Abstract
Background
Obstructive sleep apnoea (OSA) is the repetitive closure of the upper airway during sleep. This results in disturbed sleep and excessive daytime sleepiness. It is a risk factor for long‐term cardiovascular morbidity. Continuous positive airway pressure (CPAP) machines can be applied during sleep. They deliver air pressure by a nasal or oronasal mask to prevent the airway from closing, reducing sleep disturbance and improving sleep quality. Some people find them difficult to tolerate because of high pressure levels and other symptoms such as a dry mouth. Switching to machines that vary the level of air pressure required to reduce sleep disturbance could increase comfort and promote more regular use. Humidification devices humidify the air that is delivered to the upper airway through the CPAP circuit. Humidification may reduce dryness of the throat and mouth and thus improve CPAP tolerability. This updated Cochrane Review looks at modifying the delivery of positive pressure and humidification on machine usage and other clinical outcomes in OSA.
Objectives
To determine the effects of positive pressure modification or humidification on increasing CPAP machine usage in adults with OSA.
Search methods
We searched Cochrane Airways Specialised Register and clinical trials registries on 15 October 2018.
Selection criteria
Randomised parallel group or cross‐over trials in adults with OSA. We included studies that compared automatically adjusting CPAP (auto‐CPAP), bilevel positive airway pressure (bi‐PAP), CPAP with expiratory pressure relief (CPAPexp), heated humidification plus fixed CPAP, automatically adjusting CPAP with expiratory pressure relief, Bi‐PAP with expiratory pressure relief, auto bi‐PAP and CPAPexp with wakefulness detection with fixed pressure setting.
Data collection and analysis
We used standard methods expected by Cochrane. We assessed the certainty of evidence using GRADE for the outcomes of machine usage, symptoms (measured by the Epworth Sleepiness Scale (ESS)), Apnoea Hypopnoea Index (AHI), quality of life measured by Functional Outcomes of Sleep Questionnaire (FOSQ), blood pressure, withdrawals and adverse events (e.g. nasal blockage or mask intolerance). The main comparison of interest in the review is auto‐CPAP versus fixed CPAP.
Main results
We included 64 studies (3922 participants, 75% male). The main comparison of auto‐CPAP with fixed CPAP is based on 36 studies with 2135 participants from Europe, USA, Hong Kong and Australia. The majority of studies recruited participants who were recently diagnosed with OSA and had not used CPAP previously. They had excessive sleepiness (ESS: 13), severe sleep disturbance (AHI ranged from 22 to 59), and average body mass index (BMI) of 35 kg/m2. Interventions were delivered at home and the duration of most studies was 12 weeks or less. We judged that studies at high or unclear risk of bias likely influenced the effect of auto‐CPAP on machine usage, symptoms, quality of life and tolerability, but not for other outcomes.
Primary outcome
Compared with average usage of about five hours per night with fixed CPAP, people probably use auto‐CPAP for 13 minutes longer per night at about six weeks (mean difference (MD) 0.21 hours/night, 95% confidence interval (CI) 0.11 to 0.31; 31 studies, 1452 participants; moderate‐certainty evidence). We do not have enough data to determine whether auto‐CPAP increases the number of people who use machines for more than four hours per night compared with fixed CPAP (odds ratio (OR) 1.16, 95% CI 0.75 to 1.81; 2 studies, 346 participants; low‐certainty evidence).
Secondary outcomes
Auto‐CPAP probably reduces daytime sleepiness compared with fixed CPAP at about six weeks by a small amount (MD ‐0.44 ESS units, 95% CI ‐0.72 to ‐0.16; 25 studies, 1285 participants; moderate‐certainty evidence). AHI is slightly higher with auto‐CPAP than with fixed CPAP (MD 0.48 events per hour, 95% CI 0.16 to 0.80; 26 studies, 1256 participants; high‐certainty evidence), although it fell with both machine types from baseline values in the studies. Ten per cent of people in auto‐CPAP and 11% in the fixed CPAP arms withdrew from the studies (OR 0.90, 95% CI 0.64 to 1.27; moderate‐certainty evidence). Auto‐CPAP and fixed CPAP may have similar effects on quality of life, as measured by the FOSQ but more evidence is needed to be confident in this result (MD 0.12, 95% CI ‐0.21 to 0.46; 3 studies, 352 participants; low‐certainty evidence). Two studies (353 participants) provided data on clinic‐measured blood pressure. Auto‐CPAP may be slightly less effective at reducing diastolic blood pressure compared to fixed CPAP (MD 2.92 mmHg, 95% CI 1.06 to 4.77 mmHg; low‐certainty evidence). The two modalities of CPAP probably do not differ in their effects on systolic blood pressure (MD 1.87 mmHg, 95% CI ‐1.08 to 4.82; moderate‐certainty evidence). Nine studies (574 participants) provided information on adverse events such as nasal blockage, dry mouth, tolerance of treatment pressure and mask leak. They used different scales to capture these outcomes and due to variation in the direction and size of effect between the studies, the comparative effects on tolerability outcomes are uncertain (very low‐certainty evidence).
The evidence base for other interventions is smaller, and does not provide sufficient information to determine whether there are important differences between pressure modification strategies and fixed CPAP on machine usage outcomes, symptoms and quality of life. As with the evidence for the auto‐CPAP, adverse events are measured disparately.
Authors' conclusions
In adults with moderate to severe sleep apnoea starting positive airway pressure therapy, auto‐CPAP probably increases machine usage by about 13 minutes per night. The effects on daytime sleepiness scores with auto‐CPAP are not clinically meaningful. AHI values are slightly lower with fixed CPAP. Use of validated quality of life instruments in the studies to date has been limited, although where they have been used the effect sizes have not exceeded proposed clinically important differences. The adoption of a standardised approach to measuring tolerability would help decision‐makers to balance benefits with harms from the different treatment options available. The evidence available for other pressure modification strategies does not provide a reliable basis on which to draw firm conclusions. Future studies should look at the effects of pressure modification devices and humidification in people who have already used CPAP but are unable to persist with treatment.
Plain language summary
How does changing pressure in continuous positive airway pressure machines increase their usage by adults with sleep apnoea?
What is the aim of this review?
This review looks at different ways of helping people who have obstructive sleep apnoea (OSA) to use continuous positive airway pressure (CPAP) machines. OSA refers to the temporary but frequent closing and opening of the throat during sleep. Because adults with OSA do not get the sleep that they need, they can feel tired in the day and this impacts on their quality of life. They are at risk of falling asleep while carrying out their daily activities and they are at risk of having heart disease or a stroke in the long term.
Key messages
Adults who use CPAP devices that automatically vary treatment pressure probably increase use of their machines by about 13 minutes per night but it is unlikely that they will feel less tired compared with people who use fixed pressure CPAP machines. Fixed pressure CPAP is slightly better at reducing the number of episodes where the airway closes at night. We need more information to be able to assess whether there are important differences between these machines on quality of life and tolerability.
What was studied in this review?
CPAP machines are worn over the nose and mouth as people sleep at night. They blow air at a fixed pressure through the nose and mouth to keep the airway open. This makes refreshing sleep easier to achieve, but some people find the machines difficult to use regularly. They can find the level of pressure too high or they have a dry mouth when they wake up. If the pressure needed to keep the airway open is lower, the machines could be easier to use more often.
We looked for studies that compared different ways of varying the way that pressure is delivered. We have focused on studies that compared CPAP machines that automatically vary treatment pressure as the person sleeps (e.g. auto‐CPAP) with machines that deliver pressure at the same level throughout the night (fixed pressure CPAP).
What are the main results of the review?
We found 64 studies in 3922 people. Thirty‐six studies in 2135 people were relevant to the comparison of auto‐CPAP and fixed pressure CPAP. The studies were from Europe, USA, Hong Kong and Australia. Seventy‐five per cent of people recruited to the studies were men who were recently diagnosed with sleep apnoea and had no experience of using CPAP.
Compared with fixed pressure CPAP, people starting treatment with auto‐CPAP will probably use their machine by about 13 minutes more per night at around six weeks (moderate‐certainty evidence), although when we looked at the number of people who use the machines for more than four hours per night, we did not have enough information to know whether there was a difference between the different machines (low‐certainty evidence).
Auto‐CPAP probably reduces daytime symptoms by a small amount compared with fixed pressure CPAP (moderate‐certainty evidence). A similar number of people withdrew from the studies: 11% in fixed pressure CPAP and 10% with auto‐CPAP (moderate‐certainty evidence). Both machines reduced the number of times that the upper airway closed during sleep, although fixed pressure CPAP was slightly better (high‐certainty evidence). Three studies used a scale that we chose as the most relevant one to measure quality of life in sleep apnoea (the Functional Outcomes of Sleep Questionnaire). In people using the machines, the average difference between the devices on this scale was small, but there was not enough evidence to be confident about this result (low‐certainty evidence). Auto‐CPAP may be less successful in controlling blood pressure than fixed pressure CPAP, but more studies are needed to confirm this result. We are uncertain as to how people found using their devices because information on tolerability (blocked nose, dry mouth, mask leak or feeling that the pressure level was too high) was measured differently across the studies (very low‐certainty).
This Plain Language Summary is current to October 2018
Summary of findings
Background
Description of the condition
Obstructive sleep apnoea (OSA) arises from recurrent episodes of pharyngeal collapse during sleep. It disrupts sleep architecture, ventilation and cardiovascular homeostasis (Eckert 2008; Kasai 2012). As these episodes recur over time, adults with OSA experience fragmented and poor quality sleep. They report excessive daytime sleepiness, mood alterations as well as impaired cognition and memory (Gagnon 2014; Karimi 2015). Repetitive hypoxaemia is associated with increased risk of cardiovascular and cerebrovascular events (Shahar 2001; Yaggi 2005; Young 2008; Redline 2010; Drager 2011). OSA frequently coexists with systemic hypertension and, in some people, contributes to its development (Konecny 2014; Pépin 2014). Other consequences of untreated OSA include increased risk of motor vehicle accidents, higher prevalence of depression and reduced productivity at work (Tregear 2009; Jordan 2014; Hirsch Allen 2015; Karimi 2015; BaHammam 2016).
Description of the intervention
The main treatment of choice for OSA is continuous positive airway pressure (CPAP). CPAP devices apply positive pressure to the upper airway and prevent pharyngeal collapse. Consistent use of CPAP improves physiological parameters of sleep, reduces daytime sleepiness, enhances cognition and reduces motor vehicle accidents (Giles 2006; Karimi 2015). Adults with OSA are advised to use CPAP for a minimum of four hours per night; research indicates that four hours is the threshold at which symptoms of daytime sleepiness resolve (Weaver 2007).
Longitudinal studies also indicate that CPAP lowers the cardiovascular burden of OSA in people who are compliant with it (Doherty 2005; Marin 2005; Martínez‐García 2012; Myhill 2012). However, evidence from randomised studies of a beneficial effect of CPAP on cardiovascular outcomes is weak. This may be partly related to low adherence to CPAP in these studies; in the majority of randomised controlled trials (RCTs) included in a recent meta‐analysis, the average CPAP usage was < 4 hours/night (McEvoy 2016; Yu 2017). Early seminal studies had indicated that people who use CPAP for at least four hours per night obtain symptomatic benefit and, subsequently, this threshold was accepted in research studies and clinical practice as a threshold likely to meet a key goal of therapy (Kribbs 1993; Engleman 1994; Reeves Hoche 1994).
Despite the benefits associated with treatment, usage of CPAP is often low. Estimates of people who use CPAP regularly in the long term vary, but range between 29% and 85% (Pépin 1999; Weaver 2010; Rotenberg 2016; Libman 2017). Tolerating treatment with CPAP is a highly complex issue and determined by a number of factors. Disease severity, symptom relief from CPAP, underlying neurological disease or nasal anatomy play a role alongside psychological factors, such as locus of control, anxiety and depression (Wild 2004; Broström 2010a; Catcheside 2010). Finally, device‐related factors such as mask leak, skin abrasions and nasal congestion may deter use (Bollig 2010; Wickwire 2013).
Making the CPAP device more comfortable to use could also increase compliance. This could be achieved by varying the delivered airway pressure at the point of inhalation and exhalation, or by humidifying the CPAP circuit.
Pressure modification devices adjust airway pressure according to changes in airway resistance or the respiratory cycle. Automatically adjusting positive airway pressure (auto‐CPAP), bilevel positive airway pressure (bi‐PAP) or CPAP with expiratory pressure relief (CPAPexp) are modalities of pressure modification. Auto‐CPAP devices analyse inspiratory flow and titrate the airway pressure accordingly to maintain a constant airflow; thus, when airway resistance decreases (i.e. sleeping in lateral position or after weight loss), these devices reduce the pressure applied to the airway. In bi‐PAP devices, a higher pressure is applied to the airway throughout the inspiratory phase and a lower pressure is applied throughout expiration. In CPAPexp there is a decrease in airway pressure for a portion of the expiratory phase (Ryden 2014; Brown 2017; Freedman 2017).
Manufacturers have also combined multiple modalities of pressure modification into a single device e.g. auto‐CPAP combined with expiratory relief or bi‐PAP with an automatic titration mechanism (auto‐CPAP combined with bi‐PAP). These multimodality devices may have additive benefits on comfort compared to single modality devices.
Humidification devices humidify the air that is delivered to the upper airway through the CPAP circuit.
How the intervention might work
Pressure modification interventions are designed to vary and achieve the necessary pressure to maintain airway patency through the night. This has the potential to deliver the same effect on sleep disruption and symptoms but at a lower treatment pressure. By this mechanism of action, a reduction in mean pressure could minimise local side effects of CPAP, improve tolerance and increase associated usage. Bi‐PAP and C‐Flex are also designed to lower expiratory pressure and reduce the expiratory work of breathing, thereby increasing comfort. Adding humidification to the delivery of pressure in the airway might decrease side effects in the upper airway due to cold, dry airflow. Rather than change the pressure, humidity makes the pressured air delivered to the airway less likely to cause dry mouth or throat, which could improve the tolerability of CPAP. Table 5 outlines the details of each device and its associated mechanism.
1. Table of devices.
| Device | Mechanism |
| Fixed CPAP | A single pressure is set. The device attempts to maintain this during inspiration and expiration and throughout the period of use. |
| Automatically adjusting‐CPAP (auto‐CPAP) | High and low pressure limits are set. The device adjusts its pressure within these limits to try to maintain a patent (clear and open) airway. The pressure does not vary between inspiration and expiration but will vary across the period of use. |
| Bilevel positive airway pressure (Bi‐PAP) | Two pressure levels are set. The device aims to co‐ordinate with patient breaths to deliver the higher pressure throughout inspiration and the lower pressure throughout expiration. If the patient has no respiratory effort some machines will produce timed or back up breaths. |
| CPAP with expiratory pressure relief (CPAPexp) | A basic pressure is set for the period of use. The device tracks patient effort and drops from the basic pressure by a preset amount at the start of expiration, increasing back to the basic pressure at the end of expiration. The pressure drops by 1 of 3 amounts selected according to patient comfort. |
| Heated humidification | The addition of heated humidification to the CPAP circuit increases the humidity and temperature of inspired air; this aims to reduce dryness of the upper respiratory tract and improve comfort. |
| Auto‐CPAPexp | This device combines the modalities of automatically adjusting continuous positive airway pressure and expiratory pressure relief. |
| Bi‐PAPexp | This device combines the modalities of bilevel positive airway pressure and expiratory pressure relief. |
| Auto bi‐PAP with pressure relief | This device reduces the pressure delivered at the end of inspiration and pressure during the early part of expiration, combined with an automatic titration modality. |
| CPAPexp with wakefulness detection | This CPAP device with expiratory pressure relief incorporates a sensor to detect when the user is rousing from sleep. |
Why it is important to do this review
The prevalence and economic costs of OSA are likely to increase as levels of obesity increase globally (Peppard 2013; Garvey 2015; Sanna 2018). Establishing the evidence base for different types of positive pressure therapy devices will help to inform decisions aimed at reducing the health burden of poorly treated OSA. Cognitive behavioural therapy, education and increasing engagement between the patient and CPAP provider have recently been shown to increase machine use (Wozniak 2014). The last version of this review was published in 2009 (Smith 2009a), and we decided to update the review in light of a number of studies that have been published since we last assessed the evidence in this area.
Objectives
To determine the effects of positive pressure modification or humidification on increasing CPAP machine usage in adults with OSA.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) of either parallel group or cross‐over design. We included blinded and unblinded studies.
Types of participants
We included trials involving adults of either sex with a diagnosis of obstructive sleep apnoea (OSA), based on history and results of sleep studies. The sleep studies were either oximetry studies showing desaturation index (DI) of at least 5 per hour or of respiratory movements and airflow to give an Apnoea Hypopnoea Index (AHI) of at least 5 per hour.
We excluded trials assessing interventions in people with central sleep apnoea and where sleep apnoea was related to sleeping position.
Types of interventions
We included the following comparisons.
Automatically adjusting CPAP continuous positive airway pressure (auto‐CPAP) including forced oscillation technique versus fixed pressure setting CPAP (fixed CPAP).
Bilevel positive airway pressure (Bi‐PAP) versus fixed CPAP.
Continuous positive airway pressure with expiratory pressure relief (CPAPexp) versus fixed CPAP.
Heated humidification plus fixed CPAP versus fixed CPAP alone.
Automatically adjusting CPAP with expiratory pressure relief (auto‐CPAPexp) versus fixed CPAP.
Bi‐PAP with expiratory pressure relief (Bi‐PAPexp) versus fixed CPAP
Automatically adjusting Bi‐PAP (auto Bi‐PAP) versus fixed CPAP.
CPAPexp with wakefulness detection versus fixed CPAP.
We excluded studies that were conducted as short‐term laboratory‐based interventions, since they did not intend to capture the effects of interventions administered on a nightly basis at home. We excluded studies that were less than two weeks in duration because we were primarily interested in the effects of pressure modification in the context of ongoing use of CPAP.
The effects of educational and behavioural interventions are now considered in a different review (Wozniak 2014).
Types of outcome measures
Primary outcomes
Usage of CPAP, measured as initial acceptance, where data were available, and subsequent usage as measured by:
counter output that records the cumulative time that power is turned on to a CPAP machine (this does not provide information on actual time of day and duration of CPAP used each 24‐hour period);
microprocessor and monitor that measures the pressure at the mask;
subjective participant reports of the duration of CPAP use.
Data for this outcome could be measured as mean differences (MDs) in hourly use per participant per night or as the number of participants who used machines for more than four hours per night.
Secondary outcomes
Symptom scores (such as the Epworth Sleepiness Scale (ESS), Stanford Sleepiness Scale (SSS) and nasal symptoms)
Withdrawals
Quality of life or Health Status (such as the Functional Outcomes of Sleep Questionnaire (FOSQ) and Sleep Apnoea Quality of Life Index (SAQLI) scores. We analysed data from the Short‐Form 36 (SF‐36) but we did not use it as the basis for the 'Summary of findings' tables)
Sleep disruption outcomes (Apnoea Hypopnoea Index (AHI) and arousals)
Treatment pressure (for auto‐CPAP)
Blood pressure outcomes
Adverse events (most commonly measured as tolerability of treatment pressure, mask leak and nasal or oral symptoms)
Expression of preference (from cross‐over studies)
For the comparison of humidification and CPAP versus CPAP alone, we considered nasal symptoms as an additional outcome. This was intended to capture the effects of humidity directly where the mechanism of action is targeted.
Search methods for identification of studies
Electronic searches
The previously published version of this review included searches up to September 2008 (Smith 2009a). The search period for this update is September 2008 to October 2018.
We identified studies from Cochrane Airways Trials Register (Cochrane Airways 2019), which is maintained by the Information Specialist for the Group. The Cochrane Airways Trials Register contains studies identified from several sources:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL), through the Cochrane Register of Studie (CRS);
weekly searches of MEDLINE Ovid SP 1946 to date;
weekly searches of Embase Ovid SP 1974 to date;
monthly searches of PsycINFO Ovid SP 1967 to date;
monthly searches of CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature) 1937 to date;
handsearches of the proceedings of major respiratory conferences.
Studies contained in the Trials Register are identified through search strategies based on the scope of Cochrane Airways. Details of these strategies, as well as a list of handsearched conference proceedings are in Appendix 1. See Appendix 2 for the search strategy used to identify studies for this review.
We also searched the following trials registries.
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov)
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch)
Australian New Zealand Clinical Trials Registry (www.anzctr.org.au)
We searched the Cochrane Airways Trials Register and trials registries to 15 October 2018, with no restriction on type or language of publication.
Searching other resources
We reviewed reference lists of all primary studies and review articles for additional references. We contacted authors of identified trials to identify other published and unpublished studies. We checked for errata or retractions of included studies published in full text on PubMed on 8 April 2019.
Data collection and analysis
Selection of studies
Two review authors independently reviewed titles, abstracts and citations identified through electronic searching to assess potential relevance for full review. We obtained articles deemed to be of potential relevance for the review. Following scrutiny of full text, two authors independently assessed studies for inclusion based on the criteria for population, intervention, study design and outcomes (BK and DW or TL). We measured agreement by simple consensus. We resolved disagreement by involving a third party.
Data extraction and management
Two review authors extracted data from published and unpublished studies independently (BK and DW or TL). We made attempts to contact study investigators to confirm data where necessary and to provide clarification for additional information for the review.
Assessment of risk of bias in included studies
Two review authors (BK and TL) assessed the risk of bias of the included studies in terms of the process of allocation of participants to treatment groups (sequence generation and allocation concealment), blinding in relation to objective and subjective outcomes (judged as performance bias and detection bias), subsequent impact of missing data on the analysis, selective outcome reporting and other sources of bias.
We judged the studies to be at low, unclear or high risk of bias and provide supporting statements from the trial reports or correspondence, as necessary.
Measures of treatment effect
We calculated mean differences (MDs) for continuous variables measured on identical metrics for parallel and cross‐over studies. We used standardised mean differences (SMDs) to combine data from studies using different scales to measure the same outcome. For one outcome we noted that variants of the Functional Outcomes of Sleep Questionnaire (FOSQ) were used in two studies (Analysis 2.4) and so we used SMDs to combine data. We report continuous data from meta‐analyses as MDs or SMDs with 95% confidence intervals (CIs).
2.4. Analysis.
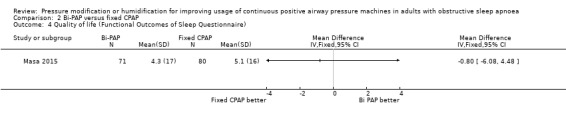
Comparison 2 Bi‐PAP versus fixed CPAP, Outcome 4 Quality of life (Functional Outcomes of Sleep Questionnaire).
For dichotomous outcomes, we calculated an odds ratio (OR) based upon the number of participants with an event versus the number of participants without an event.
Unit of analysis issues
We entered data from cross‐over studies as generic inverse variance (GIV) outcome that were either adjusted for the within‐person design or we back‐calculated the standard error (SE) from exact P values from paired t tests, if available. For continuous data we used MDs and their associated SEs. In the absence of either of these data, we used treatment group means and standard deviations (SDs) as if from parallel group trials.
One parallel study assessed the effects of four different auto‐CPAP machines compared with a device delivering fixed CPAP. We aggregated the data from the four treatment groups and used this in our analyses (Meurice 2007).
In the forest plots we have displayed sample sizes from cross‐over studies differently to parallel group studies. To generate correct total sample sizes we have entered the total sample size to the intervention arm and 0 to the corresponding control group column. This is for display purposes only and does not affect the estimated treatment effect or the error for the study.
Dealing with missing data
Where data pertaining to mean CPAP machine usage were not available from the trial reports, we contacted study authors to determine whether data could be obtained directly. We elected to impute SDs for one parallel group study for the primary outcome. We generated a simple average of the SDs for other studies because data were only reported as medians and 25th and 75th centiles.
Assessment of heterogeneity
We assessed the level of statistical variation with the I² statistic and P value for the Chi² test (Higgins 2003).
Assessment of reporting biases
We inspected publication bias visually to check for possible asymmetry for outcomes included in the 'Summary of findings' table with more than 10 studies.
Data synthesis
We combined data from studies where we judged the population, interventions and definition of outcomes to be similar, using a fixed‐effect model. We conducted a sensitivity analysis by using random‐effects modelling to determine whether variation between the studies affected the pooled estimate.
Subgroup analysis and investigation of heterogeneity
In the absence of any statistical heterogeneity for the outcomes of usage and symptoms under the comparison of auto‐CPAP and fixed CPAP, and the limited number of studies available for other outcomes, we decided not to carry out any subgroup analysis (see Differences between protocol and review for details on planned subgroups). We will reconsider our planned subgroups in future versions of the review should data become available that allows us to assess the impact of gender or baseline AHI on the outcomes of interest.
Sensitivity analysis
We could not carry out our planned sensitivity analysis to assess the risk of bias (see Differences between protocol and review). We did include the following sensitivity analyses for outcomes relevant to the 'Summary of findings' table.
We applied random‐effects modelling to assess the sensitivity of our results to the choice of statistical model. We applied this sensitivity analysis to outcomes where we had sufficient number of studies to use as the basis for an assumed distribution of effects (5 or more).
For the outcome of average machine usage we decided to include data from a large parallel group study which presented data as medians and ranges instead of means and SDs (Pépin 2016). As a sensitivity analysis, we included this study by assuming the medians to equate to means, and assigned SDs based on an average of other parallel studies.
Rating the certainty of evidence
We rated the certainty of evidence for key outcomes as high, moderate, low or very low using GRADE methods, as outlined in the GRADE Handbook (Schünemann 2013). We based these ratings on consideration of how risk of bias, inconsistency, imprecision, indirectness and publication bias impact on the findings of outcomes most relevant to decision making.
We have prioritised the comparison of auto‐CPAP and fixed CPAP over others as the basis of the conclusions of the review, but have included three other comparisons in the 'Summary of findings' tables (bi‐PAP, humidification and CPAP with expiratory pressure relief) as they are commonly used alternatives to either fixed CPAP or auto‐CPAP. The outcomes we have prioritised are based on relevance for decision making and were taken for the following latest available time points.
Average machine usage (measured as nightly average use per participant and as the number of participants achieving more than 4 hours usage per night).
Symptoms, measured by ESS.
Withdrawals.
Quality of life, measured by FOSQ.
AHI (mean number of events per hour).
Blood pressure.
Adverse events (measured by tolerability of machines).
In the text of the review we have preferentially focused reporting of follow‐up on these outcomes, where possible.
Results
Description of studies
Results of the search
See Figure 1 for the study flow diagram for searches between 2009 and 15 October 2018. From 1566 records, 64 references describing 48 potentially eligible studies were assessed for eligibility. We excluded 22 studies and included 21 studies. With the studies already included from previous searches, 64 studies met the eligibility criteria of the review. There are four ongoing studies and one completed study is awaiting assessment.
1.
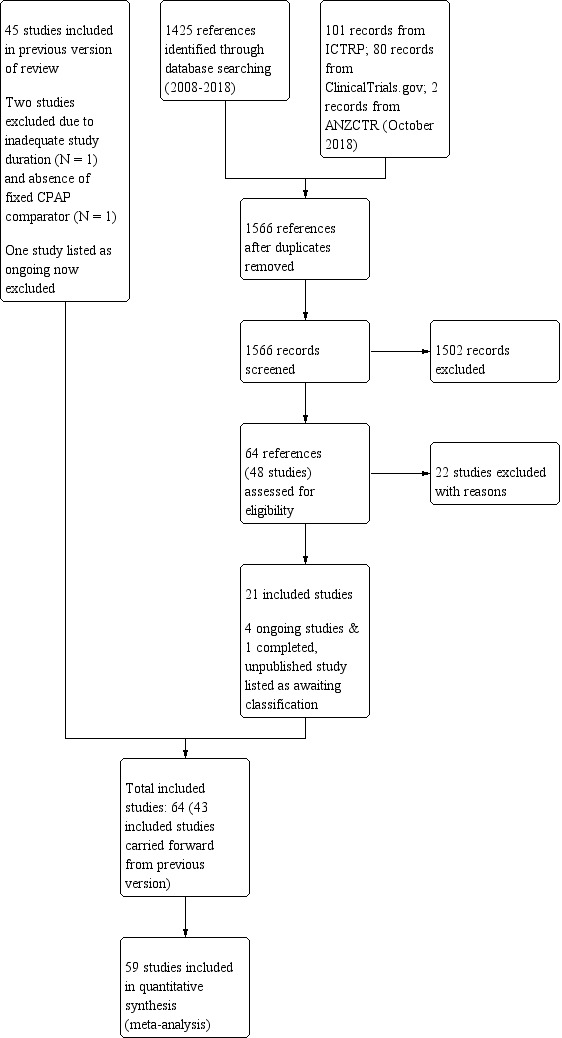
Study flow diagram: review update
Top up searches in June 2019 identified 202 records, of which we retrieved 10 for further scrutiny. Five records reported secondary analyses from one of the included studies (Masa 2015), and one referred to an excluded study (Pradeepan 2017). We also identified the published protocol for one ongoing study (NCT03428516) and conference abstract for a large completed study (NCT02749812). We assigned two records to 'Studies awaiting classification' (see below).
Included studies
Study design
Thirty studies have parallel groups and 33 have a cross‐over design. In one trial (Loube 2004), the description of methods was insufficient to determine whether the study was a parallel or cross‐over study.
Participants
The studies recruited 3922 participants who had been diagnosed with sleep apnoea and had little exposure to continuous positive airway pressure (CPAP) before study entry. The populations had similar characteristics across the seven comparisons considered by this review (Table 6). The proportion of male participants was high and ranged from 60% to 86% for the comparisons in the review. The average age of the study populations ranged between 49 and 55 and average body mass index (BMI) was between 32 kg/m2 and 35 kg/m2. Baseline sleep disruption, as measured by the Apnoea Hypopnoea Index (AHI) was severe and Epworth Sleepiness Scale (ESS) scores indicated that the study populations had excessive daytime sleepiness (11 to 16). One study recruited people with coexisting sleep apnoea and obesity hypoventilation syndrome (Masa 2015).
2. Summary of study and participant characteristics at baseline.
| Intervention arm | No. of studies (participants) | Average study duration (weeks) | Average Age | % Male participants | Average BMI (kg/m2) | Average AHI (events/hr) | Average ESS |
| Auto‐CPAP | 36 (2135) | 11 | 52 | 71 | 34 | 42 | 13 |
| Bi‐PAP | 6 (325) | 16 | 55 | 76 | 35 | 50 | 13 |
| CPAPexp | 10 (658) | 8 | 53 | 60 | 34 | 54 | 13 |
| Humidification + fixed CPAP | 6 (359) | 7 | 52 | 74 | 32 | 42 | 12 |
| Auto‐CPAPexp | 2 (188) | 14 | 49 | 77 | 34 | 39 | 11 |
| Bi‐PAP (multimodality) | 3 (187) | 12 | 54 | 86 | 32 | 41 | 10 |
| CPAPexp with wakefulness detection | 1 (70) | 4 | 51 | 69 | 36 | ‐ | 11 |
All interventions are compared with fixed CPAP. AHI: Apnoea Hypopnoea Index; BMI: body mass index; ESS: Epworth Sleepiness Scale. Averages calculated as mean of baseline means from studies contributing to each comparison. For cross‐over studies duration reflects amount of time treatment groups are exposed to one of the treatment arms, rather than the entire length of the study (conventionally double the duration of a single treatment arm exposure).
The majority of studies excluded participants who had previously used CPAP (58/64). Of the six studies actively recruiting existing users of CPAP, Jarvis 2006 included people who were established on CPAP therapy. In four studies participants were eligible if they had used CPAP but were deemed to be infrequent users (Muir 1998; Rostig 2003; Powell 2012; Gulati 2015).
Two auto‐CPAP studies selected participants who required high treatment pressure to correct sleep disturbance (Massie 2003; Noseda 2004).
Most studies were conducted in Europe and North America. A smaller number of trials were conducted in Australia (Hudgel 2000; Teschler 2000; Worsnop 2010; Hukins 2004; Jarvis 2006; Rochford 2006), Hong Kong (To 2008; Chang 2015), New Zealand (Neill 2003; Marshall 2008; Bakker 2010), and Thailand (Soudorn 2016).
The median study sample size is 40 (range 10 to 322).
Interventions
Average study duration was between 12 and 16 weeks in studies comparing auto‐CPAP, auto‐CPAPexp or bi‐PAP with fixed CPAP. Studies comparing CPAPexp or additional humidification with fixed CPAP had shorter average durations (8 and 6 weeks, respectively).
The use of standard CPAP titration protocols was common across the studies. Most were conducted over one or two nights, with the exception of Pépin 2016, where home‐based pressure titration occurred over eight nights. Extended adaptation protocols which increased the exposure of participants to CPAP devices were undertaken in two studies in order to establish optimal CPAP pressure and comfort prior to formal initiation of treatment (e.g. Senn 2003; Bloch 2018). This was used by Dolan 2008 to exclude participants who used CPAP for less than four hours per night during run‐in and by Ballard 2007 to identify and recruit people who averaged less than four hours per night to participate in the randomised phase of the study.
Automatically adjusting CPAP (auto‐CPAP)
Thirty‐six studies (2135 participants) compared auto‐CPAP with fixed CPAP (Meurice 1996; Sériès 1997; Konermann 1998; d'Ortho 2000; Hudgel 2000; Teschler 2000; Randerath 2001; Sériès 2001; Ficker 2003; Kendrick 2002; Massie 2003; Nolan 2007; Rostig 2003; Senn 2003; Hukins 2004; Hussain 2004; Marrone 2004; Noseda 2004; Resta 2004; Castronovo 2006; Jarvis 2006; Nussbaumer 2006; Rochford 2006; To 2008; West 2006; Fietze 2007; Meurice 2007; Patruno 2007; Galetke 2008; Damjanovic 2009; Vennelle 2010; Rohling 2011; Pépin 2016; Berry 2014; Chang 2015; Bloch 2018). Treatment pressures ranged from 6.2 to 10.6 in the fixed CPAP groups and were lower in the auto‐CPAP groups by about 1 cm H2O.
Bilevel positive airway pressure (Bi‐PAP)
Six studies (325 participants) compared bi‐PAP machines (excluding those with automatically adjusting and expiratory pressure mode) with fixed CPAP (Reeves‐Hoché 1995; Muir 1998; Gay 2003; Gonzalez‐Moro 2005; Gulati 2015; Masa 2015).
CPAP with expiratory pressure relief (CPAPexp)
Ten studies (658 participants) compared CPAP with expiratory pressure relief with fixed CPAP (Loube 2004; Dolan 2008; Marshall 2008; Nilius 2006; Gfüllner 2007; Modrak 2007; Wenzel 2007; Leidag 2008; Pépin 2009; Bakker 2010).
Heated humidification plus fixed CPAP
Six studies (359 participants) compared the addition of humidification to fixed CPAP with fixed CPAP (Neill 2003; Worsnop 2010; Ruhle 2011; Ryan 2009; Heiser 2010; Soudorn 2016). There was no humidification in the control groups.
Multimodality devices
Automatically adjusting CPAP with expiratory pressure relief (auto‐CPAPexp)
Two studies (188 participants) compared auto‐CPAPexp with fixed CPAP (Meurice 2009; Kushida 2011).
Bi‐PAP with expiratory pressure relief (Bi‐PAPexp) and auto Bi‐PAPexp
One study in 104 participants compared Bi‐PAPexp with fixed CPAP (Ballard 2007). Two studies evaluated auto Bi‐PAPexp with fixed CPAP (Blau 2012; Powell 2012).
CPAPexp with wakefulness detection
One study of 70 participants looked at CPAP with an expiratory pressure device that responded to the detection of wakefulness and compared it with fixed CPAP (Bogan 2017).
Outcomes
Availabilty of outcome data for our primary outcome of machine usage was high. We were able to obtain data for machine usage as either continuous or dichotomous data for 89% of studies (57/64). Symptoms measured with ESS and sleep disturbance measured with AHI were reported in 66% and 56% of studies respectively. Of 29 studies providing data on treatment pressure (45%), 24 compared auto‐CPAP with fixed CPAP.
Quality of life was reported in 23 studies (36%). Two instruments validated in sleep apnoea research were used in 11 studies (Sleep Apnoea Quality of Life Index (SAQLI) and Functional Outcomes of Sleep Questionnaire (FOSQ)) either in combination with the Short‐Form 36 (SF‐36) or on their own. For the remaining studies, only the SF‐36 was used.
There was considerable variation in the methods used to measure tolerability or adverse events in 23 studies (36%). Studies used diary records and interviews to capture effects as both dichotomous data (did or did not experience the event) or scales to rate problems with mask leak, pressure tolerance, dry mouth and nasal symptoms. Variation in how this was done prevented us from combining data in a meta‐analysis for many comparisons of interest. A number of cross‐over studies reported unadjusted dichotomous data and we could not account for this approach in our analysis due to the requirement for individual participant data.
Excluded studies
We excluded 133 studies after retrieving the full text because they did not meet the eligibility criteria of the review. Reasons for their exclusion are detailed in the 'Characteristics of excluded studies' table. On re‐examining two previously included studies against the review eligibility criteria, we determined that one of them was too short to be considered eligible for inclusion since they exposed participants to one week of treatment in a cross‐over study (Torvaldsson 2003), and the other used humidification with auto‐CPAP (Salgado 2006).
Studies awaiting classification
One study recruiting 800 participants comparing auto‐CPAP with fixed CPAP completed in 2015, but a conference abstract from 2018, reports some results (NCT02749812). Following correspondence with the study investigators we are not anticipating full availability of the results from this study before 2020. One study of a humidification device (Boyer 2019), and a secondary analysis of a CPAP compliance trial (Zamora 2019), are awaiting classification and we will determine their eligibility for future versions of the review.
Ongoing studies
Six studies are listed as ongoing. We have not been able to determine whether interim results from two studies presented as conference abstracts have been superseded by reports of completed studies (Morton 2001; Ventateswaren 2003). One published protocol pertains to a study in rescue workers who developed OSA subsequent to the collapse of the World Trade Centre in New York, USA in 2001 (NCT01753999). Two small studies evaluate auto‐CPAP (ACTRN12618000379213p; NCT01753999), and one is assessing a device that delivers pressure to the upper airway through one nostril at a time (ACTRN12617001090303).
Risk of bias in included studies
An overview of the study level judgements is provided in Figure 2.
2.
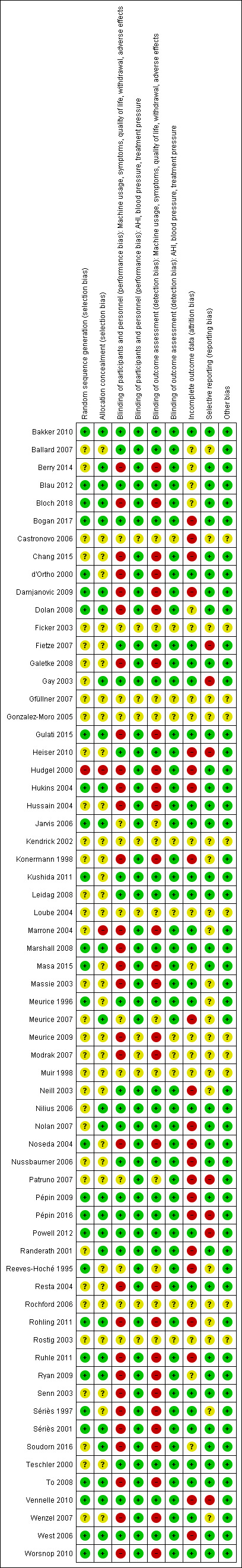
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
We considered 21 studies to be at low risk for both selection bias domains and one study had a high risk of bias for both domains (Hudgel 2000). One study was at high risk of bias in one selection bias domain only (Marrone 2004). For the remaining studies, one or both domains were at unclear risk of bias.
Blinding
We judged that the open‐label or single‐blind design used in many of the studies would likely have had a greater impact on usage outcomes, withdrawal and subjectively reported outcomes than on AHI, treatment pressure and blood pressure (see Figure 2). Measures to reduce the risk of bias arising from study personnel and participants becoming aware of treatment group assignment resulted in low risk of bias for subjective outcomes in 21 studies, a high risk of bias in 29 studies and unclear for the remainder. We judged objective outcomes to be at low risk of performance bias in 53 studies. Our 'Risk of bias' judgements for outcome assessment followed closely those we made for performance bias, given that participants would have been rating symptoms and quality of life.
Incomplete outcome data
We judged a total of 24 studies to be at a low risk of attrition bias across the outcomes of interest (see Figure 2). This was primarily where loss to follow‐up was low enough to not affect the outcomes of interest (very limited and balanced attrition or where all study participants completed). We noted that risk of attrition affected cross‐over and parallel group studies in different ways. A number of cross‐over participants who withdrew and did not cross over to the second arm of the trial were excluded from the analysis. Withdrawals for a number of long‐term parallel group studies meant that loss to follow‐up affected the long‐term outcome measurements. Twenty‐two studies had a high risk of bias and we judged the remainder as unclear.
Selective reporting
Where trial registry records have been available for a study we have been able to cross‐check prespecified outcomes against what was reported. We found evidence of selective outcome reporting for 'Summary of findings' table outcomes in seven studies (Gay 2003; Fietze 2007; Patruno 2007; Meurice 2009; Heiser 2010; Vennelle 2010; Powell 2012). The impact of this selective reporting on the overall results was limited.
Other potential sources of bias
We could not find any reason to consider the studies at high risk of other sources of bias. We could not reliably assess this domain in 11 studies, only available as conference abstracts, and judged them to be at unclear risk of bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings for the main comparison. Auto‐CPAP compared to fixed CPAP for sleep apnoea in adults.
| Auto‐CPAP compared to fixed CPAP for adults with a diagnosis of OSA | ||||||
| Patient or population: adults with a diagnosis of OSA Setting: Europe, USA, Australia and Hong Kong Intervention: automatically titrating CPAP Comparison: fixed CPAP | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Average effect or risk with fixed CPAP | Average effect or risk with auto‐CPAP | |||||
| Average machine usage (hours per night) Median follow‐up: 6 weeks |
Average nightly machine usage 5 hours per night across all CPAP arms | 0.21 hours per person per night longer
(0.11 longer to 0.31 longer) The average effect is about 13 minutes longer per person per night (6 minutes to 20 minutes) |
‐ | 1452 (31 RCTs) | ⊕⊕⊕⊝ Moderate1 | |
| Number of participants using machine for 4 or more hours per night Follow‐up: range 3 to 16 weeks |
Study population | OR 1.16 (0.75 to 1.81) |
346 (2 RCTs) |
⊕⊕⊝⊝ Low1,2 | ||
| 601 per 1000 | 636 per 1000 (530 to 732) |
|||||
| Symptoms assessed with ESS from 0 to 24 Median follow‐up: 6 weeks |
Average symptom scores ranged from 4.1 to 8.6 on the ESS | 0.44 ESS units lower (0.72 lower to 0.16 lower) | ‐ | 1285 (25 RCTs) | ⊕⊕⊕⊝ Moderate1 | MCID of between 2 to 3 has been proposed by Patel 2018. |
| Withdrawals (parallel group trials/first arm cross‐over trials) Median follow‐up: 6 weeks |
Study population | OR 0.90 (0.64 to 1.27) | 1275 (13 RCTs) | ⊕⊕⊕⊝ Moderate 2 | ||
| 110 per 1000 | 100 per 1000 (74 to 136) | |||||
| Quality of life assessed with FOSQ scale from 5 to 20 Follow‐up: range 4 to 104 weeks |
The mean quality of life score was 5.58 on the FOSQ | 0.12 FOSQ units higher (0.21 lower to 0.46 higher) | ‐ | 352 (3 RCTs) | ⊕⊕⊝⊝ Low1,3 | MCID for FOSQ has not been confirmed; a change of 1 unit has been proposed as representing a possible meaningful change (Billings 2014). |
| AHI measured by number of events/hr Median follow‐up: 6 weeks |
Average AHI ranged from 2 to 9 events per hour | 0.48 events per hour higher (0.16 higher to 0.80 higher) | ‐ | 1256 (26 RCTs) | ⊕⊕⊕⊕ High 4 | |
| Blood pressure (mmHg) Follow‐up 12 and 16 weeks |
Systolic blood pressure | ‐ | 353 (2 RCTs) | ⊕⊕⊕⊝ Moderate 5 | ||
| The mean systolic blood pressure was 133 mmHg | 1.87 mmHg higher (1.08 lower to 4.82 higher) | |||||
| Diastolic blood pressure | ‐ | 353 (2 RCTs) | ⊕⊕⊝⊝ Low 6 | |||
| The mean diastolic blood pressure was 78 mmHg | 2.92 mmHg higher (1.06 higher to 4.77 higher) | |||||
| Adverse events (machine tolerability outcomes) Follow‐up: 4 to 36 weeks |
Nine studies provided information on tolerability outcomes, but data could not be combined because they were measured and analysed inconsistently across the studies. Studies used different scales and data to report on four main outcome types: nasal blockage, dry mouth, tolerance of treatment pressure and mask leak. The direction and size of effect varied between the studies across the outcomes. |
‐ | 574 (9 RCTs) | ⊕⊝⊝⊝ Very low 7 | ||
|
*The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AHI: Apnoea Hypopnoea Index; CI: confidence interval; CPAP: continuous positive airway pressure; ESS: Epworth Sleepiness Scale; FOSQ: Functional Outcomes of Sleep Questionnaire; MCID: minimal clinically important difference; mmHg: millimetres of mercury (used to measure pressure); OR: odds ratio; OSA: obstructive sleep apnoea; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded one level due to serious risk of bias. All studies contributing data to machine usage were judged to be at unclear or high risk of bias for performance or attrition bias. 2 Downgraded one level due to serious imprecision. Wide confidence intervals include appreciable increase and slight reduction in effect. 3 Downgraded one level due to serious imprecision. Three studies contribute to the analysis with 352 participants. We believe that more trial data are needed to confirm the lack of a difference in quality of life scores. 4 We did not downgrade for inconsistency which was due to a single outlying result. Incorporating between study variation with random‐effects model did not change our interpretation of the effect (0.21 versus 0.33 events per hour). 5 Downgraded one level due to serious imprecision. Confidence interval includes small decrease and increase in BP with auto‐CPAP. 6 Downgraded two levels due to very serious inconsistency. We decided to downgrade twice for inconsistency in view of the discordant results between the studies and the likely impact this has on the confidence interval. 7 Downgraded one level due to serious risk of bias, inconsistency and imprecision. Most studies judged to have unclear or high risk of performance and detection bias, variation in reporting of data prevented meta‐analysis and many studies had small sample sizes.
Summary of findings 2. Bi‐PAP compared to fixed CPAP for sleep apnoea in adults.
| Bi‐PAP compared to fixed CPAP for improving usage of continuous positive airway pressure machines in adults with obstructive sleep apnoea | ||||||
| Patient or population: adults with a diagnosis of sleep apnoea Setting: Europe and USA Intervention: Bi‐PAP Comparison: fixed CPAP | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with fixed CPAP | Risk with Bi‐PAP | |||||
| Machine usage (hours/night) Follow‐up: 4 to 52 weeks |
Average nightly machine usage 5.5 hours per night across CPAP | 0.14 hours/night higher (0.17 lower to 0.45 higher) | ‐ | 268 (4 RCTs) | ⊕⊕⊝⊝ Low 1 2 | |
| Machine usage assessed by number of participants using machine for 4 or more hours per night ‐ not measured |
‐ | ‐ | ‐ | ‐ | ‐ | |
| Symptoms assessed with ESS from 0 to 24 Follow‐up: 4 to 12 weeks |
The mean symptoms ranged from 8 to 11 ESS units | 0.49 ESS units lower (1.46 lower to 0.48 higher) | ‐ | 226 (4 RCTs) | ⊕⊕⊝⊝ Low 1 3 | MCID of between 2 to 3 has been proposed by Patel 2018. |
| Withdrawals (parallel group trials/first arm cross‐over trials) Follow‐up: 4 to 52 weeks |
Study population | OR 0.55 (0.26 to 1.17) | 261 (3 RCTs) | ⊕⊕⊝⊝ Low 3 4 | ||
| 188 per 1000 | 113 per 1000 (57 to 213) |
|||||
| Quality of life assessed with FOSQ: scale from 5 to 20 Follow‐up: 8 weeks |
Mean change from baseline 5.1 units | 0.8 FOSQ units lower (6.08 lower to 4.48 units higher) | ‐ | 151 (1 RCT) |
⊕⊕⊝⊝ Low 3 5 | MCID for FOSQ has not been confirmed; a change of 1 unit has been proposed as representing a possible meaningful change (Billings 2014). |
| AHI measured with number of events/hr Follow‐up: 4 to 8 weeks |
The mean AHI was 6.6 events/hour | 1.36 events/hour lower (6.92 lower to 9.63 higher) | ‐ | 179 (2 RCTs) | ⊕⊕⊝⊝ Low 6 | |
| Blood pressure ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Adverse events (machine tolerability outcomes) Follow‐up: 4 to 52 weeks |
Five studies provided information on tolerability outcomes but data could not be combined because they were measured and analysed inconsistently across the studies. One study (N = 62) reported 5 withdrawals in CPAP group due to mask discomfort or device intolerance. 20 participants across both arms complained of nasal dryness. 1 trial (N = 151) reported similar rates of dry mouth (4.2% and 7.5%) and mask intolerance (11% and 10%) in Bi‐PAP and CPAP groups, respectively. Two small studies (N = 46) reported non‐specific adverse events. One reported that telephone contact did not identify the need for further interventions, and the other that there were similar rates of non‐specific adverse events. One study (N = 28) used a global treatment comfort score on a 0 to 00 VAS. There was insufficient evidence to determine whether Bi‐PAP improved comfort scores (69 versus fixed CPAP 60, P = 0.16). |
‐ | 239 (5 RCTs) | ⊕⊝⊝⊝ Very low 1 7 | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AHI: Apnoea Hypopnoea Index; Bi‐PAP: bilevel positive airway pressure; CI: confidence interval; CPAP: continuous positive airway pressure; ESS: Epworth Sleepiness Scale; FOSQ: Functional Outcomes of Sleep Questionnaire; MCID: minimal clinically important difference; OR: odds ratio; RCT: randomised controlled trial; VAS: visual analogue scale | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded one level due to serious risk of bias. Open‐label design from one study and insufficient detail available to assess blinding and allocation process in other studies.
2 Downgraded one level due to serious imprecision. Wide confidence interval including higher average usage with either device.
3 Downgraded one level due to serious imprecision. Wide confidence interval.
4 Downgraded one level due to serious risk of bias. Lack of detailed description of randomisation process and lack of blinding in two studies contributing data to the analysis.
5 Downgraded one level due to risk of bias. Single study at high risk of bias from lack of blinding.
6 Downgraded two levels due to very serious imprecision. Wide confidence interval and small sample size.
7 Downgraded due to very serious inconsistency. Variation in the measurement of tolerability across the studies meant that no data could be combined,
Summary of findings 3. CPAP with expiratory pressure relief compared to fixed CPAP for adults with obstructive sleep apnoea.
| CPAP with expiratory pressure relief compared to fixed CPAP for improving usage of CPAP machines in adults with obstructive sleep apnoea | ||||||
| Patient or population: adults with a diagnosis of sleep apnoea Setting: Europe, USA, New Zealand Intervention: CPAP with expiratory pressure relief Comparison: fixed CPAP | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with fixed CPAP | Risk with CPAP with expiratory pressure relief | |||||
| Machine usage
assessed by hours/night Follow‐up: range 2 to 24 weeks |
The mean machine usage was 5.1 hours/night | MD 0.14 hours/night higher (0.07 lower to 0.35 higher) | ‐ | 609 (9 RCTs) | ⊕⊕⊝⊝ Low 1 2 | |
| Machine usage assessed by number of participants using machine for 4 or more hours per night ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Symptoms
assessed with ESS Follow‐up: range 4 to 24 weeks |
The mean symptoms was 7 ESS | 0.17 ESS units higher (0.26 lower to 0.60 higher) | ‐ | 515 (6 RCTs) | ⊕⊕⊕⊝ Moderate 1 | MCID of between 2 to 3 has been proposed by Patel 2018. |
| Withdrawals (parallel group trials/first arm cross‐over trials) Follow‐up: 12 weeks |
Study population | OR 0.86 (0.48 to 1.55) | 298 (2 RCTs) | ⊕⊕⊝⊝ Low 3 | ||
| 201 per 1000 | 178 per 1000 (108 to 281) | |||||
| Quality of life assessed with FOSQ: scale from 5 to 20 Follow‐up: 12 weeks |
The mean quality of life was 18.7 FOSQ units | 0.4 FOSQ units lower (1.15 lower to 0.35 higher) | ‐ | 74 (1 RCT) | ⊕⊕⊝⊝ Low 4 | MCID for FOSQ has not been confirmed; a change of 1 unit has been proposed as representing a possible meaningful change (Billings 2014). |
| AHI measured by number of events/hr Follow‐up: range 6 to 12 weeks |
The mean AHI was 5.3 events/hour | 0.24 events/hour higher (0.49 lower to 0.96 higher) | ‐ | 342 (5 RCTs) | ⊕⊕⊕⊕ High | |
| Blood pressure ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Adverse events (machine tolerability outcomes) Follow‐up: range 4 to 24 weeks |
No specific measures of nasal or oral symptoms were carried out in six studies providing information on tolerability outcomes. Four studies assessed treatment comfort scores but data could not be combined. None of the studies reported that there were differences between the different treatment modes. Different measures of mask leak were made by two studies with no differences reported in either 90th percentile leak or average leak. |
‐ | 577 (6 RCTs) |
⊕⊝⊝⊝ Very low 5 6 | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AHI: Apnoea Hypopnoea Index; CI: confidence interval; CPAP: continuous positive airway pressure; ESS: Epworth Sleepiness Scale; FOSQ: Functional Outcomes of Sleep Questionnaire; MCID: minimal clinically important difference; MD: mean difference; OR: odds ratio; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded one level due to serious risk of bias. Uncertainty over study design from trials published as abstracts and open‐label design in other studies.
2 Downgraded one level due to serious imprecision. Confidence interval includes no difference and increase of up to 20 minutes per person per night.
3 Downgraded two levels due to very serious imprecision. Wide confidence intervals and small sample size.
4 Downgraded two levels due to very serious imprecision. Single study with small sample size.
5 Downgraded one level due to serious imprecision. Small sample size.
6 Downgraded two levels due to very serious inconsistency. Different study designs and approaches taken to capturing tolerability outcomes.
Summary of findings 4. Heated humidification + fixed CPAP compared to fixed CPAP alone in adults with obstructive sleep apnoea.
| Heated humidification + fixed CPAP compared to fixed CPAP alone for improving usage of CPAP machines in adults with obstructive sleep apnoea | ||||||
| Patient or population: adults with a diagnosis of sleep apnoea Setting: Europe, Australia, New Zealand and Thailand Intervention: heated humidification + fixed CPAP Comparison: fixed CPAP alone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with fixed CPAP alone | Risk with heated humidification + fixed CPAP | |||||
| Machine usage
assessed by hours per night Follow‐up: range 3 weeks to 12 weeks |
The mean machine usage was 5 hours | MD 0.37 hours higher (0.10 higher to 0.64 higher) | ‐ | 277 (6 RCTs) | ⊕⊕⊝⊝ Low 1 2 | |
| Machine usage assessed by number of participants using machine for 4 or more hours per night ‐ not measured |
‐ | ‐ | ‐ | ‐ | ‐ | |
| Symptoms
assessed with ESS Follow‐up: range 3 weeks to 12 weeks |
The mean symptoms ranged from 4 to 9 ESS | MD 0.34 ESS lower (0.93 lower to 0.26 higher) | ‐ | 184 (4 RCTs) | ⊕⊕⊝⊝ Low 3 4 | |
| Withdrawals (parallel group trials/first arm cross‐over trials) Follow‐up: median 12 weeks |
Study population | OR 1.00 (0.45 to 2.24) | 316 (3 RCTs) | ⊕⊝⊝⊝ Very low 3 5 | ||
| 159 per 1000 | 159 per 1000 (78 to 297) | |||||
| Quality of life assessed with FOSQ: scale from 5 to 20 ‐ not measured |
‐ | ‐ | ‐ | ‐ | ‐ | |
| AHI measured by number of events/hr Follow‐up: 4 weeks |
The mean AHI (events/hr) was 4.2 events/hr | MD 0.3 events/hr higher (0.95 lower to 1.55 higher) | ‐ | 44 (1 RCT) | ⊕⊕⊝⊝ Low 5 | |
| Blood pressure ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Adverse events (machine tolerability outcomes)
assessed as number of participants experiencing a blocked nose Follow‐up: mean 4 weeks |
Study population | OR 0.32 (0.16 to 0.63) | 147 (2 RCTs) | ⊕⊕⊝⊝ Low 6 | This outcome was selected from different measures of nasal symptoms. | |
| 648 per 1000 | 371 per 1000 (227 to 537) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AHI: Apnoea Hypopnoea Index; CI: confidence interval; CPAP: continuous positive airway pressure; ESS: Epworth Sleepiness Scale; FOSQ: Functional Outcomes of Sleep Questionnaire; MD: mean difference; OR: odds ratio; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded one level due to serious risk of bias. Open‐label study design in a number of studies and lack of detail regarding methods of allocation.
2 Downgraded one level due to serious imprecision. The sample size of 277 may be sufficient for effect size observed, but confirmatory studies would help to establish this more reliably.
3 Downgraded one level due to serious risk of bias, lack of blinding in one trial
4 Downgraded one level due to serious imprecision. Given baseline values and the change in both groups from using CPAP, the confidence interval includes a potentially meaningful difference of 1 unit on the ESS.
5 Downgraded two levels due to imprecision: small sample size and wide confidence intervals
6 Downgraded two levels due to very serious imprecision. Sample size across the studies is small. There were a number of different measures of nasal symptoms and the effect observed here may reflect multiplicity.
Automatically adjusting continuous positive airway pressure (auto‐CPAP) versus fixed continuous positive airway pressure (fixed CPAP)
Primary outcomes
Machine usage
Auto‐CPAP probably increases average nightly usage by about 13 minutes per person compared with fixed CPAP at a median follow‐up of six weeks (mean difference (MD) 0.21 hours/night, 95% confidence interval (CI) 0.11 to 0.31; 31 studies, 1452 participants; moderate‐certainty evidence; Analysis 1.1, Figure 3). Average machine usage in the fixed CPAP arms was about five hours per person per night. Fixed‐effect and random‐effects meta‐analysis results were identical. Data from one large parallel group trial could not be used in the primary analysis because only medians and interquartile ranges were available (Pépin 2016, N = 322). We decided to incorporate this study as a sensitivity analysis because the sample size was bigger than any others in the analysis and the direction of effect was potentially discordant. Including this study with imputed standard deviations (SDs) attenuated slightly the size of effect (MD 0.19 hours/night, 95% CI 0.10 to 0.29; 32 studies, 1774 participants; Analysis 1.2).
1.1. Analysis.
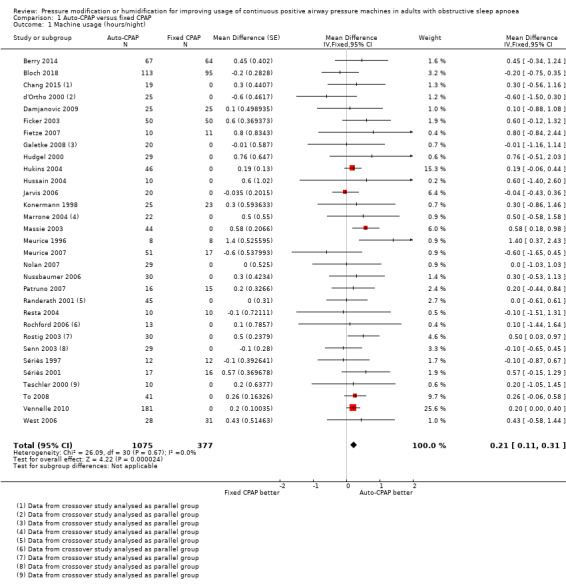
Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 1 Machine usage (hours/night).
3.
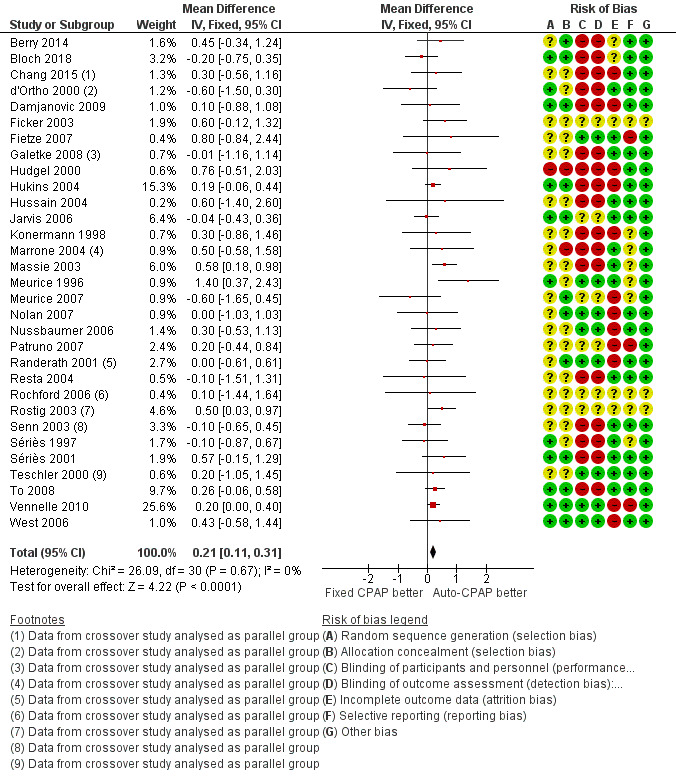
Forest plot of comparison: 1 Auto‐CPAP versus fixed CPAP, outcome: 1.1 Machine usage (hours/night).
1.2. Analysis.
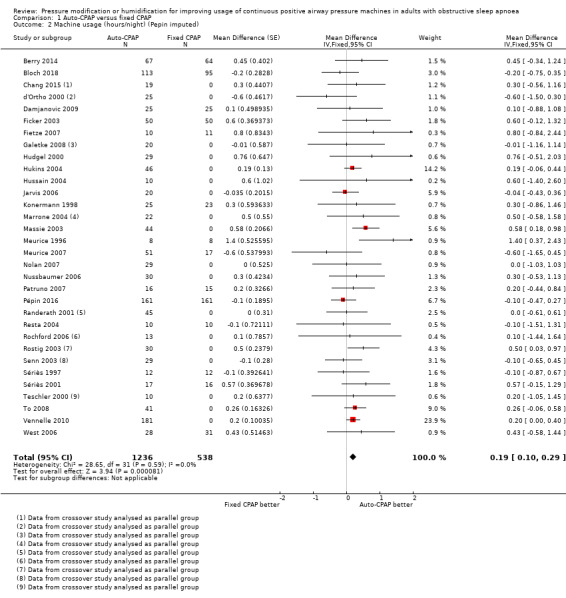
Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 2 Machine usage (hours/night) (Pepin imputed).
The number of people who use their machines for four hours or more per night may be slightly higher with auto‐CPAP, although the CIs include both substantially fewer and substantially more people with auto‐CPAP (odds ratio (OR) 1.16, 95% CI 0.75 to 1.81; 2 studies, 346 participants; low‐certainty evidence; Analysis 1.3).
1.3. Analysis.
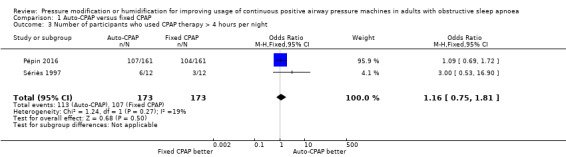
Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 3 Number of participants who used CPAP therapy > 4 hours per night.
Additional measures of usage indicate the increase in average effect could be driven by higher use on specific nights rather than incrementally greater use on all nights with auto‐CPAP (Analysis 1.4; Analysis 1.5; Analysis 1.6).
1.4. Analysis.
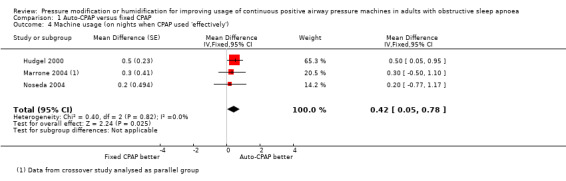
Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 4 Machine usage (on nights when CPAP used 'effectively').
1.5. Analysis.
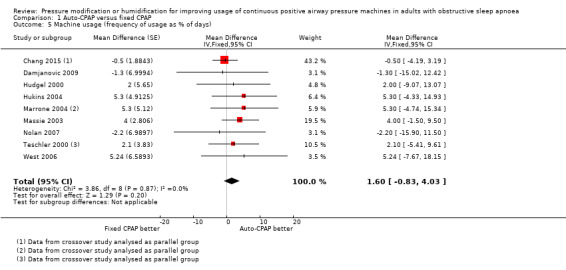
Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 5 Machine usage (frequency of usage as % of days).
1.6. Analysis.
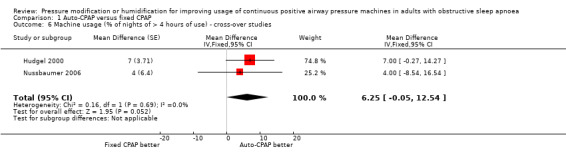
Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 6 Machine usage (% of nights of > 4 hours of use) ‐ cross‐over studies.
Secondary outcomes
Symptom scores
Auto‐CPAP probably reduces the Epworth Sleepiness Scale (ESS) score by a small degree compared with fixed CPAP at three to 16 weeks (MD ‐0.44 units, 95% CI ‐0.72 to ‐0.16; 25 studies, 1285 participants; moderate‐certainty evidence; Analysis 1.7). Average ESS scores following fixed CPAP treatment ranged from 4.1 to 8.6. There was no statistical heterogeneity and changing statistical model had no impact on the pooled result.
1.7. Analysis.
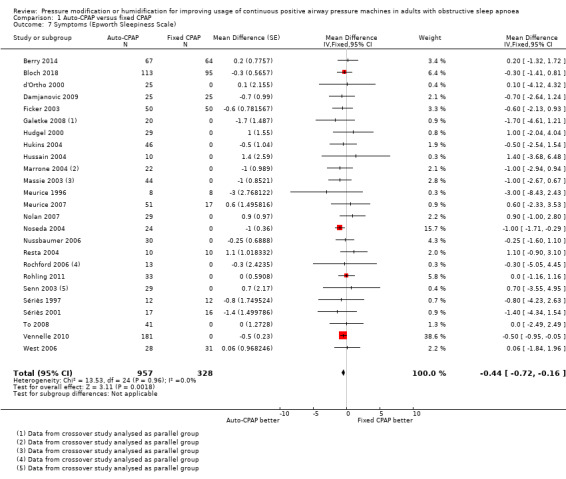
Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 7 Symptoms (Epworth Sleepiness Scale).
Withdrawals
The likelihood of withdrawal is probably similar between the two devices at six weeks (auto‐CPAP: 10% versus fixed CPAP: 11%), although the CI includes a slightly higher risk of withdrawal with both devices (OR 0.90, 95% CI 0.64 to 1.27; 13 studies, 1275 participants; moderate‐certainty evidence; Analysis 1.8).
1.8. Analysis.
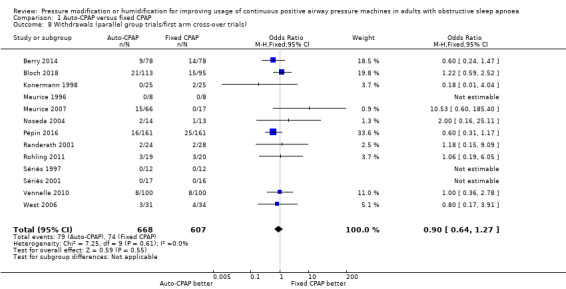
Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 8 Withdrawals (parallel group trials/first arm cross‐over trials).
Quality of life scores
Functional Outcomes of Sleep Questionnaire (FOSQ)
Quality of life measured as FOSQ may be similar between auto‐CPAP and fixed CPAP (MD 0.12 units, 95% CI ‐0.21 to 0.46; 3 studies, 352 participants; low‐certainty evidence; Analysis 1.9). There was wide variation in the follow‐up for these studies (4 to 104 weeks).
1.9. Analysis.
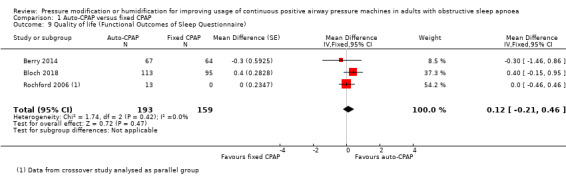
Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 9 Quality of life (Functional Outcomes of Sleep Questionnaire).
Sleep Apnoea Quality of Life Index (SAQLI)
Two trials assessing for changes in SAQLI found no significant difference in the effects of auto‐CPAP and fixed CPAP (MD ‐0.14 units, 95% CI ‐0.54 to 0.27; Analysis 1.10).
1.10. Analysis.
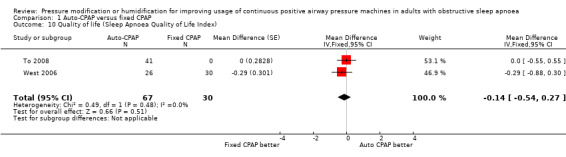
Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 10 Quality of life (Sleep Apnoea Quality of Life Index).
Short‐Form 36 (SF‐36)
Eight studies provided data on quality of life measured with the SF‐36 (Analysis 1.11). The number of studies contributing data to each domain varied from two (role physical, bodily pain, general health and social functioning) to six (vitality). Effect sizes ranged from a reduction of 6 points with auto‐CPAP on physical health domain to a 6‐point increase with auto‐CPAP on general health domain. Hukins 2004 reported no significant differences between treatment groups.
1.11. Analysis.
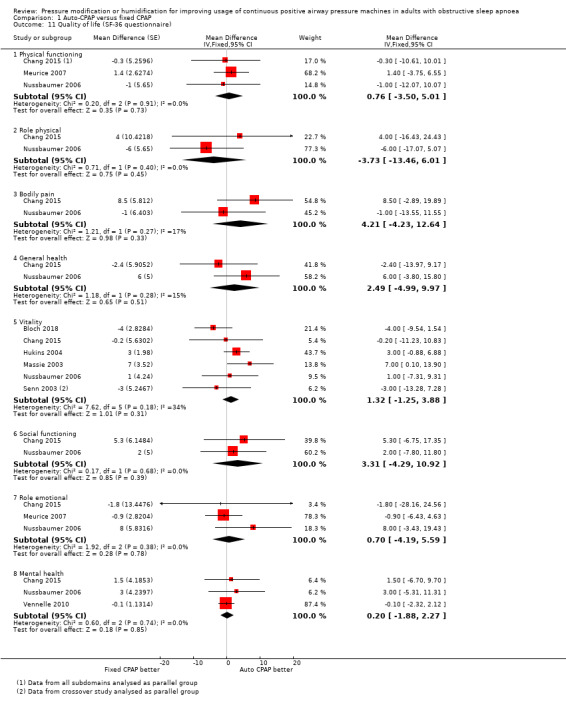
Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 11 Quality of life (SF‐36 questionnaire).
Sleep disruption
Apnoea Hypopnoea Index (AHI)
There is a slightly higher AHI with auto‐CPAP compared with fixed CPAP at six weeks (MD 0.48 events/hour, 95% CI 0.16 to 0.80; 26 studies, 1256 participants; high‐certainty evidence; Analysis 1.12). There was a high level of statistical heterogeneity that we could attribute to the inclusion of a single study (I2 = 51%; Patruno 2007). Neither excluding Patruno 2007 nor applying a random‐effects model substantially altered the direction, precision or size of the effect.
1.12. Analysis.
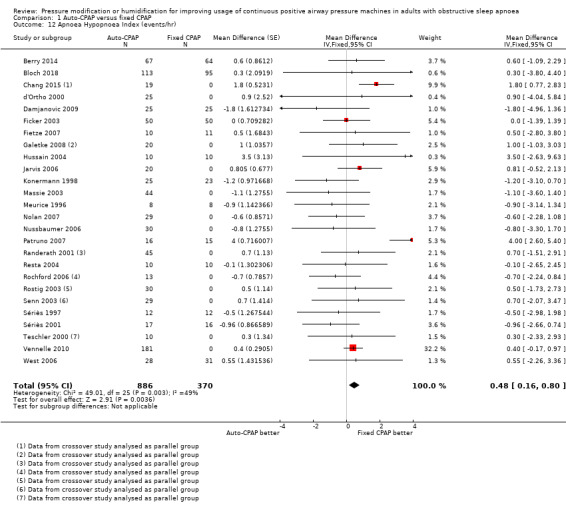
Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 12 Apnoea Hypopnoea Index (events/hr).
Arousals
There is insufficient evidence to determine the comparative effect of the devices on arousals (MD ‐0.66 events/hr, 95% CI ‐2.90 to 1.58; 4 studies, 136 participant; Analysis 1.13).
1.13. Analysis.
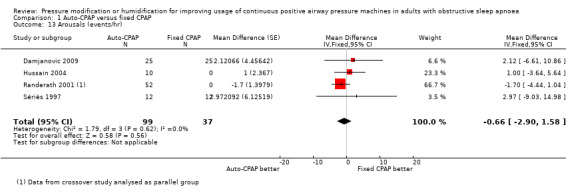
Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 13 Arousals (events/hr).
Treatment pressure
There was a high degree of statistical variation in the size and direction of differences in treatment pressures across the studies (I2 = 92%). Both fixed‐ and random‐effects models indicate lower average pressure with auto‐CPAP (fixed‐effect MD ‐1.01 cm H2O, 95% CI ‐1.17 to ‐0.84, random‐effects MD ‐1.49 cm H2O, 95% CI ‐2.12 to ‐0.85; 24 studies, 1171 participants; Analysis 1.14). Despite the different mechanisms used to deliver mask pressure between the devices, in some studies the delivered treatment pressure was equivalent between auto‐CPAP and fixed CPAP, whilst in others the mean treatment pressure in auto‐CPAP was between 3 cm H2O and 5 cm H2O lower. Differences in algorithms used by the different machines used to alter pressure (e.g. forced oscillation), variation in peak treatment pressure within study populations and the selection of participants on the basis of high treatment pressure required in others (e.g. Massie 2003; Noseda 2004), could contribute to the conflicting results.
1.14. Analysis.
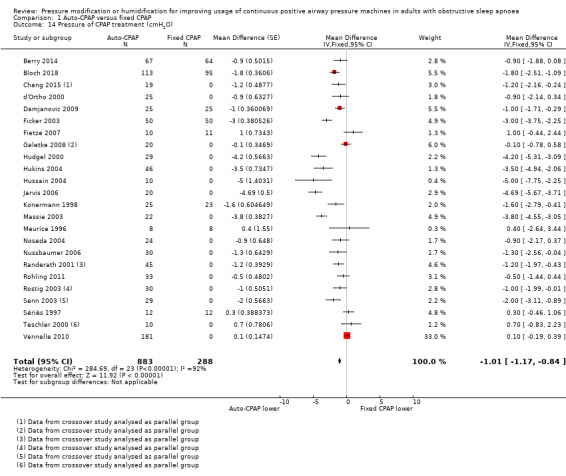
Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 14 Pressure of CPAP treatment (cmH2O).
Blood pressure
Systolic blood pressure
Our analysis could not exclude a potentially small decrease or increase in systolic blood pressure with auto‐CPAP at 12 to 16 weeks (MD 1.87 mmHg, 95% CI ‐1.08 to 4.82; 2 studies, 353 participants; moderate‐certainty evidence; Analysis 1.15). We were unable to extract data from Nolan 2007 and West 2006. Neither study reported significant differences between auto‐CPAP and fixed CPAP.
1.15. Analysis.
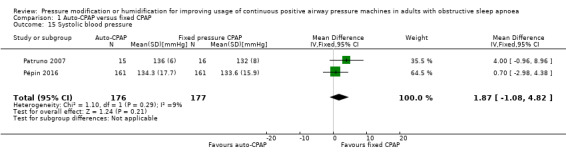
Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 15 Systolic blood pressure.
Diastolic blood pressure
Diastolic blood pressure was higher at the end of the study period with auto‐CPAP than with fixed CPAP at 12 to 16 weeks (2.92 mmHg; 95% CI 1.06 to 4.77; 2 studies; 353 participants; low‐certainty‐evidence; Analysis 1.16). Nolan 2007 reported no significant difference between auto‐CPAP and fixed CPAP but did not provide numerical values.
1.16. Analysis.
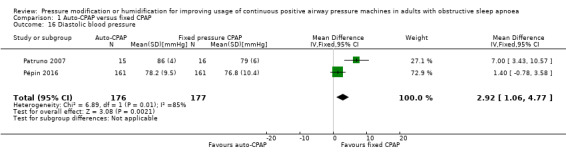
Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 16 Diastolic blood pressure.
Additional measures of blood pressure did not indicate any meaningful differences in blood pressure, namely 24‐hour mean, systolic and diastolic blood pressure: Analysis 1.17; Analysis 1.18 and Analysis 1.19); diurnal mean, systolic and diastolic blood pressure (Analysis 1.20; Analysis 1.21; Analysis 1.22), and nocturnal mean, systolic and diastolic blood pressure (Analysis 1.23; Analysis 1.24; Analysis 1.25).
1.17. Analysis.
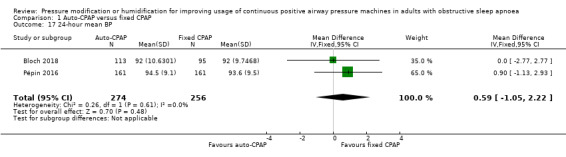
Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 17 24‐hour mean BP.
1.18. Analysis.
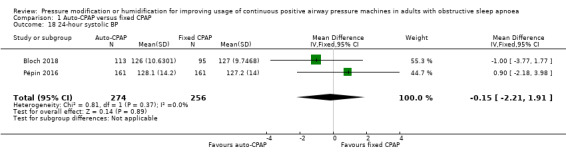
Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 18 24‐hour systolic BP.
1.19. Analysis.
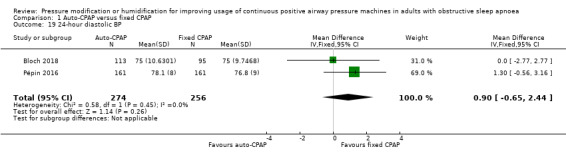
Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 19 24‐hour diastolic BP.
1.20. Analysis.
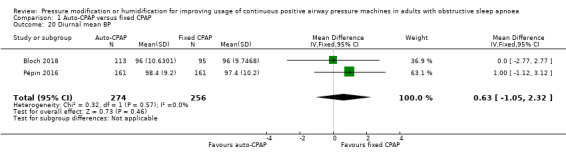
Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 20 Diurnal mean BP.
1.21. Analysis.
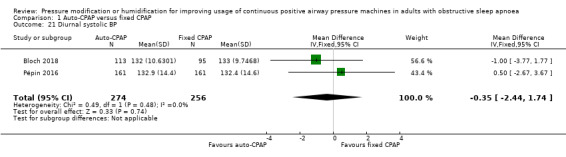
Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 21 Diurnal systolic BP.
1.22. Analysis.
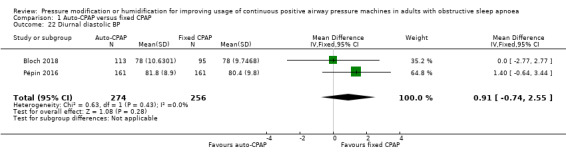
Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 22 Diurnal diastolic BP.
1.23. Analysis.
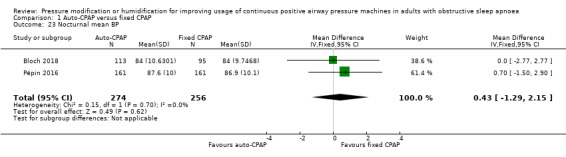
Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 23 Nocturnal mean BP.
1.24. Analysis.
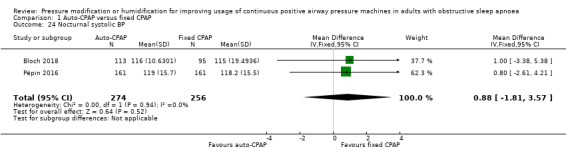
Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 24 Nocturnal systolic BP.
1.25. Analysis.
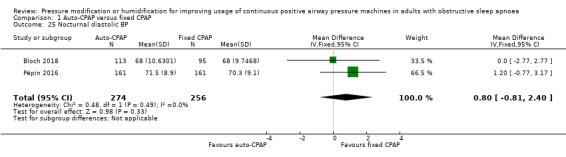
Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 25 Nocturnal diastolic BP.
Adverse events
Data on tolerability outcomes were measured and reported inconsistently across the studies. We have presented a narrative summary of the data from individual studies for each main symptom associated with machine usage. Data from one study could be presented in Analysis 1.26. For the remainder, data were presented graphically or we could not adjust data adequately for the cross‐over design.
1.26. Analysis.
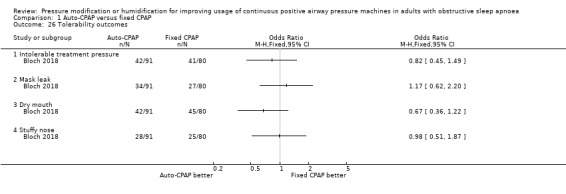
Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 26 Tolerability outcomes.
Due to the risk of bias across the studies, variation in the methods used to measure this outcome and the use of unvalidated scales for some of the studies, we rated the certainty of evidence for tolerability outcomes to be very low (see Table 1). We have been unable to obtain data from one study of 181 people that measured CPAP side effects using the Edinburgh checklist but did not report these findings (Vennelle 2010). Follow‐up ranged from 4 to 36 weeks.
Nasal blockage
Four participants in Sériès 1997 suffered nasal blockage (2 from auto‐CPAP1 group, one from auto‐CPAP2, and one from fixed CPAP), which resolved with the use of a heated humidifier. Bloch 2018 presented data from the per protocol population, reporting similar rates of nasal blockage at 2 years (31% in both groups, N = 144). Nolan 2007 presented bar charts of those experiencing blocked or runny nose during both arms of treatment (just over 40% in those treated with auto‐CPAP and just over 30% in those with fixed CPAP based on visual inspection, N = 26).
Nussbaumer 2006 reported similar scores between treatment arms by participants who rated symptoms on a visual analogue scale (VAS) (N = 38).
Dry mouth
Bloch 2018 reported lower rates of dry mouth in the auto‐CPAP group than with fixed CPAP (46% versus 56%), whereas in Nolan 2007 the direction of effect was the reverse (just under 45% versus 35% with fixed CPAP, based on visual inspection).
Paticipant‐reported symptoms were slightly lower in the auto‐CPAP arm in Nussbaumer 2006.
Tolerance of treatment pressure
Bloch 2018 reported lower rates of excessive mask pressure with auto‐CPAP than with fixed CPAP (46% versus 51%), as did Nolan 2007 (18% versus 21%).
Massie 2003 reported a significant difference between auto‐CPAP and fixed CPAP in favour of the automatic pressure mode on feeling discomfort from pressure and experiencing less trouble getting to sleep (all values P < 0.006). Randerath 2001 reported no significant differences between the two groups who were treated with both auto and fixed CPAP (no numerical values presented). d'Ortho 2000 reported little difference on an unvalidated questionnaire measuring tolerance of treatment pressure between auto‐CPAP and fixed CPAP (N = 25). In Nussbaumer 2006 participant‐rated tolerance of treatment pressure was better in the auto‐CPAP arm than during fixed CPAP treatment.
Mask leak
Bloch 2018 reported a slightly higher proportion of participants reporting mask leak in the auto‐CPAP group (37% versus 34%); Nolan 2007 presented data that indicated slightly fewer participants experiencing leak with auto‐CPAP (just over 20% versus just under 25% based on visual inspection).
Teschler 2000 reported no significant difference in mask leak between fixed CPAP (13% mask on time with leak of 0.4 L/second) and auto‐CPAP (10% mask on time with leak of 0.4 L/second). Four studies reported slightly fewer leaks as either number of leaks per person, leakage time or pressure leaked per second with auto‐CPAP compared with CPAP (Hukins 2004; West 2006; Galetke 2008; Damjanovic 2009). Nussbaumer 2006 found that mask leaks were perceived to be less problematic on auto‐CPAP than on fixed CPAP.
Participant preference
The results from the studies indicate wide variation in terms of preferences expressed by users of CPAP between the different machine types. In eight of the 14 studies reporting this outcome, on average people preferred auto‐CPAP over either fixed CPAP, or neither treatment. However, in six studies, people preferred fixed CPAP (Senn 2003; Hussain 2004; Jarvis 2006; To 2008; Vennelle 2010; Rohling 2011). In Senn 2003 the majority of participants (21/29) preferred neither treatment: but in Hussain 2004 (6/10) and To 2008 (30/41) the majority preferred treatment with fixed CPAP. In Jarvis 2006 (11/20), Vennelle 2010 (112/181) and Rohling 2011 (20/33), more participants expressed preference for fixed CPAP or no preference than preference for auto‐CPAP. We were unable to find a satisfactory explanation for this apparent discrepancy in terms of study design and technology of active interventions.
Bilevel positive airway pressure (Bi‐PAP) versus fixed CPAP
Primary outcomes
Machine usage
There is insufficient evidence to determine the effect of Bi‐PAP on average machine usage (0.14 hours/night, 95% CI ‐0.17 to 0.45; 4 studies; 268 participants; low‐certainty evidence; Analysis 2.1, Figure 4). Follow‐up ranged from 4 to 52 weeks.
2.1. Analysis.
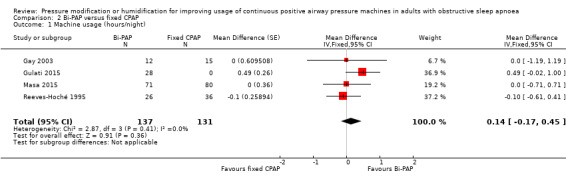
Comparison 2 Bi‐PAP versus fixed CPAP, Outcome 1 Machine usage (hours/night).
4.
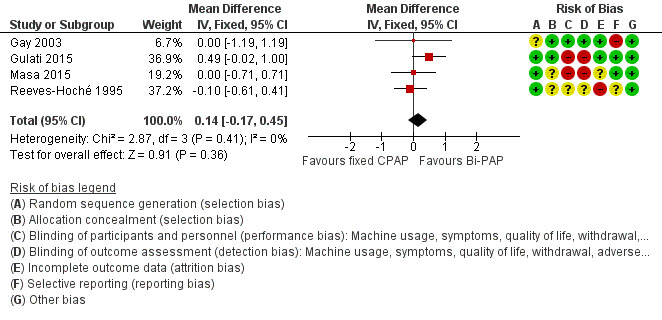
Forest plot of comparison: 2 Bi‐PAP versus fixed CPAP, outcome: 2.1 Machine usage (hours/night).
Secondary outcomes
Symptom scores
Epworth sleepiness scores (ESS) reduced from baseline with both devices in four studies with follow‐up from four to 12 weeks, but there is insufficient evidence available to determine the comparative effects of Bi‐PAP and fixed CPAP on symptoms (‐0.49 ESS units, 95% CI ‐1.46 to 0.48; 226 participants; low‐certainty evidence; Analysis 2.2).
2.2. Analysis.

Comparison 2 Bi‐PAP versus fixed CPAP, Outcome 2 Symptoms (Epworth Sleepiness Scale).
Withdrawals
There is insufficient evidence to determine whether withdrawal is more likely with Bi‐PAP (OR 0.55, 95% CI 0.26 to 1.17; 3 studies; 261 participants; low‐certainty evidence; Analysis 2.3). Follow‐up ranged from four to 52 weeks.
2.3. Analysis.
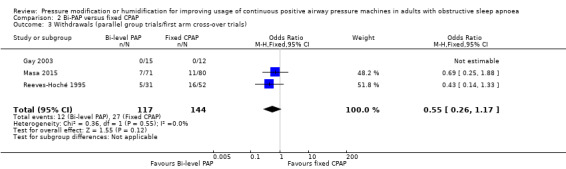
Comparison 2 Bi‐PAP versus fixed CPAP, Outcome 3 Withdrawals (parallel group trials/first arm cross‐over trials).
Quality of life scores
Functional Outcomes of Sleep Questionnaire (FOSQ)
There is insufficient evidence from one small eight‐week trial to determine the effects of Bi‐PAP on FOSQ (low‐certainty evidence, Analysis 2.4).
Sleep Apnoea Quality of Life Index (SAQLI)
There is insufficient evidence to determine the effects of Bi‐PAP on SAQLI from one study (Analysis 2.5).
2.5. Analysis.

Comparison 2 Bi‐PAP versus fixed CPAP, Outcome 5 Quality of life (Sleep Apnoea Quality of Life Index).
Short‐Form 36 (SF‐36)
There is insufficient evidence to determine the effects of Bi‐PAP on SF‐36 sub domains of physical and mental health from one study (see Analysis 2.6).
2.6. Analysis.
Comparison 2 Bi‐PAP versus fixed CPAP, Outcome 6 Quality of life (SF‐36 questionnaire).
Sleep disruption
Apnoea Hypopnoea Index (AHI)
There is insufficient evidence from two studies at four to eight weeks duration to determine the effect of Bi‐PAP on AHI (1.36 events/hour, 95% CI ‐6.92 to 9.63; 2 studies; 179 participants; Analysis 2.7).
2.7. Analysis.
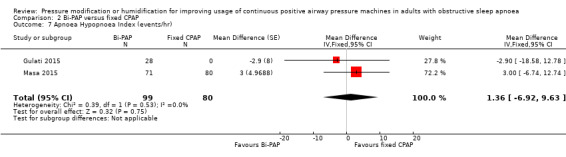
Comparison 2 Bi‐PAP versus fixed CPAP, Outcome 7 Apnoea Hypopnoea Index (events/hr).
Treatment pressure
Gulati 2015 provided a measurement of the two levels of pressure for inhalation and exhalation. Against a control mean of 11.45 cmH2O the average pressure at inhalation was 13 cmH2O and at exhalation was 4.1 cmH2O.
Participant preference
Muir 1998 reported that both CPAP and bi‐PAP were preferred by 40% of participants, while 20% did not express a preference.
Adverse events
Follow‐up across the five studies providing information for these outcomes ranged from four to 52 weeks.
Reeves‐Hoché 1995 reported five withdrawals due to either mask discomfort (n = 2) or therapy intolerance (n = 3). All were from the CPAP group. No withdrawals due to mask discomfort or therapy intolerance occurred from the bi‐PAP group. Twenty participants complained of nasal dryness (no distribution between the 2 groups reported). Three participants complained of rhinorrhoea and 15 participants complained of nasal bridge pressure (no distribution reported between the 2 groups). Masa 2015 reported similar rates of dry mouth and mask intolerance in the treatment groups (Analysis 2.9).
2.9. Analysis.
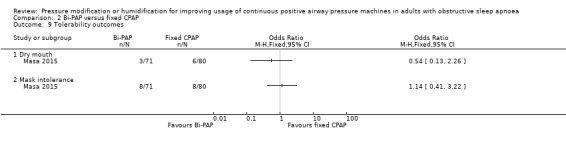
Comparison 2 Bi‐PAP versus fixed CPAP, Outcome 9 Tolerability outcomes.
Gay 2003 reported that telephone contact did not identify any complications that necessitated further interventions. Muir 1998 did not report data in terms of specific adverse events; no difference in the rate of adverse events was reported. Gulati 2015 used a global treatment comfort score on a 0‐100 VAS but there was insufficient evidence to determine the effect (Bi‐PAP: 69 versus fixed CPAP: 60, P = 0.16).
CPAP with expiratory pressure relief (CPAPexp) versus fixed CPAP
Primary outcomes
Machine usage
CPAPexp may be used more than fixed CPAP although the CI includes little or no difference in usage in studies, with a range of follow‐up of two to 24 weeks (0.14 hours/night, 95% CI ‐0.07 to 0.35; 9 studies, 609 participants; low‐certainty evidence; Analysis 3.1, Figure 5).
3.1. Analysis.
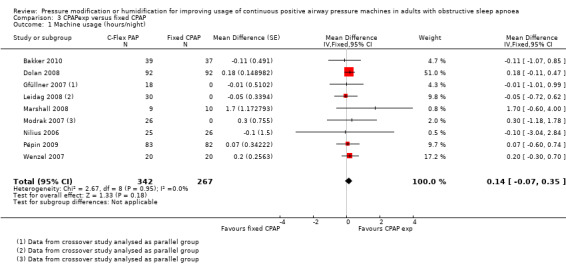
Comparison 3 CPAPexp versus fixed CPAP, Outcome 1 Machine usage (hours/night).
5.
Forest plot of comparison: 3 CPAP with expiratory pressure relief versus fixed CPAP, outcome: 3.1 Machine usage (hours/night).
Secondary outcomes
Symptom scores
The effects of CPAPexp and fixed CPAP on symptom scores are probably similar in studies with a range of follow‐up of two to 24 weeks (0.17 ESS units, 95% CI ‐0.26 to 0.60; 6 studies, 515 participants; moderate‐certainty evidence; Analysis 3.2).
3.2. Analysis.
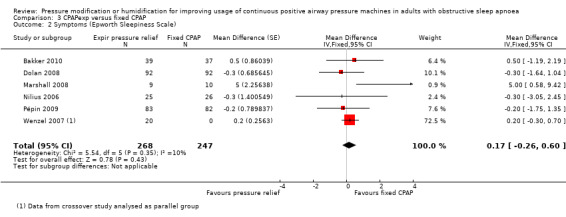
Comparison 3 CPAPexp versus fixed CPAP, Outcome 2 Symptoms (Epworth Sleepiness Scale).
Withdrawals
There is insufficient evidence to determine the effects of CPAPexp and fixed CPAP on withdrawal at 12 weeks (OR 0.86, 95% CI 0.48 to 1.55; 2 studies, 298 participants; low‐certainty evidence; Analysis 3.3).
3.3. Analysis.
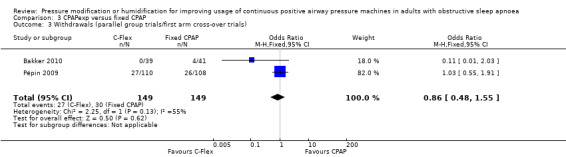
Comparison 3 CPAPexp versus fixed CPAP, Outcome 3 Withdrawals (parallel group trials/first arm cross‐over trials).
Quality of life
Functional Outcomes of Sleep Questionnaire (FOSQ)
There may be little difference in FOSQ between treatment modes based on evidence from one 12‐week study in 74 participants (‐0.40 FOSQ units, 95% CI ‐1.15 to 0.35; low‐certainty evidence; Analysis 3.4).
3.4. Analysis.

Comparison 3 CPAPexp versus fixed CPAP, Outcome 4 Quality of life (Functional Outcomes of Sleep Questionnaire).
Short‐Form 36 (SF‐36)
Two studies reported SF‐36, but only one provided data suitable for analysis. There was insufficient evidence to determine the comparative effect of these treatment modes on the different domains of the SF‐36 (Analysis 3.5).
3.5. Analysis.
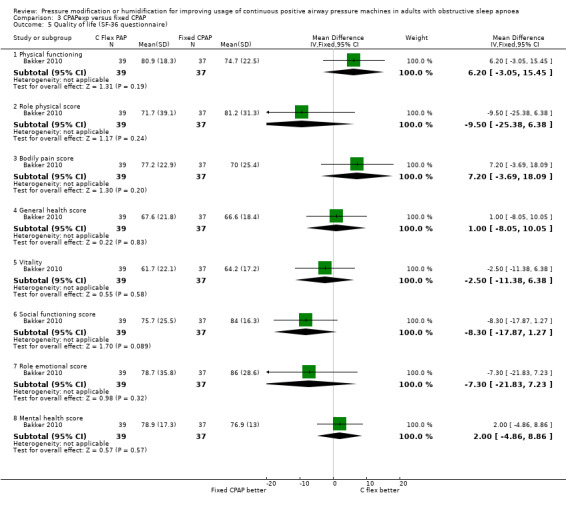
Comparison 3 CPAPexp versus fixed CPAP, Outcome 5 Quality of life (SF‐36 questionnaire).
Sleep disruption
Apnoea Hypopnoea Index (AHI)
There is little or no difference in AHI at the end of the study period ranging from four to 24 weeks (0.24 events/hr, 95% CI ‐0.49 to 0.96; 5 studies, 342 participants; high‐certainty evidence; Analysis 3.6).
3.6. Analysis.
Comparison 3 CPAPexp versus fixed CPAP, Outcome 6 Apnoea Hypopnoea Index (events/hr).
Treatment pressure
There is probably little or no difference in treatment pressure between the two groups (‐0.05cmH2O, 95% CI ‐0.63 to 0.52 cmH2O; 2 studies, 241 participants; moderate‐certainty evidence; Analysis 3.7).
3.7. Analysis.
Comparison 3 CPAPexp versus fixed CPAP, Outcome 7 Pressure of CPAP treatment (cmH2O).
Adverse events
No specific measures of nasal or oral symptoms were carried out in the trials which ranged in follow‐up from four to 24 weeks.
Four studies assessed treatment comfort scores. One parallel group trial reported data from questionnaires which indicated that CPAPexp was regarded as a more tolerable treatment than fixed CPAP by users, and led to greater satisfaction with treatment (Dolan 2008; Analysis 3.8; Analysis 3.9; Analysis 3.10). Gfüllner 2007 did not find a significant difference between the two treatments (measured as treatment comfort scores (CPAPexp: 6.9 versus fixed CPAP: 6.0). Nilius 2006 reported that after seven weeks of treatment, average tolerability scores were 15.5 in the CPAPexp group and 16.5 in the fixed CPAP group. An analysis of the individual questions after seven weeks revealed no statistically significant difference between the groups for any item. Pépin 2009 reported no statistically significant differences in assessments of side effects and comfort between the treatment modes.
3.8. Analysis.
Comparison 3 CPAPexp versus fixed CPAP, Outcome 8 Treatment satisfaction score.
3.9. Analysis.
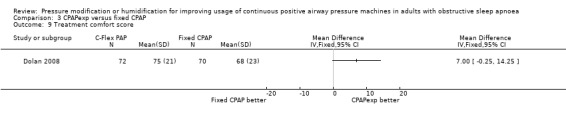
Comparison 3 CPAPexp versus fixed CPAP, Outcome 9 Treatment comfort score.
3.10. Analysis.
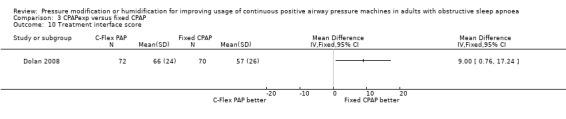
Comparison 3 CPAPexp versus fixed CPAP, Outcome 10 Treatment interface score.
Mask leak
Bakker 2010 reported no significant difference between CPAPexp and fixed CPAP in 90th percentile leak (54.8 (standard deviation (SD) 19.5) and 59.8 (SD 20.8) L/min, P = 0.30), or in average leak (43.7 (SD 14.8) and 47.6 (SD 16.8) events/h, respectively; P = 0.31). Leidag 2008 reported that leakage in fixed CPAP mode was 27.5 (SD 11.5) L/min and in CPAPexp was 28.0 (SD 10) L/min.
Particpant preference
Leidag 2008 reported that the treatment modes were equally likely to be preferred by the study participants.
Heated humidification + fixed CPAP versus fixed CPAP alone
Primary outcomes
Machine usage
Humidification may increase use of fixed CPAP by 0.37 hours per person per night, with a range of follow‐up of three to 12 weeks (95% CI 0.10 to 0.64; 6 studies, 277 participants; low‐certainty evidence; Analysis 4.1; Figure 6).
4.1. Analysis.
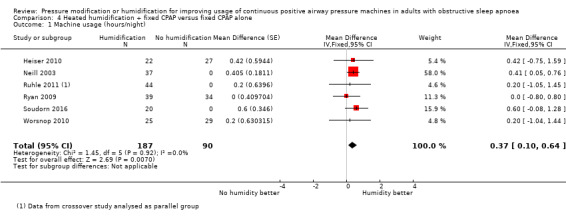
Comparison 4 Heated humidification + fixed CPAP versus fixed CPAP alone, Outcome 1 Machine usage (hours/night).
6.
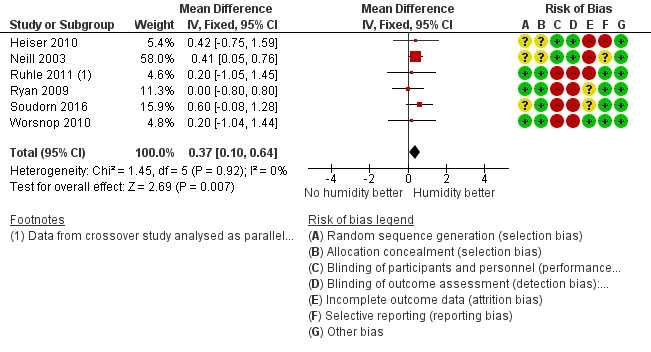
Forest plot of comparison: 4 Heated humidification + fixed pressure CPAP versus fixed pressure CPAP alone, outcome: 4.1 Machine usage (hours/night).
Secondary outcomes
Symptom scores
There may be little difference in ESS between those receiving humidification and those who did not based on four studies with a range of follow‐up of three to 12 weeks (MD ‐0.34, 95% CI ‐0.93 to 0.26; 184 participants; low‐certainty evidence; Analysis 4.2). Salgado 2006 reported no significant difference between treatments, but did not provide numerical values.
4.2. Analysis.
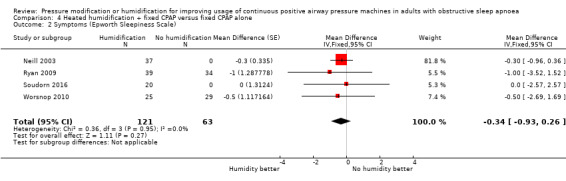
Comparison 4 Heated humidification + fixed CPAP versus fixed CPAP alone, Outcome 2 Symptoms (Epworth Sleepiness Scale).
Quality of life scores
Short‐Form 36 (SF‐36)
The effect of humidification on SF‐36 General Health Perception scores was uncertain (0.11, 95% CI ‐6.97 to 7.18; 2 studies; 124 participants, Analysis 4.5).
4.5. Analysis.
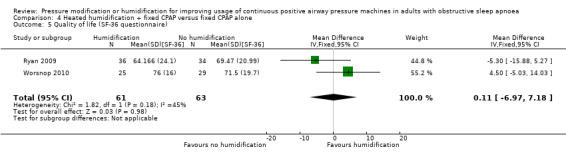
Comparison 4 Heated humidification + fixed CPAP versus fixed CPAP alone, Outcome 5 Quality of life (SF‐36 questionnaire).
Sleep disruption
Apnoea Hypopnoea Index (AHI)
AHI was measured in one small study of four weeks duration (N = 44). The effect of humidification on AHI was uncertain (0.30 events/hr higher (0.95 lower to 1.55 higher; Analysis 4.4; low‐certainty evidence).
4.4. Analysis.
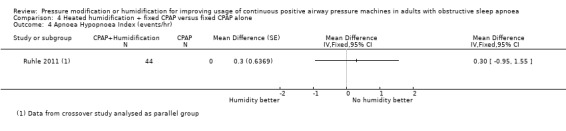
Comparison 4 Heated humidification + fixed CPAP versus fixed CPAP alone, Outcome 4 Apnoea Hypopnoea Index (events/hr).
Adverse events
Results for different unvalidated measures of nasal symptoms were reported by two studies as dichotomous data (Neill 2003; Ryan 2009), and by two studies as continuous data (Heiser 2010; Worsnop 2010). There was insufficient evidence to reliably determine the effect of additional humidification on symptoms of dry, runny, blocked or bleeding nose measured as either events (Analysis 4.6), or when rated as symptom scores (Analysis 4.7). The average duration was around four weeks.
4.6. Analysis.
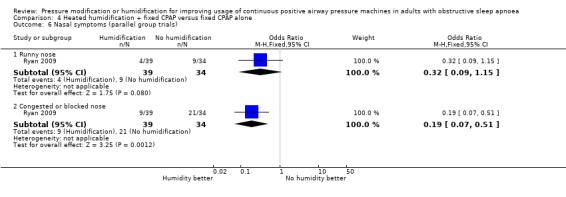
Comparison 4 Heated humidification + fixed CPAP versus fixed CPAP alone, Outcome 6 Nasal symptoms (parallel group trials).
4.7. Analysis.
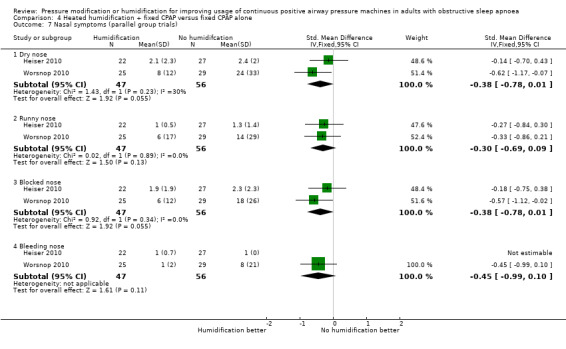
Comparison 4 Heated humidification + fixed CPAP versus fixed CPAP alone, Outcome 7 Nasal symptoms (parallel group trials).
Participant preference
Neill 2003 showed no significant difference in the number of participants expressing a preference for humidification over no humidification.
Multimodality devices versus fixed CPAP
Automatically adjusting CPAP with expiratory pressure relief (auto‐CPAPexp) versus fixed CPAP
Kushida 2011 and Meurice 2009 compared auto C‐Flex with fixed CPAP. There was no evidence of a difference in compliance between the two devices (0.03 hours, 95% CI ‐0.66 to 0.67; 2 studies, 113 participants). Results for the remaining outcomes did not provide sufficient evidence to determine the effects on symptoms, quality of life or blood pressure (Analysis 5.2; Analysis 5.3; Analysis 5.4; Analysis 5.5; Analysis 5.6; Analysis 5.7; Analysis 5.8).
5.2. Analysis.

Comparison 5 Auto‐CPAPexp versus fixed CPAP, Outcome 2 Symptoms (Epworth Sleepiness Scale).
5.3. Analysis.
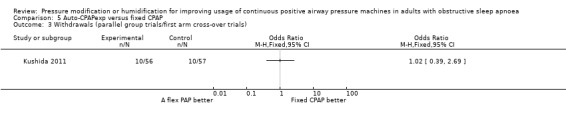
Comparison 5 Auto‐CPAPexp versus fixed CPAP, Outcome 3 Withdrawals (parallel group trials/first arm cross‐over trials).
5.4. Analysis.
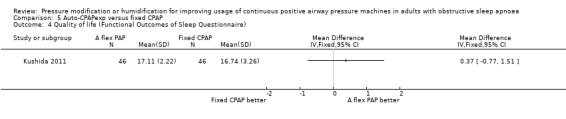
Comparison 5 Auto‐CPAPexp versus fixed CPAP, Outcome 4 Quality of life (Functional Outcomes of Sleep Questionnaire).
5.5. Analysis.
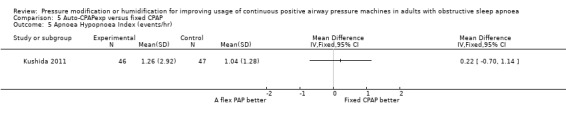
Comparison 5 Auto‐CPAPexp versus fixed CPAP, Outcome 5 Apnoea Hypopnoea Index (events/hr).
5.6. Analysis.
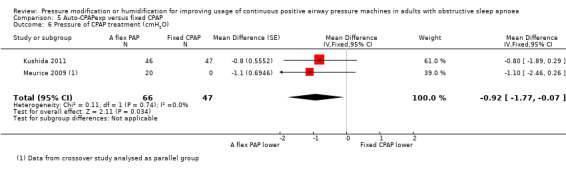
Comparison 5 Auto‐CPAPexp versus fixed CPAP, Outcome 6 Pressure of CPAP treatment (cmH2O).
5.7. Analysis.
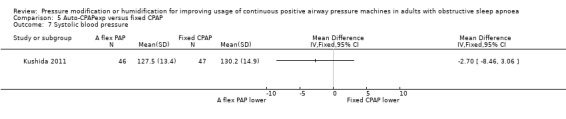
Comparison 5 Auto‐CPAPexp versus fixed CPAP, Outcome 7 Systolic blood pressure.
5.8. Analysis.
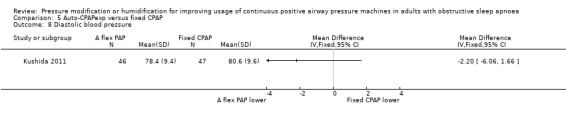
Comparison 5 Auto‐CPAPexp versus fixed CPAP, Outcome 8 Diastolic blood pressure.
Bi‐PAP with expiratory pressure relief (Bi‐PAPexp) versus fixed CPAP
Ballard 2007 compared Bi‐PAPexp (Bi‐Flex) to fixed CPAP in 104 participants who remained poorly compliant with fixed CPAP after standard interventions such as mask optimisation, humidification and sleep apnoea education; they reported increased machine use with Bi‐Flex, measured as an average of hours per night (3.7 hours versus 2.9 hours; Analysis 6.1), and as the number of participants who used the machines for more than four hours per night (Analysis 6.2). There was insufficient evidence to determine the effect on quality of life and no measurement of symptoms, withdrawals, AHI, blood pressure or adverse events.
6.1. Analysis.
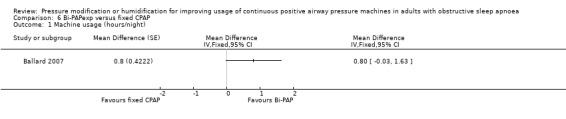
Comparison 6 Bi‐PAPexp versus fixed CPAP, Outcome 1 Machine usage (hours/night).
6.2. Analysis.
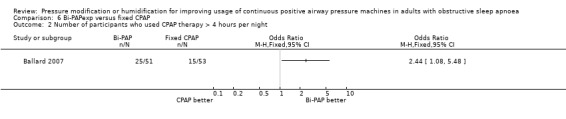
Comparison 6 Bi‐PAPexp versus fixed CPAP, Outcome 2 Number of participants who used CPAP therapy > 4 hours per night.
Automatically adjusting (auto bi‐PAP) versus fixed CPAP
Powell 2012 and Blau 2012 compared auto bi‐PAP with fixed CPAP in 83 participants with poor initial experience of CPAP. There was insufficient evidence to determine the effects on machine usage (MD 0.00 hours/night, 95% CI ‐0.70 to 0.70; Analysis 7.1), number of participants who used machines for more than four hours (Analysis 7.2), symptoms (Analysis 7.3), withdrawals (Analysis 7.4), quality of life (Analysis 7.5), and AHI (Analysis 7.6).
7.1. Analysis.
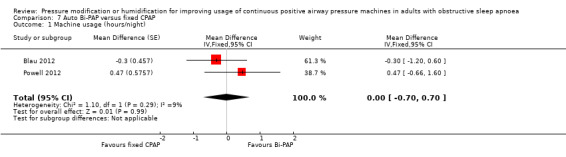
Comparison 7 Auto Bi‐PAP versus fixed CPAP, Outcome 1 Machine usage (hours/night).
7.2. Analysis.
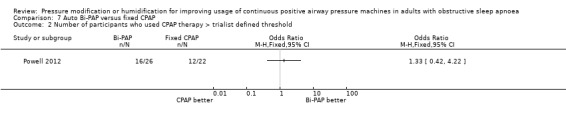
Comparison 7 Auto Bi‐PAP versus fixed CPAP, Outcome 2 Number of participants who used CPAP therapy > trialist defined threshold.
7.3. Analysis.
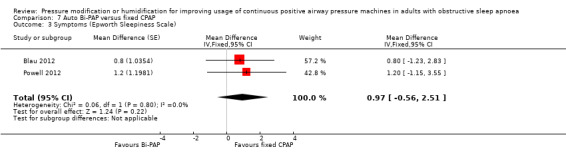
Comparison 7 Auto Bi‐PAP versus fixed CPAP, Outcome 3 Symptoms (Epworth Sleepiness Scale).
7.4. Analysis.
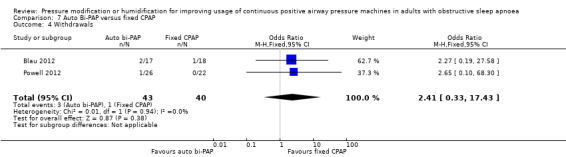
Comparison 7 Auto Bi‐PAP versus fixed CPAP, Outcome 4 Withdrawals.
7.5. Analysis.
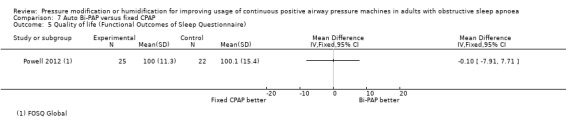
Comparison 7 Auto Bi‐PAP versus fixed CPAP, Outcome 5 Quality of life (Functional Outcomes of Sleep Questionnaire).
7.6. Analysis.
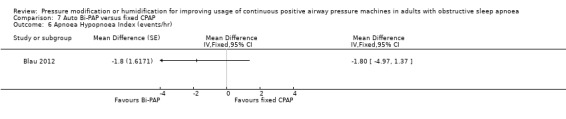
Comparison 7 Auto Bi‐PAP versus fixed CPAP, Outcome 6 Apnoea Hypopnoea Index (events/hr).
CPAP with expiratory pressure relief (CPAPexp) with wakefulness detection versus fixed CPAP
There was insufficient evidence from one study of CPAPexp designed to alter pressure as it detects wakefulness to determine the effects on machine usage, AHI, symptoms, quality of life and mask leak (Bogan 2017).
Discussion
Summary of main results
This updated systematic review includes 64 studies assessing the effect of pressure modification and humidification on clinical outcomes in adults with obstructive sleep apnoea (OSA). The addition of six studies to our main comparison has strengthened the conclusions from the previous version of this review regarding the comparative effects of automatically adjusting continuous positive airway pressure (auto‐CPAP) and CPAP with fixed pressure setting (fixed CPAP).
Auto‐CPAP probably increases machine usage by about 13 minutes per person per night when compared to fixed CPAP over three months (moderate‐certainty evidence). It probably reduces symptoms by a small amount (less than 0.5 points on the Epworth Sleepiness Scale (ESS)) over fixed CPAP (moderate‐certainty evidence). Fixed CPAP was slightly more effective at reducing the Apnoea Hypopnoea Index (AHI) (high‐certainty evidence) and it may lower diastolic blood pressure by 3 mmHg compared with auto‐CPAP (low‐certainty evidence). Variation in the approach to measuring adverse events from the devices meant that we could not determine whether the lower nightly average delivered treatment pressure of between 1 cmH2O and 3 cmH2O with auto‐CPAP had any associated impact on participant‐rated tolerability outcomes.
There is low‐certainty evidence that humidification increases average nightly machine usage, but more studies are needed to confirm this effect and to evaluate clinical outcomes, including symptoms and quality of life. The remaining studies reflect small, developing evidence bases for novel modes of pressure delivery, such as CPAP with expiratory pressure relief (CPAPexp) and automatically adjusting bilevel positive airway pressure (auto bi‐PAP).
Overall completeness and applicability of evidence
The populations recruited to the studies were predominantly male with a recent diagnosis of OSA and little previous exposure to CPAP. At baseline, the study populations had high body mass index (BMI) and AHI scores, and symptom scores indicated that they had excessive daytime sleepiness. Most of the studies to date have compared auto‐CPAP with fixed CPAP and measured machine usage, symptoms and AHI, with most studies completing measurement of outcomes at short term (i.e. 12 weeks or less). There is less information on quality of life, reflecting the relatively recent development and use of validated specific quality of life scales in OSA trials. The fragmentary nature of the evidence for tolerability outcomes, across all of the comparisons in the review, reflects different approaches to capturing information on these outcomes by the study investigators.
Auto‐CPAP versus fixed CPAP
The lack of associated clinically meaningful differences in symptoms, quality of life and AHI suggests that the average increase in machine usage of 13 minutes per night with auto‐CPAP is too small to be clinically meaningful. Our results indicate that both machine types reduce symptoms, but that the difference between them is unlikely to be clinically important. At the end of the treatment period ESS fell in both groups to between 4 and 8 from baseline values of about 13 (Table 1). The end of treatment difference of 0.5 units is smaller than the minimal clinically important difference of between 2 and 3 (Patel 2018). There is evidence of a ceiling effect in symptoms once CPAP usage exceeds four hours per night (Weaver 2007). Many participants in both groups used machines for more than four hours per night, reducing the scope for any meaningful differences in symptoms (Appendix 3). It should also be acknowledged that average study duration was between 12 and 16 weeks; therefore one should be wary of using these short‐term results to predict long‐term outcomes.
Few studies have used OSA‐specific quality of life instruments. The mean differences in Functional Outcomes of Sleep Questionnaire (FOSQ) and Sleep Apnoea Quality of Life Index (SAQLI) scores between auto‐CPAP and fixed CPAP treatment arms did not exceed minimally important differences that have been reported for these questionnaires, i.e. 1 unit (Billings 2014). From generic Short‐Form 36 (SF‐36) questionnaire scores, auto‐CPAP participants reported a bodily pain score that was 4.2 units higher, a general health score that was 2.5 units higher and a role physical score that was 3.7 units lower than fixed CPAP participants. Whilst such differences have been reported to be clinically meaningful in other conditions (Swigris 2010; Ward 2014), it is unclear whether these results would translate into meaningful differences in OSA participants. The confidence intervals (CIs) around these effects are wide, and there are no studies specifying the minimally important difference in the SF‐36 questionnaire for adults with OSA.
Inconsistent approaches to measuring tolerability across the studies made it difficult to improve our understanding of harms from both device types. Although 11 studies reported on four main tolerability outcomes (nasal blockage, dry mouth, intolerance of treatment pressure and mask leak), combining data in a single analysis for any one outcome was not possible for our main comparison. Some studies measured tolerability outcomes dichotomously (Sériès 1997; Bloch 2018); others used a visual analogue scale (VAS) to enable participants to assess the impact of various adverse events (d'Ortho 2000; Massie 2003; Nussbaumer 2006). A standardised approach to measuring CPAP tolerability has yet to become universally adopted (Boström 2010b). Building on existing efforts to arrive at an agreed approach to the definition and measurement of tolerability outcomes in CPAP research would help to improve our understanding of the relationship between tolerability, continued machine usage and symptomatic changes.
At the end of the study period, participants on fixed CPAP had a diastolic blood pressure that was almost 3 mmHg lower than participants on auto‐CPAP. It is certainly biologically plausible that fixed CPAP is more effective than auto‐CPAP in lowering diastolic blood pressure. Diastolic hypertension is thought to arise from peripheral vasoconstriction, which itself is a consequence of sympathetic activation (Pépin 2014). Auto‐CPAP has been associated with more sympathetic activation than fixed CPAP (Patruno 2014), which may lead to relative diastolic hypertension in people who are using auto‐CPAP. Furthermore, we observed a slightly higher AHI in auto‐CPAP participants, which could be associated with increased sympathetic activation.
Given the high rates of compliance, the reduction in diastolic blood pressure achieved by fixed CPAP in relation to auto‐CPAP may have implications for cardiovascular outcomes. Cook 1995 reported that a 2 mmHg drop in diastolic blood pressure could reduce risk of coronary heart disease by 6% and risk of stroke and transient ischaemic attack by 15%. Similarly, Law 2009 reported that a drop of 5 mmHg in diastolic blood pressure would reduce risk of coronary disease by 25% and risk of stroke by 36%.
On balance, we remain cautious about the effect on diastolic blood pressure. Diastolic blood pressure was one of 11 parameters of blood pressure that were analysed in this review; thus we cannot exclude multiplicity as a cause of this finding. Other parameters of blood pressure from larger sample sizes did not show similar effects (clinic systolic or 24‐hour, diurnal or nocturnal systolic, diastolic or mean blood pressure). Baguet 2013 argue that 24‐hour ambulatory blood pressure monitoring rather than office‐measured blood pressure is necessary to detect masked hypertension (i.e. normal office BP but elevated ambulatory measurements) which is present in 30% of adults with OSA and is a known risk factor for cardiovascular disease (Bobrie 2008; Angeli 2010). Our confidence in this effect was low due to very serious inconsistency; Patruno 2007 reported that fixed CPAP participants had a diastolic blood pressure that was almost 7 mmHg lower than auto‐CPAP participants; Pépin 2016 reported this difference to be less than 2 mmHg. Furthermore Pépin 2016 reported that the mean change in diastolic blood pressure from baseline did not differ between fixed CPAP and auto‐CPAP. Finally, in the nine ambulatory measurements of blood pressure made by Pépin 2016 and Bloch 2018, there were no differences between fixed CPAP and auto‐CPAP in any blood pressure parameter, either at the end of the study period or in the mean change from baseline.
There is weak consensus among investigators as to the optimal measurement of blood pressure in adults with OSA. Future research should identify which parameter of blood pressure best correlates with cardiovascular morbidity and mortality in adults with OSA. The effect on diastolic blood pressure requires further clarification given the risks associated with hypertension in OSA and recent evidence suggesting that auto‐CPAP and fixed CPAP produce different biological effects (Marrone 2018).
Effects in previous users of CPAP
People who have previously used CPAP prior to study entry are under‐represented in studies included in our review. Although we have not been able to carry out formal subgroup analysis, the results of the studies that have recruited from this population do not provide evidence of substantially different results in terms of both usage or functional outcomes. Rostig 2003 showed that auto‐CPAP improved compliance by about 30 minutes in a cross‐over trial of 30 participants who were on long‐term CPAP but whose hours of use were less than four hours per night. Ballard 2007 showed that Bi‐Flex improves machine usage by 36 minutes compared to fixed CPAP in participants with CPAP compliance of less than four hours per night, despite undergoing a CPAP optimisation intervention; however, there were no differences in FOSQ scores between the two groups. Gulati 2015 found that both Bi‐PAP or an alternative fixed CPAP device improved compliance by over two hours in participants with symptoms of pressure intolerance and fixed CPAP use of less than four hours per night; however, there were no significant differences between Bi‐PAP and alternative CPAP in terms of hours of use, ESS, Oxford Sleep Resistance (OSLER) sleep latency or SAQLI score. Powell 2012 showed that auto bi‐PAP did not improve CPAP compliance, ESS or FOSQ score in participants with a suboptimal CPAP titration.
Certainty of the evidence
The certainty of evidence varied across the different outcomes for the four comparisons we assessed. Our downgrading decisions were based on concerns about risk of bias, imprecision or inconsistency, although their impact varied across the different outcomes.
In the comparison of auto‐CPAP and fixed CPAP we downgraded the certainty of evidence for average machine usage to moderate due to risk of bias, and machine usage for four hours or more per night to low for risk of bias and imprecision (Table 1). For the remaining comparisons, we downgraded average machine usage to low for risk of bias and imprecision. We rated effects on symptoms as moderate certainty for auto‐CPAP and CPAPexp due to risk of bias (Table 1; Table 3), and as low for Bi‐PAP and humidification due to risk of bias and imprecision (Table 2; Table 4).
Across all the comparisons, the certainty of evidence for quality of life outcomes is low and this reflects the relatively recent use of validated scales in sleep apnoea trials. The very low‐certainty evidence for tolerability outcomes for all the comparisons is due to variation in how this outcome has been measured across the studies. We rated the certainty of evidence for AHI as high for the comparisons of auto‐CPAP and CPAPexp with fixed CPAP (Table 1; Table 3). We would expect the impact of long‐term attrition to be smaller on these outcomes, as the results are consistent with immediate changes to sleep disturbance which arise from using positive airway pressure, irrespective of the device. In the comparison of auto‐CPAP and fixed CPAP, we regard attrition as a less important source of uncertainty on the size of effect on diastolic blood pressure than variation in effect size between the studies (Table 1).
Potential biases in the review process
Before updating this review we made a number of changes to the methods that we had previously implemented (see Differences between protocol and review). We decided to combine data from parallel and cross‐over studies for the 2019 update of the review. We acknowledge that this decision could introduce methodological variation by combining data from short‐term within‐participant studies with parallel group studies that have a longer‐term follow‐up where between‐participant differences could attenuate over time. We used adjusted data, where possible, to account for the within‐person design of cross‐over studies. However, for a number of analyses of 'Summary of findings' table outcomes, we used unadjusted data (Analysis 1.1; Analysis 1.7; Analysis 1.12; Analysis 1.14; Analysis 3.1; Analysis 3.2; Analysis 3.6; Analysis 4.1; Analysis 4.4). It is possible that individual study weights are artificially low without a reasonable estimate of the within‐person correlation. The impact of this approach on the summary effect estimates is small, since only 10% to 15% of total sample size are affected across the analyses, and the direction and size of effect of the studies affected are aligned with the summary effect sizes. The consistent direction and size of effect for usage, symptom and AHI outcomes provides some support to our approach in combining the study designs and adjustment of the cross‐over studies.
Our decision to use a threshold of four hours as a measure of adequate machine usage could be contested. We chose this threshold as research indicates that resolution of daytime sleepiness requires a minimum of four hours of CPAP use per night (Weaver 2007). The optimal 'dose' of CPAP selected will depend on whether the intention of treatment is to modify blood pressure, subjective or objective sleepiness, functional status, as well as what individuals require (Masa 2014; Bakker 2019). Whilst it is likely that optimal dosing does indeed vary between individuals and by therapeutic aim, we have retained the view that a consistently applied threshold across a population that reflects symptomatic change reflects an important goal of treatment for adults with OSA. In the absence of more refined ways of identifying changes in individuals, we think that continuous and dichotomous measurements of machine usage provide complementary insights to inform treatment decisions.
Agreements and disagreements with other studies or reviews
Two other meta‐analyses have compared auto and fixed CPAP (Ip 2012; Xu 2012), and a third network meta‐analysis compared auto‐CPAP, fixed CPAP and oral appliances (Liu 2017). Ip 2012 and Xu 2012 report very similar effect sizes to our own, with auto‐CPAP resulting in an additional 11 minutes and 14 minutes of nightly usage, respectively. Ip 2012 also found a small decrease in ESS with auto‐CPAP and Xu 2012 reported statistically significant decreases in treatment pressure with auto‐CPAP.
Ip 2012 estimated that fixed CPAP lowered diastolic blood pressure by 8 mmHg (95% CI 4 to 11 mmHg, P < 0.001) more than auto‐CPAP. This 8 mmHg difference is markedly greater than that observed in our study (3 mmHg); the more modest effect in our study is likely due to the inclusion of Pépin 2016 which showed a more attenuated effect of fixed CPAP on diastolic blood pressure. Similar to our analysis, Ip 2012 had insufficient confidence in this effect. Liu 2017 reported no difference in the effects of fixed CPAP and auto‐CPAP on 24‐hour diastolic, 24‐hour systolic, diurnal systolic, diurnal diastolic, nocturnal systolic or nocturnal diastolic blood pressure in direct pair‐wise comparisons; office diastolic blood pressure was not included in this analysis. Interestingly, Liu 2017 ranked fixed CPAP above auto‐CPAP in its likelihood to reduce diurnal systolic (67.44% versus 45.21%) diurnal diastolic (58.75% versus 29.37%), nocturnal systolic (63.1% versus 45.93%) and nocturnal diastolic blood pressure (62.76% versus 32.57%), although no assessment of the certainty of this evidence was provided.
One meta‐analysis has been published comparing flexible titration modalities of CPAP with fixed CPAP (Bakker 2011). Despite some methodological differences relating to the eligibility of short‐term studies and separation of parallel and cross‐over studies, the effect sizes for machine usage and AHI are similar to our own.
Authors' conclusions
Implications for practice.
In adults with moderate to severe sleep apnoea who are starting positive airway pressure therapy, average machine usage is probably increased by about 13 minutes per night with automatically adjusting continuous positive airway pressure (auto‐CPAP) compared with fixed CPAP (moderate‐certainty evidence), although we do not have enough evidence to determine whether either device leads to more people using machines for four hours or more (low certainty). The reduction in daytime sleepiness associated with the average increased use of auto‐CPAP is small (moderate‐certainty evidence), and fixed CPAP is slightly more effective at reducing the Apnoea Hypopnoea Index (AHI) (high‐certainty evidence). Most of the studies to date have followed participants for shorter than 12 weeks. It is unclear whether modifying pressure in participants with mild and less pronounced sleep apnoea symptoms is beneficial. About 25% of people recruited to the studies are female. Generally, research to date has not addressed what interventions are effective in people who find CPAP difficult to tolerate.
Use of specific quality of life instruments in the studies to date has been limited, although where they have been used the effect sizes have not exceeded clinically important differences. Evidence for blood pressure outcomes is insufficient to determine the anti‐hypertensive effects between the different machine types. Tolerabillty outcomes were measured too inconsistently to further our understanding of how varying treatment pressure with auto‐CPAP changes the experience of applying positive airway pressure.
The evidence base for the remaining interventions included in the review is smaller, with a similar focus on machine usage and symptoms over quality of life. The effect on machine usage with expiratory pressure CPAP (CPAPexp) is uncertain and there is little or no difference in symptoms when compared with fixed CPAP. Although there is some evidence of higher machine usage following the addition of humidification, the certainty of evidence is low, and the effects on symptoms and tolerability are uncertain. Effects of humidification may also be affected by local ambient humidity.
Implications for research.
Study investigators and research funders initiating further research in this area should address the following issues related to the design, analysis and reporting of studies.
To date, only a small proportion of eligible trials have recruited people who have been unable to persist with CPAP. Further trials in this population are needed to inform decisions between alternative management strategies where acceptability of CPAP is lower than in the populations recruited to studies in this review.
Assessment of the effects of different pressure machines in studies that recruit a greater proportion of women and participants with lower AHIs than those in the existing studies would broaden the applicability of the evidence base.
Adoption of a standardised approach to defining machine tolerability is needed. Variation in the definition and measurement of machine tolerability has made it challenging to evaluate the harms from using different pressure modes, and to determine their relationship with machine usage. Agreement on consistent approaches to measuring mask leak, pressure tolerance, and oral and nasal symptoms would help decision‐makers to balance benefits with harms from different treatment options available. Consensus on which blood pressure parameters to measure is needed in order to better understand the relationship between blood pressure and long‐term cardiovascular outcomes.
We are aware of one large completed study (N = 800) comparing auto‐CPAP and fixed CPAP, which we expect to incorporate in future versions of this review when its results are available (NCT02749812). Two smaller ongoing studies are addressing similar outcomes to those already addressed (ACTRN12618000379213p; NCT03428516). Future studies should include longer‐term follow‐up and measure quality of life.
We have identified one ongoing study of CPAPexp (NCT01753999), and large multicentre studies in bilevel positive airway pressure (bi‐PAP), CPAPexp, humidification and automatically adjusting CPAP with expiratory pressure relief (auto‐CPAPexp) are warranted.
Studies should better report design features (e.g. blinding and allocation concealment) and be more explicit about handling data from participants who do not continue to use machines during the studies.
Given the range of treatment options that continue to emerge, head to head comparisons between different ways of modifying pressure would help to inform choice of treatment where alternatives to fixed CPAP are being considered. Such studies could also serve as a basis for a network meta‐analysis of pressure modification strategies, including a cost‐effectiveness assessment to inform local decision making.
What's new
| Date | Event | Description |
|---|---|---|
| 15 October 2018 | New search has been performed | Search run and 21 studies included. 2 studies previously included are now excluded. 4 studies are ongoing and one completed study is awaiting classification pending fuller details of methods and results. |
| 15 October 2018 | New citation required and conclusions have changed | Additional evidence has increased certainty in previous findings. New data on blood pressure have been included. We updated the methods to reflect current Cochrane standards e.g. adding a 'Summary of findings' table and PRISMA diagram. |
History
Protocol first published: Issue 3, 2001 Review first published: Issue 4, 2004
| Date | Event | Description |
|---|---|---|
| 10 September 2008 | New citation required and conclusions have changed | Review split; 28 trials added to review; new data available for primary outcomes. The main findings of the original review have been strengthened with the new data. A consistent difference in favour of auto‐titrating CPAP machines has been identified from cross‐over studies, although the estimate from parallel group trials includes no difference. Alternative pressure modification methods such as C‐Flex, bi‐level pressure and additional humidification require more research. |
| 4 September 2008 | New search has been performed | Literature search re‐run |
| 9 April 2008 | Amended | Converted to new review format |
| 29 December 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The Background and Methods sections of this review are based on a standard template used by Cochrane Airways.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS), or the Department of Health.
We are grateful to previous author Muzlifah Hanifa who initiated the protocol and was involved with study assessment, data collection and entry, and drafting of the discussion and conclusions. We are very grateful to the members of the Cochrane Airways Group editorial base who gave support with searching for studies, and to Prof John Wright for constructive comments on the protocol and the review on previous versions of the review. We are grateful for assistance given to us by study investigators who provided additional information about their studies: Konrad Bloch (Bloch 2018), Atul Gulati (Gulati 2015), Jessie Bakker (Bakker 2010), Naricha Chirakalwasan (Soudorn 2016), Evan Chang (Chang 2015), Timon Fabius (Rohling 2011), Domagoj Damjanovic (Damjanovic 2009), Maik Schroder (Nilius 2006), Silke Ryan (Ryan 2009), Nathalie Arnol and Jean‐Louis Pepin (Pépin 2009; Pépin 2016), Christopher Worsnop (Worsnop 2010), Alexander Blau (Blau 2012), Leon Rosenthal (Dolan 2008), Marie‐Pia d'Ortho (d'Ortho 2000), Nat Marshall (Marshall 2008), Adrian Kendrick (Kendrick 2002), Clifford Massie (Massie 1999; Massie 2003), Nancy Gordon (Massie 1999; Massie 2003), Sophie West and John Stradling (West 2006), Geraldine Lawless (Nolan 2007), Doug McEvoy (Morton 2001), Winfried Randerath (Randerath 2001), Frederic Sériès (Meurice 1996; Sériès 1997; Sériès 2001; Meurice 1998), Peter Cistulli (Jarvis 2006), David Hui (To 2008) and Jean‐Claude Meurice (NCT02749812).
The authors and CRG Editorial Team are grateful to the following peer and consumer reviewers for their time and comments.
Hayley Barnes, The Alfred Hospital, Melbourne.
Nathaniel Marshall, University of Sydney and the Woolcock Institute for Medical Research, Sydney.
PR Srijithesh, National Institute of Mental Health and Neurosciences, Bangalore, Karnataka, India.
Jose‐Ramon Rueda, University of the Basque Country, Lejona, Spain.
Ndi Euphrasia Ebai‐Atuh, Consumer Reviewer, Cameroon.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Trials Register
Electronic searches: core databases
| Database | Dates searched | Frequency of search |
| CENTRAL (via the Cochrane Register of Studies (CRS)) | From inception | Monthly |
| MEDLINE (Ovid) | 1946 onwards | Weekly |
| EMBASE (Ovid) | 1974 onwards | Weekly |
| PsycINFO (Ovid) | 1967 onwards | Monthly |
| CINAHL (EBSCO) | 1937 onwards | Monthly |
| AMED (EBSCO) | From inception | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the Cochrane Airways Trials Register
Sleep apnoea search
1. exp Sleep Apnea Syndromes/
2. (sleep$ adj3 (apnoea$ or apnoea$)).mp.
3. (hypopnea$ or hypopnoea$).mp.
4. OSA.mp.
5. SHS.mp.
6. OSAHS.mp.
7. or/1‐6
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomized or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases
Appendix 2. Search strategy to identify trials from the Cochrane Airways Trials Register
Database platform: Cochrane Register of Studies
Dates covered: September 2008 to October 2018
#1 SLP:MISC2 #2 MeSH DESCRIPTOR Sleep Apnea, Obstructive #3 sleep near3 (apnoea* or apnoea*) #4 (hypopnea* or hypopnoea*) #5 (OSA OR SHS OR OSAHS:TI,AB) #6 (#1 OR #2 OR #3 OR #4 OR #5) #7 humidif* #8 CPAP #9 autopap #10 Auto* NEXT CPAP #11 APAP #12 NCPAP #13 PPR #14 C* NEXT Flex #15 positive* NEAR3 pressure* #16 "expiratory pressure" #17 PEEP #18 IPB #19 IPPB #20 (continuous* OR nasal* OR inspiratory*) AND "positive airway" #21 #7 or #8 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 #22 (#6 and #21)
[Note: in search line #1, MISC1 refers to the field in the record where the reference has been coded for condition, in this case, sleep apnoea)
Search strategy used for September 2008 version
Dates covered: all years to September 2008
All records in the Specialised Register coded as 'sleep apnoea' were searched using the following terms:
(Humidif* OR CPAP OR Auto‐CPAP OR APAP OR NCPAP OR PPR OR C‐Flex OR"positive pressure" OR positive‐pressure OR "expiratory pressure" OR PEEP OR IPB OR IPPB) OR (continuous OR nasal OR inspiratory AND ("positive airway*"))
Appendix 3. Average machine usage data
| Study | Screened | Entered | Completed | % Screened | % Entered | Mean hours used (control group) |
| Bakker 2010 | 751 | 80 | 76 | 10.1 | 95 | 5.2 |
| Ballard 2007 | 204 | 104 | 104 | 51 | 100 | 2.9 |
| Berry 2014 | 240 | 156 | 131 | 54.5 | 84 | 4 |
| Blau 2012 | Not reported | 35 | 32 | N/A | 91 | 5.6 |
| Bloch 2018 | 952 | 208 | 172 | 18 | 82 | 5.5 |
| Bogan 2017 | Not reported | 70 | 65 | N/A | N/A | 4.81 |
| Castronovo 2006 | Not reported | 50 | 40 | N/A | 80 | Not reported |
| Chang 2015 | Not reported | 25 | 19 | N/A | 76 | 5.8 |
| d'Ortho 2000 | Not reported | 25 | 25 | N/A | 100 | 4.7 |
| Damjanovic 2009 | Not reported | 100 | 78 | N/A | 78 | 5.1 |
| Dolan 2008 | 222 | 184 | 142 | 64 | 76 | 5.3 |
| Ficker 2003 | Not reported | 100 | 95 | N/A | 95 | 4.8 |
| Fietze 2007 | Not reported | 21 | 21 | N/A | 100 | 4.2 |
| Galetke 2008 | Not reported | 20 | 20 | NA | 100 | 6.4 |
| Gay 2003 | Not reported | 40 | 27 | N/A | 67.5 | 5.6 |
| Gfüllner 2007 | Not reported | Not reported | 18 | N/A | N/A | 5.3 |
| Gonzalez‐Moro 2005 | Not reported | 20 | Not reported | N/A | N/A | Not reported |
| Gulati 2015 | 75 | 31 | 28 | 37.3 | 90.3 | 2.2 |
| Heiser 2010 | Not reported | 75 | 49 | N/A | 65.3 | 4.3 |
| Hudgel 2000 | Not reported | 53 | 39 | N/A | 73.6 | 5.5 |
| Hukins 2004 | 58 | 55 | 46 | 79 | 84 | 4.86 |
| Hussain 2004 | Not reported | 10 (assumed) | 10 | N/A | 100 | 3.7 |
| Jarvis 2006 | Not reported | Not reported | 20 | N/A | N/A | 6.8 |
| Kendrick 2002 | Not reported | 41 | 27 | N/A | 65.85 | Not reported |
| Konermann 1998 | Not reported | 50 | 48 | N/A | 96 | 5.6 |
| Kushida 2011 | 178 | 168 | 140 | 78.7 | 83.3 | 4.6 |
| Leidag 2008 | Not reported | 30 | 18 | N/A | 60 | 5.8 |
| Loube 2004 | Not reported | Not reported | Not reported | N/A | N/A | Not reported |
| Marrone 2004 | Not reported | 22 (assumed) | 22 | N/A | 100 | 4.4 |
| Marshall 2008 | Not reported | Not reported | 19 | N/A | NA | 3 |
| Masa 2015 | 351 | 221 (numbers reported here reflect participants screened for three treatment arms) | 200 | 57% | 90% | 5.3 |
| Massie 2003 | Not reported | 46 | 44 | N/A | 95.6 | 4.5 |
| Meurice 1996 | Not reported | 16 | 16 | N/A | 100 | 5.7 |
| Meurice 2007 | Not reported | 83 | 65 | N/A | 78 | 6.5 |
| Meurice 2009 | Not reported | 20 | 20 | N/A | 100 | 5.3 |
| Modrak 2007 | Not reported | Not reported | 26 | N/A | N/A | 5.1 |
| Muir 1998 | Not reported | Not reported | 16 | N/A | N/A | 4.9 |
| Neill 2003 | Not reported | 42 | 37 | N/A | 88 | 5.3 |
| Nilius 2006 | Not reported | 52 | 51 | N/A | 98 | 5.2 |
| Nolan 2007 | Not reported | 34 | 29 | N/A | 85 | 4.9 |
| Noseda 2004 | 93 | 27 | 24 | 26 | 89 | 5.3 (average usage on 'effective nights') |
| Nussbaumer 2006 | Not reported | 34 | 30 | N/A | 88 | 4.8 |
| Patruno 2007 | Not reported | 40 | 31 | N/A | 78 | 6.1 |
| Pépin 2009 | Not reported | 20 | 20 | N/A | 100 | 5.3 |
| Pépin 2016 | 569 | 322 | 281 | 49.4 | 87.3 | 5.2 |
| Powell 2012 | 51 | 48 | 47 | 92.2 | 97.9 | 4.4 |
| Randerath 2001 | Not reported | 52 | 47 | N/A | 90 | 5.3 |
| Reeves‐Hoché 1995 | Not reported | 83 | 62 | N/A | 75 | 5 |
| Resta 2004 | Not reported | 20 | 20 | N/A | 100 | 5.3 |
| Rochford 2006 | Not reported | N/A | 13 | N/A | N/A | 4.6 |
| Rohling 2011 | Not reported | 39 | 33 | N/A | 84.6 | 6.6 |
| Rostig 2003 | Not reported | 30 | 30 (assumed) | N/A | 100 (assumed) | 3.8 |
| Ruhle 2011 | Not reported | 44 | 44 | N/A | 100 | 4.5 |
| Ryan 2009 | 125 | 125 | 112 | 91 | 91 | 5.21 |
| Senn 2003 | Not reported | 31 | 29 | N/A | 93.55 | 7.1 |
| Sériès 1997 | 39 | 36 | 36 | 92.3 | 100 | Not reported |
| Sériès 2001 | Not reported | 48 | 48 (assumed) | N/A | 100 (assumed) | 4.2 |
| Soudorn 2016 | 104 | 20 | 20 | 19.2 | 19.2 | 5.2 |
| Teschler 2000 | Not reported | 10 | 10 | N/A | 100 | 6.1 |
| To 2008 | Not reported | 43 | 41 | N/A | 95 | 3.8 |
| Vennelle 2010 | 368 | 200 | 181 | 49.1 | 90.5 | 4 |
| Wenzel 2007 | Not reported | 20 (assumed) | 20 | NA | 100 (assumed) | 5.8 |
| West 2006 | 633 | 98 | 86 | 16 | 88 | 5.6 |
| Worsnop 2010 | Not reported | 54 | 54 (assumed) | N/A | 100 (assumed) | 4.5 |
| Abbreviations: N/A: not applicable | ||||||
Data and analyses
Comparison 1. Auto‐CPAP versus fixed CPAP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Machine usage (hours/night) | 31 | 1452 | Mean Difference (Fixed, 95% CI) | 0.21 [0.11, 0.31] |
| 2 Machine usage (hours/night) (Pepin imputed) | 32 | 1774 | Mean Difference (Fixed, 95% CI) | 0.19 [0.10, 0.29] |
| 3 Number of participants who used CPAP therapy > 4 hours per night | 2 | 346 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.75, 1.81] |
| 4 Machine usage (on nights when CPAP used 'effectively') | 3 | Mean Difference (Fixed, 95% CI) | 0.42 [0.05, 0.78] | |
| 5 Machine usage (frequency of usage as % of days) | 9 | Mean Difference (Fixed, 95% CI) | 1.60 [‐0.83, 4.03] | |
| 6 Machine usage (% of nights of > 4 hours of use) ‐ cross‐over studies | 2 | Mean Difference (Fixed, 95% CI) | 6.25 [‐0.05, 12.54] | |
| 7 Symptoms (Epworth Sleepiness Scale) | 25 | 1285 | Mean Difference (Fixed, 95% CI) | ‐0.44 [‐0.72, ‐0.16] |
| 8 Withdrawals (parallel group trials/first arm cross‐over trials) | 13 | 1275 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.64, 1.27] |
| 9 Quality of life (Functional Outcomes of Sleep Questionnaire) | 3 | 352 | Mean Difference (Fixed, 95% CI) | 0.12 [‐0.21, 0.46] |
| 10 Quality of life (Sleep Apnoea Quality of Life Index) | 2 | 97 | Mean Difference (Fixed, 95% CI) | ‐0.14 [‐0.54, 0.27] |
| 11 Quality of life (SF‐36 questionnaire) | 8 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 11.1 Physical functioning | 3 | Mean Difference (Fixed, 95% CI) | 0.76 [‐3.50, 5.01] | |
| 11.2 Role physical | 2 | Mean Difference (Fixed, 95% CI) | ‐3.73 [‐13.46, 6.01] | |
| 11.3 Bodily pain | 2 | Mean Difference (Fixed, 95% CI) | 4.21 [‐4.23, 12.64] | |
| 11.4 General health | 2 | Mean Difference (Fixed, 95% CI) | 2.49 [‐4.99, 9.97] | |
| 11.5 Vitality | 6 | Mean Difference (Fixed, 95% CI) | 1.32 [‐1.25, 3.88] | |
| 11.6 Social functioning | 2 | Mean Difference (Fixed, 95% CI) | 3.31 [‐4.29, 10.92] | |
| 11.7 Role emotional | 3 | Mean Difference (Fixed, 95% CI) | 0.70 [‐4.19, 5.59] | |
| 11.8 Mental health | 3 | Mean Difference (Fixed, 95% CI) | 0.20 [‐1.88, 2.27] | |
| 12 Apnoea Hypopnoea Index (events/hr) | 26 | 1256 | Mean Difference (Fixed, 95% CI) | 0.48 [0.16, 0.80] |
| 13 Arousals (events/hr) | 4 | 136 | Mean Difference (Fixed, 95% CI) | ‐0.66 [‐2.90, 1.58] |
| 14 Pressure of CPAP treatment (cmH2O) | 24 | 1171 | Mean Difference (Fixed, 95% CI) | ‐1.01 [‐1.17, ‐0.84] |
| 15 Systolic blood pressure | 2 | 353 | Mean Difference (IV, Fixed, 95% CI) | 1.87 [‐1.08, 4.82] |
| 16 Diastolic blood pressure | 2 | 353 | Mean Difference (IV, Fixed, 95% CI) | 2.92 [1.06, 4.77] |
| 17 24‐hour mean BP | 2 | 530 | Mean Difference (IV, Fixed, 95% CI) | 0.59 [‐1.05, 2.22] |
| 18 24‐hour systolic BP | 2 | 530 | Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐2.21, 1.91] |
| 19 24‐hour diastolic BP | 2 | 530 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.65, 2.44] |
| 20 Diurnal mean BP | 2 | 530 | Mean Difference (IV, Fixed, 95% CI) | 0.63 [‐1.05, 2.32] |
| 21 Diurnal systolic BP | 2 | 530 | Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐2.44, 1.74] |
| 22 Diurnal diastolic BP | 2 | 530 | Mean Difference (IV, Fixed, 95% CI) | 0.91 [‐0.74, 2.55] |
| 23 Nocturnal mean BP | 2 | 530 | Mean Difference (IV, Fixed, 95% CI) | 0.43 [‐1.29, 2.15] |
| 24 Nocturnal systolic BP | 2 | 530 | Mean Difference (IV, Fixed, 95% CI) | 0.88 [‐1.81, 3.57] |
| 25 Nocturnal diastolic BP | 2 | 530 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐0.81, 2.40] |
| 26 Tolerability outcomes | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 26.1 Intolerable treatment pressure | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 26.2 Mask leak | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 26.3 Dry mouth | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 26.4 Stuffy nose | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 27 Patient preference (auto‐CPAP/not auto‐CPAP) | 14 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected |
1.27. Analysis.
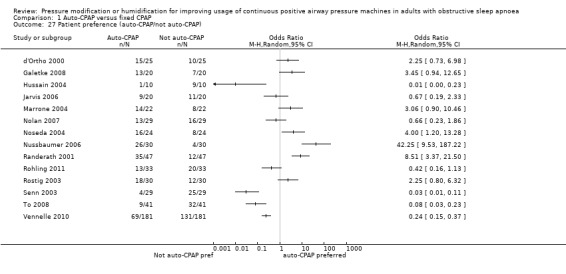
Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 27 Patient preference (auto‐CPAP/not auto‐CPAP).
Comparison 2. Bi‐PAP versus fixed CPAP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Machine usage (hours/night) | 4 | 268 | Mean Difference (Fixed, 95% CI) | 0.14 [‐0.17, 0.45] |
| 2 Symptoms (Epworth Sleepiness Scale) | 4 | 226 | Mean Difference (Fixed, 95% CI) | ‐0.49 [‐1.46, 0.48] |
| 3 Withdrawals (parallel group trials/first arm cross‐over trials) | 3 | 261 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.26, 1.17] |
| 4 Quality of life (Functional Outcomes of Sleep Questionnaire) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Quality of life (Sleep Apnoea Quality of Life Index) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 6 Quality of life (SF‐36 questionnaire) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Physical health | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Mental heath | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Apnoea Hypopnoea Index (events/hr) | 2 | 179 | Mean Difference (Fixed, 95% CI) | 1.36 [‐6.92, 9.63] |
| 8 Patient preference ‐ BiPAP/no preference or CPAP | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9 Tolerability outcomes | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.1 Dry mouth | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Mask intolerance | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Treatment comfort score | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected |
2.8. Analysis.
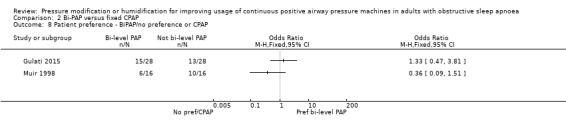
Comparison 2 Bi‐PAP versus fixed CPAP, Outcome 8 Patient preference ‐ BiPAP/no preference or CPAP.
2.10. Analysis.

Comparison 2 Bi‐PAP versus fixed CPAP, Outcome 10 Treatment comfort score.
Comparison 3. CPAPexp versus fixed CPAP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Machine usage (hours/night) | 9 | 609 | Mean Difference (Fixed, 95% CI) | 0.14 [‐0.07, 0.35] |
| 2 Symptoms (Epworth Sleepiness Scale) | 6 | 515 | Mean Difference (Fixed, 95% CI) | 0.17 [‐0.26, 0.60] |
| 3 Withdrawals (parallel group trials/first arm cross‐over trials) | 2 | 298 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.48, 1.55] |
| 4 Quality of life (Functional Outcomes of Sleep Questionnaire) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Quality of life (SF‐36 questionnaire) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Physical functioning | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | 6.20 [‐3.05, 15.45] |
| 5.2 Role physical score | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | ‐9.5 [‐25.38, 6.38] |
| 5.3 Bodily pain score | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | 7.20 [‐3.69, 18.09] |
| 5.4 General health score | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐8.05, 10.05] |
| 5.5 Vitality | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | ‐2.5 [‐11.38, 6.38] |
| 5.6 Social functioning score | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | ‐8.30 [‐17.87, 1.27] |
| 5.7 Role emotional score | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | ‐7.30 [‐21.83, 7.23] |
| 5.8 Mental health score | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐4.86, 8.86] |
| 6 Apnoea Hypopnoea Index (events/hr) | 5 | 342 | Mean Difference (Fixed, 95% CI) | 0.24 [‐0.49, 0.96] |
| 7 Pressure of CPAP treatment (cmH2O) | 2 | 241 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.63, 0.52] |
| 8 Treatment satisfaction score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Treatment comfort score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10 Treatment interface score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11 Preference | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
3.11. Analysis.
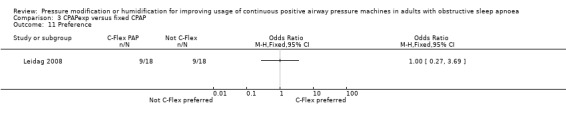
Comparison 3 CPAPexp versus fixed CPAP, Outcome 11 Preference.
Comparison 4. Heated humidification + fixed CPAP versus fixed CPAP alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Machine usage (hours/night) | 6 | 277 | Mean Difference (Fixed, 95% CI) | 0.37 [0.10, 0.64] |
| 2 Symptoms (Epworth Sleepiness Scale) | 4 | 184 | Mean Difference (Fixed, 95% CI) | ‐0.34 [‐0.93, 0.26] |
| 3 Withdrawals (parallel group trials/first arm cross‐over trials) | 3 | 209 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.45, 2.24] |
| 4 Apnoea Hypopnoea Index (events/hr) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 5 Quality of life (SF‐36 questionnaire) | 2 | 124 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐6.97, 7.18] |
| 6 Nasal symptoms (parallel group trials) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Runny nose | 1 | 73 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.09, 1.15] |
| 6.2 Congested or blocked nose | 1 | 73 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.07, 0.51] |
| 7 Nasal symptoms (parallel group trials) | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Dry nose | 2 | 103 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐0.78, 0.01] |
| 7.2 Runny nose | 2 | 103 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.69, 0.09] |
| 7.3 Blocked nose | 2 | 103 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐0.78, 0.01] |
| 7.4 Bleeding nose | 2 | 103 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.45 [‐0.99, 0.10] |
| 8 Preference | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
4.3. Analysis.
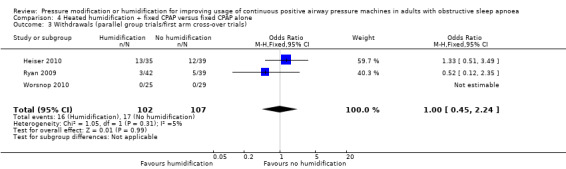
Comparison 4 Heated humidification + fixed CPAP versus fixed CPAP alone, Outcome 3 Withdrawals (parallel group trials/first arm cross‐over trials).
4.8. Analysis.
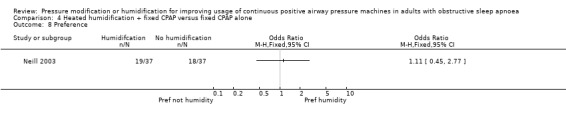
Comparison 4 Heated humidification + fixed CPAP versus fixed CPAP alone, Outcome 8 Preference.
Comparison 5. Auto‐CPAPexp versus fixed CPAP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Machine usage (hours/night) | 2 | 113 | Mean Difference (Fixed, 95% CI) | 0.03 [‐0.60, 0.67] |
| 2 Symptoms (Epworth Sleepiness Scale) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Withdrawals (parallel group trials/first arm cross‐over trials) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Quality of life (Functional Outcomes of Sleep Questionnaire) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Apnoea Hypopnoea Index (events/hr) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Pressure of CPAP treatment (cmH2O) | 2 | 113 | Mean Difference (Fixed, 95% CI) | ‐0.92 [‐1.77, ‐0.07] |
| 7 Systolic blood pressure | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Diastolic blood pressure | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
5.1. Analysis.
Comparison 5 Auto‐CPAPexp versus fixed CPAP, Outcome 1 Machine usage (hours/night).
Comparison 6. Bi‐PAPexp versus fixed CPAP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Machine usage (hours/night) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 2 Number of participants who used CPAP therapy > 4 hours per night | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Quality of life (Functional Outcomes of Sleep Questionnaire) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
6.3. Analysis.
Comparison 6 Bi‐PAPexp versus fixed CPAP, Outcome 3 Quality of life (Functional Outcomes of Sleep Questionnaire).
Comparison 7. Auto Bi‐PAP versus fixed CPAP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Machine usage (hours/night) | 2 | Mean Difference (Fixed, 95% CI) | ‐0.00 [‐0.70, 0.70] | |
| 2 Number of participants who used CPAP therapy > trialist defined threshold | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Symptoms (Epworth Sleepiness Scale) | 2 | Mean Difference (Fixed, 95% CI) | 0.97 [‐0.56, 2.51] | |
| 4 Withdrawals | 2 | 83 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.41 [0.33, 17.43] |
| 5 Quality of life (Functional Outcomes of Sleep Questionnaire) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Apnoea Hypopnoea Index (events/hr) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected |
Comparison 8. CPAPexp with wakefulness detection versus fixed CPAP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Machine usage (hours/night) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 2 Symptoms (Epworth Sleepiness Scale) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 3 Quality of life (Functional Outcomes of Sleep Questionnaire) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 4 Apnoea Hypopnoea Index (events/hr) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 5 Mask leak | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected |
8.1. Analysis.

Comparison 8 CPAPexp with wakefulness detection versus fixed CPAP, Outcome 1 Machine usage (hours/night).
8.2. Analysis.

Comparison 8 CPAPexp with wakefulness detection versus fixed CPAP, Outcome 2 Symptoms (Epworth Sleepiness Scale).
8.3. Analysis.

Comparison 8 CPAPexp with wakefulness detection versus fixed CPAP, Outcome 3 Quality of life (Functional Outcomes of Sleep Questionnaire).
8.4. Analysis.

Comparison 8 CPAPexp with wakefulness detection versus fixed CPAP, Outcome 4 Apnoea Hypopnoea Index (events/hr).
8.5. Analysis.
Comparison 8 CPAPexp with wakefulness detection versus fixed CPAP, Outcome 5 Mask leak.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bakker 2010.
| Methods | Randomised, double‐blind, parallel group study | |
| Participants | N = 76 participants (53 M/23 F). Age not reported. BMI 35.6 kg/m2; AHI 60.2; ESS 13.6 Inclusion criteria: CPAP naive Exclusion criteria: significant cardiac, respiratory, psychiatric, or sleep comorbidities |
|
| Interventions | CPAP versus C‐Flex (identical devices) Study duration: 3 months |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | 'This study was funded by Philips‐Respironics. All authors received research support from Philips‐Respironics. Philips‐Respironics manufactures and markets CPAP and C‐Flex devices.' | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization to treatment was performed prior to manual titration using a (1, 2) urn randomization procedure" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization to treatment was performed prior to manual titration using a (1, 2) urn randomization procedure" |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Low risk | Quote: "The study was a double‐blinded, parallel‐arm RCT of C‐Flex versus CPAP" Quote: "Patients were not able to access the C‐Flex menu option, and all references to "C‐Flex" on the device were covered" |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | Quote: "The study was a double‐blinded, parallel‐arm RCT of C‐Flex versus CPAP" Quote: "Patients were not able to access the C‐Flex menu option, and all references to "C‐Flex" on the device were covered" |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Low risk | Identical machine appearance unlikely to affect measurement of these outcomes |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | Quote: "The data analyst remained blinded by random 3‐digit codes being assigned to all patients" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Two patients were withdrawn".........2 patients dropped out of the CPAP group............they were replaced with additional recruitment, and therefore analysis was conducted on a per‐protocol basis" |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
Ballard 2007.
| Methods | Randomised, double‐blind, parallel group trial | |
| Participants | N = 104 participants (67 M/37 F); Age 52 years; BMI 33 kg/m2; AHI 42; ESS not reported Inclusion criteria: non‐adherent with CPAP based on 14‐day run‐in (< 4 hours/day); previous diagnosis of OSA Exclusion criteria: compliant with CPAP during run‐in |
|
| Interventions | Bi‐PAP with flexible pressure setting versus fixed CPAP setting (identical machine) Run‐in: phase 1 of study identified non‐adherent CPAP users (those using CPAP < 4 hours/day) Study duration: 12 weeks |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Quote: "This study was supported by a grant from Respironics, Inc. Respironics reimbursed National Jewish Medical and Research Center for part of Dr. Ballard’s time. Dr. Gay has received research support from ResMed. Dr. Strollo has indicated no financial conflicts of interest." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were then provided a modified positive airway pressure device (BiPAP Pro, Respironics Inc.) randomly set to either CPAP or BiFlex mode at appropriate pressure(s)" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were then provided a modified positive airway pressure device (BiPAP Pro, Respironics Inc.) randomly set to either CPAP or BiFlex mode at appropriate pressure(s)" |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Low risk | Quote: "Both patients and the investigators were blinded as to the specific mode assigned to each patient." |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | Quote: "Both patients and the investigators were blinded as to the specific mode assigned to each patient." |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Low risk | Identical machine appearance unlikely to affect measurement of these outcomes. |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | Identical machine appearance unlikely to affect measurement of these outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study reported to have been analysed on ITT principles. Unlikley to bias machine usage data but quality of life collected from completers. There was differential dropout and this may have influenced the results. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information available |
| Other bias | Low risk | No concerns identified |
Berry 2014.
| Methods | Randomised, open‐label, parallel group, singe‐centre trial | |
| Participants | N = 156 participants (145 M/11 F). Age: 59 years; BMI: 36 kg/m2; AHI: 28.5 ESS: 14.8 Inclusion criteria: AHI ≥ 10/hour; ESS ≥ 8; living within 200 miles of treatment centre; age > 18 years Exclusion criteria: previous CPAP therapy; shift work; unstable depression/psychosis; non‐adherence with medication; COPD; uncontrolled hypertension or restless legs syndrome; narcolepsy; supplemental oxygen use; congestive heart failure; nightly narcotic use; hypoventilation; neuromuscular weakness; regular sleep of < 4 hours per night; low baseline SaO2; central apnoea index > 5/hour |
|
| Interventions | Auto‐CPAP versus home PSG CPAP titration followed by fixed pressure CPAP treatment Study duration: 6 weeks |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Quote: "This study was supported by a research grant from the Res Med Foundation and an unrestricted research grant from Philips Respironics. Both grants were made to the North Florida Foundation for Research and Education. The CPAP and APAP equipment were purchased by the VA as part of the routine clinical care of the patients. The PAP setups were performed by clinical PAP respiratory therapists as part of the patient’s usual care. A registered polysomnographic technologist (CPAP titrations) and study coordinator were paid by research funding. The principal investigators received no salary support from research funding. This work was also supported by resources provided by the North Florida/South Georgia Veterans Health System, Gainesville, FL. The contents of this paper do not represent the views of the U.S. Department of Veterans Affairs or the United States Government. The authors have indicated no other financial conflicts of interest. The study was performed at the Malcom Randall VA Medical Center, Gainesville, FL." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient details available to determine process |
| Allocation concealment (selection bias) | Low risk | Quote: "The method of randomization was by opening sequential envelopes prepared by the research service." |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Study had open‐label design which likely affects usage, symptoms, quality of life attrition outcomes. |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by open‐label design. |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Impact of study design on outcome assessment related to nature of outcome. Usage, AHI and treatment pressure measured from technical readings. Symptoms, quality of life more likely affected by open‐label design. |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | Treatment pressure and AHI unlikely to be affected by open‐label design. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Balanced but potentially meaningful dropout which was slightly higher in fixed CPAP group (19/78 versus 12/78). Participants not using machines at clinic visit assumed to be non‐users and assigned values of 0 for usage outcome. Likely to be greater impact on symptoms and quality of life outcomes. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes presented according to details provided on trials registry record. |
| Other bias | Low risk | No concerns identified |
Blau 2012.
| Methods | Randomised, double‐blind, parallel group trial | |
| Participants | N = 35 participants (34 M/1 F); Age 54.2 years; BMI 30.9 kg/m2; AHI ≥ 39; ESS: 10.2 Inclusion criteria: 18 to 75 years; AHI ≥ 15; BMI < 45 kg/m²; ability to follow study specific instructions Exclusion criteria: other sleep, cardiac, pulmonary, psychiatric or neurological disorder; previous abuse of alcohol, hypnotics or drugs; previous treatment for OSA (including CPAP); inability to wear a mask |
|
| Interventions | ABRP‐PAP versus fixed CPAP Study duration: 12 weeks |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Quote: "This study was supported by an unrestricted grant from Philips Respironics, Inc. 1001 Murry Ridge Lane, Murrysville, PA, 15668, USA" Conflict of interest quote: "'Dr. Fietze, Prof. Penzel, Dr. Peter and Dr. Blau have received travel grants and honorariums for lecturing from Philips Respironics. The other authors have no significant conflicts of interest with any companies/organizations whose products or services may be discussed in this article." |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We generated a list of N = 32 uniformly distributed pseudo‐random numbers of either 0 (CPAP) or 1 (Auto bi‐level) by using the Mersenne Twister algorithm" |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation of the patient was told directly via telephone in case of randomisation from an not otherwise involved team member, situated in another campus of our university clinic." |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Low risk | Quote: "Devices were set by the study coordinators who deactivated the LCD display so that the patient and investigators did not become aware of device allocation" Quote: "There were no concrete information about characteristics of these different PAP types in the informed consent" |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | Quote: "Devices were set by the study coordinators who deactivated the LCD display so that the patient and investigators did not become aware of device allocation" |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Low risk | Quote: "The investigator making and analysing the PSG recordings on therapy and other outcome measures did not have access to information from the therapy device within their PSG montage" |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | Identical machine appearance unlikely to affect measurement of these outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3 withdrawals after allocation (1 from CPAP group and 2 from ABRP‐PAP group); these patients were not included in analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
Bloch 2018.
| Methods | Randomised, double‐blind, parallel group trial | |
| Participants | N = 208 participants (177 M/31 F). Age 55.5; BMI 32.7 kg/m2; AHI 48.4; ESS 13 Inclusion criteria: ESS ≥ 8; AHI ≥ 10/hour; age 18‐75 Exclusion criteria: psychophysiological incapacity to perform questionnaires, other sleep disorders, psychiatric disease, previous CPAP therapy, previous uvulopalatopharyngoplasty, chronic nasal obstruction, cancer, COPD, with FEV1 < 50% predicted, symptomatic cardiovascular disease, previous stroke, cheyne‐Stokes respiration, chronic pain syndromes, fibromyalgia, drug or alcohol addiction |
|
| Interventions | Auto‐CPAP versus fixed CPAP Study duration: 2 years |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Quote: "The study was supported by the Swiss National Science Foundation, the lung leagues of Zurich, St. Gallen and Thurgau and by unconditional grants from the respironics Foundation and resMed Switzerland. This was an investigator initiated trial, and the commercial companies were not involved in study design, data acquisition and analysis or writing the manuscript. competing interests KEB reports grants to his institution from Swiss National Science Foundation, Zurich lung league, respironics Foundation, resMed Switzerland, during the conduct of the study. The other authors report no competing interests." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation of participants was performed according to a 1:1 balanced block design by the study centre.......envelopes containing codes for the treatment mode and CPAP brand for 12‐24 participants were sent to participating centres as needed. The local co‐ordinator drew a paper (from an opaque envelope) with the codes for each participant." |
| Allocation concealment (selection bias) | Low risk | Quote: "...envelopes containing codes for the treatment mode and CPAP brand for 12‐24 participants were sent to participating centres as needed. The local co‐ordinator drew a paper (from an opaque envelope) with the codes for each participant." |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Quote: "True blinding of participants and clinical care‐givers was not feasible since all participants had an initial phase of autoCPAP therapy." This is likely to impact on usage data, quality of life and symptom scores. |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | Identical machine appearance unlikely to affect measurement of these outcomes. |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Quote: "True blinding of participants and clinical care‐givers was not feasible since all participants had an initial phase of autoCPAP therapy." This is likely to impact on usage data, quality of life and symptom scores |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | Identical machine appearance unlikely to affect measurement of these outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 15 out of 95 randomised to fixed CPAP group withdrew from treatment by 24 months. 21 out of 113 randomised to auto‐CPAP group withdrew from treatment by 24 months. Data were presented for ITT analysis and per protocol. Multiple imputation method used to address attrition. In view of large attrition rates there is some uncertainty over the reliability of data for usage, symptoms and quality of life. Treatment pressure and AHI unlikely to have been affected since short term measurement is informative in determining intervention effects. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
Bogan 2017.
| Methods | Randomised cross‐over study. Data analysed with paired t tests. | |
| Participants | N = 70 participants (48 M/22 F); age: 50.78; BMI: 35.93; AHI: not reported. ESS: 10.7 Inclusion criteria: 18 to 75 years of age; AHI ≥ 10 per hour; successful in‐lab polysomnography; seven hours’ sleep on most nights; bedtime midnight or earlier; fluent English speakers Exclusion criteria: use of CPAP in last 2 years; CPAP therapy contraindicated; factor or disease that might interfere with study participation (e.g. psychiatric disease, non‐adherence to medical regimens); significant sleep disorder(s) that make use of CPAP challenging; use of hypnotics and/or sedating medications; surgery of the mouth, nose, sinuses, or airways in previous 12 months; patients who are required by the nature of their employment to not comply with therapy (e.g. truck drivers, airline pilots) |
|
| Interventions | Fixed CPAPexp designed to detect transition from sleep to wakefulness versus fixed pressure CPAP Duration: 4 weeks per treatment arm |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Quote: "The authors declare that there are no conflicts of interest regarding the publication of this paper. Dr Chris Frampton is an independent, statistical consultant hired to analyse the results for this study. Medical writing assistance was provided by Anita Fitzgerald, on behalf of Fisher & Paykel Healthcare. Irene Cheung is an employed staff from Fisher & Paykel Healthcare who helped in inputting data." Study sponsored by Fisher & Paykel, manufacturers' of SensAwake expiratory relief |
|
| Notes | The type of expiratory pressure relief mechanism used in this study works differently to those evaluated by other studies in this review. We present the findings of this study separately to analyses of CPAP expiratory pressure relief devices. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Random permuted blocks were used to randomise patients into the two treatment sequence groups." |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization records were kept in a patient master log. The study coordinator set the device to the appropriate treatment arm according to the patient master log during the device setup visit." The process described is consistent with an approach that conceals the assignment from both the study personnel and the participants and so is probably adequate. |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Low risk | Quote: "Both the physician and the patient were blinded to the treatment. To ensure adequate blinding, SensAwake was turned ON in all devices and this setting displayed to the user." |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | Quote: "Both the physician and the patient were blinded to the treatment. To ensure adequate blinding, SensAwake was turned ON in all devices and this setting displayed to the user." |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Low risk | Identical machine appearance unlikely to affect measurement of these outcomes. |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | Identical machine appearance unlikely to affect measurement of these outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5 participants withdrew before completion of both arms. Data on machine usage available for participants who completed both arms. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported in accordance with trial protocol. |
| Other bias | Low risk | No significant concerns identified. |
Castronovo 2006.
| Methods | Randomised, cross‐over study. Statistical analysis approach not described | |
| Participants | N = 50 participants. 40 completed and analysed. Age: 53 years. No other baseline details reported. Inclusion criteria: severe OSA (RDI > 30) |
|
| Interventions | Auto‐CPAP versus fixed CPAP (RemStar machines set in 2 different modes) Study duration: 2 x 4 weeks |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Details not available (conference abstract) | |
| Notes | TJL wrote for confirmation of data and methods on 3 September 2008 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Unclear risk | Information not available |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Unclear risk | Quote: "Global cognitive functioning, attention, vigilance, language, memory.......were blindly assessed." No further information available to judge this |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Unclear risk | Information not available |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 20% attrition. Non‐completers not analysed |
| Selective reporting (reporting bias) | Unclear risk | Results reported in abstract form. Not all outcomes reported |
| Other bias | Unclear risk | Information not available |
Chang 2015.
| Methods | Prospective, randomised, cross‐over study. Statistical analysis methods unclear | |
| Participants | N = 19 participants (18 M/1 F). Age 46.2; BMI 30.2; AHI 59.7; ESS 9.6 Inclusion criteria: age > 20, AHI > 15, consent to wear CPAP Exclusion criteria: not consenting to positive pressure device, treatment for mood disorders such as anxiety and depression |
|
| Interventions | Auto‐CPAP versus fixed CPAP Study duration: 12 weeks |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Funding not declared. Conflict of interest: none | |
| Notes | Unadjusted values used in the analysis as the P values were high and errors were very small. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "We used single sequence of random assignments for randomisation." Not clear how sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "There was no selection bias if the patient wanted to enrol in our study." Not clear how allocation was concealed from study personnel or participants |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Study was not blinded |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Study was not blinded |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Six of the 25 enrolled OSA patients (24%; 3 received APAP first and three received CPAP first) withdrew during the first month due to intolerance to CPAP/APAP." |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
d'Ortho 2000.
| Methods | Randomised, single‐blind, cross‐over study. Method of randomisation: random sampling number tables (correspondence with trialist). All participants accounted for Data analysis: paired t test where ANOVA significant. T test used for treatment pressure. Analysis on usage not paired t test. |
|
| Participants | N = 25 participants (22 M/3 F). Mean age 57; mean AHI 57.8 Inclusion criteria: OSA confirmed by PSG; AHI > 10/hr; ATS recommended indication for CPAP treatment |
|
| Interventions | Auto‐CPAP versus fixed CPAP. No washout period Study duration: 2 x 4 week treatment arms |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Funded by Institut National de la sante et de la Recherche Medicale & by Nellcor‐Puritan Bennett. No declaration of interests provided. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sampling number tables |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Quote: "Patients were not aware of the order of administration (i.e. they were blinded to the setting mode until the first night of use after which they could easily guess which mode they were using.).........The questionnaire was completed with the help of sleep laboratory technicians who were unaware of CPAP mode." |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | Awareness of treatment group assignment unlikely to affect objective outcome data from the study |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Quote: "Patients were not aware of the order of administration (i.e. they were blinded to the setting mode until the first night of use after which they could easily guess which mode they were using.).........The questionnaire was completed with the help of sleep laboratory technicians who were unaware of CPAP mode." |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | Awareness of treatment group assignment unlikely to affect objective outcome data from the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
Damjanovic 2009.
| Methods | Randomised controlled, parallel group trial | |
| Participants | N = 100 participants (78 M/22 F); mean age 57; BMI 31 kg/m2 Inclusion criteria: AHI > 15, with or without corresponding daytime symptoms Exclusion criteria: 1. global respiratory failure; 2. central sleep apnoea syndrome; 3. severe mental or psychological impairment |
|
| Interventions | 4 groups. Autoadjusting CPAP with or without intensive support versus fixed CPAP with or without intensive support Study duration: 9 months |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Information not available (link to declarations of interest no longer live) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Pulling mixed and sealed envelopes" |
| Allocation concealment (selection bias) | Low risk | Quote: "Pulling mixed and sealed envelopes... At time of recruitment, recruiters were also unaware of allocation." |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Quote: "Patients were not told about their pressure mode, however, full blinding of pressure delivery was not possible. At time of recruitment, recruiters were also unaware of allocation." |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | Awareness of treatment group assignment unlikely to affect objective outcome data from the study |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Quote: "Outcome assessors were not blinded" |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No information on withdrawals provided |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
Dolan 2008.
| Methods | Randomised, single‐blind, parallel group trial. Method of randomisation not reported | |
| Participants | N = 184 participants (138 M/46 F); age: 48 years; ESS: 15 Inclusion criteria: newly diagnosed OSA; study participants who used PAP for 4 hours night or more in the first week of treatment were followed up; AHI > 10; ESS ≥ 10 Exclusion criteria: prior surgical procedure for OSA; significant hypoventilation; CPAP exposure; significant comorbidities |
|
| Interventions | CPAP with expiratory pressure relief (C‐Flex) versus fixed pressure CPAP Study duration: 24 weeks |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | This study was funded by Respironics. No author declarations provided. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was centralized by the sponsor. Randomization lists were generated in block sizes of 4 and 6 randomly chosen with respect to order. The actual block size of 10 comprised a block of 4 (or 6) followed by a block of 6 (or 4). Randomization cards were provided to the clinical sites at the time of study initiation." |
| Allocation concealment (selection bias) | Low risk | Quote: "Research assistants received sealed random condition assignment cards from Respironics to determine standard CPAP or C‐Flex therapy." |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Quote: "Participants were not made aware of the treatment they were assigned. This study was therefore a.......single‐blinded, controlled study". Only participants were blinded. |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | Study design unlikely to affect these outcomes. |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Study personnel were aware of treatment group assignment as the study was single‐blinded. |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | Study design unlikely to affect these outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Data were analyzed with multivariate mixed models procedures that allowed for analysis including participants with missing observations." |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
Ficker 2003.
| Methods | Randomised, parallel group study. Methods of randomisation not reported. Devices were identical in appearance. | |
| Participants | N = 100 participants. Mean age: 54.3; BMI: 31.8; AHI: 47.9; ESS: 12.6 Inclusion criteria: diurnal somnolence (≥ 8 on ESS); AHI > 10; written consent Exclusion criteria: prior CPAP therapy; central sleep apnoea or Cheyne‐Stokes respiration; severe nasal obstruction or other conditions contraindicating CPAP treatment; COPD (FEV1 < 70% predicted); congestive heart failure (NYHA III or IV) |
|
| Interventions | Auto‐CPAP (forced oscillation technique) versus fixed CPAP Conference abstract reported 8 weeks duration (published paper reported 2 nights data from laboratory studies) |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Not available (conference abstract) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Unclear risk | Information not available |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Unclear risk | Information not available |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Unclear risk | Information not available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information not available |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Unclear risk | Information not available |
Fietze 2007.
| Methods | Randomised, double‐blind, parallel group study. Participants randomised for 2 night cross‐over and retained device assigned on second night for subsequent 6‐week period. | |
| Participants | N = 21 (20 M/1 F) participants. Mean age 54.2; BMI: 30.9 kg/m2. AHI: 41.8. ESS: 12.9 Inclusion criteria: AHI > 10 or excessive sleepiness (if AHI < 10). Participants who did not have excessive sleepiness at baseline also eligible if AHI > 20 Exclusion criteria: other sleep disorders (e.g. restless leg syndrome or periodic leg movement syndrome; cardiac, pulmonary or other medical disorders;psychiatric/neurological disorders; abuse of sleep‐inducing agents or other drugs; suspected or confirmed central sleep apnoea syndrome; prior OSA treatment (e.g. CPAP, oral devices or surgery) |
|
| Interventions | Auto‐CPAP versus fixed pressure CPAP (established by manual titration after 2 night cross‐over study) Study duration: 6 weeks |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Funding quote: "This study was supported by an unrestricted grant from Respironics Inc.". No declarations reported from authors. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but no further details provided. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information available to allow judgement. |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Low risk | Same device used with different pressure settings (REMstar Auto CPAP). |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | Same device used with different pressure settings (REMstar Auto CPAP). |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Low risk | Adequate blinding procedure in place and unlikely that this has impacted on subjective outcomes. |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | Adequate blinding procedure in place and unlikely that this has impacted on objective outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 21 participants who started completed the study. |
| Selective reporting (reporting bias) | High risk | Symptoms and quality of life data measured but reported as aggregate of two treatment groups and described as not significantly different. |
| Other bias | Low risk | No concerns identified. |
Galetke 2008.
| Methods | Randomised, single‐blind, cross‐over study (participants not informed of order/setting) Statistical test: Wilcoxon test |
|
| Participants | N = 20 participants (16 M/4 F) completed and analysed. Mean age: 56 years. AHI: 33; ESS: 10.3 Inclusion criteria: new diagnosis of OSA (diagnosis established through polysomnography, AHI > 10) Exclusion criteria: COPD, congestive heart failure and other serious medical disorders |
|
| Interventions | Auto‐CPAP versus fixed pressure CPAP Same machine delivered the different treatment pressure settings Study duration: 2 x 8 weeks |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Not provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Quote: "a randomized, single‐blinded, cross‐over study." Only participants were blinded. |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by open‐label design. |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Only participants were blinded. |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by open‐label design. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
Gay 2003.
| Methods | Randomised, double‐blind, parallel group trial. Machines had settings: 'Set CPAP'; 'Set NBL' and 'A'. Assessors could alter the settings for safety reasons. Randomisation not reported | |
| Participants | N = 27 participants (22 M/5 F). Age: 44 years; BMI: 35 kg/m2; AHI: 43; ESS: 13.8 Inclusion criteria: > 18 years; AHI > 10 and < 100; ability to follow instructions and provide informed consent; willingness to return for follow‐up visit 30 days after random allocation to CPAP/BiPAP; residence within 200 miles of clinic Exclusion criteria: inability to wear a mask; prior surgical treatment for OSA; prior CPAP usage; other significant comorbidities |
|
| Interventions | Bi‐level PAP versus CPAP. Participants also given instruction via educational video on CPAP and OSA. Study duration: 30 days |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Quote: "Dr. Peter Gay received grant support for this study by Respironics Inc. (noted in manuscript)." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Low risk | Quote: "the technician selected the 'A' mode which in a double‐blinded and selective way, locked in the optimal settings for either CPAP or NBL" |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Low risk | Quote: "the technician selected the 'A' mode which in a double‐blinded and selective way, locked in the optimal settings for either CPAP or NBL.............true identification of the 'A' mode was not revealed until the end of the trial" |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by study design. |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Low risk | Quote: "the technician selected the 'A' mode which in a double‐blinded and selective way, locked in the optimal settings for either CPAP or NBL.............true identification of the 'A' mode was not revealed until the end of the trial" |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by study design. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomisation was performed prior to baseline PSG, after which a number of participants became ineligible. All participants recruited to the 2nd phase completed the study. There were no withdrawals from this study, although the sample reflects a selected population. |
| Selective reporting (reporting bias) | High risk | Data on quality of life (FOSQ) were not reported but described as 'equivalent' between two treatment arms. |
| Other bias | Low risk | No concerns identified |
Gfüllner 2007.
| Methods | Randomised, cross‐over study. Statistical analysis not clear | |
| Participants | N = 18 (15 M/3 F); mean age: 56.8 years; AHI: 41.4; BMI: 36 Inclusion criteria: non‐sleepy OSA patients |
|
| Interventions | Pressure relief CPAP versus fixed CPAP Study duration: 2 x 4 weeks |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Not available (conference abstract) | |
| Notes | TJL emailed for confirmation of methods and data on 4 September 2008 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available; requested |
| Allocation concealment (selection bias) | Unclear risk | Information not available; requested |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Unclear risk | Information not available |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Unclear risk | Information not available |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Unclear risk | Information not available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information not available |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Unclear risk | Information not available |
Gonzalez‐Moro 2005.
| Methods | Randomised parallel group study. Randomisation and blinding not described | |
| Participants | N = 20; ESS: 12. No other baseline details provided Inclusion criteria: OSA and obstructive hyperventilation syndrome Exclusion criteria: not reported |
|
| Interventions | Bi‐PAP versus fixed pressure CPAP Study duration: 12 weeks |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Not available (conference abstract) | |
| Notes | Unpublished conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Unclear risk | Information not available |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Unclear risk | Information not available |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Unclear risk | Information not available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information not available |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Unclear risk | Information not available |
Gulati 2015.
| Methods | Prospective, randomised, cross‐over study in patients who were suboptimally compliant with CPAP despite appropriate interventions. Data analysed as paired t test | |
| Participants | N = 28 participants (24 M/4 F). Mean Age 56.7 years; BMI 35 kg/m2; ESS 13.2; AHI 35 Inclusion criteria: OSA with AHI > 5, CPAP compliance < 4 hours per night for 6 weeks after CPAP prescription despite technical and educational interventions, symptoms of pressure intolerance Exclusion criteria: significant airflow obstruction (FEV1/FVC < 60%), pretreatment study showing central sleep apnoea, clinical evidence of congestive heart failure, daytime hypercapnia (PaCO2 > 6.5kPa) or previous prescription of Bi‐PAP |
|
| Interventions | Bi‐PAP versus new CPAP (brand of fixed CPAP different from the one used prior to study entry) Study duration: 2 x 4 weeks with 2 weeks washout in‐between |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Funding source: not declared; conflict of interest: none | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Simple randomisation technique was used to allocate patients into different groups." |
| Allocation concealment (selection bias) | Low risk | Quote: "(Allocation sequence concealment) was done independently by the research and development officer, so the patients and researchers were not aware of the allocation sequence." |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Quote: "Neither (participants or research personnel) were blinded as the machines used were different in each arm." |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | Outcome assessors were not blinded to treatment allocation, but the outcomes unlikely to be affected by open‐label design. |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Outcome assessors were not blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | Outcome assessors were not blinded to treatment allocation, but the outcomes unlikely to be affected by open‐label design. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "31 gave consent and were recruited. One developed a stroke during the treatment period (on Bi‐level PAP arm) and 2 others did not complete the study (one of them dropped out after first using Bi‐level PAP and the other after using the new CPAP). These subjects were excluded from the analyses." |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | No concerns identified |
Heiser 2010.
| Methods | Randomised, parallel group study | |
| Participants | N = 74 participants (M/F 60/14). Mean age 58 years; BMI 31; AHI 35; ESS 9 Inclusion criteria: newly diagnosed OSA patients (AHI > 15 on polysomnography) |
|
| Interventions | CPAP with warm air humidifier versus CPAP without warm air humidifier Study duration: 12 weeks |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Funding source: study was funded by manufacturers. Quote: "Diese Studie wurde finanziell unterstützt durch die Firmen Fisher & Paykel Healthcare und Air Products Medical GmbH" Author declaration of interests are identical to funding source |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study participants assigned according to a randomised schedule but no more detail provided about sequence of treatment group assignments |
| Allocation concealment (selection bias) | Unclear risk | No detail described. Insufficent information available to judge |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Low risk | Identical devices provided |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | Identical devices provided |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Low risk | Identical devices provided |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | Identical devices provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Non‐users excluded from the analysis |
| Selective reporting (reporting bias) | High risk | Outcome data not available for symptoms. Objective of the study differed from that of the review question, but symptoms likely to have been collected. |
| Other bias | Low risk | No concerns identified |
Hudgel 2000.
| Methods | Randomised, single‐blind, cross‐over study. Method of randomisation: hospital number (odd versus even last digit) Paired t test used for continuous data |
|
| Participants | N = 60 (53 with OSA and 7 with UARS). 21 withdrawals 2 stopped due to medical complications (not stated) and the rest did not complete the study. Further 6 did not have machine usage data. (21 M/18 F). Total number of OSA patients completing trial is 29. Data analysed for 33 patients which included 4 patients with UARS Mean age: 46 years; AHI 30; BMI: 42 kg/m2 Inclusion criteria: diagnosed OSA or UARS (confirmation by polysomnography) Exclusion criteria: prior CPAP treatment, facial/pharyngeal abnormalities requiring surgery, chronic airways disease necessitating bronchodilator usage, obesity hypoventilation syndrome, shift workers, congestive heart failure, seizure disorder, mental retardation, sedative/antidepressant/hypnotic treatment |
|
| Interventions | Auto‐CPAP versus fixed CPAP. No washout Study duration: 2 x 12 week treatment periods |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Not provided | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Hospital number (odd versus even last digit) |
| Allocation concealment (selection bias) | High risk | Study investigators likely to be aware of treatment group assignment |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Study investigators likely to be aware of treatment group assignment |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by study design |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Study investigators likely to be aware of treatment group assignment |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by open‐label design |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High withdrawal rate and non‐completers not included in analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
Hukins 2004.
| Methods | Randomised, single‐blind, cross‐over study. Method of randomisation: sealed envelopes (off‐site) Statistical analysis: paired t test |
|
| Participants | N = 55 adults (48 M/7 F) randomised (46 completed). Age: 50 years; BMI: 35; AHI: 54; ESS: 12.5 Inclusion criteria: AHI ≥ 5; optimal treatment PSG determined optimal treatment pressure; no previous home use of CPAP Exclusion criteria: significant comorbidity; complication (e.g. hypercapnic respiratory failure); non‐OSA; patients unable to use masks with Autoset T machines |
|
| Interventions | Auto‐CPAP (Autoset T) versus fixed pressure CPAP Study duration: 2 x 8‐week treatment periods |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Quote: "This was an industry supported study by ResMed Australia. Dr. Hukins received research equipment from ResMed Australia." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was performed by a laboratory scientist not involved with the study using the technique of shuffled sealed envelopes containing equal numbers of each treatment arm" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was performed by a laboratory scientist not involved with the study using the technique of shuffled sealed envelopes containing equal numbers of each treatment arm" |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Quote: "....this single‐blinded, randomised cross‐over study.......The Autoset T was used for both treatment modes in an attempt to blind the patient to the mode...Investigators were not blinded..." |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by single‐blind study design. |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Quote: "Investigators were not blinded..." |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by open‐label design. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Non‐completers not included in analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
Hussain 2004.
| Methods | Randomised, single‐blind, cross‐over study. Method of randomisation not described. Statistical test: paired t tests | |
| Participants | N = 10 (9 M/1 F). Mean age: 44.98; AHI: 47.2; BMI: 35.9; ESS: 11.1 Inclusion criteria: CPAP‐naive at baseline; symptomatic OSA (AHI > 15/h) Exclusion criteria: not described |
|
| Interventions | Auto‐CPAP versus fixed CPAP Study duration: 2 x 4‐week treatment periods (washout 2 weeks) |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Quote: "This study was funded by Respironics Inc., Murrysville, PA." Author conflicts of interest: not declared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Quote: "...this randomized, prospective, single‐blind cross‐over trial.....Patients were unaware of the treatment mode..." |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by single‐blind study design. |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Study personnel would have been aware of treatment group assignment. |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by single‐blind study design. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
Jarvis 2006.
| Methods | Randomised, cross‐over study. Statistical analysis methods unclear but paired data obtained via correspondence | |
| Participants | N = 20 participants Inclusion criteria: diagnosed with OSA; established on CPAP therapy |
|
| Interventions | Modified APAP (bi‐level pressure mode) versus fixed CPAP Study duration: 2 x 2 weeks |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Resmed sponsored the study but no other details were available. | |
| Notes | TJL emailed for confirmation of data and methods 5 September 2008. Reply from Resmed October 2008 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The study was randomised by pulling the order of treatment received 'out of a hat'" |
| Allocation concealment (selection bias) | Low risk | Quote: "The person who pulled the order was a ResMed employee independent of the study and with no knowledge of the study." |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Unclear risk | Information not available |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All completed |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
Kendrick 2002.
| Methods | Randomised, double‐blind, cross‐over study | |
| Participants | N = 41 (38M/3F). 27 completed the study. Mean age: 52.4 years; BMI: 32.3kg/m2; ESS 13.9 Eligibility criteria not provided |
|
| Interventions | Auto‐CPAP versus fixed CPAP Study duration: 2 x 2‐week treatment periods |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Not available (conference abstract) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Unclear risk | Information not available |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Unclear risk | Information not available |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Unclear risk | Information not available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information not available |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Unclear risk | Information not available |
Konermann 1998.
| Methods | Randomised, single‐blind, parallel group study. Method of randomisation not reported | |
| Participants | N = 50 participants (44 M/6F); Age 53.5. No other baseline details available | |
| Interventions | Auto‐CPAP versus fixed CPAP Study duration: 3 to 6 weeks |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Not provided | |
| Notes | Sleep study following treatment done between 3 and 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to receive either automatically adjusting or conventional nCPAP." |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Quote: "the design of the study was single‐blinded" |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Quote: "the design of the study was single‐blinded" |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Non‐completers did not contribute to the analysis (4%) |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Low risk | No concerns identified |
Kushida 2011.
| Methods | A prospective, randomised, double‐blinded, three‐arm, multicenter trial | |
| Participants | N = 168 participants (128 M/40 F). Age: 49 years; BMI: 34 kg/m2; AHI: 39; ESS: 11 Inclusion criteria: age 21‐75 years, AHI > 15/hour, able to consent, agreeable to commence CPAP as initial therapy, adequate titration within 2 weeks of enrolment Exclusion criteria: previous study participation < 30 days, > 1 titration, sedatives, medical/psychiatric illness potentially interfering with CPAP adherence, CPAP exposure < 1 year, chronic respiratory disease, upper airway surgery < 90 days, previous surgery for OSA, non‐OSA sleep disorder, excess alcohol use, shift workers |
|
| Interventions | Comparing effects of autoadjusting PAP EXPssure relief with autoadjusting PAP and fixed CPAP Study duration: 6 months (see notes) |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Quote: "Philips Respironics provided funding for this study; Drs. Kushida, Berry, Blau, Fietze, Kryger, Kuna, Pegram, and Penzel received research support for the conduct of this study through contracts between Philips Respironics and their respective institutions. Ms. Crabtree received consulting fees for statistical data analysis from Philips Respironics. Dr. Kushida has received research support from Philips Respironics, ResMed, Ventus Medical, and Pacific Medico. Dr. Berry has received research support from Philips Respironics, ResMed, and Ventus Medical. Dr. Blau has received research support from Philips Respironics, Breas, and Hoffrichter) Dr. Fietze has received research support from Philips Respironics, ResMed, Advanced Sleep Research, Breas, Hoffrichter, and Weinmann. Dr. Kryger has received research support from Ventus, and ResMed. Dr. Penzel has received research support from Philips Respironics, ResMed, Advanced Sleep Research, Breas, Hoffrichter, Somnomedics, and Weinmann." | |
| Notes | Patients in APAP group ‐ initially APAP for two weeks followed by fixed CPAP for six months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Urn randomization was used to control for the potentially confounding variables (age, gender, education, AHI, subjective sleepiness)" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make judgement |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Low risk | Quote: "The Principal Investigator (PI) and research staff administering questionnaires or interacting with the participant were blinded to randomization and the results of all participant evaluations...participants were blinded to treatment" |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Low risk | Quote: "The Principal Investigator (PI) and research staff administering questionnaires or interacting with the participant were blinded to randomization and the results of all participant evaluations" |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Fourteen participants did not receive the therapy to which they were randomized, but were included in the intention‐to‐treat analysis." |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
Leidag 2008.
| Methods | Randomised, double‐blind, cross‐over trial | |
| Participants | N = 30 participants (22 M/8 F). Age: 55.4 years, BMI 32 Inclusion criteria: clinical suspicion of OSAS with AHI > 5 on polysomnography Exclusion criteria: severe comorbidity, such as acute or chronic heart failure (NYHA grade 3 or 4), severe COPD, dementia, alcoholism, drug abuse, and age under 18 |
|
| Interventions | CPAP versus C‐Flex Study duration: 2 x 6 weeks |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Funding quote: "This study was supported by Air Products Medical GmbH." Conflicts of interest quote: "The authors had no conflicts of interest to declare in relation to this article." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make judgement |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Low risk | Quote: "The physicians and technicians performing the titration and scoring the polysomnographic data were blinded to the treatment mode which was chosen...The patients were only informed that they would receive two different modes of therapy, but they did not know if they got CPAP or C‐Flex" |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Low risk | Quote: "The physicians and technicians performing the titration and scoring the polysomnographic data were blinded to the treatment mode which was chosen" |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Twelve patients dropped out of the study (7 after C‐Flex, 5 after CPAP); 4 of them gave up the therapy completely (2 after CPAP, 2 after C‐Flex)" |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
Loube 2004.
| Methods | Randomised study. Design and method of randomisation not reported. Single‐blind trial | |
| Participants | N = 16 participants. Distribution and baseline details not reported Inclusion criteria: participants with newly diagnosed CPAP; AHI > 15; uncomplicated CPAP lab PSG Exclusion criteria: REM‐related/supine positional OSA |
|
| Interventions | C‐Flex PAP versus fixed pressure CPAP Study duration: 4 weeks |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Not available (conference abstract) | |
| Notes | Unpublished conference abstract. Details requested by email | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available; requested |
| Allocation concealment (selection bias) | Unclear risk | Information not available; requested |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Unclear risk | Information not available |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Unclear risk | Information not available |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Unclear risk | Information not available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information not available; requested |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Unclear risk | Information not available |
Marrone 2004.
| Methods | Randomised, single‐blind, cross‐over study. Method of randomisation: not described. Statistical analysis: unpaired t test | |
| Participants | N = 22 participants (21 M/1 F). Mean age 53.45; BMI: 32.9; ESS: 16.3 Inclusion criteria: newly diagnosed OSA; AHI ≥ 30 Exclusion criteria: not described |
|
| Interventions | Auto‐CPAP versus fixed CPAP Study duration: 2 x 4 weeks. No washout described |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Funding quote: "This study was supported by Air Products Medical GmbH." Conflicts of interest quote: "The authors had no conflicts of interest to declare in relation to this article." | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Machines were assigned in a single blind, random fashion." |
| Allocation concealment (selection bias) | High risk | Given that the study was single‐blind, the pressure setting of the machines may have been known by investigators |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Single‐blind study |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Single‐blind study |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | Unclear risk | No information available |
| Other bias | Low risk | No concerns identified |
Marshall 2008.
| Methods | Randomised, single‐blind, parallel group trial | |
| Participants | N = 19 participants (15 M/4 F). Mean age: 47; AHI: 78; ESS: 14 Inclusion criteria: severe OSA (AHI > 30; or symptomatic and AHI > 20) Exclusion criteria: other significant medical disorders |
|
| Interventions | C‐Flex versus fixed CPAP Study duration: 4 weeks |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Quote: "None of the authors have had any financial relationships with Respironics Inc., who are the manufacturers of the device tested. Respironics International Inc., through their New Zealand suppliers Care Medical, provided six C‐Flex machines for the purposes of this trial." | |
| Notes | Information on randomisation available from study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The sequence of allocation to treatment was determined by sleep technicians randomly picking one of a set of pre‐prepared opaque envelopes containing the treatment allocation." |
| Allocation concealment (selection bias) | Low risk | Quote: "An urn with 2 differently coloured paper clips was prepared. One clip was blindly withdrawn, and its corresponding treatment noted on a folded piece of paper in an opaque envelope which was then sealed. The clip was then replaced in the urn along with another coloured clip, which represented the other treatment. This was repeated until 20 envelopes had been produced. Because a number of patients who did not meet the entry requirements, were randomised and then withdrawn from the study, a further 5 envelopes were subsequently prepared using the same method. This method leads toward roughly equal group sizes whilst maintaining unpredictability of sequence and sample sizes." |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Study personnel would have been aware of treatment group allocation. |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Low risk | Quote: "Measurements of sleepiness and reaction times were undertaken by an investigator blinded to treatment allocation; patients were blinded to treatment allocation." |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Whilst all 19 patients were included in the primary analysis under the intention‐to‐treat principle due to missed appointments only 17 patients are included in the Epworth analysis." This is a low rate of attrition. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
Masa 2015.
| Methods | Randomised, three‐arm, parallel group | |
| Participants | N = 151 participants entered into treatment groups relevant to this review question (66 M/85 F); age: 60 years; BMI: 44; AHI: 69; ESS: 11 Inclusion criteria: 15‐80 years; AHI: > 30; no other significant sleep disorders (e.g. narcolepsy or restless leg syndrome); correctly executed 30‐minute CPAP/NIV test Exclusion criteria: significant comorbidity |
|
| Interventions | Fixed CPAP versus Non‐invasive ventilation treatment set at bilevel pressure with assured volume. Study assigned to bi‐level PAP comparison. Supplemental oxygen offered if participants met additional criteria (daytime PaO2 < 55 mmHg, with the necessary flow to maintain waking arterial oxygen saturation between 88% and 92% or PaO2 greater than or equal to 55 mmHg for at least 17 h/d). Third treatment arm consisting of a usual care control was not of interest to this review. Study duration: 3 years (for hospitalisation and withdrawal outcomes). Other outcome data reported at 8 weeks unless stated. |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Quote: "Supported by the Instituto de Salud Carlos III (Fondo de Investigaciones Sanitarias, Ministerio de Sanidad y Consumo) grant PI050402, the Spanish Respiratory Foundation 2005 (FEPAR), and Air Liquide Spain." Funders did not participate in the design or conduct of the study, analysis or interpretation of data, or manuscript preparation. The authors all declared that they had no conflicts of interest. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: participants "randomized by an electronic database (simple randomization)." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information available to judge. |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | For most outcomes of interest to the review open‐label nature of the study places the study at high risk of performance bias. |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | For most outcomes of interest to the review open‐label nature of the study places the study at high risk of detection bias. |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 10% of participants missing data at 2 months. ITT was undertaken with missing data imputed for secondary outcomes (quote): "following a multiple imputation method with iterative multivariable regression, because the missing data had characteristics compatible with a missing at random pattern." |
| Selective reporting (reporting bias) | Low risk | Outcomes of interest reported in accordance with trial registry record. |
| Other bias | Low risk | No other sources of bias identified. |
Massie 2003.
| Methods | Randomised, single‐blind, cross‐over study. Methods of randomisation not reported. Comparisons between treatment using 2‐way analysis of variance ‐ treatment order as between‐subject factor, and treatment type within‐subject factor | |
| Participants | N = 46 participants (36 M/10 F) 1 dropout and 1 data unavailable from machine. Mean age: 49; BMI: 32kg/m2 Inclusion criteria: 18 to 65 years; symptomatic OSA; AHI > 15; > 10 cmH2O to correct AHI Exclusion criteria: pre‐existing lung disease; awake resting SaO2 < 90%; 10 or more central apnoeas/hr; patients taking medication considered to interfere with sleep respiration |
|
| Interventions | Auto‐CPAP versus fixed CPAP. No washout period Study duration: 2 x 6‐week treatment periods |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Supported by a grant from ResMed Corporation. One of the authors (Neil Douglas) declared a role as medical advisor to ResMed | |
| Notes | Participants aware that machine usage was monitored; stipend offered for completion of study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Quote: ".....randomised, single‐blinded, cross‐over study....(patients) were not informed of the type of therapy they were receiving" |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Single blind study |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low rate of non‐completers excluded. One excluded from study and additional participant excluded from analysis of machine usage due to unreadable data. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
Meurice 1996.
| Methods | Randomised, parallel group study. Statistical analysis based on t tests but unadjusted data presented for all outcomes. | |
| Participants | N = 16 participants (16 M). Mean age: 54; BMI: 34.2 kg/m2; AHI: 43.6; ESS: 14.8 Inclusion criteria: diagnosis of OSA (confirmed by polysomnography; untreated OSA) Exclusion criteria: not reported |
|
| Interventions | Auto‐CPAP versus fixed CPAP Study duration: 3 weeks |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Not provided | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Low risk | Participants unaware as to group they were randomised to (CPAP machines were identical). |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Low risk | Participants unaware as to group they were randomised to (CPAP machines were identical). |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Low risk | No concerns identified |
Meurice 2007.
| Methods | Randomised, multicentre, parallel group trial | |
| Participants | N = 83. Mean age: 56 years; AHI: 52; ESS: 11.5 Inclusion criteria: new diagnosis of OSA; CPAP‐naive; AHI > 30 |
|
| Interventions | Four auto‐CPAP machines assessed:
All 4 compared against fixed pressure CPAP Study duration: 24 weeks |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Not provided | |
| Notes | Data aggregated from 4 auto‐CPAP groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The randomisation was carried out centrally..." |
| Allocation concealment (selection bias) | Low risk | Quote: "...randomly coded envelopes opened by a coordinator from envelopes batched for each centre in order to have similar proportions of patients in each group from each centre." |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Unclear risk | Information not available |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Participants who withdrew were not included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Low risk | No concerns identified |
Meurice 2009.
| Methods | Randomised, single‐blind, cross‐over trial. Statistical methods unclear | |
| Participants | N = 20. Age 57 years | |
| Interventions | Automatic‐CPAP device combined with pressure reduction during exhalation (auto C‐Flex) versus fixed CPAP Study duration: 2 x 1 month |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Not available (conference abstract) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available |
| Allocation concealment (selection bias) | Unclear risk | No information available |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Single‐blinded study |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Unclear risk | No information available |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Single‐blinded study |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Unclear risk | No information available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information available |
| Selective reporting (reporting bias) | Unclear risk | No information available. Information only available as a conference abstract. No further details about the study are available. |
| Other bias | Unclear risk | No information available |
Modrak 2007.
| Methods | Randomised, cross‐over study. Statistical analysis unclear | |
| Participants | N = 26 participants. Baseline details not available Inclusion criteria: diagnosis of OSA; CPAP‐naive |
|
| Interventions | CPAP + expiratory pressure relief versus fixed CPAP Study duration: 2 x 2 weeks |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Not available (conference abstract) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Quote: "A randomized two‐arm prospective crossover unblinded....." |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Unclear risk | Information not available |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Quote: "A randomized two‐arm prospective crossover unblinded....." |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Unclear risk | Information not available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information not available |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Unclear risk | Information not available |
Muir 1998.
| Methods | Randomised, double‐blind, cross‐over study. Method of randomisation not reported. Statisitical analysis unclear | |
| Participants | N = 16 participants. Mean age: 59 years; BMI: 31 kg/m2; AHI: 69 Inclusion criteria: previously documented OSA and poor compliance with CPAP (< 3 hours/night) |
|
| Interventions | Bi‐level PAP versus fixed CPAP Study duration: 2 x 8‐week treatment periods Pressure levels for inspiratory pressure were: 12.3 cmH2O (SD 1.8), and expiratory pressure: 7.6 cmH2O (SD 2.2) for bilevel PAP treatment, and for fixed CPAP: 9.4 cmH2O (SD 2.3) (no P value reported) |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Not available (conference abstract) | |
| Notes | Study published as conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Unclear risk | Quote: "Double‐blind, crossover study". No further information available. |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Unclear risk | We have judged the risk of bias on objective outcomes to be unclear in the absence of more detail beyond the study being double‐blind. |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Unclear risk | Quote: "Double‐blind, crossover study". No further information available. |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Unclear risk | We have judged the risk of bias on objective outcomes to be unclear in the absence of more detail beyond the study being double‐blind. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information not available |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Unclear risk | Information not available |
Neill 2003.
| Methods | Randomised, double‐blind, cross‐over study. Method of randomisation not reported. Statisical test: not reported although exact P values presented for between group tests and confidence interval reported for machine usage. | |
| Participants | N = 42 randomised (37 completed study protocol and were analysed). Mean age: 49 years. BMI: 35 kg/m2; RDI: 50; ESS: 12.1 Inclusion criteria: newly diagnosed OSA requiring treatment with CPAP Exclusion criteria: significant nasal obstruction; requirement for supplemental oxygen |
|
| Interventions | Humidification in addition to nasal CPAP versus sham humidifier in addition to nasal CPAP Study duration: 2 x 3‐week treatment periods (3 day washout) |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Quote: "This study was funded by an Otago University Research Grant." Author declarations not provided (conference abstract) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Low risk | Quote: "The authors attempted to create a placebo humidification arm in which the HC100 was used without the heating unit turned on.........An investigator blinded to research treatment interviewed the patients at the end of each treatment arm..." |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Low risk | Quote: "An investigator blinded to research treatment interviewed the patients at the end of each treatment arm..." |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Non‐completers excluded from analysis |
| Selective reporting (reporting bias) | Unclear risk | Not possible to determine based on details provided |
| Other bias | Low risk | No concerns identified |
Nilius 2006.
| Methods | Randomised, parallel group trial | |
| Participants | N = 51 participants; mean age: 57 years; AHI: 53.3 Inclusion criteria: AHI > 20 Exclusion criteria: Inability to follow instructions, failure to give informed consent, acute infection, acute cardiac disease such as acute coronary artery syndrome, NYHA grade 3 or 4 heart failure, and acute pulmonary thromboembolism |
|
| Interventions | CPAP with expiratory pressure relief versus fixed CPAP Study duration: 7 weeks |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Quote: "This study was financed by a gift from Heinen U. Lowenstein. Dr. Ruhle has received research funding from Fisher A. Paykel, Heinen U. Lowenstein, ResMed, and Weinmann, but this funding has gone into department funds. Dr. Nilius, Andreas Happel, and Ulrike Domanski have reported to the ACCP that no significant conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available; requested |
| Allocation concealment (selection bias) | Low risk | Third party responsible for randomisation and setting machines to relevant treatment mode |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Low risk | Quote: "The patients were informed that they would receive two different forms of treatment .....but were given no further details...........The technician had no information from the investigators about the modality that the patients were receiving" |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Low risk | Quote: "The physician and technicians reviewing the polysomnographic data and performing the manual CPAP titration were blinded to the treatment mode employed" |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for in analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
Nolan 2007.
| Methods | Randomised, single‐blind, cross‐over study. Statistical test: Wilcoxon matched pair test | |
| Participants | N = 34 participants (completed: 29 (26 M/3 F)). Mean age: 53 years; BMI: 29.9 kg/m2; AHI: 14.7; ESS: 12.3 Inclusion criteria: mild to moderate OSA (AHI 5‐30) Exclusion criteria: not reported |
|
| Interventions | Auto‐CPAP versus fixed pressure CPAP Study duration: 2 x 8‐week treatment periods |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Quote: "This was not an industry supported study. Drs. Nolan, Doherty, and Mc Nicholas have indicated no financial conflicts of interest." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Low risk | Quote: "An independent person not involved in the study design, protocol, or analysis assigned the devices to the patients in random order." |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Low risk | Quote: "....patients were not informed about the different technologies used in the devices.....the trial was fully blinded to the investigator performing the analysis." |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Low risk | Quote: "...the trial was fully blinded to the investigator performing the analysis." |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High rate of participants not crossing over on to subsequent treatment arm |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
Noseda 2004.
| Methods | Randomised, single‐blind, cross‐over study. Method of randomisation: random numbers table. Statistical analysis: student's t test (paired data) |
|
| Participants | N = 27 participants (23 M/4 F). Withdrawals: 3. Total completed and analysed N = 24. Mean age: 49 years; BMI: 32.3 kg/m2; AHI: 50.9; ESS 10.7 Inclusion criteria: AHI > 20/hour; MAI: > 30/hour; high variability of within night pressure to correct AHI Exclusion criteria: prior treatment with CPAP; central OSA/Cheyne Stokes; major facial abnormality; night/shift work; severe chronic heart failure/COPD; seizure disorder; mental retardation; sedative, hypnotic or antidepressant therapy; previous UPPP; prolonged hypoventilation during REM |
|
| Interventions | Auto‐CPAP versus fixed CPAP. Need for pressure assessed over a 14‐night run‐in period with auto‐CPAP. No washout period described Study duration: 2 x 8‐week treatment periods |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Not provided | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation tables |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Quote: ".....single‐blind, randomized, crossover trial........subjects were told that they would sleep with the machine functioning in two distinct modes.....no further explanation was given." |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Single‐blind nature of the study means that these outcomes are at risk of bias |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Rate of participants not crossing over could be high enough to introduce bias |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
Nussbaumer 2006.
| Methods | Randomised, cross‐over study. Double‐blinding: investigators and participants unaware as to treatment. Same machine used to deliver both treatment pressure modalities. Identical chip card given to patient which contains algorithm for either fixed or automatic titration mode. Statistical test: paired t test |
|
| Participants | N = 38 participants (30 completed the study and contributed to the analysis) (27 M/3 F). Mean age: 49 years; BMI: 31 kg/m2; ESS: 12.7; AHI: 41.1 Inclusion criteria: AHI > 10 events/hour Exclusion criteria: CHF; chronic rhinitis; other sleep disorders |
|
| Interventions | Auto‐CPAP versus fixed CPAP No washout period described Study duration: 2 x 4‐week treatment periods |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Quote: "Study supported by MADELA AG, distributors of Respironics products in Switzerland". No author conflicts provided. |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Treatments given in "random order" |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Low risk | Quote: "Patients and attending physicians were blinded to treatment modes and order of application." |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Low risk | Quote: "attending physicians were blinded to treatment modes and order of application." |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Non‐completers excluded from the analysis (N = 8) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
Patruno 2007.
| Methods | Randomised, parallel group trial | |
| Participants | N = 31 participants (25 M/ 6 F). Mean age: 48 years; BMI: 36.5kg/m2; AHI: 47; ESS: 15 Inclusion criteria: AHI > 20; ESS > 12 Exclusion criteria: not specified |
|
| Interventions | Auto‐CPAP versus fixed CPAP Study duration: 12 weeks |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Quote: "This work was supported by a University of Milan Fondo Interuniversitario per la Ricerca Scienfifica e Technologia Grant and a Minister for Instruction, University and Research Progetto di Ricerca di Interesse Nazionale 2003 grant to Dr. Montano. The authors have no financial or other potential conflicts of interest to disclose." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After CPAP titration, all subjects were randomised to receive either fixed‐level CPAP for 3 months." |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Unclear risk | Information not available |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 40 participants recruited; 9 withdrew (no information on which groups they were assigned to); 3 of these participants withdrew due to poor compliance |
| Selective reporting (reporting bias) | High risk | Data on ESS not presented: requested from authors along with details of randomisation and withdrawals |
| Other bias | Low risk | No concerns identified |
Powell 2012.
| Methods | Parallel group, randomized, double‐blind, controlled study | |
| Participants | N = 48 participants (37 M/9 F). Age: 55 years; BMI: 33 kg/m2; ESS: 9 Inclusion criteria: 21 to 75 years; AHI > 15; suboptimal CPAP titration after at least 3 hours of attempted titration Exclusion criteria: major medical or psychiatric illness; chronic respiratory failure; upper airway/ENT surgery in last 90 days; shift workers; alcohol/drug abuse within last three years; hypnotic use < 3 months; PLMI > 10 per hour; complex/central sleep apnoea; CPAP contraindication |
|
| Interventions | Auto bi‐PAP (nasal) versus fixed CPAP Study duration: 90 days |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Quote: "This study was supported by a grant from Phillips Respironics. Dr. Powell has received research support from Philips Respironics, Inc, Fisher – Paykel Healthcare, and Takeda Pharmaceuticals Dr. Malhotra has received consulting and/or research support from NIH, AHA, Philips, Medtronic, Apnex, SHC, SGS, Apnicure, Galleon, Pfizer, Merck, Cephalon, and Sepracor. Dr. Litinski has received research support from the American Sleep Medicine Foundation. Dr. Gay has received research support from Philips Respironics, Inc. Dr. Ojile has indicated no financial conflicts of interest." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Urn randomization was used for treatment group placement using the variables of gender, age, diagnostic AHI, and education" |
| Allocation concealment (selection bias) | Low risk | Quote: "Urn randomization was used for treatment group placement using the variables of gender, age, diagnostic AHI, and education" |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Low risk | Quote: "The participant, investigator, respiratory therapist, and research staff were all blinded to the therapy group" |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Low risk | Quote: "The participant, investigator, respiratory therapist, and research staff were all blinded to the therapy group" |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "A total of 48 participants were randomized into the trial......A total of 48 participants were analyzed for the primary endpoint on an intent‐to‐treat basis" |
| Selective reporting (reporting bias) | High risk | Study protocol indicates that AHI was measured but this was not reported in main trial publication. |
| Other bias | Low risk | No concerns identified |
Pépin 2009.
| Methods | Multicentre, randomised, controlled, double‐blind trial | |
| Participants | N = 218 (172 M/46 F). Age: 55 years; BMI: 31 kg/m2; AHI: 44; ESS: 11.5 Inclusion criteria: newly diagnosed sleep apnoea patients over 18 years of age who were referred for CPAP Exclusion criteria: pregnancy, medically unstable, predominantly central sleep apnoea |
|
| Interventions | Comparing effect of C‐Flex versus fixed CPAP Study duration: 3 months |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Quote: "This work was supported by grants from 'Comite ´ National des Maladies Respiratoires' and Respironics. Author conflicts not reported." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Randomization was performed by a computer‐generated schedule in random blocks of six and was stratified according to study centers' |
| Allocation concealment (selection bias) | Low risk | 'Randomization was performed by a computer‐generated schedule in random blocks of six and was stratified according to study centers' |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Low risk | 'Patients were not aware of whether they were receiving CPAP with or without C‐Flex activated'.........'The investigators who assessed outcome were unaware of the randomization status of the patients' |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Low risk | 'The investigators who assessed outcome were unaware of the randomization status of the patients and did not set up or maintain the machines' |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 'The data for all outcomes were analyzed on an intention‐to treat basis'. Balanced but high rates of withdrawal. Reasons for withdrawal include refusal to continue with treatment |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
Pépin 2016.
| Methods | Single‐centre, randomised controlled, double‐blind, parallel group trial | |
| Participants | N = 322 participants (225 M/97 F). Age: 58; BMI: 30 kg/m2 AHI: 38.8 Inclusion criteria: age: 18 to 80 years, capable of providing written informed consent, patients claiming social insurance and patients with OSA needing CPAP treatment Exclusion criteria: cardiac failure known and treated, central apnoea syndrome, patients who stopped CPAP treatment in the previous year, pregnancy, patients under guardianship, imprisoned patients, patients in hospital, patients included in another clinical study |
|
| Interventions | Fixed versus auto‐CPAP Study duration: 4 months |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Quote: "The study was funded by the Fondation Agir pour les maladies chroniques. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication." Author conflicts of interest: none declared |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was conducted by...a computer‐generated random numbers list (six patients per block)." |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was conducted by telephone call to a clinical trials statistician, independent of the study..." |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Low risk | Quote: "All patients, investigators and outcome assessment technicians were blinded to the arms to which the patients were allocated." |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Low risk | Quote: "All patients, investigators and outcome assessment technicians were blinded to the arms to which the patients were allocated." |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High and imbalanced rate of withdrawal between intervention groups. Multiplie imputation method used to replace missing values from ITT analysis. |
| Selective reporting (reporting bias) | High risk | Quality of life, as measured by SF‐36 was described as not different between the two arms. The trial registry record indicated that symptoms was also measured but this was not reported in the trial publication. |
| Other bias | Low risk | No concerns identified |
Randerath 2001.
| Methods | Randomised, cross‐over study. ANOVA test with Bonferroni correction used (for differences between baseline and treatment mode, and for differences between treatment mode) | |
| Participants | N = 52 participants (45 M/7 F). Mean age: 54.7 years; BMI: 32.4 kg/m2; AHI 35.1 Inclusion criteria: confirmed OSA by polysomnography Exclusion criteria: prior treatment with CPAP |
|
| Interventions | Auto‐CPAP versus fixed CPAP. No washout Study duration: 2 x 6‐week treatment periods 24‐hour telephone helpline was at the disposal of the participants |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Quote: "The devices were supplied by the Weinmann Company, Hamburg, Germany." No author conflicts provided | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Low risk | Quote: "Only the person who managed the randomisation knew about the order of the treatment modes in the individual patients. This person adjusted the devices to the different treatment modes." |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Low risk | Quote: "Those who did the questioning and cared for the patients in the hospital and sleep laboratory and those who evaluated results of PSG were blinded to the mode of treatment applied.........The patients were not informed about the sequence in which the two treatment modes were applied." |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Low risk | Quote: "Those who did the questioning and cared for the patients in the hospital and sleep laboratory and those who evaluated results of PSG were blinded to the mode of treatment applied." |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5/52 participants withdrew; their data were excluded from analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
Reeves‐Hoché 1995.
| Methods | Randomised, parallel group trial | |
| Participants | N = 83 (gender available for 62 completers: 45 M/17 F). Mean age: 47; BMI: 40 kg/m2; AHI: 51 Inclusion criteria: OSA diagnosed according to American Sleep Disorders Association AHI > 10; "heavy snoring"; excessive daytime sleepiness Exclusion criteria: concomitant illness requiring hospitalisation 6 months previously; psychiatric illness; pregnancy |
|
| Interventions | Bi‐PAP versus CPAP administered at home Study duration: 52 weeks Prescribed inspiratory pressure was 11 mmHg ± 0.3 and expiratory pressure was 7 mmHg ± 0.3 in the BiPAP group versus 10 mmHg ± 0.2 in the fixed CPAP group at baseline |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | 'Supported in part by Respironics'. Author conflicts not provided. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated numbers |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Unclear risk | Information not available |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Non‐completers did not contribute to analysis |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Low risk | No concerns identified |
Resta 2004.
| Methods | Randomised, parallel group trial. Single‐blinded study | |
| Participants | N = 20 participants (18 M/2 F). Mean age: 47 years; BMI: 37; ESS: 14 Inclusion criteria: untreated OSA; PSG‐confirmed diagnosis of OSA (ASDA criteria) Exclusion criteria: not reported |
|
| Interventions | Auto‐CPAP versus fixed pressure CPAP. CPAP titration undertaken manually in sleep laboratory Study duration: 4 weeks |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Not provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; other information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Quote: "...random, single‐blind fashion..." |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Single‐blind study |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
Rochford 2006.
| Methods | Randomised, cross‐over study. Statistical analysis: information not available | |
| Participants | N = 13 participants (10 M/3 F). Mean age: 48.2 years; AHI: 22.5; ESS: 11.2 Inclusion criteria: newly diagnosed OSA patients Exclusion criteria: not reported |
|
| Interventions | Auto‐CPAP (Autoset Spirit, ResMed) versus fixed CPAP Auto‐CPAP (APAP, Compumedics) versus fixed CPAP Study duration: 3 x 4‐week duration. 2‐week washout |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Not available (conference abstract) | |
| Notes | Study available as conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Unclear risk | Information not available |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Unclear risk | Information not available |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Unclear risk | Information not available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information not available |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Unclear risk | Information not available |
Rohling 2011.
| Methods | Single‐blind, randomised, cross‐over trial | |
| Participants | N = 33 participants. Mean age: 52; BMI: 30.6 kg/m2; AHI: 35; ESS: 7.5 Inclusion criteria: age > 18 years, CPAP‐naive with diagnosis of OSA, understand Dutch language, AHI > 15 events per hour with mild sleepiness or AHI > 5 events/hour with moderate/severe sleepiness Exclusion criteria: central sleep apnoea, Cheyne‐Stoke Respiration, severe nasal obstruction, facial/pharyngeal abnormalities, shift work, psychiatric disorder, heart failure, COPD, seizure disorder, pregnancy, learning disability |
|
| Interventions | Pressure restricted auto‐adapting CPAP versus fixed CPAP Study duration 2 x 12 weeks |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Not available (conference abstract) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "By block randomisation of a statistical program (Randomisation program BSR, Windows version 5.0) with a block size of 4‐4‐8" |
| Allocation concealment (selection bias) | Low risk | Quote: "Both the patient and the person who recruited the participants were unaware of the allocation sequence." |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Quote: "The trial was fully blinded to the patients. It was not possible to fully blind the investigator performing the analysis." |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Quote: "It was not possible to fully blind the investigator performing the analysis...The registered technologists were not aware of the group allocation of the patients. However they could deduct therapy as the PAP‐pressure was recorded during the sleep study." |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | In both groups 3 drop‐outs occurred. 17 subjects completed 6 weeks of CPAP with subsequently 6 weeks of RAPAP and 16 subjects completed 6 weeks of RAPAP with subsequently 6 weeks of CPAP. |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Low risk | No concerns identified |
Rostig 2003.
| Methods | Randomised, cross‐over study. Statistical analysis methods not reported. | |
| Participants | N = 30. No baseline details provided. Participants were on long‐term CPAP for OSA, but were using it for less than 4 hours per night. |
|
| Interventions | Auto‐CPAP (AutoSet T) versus fixed pressure CPAP Study duration: 2 x 4‐week treatment periods |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Not available (conference abstract) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Unclear risk | Information not available |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Unclear risk | Information not available |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Unclear risk | Information not available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information not available |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Unclear risk | Information not available |
Ruhle 2011.
| Methods | Randomised, cross‐over study | |
| Participants | N = 51 participants (gender breakdown available for 44 completers: 39 M/5 F). Age 51.5; BMI: 30.9 kg/m2; AHI: 43; ESS 10.3 Inclusion criteria: all patients referred with OSA, aged between 30 and 80 and without nasal or throat complaints Exclusion criteria: > 5 central apnoeas per hour of sleep, acute infection, NYHA III or IV heart failure, acute pulmonary embolism or acute coronary syndrome. Previous use of CPAP |
|
| Interventions | CPAP with heated humidification versus CPAP without heated humidification Study duration: 2 x 4 weeks |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Quote: "K‐H. Ruhle and G. Nilius received research funding from Fisher & Paykel Healthcare, Heinen und Löwenstein, ResMed and Weinmann. This funding has gone into department funds. The author's study was supported by a grant from Fisher & Paykel Healthcare Germany GmbH & Co. KG, 73636 Welzheim, Germany.Karl‐Josef Franke and Ulrike Domanski have no financial or other potential conflict of interest associated with this investigation." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study had a randomised, cross‐over design and used a previously prepared randomisation list. |
| Allocation concealment (selection bias) | Low risk | Quote: "The patients were told about the group allocation only after consent. The recruiting doctor was blinded to the randomisation." |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Quote: "However, it must be kept in mind that patients knew when cHH was used and this may impact on the reported symptoms" |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Quote: "The outcome assessor who calculated the study results was not blinded to the allocation." |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Fifty one patients were recruited, 7 dropped out during the course of the study, of which 5 were allocated to the humidification group and 2 in the CPAP group.....44 patients completed the study." |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | No concerns identified |
Ryan 2009.
| Methods | Randomised, parallel group trial | |
| Participants | N = 125 participants (116 M/9 F). Age: 48; BMI: 35 kg/m2; AHI: 36; ESS: 12.5 Inclusion criteria: AHI > 10, CPAP‐naive, successful nasal CPAP titration study, adequate nasal breathing Exclusion criteria: Bi‐PAP or supplemental oxygen; malignant disease; psychiatric disease; regular use of narcotics; sedatives or psychoactive substances |
|
| Interventions | Standard (dry) CPAP versus CPAP with heated humidification versus CPAP with nasal steroid spray Study duration: 4 weeks |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Quote: "This was not an industry supported study. The authors have indicated no financial conflicts of interest." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation was used to randomise patients to different treatment groups |
| Allocation concealment (selection bias) | Low risk | Randomisation occurred centrally |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Quote: "However, patients were not blinded, since blinding would be difficult to achieve and requiring the use of placebo humidification, which has also been a limitation of previous studies." |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Although the investigators administering questionnaires and downloading data from the devices were blinded to the treatment arm, participants rating symptoms and other subjective outcomes would have been aware of the treatment group. |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "All analyses were undertaken using the intention‐to‐treat principle" Quote: "Of the 123 patients participating in the study, 112 subjects (91%) completed the trial... Of the 10 patients wishing to discontinue CPAP, 5 were randomized to dry treatment, 2 were commenced on additional nasal steroid, and 3 were started on additional humidification [p = 0.207])Quote: " For dichotomous outcomes the difference in drop out was low and unlikely to affect the size or direction of effect. For machine usage and symptom scores dropouts may have influenced the results. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
Senn 2003.
| Methods | Randomised, cross‐over study. Method of randomisation not reported. Statistical methods: ANOVA. Unclear how within subject design accounted for in analysis. |
|
| Participants | N = 31 participants. Withdrawals: N = 2. (23 M/6 F). Mean age: 53 years; BMI: 33.3 kg/m2; AHI: 45.8; ESS: 14.2 Inclusion criteria: AHI > 10 per hour; CPAP‐naive |
|
| Interventions | Auto‐CPAP (DeVilbiss ‐ response to apnoeas and snoring) and AutoSet T ‐ response to apnoea and snoring + flow limitation) versus fixed CPAP Study duration: 2‐week run‐in with either auto‐CPAP device. 3 x 4 week treatment periods. |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Quote: "Supported by the Lung League of Zurich, Lung League of Schaffhausen, Lamprecht AG & Labhardt AG". Author conflicts not provided. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; no other information available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Single‐blind, cross‐over study. Quote: "Patients...... were blinded to exact study purpose and treatment modes." |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Single‐blind study |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition rate (2 participants/31); reasons for missing data given as lack of time (N = 1), and moving away from area (N = 1) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
Soudorn 2016.
| Methods | Prospective, single‐blinded, randomised, cross‐over study in climate with a high humidity level. Data analysed with paired t test | |
| Participants | N = 20 participants. (M/F 14/6). Mean age 48.9 years; BMI 28.1 kg/m2; AHI 53.7; ESS 11.5 Inclusion criteria: age > 18 years; AHI > 15 on split‐night polysomnogarphy; nasopharyngeal symptoms according to modified XERO questionnaire Exclusion criteria: > 5 central apnoeas per hour; acute infection; heart failure with NYHA class 3 or 4; acute pulmonary embolus; acute coronary syndrome; travel outside of Thailand within 2 months of study baseline pattern of split‐night PSG < 2 hours, less than optimal CPAP titration, use of humidification during split‐night study |
|
| Interventions | CPAP with heated humidification versus conventional CPAP alone Study duration: 2 x 4 weeks |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Quote: "This work was supported by the Ratchadaphiseksomphot Endowment Fund of Chulalongkorn University. All CPAP machines and related equipment were sponsored by Fisher and Paykel Healthcare Limited. However, the company had no impact on study design or interpretation of the results of the study.SR has disclosed relationships with Sanofi,Medtronic, and Merck. The other authors have disclosed no conflicts of interest." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were randomly assigned by bloc sizes of 4 to receive CPAP with or without heated humidification". Insufficient information available. |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomisation sequence was based on the random order which was in the sealed envelope prepared by our research statistician and the investigators who conducted the study and the patients were not aware of." |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Quote: "Single blind randomized cross‐over study......the subjects were blinded to the treatment arm." |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Single‐blind study design |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Twenty‐two were subjects enrolled.....Quote: "2 more subjects dropped out from the study in the first week after CPAP use, and the reasons for dropout were CPAP refusal." Rate of withdrawal could potentially have influenced some outcomes |
| Selective reporting (reporting bias) | Low risk | All outcomes reported (quality of life and AHI reported as medians so could not be used in meta‐analysis) |
| Other bias | Low risk | No concerns identified |
Sériès 1997.
| Methods | Randomised, single‐blind, parallel group study | |
| Participants | N = 36 No dropouts. Age range 36 to 65; AHI: 43.6; ESS: 15.5 Inclusion criteria: OSA confirmed by polysomnography and by clinical features; participants chosen to be treated by CPAP Exclusion criteria: life‐threatening OSA (severe hypersomnolence); OSA associated with non‐obstructive breathing disorders (narcolepsy); estimated pressure < 15 cmH2O. All participants were recruited from the Hôpital Laval sleep clinic. |
|
| Interventions | Auto‐CPAP 1 (measured effective pressure based upon polysomnography) versus Auto‐CPAP 2 (effective pressure estimated by prespecified formula) versus fixed CPAP. Data entered from Auto‐CPAP 1. Study duration: 3 weeks |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Funded in part by Pierre Medical France. Author declarations not provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table (block of 3) |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Single‐blind study |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Single‐blind study |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Low risk | No concerns identified |
Sériès 2001.
| Methods | Randomised, parallel group trial | |
| Participants | N = 48. 40 had previously participated in other trials of auto and fixed CPAP. Mean age: 48; BMI: 39.5 kg/m2 Inclusion criteria: PSG‐diagnosed OSA Exclusion criteria: corrective surgery for OSA |
|
| Interventions | Auto‐CPAP (Morphée) versus fixed CPAP Study duration: 3 weeks |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Not provided | |
| Notes | TJL emailed for details of randomisation and outcome data 8 September 2008. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "This was done using a binary randomisation list provided by our statistician who was not involved in the study." |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Some participants had previously participated in a study and could have become aware of different devices irrespective of masking treatment group assignment |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Some participants had previously participated in a study and could have become aware of different devices irrespective of masking treatment group assignment |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Most outcomes in this review unaffected by the following exclusions: Quote: "The sleep stage‐ and body position‐dependence could not be characterized in 15 patients who did not change body position or whose sleep architecture did not include REM sleep during baseline sleep recording. These subjects were therefore excluded from the comparison between sleep stages/body position‐dependent and independent patients." |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
Teschler 2000.
| Methods | Randomised, double‐blind, cross‐over study Two‐factor repeated measures analysis of variance (AVOVA). Order of testing (fixed CPAP versus auto‐CPAP first) taken as one fixed factor, mode of treatment (fixed CPAP versus auto‐CPAP as second factor) |
|
| Participants | N = 10 participants (10 M). Mean age 52 years; AHI 52.9 Inclusion criteria: > 20 AHI, residence < 50 km from clinic and newly diagnosed with OSA Exclusion criteria: co‐existing airways disease (asthma/COPD), rhinitis or cardiac failure |
|
| Interventions | Auto‐CPAP versus fixed CPAP. No washout period Study duration: 2 x 8‐week treatment periods |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Not provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; no other information available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Low risk | Quote: "The staff were blinded as to whether the machine was in auto or conventional mode. Patients were not told in which mode the machine was operating." |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Low risk | Study had double‐blind design. |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
To 2008.
| Methods | Randomised, cross‐over study. Statisitical analysis based on paired t test. | |
| Participants | N = 43 participants (2 lost to follow‐up). BMI: 28.7 kg/m2; AHI: 54.3; ESS: 13.4 Inclusion criteria: 18 to 65 years; newly diagnosed OSA (AHI > 30) Exclusion criteria: prior treatment for OSA |
|
| Interventions | Auto‐CPAP versus fixed CPAP Study duration: 2 x 8 weeks (washout: 1 week) |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Quote: "The authors declared no conflict of interest between ResMed Company and the participating institutions, which received no external funding support for this study." | |
| Notes | TJL emailed for clarification of data from study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were assigned by random table allocation into one of the two arms of the study." |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed by an investigator external to the trial. The sequence of treatment group assignment was concealed from investigators conducting the screening and ongoing assessments. |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Open‐label study |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low rate of exclusions from the analysis (N = 2) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
Vennelle 2010.
| Methods | Randomised, blinded, cross‐over trial. Statistical analysis based on paired tests. | |
| Participants | N = 200 participants (154 M/46 F). Mean age 50; BMI 34.5 kg/m2; AHI: 33; ESS 14 Inclusion criteria: ESS > 10 or sleepiness while driving; AHI > 15 on PSG or > 25 apnoeas/hypopneas per hour on limited sleep study; age 18 to 75; CPAP naive Exclusion criteria: neurological deficit compromising CPAP use; significant comorbidity; co‐existing narcolepsy/periodic limb movements; contraindication to CPAP |
|
| Interventions | Fixed pressure versus variable pressure CPAP Study duration: 2 x 6 weeks |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Quote: "This study was supported by a grant from ResMed, Poway, CA. Dr. Douglas is a shareholder in ResMed. The other authors have indicated no additional conflicts of interest. The study was proposed, designed and all analysis performed solely by the authors." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized...using a randomization schedule of balanced blocks..." |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomized...by a worker otherwise uninvolved in the trial." |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Low risk | Quote: "Patients were informed that 2 different methods of giving CPAP were to be assessed, but were not told which was the new method" Quote: "None of the staff involved in data acquisition or analysis were aware of the mode of treatment the patient was receiving" |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Low risk | Quote: "The blinded staff member conducted the follow‐up assessments unaware of the type of CPAP the patient was using at the time of assessment." |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Nineteen patients did not complete the study; at the time of dropout 9 of these were receiving fixed and 10 variable pressure CPAP" Balanced drop out which could impact on machine usage and symptoms |
| Selective reporting (reporting bias) | High risk | Side effects were rated by the Edinburgh questionnaire but not reported. |
| Other bias | Low risk | No concerns identified |
Wenzel 2007.
| Methods | Randomised, single‐blind, cross‐over study (participants not informed of order/setting). Statistical analysis based on Wicoxon methods (not specified if paired). | |
| Participants | N = 20 participants completed and analysed (16 M/4 F). AHI: 45; ESS: 10.9 Inclusion criteria: new diagnosis of OSA (diagnosis established through polysomnography) Exclusion criteria: mixed or central apnoea |
|
| Interventions | CPAPexp (C‐Flex) versus fixed pressure CPAP Same machine delivered the different treatment pressure settings Study duration: 2 x 6 weeks |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Not provided | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Quote: "....randomised, single‐blinded cross‐over study" |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Quote: "...single‐blinded cross‐over study" |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Low risk | No concerns identified |
West 2006.
| Methods | Randomised, parallel group trial. Identical machines used. Withdrawals described | |
| Participants | N = 98 participants (N considered for this review: 65 (55 M/10 F). Mean age: 47; ESS: 16. Inclusion criteria: 18 to 75 years of age; ESS > 9; proven OSA (PSG); 10 dips/hr in arterial O2 saturation; CPAP‐naive Exclusion criteria: respiratory failure requiring urgent treatment; unable to give written consent Participants were not excluded on the basis of comorbidities |
|
| Interventions | Auto‐CPAP versus algorithm established fixed CPAP Additional treatment group not considered for this review: 1 week auto‐titration followed by fixed pressure at the level of 95th centile pressure from the auto‐CPAP week data. Study duration: 24 weeks |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Quote: "ResMed UK provided part financial support for the purchase of CPAP machines for the study but was not involved in its design or analysis. D Jones was supported in part by a Helen Bearpark Scholarship from the Australasian Sleep Association and by the Sleep Apnoea Trust Association (UK). None of the authors has any conflict of interest." | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated schedule (MINIM) |
| Allocation concealment (selection bias) | Low risk | Quote: "...investigators carrying out the assessment studies were blind to their group allocation." |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Low risk | Quote: "The patients and the investigators carrying out the assessment studies were blind to their group allocation." |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | Low risk | Quote: "...the investigators carrying out the assessment studies were blind to their group allocation." |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Nine participants lost to follow‐up or excluded from the analysis: Quote: "No data were entered for those who did not attend." |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
Worsnop 2010.
| Methods | Randomised, parallel group study | |
| Participants | N = 54 participants (43 M/11 F). Mean age 55 years; AHI 46. ESS 14 Consecutive OSA patients referred for CPAP, under a program paid for by the Victorian State government, were enrolled. |
|
| Interventions | Fixed pressure CPAP + humidification versus fixed pressure CPAP alone Study duration: 12 weeks |
|
| Outcomes |
|
|
| Funding & conflicts of interest statements | Quote: "Fisher and Paykel Healthcare, Auckland, New Zealand funded this study. They were not involved in the design of this study, in the collection, analysis and interpretation of data; in the writing of the report; nor in the decision to submit the paper for publication. Author conflict of interest: None." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was concealed using a statistical randomisation package so that each subject was randomised to receive either treatment independently of the other subjects." |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was concealed using a statistical randomisation package..." |
| Blinding of participants and personnel (performance bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Quote: "It was not possible to blind patients to the type of treatment they were getting" |
| Blinding of participants and personnel (performance bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) Machine usage, symptoms, quality of life, withdrawal, adverse effects | High risk | Quote: "The one CPAP therapist saw each patient at each visit. It was not possible to blind her to the treatment that the patients were getting, but she was instructed not to make any attempts to determine what type of pump each patient was using, and to try to treat each patient in a similar manner" |
| Blinding of outcome assessment (detection bias) AHI, blood pressure, treatment pressure | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All of the fifty‐four subjects completed the study" |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
ABRP‐PAP: automatic bi‐level therapy with pressure relief; AHI: Apnoea Hypopnoea Index; ATS: American Thoracic Society; auto‐CPAP: auto‐titrating CPAP; Bi‐PAP: bilevel positive airway pressure; BMI: body mass index; COPD: chronic obstructive pulmonary disease; CPAP: continuous positive airway pressure; CPAPexp ‐ CPAP with expiratory pressure relief; DI: desaturation index; ESS: Epworth Sleepiness Scale; FEV1: forced expiratory volume in one second; FOSQ: Functional Outcomes of Sleep Questionnaire; FOT: forced oscillation technique; FVC: forced vital capacity; ITT: intention‐to treat‐analysis; MWT: Maintenance of Wakefulness Test; nCPAP: nasal CPAP; NIV: non‐invasive ventilation; NYHA: New York Heart Association; OSA: obstructive sleep apnoea; PAP: positive airway pressure; PSG: polysomnography; RDI: respiratory disturbance index; REM: rapid eye movement; SaO2: oxygen saturation; SAQLI: Sleep Apnoea Quality of Life Index; SF‐36: Short‐Form 36 quality of life questionnaire; TST: total sleep time; UARS: upper airway resistance syndrome; UPPP: WASO: wake after sleep onset
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Al Zuheibi 2013 | No fixed CPAP arm |
| Almasri 2007 | Study of different humidifying units plus CPAP |
| Aloia 2001 | CBT |
| Aloia 2004 | Review article |
| Aloia 2005 | CPAP or C‐Flex given in a sequential, non‐randomised order |
| Aloia 2005a | Not randomised |
| Anderson 2003 | Study assessing oral versus nasal interface of CPAP |
| Bachour 2004 | Study assessing chinstrap over a 2‐night laboratory titration study |
| Ball 2011 | No fixed CPAP arm; study duration 2 days |
| Bardwell 2007 | Placebo‐controlled trial |
| Becker 1991 | Non‐randomised study of treatment failure in central sleep apnoea |
| Becker 1998 | Review article |
| Berry 2002 | Review article |
| Berthon‐Jones 1996 | Non‐randomised study of APAP for OSA treatment |
| Bielicke 2008 | Comparison of effects of auto‐titrating CPAP (APAP) versus auto‐titrating CPAP with expiratory pressure relief (A‐Flex) on AHI. No fixed CPAP arm, study duration 2 nights |
| Blau 2009 | Comparison of auto‐CPAP with A‐Flex (auto‐CPAP with pressure relief during expiration) |
| Boudewyns 1999 | Non‐randomised study of CPAP treatment |
| Bradshaw 2004 | Effect of nose drops |
| Brammer 1999 | Not randomised |
| Buyse 2003 | Different algorithms of auto‐CPAP compared |
| Canisius 2007 | Inadequate duration |
| Chan 2004 | Study assessing interface chamber of CPAP |
| Chervin 1997 | Educational/psychosocial intervention |
| Chihara 2012 | No fixed CPAP arm |
| Colrain 2007 | Inadequate duration |
| Constantinidis 2000 | Non‐randomised study of nasal mucosal tissue changes with CPAP treatment |
| Coughlin 2004 | CPAP versus subtherapeutic pressure of CPAP |
| Cross 2005 | Study assessing efficacy of CPAP |
| Cumin 2011 | Overnight study only |
| Damjanovic 2005 | Educational/psychosocial support |
| Delwiche 2003 | Comparison between different auto‐CPAP devices |
| Dungan 2010 | Overnight study only; no fixed CPAP arm |
| Duntley 2005 | One‐night study |
| Duoung 2005 | One‐night study |
| e Bastos 2013 | No fixed CPAP arm |
| Engleman 1993 | Non‐randomised study of objective compliance measure of CPAP use |
| Epstein 2000 | Educational/psychosocial intervention |
| Feenstra 2005 | Assessment of nose drops on CPAP machine usage |
| Ficker 1997 | Laboratory‐based study |
| Ficker 1998 | Laboratory‐based study |
| Ficker 2000 | Laboratory‐based study |
| Fletcher 1991 | Educational/psychosocial intervention |
| Fleury 1996 | Non‐randomised study of CPAP compliance |
| Gagnadoux 1999 | Non‐randomised study on effectiveness of AutoSet to determine treatment pressure |
| Galetke 2006 | Manual versus auto‐titrating study |
| Galetke 2008a | No fixed CPAP arm |
| Galetke 2016 | Control group received humidification in addition to fixed pressure CPAP |
| Goncalves 2006 | Inadequate duration |
| Greenfield 2005 | Placebo control |
| Grote 2000 | Non‐randomised study on CPAP compliance |
| Gupta 2011 | Not a comparative trial of pressure modification devices in OSA |
| Herold 2007 | Participants randomised to receive auto‐CPAP as a titration strategy |
| Hertegonne 2003 | Laboratory‐based titration study |
| Hertegonne 2006 | Split‐night titration study |
| Horvath 2008 | Different levels of Bi‐PAP compared |
| Hosselet 1999 | Review article |
| Hoster 1996 | Laboratory‐based study |
| Hostler 2014 | No fixed CPAP arm |
| Hoy 1999 | Educational/psychosocial intervention |
| Huang 2001 | Non‐randomised study |
| Hui 2000 | Educational/psychosocial intervention |
| Hui 2001 | Non‐randomised study of CPAP effectiveness |
| Hui 2006 | Different pressure levels of CPAP compared (therapeutic and subtherapeutic) |
| Hukins 2005 | Different titration strategies compared |
| Husain 2003 | No fixed CPAP control group |
| Juhàsz 2001 | Two‐night laboratory titration study |
| Khanna 2003 | Comparison outside the focus of the review: oral versus nasal interface |
| Khayat 2007 | Participants with significant cardiac comorbidity |
| Krieger 1992 | Observational study |
| Krieger 1998 | Non‐randomised study on CPAP compliance following simplified diagnostic procedure for OSA |
| Krieger 1999 | Review article |
| Likar 1997 | Non‐randomised study of CPAP compliance |
| Liu 2007 | Inadequate duration |
| Loberes 2004 | Study assessing the effects of daytime CPAP titration |
| Lopez‐Martin 2005 | Not assessment of pressure modification |
| Loube 2003 | Laboratory based titration study |
| Mador 2005 | Randomisation between immediate provision of humidification and delayed provision of humidification |
| Mansfield 2003 | Participants randomised to CPAP or inactive control |
| Marshall 2003 | Not assessment of pressure modification |
| Masa 2004 | Different titration strategies compared |
| Massie 1999 | No control group receiving only fixed pressure CPAP |
| McArdle 2010 | Comparison of effects of manual titration versus laboratory APAP titration versus home APAP titration on CPAP compliance. Participants switched to fixed CPAP after titration study |
| McNicholas 1997 | Editorial |
| Meurice 1994 | Non‐randomised study of CPAP compliance |
| Meurice 1998 | Randomised comparison of 2 types of auto‐CPAP |
| Meurice 2007a | Study of educational interventions |
| Montserrat 2006 | Inadequate duration |
| Morley 2001 | Journal correspondence |
| Mortimore 1998 | Randomised trial comparing nose and face mask CPAP therapy |
| Mulgrew 2005 | Different diagnostic strategies compared |
| Mulgrew 2006 | Inadequate duration |
| Munoz 2009 | No fixed CPAP arm |
| Murray 2002 | Responder analysis |
| Neale 2011 | Randomised trial comparing 6 autoadapting CPAP devices in patients previously treated with fixed CPAP. Fixed CPAP arm not run concurrently with auto‐CPAP arms |
| Nolan 2006 | Randomisation between different auto‐titrating CPAP machines; data from fixed CPAP machines captured from start of trial |
| Palasiewicz 1997 | Randomised study conducted when participants were awake |
| Peach 2003 | Educational/psychosocial intervention |
| Penzel 2004 | Laboratory‐based study |
| Pevernagie 2004 | No fixed CPAP control |
| Pierce 2005 | Different APAP therapies compared |
| Pilz 2000 | Laboratory‐based study |
| Piper 2008 | Participants recruited with obesity hypoventilation syndrome |
| Planès 2003 | Randomised trial comparing auto with fixed pressure CPAP. This trial was excluded as an educational intervention administered at baseline was not standardised between the two treatment groups. Titration was also performed in different settings for auto and fixed pressure CPAP. |
| Powell 2014 | No fixed CPAP arm |
| Pradeepan 2017 | Study in people with positional OSA |
| Pépin 1995 | Non‐randomised trial on side effects of nasal CPAP therapy |
| Rains 1996 | Non‐randomised study assessing educational interventions in 4 children with OSA (PsycINFO) |
| Randerath 1999 | No fixed CPAP arm |
| Randerath 1999b | This study compared different media for informing patients about CPAP. Overnight study |
| Randerath 2001a | Laboratory‐based study |
| Randerath 2003 | No fixed CPAP arm |
| Richards 2007 | Study of CBT |
| Rosenthal 2001 | This study was excluded as participants were prescribed CPAP machines set at different hours of use (< 6.5 hours and > 7.5 hours) |
| Rosenthal 2012 | No fixed CPAP arm |
| Rubio 2015 | Inadequate duration |
| Salgado 2006 | Humidification added to APAP. No fixed pressure comparator |
| Scharf 1996 | No attempt to measure compliance |
| Sharma 1996 | Overnight study |
| Signes‐Costa 2005 | Assessment of different strategies to diagnose and manage OSA |
| Sin 2002 | Non‐randomised cohort study on the effects of a complex intervention on patient compliance with CPAP therapy |
| Speer 2012 | No fixed CPAP arm |
| Stammnitz 2004 | Laboratory‐based study |
| Suzuki 2007 | Participants randomised to auto‐CPAP or no treatment as a means of titration prior to fixed pressure CPAP |
| Taylor 2003 | Assessment of telemedicine intervention |
| Torvaldsson 2003 | Inadequate duration (2 x 1 week treatment arms) |
| van der Aa 2003 | Different titration strategies |
| Walter 2003 | No fixed CPAP arm |
| Wiese 2005 | Educational/behavioural intervention |
| Wiest 1999 | No fixed CPAP arm |
| Wiest 2002 | 2‐night titration study |
| Wimms 2013 | Comparison of S9 (humidification with autoadjusting CPAP) versus CPAP. Not a randomised trial |
AHI: Apnoea Hypopnoea Index; APAP: CBT: cognitive behavioural therapy; CPAP: continuous positive airway pressure; OSA: obstructive sleep apnoea
Characteristics of studies awaiting assessment [ordered by study ID]
Boyer 2019.
| Methods | Multicentre cross‐over study |
| Participants | N = 40 participants (23 M/17 F); Age: 62.4 years; BMI: 30.7 kg/m2; AHI: 46.7; ESS 8.6; FOSQ 10 29. Inclusion criteria: diagnosis of OSA (AHI >30 (or less than 30 if respiratory arousal index >10 events/hour); no prior treatment with CPAP; using medicine known to induce nasal dryness; previous nasal symptoms or nasal surgery. Exclusion criteria: heart failure (New York Heart Association level 3 or 4), lung disease; pregnancy; prior treatment for OSA; >5 central oxygen saturation/hour. |
| Interventions |
Study duration: 4 weeks per treatment period |
| Outcomes |
|
| Notes |
NCT02749812.
| Methods | Open‐label, randomised controlled trial |
| Participants | 800 adult participants not previously treated with CPAP. AHI in excess of 30 events per hour |
| Interventions | Auto CPAP and fixed CPAP for a period of 3 months |
| Outcomes |
The study objective is to relate effective treatment pressure with clinical outcomes and polysomnography measurements. |
| Notes | NCT02749812 record states that study completed in September 2015. Date registered as April 2016. Status update (November 2018): manuscript undergoing revision by author team. Likely publication date 2nd quarter 2019. |
Zamora 2019.
| Methods | Part of large adherence trial |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Conference abstract |
AHI: Apnoea Hypopnoea Index; BMI: body mass index; CPAP: continuous positive airway pressure; ESS: Epworth Sleepiness Scale; FOSQ: Functional Outcomes of Sleep Questionnaire; OSA: obstructive sleep apnoea
Characteristics of ongoing studies [ordered by study ID]
ACTRN12617001090303.
| Trial name or title | Comparing the usage of continuous positive airway pressure (PAP) against the RACer airway device in the treatment of obstructive sleep apnoea (OSA) in naive PAP users |
| Methods | Randomised cross‐over study |
| Participants | Planned sample size: 30. Recruitment from tertiary sleep clinic in New Zealand. Inclusion criteria: age 18 to 70 years, no prior treatment with CPAP, ability to tolerate a nasal pillow mask, AHI or RDI of > 20 events per hour |
| Interventions | Standard CPAP device or Rest‐Activity‐Cycle (RACer) system used with positive airway pressure device (RACer CPAP). The RACer device delivers pressurised air into the upper airway into one nostril at a time. Both modalities are combined in a single device, although RACer mode necessitates the use of nasal pillow. Planned duration: 4 weeks of treatment on each arm either side of a 3‐day washout period between arms. |
| Outcomes |
|
| Starting date | 20/09/2017 |
| Contact information | Dr Angela Campbell WellSleep University of Otago, Wellington 98 Churchill Drive Crofton Downs, 6035 Wellington |
| Notes |
ACTRN12618000379213p.
| Trial name or title | Auto‐titrating versus fixed continuous positive airway pressure in obesity hypoventilation syndrome |
| Methods | Randomised parallel group trial |
| Participants | Planned sample size: 40 Inclusion criteria: age 18 to 80 years; BMI > 30; PaCO2 45 mmHg to 60 mmHg; blood pH 7.35 to 7.45; no use of CPAP in the past 12 months; AHI ≥ 30 |
| Interventions | Fixed CPAP versus auto‐CPAP Study duration: 12 weeks |
| Outcomes |
|
| Starting date | May 2018 |
| Contact information | Dr Yizhong Zheng
Department of Respiratory and Sleep Medicine 50 Missenden Rd Camperdown NSW 2050 Australia |
| Notes |
Morton 2001.
| Trial name or title | The effects of humidification of nasal CPAP on adherence, compliance, patient satisfaction and symptoms: a preliminary report |
| Methods | NA |
| Participants | 80 recruited. 40 completed protocol. Mean age 52.2 (10.3). 32 M: 8 F. 5 dropped out. |
| Interventions | CPAP with humidifier versus CPAP without humidifier for 3/12 |
| Outcomes |
|
| Starting date | Not stated |
| Contact information | Sharon Morton, Flinders Medical Centre Bedford Pk. |
| Notes |
NCT01753999.
| Trial name or title | None given |
| Methods | Double‐blind cross‐over randomised trial. Randomisation sequence generated by computer |
| Participants | 400 planned as per published protocol (440 planned as per CT.gov record). Participants recruited from responders exposed to dust from collapse of World Trade Centre in 2001. Inclusion criteria: nasal resistance and sleep apnoea diagnosed by home‐based study |
| Interventions | Fixed pressure CPAP and flexible CPAP (CPAPFlex) Treatment arms scheduled to last for 4 weeks in published protocol (9 weeks according to CT.gov record) |
| Outcomes |
|
| Starting date | December 2012 |
| Contact information | |
| Notes |
NCT03428516.
| Trial name or title | Decrease in sympathetic tone in OSA patients: Is CPAP more effective than APAP? |
| Methods | Randomised parallel group |
| Participants | Planned sample size: 68 Inclusion criteria: AHI ≥ 20; daytime sleepiness; no prior exposure to CPAP Exclusion criteria: serious heart failure; central sleep apnoea index above 20% of AHI; serious comorbidity |
| Interventions | Fixed CPAP versus auto‐CPAP Study duration: 4 weeks |
| Outcomes |
|
| Starting date | 1 March 2018 |
| Contact information | Renaud Tamisier, MD, PhD University Hospital Grenoble France |
| Notes |
Ventateswaren 2003.
| Trial name or title | Not available |
| Methods | Randomised, cross‐over study |
| Participants | 5 recruited |
| Interventions | Auto‐CPAP versus CPAP for 2/52 |
| Outcomes | Heart rate variability |
| Starting date | Not stated |
| Contact information | — |
| Notes | TJL emailed for data 14 November 2008 |
AHI: Apnoea Hypopnoea Index; BMI: body mass index; CPAP: continuous positive airway pressure; ESS: Epworth Sleepiness Scale; FOSQ: Functional Outcomes of Sleep Questionnaire; OSA: obstructive sleep apnoea; PaCO2: partial pressure of arterial carbon dioxide; RDI: Respiratory Disturbance Index; SF‐36: Short‐Form 36; SAQLI: Sleep Apnoea Quality of Life Index
Differences between protocol and review
For the 2019 update of the review we have identified the following additional comparisons of interest.
Automatically adjusting CPAP with expiratory pressure relief (auto‐CPAPexp) versus fixed CPAP
Automatically adjusting bilevel positive airway pressure (auto bi‐PAP) versus fixed CPAP.
CPAP with expiratory pressure relief (CPAPexp) with wakefulness detection versus fixed CPAP.
We decided to exclude studies recruiting people with positional sleep apnoea since the pressure requirement would differ from those with non‐positional obstructive sleep apnoea (OSA).
We removed oxygen saturation (SaO2) and Respiratory Disturbance Index (RDI) as outcomes in favour of blood pressure parameters and AHI which were commonly reported and more directly addressed the objectives of our review.
We removed the risk of domain relating to monitored usage based on peer review comments for the 2019 update of the review. This is likely to be an ethical requirement in most settings where the studies were conducted and does not feature as a domain in Cochrane standards or guidance.
We applied GRADE to the findings in this review and added in remaining domains for risk of bias relating to selective other reporting and other bias.
We combined data from parallel and cross‐over studies. Having reconsidered the studies in this review, we took the view that both parallel and cross‐over studies address the same question of interest in this review with regard to machine use, symptoms, objective measures of sleep disturbance and quality of life. Data on withdrawals and adverse events have been collected from parallel studies and combined only with data from the first arm of cross‐over studies, where this could be used.
Subgroup analysis
We have not conducted any subgroup analysis due to the limited amount of statistical variation overall. Our prespecified subgroups were:
population ‐ male versus female;
baseline AHI > 20 versus < 20 per hour;
study quality ‐ high versus low (based upon the allocation concealment scores);
previous usage of CPAP.
Sensitivity analyses
We decided not to assess the impact of auto‐CPAP delivered by forced oscillation technique as part of a sensitivity analysis for the 2019 update. This was done due to the very limited number of studies using this technique.
We did not carry out the sensitivity analysis looking at awareness of monitoring. Following peer review comments received on the 2019 update of the review we have come to regard this as a feature of obtaining consent during recruitment to the studies, and not study design per se. We did not carry out a sensitivity analysis because we could not satisfactorily identify a subset of studies at low risk of bias. The multiple sources of bias across domains led us to downgrade the certainty of evidence (See Table 1).
We removed the threshold I2 for proceeding with a sensitivity analysis for random‐effects modelling. On reflection, we felt that it was better to focus on the number of studies rather than the amount of variation as the basis for exploring model choice since random‐effects modelling assumes a distribution of true, related effects. We thought that this assumption is more reasonable as the number of studies increased, irrespective of the statistical heterogeneity.
Contributions of authors
BK (2019 update): study assessment, data collection and entry, write up (results section, development of discussion and conclusion sections).
TJL: study assessment, data collection and entry, write up (results section, development of discussion and conclusion sections, abstract and plain language summary).
DRW: study assessment, data collection and entry, development of results, discussion and conclusion
IS: protocol development, development of results, discussion and conclusions.
Contributions of editorial team
Rebecca Fortescue (Co‐ordinating Editor): edited the review; advised on methodology, interpretation and content; approved the final review prior to publication. Chris Cates (Co‐ordinating Editor, Contact Editor) checked the data entry prior to the full write up of the review; advised on methodology, interpretation and content. Emma Dennett (Managing Editor): co‐ordinated the editorial process; advised on interpretation and content; edited the review. Emma Jackson (Assistant Managing Editor): conducted peer review; edited the plain language summary and reference sections of the protocol and the review. Elizabeth Stovold (Information Specialist): designed the search strategy; ran the searches; edited the search methods section.
Sources of support
Internal sources
Papworth Hospital NHS Trust, UK.
St George's, University of London, UK.
External sources
The authors declare that no such funding was received for this systematic review, Other.
Declarations of interest
Barry Kennedy: none known
Toby Lasserson is employed by Cochrane.
Dariusz Wozniak: none known
Ian Smith is an investigator on the Gulati 2015 study. Since the 2009 version of the review he has not had involvement in the assessment of bias or certainty of evidence. Recevied payments for lectures from UCB Pharma, this company markets medication for sleep disorders, but none relevant to this review.
These authors contributed equally to this work
These authors contributed equally to this work
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Bakker 2010 {published and unpublished data}
- Bakker J, Campbell A, Neill A. Randomized controlled trial comparing flexible and continuous positive airway pressure delivery: Effects on compliance, objective and subjective sleepiness and vigilance. Sleep 2010;33(4):523‐9. [PUBMED: 20394322] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker J, Campbell A, Neill A. Standard versus flexible positive airway pressure delivery: effects on compliance, objective and subjective sleepiness and vigilance. Sleep and Biological Rhythms 2009;7:A65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ballard 2007 {published data only}
- Ballard RD, Gay PC, Strollo PJ. Interventions to improve compliance in sleep apnea patients previously non‐compliant with continuous positive airway pressure. Journal of Clinical Sleep Medicine 2007;3(7):706‐12. [PMC free article] [PubMed] [Google Scholar]
- Gay PC, Ballard R, Strollo PJ. Randomized multicenter trial to improve compliance in non‐adherent OSA patients using CPAP versus novel bi‐level positive airway pressure (NBPAP) [Abstract]. Sleep 2005;28:A210. [Google Scholar]
Berry 2014 {published data only}
- Berry RB, Sriram P. Auto‐adjusting positive airway pressure treatment for sleep apnea diagnosed by home sleep testing. Journal of Clinical Sleep Medicine 2014;10(12):1269‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Blau 2012 {published and unpublished data}
- Blau A, Minx M, Diecker B, Peter JG, Glos M, Baumann G, et al. Respiration and Sleep in OSA patients treated with CPAP vs.auto bilevel pressure relief ‐ PAP. Sleep 2008;31(Suppl):A191. [Google Scholar]
- Blau A, Minx M, Diecker B, Peter JG, Glos M, Gerd B, et al. Efficacy of treatment with CPAP vs. auto bilevel pressure relief ‐ PAP. European Respiratory Journal 2007;30(Suppl 51):242s. [Google Scholar]
- Blau A, Minx M, Peter JG, Glos M, Penzel T, Baumann G, et al. Auto bi‐level pressure relief‐PAP is as effective as CPAP in OSA patients ‐ a pilot study. Sleep and Breathing 2012;16(3):773‐9. [PUBMED: 21874370] [DOI] [PubMed] [Google Scholar]
Bloch 2018 {published data only}
- Bloch KE, Huber F, Furian M, Latshang TD, Lo Cascio CM, Nussbaumer‐Ochsner Y, et al. Autoadjusted versus fixed CPAP for obstructive sleep apnoea: a multicentre, randomised equivalence trial. Thorax 2018;73(2):174‐84. [DOI: 10.1136/thoraxjnl-2016-209699] [DOI] [PubMed] [Google Scholar]
- Bloch KE, Latshang TD, Nussbaumer‐Ochsner Y, Kohler M, Thurnheer R, Laube I, et al. Equivalence of autoCPAP and fixed CPAP in the long‐term treatment of obstructive sleep apnea syndrome: a randomized, controlled multicenter trial. Chest 2010;138:886A. [Google Scholar]
Bogan 2017 {published data only}
- Bogan RK, Wells C. A randomized crossover trial of a pressure relief technology (SensAwake TM) in continuous positive airway pressure to treat obstructive sleep apnea. Journal of Sleep Research 2017;2017:3978073. [DOI: 10.1155/2017/3978073] [DOI] [PMC free article] [PubMed] [Google Scholar]
Castronovo 2006 {published data only}
- Castronovo V, Marelli S, Cappa S, Fossati A, Fantni ML, Uyanik O, et al. P386 Cognitive functioning in obstructive sleep apnea (OSA) patients: effect of AutoCPAP verses fixed pressure CPAP. Sleep Medicine 2006;7:81s. [DOI: 10.1016/j.sleep.2006.07.195] [DOI] [Google Scholar]
Chang 2015 {published data only (unpublished sought but not used)}
- Chang ET, Shen YC, Wang HM. Comparison of improvement in quality of life between continuous positive airway pressure and autotitrating positive airway pressure treatment for obstructive sleep apnoea: A randomised crossover study. Tzu Chi Medical Journal 2015;27:110‐12. [DOI: ] [Google Scholar]
d'Ortho 2000 {published data only}
- d'Ortho MP, Grilier‐Lanoir V, Levy P, Goldenberg F, Corriger E, Harf A, et al. Constant versus automatic continuous positive airway pressure therapy. Chest 2000;118(4):1010‐17. [DOI] [PubMed] [Google Scholar]
Damjanovic 2009 {published and unpublished data}
- Damjanovic D, Fluck A, Bremer H, Muller‐Quernheim J, Idzko M, Sorichter S. Compliance in sleep apnoea therapy: influence of home care support and pressure mode. European Respiratory Journal 2009;33(4):804‐11. [PUBMED: 19129293] [DOI] [PubMed] [Google Scholar]
- Klein C, Damjanovic D, Idzko M, Seuthe B, Walterspacher S, Kabitz H, et al. Compliance in sleep apnoea therapy: influence of home care support and pressure mode ‐ a 5‐year follow‐up. Journal of Sleep Research 2010;19 (Suppl 2):20. [Google Scholar]
Dolan 2008 {published data only}
- Dolan DC, Okonkwo R, Gfullner F, Hansbrough JR, Strobel RJ, Rosenthal L. Longitudinal comparison study of pressure relief (C‐Flex™) vs. CPAP in OSA patients. Sleep and Breathing 2009;13(1):73‐7. [DOI: 10.1007/s11325-008-0199-1] [DOI] [PubMed] [Google Scholar]
- Rosenthal L, Hansbrough J, Zachek M, Gfuellner F, Betz S, Nash M, et al. International multi‐center long term study of treatment satisfaction and compliance in OSA, CPAP with expiratory pressure relief versus conventional CPAP [Abstract]. Sleep 2005;28:A180. [Google Scholar]
- Rosenthal L, Hansbrough J, Zachek M, Gfuellner F, Betz S, Pla‐Ferrer T, et al. International multi‐center CPAP study of split‐night titration and expiratory pressure relief long term effects on compliance and subjective satisfaction [Abstract]. Sleep 2005;28:A211. [Google Scholar]
- Rosenthal LR, Gfuenier F, Hansbrough JR, Zachek M, Strobel R. Does expiratory pressure relief influence adherence? A multicenter trial of CFLEX versus CPAP therapy [Abstract]. American Thoracic Society International Conference; 2005 May 20‐25; San Diego. 2005:C29; Poster: 524.
Ficker 2003 {unpublished data only}
- Ficker JH, Clarenbach CF, Neukichner C, Fuchs FS, Wiest GH, Pous Schahin S, et al. Auto‐CPAP therapy based on the forced oscillation technique. Biomedizinische Technik 2003;48(3):68‐73. [DOI] [PubMed] [Google Scholar]
- Ficker JH, Clarenbach CF, Neukirchner C, Fuchs FS, Wiest GH, Schahin SP, et al. Efficacy and compliance of autoadjusting CPAP therapy based on the forced oscillation technique. American Thoracic Society 98th International Conference; 2002 May 17‐22; Georgia. 2002:B21.
Fietze 2007 {published data only}
- Fietze I, Glos M, Moebus I, Witt C, Penzel T, Baumann G. Automatic pressure titration with APAP Is as effective as manual titration with CPAP in patients with obstructive sleep apnea. Respiration 2007;74:279–86. [DOI: 10.1159/000100364] [DOI] [PubMed] [Google Scholar]
Galetke 2008 {published data only}
- Galetke W, Anduleit N, Richter K, Stieglitz S, Randerath WJ. Comparison of automatic and continuous positive airway pressure in a night‐by‐night analysis: a randomized, crossover study. Respiration 2008;75(2):163‐9. [DOI] [PubMed] [Google Scholar]
Gay 2003 {unpublished data only}
- Gay PC, Herold DL, Olson EJ. A randomized, double‐blind clinical trial comparing continuous positive airway pressure with a novel bilevel pressure system for treatment of obstructive sleep apnea syndrome. Sleep 2003;26(7):864‐70. [DOI] [PubMed] [Google Scholar]
Gfüllner 2007 {published data only}
- Gfüllner F, Montalvan S, Weber G, Fuchtenbusch M, Pfeifer M. Randomized crossover study of treatment compliance and satisfaction in non‐sleepy patients with obstructive sleep apnea: CPAP with pressure relief during exhalation vs. conventional CPAP. Sleep 2007;30(Suppl):A185. [Google Scholar]
Gonzalez‐Moro 2005 {published data only}
- Gonzalez‐Moro JMR, Hernandez‐Vazquez J, Lucas‐Ramos P, Lopez‐Martin S, Rubio‐Socorro Y, Miguel‐Diez J. Management of patients with obstructive sleep apnea syndrome (OSAS) and obesity hypoventilation syndrome (OHS): CPAP vs BIPAP: a short term study [Abstract]. American Thoracic Society 2005 International Conference; May 20‐25; San Diego. 2005:C29; Poster: 525.
Gulati 2015 {published data only}
- Gulati A, Oscroft N, Chadwick R, Ali M, Smith I. The impact of changing people with sleep apnea using CPAP less than 4 h per night to a Bi‐level device. Respiratory Medicine 2015;109(6):778‐83. [DOI] [PubMed] [Google Scholar]
Heiser 2010 {published data only}
- Heiser C, Sommer JU, Stern‐Straeter J, Tillmann H‐C, Hormann K, Maurer JT, et al. Influence of air humidification on the acceptance of continuous positive airway pressure in patients with obstructive sleep apnea [Einfluss der Atemluftbefeuchtung auf die Akzeptanz der CPAP‐Therapie bie Patienten mit obstruktiver Schlafapnoe]. Somnologie 2010;14(4):282‐90. [DOI: 10.1007/s11818-010-0488-3] [DOI] [Google Scholar]
Hudgel 2000 {published data only}
- Hudgel DW, Fung C. A long‐term randomized, crossover comparison of auto‐titrating and standard nasal continuous airway pressure. Sleep 2000;23(5):645‐8. [PubMed] [Google Scholar]
Hukins 2004 {published data only}
- Hukins C. Comparative study of auto‐titrating and fixed‐pressure CPAP in the home: a randomized, single‐blind crossover study. Sleep 2004;27(8):1512‐7. [DOI] [PubMed] [Google Scholar]
- Hukins C, Davies K. Randomised controlled trial of auto‐titrating versus fixed pressure CPAP. Annual Scientific Meeting of the Thoracic Society of Australia and New Zealand; 2001 March 16‐21; Brisbane. 2001:A88.
Hussain 2004 {published data only}
- Hussain SF, Love L, Burt H, Fleetham JA. A randomized trial of auto‐titrating CPAP and fixed CPAP in the treatment of obstructive sleep apnea‐hypopnea. Respiratory Medicine 2004;98(4):330‐3. [DOI] [PubMed] [Google Scholar]
Jarvis 2006 {published data only}
- Hansford A. Individual patient data (as supplied 2008). (Data on file).
- Jarvis R, Hansford A, Little C, Qian J, Cistulli P. The clinical efficacy and comfort of modified automatic positive airway pressure (APAP) mode compared with continuous pressure (CPAP). European Respiratory Journal 2006;28(Suppl 50):410s. [Google Scholar]
Kendrick 2002 {unpublished data only}
- Kendrick AH, Wiltshire N, Harper A, Buchanan F, Catterall JR. Double‐blind randomized controlled trial of autotitrating versus fixed continuous airways pressure (CPAP) in CPAP naive patients. www.abstracts‐on‐line.com/abstracts/ATS 2002; Vol. D26:poster 910.
- Wiltshire N, Harper A, Buchanan F, Catterall JR, Kendrick AH. Autotitrating versus fixed pressure continuous positive airway pressure (CPAP): double‐blind randomized controlled trial in CPAP naive patients. Thorax 2001;Suppl iii:28. [Google Scholar]
Konermann 1998 {published data only}
- Konermann M, Sanner BM, Vyleta M, Laschewski F, Groetz J, Sturm A, et al. Use of conventional and self‐adjusting nasal continuous positive airway pressure for treatment of severe obstructive sleep apnea syndrome. Chest 1998;113(4):714‐8. [DOI] [PubMed] [Google Scholar]
Kushida 2011 {published data only}
- Kushida CA, Berry RB, Blau A, Crabtree T, Fietze I, Kryger MH, et al. Positive airway pressure initiation: a randomized controlled trial to assess the impact of therapy mode and titration process on efficacy, adherence, and outcomes. Sleep 2011;34(8):1083‐92. [PUBMED: 21804670] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzel T, Dimitrova P, Berry RB, Blau A, Crabtree T, Fietze I, et al. Comparison of efficacy, adherence and functional outcomes with automatically adjusted positive airway pressure with A‐flex®. Sleep Medicine 2011;12 (Suppl 1):S44. [Google Scholar]
Leidag 2008 {published and unpublished data}
- Leidag M, Hader C, Keller T, Meyer Y, Rasche K. Mask leakage in continuous positive airway pressure and C‐Flex. Journal of Physiology and Pharmacology 2008;59 (Suppl 6):401‐6. [PUBMED: 19218664] [PubMed] [Google Scholar]
Loube 2004 {published data only}
- Loube D, Ball N. Comparison of compliance with proportional positive airway pressure (c‐flex) to continuous positive airway pressure treatment of obstructive sleep apnea [Abstract]. Sleep 2004;27:A228. [Google Scholar]
Marrone 2004 {published data only}
- Marrone O, Resta O, Salvaggio A, Giliberti T, Stefano A, Insalaco G. Preference for fixed or automatic CPAP in patients with obstructive sleep apnea syndrome. Sleep Medicine 2004;5(3):247‐51. [DOI] [PubMed] [Google Scholar]
Marshall 2008 {published data only}
- Marshall NS, Campbell AJ, O'Keefe KM, Neill AM. Randomised controlled trial of a novel self‐modulating CPAP (c‐flex) compared to CPAP in severe obstructive sleep apnoea (OSA). Internal Medicine Journal 2006;36:A31. [Google Scholar]
- Marshall NS, Neill AM, Campbell AJ. Randomised trial of compliance with flexible (C‐Flex) and standard continuous positive airway pressure for severe obstructive sleep apnea. Sleep and Breathing 2008;12:393‐6. [DOI: 10.1007/s11325-008-0189-3] [DOI] [PubMed] [Google Scholar]
- Neill AM, Campbell AJ, Marshall NS. Randomized controlled trial of compliance with flexible and standard continuous positive airway pressure for severe obstructive sleep apnea. Proceedings of the American Thoracic Society 2006;2:A869. [Google Scholar]
Masa 2015 {published data only}
- Corral J, Mogollon MA, Sanchez‐Quiroga MA, Terreros Gomez J, Romero A, Caballero C, et al. Echocardiographic changes with non‐invasive ventilation and CPAP in obesity hypoventilation syndrome. Thorax 2018;73(4):361‐8. [DOI] [PubMed] [Google Scholar]
- Lopez‐Jimenez MJ, Masa JF, Corral J, Teran J, Ordaz E, Troncoso MF, et al. Mid‐ and long‐term efficacy of non‐invasive ventilation in obesity hypoventilation syndrome: the Pickwick study. Archivos de Bronconeumologia 2016;52(3):158‐65. [DOI] [PubMed] [Google Scholar]
- Masa JF, Corral J, Alonso ML, Ordax E, Troncoso MF, Gonzalez M, et al. Efficacy of different treatment alternatives for obesity hypoventilation syndrome. American Journal of Respiratory and Critical Care Medicine 2015;192(1):86‐95. [DOI] [PubMed] [Google Scholar]
- Masa JF, Corral J, Alonso ML, Ordaz E, Troncoso MF, Gonzalez M, et al. Efficacy of different treatment alternatives for obesity hypoventilation syndrome: Pickwick Study (online data supplement). www.atsjournals.org/doi/suppl/10.1164/rccm.201410‐1900OC/suppl_file/masa_data_supplement.pdf (accessed prior to 18 March 2019). [DOI: 10.1164/rccm.201410-1900OC] [DOI]
- Masa JF, Mokhlesi B, Benitez I, Gomez de Terreros FJ, Sanchez‐Quiroga MA, Romero A, et al. Long‐term clinical effectiveness of continuous positive airway pressure therapy versus non‐invasive ventilation therapy in patients with obesity hypoventilation syndrome: a multicentre, open‐label, randomised controlled trial. Lancet 2019;393(10182):1721‐32. [DOI] [PubMed] [Google Scholar]
- Quiroga AS, Mogollon MV, Terreros Gomez J, Corral J, Romero A, Caballero C, et al. Echocardiographic changes with positive airway pressure in obesity hypoventilation syndrome. Pickwick study. European Respiratory Journal. 2017:PA4730.
- Quiroga AS, Mokhlesi B, Penafiel JC, Alvarez ML, Carbajo EO, Acevedo MF, et al. Long term positive airway pressure effectiveness in obesity hypoventilation syndrome. Pickwick study results. European Respiratory Journal. 2018; Vol. 52:OA5414.
Massie 2003 {published and unpublished data}
- Massie C, Hart R, Douglas N. Comparison between automatic and manual continuous positive airway pressure (CPAP) therapy using the Autoset T. Amercian Journal of Respiratory and Critical Care Medicine 2003;D26:911. [DOI] [PubMed] [Google Scholar]
- Massie CA, McArdle N, Hart RW, Schmidt‐Nowara WW, Lankford A, Hudgel DW, et al. Comparison between automatic and fixed positive airways pressure therapy in the home. American Journal of Respiratory Critical Medicine 2003;167(1):20‐3. [DOI] [PubMed] [Google Scholar]
Meurice 1996 {published data only}
- Meurice JC, Marc I, Sériès F. Efficacy of auto‐CPAP in the treatment of obstructive sleep apnea/hypopnea syndrome. American Journal of Respiratory Critical Care Medicine 1996;153:794‐8. [DOI] [PubMed] [Google Scholar]
Meurice 2007 {published data only}
- Meurice JC, Cornette A, Philip‐Joet F, Pepin JL, Escourrou P, Ingrand P, et al. Evaluation of autoCPAP devices in home treatment of sleep apnea/hypopnea syndrome. Sleep Medicine 2007;8(7‐8):695‐703. [DOI] [PubMed] [Google Scholar]
Meurice 2009 {published data only}
- Meurice JC, Paquereau J, Tamisier R, Levrat V, Pepin JL. Randomized controlled crossover trial comparing CPAP with an autoCPAP device combined with pressure reduction during exhalation (autoC‐Flex™). European Respiratory Journal 2009;34 (Suppl 53):803s. [Google Scholar]
Modrak 2007 {published data only}
- Modrak J, Greenblatt D. A prospective randomized crossover trial to obtain objective comparison between initial continuous positive airway pressure (CPAP) use with and without expiratory pressure relief in patients with obstructive sleep apnea (OSA). Sleep 2007;30(Suppl):A206. [Google Scholar]
Muir 1998 {unpublished data only}
- Muir JF, Verin E, Portier F, Benichou P, Lofaso F, Martin F, et al. Bilevel (BLPV) versus continuous (CPAP) nasal positive airway pressure in obstructive sleep apnea syndrome (OSAS) patients with previous poor compliance to CPAP. American Journal of Respiratory and Critical Care Medicine 1998;157(3 Suppl):A344. [Google Scholar]
- Muir JF, Verin E, Portier F, Benichou P, Lofaso F, Martin F, et al. Bilevel (BPAP) versus continuous (CPAP) nasal positive airway pressure in OSAS patients with previous poor compliance to CPAP. European Respiratory Journal 1997;10(Suppl 25):187S. [Google Scholar]
Neill 2003 {published data only}
- Neill AM, Wai HS, Bannan SPT, Beasley CR, Weatherall M, Campbell AJ. Humidified nasal continuous positive airway pressure in obstructive sleep apnoea. European Respiratory Journal 2003;22(2):258‐62. [DOI] [PubMed] [Google Scholar]
Nilius 2006 {published data only}
- Nilius G, Happel A, Domanski U, Ruhle KH. Pressure‐relief continuous positive airway pressure vs constant continuous positive airway pressure: a comparison of efficacy and compliance. Chest 2006;130(4):1018‐24. [DOI] [PubMed] [Google Scholar]
Nolan 2007 {published data only}
- Nolan GM, Doherty LS, Goodman P, McNicholas WT. Comparison of auto‐adjusting and fixed pressure positive airway pressure therapy in patients with mild to moderate sleep apnoea syndrome (OSAS). European Respiratory Society 13th Annual Congress; 2003 Sep 28‐29; Vienna. 2003:P676.
- Nolan GM, Doherty LS, McNicholas WT. Auto‐adjusting versus fixed positive pressure therapy in mild to moderate obstructive sleep apnoea. Sleep 2007;30(2):189‐94. [DOI] [PubMed] [Google Scholar]
Noseda 2004 {published data only}
- Noseda A, Kempenaers C, Kerkhofs M, Braun S, Linkowski P, Jann E. Constant versus auto‐continuous positive airway pressure in patients with sleep apnea hypopnea syndrome and a high variability in pressure requirement. Chest 2004;126(1):31‐7. [DOI] [PubMed] [Google Scholar]
Nussbaumer 2006 {published data only}
- Nussbaumer Y, Bloch KE, Genser T, Thurnheer R. Equivalence of auto‐adjusted and constant continuous positive airway pressure in home treatment of sleep apnea. Chest 2006;129(3):638‐43. [DOI] [PubMed] [Google Scholar]
Patruno 2007 {published data only}
- Patruno V, Aiolfi S, Costantino G, Murgia R, Selmi C, Malliani A, et al. Fixed and auto‐adjusting continuous positive airway pressure treatments are not similar in reducing cardiovascular risk factors in patients with obstructive sleep apnea. Chest 2007;131(5):1393‐9. [DOI] [PubMed] [Google Scholar]
- Patruno V, Cazzola K, Vicenzi A, Porta A, Tobaldini E, Aiofi S, et al. Differential effects of continuous positive airway pressure and auto adjusting continuous positive pressure therapy on obstructive sleep apnea patients preliminary results [Abstract]. Sleep 2005;28:A169. [Google Scholar]
Pépin 2009 {published and unpublished data}
- Pepin JL, Muir JF, Gentina T, Dauvilliers Y, Tamisier R, Sapene M, et al. Pressure reduction during exhalation in sleep apnea patients treated by Continuous positive airway pressure (CPAP). European Respiratory Journal 2008;32 (Suppl 52):41s. [DOI] [PubMed] [Google Scholar]
- Pépin JL, Muir JF, Gentina T, Dauvilliers Y, Tamisier R, Sapene M, et al. Pressure reduction during exhalation in sleep apnea patients treated by continuous positive airway pressure. Chest 2009;136(2):490‐7. [PUBMED: 19567496] [DOI] [PubMed] [Google Scholar]
Pépin 2016 {published data only}
- Pepin JL, Baguet JP, Tamisier R, Lepaulle B, Arbib F, Arnol N, et al. Fixed pressure (FP) versus auto‐adjusting continuous positive airway pressure (autoCPAP): Comparison of efficacy in reducing blood pressure, a randomized controlled trial. American Journal of Respiratory and Critical Care Medicine 2014;189:A2411. [Google Scholar]
- Pepin JL, Baguet JP, Tamisier R, Lepaulle B, Arbib F, Arnol N, et al. Fixed pressure (FP) versus auto‐adjusting continuous positive airway pressure (autoCPAP): Comparison of efficacy in reducing blood pressure, a randomized controlled trial. European Respiratory Journal. 2013; Vol. 42 (Suppl 57):40s.
- Pépin JL, Tamisier R, Baguet JP, Lepaulle B, Arbib F, Arnol N, et al. Fixed‐pressure CPAP versus auto‐adjusting CPAP:comparison of efficacy on blood pressure in obstructive sleep apnoea, a randomised clinical trial. Thorax 2016;71:726‐33. [DOI: 10.1136/thoraxjnl-2015-207700] [DOI] [PubMed] [Google Scholar]
Powell 2012 {published data only}
- Powell ED, Gay PC, Ojile JM, Litinski M, Malhotra A. A pilot study assessing adherence to auto‐bilevel following a poor initial encounter with CPAP. Journal of Clinical Sleep Medicine 2012;8(1):43‐7. [PUBMED: 22334808] [DOI] [PMC free article] [PubMed] [Google Scholar]
Randerath 2001 {published and unpublished data}
- Randerath WJ, Schraeder O, Galetke W, Feldmeyer F, Ruhle KH. Autoadjusting CPAP therapy based on impedance efficacy, compliance and acceptance. American Journal of Respiratory and Critical Care Medicine 2001;163:652‐7. [DOI] [PubMed] [Google Scholar]
- Randerath WJ, Schräder O, Galetke W, Rühle K‐H. A prospective randomised cross‐over study on the effects acceptance and compliance of an automatic CPAP device on the forced oscillation technique (APAP‐fot) compared with constant CPAP. European Respiratory Journal 2000;16(Suppl 31):508s. [Google Scholar]
Reeves‐Hoché 1995 {published data only}
- Reeves‐Hoché MK, Hudgel DW, Meck R, Witteman R, Ross A, Zwillich CW. Continuous versus bilevel positive airway pressure for obstructive sleep apnea. American Journal of Respiratory Critical Care Medicine 1995;151:443‐9. [DOI] [PubMed] [Google Scholar]
Resta 2004 {published data only}
- Resta O, Carratu P, Depalo A, Giliberti T, Ardito M, Marrone O, et al. Effects of fixed compared to automatic CPAP on sleep in Obstructive Sleep Apnoea Syndrome. Monaldi Archives of Chest Disease 2004;61(3):153‐6. [DOI] [PubMed] [Google Scholar]
Rochford 2006 {published data only}
- Rochford PD, Collins AL, Howard ME, Pierce PJ. Comparison of two auto‐titrating CPAP devices with fixed CPAP in obstructive sleep apnoea (OSA). Internal Medicine Journal 2006;36:A32. [Google Scholar]
Rohling 2011 {published data only}
- Rohling L, Eijsvogel M. Alternative method for non‐invasive automatic positive airway pressure therapy in OSAS patients. European Respiratory Journal 2011;38 (Suppl 55):711s. [Google Scholar]
Rostig 2003 {unpublished data only}
- Rostig S, Synofik C, Peter JH, Vogelmeier C, Becker HF. [B026] [Poster: 707] Auto‐adjusting versus conventional nCPAP therapy in obstructive sleep apnea patients with low treatment compliance. www.abstracts2view.com. 2003.
- Rostig S, Synofzik C, Peter JH, Vogelmeier C, Becker HF. Auto‐adjusting versus conventional nCPAP therapy in sleep apnea patients with low treatment compliance [Abstract]. Sleep Medicine 2003;4(Suppl 1):S40. [Google Scholar]
Ruhle 2011 {published and unpublished data}
- Nilius G, Franke KJ, Domanski U, Ruehle KH. Prospectively randomised comparison of CPAP without and with controlled heated humidification with tube heater. European Respiratory Journal 2007;30 (Suppl 51):265s. [Google Scholar]
- Ruhle KH, Franke KJ, Domanski U, Nilius G. Quality of life, compliance, sleep and nasopharyngeal side effects during CPAP therapy with and without controlled heated humidification. Sleep and Breathing 2011;15(3):479‐85. [PUBMED: 20503074] [DOI] [PubMed] [Google Scholar]
Ryan 2009 {published and unpublished data}
- Ryan S, Doherty LS, Nolan GM, McNicholas WT. Effects of heated humidification and topical steroids on compliance, nasal symptoms, and quality of life in patients with obstructive sleep apnea syndrome using nasal continuous positive airway pressure.. Journal of Clinical Sleep Medicine 2009;15(5):422‐7. [PUBMED: 19961025] [PMC free article] [PubMed] [Google Scholar]
Senn 2003 {published and unpublished data}
- Senn O, Brack T, Matthews F, Russi EW, Bloch KE. Randomized controlled cross‐over trial of two different auto‐CPAP devices for obstructive sleep apnea therapy. American Thoracic Society 98thI International Conference; 2002 May 17‐22; Georgia. 2002; Vol. [D26]:[Poster: 912].
- Senn O, Brack T, Matthews F, Russi EW, Bloch KE. Randomized short‐term trial of two auto‐CPAP devices versus fixed continuous positive airway pressure for the treatment of sleep apnea. American Journal of Respiratory Critical Care Medicine 2003;168:1506‐11. [DOI] [PubMed] [Google Scholar]
Sériès 1997 {published and unpublished data}
- Sériès F, Marc I. Efficacy of automatic continuous positive airway pressure therapy that uses an estimated required pressure in the treatment of the obstructive sleep apnea syndrome. Annals of Internal Medicine 1997;127(8):588‐95. [DOI] [PubMed] [Google Scholar]
Sériès 2001 {published data only}
- Series F, Marc I. Importance of sleep stage‐ and body position‐dependence of sleep apnoea in determining benefits to auto‐CPAP therapy. European Respiratory Journal 2001;18(1):170‐5. [DOI] [PubMed] [Google Scholar]
Soudorn 2016 {published data only}
- Soudorn C, Muntham D, Reutrakul S, Chirakalwasan N. Effect of heated humidification on CPAP therapy adherence in subjects with obstructive sleep apnea with nasopharyngeal symptoms. Respiratory Care 2016;61(9):1151–9. [DOI] [PubMed] [Google Scholar]
Teschler 2000 {published data only}
- Teschler H, Wessendorf TE, Farhat AA, Konietzko N, Berthon‐Jones M. Two months auto‐adjusting versus conventional nCPAP for obstructive sleep apnoea syndrome. European Respiratory Journal 2000;15(6):990‐5. [DOI] [PubMed] [Google Scholar]
To 2008 {published data only}
- To KW, Chan WC, Choo KL, Lam WK, Wong KK, Hui DS. A randomized cross‐over study of auto‐continuous positive airway pressure versus fixed‐continuous positive airway pressure in patients with obstructive sleep apnoea. Respirology 2008;13(1):79‐86. [DOI] [PubMed] [Google Scholar]
- To KW, Chan WC, Choo KL, Wong KK, Hui DSC. Randomized prospective cohort of auto continuous positive airway pressure (auto‐CPAP) versus fixed continuous positive airway pressure (Fixed‐CPAP) in Chinese patients with obstructive sleep apnea. American Thoracic Society International Conference; May 19‐24; San Diego, California. 2006:A868.
Vennelle 2010 {published and unpublished data}
- Vennelle M, White S, Riha RL, Mackay TW, Engleman HM, Douglas NJ. Randomized controlled trial of variable‐pressure versus fixed‐pressure continuous positive airway pressure (CPAP) treatment for patients with obstructive sleep apnea/hypopnea syndrome (OSAHS). Sleep 2010;33(2):267‐71. [PUBMED: 20175411] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wenzel 2007 {published data only}
- Wenzel M, Kerl J, Dellweg D, Barchfeld T, Wenzel C, Kohler O. Expiratory pressure reduction (C‐Flex method) versus fix CPAP in the therapy for obstructive sleep apnoea. Pneumologie 2007;61(11):692‐6. [DOI] [PubMed] [Google Scholar]
West 2006 {published data only}
- West SD, Jones DR, Stradling J. Comparison of three ways to determine and deliver pressure during nasal CPAP therapy for obstructive sleep apnoea. Thorax 2005;61(3):226‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West SD, Jones DR, Stradling JR. A comparison of three ways to start nasal continuous positive airways pressure treatment for obstructive sleep apnoea [Abstract]. American Thoracic Society International Conference; May 20‐25; San Diego, California. 2005:532.
- West SD, Jones DR, Stradling JR. Comparison of three ways to determine and deliver pressure during nasal CPAP therapy for obstructive sleep apnoea. Thorax 2006;61(3):226‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Worsnop 2010 {published and unpublished data}
- Worsnop C, Miseski S, Rochford P. Humidification of continuous positive airway pressure treatment in sleep apnoea reduces nasal symptoms [Abstract]. Proceedings of the American Thoracic Society 2003;3:P178. [Google Scholar]
- Worsnop CJ, Miseski S, Rochford PD. Routine use of humidification with nasal continuous positive airway pressure. Internal Medicine Journal 2010;40(9):650‐6. [PUBMED: 19460056] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Almasri 2007 {published data only}
- Almasri E, Kline L. The addition of heated wall tubing provides more humidity and comfort than standard heated humidifier CPAP units. Sleep 2007;30(Suppl):A190. [Google Scholar]
Aloia 2001 {published data only}
- Aloia MS, Dio L, Ilnicky N, Perlis ML, Greenblatt DW, Giles DE. Improving compliance with nasal CPAP in older adults with OAHS. Sleep and Breathing 2001;5(1):13‐21. [DOI] [PubMed] [Google Scholar]
- Aloia MS, Ilnicky N, Dio P, Perlis M, Greenblatt DW, Giles DE. Neuropsychological changes and treatment compliance in older adults. Journal of Psychosomatic Research 2003;54(1):71‐6. [DOI] [PubMed] [Google Scholar]
Aloia 2004 {published data only}
- Aloia MS, Arnedt JT, Riggs RL, Hecht J, Borelli B. Clinical management of poor adherence to CPAP: motivational enhancement. Behavioural Sleep Medicine 2004;2(4):205‐22. [DOI] [PubMed] [Google Scholar]
Aloia 2005 {published data only}
- Aloia MS, Arnedt J, Zimmerman M, Skrekas J, Harris S, Carlise C, et al. Combined therapy to improve adherence to CPAP [Abstract]. Sleep 2005;28 (Suppl):A192. [Google Scholar]
Aloia 2005a {published data only}
- Aloia MS, Stanchina M, Arnedt JT, Malhotra A, Millman RP. Treatment adherence and outcomes in flexible versus standard continuous positive airway pressure therapy. Chest 2005;127(6):2085‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Al Zuheibi 2013 {published data only}
- Al Zuheibi T, Al Abri M. Effects of three modes of respironics auto CPAP machines in patients with OSAHS. Sleep Medicine 2013;14 (Suppl 1):e316‐17. [DOI: ] [Google Scholar]
Anderson 2003 {published data only}
- Anderson FE, Kingshott RN, Taylor DR, Jones DR, Kline LR, Whyte KF. A randomized crossover efficacy trial of oral CPAP (Oracle) compared with nasal CPAP in the management of obstructive sleep apnea. Sleep 2003;26(6):721‐6. [DOI] [PubMed] [Google Scholar]
Bachour 2004 {published data only}
- Bachour A, Hurmerinta K, Maasilta P. Mouth closing device (chinstrap) reduces mouth leak during nasal CPAP. Sleep Medicine 2004;5(3):261‐7. [DOI] [PubMed] [Google Scholar]
Ball 2011 {published data only}
- Ball N, Gordon N, Casal E, Parish J. Evaluation of auto bi‐level algorithm to treat pressure intolerance in obstructive sleep apnea. Sleep and Breathing 2011;15(3):301‐9. [DOI] [PubMed] [Google Scholar]
Bardwell 2007 {published data only}
- Bardwell WA, Loredo JS, Ancoli‐Israel S, Dimsdale JE. Effects of two weeks nocturnal oxygen supplementation and continuous positive airway pressure treatment on psychological symptoms in patients with obstructive sleep apnea a randomized placebo controlled study [Abstract]. Sleep 2005;28 (Suppl):A160. [DOI] [PubMed] [Google Scholar]
- Bardwell WA, Norman D, Ancoli‐Israel S, Loredo JS, Lowery A, Lim W, et al. Effects of 2‐week nocturnal oxygen supplementation and continuous positive airway pressure treatment on psychological symptoms in patients with obstructive sleep apnea: a randomized placebo‐controlled study. Behavioral Sleep Medicine 2007;5(1):21‐38. [DOI] [PubMed] [Google Scholar]
- Norman D, Loredo JS, Nelesen RA, Ancoli‐Israel S, Mills PJ, Ziegler MG, et al. Effects of two weeks of continuous positive airway pressure (CPAP) versus supplemental oxygen on 24‐hour ambulatory blood pressure: a placebo‐controlled study. Proceedings of the American Thoracic Society 2006;3:A448. [DOI] [PubMed] [Google Scholar]
Becker 1991 {published data only}
- Becker H, Fett I, Nees E, Peter JH, Wichert VP. Treatment of primary and secondary therapeutic failures of nCPAP therapy in sleep apnoea [Behandlung primärer und sekundärer Therapieversager der nCPAP‐Behandlung bei Patienten mit Schalfapnoe]. Pneumologie 1991;45:301‐5. [PubMed] [Google Scholar]
Becker 1998 {published data only}
- Becker HF. Experience with new auto‐titrating nasal continuous positive airway pressure machines. Monaldi Archives for Chest Disease 1998;53(5):582‐5. [PubMed] [Google Scholar]
Berry 2002 {published data only}
- Berry RB, Parish JM, Hartse KM. The use of auto‐titrating continuous positive airway pressure for treatment of adult obstructive sleep apnea. Sleep 2002;25(2):148‐73. [PubMed] [Google Scholar]
Berthon‐Jones 1996 {published data only}
- Berthon‐Jones M, Lawrence S, Sullivan CE, Grunstein R. Nasal continuous positive airway pressure treatment. Sleep 1996;19(9):S131‐5. [DOI] [PubMed] [Google Scholar]
Bielicke 2008 {published data only}
- Bielicke K, Blau A, Diecker B, Penzel T, Baumann G, Fietze I. Efficacy of OSA treatment with a new Auto‐CPAP mode. European Respiratory Journal 2008;32 (Suppl 52):121s. [Google Scholar]
Blau 2009 {published data only}
- Blau A, Gentina T, Bielicke G, Penzel T, Baumann G, Fietze I. Efficacy of auto‐CPAP with pressure relief during exhalation (A‐Flex) during the treatment of OSA. European Respiratory Journal 2009;34 (Suppl 53):E4534. [Google Scholar]
Boudewyns 1999 {published data only}
- Boudewyns A, Grillier‐Lanoir V, Willemen MJ, Cock WA, Heyning PH, Backer WA. Two months follow‐up of auto‐CPAP treatment in patients with obstructive sleep apnoea. Thorax 1999;54(2):147‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bradshaw 2004 {published data only}
- Bradshaw DA, Murphy DP, Ruff GA. The effects of oral zolpidem on nasal continuous positive airway pressure (CPAP) compliance in the treatment of obstructive sleep apnea (OSA) [Abstract]. Sleep 2004;27 (Suppl):A182. [Google Scholar]
Brammer 1999 {published data only}
- Brammer PA, Lewis RA. The use of auto‐CPAP in long term users of fixed pressure CPAP. Thorax 1999;54(Suppl 3):A48 P117. [Google Scholar]
Buyse 2003 {published data only}
- Buyse B, Cauberghs M, Demedts M. Comparison of 2 auto CPAP algorithms: apnea‐hypopnea‐flow limitation versus airway impedance [Abstract]. European Respiratory Journal 2003;22(Suppl 45):P670. [Google Scholar]
- Buyse B, Cauberghs M, Demedts M. Study of 2 auto CPAP's based on flow limitation but with different working algorithm [Abstract]. European Respiratory Journal 2003;22(Suppl 45):P669. [Google Scholar]
Canisius 2007 {published data only}
- Canisius S, Ploch T, Loh A, Vogelmeier C, Jerrentrup A. Comparison of therapeutic equivalence of the auto‐CPAP device 'Delphinus' with conventional nCPAP therapy [Vergleich der therapeutischen Äquivalenz des automatischen nCPAP‐Gerätes "Delphinus(r)" mit der konventionellen nCPAP‐Therapie]. Somnologie 2007;11(1):28‐34. [Google Scholar]
- Jerrentrup L, Canisius S, Wilhelm S, Kesper K, Ploch T, Vogelmeier C, et al. Work of breathing in fixed and pressure relief continuous positive airway pressure (C‐Flex™): a post hoc analysis. Respiration 2017;93:23‐31. [DOI] [PubMed] [Google Scholar]
Chan 2004 {published data only}
- Chan J, Tedjasaputra I, Chan CS. Improving CPCP compliance and patient comfort with the mirage ACTiva system [Abstract]. American Thoracic Society 100th International Conference; 2004 May 21‐26; Orlando. 2004:C65 Poster K3.
Chervin 1997 {published data only}
- Chervin RD, Theut S, Bassetti C, Aldrich MS. Compliance with nasal CPAP can be improved by simple interventions. Sleep 1997;20(4):284‐9. [DOI] [PubMed] [Google Scholar]
Chihara 2012 {published data only}
- Chihara Y, Tsuboi T, Hitomi T, Azuma M, Murase K, Toyama Y, et al. Flexible positive airway pressure improves treatment adherence compared with auto‐adjusting PAP. Sleep 2013;36(2):229‐36. [PUBMED: 23372270] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chihara Y, Tsuboi T, Hitomi T, Azumi M, Murase K, Toyama Y, et al. Flexible positive airway pressure improves treatment adherence compared with auto‐adjusting PAP. European Respiratory Journal 2012;40 (Suppl 56):702s. [DOI] [PMC free article] [PubMed] [Google Scholar]
Colrain 2007 {published data only}
- Colrain I, Brooks S, Black J. Evaluation of a new nasal expiratory positive airway pressure (EPAP) device for the treatment of snoring and obstructive sleep apnea syndrome. Sleep 2007;30(Suppl):A191. [Google Scholar]
Constantinidis 2000 {published data only}
- Constanidis J, Knobber D, Steinhart H, Kuhn J, Iro H. Morphological and functional alterations in the nasal mucosa following nCPAP therapy [Morphologische und funktionelle Veränderungen der Nasenshleimhaut nach nCPAP‐Therapie]. HNO 2000;48(10):747‐52. [DOI] [PubMed] [Google Scholar]
Coughlin 2004 {published data only}
- Coughlin SR, Mugarza JA, Mawdsley L, Wilding JP, Calverley PM. Continuous positive airways pressure treatment reduces the cardiovascular risk factors associated with obstructive sleep apnoea [Abstract]. American Thoracic Society 100th International Conference; 2004 May 21‐26; Orlando. 2004:C99 Poster 111.
Cross 2005 {published data only}
- Cross MD, Vennelle M, Engleman HM, Douglas NJ. Predictors of poor titration and outcome in patients with obstructive sleep apnea/hypopnea syndrome (OSAHS) after home or hospital automated CPAP titration [Abstract]. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California. 2005:C58; Poster: C48.
Cumin 2011 {published data only}
- Cumin D, Whiting D, Malla A, Gerred A, Dungan G. Randomised, cross‐over evaluation of a novel implementation of pressure relief technology ‐ SensAwake and fixed pressure CPAP. Sleep Medicine. 2011; Vol. 12 (Suppl 1):S75.
Damjanovic 2005 {published data only}
- Damjanovic D, Fluck A, Idzko M, Muller‐Quernheim J, Sorichter S. Nasal CPAP in the therapy of OSAS ‐ does a closer patient guidance and support increase compliance?. European Respiratory Journal 2005;26(Suppl 49):4521. [Google Scholar]
Delwiche 2003 {published data only}
- Delwiche JP, Evrard G, Delaunois L. Auto nCPAP or not auto nCPAP [Abstract]. European Respiratory Journal 2003;22(Suppl 45):P675. [Google Scholar]
Dungan 2010 {published data only}
- Dungan G, Marshall N, Hoyos C, Yee B, Grunstein R. Randomised controlled trial of a device modification for reducing autoCPAP pressure during episodes of apparent wakefulness in OSA patients. Sleep and Biological Rhythms 2010;8:A79. [Google Scholar]
Duntley 2005 {published data only}
- Duntley S, Morrissey A, Doerr C, Svoboda J, Duntley L, Kampelman J. Flexible CPAP with expiratory pressure relief an in laboratory polysomnographic comparison with conventional CPAP [Abstract]. Sleep 2005;28:A182. [Google Scholar]
Duoung 2005 {published data only}
- Duong M, Jayaram L, Camfferman D, Catcheside P, Mykytyn I, McEvoy RD. Use of heated humidification during nasal CPAP titration in obstructive sleep apnoea syndrome. European Respiratory Journal 2005;26(4):679‐85. [DOI] [PubMed] [Google Scholar]
e Bastos 2013 {published data only}
- Bastos HN, Castro AS, Pinto T, Marinho A, Sucena M, Drummond M, et al. Randomized short–term trial of high span versus low span APAP for the treatment of obstructive sleep apnea. European Respiratory Journal 2013;42 (Suppl 57):737s. [Google Scholar]
Engleman 1993 {published data only}
- Engleman HM, Douglas NJ. CPAP compliance. Sleep 1993;16(8 Suppl):s114. [DOI] [PubMed] [Google Scholar]
Epstein 2000 {unpublished data only}
- Epstein L, Graham L, Turner A, Larkin E, Garshick E, Ayas N, et al. Comparison of two methods for achieving CPAP compliance. American Journal of Respiratory and Critical Care Medicine 2000;161(3 Suppl):A358. [Google Scholar]
Feenstra 2005 {published data only}
- Feenstra J, Rixon K, Hukins C. A randomised single‐blinded cross over trial of sesame oil ("NOZIL") for the treatment of nasal symptoms with CPAP [Abstract]. Respirology 2005;10 (Suppl):A90. [Google Scholar]
Ficker 1997 {published data only}
- Ficker JH, Wiest GH, Lehnert G, Fischer CJ, Katalinic A, Hahn EG. Auto‐CPAP treatment of obstructive sleep apnoea ["Auto‐CPAP"‐Therapie des obstruktiven Schlafapnoe‐Syndroms]. Deutsche Medizinische Wochenschrift 1997;122:1482‐8. [DOI] [PubMed] [Google Scholar]
Ficker 1998 {published data only}
- Ficker JH, Wiest GH, Lehnert G, Wiest B, Hahn EG. Evaluation of an auto‐CPAP device for treatment of obstructive sleep apnoea. Thorax 1998;53:643‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ficker 2000 {published data only}
- Ficker JH, Fuchs FS, Wiest GH, Asshoff G, Schmelzer AH, Hahn EG. An auto‐continuous positive airway pressure device controlled exclusively by the forced oscillation technique. European Respiratory Journal 2000;16(5):914‐20. [DOI] [PubMed] [Google Scholar]
Fletcher 1991 {published data only}
- Fletcher EC, Luckett RA. The effect of positive reinforcement on hourly compliance in nasal continuous positive airway pressure users with obstructive sleep apnea. American Review of Respiratory Disease 1991;134:936‐41. [DOI] [PubMed] [Google Scholar]
Fleury 1996 {published data only}
- Fleury B, Rakotonanahary D, Hausser‐Hauw C, Lebeau B, Guilleminault C. Objective patient compliance in long‐term use of nCPAP. European Respiratory Journal 1996;9:2356‐9. [DOI] [PubMed] [Google Scholar]
Gagnadoux 1999 {published data only}
- Gagnadoux F, Rakotonanahary D, Martins de Araujo MT, Barros‐Vieira S, Fleury B. Long‐term efficacy of fixed CPAP recommended by Autoset (TM) for OSAS. Sleep 1999;22(8):1095‐9. [DOI] [PubMed] [Google Scholar]
Galetke 2006 {published data only}
- Galetke W, Richter K, Anduleit N, Randerath W. Automatic CPAP titration based on forced oscillation technique flow and snore signals in the obstructive sleep apnea syndrome. European Respiratory Journal 2006;28(Suppl 50):411. [Google Scholar]
Galetke 2008a {published data only}
- Galetke W, Stieglitz S, Priegnitz C, Schaefer T, Randerath W. Comparison of standard humidification and humidification with a heated breathing tube in CPAP therapy of obstructive sleep apnea. European Respiratory Journal 2008;32 (Suppl 52):121s. [Google Scholar]
Galetke 2016 {published data only}
- Galetke W, Nothofer E, Priegnitz C, Anduleit N, Randerath W. Effect of a heated breathing tube on efficacy, adherence and side effects during continuous positive airway pressure therapy in obstructive sleep apnea. Respiration 2016;91(1):18‐25. [DOI] [PubMed] [Google Scholar]
Goncalves 2006 {published data only}
- Goncalves MR, Carrondo CM, Amorim J, Winck JC. Comparison of two different modes during bi‐level positive pressure ventilation in patients with restrictive disorders: spontaneous versus assist control. European Respiratory Journal 2006;28(Suppl 50):183s. [Google Scholar]
Greenfield 2005 {published data only}
- Greenfield D, Gehrman P, Linn M, Liu LQ, Corey‐Bloom J, Ancoli‐Israel S. CPAP compliance in mild‐moderate Alzheimer's patients with SDB [Abstract]. Sleep 2003;26 (Suppl):A154. [Google Scholar]
Grote 2000 {published data only}
- Grote L, Hedner J, Grunstein R, Kraiczi H. Therapy with nCPAP: incomplete elimination of sleep related breathing disorder. European Respiratory Journal 2000;16(5):921‐7. [DOI] [PubMed] [Google Scholar]
Gupta 2011 {published data only}
- Gupta S, Bollavaram N, Knapik S. At home patient directed daytime continuous positive airway pressure (CPAP) mask acclimatization prior to starting CPAP therapy. Chest 2011;140 (4_MeetingAbstracts):824A. [Google Scholar]
Herold 2007 {published data only}
- Herold J, Ebert T, Steinmann A, Roessner E, Ficker J. Randomized study of an auto titration device (Somnoset) versus manual CPAP titration. European Respiratory Journal 2007;30(Suppl 51):473. [Google Scholar]
Hertegonne 2003 {published data only}
- Hertegonne KB, Proot PM, Pauwels RA, Pevernagie DA. Comfort and pressure profiles of two auto‐adjustable positive airway pressure devices: a technical report. Respiratory Medicine 2003;97(8):903‐8. [DOI] [PubMed] [Google Scholar]
Hertegonne 2006 {published data only}
- Hertegonne K, Volna J, Portier S, Maele G, Pevernagie D. Titration procedures in CPAP‐therapy: auto‐CPAP or prediction formula?. Sleep Medicine 2006;7:80s. [DOI] [PubMed] [Google Scholar]
Horvath 2008 {published data only}
- Horvath T, Szkaes Z. Short term compliance with auto versus bi‐level BIPAP. Sleep 2008;31(Suppl):A188. [Google Scholar]
Hosselet 1999 {published data only}
- Hosselet JJ. Sef‐adjusted continuous positive pressure and treatment of obstructive respiratory sleep disorders [Pression positive continue auto‐pilotée dans la titration et le traitement des troubles respiratoires obstructifs du sommeil]. Revue des Maladies Respiratoires 1999;16(5):799‐808. [PubMed] [Google Scholar]
Hoster 1996 {published data only}
- Hoster M, Schlenker E, Ruhle KH. Effect of automatically titrated CPAP systems on sleep and respiration in sleep apnoea syndrome [Einfluß automatisch titrierender CPAP‐Systeme auf Schlaf und Atmung bei SAS]. Wiener Medizinische Wochenschrift 1996;146:13‐4. [PubMed] [Google Scholar]
Hostler 2014 {published data only}
- Hostler J, Sheikh K, Khramtsov A, Andrada T, Foster B, Puderbaugh A, et al. The effects of A‐Flex on Auto‐PAP adherence and efficacy. Sleep 2014;37 (Abstract Supplement):A108. [Google Scholar]
Hoy 1999 {published data only}
- Hoy CJ, Vennelle M, Kingshott RN, Engleman HM, Douglas NJ. Can intensive support improve continuous positive airway pressure use in patients with the sleep apnea/hypopnea syndrome?. American Journal of Respiratory Critical Care Medicine 1999;159(4):1096‐100. [DOI] [PubMed] [Google Scholar]
Huang 2001 {published data only}
- Huang R, Huang XZ, Xiao Y. Evaluation of an auto‐CPAP device for treatment of obstructive sleep apnea. Beijing Medical Journal 2001;23(6):350‐2. [Google Scholar]
Hui 2000 {published data only}
- Hui DS, Chan JK, Choy DK, Ko FW, Li TS, Leung RC, et al. Effects of augmented continuous positive airway pressure education and support on compliance and outcome in a Chinese population. Chest 2000;117(5):1410‐15. [DOI] [PubMed] [Google Scholar]
Hui 2001 {published data only}
- Hui DS, Choy DK, Li TS, Ko FW, Wong KW, Chan JK, et al. Determinants of continuous positive airway pressure compliance in a group of Chinese patients with obstructive sleep apnea. Chest 2001;120:170‐6. [DOI] [PubMed] [Google Scholar]
Hui 2006 {published data only}
- Hui DS, To KW, Ko FW, Fok JP, Chan MC, Ngai JC, et al. A randomized sub‐therapeutic CPAP controlled study of the effects of nasal CPAP on 24‐hour systemic blood pressure in obstructive sleep apnoea syndrome. Proceedings of the American Thoracic Society 2006;1:A870. [Google Scholar]
Hukins 2005 {published data only}
- Hukins C, Popovic J, Davies K. Randomised controlled trial of arbitrary pressure CPAP. Thoracic Society of Australia and New Zealand Annual Scientific Meeting; 2001 March 16‐21; Brisbane. 2001:A88.
- Hukins CA. Arbitrary pressure continuous positive airway pressure for obstructive sleep apnea syndrome. American Journal of Respiratory and Critical Care Medicine 2005;171(5):500‐5. [DOI] [PubMed] [Google Scholar]
Husain 2003 {published data only}
- Husain AM. Evaluation and comparison of Tranquility and AutoSet T auto‐titrating CPAP machines. Journal of Clinical Neurophysiology 2003;20(4):291‐5. [DOI] [PubMed] [Google Scholar]
Juhàsz 2001 {published data only}
- Juhàsz J, Becker H, Cassel W, Rostig S, Peter JH. Proportional positive airway pressure: a new concept to treat obstructive sleep apnea. European Respiratory Journal 2001;17:467‐73. [DOI] [PubMed] [Google Scholar]
Khanna 2003 {published data only}
- Khanna R, Kline LR. A prospective 8 week trial of nasal interfaces versus a novel oral interface (Oracle) for treatment of obstructive sleep apnea hypopnea syndrome. Sleep Medicine 2003;4(4):333‐8. [DOI] [PubMed] [Google Scholar]
Khayat 2007 {published data only}
- Khayat RN, Roy M, Abraham WT. Comparison between CPAP and Bi‐Flex(r) in the treatment of obstructive sleep apnea (OSA) in patients with systolic heart failure. American Thoracic Society International Conference; 2007 May 18‐23; San Francisco. 2007:Poster 621.
Krieger 1992 {published data only}
- Krieger J. Long‐term compliance with nasal continuous positive airway pressure (CPAP) in obstructive sleep apnea patients and non‐apneic snorers. Sleep 1992;15(6):S42‐6. [DOI] [PubMed] [Google Scholar]
Krieger 1998 {published data only}
- Krieger J, Sforza E, Petiau C, Weiss T. Simplified diagnostic procedure for obstructive sleep apnoea syndrome: lower subsequent compliance with CPAP. European Respiratory Journal 1998;12(4):776‐9. [DOI] [PubMed] [Google Scholar]
Krieger 1999 {published data only}
- Krieger J. Therapeutic use of auto‐CPAP. Sleep Medicine Review 1999;3(2):159‐74. [DOI] [PubMed] [Google Scholar]
Likar 1997 {published data only}
- Likar LL, Panciera M, Erickson AD. Group education sessions and compliance in nasal continuous positive airways pressure users with obstructive sleep apnoea. Chest 1997;111:936‐41. [DOI] [PubMed] [Google Scholar]
Liu 2007 {published data only}
- Liu J‐X, Zhu Y. Improvement in therapeutic pressure parameters and symptoms in patients with obstructive sleep apnea hypopnea syndrome after intervention with two types of devices for automatic and continuous regulation of the positive airways pressure. Journal of Clinical Rehabilitative Tissue Engineering Research 2007;11(9):1675‐8. [Google Scholar]
Loberes 2004 {published data only}
- Lloberes P, Rodriguez B, Roca A, Sagales MT, Calzada MD, Gimenez S, et al. Comparison of conventional nighttime with automatic or manual daytime CPAP titration in unselected sleep apnea patients: study of the usefulness of daytime titration studies. Respiratory Medicine 2004;98(7):619‐25. [DOI] [PubMed] [Google Scholar]
Lopez‐Martin 2005 {published data only}
- Lopez‐Martin S, Sanchez‐Munoz G, Gonzalez‐Moro JM, Miguel‐Diez J, Pedraza‐Serrano F, Lucas‐Ramos P. CPAP treatment compliance in patients with obstructive sleep apnea syndrome (OSAS) does it improve when the treatment is inhaled under supervision [Abstract]. American Thoracic Society 2005 International Conference; May 20‐25; San Diego. 2005:C29; Poster: 533.
Loube 2003 {published data only}
- Loube DI, Ball NJ, Baker TJ. A comparison of a novel positive airway pressure therapy mode to continuous positive airway pressure for adult obstructive sleep apnea treatment [Abstract]. Sleep 2003;26:A253. [Google Scholar]
- Loube DI, Ball NJ, Baker TJ. Comparison of proportional positive airway pressure to continuous positive airway pressure for obstructive sleep apnea treatment [abstract]. American Thoracic Society 99th International Conference; 2003 May 16‐21; Seattle. 2003.
Mador 2005 {published data only}
- Mador MJ, Krauza M, Pervez A, Pierce D, Braun M. Effect of heated humidification on compliance and quality of life in patients with sleep apnea using nasal continuous positive airway pressure. Chest 2005;128(4):2151‐8. [DOI] [PubMed] [Google Scholar]
- Mador MJ, Krauza M, Pervez A, Pierce D, Braun M. Effect on heated humidification on nasal CPAP compliance and quality of life in patients with sleep apnea [Abstract]. American Thoracic Society 2005 International Conference; May 20‐25; San Diego 2005;C58:Poster: C52. [Google Scholar]
Mansfield 2003 {unpublished data only}
- Mansfield DR, Gollogly NC, Bergin P, Kaye DW, Naughton MT. Cardiomyopathy, (obstructive) apnea and trial of nasal positive airway pressure (CATNAP) study [abstract]. American Thoracic Society 99th International Conference; 2003 May 16‐21; Seattle. 2003.
Marshall 2003 {published data only}
- Marshall MJ, Scammels C, Lowe S. Does proactive intervention influence compliance on continuous positive airway pressure therapy (CPAP)?. Respiratory Care 2003;48(11):1094. [Google Scholar]
Masa 2004 {published data only}
- Masa JF, Jimenez A, Duran J, Capote F, Monasterio C, Mayos M, et al. Alternative methods of titrating continuous positive airway pressure: a large multicenter study. American Journal of Respiratory and Critical Care Medicine 2004;170(11):1218‐24. [DOI] [PubMed] [Google Scholar]
- Masa JF, Rubio M, Jimenez A, Duran J, Capote F, Monasterio C, et al. Efficacy of CPAP obtained by means of automatic and mathematical titration (exploratory data) [Abstract]. European Respiratory Journal 2003;22(Suppl 45):P668. [Google Scholar]
Massie 1999 {published data only}
- Massie CA, Hart RW, Peralez K, Richards GN. Effects of humidification on nasal symptoms and compliance in sleep apnea patients using continuous positive airway pressure. Chest 1999;116(2):403‐8. [DOI] [PubMed] [Google Scholar]
McArdle 2010 {published data only}
- McArdle N, Singh B, Murphy M, Gain KR, Maguire C, Mutch S, et al. Continuous positive airway pressure titration for obstructive sleep apnoea: automatic versus manual titration. Thorax 2010;65:606‐11. [DOI: 10.1136/thx.2009.116756] [DOI] [PubMed] [Google Scholar]
McNicholas 1997 {published data only}
- McNicholas WT. Sleep apnoea syndrome today: much done, more to do. European Respiratory Journal 1997;10(5):969‐70. [DOI] [PubMed] [Google Scholar]
Meurice 1994 {published data only}
- Meurice JC, Dore P, Paquereau J, Neau JP, Ingrand P, Chavagnat JJ, et al. Predictive factors of long‐term compliance with nasal continuous positive airways pressure treatment in sleep apnea syndrome. Chest 1994;105(2):429‐33. [DOI] [PubMed] [Google Scholar]
Meurice 1998 {published data only}
- Meurice JC, Paquereau J, Denjean A, Patte F, Sériès F. Influence of correction of flow limitation on continuous positive airway pressure efficiency in sleep apnoea/hypopnoea syndrome. European Respiratory Journal 1998;11:1121‐7. [DOI] [PubMed] [Google Scholar]
Meurice 2007a {published data only}
- Meurice J‐C, Ingrand P, Portier F, Arnulf I, Rakotonanahari D, Fournier E, et al. A multicentre trial of education strategies at CPAP induction in the treatment of severe sleep apnoea‐hypopnoea syndrome. Sleep Medicine 2007;8(1):37‐42. [DOI] [PubMed] [Google Scholar]
Montserrat 2006 {published data only}
- Montserrat JM, Prieto R, Puig M, Carrion M, Leon C, Hernandez L. Comparison between fixed and automatic continuous positive airway pressure (CPAP) in patients with nose problems after starting treatment with CPAP. Sleep Medicine 2006;7:S79. [Google Scholar]
Morley 2001 {published data only}
- Morley CJ, Davis P, Doyle L. Continuous positive airway pressure: randomized, controlled trial in Australia. Pediatrics 2001;108(6):1383. [DOI] [PubMed] [Google Scholar]
Mortimore 1998 {published data only}
- Mortimore IL, Whittle AT, Douglas NJ. Comparison of nose and face mask CPAP therapy for sleep apnoea. Thorax 1998;53(4):290‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mulgrew 2005 {published data only}
- Mulgrew AT, Fox N, Cortes L, Pamplin C, Ayas NT, Ryan CF. A randomized study to compare inpatient polysomnography (PSG) with outpatient diagnosis and CPAP titration in patients with a high probability of obstructive sleep apnea (OSA) [Abstract]. American Thoracic Society 2005 International Conference; May 20‐25; San Diego. 2005:C29; Poster: 530.
Mulgrew 2006 {published data only}
- Mulgrew AT, Cheema R, Fleetham J, Fox N, Ayas NT. Evaluation of performance and patient satisfaction using AutoCPAP with C‐Flex versus standard CPAP. American Thoracic Society 2006 International Conference; May 19‐24; San Diego 2006:A869.
- Mulgrew AT, Cheema R, Fleetham J, Ryan CF, Ayas NT. Efficacy and patient satisfaction with auto‐adjusting CPAP with variable expiratory pressure vs standard CPAP: a two‐night randomized crossover trial. Sleep and Breathing 2007;11(1):31‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Munoz 2009 {published data only}
- Muñoz MJ, Mosteiro M, Torres ML, Gil C, Cobas A. Comparative study of efficacy in control of respiratory events by three models of APAP. European Respiratory Journal 2009;34 (Suppl 53):804s. [Google Scholar]
Murray 2002 {published data only}
- Murray M, Vennelle M, Kingshott R, Hoy C, Engleman HM, Douglas NJ. Psychosocial determinates of CPAP use: analysis of randomized trials. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A722. [Google Scholar]
Neale 2011 {published data only}
- Neale S, Buchanan F, Allaouat N, Catterall JR, Kendrick AH. Randomized trial of 6 auto‐adapting CPAP devices. European Respiratory Journal 2011;38 (Suppl 55):575s. [Google Scholar]
- Neale S, Buchanan F, Allaouat N, Catterall JR, Kendrick AH. Randomized trial of 6 auto‐adapting CPAP devices: Effects on quality of life. European Respiratory Journal 2011;38 (Suppl 55):576s. [Google Scholar]
Nolan 2006 {published data only}
- Nolan GM, Ryan S, O'Connor M, McNichols WTM. Patient evaluation of three auto‐adjusting positive airway pressure (APAP) devices [Abstract]. European Respiratory Journal 2004;24(Suppl 48):566s. [Google Scholar]
- Nolan GM, Ryan S, O'Connor TM, McNicholas WT. Comparison of three auto‐adjusting positive pressure devices in patients with sleep apnoea. European Respiratory Journal 2006;28(1):159‐64. [DOI] [PubMed] [Google Scholar]
Palasiewicz 1997 {published data only}
- Palasiewicz G, Sliwinksi P, Koziej M, Zielinski J. Acute effects of CPAP and BiPAP breathing on pulmonary haemodynamics in patients with obstructive sleep apnoea. Monaldi Archive of Chest Disease 1997;52(5):440‐3. [PubMed] [Google Scholar]
- Sliwinksi P, Palasiewicz G, Hawrylkiewicz I, Koziej M, Zielinksi J. The effects of CPAP and BiPAP breathing on pulmonary haemodynamics in patients with OSAS. European Respiratory Journal 1996;9(Suppl 23):154s‐5s. [Google Scholar]
Peach 2003 {published data only}
- Peach RF, Jelic S, Zhong X, Basner RC, Zimmerman BJ. Effect of self‐regulation and self‐monitoring on CPAP adherence in obstructive sleep apnea (OSA) [Abstract]. Sleep 2003;26:A263. [Google Scholar]
Penzel 2004 {published data only}
- Penzel T, Kesper K, Ploch T, Canisius S, Pinnow I, Heitman J, et al. Inspiratory flow limitation during NREM and REM sleep investigated under CPAP and C‐flex conditions [Abstract]. Sleep 2004;27:A191. [Google Scholar]
Pépin 1995 {published data only}
- Pépin JL, Leger P, Veale D, Langevin B, Robert D, Levy P. Side effects of nasal continuous positive airways pressure in sleep apnea syndrome: study of 193 patients in two French sleep centers. Chest 1995;107(2):375‐81. [DOI] [PubMed] [Google Scholar]
Pevernagie 2004 {published data only}
- Pevernagie DA, Proot PM, Hertegonne KB, Neyens MC, Hoornaert KP, Pauwels RA. Efficacy of flow‐ versus impedance‐guided auto‐adjustable continuous positive airway pressure: a randomized cross‐over trial. Chest 2004;126(1):25‐30. [DOI] [PubMed] [Google Scholar]
Pierce 2005 {published data only}
- Pierce RJ, Collins AL, Howard M, Rochford PD. Comparison of two CPAP auto‐titration therapies in OSA [Abstract]. American Thoracic Society International Conference; May 20‐25; San Diego. 2005:C29; Poster: 529.
- Pierce RJ, Collins AL, Howard M, Rochford PD. Comparison of two CPAP auto‐titration therapies in OSA [Abstract]. Respirology 2005;10(Suppl):A89. [Google Scholar]
Pilz 2000 {published data only}
- Pilz K, Thalhofer S, Meissner P, Dorow P. Improvement of CPAP therapy by a self‐adjusting system. Sleep and Breathing 2000;4(4):169‐72. [DOI] [PubMed] [Google Scholar]
Piper 2008 {published data only}
- Piper AJ, Wang D, Yee BJ, Barnes DJ, Grunstein RR. Randomised trial of CPAP versus bilevel support in the treatment of obesity hypoventilation syndrome without severe nocturnal desaturation. Thorax 2008;63(5):395‐401. [DOI] [PubMed] [Google Scholar]
- Piper AJ, Wang D, Yee BJ, Barnes DJ, Grunstein RR. Randomised trial of CPAP vs bilevel support in the initial management of patients with obesity hypoventilation syndrome. Sleep and Biological Rhythms 2006;4(Suppl 1):A11. [Google Scholar]
Planès 2003 {published data only}
- Planès C, D'Ortho M‐P, Foucher A, Berkani M, Leroux K, Essalhi M, et al. Efficacy and cost of home‐initiated auto‐CPAP versus conventional nCPAP. Sleep 2003;26(2):156‐60. [DOI] [PubMed] [Google Scholar]
Powell 2014 {published data only}
- Powell ED, Andry JM, Whitney C, Miller CJ, Hames K, Bowman BR. An equivalence study comparing a new lightweight AutoPAP device to an established AutoPAP device. Sleep 2014;37 (Abstract Supplement):A117. [Google Scholar]
Pradeepan 2017 {published and unpublished data}
- Pradeepan S. Fixed versus automatic positive airway pressure therapy for positional obstructive sleep apnoea‐a double‐blind, randomised trial. Journal of Sleep Research 2017;26:69‐70. [Google Scholar]
- Pradeepan S, Yates N, Suthers B, Hensley M. Fixed versus automatic positive airway pressure therapy for positional obstructive sleep apnoea‐a double‐blind, randomised trial. Sleep. 2018; Vol. 41:A209‐10.
Rains 1996 {published data only}
- Rains JC. Treatment of obstructive sleep apnea in pediatric patients: behavioral intervention for compliance with nasal continuous positive airway pressure. Clinical Paediatrics 1996;34(10):535‐41. [DOI] [PubMed] [Google Scholar]
Randerath 1999 {published data only}
- Randerath WJ, Parys K, Feldmeyer F, Sanner B, Rühle KH. Self‐adjusting nasal continuous airway pressure therapy based on measurement of impedance. Chest 1999;116(4):991‐9. [DOI] [PubMed] [Google Scholar]
Randerath 1999b {published data only}
- Randerath WJ, Kujumdshieva B, Kroll B, Schwickert M, Dingemann A, Ruhle K‐H. Influence of audiovisual and print media on patient information in sleep apnea syndrome. Somnologie 1999;3(2):57‐61. [Google Scholar]
Randerath 2001a {published data only}
- Randerath WJ, Galetke W, David M, Siebrecht H, Sanner B, Rühle KH. Prospective randomized comparison of impedance‐controlled auto‐continuous positive airway pressure (APAPfot) with constant CPAP. Sleep Medicine 2001;2:115‐24. [DOI] [PubMed] [Google Scholar]
Randerath 2003 {published data only}
- Randerath WJ, Galetke W, Ruehle KH. Auto‐adjusting CPAP based on impedance versus bilevel pressure in difficult‐to‐treat sleep apnea syndrome: a prospective randomized crossover study. Medical Science Monitor 2003;9(8):CR353‐8. [PubMed] [Google Scholar]
Richards 2007 {published data only}
- Richards D, Bartlett DJ, Wong K, Malouff J, Grunstein RR. Increased adherence to CPAP with a group cognitive behavioral treatment intervention: a randomized trial. Sleep 2007;30(5):635‐40. [DOI] [PubMed] [Google Scholar]
- Richards DL, Bartlett D, Malouff J, Grunstein R. The role of CBT in CPAP adherence. Internal Medicine Journal 2006;36:A31. [Google Scholar]
Rosenthal 2001 {published data only}
- Rosenthal L. The results of CPAP therapy under two adherence schedules. Journal for Respiratory Care Practitioners 2001;14(4):27‐8. [Google Scholar]
Rosenthal 2012 {published data only}
- Rosenthal L, Woidtke R, Andry J, Rafati S, Garcia M, Gordon N. Nocturnal oxygen saturation in osa subjects treated with auto‐PAP: comparison of exhalation pressure relief to standard pressure delivery. Sleep 2012;35 (Abstract Supplement):A173. [Google Scholar]
Rubio 2015 {published data only}
- Haba‐Rubio J, Petitpierre NJ, Cornette F, Tobback N, Vat S, Giallourou T, et al. Oscillating positive airway pressure versus CPAP for the treatment of obstructive sleep apnea. Frontiers in Medicine 2015;2(29):10.3389/fmed.2015.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Salgado 2006 {published data only}
- Salgado S, Boleo‐Tome J, Dias R, Canhao C, Teixeira J, Feliciano A, et al. Impact of humidification on compliance and side effects in obstructive sleep apnea (OSA) patients under auto continuous positive airway pressure (ACPAP) therapy. European Respiratory Journal 2006;28(Suppl 50):409s. [Google Scholar]
- Salgado S, Boleo‐Tomé J, Dias R, Canhão C, Teixeira J, Feliciano A, et al. Impact of humidification on compliance and side effects in obstructive sleep apnea (OSA) patients under auto continuous positive airway pressure (ACPAP) therapy. Revista Portuguesa de Pneumologia 2006;12(6 Suppl 1):55. [Google Scholar]
- Salgado SM, Boleo‐Tome JP, Canhao JM, Dias AR, Teixeira JI, Pinto PM, et al. Impact of heated humidification with automatic positive airway pressure in obstructive sleep apnea therapy. Jornal Brasileiro de Pneumologia 2008;34(9):690‐4. [PUBMED: 18982206] [DOI] [PubMed] [Google Scholar]
Scharf 1996 {published data only}
- Scharf MB, Brannen DE, McDannold MD, Berkowitz DV. Computerized adjustable versus fixed NCPAP treatment of obstructive sleep apnoea. Sleep 1996;19(6):491‐6. [DOI] [PubMed] [Google Scholar]
Sharma 1996 {published data only}
- Sharma S, Wali S, Pouliot Z, Peters M, Neufeld H, Kryger M. Treatment of obstructive sleep apnea with a self‐titrating continuous positive airway pressure (CPAP) system. Sleep 1996;19(6):497‐501. [DOI] [PubMed] [Google Scholar]
Signes‐Costa 2005 {published data only}
- Signes‐Costa J, Chiner E, Andreu AL, Pastor E, Llombart M, Gomez‐Merino E, et al. Patient compliance with CPAP home based diagnosis and review preliminary report [Abstract]. European Respiratory Journal 2004;24(Suppl 48):567s. [Google Scholar]
Sin 2002 {published data only}
- Sin D, Mayers I, Man GC, Pawluck L. Long‐term compliance rates to continuous positive airways pressure in obstructive sleep apnoea. Chest 2002;121:430‐5. [DOI] [PubMed] [Google Scholar]
Speer 2012 {published data only}
- Speer TK, Webb R. Effect of heated wall tubing with heated humidification on PAP usage at 30 days post PAP initiation. Sleep 2012;35 (Suppl):A166. [Google Scholar]
Stammnitz 2004 {published data only}
- Stammnitz A, Jerrentrup A, Penzel T, Peter JH, Vogelmeier C, Becker HF. Automatic CPAP titration with different self‐setting devices in patients with obstructive sleep apnoea. European Respiratory Journal 2004;24(2):273‐8. [DOI] [PubMed] [Google Scholar]
Suzuki 2007 {published data only}
- Suzuki M, Saigusa H, Furukawa T. Comparison of sleep parameters at titration and subsequent compliance between CPAP‐pretreated and non‐CPAP‐pretreated patients with obstructive sleep apnea. Sleep Medicine 2007;8(7‐8):773‐8. [DOI] [PubMed] [Google Scholar]
Taylor 2003 {unpublished data only}
- Taylor YL. A comparison between a telemedicine and traditional management model of care with nasal continuous positive airway pressure use among individuals with obstructive sleep apnea syndrome [Dissertation]. Washington: The George Washington University, 2003. [Google Scholar]
- Taylor YL, Eliasson A, Kristo D, Andrala T, Bigott T, Ephraim P, et al. Can telemedicine improve compliance with nasal CPAP? [Abstract]. Sleep 2003;26:A263. [Google Scholar]
Torvaldsson 2003 {unpublished data only}
- Torvaldsson S, Grote L, Peker Y, Hedner J. A comparison of two different auto‐titrating CPAP devices and constant CPAP in patients with obstructive sleep apnea. American Thoracic Society 99th International Conference; 2003 May 16‐21; Seattle. 2003:[B026] [Poster: 719].
- Torvaldsson S, Grote L, Peker Y, Hedner J. A comparison of two different auto‐titrating CPAP devices and constant CPAP in patients with obstructive sleep apnoea. European Respiratory Journal 2003;22(Suppl 45):Abstract P673. [Google Scholar]
van der Aa 2003 {published data only}
- Aa H, Meijer PM, Meinesz AF, Hoeven H, Wijkstra PJ. Titration of CPAP without polysomnography is equally effective as auto‐CPAP titration in patients with OSAS [Abstract]. European Respiratory Journal 2003;22(Suppl 45):P679. [Google Scholar]
Walter 2003 {published data only}
- Walter TJ, Samadder G, Strausbaugh B, Brant L. A comparison of auto‐adjusting CPAP, bilevel CPAP, and standard CPAP therapy in patients with REM related obstructive sleep apnea [Abstract]. Sleep 2003;26:A208. [Google Scholar]
Wiese 2005 {published data only}
- Wiese HJ, Boethel C, Phillips B, Wilson JF, Peters J, Viggiano T. CPAP compliance: video education may help!. Sleep Medicine 2005;6:171‐4. [DOI] [PubMed] [Google Scholar]
Wiest 1999 {published data only}
- Wiest GH, Lehnert G, Brück WM, Meyer M, Hahn EG, Ficker JH. A heated humidifier reduces upper‐airway dryness during continuous positive airway pressure therapy. Respiratory Medicine 1999;93(1):21‐6. [DOI] [PubMed] [Google Scholar]
Wiest 2002 {published data only}
- Wiest GH, Harsch IA, Fuchs FS, Kitzbichler S, Bogner K, Brueckl WM, et al. Initiation of CPAP therapy for OSA: does prophylactic humidification during CPAP pressure titration improve initial patient acceptance and comfort?. Respiration 2002;69(5):406‐12. [DOI] [PubMed] [Google Scholar]
Wimms 2013 {published data only}
- Wimms AJ, Richards GN, Benjafield AV. Assessment of the impact on compliance of a new CPAP system in obstructive sleep apnea. Sleep and Breathing 2013;17(1):69‐76. [PUBMED: 22286779] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Boyer 2019 {published data only}
- Study to predict the benefits of first‐line humidification use and comparison of the effect of ThermoSmart™ and no humidification on adherence. clinicaltrials.gov/ct2/show/study/NCT01742949 (first received 6 December 2012).
- Boyer L, Philippe C, Covali‐Noroc A, Dalloz MA, Rouvel‐Tallec A, Maillard D, et al. OSA treatment with CPAP: randomized crossover study comparing tolerance and efficacy with and without humidification by ThermoSmart(). Clinical Respiratory Journal 2019;13(6):384‐90. [DOI] [PubMed] [Google Scholar]
NCT02749812 {unpublished data only}
- Bironneau V, Gagnadoux F, Ingrand P, Pontier S, Iamandi C, Portel L, et al. [Fixed‐pressure CPAP versus auto‐adjusting CPAP : comparison of efficacy in obstructive sleep apnoea (OSAS) according to the individual level of efficient pressure variability]. European Respiratory Journal. 2018:PA2252.
- Bironneau V, Loustonneau E, Pontier S, Gagnadoux F, Iamandi I, Portel L, et al. Is it possible to determine the type of positive pressure device (constant or auto‐titrating) to use in the treatment of OSA? [Et s’il était possible de prédire le type d’appareil de PPC (constant ou autopiloté) à utiliser dans le traitement du SAHOS?]. Revue des Maladies Respiratoires 2016;33 (Suppl):A12‐13. [Google Scholar]
- NCT02749812. Interest of treatment of obstructive sleep apnea syndrome by constant CPAP and auto CPAP (PREDIVARIUS) [Beneficial effects of obstructive sleep apnea syndrome (OSAS) treatment by automatic continuous positive airway pressure (APAP) vs constant continuous positive airway pressure (constant CPAP) according to the level of the efficient pressure and its variability. A multicentric randomized trial]. clinicaltrials.gov/ct2/show/NCT02749812 (first received 25 April 2016).
Zamora 2019 {unpublished data only}
- Zamora T, Deering S, Stepnowsky CJ. The sleep apnea quality of life index as a patient‐centered measure. Sleep. 2019:A213.
References to ongoing studies
ACTRN12617001090303 {unpublished data only}
- ACTRN12617001090303. Comparing the usage of continuous positive airway pressure (PAP) against the RACer airway device in the treatment of obstructive sleep apnoea (OSA) in naive PAP users [Continuous positive airway pressure versus RACer airway device in the treatment of obstructive sleep apnoea ‐ adherence in naive PAP users]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=372904 (first received 14 August 2017).
ACTRN12618000379213p {unpublished data only}
- ACTRN12618000379213p. Comparing auto‐titrating continuous positive airway pressure device with fixed continuous positive airway pressure device in improvement in hypercapnia among patients with obesity hypoventilation syndrome. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=374259 (first received 14 March 2018).
Morton 2001 {published and unpublished data}
- Morton S, Rowland S, Deverson Q, McEvoy RD. The effects of humidification of nasal CPAP on adherence, compliance, patient satisfaction and symptoms: a preliminary report. Annual Scientific Meeting of the Thoracic Society of Australia and New Zealand; 2001 16‐21 March, Brisbane. 2001.
NCT01753999 {published data only}
- Ayappa I, Sunderram J, Black K, Twumasi A, Udasin I, Harrison D, et al. A comparison of CPAP and CPAPFLEX in the treatment of obstructive sleep apnea in World Trade Center responders: study protocol for a randomized controlled trial. Trials 2015;16:403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducca EL, Twumasi A, Gumb T, Perez A, Lewis CP, Patel R, et al. Physiological and psychological factors influencing continuous positive airway pressure (CPAP) use in world trade center (WTC) responders with obstructive sleep apnea (OSA). Sleep 2016;39:A158. [Google Scholar]
NCT03428516 {published data only}
- NCT03428516. Decrease obstructive sleep apnea (OSA) sympathetic tone: impact of APAP vs CPAP (APAP‐CPAP) [Decrease in sympathetic tone in OSA patients: is CPAP more effective than APAP ?]. clinicaltrials.gov/ct2/show/NCT03428516 (first received 9 February 2018).
- Treptow E, Pepin JL, Bailly S, Levy P, Bosc C, Destors M, et al. Reduction in sympathetic tone in patients with obstructive sleep apnoea: is fixed CPAP more effective than APAP? A randomised, parallel trial protocol. BMJ Open 2019;9(4):e024253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ventateswaren 2003 {published data only}
- Ventateswaran S, Hukins C, Duce B. Auto titrating continuous positive airways pressure (auto‐CPAP): comparisons of the autonomic markers of arousals with this treatment compared to fixed pressure CPAP treatment. Proceedings of the Thoracic Society of Australia and New Zealand Annual Scientific Meeting; 2003 April 4‐9; Adelaide. 2003; Vol. 8:A11.
Additional references
Angeli 2010
- Angeli F, Reboldi G, Verdecchia P. Masked hypertension: evaluation, prognosis, and treatment. American Journal of Hypertension 2010;23(9):941‐8. [DOI] [PubMed] [Google Scholar]
Baguet 2013
- Baguet JP, Boutin I, Barone‐Rochette G, Levy P, Tamisier R, Pierre H, et al. Hypertension diagnosis in obstructive sleep apnea: self or 24‐hour ambulatory blood pressure monitoring?. International Journal of Cardiology 2013;167(5):2346‐7. [DOI] [PubMed] [Google Scholar]
BaHammam 2016
- BaHammam AS, Kendzerska T, Gupta R, Ramasubramanian C, Neubauer DN, Narasimhan M, et al. Comorbid depression in obstructive sleep apnea: an under‐recognized association. Sleep and Breathing 2016;20(2):447‐56. [DOI] [PubMed] [Google Scholar]
Bakker 2011
- Bakker JP, Marshall NS. Flexible pressure delivery modification of continuous positive airway pressure for obstructive sleep apnea does not improve compliance with therapy: systematic review and meta‐analysis. Chest 2011;139(6):1322‐30. [DOI] [PubMed] [Google Scholar]
Bakker 2019
- Bakker JP, Weaver TE, Parthasarathy S, Aloia MS. Adherence to CPAP. What should we be aiming for, and how can we get there?. Chest 2019;155(6):1272‐87. [DOI: 10.1016/j.chest.2019.01.012] [DOI] [PubMed] [Google Scholar]
Billings 2014
- Billings ME, Rosen CL, Auckley D, Benca R, Foldvary‐Schaefer N, Iber C, et al. Psychometric performance and responsiveness of the functional outcomes of sleep questionnaire and sleep apnea quality of life instrument in a randomized trial: the HomePAP study. Sleep 2014;37(12):2017‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bobrie 2008
- Bobrie G, Clerson P, Ménard J, Postel‐Vinay N, Chatellier G, Plouin PF. Masked hypertension: a systematic review. Journal of Hyerptension 2008;26(9):1715‐25. [DOI] [PubMed] [Google Scholar]
Bollig 2010
- Bollig SM. Encouraging CPAP adherence: it is everyone's job. Respiratory Care 2010;55(9):1230‐9. [PubMed] [Google Scholar]
Boström 2010b
- Broström A, Arestedt KF, Nilsen P, Strömberg A, Ulander M, Svanborg E. The side‐effects to CPAP treatment inventory: the development and initial validation of a new tool for the measurement of side‐effects to CPAP treatment. Sleep 2010;19(4):603‐11. [DOI] [PubMed] [Google Scholar]
Broström 2010a
- Broström A, Nilsen P, Johansson P, Ulander M, Strömberg A, Svanborg E, et al. Putative facilitators and barriers for adherence to CPAP treatment in patients with obstructive sleep apnea syndrome: a qualitative content analysis. Sleep Medicine 2010;11(2):126‐30. [DOI] [PubMed] [Google Scholar]
Brown 2017
- Brown LK, Javaheri S. Positive airway pressure device technology past and present: what's in the "Black Box"?. Sleep Medicine Clinic 2017;12(4):501‐15. [DOI] [PubMed] [Google Scholar]
Catcheside 2010
- Catcheside P. Predictors of continuous positive airway pressure adherence. F1000 Medicine Reports 2010;2:70. [DOI: 10.3410/M2-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cochrane Airways 2019
- Cochrane Airways Trials Register. airways.cochrane.org/trials‐register (accessed 8 April 2019).
Cook 1995
- Cook NR, Cohen J, Hebert PR, Taylor JO, Hennekens CH. Implications of small reductions in diastolic blood pressure for primary prevention. Archives of Internal Medicine 1995;155(7):701‐9. [PubMed] [Google Scholar]
Doherty 2005
- Doherty LS, Kiely JL, Swan V, McNicholas WT. Long‐term effects of nasal continuous positive airway pressure therapy on cardiovascular outcomes in sleep apnea syndrome. Chest 2005;127(6):2076‐84. [DOI] [PubMed] [Google Scholar]
Drager 2011
- Drager LF, Polotsky VY, Lorenzi‐Filho G. Obstructive sleep apnea: an emerging risk factor for atherosclerosis. Chest 2011;140(2):534‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eckert 2008
- Eckert DJ, Malhotra A. Pathophysiology of adult obstructive sleep apnea. Procedings of the American Thoracic Society 200;5(2):144‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Engleman 1994
- Engleman HM, Martin SE, Douglas NJ. Compliance with CPAP therapy in patients with the sleep apnoea/hypopnoea syndrome. Thorax 1994;49(3):263‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Freedman 2017
- Freedman N. Treatment of obstructive sleep apnea: choosing the best positive airway pressure device. Sleep Medicine Clinics 2017;12(4):529‐42. [DOI] [PubMed] [Google Scholar]
Gagnon 2014
- Gagnon K, Baril AA, Gagnon JF, Fortin M, Décary A, Lafond C, et al. Cognitive impairment in obstructive sleep apnea. Pathologie Biologie 2014;62(5):233‐40. [DOI] [PubMed] [Google Scholar]
Garvey 2015
- Garvey JF, Pengo MF, Drakatos P, Kent BD. Epidemiological aspects of obstructive sleep apnea. Journal of Thoracic Disease 2015;7(5):920–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Giles 2006
- Giles TL, Lasserson TJ, Smith BJ, White J, Wright J, Cates CJ. Continuous positive airways pressure for obstructive sleep apnoea. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD001106.pub3] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hirsch Allen 2015
- Hirsch Allen AJ, Bansback N, Ayas NT. The effect of OSA on work disability and work‐related injuries. Chest 2015;147(5):1422‐28. [DOI] [PubMed] [Google Scholar]
Ip 2012
- Ip S, D'Ambrosio C, Patel K, Obadan N, Kitsios GD, Chung M, et al. Auto‐titrating versus fixed continuous positive airway pressure for the treatment of obstructive sleep apnea: a systematic review with meta‐analyses. Systematic Reviews 2012;1:20. [DOI: 10.1186/2046-4053-1-20] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jordan 2014
- Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet 2014;383(9918):736‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karimi 2015
- Karimi M, Hedner J, Häbel H, Nerman O, Grote L. Sleep apnea‐related risk of motor vehicle accidents is reduced by continuous positive airway pressure: Swedish Traffic Accident Registry data. Sleep 2015;38(3):341‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kasai 2012
- Kasai T, Floras JS, Bradley TD. Sleep apnea and cardiovascular disease: a bidirectional relationship. Circulation 2012;126(12):1495‐510. [DOI] [PubMed] [Google Scholar]
Konecny 2014
- Konecny T, Kara T, Somers VK. Obstructive Sleep Apnea and Hypertension. Hypertension 2014;63:203‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kribbs 1993
- Kribbs NB, Pack AI, Kline LR, Getsy JE, Schuett JS, Henry JN, et al. Effects of one night without nasal CPAP treatment on sleep and sleepiness in patients with obstructive sleep apnea. American Review of Respiratory Disease 1993;147(5):1162‐8. [DOI] [PubMed] [Google Scholar]
Law 2009
- Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta‐analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 2009;338:b1665. [DOI: 10.1136/bmj.b1665] [DOI] [PMC free article] [PubMed] [Google Scholar]
Libman 2017
- Libman E, Bailes S, Fichten CS, Rizzo D, Creti L, Baltzan M, et al. CPAP treatment adherence in women with obstructive sleep apnea. Sleep Disorders 2017;2017:2760650. [DOI: 10.1155/2017/2760650] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2017
- Liu T, Li W, Zhou H, Wang Z. Verifying the relative efficacy between continuous positive airway pressure therapy and its alternatives for obstructive sleep apnea: a network meta‐analysis. Frontiers in Neurology 2017;8:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marin 2005
- Marin J, Carrizo S, Vicente E, Agusti A. Long‐term cardiovascular outcomes in men with obstructive sleep apnoea‐hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005;365(9464):1046‐53. [DOI] [PubMed] [Google Scholar]
Marrone 2018
- Marrone O, Cibella F, Pépin JL, Grote L, Verbraecken J, Saaresranta T, et al. Fixed but not autoadjusting positive airway pressure attenuates the time‐dependent decline in glomerular filtration rate in patients with OSA. Chest 2018;154(2):326‐34. [DOI: 10.1016/j.chest.2018.04.020] [DOI] [PubMed] [Google Scholar]
Martínez‐García 2012
- Martínez‐García MA, Campos‐Rodríguez F, Catalán‐Serra P, Soler‐Cataluña JJ, Almeida‐Gonzalez C, Cruz Morón I, et al. Cardiovascular mortality in obstructive sleep apnea in the elderly: role of long‐term continuous positive airway pressure treatment: a prospective observational study. American Journal of Respiratory and Critical Care Medicine 2012;186(9):909‐16. [DOI] [PubMed] [Google Scholar]
Masa 2014
- Masa JF, Corral‐Peñafiel J. Should use of 4 hours continuous positive airway pressure per night be considered acceptable compliance? . European Respiratory Journal 2014;44:1119‐20. [DOI: 10.1183/09031936.00121514] [DOI] [PubMed] [Google Scholar]
McEvoy 2016
- McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, et al. SAVE Investigators and Co‐ordinators. CPAP for prevention of cardiovascular events in obstructive sleep apnea. New England Journal of Medicine 2016;375(10):919‐31. [DOI: 10.1056/NEJMoa1606599] [DOI] [PubMed] [Google Scholar]
Myhill 2012
- Myhill PC, Davis WA, Peters KE, Chubb SA, Hillman D, Davis TM. Effect of continuous positive airway pressure therapy on cardiovascular risk factors in patients with type 2 diabetes and obstructive sleep apnea. Journal of Clinical Endocrinology and Metabolism 2012;97(11):4212‐8. [DOI] [PubMed] [Google Scholar]
Patel 2018
- Patel S, Kon SS, Nolan CM, Barker RE, Simonds AK, Morrell MJ, et al. The Epworth Sleepiness Scale: minimum clinically important difference in obstructive sleep apnea. American Journal of Respiratory and Critical Care Medicine 2018;197(7):961‐3. [DOI: 10.1164/rccm.201704-0672LE] [DOI] [PMC free article] [PubMed] [Google Scholar]
Patruno 2014
- Patruno V, Tobaldini E, Bianchi AM, Mendez MO, Coletti O, Costantino G, et al. Acute effects of autoadjusting and fixed continuous positive airway pressure treatments on cardiorespiratory coupling in obese patients with obstructive sleep apnea. European Journal of Internal Medicine 2014;25(2):164‐8. [DOI] [PubMed] [Google Scholar]
Peppard 2013
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep‐disordered breathing in adults. American Journal of Epidemiology 2013;177(9):1006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pépin 1999
- Pépin JL, Krieger J, Rodenstein D, Cornette A, Sforza E, Delguste P, et al. Effective compliance during the first 3 months of continuous positive airway pressure. Respiratory and Critical Care Medicine 1999;130(4):1124‐9. [DOI] [PubMed] [Google Scholar]
Pépin 2014
- Pépin JL, Borel AL, Tamisier R, Baguet JP, Levy P, Dauvilliers Y. Hypertension and sleep: overview of a tight relationship. Sleep Medicine Reviews 2014;18(6):509‐19. [DOI] [PubMed] [Google Scholar]
Redline 2010
- Redline S, Yenokyan G, Gottlieb DJ, Shahar E, O'Connor GT, Resnick HE, et al. Obstructive sleep apnea‐hypopnea and incident stroke: the sleep heart health study. American Journal of Respiratory and Critical Care Medicine 2010;182(2):269‐77. [DOI: 10.1164/rccm.200911-1746OC] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reeves Hoche 1994
- Reeves‐Hoche MK, Meck R, Zwillich CW. Nasal CPAP: an objective evaluation of patient compliance. American Journal of Respiratory and Critical Care Medicine 1994;149(1):149‐54. [DOI] [PubMed] [Google Scholar]
Rotenberg 2016
- Rotenberg BW, Vicini C, Pang EB, Pang KP. Reconsidering first‐line treatment for obstructive sleep apnea: a systematic review of the literature. Journal of Otolaryngology 2016;45:23. [DOI: 10.1186/s40463-016-0136-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ryden 2014
- Ryden A, Bando JM, Aysola RS. Auto‐adjusting and advanced positive airway pressure therapeutic modalities. Seminars in Respiratory and Critical Care Medicine 2014;35(5):596‐603. [DOI] [PubMed] [Google Scholar]
Sanna 2018
- Sanna A, Lacedonia D. OSAS: its burden increases, not enough the awareness. Multidisciplinary Respiratory Medicine 2018;13:42. [DOI: 10.1186/s40248-018-0156-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Shahar 2001
- Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Nieto FJ, et al. Sleep‐disordered breathing and cardiovascular disease: cross‐sectional results of the Sleep Heart Health Study. American Journal of Respiratory and Critical Care Medicine 2001;163(1):19‐25. [DOI] [PubMed] [Google Scholar]
Swigris 2010
- Swigris JJ, Brown KK, Behr J, du Bois RM, King TE, Raghu G, et al. The SF‐36 and SGRQ: validity and first look at minimum important differences in IPF. Respiratory Medicine 2010;104(2):296‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tregear 2009
- Tregear S, Reston J, Schoelles K, Phillips B. Obstructive sleep apnea and risk of motor vehicle crash: systematic review and meta‐analysis. Journal of Clinical Sleep Medicine 2009;5(6):578‐81. [PMC free article] [PubMed] [Google Scholar]
Ward 2014
- Ward MM, Guthrie LC, Alba MI. Clinically important changes in short form 36 health survey scales for use in rheumatoid arthritis clinical trials: the impact of low responsiveness. Arthritis Care and Research 2014;66(12):1783‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weaver 2007
- Weaver TE, Maislin G, Dinges DF, Bloxham T, George CF, Greenberg H, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep 2007;30(6):711‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weaver 2010
- Weaver TE, Sawyer AM. Adherence to continuous positive airway pressure treatment for obstructive sleep apnoea: implications for future interventions. Indian Journal of Medical Research 2010;131:245‐58. [PMC free article] [PubMed] [Google Scholar]
Wickwire 2013
- Wickwire EM, Lettieri CJ, Cairns AA, Collop NA. Maximizing positive airway pressure adherence in adults: a common‐sense approach. Chest 2013;144(2):680‐93. [DOI] [PubMed] [Google Scholar]
Wild 2004
- Wild MR, Engleman HM, Douglas NJ, Espie CA. Can psychological factors help us to determine adherence to CPAP? A prospective study. European Respiratory Journal 2004;24:461‐5. [DOI] [PubMed] [Google Scholar]
Wozniak 2014
- Wozniak DR, Lasserson TJ, Smith I. Educational, supportive and behavioural interventions to improve usage of continuous positive airway pressure machines in adults with obstructive sleep apnoea. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD007736.pub2] [DOI] [PubMed] [Google Scholar]
Xu 2012
- Xu T, Li T, Wei D, Feng Y, Xian L, Wu H, et al. Effect of automatic versus fixed continuous positive airway pressure for the treatment of obstructive sleep apnea: an up‐to‐date meta‐analysis. Sleep and Breathing 2012;16(4):1017‐26. [DOI] [PubMed] [Google Scholar]
Yaggi 2005
- Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. New England Journal of Medicine 2005;353(19):2034‐41. [DOI] [PubMed] [Google Scholar]
Young 2008
- Young T, Finn L, Peppard PE, Szklo‐Coxe M, Austin D, Nieto FJ, et al. Sleep disordered breathing and mortality: eighteen‐year follow‐up of the Wisconsin sleep cohort. Sleep 2008;31(8):1071‐8. [PMC free article] [PubMed] [Google Scholar]
Yu 2017
- Yu J, Zhou Z, McEvoy RD, Anderson CS, Rodgers A, Perkovic V, Neal B. Association of positive airway pressure with cardiovascular events and death in adults with sleep apnea: a systematic review and meta‐analysis. JAMA 2017;318(2):156‐66. [DOI: 10.1001/jama.2017.7967] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Haniffa 2004
- Haniffa M, Lasserson TJ, Smith I. Interventions to improve compliance with continuous positive airway pressure for obstructive sleep apnoea. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD003531.pub2] [DOI] [PubMed] [Google Scholar]
Smith 2009a
- Smith I, Lasserson TJ. Pressure modification for improving usage of continuous positive airway pressure machines in adults with obstructive sleep apnoea. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD003531.pub3] [DOI] [PubMed] [Google Scholar]


