ABSTRACT
The superior longitudinal fasciculus/arcuate white matter complex (SLF/AC) is the largest and most complex white matter tract of the human cerebrum with multiple inter-linked connections encompassing multiple cognitive functions such as language, attention, memory, emotion, and visuospatial function. However, little is known regarding the overall connectivity of this complex. Recently, the Human Connectome Project parcellated the human cortex into 180 distinct regions. Utilizing diffusion spectrum magnetic resonance imaging tractography coupled with the human cortex parcellation data presented earlier in this supplement, we aim to describe the macro-connectome of the SLF/AC in relation to the linked parcellations present within the human cortex. The purpose of this study is to present this information in an indexed, illustrated, and tractographically aided series of figures and tables for anatomic and clinical reference.
Keywords: Anatomy, Cerebrum, Connectivity, DTI, Functional connectivity, Human, Parcellations
ABBREVIATIONS
- AF
arcuate fasciculus
- DSI
diffusion spectrum imaging
- DTI
diffusion tensor imaging
- IPL
inferior parietal lobule
- MR
magnetic resonance
- ROI
region of interest
- SLF
superior longitudinal fasciculus
- SLF/AC
superior longitudinal fasciculus/arcuate white matter complex
Having finished characterizing the structural and functional connectivity of all 180 cortical regions described in the Glasser study,1 we now turn our attention to the specific tractographic fiber connections described in the first nine chapters of this supplement. Using these data, we define the sets of parcellations that integrate within the human brain to form eight large white matter tracts, including the superior longitudinal fasciculus (SLF), inferior longitudinal fasciculus, middle longitudinal fasciculus, inferior fronto-occipital fasciculus, frontal aslant tract, uncinate, cingulum, and vertical occipital fasciculus. We begin with the SLF that for our purposes includes the arcuate fasciculus (AF), making this the largest and most complex long-range fiber bundle in the brain.
The superior longitudinal fasciculus/arcuate white matter complex (SLF/AC) is the largest white matter fiber grouping in the cerebrum, is present in each hemisphere, and is well known for its connections in the perisylvian, parietal, and occipital cortices.2,3 It is well described that the primary functional role associated with the SLF/AC is transmission of speech and language.4 It is also believed that the SLF plays a role in language transitioning (ie bilingualism).5 Other functional associations of the SLF/AC have also been described including crucial roles in attention, memory, emotion, language, visuospatial processing, and numerical cognition.6-8
Although diffusion tensor imaging (DTI) studies have elucidated the structural anatomy of the SLF/AC, little is known about its various cortical terminations.9 Recently, the Human Connectome Project published parcellation data redefining the human cortex.1 This provides a unique opportunity to elucidate the macro-connectome of the human cerebrum, in that high-resolution DTI tractography has been shown to accurately illustrate the anatomy and microstructure of the SLF/AC.10 In this study, we utilized diffusion spectrum imaging (DSI) tractography in conjunction with the Glasser parcellation scheme to illustrate the macro-connectivity of the parcellations integrating within the confines of the SLF/AC. The purpose of this study is to present the structural connectivity of the SLF/AC in an indexed, illustrated, and tractographically aided series of figures and tables for anatomic and clinical reference.
METHODS
Identification of Relevant Cortical Regions
The parcellation data entries within the first nine chapters of this supplement were reviewed to determine the specific cortical regions with structural connectivity in the distribution of the SLF. These data were tabulated, and connections between individual parcellations within the SLF were recorded. These results served as the basis for constructing simplified tractography maps of the SLF and performing deterministic tractography.
Deterministic Tractography
Publicly available imaging data from the Human Connectome Project was obtained for this study from the HCP database (http://humanconnectome.org, release Q3). Diffusion imaging with corresponding T1-weighted images from 10 healthy, unrelated controls were analyzed (subjects IDs: 100307, 103414, 105115, 110411, 111312, 113619, 115320, 117112, 118730, 118932). A multishell diffusion scheme was used, and the b-values were 990, 1985, and 1980 s/mm2. Each b-value was sampled in 90 directions. The in-plane resolution was 1.25 mm. The diffusion data were reconstructed using generalized q-sampling imaging with a diffusion sampling length ratio of 1.25.11
We performed brain registration to montreal neurologic institute space, wherein imaging is warped to fit a standardized brain model comparison between subjects. Tractography was performed in DSI studio using a region of interest approach to initiate fiber tracking from a user-defined seed region. A two region of interest (ROI) approach was used to isolate tracts. Voxels within each ROI were automatically traced with a maximum angular threshold of 45°. When a voxel was approached with no tract direction or a direction change of greater than 45 degrees, the tract was halted. Tractography was stopped after reaching a maximum length of 800 mm. In some instances, exclusion ROIs were placed to exclude obvious spurious tracts that were not involved in the white matter pathway of interest. Tractographic results are shown only for regions of interest within the left cerebral hemisphere.
CONNECTIVITY OVERVIEW
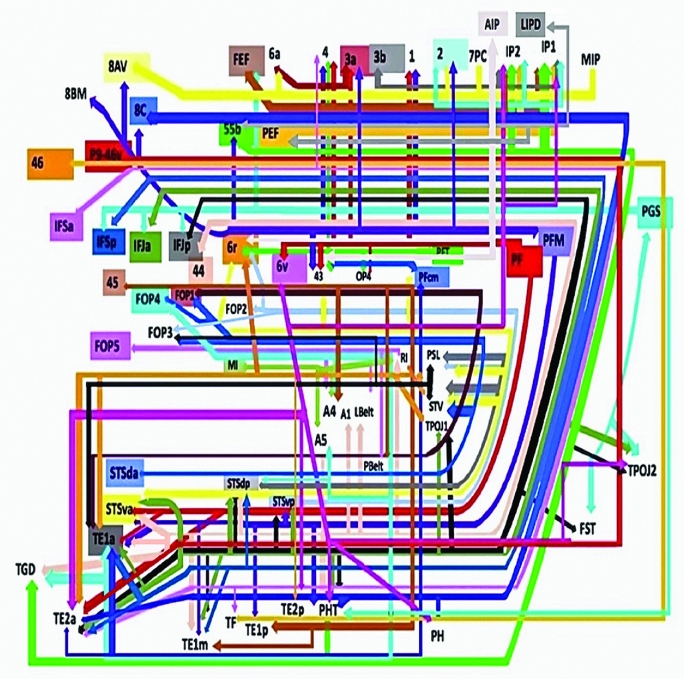
Presented in Figure 1, we demonstrate the functionally relevant and anatomically connected cerebral parcellation data that integrates within the confines of the SLF/AC. In order to simplify this complex data set further, we divided the SLF/AC into three subunits based on the literature: SLF I, SLF II, and SLF III combined with the arcuate (Figures 2-4).12 Pertinent examples of tractographically connected parcellations are represented for each subdivision (Figures 5-14). It should be noted that the figures and tables presented in this study do not imply directionality. Instead, supposed information transit is utilized as a simplified means for connectivity description and reference. Given the variability between visual and descriptive renditions of the divisions of the SLF/AC, certain instances of overlap between parcellations are inevitable. The following subdivisions do not stand to concretely separate this tract. They are merely represented in this regard for ease of reference.
FIGURE 1.
Tract map showing all structural connections that integrate within the SLF/AF. Connections between cortical areas are color-coded based on the parcellation of origin (eg black arrows indicate structural connections from origin IFJp to areas TPOJ2, TOPJ1, FST, PHT, STSvp, STSdp, TE1m, and TE2a). Note that arrows are not meant to imply the direction of information transmit.
FIGURE 2.
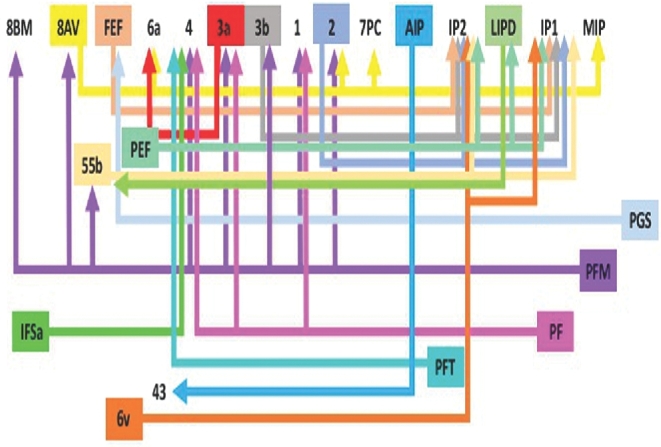
Tract map of SLF I. Connections between cortical areas are color-coded based on the parcellation of origin. Note that arrows are not meant to imply the direction of information transmit.
FIGURE 4.
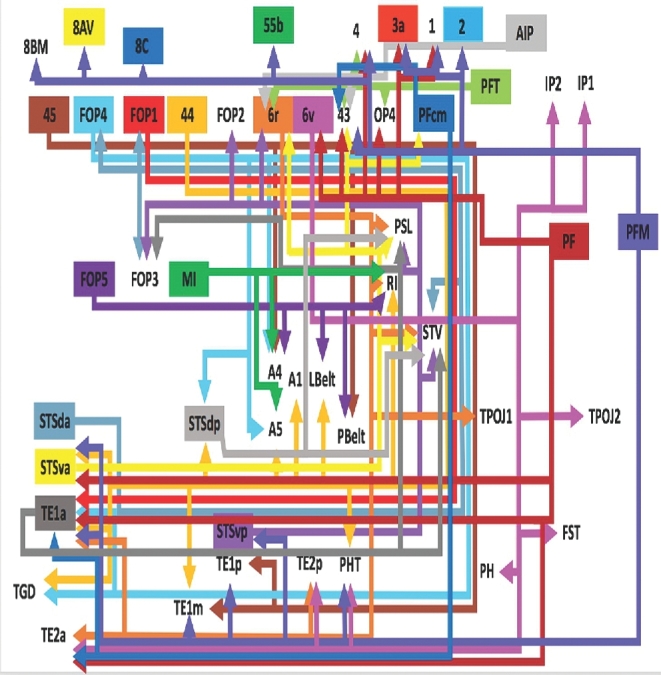
Tract map of SLF III and the AF. Connections between cortical areas are color-coded based on the parcellation of origin. Note that arrows are not meant to imply the direction of information transmit.
FIGURE 5.
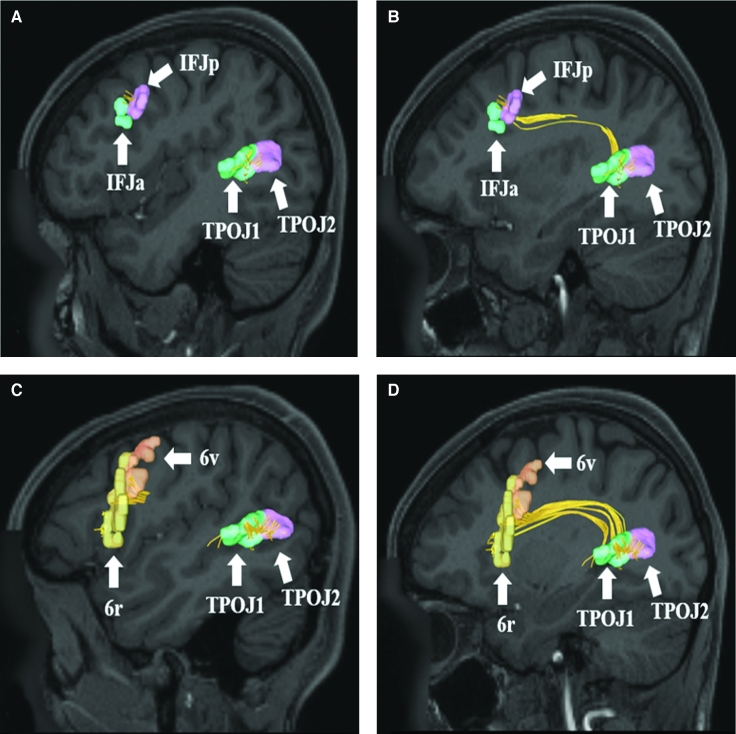
A and B, SLF connections from temporal-parietal-occipital junction parcellations (TPOJ1, TPOJ2) to areas IFJa and IFJp in the inferior frontal sulcus. Connections are shown in the left cerebral hemisphere on T1-weighted magnetic resonance (MR) images in the sagittal plane. C and D, SLF connections from temporal-parietal-occipital junction parcellations (TPOJ1 and TPOJ2) to areas 6r and 6v in the inferior frontal gyrus. Connections are shown in the left cerebral hemisphere on T1-weighted MR images in the sagittal plane. All parcellations are identified with white arrows and corresponding labels. The SLF can be seen coursing superiorly before bending craniocaudally to terminate in regions of the frontal lobe.
FIGURE 14.
SLF connections from inferior frontal sulcus area PFm. Area PFm demonstrates structural connections to A and B, areas 2, 4, 55b, and 8Av anteriorly and C and D, areas STSvp, TE1m, TE1p, and PHT inferiorly. These connections are shown in the left cerebral hemisphere on T1-weighted MR images in the A and C, sagittal and B and D, axial planes, respectively. All parcellations are identified with white arrows and corresponding labels.
SLF Connectivity
Table lists the tractographically connected parcellations within the SLF/AF complex as a whole. Figure 1 illustrates the macro-connectome of these connections. Arrows are included for reference only. Directionality of information transit is not meant to be assumed or implied.
TABLE.
Regions Integrating Within the SLF
| Original parcellation | Terminations |
|---|---|
| 2 | IP1 |
| IP2 | |
| PFm | |
| 3a | 6a |
| 3b | IP1 |
| IP2 | |
| 6r | PSL |
| RI | |
| STV | |
| TE1a | |
| TE2a | |
| TE2p | |
| TPOJ1 | |
| 6v | FST |
| IP1 | |
| IP2 | |
| PH | |
| PHT | |
| TE2a | |
| TE2p | |
| TPOJ2 | |
| 8AV | 2 |
| 6a | |
| 7PC | |
| MIP | |
| 8C | PH |
| PHT | |
| TE2a | |
| 44 | A1 |
| A5 | |
| LBelt | |
| PHT | |
| RI | |
| STSdp | |
| STSva | |
| TE1a | |
| TE1m | |
| TGd | |
| 45 | A4 |
| PBelt | |
| TE1m | |
| TE1p | |
| 46 | TF |
| 55b | IP1 |
| IP2 | |
| PHT | |
| TGd | |
| AIP | 6r |
| 43 | |
| FEF | IP1 |
| IP2 | |
| FOP1 | TE1a |
| FOP4 | A4 |
| A5 | |
| STSdp | |
| TE1a | |
| TGd | |
| FOP5 | A4 |
| LBelt | |
| PBelt | |
| RI | |
| IFJa | PHT |
| STSdp | |
| STSva | |
| STSvp | |
| TE1a | |
| TE1m | |
| TE2a | |
| TPOJ1 | |
| TPOJ2 | |
| IFJp | FST |
| PHT | |
| STSdp | |
| STSvp | |
| TE1m | |
| TE2a | |
| TPOJ1 | |
| TPOJ2 | |
| IFSa | 4 |
| TE2a | |
| TF | |
| IFSp | STSdp |
| TE1a | |
| TE1m | |
| TE2a | |
| LIPd | 55b |
| PEF | |
| MI | A4 |
| A5 | |
| RI | |
| p9-46v | TE2a |
| PEF | IP1 |
| IP2 | |
| PF | 1 |
| 3a | |
| 4 | |
| 6v | |
| 43 | |
| OP4 | |
| STSva | |
| TE1a | |
| TE2a | |
| PFcm | 43 |
| TE1a | |
| TE2a | |
| PFm | 1 |
| 2 | |
| 3a | |
| 4 | |
| 8AV | |
| 8BM | |
| 8C | |
| 43 | |
| 55b | |
| STSva | |
| STSvp | |
| TE1a | |
| TE1m | |
| TE1p | |
| PFt | 4 |
| 6r | |
| 43 | |
| OP4 | |
| PGs | FEF |
| FST | |
| IFJa | |
| IFJp | |
| IFSp | |
| PHT | |
| TPOJ2 | |
| STSda | FOP1 |
| FOP3 | |
| FOP4 | |
| STV | |
| STSdp | IFSp |
| STV | |
| PSL | |
| STSva | 6r |
| 43 | |
| PFcm | |
| PSL | |
| STV | |
| STSvp | 6r |
| FOP2 | |
| FOP3 | |
| PSL | |
| STV | |
| TE1a | FOP3 |
| PSL | |
| STV |
SLF I Connectivity
The SLF is classically divided into four distinct parts in human brains.12 SLF I connects parts of the superior parietal lobule with secondary motor areas, including the supplementary motor area, in the superior frontal gyrus.13 Connectivity for this subdivision is represented graphically in Figure 2. In short, connections in this region involve the medial complex of the SLF towards the medial interparietal sulcus, connecting areas underlying the superior frontal gyrus and the superior parietal lobule.
SLF II Connectivity
SLF II classically connects parts of the inferior parietal lobule (IPL) to the dorsolateral prefrontal cortex, including parts of the middle frontal gyrus.12 Connectivity for this subdivision is represented graphically in Figure 3. In short, connections in this region involve the posterolateral complex of the SLF, inferior to SLF I, connecting the parcellation of the middle frontal gyrus to the IPL.
FIGURE 3.
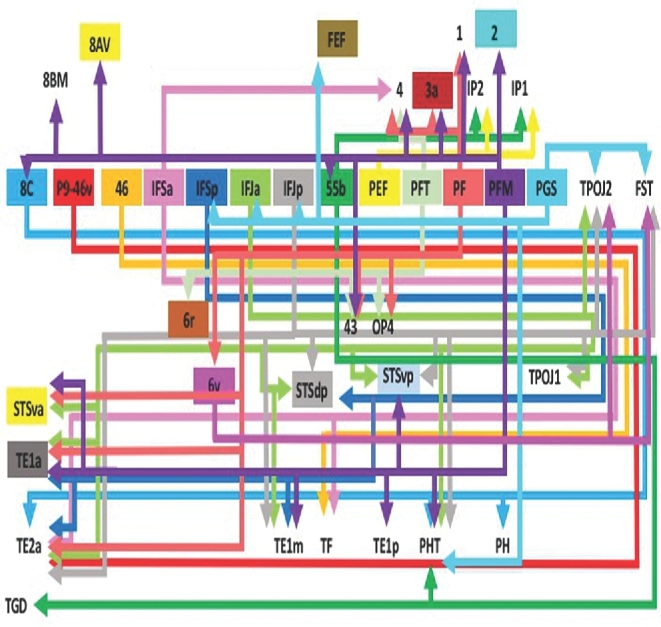
Tract map of SLF II. Connections between cortical areas are color-coded based on the parcellation of origin. Note that arrows are not meant to imply the direction of information transmit.
SLF III/Arcuate Connectivity
SLF III classically runs within the frontal and parietal opercula, connecting parts of the IPL to the ventral prefrontal cortex, including parts of the inferior frontal gyrus.12 Connectivity for this subdivision is represented graphically in Figure 4. In short, connections in this region involve the fronto-parietal operculum and its various connections to the insula. For simplicity, the arcuate complex is included in our depictions of SLF III. The AF is classically considered the longest subdivision of the SLF, connecting the frontal operculum and preopercular parcellations to those in the temporal lobe.12 Numerous connections to the perisylvian region are present in this scheme.
FIGURE 6.
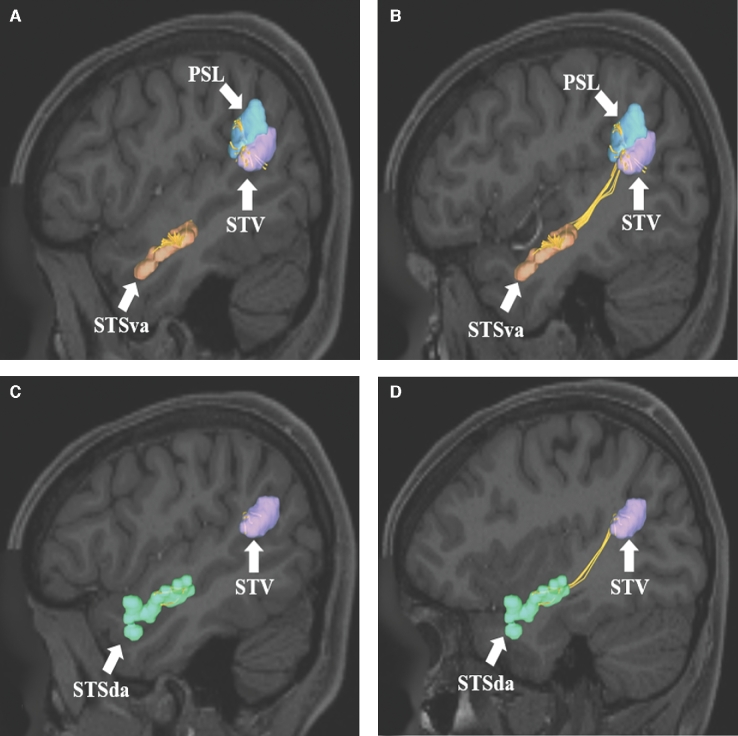
SLF connections from A and B, STSva and C and D, STSda to areas PSL and STV of the IPL. Connections are shown in the left cerebral hemisphere on T1-weighted MR images in the sagittal plane. All parcellations are identified with white arrows and corresponding labels. The SLF can be seen coursing posteriorly and superiorly within the subcortical white matter of the temporal before terminating in the perisylvian gray matter of the IPL.
FIGURE 7.
Aggregate of all SLF connections from the superior temporal sulcus regions (STSva, STSda, STSvp, and STSdp) to areas PSL and STV of the IPL. Connections are shown in the left cerebral hemisphere on T1-weighted MR images in the A, sagittal, B, axial, and C, oblique-sagittal planes. All parcellations are identified with white arrows and corresponding labels. The SLF can be easily seen coursing posteriorly and superiorly within the subcortical white matter of the temporal before terminating in the perisylvian gray matter of the IPL.
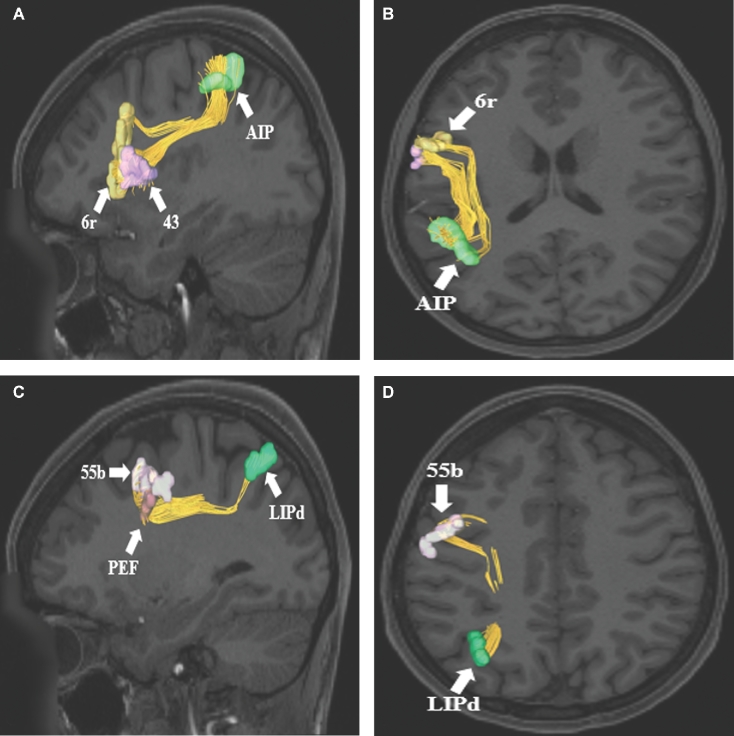
FIGURE 8.
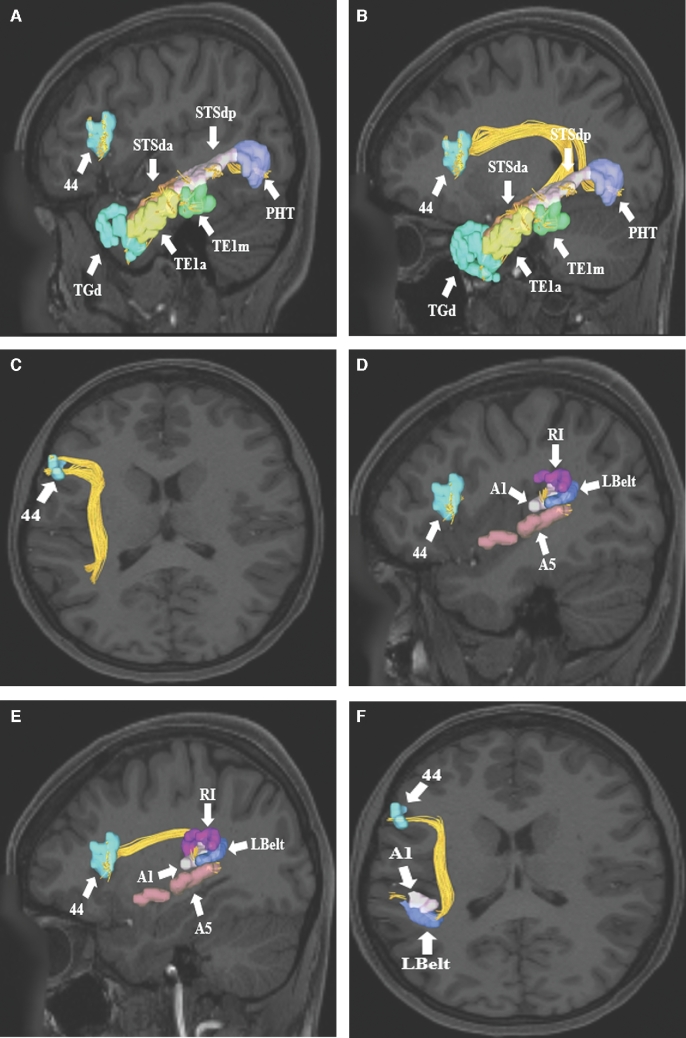
A-C, SLF connections from area 44 in the inferior frontal gyrus to parts of temporal lobe. Connections are shown in the left cerebral hemisphere on T1-weighted MR images in the A and B, sagittal and C, axial planes. Area 44 exhibits structural connections via the SLF to temporal regions TGd, TE1a, TE1m, STSda, STSdp, and PHT in this subject brain. D-F, Area 44 also demonstrates structural connections to areas A5, A1, LBelt, and RI in the superior temporal gyrus and posterior insular cortex. Connections are shown in the left cerebral hemisphere on T1-weighted MR images in the D and E, sagittal, and F, axial planes. All parcellations are identified with white arrows and corresponding labels. The SLF can be seen coursing posteriorly before bending 90° to enter the subcortical white matter of the temporal lobe and terminate in the aforementioned parcellations.
FIGURE 9.
SLF connections from parietal parcellation A and B, AIP to regions 6r and 43 of the inferior frontal gyrus. Connections are shown in the left cerebral hemisphere on T1-weighted MR images in the A, sagittal and B, axial planes. C and D, SLF connections from parietal parcellation LIPd to regions 55b and PEF of the frontal lobe. Connections are shown in the left cerebral hemisphere on T1-weighted MR images in the C, sagittal and D, axial planes. All parcellations are identified with white arrows and corresponding labels. The SLF can courses inferiorly from the gray matter of the parietal lobe and gradually curves 90 degrees to terminate in different parts of the frontal lobe.
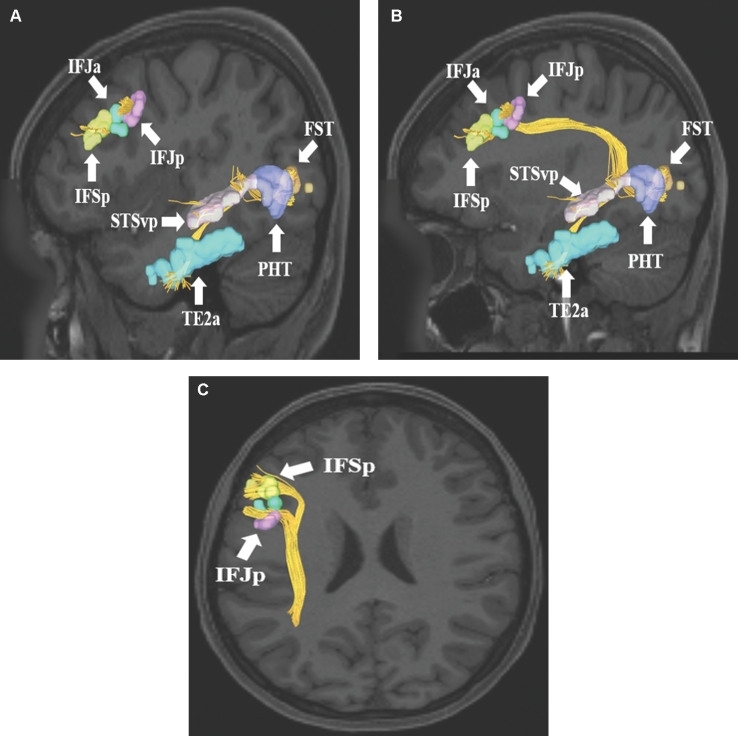
FIGURE 10.
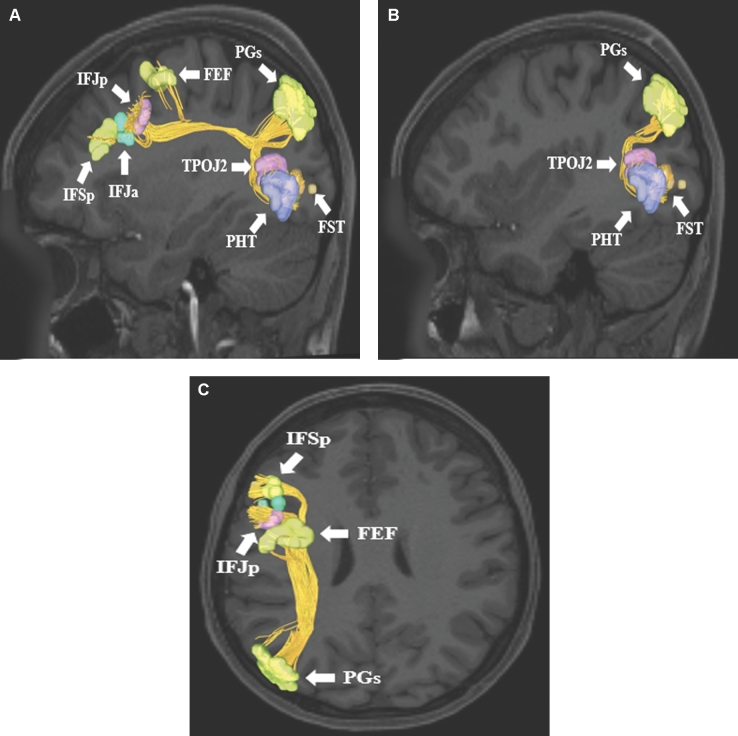
SLF connections from inferior parietal lobule area PGs. Area PGs has connections to frontal lobe parcellations (IFSp, IFJa, IFJp, and FEF) and lateral occipital lobe parcellations (TPOJ2, PHT, and FST). Connections are shown in the left cerebral hemisphere on T1-weighted MR images in the A and B, sagittal and C, axial planes. All parcellations are identified with white arrows and corresponding labels.
FIGURE 11.
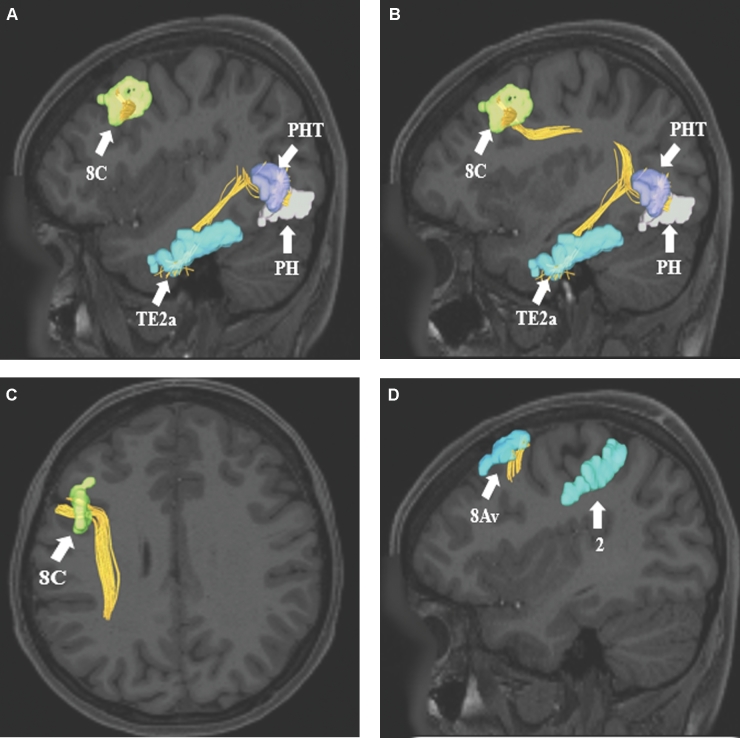
SLF connections from frontal lobe parcellations A-C, 8C and D, 8Av. Area 8C has connections to regions PHT, PH, and TE2a. These connections are shown in the left cerebral hemisphere on T1-weighted MR images in the A and B, sagittal and C, axial planes. In contrast, area 8Av has connections to regions 6a, 2, and MIP. These connections are shown in the left cerebral hemisphere on T1-weighted MR images in the A and B, sagittal and C, axial planes. All parcellations are identified with white arrows and corresponding labels.
FIGURE 12.
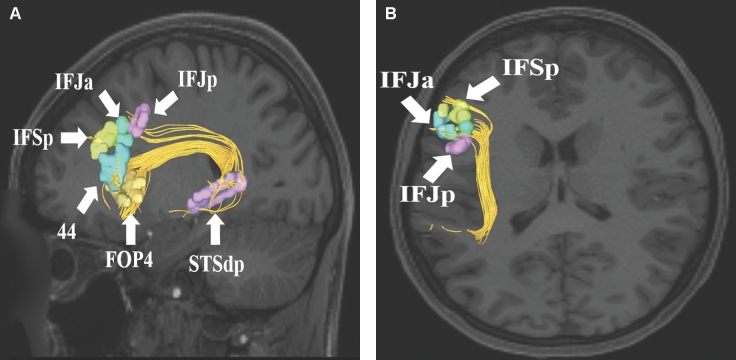
SLF connections from superior temporal sulcus area STSdp. Area STSdp has connections to regions IFJp, IFJa, IFSp, 44, and FOP4. These connections are shown in the left cerebral hemisphere on T1-weighted MR images in the A, sagittal and B, axial planes. All parcellations are identified with white arrows and corresponding labels.
FIGURE 13.
SLF connections from inferior frontal sulcus areas IFSp, IFJa, and IFJp. These areas demonstrate variable connectivity to parts of the lateral occipital and temporal lobes. Their connections are shown in the left cerebral hemisphere on T1-weighted MR images in the A and B, sagittal and C, axial planes. All parcellations are identified with white arrows and corresponding labels.
DISCUSSION
In this study, we describe the most detailed map to date regarding macro-connectivity of the SLF/AC and its relevant cerebral parcellations. It can be surmised from this data that actionable future studies and surgical planning may be better outlined. Our data confirm the results of others,12,14-17 our descriptive study supports supposed connectivity within the confines of the human cerebrum. Anatomic models of the SLF/AC generally begin at the caudal portion of the superior temporal gyrus, arch posteriorly and then caudally over the sylvian fissure to end within the frontal lobe.18,19
Historically, the SLF/AC was thought to encompass a single entity and route of transmission void of multiple subunits.12 However, recent evidence suggests the SLF can be divided into multiple subcomponents which can be described based on anatomic structures they connect.12,14-17 The SLF has also been divided into a superficial anterior segment connecting the supramarginal gyrus and superior temporal gyrus with the precentral gyrus and lateral frontal cortex, as well as a superficial posterior segment connecting the posterior portion of the middle temporal gyrus and supramarginal gyrus. Included within this paradigm is a long segment, otherwise called the AF, connecting the middle and inferior temporal gyri with the precentral, inferior, and middle frontal gyri.9,19
From a purely structural standpoint, it is reasonable to hypothesize that the SLF/AC would play a critical role in language function given its known connections to the posterior language areas and the canonical Broca's area.20 Studies regarding ideomotor apraxia discovered in the 1980s led to the idea that the SLF/AC is at least in part involved in speech articulation.21 Specifically, as illustrated during awake cortical and subcortical mapping studies, excellent correlation exists with the cortical boundary of the SLF and demonstrable speech function.22 Electrical stimulation of the SLF has also been shown to elicit syntactic and phonemic disorders (ie, paraphasias) in the dominant cerebral hemisphere.23 In addition, the language network has long been thought of as comprising a dorsal and ventral stream in which the dorsal pathway involves the SLF/AF complex.24 Damage to the SLF/AF is associated with nonfluent variants of primary progressive aphasia, again suggesting that the SLF is critical to normal language fuction.24 Finally, the dorsal phonological system, with deficits characterized by phonemic paraphasia, has been demonstrated to be integrated within the SLF, specifically localizing to the area of the IPL with connections between the supramarginal gyrus and ventral premotor cortex.25
On the nondominant side, the SLF is thought to play a role in visuospatial awareness as well as attentional selection of sensory content. This is further reinforced secondary to studies demonstrating hemi-neglect subsequent to damage to the inferior parietal lobe and SLF as it runs in this region.26-32 The SLF may also play an important role in the modulation of audio-spatial information.7
CONCLUSION
The SLF/AC is an incredibly complex white matter tract connecting multiple regions of the human cerebrum. It is critical in the transmission and processing of multiple types of data for the proper execution of cognitive tasks such as language, attention, memory, emotion, and visuospatial function. Further, sub-tract-guided functional and anatomic studies are needed to enhance our understanding of the functional connectivity of the human cerebrum. Our tractographic map of this white matter bundle can serve as a reference point for these future studies.
Disclosures
Synaptive Medical assisted in the funding of all 18 chapters of this supplement. No other funding sources were utilized in the production or submission of this work.
Acknowledgments
Data were provided [in part] by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University. We would also like to thank Brad Fernald, Haley Harris, and Alicia McNeely of Synaptive Medical for their assistance in constructing the network figures for Chapter 18 and for coordinating the completion and submission of this supplement.
REFERENCES
- 1. Glasser MF, Coalson TS, Robinson EC et al. . A multi-modal parcellation of human cerebral cortex. Nature. 2016;536(7615):171-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jellison BJ, Field AS, Medow J, Lazar M, Salamat MS, Alexander AL. Diffusion tensor imaging of cerebral white matter: a pictorial review of physics, fiber tract anatomy, and tumor imaging patterns. AJNR Am J Neuroradiol. 2004;25(3):356-369. [PMC free article] [PubMed] [Google Scholar]
- 3. Schmahmann JD, Smith EE, Eichler FS, Filley CM. Cerebral white matter: neuroanatomy, clinical neurology, and neurobehavioral correlates. Ann N Y Acad Sci. 2008;1142:266-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Axer H, Klingner CM, Prescher A. Fiber anatomy of dorsal and ventral language streams. Brain Lang. 2013;127(2):192-204. [DOI] [PubMed] [Google Scholar]
- 5. Moritz-Gasser S, Duffau H. Cognitive processes and neural basis of language switching: proposal of a new model. Neuroreport. 2009;20(18):1577-1580. [DOI] [PubMed] [Google Scholar]
- 6. Mesulam MM. From sensation to cognition. Brain. 1998;121 (6):1013-1052. [DOI] [PubMed] [Google Scholar]
- 7. Petrides M, Pandya DN. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur J Neurosci. 2002;16(2):291-310. [DOI] [PubMed] [Google Scholar]
- 8. Moeller K, Willmes K, Klein E. A review on functional and structural brain connectivity in numerical cognition. Front Hum Neurosci. 2015;9:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Martino J, De Witt Hamer PC, Berger MS et al. . Analysis of the subcomponents and cortical terminations of the perisylvian superior longitudinal fasciculus: a fiber dissection and DTI tractography study. Brain Struct Funct. 2013;218(1):105-121. [DOI] [PubMed] [Google Scholar]
- 10. Kamali A, Flanders AE, Brody J, Hunter JV, Hasan KM. Tracing superior longitudinal fasciculus connectivity in the human brain using high resolution diffusion tensor tractography. Brain Struct Funct. 2014;219(1):269-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yeh FC, Wedeen VJ, Tseng WY. Generalized q-sampling imaging. IEEE Trans Med Imaging. 2010;29(9):1626-1635. [DOI] [PubMed] [Google Scholar]
- 12. Makris N, Kennedy DN, McInerney S et al. . Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex. 2005;15(6):854-869. [DOI] [PubMed] [Google Scholar]
- 13. Jang SH, Hong JH. The anatomical characteristics of superior longitudinal fasciculus I in human brain: diffusion tensor tractography study. Neurosci Lett. 2012;506(1):146-148. [DOI] [PubMed] [Google Scholar]
- 14. Catani M, Jones DK. Perisylvian language networks of the human brain. Ann Neurol. 2005;57(1):8-16. [DOI] [PubMed] [Google Scholar]
- 15. Frey S, Campbell JS, Pike GB, Petrides M. Dissociating the human language pathways with high angular resolution diffusion fiber tractography. J Neurosci. 2008;28(45):11435-11444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Makris N, Papadimitriou GM, Kaiser JR, Sorg S, Kennedy DN, Pandya DN. Delineation of the middle longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex. 2009;19(4):777-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang Y, Zhang J, Oishi K et al. . Atlas-guided tract reconstruction for automated and comprehensive examination of the white matter anatomy. Neuroimage. 2010;52(4):1289-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Christidi F, Karavasilis E, Samiotis K, Bisdas S, Papanikolaou N. Fiber tracking: A qualitative and quantitative comparison between four different software tools on the reconstruction of major white matter tracts. Eur J Radiol Open. 2016;3:153-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Potapov AA, Goryainov SA, Zhukov VY et al. . [The long-associative pathway of the white matter: modern view from the perspective of neuroscience]. Zh Vopr Neirokhir Im N N Burdenko. 2014;78(5):66-77; discussion 77. [PubMed] [Google Scholar]
- 20. Bernal B, Altman N. The connectivity of the superior longitudinal fasciculus: a tractography DTI study. Magn Reson Imaging. 2010;28(2):217-225. [DOI] [PubMed] [Google Scholar]
- 21. De Renzi E. [Agnosia]. Recenti Prog Med. 1989;80(12):633-637. [PubMed] [Google Scholar]
- 22. Davtian M, Ulmer JL, Mueller WM, Gaggl W, Mulane MP, Krouwer HG. The superior longitudinal fasciculus and speech arrest. J Comput Assist Tomogr. 2008;32(3):410-414. [DOI] [PubMed] [Google Scholar]
- 23. Wilson SM, Galantucci S, Tartaglia MC, Gorno-Tempini ML. The neural basis of syntactic deficits in primary progressive aphasia. Brain Lang. 2012;122(3):190-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Agosta F, Galantucci S, Canu E et al. . Disruption of structural connectivity along the dorsal and ventral language pathways in patients with nonfluent and semantic variant primary progressive aphasia: a DT MRI study and a literature review. Brain Lang. 2013;127(2):157-166. [DOI] [PubMed] [Google Scholar]
- 25. Maldonado IL, Moritz-Gasser S, Duffau H. Does the left superior longitudinal fascicle subserve language semantics? A brain electrostimulation study. Brain Struct Funct. 2011;216(3):263-274. [DOI] [PubMed] [Google Scholar]
- 26. Hoeft F, Barnea-Goraly N, Haas BW et al. . More is not always better: increased fractional anisotropy of superior longitudinal fasciculus associated with poor visuospatial abilities in Williams syndrome. J Neurosci. 2007;27(44):11960-11965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Naito E, Amemiya K, Morita T. [Parietal cortices and body information]. Brain Nerve. 2016;68(11):1313-1320. [DOI] [PubMed] [Google Scholar]
- 28. Ptak R. The frontoparietal attention network of the human brain. Neuroscientist. 2012;18(5):502-515. [DOI] [PubMed] [Google Scholar]
- 29. Chechlacz M, Rotshtein P, Hansen PC, Deb S, Riddoch MJ, Humphreys GW. The central role of the temporo-parietal junction and the superior longitudinal fasciculus in supporting multi-item competition: evidence from lesion-symptom mapping of extinction. Cortex. 2013;49(2):487-506. [DOI] [PubMed] [Google Scholar]
- 30. Mesulam MM. A cortical network for directed attention and unilateral neglect. Ann Neurol. 1981;10(4):309-325. [DOI] [PubMed] [Google Scholar]
- 31. Shinoura N, Suzuki Y, Yamada R, Tabei Y, Saito K, Yagi K. Damage to the right superior longitudinal fasciculus in the inferior parietal lobe plays a role in spatial neglect. Neuropsychologia. 2009;47(12):2600-2603. [DOI] [PubMed] [Google Scholar]
- 32. Posner MI, Walker JA, Friedrich FJ, Rafal RD. Effects of parietal injury on covert orienting of attention. J Neurosci. 1984;4(7):1863-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]