ABSTRACT
In this supplement, we seek to show a comprehensive anatomic atlas of the human cerebrum demonstrating all 180 distinct regions comprising the cerebral cortex. The location, functional connectivity, and structural connectivity of these regions are outlined, and where possible a discussion is included of the functional significance of these areas. In this chapter, we specifically address regions integrating to form the inferior longitudinal fasciculus.
Keywords: Anatomy, Cerebrum, Connectivity, DTI, Functional connectivity, Human, Parcellations
ABBREVIATIONS
- DSI
diffusion spectrum imaging
- ILF
inferior longitudinal fasciculus
- MR
magnetic resonance
- PPA
primary progressive aphasia
- ROI
region of interest
- SLF/AC
superior longitudinal fasciculus/arcuate fasciculus complex
The inferior longitudinal fasciculus (ILF) is one of eight major white matter tract bundles connecting key cortical areas of the cerebrum. It was first described by Burdach in 1822.1 The ILF connects regions in the occipital lobe to those in the temporal lobe,2,3 generally originating ventromedially from the fusiform gyrus and dorsomedially from the cuneus as it projects to medial, lateral, and anterior temporal regions.4 These temporal regions are close to the amygdala and hippocampus.4 As it courses from the occipital to the temporal lobe, the ILF passes adjacent to the lateral walls of the temporal and occipital horns of the lateral ventricle.5
Putative functions of the ILF include roles in object and facial recognition, discrimination, and memory.6 Damage to this white matter pathway is also known to cause object recognition deficits.6 Despite its seemingly important roles in visual processing and object recognition, the areas of the cerebral cortex that give rise to the ILF have not been established or described in any great detail. This lack of anatomic specificity poses challenges to clinicians and scientists alike.
In this paper, we aim to show the cortical regions that connect to and integrate within the ILF. We delineate its boundaries using the cortical parcellation scheme presented earlier in this supplement. Through diffusion spectrum imaging (DSI), we show the tractography of the ILF arising from its relevant cortical parcellations, and show a simplified tract map summarizing those regions with white matter connections specific to the ILF. The purpose of this study is to present the structural connectivity of the ILF in an indexed, illustrated, and tractographically aided series of figures and tables for anatomic and clinical reference.
METHODS
Identification of Relevant Cortical Region
The parcellation data entries within the first nine chapters of this supplement were reviewed to determine the specific cortical regions with structural connectivity in the distribution of the ILF. These data were tabulated, and connections between individual parcellations within the ILF were recorded. These results served as the basis for constructing a simplified tractography map of the ILF and performing deterministic tractography.
Deterministic Tractography
Publicly available imaging data from the Human Connectome Project was obtained for this study from the HCP database (http://humanconnectome.org, release Q3). Diffusion imaging with corresponding T1-weighted images from 10 healthy, unrelated controls were analyzed (subjects IDs: 100307, 103414, 105115, 110411, 111312, 113619, 115320, 117112, 118730, 118932). A multishell diffusion scheme was used, and the b-values were 990, 1985, and 1980 s/mm2. Each b-value was sampled in 90 directions. The in-plane resolution was 1.25 mm. The diffusion data was reconstructed using generalized q-sampling imaging with a diffusion sampling length ratio of 1.25.7
We performed brain registration to MNI space, wherein imaging is warped to fit a standardized brain model comparison between subjects. Tractography was performed in DSI studio using a region of interest approach to initiate fiber tracking from a user-defined seed region. A two region of interest approach was used to isolate tracts. Voxels within each ROI were automatically traced with a maximum angular threshold of 45°. When a voxel was approached with no tract direction or a direction change of greater than 45°, the tract was halted. Tractography was stopped after reaching a maximum length of 800 mm. In some instances, exclusion ROIs were placed to exclude obvious spurious tracts that were not involved in the white matter pathway of interest. Tractographic results are shown only for regions of interest within the left cerebral hemisphere.
CONNECTIVITY OVERVIEW
Seven parcellations of the temporal lobe and one from the posterior parietal lobe demonstrate fiber tractography in the distribution of the ILF: PEEC, STGa, TA2, TE1a, TF, TGd, TGv, and PGp. Table summarizes the relevant cortical regions that integrate to form the ILF. These regions show variable connections to early visual processing areas (V1, V2, V3, and V4), late visual processing areas (V6, V7, V8, and FFC), the parahippocampal gyrus (VMV1, VMV2, PH, and PHA3), and lateral occipital and temporal-parietal-occipital junction areas (LO3, TPOJ3, MT, and MST). Figure 1 illustrates the relevant structural connectivity of the cerebral parcellation data within the confines of the ILF. In addition, Figures 2-4 illustrate key DSI-based connectivity examples chosen for the strength and breadth of linked parcellation data. It should be noted that the figures and tables presented in this study do not imply directionality. Instead, supposed information transit is utilized as a simplified means for connectivity description.
TABLE.
Regions Integrating Within the ILF
| Original parcellation | Terminations |
|---|---|
| PEEC | PHA2 |
| TPOJ3 | |
| VMV3 | |
| V1 | |
| PGp | FFC |
| PH | |
| TA2 | 52 |
| A5 | |
| MBelt | |
| PI | |
| STGa | |
| V1 | |
| V2 | |
| V3 | |
| V6a | |
| TE1a | V1 |
| V2 | |
| TGd | PH |
| PHA3 | |
| V1 | |
| V2 | |
| V3 | |
| V7 | |
| VMV1 | |
| TGv | LO3 |
| MST | |
| PH | |
| TPOJ3 | |
| V1 | |
| V2 | |
| V3 | |
| V4 | |
| V7 | |
| V8 | |
| VMV2 | |
| VVC | |
| TF | MT |
| PGp | |
| TPOJ3 | |
| V2 | |
| V3 | |
| V3a | |
| V3b | |
| V4 | |
| STGa | V3 |
FIGURE 1.
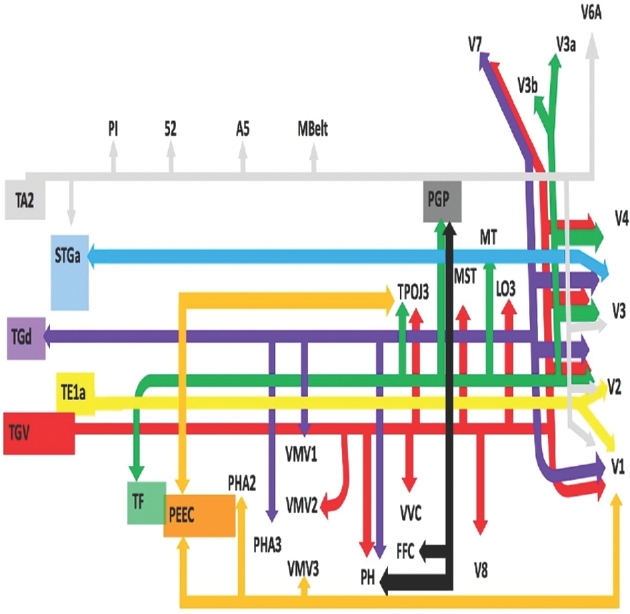
Simplified tract map showing the structural connections that integrate within the ILF. Connections between cortical areas are color-coded based on the parcellation of origin (eg purple arrows indicate structural connections from origin TGd to areas V1, V2, V3, V7, PH, PHA3, and VMV1). Note that arrows are not meant to imply the direction of information transmit.
FIGURE 2.
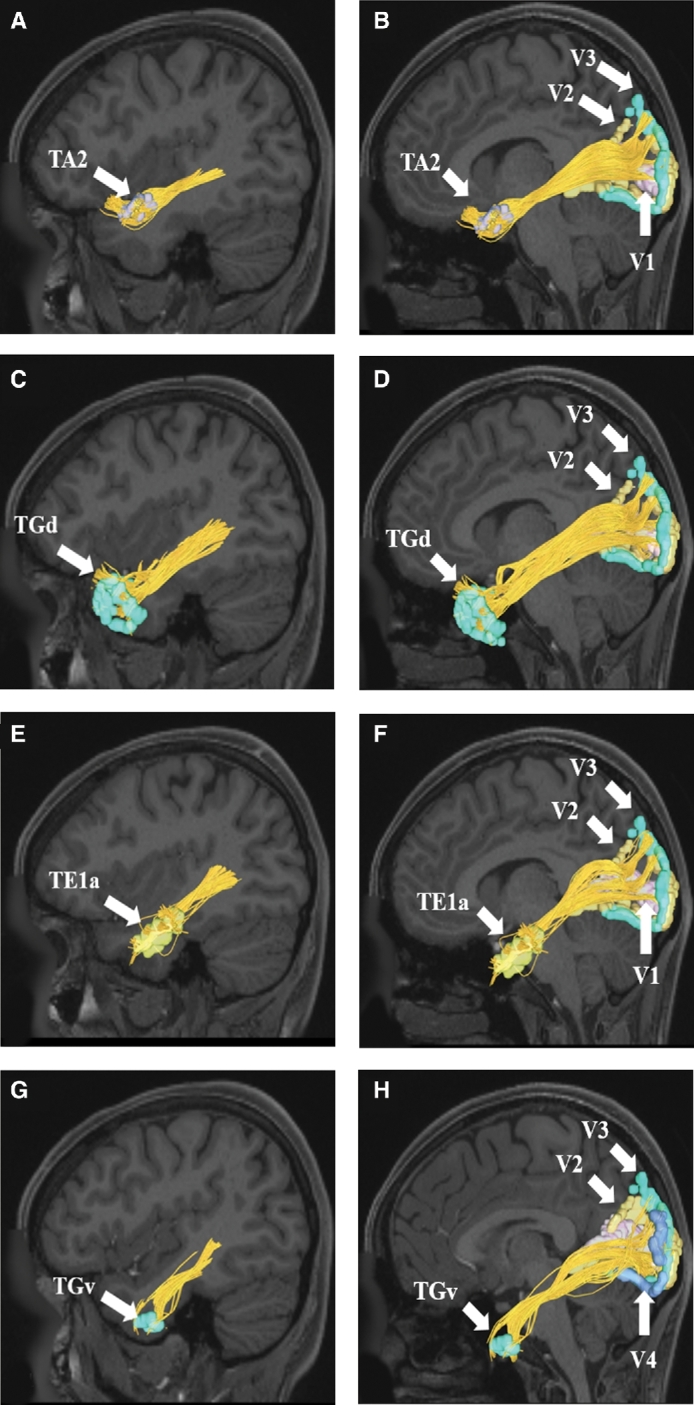
ILF connections from anterior temporal lobe regions A and B, TA2, C and D, TGd, E and F, TE1a, and G and H, TGv to early visual processing regions V1, V2, V3, and V4 in the left cerebral hemisphere. Connections are shown on T1-weighted magnetic resonance (MR) images in the sagittal plane. All parcellations are indicated with white arrows and corresponding labels. The ILF is seen coursing from the temporal regions posteriorly to the occipital lobe.
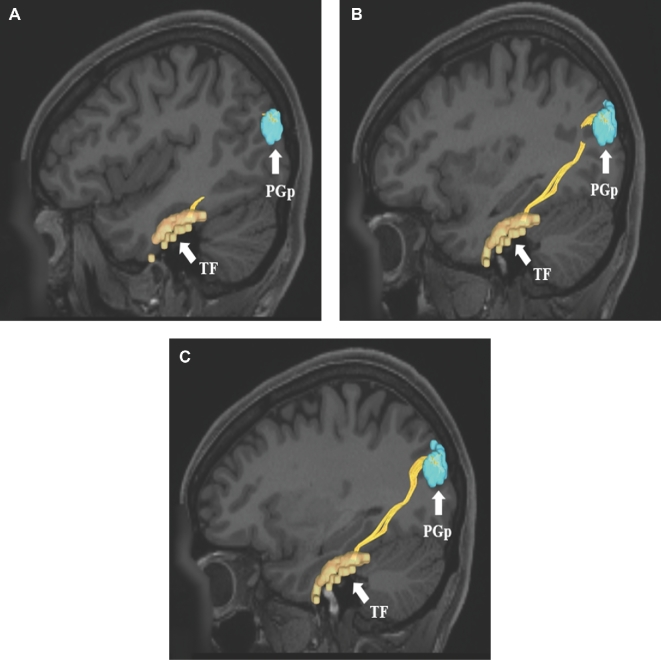
FIGURE 4.
A-C, ILF connections from temporal lobe area TF to the inferior parietal lobule (area PGp). Connections are shown in the left cerebral hemisphere on T1-weighted MR images in the sagittal plane. All parcellations are indicated with white arrows and corresponding labels. The ILF is seen coursing from area TF posteriorly to the inferior parietal lobe.
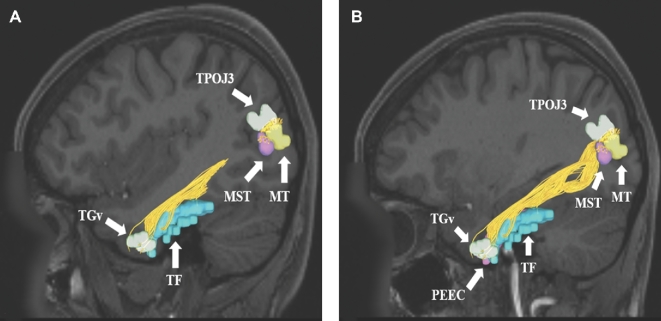
FIGURE 3.
A and B, ILF connections from temporal regions TGv, TF, and PEEC to the temporal-parietal-occipital junction (TPOJ3) and lateral occipital lobe (MT and MST). Connections are shown in the left cerebral hemisphere on T1-weighted MR images in the sagittal plane. All parcellations are indicated with white arrows and corresponding labels. The ILF is seen coursing from the temporal regions posteriorly to the occipital lobe.
DISCUSSION
In this study, we used the Glasser parcellation scheme and deterministic tractography to construct a tractography-based model of the ILF.8 It is our aim to move towards a more precise, anatomically specific model of the ILF for use in future studies. Here, we present our atlas of the parcellations with fiber tractography confined within the ILF and review the functional significance of this white matter tract.
The ILF, while readily identified in both cerebral hemispheres, exhibits differential fractional anisotropy bilaterally.2 Several studies have shown that ILF degeneration may cause deficits related to object recognition,2,9-14 and an association between lower ILF fractional anisotropy scores and higher levels of clinical impairment have been reported.2 Increasing damage to the ILF is also associated with more severe neurologic symptomatology.2 Electrical stimulation study of the ILF has also demonstrated impaired object recognition and reading ability in patients with brain tumors.15
Beyond its role in objection recognition processing, the ILF has been implicated more generally in global cerebral visual processing and facial recognition.2,11,12,14,16,17 Visual memory disturbances have been reported following right ILF disconnection,18 suggesting a role for this white matter pathway in visual agnosias, including prosopagnosia, when the tract is damaged or disconnected. Patients with a history of visual hallucinations also demonstrate decreased white matter tract integrity of the left ILF coupled with significantly reduced fractional anisotropy scores.19
While there is an abundance of evidence implicating the ILF in critical visual processes, its role in human language function remains controversial. Some groups have demonstrated a role for the ILF in normal language functioning.2,10,11,20 However, compared to the superior longitudinal fasciculus/arcuate fasciculus complex (SLF/AC), electrical stimulation of the ILF was not strongly associated with phonological errors, such as conduction aphasias, as seen with stimulation of the SLF/AC.10 In addition, no naming disturbances were reported relative to disconnecting or stimulating the ILF.10 Despite this, some groups have proposed that the ILF is part of the ventral semantic processing stream,14,21,22 effectively serving as a pathway for the indirect transfer of semantic information during language processing tasks. Lending credence to the idea that the ILF subserves some aspects of ventral semantic language function is one study that demonstrated that patients with semantic variants of primary progressive aphasia (PPA) exhibited more severe damage to left ventral tracts, including the uncinate and ILF, compared to patients with nonfluent variants of PPA who demonstrated more severe damage to dorsal semantic tracts, namely the superior longitudinal fasciculus.20
Structural abnormalities of the ILF, identified in part via diffusion tensor imaging-based axonal tracking,23 have also been reported in a variety of neuropsychiatric disease states. For example, impairments in the right ILF were identified in amyotrophic lateral sclerosis patients compared to a healthy human control cohort in relation to emotional processing tasks.16 A second study has shown that there are measurable differences in the right ILF in patients with autism spectrum disorder.24 ILF tract abnormalities have also been observed in semantic dementia patients,25 who exhibited white matter abnormalities in the uncinate and ILF.25 Finally, it has been suggested that structural brain abnormalities increase the risk of alcohol dependence in patients with abnormal neural networks that include the ILF.26
Reading capability has also been linked to the ILF.4,11,14,21,27 Some studies show that poorer reading capability could be related to lower fractional anisotropy scores in the left temporoparietal and frontal areas.4 In another study, electrostimulation of the ILF induced reading disturbances.14 In that study, poorer reading skills correlated with lower fractional anisotropy scores.14 In most of these studies, however, the corona radiata and left arcuate fasciculus have been identified as the primary tracts related to impaired reading ability, as opposed to the ILF.4,11,14,21
CONCLUSION
The ILF is a major white matter tract that connects occipital and temporal lobe parcellations. While the precise nature of the structural changes that can affect the ILF have yet to be fully characterized, it is evident that this white matter tract is not merely a visual processing stream. Instead it is involved in visual processing tasks, language/semantic function, emotional regulation, reading, and neuropsychiatric disease states. Further sub-tract-guided functional and anatomic studies are needed to enhance our understanding of the functional and structural connectivity of this white matter bundle to characterize its role in the human connectome.
Disclosures
Synaptive Medical assisted in the funding of all 18 chapters of this supplement. No other funding sources were utilized in the production or submission of this work.
Acknowledgments
Data were provided [in part] by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University. We would also like to thank Brad Fernald, Haley Harris, and Alicia McNeely of Synaptive Medical for their assistance in constructing the network figures for Chapter 18 and for coordinating the completion and submission of this supplement.
REFERENCES
- 1. Potapov AA, Goryainov SA, Zhukov VY et al. . [The long-associative pathway of the white matter: modern view from the perspective of neuroscience]. Zhurnal Voprosy Neirokhirurgii Imeni N N Burdenko. 2014;78(5):66-77; . [PubMed] [Google Scholar]
- 2. Ortibus E, Verhoeven J, Sunaert S, Casteels I, de Cock P, Lagae L. Integrity of the inferior longitudinal fasciculus and impaired object recognition in children: a diffusion tensor imaging study. Dev Med Child Neurol. 2012;54(1):38-43. [DOI] [PubMed] [Google Scholar]
- 3. Lazar M, Weinstein DM, Tsuruda JS et al. . White matter tractography using diffusion tensor deflection. Hum Brain Mapp. 2003;18(4):306-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vandermosten M, Boets B, Wouters J, Ghesquiere P. A qualitative and quantitative review of diffusion tensor imaging studies in reading and dyslexia. Neurosci Biobehav Rev. 2012;36(6):1532-1552. [DOI] [PubMed] [Google Scholar]
- 5. Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. 2002;17(1):77-94. [DOI] [PubMed] [Google Scholar]
- 6. Schmahmann JD, Pandya DN, Wang R et al. . Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130(3):630-653. [DOI] [PubMed] [Google Scholar]
- 7. Yeh FC, Wedeen VJ, Tseng WY. Generalized q-sampling imaging. IEEE Trans Med Imaging. 2010;29(9):1626-1635. [DOI] [PubMed] [Google Scholar]
- 8. Glasser MF, Coalson TS, Robinson EC et al. . A multi-modal parcellation of human cerebral cortex. Nature. 2016;536(7615):171-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fernandez Coello A, Duvaux S, De Benedictis A, Matsuda R, Duffau H. Involvement of the right inferior longitudinal fascicle in visual hemiagnosia: a brain stimulation mapping study. J Neurosurg. 2013;118(1):202-205. [DOI] [PubMed] [Google Scholar]
- 10. Mandonnet E, Nouet A, Gatignol P, Capelle L, Duffau H. Does the left inferior longitudinal fasciculus play a role in language? A brain stimulation study. Brain. 2007;130(3):623-629. [DOI] [PubMed] [Google Scholar]
- 11. Liao Y, Huang X, Wu Q et al. . Is depression a disconnection syndrome? Meta- analysis of diffusion tensor imaging studies in patients with MDD. J Psychiatry Neurosci. 2013;38(1):49-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tusa RJ, Ungerleider LG. The inferior longitudinal fasciculus: a reexamination in humans and monkeys. Ann Neurol. 1985;18(5):583-591. [DOI] [PubMed] [Google Scholar]
- 13. Latini F. New insights in the limbic modulation of visual inputs: the role of the inferior longitudinal fasciculus and the Li-Am bundle. Neurosurg Rev. 2015;38(1):179-190; discussion 189-190. [DOI] [PubMed] [Google Scholar]
- 14. Dick AS, Bernal B, Tremblay P. The Language Connectome. Neuroscientist. 2014;20(5):453-467. [DOI] [PubMed] [Google Scholar]
- 15. Jimenez de la Pena M, Gil Robles S, Recio Rodriguez M, Ruiz Ocana C, Martinez de Vega V. Cortical and subcortical mapping of language areas: correlation of functional MRI and tractography in a 3T scanner with intraoperative cortical and subcortical stimulation in patients with brain tumors located in eloquent areas. Radiologia. 2013;55(6):505-513. [DOI] [PubMed] [Google Scholar]
- 16. Sedda A. Disorders of emotional processing in amyotrophic lateral sclerosis. Curr Opin Neurol. 2014;27(6):659-665. [DOI] [PubMed] [Google Scholar]
- 17. Grossi D, Soricelli A, Ponari M et al. . Structural connectivity in a single case of progressive prosopagnosia: the role of the right inferior longitudinal fasciculus. Cortex. 2014;56:111-120. [DOI] [PubMed] [Google Scholar]
- 18. Shinoura N, Suzuki Y, Tsukada M et al. . Impairment of inferior longitudinal fasciculus plays a role in visual memory disturbance. Neurocase. 2007;13(2):127-130. [DOI] [PubMed] [Google Scholar]
- 19. Ashtari M, Cottone J, Ardekani BA et al. . Disruption of white matter integrity in the inferior longitudinal fasciculus in adolescents with schizophrenia as revealed by fiber tractography. Arch Gen Psychiatry. 2007;64(11):1270-1280. [DOI] [PubMed] [Google Scholar]
- 20. Agosta F, Galantucci S, Canu E et al. . Disruption of structural connectivity along the dorsal and ventral language pathways in patients with nonfluent and semantic variant primary progressive aphasia: A DT MRI study and a literature review. Brain Lang. 2013;127(2):157-166. [DOI] [PubMed] [Google Scholar]
- 21. Martino J, De Lucas EM. Subcortical anatomy of the lateral association fascicles of the brain: A review. Clin Anat 2014;27(4):563-569. [DOI] [PubMed] [Google Scholar]
- 22. Dick AS, Tremblay P. Beyond the arcuate fasciculus: consensus and controversy in the connectional anatomy of language. Brain. 2012;135(12):3529-3550. [DOI] [PubMed] [Google Scholar]
- 23. Mori S, Kaufmann WE, Davatzikos C et al. . Imaging cortical association tracts in the human brain using diffusion-tensor-based axonal tracking. Magn Reson Med. 2002;47(2):215-223. [DOI] [PubMed] [Google Scholar]
- 24. Koldewyn K, Yendiki A, Weigelt S et al. . Differences in the right inferior longitudinal fasciculus but no general disruption of white matter tracts in children with autism spectrum disorder. Proc Natl Acad Sci U S A. 2014;111(5):1981-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Whitwell JL, Avula R, Senjem ML et al. . Gray and white matter water diffusion in the syndromic variants of frontotemporal dementia. Neurology. 2010;74(16):1279-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Seigneurie AS, Guerin Langlois C, Limosin F. [Cognitive vulnerability to alcohol dependence: related neuroanatomic endophenotypes]. Encephale. 2013;39(5):320-325. [DOI] [PubMed] [Google Scholar]
- 27. Gil-Robles S, Carvallo A, Jimenez Mdel M et al. . Double dissociation between visual recognition and picture naming: A study of the visual language connectivity using tractography and brain stimulation. Neurosurgery. 2013;72(4):678-686. [DOI] [PubMed] [Google Scholar]