Abstract
Chronic Myeloid Leukemia (CML) is a disease arising in stem cells expressing the BCR-ABL oncogenic tyrosine kinase that transforms one Hematopoietic stem/progenitor Cell into a Leukemic Stem Cell (LSC) at the origin of differentiated and proliferating leukemic cells in the bone marrow (BM). CML-LSCs are recognized as being responsible for resistances and relapses that occur despite the advent of BCR-ABL-targeting therapies with Tyrosine Kinase Inhibitors (TKIs). LSCs share a lot of functional properties with Hematopoietic Stem Cells (HSCs) although some phenotypical and functional differences have been described during the last two decades. Subverted mechanisms affecting epigenetic processes, apoptosis, autophagy and more recently metabolism and immunology in the bone marrow microenvironment (BMM) have been reported. The aim of this review is to bring together the modifications and molecular mechanisms that are known to account for TKI resistance in primary CML-LSCs and to focus on the potential solutions that can circumvent these resistances, in particular those that have been, or will be tested in clinical trials.
Keywords: chronic myeloid leukemia, leukemic stem cells, resistance, tyrosine kinase inhibitors, microenvironment, apoptosis, autophagy, metabolism, epigenetic, clinical trials
1. Introduction
Chronic Myeloid Leukemia (CML) is a myeloproliferative neoplasm characterized by a chromosomic translocation between chromosomes 9 and 22 at a very early stem/progenitor cell level, with the emergence of the well-known chromosome of Philadelphia. The oncogenic event fuses parts of the gene for the c-ABL tyrosine kinase downstream of the BCR gene. This creates the constitutively active BCR-ABL tyrosine kinase, at the root of the disease. BCR-ABL supports initiation and progression of CML through a plethora of signaling pathways [1]. If left untreated, CML rapidly evolves from a chronic phase into a blast crisis with a massive accumulation of myeloid cells in the BM and the blood. This uncontrolled proliferation of Philadelphia positive cells (Ph+) supplants normal hematopoiesis, with a gradual replacement of normal blood cells.
The very first treatments developed with Hydroxyurea, Busulfan or Interferon-Alpha (IFN-α)-based therapies have shown their limitation to affect BCR-ABL proliferative cells and thereby to keep the disease in check [2]. CML was the first cancer to benefit from a targeted therapy in the early 2000s with STI571/Imatinib, a tyrosine kinase inhibitor (TKI), that specifically blocks ABL activity. This treatment dramatically improved the therapeutic outcome of the patients, with 95% of them achieving a complete hematological remission (CHR) [3]. Furthermore, second- (Dasatinib/BMS354825, Nilotinib/AMN107, Bosutinib/SKI-606) and third- (Ponatinib/AP24534) generation TKIs have been designed to bypass primary and secondary resistances to Imatinib [4]. The rise of these TKIs has drastically improved CML patients’ outcome and survival, redefining CML from an incurable disease to a manageable one. While TKIs, especially the second-generation ones, are very efficient to eliminate blasts, they remain nonetheless toxic for healthy cells in the long run with numerous side effects affecting the gastrointestinal tract or the cardiovascular system [5].
A discontinuation of Imatinib has therefore been tested once the disease is undetectable at the molecular level. Unfortunately, half of the patients in this study relapsed within two years [6], supporting the idea of a residual disease sustained by a discrete population of Leukemic Stem Cells (LSCs), that are insensitive to treatments, capable to self-maintain and to reinitiate the disease in the long-term. Therefore, successfully achieving a cure requires the elimination of LSCs. Most of the time, LSCs are in a quiescent state in the bone marrow (BM) and thus insensitive to TKI monotherapy. This is why during the last decade, many research groups have been deciphering the pathways involved in LSC maintenance and expansion, to propose numerous pertinent approaches to eradicate them specifically.
Most dysregulations connected to TKI resistance in CML are exclusively observed on cell lines, but some of them were also found in primary CD34+ CML cells.
The present review is focused on TKI-resistance processes observed ex-vivo for which pharmacological targeting has been demonstrated to resensitize LSCs to TKIs (Table 1) eventually given rise to clinical trials (Table 2), summarized in a global overview (Figure 1).
Table 1.
Chronic Myeloid Leukemia (CML) Treatments with Ex-Vivo Evidences of Effectiveness either in Combination with tyrosine kinase inhibitor (TKIs) or Alone.
| Scheme Number | Treatment 1 | Treatment 2 | Pathway | References |
|---|---|---|---|---|
| 1 | Ponatinib | Asciminib | BCR-ABL1 allosteric inhibitor | [7] |
| 2 | Imatinib Dasatinib Nilotinib |
GSK343 | EZH2 inhibitor | [8] |
| 3 | Imatinib | LBH589 | HDAC inhibitor | [9] |
| 4 | Nilotinib | LBH589 | HDAC inhibitor | [10] |
| 5 | Imatinib | LAQ824 | HDAC inhibitor | [11] |
| 6 | Imatinib | Hydralazine and Magnesium Valporate | HDAC inhibitors | [12] |
| 7 | Imatinib | Tenovin-6 | SIRT1 inhibitor | [13] |
| 8 | Nilotinib | TV39OH | SIRT1 inhibitor | [14] |
| 9 | Imatinib | miR30a inhibition | Genetic repressor/activator | [15] |
| 10 | Imatinib | miR-202 overexpression | Inhibition of hexokinase 2 (HK2) and glycolysis | [16] |
| 11 | Imatinib | miR-486 | Genetic repressor/activator | [17] |
| 12 | Imatinib | Ovatodiolide | miR-155 upregulation/PI3K/mTOR inhibition | [18] |
| 13 | Imatinib | RI-BPI | BCL6 inhibitor | [19] |
| 14 | Imatinib | FX1 | BCL6 inhibitor | [20] |
| 15 | Imatinib | A-1210477 | MCL-1 inhibitor | [20] |
| 16 | Dasatinib | Sabutoclax | Pan-BCL2 inhibitor | [21] |
| 17 | Imatinib | ABT-737 | BCL2 and Bcl-xL inhibitor | [22,23] |
| 18 | Nilotinib | Nutlin3a ABT-737 |
MDM2 inhibitor BCL2 and Bcl-xL inhibitor |
[24] |
| 19 | Imatinib | DS-5272 | MDM2 inhibitor | [25] |
| 20 | Imatinib | ABT-199 (Venetoclax) | BCL2 inhibitor | [26] |
| 21 | Nilotinib | ABT-199 (Venetoclax) | BCL2 inhibitor | [27] |
| 22 | Imatinib Nilotinib Dasatinib |
Chloroquine Bafilomycin A1 |
Autophagy inhibitors | [9,28] |
| 23 | Imatinib | Chloroquine Bafilomycin A1 |
Autophagy inhibitors | [29] |
| 24 | Imatinib Nilotinib Dasatinib |
Clarithromycin | Autophagy inhibitor | [30] |
| 25 | Imatinib | Spautin-1 | Autophagy inhibitor | [31] |
| 26 | Ponatinib | Vismodegib (GDC-0449) | Smo antagonist Hedgehog pathway |
[32] |
| 27 | Nilotinib | Lys05 | Autophagy inhibitor | [33] |
| 28 | Nilotinib | PIK-III | VPS34 inhibitor Autophagy |
[33] |
| 29 | Imatinib | PTC-209 | BMI1 inhibitor | [34] |
| 30 | Nilotinib | WNT974 | PORCN selective inhibitor Wnt pathway |
[35] |
| 31 | Nilotinib | PRI-724 | CBP inhibitor Wnt/β-catenin inhibition |
[36] |
| 32 | Nilotinib | XL880 R428 |
AXL inhibitors | [37] |
| 33 | Imatinib | Plerifaxor (ADM3100) | CXCR4 antagonist | [34] |
| 34 | Imatinib Nilotinib |
Plerifaxor (ADM3100) | CXCR4 antagonist | [38] |
| 35 | Nilotinib | Plerifaxor (ADM3100) | CXCR4 antagonist | [39] |
| 36 | Imatinib | BTK140 | CXCR4 antagonist | [40] |
| 37 | Imatinib | Filgotinib Itacitinib | JAK1 specific inhibitors, JAK1/STAT3 pathway | [41] |
| 38 | Imatinib | Wogonin | STAT5 pathway inhibitor | [42] |
| 39 | Imatinib | Tigecycline | Mitochondrial ribosome protein translation and respiratory chain | [43] |
| 40 | Nilotinib | SR18292 | PGC-1α inhibitor/SIRT1 pathway inhibition | [14] |
| 41 | Imatinib Dasatinib |
Ivermectin | mTOR inhibitor | [44] |
| 42 | Imatinib | Anti-CD70 | Wnt/β-catenin inhibition | [45] |
| 43 | Imatinib | Sonidegib (LDE225) | Smo antagonist Hedgehog pathway inhibition |
[46] |
| 44 | Imatinib | Ly364947 | TGF-βRI inhibitor TGFβ/Activin/NODAL pathway | [47] |
| 45 | Imatinib Dasatinib Ponatinib |
EW-7197 | TGF-β signaling inhibitor | [11] |
| 46 | Dasatinib | BMS-911543 | JAK2 inhibitor | [48] |
| 47 | Dasatinib | Niclosamide | ERK/MNK1/eIF4E pathway inhibition | [49] |
| 48 | Imatinib | SB216763 | GSK-3 specific inhibitor | [50] |
| 49 | Imatinib Nilotinib |
PD146176 | 15-LO inhibition/ arachidonic acid -leukotriene pathway inhibition | [51] |
| 50 | none | EZH2 CRISPR/Cas9 invalidation | EZH2 inhibition | [52] |
| 51 | none | Pracinostat | HDAC inhibitor | [53] |
| 52 | none | PJ-68 | Methyltransferase inhibitor (PRMT5) Wnt/β-catenin pathway inhibition |
[54] |
| 53 | none | Dactolisib (BEZ235) Chloroquine |
PI3K/mTOR inhibitor Autophagy inhibitor |
[55] |
| 54 | none | Vismodegib (GDC-0449) | Smo antagonistHH pathway inhibition | [56] |
| 55 | none | Resveratrol | Autophagic cell death induction | [57] |
| 56 | none | IFN-α Ruxolitinib |
MHC II increased expression JAK1/2 inhibitor |
[58] |
| 57 | none | shATG7 | Mitochondrial ROS and oxidative phosphorylation increase Autophagy inhibition |
[59] |
| 58 | none | miR-326 overexpression | Downregulation of SmoHH pathway inhibition | [60] |
| 59 | none | PD184352 BMS-21462 |
MEK specific inhibitor Farnesyl transferase inhibitor |
[61] |
| 60 | none | CGP57380 | MNK1 inhibitor ERK/MNK1/eIF4E pathway inhibition | [62] |
| 61 | none | LY255283 | BLT2 inhibitor/arachidonic acid -leukotriene pathway inhibition | [63] |
| 62 | none | RG7112/7388 CPI-203/0610 |
HMD2 inhibitors (p53 inhibition) BET inhibitors (c-Myc inhibition) |
[64] |
Table 2.
Clinical Trials Involving CML Patients Treated either with Investigational Molecules in Combination with TKIs or Alone.
| Scheme Number |
Treatment 1 | Treatment 2 | Pathway | Investigation | Phase | Identifier | First Posted | Status |
|---|---|---|---|---|---|---|---|---|
| 1 | Imatinib Nilotinib Dasatinib |
Asciminib | BCR-ABL1 allosteric inhibitor | Frontline combination in CP- CML | II | NCT03906292 | 2019 | Recruiting |
| 2 | none | Asciminib | BCR-ABL1 allosteric inhibitor | Efficacy of ABL001 versus Bosutinib in CP-CML patients previously treated with TKIs | III | NCT03106779 | 2017 | Recruiting |
| 3 | Dasatinib | Asciminib | BCR-ABL1 allosteric inhibitor | Combination in CML in lymphoid blast crisis | I | NCT03595917 | 2018 | Recruiting |
| 4 | Imatinib | Asciminib | BCR-ABL1 allosteric inhibitor | Efficacy and safety of combination in patients with CP-CML | II | NCT03578367 | 2018 | Recruiting |
| 5 | Imatinib Nilotinib Dasatinib |
Asciminib | BCR-ABL1 allosteric inhibitor | Oral ABL001 in CML Patients | I | NCT02081378 | 2014 | Recruiting |
| 6 | Imatinib | Cytarabine | DNA synthesis inhibitor | Combination in patients with CML | II | NCT00022490 | 2003 | Terminated |
| 7 | Imatinib | Cytarabine | DNA synthesis inhibitor | Combination in patients with CML | I/II | NCT00015834 | 2003 | Completed |
| 8 | Dasatinib | SAHA (Vorinostat) |
HDAC inhibitor | Combination in treating patients with accelerated phase or BP- CML | I | NCT00816283 | 2009 | Completed |
| 9 | Imatinib | Panobinostat (LBH589) |
HDAC inhibitor | Safety and tolerability of LBH589 combined with imatinib in CML patients in MCR | I | NCT00686218 | 2008 | Completed |
| 10 | Imatinib | Decitabine | DNA methyltransferase inhibitor | Combination in patients with CML | II | NCT00054431 | 2003 | Completed |
| 11 | Dasatinib | Venetoclax | BCL2 inhibitor | Combination in treating patients with BCR-ABL1 positive early chronic phase | II | NCT02689440 | 2016 | Recruiting |
| 12 | Ponatinib | Venetoclax Dexamethasone | BCL2 inhibitor Anti-inflammatory |
Triple combination in BCR-ABL positive relapsed CML | I/II | NCT03576547 | 2018 | Recruiting |
| 13 | Imatinib | Oblimersen | bcl-2 antisense oligodeoxynucleotide | Oblimersen and Imatinib in treating patients with CML | II | NCT00049192 | 2003 | Completed |
| 14 | Imatinib | Hydroxychloroquine | Autophagy inhibitor | Effectiveness of combination on BCR/ABL levels in CML patients in MCR | II | NCT01227135 | 2010 | Unknown |
| 15 | Imatinib | Everolimus (RAD001) | mTOR inhibitor | Combination in patients in CP-CML who are not in CCR after previous Imatinib | I/II | NCT00093639 | 2004 | Completed |
| 16 | Imatinib | Temsirolimus | mTOR inhibitor | Temsirolimus and Imatinib in treating patients with CML | I | NCT00101088 | 2005 | Terminated |
| 17 | Dasatinib | PF04449913 (Glasdegib) |
Smo antagonist HH inhibition |
Study of PF-04449913 in select hematologic malignancies | I | NCT00953758 | 2009 | Completed |
| 18 | Imatinib | Interferon-α | Immunomodulatory effect | INF-α and Imatinib in CML patients | II | NCT00045422 | 2003 | Completed |
| 19 | Imatinib | Pioglitazone | PPARγ agonist STAT5 inhibition |
Combination in patients with relapsed CML | II | NCT02767063 | 2016 | Terminated |
| 20 | Imatinib | Pioglitazone | PPARγ agonist STAT5 inhibition |
Efficiency of combination to treat CML | II | NCT02687425 | 2016 | Unknown |
| 21 | TKIs | Pioglitazone | PPARγ agonist STAT5 inhibition |
Pioglitazone and TKI in patients with relapsed CML | II | NCT02730195 | 2019 | Terminated |
| 22 | Dasatinib | Cyclosporine | IL-2 inhibitor | Combination in patients with CML refractory or intolerant to imatinib | I | NCT01426334 | 2011 | Terminated |
| 23 | Pioglitazone | Avelumab (Anti-PD-L1) | PPARγ agonist STAT5 inhibition PD-1/PD-L1 inhibition |
Therapies in combination or sequentially with TKIs in CP-CML patients in CCR (ACTIW) | I/II | NCT02767063 | 2016 | Recruiting |
| 24 | Imatinib Nilotinib Dasatinib |
Pembrolizumab (Anti-PD-1) | PD-1/PD-L1 inhibition | Pembrolizumab and TKIs in CML patients with persistently detectable MRD | II | NCT03516279 | 2018 | Recruiting |
| 25 | Dasatinib Nilotinib |
Ruxolitinib | JAK1/2 selective inhibitor | Ruxolitinib phosphate and Dasatinib or Nilotinib in treating CML patients | II | NCT03654768 | 2018 | Recruiting |
| 26 | Nilotinib | Ruxolitinib | JAK1/2 selective inhibitor | Ruxolitinib in treating participants with CML with MRD after TKIs | I/II | NCT01751425 | 2012 | Active, not recruiting |
| 27 | Nilotinib | Ruxolitinib | JAK1/2 selective inhibitor | Nilotinib/Ruxolitinb therapy for TKI resistant Ph-Leukemia | I//II | NCT01914484 | 2013 | Unknown |
| 28 | Nilotinib | Ruxolitinib | JAK1/2 selective inhibitor | Combination in CP-CML Patients | I | NCT01702064 | 2012 | Completed |
| 29 | Nilotinib | Peginterferon α2b | Immunomodulatory effect | Evaluation of TKI and INF-α | III | NCT01657604 | 2012 | Active, not recruiting |
| 30 | Imatinib Nilotinib |
Peginterferon α2b | Immunomodulatory effect | Imatinib or Nilotinib with Pegylated Interferon-α2b in CML | II | NCT00573378 | 2007 | Withdrawn |
| 31 | Dasatinib | Peginterferon α2b | Immunomodulatory effect | Safety and efficacy of combination in newly diagnosed CML (NordCML007) | II | NCT01725204 | 2012 | Completed |
| 32 | Bosutinib | Ropeginterferon | Immunomodulatory effect | Long-acting low dose Ropeginterferon for CML treated with Bosutinib from diagnosis | II | NCT03831776 | 2019 | Recruiting |
| 33 | Nilotinib | Sonidegib (LDE225) | Smo antagonist HH inhibition |
Combination in CML patients who developed resistance to prior therapy | I | NCT01456676 | 2011 | Completed |
| 34 | Dasatinib | BMS-833923 | Smo antagonist HH inhibition |
Combination therapy in CML | I/II | NCT01218477 | 2010 | Completed |
| 35 | Dasatinib | BMS833923 | Smo antagonist HH inhibition |
Dasatinib combo with Smo antagonist | II | NCT01357655 | 2011 | Terminated |
| 36 | Dasatinib | PF04449913 | Smo antagonist HH inhibition |
Study in select hematologic malignancies | I | NCT00953758 | 2009 | Completed |
| 37 | Imatinib | Zarnestra | Farnesyltransferase inhibitor | Zarnestra and Gleevec in CP- CML | I | NCT00040105 | 2002 | Completed |
| 38 | Imatinib | Lonafarnib | Farnesyltransferase inhibitor | Lonafarnib and Gleevec in CML | I | NCT00047502 | 2002 | Completed |
| 39 | none | Sorafenib (BAY43-9006) | Raf/VEGFR/PDGFR inhibitor | Raf kinase inhibitor BAY 43-9006 in CML patients resistant to Gleevec | II | NCT00661180 | 2008 | Completed |
| 40 | Imatinib | Vatalanib (PTK 787) | VEGFR inhibitor | Combination in patients with BP-CML | I/II | NCT00088231 | 2004 | Completed |
| 41 | Dasatinib | BP1001 | Grb2 inhibitor | Combination of liposomal Grb2 antisense oligonucleotide with Dasatinib in CML patients | I/II | NCT02923986 | 2016 | Recruiting |
| 42 | none | MK0457 | Aurora kinase inhibition | MK0457 in CML patients (0457-003) | I | NCT00111683 | 2005 | Completed |
| 43 | Dasatinib | MK0457 | Aurora kinase inhibition | Evaluation of efficacy and safety in patients with CML | I | NCT00500006 | 2007 | Terminated |
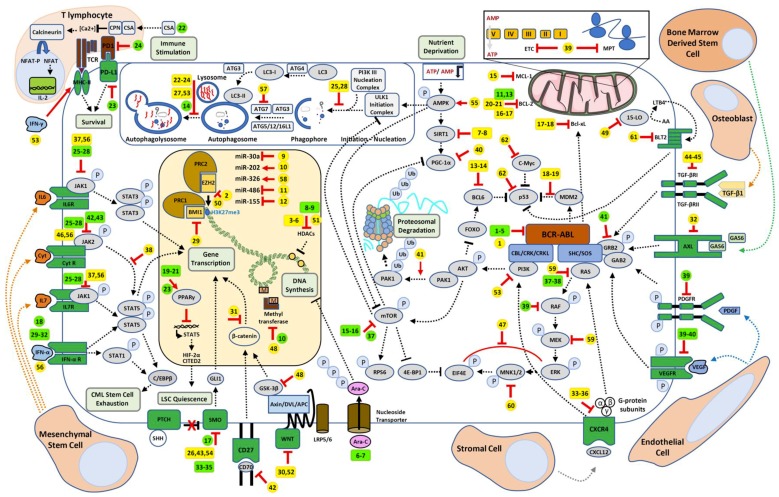
Figure 1.
Chronic Myeloid Leukemia (CML) Leukemic Stem Cells (LSC) pathways involved in tyrosine kinase inhibitor (TKI) resistance and potential therapeutic targets to impair them. LSC (in the center) is represented within its microenvironment and key interactions with different bone-marrow cells are shown. This figure is coupled with Table 1 for ex-vivo candidate molecules (yellow tags) and Table 2 for clinical trials involving candidate molecules (green tags) with their respective mode of action (red symbols).
2. Leukemic Stem Cells: Cannot See the Wood for the Trees
LSCs were first identified in Acute Myeloid Leukemia (AML) within the CD34+/CD38− cell population and defined as cells that can engraft and initiate AML in a NOD/SCID immunodeficient host mouse [65]. They have the ability to self-renew and also to produce differentiation-impaired leukemic blasts [65]. LSCs have also been described in other leukemias such as CML [66] or B-lineage acute lymphoblastic leukemia [67], and Cancer Stem Cells (CSCs) have been identified in numerous solid cancers such as breast [68], colorectal [69] or brain [70] cancers, unravelling their crucial importance in cancerology.
LSCs that also referred as Leukemia-Initiating Cells (LICs), represent a very small subset of CML cells, residing in the very complex microenvironment of the BM. Contrary to proliferating leukemic cells, LSCs have been shown to be “BCR-ABL oncogene independent” as demonstrated by their capacity to maintain and reinitiate the disease even after BCR-ABL activity has been shut down in the SCLtTA/BCR-ABL double transgenic CML mouse model [71]. Furthermore, despite a continuous inhibition of BCR-ABL by TKIs (Imatinib, Dasatinib or Nilotinib), primary CML stem/progenitors are able to self-maintain in the presence of a cocktail of cytokines [72]. Since LSCs emerge from HSCs, the two types of cells cannot be formerly distinguished from each other from their phenotype. The direct identification of LSCs and evaluation of their numbers in BM or blood sample is not feasible yet, and the gold standard model to study LSCs remains the serial transplantation in recipient mice, of samples containing LSCs, followed by monitoring the development of leukemia [73].
Many studies have found that specific biochemical dysregulations in LSCs can contribute to their resistance to TKI treatments, resulting in their persistence in the bone marrow microenvironment (BMM). It is currently accepted that bi-therapies combining TKIs with various drugs that target these dysregulations could lead to elimination of LSCs.
3. BCR-ABL Dependent and Independent Resistances
3.1. BCR-ABL-Dependent Resistances and Alternative Solutions
Emergence of point mutations in BCR-ABL, essentially in the different structural domains of ABL1: Phosphate-binding loop (P-loop), Activation-loop (A-loop), SRC Homology 2/3 Domain (SH2/SH3) and catalytic domains represent the most encountered forms of BCR-ABL-dependent resistances [74]. They are usually the consequence of long-term adaptation of leukemic cells upon TKI treatments. Those mutations are mostly single ones although double mutants have also been identified, with some of them refractory to all TKIs. Some mutations can confer resistance to some or to all TKIs. For instance, the gatekeeper mutations affecting threonine 315: T315I and T315V are only sensitive to Ponatinib, the third generation of TKIs. As the disease progresses towards accelerated or blast crisis phases, genomic instabilities can favor double mutations on BCR-ABL such as T315I/G250E, T315I/Q252H, T315I/Y253H, T315I/E255V, T315I/F311, T315I/M351T, T315I/F359V, T315I/H396R or T315I/E453K which are associated with poor prognosis as most of them generate insensitivity to all available TKIs [75].
Imatinib, the first synthetized TKI, targets the ATP binding site of the AB1 catalytic domain in a type-II-dependent fashion, stabilizing the catalytically inactive conformation of the kinase to induce apoptosis of CML cells. The emergence of 2nd generation TKI was a necessity to treat first-line-Imatinib refractory patients and Dasatinib, Nilotinib and Bosutinib were developed to solve this issue. Dasatinib is a type-I kinase inhibitor (only affecting the catalytically active conformation) while the two other TKIs are type-II kinase inhibitors like Imatinib. In an attempt to treat BCR-ABL T315I refractory patients, Ponatinib was developed to target the active conformation of T315I BCR-ABL using a structure- design strategy. However, some BCR-ABL double mutants still appeared resistant to Ponatinib [75]. Asciminib is the last developed TKI and also the first allosteric BCR-ABL inhibitor that is effective against any mutation in the ATP binding site [76]. Furthermore, combination of Asciminib with Ponatinib is highly efficient against BCR-ABL compound mutants [7]. Indeed, this molecule is currently in five recruiting clinical trials (NCT03906292, NCT03106779, NCT03595917, NCT03578367 and NCT02081378).
In addition to BCR-ABL point mutations, a higher expression of BCR-ABL can induce TKI resistance as observed for CD34+/BCR-ABLHIGH expressing cells [77]. In the same way, the genomic instability that goes with CML progression towards late phases further increases the occurrence of BCR-ABL mutations. Furthermore, BCR-ABL is known to trigger DNA damages (double-strand breaks) via reactive oxygen species (ROS) stimulation [78] linked to PI3K/mTOR activation [79], which further increases mutagenesis by promoting the emergence of additional mutations.
3.2. BCR-ABL-Independent Resistances
Targeting DNA synthesis with the anti-metabolite cytarabine (NCT00022490, NCT00015834) has been first considered as a broad approach to counteract BCR-ABL-independent resistances in CML. During the last two decades, the description at a molecular level of diverse BCR-ABL-independent resistance mechanisms, led to the identification of dysregulated signaling pathways in LSCs. Those dysregulations have paved the way for precise pharmacological interventions to resensitize resistant CML cells to TKIs, even in the case of the T315I “hell” mutation. Several examples are presented below with a specific focus on mechanisms allowing the maintenance of CML-LSCs, and for most cases, on potential therapeutic molecules to target them.
3.2.1. Drug Transporters
LSCs resistance to chemotherapies or TKIs can be partly explained by the high expression of different members of the ATP-binding cassette (ABC) family of transporters able to export out of cancer cells a wide range of molecules, thereby decreasing their therapeutic potential. The high expression of ABCB1 (MDR1, P-glycoprotein), ABCC1 (multidrug resistance protein, MRP1) and ABCG2 (breast cancer resistance protein; BCRP) transporters confers multidrug resistance (MDR) to LSCs. For example, ABCG2 has been shown to efflux Imatinib from cells [80] and to control its delivery in-vivo [81]. Efflux-mediated resistance was shown not to be specific to Imatinib but also to apply to other TKIs [82].
3.2.2. Targeting Epigenetic Dysregulation in CML
Epigenetic modifications at the chromatin control gene expression during proliferation and differentiation of stem cells. Interestingly, an abundant literature reports that the epigenetic signature of cancer stem cells is quite different from their normal counterpart highlighting that an altered epigenetic program may play an important role in cancer progression.
Because numerous epigenetic mechanisms have been reported to be altered during CML progression [83,84] or reprogrammed after TKI therapy [85], their modulation has to be considered in order to counteract with resistance as a means of eradicating residual disease [86].
Proteins of the polycomb family that are part of the Polycomb Repressive Complexes 1 and 2 (PRC1/PRC2) function as repressors of gene expression via chromatin modifications [87]. These two complexes have been involved in the progression of both solid tumors and hematological malignancies [88]. Their epigenetic regulatory function can be affected by alterations in the genes coding for the different components of each complex or through regulation of associated protein partners.
BMI1 a polycomb protein, part of the PRC1 complex, plays an important role in supporting self-renewal of both HSC and LSCs [89]. While there is no clear demonstration of the involvement of BMI1 in CML initiation, the polycomb clearly cooperates with BCR-ABL when introduced in CD34+ CML cells to stimulate their proliferation and self-renewal [90]. Furthermore, BMI1 was shown to be overexpressed in CML vs control subjects [91] with an increased expression that mirrors disease progression [91,92] and could thus represent a molecular marker for CML prognosis prediction [93] Moreover, BMI1 could be a potential target. Indeed, CD34+ CML cells treated with the BMI1 inhibitor PTC-209, failed to achieve clonogenicity [92] leaving hope for a possible targeting of LSCs.
EZH2, another important polycomb protein, member of the PRC2 complex, was also described as being implicated in CML-LSCs maintenance after mutation or overexpression that triggered an epigenetic reprogramming. Targeting EZH2 by CRISPR/Cas9-mediated gene editing [52] is an efficient strategy to target LICs, and the specific inhibitor GSK343 [8] sensitizes LSCs to TKIs.
ASXL1 that associates with PRC2 is well known to regulate epigenetic marks and transcription. The ASXL1 gene is frequently mutated in CML diagnosed-patients and often correlated with poor prognosis [94,95], suggesting a role in the progression of the disease [96,97] and could thus represent a future attractive target.
The Histone deacetylase (HDAC) family represents another class of epigenetic regulators that have been extensively described for their implication in cancer biogenesis and in cancer stem cells maintenance. In the case of CML, the first HDAC inhibitor used with success in combination with a TKI to target LSCs is the class I and II dual-HDAC inhibitor SAHA/Vorinostat (Suberoylanilide hydroxamic acid) that was found to enhance Imatinib-induced apoptosis of CD34+ CML cells [98]. Other HDAC inhibitors like LBH589 can be combined with Imatinib [83], Nilotinib [10] or LAQ824 to eliminate Imatinib-refractory primary CML cells [83,99]. Furthermore, LBH589 and LAQ824 combined with a TKI gave interesting results in Imatinib-resistant CML cells harboring the BCR-ABL T315I mutation [99,100]. The class I, II and IV HDAC inhibitor Pracinostat, corrects pre-mRNA splicing of the BIM pro-apoptotic protein to overcome BIM deletion polymorphism-induced TKI resistance observed in certain CML cases [53]. Preclinical in-vitro studies have shown that the combination of Vorinostat with Dasatinib leads to the death of CML primary cells and can also attenuate the levels of expression of E255K and T315I-BCR-ABL mutants [10]. Combination of HDAC inhibitors (Hydralazine and Magnesium Valproate) with Imatinib gave promising clinical results on Imatinib non-responders [12], and several TKIs/HDAC inhibitors combinations such as Imatinib and Panobinostat (pan HDAC inhibitor): NCT00686218; or Dasatinib and Vorinostat: NCT00816283 were evaluated in clinical trials.
Among the SIRT group that is part of the HDAC family, SIRT1, a NAD-dependent deacetylase has been described, first to be overexpressed in human CML-LSCs [13] and second, to play a role in the regulation of both HSCs and LSCs [101,102]. Furthermore, Wang et al. described that histone deacetylation by SIRT1 was implicated in the acquisition of genetic mutations in BCR-ABL associated with TKI resistance and that targeting SIRT1 can be a good strategy to overcome these drug resistances [101]. The specific inhibition of SIRT1 by Tenovin-6 [13] or TV39OH [14] enhances p53 anti-cancer functions via increased acetylation and therefore combination with Imatinib or Nilotinib respectively leads to LSCs targeting.
Activity of promoters can be regulated by methylation-demethylation processes. The role of methylation in CML was already suspected in the pre-TKI era when silencing of cadherin 13, a mediator of calcium-dependent cell-cell adhesion, was demonstrated to correlate with response toIFN-α [103]. Besides, methylation can serve as a prognostic tool. Indeed, the methylation status of the BIM promoter, is associated with an unfavorable prognosis in CML diagnosed patients [104], while an increased BCR promoter methylation correlates with a more favorable response to Imatinib [105].
Otherwise, methylation of some gene promoters was reported to be implicated in CML progression like the one for SOCS1 that stimulates the JAK/STAT activity [29,106] or that of the ATP-dependent RNA helicase DDX43 [107]. Progression of the disease was also demonstrated to correlate with methylation of the promoter for genes implicated in cell-cycle progression (p15 and p16) [108,109,110], apoptosis (p14 ARF) [109,110], p53 [110] and DAPK [110,111]. TKI resistance has been associated with methylation of several promoters for sFRP1 in the Wnt pathway [112], or of the Src suppressor gene PDLIM4 [113], and of HOXA4. Studies in CD34+ progenitor cells indicate that methylation particularly concerns tumor suppressor genes and occurs very early during CML transformation before being markedly decreased in the last phase of the disease. More recently, another study examined the DNA methylation profile of CD34+/CD15− in early CP-CML patients’ cells and described 18 genes that could be aberrantly repressed upon hypermethylation and 81 upon hypomethylation [114]. This suggests that demethylating agents could be more effective in the first phase of the disease [115]. Interestingly, the PRMT5 methyltransferase, overexpressed in CML-LSCs, can be targeted with the small-molecule inhibitor PJ-68, abrogating the Wnt/β-catenin pathway and leading to the elimination of LSCs [54].
Most of the molecules that modify promoter methylation have only been tested alone. Nevertheless, a phase II study that combined decitabine with Imatinib on a 28-patient CML cohort, showed an improvement in the survival for non-responder patients in advanced phase CML without BCR-ABL kinase mutation [116].
The non-coding RNAs of the long non-coding (lncRNAs) or the miRNAs families represent a recently discovered epigenetic process regulating mRNA levels and protein translation via different mechanisms [117].
Today, only few reports have emerged concerning dysregulations of lncRNAs in CML, although none have been described yet for LSCs. Nevertheless, there is some hope for the development of new therapeutic weapons in the near future. For instance, the lncRNA-HULK which is aberrantly expressed in CML, positively correlates with clinical evolution of the disease, and its down-regulation is sufficient to trigger apoptosis of leukemic cells by repression of c-MYC and BCL-2 [118]. The lncRNA-PLIN2 also upregulated in CML patients by a CEBPA induced-mechanism, increased in turn the expression of AKT, p-AKT, GSK-3β, β-catenin and Axin2/conductin, and could promote tumor growth by activating GSK3 and Wnt/β-catenin signaling in-vivo [119]. The expression level of lncRNA-BGL3 strongly increases following TKI treatment because of a BCR-ABL-dependent repression mechanism involving a c-MYC-dependent methylation [120]. Through its transcriptional interference activity, the lncRNA-MEG3 can regulate miR-21 expression, influencing the levels of MMP-2, MMP-9, BCL-2 and BAX and regulating proliferation and apoptosis [121].
The dysregulation of some miRNAs expression has been frequently reported in CML patients, in the last decade [122,123,124,125], presenting them as potential biomarkers [126] and opening new therapeutic options [86]. A group of 19 miRNAs was identified to predict clinical resistance to Imatinib in newly diagnosed CML [127] and more recently, 8 dysregulated miRNAs were described to be involved in the BCR-ABL-independent resistance to TKI in CML [128]. Moreover, targeting miR30a decreases autophagy and therefore sensitizes primary LMC cells to Imatinib [15], while overexpression of miR-203 overcomes TKI-resistance in-vitro [129]. Furthermore, overexpression of miR-202 inhibits hexokinase 2 (HK2) and glycolysis which resensitizes Imatinib-resistant CML cells [16]. Also, inhibition of miR-486, overexpressed in CML progenitors interfered with their growth and survival and sensitized them to Imatinib [17]. The inhibition of BCR-ABL by TKIs is also responsible for increased endogenous miR-126 levels, favoring LSCs quiescence and persistence [130]. Recently, a study showed that combining Imatinib with Ovatodiolide, a natural diterpenoid that up-regulates miR-155 to inhibit PI3K/mTOR, efficiently induced cell death of CD34+/CD38− CML cells. [18].
3.2.3. Apoptosis Defects in Resistance
Numerous genes implicated in apoptotic induction were found to be repressed or mutated in CML cells such as the tumor suppressor gene p53 [131], the pro-apoptotic members BIM [104] and BAD [132] or members of the anti-apoptotic BCL2 family [132,133,134,135] like BCL2, Bcl-xL and MCL1. BCR-ABL is a potent cell death inhibitor and was demonstrated to promote CML progression by preventing formation of the caspase-9 apoptosome [136].
Targeting the BCL6/p53 axis could be one option, as BCL6 represses p53. This was achieved on primary human CD34+/CD38− CML stem cells using a peptide inhibitor of BCL6 (RI-BPI) [19]. TKI resistance was also described to be associated with a STAT1-dependent upregulation of BCL6 in CD34+ CML stem cells and a combination of TKIs with inhibitors of BCL6 (FX1) or MCL1 (A-1210477) is sufficient to overcome this resistance in LSCs [20].
During the last decade, many studies have addressed the potential of targeting BCL2 family members in combination with one TKI, producing convincing results. The pan-BCL2 inhibitor Sabutoclax, associated with BCR-ABL inhibition with Dasatinib, rendered BM-resident blast crisis LSCs, sensitive to the TKI, suggesting a possible elimination of dormant LSCs [21]. Similarly, the BCL2/Bcl-xL inhibitor ABT-737 associated with Imatinib demonstrated strong synergism to induce cell death of both proliferating and quiescent CD34+/CD38− TKI-insensitive CML cell populations [22,23]. In two other studies, a cell death response was triggered on primary CML CD34+ quiescent cells by a triple combination associating ABT-737 to block BCL2 and Bcl-xL, Nilotinib to inhibit BCR-ABL and a MDM2 inhibitor such as Nutlin3a [24] or DS-5272 [25] to restore p53 activation.
Association of the BH3 mimetic ABT-199 (Venetoclax), a more specific BCL-2 inhibitor, with either Imatinib [26] or Nilotinib [27] synergistically induced death of quiescent stem/progenitor primary CML CD34+ cells. Furthermore, the association of Dasatinib with ABT-199/Venetoclax will be evaluated on a phase II clinical trial (NCT02689440) that is recently recruiting. A tri-therapy combining Ponatinib, Venetoclax and Dexamethasone will also be tested in the NCT03576547 trial. Interestingly, Imatinib combined with BCL-2 antisense oligodeoxynucleotide (Oblimersen) was evaluated in a phase II clinical trial (NCT00049192).
3.2.4. Role of Autophagy in CML Resistances
Autophagy enables cells to degrade and recycle their intracellular components in lysosomes, to avoid cell death, being a protective cellular response that occurs under stress conditions and in particular during nutrient depletion [137].
The first evidence of a possible role of autophagy in CML initiation and progression came with the observation that an increased autophagic response occurred in primary CML cells in response to Imatinib and that co-treatment with inhibitors (Chloroquine, Bafilomycin A1) of late stage autophagy, resulted in the elimination of phenotypically and functionally defined CML stem cells [9,28]. Since, numerous studies have demonstrated that chloroquine and other lysosomal inhibitors induce LSCs elimination [39,138]. Furthermore, the combination of hydroxychloroquine with Imatinib is being evaluated in a clinical trial (NCT01227135).
It was then reported that autophagy genes from the ATG family, such as beclin1 and atg5, are overexpressed in CD34+ cells from chronic-phase CML patients [139] and could be repressed by miR-30a, whose expression decreased after Imatinib treatment [15]. This suggests that the TKI treatment favors a protective autophagic process, arguing in favor of the blockade of this process to render the TKI treatment fully efficient. Carella et al. have thus demonstrated that the combination of TKIs with the antibiotic Clarithromycin, that induced accumulation of LC3-II without affecting mTOR or AKT pathways, shows remarkable responses in high-risk and advanced CML-patients [30]. Spautin-1, an autophagy inhibitor which acts on Beclin-1 expression after inactivation of VSP34-AKT complexes, was shown to enhance Imatinib-induced primary CML cells apoptosis [31]. BCR-ABL was reported to control autophagy through PI3K/AKT/FOXO4 signaling, upregulating ATF5 expression that, in turn, stimulates mTOR transcription [140]. In this context, the PI3K/AKT/FOXO4/mTOR pathway was suggested as a druggable axis for TKI-resistant CML patients [140]. The mTOR pathway has been targeted in the clinical trials by combining Imatinib with Everolimus (NCT00093639) and Temsorilimus (NCT00101088) respectively. The PI3K/mTOR inhibitor BEZ235 used as a monotherapy, or in combination with Chloroquine, induced apoptosis in TKI-unresponsive primary CML patients’ cells [55].
Interestingly, other signaling pathways involved in the regulation of CML-LSCs self-renewal such as the Sonic Hedgehog pathway (Shh) [56] and BMI1 [92], were also reported to implicate autophagy modulation. Pharmacological inhibition of the Shh pathway by the Smo antagonist Vismodegib (GDC-0449) was associated with a markedly induced autophagy of CML cells whose simultaneous inhibition potently killed Imatinib-sensitive and -resistant BCR-ABL+ cells [56]. In addition, Vismodegib combined with Ponatinib, eliminated therapy-resistant T315I BCR-ABL cells [32]. The other Shh inhibitor PF04449913 associated with Dasatinib in a clinical trial did not exert toxicity on normal cells [141] (NCT00953758).
More recently, two second generation autophagy inhibitors, Lys05 a highly potent lysosometric agent and PIK-III a selective inhibitor of VPS34, demonstrated synergistic effects with TKIs to reduce primary CML LSCs quiescence, compared to the first-generation autophagy inhibitor Hydroxychloroquine [33].
One can note that autophagy inhibition could have beneficial roles on other CML treatments. The plant phytoalexin Resveratrol was notably reported to induce autophagic cell death in primary CML-CD34+ [57]. It was also reported by our group that the PRC1 component protein BMI1, that plays a key role in LSC self-renewal, prevented a protective CCNG2-dependent autophagy mechanism, that likely represents a means to support CML progression to the acute phase. Inhibition of BMI1 by the pharmacological drug PTC-209, synergized with Imatinib to strongly decrease the clonogenic properties of CD34+ CML cells [92].
It is well known that limited oxygen availability in the microenvironment generates a gradient of oxygen from the blood vessel to hypoxic areas which regulates mTOR that in turn inhibits autophagy [142]. In this context, Ianniciello et al., blocked autophagy using the Vps34 inhibitor in both healthy and CML-CD34+ and demonstrated that only healthy cells retained their capacity to proliferate after leaving hypoxia-induced quiescence, highlighting that autophagy is required for CD34+ CML cells [143].
3.2.5. Influence of the Microenvironment on CML Resistance
The microenvironment plays a major supportive role in hematological malignancies, during leukemogenesis, but also in the appearance of resistance and relapse. The medullary microenvironment represents a complex set of vascular, nerve, immune cells, that shape not only the structure and the plasticity of the BM but also its functions. In particular, interactions of HSCs with the BM niche are fundamental to both maintain the stem cell reservoir and to produce cells for all blood lineages after asymmetric division [144]. Similarly, preservation of quiescent CML-LSCs by the BMM is essential [145] and alterations in the microenvironmental regulation by leukemic cells can play a part in the modification of the CML-LSCs response to TKIs [146].
The therapeutic efficacy of interferon-α (IFN-α) treatment was early explained by the normalization of the interactions between CML progenitors and their BMM [147] in part after restoration of a β1 integrin-mediated inhibition of hematopoietic progenitor proliferation by BM [148]. IFN-α was then clinically tested in combination with Imatinib in a phase II clinical trial (NCT00045422).
The homing of BCR-ABL+ LSCs/progenitors to recipient marrow requires numerous physical interactions. CD44 expression was reported to be increased in BCR-ABL+ stem/progenitor cells, and by engaging functional E-selectin ligands, to improve homing and engraftment of leukemic cells [149]. Furthermore, destruction of selectin ligands by neuraminidase on leukemic progenitors reduced their engraftment [150].
Wnt/β-catenin signaling from the BMM was also shown to contribute to the preservation of CML-LSCs after TKI treatment [151]. Because secretion of Wnt ligands requires their modification by the O-acyl transferase Porcupine (PORCN), the effects of the selective PORCN inhibitor WNT974 were investigated. WNT974 efficiently blocked the Wnt pathway in primary human CML cells and in association with Nilotinib, strongly decreased the colony-forming potential of CML stem/progenitors [35]. The novel Wnt/β-catenin signaling inhibitor PRI-724, associated with Nilotinib synergistically killed TKI-resistant primary BC-CML cells even under leukemia/mesenchymal stromal cells (MSCs) co-culture conditions [36]. Besides, another mechanism of resistance of CML-LSCs to TKI treatment by MSCs was identified through N-cadherin receptors and Wnt-β-catenin signaling [152]. It was reported that a paracrine loop promoted self-renewal of LSCs, by stabilizing β-catenin, involving the overexpressed AXL tyrosine kinase receptor on primary CML CD34+ cells and its ligand Gas6 secreted by MSCs [37]. In this context, pharmacological inhibition of AXL by the small molecules XL880 or R428, in combination with Nilotinib, strongly inhibited the growth of LSCs [37].
The bone morphogenetic protein (BMP) pathway represents another level of control of the BMM on LSCs with an over production of BMP2 and BMP4 that contributes to the dysregulation of intracellular BMP signaling in primary CP-CML samples [153]. In contrast to patients in complete cytogenetic remission, LSCs and progenitors from TKI-resistant patients displayed higher levels of BMPR1b expression and the mesenchymal cells of TKI-resistant patients increased BMP4 production, depicting a BMP autocrine loop that could account for the resistance to TKI treatment [154]. These results suggest that new pharmacological molecules against the BMP pathway could target CML-LSCs in their niche.
The CXCR4/CXCL12 axis provides survival-enhancing traits to myeloid progenitor cells and was suspected of playing a similar function in CML [155]. A high level expression of CXCR4 and of CXCL12 was notably reported in CML cells and mesenchymal stromal cells respectively [34]. Moreover, Imatinib or Nilotinib treatments of BCR-ABL+ cells induced an increased surface expression of CXCR4 [38]. Disrupting the CXCR4/CXCL12 axis by the selective CXCR4 antagonists Plerifaxor (ADM3100) [34,38,156] or BTK140 [40] lead to increase of leukemic cells’ sensitivity to TKIs. It was recently reported that CML-LSCs express the cytokine-targeting surface enzyme dipeptidylpeptidase-IV (DPPIV/CD26) that can disrupt SDF-1/CXCR4 interaction accounting for the extramedullary spread of CML-LSCs [157]. However, the specific CD26 inhibitor Vildagliptin in combination with Nilotinib did not reveal any significant cooperative effect on engraftment of CML cells in NSG mice [158].
Paracrine communication within the BMM is also crucial. For instance, BCR-ABL induced interleukin-6 (IL6) expression by BM stromal cells that in turn sustains CML development in engrafted mice [159]. This IL6 production can be the result of reprogrammed stromal cells in response to leukemic cells [160] and is suspected to decrease the self-renewal of normal progenitors. Welner et al. demonstrated that a specific IL6-blocking antibody of protected human CD34+ cells when exposed to CML cells and that this approach might be an effective therapy in drug-resistant CML [161].
In line with this observation, Kuepper et al. reported that IL6 could be a trigger of a JAK1-STA3 signaling pathway activation leading to the persistence of CML-LSCs; in this context, the use of JAK1 specific inhibitors (Filgotinib and Itacitinib) in combination with BCR-ABL inhibition induced apoptosis in quiescent CML-LSCs [41]. Other recent studies performed on isolated BM-derived mesenchymal stem cells (MSCs) from CML patients, showed that interleukin-7 (IL7) is highly secreted by MSCs from Imatinib-resistant patients compared to responsive patients, and that IL7 elicited Imatinib and Nilotinib resistance via a BCR-ABL independent activation of JAK1/STAT5 signaling. This opens the way for treatments combining TKIs with IL7/JAK1/STAT5 inhibitors to manage CML [162]. In this context, direct or indirect inhibition of STAT5 has been a subject of great interest. The flavonoid-like chemical compound Wogonin potentiated the inhibitory effect of Imatinib on leukemia development by suppressing STAT5 pathway in primary CML CD34+ cells [42]. Prost et al. used peroxysome proliferator-activated receptor-γ (PPARγ) agonists from the glitazone family and observed a marked disappearance of the CML-LSC pool after a decrease of STAT5 expression. Importantly, three patients receiving pioglitazone achieved sustained CMR, for up to 4.7 years [163]; NCT02767063). Two other phase II clinical trials have evaluated the efficiency of pioglitazone with Imatinib (NCT02687425, NCT02730195). Another study specified that pioglitazone could be combined with any of the first-, second- or third-generation TKIs to be effective against progenitor cells from both early and advanced stages CML [164].
The BMM presents a complex gradient of oxygen distribution, that differentially impacts both HSCs and LSCs [165]. LSCs are well adapted to this environment and can balance between self-renewal in low oxygen zones near the endosteal niche or engage in differentiation close to the vascular niches. The hypoxia-inducible transcription factor HIF1α that triggers an adaptive response to low oxygen concentration is required for the maintenance and survival of CML-LSCs [166]. Furthermore, physiologic hypoxia promotes maintenance of CML-LSCs independently of BCR-ABL activity [167], suggesting that targeting HIF1α in combination with a TKI could lead to complete eradication of LSCs.
3.2.6. Functional Cross-Talks between the Microenvironment and Immunotherapy in CML
CML development is associated with an alteration of immune responses [168] and a better knowledge in the immunological composition of the CML BMM led to the design of a novel risk stratification model predicting patient’s response to TKI [169]. High-risk CML patients were shown to have an increased number of regulatory T cells (Treg) [170], and to highly express myeloid-derived suppressor cell (MDSC)-associated arginase 1 that can inhibit T-cell function [171]. Furthermore, as CD34+/CD38− human CML cells express high levels of the CD25/IL2 receptor, inhibition of IL2 synthesis by cyclosporine in combination with Dasatinib was tested in a phase I clinical trial with the hope to eliminate CD25+ LSCs (NCT01426334).
On the other hand, CD34+ LSCs from CML patients were shown to express programmed death receptor ligand 1 (PD-L1) at diagnosis [171] whose expression was further upregulated in response to IFN-γ which could represent an interesting opportunity for T-cell immunotherapy of CML [172]. This is why some recent clinical trials are evaluating the benefit to block the PD-1/PD-L1 interaction by anti-PD-L1 antibodies on CML patients, either in combination with pioglitazone (Avelumab, NCT02767063) or Imatinib, Nilotinib or Dasatinib (Pembrolizumab, NCT03516279).
As for numerous cancer types, expression of MHC-II antigens was shown to be decreased on CML cells [58] which can participate in an immune evasion of CML cells. Tarafdar et al. have demonstrated that IFN-γ treatment of CML stem/progenitor cells induced the re-expression of MHC-II antigens, that were down-regulated by JAK1/2-mediated signals from either CML cells and/or by the CML-modified microenvironment. This suggests that associating IFN-γ with the JAK1/2 inhibitor Ruxolitinib (RUX) could favor potential immunomodulatory-based therapies [58].
Three clinical trials associating RUX and TKIs are currently in recruiting phase (NCT03654768, NCT01751425, NCT01914484) and one is terminated with encouraging results ([173], NCT01702064).
Before the use of TKIs, IFN-α treatment was the first-line therapy for CML, with some evidences of associated-immune activation particularly at the level of NK-cell cytotoxicity [174,175]. Burchert et al. found that IFN-α therapy was associated with an increased expression of leukemia-associated antigen proteinase-3 suggesting that the induction of proteinase-3-specific CTLs may contribute to sustained remission after Imatinib discontinuation [176].
Long time after being replaced by TKIs, IFN-α aroused renewed interest in CML therapy as witnessed by numerous clinical trials combining this cytokine with TKIs that show promising results [177,178]: Imatinib/IFN-α (NCT00045422; NCT00573378), Nilotinib/IFN-α (NCT01657604; NCT00573378), Dasatinib/IFN-α NCT01725204) and Bosutinib/IFN-α (NCT03831776).
3.2.7. Tumor Microenvironment Impacts CML Cell’s Metabolism
LSCs from hematologic disorders establish narrow links with their stroma as a way to regulate their metabolism and redox state which are both crucial for leukemogenesis as described previously [179]. CML cells in their stromal microenvironment express specific metabolic features that represent opportunities for new treatment approaches, some of which are already being tested in clinical trials.
Earlier, it was reported that CML patients either sensitive or resistant to Imatinib showed heterogenous metabolic responses compared to their normal counterparts [180]. More recently, using liquid chromatography spectrometric metabolite profiling coupled with a multivariate statistical method, Karlikova et al. identified modifications of the metabolome of CML patients treated with TKIs, highlighting major changes in glycolysis, the citric acid cycle and amino acid metabolism [181]. Furthermore, they observed differences in the levels of amino acids and acylcarnitines between Imatinib-responder and non-responder CML patients [181]. Hattori et al. reported, in human and mouse CML models, that progression of the disease depended on a highly activated and functional expression of BCAT1, a cytosolic aminotransferase for branched-chain amino acids (BCAAs) [182]. Interestingly, eradication of LSCs has been demonstrated after targeting the amino acid metabolism in AML [183] but this LSC metabolic vulnerability has yet to be demonstrated in CML.
Besides AA metabolism, glycolysis, citric acid cycle and oxidative phosphorylation constitute numerous alternative therapies for CML. Indeed, it was shown that primary CD34+ CML cells depend on upregulated metabolism for their survival as demonstrated by stable isotope-assisted metabolomics and functional assays, and that the combination of Imatinib with the antibiotic tigecycline, that inhibits mitochondrial protein translation, selectively eliminates CML-LSCs [43]. SIRT1, as mentioned earlier, contributes to CML-LSCs maintenance and TKI resistance. Abraham et al. explained, by using the PGC-1α inhibitor SR18292, that the transcriptional coactivator and SIRT1-substrate, PGC-1α mediated these biological aspects by increasing oxidative phosphorylation [14]. In the same study, SR18292 strongly reduced oxygen consumption rate (OCR) on CML CD34+ without affecting extracellular acidification rate (ECAR) and combination with Nilotinib highly increased apoptosis and reduced clonogenicity of the leukemic cells [14].
Autophagy can also be connected to metabolism in CML cells. Another antibiotic, Ivermectin, well known for inducing autophagy by interfering with the Akt/mTOR pathway in other cancers [184], was found to induce caspase-dependent apoptosis of CML cells following induction of mitochondrial dysfunction and oxidative stress by respiratory complex I inhibition. This molecule, in combination either with Imatinib or Dasatinib, increased primary CML CD34+ cell death [44]. In addition, Karvela et al. demonstrated that pharmacological inhibition of autophagy or downregulation of ATG7, induced a decrease in glycolysis, an increase in oxidative phosphorylation and mitochondrial ROS accumulation thus highlighting ATG7 as a potent therapeutic target in CML [59].
Besides the BMM, Ye et al. reported that a subpopulation of LSCs expressing CD36 fatty acid transporter can use gonadal adipose tissue (GAT) as an extra-medullary niche to fuel their metabolism and evade chemotherapy [185].
Interestingly, BCL2 inhibition was demonstrated to reduce oxidative phosphorylation and selectively eradicate in AML-LSCs [186]. The previously described efficacy of BCL2 inhibition [25] in CML could be partially explained by this mechanism.
3.2.8. Targeting Signaling Pathways Connected to Key Leukemic Stem Cells Features
Self-renewal of LSCs is regulated by multiple pathways such as the Wnt, Shh, BMI1 pathways and is highly dependent on the tumor microenvironment as previously described. Blocking this fundamental stem cell function through inhibition of these pathways is an obvious strategy. There has been an accumulation of studies showing a strong role of Wnt/β-catenin in blastic transformation [187], in survival of BCR-ABL leukemic cells [188] and in self-renewal of CML-LSCs [189]. Interestingly, CML-LSCs were shown to express CD27 that responds to the tumor necrosis factor (TNF) family ligand CD70 to increase expression of Wnt target genes [190]. Furthermore, because expression of CD70 is restricted to activated lymphocytes and dendritic cells its engagement could account for an adaptive immune response to leukemia progression [190]. More recently, Riether et al. demonstrated that TKIs induced the expression of CD70 in LSCs triggering CD27 signaling in a cell-autonomous and/or paracrine manner. The blockade of CD70 by a specific antibody combined with Imatinib effectively eliminated human CD34+ CML progenitor/stem cells [45]. Despite all these possibilities, the only pharmacological approach tested on primary CML CD34+ cells are the selective PORCN inhibitor, WNT974 described above [35].
Smoothened (Smo), a component of the Hedgehog (Shh) pathway involved in LSCs self-renewal, was considered as a promising target to eliminate LSCs despite its important role in the maintenance of normal HSCs [191]. Indeed, two different studies demonstrated that targeting the Shh pathway may provide some benefits. It was first observed that overexpression of miR-326 downregulated Smo to induce an elevated rate of apoptosis in CML CD34+ cells [60]. In addition, inhibition of Shh by the Smo antagonist LDE225 (Sonidegib) in combination with Imatinib, reduced the self-renewal capacity of CD34+ CP-CML and their engraftment in NSG mice, without affecting HSCs [46]. Sonidegib was also combined with Nilotinib in the treatment of chronic or accelerated phase in patients who developed resistance to first-line therapy (NCT01456676). Besides, the specific targeting of Smo has been evaluated in clinical trials in combination with Dasatinib (BMS-833923; NCT01218477 and NCT01357655) (PF04449913; NCT00953758).
BCR-ABL controls many signaling pathways some of which are essential for resistance to TKI-induced apoptosis: PI3K/AKT [192], JAK/STAT [193,194], or Ras/MEK/ERK [195] and represent therefore good secondary targetable candidates to reach a cure for CML [196].
AKT can inhibit FOXO-mediated transcription of genes required for apoptosis such as Bim [197]. More precisely, Naka et al. describe that FOXO3a plays an essential role in the maintenance of CML-LSCs in a CML-like myeloproliferative disease mouse model [47] and that TGF-β activates AKT in LSCs by controlling FOXO3a localization [47]. Furthermore, the association of the TGF-β inhibitors, Ly364947 with Imatinib [47] or EW-7197 with Imatinib, Dasatinib or Ponatinib [11] was efficient to respectively decrease the maintenance of LSCs and to eliminate CML leukemia-initiating cells.
As discussed above in the autophagy section, the clinically well tolerated mTOR inhibitor RAD001 was used to block Imatinib-induced AKT activation that mediates survival during the early phase of Imatinib resistance [198].
Janus kinase 2 (JAK2) has recently emerged as an attractive target to improve TKI treatment in CML as demonstrated in-vitro [199]. Moreover, the JAK2 inhibitor BMS-911543 selectively targets CML-LSCs without affecting healthy progenitor/stem cells and its association with Dasatinib effectively eliminated primary TKI-insensitive CML-LSCs [48]. The other JAK2 inhibitor RUX was successfully combined with Nilotinib to lead to a strong reduction in primitive quiescent CML stem cells [50].
The Ras/MEK/ERK pathway can be targeted at different levels and many molecules have been tested in combination with TKIs. Farnesyl transferase inhibitors such as BMS-214662 (that inactivate Ras by inhibiting its recruitment to the plasma membrane) have been associated with Imatinib in a phase I clinical trial (Zarnestra; NCT00040105 and Lonafarnib; NCT00047502). MEK could also be a potential therapeutic target, as its specific MEK inhibitor PD184352, when combined with BMS-214662 increased apoptosis in TKI-unresponsive CD34+ CML cells [61]. Lonafernib that inhibits Raf was evaluated in a phase I clinical trial (NCT00047502). Imatinib was also tested with the dual Raf/VEGFR inhibitor Sorafenib in a phase II clinical trial (NCT00661180) and with the VEGFR inhibitor Vatalanib in a phase I/II study (NCT00088231). Grb2 is a potent activator of ERK1 and ERK2 in Ph+ leukemic cells [200], and the use of BP1001, a liposome-packaged Grb2 antisense oligonucleotide is well tolerated in CML patients [201] and is evaluated in combination with Dasatinib in a phase I/II clinical trial (NCT02923986).
Niclosamide, an FDA-approved anthelmintic drug, inhibits the overexpressed ERK/MNK1/eIF4E pathway which makes BP-CML more sensitive to Dasatinib [49]. The MNK inhibitor CGP57380 was as efficient as Dasatinib to impair LSCs function [62]. Activation of β-catenin was linked to CML drug resistance through BCR-ABL impaired binding to GSK3β (glycogen synthase kinase 3β). The specific GSK3 inhibitor SB216763, associated with Imatinib led to an almost complete suppression of primary CML progenitors/stem cells but failed to do so when combined with Dasatinib [202].
The dysregulation of the serine/threonine Aurora kinases (A, B and C) activity is well known to induce chromosome instability and aberrant mitosis in hematologic malignancies [203]. Targeting this pathway in CML, and particularly in TKI-resistant patients, emerged quite early. In this line, the pan-Aurora Kinase inhibitor MK-0457 (VX-680), has been investigated for the treatment of resistant CML patients harboring the BCR-ABL mutation T315I with encouraging results at a dose that did not induce side effects [204] while 44% of the patients had hematological responses [205], and cytogenetic responses were observed in 14% of patients in a phase II study ([206]; NCT00111683). A phase I study also evaluated the combination of Dasatinib and MK-0457 on CML patients (NCT00500006).
On the other hand, the arachidonic acid (AA)-leukotriene (LT) pathway has been demonstrated to be implicated in the LSCs survival. Alox15, that encodes for the 15-lipoxygenase (15-LO), has been shown to play an essential role in the functional regulation of CML-LSCs and specific inhibition of 15-LO by PD146176 leads to human CML CD34+ death with a synergy when combined either with imatinib or nilotinib [51]. Among the products of 15-LO, LTB4 can stimulate BLT2 receptor whose expression is significantly increased in CML CD34+ stem/progenitors, and inhibition of this pathway by the BLT2-specific inhibitor LY255283 induced apoptosis and inhibited self-renewal capacity of CD34+ cells from TKI-resistant BP-CML patients [63].
4. Conclusions
CML has benefited from three generations of TKIs, which have transformed the leukemia into a perfectly controlled disease but for which interruption of the monotherapy is often associated with relapse due to persisting LSCs.
Numerous studies have identified molecular and functional dysregulations in LSCs as well as specific relationships between CML cells and their medullary tissue environment, which can also be involved in the emergence of TKI resistance. The development of molecules targeting these different situations has frequently demonstrated that it was possible to improve the sensitivity of LSCs to TKIs supporting the development of combinatorial treatments. Association of TKI with other drugs recently emerged as stronger solutions to eliminate LSCs that in some cases are being investigated in clinical trials.
Thus, in the past two decades, studies have first shown that it may be interesting to target epigenetic and apoptotic functions. Numerous ex-vivo studies have also described the autophagic survival process as a potential target leading to LSCs eradication that should soon be tested in clinical trials. The most recent insights concern metabolic differences between LSCs and HSCs, like mitochondrial bioenergetics and indirect or direct targeting of the mitochondrial respiratory chain have already been tested on primary CML cells and need to be evaluated in clinical investigations.
Other new strategies aimed at targeting the tumor microenvironment, mostly inspired from ex-vivo experiments, have already made their way to clinical trials. The emerging success of immunotherapies targeting immune checkpoints in other cancers will probably be an additional option to improve the efficacy of TKIs in CML.
Recent technological advances are allowing new studies deciphering the heterogeneity between cancer cells and the establishment of predictive models for TKIs response. Current developments in single-cell transcriptomic analysis identified a blast-crisis specific stem cell population resistant to a TKI treatment [207].
NGS is now the gold standard method to detect early occurrences of kinase domain mutations, that helps to predict relapses [208,209].
In parallel, Single Cell Analysis is a promising emerging approach to identify genome-scale molecular information at the single cell level. By combining transcriptomic and proteomic analyses, Abraham et al. showed, at the CML patient level the therapeutic potential of RG7112/7388 (HDM2 inhibitors) and CPI-203/0610 (BET inhibitors) combination to target p53 and c-MYC respectively in LSC maintenance [64].
With up-to-date technologies, the fundamental knowledge about the mechanisms which govern LSCs response to TKIs according to individuals will improve. We can then expect in the near future that multi-drug regimens, developed around TKIs, can be proposed to tackle the different molecular or cellular pathways associated with TKI resistance that have been described here, aiming ultimately at the development of personalized treatments.
Abbreviations
| 15-LO | 15-Lipoxygenase |
| 4E-BP1 | Eukaryotic translation initiation factor 4E-binding protein 1 |
| A-loop | Activation-loop |
| AA | Arachidonic acid |
| ABC | ATP-binding cassette |
| ABCB1/MCR1 | Multidrug resistance protein 1/Permeability glycoprotein |
| ABCC1/MRP1 | Multidrug resistance-associated protein 1 |
| ABCG2/BCRP | ATP-binding cassette super-family G member 2 |
| AKT/PKB | Protein kinase B |
| AML | Acute Myeloid Leukemia |
| AMP | Adenosine Monophosphate |
| AMPK | AMP-activated protein kinase |
| AP | Adenomatous Polyposis Coli |
| Ara-C | Cytarabine |
| ATG | Autophagy related genes |
| ATP | Adenosine Tri-Phosphate |
| AXL | Tyrosine-protein kinase receptor UFO |
| BCL-2 | B-cell lymphoma 2 |
| BCL6 | B-cell lymphoma 6 |
| Bcl-xL | B-cell lymphoma-extra large |
| BCR-ABL | Breakpoint Cluster Region-Abelson murine leukemia viral oncogene homolog 1 |
| BET | Bromodomain Extra-Terminal motif |
| BH3 | Bcl-2 homology domain 3 |
| BIM | Bcl-2-like protein 11 |
| BLT2 | Leukotriene B4 receptor 2 |
| BM | Bone Marrow |
| BMI1 | B cell-specific Moloney murine leukemia virus integration site 1 |
| BMM | Bone marrow microenvironment |
| BMP | Bone morphogenetic proteins |
| BP-CML | Blastic phase chronic myeloid leukemia |
| c-ABL | Abelson murine leukemia viral oncogene homolog 1 |
| C/EBPβ | CCAAT/Enhancer-binding Protein beta |
| CBL | Casitas B-lineage Lymphoma |
| CCNG2 | Cyclin G2 |
| CCR | Complete Cytogenetic Remission |
| CHR | Complete Hematological Remission |
| CITED2 | Cbp/p300-interacting transactivator 2 |
| CpN | Cyclophilin |
| CML | Chronic Myeloid Leukemia |
| CQ | Chloroquine |
| CRK | CT10 Regulator of Kinase |
| CRKL | Crk-like protein |
| CsA | Cyclosporine |
| CXCL12 | C-X-C motif chemokine 12 |
| CXCR4 | C-X-C chemokine receptor type 4 |
| Cyt | Cytokines |
| CytR | Cytokine receptors |
| DNA | Deoxyribonucleic acid |
| DVL | Dishevelled |
| ECAR | Extracellular Acidification Rate |
| EIF4E | Eukaryotic translation initiation factor 4E |
| ERK | Extracellular signal-regulated kinases |
| ETC | Electron Transport Chain |
| EZH2 | Enhancer Of Zeste 2 Polycomb Repressive Complex 2 Subunit |
| FOXO | Forkhead box protein O |
| GAB2 | GRB2-associated-binding protein 2 |
| GAS6 | Growth arrest–specific 6 |
| GLI1 | Glioma-associated oncogene homologue 1 |
| GRB2 | Growth factor receptor-bound protein 2 |
| GSK-3β | Glycogen Synthase Kinase 3 Beta |
| H3K27me3 | Histone H3 trimethylase activity at lysine 27 |
| HCQ | Hydroxychloroquine |
| HIF-2α | Hypoxia-inducible factor-2 alpha |
| HDAC | Histone deacetylase |
| HSC | Hematopoietic Stem Cell |
| IFN | Interferon |
| IL | Interleukin |
| ILR | Interleukin Receptor |
| JAK | Janus-family tyrosine kinase |
| LC3 | microtubule-associated protein 1 light chain 3 |
| LIC | Leukemia-Initiating Cells |
| LRP5/6 | Low density lipoprotein receptor-related protein 5/6 |
| LSC | Leukemic Stem Cell |
| LT | Leukotriene |
| LTB4 | Leukotriene B4 |
| MCL-1 | induced myeloid leukemia cell differentiation protein |
| MCR | Major Cytogenetic Remission |
| MDR | Multidrug Resistance |
| MDM2 | Mouse double minute 2 homolog |
| MEK | Mitogen-activated protein kinase kinase; |
| MHC | Major histocompability complex |
| miRNA | Micro ribo nucleic acid; |
| MNK | MAP kinase-interacting serine/threonine-protein kinase |
| MPT | Mitochondrial protein translation |
| mTOR | mechanistic Target Of Rapamycin |
| NFAT | Nuclear factor of activated T-cells |
| NSG (NOD/SCID) | Nonobese diabetic/severe combined immunodeficient |
| OCR | Oxygen Consumption Rate |
| P-loop | Phosphate-binding loop |
| PAK1 | Serine/threonine-protein kinase 1 |
| PD-1 | Programmed cell death 1 |
| PD-L1 | Programmed death-ligand 1 |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PI3K-III | Phosphoinositide 3-kinase class III |
| PORCN | Porcupine O-acyltransferase |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| PRC | Protein regulator of cytokinesis |
| PTCH | Protein patched homolog 1 |
| RAF | Rapidly accelerated fibrosarcoma kinases |
| RPS6 | Ribosomal protein S6 |
| SH2/SH3 | SRC Homology 2/3 Domain |
| SHC | SH2-contaltining collagen-related proteins |
| SHH | Sonic Hedgehog |
| SIRT1 | Sirtuin 1 |
| SMO | Smoothened |
| SOS | Son Of Sevenless guanine nucleotide exchange factor |
| STAT | Signal transducer and activator of transcription; |
| TCR | T cell receptor |
| TGF-β1 | Transforming growth factor beta 1 |
| TGF-βR | abrogated transforming growth factor beta receptor |
| TKI | Tyrosine Kinase Inhibitor |
| TNF | Tumor Necrosis Factor |
| Ub | Ubiquitin |
| ULK1 | unc-51 like autophagy activating kinase 1 |
| VEGF | Vascular endothelial growth factor |
| VEGFR | Vascular endothelial growth factor receptor |
| WNT | Wingless-related integration site |
Funding
This work was supported in part by FiLMC-Pfizer grant and by INSERM. Fabien Muselli has been supported by a grant from the French Ministry of Research through Université Côte d’Azur and a 4th year PhD grant from the Ligue Nationale Contre le Cancer (LNCC).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Cilloni D., Saglio G. Molecular pathways: BCR-ABL. Clin. Cancer Res. 2012;18:930–937. doi: 10.1158/1078-0432.CCR-10-1613. [DOI] [PubMed] [Google Scholar]
- 2.Stone R.M. Optimizing treatment of chronic myeloid leukemia: A rational approach. Oncologist. 2004;9:259–270. doi: 10.1634/theoncologist.9-3-259. [DOI] [PubMed] [Google Scholar]
- 3.Druker B.J., Talpaz M., Resta D.J., Peng B., Buchdunger E., Ford J.M., Lydon N.B., Kantarjian H., Capdeville R., Ohno-Jones S., et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N. Engl. J. Med. 2001;344:1031–1037. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 4.Kaleem B., Shahab S., Ahmed N., Shamsi T.S. Chronic Myeloid Leukemia--Prognostic Value of Mutations. Asian Pac. J. Cancer Prev. 2015;16:7415–7423. doi: 10.7314/APJCP.2015.16.17.7415. [DOI] [PubMed] [Google Scholar]
- 5.Caldemeyer L., Dugan M., Edwards J., Akard L. Long-Term Side Effects of Tyrosine Kinase Inhibitors in Chronic Myeloid Leukemia. Curr. Hematol. Malig. Rep. 2016;11:71–79. doi: 10.1007/s11899-016-0309-2. [DOI] [PubMed] [Google Scholar]
- 6.Mahon F.X., Rea D., Guilhot J., Guilhot F., Huguet F., Nicolini F., Legros L., Charbonnier A., Guerci A., Varet B., et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: The prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11:1029–1035. doi: 10.1016/S1470-2045(10)70233-3. [DOI] [PubMed] [Google Scholar]
- 7.Eide C.A., Zabriskie M.S., Savage Stevens S.L., Antelope O., Vellore N.A., Than H., Schultz A.R., Clair P., Bowler A.D., Pomicter A.D., et al. Combining the Allosteric Inhibitor Asciminib with Ponatinib Suppresses Emergence of and Restores Efficacy against Highly Resistant BCR-ABL1 Mutants. Cancer Cell. 2019;36:431–443 e435. doi: 10.1016/j.ccell.2019.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott M.T., Korfi K., Saffrey P., Hopcroft L.E., Kinstrie R., Pellicano F., Guenther C., Gallipoli P., Cruz M., Dunn K., et al. Epigenetic Reprogramming Sensitizes CML Stem Cells to Combined EZH2 and Tyrosine Kinase Inhibition. Cancer Discov. 2016;6:1248–1257. doi: 10.1158/2159-8290.CD-16-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellodi C., Lidonnici M.R., Hamilton A., Helgason G.V., Soliera A.R., Ronchetti M., Galavotti S., Young K.W., Selmi T., Yacobi R., et al. Targeting autophagy potentiates tyrosine kinase inhibitor-induced cell death in Philadelphia chromosome-positive cells, including primary CML stem cells. J. Clin. Investig. 2009;119:1109–1123. doi: 10.1172/JCI35660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiskus W., Pranpat M., Balasis M., Bali P., Estrella V., Kumaraswamy S., Rao R., Rocha K., Herger B., Lee F., et al. Cotreatment with vorinostat (suberoylanilide hydroxamic acid) enhances activity of dasatinib (BMS-354825) against imatinib mesylate-sensitive or imatinib mesylate-resistant chronic myelogenous leukemia cells. Clin. Cancer Res. 2006;12:5869–5878. doi: 10.1158/1078-0432.CCR-06-0980. [DOI] [PubMed] [Google Scholar]
- 11.Naka K., Ishihara K., Jomen Y., Jin C.H., Kim D.H., Gu Y.K., Jeong E.S., Li S., Krause D.S., Kim D.W., et al. Novel oral transforming growth factor-beta signaling inhibitor EW-7197 eradicates CML-initiating cells. Cancer Sci. 2016;107:140–148. doi: 10.1111/cas.12849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cervera E., Candelaria M., Lopez-Navarro O., Labardini J., Gonzalez-Fierro A., Taja-Chayeb L., Cortes J., Gordillo-Bastidas D., Duenas-Gonzalez A. Epigenetic therapy with hydralazine and magnesium valproate reverses imatinib resistance in patients with chronic myeloid leukemia. Clin. Lymphoma Myeloma Leuk. 2012;12:207–212. doi: 10.1016/j.clml.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Li L., Wang L., Li L., Wang Z., Ho Y., McDonald T., Holyoake T.L., Chen W., Bhatia R. Activation of p53 by SIRT1 inhibition enhances elimination of CML leukemia stem cells in combination with imatinib. Cancer Cell. 2012;21:266–281. doi: 10.1016/j.ccr.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abraham A., Qiu S., Chacko B.K., Li H., Paterson A., He J., Agarwal P., Shah M., Welner R., Darley-Usmar V.M., et al. SIRT1 regulates metabolism and leukemogenic potential in CML stem cells. J. Clin. Investig. 2019;130:2685–2701. doi: 10.1172/JCI127080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu Y., Yang L., Zhao M., Zhu S., Kang R., Vernon P., Tang D., Cao L. Targeting microRNA-30a-mediated autophagy enhances imatinib activity against human chronic myeloid leukemia cells. Leukemia. 2012;26:1752–1760. doi: 10.1038/leu.2012.65. [DOI] [PubMed] [Google Scholar]
- 16.Deng Y., Li X., Feng J., Zhang X. Overexpression of miR-202 resensitizes imatinib resistant chronic myeloid leukemia cells through targetting Hexokinase 2. Biosci. Rep. 2018;38 doi: 10.1042/BSR20171383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L.S., Li L., Li L., Chu S., Shiang K.D., Li M., Sun H.Y., Xu J., Xiao F.J., Sun G., et al. MicroRNA-486 regulates normal erythropoiesis and enhances growth and modulates drug response in CML progenitors. Blood. 2015;125:1302–1313. doi: 10.1182/blood-2014-06-581926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tu Y.X., Wang S.B., Fu L.Q., Li S.S., Guo Q.P., Wu Y., Mou X.Z., Tong X.M. Ovatodiolide targets chronic myeloid leukemia stem cells by epigenetically upregulating hsa-miR-155, suppressing the BCR-ABL fusion gene and dysregulating the PI3K/AKT/mTOR pathway. Oncotarget. 2018;9:3267–3277. doi: 10.18632/oncotarget.23231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hurtz C., Hatzi K., Cerchietti L., Braig M., Park E., Kim Y.M., Herzog S., Ramezani-Rad P., Jumaa H., Muller M.C., et al. BCL6-mediated repression of p53 is critical for leukemia stem cell survival in chronic myeloid leukemia. J. Exp. Med. 2011;208:2163–2174. doi: 10.1084/jem.20110304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madapura H.S., Nagy N., Ujvari D., Kallas T., Krohnke M.C.L., Amu S., Bjorkholm M., Stenke L., Mandal P.K., McMurray J.S., et al. Interferon gamma is a STAT1-dependent direct inducer of BCL6 expression in imatinib-treated chronic myeloid leukemia cells. Oncogene. 2017;36:4619–4628. doi: 10.1038/onc.2017.85. [DOI] [PubMed] [Google Scholar]
- 21.Goff D.J., Court Recart A., Sadarangani A., Chun H.J., Barrett C.L., Krajewska M., Leu H., Low-Marchelli J., Ma W., Shih A.Y., et al. A Pan-BCL2 inhibitor renders bone-marrow-resident human leukemia stem cells sensitive to tyrosine kinase inhibition. Cell Stem Cell. 2013;12:316–328. doi: 10.1016/j.stem.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mak D.H., Wang R.Y., Schober W.D., Konopleva M., Cortes J., Kantarjian H., Andreeff M., Carter B.Z. Activation of apoptosis signaling eliminates CD34+ progenitor cells in blast crisis CML independent of response to tyrosine kinase inhibitors. Leukemia. 2012;26:788–794. doi: 10.1038/leu.2011.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Airiau K., Mahon F.X., Josselin M., Jeanneteau M., Turcq B., Belloc F. ABT-737 increases tyrosine kinase inhibitor-induced apoptosis in chronic myeloid leukemia cells through XIAP downregulation and sensitizes CD34+ CD38(-) population to imatinib. Exp. Hematol. 2012;40:367–378.e362. doi: 10.1016/j.exphem.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Carter B.Z., Mak P.Y., Mak D.H., Ruvolo V.R., Schober W., McQueen T., Cortes J., Kantarjian H.M., Champlin R.E., Konopleva M., et al. Synergistic effects of p53 activation via MDM2 inhibition in combination with inhibition of Bcl-2 or Bcr-Abl in CD34+ proliferating and quiescent chronic myeloid leukemia blast crisis cells. Oncotarget. 2015;6:30487–30499. doi: 10.18632/oncotarget.5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carter B.Z., Mak P.Y., Mu H., Wang X., Tao W., Mak D.H., Dettman E.J., Cardone M., Zernovak O., Seki T., et al. Combined inhibition of MDM2 and Bcr-Abl tyrosine kinase targets chronic myeloid leukemia stem/progenitor cells in a murine model. Haematologica. 2019 doi: 10.3324/haematol.2019.219261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ko T.K., Chuah C.T., Huang J.W., Ng K.P., Ong S.T. The BCL2 inhibitor ABT-199 significantly enhances imatinib-induced cell death in chronic myeloid leukemia progenitors. Oncotarget. 2014;5:9033–9038. doi: 10.18632/oncotarget.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carter B.Z., Mak P.Y., Mu H., Zhou H., Mak D.H., Schober W., Leverson J.D., Zhang B., Bhatia R., Huang X., et al. Combined targeting of BCL-2 and BCR-ABL tyrosine kinase eradicates chronic myeloid leukemia stem cells. Sci. Transl. Med. 2016;8:355ra117. doi: 10.1126/scitranslmed.aag1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calabretta B., Salomoni P. Inhibition of autophagy: A new strategy to enhance sensitivity of chronic myeloid leukemia stem cells to tyrosine kinase inhibitors. Leuk. Lymphoma. 2011;52:54–59. doi: 10.3109/10428194.2010.546913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pena M.C.R., Pardini M.I.M.C., Colturato V.A.R., Pinheiro N.A. Methylation status of the SOCS 1 and JUNB genes in chronic myeloid leukemia patients. Rev. Bras. Hematol. Hemoter. 2009;31:147–152. doi: 10.1590/S1516-84842009005000050. [DOI] [Google Scholar]
- 30.Carella A.M., Beltrami G., Catania G., Pica G., Ghiggi C., Garuti A., Carella A. Inhibition of autophagy with clarithromycin: A new strategy to enhance sensitivity of CML stem cells to tyrosine kinase inhibitors. Leuk. Suppl. 2012;1:S49–S50. doi: 10.1038/leusup.2012.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shao S., Li S., Qin Y., Wang X., Yang Y., Bai H., Zhou L., Zhao C., Wang C. Spautin-1, a novel autophagy inhibitor, enhances imatinib-induced apoptosis in chronic myeloid leukemia. Int. J. Oncol. 2014;44:1661–1668. doi: 10.3892/ijo.2014.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katagiri S., Tauchi T., Okabe S., Minami Y., Kimura S., Maekawa T., Naoe T., Ohyashiki K. Combination of ponatinib with Hedgehog antagonist vismodegib for therapy-resistant BCR-ABL1-positive leukemia. Clin. Cancer Res. 2013;19:1422–1432. doi: 10.1158/1078-0432.CCR-12-1777. [DOI] [PubMed] [Google Scholar]
- 33.Baquero P., Dawson A., Mukhopadhyay A., Kuntz E.M., Mitchell R., Olivares O., Ianniciello A., Scott M.T., Dunn K., Nicastri M.C., et al. Targeting quiescent leukemic stem cells using second generation autophagy inhibitors. Leukemia. 2019;33:981–994. doi: 10.1038/s41375-018-0252-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vianello F., Villanova F., Tisato V., Lymperi S., Ho K.K., Gomes A.R., Marin D., Bonnet D., Apperley J., Lam E.W., et al. Bone marrow mesenchymal stromal cells non-selectively protect chronic myeloid leukemia cells from imatinib-induced apoptosis via the CXCR4/CXCL12 axis. Haematologica. 2010;95:1081–1089. doi: 10.3324/haematol.2009.017178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agarwal P., Zhang B., Ho Y., Cook A., Li L., Mikhail F.M., Wang Y., McLaughlin M.E., Bhatia R. Enhanced targeting of CML stem and progenitor cells by inhibition of porcupine acyltransferase in combination with TKI. Blood. 2017;129:1008–1020. doi: 10.1182/blood-2016-05-714089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou H., Mak P.Y., Mu H., Mak D.H., Zeng Z., Cortes J., Liu Q., Andreeff M., Carter B.Z. Combined inhibition of beta-catenin and Bcr-Abl synergistically targets tyrosine kinase inhibitor-resistant blast crisis chronic myeloid leukemia blasts and progenitors in vitro and in vivo. Leukemia. 2017;31:2065–2074. doi: 10.1038/leu.2017.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin Y., Nie D., Li J., Du X., Lu Y., Li Y., Liu C., Zhou J., Pan J. Gas6/AXL Signaling Regulates Self-Renewal of Chronic Myelogenous Leukemia Stem Cells by Stabilizing beta-Catenin. Clin. Cancer Res. 2017;23:2842–2855. doi: 10.1158/1078-0432.CCR-16-1298. [DOI] [PubMed] [Google Scholar]
- 38.Dillmann F., Veldwijk M.R., Laufs S., Sperandio M., Calandra G., Wenz F., Zeller J., Fruehauf S. Plerixafor inhibits chemotaxis toward SDF-1 and CXCR4-mediated stroma contact in a dose-dependent manner resulting in increased susceptibility of BCR-ABL+ cell to Imatinib and Nilotinib. Leuk. Lymphoma. 2009;50:1676–1686. doi: 10.1080/10428190903150847. [DOI] [PubMed] [Google Scholar]
- 39.Helgason G.V., Karvela M., Holyoake T.L. Kill one bird with two stones: Potential efficacy of BCR-ABL and autophagy inhibition in CML. Blood. 2011;118:2035–2043. doi: 10.1182/blood-2011-01-330621. [DOI] [PubMed] [Google Scholar]
- 40.Beider K., Darash-Yahana M., Blaier O., Koren-Michowitz M., Abraham M., Wald H., Wald O., Galun E., Eizenberg O., Peled A., et al. Combination of imatinib with CXCR4 antagonist BKT140 overcomes the protective effect of stroma and targets CML in vitro and in vivo. Mol. Cancer. 2014;13:1155–1169. doi: 10.1158/1535-7163.MCT-13-0410. [DOI] [PubMed] [Google Scholar]
- 41.Kuepper M.K., Butow M., Herrmann O., Ziemons J., Chatain N., Maurer A., Kirschner M., Maie T., Costa I.G., Eschweiler J., et al. Stem cell persistence in CML is mediated by extrinsically activated JAK1-STAT3 signaling. Leukemia. 2019;33:1964–1977. doi: 10.1038/s41375-019-0427-7. [DOI] [PubMed] [Google Scholar]
- 42.Xu X., Zhang X., Liu Y., Yang L., Huang S., Lu L., Wang S., Guo Q., Zhao L. BM microenvironmental protection of CML cells from imatinib through Stat5/NF-kappaB signaling and reversal by Wogonin. Oncotarget. 2016;7:24436–24454. doi: 10.18632/oncotarget.8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuntz E.M., Baquero P., Michie A.M., Dunn K., Tardito S., Holyoake T.L., Helgason G.V., Gottlieb E. Targeting mitochondrial oxidative phosphorylation eradicates therapy-resistant chronic myeloid leukemia stem cells. Nat. Med. 2017;23:1234–1240. doi: 10.1038/nm.4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang J., Xu Y., Wan H., Hu J. Antibiotic ivermectin selectively induces apoptosis in chronic myeloid leukemia through inducing mitochondrial dysfunction and oxidative stress. Biochem. Biophys. Res. Commun. 2018;497:241–247. doi: 10.1016/j.bbrc.2018.02.063. [DOI] [PubMed] [Google Scholar]
- 45.Riether C., Schurch C.M., Flury C., Hinterbrandner M., Druck L., Huguenin A.L., Baerlocher G.M., Radpour R., Ochsenbein A.F. Tyrosine kinase inhibitor-induced CD70 expression mediates drug resistance in leukemia stem cells by activating Wnt signaling. Sci. Transl. Med. 2015;7:298ra119. doi: 10.1126/scitranslmed.aab1740. [DOI] [PubMed] [Google Scholar]
- 46.Irvine D.A., Zhang B., Kinstrie R., Tarafdar A., Morrison H., Campbell V.L., Moka H.A., Ho Y., Nixon C., Manley P.W., et al. Deregulated hedgehog pathway signaling is inhibited by the smoothened antagonist LDE225 (Sonidegib) in chronic phase chronic myeloid leukaemia. Sci. Rep. 2016;6:25476. doi: 10.1038/srep25476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Naka K., Hoshii T., Muraguchi T., Tadokoro Y., Ooshio T., Kondo Y., Nakao S., Motoyama N., Hirao A. TGF-beta-FOXO signalling maintains leukaemia-initiating cells in chronic myeloid leukaemia. Nature. 2010;463:676–680. doi: 10.1038/nature08734. [DOI] [PubMed] [Google Scholar]
- 48.Lin H., Chen M., Rothe K., Lorenzi M.V., Woolfson A., Jiang X. Selective JAK2/ABL dual inhibition therapy effectively eliminates TKI-insensitive CML stem/progenitor cells. Oncotarget. 2014;5:8637–8650. doi: 10.18632/oncotarget.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Z., Li Y., Lv C., Wang L., Song H. Anthelmintic drug niclosamide enhances the sensitivity of chronic myeloid leukemia cells to dasatinib through inhibiting Erk/Mnk1/eIF4E pathway. Biochem. Biophys. Res. Commun. 2016;478:893–899. doi: 10.1016/j.bbrc.2016.08.047. [DOI] [PubMed] [Google Scholar]
- 50.Gallipoli P., Cook A., Rhodes S., Hopcroft L., Wheadon H., Whetton A.D., Jorgensen H.G., Bhatia R., Holyoake T.L. JAK2/STAT5 inhibition by nilotinib with ruxolitinib contributes to the elimination of CML CD34+ cells in vitro and in vivo. Blood. 2014;124:1492–1501. doi: 10.1182/blood-2013-12-545640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen Y., Peng C., Abraham S.A., Shan Y., Guo Z., Desouza N., Cheloni G., Li D., Holyoake T.L., Li S. Arachidonate 15-lipoxygenase is required for chronic myeloid leukemia stem cell survival. J. Clin. Investig. 2014;124:3847–3862. doi: 10.1172/JCI66129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xie H., Peng C., Huang J., Li B.E., Kim W., Smith E.C., Fujiwara Y., Qi J., Cheloni G., Das P.P., et al. Chronic Myelogenous Leukemia- Initiating Cells Require Polycomb Group Protein EZH2. Cancer Discov. 2016;6:1237–1247. doi: 10.1158/2159-8290.CD-15-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rauzan M., Chuah C.T., Ko T.K., Ong S.T. The HDAC inhibitor SB939 overcomes resistance to BCR-ABL kinase Inhibitors conferred by the BIM deletion polymorphism in chronic myeloid leukemia. PLoS ONE. 2017;12:e0174107. doi: 10.1371/journal.pone.0174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jin Y., Zhou J., Xu F., Jin B., Cui L., Wang Y., Du X., Li J., Li P., Ren R., et al. Targeting methyltransferase PRMT5 eliminates leukemia stem cells in chronic myelogenous leukemia. J. Clin. Investig. 2016;126:3961–3980. doi: 10.1172/JCI85239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mitchell R., Hopcroft L.E.M., Baquero P., Allan E.K., Hewit K., James D., Hamilton G., Mukhopadhyay A., O’Prey J., Hair A., et al. Targeting BCR-ABL-Independent TKI Resistance in Chronic Myeloid Leukemia by mTOR and Autophagy Inhibition. J. Natl. Cancer Inst. 2018;110:467–478. doi: 10.1093/jnci/djx236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zeng X., Zhao H., Li Y., Fan J., Sun Y., Wang S., Wang Z., Song P., Ju D. Targeting Hedgehog signaling pathway and autophagy overcomes drug resistance of BCR-ABL-positive chronic myeloid leukemia. Autophagy. 2015;11:355–372. doi: 10.4161/15548627.2014.994368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Puissant A., Robert G., Fenouille N., Luciano F., Cassuto J.P., Raynaud S., Auberger P. Resveratrol promotes autophagic cell death in chronic myelogenous leukemia cells via JNK-mediated p62/SQSTM1 expression and AMPK activation. Cancer Res. 2010;70:1042–1052. doi: 10.1158/0008-5472.CAN-09-3537. [DOI] [PubMed] [Google Scholar]
- 58.Tarafdar A., Hopcroft L.E., Gallipoli P., Pellicano F., Cassels J., Hair A., Korfi K., Jorgensen H.G., Vetrie D., Holyoake T.L., et al. CML cells actively evade host immune surveillance through cytokine-mediated downregulation of MHC-II expression. Blood. 2017;129:199–208. doi: 10.1182/blood-2016-09-742049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karvela M., Baquero P., Kuntz E.M., Mukhopadhyay A., Mitchell R., Allan E.K., Chan E., Kranc K.R., Calabretta B., Salomoni P., et al. ATG7 regulates energy metabolism, differentiation and survival of Philadelphia-chromosome-positive cells. Autophagy. 2016;12:936–948. doi: 10.1080/15548627.2016.1162359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Babashah S., Sadeghizadeh M., Hajifathali A., Tavirani M.R., Zomorod M.S., Ghadiani M., Soleimani M. Targeting of the signal transducer Smo links microRNA-326 to the oncogenic Hedgehog pathway in CD34+ CML stem/progenitor cells. Int. J. Cancer. 2013;133:579–589. doi: 10.1002/ijc.28043. [DOI] [PubMed] [Google Scholar]
- 61.Pellicano F., Simara P., Sinclair A., Helgason G.V., Copland M., Grant S., Holyoake T.L. The MEK inhibitor PD184352 enhances BMS-214662-induced apoptosis in CD34+ CML stem/progenitor cells. Leukemia. 2011;25:1159–1167. doi: 10.1038/leu.2011.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lim S., Saw T.Y., Zhang M., Janes M.R., Nacro K., Hill J., Lim A.Q., Chang C.T., Fruman D.A., Rizzieri D.A., et al. Targeting of the MNK-eIF4E axis in blast crisis chronic myeloid leukemia inhibits leukemia stem cell function. Proc. Natl. Acad. Sci. USA. 2013;110:E2298–E2307. doi: 10.1073/pnas.1301838110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xiao M., Ai H., Li T., Rajoria P., Shahu P., Li X. Targeting of the BLT2 in chronic myeloid leukemia inhibits leukemia stem/progenitor cell function. Biochem. Biophys. Res. Commun. 2016;472:610–616. doi: 10.1016/j.bbrc.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 64.Abraham S.A., Hopcroft L.E., Carrick E., Drotar M.E., Dunn K., Williamson A.J., Korfi K., Baquero P., Park L.E., Scott M.T., et al. Dual targeting of p53 and c-MYC selectively eliminates leukaemic stem cells. Nature. 2016;534:341–346. doi: 10.1038/nature18288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bonnet D., Dick J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 66.Holyoake T., Jiang X., Eaves C., Eaves A. Isolation of a highly quiescent subpopulation of primitive leukemic cells in chronic myeloid leukemia. Blood. 1999;94:2056–2064. doi: 10.1182/blood.V94.6.2056. [DOI] [PubMed] [Google Scholar]
- 67.Uckun F.M., Sather H., Reaman G., Shuster J., Land V., Trigg M., Gunther R., Chelstrom L., Bleyer A., Gaynon P., et al. Leukemic cell growth in SCID mice as a predictor of relapse in high-risk B-lineage acute lymphoblastic leukemia. Blood. 1995;85:873–878. doi: 10.1182/blood.V85.4.873.bloodjournal854873. [DOI] [PubMed] [Google Scholar]
- 68.Al-Hajj M., Wicha M.S., Benito-Hernandez A., Morrison S.J., Clarke M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.O’Brien C.A., Pollett A., Gallinger S., Dick J.E. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 70.Singh S.K., Hawkins C., Clarke I.D., Squire J.A., Bayani J., Hide T., Henkelman R.M., Cusimano M.D., Dirks P.B. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 71.Hamilton A., Helgason G.V., Schemionek M., Zhang B., Myssina S., Allan E.K., Nicolini F.E., Muller-Tidow C., Bhatia R., Brunton V.G., et al. Chronic myeloid leukemia stem cells are not dependent on Bcr-Abl kinase activity for their survival. Blood. 2012;119:1501–1510. doi: 10.1182/blood-2010-12-326843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Corbin A.S., Agarwal A., Loriaux M., Cortes J., Deininger M.W., Druker B.J. Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity. J. Clin. Investig. 2011;121:396–409. doi: 10.1172/JCI35721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Clarke C.J., Holyoake T.L. Preclinical approaches in chronic myeloid leukemia: From cells to systems. Exp. Hematol. 2017;47:13–23. doi: 10.1016/j.exphem.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Milojkovic D., Apperley J. Mechanisms of Resistance to Imatinib and Second-Generation Tyrosine Inhibitors in Chronic Myeloid Leukemia. Clin. Cancer Res. 2009;15:7519–7527. doi: 10.1158/1078-0432.CCR-09-1068. [DOI] [PubMed] [Google Scholar]
- 75.Zabriskie M.S., Eide C.A., Tantravahi S.K., Vellore N.A., Estrada J., Nicolini F.E., Khoury H.J., Larson R.A., Konopleva M., Cortes J.E., et al. BCR-ABL1 compound mutations combining key kinase domain positions confer clinical resistance to ponatinib in Ph chromosome-positive leukemia. Cancer Cell. 2014;26:428–442. doi: 10.1016/j.ccr.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schoepfer J., Jahnke W., Berellini G., Buonamici S., Cotesta S., Cowan-Jacob S.W., Dodd S., Drueckes P., Fabbro D., Gabriel T., et al. Discovery of Asciminib (ABL001), an Allosteric Inhibitor of the Tyrosine Kinase Activity of BCR-ABL1. J. Med. Chem. 2018;61:8120–8135. doi: 10.1021/acs.jmedchem.8b01040. [DOI] [PubMed] [Google Scholar]
- 77.Barnes D.J., Palaiologou D., Panousopoulou E., Schultheis B., Yong A.S., Wong A., Pattacini L., Goldman J.M., Melo J.V. Bcr-Abl expression levels determine the rate of development of resistance to imatinib mesylate in chronic myeloid leukemia. Cancer Res. 2005;65:8912–8919. doi: 10.1158/0008-5472.CAN-05-0076. [DOI] [PubMed] [Google Scholar]
- 78.Sattler M., Verma S., Shrikhande G., Byrne C.H., Pride Y.B., Winkler T., Greenfield E.A., Salgia R., Griffin J.D. The BCR/ABL tyrosine kinase induces production of reactive oxygen species in hematopoietic cells. J. Biol. Chem. 2000;275:24273–24278. doi: 10.1074/jbc.M002094200. [DOI] [PubMed] [Google Scholar]
- 79.Kim J.H., Chu S.C., Gramlich J.L., Pride Y.B., Babendreier E., Chauhan D., Salgia R., Podar K., Griffin J.D., Sattler M. Activation of the PI3K/mTOR pathway by BCR-ABL contributes to increased production of reactive oxygen species. Blood. 2005;105:1717–1723. doi: 10.1182/blood-2004-03-0849. [DOI] [PubMed] [Google Scholar]
- 80.Burger H., van Tol H., Boersma A.W., Brok M., Wiemer E.A., Stoter G., Nooter K. Imatinib mesylate (STI571) is a substrate for the breast cancer resistance protein (BCRP)/ABCG2 drug pump. Blood. 2004;104:2940–2942. doi: 10.1182/blood-2004-04-1398. [DOI] [PubMed] [Google Scholar]
- 81.Breedveld P., Pluim D., Cipriani G., Wielinga P., van Tellingen O., Schinkel A.H., Schellens J.H. The effect of Bcrp1 (Abcg2) on the in vivo pharmacokinetics and brain penetration of imatinib mesylate (Gleevec): Implications for the use of breast cancer resistance protein and P-glycoprotein inhibitors to enable the brain penetration of imatinib in patients. Cancer Res. 2005;65:2577–2582. doi: 10.1158/0008-5472.CAN-04-2416. [DOI] [PubMed] [Google Scholar]
- 82.Dohse M., Scharenberg C., Shukla S., Robey R.W., Volkmann T., Deeken J.F., Brendel C., Ambudkar S.V., Neubauer A., Bates S.E. Comparison of ATP-binding cassette transporter interactions with the tyrosine kinase inhibitors imatinib, nilotinib, and dasatinib. Drug Metab. Dispos. 2010;38:1371–1380. doi: 10.1124/dmd.109.031302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang B., Strauss A.C., Chu S., Li M., Ho Y., Shiang K.D., Snyder D.S., Huettner C.S., Shultz L., Holyoake T., et al. Effective targeting of quiescent chronic myelogenous leukemia stem cells by histone deacetylase inhibitors in combination with imatinib mesylate. Cancer Cell. 2010;17:427–442. doi: 10.1016/j.ccr.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kinstrie R., Karamitros D., Goardon N., Morrison H., Hamblin M., Robinson L., Clark R.E., Copland M., Vyas P. Heterogeneous leukemia stem cells in myeloid blast phase chronic myeloid leukemia. Blood Adv. 2016;1:160–169. doi: 10.1182/bloodadvances.2016000810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Holyoake T.L., Vetrie D. The chronic myeloid leukemia stem cell: Stemming the tide of persistence. Blood. 2017;129:1595–1606. doi: 10.1182/blood-2016-09-696013. [DOI] [PubMed] [Google Scholar]
- 86.Koschmieder S., Vetrie D. Epigenetic dysregulation in chronic myeloid leukaemia: A myriad of mechanisms and therapeutic options. Semin. Cancer Biol. 2018;51:180–197. doi: 10.1016/j.semcancer.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 87.Simon J.A., Kingston R.E. Mechanisms of polycomb gene silencing: Knowns and unknowns. Nat. Rev. Mol. Cell Biol. 2009;10:697–708. doi: 10.1038/nrm2763. [DOI] [PubMed] [Google Scholar]
- 88.Laugesen A., Helin K. Chromatin repressive complexes in stem cells, development, and cancer. Cell Stem Cell. 2014;14:735–751. doi: 10.1016/j.stem.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 89.Lessard J., Sauvageau G. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature. 2003;423:255–260. doi: 10.1038/nature01572. [DOI] [PubMed] [Google Scholar]
- 90.Rizo A., Horton S.J., Olthof S., Dontje B., Ausema A., van Os R., van den Boom V., Vellenga E., de Haan G., Schuringa J.J. BMI1 collaborates with BCR-ABL in leukemic transformation of human CD34+ cells. Blood. 2010;116:4621–4630. doi: 10.1182/blood-2010-02-270660. [DOI] [PubMed] [Google Scholar]
- 91.Saudy N.S., Fawzy I.M., Azmy E., Goda E.F., Eneen A., Abdul Salam E.M. BMI1 gene expression in myeloid leukemias and its impact on prognosis. Blood Cells Mol. Dis. 2014;53:194–198. doi: 10.1016/j.bcmd.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 92.Mourgues L., Imbert V., Nebout M., Colosetti P., Neffati Z., Lagadec P., Verhoeyen E., Peng C., Duprez E., Legros L., et al. The BMI1 polycomb protein represses cyclin G2-induced autophagy to support proliferation in chronic myeloid leukemia cells. Leukemia. 2015;29:1993–2002. doi: 10.1038/leu.2015.112. [DOI] [PubMed] [Google Scholar]
- 93.Mohty M., Yong A.S., Szydlo R.M., Apperley J.F., Melo J.V. The polycomb group BMI1 gene is a molecular marker for predicting prognosis of chronic myeloid leukemia. Blood. 2007;110:380–383. doi: 10.1182/blood-2006-12-065599. [DOI] [PubMed] [Google Scholar]
- 94.Branford S., Wang P., Yeung D.T., Thomson D., Purins A., Wadham C., Shahrin N.H., Marum J.E., Nataren N., Parker W.T., et al. Integrative genomic analysis reveals cancer-associated mutations at diagnosis of CML in patients with high-risk disease. Blood. 2018;132:948–961. doi: 10.1182/blood-2018-02-832253. [DOI] [PubMed] [Google Scholar]
- 95.Boultwood J., Perry J., Zaman R., Fernandez-Santamaria C., Littlewood T., Kusec R., Pellagatti A., Wang L., Clark R.E., Wainscoat J.S. High-density single nucleotide polymorphism array analysis and ASXL1 gene mutation screening in chronic myeloid leukemia during disease progression. Leukemia. 2010;24:1139–1145. doi: 10.1038/leu.2010.65. [DOI] [PubMed] [Google Scholar]
- 96.Menezes J., Salgado R.N., Acquadro F., Gomez-Lopez G., Carralero M.C., Barroso A., Mercadillo F., Espinosa-Hevia L., Talavera-Casanas J.G., Pisano D.G., et al. ASXL1, TP53 and IKZF3 mutations are present in the chronic phase and blast crisis of chronic myeloid leukemia. Blood Cancer J. 2013;3:e157. doi: 10.1038/bcj.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yang H., Kurtenbach S., Guo Y., Lohse I., Durante M.A., Li J., Li Z., Al-Ali H., Li L., Chen Z., et al. Gain of function of ASXL1 truncating protein in the pathogenesis of myeloid malignancies. Blood. 2018;131:328–341. doi: 10.1182/blood-2017-06-789669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nimmanapalli R., Fuino L., Stobaugh C., Richon V., Bhalla K. Cotreatment with the histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA) enhances imatinib-induced apoptosis of Bcr-Abl-positive human acute leukemia cells. Blood. 2003;101:3236–3239. doi: 10.1182/blood-2002-08-2675. [DOI] [PubMed] [Google Scholar]
- 99.Nimmanapalli R., Fuino L., Bali P., Gasparetto M., Glozak M., Tao J., Moscinski L., Smith C., Wu J., Jove R., et al. Histone deacetylase inhibitor LAQ824 both lowers expression and promotes proteasomal degradation of Bcr-Abl and induces apoptosis of imatinib mesylate-sensitive or -refractory chronic myelogenous leukemia-blast crisis cells. Cancer Res. 2003;63:5126–5135. [PubMed] [Google Scholar]
- 100.Fiskus W., Pranpat M., Bali P., Balasis M., Kumaraswamy S., Boyapalle S., Rocha K., Wu J., Giles F., Manley P.W., et al. Combined effects of novel tyrosine kinase inhibitor AMN107 and histone deacetylase inhibitor LBH589 against Bcr-Abl-expressing human leukemia cells. Blood. 2006;108:645–652. doi: 10.1182/blood-2005-11-4639. [DOI] [PubMed] [Google Scholar]
- 101.Wang Z., Yuan H., Roth M., Stark J.M., Bhatia R., Chen W.Y. SIRT1 deacetylase promotes acquisition of genetic mutations for drug resistance in CML cells. Oncogene. 2013;32:589–598. doi: 10.1038/onc.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yuan H., Wang Z., Li L., Zhang H., Modi H., Horne D., Bhatia R., Chen W. Activation of stress response gene SIRT1 by BCR-ABL promotes leukemogenesis. Blood. 2012;119:1904–1914. doi: 10.1182/blood-2011-06-361691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Roman-Gomez J., Castillejo J.A., Jimenez A., Cervantes F., Boque C., Hermosin L., Leon A., Granena A., Colomer D., Heiniger A., et al. Cadherin-13, a mediator of calcium-dependent cell-cell adhesion, is silenced by methylation in chronic myeloid leukemia and correlates with pretreatment risk profile and cytogenetic response to interferon alfa. J. Clin. Oncol. 2003;21:1472–1479. doi: 10.1200/JCO.2003.08.166. [DOI] [PubMed] [Google Scholar]
- 104.San Jose-Eneriz E., Agirre X., Jimenez-Velasco A., Cordeu L., Martin V., Arqueros V., Garate L., Fresquet V., Cervantes F., Martinez-Climent J.A., et al. Epigenetic down-regulation of BIM expression is associated with reduced optimal responses to imatinib treatment in chronic myeloid leukaemia. Eur. J. Cancer. 2009;45:1877–1889. doi: 10.1016/j.ejca.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 105.Koh Y., Kim D.Y., Park S.H., Byun H.M., Kim I., Yoon S.S., Kim B.K., Park E., Yang A.S., Park S. Increased BCR promoter DNA methylation status strongly correlates with favorable response to imatinib in chronic myeloid leukemia patients. Oncol. Lett. 2011;2:181–187. doi: 10.3892/ol.2010.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu T.C., Lin S.F., Chang J.G., Yang M.Y., Hung S.Y., Chang C.S. Epigenetic alteration of the SOCS1 gene in chronic myeloid leukaemia. Br. J. Haematol. 2003;123:654–661. doi: 10.1046/j.1365-2141.2003.04660.x. [DOI] [PubMed] [Google Scholar]
- 107.Chen Q., Lin J., Qian J., Deng Z.Q., Qian W., Yang J., Li Y., Chen X.X., Ma Y.J., Ma J.C., et al. The methylation status of the DDX43 promoter in Chinese patients with chronic myeloid leukemia. Genet. Test. Mol. Biomark. 2013;17:508–511. doi: 10.1089/gtmb.2012.0530. [DOI] [PubMed] [Google Scholar]
- 108.Ahmad I., Mir R., Javid J., Farooq S., Yadav P., Zuberi M., Masroor M., Gupta N., Ray P.C., Saxena A. Epigenetic Silencing of P16 (INK4a) Gene by Promoter Hypermethylation in Chronic Myelogenous Leukemia. Clin. Lymphoma Myeloma Leuk. 2014;14:S139. doi: 10.1016/j.clml.2014.06.072. [DOI] [Google Scholar]
- 109.Nagy E., Beck Z., Kiss A., Csoma E., Telek B., Konya J., Olah E., Rak K., Toth F.D. Frequent methylation of p16INK4A and p14ARF genes implicated in the evolution of chronic myeloid leukaemia from its chronic to accelerated phase. Eur. J. Cancer. 2003;39:2298–2305. doi: 10.1016/S0959-8049(03)00552-5. [DOI] [PubMed] [Google Scholar]
- 110.Bodoor K., Haddad Y., Alkhateeb A., Al-Abbadi A., Dowairi M., Magableh A., Bsoul N., Ghabkari A. DNA hypermethylation of cell cycle (p15 and p16) and apoptotic (p14, p53, DAPK and TMS1) genes in peripheral blood of leukemia patients. Asian Pac. J. Cancer Prev. 2014;15:75–84. doi: 10.7314/APJCP.2014.15.1.75. [DOI] [PubMed] [Google Scholar]
- 111.Qian J., Wang Y.L., Lin J., Yao D.M., Xu W.R., Wu C.Y. Aberrant methylation of the death-associated protein kinase 1 (DAPK1) CpG island in chronic myeloid leukemia. Eur. J. Haematol. 2009;82:119–123. doi: 10.1111/j.1600-0609.2008.01178.x. [DOI] [PubMed] [Google Scholar]
- 112.Pehlivan M., Sercan Z., Sercan H.O. sFRP1 promoter methylation is associated with persistent Philadelphia chromosome in chronic myeloid leukemia. Leuk. Res. 2009;33:1062–1067. doi: 10.1016/j.leukres.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 113.Jelinek J., Gharibyan V., Estecio M.R., Kondo K., He R., Chung W., Lu Y., Zhang N., Liang S., Kantarjian H.M., et al. Aberrant DNA methylation is associated with disease progression, resistance to imatinib and shortened survival in chronic myelogenous leukemia. PLoS ONE. 2011;6:e22110. doi: 10.1371/journal.pone.0022110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Maupetit-Mehouas S., Court F., Bourgne C., Guerci-Bresler A., Cony-Makhoul P., Johnson H., Etienne G., Rousselot P., Guyotat D., Janel A., et al. DNA methylation profiling reveals a pathological signature that contributes to transcriptional defects of CD34(+) CD15(-) cells in early chronic-phase chronic myeloid leukemia. Mol. Oncol. 2018;12:814–829. doi: 10.1002/1878-0261.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Janssen J.J., Denkers F., Valk P., Cornelissen J.J., Schuurhuis G.J., Ossenkoppele G.J. Methylation patterns in CD34 positive chronic myeloid leukemia blast crisis cells. Haematologica. 2010;95:1036–1037. doi: 10.3324/haematol.2009.015693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Oki Y., Kantarjian H.M., Gharibyan V., Jones D., O’Brien S., Verstovsek S., Cortes J., Morris G.M., Garcia-Manero G., Issa J.P. Phase II study of low-dose decitabine in combination with imatinib mesylate in patients with accelerated or myeloid blastic phase of chronic myelogenous leukemia. Cancer. 2007;109:899–906. doi: 10.1002/cncr.22470. [DOI] [PubMed] [Google Scholar]
- 117.Pelechano V., Steinmetz L.M. Gene regulation by antisense transcription. Nat. Rev. Genet. 2013;14:880–893. doi: 10.1038/nrg3594. [DOI] [PubMed] [Google Scholar]
- 118.Lu Y., Li Y., Chai X., Kang Q., Zhao P., Xiong J., Wang J. Long noncoding RNA HULC promotes cell proliferation by regulating PI3K/AKT signaling pathway in chronic myeloid leukemia. Gene. 2017;607:41–46. doi: 10.1016/j.gene.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 119.Sun C., Luan S., Zhang G., Wang N., Shao H., Luan C. CEBPA-mediated upregulation of the lncRNA PLIN2 promotes the development of chronic myelogenous leukemia via the GSK3 and Wnt/beta-catenin signaling pathways. Am. J. Cancer Res. 2017;7:1054–1067. [PMC free article] [PubMed] [Google Scholar]
- 120.Guo G., Kang Q., Zhu X., Chen Q., Wang X., Chen Y., Ouyang J., Zhang L., Tan H., Chen R., et al. A long noncoding RNA critically regulates Bcr-Abl-mediated cellular transformation by acting as a competitive endogenous RNA. Oncogene. 2015;34:1768–1779. doi: 10.1038/onc.2014.131. [DOI] [PubMed] [Google Scholar]
- 121.Li Z., Yang L., Liu X., Nie Z., Luo J. Long noncoding RNA MEG3 inhibits proliferation of chronic myeloid leukemia cells by sponging microRNA21. Biomed. Pharm. 2018;104:181–192. doi: 10.1016/j.biopha.2018.05.047. [DOI] [PubMed] [Google Scholar]
- 122.Agirre X., Jimenez-Velasco A., San Jose-Eneriz E., Garate L., Bandres E., Cordeu L., Aparicio O., Saez B., Navarro G., Vilas-Zornoza A., et al. Down-regulation of hsa-miR-10a in chronic myeloid leukemia CD34+ cells increases USF2-mediated cell growth. Mol. Cancer Res. 2008;6:1830–1840. doi: 10.1158/1541-7786.MCR-08-0167. [DOI] [PubMed] [Google Scholar]
- 123.Flamant S., Ritchie W., Guilhot J., Holst J., Bonnet M.L., Chomel J.C., Guilhot F., Turhan A.G., Rasko J.E. Micro-RNA response to imatinib mesylate in patients with chronic myeloid leukemia. Haematologica. 2010;95:1325–1333. doi: 10.3324/haematol.2009.020636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Machova Polakova K., Lopotova T., Klamova H., Burda P., Trneny M., Stopka T., Moravcova J. Expression patterns of microRNAs associated with CML phases and their disease related targets. Mol. Cancer. 2011;10:41. doi: 10.1186/1476-4598-10-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhu X., Lin Z., Du J., Zhou X., Yang L., Liu G. Studies on microRNAs that are correlated with the cancer stem cells in chronic myeloid leukemia. Mol. Cell Biochem. 2014;390:75–84. doi: 10.1007/s11010-013-1958-2. [DOI] [PubMed] [Google Scholar]
- 126.Kotagama K., Chang Y., Mangone M. miRNAs as Biomarkers in Chronic Myelogenous Leukemia. Drug Dev. Res. 2015;76:278–285. doi: 10.1002/ddr.21266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.San Jose-Eneriz E., Roman-Gomez J., Jimenez-Velasco A., Garate L., Martin V., Cordeu L., Vilas-Zornoza A., Rodriguez-Otero P., Calasanz M.J., Prosper F., et al. MicroRNA expression profiling in Imatinib-resistant Chronic Myeloid Leukemia patients without clinically significant ABL1-mutations. Mol. Cancer. 2009;8:69. doi: 10.1186/1476-4598-8-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Salati S., Salvestrini V., Carretta C., Genovese E., Rontauroli S., Zini R., Rossi C., Ruberti S., Bianchi E., Barbieri G., et al. Deregulated expression of miR-29a-3p, miR-494-3p and miR-660-5p affects sensitivity to tyrosine kinase inhibitors in CML leukemic stem cells. Oncotarget. 2017;8:49451–49469. doi: 10.18632/oncotarget.17706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li Y., Yuan Y., Tao K., Wang X., Xiao Q., Huang Z., Zhong L., Cao W., Wen J., Feng W. Inhibition of BCR/ABL protein expression by miR-203 sensitizes for imatinib mesylate. PLoS ONE. 2013;8:e61858. doi: 10.1371/journal.pone.0061858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang B., Nguyen L.X.T., Li L., Zhao D., Kumar B., Wu H., Lin A., Pellicano F., Hopcroft L., Su Y.L., et al. Bone marrow niche trafficking of miR-126 controls the self-renewal of leukemia stem cells in chronic myelogenous leukemia. Nat. Med. 2018;24:450–462. doi: 10.1038/nm.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Di Bacco A., Keeshan K., McKenna S.L., Cotter T.G. Molecular abnormalities in chronic myeloid leukemia: Deregulation of cell growth and apoptosis. Oncologist. 2000;5:405–415. doi: 10.1634/theoncologist.5-5-405. [DOI] [PubMed] [Google Scholar]
- 132.Fernandez-Luna J.L. Bcr-Abl and inhibition of apoptosis in chronic myelogenous leukemia cells. Apoptosis. 2000;5:315–318. doi: 10.1023/A:1009623222534. [DOI] [PubMed] [Google Scholar]
- 133.Tzifi F., Economopoulou C., Gourgiotis D., Ardavanis A., Papageorgiou S., Scorilas A. The Role of BCL2 Family of Apoptosis Regulator Proteins in Acute and Chronic Leukemias. Adv. Hematol. 2012;2012 doi: 10.1155/2012/524308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Guillem V., Amat P., Collado M., Cervantes F., Alvarez-Larran A., Martinez J., Tormo E., Eroles P., Solano C., Hernandez-Boluda J.C. BCL2 gene polymorphisms and splicing variants in chronic myeloid leukemia. Leuk. Res. 2015;39:1278–1284. doi: 10.1016/j.leukres.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 135.Kim D.H., Xu W., Ma C., Liu X., Siminovitch K., Messner H.A., Lipton J.H. Genetic variants in the candidate genes of the apoptosis pathway and susceptibility to chronic myeloid leukemia. Blood. 2009;113:2517–2525. doi: 10.1182/blood-2008-07-169110. [DOI] [PubMed] [Google Scholar]
- 136.Deming P.B., Schafer Z.T., Tashker J.S., Potts M.B., Deshmukh M., Kornbluth S. Bcr-Abl-mediated protection from apoptosis downstream of mitochondrial cytochrome c release. Mol. Cell Biol. 2004;24:10289–10299. doi: 10.1128/MCB.24.23.10289-10299.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bento C.F., Renna M., Ghislat G., Puri C., Ashkenazi A., Vicinanza M., Menzies F.M., Rubinsztein D.C. Mammalian Autophagy: How Does It Work? Annu. Rev. Biochem. 2016;85:685–713. doi: 10.1146/annurev-biochem-060815-014556. [DOI] [PubMed] [Google Scholar]
- 138.Rothe K., Porter V., Jiang X. Current Outlook on Autophagy in Human Leukemia: Foe in Cancer Stem Cells and Drug Resistance, Friend in New Therapeutic Interventions. Int. J. Mol. Sci. 2019;20:461. doi: 10.3390/ijms20030461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rothe K., Lin H., Lin K.B., Leung A., Wang H.M., Malekesmaeili M., Brinkman R.R., Forrest D.L., Gorski S.M., Jiang X. The core autophagy protein ATG4B is a potential biomarker and therapeutic target in CML stem/progenitor cells. Blood. 2014;123:3622–3634. doi: 10.1182/blood-2013-07-516807. [DOI] [PubMed] [Google Scholar]
- 140.Sheng Z., Ma L., Sun J.E., Zhu L.J., Green M.R. BCR-ABL suppresses autophagy through ATF5-mediated regulation of mTOR transcription. Blood. 2011;118:2840–2848. doi: 10.1182/blood-2010-12-322537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Martinelli G., Oehler V.G., Papayannidis C., Courtney R., Shaik M.N., Zhang X., O’Connell A., McLachlan K.R., Zheng X., Radich J., et al. Treatment with PF-04449913, an oral smoothened antagonist, in patients with myeloid malignancies: A phase 1 safety and pharmacokinetics study. Lancet Haematol. 2015;2:e339–e346. doi: 10.1016/S2352-3026(15)00096-4. [DOI] [PubMed] [Google Scholar]
- 142.DeYoung M.P., Horak P., Sofer A., Sgroi D., Ellisen L.W. Hypoxia regulates TSC1/2-mTOR signaling and tumor suppression through REDD1-mediated 14-3-3 shuttling. Genes Dev. 2008;22:239–251. doi: 10.1101/gad.1617608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ianniciello A., Dumas P.Y., Drullion C., Guitart A., Villacreces A., Peytour Y., Chevaleyre J., Brunet de la Grange P., Vigon I., Desplat V., et al. Chronic myeloid leukemia progenitor cells require autophagy when leaving hypoxia-induced quiescence. Oncotarget. 2017;8:96984–96992. doi: 10.18632/oncotarget.18904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schepers K., Campbell T.B., Passegue E. Normal and leukemic stem cell niches: Insights and therapeutic opportunities. Cell Stem Cell. 2015;16:254–267. doi: 10.1016/j.stem.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shah M., Bhatia R. Preservation of Quiescent Chronic Myelogenous Leukemia Stem Cells by the Bone Marrow Microenvironment. Adv. Exp. Med. Biol. 2018;1100:97–110. doi: 10.1007/978-3-319-97746-1_6. [DOI] [PubMed] [Google Scholar]
- 146.Zhang B., Ho Y.W., Huang Q., Maeda T., Lin A., Lee S.U., Hair A., Holyoake T.L., Huettner C., Bhatia R. Altered microenvironmental regulation of leukemic and normal stem cells in chronic myelogenous leukemia. Cancer Cell. 2012;21:577–592. doi: 10.1016/j.ccr.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bhatia R., Wayner E.A., McGlave P.B., Verfaillie C.M. Interferon-alpha restores normal adhesion of chronic myelogenous leukemia hematopoietic progenitors to bone marrow stroma by correcting impaired beta 1 integrin receptor function. J. Clin. Investig. 1994;94:384–391. doi: 10.1172/JCI117333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bhatia R., McCarthy J.B., Verfaillie C.M. Interferon-alpha restores normal beta 1 integrin-mediated inhibition of hematopoietic progenitor proliferation by the marrow microenvironment in chronic myelogenous leukemia. Blood. 1996;87:3883–3891. doi: 10.1182/blood.V87.9.3883.bloodjournal8793883. [DOI] [PubMed] [Google Scholar]
- 149.Krause D.S., Lazarides K., von Andrian U.H., Van Etten R.A. Requirement for CD44 in homing and engraftment of BCR-ABL-expressing leukemic stem cells. Nat. Med. 2006;12:1175–1180. doi: 10.1038/nm1489. [DOI] [PubMed] [Google Scholar]
- 150.Krause D.S., Lazarides K., Lewis J.B., von Andrian U.H., Van Etten R.A. Selectins and their ligands are required for homing and engraftment of BCR-ABL1+ leukemic stem cells in the bone marrow niche. Blood. 2014;123:1361–1371. doi: 10.1182/blood-2013-11-538694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hu Y., Chen Y., Douglas L., Li S. beta-Catenin is essential for survival of leukemic stem cells insensitive to kinase inhibition in mice with BCR-ABL-induced chronic myeloid leukemia. Leukemia. 2009;23:109–116. doi: 10.1038/leu.2008.262. [DOI] [PubMed] [Google Scholar]
- 152.Zhang B., Li M., McDonald T., Holyoake T.L., Moon R.T., Campana D., Shultz L., Bhatia R. Microenvironmental protection of CML stem and progenitor cells from tyrosine kinase inhibitors through N-cadherin and Wnt-beta-catenin signaling. Blood. 2013;121:1824–1838. doi: 10.1182/blood-2012-02-412890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Laperrousaz B., Jeanpierre S., Sagorny K., Voeltzel T., Ramas S., Kaniewski B., Ffrench M., Salesse S., Nicolini F.E., Maguer-Satta V. Primitive CML cell expansion relies on abnormal levels of BMPs provided by the niche and on BMPRIb overexpression. Blood. 2013;122:3767–3777. doi: 10.1182/blood-2013-05-501460. [DOI] [PubMed] [Google Scholar]
- 154.Grockowiak E., Laperrousaz B., Jeanpierre S., Voeltzel T., Guyot B., Gobert S., Nicolini F.E., Maguer-Satta V. Immature CML cells implement a BMP autocrine loop to escape TKI treatment. Blood. 2017;130:2860–2871. doi: 10.1182/blood-2017-08-801019. [DOI] [PubMed] [Google Scholar]
- 155.Agarwal P., Isringhausen S., Li H., Paterson A.J., He J., Gomariz A., Nagasawa T., Nombela-Arrieta C., Bhatia R. Mesenchymal Niche-Specific Expression of Cxcl12 Controls Quiescence of Treatment-Resistant Leukemia Stem Cells. Cell Stem Cell. 2019;24:769–784.e766. doi: 10.1016/j.stem.2019.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Weisberg E., Azab A.K., Manley P.W., Kung A.L., Christie A.L., Bronson R., Ghobrial I.M., Griffin J.D. Inhibition of CXCR4 in CML cells disrupts their interaction with the bone marrow microenvironment and sensitizes them to nilotinib. Leukemia. 2012;26:985–990. doi: 10.1038/leu.2011.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Herrmann H., Sadovnik I., Cerny-Reiterer S., Rulicke T., Stefanzl G., Willmann M., Hoermann G., Bilban M., Blatt K., Herndlhofer S., et al. Dipeptidylpeptidase IV (CD26) defines leukemic stem cells (LSC) in chronic myeloid leukemia. Blood. 2014;123:3951–3962. doi: 10.1182/blood-2013-10-536078. [DOI] [PubMed] [Google Scholar]
- 158.Willmann M., Sadovnik I., Eisenwort G., Entner M., Bernthaler T., Stefanzl G., Hadzijusufovic E., Berger D., Herrmann H., Hoermann G., et al. Evaluation of cooperative antileukemic effects of nilotinib and vildagliptin in Ph(+) chronic myeloid leukemia. Exp. Hematol. 2018;57:50–59.e56. doi: 10.1016/j.exphem.2017.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Reynaud D., Pietras E., Barry-Holson K., Mir A., Binnewies M., Jeanne M., Sala-Torra O., Radich J.P., Passegue E. IL-6 controls leukemic multipotent progenitor cell fate and contributes to chronic myelogenous leukemia development. Cancer Cell. 2011;20:661–673. doi: 10.1016/j.ccr.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Schepers K., Pietras E.M., Reynaud D., Flach J., Binnewies M., Garg T., Wagers A.J., Hsiao E.C., Passegue E. Myeloproliferative neoplasia remodels the endosteal bone marrow niche into a self-reinforcing leukemic niche. Cell Stem Cell. 2013;13:285–299. doi: 10.1016/j.stem.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Welner R.S., Amabile G., Bararia D., Czibere A., Yang H., Zhang H., Pontes L.L., Ye M., Levantini E., Di Ruscio A., et al. Treatment of chronic myelogenous leukemia by blocking cytokine alterations found in normal stem and progenitor cells. Cancer Cell. 2015;27:671–681. doi: 10.1016/j.ccell.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhang X., Tu H., Yang Y., Jiang X., Hu X., Luo Q., Li J. Bone marrow-derived mesenchymal stromal cells promote resistance to tyrosine kinase inhibitors in chronic myeloid leukemia via the IL-7/JAK1/STAT5 pathway. J. Biol. Chem. 2019;294:12167–12179. doi: 10.1074/jbc.RA119.008037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Prost S., Relouzat F., Spentchian M., Ouzegdouh Y., Saliba J., Massonnet G., Beressi J.P., Verhoeyen E., Raggueneau V., Maneglier B., et al. Erosion of the chronic myeloid leukaemia stem cell pool by PPARgamma agonists. Nature. 2015;525:380–383. doi: 10.1038/nature15248. [DOI] [PubMed] [Google Scholar]
- 164.Glodkowska-Mrowka E., Manda-Handzlik A., Stelmaszczyk-Emmel A., Seferynska I., Stoklosa T., Przybylski J., Mrowka P. PPARgamma ligands increase antileukemic activity of second- and third-generation tyrosine kinase inhibitors in chronic myeloid leukemia cells. Blood Cancer J. 2016;6:e377. doi: 10.1038/bcj.2015.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhou H.S., Carter B.Z., Andreeff M. Bone marrow niche-mediated survival of leukemia stem cells in acute myeloid leukemia: Yin and Yang. Cancer Biol. Med. 2016;13:248–259. doi: 10.20892/j.issn.2095-3941.2016.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhang H., Li H., Xi H.S., Li S. HIF1alpha is required for survival maintenance of chronic myeloid leukemia stem cells. Blood. 2012;119:2595–2607. doi: 10.1182/blood-2011-10-387381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ng K.P., Manjeri A., Lee K.L., Huang W., Tan S.Y., Chuah C.T., Poellinger L., Ong S.T. Physiologic hypoxia promotes maintenance of CML stem cells despite effective BCR-ABL1 inhibition. Blood. 2014;123:3316–3326. doi: 10.1182/blood-2013-07-511907. [DOI] [PubMed] [Google Scholar]
- 168.Ilander M., Kreutzman A., Mustjoki S. IFNalpha induces prolonged remissions modeling curative immunologic responses in chronic myeloid leukemia. Oncoimmunology. 2014;3:e28781. doi: 10.4161/onci.28781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bruck O., Blom S., Dufva O., Turkki R., Chheda H., Ribeiro A., Kovanen P., Aittokallio T., Koskenvesa P., Kallioniemi O., et al. Immune cell contexture in the bone marrow tumor microenvironment impacts therapy response in CML. Leukemia. 2018;32:1643–1656. doi: 10.1038/s41375-018-0175-0. [DOI] [PubMed] [Google Scholar]
- 170.Bachy E., Bernaud J., Roy P., Rigal D., Nicolini F.E. Quantitative and functional analyses of CD4(+) CD25(+) FoxP3(+) regulatory T cells in chronic phase chronic myeloid leukaemia patients at diagnosis and on imatinib mesylate. Br. J. Haematol. 2011;153:139–143. doi: 10.1111/j.1365-2141.2010.08453.x. [DOI] [PubMed] [Google Scholar]
- 171.Christiansson L., Soderlund S., Svensson E., Mustjoki S., Bengtsson M., Simonsson B., Olsson-Stromberg U., Loskog A.S. Increased level of myeloid-derived suppressor cells, programmed death receptor ligand 1/programmed death receptor 1, and soluble CD25 in Sokal high risk chronic myeloid leukemia. PLoS ONE. 2013;8:e55818. doi: 10.1371/journal.pone.0055818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Riether C., Gschwend T., Huguenin A.L., Schurch C.M., Ochsenbein A.F. Blocking programmed cell death 1 in combination with adoptive cytotoxic T-cell transfer eradicates chronic myelogenous leukemia stem cells. Leukemia. 2015;29:1781–1785. doi: 10.1038/leu.2015.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sweet K., Hazlehurst L., Sahakian E., Powers J., Nodzon L., Kayali F., Hyland K., Nelson A., Pinilla-Ibarz J. A phase I clinical trial of ruxolitinib in combination with nilotinib in chronic myeloid leukemia patients with molecular evidence of disease. Leuk. Res. 2018;74:89–96. doi: 10.1016/j.leukres.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pawelec G., Da Silva P., Max H., Kalbacher H., Schmidt H., Bruserud O., Zugel U., Baier W., Rehbein A., Pohla H. Relative roles of natural killer- and T cell-mediated anti-leukemia effects in chronic myelogenous leukemia patients treated with interferon-alpha. Leuk. Lymphoma. 1995;18:471–478. doi: 10.3109/10428199509059647. [DOI] [PubMed] [Google Scholar]
- 175.de Castro F.A., Palma P.V., Morais F.R., Simoes B.P., Carvalho P.V., Ismael S.J., Lima C.P., Voltarelli J.C. Immunological effects of interferon-alpha on chronic myelogenous leukemia. Leuk. Lymphoma. 2003;44:2061–2067. doi: 10.1080/1042819031000110973. [DOI] [PubMed] [Google Scholar]
- 176.Burchert A., Muller M.C., Kostrewa P., Erben P., Bostel T., Liebler S., Hehlmann R., Neubauer A., Hochhaus A. Sustained molecular response with interferon alfa maintenance after induction therapy with imatinib plus interferon alfa in patients with chronic myeloid leukemia. J. Clin. Oncol. 2010;28:1429–1435. doi: 10.1200/JCO.2009.25.5075. [DOI] [PubMed] [Google Scholar]
- 177.Preudhomme C., Guilhot J., Nicolini F.E., Guerci-Bresler A., Rigal-Huguet F., Maloisel F., Coiteux V., Gardembas M., Berthou C., Vekhoff A., et al. Imatinib plus peginterferon alfa-2a in chronic myeloid leukemia. N. Engl. J. Med. 2010;363:2511–2521. doi: 10.1056/NEJMoa1004095. [DOI] [PubMed] [Google Scholar]
- 178.Simonsson B., Gedde-Dahl T., Markevarn B., Remes K., Stentoft J., Almqvist A., Bjoreman M., Flogegard M., Koskenvesa P., Lindblom A., et al. Combination of pegylated IFN-alpha2b with imatinib increases molecular response rates in patients with low- or intermediate-risk chronic myeloid leukemia. Blood. 2011;118:3228–3235. doi: 10.1182/blood-2011-02-336685. [DOI] [PubMed] [Google Scholar]
- 179.Nemkov T., D’Alessandro A., Reisz J.A. Metabolic underpinnings of leukemia pathology and treatment. Cancer Rep. 2019;2:e1139. doi: 10.1002/cnr2.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.A J., Qian S., Wang G., Yan B., Zhang S., Huang Q., Ni L., Zha W., Liu L., Cao B., et al. Chronic myeloid leukemia patients sensitive and resistant to imatinib treatment show different metabolic responses. PLoS ONE. 2010;5:e13186. doi: 10.1371/journal.pone.0013186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Karlikova R., Siroka J., Friedecky D., Faber E., Hrda M., Micova K., Fikarova I., Gardlo A., Janeckova H., Vrobel I., et al. Metabolite Profiling of the Plasma and Leukocytes of Chronic Myeloid Leukemia Patients. J. Proteome Res. 2016;15:3158–3166. doi: 10.1021/acs.jproteome.6b00356. [DOI] [PubMed] [Google Scholar]
- 182.Hattori A., Tsunoda M., Konuma T., Kobayashi M., Nagy T., Glushka J., Tayyari F., McSkimming D., Kannan N., Tojo A., et al. Cancer progression by reprogrammed BCAA metabolism in myeloid leukaemia. Nature. 2017;545:500–504. doi: 10.1038/nature22314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Jones C.L., Stevens B.M., D’Alessandro A., Reisz J.A., Culp-Hill R., Nemkov T., Pei S., Khan N., Adane B., Ye H., et al. Inhibition of Amino Acid Metabolism Selectively Targets Human Leukemia Stem Cells. Cancer Cell. 2018;34:724–740 e724. doi: 10.1016/j.ccell.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Dou Q., Chen H.N., Wang K., Yuan K., Lei Y., Li K., Lan J., Chen Y., Huang Z., Xie N., et al. Ivermectin Induces Cytostatic Autophagy by Blocking the PAK1/Akt Axis in Breast Cancer. Cancer Res. 2016;76:4457–4469. doi: 10.1158/0008-5472.CAN-15-2887. [DOI] [PubMed] [Google Scholar]
- 185.Ye H., Adane B., Khan N., Sullivan T., Minhajuddin M., Gasparetto M., Stevens B., Pei S., Balys M., Ashton J.M., et al. Leukemic Stem Cells Evade Chemotherapy by Metabolic Adaptation to an Adipose Tissue Niche. Cell Stem Cell. 2016;19:23–37. doi: 10.1016/j.stem.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lagadinou E.D., Sach A., Callahan K., Rossi R.M., Neering S.J., Minhajuddin M., Ashton J.M., Pei S., Grose V., O’Dwyer K.M., et al. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell. 2013;12:329–341. doi: 10.1016/j.stem.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hu J., Feng M., Liu Z.L., Liu Y., Huang Z.L., Li H., Feng W.L. Potential role of Wnt/beta-catenin signaling in blastic transformation of chronic myeloid leukemia: Cross talk between beta-catenin and BCR-ABL. Tumour Biol. 2016;39 doi: 10.1007/s13277-016-5413-3. [DOI] [PubMed] [Google Scholar]
- 188.Gregory M.A., Phang T.L., Neviani P., Alvarez-Calderon F., Eide C.A., O’Hare T., Zaberezhnyy V., Williams R.T., Druker B.J., Perrotti D., et al. Wnt/Ca2+/NFAT signaling maintains survival of Ph+ leukemia cells upon inhibition of Bcr-Abl. Cancer Cell. 2010;18:74–87. doi: 10.1016/j.ccr.2010.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zhao C., Blum J., Chen A., Kwon H.Y., Jung S.H., Cook J.M., Lagoo A., Reya T. Loss of beta-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell. 2007;12:528–541. doi: 10.1016/j.ccr.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Schurch C., Riether C., Matter M.S., Tzankov A., Ochsenbein A.F. CD27 signaling on chronic myelogenous leukemia stem cells activates Wnt target genes and promotes disease progression. J. Clin. Investig. 2012;122:624–638. doi: 10.1172/JCI45977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhao C., Chen A., Jamieson C.H., Fereshteh M., Abrahamsson A., Blum J., Kwon H.Y., Kim J., Chute J.P., Rizzieri D., et al. Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature. 2009;458:776–779. doi: 10.1038/nature07737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Jain S.K., Langdon W.Y., Varticovski L. Tyrosine phosphorylation of p120cbl in BCR/abl transformed hematopoietic cells mediates enhanced association with phosphatidylinositol 3-kinase. Oncogene. 1997;14:2217–2228. doi: 10.1038/sj.onc.1201049. [DOI] [PubMed] [Google Scholar]
- 193.Chai S.K., Nichols G.L., Rothman P. Constitutive activation of JAKs and STATs in BCR-Abl-expressing cell lines and peripheral blood cells derived from leukemic patients. J. Immunol. 1997;159:4720–4728. [PubMed] [Google Scholar]
- 194.de Groot R.P., Raaijmakers J.A., Lammers J.W., Koenderman L. STAT5-Dependent CyclinD1 and Bcl-xL expression in Bcr-Abl-transformed cells. Mol. Cell Biol. Res. Commun. 2000;3:299–305. doi: 10.1006/mcbr.2000.0231. [DOI] [PubMed] [Google Scholar]
- 195.McCubrey J.A., Steelman L.S., Abrams S.L., Bertrand F.E., Ludwig D.E., Basecke J., Libra M., Stivala F., Milella M., Tafuri A., et al. Targeting survival cascades induced by activation of Ras/Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways for effective leukemia therapy. Leukemia. 2008;22:708–722. doi: 10.1038/leu.2008.27. [DOI] [PubMed] [Google Scholar]
- 196.Danisz K., Blasiak J. Role of anti-apoptotic pathways activated by BCR/ABL in the resistance of chronic myeloid leukemia cells to tyrosine kinase inhibitors. Acta Biochim. Pol. 2013;60:503–514. doi: 10.18388/abp.2013_2014. [DOI] [PubMed] [Google Scholar]
- 197.Fu Z., Tindall D.J. FOXOs, cancer and regulation of apoptosis. Oncogene. 2008;27:2312–2319. doi: 10.1038/onc.2008.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Burchert A., Wang Y., Cai D., von Bubnoff N., Paschka P., Muller-Brusselbach S., Ottmann O.G., Duyster J., Hochhaus A., Neubauer A. Compensatory PI3-kinase/Akt/mTor activation regulates imatinib resistance development. Leukemia. 2005;19:1774–1782. doi: 10.1038/sj.leu.2403898. [DOI] [PubMed] [Google Scholar]
- 199.Samanta A., Perazzona B., Chakraborty S., Sun X., Modi H., Bhatia R., Priebe W., Arlinghaus R. Janus kinase 2 regulates Bcr-Abl signaling in chronic myeloid leukemia. Leukemia. 2011;25:463–472. doi: 10.1038/leu.2010.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Modi H., Li L., Chu S., Rossi J., Yee J.K., Bhatia R. Inhibition of Grb2 expression demonstrates an important role in BCR-ABL-mediated MAPK activation and transformation of primary human hematopoietic cells. Leukemia. 2011;25:305–312. doi: 10.1038/leu.2010.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ohanian M., Tari Ashizawa A., Garcia-Manero G., Pemmaraju N., Kadia T., Jabbour E., Ravandi F., Borthakur G., Andreeff M., Konopleva M., et al. Liposomal Grb2 antisense oligodeoxynucleotide (BP1001) in patients with refractory or relapsed haematological malignancies: A single-centre, open-label, dose-escalation, phase 1/1b trial. Lancet Haematol. 2018;5:e136–e146. doi: 10.1016/S2352-3026(18)30021-8. [DOI] [PubMed] [Google Scholar]
- 202.Reddiconto G., Toto C., Palama I., De Leo S., de Luca E., De Matteis S., Dini L., Passerini C.G., Di Renzo N., Maffia M., et al. Targeting of GSK3beta promotes imatinib-mediated apoptosis in quiescent CD34+ chronic myeloid leukemia progenitors, preserving normal stem cells. Blood. 2012;119:2335–2345. doi: 10.1182/blood-2011-06-361261. [DOI] [PubMed] [Google Scholar]
- 203.Li J.J., Li S.A. Mitotic kinases: The key to duplication, segregation, and cytokinesis errors, chromosomal instability, and oncogenesis. Pharmacol. Ther. 2006;111:974–984. doi: 10.1016/j.pharmthera.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 204.Giles F.J., Cortes J., Jones D., Bergstrom D., Kantarjian H., Freedman S.J. MK-0457, a novel kinase inhibitor, is active in patients with chronic myeloid leukemia or acute lymphocytic leukemia with the T315I BCR-ABL mutation. Blood. 2007;109:500–502. doi: 10.1182/blood-2006-05-025049. [DOI] [PubMed] [Google Scholar]
- 205.Giles F.J., Swords R.T., Nagler A., Hochhaus A., Ottmann O.G., Rizzieri D.A., Talpaz M., Clark J., Watson P., Xiao A., et al. MK-0457, an Aurora kinase and BCR-ABL inhibitor, is active in patients with BCR-ABL T315I leukemia. Leukemia. 2013;27:113–117. doi: 10.1038/leu.2012.186. [DOI] [PubMed] [Google Scholar]
- 206.Seymour J.F., Kim D.W., Rubin E., Haregewoin A., Clark J., Watson P., Hughes T., Dufva I., Jimenez J.L., Mahon F.X., et al. A phase 2 study of MK-0457 in patients with BCR-ABL T315I mutant chronic myelogenous leukemia and philadelphia chromosome-positive acute lymphoblastic leukemia. Blood Cancer J. 2014;4:e238. doi: 10.1038/bcj.2014.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Giustacchini A., Thongjuea S., Barkas N., Woll P.S., Povinelli B.J., Booth C.A.G., Sopp P., Norfo R., Rodriguez-Meira A., Ashley N., et al. Single-cell transcriptomics uncovers distinct molecular signatures of stem cells in chronic myeloid leukemia. Nat. Med. 2017;23:692–702. doi: 10.1038/nm.4336. [DOI] [PubMed] [Google Scholar]
- 208.Kizilors A., Crisa E., Lea N., Passera R., Mian S., Anwar J., Best S., Nicolini F.E., Ireland R., Aldouri M., et al. Effect of low-level BCR-ABL1 kinase domain mutations identified by next-generation sequencing in patients with chronic myeloid leukaemia: A population-based study. Lancet Haematol. 2019;6:e276–e284. doi: 10.1016/S2352-3026(19)30027-4. [DOI] [PubMed] [Google Scholar]
- 209.Soverini S., Mancini M., Bavaro L., Cavo M., Martinelli G. Chronic myeloid leukemia: The paradigm of targeting oncogenic tyrosine kinase signaling and counteracting resistance for successful cancer therapy. Mol. Cancer. 2018;17:49. doi: 10.1186/s12943-018-0780-6. [DOI] [PMC free article] [PubMed] [Google Scholar]