ABSTRACT
Research on the interplay between iron and copper metabolism in humans began to flourish in the mid-20th century, and diseases associated with dysregulated homeostasis of these essential trace minerals are common even today. Iron deficiency is the most frequent cause of anemia worldwide, leading to significant morbidity, particularly in developing countries. Iron overload is also quite common, usually being the result of genetic mutations which lead to inappropriate expression of the iron-regulatory hormone hepcidin. Perturbations of copper homeostasis in humans have also been described, including rare genetic conditions which lead to severe copper deficiency (Menkes disease) or copper overload (Wilson disease). Historically, the common laboratory rat (Rattus norvegicus) was the most frequently utilized species to model human physiology and pathophysiology. Recently, however, the development of genetic-engineering technology combined with the worldwide availability of numerous genetically homogenous (i.e., inbred) mouse strains shifted most research on iron and copper metabolism to laboratory mice. This created new opportunities to understand the function of individual genes in the context of a living animal, but thoughtful consideration of whether mice are the most appropriate models of human pathophysiology was not necessarily involved. Given this background, this review is intended to provide a guide for future research on iron- and copper-related disorders in humans. Generation of complementary experimental models in rats, swine, and other mammals is now facile given the advent of newer genetic technologies, thus providing the opportunity to accelerate the identification of pathogenic mechanisms and expedite the development of new treatments to mitigate these important human disorders.
Keywords: iron deficiency, iron-deficiency anemia, hepcidin, hereditary hemochromatosis, β-thalassemia, copper deficiency, Menkes disease, Wilson disease, anemia
Introduction
Recently developed genetic-engineering tools stand ready to open new vistas in iron and copper homeostasis. An easy-to-use genome-editing technology, assigned the unwieldy and uninformative acronym CRISPR-Cas9 (for clustered regularly interspaced short palindromic repeats–CRISPR-associated protein 9), allows researchers readily to generate genetically modified experimental models in almost any species—CRISPR-Cas9 and a guide RNA enable very specific modifications of DNA sequence (1–3). This is a critical advance because earlier molecular techniques for mammals were largely constrained to mice and harder to master than CRISPR-Cas9 technology. Thus, now is an appropriate time to summarize currently available animal models of iron- and copper-related diseases in humans to guide the employment of new tools to advance our understanding of the molecular pathogenesis of each disorder and expedite the development of new therapeutic approaches. In this article, we will summarize existing experimental models that faithfully mimic the pathophysiology of human disease, and also identify areas where the development of new animal models could improve translational potential. The issues considered herein are particularly salient given the dramatic shift to using laboratory mice as experimental models in biomedical research over the past 20–30 y (4), and several notable investigative failures where observations made in mice did not translate to humans (5–7).
Background
Iron and copper are essential dietary constituents for humans and other mammals. Deficiencies or excesses of both elements result in significant morbidity, underscoring the importance of thoroughly understanding the molecular mechanisms that regulate iron and copper homeostasis. Iron deficiency (ID) is the most common cause of anemia worldwide, and also quite common in the United States. Globally, dietary iron insufficiency (and low iron bioavailability), often combined with chronic inflammation due to bacterial or parasitic infections, underlies most cases of ID and iron deficiency anemia (IDA). In the United States, IDA most commonly occurs in those with limited economic means and is typically associated with increased demand for iron (e.g., in pregnancy and during periods of rapid growth) or malabsorptive disorders (e.g., in Crohn's disease and ulcerative colitis), and after gastric-bypass surgery. Iron metabolism is also frequently perturbed in those afflicted with autoimmune diseases such as rheumatoid arthritis, and in chronic kidney disease and cancer patients, leading to the development of the anemia of inflammation (AI). Transactivation of the hepcidin antimicrobial peptide gene (HAMP; encoding the iron-regulatory hormone hepcidin) in hepatocytes by proinflammatory cytokines (e.g., IL-6) leads to increases in circulating concentrations of the hormone. Hepcidin decreases serum iron concentrations by inhibiting intestinal iron absorption and decreasing iron release from body stores (e.g., in macrophages), thus contributing to the pathogenesis of AI.
Conditions associated with excess (pathologic) body iron burden, such as hereditary hemochromatosis (HH) and β-thalassemia, have also been frequently described in humans. HH occurs most commonly as a result of mutations in genes encoding proteins that transactivate the HAMP gene in hepatocytes [i.e., homeostatic iron regulator (HFE), transferrin receptor 2 (TFR2), hemojuvelin (HJV)]. Dysfunction of these proteins leads to low circulating hepcidin concentrations, which results in excessive intestinal iron absorption and concomitant serum and tissue iron accumulation (which cannot be corrected because no active, regulated iron excretory system exists in humans or other mammals). Iron overload occurs in patients with β-thalassemia often due to frequent blood transfusion, but also due to dysregulation of HAMP transcription leading to inappropriately low production of hepcidin that can occur in the absence of transfusion. As in the case of HH, this dysregulation causes excessive absorption of enteral iron and systemic iron overload.
Copper imbalance also perturbs normal homeostasis in humans. Although dietary copper insufficiency is uncommon, severe copper deficiency occurs in Menkes disease (MD), which is caused by mutations in the gene encoding a copper transporter [ATPase copper transporting α (ATP7A)]. Impaired ATP7A function blunts intestinal copper absorption, leading to severe systemic copper depletion and dysfunction of various cuproenzymes in different tissues (e.g., brain). Mutations in the gene encoding a similar copper transporter, ATP7B (ATPase copper transporting β), underlie the pathogenesis of copper overload in patients with Wilson disease (WD). Patients afflicted with this rare genetic disorder have impaired biliary copper excretion, leading to copper accumulation in the liver and eventually other tissues, ultimately causing oxidative damage and organ dysfunction.
Although rats were the primary model used in biomedical research in past decades, the advent of genetic-engineering techniques specifically devoted to mice resulted in a dramatic shift towards the development of mouse models. However, mice may not always be the best model organism to mimic accurately the complex pathophysiology of human disease. The motives of lower costs and the ready availability of inbred strains harboring genetic modifications do not constitute scientific rationales for choosing mice. In this review, we summarize current experimental models for iron- and copper-related research to highlight the strengths and limitations of each. Our intent is to aid scientists in choosing the appropriate experimental models to use to obtain translatable results relevant to human health and disease.
IDA
Acquired and genetic causes in humans
ID is the most prevalent cause of anemia in humans worldwide and IDA is the most severe manifestation of chronic iron depletion. IDA is characterized by small (i.e., microcytic) and pale (i.e., hypochromic) RBCs with an impaired ability to transport oxygen. IDA is most commonly an acquired condition, but rare genetic mutations may also lead to severe iron depletion. Causes of acquired IDA include: 1) dietary iron insufficiency (with or without concurrent inflammation); 2) the inability to assimilate adequate dietary iron due to impaired iron absorption (e.g., resulting from intestinal pathologies or gastric-bypass surgery); and 3) increased demand for iron (e.g., during periods of rapid growth and in pregnancy). Rare genetic conditions in humans may also lead to IDA, including mutations in the genes encoding transferrin (TF) (8) and divalent metal-ion transporter 1 (DMT1) (Figure 1). TF is the major iron-binding (transport) protein in blood. Lack of fully functional TF impairs iron delivery (from the gut and stores) to developing erythroblasts of the bone marrow, thus leading to iron-restricted erythropoiesis and consequent IDA. DMT1 is required for intestinal iron absorption (9, 10), and for cellular iron acquisition from circulating diferric-TF via transferrin receptor 1 (TFR1), most notably by developing RBCs (Figure 1). Lack of DMT1 function thus impairs iron import into duodenal enterocytes plus uptake of iron from the blood by erythroblasts. Although human SLC11A2 (encoding DMT1) mutations are rare, manifestations are severe, including IDA, and paradoxically, in some patients, hepatic (and systemic) iron overload (14). This latter effect may be due to incomplete inactivation of DMT1, or alternative pathways of iron import, that allow assimilation of small amounts of dietary iron. Because this absorbed iron cannot be efficiently utilized for hemoglobin (Hb) synthesis by erythrocyte precursors (due to DMT1 dysfunction), serum iron increases and eventually exceeds the TF-binding capacity, thus resulting in the appearance of highly reactive, non-transferrin-bound iron (NTBI). Serum NTBI is taken up nonspecifically into the liver and other tissues, with possible pathological outcomes. One patient harboring a mutation in SLC11A2 had a completely null phenotype (without liver iron loading) (15), emphasizing the necessity of intestinal DMT1 for absorption of dietary iron.
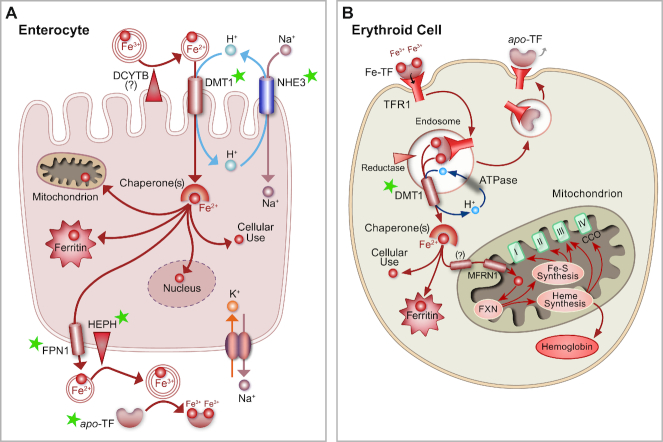
FIGURE 1.
Iron acquisition and metabolism in enterocytes and erythroid cells. Green stars highlight proteins associated with genetic defects that can cause IDA and other iron dyshomeostasis, as discussed in the text. (A) Duodenal enterocyte showing the main proteins involved in the absorption of dietary nonheme iron. A ferrireductase (possibly DCYTB) and DMT1 are depicted on the apical (upper) surface of the cell. Also shown is NHE3, which may directly provide protons for cotransport with ferrous iron via DMT1. The sodium gradient is maintained by the Na+/K+ ATPase, depicted on the basolateral surface. In enterocytes, iron is probably bound by chaperones, like poly-r(C)-binding proteins 1 and 2 (PCBP1/2) 1/2) (11, 12), and distributed into the cytosol (for cellular use), the mitochondria, or the nucleus, or stored in ferritin. Iron that transits the cell is exported by FPN1, oxidized by HEPH [and/or other (unknown) oxidases], and bound to apo-TF for distribution to the liver. (B) Mutations in SLC11A2 (encoding DMT1) also disrupt iron acquisition by developing erythroid cells; the defect here contributes further to the genetic iron deficiency. DMT1 is required for iron uptake by these cells via its role in the transferrin cycle. When DMT1 is dysfunctional, erythroid cells do not accumulate sufficient iron, impairing their ability to produce hemoglobin, and contributing to the development of IDA. In this cell type, DMT1 may also be required for iron import into mitochondria (as indicated by “?”), as recently suggested by Wolff et al. (13). MFRN1 and FXN also participate in mitochondrial iron metabolism, and heme iron produced in the mitochondrion may be utilized by cytochrome C oxidase. DCYTB, duodenal cytochrome B; DMT1, divalent metal-ion transporter 1; FPN1, ferroportin 1; FXN, frataxin; HEPH, hephaestin; IDA, iron-deficiency anemia; MFRN1, mitoferrin 1; NHE3, sodium-hydrogen exchanger 3; SLC, solute carrier; TF, transferrin.
Moreover, another rare genetic condition which is typified by severe IDA has been described in humans: iron-refractory, iron-deficiency anemia (IRIDA). This disorder is caused by mutations in the transmembrane protease, serine 6 (TMPRSS6) gene that encodes matriptase 2 (16), which is a membrane-bound serine protease that suppresses expression of hepcidin in hepatocytes when body iron stores are depleted and during anemia (with concurrent tissue hypoxia) (17) (Figure 2).
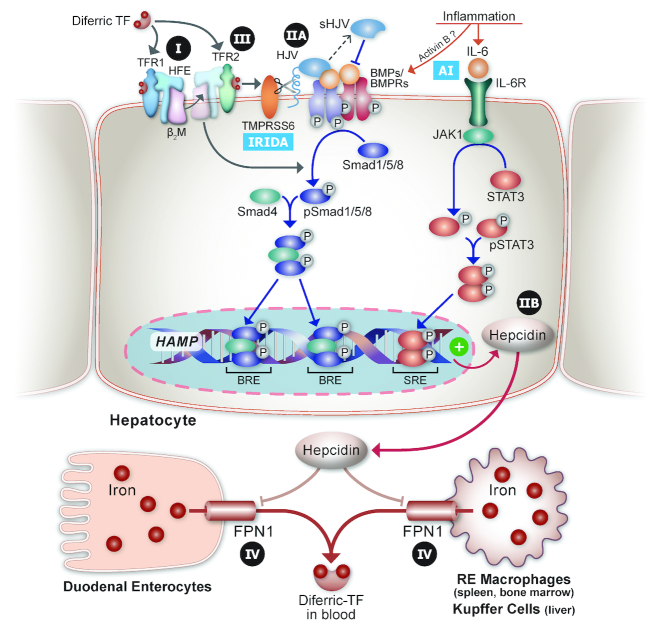
FIGURE 2.
Genetic disorders of iron metabolism in humans involving dysregulation of the HAMP/hepcidin/FPN1 axis. The molecular machinery that regulates transactivation of the HAMP gene is depicted. Under basal conditions, diferric-TF interacts with a complex of TFR1/HFE/β2M on the surface of hepatocytes. When body iron amounts are elevated and transferrin saturation increases, a new complex forms between diferric-TF/TFR2/HFE/β2M; then, this complex interacts with cell surface BMP ligands/BMP receptors and stimulates BMP/SMAD protein family signaling to increase HAMP expression. Mutations in the genes encoding HFE and TFR2 lead to inappropriately low hepcidin production and underlie type I and type III HH, respectively (see Table 1). HJV is required for proper BMP/BMPR signaling to HAMP, and mutations in the gene encoding HJV underlie type IIA HH. Other types of HH occur from mutations in HAMP (type IIB) that impair hepcidin production or lead to dysfunction of the hepcidin protein, and in SLC40A1 (encoding FPN1), leading to “ferroportin disease,” or type IV HH. Also indicated are the influences of inflammation on HAMP transcription, which underlie the AI, and mutations in TMPRSS6, which cause IRIDA because the ability to downregulate HAMP expression via cleaving HJV from the plasma membrane is abolished. Lastly, hepcidin, secreted by hepatocytes into the circulation, decreases FPN1 protein concentrations on the surface of enterocytes and RE macrophages (including hepatic Kupffer cells), thus inhibiting intestinal iron absorption and iron release from stores, to lower serum iron. AI, anemia of inflammation; BMP, bone morphogenetic protein; BMPR, bone morphogenetic protein receptor; BRE, BMP response element; FPN1, ferroportin 1; HAMP, hepcidin antimicrobial peptide gene; HFE, homeostatic iron regulator; HH, hereditary hemochromatosis; HJV, hemojuvelin; IRIDA, iron-refractory, iron-deficiency anemia; JAK, Janus kinase; RE, reticuloendothelial; SLC, solute carrier; SMAD, small mothers against decapentaplegic; SRE, SMAD response element; STAT, signal transducer and activator of transcription proteins; TF, transferrin; TFR, transferrin receptor; TMPRSS6, transmembrane protease, serine 6; β2M, β2-microglobulin.
Animal models of nutritional ID
To mimic dietary iron insufficiency [or nutritional iron deficiency (NID)] in humans, which very frequently results in anemia, experimental animals can be fed defined diets with low iron content. Several mammalian species have been utilized for such investigations (as described below), but laboratory rodents have been most commonly used. Rats (in general) are more susceptible to developing IDA due to dietary iron deprivation than are some commonly used laboratory mouse strains (e.g., C57BL6). This difference may be due to the evolution of rats as compared with mice, 2 species that diverged between 12 and 24 million years ago. Although both species are considered omnivorous, mice prefer grains, fruits, and seeds, whereas rats tend to be more opportunistic, also eating meat and animal products (when available). Because iron bioavailability may be greater from these latter foods, which contain heme iron (in addition to nonheme iron), mice may have evolved to retain body iron more tightly than rats. Given that some commonly used strains of laboratory mice are less likely to develop IDA due to dietary iron restriction, many investigators have utilized rats for such investigations (references provided here are examples, randomly selected out of literally hundreds in the scientific literature) (18–27). Distinct differences in the severity of ID have, however, been noted between rat strains (e.g., Fischer 344, Wistar, and Sprague Dawley) (28, 29). NID has also been modeled in dogs, with clinical manifestations similar to humans (30). Pigs are also an excellent model for studies of human iron metabolism because they are true omnivores that can assimilate dietary heme and nonheme iron, and have similarities to humans in the structure and function of the gastrointestinal tract (31). In one recent report, severe IDA was corrected in piglets by providing dietary Hb (32). Rhesus monkeys are also an excellent model of IDA given similarities to humans regarding gestational physiology and brain development. NID was induced in monkeys to assess the usefulness of serum ferritin as a biomarker of iron status (33). Interestingly, IDA may also occur spontaneously in monkeys, like in humans, affecting activity and emotionality (34).
Animal models of IDA during pregnancy
Pregnancy frequently leads to IDA in humans owing to increased demand for iron (from expansion of the maternal blood supply and to supply the growing fetal-placental unit). This so-called “anemia of pregnancy” has been modeled in rats and mice. In rodents, however, IDA does not naturally occur during pregnancy (at least under laboratory conditions), so low-iron feeding is typically required. Rats were used to study IDA during pregnancy, with similar pathophysiological outcomes to humans (23, 35). Pregnant mice fed low-iron diets also faithfully mimicked human IDA (36). The guinea pig is also an appropriate model for studying the effect of ID on brain development, because a critical period of cerebral maturation occurs prenatally (as in humans). IDA during pregnancy was recently modeled in guinea pigs. Notably, offspring born to ID dams had impaired auditory function (37). Similar studies have been done using Rhesus monkeys that were deprived of dietary iron during pregnancy (38). Outcomes showed that IDA during pregnancy can compromise hematological parameters of the offspring at birth.
Experimental models of genetic IDA
Rodent models of genetic IDA also exist. For example, microcytic anemia (mk) mice (39) and Belgrade (b) rats (40) harbor inactivating mutations in the Slc11a2 gene, causing severe IDA (41, 42) due to diminished duodenal iron uptake (39) and ineffective TF cycling (40, 43). In a remarkable coincidence occurring despite evident evolutionary divergence between rats and mice, the same G185R mutation in the Slc11a2 gene occurred in mk mice and b rats. Although these models each have specific aspects of IDA, the mutations also generate noteworthy differences from the nutritional disorder. For example, the mk mouse and b rat retain more iron (and possibly other metals) in the gut lumen because DMT1 ordinarily imports iron, but it fails to do so effectively in these mutant rodents. Motivated by these observations, Andrews and colleagues (44) generated global and intestine-specific Slc11a2 knockout (KO) mice; both exhibit severe systemic iron depletion and IDA. The sex-linked anemia (sla) mouse, harboring a spontaneously occurring mutation in the gene encoding the ferroxidase hephaestin (HEPH), is another model of genetic ID. Additional experimental models were also recently created and characterized, including global and intestine-specific HEPH KO mice (45, 46). In duodenal enterocytes, HEPH oxidizes ferrous iron after export by ferroportin 1 (FPN1), thus allowing ferric iron binding to TF in the lamina propria of intestinal villi for distribution to the liver in the portal blood circulation (47) (Figure 1). Lack of HEPH function leads to impaired iron efflux from duodenal enterocytes, and consequent systemic ID. Importantly, however, humans carrying inactivating mutations in the gene encoding HEPH have not been reported to date, so the translational significance of these experimental observations is unclear. Another model of genetic ID is the hypotransferrinemia (hpx) mouse, which does not produce any functional transferrin (48); although extremely rare, humans with a similar phenotype also exist (49). Lack of TF impairs iron delivery to developing RBCs in the bone marrow, thus impairing erythropoiesis and leading to the development of IDA. Furthermore, mice lacking other intestinal iron transport–related proteins also develop severe ID and/or IDA, including the apically expressed, sodium-hydrogen exchanger 3 (NHE3), which presumably provides the protons required by DMT1 to transport ferrous iron into enterocytes (9, 50), and the iron exporter FPN1 (51) (Figure 1). In addition, mice lacking the membrane-bound serine protease matriptase-2 (encoded by the Tmprss6 gene) (Figure 2) develop a severe IDA, designated IRIDA (52). Matriptase-2 is apparently involved in downregulating HAMP gene expression in hepatocytes (53); thus, lack of serine protease activity leads to inappropriately elevated hepcidin expression, which blunts intestinal iron absorption (resulting in IDA).
Conclusions: experimental animal models of IDA
In summary, human ID and IDA have been successfully modeled in several different mammalian species. Dietary iron insufficiency, or NID, has been modeled in mice, rats, dogs, and monkeys. Rats may be the most practical experimental model, given that mice are more resistant (in general) to developing dietary iron insufficiency–related IDA. Pigs and monkeys are perhaps the best models; however, pigs are very expensive to purchase, house, and maintain, and the utility of using nonhuman primates for biomedical research is also constrained by financial (and ethical) considerations. Models of the anemia of pregnancy have also been created and characterized in many of the same species (plus guinea pigs). Many of these experimental models have shown quite similar outcomes to ID during pregnancy in humans. Experimental models of genetic causes of ID and IDA in humans have also been described. In some cases, spontaneous mutants have been shown to have similar phenotypes to humans carrying mutations in the same genes (e.g., SLC11A2, TF, and TMPRSS6). In other cases, genetic-engineering techniques have been utilized to inactivate genes of interest, such as in global and intestine-specific Heph KO mice, for which no known human correlate exists. Collectively, these animal models have advanced our understanding of the negative physiological outcomes associated with body iron depletion and have further provided valuable tools to facilitate the development of new and better approaches to supplement iron in at-risk individuals and populations.
Iron Overload: HH
HH designates a group of related genetic disorders caused by mutations in genes that encode hepcidin (HAMP) or proteins that transactivate the HAMP gene in hepatocytes (54), including HFE, TFR2, and HJV. Another cause of HH is mutations in the gene encoding the iron exporter FPN1 (SLC40A1) (Table 1). The molecular interactions for HH are represented in Figure 2. Hepcidin lowers serum iron by blocking iron export from duodenal enterocytes and iron release from reticuloendothelial (RE) macrophages (which store excess iron) and hepatocytes. Physical interactions between hepcidin and FPN1 at the cell surface cause the transporter to be internalized and degraded. HH is characterized by inappropriately low concentrations of hepcidin (given body iron status) or impaired hepcidin-mediated regulation of FPN1 protein concentrations, resulting in elevated intestinal iron absorption and concomitant iron accumulation in many tissues (e.g., heart, liver, joints, endocrine organs, gonads, and skin). Pathological iron loading develops because the ability to store excess iron is limited, and no active means to excrete excess body iron exists in mammals. Moreover, because excess (unbound) iron promotes production of damaging oxygen free radicals, prolonged iron overload can cause serious health problems, including cardiomyopathy, diabetes, osteoarthritis, cirrhosis and hepatocellular carcinoma, impotence, and hypothyroidism.
TABLE 1.
Mouse models of HH and comparison with the human disease1
| Human HH type (mutant gene) | Mouse model | Mouse phenotype | Human phenotype | References |
|---|---|---|---|---|
| Type 1 (HFE) | Hfe −/− Hfe C282Y/C282Y Hfe C282Y/H63D | Decreased hepcidin, elevated TSAT, increased body iron (liver), increased intestinal Fe absorption, cardiac hypertrophy; no hepatic fibrosis or cirrhosis | Adult-onset; liver disease usually predominates, but endocrine disorders, cardiac problems, and joint disease also occur | (55–58) |
| Type 2A (HJV) | Hjv −/− | Decreased hepcidin, elevated TSAT, increased body iron (liver, pancreas, heart), splenic iron sparing; do not develop diabetes or cardiomyopathy | Juvenile-onset; cardiomyopathy and endocrinopathies predominate; parenchymal iron accumulation in liver | (59, 60) |
| Type 2B (HAMP) | Hamp1 −/− | No hepcidin, increased body iron (liver, pancreas, heart), splenic iron sparing, centrolobular accumulation of liver iron | Juvenile-onset; similar to Type 2A | (61, 62) |
| Type 3 (TFR2) | Tfr2 −/− | Decreased hepcidin, elevated TSAT, increased hepatic iron, splenic iron sparing | Adult-onset; similar to Type 1 | (63, 64) |
| Type 4 (SLC40A1; encoding FPN1) | Fpn ffe | Iron loading in hepatic Kupffer cells, high serum ferritin, low TSAT | “Ferroportin disease”; macrophage iron trapping, low TSAT, anemia | (65) |
ffe, Flatiron; FPN, ferroportin 1; HAMP, hepcidin antimicrobial peptide gene; HFE, homeostatic iron regulator; HH, hereditary hemochromatosis; HJV, hemojuvelin; SLC, solute carrier; TFR, transferrin receptor; TSAT, transferrin saturation.
Animal models of HH
Four types of HH have been described in humans (Table 1). Mice were genetically engineered to mimic the human disease (listed in Table 1); however, a major limitation of these mouse models is the lack of late- or end-stage disease manifestations that typify human HH (as described above). This lack may be due to the fact that the pathophysiologic outcomes of aging are quite distinct in mice as compared with humans, with mice having a much shorter life span, a smaller body size, and a higher basal metabolic rate. In the case of iron overload, oxygen free radical–mediated cellular and tissue damage may take many years to affect organ function negatively; thus, excess iron deposited in human tissues for several years or decades impairs physiologic function, yet iron accumulation in mouse tissues does not have sufficient time to cause such metabolic disturbances. The pattern of iron loading is also distinct in mouse models of HH, further complicating the situation. For example, iron loads in the exocrine pancreas of mice, whereas in humans, iron also accumulates in the endocrine pancreas (eventually impairing β-cell function and resulting in the development of diabetes mellitus). Furthermore, mice do not absorb and utilize dietary iron derived from heme, as humans do; therefore, a possible role for heme iron in iron loading cannot be assessed in mice. In addition, mice derive ≤50% of iron to support erythropoiesis from the diet, whereas erythropoietic iron is mostly derived from recycling in humans (66, 67). Mice may thus not be the ideal species in which to model human HH. Accordingly, a rat model of HH has also been described, Hsd:HHCL Wistar rats, which were found to have a mutation in TFR2, leading to inappropriately low hepcidin production and the development of periportal, hepatocellular iron accumulation (68). Iron status and tissue pathology over the life span have not yet been studied in this unique rat strain, however, so long-term pathophysiological outcomes associated with iron loading in this model are unknown. Given some of these notable limitations of existing experimental models of HH, the development of additional, complementary models, perhaps in rats or other mammalian species, is warranted.
Dietary iron overload: rat and mouse models
In humans, iron loading due to excessive intake of dietary iron is uncommon because mechanisms exist to limit intestinal iron absorption (e.g., increased production of ferritin, which binds excess iron within enterocytes and prevents it from being absorbed, plus regulatory pathways defined by the mutants described in Table 1). Moreover, humans are not typically exposed to iron at high enough concentrations to bypass these normal protective mechanisms; except, for example, in the case of accidental ingestion of iron supplements. Elevated iron supplementation has, however, been commonly used in experimental animals to induce iron overload and mimic human iron-loading disorders (69, 70). Typical iron concentrations utilized vary but can be as high as 2–3% (20,000–30,000 ppm). Dietary iron at these concentrations presumably saturates active-transport mechanisms and then leads to nonspecific, unregulated iron movement through the intestinal epithelium (possibly via the paracellular pathway). This experimental iron-loading approach, however, is not without possible negative physiological outcomes associated with the absorption of other dietarily essential minerals, such as Cu and Zn (71). For example, dietary iron loading caused copper depletion in mice (72), thus increasing copper requirements (73). This dietary competition contrasts with hepatic accumulation of zinc in HH (74). Speculatively, this contrast could reflect metal ion competition for functional DMT1 and/or FPN1 in the dietary loading or increased expression of 1 or both transporters in HH. It is well established that DMT1 is relatively promiscuous in transporting metal ions (75, 76), and such a situation could apply also to FPN1. Consumption of a diet containing 0.25% carbonyl iron (i.e., 2500 ppm) for 2 wk induced a maximal increase in hepatic hepcidin expression in mice, with no further increase noted at concentrations ≤2% (77). Dietary iron overload in mice also induced ferroptosis, a recently defined form of nonapoptotic cell death (78), and increased oxidative stress and caused cardiac hypertrophy (79). Another experimental approach to induce iron overload is intraperitoneal (IP) injection of iron dextran. A recent investigation compared the effect of enteral (oral) and parenteral (injection) routes of iron administration on hepcidin production. Interestingly, hepcidin expression increased within 1 wk in response to dietary (enteral) iron in mice, whereas the response to pharmacological (parenteral) iron administration was markedly different, with no increase in hepcidin expression noted ≤7 d after treatment (80).
Dietary iron-loading experiments have also been frequently done with rats. Consistent with studies in mice, rats fed a high-iron diet developed copper deficiency, again suggesting that high dietary iron increases the requirement for copper (81, 82). High-iron (and calcium) consumption perturbed zinc metabolism in rats, but the relative influence of iron versus calcium was not experimentally determined (83). Furthermore, high-iron feeding impaired absorption of manganese from the lungs to the blood, after intratracheal Mn administration (84). Also, consumption of a high-iron diet (and IP and intravenous iron treatments) led to iron loading mainly in RE macrophages and hepatocytes (85). In another iron-loading study, parenteral iron (in the form of IP RBCs) contributed more to spleen iron loading, but high dietary (carbonyl) iron contributed more to liver iron loading (86).
In conclusion, although dietary iron loading is uncommon in humans, this experimental approach is commonly used for rodents. Whereas excessive iron transfer through the gut epithelium and concomitant tissue iron accumulation occur when dietary iron is high (mimicking some aspects of genetic iron-loading disorders in humans), absorption of other essential minerals may be impaired (Cu, Zn, Mn), confounding data interpretation and possibly differing from what happens in HH. Moreover, the pattern of iron loading may be distinct based upon whether the excess iron is provided enterally or parenterally. In general though, dietary iron-loading studies done in mice and rats have shown comparable pathologic outcomes, with no clear advantage emerging in either species. Given that dietary (or parenteral) iron loading only mimics some aspects of relevant human diseases (and is potentially fraught with experimental confounders), there is no clear rationale for using similar experimental approaches in other mammalian species.
β-Thalassemia: An Iron-Loading Anemia
Hb in adult mammals, including humans, consists of 2 α-globin chains and 2 β-globin chains, encoded by distinct α- and β-globin genes on separate chromosomes (87). Frequently, the genes exist as duplicates; for example, humans have 2 nonallelic α-globin genes that produce identical α-globin chains. The human β-globin gene cluster also results in the production of 2 β-like-globin chains, δ and β, in adults, with the β gene accounting for 97–98% of total β-like-globin. β-Thalassemia is caused by a variety of mutations in the HBB (β) gene, which result in partial (i.e., β+-thalassemia) or nearly complete (i.e., β0-thalassemia) deficiency in the synthesis of β-globin chains, leading to Hb dysfunction and consequent severe anemia. The disease has been characterized as either β-thalassemia major or β-thalassemia intermedia, with the phenotype of the former being more severe than the latter. Importantly though, individuals with both β0-thalassemia and β+-thalassemia have been diagnosed with β-thalassemia major, and β-thalassemia intermedia, so this distinction between different forms of the disease depends on clinical not molecular criteria. For example, a mutation that deletes the entire β-globin gene results in a β0-thalassemia; however, it presents as thalassemia intermedia if there is sufficient expression of the γ-globin chain of fetal Hb to compensate for the lack of β-globin chains, or it presents as thalassemia major if γ-globin expression fails to do so. The clinical presentation is also affected by the extent of α-globin expression; the greater the imbalance of α and β, the more likely that the presentation will be thalassemia major. The treatment used for thalassemia major is frequent blood transfusion, but this results in systemic iron overload due, in part, to the lack of an excretory pathway for excess iron. In β-thalassemia intermedia, where blood transfusions are unnecessary or less frequent, iron overload can still occur due to inappropriately reduced expression of liver-derived hepcidin, and concomitant pathological increases in intestinal iron absorption (see below for more information) (88).
Mouse models of β-thalassemia
Many mouse models of human β-thalassemia have been developed over the past few decades (89). In adult mice, 2 β-globin genes are expressed, b1 and b2. The b1 gene accounts for ∼80% of total β-globin chains, whereas the b2 gene contributes ∼20%; hence, the designations βmajor (for the b1 gene) and βminor (for the b2 gene). The βmajor gene was mutated in mice by inserting a bacterial gene into exon 2, creating Hbbth-2 mice. Mice homozygous for this insertional disruption die in the perinatal period of severe anemia (90). Interestingly, when the βmajor gene was deleted in mice (Hbbth-1), homozygotes survived into adulthood and were less severely anemic than in Hbbth-2 mice (91). These disparate results may be explained by the complex process of control of gene expression by an upstream locus control region. Young Hbbth-1 mice exhibited elevated intestinal iron absorption (92) and progressive tissue iron accumulation in the absence of blood transfusions (which were not required to preserve life) (93). Collectively, the authors of these articles suggested that these mouse models of β-thalassemia closely mimicked the human disease.
Mice have also been created that lack both adult β-globin genes, modeling β-zero (β0) thalassemia in humans (94). Heterozygotes were viable and fertile, but severely anemic, whereas homozygotes died in utero. Other investigators utilized a similar approach, and demonstrated that mice lacking both adult β-globin genes (homozygous Hbbth-3 mice) died in the perinatal period, and although heterozygotes appeared normal, their hematological indexes reflected severe thalassemia (95). Interestingly, heterozygotes also developed spontaneous iron overload in spleen, liver, and kidneys. Comparison of such heterozygotes to homozygotes that lacked the βmajor gene demonstrated that iron overload was more progressive and substantial in the heterozygous Hbbth-3 mice than in the homozygous less severely anemic Hbbth-2 mice (96). It is likely that the severity of the anemia drives the amount of iron overload in these mouse models. Many additional mouse models of β-thalassemia have been generated, but most have not been carefully compared for iron overloading as in the case just cited. We describe them briefly below because they do provide an opportunity to confirm this argument.
One such mouse line contains a single copy of the human βIVS-2-654 gene, representing a common β-globin C to T splicing mutation (at nucleotide 654 of exon 2), in place of the 2 mouse adult β-globin genes (i.e., Hbbth-4 mice) (97). Homozygous mice carrying this mutant gene fail to survive postnatally, but heterozygotes showed features of β-thalassemia intermedia. A humanized mouse model was also created for a common β0-thalassemia mutation (98) with a 4-bp deletion in the intact human β-globin locus on the Hbbth-3 background (so lacking both mouse β-globin genes and referred to as DH△4bp mice). Heterozygous DH△4bp mice model the key features of thalassemia at the phenotypic level. Another mouse model was designed to mimic the G to A mutation at codon 26 of the human β-globin gene (99). This results in “hemoglobin E” (HbE) disease, which is typified by a mild anemia. HbE is a functional, but unstable structural Hb variant where the mutation not only results in E26K but also in an alternative splice site that diminishes normal splicing and thus β-globin production. These humanized mice carry the HbE (βE) mutation in the context of the human β-globin locus (again on the Hbbth-3 background) and display hematological abnormalities consistent with HbE homozygosity in humans.
Another report described the generation of a humanized mouse model carrying the common IVSI-110 splicing mutation within the human β-globin locus (also on the Hbbth-3 background) (100). Mice carrying the IVSI-110β mutation showed a 90% decrease in human β-globin chain synthesis, due to aberrant splicing. The authors concluded that the humanized IVSI-110 mouse model accurately recapitulates the splicing defect found in comparable β-thalassemia patients. Lastly, another investigation reported the generation of a humanized mouse model of β-thalassemia major (or Cooley's anemia) by targeted gene replacement in embryonic stem cells (101). The adult mouse β-globin genes were replaced with a delayed switching human γ- to β0-globin gene cassette to create γβ0 knockin (KI) mice. This is an important advance because the developmental timing of Hb switching in humans and mice is different, and mice lack a true fetal Hb. Heterozygous γβ0 mice developed β-thalassemia intermedia. When these mice were bred to human α-globin KI mice, the animals survived solely on fetal Hb during fetal life but died of severe anemia upon completion of the Hb switch after birth (unless therapeutic intervention ensued). New transfusion strategies and chelation therapies could be tested in these mice. This model is also ideal for developing and testing experiemental approaches to reactivate the silenced human γ-globin gene by trans-acting factors or small molecules.
In conclusion, in recent years, mouse models have become available for multiple β-thalassemia disorders reported in the primary scientific literature. The structural and functional conservation of Hb in mammals (in general) has made the laboratory mouse a useful organism in which to study the Hb protein and the individual globin genes. Collectively, these investigations have generated animal models that display many features of β-thalassemia in humans, and abnormal iron status (in the absence of a need for blood transfusions) is clearly associated with some models (92, 93, 96, 102). Substantive differences in the expression of human and mouse globin genes during development have also revealed limitations of the mouse as a model of the human disease. Moreover, given notable physiological differences between mice and humans (6), the development of additional β-thalassemia models in other species could provide novel insight into how iron homeostasis is perturbed by mutations in the β-globin genes.
Management of aberrant iron metabolism in mouse models of β-thalassemia
Iron overload occurs in human β-thalassemia owing to frequent blood transfusion and/or inappropriately low production of the iron-regulatory hormone hepcidin. In β-thalassemia intermedia, patients typically do not require regular blood transfusions (103). Even without the excess iron from transfusions, pathological suppression of hepcidin expression results in excessive iron absorption in patients with β-thalassemia (104, 105). This likely occurs due to the predominate influence of enhanced erythropoietic demand which decreases HAMP gene transcription (thus overriding the positive effect that iron loading has on HAMP expression). Hepcidin suppression may be mediated via the erythroid iron regulator, erythroferrone, which decreases HAMP gene expression in hepatocytes when erythroid demand is elevated (106). Furthermore, studies in mouse models of β-thalassemia intermedia (e.g., in Hbbth-1/th-1 and Hbbth-3/+ mice) demonstrated that transgenic overexpression of hepcidin prevents iron overload and improves erythropoiesis (107, 108). Also, KO of TMPRSS6 (encoding matriptase-2, which negatively regulates HAMP gene transcription in hepatocytes) in a mouse model of β-thalassemia increased hepcidin concentrations, prevented iron loading, and improved erythropoiesis (109, 110). Collectively, these investigations provided the impetus for the development of complementary approaches to mitigate iron overload in β-thalassemia patients with suppression of hepcidin expression, such as mini-hepcidins, which are short (functional) peptides based on the 7–9 N-terminal amino acids of hepcidin (111).
AI
AI (or the anemia of chronic disease) is the most prevalent cause of anemia in hospitalized and chronically ill patients, and in those who have long-term inflammatory conditions such as infection, autoimmune disease, chronic kidney disease, and cancer (112, 113). Clinically, it is classified as a normochromic, normocytic (or microcytic) anemia that is usually mild, with serum Hb concentrations 8–9.5 g/dL (note that the normal range is 13.5–17.5 g/dL for adult men and 12.0–13.5 g/dL for women). Patients with AI also typically have a low reticulocyte count and low serum iron, leading to a decrease in transferrin saturation with normal to marginal iron stores (114). Hepatocyte hepcidin production increases owing to proinflammatory cytokine signaling, most prominently mediated by IL-6 (115). Increased circulating hepcidin blocks iron release (via FPN1) from duodenal enterocytes and RE macrophages, ultimately leading to hypoferremia, iron-restricted erythropoiesis, anemia, and tissue hypoxia. The proinflammatory milieu also interferes with the production (or activity) of erythropoietin, leading to a suppression of erythropoietic activity. Decreased erythrocyte survival, mediated by multiple pathological mechanisms, also typifies AI.
Animal models of AI
AI is a multifactorial disease affecting multiple organ systems; thus, animal models have been developed using a variety of experimental approaches. Not surprisingly, many investigators have modeled human AI in mice (116), although there are several limitations of using mice for such studies. For example, mouse erythrocytes have a smaller mean corpuscular volume, lower oxygen affinity, and a shorter half-life, leading to more active erythropoiesis in mice with a higher basal reticulocyte count. Other notable differences between mouse and human erythroid cells include variations in membrane protein structure and function, glucose utilization, vitamin C metabolism, molecular signaling pathways, and regulation of ion content (117). Furthermore, genome-wide transcriptome profiling revealed species-specific profiles and nonconservation of regulatory RNAs (e.g., long noncoding RNAs) when comparing human and mouse erythroid cells. Also, mice mount a more robust reticulocyte response to various inflammatory stimuli, and extramedullary (stress) erythropoiesis occurs more extensively in mice.
Despite these notable differences in mouse and human physiology, chronic inflammation (modeling AI) has been induced in laboratory mice using several approaches, including IP injection of heat-killed Brucella abortus (118–120), zymosan/LPS (121), or LPS (122, 123); subcutaneous injection of turpentine oil (124–126); and injection of Staphylococcus epidermidis–coated beads into the peritoneum (116). Oral delivery of dextran sulfate sodium via drinking water induces chronic colitis (127), providing another potentially useful AI model related to inflammatory bowel disease. Injection of Freund's complete adjuvant to generate collagen-induced arthropathy has also been used to model the AI associated with rheumatoid arthritis (127). Cecal ligation and puncture is another approach to induce chronic inflammation; this procedure, however, is difficult to master and time consuming (127, 128). Moreover, transgenic mice overexpressing the proinflammatory cytokine IL-6 (129) or hepcidin (130) have also been utilized as models of human AI.
AI has also been induced in rats. Limitations similar to those described above for mice may also apply to rat models of AI, but the challenge has not been so thoroughly examined in the scientific literature as it has for mice. Rat models include IP injection of group A streptococcal peptidoglycan-polysaccharide (131–133) and repeated injection of Freund's complete adjuvant (116). AI has also been modeled in other species, including dogs (by repeated subcutaneous injection of turpentine oil) (134) and nonhuman primate models (by subcutaneous injection of human IL-6 in Cynomolgus monkeys) (135, 136).
In conclusion, AI is a multifactorial disease, so many animal models have been developed to mimic uniquely one aspect of the human disease. The most commonly used species for modeling human AI is the laboratory mouse. Although there are notable limitations to using mice for these investigations, there are several reasons why researchers have focused in the past 2–3 decades on mouse models [as reviewed in (116)], including the abundance of (inbred) genetically engineered mice available for biomedical research. Using rats may offer some advantages (compared with using mice), as discussed above, but research on AI in rats has lagged considerably behind mouse studies. Although the pig has not to our knowledge been used for AI studies, its similarities to humans do make it potentially attractive in this regard. Nonhuman primates would likely be superior to rats, mice, or swine to model AI, but again, ethical and financial concerns have limited their use to date.
MD: Genetic Copper Deficiency
Copper is a necessary cofactor for numerous enzymes in humans (i.e., cuproenzymes), but it is toxic when in excess due to Fenton-like chemistry which produces reactive oxygen radicals. Body copper concentrations must thus be tightly regulated. MD (or Menkes syndrome) is an inherited disorder in which copper homeostasis is perturbed (Figure 3) with grave pathophysiological outcomes. MD is characterized by failure to thrive (impaired neonatal growth); sparse, kinky hair; and neurodegeneration. Other characteristics include hypotonia, seizures, developmental delay, and intellectual impairment. MD symptoms typically emerge during infancy and affected children frequently die before 6 y of age. In some individuals with MD, early intervention with supplemental copper attenuates disease progression. Occipital horn syndrome (OHS) is a less severe form of the disease with a later onset of symptoms (early- to mid-childhood). OHS is characterized by abnormal calcium deposits in the occipital bone (at the base of the skull), loose skin and joints, and coarse hair.
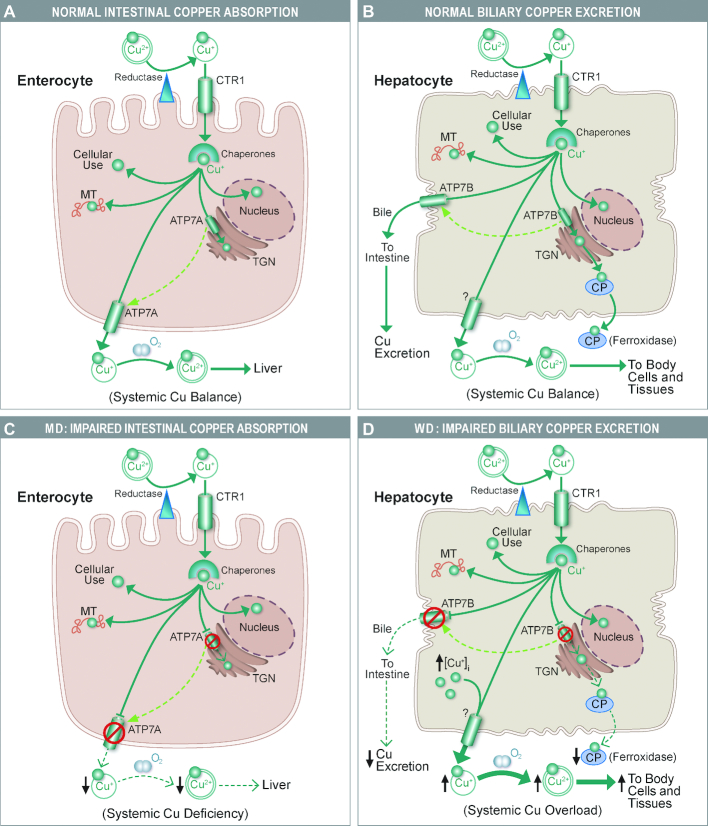
FIGURE 3.
Altered copper homeostasis in enterocytes and hepatocytes underlies MD and WD, respectively. (A, B) Normal copper homeostasis in enterocytes and hepatocytes; (C, D) perturbations that occur in MD and WD. The ATP7A protein in enterocytes is required to deliver copper to support cuproenzyme synthesis in the TGN, and when copper is in excess, it traffics to the basolateral membrane and functions in copper export. In MD, when mutations in the gene encoding ATP7A lead to the production of a dysfunctional protein, absorption of dietary copper is impaired, ultimately leading to severe systemic copper deficiency and notable pathophysiologic changes (C). An analogous protein expressed in hepatocytes, ATP7B, similarly functions in copper delivery to the TGN to support the production of CP, which is secreted into the circulation and functions mainly in iron metabolism. When hepatic copper is in excess, ATP7B traffics to the canalicular membrane where it excretes copper into the biliary tree. Excreted copper is presumably complexed with bile salts (and thus unavailable for reabsorption) and eliminated in the feces. In WD, the mutant ATP7B protein can no longer efficiently pump excess copper into the bile, so copper accumulates in hepatocytes, eventually exceeding the storage capacity of the liver and spilling out into the systemic circulation (leading to excess copper accumulation in some tissues) (D). CP production is also impaired, which disrupts iron homeostasis in WD patients (e.g., causing liver iron loading). ATP7A, ATPase copper transporting α; ATP7B, ATPase copper transporting β; CP, ceruloplasmin; CTR1, copper transporter 1; MD, Menkes disease; MT, metallothoinein; TGN, trans-Golgi network; WD, Wilson disease.
MD is caused by mutations in the gene encoding the ATP7A copper transporter, which is a dual-function protein. One function relates to the synthesis of cuproenzymes in the secretory pathway in the trans-Golgi. During intracellular copper excess, ATP7A traffics to the basolateral membrane and performs its second function, copper export from the cell. ATP7A plays a predominant role in intestinal copper absorption, renal copper reabsorption, and copper transport across the blood–brain barrier. Dysfunction of intestinal ATP7A results in severe systemic copper deficiency, with characteristically low copper in brain and other tissues. Decreased copper concentrations may reduce the activity of numerous copper-containing enzymes that contribute to the structure and function of bone, skin, hair, the vasculature, and the central nervous system. The pathological manifestations of MD (and OHS) are likely caused by low activity of these cuproenzymes. In humans, ∼370 ATP7A mutations have been identified, resulting in variable clinical manifestations (137). Full-term pregnancies and live births typically occur even with the most severe mutations.
Animal models of MD
Experimental animal models of MD exist most commonly in mice, owing to the identification of spontaneously occurring mutants. The advent of genetic-engineering techniques (that were perfected first in mice) led to the development of additional MD mouse models. Mice with mutations in the Atp7a gene are called mottled mutants, reflecting the X-linked nature of the mutation and “random” X-chromosome inactivation leading to a distinct pattern of coat pigmentation in heterozygous females. There are several mouse strains harboring different mutations. Mottled mice were first described in 1953, but it was not until 1974 that primary defects in copper transport were established as a disease-causing abnormality. Today, 109 mottled alleles have been identified; ∼50% of them resulted from gene trapping manipulations. Many other mottled mutants arose spontaneously, but several were the result of exposing mice to mutagenic agents (toxic chemicals or radiation). Mottled males, being hemizygous, exhibit a severe, often lethal, phenotype.
Many male mice carrying Atp7a mutations die in utero, due to severe defects in copper homeostasis. These include the mottled spot (Atp7amo-spot) mutants, which have a genomic deletion of exons 11–14 (138). Mottled candy (Atp7amo-ca) mice carry a disruptive insertion in exon 10 (138). In another (more severe) MD model, Atp7aMo-Tohm, hemizygous males typically die at around 11 days of gestation (139). In Atp7amo11-H (11H) mutants, embryonic lethality was observed, likely due to mis-localization of the ATP7A protein to the endoplasmic reticulum, impaired glycosylation, and reduced copper delivery to the secretory pathway in the trans-Golgi (140). In mottled-dappled (Atp7amo-dp) mice, which harbor a large deletion in the 5' region of the Atp7a gene, hemizygous males usually die at around embryonic day 17. Notable pathological outcomes are bending and thickening of the ribs and distortions of the pectoral and pelvic girdles and limbs (141).
Another group of mutant mice, with less severe phenotypes, are widely used for studies of copper metabolism and serve as models of “classical” MD in humans. These include the brindled, mosaic, and macular strains, in which ATP7A activity is significantly reduced but not completely abolished. Brindled mice (Atp7amo-br) carry a 6-bp, in-frame deletion in exon 11 (142), are hypopigmented, and die ∼15 d after birth; however, mutants can be rescued by IP copper treatment before postnatal day 10 (143). Mosaic mice (Atp7amo-ms) harbor a G to C nucleotide transversion in exon 15 of the Atp7a gene, resulting in an Arg to Pro substitution in the highly conserved sixth transmembrane domain of the protein. These mice, which load excess copper in the small intestine and kidney, and have low copper in the brain, liver, and heart, normally die in the second week of life (144). Macular mice (Atp7amo-ml) carry a T to C bp transition at position 4223, with a Ser to Pro substitution in the eighth transmembrane domain (145). Hemizygous males began to show depigmentation and curly whiskers around postnatal day 3, then seizures and ataxia are observed around day 8 and mice die shortly thereafter (median age at death: ∼15 d) (146).
Other MD mouse models are yet less severe (and resemble the phenotype of OHS in humans), allowing male mice to survive to maturity. One such strain, viable brindled (Atp7amo-vbr) mice, carry a mutation that alters the phosphorylation domain in the ATP7A protein (142). These mutants succumb to blood vessel rupture between 50 and 100 d after birth (147). The molecular defect seems to relate to constitutive trafficking of the ATP7A protein to the plasma membrane (due to hyperphosphorylation), which reduces copper delivery to the trans-Golgi to support cuproenzyme synthesis (140). Blotchy (Atp7amo-blo) mice, which show the longest survival, harbor a splice site mutation in exon 11 (142, 148) and succumb to blood vessel rupture at ≥150 d after birth (147). Conditional KOs of the Atp7a gene have also been described (149). Another strain, Atp7a (T985I/Y) mice, have a conditional KI of Atp7a (T985I), the orthologue of the human ATP7A (T994I) (a mutation associated with a less severe, related disease in humans, referred to as X-linked distal hereditary motor neuropathy). These mice display altered Cu concentrations within the peripheral and central nervous systems, an increased diameter of muscle fibers, and altered myogenin and myostatin gene expression (150).
In summary, to our knowledge, MD has been modeled only in laboratory mice. In these naturally occurring mutants, and genetically engineered mice, a spectrum of copper deficiency–related phenotypes is observed, allowing them to serve as useful models for the wide variety of human phenotypes associated with ATP7A mutations. Given vast differences in mouse and human physiology, due to millions of years of divergent evolution, the development of alternative and complementary models in other commonly utilized experimental species could expedite the development of molecular therapies to treat humans with this group of devastating ATP7A-related disorders. It may be rewarding to now use CRISPR-Cas9 to develop models in pigs and/or primates as well to reflect the variety of MD symptoms more closely.
WD: Genetic Copper Overload
WD is an autosomal recessive, inherited disorder, in which biliary copper excretion is impaired, ultimately resulting in pathological copper accumulation in the liver, brain, and eyes (151). Disease manifestations may be noted as early as age 6 y or as late as 45 y, but it more typically begins during the teenage years. Pathological features include liver disease and neuropsychiatric problems. Notable symptoms of WD include jaundice (with characteristic yellowing of the skin or whites of the eyes), lethargy, hypophagia, and abdominal swelling. Liver disease is usually the initial feature in children and young adults; however, those diagnosed at older ages frequently have only very mild liver disease. Neurological and psychiatric problems are often the initial features of adult-onset WD, as manifested by clumsiness, tremors, difficulty walking and speaking, impaired cognitive function, depression, anxiety, and mood swings. Many patients with WD display copper deposits in the cornea of the eye, which form green-to-brownish rings, often called Kayser–Fleischer rings, considered a pathognomonic sign and frequently resulting in abnormalities in eye movements.
WD is caused by mutations in the ATP7B gene, encoding a copper efflux transporter, ATP7B, a paralog of the ATP7A copper transporter. ATP7B functions in copper transport into the trans-Golgi to support the production of cuproenzymes in the secretory pathway, including ceruloplasmin (CP; a circulating ferroxidase), in addition to copper export into the bile at the canicular surface of hepatocytes (Figure 3). ATP7B-mediated copper transport into the bile represents the main excretory route for copper and the principle homeostatic checkpoint for whole-body copper homeostasis. With ATP7B dysfunction, copper accumulates to excessive concentrations in the liver, and eventually when the storage capacity of the liver is surpassed, copper spills out into the circulation. Copper then deposits in other tissues, most prominently in the brain and eye, causing oxidative stress and eventually tissue damage. Treatments used for WD include copper chelators and supplemental dietary zinc (which blocks intestinal copper absorption).
Animal models of WD
One spontaneously occurring mouse model of WD is the toxic milk (tx) mouse. Notable features of these mutant mice include the production of milk with low copper content, hepatic copper accumulation (with most being bound to metallothionein), enlarged hepatocytes with abnormal nuclei, and decreased hepatic CP production (152). Note that CP is a circulating ferroxidase that is required for iron export from some tissues (e.g., liver). ATP7B KO mice have also been generated. These mice display gradual accumulation of hepatic copper (with eventual fibrosis and cirrhosis), with increased copper content also observed in kidney and brain. During pregnancy, copper also accumulates in the placenta (reflecting impaired copper delivery to the developing fetuses) and lactating mammary glands (reflecting impaired copper transport into milk), both contributing to the development of severe copper deficiency in the pups (153). A rat model of WD has also been described, the Long Evans Cinnamon (LEC) rat. This rat strain has a spontaneous mutation in the Atp7b gene, resulting in hepatic copper accumulation and development of acute hepatitis by 4 mo of age, and low plasma CP (which perturbs iron metabolism). The hepatitis can be prevented in these rats with the use of a copper chelator (D-penicillamine). LEC rats also develop hepatocellular carcinoma, which is rare in patients with WD (154, 155). Further, these rats rarely develop prominent neurologic abnormalities (that typify adult-onset human WD) and Kayser–Fleischer (corneal) rings have not been documented. Another rat strain, LPP rats, were generated because LEC rats carry mutations in Atp7b and an adjacent gene, serotonin N-acetyltransferase, which is involved in melatonin production. LPP rats gradually accumulate hepatic copper and develop biochemical signatures of liver disease at ∼90 d of age (156). Canine models of WD have also been described, including the Labrador Retriever which displays centrilobular hepatic copper accumulation. Like rodent models of WD, however, these dogs do not develop neurological disease nor evidence of corneal copper deposition (157). Another naturally occurring, related canine model is the Bedlington Terrier. These dogs have a mutation in copper metabolism domain containing 1 (Commd1). The COMMD1 protein regulates stability of the ATP7B protein in hepatocytes (and possibly other cells). When the COMMD1 protein is dysfunctional, ATP7B production is low, leading to impaired biliary copper excretion and concomitant hepatic copper overload (158).
In conclusion, multiple animal models of WD have been described in several mammalian species. The human disease has a complex phenotype, and only some aspects of it are manifest in experimental models of WD. Some end-stage disease manifestations do not occur in these experimental models, including copper loading in brain and cornea (and resulting pathologic outcomes). The generation of additional, complementary models in other mammalian species is thus warranted. In this case, despite the challenges articulated in preceding sections, development of swine or nonhuman primate models of WD could be a fruitful approach.
Copper-Deficiency Anemia
Dietary copper insufficiency is uncommon in humans, but copper deficiency does occur in patients with MD. Experimentally, copper depletion is associated with an ID-like anemia, but the underlying molecular perturbations are unclear. Multicopper ferroxidases, HEPH and CP, may be produced at low concentrations during copper depletion, thus impairing iron release from intestinal enterocytes and RE macrophages, causing low serum iron. Experimental copper deficiency also impairs Hb synthesis, causing anemia (159), which may be secondary to hypoferremia (due to low HEPH- and CP-mediated ferroxidase activity); however, a primary, copper-related defect in Hb synthesis in developing RBCs cannot be ruled out (e.g., perhaps related to ferrochelatase activity) (160). Copper-deficiency anemia (CDA) has been modeled in mice. Pregnant Hsd:ICR (CD-1), outbred albino mice were fed a copper-deficient diet containing 0.36 mg Cu/kg (normal = 6–7 mg/kg); offspring were anemic (161). HIF2α, a hypoxia-inducible transcription factor, was activated in mice fed a diet containing low copper (0.3–0.7 ppm), inducing expression of genes involved in intestinal iron absorption (162). Mice fed a copper-deficient diet had decreased HEPH activity, likely suppressing iron release from duodenal enterocytes (163). Interestingly, female mice developed a more extreme form of CDA than males (164).
Genetic models of copper deficiency also exist. For example, KO of copper transporter 1 (Ctr1—the main copper importer in the intestine) in mice produced embryonic lethality. Intestine-specific Ctr1 KO mice showed poor growth and premature death at ∼10 d of age, but could be rescued by IP injection of CuSO4 (165). Rat models of copper deficiency also exist. After pregnant Holtzman rats were fed a low-copper diet (0.36 mg Cu/kg), offspring were anemic with low brain and plasma iron (161). Hepcidin expression was also low in these rats, and FPN1 protein concentrations were high (166). Sprague Dawley rats fed a low-copper diet (<0.3 mg Cu/kg) had very low CP activity; however, Fe supplementation did not alleviate the noted anemia (167). Moreover, copper-deficient rats of both sexes had lower HEPH protein concentrations and impaired Fe absorption. These physiologic perturbations were reversed by repletion of dietary copper (168). In a classical precedent, pigs fed a low-copper diet developed microcytic, hypochromic anemia, which could be reversed by Cu supplementation, but not by iron (169, 170).
In conclusion, rats and pigs showed an anemic phenotype similar to human infants with inherited copper deficiency (i.e., MD patients), with lower plasma iron concentrations; however, in copper-deficient mice, plasma iron concentrations were not always decreased by copper depletion. Hence, rats and swine may be the models of choice for studies on CDA.
Conclusions
In this review, we highlighted animal models of widespread and rarer human disorders of iron and copper homeostasis that have continuing utility, plus emphasized situations where development of new experimental models could be advantageous. Many useful mammalian models of disturbances in iron and copper homeostasis already exist; however, a large fraction of these are mice. Although mice are less expensive to raise and maintain than nearly all other mammals, mice may not necessarily be the best models of human physiology and pathophysiology. The chief reasons that mice have been at the forefront of biomedical research in recent decades is that KO, KI, and transgenic strains have been much easier to produce than in other mammals, with an additional contribution derived from long-standing inbred strains of mice which provide a genetically uniform background for experimentation. Recently perfected genetic-engineering methods, however, encourage the development of new models in other species, some in outbred strains which more closely model the human condition. Given the advent of these newer techniques to induce heritable, genomic mutations, it seems likely that complementary models of human iron- and copper-related disorders will be developed in coming years. Collectively, we anticipate that these new models of human pathophysiology will provide significant advancements in our understanding of the molecular underpinnings of these (and other) important disorders.
Acknowledgments
The authors’ responsibilities were as follows—JFC: received the initial invitation to write a review on this topic; XW: conducted the original literature review, but MDG and JFC also identified references; XW: drafted an initial outline with MDG and JFC revising and rewriting the initial submission and resubmission; and all authors: read and approved the final manuscript.
Notes
Supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant R01 DK074867 (to JFC), and NIDDK and Office of Dietary Supplements grant R01 DK109717 (to JFC).
Author disclosures: XW, MDG, and JFC, no conflicts of interest.
The thalassemia section is dedicated to Sir David Weatherall who passed away 8 December, 2018. One of us (MDG) was privileged to have postdoctoral training at the Johns Hopkins Medical Institutions when David Weatherall was also there on his first sabbatical from the UK. David Weatherall had already acquired incredible expertise on the thalassemias that he generously shared. He developed (with Clegg and Naughton) a method for separating α- and β-globin chains during this period. The technique proved valuable not only for characterizing the thalassemias, but also for studying the globins of many mammalian species.
Abbreviations used: AI, anemia of inflammation; ATP7A, ATPase copper transporting α; ATP7B, ATPase copper transporting β; Cas9, CRISPR-associated protein 9; CDA, copper-deficiency anemia; Commd1, copper metabolism domain containing 1; CP, ceruloplasmin; CRISPR, clustered regularly interspaced short palindromic repeats; DMT1, divalent metal-ion transporter 1; FPN1, ferroportin 1; HAMP, hepcidin antimicrobial peptide gene; Hb, hemoglobin; HEPH, hephaestin; HFE, homeostatic iron regulator; HH, hereditary hemochromatosis; HJV, hemojuvelin; ID, iron deficiency; IDA, iron deficiency anemia; IP, intraperitoneal; IRIDA, iron-refractory, iron-deficiency anemia; KI, knockin; KO, knockout; LEC, Long Evans Cinnamon; MD, Menkes disease; NID, nutritional iron deficiency; NTBI, non-transferrin-bound iron; OHS, occipital horn syndrome; RE, reticuloendothelial; SLC, solute carrier; TF, transferrin; TFR, transferrin receptor; TMPRSS6, transmembrane protease, serine 6; WD, Wilson disease.
References
- 1. Haurwitz RE, Jinek M, Wiedenheft B, Zhou K, Doudna JA. Sequence- and structure-specific RNA processing by a CRISPR endonuclease. Science. 2010;329:1355–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang F, Wen Y, Guo X. CRISPR/Cas9 for genome editing: progress, implications and challenges. Hum Mol Genet. 2014;23:R40–6. [DOI] [PubMed] [Google Scholar]
- 3. Doudna J, Mali P. CRISPR-Cas: a laboratory manual. New York: Cold Spring Harbor Laboratory Press; 2016. [Google Scholar]
- 4. Homberg JR, Wohr M, Alenina N. Comeback of the rat in biomedical research. ACS Chem Neurosci. 2017;8:900–3. [DOI] [PubMed] [Google Scholar]
- 5. Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L et al.. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013;110:3507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Perlman RL. Mouse models of human disease: an evolutionary perspective. Evol Med Public Health. 2016:170–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iannaccone PM, Jacob HJ. Rats! Dis Model Mech. 2009;2:206–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beutler E, Gelbart T, Lee P, Trevino R, Fernandez MA, Fairbanks VF. Molecular characterization of a case of atransferrinemia. Blood. 2000;96:4071–4. [PubMed] [Google Scholar]
- 9. Shawki A, Anthony SR, Nose Y, Engevik MA, Niespodzany EJ, Barrientos T, Ohrvik H, Worrell RT, Thiele DJ, Mackenzie B. Intestinal DMT1 is critical for iron absorption in the mouse but is not required for the absorption of copper or manganese. Am J Physiol Gastrointest Liver Physiol. 2015;309:G635–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang X, Zhang M, Flores SRL, Woloshun RR, Yang C, Yin L, Xiang P, Xu X, Garrick MD, Vidyasagar S et al.. Oral gavage of ginger nanoparticle-derived lipid vectors carrying Dmt1 siRNA blunts iron loading in murine hereditary hemochromatosis. Mol Ther. 2019;27:493–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ryu MS, Zhang D, Protchenko O, Shakoury-Elizeh M, Philpott CC. PCBP1 and NCOA4 regulate erythroid iron storage and heme biosynthesis. J Clin Invest. 2017;127:1786–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shi H, Bencze KZ, Stemmler TL, Philpott CC. A cytosolic iron chaperone that delivers iron to ferritin. Science. 2008;320:1207–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wolff NA, Garrick MD, Zhao L, Garrick LM, Ghio AJ, Thevenod F. A role for divalent metal transporter (DMT1) in mitochondrial uptake of iron and manganese. Sci Rep. 2018;8:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mims MP, Guan YL, Pospisilova D, Priwitzerova M, Indrak K, Ponka P, Divoky V, Prchal JT. Identification of a human mutation of DMT1 in a patient with microcytic anemia and iron overload. Blood. 2005;105:1337–42. [DOI] [PubMed] [Google Scholar]
- 15. Blanco E, Kannengiesser C, Grandchamp B, Tasso M, Beaumont C. Not all DMT1 mutations lead to iron overload. Blood Cells Mol Dis. 2009;43:199–201. [DOI] [PubMed] [Google Scholar]
- 16. Finberg KE, Heeney MM, Campagna DR, Aydinok Y, Pearson HA, Hartman KR, Mayo MM, Samuel SM, Strouse JJ, Markianos K et al.. Mutations in TMPRSS6 cause iron-refractory iron deficiency anemia (IRIDA). Nat Genet. 2008;40:569–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Melis MA, Cau M, Congiu R, Sole G, Barella S, Cao A, Westerman M, Cazzola M, Galanello R. A mutation in the TMPRSS6 gene, encoding a transmembrane serine protease that suppresses hepcidin production, in familial iron deficiency anemia refractory to oral iron. Haematologica. 2008;93:1473–9. [DOI] [PubMed] [Google Scholar]
- 18. Beard JL, Zhan CS, Brigham DE. Growth in iron-deficient rats. Proc Soc Exp Biol Med. 1995;209:65–72. [DOI] [PubMed] [Google Scholar]
- 19. Hess SY, Zimmermann MB, Arnold M, Langhans W, Hurrell RF. Iron deficiency anemia reduces thyroid peroxidase activity in rats. J Nutr. 2002;132:1951–5. [DOI] [PubMed] [Google Scholar]
- 20. Dallman PR, Siimes MA, Manies EC. Brain iron: persistent deficiency following short-term iron deprivation in the young rat. Br J Haematol. 1975;31:209–15. [DOI] [PubMed] [Google Scholar]
- 21. Baggs RB, Miller SA. Nutritional iron deficiency as a determinant of host resistance in the rat. J Nutr. 1973;103:1554–60. [DOI] [PubMed] [Google Scholar]
- 22. Siimes MA, Refino C, Dallman PR. Manifestation of iron deficiency at various levels of dietary iron intake. Am J Clin Nutr. 1980;33:570–4. [DOI] [PubMed] [Google Scholar]
- 23. Andersen HS, Gambling L, Holtrop G, McArdle HJ. Maternal iron deficiency identifies critical windows for growth and cardiovascular development in the rat postimplantation embryo. J Nutr. 2006;136:1171–7. [DOI] [PubMed] [Google Scholar]
- 24. Chen OS, Schalinske KL, Eisenstein RS. Dietary iron intake modulates the activity of iron regulatory proteins and the abundance of ferritin and mitochondrial aconitase in rat liver. J Nutr. 1997;127:238–48. [DOI] [PubMed] [Google Scholar]
- 25. Cottin SC, Gambling L, Hayes HE, Stevens VJ, McArdle HJ. Pregnancy and maternal iron deficiency stimulate hepatic CRBPII expression in rats. J Nutr Biochem. 2016;32:55–63. [DOI] [PubMed] [Google Scholar]
- 26. Pinero DJ, Li NQ, Connor JR, Beard JL. Variations in dietary iron alter brain iron metabolism in developing rats. J Nutr. 2000;130:254–63. [DOI] [PubMed] [Google Scholar]
- 27. Tran PV, Fretham SJ, Wobken J, Miller BS, Georgieff MK. Gestational-neonatal iron deficiency suppresses and iron treatment reactivates IGF signaling in developing rat hippocampus. Am J Physiol Endocrinol Metab. 2012;302:E316–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rao J, Jagadeesan V. Development of a rat model for iron deficiency and toxicological studies: comparison among Fischer 344, Wistar, and Sprague Dawley strains. Lab Anim Sci. 1995;45:393–7. [PubMed] [Google Scholar]
- 29. Kasaoka S, Yamagishi H, Kitano T. Differences in the effect of iron-deficient diet on tissue weight, hemoglobin concentration and serum triglycerides in Fischer-344, Sprague-Dawley and Wistar rats. J Nutr Sci Vitaminol (Tokyo). 1999;45:359–66. [DOI] [PubMed] [Google Scholar]
- 30. Fry MM, Kirk CA. Reticulocyte indices in a canine model of nutritional iron deficiency. Vet Clin Pathol. 2006;35:172–81. [DOI] [PubMed] [Google Scholar]
- 31. Patterson JK, Lei XG, Miller DD. The pig as an experimental model for elucidating the mechanisms governing dietary influence on mineral absorption. Exp Biol Med. 2008;233:651–64. [DOI] [PubMed] [Google Scholar]
- 32. Staron R, Lipinski P, Lenartowicz M, Bednarz A, Gajowiak A, Smuda E, Krzeptowski W, Pieszka M, Korolonek T, Hamza I et al.. Dietary hemoglobin rescues young piglets from severe iron deficiency anemia: duodenal expression profile of genes involved in heme iron absorption. PLoS One. 2017;12:e0181117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sreeramulu D, Qadri S, Nair KM, Sivakumar B. Induction of dietary iron deficiency in rhesus monkeys: sequential changes in serum ferritin and other biochemical indicators of iron status. Ann Nutr Metab. 1994;38:322–30. [DOI] [PubMed] [Google Scholar]
- 34. Golub MS, Hogrefe CE, Widaman KF, Capitanio JP. Iron deficiency anemia and affective response in rhesus monkey infants. Dev Psychobiol. 2009;51:47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hossain MB, Kelleher SL, Lonnerdal B. Maternal iron and zinc supplementation during pregnancy affects body weight and iron status in rat pups at weaning. J Nutr. 2011;141:798–804. [DOI] [PubMed] [Google Scholar]
- 36. Hubbard AC, Bandyopadhyay S, Wojczyk BS, Spitalnik SL, Hod EA, Prestia KA. Effect of dietary iron on fetal growth in pregnant mice. Comp Med. 2013;63:127–35. [PMC free article] [PubMed] [Google Scholar]
- 37. Jougleux JL, Rioux FM, Church MW, Fiset S, Surette ME. Mild maternal iron deficiency anemia during pregnancy and lactation in guinea pigs causes abnormal auditory function in the offspring. J Nutr. 2011;141:1390–5. [DOI] [PubMed] [Google Scholar]
- 38. Golub MS, Hogrefe CE, Tarantal AF, Germann SL, Beard JL, Georgieff MK, Calatroni A, Lozoff B. Diet-induced iron deficiency anemia and pregnancy outcome in rhesus monkeys. Am J Clin Nutr. 2006;83:647–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fleming MD, Trenor CI, Su MA, Foernzler D, Beier DR, Dietrich WF, Andrews NC. Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nature Genet. 1997;16:383–6. [DOI] [PubMed] [Google Scholar]
- 40. Fleming MD, Romano MA, Su MA, Garrick LM, Garrick MD, Andrews NC. Nramp2 is mutated in the anemic Belgrade (b) rat: evidence of a role for Nramp2 in endosomal iron transport. Proc Natl Acad Sci U S A. 1998;95:1148–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Farcich EA, Morgan EH. Diminished iron acquisition by cells and tissues of Belgrade laboratory rats. Am J Physiol. 1992;262:R220–4. [DOI] [PubMed] [Google Scholar]
- 42. Wallace DF. The regulation of iron absorption and homeostasis. Clin Biochem Rev. 2016;37:51–62. [PMC free article] [PubMed] [Google Scholar]
- 43. Garrick MD, Gniecko K, Liu Y, Cohan DS, Garrick LM. Transferrin and the transferrin cycle in Belgrade rat reticulocytes. J Biol Chem. 1993;268:14867–74. [PubMed] [Google Scholar]
- 44. Gunshin H, Fujiwara Y, Custodio AO, Direnzo C, Robine S, Andrews NC. Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver. J Clin Invest. 2005;115:1258–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Doguer C, Ha JH, Gulec S, Vulpe CD, Anderson GJ, Collins JF. Intestinal hephaestin potentiates iron absorption in weanling, adult, and pregnant mice under physiological conditions. Blood Adv. 2017;1:1335–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fuqua BK, Lu Y, Darshan D, Frazer DM, Wilkins SJ, Wolkow N, Bell AG, Hsu J, Yu CC, Chen H et al.. The multicopper ferroxidase hephaestin enhances intestinal iron absorption in mice. PLoS One. 2014;9:e98792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bannerman RM, Bannerman CE, Kingston PJ. Hereditary iron deficiency: X-linked anemia (sla) in newborn and suckling mice. Br J Haematol. 1973;25:280. [PubMed] [Google Scholar]
- 48. Trenor CC, Campagna DR, Sellers VM, Andrews NC, Fleming MD. The molecular defect in hypotransferrinemic mice. Blood. 2000;96:1113–8. [PubMed] [Google Scholar]
- 49. Heilmeyer L, Keller W, Vivell O, Keiderling W, Betke K, Woehler F, Schultze HE. [Congenital atransferrinemia in a 7-year-old girl]. Dtsch Med Wochenschr. 1961;86:1745–51. [DOI] [PubMed] [Google Scholar]
- 50. Shawki A, Engevik MA, Kim RS, Knight PB, Baik RA, Anthony SR, Worrell RT, Shull GE, Mackenzie B. Intestinal brush-border Na+/H+ exchanger-3 drives H+-coupled iron absorption in the mouse. Am J Physiol Gastrointest Liver Physiol. 2016;311:G423–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S, Andrews NC. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005;1:191–200. [DOI] [PubMed] [Google Scholar]
- 52. Folgueras AR, de Lara FM, Pendas AM, Garabaya C, Rodriguez F, Astudillo A, Bernal T, Cabanillas R, Lopez-Otin C, Velasco G. Membrane-bound serine protease matriptase-2 (Tmprss6) is an essential regulator of iron homeostasis. Blood. 2008;112:2539–45. [DOI] [PubMed] [Google Scholar]
- 53. Silvestri L, Pagani A, Nai A, De Domenico I, Kaplan J, Camaschella C. The serine protease matriptase-2 (TMPRSS6) inhibits hepcidin activation by cleaving membrane hemojuvelin. Cell Metab. 2008;8:502–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pietrangelo A. Hereditary hemochromatosis: pathogenesis, diagnosis, and treatment. Gastroenterol. 2010;139:393–408. [DOI] [PubMed] [Google Scholar]
- 55. Bahram S, Gilfillan S, Kuhn LC, Moret R, Schulze JB, Lebeau A, Schumann K. Experimental hemochromatosis due to MHC class IHFE deficiency: immune status and iron metabolism. Proc Natl Acad Sci U S A. 1999;96:13312–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhou XY, Tomatsu S, Fleming RE, Parkkila S, Waheed A, Jiang JX, Fei Y, Brunt EM, Ruddy DA, Prass CE et al.. HFE gene knockout produces mouse model of hereditary hemochromatosis. Proc Natl Acad Sci U S A. 1998;95:2492–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fleming RE, Holden CC, Tomatsu S, Waheed A, Brunt EM, Britton RS, Bacon BR, Roopenian DC, Sly WS. Mouse strain differences determine severity of iron accumulation in Hfe knockout model of hereditary hemochromatosis. Proc Natl Acad Sci USA. 2001;98:2707–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lebeau A, Frank J, Biesalski HK, Weiss G, Srai SKS, Simpson RJ, McKie AT, Bahram S, Gilfillan S, Schumann K. Long-term sequelae of HFE deletion in C57BL/6 × 129/O1a mice, an animal model for hereditary haemochromatosis. Eur J Clin Invest. 2002;32:603–12. [DOI] [PubMed] [Google Scholar]
- 59. Huang FW, Pinkus JL, Pinkus GS, Fleming MD, Andrews NC. A mouse model of juvenile hemochromatosis. J Clin Invest. 2005;115:2187–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Niederkofler V, Salie R, Arber S. Hemojuvelin is essential for dietary iron sensing, and its mutation leads to severe iron overload. J Clin Invest. 2005;115:2180–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nicolas G, Bennoun M, Devaux I, Beaumont C, Grandchamp B, Kahn A, Vaulont S. Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad Sci U S A. 2001;98:8780–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lesbordes-Brion JC, Viatte L, Bennoun M, Lou DQ, Ramey G, Houbron C, Hamard G, Kahn A, Vaulont S. Targeted disruption of the hepcidin 1 gene results in severe hemochromatosis. Blood. 2006;108:1402–5. [DOI] [PubMed] [Google Scholar]
- 63. Wallace DF, Summerville L, Lusby PE, Subramaniam VN. First phenotypic description of transferrin receptor 2 knockout mouse, and the role of hepcidin. Gut. 2005;54:980–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Fleming RE, Ahmann JR, Migas MC, Waheed A, Koeffler HP, Kawabata H, Britton RS, Bacon BR, Sly WS. Targeted mutagenesis of the murine transferrin receptor-2 gene produces hemochromatosis. Proc Natl Acad Sci U S A. 2002;99:10653–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zohn IE, De Domenico I, Pollock A, Ward DM, Goodman JF, Liang XY, Sanchez AJ, Niswander L, Kaplan J. The flatiron mutation in mouse ferroportin acts as a dominant negative to cause ferroportin disease. Blood. 2007;109:4174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chappelle E, Gabrio BW, Stevens AR Jr, Finch CA. Regulation of body iron content through excretion in the mouse. Am J Physiol. 1955;182:390–2. [DOI] [PubMed] [Google Scholar]
- 67. Parmar JH, Davis G, Shevchuk H, Mendes P. Modeling the dynamics of mouse iron body distribution: hepcidin is necessary but not sufficient. BMC Syst Biol. 2017;11:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bartnikas TB, Wildt SJ, Wineinger AE, Schmitz-Abe K, Markianos K, Cooper DM, Fleming MD. A novel rat model of hereditary hemochromatosis due to a mutation in transferrin receptor 2. Comp Med. 2013;63:143–55. [PMC free article] [PubMed] [Google Scholar]
- 69. Bacon BR, Brittenham GM, Tavill AS, McLaren CE, Park CH, Recknagel RO. Hepatic lipid peroxidation in vivo in rats with chronic dietary iron overload is dependent on hepatic iron concentration. Trans Assoc Am Physicians. 1983;96:146–54. [PubMed] [Google Scholar]
- 70. Park CH, Bacon BR, Brittenham GM, Tavill AS. Pathology of dietary carbonyl iron overload in rats. Lab Invest. 1987;57:555–63. [PubMed] [Google Scholar]
- 71. Whittaker P. Iron and zinc interactions in humans. Am J Clin Nutr. 1998;68:442S–6S. [DOI] [PubMed] [Google Scholar]
- 72. Ha JH, Doguer C, Collins JF. Consumption of a high-iron diet disrupts homeostatic regulation of intestinal copper absorption in adolescent mice. Am J Physiol Gastrointest Liver Physiol. 2017;313:G353–G60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wang T, Xiang P, Ha JH, Wang XY, Doguer C, Flores SRL, Kang YJ, Collins JF. Copper supplementation reverses dietary iron overload-induced pathologies in mice. J Nutr Biochem. 2018;59:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Adams PC, Bradley C, Frei JV. Hepatic zinc in hemochromatosis. Clin Invest Med. 1991;14:16–20. [PubMed] [Google Scholar]
- 75. Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–8. [DOI] [PubMed] [Google Scholar]
- 76. Garrick MD, Dolan KG, Ghio A, Horbinski C, Higgins D, Porubcin M, Moore EG, Hainsworth LN, Umbreit JN, Conrad ME et al.. DMT1 (divalent metal transporter 1): a mammalian transporter for multiple metals. BioMetals. 2003;16:41–54. [DOI] [PubMed] [Google Scholar]
- 77. Rishi G, Secondes ES, Subramaniam VN. Hemochromatosis: evaluation of the dietary iron model and regulation of hepcidin. Biochim Biophys Acta Mol Basis Dis. 2018;1864:2550–6. [DOI] [PubMed] [Google Scholar]
- 78. Wang H, An P, Xie EJ, Wu Q, Fang XX, Gao H, Zhang ZZ, Li YZ, Wang XD, Zhang J et al.. Characterization of ferroptosis in murine models of hemochromatosis. Hepatology. 2017;66:449–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sukumaran A, Chang J, Han M, Mintri S, Khaw BA, Kim J. Iron overload exacerbates age-associated cardiac hypertrophy in a mouse model of hemochromatosis. Sci Rep. 2017;7:5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Daba A, Gkouvatsos K, Sebastiani G, Pantopoulos K. Differences in activation of mouse hepcidin by dietary iron and parenterally administered iron dextran: compartmentalization is critical for iron sensing. J Mol Med. 2013;91:95–102. [DOI] [PubMed] [Google Scholar]
- 81. Klevay LM. Iron overload can induce mild copper deficiency. J Trace Elem Med Biol. 2001;14:237–40. [DOI] [PubMed] [Google Scholar]
- 82. Ha JH, Doguer C, Wang X, Flores SR, Collins JF. High-iron consumption impairs growth and causes copper-deficiency anemia in weanling Sprague-Dawley rats. PloS One. 2016;11:e0161033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jayalakshmi S, Platel K. Compromised zinc status of experimental rats as a consequence of prolonged iron & calcium supplementation. Indian J Med Res. 2016;143:238–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Thompson K, Molina R, Donaghey T, Brain JD, Wessling-Resnick M. The influence of high iron diet on rat lung manganese absorption. Toxicol Appl Pharmacol. 2006;210:17–23. [DOI] [PubMed] [Google Scholar]
- 85. Papanastasiou DA, Vayenas DV, Vassilopoulos A, Repanti M. Concentration of iron and distribution of iron and transferrin after experimental iron overload in rat tissues in vivo: study of the liver, the spleen, the central nervous system and other organs. Pathol Res Pract. 2000;196:47–54. [DOI] [PubMed] [Google Scholar]
- 86. Wania CA, Macey DJ, Webb J, Hall PD, St Pierre TG. Effects of prolonged iron loading in the rat using both parenteral and dietary routes. Biometals. 1999;12:103–13. [DOI] [PubMed] [Google Scholar]
- 87. Garrick MD, Garrick LM. Hemoglobins and globin genes. In: Agar NS, Board PG, editors. The red cells of domestic mammals. Amsterdam: Elsevier/ North Holland; 1983. pp. 165–200. [Google Scholar]
- 88. Kearney SL, Nemeth E, Neufeld EJ, Thapa D, Ganz T, Weinstein DA, Cunningham MJ. Urinary hepcidin in congenital chronic anemias. Pediatr Blood Cancer. 2007;48:57–63. [DOI] [PubMed] [Google Scholar]
- 89. McColl B, Vadolas J. Animal models of β-hemoglobinopathies: utility and limitations. J Blood Med. 2016;7:263–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Shehee WR, Oliver P, Smithies O. Lethal thalassemia after insertional disruption of the mouse major adult beta-globin gene. Proc Natl Acad Sci USA. 1993;90:3177–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Skow L, Burkhart B, Johnson F, Popp R, Popp D, Goldberg S, Anderson W, Barnett L, Lewis S. A mouse model for β-thalassemia. Cell. 1983;34:1043–52. [DOI] [PubMed] [Google Scholar]
- 92. Van Wyck D, Tancer M, Popp R. Iron homeostasis in beta-thalassemic mice. Blood. 1987;70:1462–5. [PubMed] [Google Scholar]
- 93. Garrick LM, Strano-Paul L, Hoke JE, Kirdani-Ryan LA, Alberico RA, Everett MM, Bannerman RM, Garrick MD. Tissue iron deposition in untransfused beta thalassemic mice. Exp Hematol. 1989;17:423–8. [PubMed] [Google Scholar]
- 94. Ciavatta DJ, Ryan TM, Farmer SC, Townes TM. Mouse model of human beta zero thalassemia: targeted deletion of the mouse beta maj- and beta min-globin genes in embryonic stem cells. Proc Natl Acad Sci USA. 1995;92:9259–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Yang B, Kirby S, Lewis J, Detloff PJ, Maeda N, Smithies O. A mouse model for beta 0-thalassemia. Proc Natl Acad Sci USA. 1995;92:11608–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. De Franceschi L, Daraio F, Filippini A, Carturan S, Muchitsch E, Roetto A, Camaschella C. Liver expression of hepcidin and other iron genes in two mouse models of beta-thalassemia. Haematologica. 2006;91:1336–42. [PubMed] [Google Scholar]
- 97. Lewis J, Yang BL, Kim R, Sierakowska H, Kole R, Smithies O, Maeda N. A common human β globin splicing mutation modeled in mice. Blood. 1998;91:2152–6. [PubMed] [Google Scholar]
- 98. Jamsai D, Zaibak F, Khongnium W, Vadolas J, Voullaire L, Fowler KJ, Gazeas S, Fucharoen S, Williamson R, Ioannou PA. A humanized mouse model for a common β0-thalassemia mutation. Genomics. 2005;85:453–61. [DOI] [PubMed] [Google Scholar]
- 99. Jamsai D, Zaibak F, Vadolas J, Voullaire L, Fowler KJ, Gazeas S, Peters H, Fucharoen S, Williamson R, Ioannou PA. A humanized BAC transgenic/knockout mouse model for HbE/β-thalassemia. Genomics. 2006;88:309–15. [DOI] [PubMed] [Google Scholar]
- 100. Vadolas J, Nefedov M, Wardan H, Mansooriderakshan S, Voullaire L, Jamsai D, Williams R, Ioannou PA. Humanized β-thalassemia mouse model containing the common IVSI-110 splicing mutation. J Biol Chem. 2006;281:7399–405. [DOI] [PubMed] [Google Scholar]
- 101. Huo YL, McConnell SC, Yang S, Zhang TT, Sun CW, Wu LC, Ryan TM. Humanized mouse model of Cooley's anemia. J Biol Chem. 2009;284:4889–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kautz L, Jung G, Du X, Gabayan V, Chapman J, Nasoff M, Nemeth E, Ganz T. Erythroferrone contributes to hepcidin suppression and iron overload in a mouse model of β-thalassemia. Blood. 2015;126:2031–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Taher AT, Musallam KM, Cappellini MD, Weatherall DJ. Optimal management of β thalassaemia intermedia. Br J Haematol. 2011;152:512–23. [DOI] [PubMed] [Google Scholar]
- 104. Papanikolaou G, Tzilianos M, Christakis JI, Bogdanos D, Tsimirika K, MacFarlane J, Goldberg YP, Sakellaropoulos N, Ganz T, Nemeth E. Hepcidin in iron overload disorders. Blood. 2005;105:4103–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Origa R, Galanello R, Ganz T, Giagu N, Maccioni L, Faa G, Nemeth E. Liver iron concentrations and urinary hepcidin in β-thalassemia. Haematologica. 2007;92:583–8. [DOI] [PubMed] [Google Scholar]
- 106. Kautz L, Jung G, Du X, Gabayan V, Chapman J, Nasoff M, Nemeth E, Ganz T. Erythroferrone contributes to hepcidin suppression and iron overload in a mouse model of β-thalassemia. Blood. 2015;126:2031–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Nicolas G, Viatte L, Lou DQ, Bennoun M, Beaumont C, Kahn A, Andrews NC, Vaulont S. Constitutive hepcidin expression prevents iron overload in a mouse model of hemochromatosis. Nat Genet. 2003;34:97–101. [DOI] [PubMed] [Google Scholar]
- 108. Gardenghi S, Ramos P, Marongiu MF, Melchiori L, Breda L, Guy E, Muirhead K, Rao N, Roy CN, Andrews NC et al.. Hepcidin as a therapeutic tool to limit iron overload and improve anemia in β-thalassemic mice. J Clin Invest. 2010;120:4466–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Finberg KE, Whittlesey RL, Andrews NC. Tmprss6 is a genetic modifier of the Hfe-hemochromatosis phenotype in mice. Blood. 2011;117:4590–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Nai A, Pagani A, Mandelli G, Lidonnici MR, Silvestri L, Ferrari G, Camaschella C. Deletion of TMPRSS6 attenuates the phenotype in a mouse model of β-thalassemia. Blood. 2012;119:5021–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Casu C, Oikonomidou PR, Chen H, Nandi V, Ginzburg Y, Prasad P, Fleming RE, Shah YM, Valore EV, Nemeth E et al.. Minihepcidin peptides as disease modifiers in mice affected by β-thalassemia and polycythemia vera. Blood. 2016;128:265–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Theurl I, Aigner E, Theurl M, Nairz M, Seifert M, Schroll A, Sonnweber T, Eberwein L, Witcher DR, Murphy AT et al.. Regulation of iron homeostasis in anemia of chronic disease and iron deficiency anemia: diagnostic and therapeutic implications. Blood. 2009;113:5277–86. [DOI] [PubMed] [Google Scholar]
- 113. Weiss G, Ganz T, Goodnough LT. Anemia of inflammation. Blood. 2019;133:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–23. [DOI] [PubMed] [Google Scholar]
- 115. Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Rivera S, Ganz T. Animal models of anemia of inflammation. Sem Hematol. 2009;46:351–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. An X, Schulz VP, Mohandas N, Gallagher PG. Human and murine erythropoiesis. Curr Opin Hematol. 2015;22:206–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Sasu BJ, Cooke KS, Arvedson TL, Plewa C, Ellison AR, Sheng J, Winters A, Juan T, Li HY, Begley CG et al.. Antihepcidin antibody treatment modulates iron metabolism and is effective in a mouse model of inflammation-induced anemia. Blood. 2010;115:3616–24. [DOI] [PubMed] [Google Scholar]
- 119. Cooke KS, Hinkle B, Salimi-Moosavi H, Foltz I, King C, Rathanaswami P, Winters A, Steavenson S, Begley CG, Molineux G et al.. A fully human anti-hepcidin antibody modulates iron metabolism in both mice and nonhuman primates. Blood. 2013;122:3054–61. [DOI] [PubMed] [Google Scholar]
- 120. Kim A, Fung E, Parikh SG, Valore EV, Gabayan V, Nemeth E, Ganz T. A mouse model of anemia of inflammation: complex pathogenesis with partial dependence on hepcidin. Blood. 2014;123:1129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lasocki S, Millot S, Andrieu V, Letteron P, Pilard N, Muzeau F, Thibaudeau O, Montravers P, Beaumont C. Phlebotomies or erythropoietin injections allow mobilization of iron stores in a mouse model mimicking intensive care anemia. Crit Care Med. 2008;36:2388–94. [DOI] [PubMed] [Google Scholar]
- 122. Montosi G, Corradini E, Garuti C, Barelli S, Recalcati S, Cairo G, Valli L, Pignatti E, Vecchi C, Ferrara F et al.. Kupffer cells and macrophages are not required for hepatic hepcidin activation during iron overload. Hepatology. 2005;41:545–52. [DOI] [PubMed] [Google Scholar]
- 123. Wallace DF, Subramaniam VN. Analysis of IL-22 contribution to hepcidin induction and hypoferremia during the response to LPS in vivo. Int Immunol. 2015;27:281–7. [DOI] [PubMed] [Google Scholar]
- 124. Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, Beaumont C, Kahn A, Vaulont S. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110:1037–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Prince OD, Langdon JM, Layman AJ, Prince IC, Sabogal M, Mak HH, Berger AE, Cheadle C, Chrest FJ, Yu Q et al.. Late stage erythroid precursor production is impaired in mice with chronic inflammation. Haematologica. 2012;97:1648–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Langdon JM, Yates SC, Femnou LK, McCranor BJ, Cheadle C, Xue QL, Vaulont S, Civin CI, Walston JD, Roy CN. Hepcidin-dependent and hepcidin-independent regulation of erythropoiesis in a mouse model of anemia of chronic inflammation. Am J Hematol. 2014;89:470–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Schubert TEO, Obermaier F, Ugocsai P, Mannel DN, Echtenacher B, Hofstadter F, Haerle P. Murine models of anaemia of inflammation: extramedullary haematopoiesis represents a species specific difference to human anaemia of inflammation that can be eliminated by splenectomy. Int J Immunopathol Pharmacol. 2008;21:577–84. [DOI] [PubMed] [Google Scholar]
- 128. Schubert T, Echtenacher B, Hofstadter F, Mannel DN. TNF-independent development of transient anemia of chronic disease in a mouse model of protracted septic peritonitis. Lab Invest. 2003;83:1743–50. [DOI] [PubMed] [Google Scholar]
- 129. Katsume A, Saito H, Yamada Y, Yorozu K, Ueda O, Akamatsu K, Nishimoto N, Kishimoto T, Yoshizaki K, Ohsugi Y. Anti-interleukin 6 (IL-6) receptor antibody suppresses Castleman's disease like symptoms emerged in IL-6 transgenic mice. Cytokine. 2002;20:304–11. [DOI] [PubMed] [Google Scholar]
- 130. Roy CN, Mak HH, Akpan I, Losyev G, Zurakowski D, Andrews NC. Hepcidin antimicrobial peptide transgenic mice exhibit features of the anemia of inflammation. Blood. 2007;109:4038–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Sartor RB, Anderle SK, Rifai N, Goo DA, Cromartie WJ, Schwab JH. Protracted anemia associated with chronic, relapsing systemic inflammation induced by arthropathic peptidoglycan-polysaccharide polymers in rats. Infect Immun. 1989;57:1177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Coccia MA, Cooke K, Stoney G, Pistillo J, Del Castillo J, Duryea D, Tarpley JE, Molineux G. Novel erythropoiesis stimulating protein (darbepoetin alfa) alleviates anemia associated with chronic inflammatory disease in a rodent model. Exp Hematol. 2001;29:1201–9. [DOI] [PubMed] [Google Scholar]
- 133. Theurl I, Schroll A, Sonnweber T, Nairz M, Theurl M, Willenbacher W, Eller K, Wolf D, Seifert M, Sun CC et al.. Pharmacologic inhibition of hepcidin expression reverses anemia of chronic inflammation in rats. Blood. 2011;118:4977–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Chikazawa S, Nakazawa T, Hori Y, Hoshi F, Kanai K, Ito N, Orino K, Watanabe K, Higuchi S. Change in serum ferritin concentration in experimentally induced anemia of chronic inflammation in dogs. J Vet Med Sci. 2013;75:1419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Uchiyama Y, Koike N, Mihara M. Anemia in monkey collagen-induced arthritis is correlated with serum IL-6, but not TNFα. Rheumatol Internat. 2008;28:879–83. [DOI] [PubMed] [Google Scholar]
- 136. Schwoebel F, van Eijk LT, Zboralski D, Sell S, Buchner K, Maasch C, Purschke WG, Humphrey M, Zollner S, Eulberg D et al.. The effects of the anti-hepcidin Spiegelmer NOX-H94 on inflammation-induced anemia in cynomolgus monkeys. Blood. 2013;121:2311–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Danks DM. Of mice and men, metals and mutations. J Med Genet. 1986;23:99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Cunliffe P, Reed V, Boyd Y. Intragenic deletions at Atp7a in mouse models for Menkes disease. Genomics. 2001;74:155–62. [DOI] [PubMed] [Google Scholar]
- 139. Mototani Y, Miyoshi I, Okamura T, Moriya T, Meng Y, Pei XY, Kameo S, Kasai N. Phenotypic and genetic characterization of the Atp7a Mo-Tohm mottled mouse: a new murine model of Menkes disease. Genomics. 2006;87:191–9. [DOI] [PubMed] [Google Scholar]
- 140. Kim BE, Petris MJ. Phenotypic diversity of Menkes disease in mottled mice is associated with defects in localisation and trafficking of the ATP7A protein. J Med Genetic. 2007;44:641–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Haddad MR, Patel KD, Sullivan PH, Goldstein DS, Murphy KM, Centeno JA, Kaler SG. Molecular and biochemical characterization of Mottled-dappled, an embryonic lethal Menkes disease mouse model. Mol Genet Metab. 2014;113:294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Reed V, Boyd Y. Mutation analysis provides additional proof that mottled is the mouse homologue of Menkes’ disease. Hum Mol Genet. 1997;6:417–23. [DOI] [PubMed] [Google Scholar]
- 143. Grimes A, Hearn CJ, Lockhart P, Newgreen DF, Mercer JFB. Molecular basis of the brindled mouse mutant (Mo br): a murine model of Menkes disease. Hum Mol Genet. 1997;6:1037–42. [DOI] [PubMed] [Google Scholar]
- 144. Lenartowicz M, Grzmil P, Shoukier M, Starzynski R, Marciniak M, Lipinski P. Mutation in the CPC motif-containing 6th transmembrane domain affects intracellular localization, trafficking and copper transport efficiency of ATP7A protein in mosaic mutant mice—an animal model of Menkes disease. Metallomics. 2012;4:197–204. [DOI] [PubMed] [Google Scholar]
- 145. Murata Y, Kodama H, Abe T, Ishida N, Nishimura M, Levinson B, Gitschier J, Packman S. Mutation analysis and expression of the mottled gene in the macular mouse model of Menkes disease. Ped Res. 1997;42:436–42. [DOI] [PubMed] [Google Scholar]
- 146. Yamano T, Shimada M, Kawasaki H, Onaga A, Nishimura M. Clinico-pathological study on macular mutant mouse. Acta Neuropathol. 1987;72:256–60. [DOI] [PubMed] [Google Scholar]
- 147. Rowe DW, McGoodwin EB, Martin GR, Sussman MD, Grahn D, Faris B, Franzblau C. A sex-linked defect in the cross-linking of collagen and elastin associated with the mottled locus in mice. J Exp Med. 1974;139:180–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Das S, Levinson B, Vulpe C, Whitney S, Gitschier J, Packman S. Similar splicing mutations of the Menkes/mottled copper-transporting ATPase gene in occipital horn syndrome and the blotchy mouse. Am J Hum Genet. 1995;56:570–6. [PMC free article] [PubMed] [Google Scholar]
- 149. Wang Y, Zhu S, Weisman GA, Gitlin JD, Petris MJ. Conditional knockout of the Menkes disease copper transporter demonstrates its critical role in embryogenesis. PloS One. 2012;7:e43039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Perez-Siles G, Grant A, Ellis M, Ly C, Kidambi A, Khalil M, Llanos RM, La Fontaine S, Strickland AV, Züchner S et al.. Characterizing the molecular phenotype of an Atp7a T985I conditional knock in mouse model for X-linked distal hereditary motor neuropathy (dHMNX). Metallomics. 2016;8:981–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Reed E, Lutsenko S, Bandmann O. Animal models of Wilson disease. J Neurochem. 2018;146:356–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Theophilos MB, Cox DW, Mercer JFB. The toxic milk mouse is a murine model of Wilson disease. Hum Mol Genet. 1996;5:1619–24. [DOI] [PubMed] [Google Scholar]
- 153. Buiakova OI, Xu J, Lutsenko S, Zeitlin S, Das K, Das S, Ross BM, Mekios C, Scheinberg IH, Gilliam TC. Null mutation of the murine ATP7B (Wilson disease) gene results in intracellular copper accumulation and late-onset hepatic nodular transformation. Hum Mol Genet. 1999;8:1665–71. [DOI] [PubMed] [Google Scholar]
- 154. Wu J, Forbes JR, Chen HS, Cox DW. The LEC rat has a deletion in the copper transporting ATPase gene homologous to the Wilson disease gene. Nature Genet. 1994;7:541–5. [DOI] [PubMed] [Google Scholar]
- 155. Li Y, Togashi Y, Sato S, Emoto T, Kang JH, Takeichi N, Kobayashi H, Kojima Y, Une Y, Uchino J. Spontaneous hepatic copper accumulation in Long-Evans Cinnamon rats with hereditary hepatitis. A model of Wilson's disease. J Clin Invest. 1991;87:1858–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Ahmed S, Deng H, Borjigin J. A new strain of rat for functional analysis of PINA. Mol Brain Res. 2005;137:63–9. [DOI] [PubMed] [Google Scholar]
- 157. Fieten H, Gill Y, Martin AJ, Concilli M, Dirksen K, van Steenbeek FG, Spee B, van den Ingh T, Martens E, Festa P et al.. The Menkes and Wilson disease genes counteract in copper toxicosis in Labrador retrievers: a new canine model for copper-metabolism disorders. Dis Mod Mech. 2016;9:25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. de Bie P, van de Sluis B, Burstein E, van den Berghe PVE, Muller P, Berger R, Gitlin JD, Wijmenga C, Klomp LWJ. Distinct Wilson's disease mutations in ATP7B are associated with enhanced binding to COMMD1 and reduced stability of ATP7B. Gastroenterology. 2007;133:1316–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Myint ZW, Oo TH, Thein KZ, Tun AM, Saeed H. Copper deficiency anemia: review article. Ann Hematol. 2018;97:1527–34. [DOI] [PubMed] [Google Scholar]
- 160. Wagner GS, Tephly TR. A possible role of copper in the regulation of heme biosynthesis through ferrochelatase. Adv Exp Med Biol. 1975;58:343–54. [DOI] [PubMed] [Google Scholar]
- 161. Pyatskowit JW, Prohaska JR. Copper deficient rats and mice both develop anemia but only rats have lower plasma and brain iron levels. Comp Biochem Physiol C Toxicol Pharmacol. 2008;147:316–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Matak P, Zumerle S, Mastrogiannaki M, El Balkhi S, Delga S, Mathieu JR, Canonne-Hergaux F, Poupon J, Sharp PA, Vaulont S et al.. Copper deficiency leads to anemia, duodenal hypoxia, upregulation of HIF-2α and altered expression of iron absorption genes in mice. PLoS One. 2013;8:e59538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Chen HJ, Huang G, Su T, Gao H, Attieh ZK, McKie AT, Anderson GJ, Vulpe CD. Decreased hephaestin activity in the intestine of copper-deficient mice causes systemic iron deficiency. J Nutr. 2006;136:1236–41. [DOI] [PubMed] [Google Scholar]
- 164. Lynch SM, Klevay LM. Contrasting effects of a dietary copper deficiency in male and female mice. Proc Soc Exp Biol Med. 1994;205:190–6. [DOI] [PubMed] [Google Scholar]
- 165. Nose Y, Kim BE, Thiele DJ. Ctr1 drives intestinal copper absorption and is essential for growth, iron metabolism, and neonatal cardiac function. Cell Metab. 2006;4:235–44. [DOI] [PubMed] [Google Scholar]
- 166. Jenkitkasemwong S, Broderius M, Nam H, Prohaska JR, Knutson MD. Anemic copper-deficient rats, but not mice, display low hepcidin expression and high ferroportin levels. J Nutr. 2010;140:723–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Reeves PG, DeMars LCS. Signs of iron deficiency in copper-deficient rats are not affected by iron supplements administered by diet or by injection. J Nutr Biochem. 2006;17:635–42. [DOI] [PubMed] [Google Scholar]
- 168. Reeves PG, DeMars LCS. Repletion of copper-deficient rats with dietary copper restores duodenal hephaestin protein and iron absorption. Exp Biol Med. 2005;230:320–5. [DOI] [PubMed] [Google Scholar]
- 169. Lahey ME, Gubler CJ, Chase MS, Cartwright GE, Wintrobe MM. Studies on copper metabolism II. Hematologic manifestations of copper deficiency in swine. Blood. 1952;7:1053–74. [PubMed] [Google Scholar]
- 170. Gipp WF, Pond WG, Tasker J, Van Campen D, Krook L, Visek WJ. Influence of level of dietary copper on weight gain. Hematology and liver copper and iron storage of young pigs. J Nutr. 1973;103:713–9. [DOI] [PubMed] [Google Scholar]