite-treated fish than in that of nitrate-treated fish. There may be toxicity inherent in these higher circulating concentrations of nitrite, perhaps due to nitrite-mediated oxidation of hemoglobin, leading to methemoglobinemia (39, 76), which we were unable to quantify due to limited blood volumes. To this end, potential methemoglobinemia may induce a hypoxic state, resulting in a hormone-mediated stress response that stimulates lipolysis (78, 79). Likewise, the presence of mild gill irritation, associated with nitrite exposure, may also contribute to the increased oxygen cost of exercise (39, 76).
In conclusion, we demonstrated that nitrate treatment decreased the oxygen cost of exercise independently of changes in skeletal muscle mitochondrial functions or abundances. Nitrate treatment was associated with changes in the relative abundances of metabolic fuels for energy production, both at rest and after exercise. Nitrate-induced differences in the net utilization of different fuel sources (such as glycolytic and TCA intermediates, fatty acids, lactate, and ketone bodies) may have contributed to improvements in exercise performance. While nitrite had similar effects as nitrate on the abundances of metabolites at rest, the data suggest that nitrite treatment leads to the net depletion of fatty acids for energy production during exercise.
Supplementary Material
Acknowledgments
We thank Carrie L Barton, Eric Johnson, Kimberly Hayward (Sinnhuber Aquatic Research Laboratory), Claudia Maier [Department of Chemistry and Oregon State University (OSU) Mass Spectrometry Center], Jeffrey Morré (OSU Mass Spectrometry Center), and Jaewoo Choi (Linus Pauling Institute Analytical Services Core) for technical assistance and advice. The authors’ responsibilities were as follows—ERA, LMB, LT, MMR, RLT, JFS, NGH: designed the research; ERA, LMB, LSM, LT, CRL, SS, MCP, RMK, MG-J, SEE, HDS, SAN, MMR: conducted research and analyzed data; SS, SAN, MMR, RLT, JFS, NGH: provided essential reagents; ERA, LMB: wrote the paper; NGH: had primary responsibility for the final content; and all authors: read and approved the final manuscript.
Notes
This work was supported in part by the Celia Strickland and G Kenneth Austin III Endowment; the Oregon Agricultural Experimental Station; the Oregon State University Environmental Health Sciences Center; the Oregon Clinical & Translational Research Institute grant KL2TR002370 and award UL1TR002369; the National Institutes of Health grants S10 RR027878 and K01DK103829; and NIEHS Environmental Health Sciences grant P30 ES000210.
Author disclosures: ERA, LMB, LSM, LT, CRL, SS, MCP, RMK, MG-J, SEE, HDS, SAN, MMR, RLT, JFS, and NGH, no conflicts of interest.
Supplemental Methods, Supplemental Figures 1–3, and Supplemental Tables 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: FDR, false discovery rate; Nos, NO synthase; OSU, Oregon State University; P:O, ratio of ATP production to O2 consumption; TCA, tricarboxylic acid; Xor, xanthine oxidoreductase.
References
- 1. Peeling P, Binnie MJ, Goods PSR, Sim M, Burke LM. Evidence-based supplements for the enhancement of athletic performance. Int J Sport Nutr Exerc Metab. 2018;28(2):178–87. [DOI] [PubMed] [Google Scholar]
- 2. Romijn JA, Coyle EF, Sidossis LS, Gastaldelli A, Horowitz JF, Endert E, Wolfe RR. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol. 1993;265(1):E380–91. [DOI] [PubMed] [Google Scholar]
- 3. van Loon LJ, Greenhaff PL, Constantin-Teodosiu D, Saris WH, Wagenmakers AJ. The effects of increasing exercise intensity on muscle fuel utilisation in humans. J Physiol. 2001;536(1):295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burke LM, Hawley JA.. Swifter, higher, stronger: what's on the menu?. Science. 2018;362(6416):781–7. [DOI] [PubMed] [Google Scholar]
- 5. Egan B, Zierath JR.. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013;17(2):162–84. [DOI] [PubMed] [Google Scholar]
- 6. Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol (Oxf). 2007;191(1):59–66. [DOI] [PubMed] [Google Scholar]
- 7. Bailey SJ, Winyard P, Vanhatalo A, Blackwell JR, Dimenna FJ, Wilkerson DP, Tarr J, Benjamin N, Jones AM. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J Appl Physiol (1985). 2009;107(4):1144–55. [DOI] [PubMed] [Google Scholar]
- 8. Affourtit C, Bailey SJ, Jones AM, Smallwood MJ, Winyard PG. On the mechanism by which dietary nitrate improves human skeletal muscle function. Front Physiol. 2015;6;211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maughan RJ, Burke LM, Dvorak J, Larson-Meyer DE, Peeling P, Phillips SM, Rawson ES, Walsh NP, Garthe I, Geyer H et al.. IOC consensus statement: dietary supplements and the high-performance athlete. Int J Sport Nutr Exerc Metab. 2018;28(2):104–25. [DOI] [PubMed] [Google Scholar]
- 10. Jones AM. Dietary nitrate supplementation and exercise performance. Sports Med. 2014;44(Suppl 1):35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Govoni M, Jansson EA, Weitzberg E, Lundberg JO. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide. 2008;19(4):333–7. [DOI] [PubMed] [Google Scholar]
- 12. Lundberg JO, Govoni M.. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic Biol Med. 2004;37(3):395–400. [DOI] [PubMed] [Google Scholar]
- 13. Lundberg JO, Weitzberg E, Lundberg JM, Alving K. Intragastric nitric oxide production in humans: measurements in expelled air. Gut. 1994;35(11):1543–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Feelisch M, Fernandez BO, Bryan NS, Garcia-Saura MF, Bauer S, Whitlock DR, Ford PC, Janero DR, Rodriguez J, Ashrafian H. Tissue processing of nitrite in hypoxia: an intricate interplay of nitric oxide-generating and -scavenging systems. J Biol Chem. 2008;283(49):33927–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Denninger JW, Marletta MA.. Guanylate cyclase and the .No/cGMP signaling pathway. Biochim Biophys Acta. 1999;1411(2–3):334–50. [DOI] [PubMed] [Google Scholar]
- 16. Gee LC, Ahluwalia A. Dietary nitrate lowers blood pressure: epidemiological, pre-clinical experimental and clinical trial evidence. Curr Hypertens Rep. 2016;18(2):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roberts CK, Barnard RJ, Scheck SH, Balon TW. Exercise-stimulated glucose transport in skeletal muscle is nitric oxide dependent. Am J Physiol. 1997;273(1):E220–5. [DOI] [PubMed] [Google Scholar]
- 18. Bradley SJ, Kingwell BA, McConell GK. Nitric oxide synthase inhibition reduces leg glucose uptake but not blood flow during dynamic exercise in humans. Diabetes. 1999;48(9):1815–21. [DOI] [PubMed] [Google Scholar]
- 19. Baron AD. Hemodynamic actions of insulin. Am J Physiol. 1994;267(2):E187–202. [DOI] [PubMed] [Google Scholar]
- 20. Kina-Tanada M, Sakanashi M, Tanimoto A, Kaname T, Matsuzaki T, Noguchi K, Uchida T, Nakasone J, Kozuka C, Ishida M et al.. Long-term dietary nitrite and nitrate deficiency causes the metabolic syndrome, endothelial dysfunction and cardiovascular death in mice. Diabetologia. 2017;60(6):1138–51. [DOI] [PubMed] [Google Scholar]
- 21. Bhaswant M, Brown L, McAinch AJ, Mathai ML. Beetroot and sodium nitrate ameliorate cardiometabolic changes in diet-induced obese hypertensive rats. Mol Nutr Food Res. 2017;61:1700478. [DOI] [PubMed] [Google Scholar]
- 22. Gheibi S, Jeddi S, Carlstrom M, Gholami H, Ghasemi A. Effects of long-term nitrate supplementation on carbohydrate metabolism, lipid profiles, oxidative stress, and inflammation in male obese type 2 diabetic rats. Nitric Oxide. 2018;75:27–41. [DOI] [PubMed] [Google Scholar]
- 23. Ashworth A, Bescos R. Dietary nitrate and blood pressure: evolution of a new nutrient? Nutr Res Rev. 2017;30(2):208–19. [DOI] [PubMed] [Google Scholar]
- 24. Hord NG, Tang Y, Bryan NS. Food sources of nitrates and nitrites: the physiologic context for potential health benefits. Am J Clin Nutr. 2009;90(1):1–10. [DOI] [PubMed] [Google Scholar]
- 25. Hernandez A, Schiffer TA, Ivarsson N, Cheng AJ, Bruton JD, Lundberg JO, Weitzberg E, Westerblad H. Dietary nitrate increases tetanic [Ca2+]i and contractile force in mouse fast-twitch muscle. J Physiol. 2012;590(15):3575–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fulford J, Winyard PG, Vanhatalo A, Bailey SJ, Blackwell JR, Jones AM. Influence of dietary nitrate supplementation on human skeletal muscle metabolism and force production during maximum voluntary contractions. Pflugers Arch. 2013;465(4):517–28. [DOI] [PubMed] [Google Scholar]
- 27. Ferguson SK, Hirai DM, Copp SW, Holdsworth CT, Allen JD, Jones AM, Musch TI, Poole DC. Effects of nitrate supplementation via beetroot juice on contracting rat skeletal muscle microvascular oxygen pressure dynamics. Respir Physiol Neurobiol. 2013;187(3):250–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Roberts LD, Ashmore T, McNally BD, Murfitt SA, Fernandez BO, Feelisch M, Lindsay R, Siervo M, Williams EA, Murray AJ et al.. Inorganic nitrate mimics exercise-stimulated muscular fiber-type switching and myokine and gamma-aminobutyric acid release. Diabetes. 2017;66(3):674–88. [DOI] [PubMed] [Google Scholar]
- 29. Larsen FJ, Schiffer TA, Borniquel S, Sahlin K, Ekblom B, Lundberg JO, Weitzberg E. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab. 2011;13(2):149–59. [DOI] [PubMed] [Google Scholar]
- 30. Larsen FJ, Schiffer TA, Ekblom B, Mattsson MP, Checa A, Wheelock CE, Nystrom T, Lundberg JO, Weitzberg E. Dietary nitrate reduces resting metabolic rate: a randomized, crossover study in humans. Am J Clin Nutr. 2014;99(4):843–50. [DOI] [PubMed] [Google Scholar]
- 31. Whitfield J, Ludzki A, Heigenhauser GJ, Senden JM, Verdijk LB, van Loon LJ, Spriet LL, Holloway GP. Beetroot juice supplementation reduces whole body oxygen consumption but does not improve indices of mitochondrial efficiency in human skeletal muscle. J Physiol. 2016;594(2):421–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hargreaves M, Spriet LL. Exercise metabolism: fuels for the fire. Cold Spring Harb Perspect Med. 2018;8(8):a029744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ashmore T, Roberts LD, Morash AJ, Kotwica AO, Finnerty J, West JA, Murfitt SA, Fernandez BO, Branco C, Cowburn AS et al.. Nitrate enhances skeletal muscle fatty acid oxidation via a nitric oxide-cGMP-PPAR-mediated mechanism. BMC Biol. 2015;13:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, Collins JE, Humphray S, McLaren K, Matthews L et al.. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496(7446):498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Palstra AP, Rovira M, Rizo-Roca D, Torrella JR, Spaink HP, Planas JV. Swimming-induced exercise promotes hypertrophy and vascularization of fast skeletal muscle fibres and activation of myogenic and angiogenic transcriptional programs in adult zebrafish. BMC Genomics. 2014;15:1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Palstra AP, Tudorache C, Rovira M, Brittijn SA, Burgerhout E, van den Thillart GE, Spaink HP, Planas JV. Establishing zebrafish as a novel exercise model: swimming economy, swimming-enhanced growth and muscle growth marker gene expression. PLoS One. 2010;5(12):e14483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bugel SM, Tanguay RL, Planchart A. Zebrafish: a marvel of high-throughput biology for 21st century toxicology. Curr Environ Health Rep. 2014;1(4):341–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Simmonds AIM, Miln C, Seebacher F. Zebrafish (danio rerio) as a model for sprint exercise training. Zebrafish. 2018;16(1):1–7. [DOI] [PubMed] [Google Scholar]
- 39. Jensen FB. Nitric oxide formation from nitrite in zebrafish. J Exp Biol. 2007;210(19):3387–94. [DOI] [PubMed] [Google Scholar]
- 40. Lepiller S, Franche N, Solary E, Chluba J, Laurens V. Comparative analysis of zebrafish nos2a and nos2b genes. Gene. 2009;445(1–2):58–65. [DOI] [PubMed] [Google Scholar]
- 41. Axton ER, Hardardt EA, Stevens JF. Stable isotope-assisted LC-MS/MS monitoring of glyceryl trinitrate bioactivation in a cell culture model of nitrate tolerance. J Chromatogr B Analyt Technol Biomed Life Sci. 2016;1019:156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Learmonth C, Carvalho AP.. Acute and chronic toxicity of nitrate to early life stages of zebrafish—setting nitrate safety levels for zebrafish rearing. Zebrafish. 2015;12(4):305–11. [DOI] [PubMed] [Google Scholar]
- 43. Simmons AE, Karimi I, Talwar M, Simmons TW. Effects of nitrite on development of embryos and early larval stages of the zebrafish (danio rerio). Zebrafish. 2012;9(4):200–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Babaei F, Ramalingam R, Tavendale A, Liang Y, Yan LS, Ajuh P, Cheng SH, Lam YW. Novel blood collection method allows plasma proteome analysis from single zebrafish. J Proteome Res. 2013;12(4):1580–90. [DOI] [PubMed] [Google Scholar]
- 45. Piknova B, Schechter AN.. Measurement of nitrite in blood samples using the ferricyanide-based hemoglobin oxidation assay. Methods Mol Biol. 2011;704:39–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Conley MN, Roberts C, Sharpton TJ, Iwaniec UT, Hord NG. Increasing dietary nitrate has no effect on cancellous bone loss or fecal microbiome in ovariectomized rats. Mol Nutr Food Res. 2017;61(5):1600372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Masse AJ, Thomas JK, Janz DM. Reduced swim performance and aerobic capacity in adult zebrafish exposed to waterborne selenite. Comp Biochem Physiol C Toxicol Pharmacol. 2013;157(3):266–71. [DOI] [PubMed] [Google Scholar]
- 48. Marit JS, Weber LP. Persistent effects on adult swim performance and energetics in zebrafish developmentally exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Aquat Toxicol. 2012;106:131–9. [DOI] [PubMed] [Google Scholar]
- 49. Thomas JK, Janz DM. Dietary selenomethionine exposure in adult zebrafish alters swimming performance, energetics and the physiological stress response. Aquat Toxicol. 2011;102(1–2):79–86. [DOI] [PubMed] [Google Scholar]
- 50. Lanza IR, Nair KS. Functional assessment of isolated mitochondria in vitro. Methods Enzymol. 2009;457:349–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Miller BF, Wolff CA, Peelor FF 3rd, Shipman PD, Hamilton KL. Modeling the contribution of individual proteins to mixed skeletal muscle protein synthetic rates over increasing periods of label incorporation. J Appl Physiol (1985). 2015;118(6):655–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Watson GW, Wickramasekara S, Fang Y, Maier CS, Williams DE, Dashwood RH, Perez VI, Ho E. HDAC6 activity is not required for basal autophagic flux in metastatic prostate cancer cells. Exp Biol Med (Maywood). 2016;241(11):1177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kirkwood JS, Lebold KM, Miranda CL, Wright CL, Miller GW, Tanguay RL, Barton CL, Traber MG, Stevens JF. Vitamin C deficiency activates the purine nucleotide cycle in zebrafish. J Biol Chem. 2012;287(6):3833–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Xia J, Wishart DS.. Using metaboanalyst 3.0 for comprehensive metabolomics data analysis. Curr Protoc Bioinformatics. 2016;55(1):14.10.1–14.10.91. [DOI] [PubMed] [Google Scholar]
- 55. Barupal DK, Fiehn O. Chemical similarity enrichment analysis (chemrich) as alternative to biochemical pathway mapping for metabolomic datasets. Sci Rep. 2017;7(1):14567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mo L, Wang Y, Geary L, Corey C, Alef MJ, Beer-Stolz D, Zuckerbraun BS, Shiva S. Nitrite activates AMP kinase to stimulate mitochondrial biogenesis independent of soluble guanylate cyclase. Free Radic Biol Med. 2012;53(7):1440–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vaughan RA, Gannon NP, Carriker CR. Nitrate-containing beetroot enhances myocyte metabolism and mitochondrial content. J Tradit Complement Med. 2016;6(1):17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Parker BA, Walton CM, Carr ST, Andrus JL, Cheung ECK, Duplisea MJ, Wilson EK, Draney C, Lathen DR, Kenner KB et al.. Beta-hydroxybutyrate elicits favorable mitochondrial changes in skeletal muscle. Int J Mol Sci. 2018;19(8):2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Evans M, Cogan KE, Egan B. Metabolism of ketone bodies during exercise and training: physiological basis for exogenous supplementation. J Physiol. 2017;595(9):2857–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Puchalska P, Crawford PA. Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab. 2017;25(2):262–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Brooks GA. Energy flux, lactate shuttling, mitochondrial dynamics, and hypoxia. Adv Exp Med Biol. 2016;903:439–55. [DOI] [PubMed] [Google Scholar]
- 62. Pan JW, Telang FW, Lee JH, de Graaf RA, Rothman DL, Stein DT, Hetherington HP. Measurement of beta-hydroxybutyrate in acute hyperketonemia in human brain. J Neurochem. 2001;79(3):539–44. [DOI] [PubMed] [Google Scholar]
- 63. Yang L, Gao L, Nickel T, Yang J, Zhou J, Gilbertsen A, Geng Z, Johnson C, Young B, Henke C et al.. Lactate promotes synthetic phenotype in vascular smooth muscle cells. Circ Res. 2017;121(11):1251–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Halestrap AP. Monocarboxylic acid transport. Compr Physiol. 2013;3(4):1611–43. [DOI] [PubMed] [Google Scholar]
- 65. Kashiwaya Y, Sato K, Tsuchiya N, Thomas S, Fell DA, Veech RL, Passonneau JV. Control of glucose utilization in working perfused rat heart. J Biol Chem. 1994;269(41):25502–14. [PubMed] [Google Scholar]
- 66. Sato K, Kashiwaya Y, Keon CA, Tsuchiya N, King MT, Radda GK, Chance B, Clarke K, Veech RL. Insulin, ketone bodies, and mitochondrial energy transduction. FASEB J. 1995;9(8):651–8. [DOI] [PubMed] [Google Scholar]
- 67. Koeslag JH, Noakes TD, Sloan AW. Post-exercise ketosis. J Physiol. 1980;301:79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Winder WW, Baldwin KM, Holloszy JO. Exercise-induced increase in the capacity of rat skeletal muscle to oxidize ketones. Can J Physiol Pharmacol. 1975;53(1):86–91. [DOI] [PubMed] [Google Scholar]
- 69. Ashmore T, Fernandez BO, Branco-Price C, West JA, Cowburn AS, Heather LC, Griffin JL, Johnson RS, Feelisch M, Murray AJ. Dietary nitrate increases arginine availability and protects mitochondrial complex I and energetics in the hypoxic rat heart. J Physiol. 2014;592(21):4715–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hellsten Y, Hansson HA, Johnson L, Frandsen U, Sjodin B. Increased expression of xanthine oxidase and insulin-like growth factor I (IGF-I) immunoreactivity in skeletal muscle after strenuous exercise in humans. Acta Physiol Scand. 1996;157(2):191–7. [DOI] [PubMed] [Google Scholar]
- 71. Piknova B, Park JW, Swanson KM, Dey S, Noguchi CT, Schechter AN. Skeletal muscle as an endogenous nitrate reservoir. Nitric Oxide. 2015;47:10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Piknova B, Park JW, Kwan Jeff Lam K, Schechter AN. Nitrate as a source of nitrite and nitric oxide during exercise hyperemia in rat skeletal muscle. Nitric Oxide. 2016;55:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Weber JM, Choi K, Gonzalez A, Omlin T. Metabolic fuel kinetics in fish: swimming, hypoxia and muscle membranes. J Exp Biol. 2016;219(2):250–8. [DOI] [PubMed] [Google Scholar]
- 74. Ivarsson N, Schiffer TA, Hernandez A, Lanner JT, Weitzberg E, Lundberg JO, Westerblad H. Dietary nitrate markedly improves voluntary running in mice. Physiol Behav. 2017;168:55–61. [DOI] [PubMed] [Google Scholar]
- 75. Tenopoulou M, Doulias PT, Nakamoto K, Berrios K, Zura G, Li C, Faust M, Yakovishina V, Evans P, Tan L et al.. Oral nitrite restores age-dependent phenotypes in eNOS-null mice. JCI Insight. 2018;3(16):e122156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Jensen FB. Nitrite disrupts multiple physiological functions in aquatic animals. Comp Biochem Physiol A Mol Integr Physiol. 2003;135(1):9–24. [DOI] [PubMed] [Google Scholar]
- 77. Voslarova E, Pištěková V, Svobodova Z, Bedanova I. Nitrite toxicity to danio rerio: effects of subchronic exposure on fish growth. Acta Veterinaria Brno. 2008;77:455–60. [PubMed] [Google Scholar]
- 78. Gattuso A, Garofalo F, Cerra MC, Imbrogno S. Hypoxia tolerance in teleosts: implications of cardiac nitrosative signals. Front Physiol. 2018;9:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Van den Thillart G, Vianen G, Zaagsma J. Adrenergic regulation of lipid mobilization in fishes; a possible role in hypoxia survival: fish growth and metabolism. Environmental, nutritional and hormonal regulation. Fish Physiol Biochem. 2002;27(1):189–204. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.