Abstract
Background
Prognostic models are needed that reflect contemporary practice for men with metastatic castration-resistant prostate cancer (mCRPC). We sought to identify predictive and prognostic variables for overall survival (OS) in chemotherapy-naïve men with mCRPC treated with enzalutamide.
Patients and methods
Patients from the PREVAIL trial database (enzalutamide versus placebo) were randomly split 2 : 1 into training (n = 1159) and testing (n = 550) sets. Using the training set, 23 predefined variables were analyzed and a multivariable model predicting OS was developed and validated in an independent testing set.
Results
Patient characteristics and outcomes were well balanced between training and testing sets; median OS was 32.7 months in each. The final validated multivariable model included 11 independent prognostic variables. Median OS for low-, intermediate-, and high-risk groups (testing set) defined by prognostic risk tertiles were not yet reached (NYR) (95% CI NYR–NYR), 34.2 months (31.5–NYR), and 21.1 months (17.5–25.0), respectively. Hazard ratios (95% CI) for OS in the low- and intermediate-risk groups versus high-risk group were 0.20 (0.14–0.29) and 0.40 (0.30–0.53), respectively. Secondary outcomes of response and progression differed widely in model-defined risk groups. Enzalutamide improved outcomes in all prognostic risk groups.
Conclusions
Our validated prognostic model incorporates variables routinely collected in chemotherapy-naïve men with mCRPC treated with enzalutamide, identifying subsets of patients with widely differing survival outcomes that provide useful information for external validation, patient care, and clinical trial design.
Trial registration
ClinicalTrials.gov: NCT01212991.
Keywords: prognostic variables, overall survival, nomogram, enzalutamide, metastatic castration-resistant prostate cancer, multivariable model
Key Message
We developed and validated a prognostic model for overall survival in chemotherapy-naïve men with mCRPC treated with enzalutamide, identifying risk groups with a wide range of clinical outcomes.
Introduction
The majority of men who develop lethal prostate cancer will first develop metastatic castration-resistant prostate cancer (mCRPC) [1]. Despite treatments that improve survival [2–11], heterogeneity between patients in disease biology and burden account for significant differences in treatment outcomes.
Prognostic models for survival have been developed and utilized for patient care and clinical trial design [12, 13]. Prior studies identified independent prognostic variables for survival in men with mCRPC treated with docetaxel or abiraterone acetate [13–17], including both tumor and host factors. How these prognostic factors together perform in predicting outcomes in chemotherapy-naïve mCRPC men treated with the novel androgen receptor (AR)-directed therapies enzalutamide or abiraterone, as is often current practice, is unknown, and validated prognostic models are needed.
Enzalutamide is a second-generation AR inhibitor approved for the treatment of men with mCRPC. In the randomized, phase III PREVAIL trial, enzalutamide significantly reduced the risk of death by 29% [hazard ratio (HR) 0.71; P < 0.001] compared with placebo [2]. By analyzing patients from PREVAIL, we sought to validate and identify novel prognostic and predictive variables associated with survival in chemotherapy-naïve asymptomatic to minimally symptomatic men with mCRPC treated with enzalutamide.
Patients and methods
Patients and study design
The full methodology and results of the international, randomized, double-blind, placebo-controlled, phase III PREVAIL trial (NCT01212991) have been previously reported (also see supplementary Methods, available at Annals of Oncology online) [2].
Data sets
The primary end point used for the model was overall survival (OS), using an updated analysis after 784 deaths (46%, data cutoff 1 June 2014) [18]; all secondary end points used the planned interim data cut at 16 September 2013. Patients from PREVAIL (both enzalutamide and placebo arms) were randomly divided 2 : 1 into the training and testing sets for this analysis (supplementary Figure S1, available at Annals of Oncology online).
From the data available in the PREVAIL data set, 23 variables were selected for analysis (supplementary Table S1, available at Annals of Oncology online), based on previous work demonstrating their potential importance in mCRPC outcomes [13, 14, 17, 19, 20]. Skewed data were log transformed or coded as categorical. Correlations were examined using the Spearman correlation method.
Model building
Categorical and/or continuous variables associated with the 23 selected variables with data available in the PREVAIL data set (supplementary Table S1, available at Annals of Oncology online) were analyzed using stepwise Cox proportional hazards model and adaptive least absolute shrinkage and selection operator (ALASSO), with the hypothesis that results could be considered robust if both methods identified the same or similar sets of variables. Using the training set, 23 variables were analyzed in a stepwise Cox proportional hazards model and a penalized Cox proportional hazards model using ALASSO penalty [2, 21]. The absolute shrinkage property of the ALASSO method results in more stable variable selection [21, 22]. The Akaike Information Criterion (AIC) [23] was used to examine which variables contributed most to the final model. Model building followed a prospectively defined plan in which prognostic discrimination and parsimony were balanced with the need to include variables previously established and validated in the mCRPC setting to maximize generalizability. A predictive treatment interaction term was created for each candidate variable and was tested in the proportional hazards regression model for improvement in OS with enzalutamide relative to placebo.
Model validation
A multivariable Cox proportional hazards model predicting OS using the training set was developed. The HR and 95% confidence interval (CI) were computed for each potentially prognostic variable. Predictive accuracy was assessed in the testing set using time-dependent area under the curve (tAUC) and calibration assessed by plotting the predicted probability of death at 3-month intervals between 6 and 39 months versus observed probability.
A risk score was developed from the multivariable model using the training set. Based on risk scores, the testing set was divided at the median cut point (high- and low-risk groups) and by tertiles (high-, intermediate-, and low-risk groups). The OS in the training and testing sets was analyzed using Kaplan–Meier methodology. In the testing set risk groups, the primary end point of OS and secondary end points of radiographic progression-free survival (PFS) and prostate-specific antigen (PSA)–PFS were analyzed using Kaplan–Meier methodology. Analyses were carried out using SAS software (version 9.4; SAS Institute, Cary, NC) and R glmnet and R survAUC packages, R version 3.2.2 [24].
Results
Patients
Baseline demographics, medical history, disease characteristics, and distribution of treatment assignments (enzalutamide or placebo) were balanced between the training set (n = 1159) and the testing set (n = 550; supplementary Table S2, available at Annals of Oncology online). Eight patients from the full PREVAIL population (n = 1717) were excluded from this analysis because of missing baseline values for continuous variables. At the data cutoff date, median OS times of the training and testing sets were an estimated 32.7 months (95% CI 31.3–35.5) and 32.7 months, respectively [95% CI 30.9–not yet reached (NYR); supplementary Figure S2, available at Annals of Oncology online].
Prognostic variable selection
The stepwise model identified 14 prognostic variables for OS, whereas ALASSO identified 13. All variables identified with ALASSO were identified with the stepwise model: albumin, alkaline phosphatase (ALP), Eastern Cooperative Oncology Group performance status, hemoglobin, lactate dehydrogenase (LDH), neutrophil-to-lymphocyte ratio (NLR), number of bone metastases, presence of pain, pattern of spread (visceral versus bone versus lymph node only), PSA, time from diagnosis to randomization, treatment (enzalutamide versus placebo), and type of progression at study entry (PSA only versus radiographic). The only variable not identified by ALASSO was de novo metastatic (M1) disease.
Before modeling, we tested for the presence of a treatment–biomarker interaction with all prognostic variables. Each treatment interaction term was not significant (P > 0.05), indicating that enzalutamide treatment was independently associated with improved survival in all prognostic biomarker–defined groups; thus no predictive biomarkers for enzalutamide efficacy were identified or included in the model.
Given the need for parsimony in model development, we carried out AIC analysis using a stepwise model. The AIC method avoids overfitting, and the decrease in AIC score began to flatten at nine parameters (supplementary Figure S3, available at Annals of Oncology online), indicating that albumin, LDH, de novo M1 disease, NLR, number of bone metastases, presence of pain, PSA, time from diagnosis to randomization, and treatment were the strongest prognostic variables.
The nine variables identified by AIC analysis did not include visceral pattern of spread, which previously has been demonstrated to be strongly prognostic of survival in men with mCRPC [14, 15, 17, 25, 26]. Likewise, neither ALP nor hemoglobin was included in the AIC analysis, despite their inclusion in the ALASSO and stepwise models and previous validation studies [13, 14]. In our data set, ALP had a moderate correlation with the number of bone metastases, and PSA and hemoglobin had moderate correlation with albumin (supplementary Table S3, available at Annals of Oncology online). However, to develop a clinically relevant model and potentially improve external validity, we included pattern of spread, ALP, and hemoglobin in our final model. Additionally, because our initial hypothesis was that variables identified by both ALASSO and the stepwise models would be more robust, we opted to remove de novo M1 disease, which was moderately correlated with time from diagnosis (r = −0.49; supplementary Table S3, available at Annals of Oncology online), a variable captured in both models and AIC analysis. Thus, the final model contained 11 prognostic variables: albumin, ALP, hemoglobin, LDH, NLR, number of bone metastases, presence of pain, pattern of spread, PSA, time from diagnosis to randomization, and treatment. Importantly, our model did not include 12 variables due to lack of statistical significance or model improvement, including type of progression, number of prior secondary hormonal therapies, PSA doubling time, age, baseline corticosteroid use, or prior prostatectomy.
Multivariable model for predicting OS
In a multivariable Cox proportional hazards model performed on the training set, all 11 variables were significantly associated with OS (Table 1). Concordance for predicting OS as assessed by tAUC score for the 11-variable model was 0.74 in the testing validation set (supplementary Figure S4, available at Annals of Oncology online).
Table 1.
Multivariable analysis of prognostic variables for the training set
| Prognostic variable | Definition | HR (95% CI) |
|---|---|---|
| Albumin | Continuous, per point rise in μg/dl | 0.71 (0.53–0.95) |
| ALP | <ULN versus ≥ULN | 0.79 (0.64–0.98) |
| Number of bone metastases | <10 versus ≥10 | 0.68 (0.56–0.83) |
| Hemoglobin | Continuous, g/dl | 0.85 (0.78–0.92) |
| LDH | <ULN versus ≥1 × ULN | 0.67 (0.54–0.82) |
| NLR | <2.5 versus ≥2.5 | 0.69 (0.57–0.82) |
| Pain score | 0–1 versus ≥2 (linear scale) | 0.77 (0.64–0.92) |
| Pattern of spread | No liver metastases versus any liver metastases | 0.49 (0.35–0.69) |
| Loge PSA | Continuous, loge of baseline PSA ng/ml | 1.23 (1.14–1.32) |
| Time from diagnosis to randomization | Continuous, months | 0.997 (0.995–0.998) |
| Treatment | Enzalutamide versus placebo | 0.68 (0.57–0.81) |
ALP, alkaline phosphatase; CI, confidence interval; HR, hazard ratio; LDH, lactate dehydrogenase; NLR, neutrophil-to-lymphocyte ratio; PSA, prostate-specific antigen; ULN, upper limit of normal.
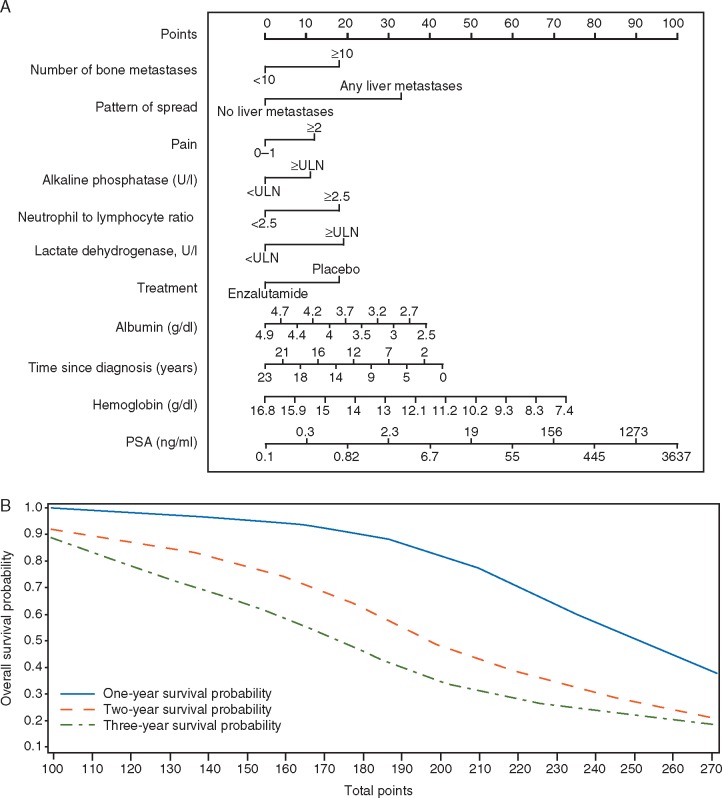
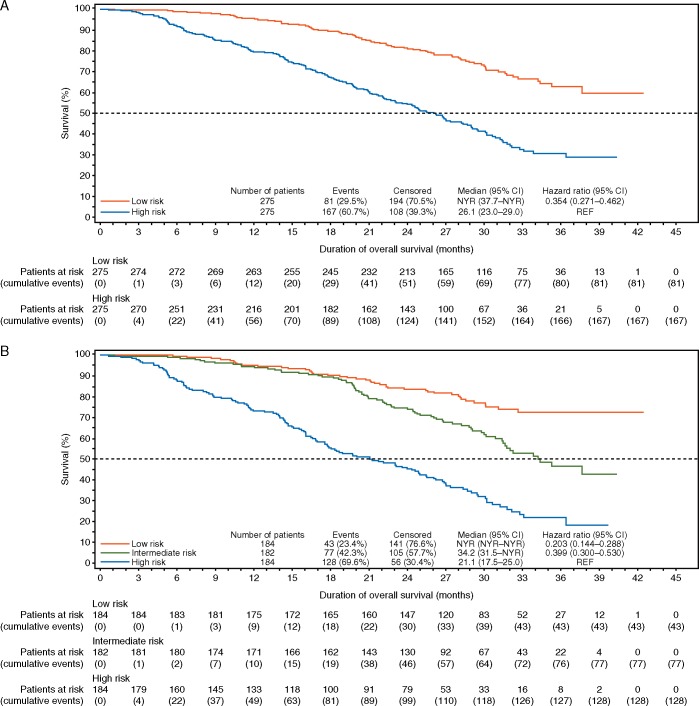
A risk score was calculated using regression coefficients from the training set for the 11 variables. An algorithm was developed accordingly (supplementary Table S4, available at Annals of Oncology online) and a nomogram was produced in Figure 1. Patients in the testing set were stratified as being at high or low risk of death based on median risk score and also stratified as high, intermediate, or low risk based on risk score tertiles. The 11-variable model provided a significant separation between low-risk and high-risk patients (HR 0.35; 95% CI 0.27–0.46; Figure 2A) and between low-risk (HR 0.20; 95% CI 0.14–0.29) and intermediate-risk (HR 0.40; 95% CI 0.30–0.53) versus high-risk patients (Figure 2B). The full model description is provided in supplementary Table S4, available at Annals of Oncology online.
Figure 1.
A nomogram is used to assign each of 11 prognostic factors with a point range from 0 to 100 in a graphic interface, based on the estimated regression coefficients from the final multivariable Cox proportional hazards model predicting overall survival (OS). The nomogram results match with the algorithm. The 1-, 2-, and 3-year survival probability versus total points were also generated to allow the clinical partition to predict OS. The nomogram was developed using SAS® enterprise version 7.1 and is based on the following article: http://support.sas.com/resources/papers/proceedings13/264-2013.pdf. Instructions to physicians: All of the 11 prognostic factors should be available before using this model. Start from the second top axis by identifying the number of bone metastases. Draw a vertical line to the points axis (top line) to represent the number of prognostic points the patients will receive for number of bone metastases. Do the same for the other prognostic variables. Once all prognostic points for the predictors have been determined, add up the prognostic points for each prognostic variable. On the basis of the total points, one can determine the 1-year survival probability by drawing a vertical line from the total points x-axis to the survival probability. The same process can be performed to estimate the 2-year and 3-year survival probability.
Figure 2.
Analysis of overall survival based on the 11-variable model. Analyses in (A) high- and low-risk patients as stratified by median risk score and (B) high-, intermediate-, and low-risk patients as stratified by tertiles of the risk score. CI, confidence interval; NYR, not yet reached; REF, reference.
Next, we examined the impact of enzalutamide treatment on men in both low- and high-risk prognostic groups of the testing set. Median OS among low-risk patients was NYR with both enzalutamide and placebo, whereas among high-risk patients, median OS was 27.4 and 24.9 months, respectively. Among low-risk patients, median radiographic PFS was 27.5 months with enzalutamide and 8.2 months with placebo (Table 2; supplementary Figure S5A, available at Annals of Oncology online) and in high-risk patients, this was 16.6 and 5.3 months, respectively (Table 2; supplementary Figure S5A, available at Annals of Oncology online). Median PSA–PFS and radiographic and PSA responses also differed significantly by risk groups (Table 2; supplementary Figure S5B, available at Annals of Oncology online).
Table 2.
Summary of primary and secondary end points in the testing set
| Using the 11-variable multivariable model | ||||
|---|---|---|---|---|
| Outcome | Low-risk patients | High-risk patients | ||
| (<median risk score) |
(>median risk score) |
|||
| Overalla | Enzalutamide | Overalla | Enzalutamide | |
| (n = 275) | (n = 168) | (n = 275) | (n = 112) | |
| Median OS, months (95% CI) | NYR (37.7–NYR) | NYR (37.7–NYR) | 26.1 (23.0–29.0) | 27.4 (22.7–30.2) |
| Median radiographic PFS, months (95% CI) | 19.1 (16.4–24.6) | 27.5 (19.1–30.7) | 8.4 (6.0–11.0) | 16.6 (13.8–23.6) |
| Median PSA–PFS, months (95% CI) | 8.4 (8.1–11.1) | 16.4 (13.7–16.8) | 4.6 (3.7–5.6) | 8.3 (8.3–11.1) |
| Best overall soft-tissue response, n/Nb (%) [95% CI] | 47/117 (40) | 43/66 (65) | 35/134 (26) | 33/64 (52) |
| [31.2–49.6] | [52.4–76.5] | [18.9–34.4] | [38.7–64.3] | |
| Complete soft-tissue response, n/Nb (%) | 21/117 (18) | 20/66 (30) | 10/134 (7) | 9/64 (14) |
| Partial soft-tissue response, n/Nb (%) | 26/117 (22) | 23/66 (35) | 25/134 (19) | 24/64 (38) |
| Confirmed PSA decline ≥30%, n/Nc (%) | 154/268 (57) | 148/165 (90) | 88/253 (35) | 85/109 (78) |
| Confirmed PSA decline ≥50%, n/Nc (%) | 145/268 (54) | 140/165 (85) | 81/253 (32) | 78/109 (72) |
| Confirmed PSA decline ≥90%, n/Nc (%) | 88/268 (33) | 87/165 (53) | 50/253 (20) | 49/109 (45) |
| Using the numerically based risk group stratificationd | ||||||
|---|---|---|---|---|---|---|
| Low-risk patients | Intermediate-risk patients | High-risk patients | ||||
| (0–3 risk variables at baseline) |
(4–6 risk variables at baseline) |
(7–10 risk variables at baseline) |
||||
| Overalla | Enzalutamide | Overalla | Enzalutamide | Overalla | Enzalutamide | |
| (n = 244) | (n = 152) | (n = 233) | (n = 103) | (n = 73) | (n = 25) | |
| Median OS, months (95% CI) | NYR (37.7–NYR) | NYR (37.7–NYR) | 30.1 (27.0–32.2) | 30.1 (27.0–33.1) | 16.5 (13.5–18.9) | 16.6 (14.3–22.1) |
| Median radiographic PFS, months (95% CI) | 19.1 (16.5–24.7) | 27.5 (19.1–30.7) | 11.0 (8.2–15.7) | 22.0 (15.7–27.9) | 5.4 (3.5–8.1) | 8.5 (5.4–16.6) |
| Median PSA–PFS, months (95% CI) | 8.4 (8.1–11.1) | 14.5 (11.3–19.1) | 4.6 (3.7–8.2) | 11.0 (8.3–11.3) | 3.7 (2.9–4.7) | 5.6 (3.7–8.3) |
| Best overall soft-tissue response, n/Nb (%) [95% CI] | 46/111 (41) | 43/66 (65) | 32/102 (31) | 29/49 (59) | 4/38 (11) | 4/15 (27) |
| [32.2–51.2] | [52.4–76.5] | [22.6–41.3] | [44.2–73.0] | [2.9–24.8] | [7.8–55.1] | |
| Complete soft-tissue response, n/Nb (%) | 23/111 (21) | 22/66 (33) | 7/102 (7) | 6/49 (12) | 1/38 (3) | 1/15 (7) |
| Partial soft-tissue response, n/Nb (%) | 23/111 (21) | 21/66 (32) | 25/102 (25) | 23/49 (47) | 3/38 (8) | 3/15 (20) |
| Confirmed PSA decline ≥30%, n/Nc (%) | 140/240 (58) | 136/150 (91) | 88/223 (39) | 84/102 (82) | 14/58 (24) | 13/22 (59) |
| Confirmed PSA decline ≥50%, n/Nc (%) | 133/240 (55) | 129/150 (86) | 82/223 (37) | 79/102 (77) | 11/58 (19) | 10/22 (45) |
| Confirmed PSA decline ≥90%, n/Nc (%) | 82/240 (34) | 81/150 (54) | 53/223 (24) | 52/102 (51) | 3/58 (5) | 3/22 (14) |
Enzalutamide and placebo treatment included in model.
N represents the number of patients with measurable disease at baseline.
N represents the number of patients with at least one postbaseline PSA assessment.
Risk variables: PSA >50 ng/ml, time from diagnosis <60 months, albumin <4.0 g/dl, hemoglobin <12.5 g/dl, NLR ≥2.5, any liver metastases, pain score ≥2, LDH ≥ ULN, ≥10 bone metastases, ALP ≥ ULN, placebo treatment (i.e. natural history).
NYR, not yet reached; OS, overall survival; PFS, progression-free survival.
Risk group development
We examined the utility of categorizing patients into three risk groups based on the number of risk variables from our 11-variable model present at baseline, including enzalutamide or placebo treatment. Clinically relevant cutoffs based on median values or lower limit of normal in the testing set were used for variables with continuous variables (see supplementary Results and Figure S6, available at Annals of Oncology online, and Table 2), which also provided broad discrimination of all clinical outcomes.
Discussion
We developed a clinically useful prognostic model for OS in chemotherapy-naïve men with mCRPC treated with enzalutamide. In addition to treatment, this model contains eight traditional prognostic variables associated with survival (i.e. albumin, ALP, hemoglobin, LDH, number of bone metastases, presence of pain, pattern of spread, and PSA), and incorporates two novel independent prognostic variables relevant to this minimally symptomatic setting: NLR and time since diagnosis. A shorter time from diagnosis to randomization was independently associated with shorter OS in PREVAIL, reflecting metastatic disease at diagnosis or short duration of castration sensitivity. We validated our 11-variable model in an independent testing data set and demonstrated a high level of prognostic accuracy, similar to clinically useful models previously validated in the docetaxel or post-docetaxel mCRPC treatment settings [13–15]. In addition, our model provides discrimination for key secondary outcomes in this setting, including PFS, PSA decline, and radiographic response. Interestingly, we did not identify specific predictors of enzalutamide benefit, indicating that enzalutamide improved outcomes regardless of prognostic risk group.
This prognostic model provides several advantages to those previously available because it was developed and validated in a contemporary treatment setting with first-line enzalutamide in chemotherapy-naïve men with mCRPC, reflecting current practice. Although we did not specifically validate our model in men treated with abiraterone, the inclusion of established risk factors and the similar survival times with abiraterone and enzalutamide in this setting suggests that this model may provide valuable and generalizable prognostic information to physicians and patients who are treated with first-line novel AR pathway inhibition. Further external validation of this model is critical for generalizability, however. Additionally, we utilized multiple methods for model development and incorporated robust clinical variables previously validated in other treatment settings in the literature, which broadens the clinical applicability of our model. Furthermore, we incorporated enzalutamide and placebo treatment into our model to account for the natural history of mCRPC, which should provide useful information for future clinical trial designs, stratification schemes, and identification of risk groups for trial eligibility and outcome assessments. Finally, our model was developed based on OS, the gold standard end point for regulatory decisions and patient outcomes for mCRPC.
Our model is not without limitations. Notably, the PREVAIL data set lacked tissue biopsies, circulating tumor cell (CTC), or cell-free DNA/RNA biomarkers for inclusion in our model. Biomarkers such as CTC enumeration, genomic alterations, AR splice variants, and AR copy number may help better predict survival and should be incorporated into future studies [27–30]. Despite this, our model identified risk groups with widely differing survival times, illustrating the heterogeneity of outcomes among men with chemotherapy-naïve mCRPC based on readily available clinical parameters.
It should be noted that all validated prognostic models have limitations in informing clinical practice. Although the variables included have strong biologic rationale and independent validation, outcomes for individuals in contemporary practice may differ from those in clinical trial populations, and external validation is recommended in a broader, nontrial population of men with mCRPC. Accordingly, the prognostic model presented in this paper, and in general, should not displace the well-informed clinical judgment of healthcare professionals treating individual patients. However, knowledge of prognosis may aid decisions regarding the aggressiveness with which to pursue active therapy for mCRPC and should help shape trial designs that utilize combinations with AR-directed therapies, using more aggressive approaches for men with high-risk mCRPC who may benefit from combination therapies. We did not incorporate posttreatment PSA declines or radiographic responses in our model, despite their strong associations with survival [14, 20, 31], in order to develop a purely pretreatment survival model. However, ongoing work is characterizing updated prognosis based on responses after enzalutamide treatment. Finally, early use of abiraterone, apalutamide, and enzalutamide in men with nonmetastatic CRPC and metastatic hormone-sensitive prostate cancer may affect the utility and future generalizability of this model.
The independent prognostic value of NLR was a novel finding that may reflect underlying tumor-associated inflammation or host immune response. Thus, incorporation of NLR, available from a complete blood count with differential, may prove useful for risk stratification in asymptomatic men with mCRPC. Recent data support use of NLR in other cancer settings [32] and, along with albumin and functional status, provide useful measures of host response important in determining long-term survival. Here, we used a cutoff of 2.5 for NLR, similar to that previously evaluated in men with mCRPC [17], but more relevant in this minimally symptomatic setting.
Conclusion
In summary, our prognostic model was constructed and validated using data routinely collected in chemotherapy-naïve men with mCRPC treated with enzalutamide, identifying subsets of patients with widely differing survival outcomes. This model has potential clinical utility for individual and trial-level survival, potential outcomes prognostication, and clinical trial design of novel treatment approaches in this population.
Supplementary Material
Acknowledgements
Medical writing and editorial support, funded by Medivation, which was acquired by Pfizer in September 2016, and Astellas Pharma, Inc, were provided by Nathan Yardley, PhD, Edwin Thrower, PhD, and Shannon Davis of Ashfield Healthcare Communications.
Funding
Medivation, which was acquired by Pfizer in September 2016; and Astellas Pharma, Inc (the co-developers of enzalutamide; no grant number applies). AJA receives support from the Duke Cancer Center's P30 grant CA014236 and the US Department of Defense Prostate Cancer Clinical Trials Consortium (DOD PCCTC) award W81XWH-14-2-0179.
Disclosure
AJA reports honoraria from Dendreon and Sanofi; an advisory role for Bayer, Dendreon, Eisai, Janssen Biotech, Medivation, Novartis, and Sanofi; membership on a speakers bureau for Dendreon and Sanofi; research funding to his institution from Astellas Pharma, Bayer, Bristol-Myers Squibb, Dendreon, Gilead Sciences, Janssen Oncology, Medivation, Novartis, Pfizer, and Sanofi; intellectual property for CTC novel capture technology belonging to his institution; and travel expenses from Dendreon, Janssen Biotech, Medivation, and Sanofi. PL is an employee of Pfizer. CSH reports an advisory role for Asana Biosciences, Astellas Pharma, Bayer, Blue Earth Diagnostics, Churchill Pharmaceuticals, Clovis Oncology, Dendreon, Emergent BioSolutions, Endocyte, Ferring, Medivation, MorphoSys, Orion Corporation, and Parexel; research funding to her institution from Algeta/Bayer, Aragon Pharmaceuticals, AstraZeneca, Bayer, Dendreon, Emergent BioSolutions, Genentech, Medivation, Pfizer, and Sanofi; and travel expenses from Asana Biosciences, Astellas Pharma, Bayer, Blue Earth Diagnostics, Churchill Pharmaceuticals, Clovis Oncology, Dendreon, Emergent BioSolutions, Emergent BioSolutions, Endocyte, and Ferring. CNS reports an advisory role or honoraria from Astellas Pharma, Clovis Oncology, Janssen, Pfizer, Bayer, and Sanofi/Genzyme. GS reports an advisory role for Agensys, Amgen, Argos Therapeutics, AstraZeneca, Bayer, Bristol-Myers Squibb, Eisai, Exelixis, Genentech, Janssen, Merck, Novartis, Pfizer, and Sanofi; membership on a speakers bureau for Clinical Care Options/NCCN; and research funding to his institution from Bayer, Boehringer Ingelheim, Celgene, Merck, and Onyx. BT reports honoraria from Amgen, Astellas Pharma, Bayer, Ferring, Janssen, and Sanofi; an advisory role for Astellas Pharma, Bayer, Ferring, Janssen, Sanofi, Steba Biotech, and Takeda; membership on a speakers bureau for Amgen and Janssen; research funding to his institution from Ferring; and travel expenses from Amgen, Astellas Pharma, Bayer, Ferring, Janssen, and Sanofi. AJT reports honoraria from Astellas Pharma; travel/conference support from Sanofi and Janssen; and an advisory role for Bristol-Myers Squibb, Merck Sharp & Dohme, Janssen, Sanofi, and Roche (all with compensation to his institution). KF reports honoraria from Astellas Pharma, Janssen, and Sanofi and an advisory role for Astellas Pharma, Bayer, Janssen, Orion, and Sanofi. DP is an employee of Astellas Pharma Europe. EKW was an employee of Medivation, which was acquired by Pfizer in September 2016, at the time of this work. AK is an employee of Astellas Pharma and reports stock in Abbott and AbbVie and intellectual property in Abbott. TMB reports stock and/or other ownership interests in Salarius Pharmaceuticals; an advisory role for AstraZeneca, Churchill Pharmaceuticals, Dendreon, Janssen Biotech, Janssen Japan, Janssen Oncology, Janssen Research & Development, Johnson & Johnson, and Roche; and research funding to his institution from Astellas Pharma, Boehringer Ingelheim, Bristol-Myers Squibb, Dendreon, Janssen Research & Development, Medivation, OncoGenex, Sotio, and Theraclone Sciences.
References
- 1. Ferlay J, Soerjomataram I, Ervik M. et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11, 2016. http://globocan.iarc.fr (31 May 2018, date last accessed).
- 2. Beer TM, Armstrong AJ, Rathkopf DE. et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 2014; 371(5): 424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Scher HI, Fizazi K, Saad F. et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012; 367(13): 1187–1197. [DOI] [PubMed] [Google Scholar]
- 4. Fizazi K, Scher HI, Molina A. et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2012; 13(10): 983–992. [DOI] [PubMed] [Google Scholar]
- 5. Kantoff PW, Higano CS, Shore ND. et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010; 363(5): 411–422. [DOI] [PubMed] [Google Scholar]
- 6. Ryan CJ, Smith MR, Fizazi K. et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2015; 16(2): 152–160. [DOI] [PubMed] [Google Scholar]
- 7. de Bono JS, Oudard S, Ozguroglu M. et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 2010; 376(9747): 1147–1154. [DOI] [PubMed] [Google Scholar]
- 8. Parker C, Nilsson S, Heinrich D. et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013; 369(3): 213–223. [DOI] [PubMed] [Google Scholar]
- 9. Ryan CJ, Smith MR, de Bono JS. et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 2013; 368(2): 138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Horwich A, Hugosson J, de Reijke T. et al. Prostate cancer: ESMO consensus conference guidelines 2012. Ann Oncol 2013; 24(5): 1141–1162. [DOI] [PubMed] [Google Scholar]
- 11. Gillessen S, Attard G, Beer TM. et al. Management of patients with advanced prostate cancer: the report of the Advanced Prostate Cancer Consensus Conference APCCC 2017. Eur Urol 2018; 73(2): 178–211. [DOI] [PubMed] [Google Scholar]
- 12. Halabi S, Kelly WK, Ma H. et al. Meta-analysis evaluating the impact of site of metastasis on overall survival in men with castration-resistant prostate cancer. J Clin Oncol 2016; 34(14): 1652–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Halabi S, Lin CY, Kelly WK. et al. Updated prognostic model for predicting overall survival in first-line chemotherapy for patients with metastatic castration-resistant prostate cancer. J Clin Oncol 2014; 32(7): 671–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Armstrong AJ, Garrett-Mayer ES, Yang YC. et al. A contemporary prognostic nomogram for men with hormone-refractory metastatic prostate cancer: a TAX327 study analysis. Clin Cancer Res 2007; 13(21): 6396–6403. [DOI] [PubMed] [Google Scholar]
- 15. Chi KN, Kheoh T, Ryan CJ. et al. A prognostic index model for predicting overall survival in patients with metastatic castration-resistant prostate cancer treated with abiraterone acetate after docetaxel. Ann Oncol 2016; 27(3): 454–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ryan CJ, Kheoh T, Li J. et al. Prognostic index model for progression-free survival in chemotherapy-naïve metastatic castration-resistant prostate cancer treated with abiraterone acetate plus prednisone. Clin Genitourin Cancer 2018; 16(1): 72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Templeton AJ, Pezaro C, Omlin A. et al. Simple prognostic score for metastatic castration-resistant prostate cancer with incorporation of neutrophil-to-lymphocyte ratio. Cancer 2014; 120(21): 3346–3352. [DOI] [PubMed] [Google Scholar]
- 18. Beer TM, Armstrong AJ, Rathkopf D. et al. Enzalutamide in men with chemotherapy-naive metastatic castration-resistant prostate cancer: extended analysis of the phase 3 PREVAIL study. Eur Urol 2017; 71(2): 151–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Halabi S, Small EJ, Kantoff PW. et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol 2003; 21(7): 1232–1237. [DOI] [PubMed] [Google Scholar]
- 20. Sonpavde G, Pond GR, Berry WR. et al. The association between radiographic response and overall survival in men with metastatic castration-resistant prostate cancer receiving chemotherapy. Cancer 2011; 117(17): 3963–3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tibshirani R. The lasso method for variable selection in the Cox model. Stat Med 1997; 16(4): 385–395. [DOI] [PubMed] [Google Scholar]
- 22. Zhang HH, Lu W.. Adaptive Lasso for Cox's proportional hazards model. Biometrika 2007; 94(3): 691–703. [Google Scholar]
- 23. Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr 1974; 19(6): 716–723. [Google Scholar]
- 24.The R Foundation. The R project for statistical computing. 2016. https://www.r-project.org/ (31 May 2018, date last accessed).
- 25. Pond GR, Sonpavde G, de Wit R. et al. The prognostic importance of metastatic site in men with metastatic castration-resistant prostate cancer. Eur Urol 2014; 65(1): 3–6. [DOI] [PubMed] [Google Scholar]
- 26. Armstrong AJ, Tannock IF, de Wit R. et al. The development of risk groups in men with metastatic castration-resistant prostate cancer based on risk factors for PSA decline and survival. Eur J Cancer 2010; 46(3): 517–525. [DOI] [PubMed] [Google Scholar]
- 27. Antonarakis ES, Lu C, Wang H. et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med 2014; 371(11): 1028–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Bono JS, Scher HI, Montgomery RB. et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res 2008; 14(19): 6302–6309. [DOI] [PubMed] [Google Scholar]
- 29. Romanel A, Gasi Tandefelt D, Conteduca V. et al. Plasma AR and abiraterone-resistant prostate cancer. Sci Transl Med 2015; 7(312): 312re10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Robinson D, Van Allen EM, Wu YM. et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015; 161(5): 1215–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Armstrong AJ, Saad F, Phung D. et al. Clinical outcomes and survival surrogacy studies of prostate-specific antigen declines following enzalutamide in men with metastatic castration-resistant prostate cancer previously treated with docetaxel. Cancer 2017; 123(12): 2303–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Templeton AJ, McNamara MG, Seruga B. et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014; 106(6): dju124. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.