Abstract
Neutrophils are key to host defence, and impaired neutrophil function predisposes to infection with an array of pathogens, with Staphylococcus aureus a common and sometimes life-threatening problem in this setting. Both infiltrating immune cells and replicating bacteria consume oxygen, contributing to the profound tissue hypoxia that characterises sites of infection. Hypoxia in turn has a dramatic effect on both neutrophil bactericidal function and the properties of S. aureus, including the production of virulence factors. Hypoxia thereby shapes the host–pathogen interaction and the progression of infection, for example promoting intracellular bacterial persistence, enabling local tissue destruction with the formation of an encaging abscess capsule, and facilitating the establishment and propagation of bacterial biofilms which block the access of host immune cells. Elucidating the molecular mechanisms underlying host–pathogen interactions in the setting of hypoxia will enable better understanding of persistent and recalcitrant infections due to S. aureus and may uncover novel therapeutic targets and strategies.
Keywords: neutrophils, host-pathogen interaction, hypoxia, Staphylococcus aureus
1. Introduction
Our understanding of host–pathogen interactions has gradually evolved, from the initial concept of an invader overcoming or being defeated by host defences to a more complex and flexible relationship with microbes, where outcomes may result in damage, benefit or be entirely neutral. There are thus a range of ‘interaction’ states including colonisation, commensalism and disease, and the same microbe may shift between these states depending on the precise microenvironment in which the interaction between host and microbe occurs. In this review, we explore the impact of reduced oxygen availability (‘hypoxia’) on the interaction between Staphylococcus aureus (S. aureus) and our most abundant innate immune cell, the neutrophil.
S. aureus is a Gram-positive coccal bacterium, which colonises around 30% of the population worldwide [1]. It causes frequent localised infections of the skin, but can also induce life-threatening human infection if mucosal surfaces are breached. S. aureus possesses numerous virulence factors which help it cross the skin barrier and access deeper tissues, thereby causing a spectrum of diseases from local skin and soft tissue infections (SSTI including superficial abscesses, wound infections, and cellulitis) to more severe and invasive conditions with a high morbidity and mortality, such as osteomyelitis, joint infections, endocarditis and bacteraemia/septicaemia [2]. Invasive infection may be enhanced by the ability of other skin commensals to act as ‘pro-infectious agents’, dramatically reducing the S. aureus infectious dose required to initiate disease [3]. S. aureus has a propensity to infect medical devices, including intravenous cannulae [4] and prosthetic joints or heart valves [5], promoted by its ability to form biofilms. The importance of S. aureus as a human pathogen is enhanced by its multidrug resistance profile; there are strains resistant to most available antibiotics e.g., methicillin-resistant S. aureus (MRSA) and vancomycin-resistant S. aureus (VRSA) [6].
In addition to its ability to respond to evolutionary pressures from antibiotics, S. aureus has also evolved to combat professional phagocytic cells such as polymorphonuclear leukocytes (PMNs or neutrophils). Neutrophils are crucial for human defence against staphylococcal infections, as highlighted when neutrophil function is defective (see below). However, S. aureus may lyse the engulfing phagocyte, or persist inside even fully competent neutrophils despite the high-grade bactericidal functions these cells command [7]. Many factors contribute to the resistance of S. aureus to host-mediated killing, including the propensity of this pathogen to infect areas of relative tissue hypoxia. Whilst in healthy tissues, the oxygen tension is commonly 20–70 mm Hg (2.5–9% O2), infection sites show much lower oxygen levels <10 mm Hg (<1% oxygen) [8]. The efficacy of innate immune cells to handle this pathogen is thus at least partly dependent on their ability to operate in a low-oxygen milieu and overcome S. aureus adaptations to the local immune and environmental pressures.
There is incomplete understanding of interactions between innate immune cells and S. aureus, and how these interactions may result in pathogen death, containment or dissemination. Studying host–pathogen interaction under conditions that resemble those present in humans during bacterial invasion, such as hypoxia, will promote better understanding of how this important pathogen establishes and perpetuates infection.
2. Neutrophil Killing Mechanisms
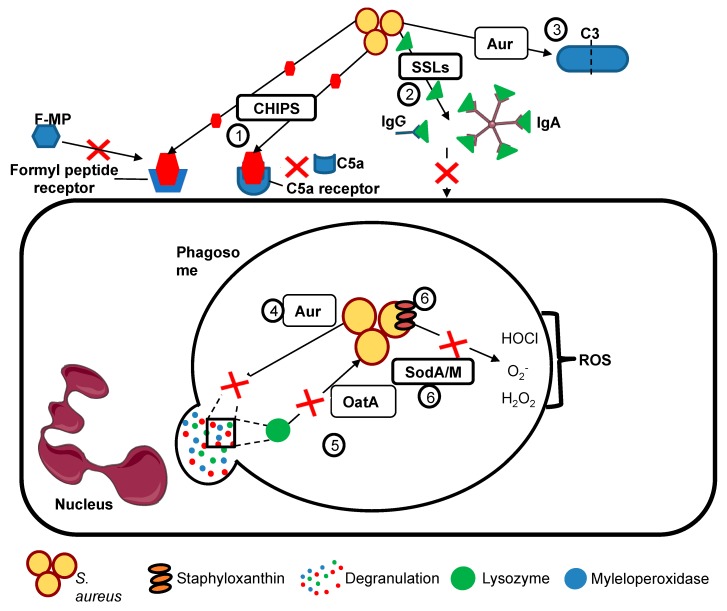
Neutrophils are rapidly responsive, but short-lived, bone marrow-derived innate immune cells. They are the most abundant circulating leucocytes, crucial to the host response against invading pathogens. Neutrophils are not tissue residents but transmigrate on demand to the site of an infection, where they phagocytose and kill invading pathogens, by deployment of pre-formed antimicrobial granules, by the de novo generation of reactive oxygen species (ROS) and by the release of chromatin decorated with histones and granule proteins such as myeloperoxidase (NETosis); these mechanisms and how they are modulated by hypoxia are discussed in more detail below. S. aureus combats these innate immune functions by the release of a range of virulence factors, examples of which are shown in Figure 1 and Table 1. These include (but are not limited to) staphylococcal superantigen-like (SSL) proteins, which bind immunoglobulins and complement components to block opsonisation, and chemotaxis inhibitory protein of S. aureus (CHIPS), which prevents binding of chemoattractants such as formylated peptides to neutrophil receptors (Figure 1).
Figure 1.
S. aureus avoids engulfment and killing by neutrophils. S. aureus avoids killing by neutrophils by preventing phagocytosis and resisting internal killing mechanisms using a number of strategies, including: (1) Neutrophil chemotaxis is inhibited by chemotaxis inhibitory protein of S. aureus (CHIPS), which prevents binding of chemoattractants such as activated complement and bacterial formylated peptides (F-MP) to neutrophil C5a and formyl peptide receptors. (2) Staphylococcal superantigen-like proteins (SSLs) bind IgG and IgA preventing their adherence to neutrophils and hence blocking opsonisation. Aureolysin prevents (3) complement activation by cleaving C3, blocking C3a activation. Granule-derived antimicrobial peptides such as lysozyme or MPO myeloperoxidase are also cleaved by (4) aureolysin (Aur). (5) S. aureus is protected from degradation by lysozymes through modification of peptidoglycan by O-acetyltransferase (OatA). (6) There are multiple systems to combat ROS including antioxidants such as SodA/SodM and Staphyloxanthin, which protect staphylococcus from the oxidative stress due to ROS. This figure was created using Servier Medical Art templates, which are licensed under a Creative Commons Attribution 3.0 Unported License; https://smart.servier.com.
Table 1.
Examples of S. aureus virulence factors relevant to immune evasion.
| Group | Virulence Factor | Mechanism |
|---|---|---|
| Prevention of phagocyte recognition by opsonisation and hence reduction of phagocytosis | Protein A (SpA) | Cross links Fab domain of IgM and binds Fcγ domain of immunoglobulin G. |
| Clumping factor A (ClfA) | Fibrinogen-binding surface protein causing platelet aggregation. Antiphagocytic effect with or without presence of fibrinogen. | |
| Staphylococcal complement inhibitor (SCIN) | Inhibits C3 complement convertase by preventing the C3b generation. | |
| Aureolysin | Anti-protease blocks C3 complement activity through cleaving C3 blocking C3a activation of neutrophils. Also cleaves granule-derived antimicrobial peptides. | |
| Induction of phagocyte damage and death | Panton-Valentine leukocidin (PVL) and other leukocidins such as gamma-haemolysin and LukED | Triggers apoptosis and necrosis of cells, initiated by pore formation. |
| Phenol-soluble modulins (PSMs) | Cause lysis of blood cells, assists in the structuring and dispersal of biofilms. | |
| Alpha-haemolysin (alpha toxin) | Forms pores in cells through its interaction with the ADAM10 receptor, resulting in cell lysis. | |
| Prevention of neutrophil chemotaxis and recruitment to sites of staphylococcal infection | Chemotaxsis inhibitory protein (CHIPs) | Blocks chemotaxis towards C5a and formylated peptides by binding to neutrophil C5a receptors formyl peptide receptors, preventing neutrophil recruitment to sites of staphylococcal infection. |
| Extracellular adherence protein (Eap) | Blocks complement activation and neutrophil adhesion to activated endothelium inhibiting neutrophil recruitment; suppresses NETosis. | |
| Staphylococcal superantigen like (SSLs) | A group of structurally similar antigens with functions including binding IgA, IgG, matrix metalloproteinases amd neutrophil adhesion molecules, which act together to inhibit neutrophil recruitment to staphylococcal infection. | |
| Evasion of phagocyte killing | OatA | Catalysing the O-acetylation of peptidoglycan in the Staphylococcal cell wall, rendering it insensitive to lysozyme (which is secreted by phagocytes and constitutively present in secretions such as tears). |
| SodA/M | Superoxide dismutases provide resistance to reactive oxygen species (ROS) produced by neutrophils including superoxide. | |
| Staphyloxanthin | A carotenoid which provides protection against oxidative stress and ROS | |
| Phenol-soluble modulins (PSMs) |
Cause cell lysis, aid in biofilm development and stimulate inflammation. |
Micro-organisms present a range of pathogen-associated molecular patterns (PAMPs) and are recognised by receptors, e.g., toll-like receptors (TLRs), which are present on the surface of immune cells including neutrophils [9]. PMNs recognise multiple pathogen-associated molecules whether integral or secreted by Gram-positive or Gram-negative bacteria. These include peptidoglycan (sensed by multiple pattern-recognition receptors, including nucleotide-binding oligomerization domain-containing protein 1 (NOD1), NOD2, NOD-, LRR- and pyrin domain-containing 3 (NLRP3) and peptidoglycan recognition protein 1 (PGLYRP1)), lipoteichoic acids (Toll-like receptor (TLR) 2 ligand), lipopolysaccharide (recognised by the TLR4 and myeloid differentiation factor 2 (MD-2) complex) or flagellin (TLR5 ligand). Other bacterial products including formylated peptides (F-MPs: the exemplar is N-formyl-methionyl-leucyl-phenylalanine, fMLP) interact via dedicated formyl peptide receptors to recruit neutrophils to the site of infection and subsequently activate their bactericidal functions. Opsonins such as complement fragments and antibodies bind the pathogen and interact with phagocyte receptors. Pathogen recognition is essential for subsequent phagocytosis, which is further facilitated by opsonisation.
Phagocytosis is a complex process linking cell surface receptors to alterations in membrane conformation and the actin cytoskeleton. Phagocytosed pathogens are enclosed in a membrane-bound phagosome, within which most (but not all) microorganisms are effectively killed (Figure 1) by the combination of degranulation of antimicrobial agents (from pre-formed neutrophil granule populations including a range of proteases and enzymes such as elastase, myeloperoxidase (MPO) and the cathepsins, iron sequestrators such as lactoferrin, and a plethora of other bactericidal molecules) and by the assembly of the NADPH oxidase complex at the phagosomal membrane enabling ROS production, the ‘oxidative burst’, and formation of reactive nitrogen species such as nitric oxide (NO). If the internalisation of pathogen is not feasible, neutrophils may release NETs in an an attempt to kill invaders extracellularly; however this strategy is not always successful [10].
3. Neutrophil Dysfunction and Staphylococcal Infection
Several inherited and acquired neutrophil disorders have been described (examples listed in Table 2; [11,12,13,14,15,16,17,18,19]), often leading to life-threating infections; S. aureus is a prominent pathogen in these settings, suggesting neutrophils are key in restricting its pathogenicity. Patients with such defects often receive continuous anti-staphylococcal antibiotic prophylaxis.
Table 2.
Neutrophil Disorders and Infection.
| Disease | Defect | PMN Dysfunction | Clinical Outcomes |
|---|---|---|---|
| Neutropenia | Decreased PMN numbers, either congenital (e.g., elastase deficiency) or acquired (most commonly drug-induced such as cancer chemotherapy). | Insufficient PMN numbers to respond to invading pathogens, life-threatening Gram-negative and Gram-positive infections. | Life-threatening infections during periods of neutropenia, susceptibility reduced when neutrophil count recovers. |
| Chronic granulomatous disease (CGD) | Mutations in NADPH oxidase components; reduced or absent ROS formation. | Reduced killing of certain pathogens e.g., Staphylococcus aureus, Aspergillus fumigatus, Gram- negative bacilli. | Life-threatening infections with Staphylococcus and Apergillus; aberrant healing (granulomas). |
| Hyper IgE Syndrome (formerly Job’s Syndrome) | Mutations in STAT3 (signal transducer and activator of transcription 3) or DOCK 8 (Dedicator of cytokinesis 8) or TYK2 leading to impaired T cell function and diminished neutrophil chemotaxis | Reduced killing of certain pathogens e.g., Staphylococcus aureus, Aspergillus fumigatus. | Staphylococcal and fungal skin infections, pulmonary and joint infections, ‘cold’ abscess formation (reduced cytokine release). |
| Myeloperoxidase deficiency | Decreased or lack of MPO/HOCl system required to generate the full range of ROS. | Increased chronic conditions mediated by adaptive immunity, decreased NET killing of microbes. | Susceptibility to chronic infections caused by Candida albicans, S. aureus. |
| SGD (Specific Granule Deficiency) | Absence of specific granules, bilobed neutrophils nuclei. Altered content of other granule populations. | Impaired chemotaxis, aberrant granule organisation, reduced respiratory burst, and deficient bactericidal activity (mainly to S. aureus). | Staphylococcal skin infections, aberrant skin lesion healing. |
| Chediak Higashi Syndrome | Mutations in lysosomal trafficking regulator (LYST) leading to failure of lysosomal trafficking in neutrophils and other cells | Giant granules, impaired phagocytosis and phagosomal maturation, oxidative burst and degranulation | Albinism, neurological defects, coagulopathy, recurrent skin (staphylococcal) infections and respiratory infection |
The importance of the neutrophil in the anti-staphylococcal immunity was highlighted when chronic granulomatous disease (CGD) was first described and its cause identified [20]. Neutrophils from CGD patients are unable to produce ROS from molecular oxygen, because of mutations in components of NADPH oxidase enzyme. CGD patients are highly susceptible to infections caused by microorganisms such as Staphylococcus spp., Aspergillus spp., Salmonella spp., and Serratia spp. [21], as the defective oxidative burst leads to a failure of pathogen killing. Other disorders of neutrophil function likewise confer susceptibility to S. aureus infection [11,12,13,14,15,16,17,18,19], underscoring the importance of these cells in host–pathogen interactions.
4. Physiological and Pathological Hypoxia
Hypoxia represents an imbalance between oxygen supply and demand. Oxygen gradients exist within and across tissues; this ‘physiological hypoxia’ is heightened by disease processes such as inflammation and infection, leading to ‘pathological hypoxia’. Local tissue ‘hypoxia’ is in fact normal in the healthy organism. The oxygen level in tissue environments differs considerably from that of inspired air (pO2 about 160 mmHg). Circulating neutrophils repeatedly transit between a pO2 of approximately 100 mmHg in main arteries, 50 mmHg in arterioles and 20–30 mmHg in capillaries. Since oxygen must diffuse from capillaries to the surrounding environments, the oxygen tension in normal tissues can be even lower, leading to ‘physiological hypoxia’. Tissue oxygenation can be measured using micro-electrodes or by staining with compounds such as pimonidazole, which binds to thiol groups at oxygen tensions below 10 mmHg, and the OxyLite PO2 system (which determines the O2-dependent fluorescent lifetime of ruthenium chloride); for example, the pO2 in the healthy thymus is approximately 10 mm Hg and around 16 mm Hg in the spleen [22].
Tissue oxygenation status may be compromised by a range of pathological processes, such as atherosclerosis (reduced delivery of oxygenated blood by narrowed vessels) and malignancy (abnormal blood vessels and increased diffusion distance). Importantly, neutrophilic infiltration induces ‘inflammatory hypoxia’. In a sterile colitis model, infiltrating PMNs depleted local epithelial oxygen levels by consuming oxygen to fuel the oxidative burst [23]. In the setting of infection, bacteria also contributes to tissue hypoxia, for example S. aureus depleted dissolved oxygen in a skin infection model [24] and stabilisation of HIF1α (a marker of hypoxia, see below) has been demonstrated in human skin biopsies in both keratinocytes and infiltrating neutrophils in the setting of S. aureus skin infection [25]. Together, neutrophilic inflammation and bacterial replication contribute to the establishment of ‘pathological hypoxia’.
Thus, neutrophils must sense local oxygen levels and adapt to operate within hypoxic environments in order to control infection by S. aureus; hence it is important to establish the impact of hypoxia on key neutrophil microbicidal functions and on bacterial virulence determinants. Of note, most data on neutrophil and bacterial function have been collected by studying isolated cells cultured in atmospheric oxygen (160 mmHg), far in excess of the oxygen levels in the relevant tissue environment.
5. Mammalian Oxygen Sensing and Response to Hypoxia
The mammalian molecular response to hypoxia is largely mediated by the family of hypoxia-inducible factor (HIF) transcription factors and has been summarised in detail elsewhere [26]. In brief, HIF-α stability is post-transcriptionally regulated by molecular oxygen, via oxygen-sensitive prolyl hydroxylases (PHDs). There are three HIFs and three PHDs, and it is now appreciated that they have somewhat differing roles in regulating neutrophil function and metabolic responses in hypoxic settings [27]. When oxygen is available, PHDs are catalytically active and hydroxylate HIF-α, targeting it for proteasomal degradation. Another oxygen-dependent enzyme, Factor Inhibiting HIF (FIH), hydroxylates asparagine residues to block association with transcriptional co-activators CBP/p300 [28]. When molecular oxygen in the cytoplasm is reduced, PHD and FIH are inactive, HIF-1α subunits accumulate in the cytoplasm, heterodimerise with constitutively expressed HIF-1β and recruit p300/CBP coactivator proteins. This regulatory complex transmigrates to the nucleus and binds to hypoxia responsive elements (HREs) to control the transcription of target genes which enact cellular hypoxic responses.
Although HIFs are often referred to as the master regulators of hypoxic responses, it is important to note that HIFs can also be activated by inflammatory signals independent of hypoxia (for example, bacterial lipopolysaccharide mediated a transcription-dependent upregulation of HIF-1α expression in macrophages [29]), and it is increasingly recognised that some effects of hypoxia are independent of HIFs (e.g., hypoxia-enhanced degranulation [30]). Hence a neutrophil migrating into inflamed tissues will experience variable oxygen tensions, leading to a range of HIF-dependent and HIF-independent effects in a dynamic and context-dependent fashion.
6. S. aureus Responses to Adverse Environmental Conditions
Pathogens such as S. aureus evolve to exploit local conditions and circumvent immune-mediated killing, with the emergence of bacterial strains possessing a wide range of virulence factors; it is thus of importance to study the host–pathogen interaction including such virulent bacterial strains rather than concentrating on single laboratory-adapted strains. Staphylococcal virulence factors (see Table 1) have been reviewed in detail elsewhere [31] and include substances that combat phagocyte mobilisation, recognition and phagocytosis; toxins to destroy host immune cells, factors which detoxify host bactericidal peptides, proteases and ROS; immune modulators that enable phagosomal escape; and elements to allow adaptation to adverse conditions such as hypoxia (see below). Several S. aureus strains can survive intracellularly (Figure 2), either by reducing virulence (Small Colony Variants or SCVs, discussed in more detail below), by ‘defusing’ the phagosomal arsenal (eg by secretion of catalase) or by escaping from the phagosome (reviewed in [32]); such adaptations may be driven by external conditions, either in the extracellular or intracellular environment. S. aureus has been found to remain viable and virulent inside murine PMNs, within larger vacuoles called “spacious phagosomes” or inside small vacuoles termed “tight phagosomes” [33]; those within spacious phagosomes (suggested to have formed via macropinocytosis rather than true phagocytosis) seemed more likely to survive, perhaps due to failure to deploy the normal killing mechanisms in this setting. Hypoxic neutrophils or neutrophils isolated from patients with CGD were able to contain, but not kill ingested S. aureus [34], again highlighting the importance of the oxidative burst in the execution of this pathogen.
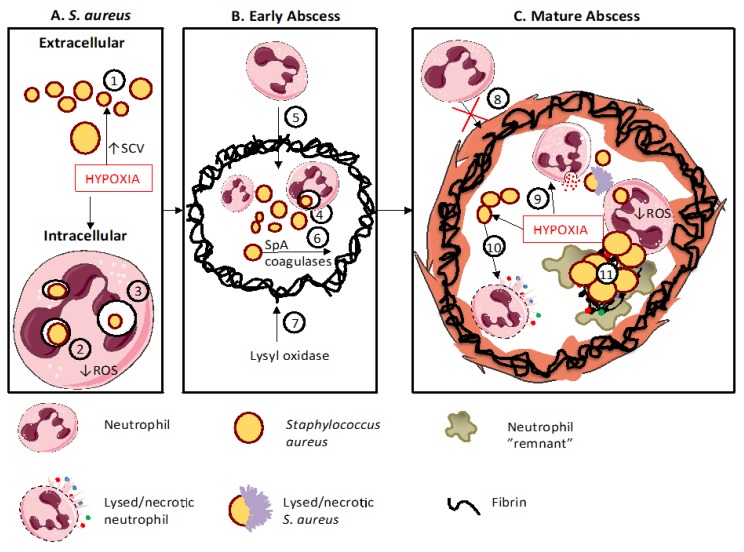
Figure 2.
Neutrophils and hypoxia contribute to abscess formation and development. Panel A depicts the interaction between neutrophil and S. aureus in a typical hypoxic environment, panel B the early stages in abscess formation and panel C the role of hypoxia in establishing the mature abscess and preventing the resolution of infection and inflammation. A. A hypoxic micro-environment promotes the emergence of small colony variants (SCVs, 1) which, together with decreased neutrophil ROS generation consequent to a lack of molecular oxygen (2) promotes enhanced intracellular persistence (3). B. An early abscess develops from an extracellular bacterium or from an intracellular S. aureus (4), which has been carried from a distal site. Neutrophils can enter the early abscess, (5) but formation of a fibrin capusle is instigated by S. aureus factors such as SpA and coagulases (6) and by HIF-dependent lysyl oxidase from surrounding cells (7). C. Once formed, the mature abscess capsule prevents further neutrophil infiltration (8). The highly hypoxic conditions alter neutrophil processes by enhancing degranulation (9) and enhanced S. aureus secretion of leukocidins (10) to induce neutrophil lysis. The mature abscess contains products from necrotic and degranulated neutrophils, multiplying S. aureus and persisting intracellular S. aureus within neutrophils (11). This figure was created using Servier Medical Art templates, which are licensed under a Creative Commons Attribution 3.0 Unported License; https://smart.servier.com.
In common with many bacteria, S. aureus senses and responds to environmental changes via two-component regulatory systems, comprising a histidine kinase that senses a specific environmental stimulus and a corresponding response regulator, which controls the differential expression of target genes. S. aureus controls the production of virulence factors through two-component regulatory loci including sae (S. aureus exoprotein expression), agr (accessory gene regulator), and also ssrAB (staphylococcal respiratory response AB) and airSR (anaerobic iron-sulfur cluster-containing redox sensor regulator); the latter two systems sense hypoxia, allowing metabolic adaptation and the secretion of virulence factors such as staphyloxanthin (a carotenoid which provides protection against ROS) to combat oxidative stress [35,36]. The secretory phenotype also depends on bacterial density, detected by quorum sensing mechanisms. At low densities (e.g., in early infection), S. aureus adopts the “adhesive” phenotype, with upregulation of surface adhesion molecules to facilitate attachment and biofilm formation. Later in infection, as determined by local conditions such as hypoxia, staphylococci can switch to an “invasive” phenotype secreting toxins and proteases to promote bacterial dissemination to distant tissues [35,37]. Thus S. aureus adjust their expression of virulence factors according to environmental conditions.
7. The Impact of Hypoxia on Neutrophil Bactericidal Functions
In vitro studies of human neutrophils suggest that functions underpinning recruitment, such as polarisation and chemotaxis, are fully sustained at low-oxygen levels [38]. Thus a hypoxic environment will not prevent the mobilisation of PMN from circulation in response to infection. This was indeed confirmed in mouse models employing genetic/pharmacological manipulation of the HIF-signalling pathways [39,40] and in animals exposed to acute hypoxia [41]. However, in the whole animal studies, despite equivalent neutrophil recruitment, acute hypoxia dramatically worsened the outcome of the host–pathogen interaction with neutrophil-dependent hypothermia and cardiac compromise, whilst preconditioning animals through longer exposures to hypoxia prior to infection was protective [41]. These complex effects were underpinned by changes in neutrophil metabolism and underscore the need to translate in vitro observations on isolated cells to more clinically relevant systems.
Neutrophil phagocytosis has been reported to be either unchanged [38] or increased [42] by hypoxia. These opposing observations likely reflect methodological differences; Fritzenwanger et al. [42] isolated blood samples from subjects exposed to hypoxia, but isolated the cells in atmospheric oxygen used zymosan as prey. In contrast, McGovern et al. [38] isolated blood from volunteers and then incubated the isolated cells in hypoxic conditions, adding S. aureus when the cells had ‘acclimatised’ to hypoxia. However, these studies both provide evidence that hypoxic impairment of neutrophil bactericidal activity does not relate to impaired ingestion of prey.
The function of the NADPH oxidase (defective in CGD) is to generate ROS at the phagosome; together with the discharge of granule proteases, this generates an environment that is hostile to bacterial survival [43]. ROS comprise a range of unstable oxygen free radicals with O2•− (superoxide anion), O2 (singlet oxygen) and OH• (hydroxyl radical), as well as more stable and freely diffusible radical and non-radical oxidants, such as NO• (nitric oxide) and H2O2 (generation of the latter radical requiring myeloperoxidase from the neutrophil granules). Molecular oxygen is required for generation of ROS, and is thus an important mediator of neutrophil microbicidal function [44] including the killing of S. aureus [38]. Whilst neither genetic nor pharmacologic manipulation of HIF modulated the oxidative burst [39,40], hypoxia sufficient to limit the availability of oxygen as a substrate for the NADPH oxidase can compromise the ability to kill pathogens such as S. aureus in an HIF-independent fashion [38]. Thus, it is important to remember that manipulation of HIF in experimental systems does not always recapitulate the effects of hypoxia, particularly in the acute setting.
Granule proteins are key to the effective killing of many pathogens, and hypoxia may modulate degranulation responses in a number of ways. Firstly, neutrophils develop in a hypoxic niche in the bone marrow [45], and HIF-dependent transcription regulates the expression of key bactericidal proteases and peptides including neutrophil elastase, cathepsin G and cathelicidin [39]. Secondly, in vitro exposure to acute hypoxia increased the stimulated release of neutrophil microbicidal proteins and proteases, including myeloperoxidase (MPO), lactoferrin, metalloproteinase-9 (MMP-9) and elastase [30]. This may impact tissue destruction and be relevant to certain aspects of staphylococcal pathology, for example abscess formation (see below). Thirdly, hypoxia may be relevant to the release of neutrophil extracellular traps (NETs), which consist of chromatin decorated with histones and granule proteins and can bind to, restrain and kill both Gram-positive and -negative bacteria [46]. NETosis is a form of neutrophil death, which differs from apoptosis or necrosis and is predominantly ROS-dependent; CGD neutrophils form few NETs with aberrant morphology [47]. Despite this, NET formation in response to viable S. aureus (wild-type or nuclease-deficient strains) was retained under hypoxia (1% oxygen), suggesting that even trace levels of ROS suffice to signal NETs release [48] in response to relevant stimuli.
Since hypoxia impairs phagocyte function, exposure to high oxygen tensions has been suggested as a means of promoting infection resolution. Hyperbaric oxygen therapy (HBOT) has been used in a range of experimental and therapeutic settings, including staphylocoocal infection; such systemic hyperoxia will have complex effects on host cells (including phagocytes) and on infecting bacteria. HBOT has been shown to enhance antibiotic efficiency in a rat model of infective endocarditis [49], but not in a mouse model of implant-associated osteomyelitis [50]. The reason for this difference and other conflicting reports in the literature is not clear, but might relate in part to the different model systems and perhaps to the ability of hyperbaric oxygen to increase oxygen tensions locally in vegetations (immediately bathed in oxygenated blood) as opposed to deep within hypoxic bone tissues or other infection sites.
Whilst acute hypoxia modulates neutrophil ROS generation and degranulation responses, more prolonged hypoxia has dramatic effects on neutrophil metabolism and survival. Prolonged stabilisation of HIF1α leads to enhanced glycolytic flux and ATP pools (reviewed in [51]) and diminished neutrophil apoptosis [52]. An important insight was garnered from studying a S. aureus murine skin infection in mice placed in different oxygen environments; as noted above, systemic hypoxia converted a minor superficial staphylococcal infection to near-universal with rapid fatality. Enhanced bacterial replication was not the cause of this lethal effect, and preconditioning animals in hypoxia rescued outcomes. The phenotype largely resided in the neutrophil’s response to hypoxia, with HIF1α activation leading to elevated neutrophil glucose requirements, resulting in worse disease outcome in hypoxemic animals such as cardiac failure and hypoglycaemia [41]. Whilst a detailed discussion of such metabolic re-programming in the setting of infection and hypoxia is beyond the scope of this review, the outcomes are pro-inflammatory and usually detrimental to the host.
8. The Role of Neutrophils and Hypoxia in Shaping Staphylococcal Infections
Both microbes and immune cells contribute to hypoxia at the site of infection and their response to this environmental pressure helps to shape the progress of infection. As outlined above, hypoxia has a wide and dynamic range of impacts on neutrophil functionality, which may compromise bactericidal function, increase tissue damage and impair inflammation resolution. S. aureus possesses a repertoire of environmental sensors and regulatory proteins that interact to detect and respond to low oxygen states, both by activating genes required to handle hypoxic stress and by increasing expression of toxins and proteases (reviewed in [35]). For example, supernatants prepared from S. aureus induced cytotoxicity in a range of mammalian cells (including neutrophil-like HL-60 cells) that significantly increased if the bacteria were cultured under lower oxygenation [53]. Although the two-component environmental sensor SsrAB was shown to be key to this response, the range of hypoxia-upregulated virulence factors was not further delineated in this study. S. aureus increases the transcription of the intercellular adhesin (ica) cluster, leading to increased polysaccharide intercellular adhesin (PIA) production and protecting the bacteria against non-oxidative neutrophil killing mechanisms and promoting biofilm formation in anoxic conditions [54]. Lack of available oxygen limits ATP synthesis and results in the inability to replenish NADH/NAD+ pools, restricting growth and promoting the emergence of SCVs [55]. SCVs are enabled with respect to intracellular persistence, antimicrobial resistance, immune evasion and biofilm formation, and have been shown to emerge in the setting of chronic staphylococcal infections [56].
In this section, we describe how hypoxia and the host immune response interact with this organism to cause two typical clinical syndromes of staphylococcal infection, namely abscess formation and infection of prosthetic material.
9. Staphylococcal Abscess Formation
Abscesses are the archetypal manifestation of S. aureus infections and both host and pathogen factors contribute to their formation (see Figure 2).
Patients with impaired neutrophil function have increased risk of skin infection; patients with Hyper IgE syndrome are particularly prone to staphylococcal skin and lung abscesses [14], although other cellular and cytokine defects may contribute to this susceptibility [57]. Abscess formation is also a frequent consequence of disseminated infection, and in a zebrafish model, such abscesses were shown to arise from small numbers or even single bacteria carried to distant sites whilst residents within host neutrophils [58]. Thus, neutrophils may function as “Trojan” carriers of infection and factors such as hypoxia [37], which favour intracellular persistence, and will encourage such metastatic infection (Figure 2A).
A range of bacterial toxins including Staphylococcal protein A (SpA) and coagulases contribute to early abscess formation [59,60]. Coagulases allow the pathogen to usurp the host coagulation system, forming fibrin deposits which eventually mature to act as a barrier against immune cells, preventing their penetration into the developing abscess [60], where the pathogen replicates without interference. The fibrin capsule matures by cross-linking of components and deposition of collagen. Lysyl oxidase (an amine oxidase required for biosynthetic cross-linking of extracellular matrix components) was found to be upregulated in an HIF-dependent fashion [61]. It is abundantly expressed in human and murine staphylococcal abscesses, especially adjacent to the capsule (although the precise cell type(s) expressing LOX in vivo were not defined). Also, inhibition of LOX reduced collagenisation of the abscess capsule and led to more diffuse infection [61]. These processes are represented in Figure 2B.
Neutrophils are recruited to combat infection, but S. aureus secretes a number of leukocidins and haemolysins to lyse neutrophils, and their release may be enhanced in hypoxic settings [61]. Virulence factors such as SarA, which may also contribute to abscess formation, are likewise induced by hypoxia [62]. As infection progresses, the abscess consists of a large mass of pathogens at the central part of the lesion, a layer of necrotic PMNs, followed by other necrotic cells and/or healthy cells and surrounded by a dense fibrin capsule [59,60]. This is a hypoxic environment [25], and in addition to the effects noted on bacteria, hypoxia impairs the capacity to mount a neutrophil respiratory burst to kill ingested S. aureus [38]. Also, augmented secretion of proteases by hypoxic neutrophils [30] will contribute to host tissue breakdown and bacterial lysis. Thus, hypoxia acts on both bacteria and host to regulate the establishment and maturation of an abscess cavity (Figure 2C).
10. Staphylococcal Biofilms and Infection of Prosthetic Material
S. aureus has a propensity to cause infections on indwelling prosthetic materials, for example intravenous cannulae, joint replacements and artificial heart valves. Most prosthetic device infections are thought to result from skin flora contamination during implantation or tracking of infection from the subcutaneous portion of the device to deeper sites. The ability of S. aureus to form biofilms (surface-associated collections of bacteria embedded within a slimy matrix composed of a polymeric assembly of polysaccharides, proteins, lipids and DNA) is key to its ability to colonise and cause persistent infection of such devices (See Figure 3). Oxygen consumption by microbes and immune cells renders biofilms profoundly hypoxic [63].
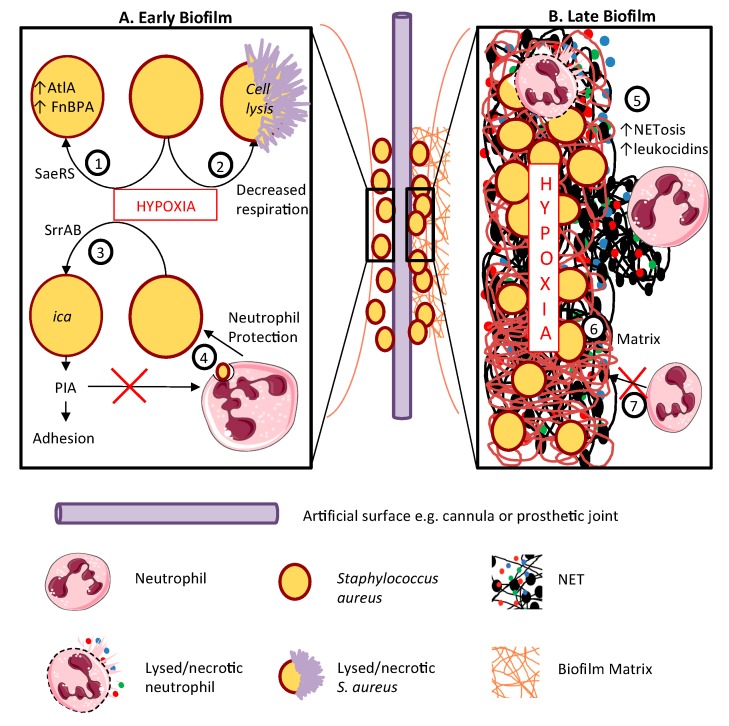
Figure 3.
Hypoxia enhances staphylococcal biofilm development. Biofilms are most likely to form on prosthetic surfaces such as intravenous cannulae or replacement joints or heart valves. The host and pathogen processes contributing to biofilm formation are depicted in the left-hand panel A. Panel B (right) depicts the role of hypoxia in biofilm maintenance and progression. A. In early biofilm development, hypoxia activates the SaeRS 2- component system, increasing AtlA and FnBPA production (1); decreases staphylococcal respiration, potentially resulting in cell death and lysis (2); and activates the PIA (polysaccharide intercellular adhesion encoding operon (ica) via the SrrAB regulator (3), increasing biofilm adhesion. PIA accumulation also impairs neutrophil non-oxidative killing (4). B. Mature biofilms are profoundly hypoxic, promoting NETosis and leukocidin production (5) adding to the polymeric biofilm matrix of polysaccharides, proteins and lipids, entangling multiplying staphylococci (6). This matrix blocks the entry of neutrophils into the biofilm (7). This figure was created using Servier Medical Art templates, which are licensed under a Creative Commons Attribution 3.0 Unported License; https://smart.servier.com.
Importantly, S. aureus increases biofilm formation dramatically in response to hypoxia [64,65]. The mechanism by which hypoxia promotes biofilm formation is imperfectly understood and likely multi-factorial (see Figure 3A). Hypoxic activation of the SaeRS 2-component regulatory system results in increased expression of AtlA (peptidoglycan hydrolase), and fibronectin-binding protein A was shown to be required for biofilm formation, with diminished bacterial respiration leading to programmed cell lysis and promoting biofilm development [64]. The key adhesin responsible for the accumulation phase of Staphylococcal biofilms is the polysaccharide intercellular adhesin (PIA), encoded by the ica operon; PIA expression is up-regulated in low oxygen environments under the control of the staphylococcal respiratory response regulator, SrrAB [54].
Established biofilms (Figure 3B) are resistant to antibiotics and to neutrophils [66], skewing these cells towards NETosis, which may encourage biofilm development [10] at least in part by the production of leukocidins, which promote neutrophil lysis and/or NETosis. Interestingly, the outcome of the neutrophil:biofilm interaction depends on its stage of development; in a mouse model, neutrophils conferred early protection against biofilm attachment to implanted devices, but later in the process, IL-1β promoted marked neutrophilic infiltration but increased bacterial load [67]. Thus neutrophils may fail to control or even encourage bacterial growth within the biofilm but prevent local spread or dissemination of infection. In a model of prosthetic joint infection, the interaction of neutrophils with the staphylococcal biofilm led to pro-inflammatory cytokine production, triggering the formation and activation of osteoclasts, bone resorption and prosthesis loosening [68].
11. Conclusions
Staphylococcal infections remain a major cause of human morbidity and mortality. Infection and inflammation enhance tissue hypoxia, profoundly influencing the host–pathogen interaction, with both S. aureus and neutrophils responding to low oxygen availability. Whilst outcomes will be determined by the precise local conditions, hypoxia may limit bacterial growth and promote bacterial persistence, whilst modulating neutrophil function to engage infection but not to eradicate it. However, such intracellular persistence may, in some settings, allow neutrophils to promote staphylococcal dissemination, and may promote biofilm formation to exclude immune cells from the infective site. Further exploring the molecular mechanisms underlying host–pathogen interactions in the setting of hypoxia may lead to new strategies to treat persistent and recalcitrant infections due to S. aureus.
Acknowledgments
The authors thank Michael Brockhurst (Department of Animal and Plant Sciences, University of Sheffield) for critical appraisal of this manuscript.
Funding
This work was funded by the Florey Institute, Medical Research Foundation, British Lung Foundation PPRG16-13 and MRC MR/No2995X/1.
Disclosure Statement
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Thammavongsa V., Kim H.K., Missiakas D., Schneewind O. Staphylococcal manipulation of host immune responses. Nat. Rev. Microbiol. 2015;13:529–543. doi: 10.1038/nrmicro3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tong S.Y., Davis J.S., Eichenberger E., Holland T.L., Fowler V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015;28:603–661. doi: 10.1128/CMR.00134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boldock E., Surewaard B.G.J., Shamarina D., Na M., Fei Y., Ali A., Williams A., Pollitt E.J.G., Szkuta P., Morris P., et al. Human skin commensals augment Staphylococcus aureus pathogenesis. Nat. Microbiol. 2018;3:881–890. doi: 10.1038/s41564-018-0198-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas M.G., Morris A.J. Cannula-associated Staphylococcus aureus bacteraemia: Outcome in relation to treatment. Intern. Med. J. 2005;35:319–330. doi: 10.1111/j.1445-5994.2005.00823.x. [DOI] [PubMed] [Google Scholar]
- 5.Galar A., Weil A.A., Dudzinski D.M., Muñoz P., Siedner M.J. Methicillin-Resistant Staphylococcus aureus Prosthetic Valve Endocarditis: Pathophysiology, Epidemiology, Clinical Presentation, Diagnosis, and Management. Clin. Microbiol. Rev. 2019;32 doi: 10.1128/CMR.00041-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gardete S., Tomasz A. Mechanisms of vancomycin resistance in Staphylococcus aureus. J. Clin. Investig. 2014;124:2836–2840. doi: 10.1172/JCI68834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu T., Porter A.R., Kennedy A.D., Kobayashi S.D., DeLeo F.R. Phagocytosis and killing of Staphylococcus aureus by human neutrophils. J. Innate Immun. 2014;6:639–649. doi: 10.1159/000360478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simmen H.P., Blaser J. Analysis of pH and pO2 in abscesses, peritoneal fluid, and drainage fluid in the presence or absence of bacterial infection during and after abdominal surgery. Am. J. Surg. 1993;166:24–27. doi: 10.1016/S0002-9610(05)80576-8. [DOI] [PubMed] [Google Scholar]
- 9.Parker L.C., Whyte M.K., Dower S.K., Sabroe I. The expression and roles of Toll-like receptors in the biology of the human neutrophil. J. Leukoc. Biol. 2005;77:886–892. doi: 10.1189/jlb.1104636. [DOI] [PubMed] [Google Scholar]
- 10.Bhattacharya M., Berends E.T.M., Chan R., Schwab E., Roy S., Sen C.K., Torres V.J., Wozniak D.J. Staphylococcus aureus biofilms release leukocidins to elicit extracellular trap formation and evade neutrophil-mediated killing. Proc. Natl. Acad. Sci. USA. 2018;115:7416–7421. doi: 10.1073/pnas.1721949115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howard M.W., Strauss R.G., Johnston R.B., Jr. Infections in patients with neutropenia. Am. J. Dis. Child. 1977;131:788–790. doi: 10.1001/archpedi.1977.02120200070015. [DOI] [PubMed] [Google Scholar]
- 12.Buvelot H., Posfay-Barbe K.M., Linder P., Schrenzel J., Krause K.H. Staphylococcus aureus, phagocyte NADPH oxidase and chronic granulomatous disease. FEMS Microbiol. Rev. 2017;41:139–157. doi: 10.1093/femsre/fuw042. [DOI] [PubMed] [Google Scholar]
- 13.Bortoletto P., Lyman K., Camacho A., Fricchione M., Khanolkar A., Katz B.Z. Chronic Granulomatous Disease: A Large, Single-center US Experience. Pediatr. Infect. Dis. J. 2015;34:1110–1114. doi: 10.1097/INF.0000000000000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hill H.R., Ochs H.D., Quie P.G., Clark R.A., Pabst H.F., Klebanoff S.J., Wedgwood R.J. Defect in neutrophil granulocyte chemotaxis in Job’s syndrome of recurrent “cold” staphylococcal abscesses. Lancet. 1974;2:617–619. doi: 10.1016/S0140-6736(74)91942-4. [DOI] [PubMed] [Google Scholar]
- 15.Parry M.F., Root R.K., Metcalf J.A., Delaney K.K., Kaplow L.S., Richar W.J. Myeloperoxidase deficiency: Prevalence and clinical significance. Ann. Intern. Med. 1981;95:293–301. doi: 10.7326/0003-4819-95-3-293. [DOI] [PubMed] [Google Scholar]
- 16.Strauss R.G., Bove K.E., Jones J.F., Mauer A.M., Fulginiti V.A. An anomaly of neutrophil morphology with impaired function. N. Engl. J. Med. 1974;290:478–484. doi: 10.1056/NEJM197402282900903. [DOI] [PubMed] [Google Scholar]
- 17.Kyme P., Thoennissen N.H., Tseng C.W., Thoennissen G.B., Wolf A.J., Shimada K., Krug U.O., Lee K., Müller-Tidow C., Berdel W.E., et al. C/EBPε mediates nicotinamide-enhanced clearance of Staphylococcus aureus in mice. J. Clin. Investig. 2012;122:3316–3329. doi: 10.1172/JCI62070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Introne W., Boissy R.E., Gahl W.A. Clinical, molecular, and cell biological aspects of Chediak-Higashi syndrome. Mol. Genet. Metab. 1999;68:283–303. doi: 10.1006/mgme.1999.2927. [DOI] [PubMed] [Google Scholar]
- 19.Bellinati-Pires R., Salgado M.M., Joazeiro P.P., Carneiro-Sampaio M.M. Delayed phagocytosis and bacterial killing in Chediak-Higashi syndrome neutrophils detected by a fluorochrome assay. Ultrastructural aspects. Mem. Inst. Oswaldo Cruz. 1992;87:575–581. doi: 10.1590/S0074-02761992000400018. [DOI] [PubMed] [Google Scholar]
- 20.Curnutte J.T., Whitten D.M., Babior B.M. Defective superoxide production by granulocytes from patients with chronic granulomatous disease. N. Engl. J. Med. 1974;290:593–597. doi: 10.1056/NEJM197403142901104. [DOI] [PubMed] [Google Scholar]
- 21.Winkelstein J.A., Marino M.C., Johnston R.B., Jr., Boyle J., Curnutte J., Gallin J.I., Malech H.L., Holland S.M., Ochs H., Quie P., et al. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine. 2000;79:155–169. doi: 10.1097/00005792-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Braun R.D., Lanzen J.L., Snyder S.A., Dewhirst M.W. Comparison of tumor and normal tissue oxygen tension measurements using OxyLite or microelectrodes in rodents. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H2533–H2544. doi: 10.1152/ajpheart.2001.280.6.H2533. [DOI] [PubMed] [Google Scholar]
- 23.Campbell E.L., Bruyninckx W.J., Kelly C.J., Glover L.E., McNamee E.N., Bowers B.E., Bayless A.J., Scully M., Saeedi B.J., Golden-Mason L., et al. Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity. 2014;40:66–77. doi: 10.1016/j.immuni.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lone A.G., Atci E., Renslow R., Beyenal H., Noh S., Fransson B., Abu-Lail N., Park J.J., Gang D.R., Call D.R. Staphylococcus aureus induces hypoxia and cellular damage in porcine dermal explants. Infect. Immun. 2015;83:2531–2541. doi: 10.1128/IAI.03075-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Werth N., Beerlage C., Rosenberger C., Yazdi A.S., Edelmann M., Amr A., Bernhardt W., Von Eiff C., Becker K., Schafer A., et al. Activation of hypoxia inducible Factor 1 is a general phenomenon in infections with human pathogens. PLoS ONE. 2010;5:e11576. doi: 10.1371/journal.pone.0011576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koyasu S., Kobayashi M., Goto Y., Hiraoka M., Harada H. Regulatory mechanisms of hypoxia-inducible factor 1 activity: Two decades of knowledge. Cancer Sci. 2018;109:560–571. doi: 10.1111/cas.13483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watts E.R., Walmsley S.R. Inflammation and Hypoxia: HIF and PHD Isoform Selectivity. Trends Mol. Med. 2019;25:33–46. doi: 10.1016/j.molmed.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Lando D., Peet D.J., Whelan D.A., Gorman J.J., Whitelaw M.L. Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science. 2002;295:858–861. doi: 10.1126/science.1068592. [DOI] [PubMed] [Google Scholar]
- 29.Blouin C.C., Pagé E.L., Soucy G.M., Richard D.E. Hypoxic gene activation by lipopolysaccharide in macrophages: Implication of hypoxia-inducible factor 1alpha. Blood. 2004;103:1124–1130. doi: 10.1182/blood-2003-07-2427. [DOI] [PubMed] [Google Scholar]
- 30.Hoenderdos K., Lodge K.M., A Hirst R., Chen C., Palazzo S.G.C., Emerenciana A., Summers C., Angyal A., Porter L., Juss J.K., et al. Hypoxia upregulates neutrophil degranulation and potential for tissue injury. Thorax. 2016;71:1030–1038. doi: 10.1136/thoraxjnl-2015-207604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guerra F.E., Borgogna T.R., Patel D.M., Sward E.W., Voyich J.M. Epic Immune Battles of History: Neutrophils vs. Staphylococcus aureus. Front. Cell Infect. Microbiol. 2017;7:286–305. doi: 10.3389/fcimb.2017.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horn J., Stelzner K., Rudel T., Fraunholz M. Inside job: Staphylococcus aureus host-pathogen interactions. Int. J. Med. Microbiol. 2018;308:607–624. doi: 10.1016/j.ijmm.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 33.Gresham H.D., Lowrance J.H., Caver T.E., Wilson B.S., Cheung A.L., Lindberg F.P. Survival of Staphylococcus aureus inside neutrophils contributes to infection. J. Immunol. 2000;164:3713–3722. doi: 10.4049/jimmunol.164.7.3713. [DOI] [PubMed] [Google Scholar]
- 34.Leliefeld P.H.C., Pillay J., Vrisekoop N., Heeres M., Tak T., Kox M., Rooijakkers S.H.M., Kuijpers T.W., Pickkers P., Leenen L.P.H., et al. Differential antibacterial control by neutrophil subsets. Blood Adv. 2018;2:1344–1355. doi: 10.1182/bloodadvances.2017015578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balasubramanian D., Harper L., Shopsin B., Torres V.J. Staphylococcus aureus pathogenesis in diverse host environments. Pathog. Dis. 2017;75 doi: 10.1093/femspd/ftx005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hall J.W., Yang J., Guo H., Ji Y. The Staphylococcus aureus AirSR Two-Component System Mediates Reactive Oxygen Species Resistance via Transcriptional Regulation of Staphyloxanthin Production. Infect. Immun. 2017;85 doi: 10.1128/IAI.00838-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dunman P.M., Murphy E., Haney S., Palacios D., Tucker-Kellogg G., Wu S., Brown E.L., Zagursky R.J., Shlaes D., Projan S.J. Transcription profiling-based identification of Staphylococcus aureus genes regulated by the agr and/or sarA loci. J. Bacteriol. 2001;183:7341–7353. doi: 10.1128/JB.183.24.7341-7353.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGovern N.N., Cowburn A.S., Porter L., Walmsley S.R., Summers C., Thompson A.A.R., Anwar S., Willcocks L.C., Whyte M.K.B., Condliffe A.M., et al. Hypoxia selectively inhibits respiratory burst activity and killing of Staphylococcus aureus in human neutrophils. J. Immunol. 2011;186:453–463. doi: 10.4049/jimmunol.1002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peyssonnaux C., Datta V., Cramer T., Doedens A., Theodorakis E.A., Gallo R.L., Hurtado-Ziola N., Nizet V., Johnson R.S. HIF-1alpha expression regulates the bactericidal capacity of phagocytes. J. Clin. Investig. 2005;115:1806–1815. doi: 10.1172/JCI23865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zinkernagel A.S., Peyssonnaux C., Johnson R.S., Nizet V. Pharmacologic augmentation of hypoxia-inducible factor-1alpha with mimosine boosts the bactericidal capacity of phagocytes. J. Infect. Dis. 2008;197:214–217. doi: 10.1086/524843. [DOI] [PubMed] [Google Scholar]
- 41.Thompson A.A., Dickinson R.S., Murphy F., Thomson J.P., Marriott H.M., Tavares A., Willson J., Williams L., Lewis A., Mirchandani A., et al. Hypoxia determines survival outcomes of bacterial infection through HIF-1alpha dependent re-programming of leukocyte metabolism. Sci. Immunol. 2017;2:eaal2861. doi: 10.1126/sciimmunol.aal2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fritzenwanger M., Jung C., Goebel B., Lauten A., Figulla H.R. Impact of short-term systemic hypoxia on phagocytosis, cytokine production, and transcription factor activation in peripheral blood cells. Mediat. Inflamm. 2011;2011:429501. doi: 10.1155/2011/429501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reeves E.P., Nagl M., Godovac-Zimmermann J., Segal A.W. Reassessment of the microbicidal activity of reactive oxygen species and hypochlorous acid with reference to the phagocytic vacuole of the neutrophil granulocyte. J. Med. Microbiol. 2003;52:643–651. doi: 10.1099/jmm.0.05181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Babior B.M., Kipnes R.S., Curnutte J.T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J. Clin. Investig. 1973;52:741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eliasson P., Jonsson J.I. The hematopoietic stem cell niche: Low in oxygen but a nice place to be. J. Cell Physiol. 2010;222:17–22. doi: 10.1002/jcp.21908. [DOI] [PubMed] [Google Scholar]
- 46.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S., Weinrauch Y., Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 47.Fuchs T.A., Abed U., Goosmann C., Hurwitz R., Schulze I., Wahn V., Weinrauch Y., Brinkmann V., Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Branitzki-Heinemann K., Möllerherm H., Völlger L., Husein D.M., de Buhr N., Blodkamp S., Reuner F., Brogden G., Naim H.Y., von Köckritz-Blickwede M. Formation of Neutrophil Extracellular Traps under Low Oxygen Level. Front. Immunol. 2016;7:518. doi: 10.3389/fimmu.2016.00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lerche C.J., Christophersen L.J., Kolpen M., Nielsen P.R., Trøstrup H., Thomsen K., Hyldegaard O., Bundgaard H., Jensen P.Ø., Høiby N., et al. Hyperbaric oxygen therapy augments tobramycin efficacy in experimental Staphylococcus aureus endocarditis. Int. J. Antimicrob. Agents. 2017;50:406–412. doi: 10.1016/j.ijantimicag.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 50.Jørgensen N.P., Hansen K., Andreasen C.M., Pedersen M., Fuursted K., Meyer R.L., Petersen E. Hyperbaric Oxygen Therapy is Ineffective as an Adjuvant to Daptomycin with Rifampicin Treatment in a Murine Model of Staphylococcus aureus in Implant-Associated Osteomyelitis. Microorganisms. 2017;5:21. doi: 10.3390/microorganisms5020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sadiku P., Walmsley S.R. Hypoxia and the regulation of myeloid cell metabolic imprinting: Consequences for the inflammatory response. EMBO Rep. 2019;20:e47388. doi: 10.15252/embr.201847388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hannah S., Mecklenburgh K., Rahman I., Bellingan G.J., Greening A., Haslett C., Chilvers E.R. Hypoxia prolongs neutrophil survival in vitro. FEBS Lett. 1995;372:233–237. doi: 10.1016/0014-5793(95)00986-J. [DOI] [PubMed] [Google Scholar]
- 53.Wilde A.D., Snyder D.J., Putnam N.E., Valentino M.D., Hammer N.D., Lonergan Z.R., Hinger S.A., Aysanoa E.E., Blanchard C., Dunman P.M., et al. Bacterial Hypoxic Responses Revealed as Critical Determinants of the Host-Pathogen Outcome by TnSeq Analysis of Staphylococcus aureus Invasive Infection. PLoS Pathog. 2015;11:e1005341. doi: 10.1371/journal.ppat.1005341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ulrich M., Bastian M., Cramton S.E., Ziegler K., Pragman A.A., Bragonzi A., Memmi G., Wolz C., Schlievert P.M., Cheung A., et al. The staphylococcal respiratory response regulator SrrAB induces ica gene transcription and polysaccharide intercellular adhesin expression, protecting Staphylococcus aureus from neutrophil killing under anaerobic growth conditions. Mol. Microbiol. 2007;65:1276–1287. doi: 10.1111/j.1365-2958.2007.05863.x. [DOI] [PubMed] [Google Scholar]
- 55.Christmas B.A.F., Rolfe M.D., Rose M., Green J. Staphylococcus aureus adaptation to aerobic low-redox-potential environments: Implications for an intracellular lifestyle. Microbiology. 2019;165:779–791. doi: 10.1099/mic.0.000809. [DOI] [PubMed] [Google Scholar]
- 56.Kahl B.C., Becker K., Löffler B. Clinical Significance and Pathogenesis of Staphylococcal Small Colony Variants in Persistent Infections. Clin. Microbiol. Rev. 2016;29:401–427. doi: 10.1128/CMR.00069-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Myles I.A., Anderson E.D., Earland N.J., Zarember K.A., Sastalla I., Williams K.W., Gough P., Moore I.N., Ganesan S., Fowler C.J., et al. TNF overproduction impairs epithelial staphylococcal response in hyper IgE syndrome. J. Clin. Investig. 2018;128:3595–3604. doi: 10.1172/JCI121486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prajsnar T.K., Hamilton R., Garcia-Lara J., McVicker G., Williams A., Boots M., Foster S.J., Renshaw S.A. A privileged intraphagocyte niche is responsible for disseminated infection of Staphylococcus aureus in a zebrafish model. Cell Microbiol. 2012;14:1600–1619. doi: 10.1111/j.1462-5822.2012.01826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cheng A.G., Kim H.K., Burts M.L., Krausz T., Schneewind O., Missiakas D.M. Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues. FASEB J. 2009;23:3393–3404. doi: 10.1096/fj.09-135467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheng A.G., McAdow M., Kim H.K., Bae T., Missiakas D.M., Schneewind O. Contribution of coagulases towards Staphylococcus aureus disease and protective immunity. PLoS Pathog. 2010;6:e1001036. doi: 10.1371/journal.ppat.1001036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beerlage C., Greb J., Kretschmer D., Assaggaf M., Trackman P.C., Hansmann M.L., Bonin M., Eble J.A., Peschel A., Brüne B., et al. Hypoxia-inducible factor 1-regulated lysyl oxidase is involved in Staphylococcus aureus abscess formation. Infect. Immun. 2013;81:2562–2573. doi: 10.1128/IAI.00302-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chan P.F., Foster S.J. Role of SarA in virulence determinant production and environmental signal transduction in Staphylococcus aureus. J. Bacteriol. 1998;180:6232–6241. doi: 10.1128/jb.180.23.6232-6241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu Y., Klapper I., Stewart P.S. Hypoxia arising from concerted oxygen consumption by neutrophils and microorganisms in biofilms. Pathog. Dis. 2018;76 doi: 10.1093/femspd/fty043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mashruwala A.A., Guchte A.V., Boyd J.M. Impaired respiration elicits SrrAB-dependent programmed cell lysis and biofilm formation in Staphylococcus aureus. Elife. 2017;6:e23845. doi: 10.7554/eLife.23845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pabst B., Pitts B., Lauchnor E., Stewart P.S. Gel-Entrapped Staphylococcus aureus Bacteria as Models of Biofilm Infection Exhibit Growth in Dense Aggregates, Oxygen Limitation, Antibiotic Tolerance, and Heterogeneous Gene Expression. Antimicrob. Agents Chemother. 2016;60:6294–6301. doi: 10.1128/AAC.01336-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Günther F., Wabnitz G.H., Stroh P., Prior B., Obst U., Samstag Y., Wagner C., Hänsch G.M. Host defence against Staphylococcus aureus biofilms infection: Phagocytosis of biofilms by polymorphonuclear neutrophils (PMN) Mol. Immunol. 2009;46:1805–1813. doi: 10.1016/j.molimm.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 67.Gutierrez Jauregui R., Fleige H., Bubke A., Rohde M., Weiss S., Förster R. IL-1β Promotes Staphylococcus aureus Biofilms on Implants in vivo. Front. Immunol. 2019;10:1082. doi: 10.3389/fimmu.2019.01082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Josse J., Valour F., Maali Y., Diot A., Batailler C., Ferry T., Laurent F. Interaction Between Staphylococcal Biofilm and Bone: How Does the Presence of Biofilm Promote Prosthesis Loosening? Front. Microbiol. 2019;10:1602. doi: 10.3389/fmicb.2019.01602. [DOI] [PMC free article] [PubMed] [Google Scholar]